Physical properties of ternary chloro-perovskites KTCl3 (T = Ge, Al) for optoelectronic applications
-
Muhammad Tahir
, Mohamed Hussien
Abstract
This study uses density functional theory to investigate various properties of ternary chloro-perovskites, KTCl3 (T = Ge or Al). Both compounds have a cubic structure with lattice parameters of 5.159 Å for KAlCl3 and 5.378 Å for KGeCl3. KGeCl3 has a direct band gap of 1.88 eV, while KAlCl3 exhibits metallic behavior due to aluminum states crossing the energy level, indicating the material’s ability to conduct electricity. Analysis of the electronic structure shows that the electronic properties of KGeCl3 are primarily influenced by the interactions of germanium and chlorine orbitals, while those of KAlCl3 are dominated by aluminum orbitals. Optical properties reveal that these materials exhibit strong absorption and high reflectivity in the ultraviolet and visible light range. These properties suggest potential use in optoelectronic applications. Their mechanical stability is supported by elastic constant analysis, which shows their ductile nature and anisotropic behavior, as indicated by the bulk modulus, shear modulus, Young’s modulus, and Poisson’s ratio. Pugh’s ratio values of 2.2711 and 2.5035 for KAlCl3 and KGeCl3, respectively, confirm ductility, while Poisson’s ratio suggests a dominant ionic bonding character. These findings provide significant information on the material’s potential applications.
1 Introduction
Perovskites ABX3, in which X is an anion and A and B are two cations, can result from arranging atoms in a variety of ways. This is because most elements in the periodic table have the ability to exchange for ones in the A and B locations. Six X anions coordinate with the B-site cations, which are located in the center of the octahedron in a unit cell, while A cations are located in the upper corner of the cube [1,2]. Researchers have conducted a significant amount of research on the ABX3 perovskites due to their enormous number of structural families with specific properties, which have allowed them to find substantial roles in various potential applications [3,4]. Recently, halide perovskite from I–IV–VII materials has shown amazing results for their expected applications as a photocatalytic cell absorber, as insulating materials, and semiconducting behavior. Cheng et al. investigated a new variety of all-solid-state and inorganic photocatalytic cell system that comprises the P-type direct band gap semiconducting CsSnI3 with n-type TiO2 with N719 dye, exhibiting a variant ratio of up to 11.3%. Similarly, Lee et al. investigated an inexpensive solution based on solar cells, which depends on an extremely crystalline absorbing material (CH3NH3PbI2Cl) with high-frequency light or equal to infrared absorptivity, which has a more power efficiency of 11.9% in a single cell device in full sunlight [5]. Recently, Bursschka et al. prepared solar cells based on CH3NH3PbI3/TiO3 with a power conversion efficiency of up to 15% and high stability gain. Daniel et al. studied LiBaF3 and reported that such types of compounds are good for energy storing devices. All the newly conducted experiments showed that such types of materials are important for solar cells as absorbing materials. Yet, theoretical reports on this type of material were quite incomplete. Borriello et al. reported the structural and electronic properties of tin-based ABX3 compounds. Murtaza and Ahmad studied the structural and optoelectronic properties of cubic perovskites CsPbX3 (X = Cl, Br, I) [6]. Cheng et al. also studied the electronic and structural properties of lead-based ABX3 compounds. Recently, Mosconi et al. studied the different aspects of CH3NH3PbX3 and mixed halide perovskites. Ternary halides have been the perovskite crystal of interest for some time now because of their expanding applications in the electrical, magnetic, and superionic domains [7,8]. It has recently been discovered that CsPbI3 and CsPbBr3 metal halides exhibit excellent photo, thermal, and moisture-resistant properties. Long-term stability of the perovskite structural alloy MAPbBr3 was obtained by Kulbak et al. by substituting the organic MA+ cation with Cs to generate CsPbBr3. In recent years, significant advancements have been made in the study of chloro-perovskites for optoelectronic applications. Recent studies have explored the structural, electronic, and optical properties of various chloro-perovskite compounds, highlighting their potential for high-efficiency solar cells, photodetectors, and other optoelectronic devices [9,10,11]. Our study aims to contribute to this growing body of research by investigating the properties of KTCl3 (T = Ge, Al) and comparing them with state-of-the-art materials to highlight their unique advantages and potential applications. In this study, we report a broad study of the structural, electronic, optical, and elastic properties of the cubic perovskites KTCl3 (T = Ge or Al) using the FP-LAPW approach based on density functional theory (DFT). We also report the optical properties of these materials, which are best for photocatalytic cells with a perfect absorber with good optical absorption [12,13].
2 Computational methodology
In this study, first-principles calculations using the FP-LAPW method are implemented in the WEIN2k code based on DFT. In this method, the augmented plane wave and the solution to the KohnSham equation are performed self-consistently, and a local orbital basis set is organized to show the electronic band structure for all atoms and their related orbitals. All the calculations were converged with respect to the size of the basis set and Brillouin zone (BZ) sampling [14,15]. A tetrahedron technique is used to determine the BZ integration within the self-consistency cycle performance. The correlation and exchange effects treated within the three potentials are the modified BeckeJohnson exchange potential (mBJ) and gradient approximation (GGA) based on the FP-LAPW method [16,17]. Many DFT scientists used this technique. The k-mesh points used in the BZ are 20 × 20 × 20 for a well-conserved self-consistency cycle. The energy cut-off is set to 10−5 Ry, as suggested previously [18]. The convergence parameter R MT K max, which controls the size of the basis sets, is set to 7, which indicates convergence, where K max is the plane wave cutoff and R MT is the smallest of all the atomic sphere radii. The G max parameter was selected to be 12 a.u−1.
3 Results and discussion
3.1 Structural properties
KTCl3 (T = Ge or Al) has a cubic perovskite structure described by the formula ABCl3, as displayed in Figure 1. Potassium (K) is at the A-site, while aluminum (Al) or germanium (Ge) is at the B-site. In both compounds, K is located at (0, 0, 0), and Cl is located at (0.5, 0.5, 0.5). Al and Ge share the same (0.5, 0.5, 0.5) position (Table 1). Both compounds have the same Pm3-m (221) space group, indicating a cubic unit cell arrangement [19,20,21,22]. The elemental configuration for atoms is K: (1s2, 2s2, 2p6, 3s2, 3p6, 3d1), Cl: (1s2, 2s2, 2p6, 3s2, 3p5), Al: (1s2, 2s2, 2p6, 3s2, 2p1), and Ge: (1s2, 2s2, 2p6, 3s2, 3p6, 3d10, 4s2, 4p2).
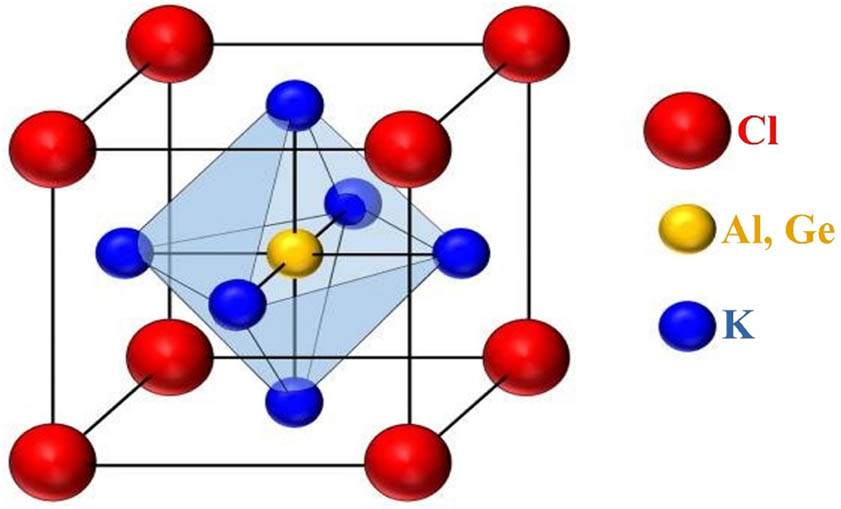
Crystal structure of KTCl3 (T = Ge or Al) perovskites.
Unit cell structural parameters of KTCl3 (T = Ge or Al)
| Atoms | Coordination number | Wyckoff position | Multiplicity | Symmetry | Coordinates |
|---|---|---|---|---|---|
| A (K) | 12 | a | 1 | m-3m | (0.0, 0.0, 0.0) |
| B (Al/Ge) | 6 | b | 1 | m-3m | (0.5, 0.5, 0.5) |
| X (Cl) | 2 | c | 4 | 4/m m.m | (0.5, 0.0, 0.5) |
| (0.5, 0.0, 0.5) | |||||
| (0.5, 0.0, 0.5) |
Table 2 shows the calculated properties used to assess the structural stability of KTCl3 (T = Ge or Al), such as lattice constant (a 0), bulk modulus (B), bulk modulus pressure derivative (B′), equilibrium volume (V 0), and ground-state energy (E 0). KGeCl3 has a larger lattice constant (a 0) of 5.378 Å, a smaller bulk modulus (B) of 27.3900 GPa, and an equilibrium volume (V 0) of 996.41 a.u.3 KAlCl3 has a smaller lattice constant (a 0) of 5.159 Å, a larger bulk modulus (B) of 31.5846 GPa, and an equilibrium volume (V 0) of 891.71 a.u.3, as shown in Table 2. The bulk modulus pressure derivative (B′) is the same for both compounds at 5.0000. The ground-state energy (E 0) is lower for KAlCl3 (−4459.40 Ry) compared to KGeCl3 (−8171.90 Ry) (Figure 2).
