Modeling open surgery in mice to explore peritoneal damage, carbon dioxide humidification and desmoidogenesis
-
Timothy Chittleborough
Abstract
Background
The exposure of the peritoneum to desiccation during surgery generates lasting damage to the mesothelial lining which impacts inflammation and tissue repair. We have previously explored open abdominal surgery in mice subjected to passive airflow however, operating theatres employ active airflow. Therefore, we sought an engineering solution to recapitulate the active airflow in mice. Similarly, to the passive airflow studies we investigated the influence of humidified-warm carbon dioxide (CO2) on this damage in the context of active airflow. Additionally, we addressed the controversial role of surgery in exacerbating desmoidogenesis in a mouse model of familial adenomatous polyposis.
Methods
An active airflow mouse-operating module manufactured to produce the equivalent downdraft airflow to that of a modern operating theatre was employed. We quantified mesothelial cell integrity by scanning electron microscopy (SEM) sampled from the peritoneal wall that was subjected to mechanical damage or not, with and without the delivery of humidified-warm CO2. To explore the role of open and laparoscopic surgery in the process of desmoidogenesis we crossed Apcmin/+ C57Bl/6 mice with p53+/− mice to generate animals that developed desmoid tumors with 100% penetrance.
Results
One hour of active airflow generates substantial damage to peritoneal mesothelial cells and their microvilli as measured at 24 h post intervention, which is significantly greater than that generated by passive airflow. Use of humidified-warm CO2 mostly protects the mesothelium that had not experienced additional mechanical (surgical) damage at 24 h. Maximal damage was evident in all treatment groups regardless of flow or use of gas. At day 10 mechanically-damaged peritoneum remains in mice but is essentially repaired in the gas-treated groups. Regarding desmoidogenesis, operating procedures did not increase the frequency of desmoid tumors but their frequency correlated with time following surgery but not age of mice.
Conclusions
Active airflow generates more peritoneal damage than passive airflow and is reduced significantly by the use of humidified-warm CO2. Introduced peritoneal damage is largely repaired in mice by day 10 with gas. Desmoid tumor incidence is not increased substantially by surgery itself but rises over time following surgery compared to non-surgery mice.
Introduction
Surgery employing laparotomy involves exposure of the abdomen and its associated visceral and peritoneal tissue surfaces to the risk of surface desiccation. This exposure is not passive as the modern operating theatre employs active filtered air exchange of approximately 20 room volumes per hour [1] leading to desiccation [2] and heat loss [3]. The visceral and parietal peritoneal surfaces are covered by an integral layer of mesothelial cells augmented by a lawn of apical microvilli [4]. By using mouse models, such desiccation is readily evident and measureable by documenting changes to the cellular architecture [5]. Such damage to the peritoneum exacerbated the propensity of cancer cells to implant with the potential to initiate peritoneal carcinomatosis [6].
In an attempt to address and to minimize open surgery-associated peritoneal damage, we have explored the use of humidified-warm carbon dioxide (HWCO2) delivered via a bespoke mini gas perfusion device. In this passive airflow model, we reported protection of the peritoneal surfaces by using HWCO2 [5], similar to that employed in human open surgery [7]. Here, we advanced our investigation of peritoneal damage by engineering a surgery theatre set-up designed for mice whereby airflow was instead delivered by active airflow. The consequence of greater airflow was increased peritoneal damage compared to passive airflow yet this exacerbated damage was minimized by co-delivery of HWCO2.
Previously, we investigated the impact of peritoneal damage on tumor cell implantation where exogenous cancer cells were introduced into the peritoneal cavity [5, 6]. To examine a different aspect of surgery impact on tumorigenesis here we used a genetic model (Apcmin/+:p53KO) predisposed to endogenous abdominal wall desmoid tumors, desmoidogenesis [8, 9]. This model was of interest because abdominal surgery significantly increases the risk of development of this neoplasm in patients with familial adenomatous polyposis (FAP) [10, 11, 12, 13, 14]. Furthermore, desmoid tumors cause serious morbidity and mortality in patients with FAP, particularly following prophylactic colectomy [10, 14]. Accordingly, we used four approaches: open and laparoscopic interventions, with or without HWCO2 to address the consequences of these different surgical methods on desmoidogenesis.