Data computed from optimized crystal unit cells of KTCl3 (T = Ge or Al) perovskites
| Crystals | Lattice constant (a 0) (Å) | Bulk modulus (B) (GPa) | Bulk modulus derivative (B′) | Equilibrium volume (V 0) (a.u.3) | Ground-state energy (E 0) (Ry) |
|---|---|---|---|---|---|
| KGeCl3 | 5.378 | 27.3900 | 5.0000 | 996.41 | −8171.90 |
| KAlCl3 | 5.159 | 31.5846 | 5.0000 | 891.71 | −4459.40 |
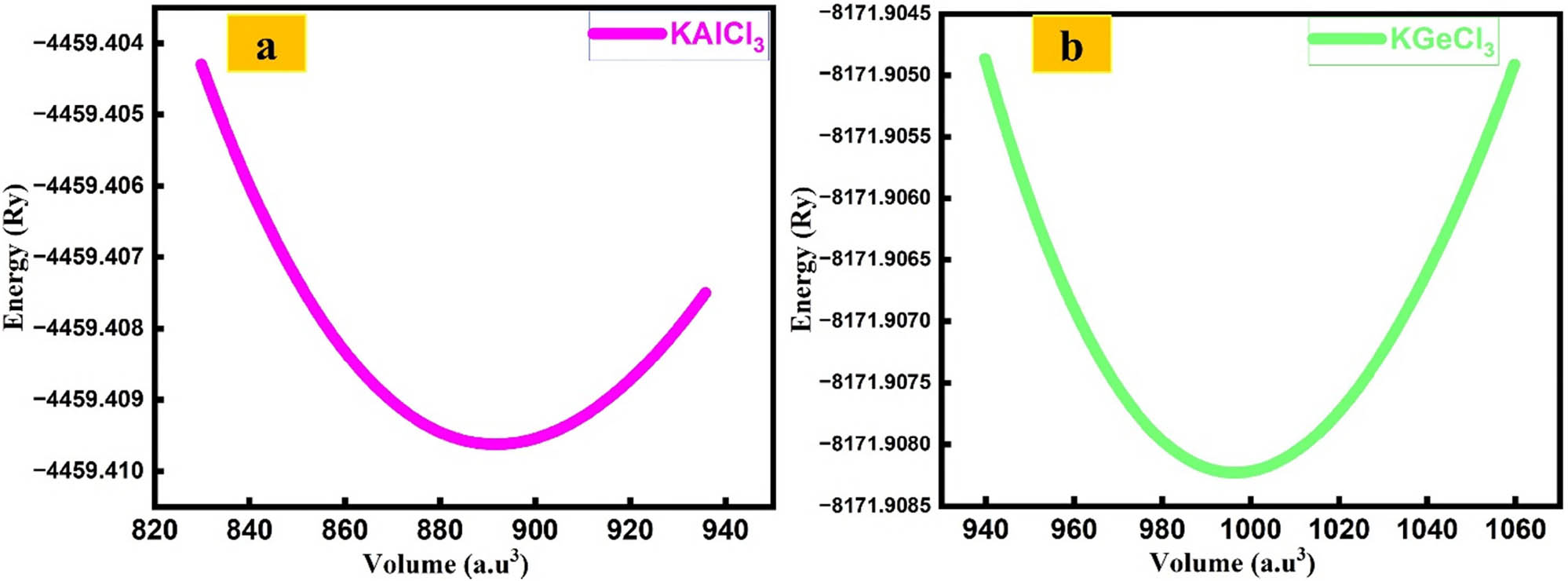
Optimization curve of KTCl3 (T = Ge or Al) perovskites. : (a) KAlCl3 and (b) KGeCl3.
The ability of a material to resist compression, known as its bulk modulus (B), is higher for KAlCl3 (31.5846 GPa) compared to KGeCl3 (27.3900 GPa). This indicates that KAlCl3 is mechanically more rigid and less prone to compression. Furthermore, the bulk modulus pressure derivative (B′) for both compounds is similar (5.0000), which aligns with typical values for chloro-perovskites. This similarity suggests that both compounds exhibit stability under low-pressure conditions [23,24].
The most stable electronic arrangement for KGeCl3 (ground-state energy: −8171.90 Ry) requires less energy than that of KAlCl3 (−4459.40 Ry). This difference indicates that KGeCl3 has a more stable electronic structure and is easier to form. The lower formation energy of KGeCl3 suggests that it is structurally less dense and more mechanically flexible than KAlCl3. In summary, KGeCl3 has a lower formation energy, making it a softer material, while KAlCl3 is structurally denser and more rigid. These differences affect their potential applications, with KGeCl3 being more suitable for electronic and optoelectronic uses due to its lower formation energy and better adaptability [25,26,27].
3.2 Electronic properties
The electronic properties of KTCl3 (T = Ge or Al) differ significantly. KGeCl3 has a direct band gap of 1.88 eV, meaning it can effectively convert light into electricity (Figure 3). This property makes it a good choice for optoelectronic devices like solar cells, photodetectors, and lights. The direct band gap allows for efficient movement of electrons between different energy levels, enhancing its ability to generate or emit light. The analysis of the density of available energy states (DOS) shows that potassium (K) slightly affects the valence band, with minor contributions from specific energy states, as shown in Figure 4. Germanium (Ge), on the other hand, significantly influences both the valence and conduction bands. Ge contributes to the valence band with one type of energy state, while it has a prominent contribution to the conduction band with a different type of state. This indicates Ge’s importance in the material’s ability to conduct charge. Chlorine (Cl) makes its presence felt primarily in the valence band, with two types of states dominating. In contrast, a different Cl state plays a significant role in the conduction band, highlighting its influence on electronic transitions. The substantial contributions from both Ge and Cl orbitals underscore KGeCl3’s effectiveness as a semiconductor material.
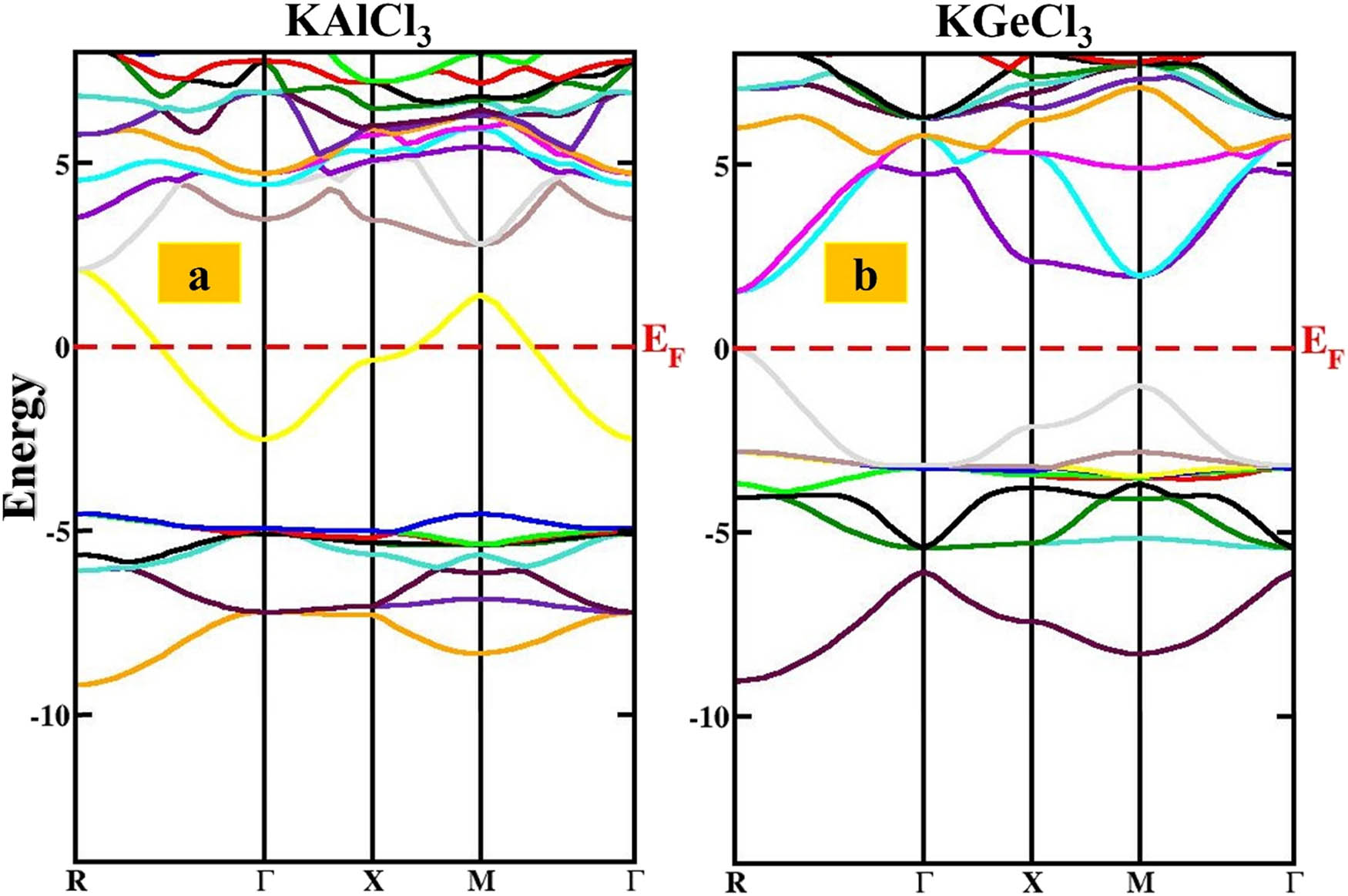
Electronic band structures of KTCl3 (T = Ge, Al) perovskites: (a) KAlCl3 and (b) KGeCl3.
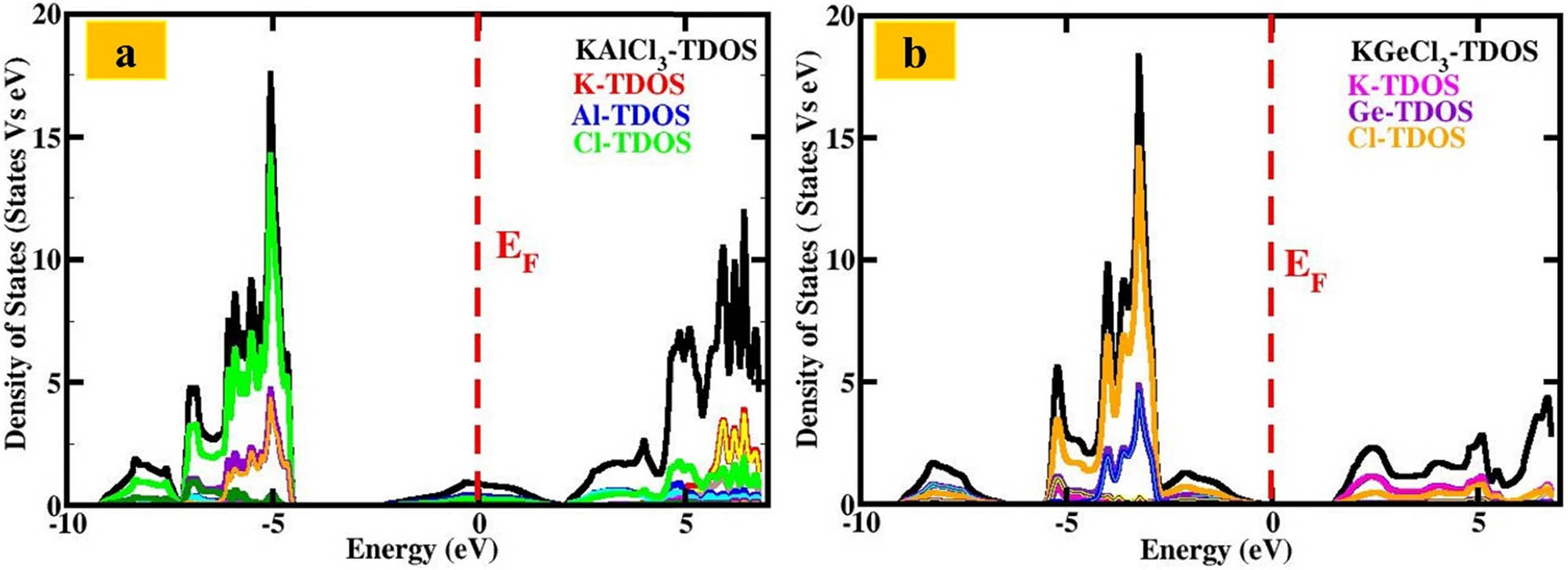
Total and partial density of states (DOS) versus energy for KTCl3 (T = Ge, Al) perovskites: (a) KAlCl3 and (b) KGeCl3.
In contrast, KAlCl3 has no band gap, making it an excellent electrical conductor (Figure 3). This property is ideal for electronics applications, such as interconnects and charge transport layers. The analysis of the density of states (DOS) reveals that potassium (K) contributes significantly to the valence band, primarily through the K (red) state, with additional contributions from K-d (yellow) and K-f (brown) states, as displayed in Figure 4. Aluminum (Al) plays a crucial role in conductivity, with its blue electronic state crossing the Fermi level. This direct connection between the valence and conduction bands forms a peak in the conduction band, confirming the metallic nature of KAlCl3. Additionally, the Al-p (cyan) state contributes to the metallic character. The element chlorine (Cl) plays a crucial role in the energy band structure of the material KAlCl3, influencing its electrical properties. Various Cl states contribute to the valence band, the energy range where electrons participate in electrical conduction. The Cl-green, Cl-p (violet), and Cl-d (green) states have a dominant presence in this band, while the Cl-f (orange) state is positioned slightly higher in energy. The presence of these chlorine states enhances the metallic characteristics of KAlCl3, giving it excellent electrical conductivity. As a result, KAlCl3 is well-suited for applications in conductive coatings, electrode materials, and electronics, where efficient charge transport is essential. KGeCl3’s semiconductor properties make it suitable for optics and solar power due to its ability to conduct electricity only under specific conditions. On the other hand, KAlCl3’s metallic properties make it a promising choice for coatings, circuits, and transporting electrical charges. KGeCl3’s Ge and Cl atoms enable electron movement, making it ideal for devices that convert light into electricity. In contrast, KAlCl3’s Al gives it high electrical conductivity, making it useful for electrical components and as a conductor. These properties make KTCl3 (T = Ge or Al) crucial for developing advanced electronics and optical technologies.
3.2.1 Optical properties
3.2.1.1 Real and imaginary parts of the dielectric function
The dielectric response of KTCl3 (T = Ge, Al) reveals their optical behavior, where ε 1 represents light refraction and ε 2 denotes absorption due to electronic transitions [28,29,30,31,32,33].
The optical response of potassium-based trichlorides, KAlCl3 and KGeCl3, is evaluated through their complex dielectric functions, offering insight into their suitability for optoelectronic applications. The real part of the dielectric function (ε 1) for KAlCl3 exhibits an initial value of 3.30 at 3.25 eV, decreases to 2.91 at 6.33 eV, and increases to 3.25 at 9.51 eV, indicating moderate dispersion and wavelength-dependent refractive behavior. In comparison, KGeCl3 demonstrates a significantly higher initial ε 1 of 4.92 at 2.06 eV, declining to 2.06 at 5.41 eV and increasing again to 3.34 at 9.82 eV, suggesting higher polarizability and enhanced refractive index attributes favorable for high-index optical components. The imaginary part of the dielectric function (ε 2), which describes photon absorption due to electronic transitions, further distinguishes the materials (Figure 5).
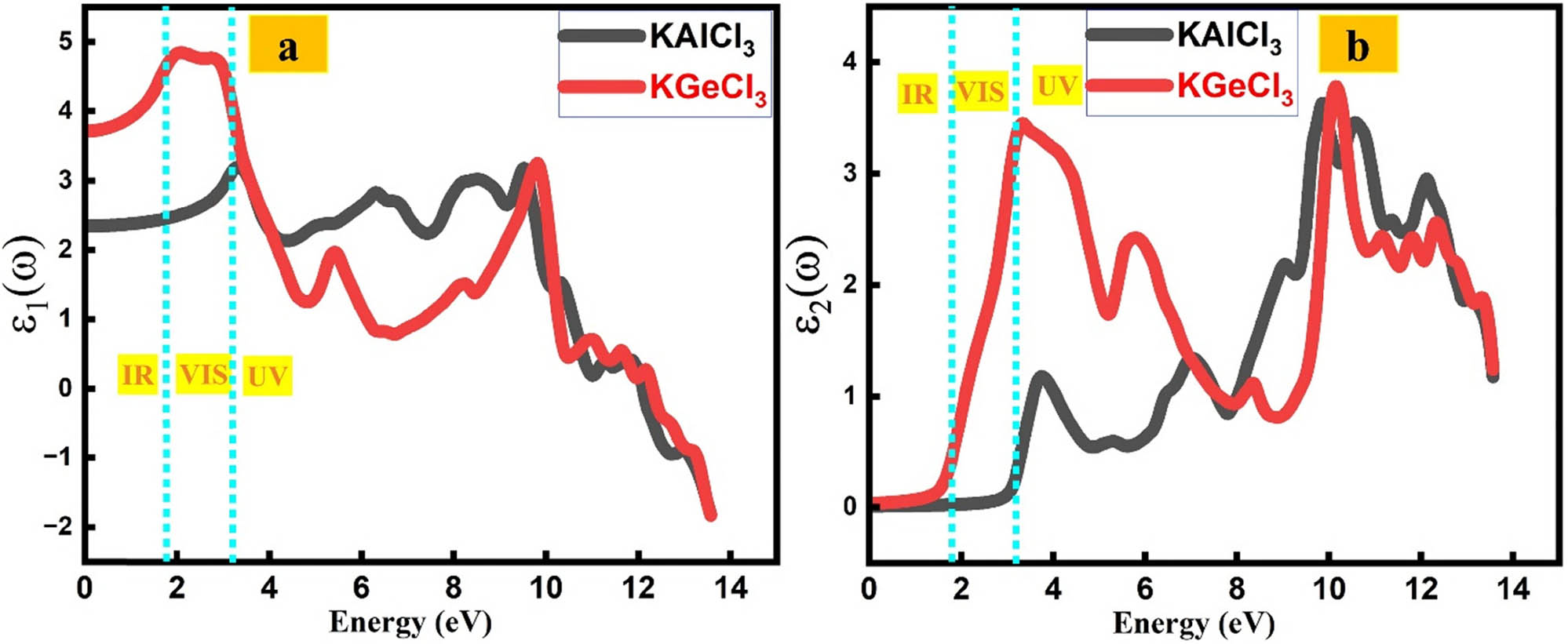
(a) Real part of the dielectric function of KTCl3 (T = Ge or Al) perovskites. (b) Imaginary part of the dielectric function of KTCl3 (T = Ge or Al) perovskites.
KAlCl3 shows moderate absorption at 3.75 eV (ε 2 = 1.23), a pronounced UV absorption at 9.84 eV (ε 2 = 3.68), and a weaker peak at 12.10 eV (ε 2 = 3.01), signifying its applicability in UV photonics and filtering. Conversely, KGeCl3 features a strong absorption peak in the near-UV region at 3.33 eV (ε 2 = 3.50), a dip at 5.75 eV (ε 2 = 2.48), followed by renewed absorption at 10.15 eV (ε 2 = 3.82), indicating robust interaction with a broader energy range. These findings highlight KGeCl3’s potential for tunable photonic devices and high-refractive-index optics, while KAlCl3 offers thermal and optical stability suitable for components such as capacitors, waveguides, and dielectric layers.
3.2.1.2 Optical conductivity and absorption coefficient
The electronic and optical behavior of KTCl3 (T = Ge, Al) is governed by their conductivity and light absorption characteristics [34,35,36].
KAlCl3 demonstrates a notable increase in electrical conductivity with increasing photon energy, starting from a modest 668 Ω−1 cm−1 at 3.74 eV and peaking at 5,007 Ω−1 cm−1 at 10.62 eV, as shown in Figure 6(a). This trend indicates strong photon-induced carrier excitation and enhanced charge transport under high-energy illumination. The conductivity remains elevated (4,898 Ω−1 cm−1 at 12.12 eV), suggesting its potential for integration into high-frequency optoelectronic devices, such as UV photodetectors and dielectric components in optical systems. In comparison, KGeCl3 exhibits higher initial conductivity (1,862 Ω−1 cm−1 at 4.33 eV) and peaks at 5,228 Ω−1 cm−1 at 10.20 eV, with a slight decrease to 4,300 Ω−1 cm−1 at 12.33 eV. These characteristics confirm its excellent charge mobility and suitability for high-speed and UV-range electronic applications.