Materials and methods
Mouse surgery studies
Female BALB/c mice aged 7–12 weeks old, weighing 17–25 g were used according to the Institutional Animal Ethics Committee approval and the National Health and Medical Research Council of Australia as previously described [5]. Mice were purchased from the ARC (Perth, Australia) and housed in the PMCC facility with a 12 h light-dark cycle allowing ad libitum access to food and water. The delivery of HWCO2 was via a HumiGard (Fisher and Paykel Healthcare, New Zealand) recalibrated to produce a pressure of 2 mmHg appropriate to mice (Figure 1(a)).

Mouse surgery set up, engineered devices and effect of humidified-warm CO2.
Normal BALB/c mice were anesthetized, intubated and placed on a warming pad as for insufflated mice used in previous laparoscopic studies [6] with or without a miniaturized gas diffuser designed for open surgery studies (Figure 1(b)). For these laparotomy investigations, mice were randomized to two groups: (i) open surgery with exposure to active airflow and (ii) open surgery with active airflow plus H-WCO2 diffusion using a purpose engineered operating suite (Figure 1(c)). Replicating surgical trauma on the peritoneal abdominal wall has been described [5]. Briefly, peritoneal wall damage was simulated using sterile plastic serrated forceps on one side of the peritoneum. This was performed by a sole investigator whereby the mini gas diffuser was removed and the sterile plastic forceps used to abrade the mesothelial layer with five strokes to the right-sided wall at the 30 min time point standardized to the middle of the peritoneum approximately 1 cm from the abdominal incision. Peritoneal wall from the left side was used as a non-surgical intervention control. C57BL/6 mice (see further details below) were similarly managed to above but were ventilated with a nose cone (instead of intubated) due to the small size of the Apcmin/+:p53+/−mice.
Equipment
To establish operating room conditions that accurately replicate that employed in the operating theatre a custom miniature laminar flow system was developed. Laminar flow was created with a perforated ceiling and a computer-cooling fan mounted directly above the mouse operating space. Flow velocity tested with a hot wire anemometer and calibrated to be 0.5 m/sec in accordance with the DIN standard 1946 for hospital ventilation. Fisher and Paykel Healthcare engineered an open-sided box constructed from Perspex with the aim of positioning the fan over the anesthetized mouse. Thus, the “mouse” operating suite recapitulated the environment used for patients (Figure 1(c)). Mouse vital signs were monitored as previously described as well as tissue analyses [5, 6]. Briefly, for perfusion indices presented in this study these were determined using an inbuilt pulse oximeter and SpO2 sensor (PhysioSuite™; Kent Scientific, Torrington, CT, USA) which analyzes the rate of change between oxygenated hemoglobin and reduced hemoglobin as arterial oxygen saturation expressed as a percentage.
Desmoidogenesis studies
A model of desmoidogenesis was developed by crossing of ApcMin/+ mice [15, 16] with p53+/− mice on a C57Bl/6 background based upon a similar model using a different Apc mutation reported previously [9]. Due to breeding constraints due to reduced viability of female p53−/− mice [17] only male mice were evaluated. Genotyping by polymerase chain reactions for the p53 exon deletion used the following primers: p53x6s 5ʹ-TTATGAGCCACCCGAGGT-3ʹ; p53x7as 5ʹ-TATACTCAGAGCCGGCCT-3ʹ; neo18.5 5ʹ-TCCTCGTGCTTTACGGTATC-3ʹ, reaction ratio 1:1:2 to generate product of 600 bp (KO) and 450 bp (WT), respectively. For the Apcmin mutation detection PCRs employed the following primers: MAPCMT 5ʹ-TGAGAAAGACAGAAGTTA-3ʹ; MAPC15 5ʹ-TTCCACTTTGGCATAAGG-3ʹ; MAPC9 5ʹ-GCCATCCCTTCACGTTAG-3ʹ to generate 600 bp (WT) and 300 bp (min allele) products, respectively. In these studies, no tissue was removed and the only intervention was either laparoscopy or laparotomy in order to determine the impact of surgical approach on subsequent desmoid risk. Desmoid burden was quantified macroscopically (number of lesions present) as well as microscopically at sites of surgical trauma (i. e. laparotomy wound).
Semi-quantitative analyses
Mice were culled at 24 h and 10 days. Scanning electron microscopy (SEM) was used to evaluate changes to morphology and alterations quantified using a customary scale adapted from the H-score method. Essentially, microvilli were evaluated per cell as normal, damaged or shortened and absent. The proportions were expressed as a percentage of the total. For mesothelial cells, integrity cells were scored as normal, retracted or bulging expressed as a percentage of the total [6].
Statistical analyses
Data are expressed as mean±standard error of the mean evaluated using GraphPad® Prism 6 (La Jolla, CA, USA) and analyzed using 1- or 2-way ANOVA with Tukey’s multiple comparisons test or two-tailed unpaired t-test. A p-value of less than 0.05 was considered statistically significant.
Results
Design of animal operating suite
Laminar flow ventilation is standard in current day operating rooms. Investigations have demonstrated the efficacy of the system to reduce the number of particles and bacteria in the operating room air. To achieve effective laminar flow, highly filtered air is blown with uniform velocity in single parallel flow lines over the target area. The air may be “changed” more than 300 times per hour at a flow velocity of 0.2–0.5 m/s. This continual flow is a relevant variable when recapitulating the operating environment in animal models of open abdominal surgery as it may impact the local temperature of the wound and the evaporative capacity of the operating environment has a desiccating effect on abdominal surfaces [5, 18].
To explore different modes of open surgery for testing the effects of HWCO2 on the peritoneum mice were subjected to intubation and anesthesia as previously described [6] with an option to deliver HWCO2 via a proprietary humidification device shown in Figure 1(a). A laparotomy model was developed where mice were exposed to ambient and passive airflow with and without provision of HWCO2 delivered via a gas diffusion device (Figure 1(b)) [5]. To recapitulate active airflow indicative of a modern operating theatre a Perspex® box structure was engineered to provide a constant down draft of ambient air to mice undergoing laparotomy (Figure 1(c)) with or without the gas diffusor device.
Temperature maintenance under active airflow
As all mice were the same strain (BALB/c) and balanced for gender the effects of various surgery modalities could be robustly compared within new, and against previously published data [5]. A rectal probe was employed to as a reliable method of recording core body temperature over time [19]. The current study focused on the active airflow surgery model finding that HWCO2 allows the maintenance of statistically higher stable core body temperature for the first 15 min for each 5-min interval from the beginning and overall for all time points of the procedure compared to active airflow alone (Figure 1(d)). However, the temperatures measured previously in mice undergoing laparoscopy [6] and laparotomy [5] were all higher by approximately 1 °C (37.5 °C) where HWCO2 was used. These data indicate that the active flow of air into an open abdomen is most effective at lowering core body temperature in mice.
Total body perfusion under active airflow
We monitored vital signs in all mice over the procedure and collected perfusion at the distant hind paw as previously described [5] to find that HWCO2 allowed for significantly increased perfusion compared to active airflow alone (Figure 1(e)). The degree of perfusion was reduced by approximately one-third when compared to passive flow data but comparable to the passive flow with additional HWCO2 [5].
Measuring peritoneal integrity after laparotomy under active airflow
At 24 h, post-surgery SEM micrographs reveal extensive loss of microvilli under active flow conditions (Figure 2(a)) while microvillus integrity was essentially comparable to baseline when HWCO2 was employed (Figure 2(b)). The quantitative measures of extent of damage are presented in Figure 2(c). Historical data from passive airflow work [5] have been included to emphasize the effects of active vs. passive flow. Although this previous study by definition was conducted at an earlier time the strain of mouse, age range, gender mix and experimental team were comparable. When mechanical damage was introduced to one side of the peritoneal wall and evaluated 24 h later all groups displayed maximal microvilli damage (Figure 2(d)).

Comparing peritoneal damage following laparotomy at 24 h post-treatment.
Figure 2(a) and 2(e) highlights the separation of cells from each other across the mesothelial cell sheet that typically lines the peritoneum. This effect was most pronounced in the active airflow group and the delaminating/retraction of cell was reduced when HWCO2 was used (Figure 2(f) and (g)). By contrast, the extent of mesothelial damage was pervasive in all groups when mechanical damage was assessed (Figure 2(h)).