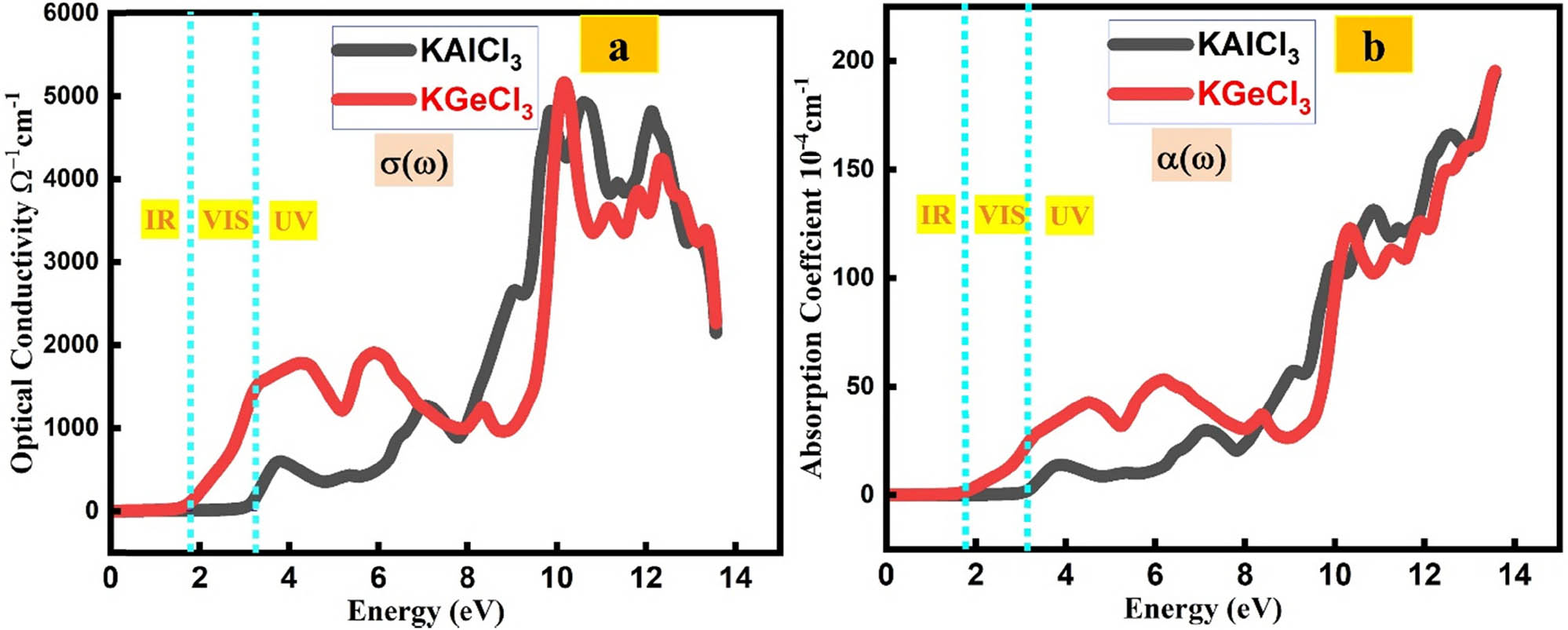
(a) Optical conductivity of KTCl3 (T = Ge or Al). (b) Absorption coefficient of KTCl3 (T = Ge or Al).
Optical absorption behavior, represented by the absorption coefficient in Figure 6(b), further distinguishes the two materials. KAlCl3 shows significant absorption in the near-UV region, with a peak value of 16.95 at 3.78 eV, making it suitable for optical coatings and UV-sensitive materials. However, its absorption decreases at higher energies (1.34 at 10.87 eV and 1.97 at 13.60 eV), limiting its performance in the deep-UV range. In contrast, KGeCl3 exhibits superior absorption across a broader energy spectrum, with values increasing from 45.81 (4.56 eV) to 125.60 (10.31 eV), reaching a maximum of 187.76 at 13.58 eV. This strong and sustained absorption indicates its potential for high-performance UV photodetectors, protective coatings, and solar-energy-harvesting technologies.
3.2.1.3 Reflection and extinction coefficients
KTCl3 (T = Ge, Al) shows promising photonic potential, as indicated by its reflection and extinction coefficients [28,37]. The extinction coefficient indicates how a material absorbs and scatters light, affecting transmission and energy loss in optical devices [38,39].
The optical reflectivity of KTCl3 (T = Al, Ge) exhibits strong energy dependence, as illustrated in Figure 7(a). For KAlCl3, reflectivity is relatively low (9.6%) at 3.56 eV, enhancing its transparency in the lower energy range. However, it increases significantly with photon energy, reaching 23% at 10.90 eV and peaking at 60% around 13.58 eV, indicating potential for energy-selective optical coatings and antireflective applications. In contrast, KGeCl3 demonstrates higher initial reflectivity (13.2% at 3.21 eV), with sustained moderate reflection across the spectrum and a sharp increase to 58% at 13.58 eV, making it suitable for high-reflectance mirrors and UV shielding in optoelectronic systems.
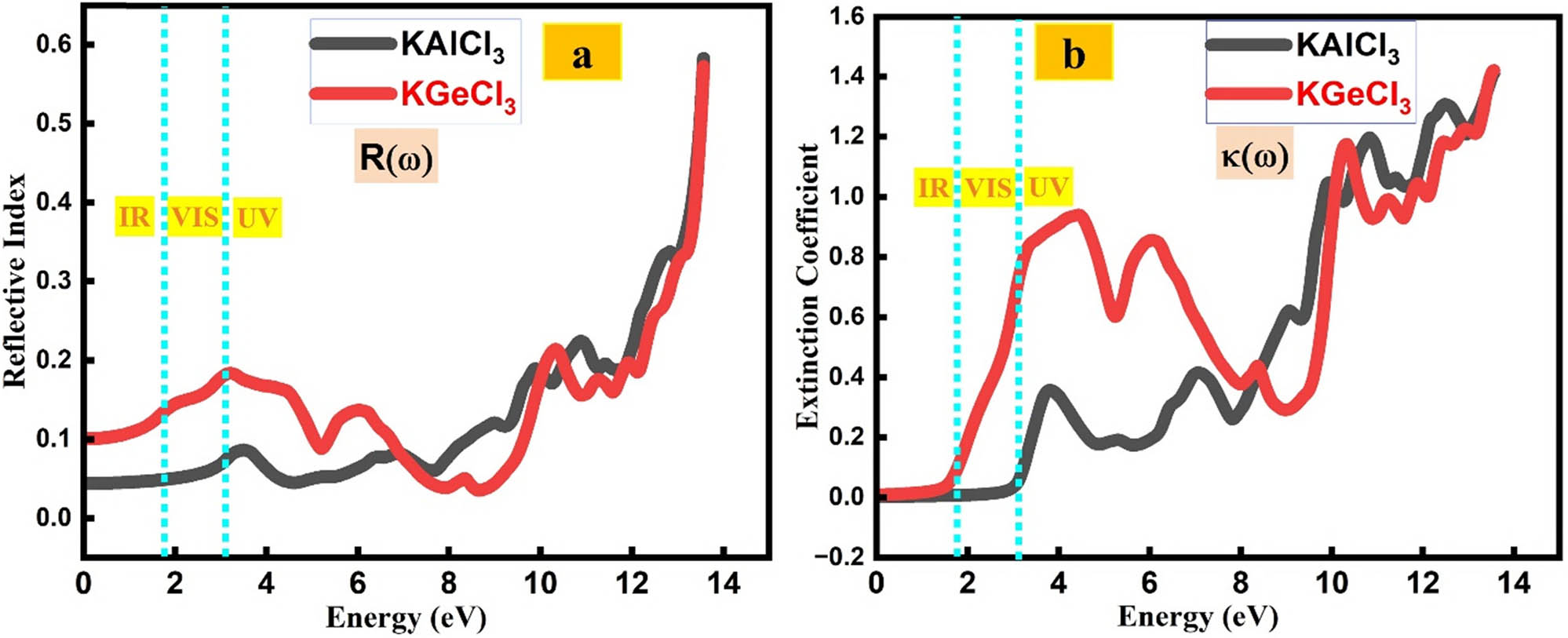
(a) Reflective index of KTCl3 (T = Ge or Al). (b) Extinction coefficient of KTCl3 (T = Ge or Al).
The extinction coefficient κ(ω), shown in Figure 7(b), quantifies a material’s ability to attenuate light via absorption and scattering. For KAlCl3, κ increases from 0.37 at 3.82 eV to 1.43 at 13.60 eV, indicating strong absorption in the high-energy UV region. KGeCl3 shows consistently higher κ values across the same range, beginning at 0.95 (4.44 eV) and reaching 1.43 at 13.56 eV. Its broad and gradual increase suggests enhanced light attenuation, making KGeCl3 more effective for UV-blocking applications, optical filters, and light management components in photonic devices.
3.2.1.4 Refractive index and energy loss function (ELF)
The refractive index and ELF of KTCl3 (T = Ge, Al) indicate their light-bending and energy-loss characteristics, essential for optical device design [34,37,40]. The ELF reflects energy loss from electron interactions, revealing plasmonic and dielectric behavior for advanced tech applications.
The refractive index n(ω) of KTCl3 (T = Al, Ge), shown in Figure 8(a), reveals their light-bending capabilities across the photon energy spectrum. KAlCl3 exhibits a maximum refractive index of ∼1.83 at low energy (∼2.62 eV), which gradually decreases with increasing energy, indicating normal dispersion behavior. KGeCl3 shows a higher peak value of ∼2.23 at 2.64 eV, suggesting stronger light confinement and enhanced optical density. These refractive characteristics make both compounds suitable for integrated photonic components such as lenses, waveguides, and optical coatings where precise light propagation is critical.