Longer term measures of peritoneal integrity
By day 10, microvilli and mesothelial cell integrity is essentially normal in all groups of mice regardless of HWCO2 delivery (Figure 3(a) and (c)). However, as previously discovered the introduction of mechanical damage at the time of surgery has an enduring impact on microvilli restoration and mesothelial cell integrity (Figure 3(b) and 3(d)). By both cellular indicators, the use of HWCO2 appears to accelerate or perhaps allow tissue repair such that base line normal cellular SEM micrographs were observed at day 10. These data suggest that HWCO2 provides protection to both exposed and additionally mechanically damaged peritoneum over the 1 h of operating time.
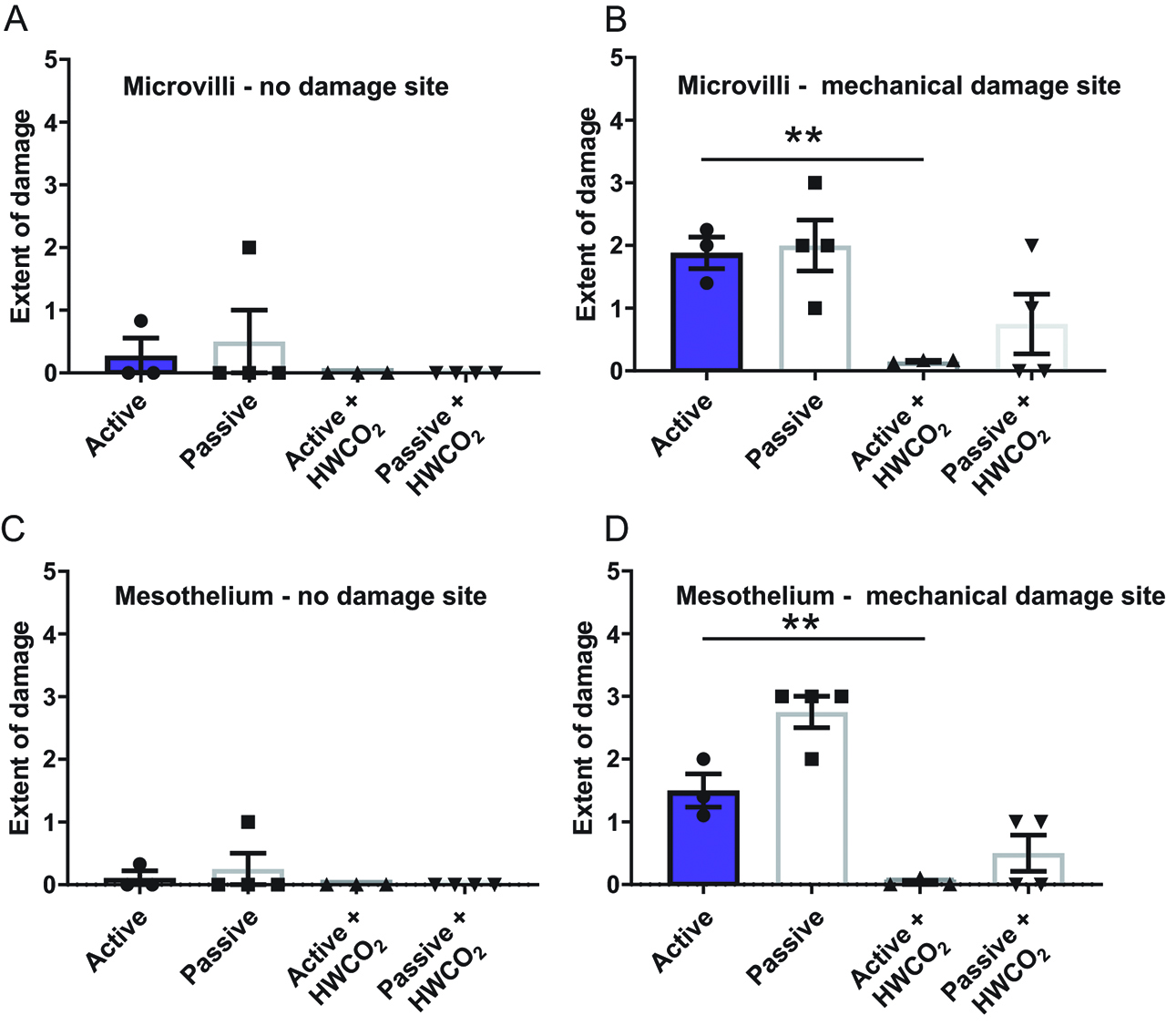
Comparing peritoneal damage following laparotomy at 10-day post-treatment.