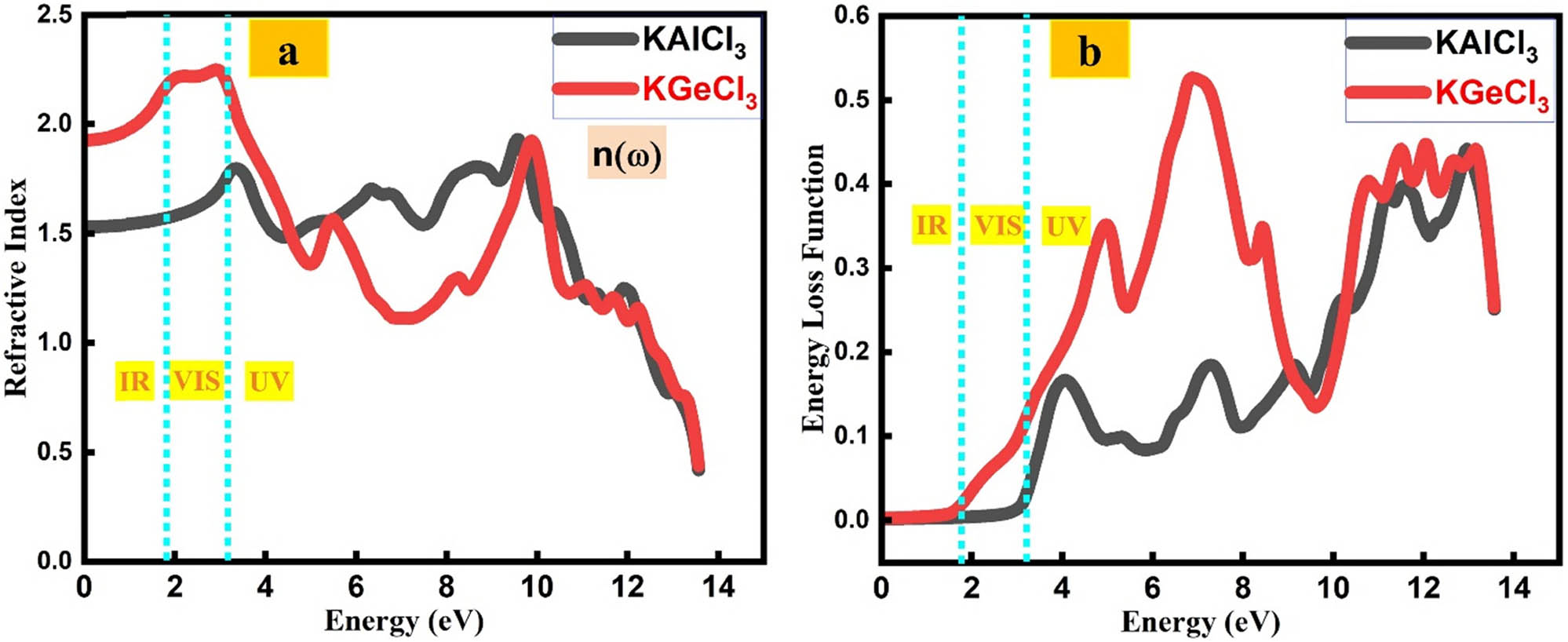
(a) Refractive index of KTCl3 (T = Ge or Al). (b) ELF of KTCl3 (T = Ge or Al).
The energy loss function ELF(ω), plotted in Figure 8(b), quantifies the energy dissipated by fast electrons traversing the material, primarily associated with plasma resonance peaks. KAlCl3 shows a prominent ELF peak at 12.65 eV, while KGeCl3 exhibits its maximum around 13.12 eV, corresponding to bulk plasmon excitations. These peaks indicate strong electron-energy loss near these energies, critical for understanding dielectric screening and charge carrier dynamics. The distinct ELF profiles suggest potential applications in plasmonic devices, electron microscopy coatings, and high-frequency dielectric components.
3.3 Elastic properties
The elastic constants (C ij ) measure how a material reacts to stress, specifically its tendency to deform and recover its shape when stress is applied and removed. For cubic crystals like KTCl3 (T = Ge or Al), three key constants (C 11, C 12, and C 44) govern their mechanical properties [41,42,43]. These constants are calculated using the Charpin method within the WIEN2K program. To ensure mechanical stability, the elastic constants must meet specific conditions: (C 11 – C 12) > 0, (C 11 + 2C 12) > 0, and C 44 > 0. Additionally, the condition C 12 < B < C 11 confirms the mechanical stability of these cubic compounds, where B is the bulk modulus. As there are no existing data on the elastic properties of KTCl3 (T = Ge or Al), our calculated values can provide a reference for future research [31]. Using the following equations, we determined the mechanical parameters: anisotropy factor (A), shear modulus (G), Young’s modulus (E), and Poisson’s ratio (υ):
The shear modulus of the materials is represented by G [43], with Gᵣ and Gᵥ representing the lower and upper bounds, respectively. The anisotropy factor (A) measures the material’s departure from isotropy (A = 1) or indicates anisotropy (A ≠ 1). Our results show that KAlCl3 (A = 0.65) and KGeCl3 (A = 0.36) are anisotropic, as indicated by their A values [44,45].
Young’s modulus (E) measures material stiffness [46], with higher values indicating stiffer materials. Poisson’s ratio (ν) gives insights into bonding characteristics. Values of ν below 0.1 indicate strong covalent bonding, while values around 0.25 suggest ionic character. Our calculations show ν as 0.56 for KAlCl3 and 0.23 for KGeCl3, suggesting both materials have significant ionic bonding contributions.
The Pugh’s ratio (B/G) helps predict a material’s behavior as ductile or brittle. Ductile behavior is indicated by a Pugh’s ratio greater than 1.75. In the case of KAlCl3 and KGeCl3, their respective Pugh’s ratios are 2.2711 and 2.5035, which both exceed the ductile threshold. This indicates that both compounds exhibit mechanical ductility [47] (Table 3).
Elastic properties of KTCl3 (T = Ge or Al)
| Compound | C11 (GPa) | C12 (GPa) | C44 (GPa) | B (GPa) | A | G (GPa) | E (GPa) | Y | B/G |
|---|---|---|---|---|---|---|---|---|---|
| KAlCl3 | 35.1975 | 27.3021 | 19.32 | 80.65 | 0.65 | 35.51 | 210 | 0.56 | 2.2711 |
| KGeCl3 | 58.64 | 36.42 | 22.44 | 70.45 | 0.36 | 28.14 | 170 | 0.23 | 2.5035 |
The different ways KTCl3 (T = Ge or Al) react to being stretched or squished (their elastic properties) give us a better understanding of how they move and remain stable. These properties vary depending on the direction of the force, as shown in detailed visualizations of their stiffness (Young’s modulus), resistance to shearing (shear modulus), and tendency to change shape when stretched (Poisson’s ratio). KAlCl3 exhibits highly variable stiffness in different directions, as seen in the star-shaped Young’s modulus plot (Figure 9(a)). When force is applied, the material’s response differs based on the direction. This asymmetry is also evident in the 2D projection (Figure 9(b)). Similarly, the shear modulus plots (Figure 9(c) and (d)) show varying resistance to deformation forces, indicating non-uniform deformation behavior. The Poisson’s ratio plots (Figure 9(e) and (f)) further highlight this anisotropic behavior, indicating that KAlCl3’s deformation pattern is strongly influenced by its crystal structure.
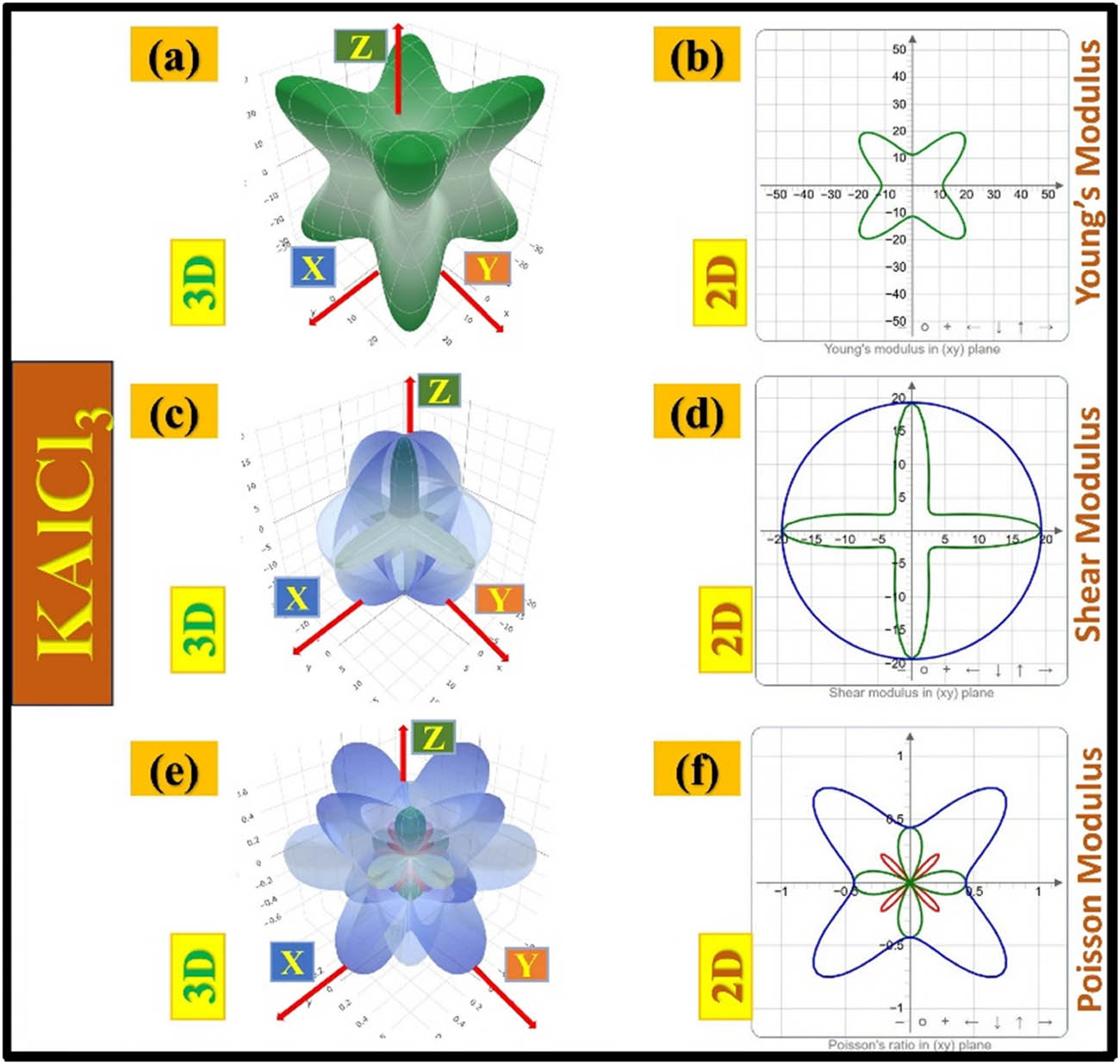
Anisotropic elastic properties of KAlCl3, illustrating variations in Young’s modulus, shear modulus, and Poisson’s ratio through 3D and 2D representations.
KGeCl3 has a more symmetrical and cubic-like distribution of its Young’s modulus in three dimensions (Figure 10(a)), indicating less anisotropy than KAlCl3. Its 2D projection of Young’s modulus (Figure 10(b)) shows less directional variation, suggesting a more balanced stiffness response in different crystal orientations. The shear modulus plots (Figure 10(c) and (d)) show that KGeCl3 resists deformation more uniformly than KAlCl3. The Poisson’s ratio distribution (Figure 10(e) and (f)) confirms this pattern, supporting the idea that KGeCl3’s elastic response is more uniform in different directions. Both materials exhibit anisotropy, but KGeCl3 has a more balanced response.
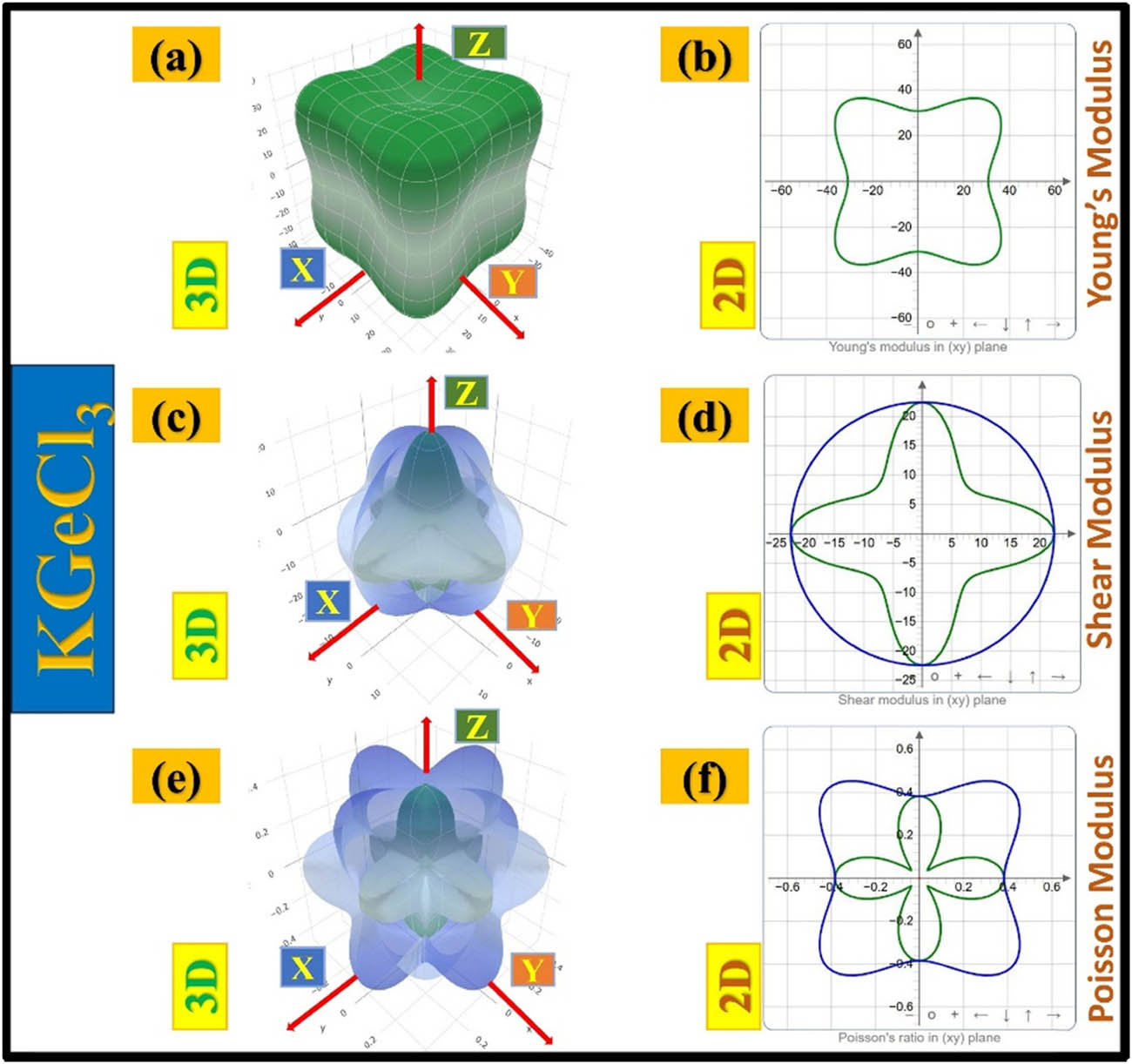
Anisotropic elastic properties of KGeCl3, illustrating variations in Young’s modulus, shear modulus, and Poisson’s ratio through 3D and 2D representations.
Based on calculated properties, KAlCl3 and KGeCl3 display direction-dependent behavior, are mechanically stable, and can be deformed without breaking. The higher Young’s modulus of KAlCl3 indicates it is stiffer than KGeCl3. The Poisson’s ratio supports the idea that the interactions within these materials are mainly ionic. These results enhance our understanding of the mechanical stability and possible uses of KTCl3 (T = Ge or Al) in different technologies.
3.4 Comparison with state-of-the-art materials
To better contextualize our findings, we have compared the properties of KTCl3 (T = Ge, Al) with those of well-known materials used in optoelectronic applications, such as CsPbBr3, MAPbI3, and other relevant chloro-perovskites. Table 4 summarizes the key properties of these materials, including band gaps, absorption coefficients, and mechanical properties.
Comparative study of KTCl3 (T = Ge, Al) with other compounds
| Material | Band gap (eV) | Absorption coefficient (cm−1) | Bulk modulus (GPa) | Shear modulus (GPa) | Reference |
|---|---|---|---|---|---|
| KGeCl3 | 1.88 | 187.76 | 27.39 | 28.14 | This study |
| KAlCl3 | Metallic | 16.95 | 31.58 | 35.51 | This study |
| CsPbBr3 | 2.30 | 150.00 | 30.00 | 30.00 | [48] |
| MAPbI3 | 1.56 | 120.00 | 25.00 | 20.00 | [49] |
| CsGeCl3 | 1.80 | 160.00 | 28.00 | 29.00 | [50] |
KGeCl3 exhibits a direct band gap of 1.88 eV, which is comparable to CsPbBr3 (2.30 eV) and MAPbI3 (1.56 eV), making it suitable for optoelectronic applications. Its high absorption coefficient (187.76 cm−1) indicates strong light absorption, particularly in the UV-visible range, which is advantageous for solar cells and photodetectors. Mechanically, KGeCl3 shows a bulk modulus of 27.39 GPa and a shear modulus of 28.14 GPa, indicating good mechanical stability and ductility. These properties make KTCl3 (T = Ge, Al) promising candidates for optoelectronic applications, offering a balance of electronic, optical, and mechanical properties that are competitive with state-of-the-art materials.
4 Conclusion
DFT calculations were performed to investigate the structural, electronic, optical, and elastic properties of KTCl3 (T = Ge, Al). Both compounds are structurally stable, crystallizing in a cubic phase. Electronic band structure analysis shows that KGeCl3 is a direct bandgap semiconductor with a gap of 1.88 eV, while KAlCl3 exhibits metallic behavior due to Al-derived states crossing the Fermi level. The projected DOS reveals that Ge and Cl orbitals dominate the valence and conduction bands in KGeCl3, whereas Al states primarily contribute to the conduction in KAlCl3. Optical properties, including the dielectric function, absorption coefficient, reflectivity, and refractive index, indicate strong photon absorption in the UV-visible region, suggesting their applicability in optoelectronic devices such as UV detectors and photonic coatings. The calculated optical conductivity further supports their potential for high-frequency electronic applications. Elastic property analysis confirms mechanical stability and ductility, with the B/G and Poisson’s ratios indicating ionic bonding and elastic anisotropy. Although the Tran–Blaha modified Becke–Johnson (TB-mBJ) potential improves bandgap predictions compared to standard GGA, this study did not employ hybrid functionals such as HSE06, which are known to provide more accurate electronic and optical descriptions due to the inclusion of non-local exchange. Moreover, excitonic effects were not considered and may influence absorption near the band edge. Despite these limitations, the results provide valuable insights and establish KTCl3 (T = Ge, Al) as promising candidates for optoelectronic, dielectric, and mechanical device applications.
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this research work under the Research Support Program for the Large Group Project at King Khalid University, Kingdom of Saudi Arabia, through the project number RGP2/60/46.
-
Funding information: This article was funded by Deanship of Research and Graduate Studies at King Khalid University under the Research Support Program for the large group project through the project number RGP2/60/46.