Development of a desmoid tumor model in mice
To generate a model of desmoidogenesis ApcMin/:p53−/− mice were bred on a C57BL/6 background. The mouse model allowed one hundred percent penetrance of desmoid tumors. Mice were culled due to illness that most typically involved anemia associated with bloody stools or morbid behavior. This pathology is typical of ApcMin/+ mice that have developed intestinal adenomas which were subsequently confirmed by examination of the large and small intestines. Co-incident with adenoma formation were the development of desmoid tumors on the abdominal walls of ApcMin/:p53−/− (Figure 4(a)) but not ApcMin/:p53+/− or ApcMin/+ mice. Such tumors are highlighted by staining fresh specimens with Fast Green (Figure 4(b)) and were further characterized by scanning electron microscopy (Figure 4(c) and 4(d)). Clinical features of mice evaluated in this study are reported in Table 1.

Development of a mouse model for the study of the effects of surgery on desmoid tumorigenesis.
Characteristic of clinical features and desmoid tumor burden in Apcmin/+:p53−/− mice.
| Apcmin/+:p53−/− | Age at surgery | Operative findings | Reason for culling | Age at death | Days post-surgery at death | Desmoid tumor burden | Adenoma burden (small, large bowel) | Other findings |
|---|---|---|---|---|---|---|---|---|
| 1: Laparoscopy, dry-cold CO2 | 52 | N/A | Anemia | 76 | 24 | 5. | 0, 3 | Mesenteric mass – pancreatic acinar cell carcinoma |
| 57 | N/A | Anemia | 112 | 55 | 18 | 14, 1 | Mesenteric mass – lymphoproliferative | |
| 43 | N/A | Enlarging neck mass | 77 | 34 | 8 | 4, 3 | Enlarging neck mass ~1.5 cm in size – sarcomatous. Mesenteric mass – lymphoproliferative | |
| 2: Laparoscopy, humidified-warm CO2 | 53 | N/A | Anemia | 113 | 60 | 10 | 17, 3 | Intussusception. Lesion on hind leg muscle – peripheral desmoid |
| 42 | N/A | Anemia | 97 | 55 | 27 | 16, 4 | Mesenteric mass – lymphoproliferative | |
| 47 | N/A | Enlarging LIF mass | 103 | 56 | 19 | 11, 1 | 5 Retroperitoneal desmoids. LIF mass – angiosarcoma | |
| 3: Laparotomy, conventional | 56 | 10 desmoids | Prolapse | 72 | 16 | 10. | 3, 6 | Pancreatic nodule – pancreatic acinar cell carcinoma |
| 54 | 10 desmoids | Tachypnea | 99 | 45 | 14 | 19, 0 | Grossly enlarged thymus – lymphoma; 2 retroperitoneal desmoids | |
| 46 | 15 desmoids | Anemia | 109 | 63 | 14 | 12, 3 | Mesenteric mass – lymphoproliferative, multiple intussusceptions, 6 retroperitoneal desmoids | |
| 4: Laparotomy, humidified-warm CO2 | 48 | 13 desmoids | Anemia | 96 | 48 | 12 | 19, 2 | 3 retroperitoneal desmoids. Mesenteric mass – lymphoproliferative |
| 47 | 7 desmoids | Anemia | 85 | 38 | 9 | 16, 2 | Mesenteric mass – lymphoproliferative | |
| 44 | 9 desmoids | Anemia | 105 | 61 | 19 | 11, 4 | Grossly enlarged thymus – lymphoma |
ApcMin/+:p53−/− mice had a slightly reduced time to development of disease compared to ApcMin/+ mice while the p53+/− genotype afforded extended disease-free survival (Figure 4(e)) and more rapidly than expected for mice on a p53+/− or p53−/− background alone [17]. The small and large intestinal adenomas were observed in all mice with the mutant Apc allele. However, only p53−/− genotypes had the additional burden of desmoid tumors (Figure 4(f)). Of note only p53−/− male mice were investigated in these studies due to the general low yield of p53−/− female mice. This sex bias presents a limitation of this pre-clinical study because desmoid tumors are twice as frequent in females vs. males [20].