-
Author contributions: Muhammad Tahir, Wafa Mohammed Almalki, Nasir Rahman, Mudasser Husain, Mohamed Hussien, and Vineet Tirth wrote the manuscript. Khamael M. Abualnaja, Abid Ali Khan, Amir Ullah, Rajwali Khan, and Aurangzeb Khan prepared the figures. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Khalid S, Fahad S, Khan J, Sun X, Khenata R, Huang W, et al. Understanding the structural, electronic and optical properties of CuXY2 (X = Si, Ge, Y = P, As): A DFT + U approach. Optik (Stuttg). 2020;221:165212.10.1016/j.ijleo.2020.165212Search in Google Scholar
[2] Ullah A, Rahman N, Husain M, Almalki WM, Hussien M, Tirth V, et al. DFT study of structural, elastic and optoelectronic properties of binary X2Se (X = Cd, Zn) chalcogenides. Inorg Chem Commun. 2025;174:113985.10.1016/j.inoche.2025.113985Search in Google Scholar
[3] Roknuzzaman M, Alarco JA, Wang H, Du A, Tesfamichael T, Ostrikov KK. Ab initio atomistic insights into lead-free formamidinium based hybrid perovskites for photovoltaics and optoelectronics. Comput Mater Sci. 2019;169:109118.10.1016/j.commatsci.2019.109118Search in Google Scholar
[4] Roknuzzaman M, Ostrikov KK, Wasalathilake KC, Yan C, Wang H, Tesfamichael T. Insight into lead-free organic-inorganic hybrid perovskites for photovoltaics and optoelectronics: A first-principles study. Org Electron. 2018;59:99–106.10.1016/j.orgel.2018.04.051Search in Google Scholar
[5] Tseng Y-T, Wu CC. Photovoltaic performance of CH3NH3PbI2Cl perovskite solar cell. Appl Funct Mater. 2022;2(2):4–20.10.35745/afm2022v02.02.0003Search in Google Scholar
[6] Murtaza G, Ahmad I. First principle study of the structural and optoelectronic properties of cubic perovskites CsPbM3 (M=Cl, Br, I) . Phys B: Condens Matte. 201;17(1):3222–9.10.1016/j.physb.2011.05.028Search in Google Scholar
[7] Korba SA, Meradji H, Ghemid S, Bouhafs B. First principles calculations of structural, electronic and optical properties of BaLiF3. Comput Mater Sci. 2009;44(4):1265–71.10.1016/j.commatsci.2008.08.012Search in Google Scholar
[8] Boumriche A, Gesland JY, Bulou A, Rousseau M, Fourquet JL, Hennion B. Structure and dynamics of the inverted perovskite BaLiF3. Solid State Commun. 1994;91(2):125–8.10.1016/0038-1098(94)90268-2Search in Google Scholar
[9] Sarhani ME, Dahame T, Belkhir ML, Bentria B, Begagra A. AB-INITIO study of electronic, mechanical, optical and thermoelectric properties of KGeCl3 for photovoltaic application. Heliyon. 2023;9(9):e19808.10.1016/j.heliyon.2023.e19808Search in Google Scholar PubMed PubMed Central
[10] Tarekuzzaman M, Ishraq MH, Parves MS, Rayhan MA, Ahmad S, Rasheduzzaman M, et al. An in-depth investigation of lead-free KGeCl 3 perovskite solar cells employing optoelectronic, thermomechanical, and photovoltaic properties: DFT and SCAPS-1D frameworks. Phys Chem Chem Phys. 2024;26(43):27704–34.10.1039/D4CP02974GSearch in Google Scholar
[11] Islam MR, Mojumder MRH, Islam ASMJ, Alom MZ. Strain-driven tunability of the optical, electronic, and mechanical properties of lead-free inorganic CsGeCl3 perovskites. Phys Scr. 2022;97(12):125817.10.1088/1402-4896/ac9e25Search in Google Scholar
[12] Hao F, Stoumpos CC, Cao DH, Chang RPH, Kanatzidis MG. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat Photonics. 2014;8(6):489–94.10.1038/nphoton.2014.82Search in Google Scholar
[13] Jithin PV, Bitla Y, Patidar MM, Ganesan V, Sankaran KJ, Kurian J. Enhanced magnetoresistance and evolution of Griffiths-like phase in La1−x Cax MnO3 (x = 0.4, 0.5) nanoparticles. J Nanopart Res. 2023;25(10):207.10.1007/s11051-023-05847-7Search in Google Scholar
[14] Mubarak AA, Mousa AA. The electronic and optical properties of the fluoroperovskite BaXF3 (X = Li, Na, K, and Rb) compounds. Comput Mater Sci. 2012;59:6–13.10.1016/j.commatsci.2012.02.020Search in Google Scholar
[15] Mousa AA, Mahmoud NT, Khalifeh JM. The electronic and optical properties of the fluoroperovskite XLiF3 (X = Ca, Sr, and Ba) compounds. Comput Mater Sci. 2013;79:201–5.10.1016/j.commatsci.2013.06.016Search in Google Scholar
[16] Yalcin BG, Salmankurt B, Duman S. Investigation of structural, mechanical, electronic, optical, and dynamical properties of cubic BaLiF3, BaLiH3, and SrLiH3. Mater Res Express. 2016;3(3):36301.10.1088/2053-1591/3/3/036301Search in Google Scholar
[17] Suchikova Y, Kovachov S, Bohdanov I, Karipbayev ZT, Zhydachevskyy Y, Lysak A, et al. Advanced synthesis and characterization of CdO/CdS/ZnO heterostructures for solar energy applications. Materials (Basel). 2024;17(7):1566.10.3390/ma17071566Search in Google Scholar PubMed PubMed Central
[18] Chowdhury N, Riesen N, Riesen H. Efficient generation of stable Sm2+ in nanocrystalline BaLiF3: Sm3+ by UV-and X-irradiation. J Phys Chem C. 2019;123(41):25477–81.10.1021/acs.jpcc.9b07230Search in Google Scholar
[19] Pingak RK, Bouhmaidi S, Setti L. Investigation of structural, electronic, elastic and optical properties of Ge-halide perovskites NaGeX3 (X = Cl, Br and I): A first-principles DFT study. Phys B Condens Matter. 2023;663:415003.10.1016/j.physb.2023.415003Search in Google Scholar
[20] Lynn MO, Ologunagba D, Dangi BB, Kattel S. Density functional theory study of bulk properties of transition metal nitrides. Phys Chem Chem Phys. 2023;25(6):5156–63.10.1039/D2CP06082ESearch in Google Scholar
[21] Sholihun S, Kadarisman HP, Nurwantoro P. Density-functional-theory calculations of formation energy of the nitrogen-doped diamond. Indones J Chem. 2018;18(4):749–54.10.22146/ijc.26785Search in Google Scholar
[22] Azam S, Goumri-Said S, Khan SA, Kanoun MB. Electronic, optical and thermoelectric properties of new metal-rich homological selenides with palladium–indium: Density functional theory and Boltzmann transport model. J Phys Chem Solids. 2020;138:109229.10.1016/j.jpcs.2019.109229Search in Google Scholar
[23] Hutama AS, Huang H, Kurniawan YS. Investigation of the chemical and optical properties of halogen-substituted N-methyl-4-piperidone curcumin analogs by density functional theory calculations. Spectrochim Acta Part A Mol Biomol Spectrosc. 2019;221:117152.10.1016/j.saa.2019.117152Search in Google Scholar PubMed
[24] Hauwali NUJ, Syuhada I, Rosikhin A, Winata T. Fundamental properties of parallelogram graphene nanoflakes: A first principle study. Mater Today Proc. 2021;44:3305–8.10.1016/j.matpr.2020.11.532Search in Google Scholar
[25] Pradipta MF, Pranowo HD, Alfiyah V, Hutama AS. Theoretical study of oxygen atom adsorption on a polycyclic aromatic hydrocarbon using density-functional theory. Indones J Chem. 2021;21(5):1072–85.10.22146/ijc.53583Search in Google Scholar
[26] Jena AK, Kulkarni A, Miyasaka T. Halide perovskite photovoltaics: Background, status, and future prospects. Chem Rev. 2019;119(5):3036–103.10.1021/acs.chemrev.8b00539Search in Google Scholar PubMed
[27] Mubarak AA, Al-Omari S. First-principles calculations of two cubic fluoropervskite compounds: RbFeF3 and RbNiF3. J Magn Magn Mater. 2015;382:211–8.10.1016/j.jmmm.2015.01.073Search in Google Scholar
[28] Ahmad R, Mehmood N. A density functional theory investigations of half-heusler compounds RhVZ (Z = P, As, Sb). J Supercond Nov Magn. 2018;31(5):1577–86.10.1007/s10948-017-4370-4Search in Google Scholar
[29] Khan NU, Abdullah N, Khan UA, Tirth V, Al-Humaidi JY, Refat MS, et al. Investigation of structural, opto-electronic and thermoelectric properties of titanium based chloro-perovskites XTiCl3 (X = Rb, Cs): A first-principles calculations. RSC Adv. 2023 Feb;13(9):6199–209.10.1039/D3RA00200DSearch in Google Scholar
[30] Azeem W, Shahzad MK, Wong YH, Tahir MB. Ab-initio calculations for the study of the hydrogen storage properties of CsXH3 (X = Co, Zn) perovskite-type hydrides. Int J Hydrog Energy. 2024;50:305–13.10.1016/j.ijhydene.2023.07.072Search in Google Scholar
[31] Khan I, Ullah A, Rahman N, Husain M, Tirth V, Sohail M. First principle study of structural and optoelectronic properties of ZnLiX3 (X = Cl or F) perovskites. Results Phys. 2024;66:108019.10.1016/j.rinp.2024.108019Search in Google Scholar
[32] Pei Z, Leng K, Xia W, Lu Y, Wu H, Zhu X. Structural characterization, dielectric, magnetic and optical properties of double perovskite Bi2FeMnO6 ceramics. J Magn Magn Mater. 2020;508:166891.10.1016/j.jmmm.2020.166891Search in Google Scholar
[33] Kim H, Han JS, Choi J, Kim SY, Jang HW. Halide perovskites for applications beyond photovoltaics. Small Methods. 2018;2(3):1700310.10.1002/smtd.201700310Search in Google Scholar
[34] Rahman N, Husain M, Yang J, Sajjad M, Murtaza G, Ul Haq M, et al. First principle study of structural, electronic, optical and mechanical properties of cubic fluoro-perovskites:(CdXF3, X = Y, Bi). Eur Phys J Plus. 2021;136(3):1–11.10.1140/epjp/s13360-021-01177-6Search in Google Scholar