Effect of surgery approach on desmoid tumor formation
To evaluate the effect of laparoscopic and laparotomy surgery on the degree of desmoid formation in ApcMin/+:p53−/− mice animals were culled when declared by an independent animal technician blinded to the genotype and surgery approach. Similar tumor burdens were found in the no-intervention and surgery groups (Figure 4(f)).
The humidified-warm (HW) and dry-cold (DC) CO2 groups were pooled according to surgery approach whereby there were no significant relationship between desmoid tumor burden and age at cull (Figure 5(a)). However, when the desmoid tumors were evaluated in terms of days post-surgery there was a trend toward an increased burden in relation to time following surgery. By contrast, adenoma burden in ApcMin/+:p53−/− mice increased with age as expected as well as with time from surgery (Supplemental Figure 1). There was no significant relationship between adenoma tumor burden, CO2 type or surgery modality although there was perhaps a trend toward more adenomas in mice that received HWCO2 but these mice were generally older at the time of cull consistent with an increase in adenomatogenesis with age in the Apcmin/+ model. When all genotypes were considered collectively vs. HWCO2 and either DCCO2 or passive airflow there was no significant differences in time to cull (data not shown).

Desmoid tumor burden increases with time following surgery but not age.
Discussion
To explore whether there might be a role for HWCO2 in peritoneal tissue protection we have developed a series of mouse surgery platforms that cover laparoscopic [6] and open surgery [5]. Mice are not perfect for the modeling of human pathology but the ultrastructure, cell biology and gene expression profiles of mouse and human peritoneal tissues are very similar [21]. However, individual mouse to mouse, particularly on a strain-by-strain basis, are genetically and phenotypically uniform allowing for studies with tight experimental design.
Our previous open surgery studies employed passive airflow where HWCO2 led to measurable and significant tissue protection of the peritoneum [5]. Here we added the sophistication of active airflow to model the effects of the active airflow indicative of the human operating suite. This change to active airflow increased the degree of damage compared to passive airflow. Similarly, HWCO2 delivered into the abdominal cavity during open surgery allowed maintenance of normothermia and oxygen perfusion in mice over the 1-h period of the study.
At 24 h post-surgery active airflow by all measures showed increased tissue damage at the cellular and subcellular level well above base line. This was most notably when microvilli integrity was evaluated. HWCO2 use reduced this damage as well as that observed when assessing mesothelial cell connectivity.
Parallel studies at 24 h post introduced mechanical peritoneal wall damage highlighted the persistence of this tissue insult that was beyond the ability of HWCO2 to ameliorate. By day 10, the effects of open surgery on peritoneal cellular architecture are no longer distinguishable from non-surgery mice but this was not the case where mechanical damage was introduced. As we observed previously HWCO2 during the 1 h of open-surgery led to substantial restoration of peritoneal integrity. These data replicate our previous experience where active airflow was used [5]. The mechanism(s) that are at work to accelerate repair or reduce the impact of damage post-surgery time remain unresolved. One possibility we have considered is that the increased perfusion of gases in the whole animal may influence this tissue repair. This is an area of ongoing research. Nevertheless, these comparative studies reinforce the observation that HWCO2 both reduces immediate tissue damage associated with desiccation as well as positively influencing tissue repair in the case were damage is actively introduced.
Having established a robust and reproducible platform for exploring the effects of different surgery modalities, we then addressed an outstanding surgery question in the context of desmoidogenesis. The unresolved issue of the role of abdominal surgical trauma associated with prophylactic colectomy as It relates to subsequent desmoidogenesis in familial adenomatous polyposis (FAP), is difficult to model and the effects of different surgery, complex to measure. Here, we developed a mouse model, which recapitulates that of FAP patients who are predisposed to desmoid tumors. Desmoidogenesis had been explored previously using a different Apc mutation (Apc1638N) that is associated with a longer latency to illness that ApcMin/+ mice and where the crossing with p53 KO mice further accelerated tumor formation and particularly burden [9].
There is an established risk of desmoids in FAP [11]. Conversely, there is growing evidence that laparoscopic surgery is less prone to desmoidogenesis in FAP patients [12, 14]. As we explored multiple surgery approaches in mice predisposed to desmoids the sample size in each cohort of mice was small. Nevertheless, the mouse model we employed allowed the investigation of surgical intervention (open and laparoscopic) and desmoidogenesis whereby as in patients with FAP there was a significant positive correlation between time from surgery and increased desmoid tumor burden.
In conclusion, the use of mice provides an opportunity to explore the effects of different modes of surgery along with the role of different kinds of CO2. With regard to peritoneal damage, open surgery under conditions that recapitulate the airflow indicative of a modern operating theatre generates substantive damage. HWCO2 perfused into the abdomen of mice under such operating conditions significantly reduced tissue damage. Perhaps more interesting, HWCO2 use at the time of additional, focused and simulated surgical-induced damage did not affect the burden of damage 1 day later but had a substantive effect at day 10. The mechanism that underpins this effect is unclear.
The development of an operating platform, which recapitulates that used for patients, allowed the investigation of an alleged surgery-associated tumor model that is desmoidogenesis. The association was replicated and no association with CO2 type was evident nor did HWCO2 provide any substantive protection. Overall, these model platforms for investigating surgery modalities under tightly controlled conditions using genetically defined and well mice inform the design of studies in larger mammals and patients.
Acknowledgments
We thank the animal facilities staff for their professional assistance in managing our animals.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This work was supported by the National Health and Medical Research Council (Funder Id: http://dx.doi.org/10.13039/501100000925, Grant Number: APP1140147). Fisher and Paykel Healthcare (FPHC) provided the equipment that was essential for this study and the engineering of the mouse-operating module. RGR, TC and ACL have had travel support in the past from FPHC.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect 2002;51:79–84.10.1053/jhin.2002.1217Search in Google Scholar PubMed
2. Persson M, van der Linden J. Intraoperative field flooding with warm humidified CO2 may help to prevent adhesion formation after open surgery. Med Hypotheses 2009;73:521–3.10.1016/j.mehy.2009.06.009Search in Google Scholar PubMed
3. Kaudasch G, Schempp P, Skierski P, Turner E. The effect of convection warming during abdominal surgery on the early postoperative heat balance. Anaesthesist 1996;45:1075–81.10.1007/s001010050342Search in Google Scholar PubMed
4. Lindic J, Psenicnik M, Bren A, Gucek A, Ferluga D, Kveder R. The morphology of parietal peritoneum: a scanning electron micrograph study. Adv Perit Dial 1993;9:36–8.Search in Google Scholar PubMed
5. Carpinteri S, Sampurno S, Malaterre J, Millen R, Dean M, Kong J, et al. Experimental study of delivery of humidified-warm carbon dioxide during open abdominal surgery. Br J Surg 2018;105:597–605.10.1002/bjs.10685Search in Google Scholar PubMed
6. Carpinteri S, Sampurno S, Bernardi MP, Germann M, Malaterre J, Heriot A, et al. Peritoneal tumorigenesis and inflammation are ameliorated by humidified-warm carbon dioxide insufflation in the mouse. Ann Surg Oncol 2015;22:1540–7.10.1245/s10434-015-4508-1Search in Google Scholar
7. Frey J, Holm M, Janson M, Egenvall M, van der Linden J. Relation of intraoperative temperature to postoperative mortality in open colon surgery-an analysis of two randomized controlled trials. Int J Colorectal Dis 2016;31:519–24.10.1007/s00384-015-2467-4Search in Google Scholar PubMed
8. Halberg RB, Chen X, Amos-Landgraf JM, White A, Rasmussen K, Clipson L, et al. The pleiotropic phenotype of Apc mutations in the mouse: allele specificity and effects of the genetic background. Genetics 2008;180:601–9.10.1534/genetics.108.091967Search in Google Scholar PubMed
9. Smits R, van der Houven van Oordt W, Luz A, Zurcher C, Jagmohan-Changur S, Breukel C, et al. Apc1638N: a mouse model for familial adenomatous polyposis-associated desmoid tumors and cutaneous cysts. Gastroenterology 1998;114:275–83.10.1016/S0016-5085(98)70478-0Search in Google Scholar PubMed
10. Arvanitis ML, Jagelman DG, Fazio VW, Lavery IC, McGannon E. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum 1990;33:639–42.10.1007/BF02150736Search in Google Scholar PubMed
11. Saito Y, Hinoi T, Ueno H, Kobayashi H, Konishi T, Ishida F, et al. Risk factors for the development of desmoid tumor after colectomy in patients with familial adenomatous polyposis: multicenter retrospective cohort study in Japan. Ann Surg Oncol 2016;23:559–65.10.1245/s10434-016-5380-3Search in Google Scholar PubMed
12. Sinha A, Burns EM, Latchford A, Clark SK. Risk of desmoid formation after laparoscopic versus open colectomy and ileorectal anastomosis for familial adenomatous polyposis. BJS Open 2018;2:452–5.10.1002/bjs5.90Search in Google Scholar PubMed PubMed Central
13. Ueno H, Kobayashi H, Konishi T, Ishida F, Yamaguchi T, Hinoi T, et al. Prevalence of laparoscopic surgical treatment and its clinical outcomes in patients with familial adenomatous polyposis in Japan. Int J Clin Oncol 2016;21:713–22.10.1007/s10147-016-0953-5Search in Google Scholar PubMed
14. Vitellaro M, Sala P, Signoroni S, Radice P, Fortuzzi S, Civelli EM, et al. Risk of desmoid tumours after open and laparoscopic colectomy in patients with familial adenomatous polyposis. Br J Surg 2014;101:558–65.10.1002/bjs.9411Search in Google Scholar PubMed
15. Moser AR, Dove WF, Roth KA, Gordon JI. The Min (multiple intestinal neoplasia) mutation: its effect on gut epithelial cell differentiation and interaction with a modifier system. J Cell Biol 1992;116:1517–26.10.1083/jcb.116.6.1517Search in Google Scholar PubMed PubMed Central
16. Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 1992;256:668–70.10.1126/science.1350108Search in Google Scholar PubMed
17. Donehower LA. The p53-deficient mouse: a model for basic and applied cancer studies. Semin Cancer Biol 1996;7:269–78.10.1006/scbi.1996.0035Search in Google Scholar PubMed
18. Marshall JK, Lindner P, Tait N, Maddocks T, Riepsamen A, van der Linden J. Intra-operative tissue oxygen tension is increased by local insufflation of humidified-warm CO2 during open abdominal surgery in a rat model. PLoS One 2015;10:e0122838.10.1371/journal.pone.0122838Search in Google Scholar PubMed PubMed Central
19. Meyer CW, Ootsuka Y, Romanovsky AA. Body temperature measurements for metabolic phenotyping in mice. Front Physiol 2017;8:520.10.3389/fphys.2017.00520Search in Google Scholar PubMed PubMed Central
20. Bonvalot S, Desai A, Coppola S, Le Pechoux C, Terrier P, Domont J, et al. The treatment of desmoid tumors: a stepwise clinical approach. Ann Oncol 2012;23:x158–66.10.1093/annonc/mds298Search in Google Scholar PubMed
21. Mutsaers SE, Wilkosz S. Structure and function of mesothelial cells. Cancer Treat Res 2007;134:1–19.10.1007/978-0-387-48993-3_1Search in Google Scholar PubMed
Supplementary Material
The online version of this article offers supplementary material (DOI:https://doi.org/10.1515/pp-2019-0023).
© 2019 Chittleborough et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Research Articles
- CRS and HIPEC in patients with peritoneal metastasis secondary to colorectal cancer: The small-bowel PCI score as a predictor of survival
- Perceptions in the management of colorectal peritoneal metastases: A bi-national survey of colorectal surgeons
- Systemic complications of the bidirectional intraoperative chemotherapy with intravenous ifosfamide and hyperthermic intraperitoneal chemotherapy (HIPEC) using cisplatin plus doxorubicin
- Impact of extent of parietal peritonectomy on oncological outcome after cytoreductive surgery and HIPEC
- Modeling open surgery in mice to explore peritoneal damage, carbon dioxide humidification and desmoidogenesis
Articles in the same Issue
- Research Articles
- CRS and HIPEC in patients with peritoneal metastasis secondary to colorectal cancer: The small-bowel PCI score as a predictor of survival
- Perceptions in the management of colorectal peritoneal metastases: A bi-national survey of colorectal surgeons
- Systemic complications of the bidirectional intraoperative chemotherapy with intravenous ifosfamide and hyperthermic intraperitoneal chemotherapy (HIPEC) using cisplatin plus doxorubicin
- Impact of extent of parietal peritonectomy on oncological outcome after cytoreductive surgery and HIPEC
- Modeling open surgery in mice to explore peritoneal damage, carbon dioxide humidification and desmoidogenesis