[35] van Blaaderen JJ, van den Brekel LA, Krämer KW, Dorenbos P. Scintillation and Optical Characterization of CsCu2I3 Single Crystals from 10 to 400 K. Chem Mater. 2023;35(22):9623–31.10.1021/acs.chemmater.3c01810Search in Google Scholar PubMed PubMed Central
[36] Mandal S, Sarkar P. Physical insights into the ultralow lattice thermal conductivity and high thermoelectric performance of bulk LiMTe 2 (M = Al, Ga). J Mater Chem C. 2023;11(40):13691–706.10.1039/D3TC02314ASearch in Google Scholar
[37] Chaba Mouna S, Radjai M, Bouhemadou A, Rahman MA, Kara H, Houatis D, et al. Structural, electronic, and optical characteristics of BaXCl3 (X = Li, Na) perovskites. Mater Sci Eng B. 2024;308:117578.10.1016/j.mseb.2024.117578Search in Google Scholar
[38] Mubarak AA. Ab initio study of the structural, electronic and optical properties of the fluoropervskite SrXF3 (X = Li, Na, K and Rb) compounds. Comput Mater Sci. 2014;81:478–82.10.1016/j.commatsci.2013.08.055Search in Google Scholar
[39] Ephraim Babu K, Murali N, Vijaya Babu K, Taddesse Shibeshi P, Veeraiah V. Structural, elastic, electronic, and optical properties of cubic perovskite CsCaCl3 compound: An ab initio study. Acta Phys Pol A. 2014;125(5):1179–85.10.12693/APhysPolA.125.1179Search in Google Scholar
[40] Arjun U, Ranjith KM, Jesche A, Hirschberger F, Sarma DD, Gegenwart P. Efficient adiabatic demagnetization refrigeration to below 50 mK with Ultrahigh-Vacuum-Compatible Ytterbium Diphosphates A YbP 2 O 7 (A = Na, K). Phys Rev Appl. 2023;20(1):14013.10.1103/PhysRevApplied.20.014013Search in Google Scholar
[41] Ullah W, Nasir R, Husain M, Rahman N, Ullah H, Sfina N, et al. Revealing the remarkable structural, electronic, elastic, and optical properties of Zn-based fluoropervskite ZnXF3 (x = Sr, Ba) employing DFT. Indian J Phys. 2024;1–12.10.1007/s12648-024-03146-ySearch in Google Scholar
[42] Jamal M, Bilal M, Ahmad I, Jalali-Asadabadi S. IRelast package. J Alloy Compd. 2018;735:569–79.10.1016/j.jallcom.2017.10.139Search in Google Scholar
[43] Husain M, Ahmad MS, Rahman N, Sajjad M, Rauf A, Habib A, et al. First principle study of the structural, electronic, and mechanical properties of cubic fluoroperovskites: (ZnXF 3, X = Y, Bi). Fluoride. 2020;53(4):657–67.Search in Google Scholar
[44] Pitriana P, Wungu TDK, Hidayat R. The characteristics of band structures and crystal binding in all-inorganic perovskite APbBr3 studied by the first principle calculations using the Density Functional Theory (DFT) method. Results Phys. 2019;15:102592.10.1016/j.rinp.2019.102592Search in Google Scholar
[45] Ullah A, Rahman N, Husain M, Abualnaja KM, Alosaimi G, Belhachi S, et al. Unlocking the physical properties of RbXBr3 (X = Ba, Be) halide perovskites for potential applications: DFT study. Inorg Chem Commun. 2025;173:113879.10.1016/j.inoche.2024.113879Search in Google Scholar
[46] Harmel M, Khachai H, Haddou A, Khenata R, Murtaza G, Abbar B, et al. Ab initio study of the mechanical, thermal and optoelectronic properties of the cubic CsBaF3. Acta Phys Pol A. 2015;128(1):34–42.10.12693/APhysPolA.128.34Search in Google Scholar
[47] Johannes AZ. Simulasi Perubahan Densitas Muatan Adsorpsi Atom Hidrogen-Grafena Dengan Teori Fungsi Kerapatan. J Fis Fis Sains Dan Apl. 2018;3(2):179–84.10.35508/fisa.v3i2.624Search in Google Scholar
[48] Ezzeldien M, Al-Qaisi S, Alrowaili ZA, Alzaid M, Maskar E, Es-Smairi A, et al. Electronic and optical properties of bulk and surface of CsPbBr3 inorganic halide perovskite a first principles DFT 1/2 approach. Sci Rep. 2021;11(1):20622.10.1038/s41598-021-99551-ySearch in Google Scholar PubMed PubMed Central
[49] Rahman TB, Rahman MM, Amir-Al Zumahi SM, Islam MR, Rahman MM. Strain-tuned structural, optoelectronic and dielectric properties of cubic MAPbI3 perovskite driven by SOC using first-principles theory. Solid State Commun. 2024;394:115728.10.1016/j.ssc.2024.115728Search in Google Scholar
[50] El Akkel M, Dahbi S, Benyoussef S, Tahiri N, Ez-Zahraouy H. First-principles studies of structural, vibrational, elastic, optoelectronic, and thermoelectric properties of doped, alloyed, and strained CsGeCl3 for photovoltaic applications. Sol Energy. 2024;282:112903.10.1016/j.solener.2024.112903Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Single-step fabrication of Ag2S/poly-2-mercaptoaniline nanoribbon photocathodes for green hydrogen generation from artificial and natural red-sea water
- Abundant new interaction solutions and nonlinear dynamics for the (3+1)-dimensional Hirota–Satsuma–Ito-like equation
- A novel gold and SiO2 material based planar 5-element high HPBW end-fire antenna array for 300 GHz applications
- Explicit exact solutions and bifurcation analysis for the mZK equation with truncated M-fractional derivatives utilizing two reliable methods
- Optical and laser damage resistance: Role of periodic cylindrical surfaces
- Numerical study of flow and heat transfer in the air-side metal foam partially filled channels of panel-type radiator under forced convection
- Water-based hybrid nanofluid flow containing CNT nanoparticles over an extending surface with velocity slips, thermal convective, and zero-mass flux conditions
- Dynamical wave structures for some diffusion--reaction equations with quadratic and quartic nonlinearities
- Solving an isotropic grey matter tumour model via a heat transfer equation
- Study on the penetration protection of a fiber-reinforced composite structure with CNTs/GFP clip STF/3DKevlar
- Influence of Hall current and acoustic pressure on nanostructured DPL thermoelastic plates under ramp heating in a double-temperature model
- Applications of the Belousov–Zhabotinsky reaction–diffusion system: Analytical and numerical approaches
- AC electroosmotic flow of Maxwell fluid in a pH-regulated parallel-plate silica nanochannel
- Interpreting optical effects with relativistic transformations adopting one-way synchronization to conserve simultaneity and space–time continuity
- Modeling and analysis of quantum communication channel in airborne platforms with boundary layer effects
- Theoretical and numerical investigation of a memristor system with a piecewise memductance under fractal–fractional derivatives
- Tuning the structure and electro-optical properties of α-Cr2O3 films by heat treatment/La doping for optoelectronic applications
- High-speed multi-spectral explosion temperature measurement using golden-section accelerated Pearson correlation algorithm
- Dynamic behavior and modulation instability of the generalized coupled fractional nonlinear Helmholtz equation with cubic–quintic term
- Study on the duration of laser-induced air plasma flash near thin film surface
- Exploring the dynamics of fractional-order nonlinear dispersive wave system through homotopy technique
- The mechanism of carbon monoxide fluorescence inside a femtosecond laser-induced plasma
- Numerical solution of a nonconstant coefficient advection diffusion equation in an irregular domain and analyses of numerical dispersion and dissipation
- Numerical examination of the chemically reactive MHD flow of hybrid nanofluids over a two-dimensional stretching surface with the Cattaneo–Christov model and slip conditions
- Impacts of sinusoidal heat flux and embraced heated rectangular cavity on natural convection within a square enclosure partially filled with porous medium and Casson-hybrid nanofluid
- Stability analysis of unsteady ternary nanofluid flow past a stretching/shrinking wedge
- Solitonic wave solutions of a Hamiltonian nonlinear atom chain model through the Hirota bilinear transformation method
- Bilinear form and soltion solutions for (3+1)-dimensional negative-order KdV-CBS equation
- Solitary chirp pulses and soliton control for variable coefficients cubic–quintic nonlinear Schrödinger equation in nonuniform management system
- Influence of decaying heat source and temperature-dependent thermal conductivity on photo-hydro-elasto semiconductor media
- Dissipative disorder optimization in the radiative thin film flow of partially ionized non-Newtonian hybrid nanofluid with second-order slip condition
- Bifurcation, chaotic behavior, and traveling wave solutions for the fractional (4+1)-dimensional Davey–Stewartson–Kadomtsev–Petviashvili model
- New investigation on soliton solutions of two nonlinear PDEs in mathematical physics with a dynamical property: Bifurcation analysis
- Mathematical analysis of nanoparticle type and volume fraction on heat transfer efficiency of nanofluids
- Creation of single-wing Lorenz-like attractors via a ten-ninths-degree term
- Optical soliton solutions, bifurcation analysis, chaotic behaviors of nonlinear Schrödinger equation and modulation instability in optical fiber
- Chaotic dynamics and some solutions for the (n + 1)-dimensional modified Zakharov–Kuznetsov equation in plasma physics
- Fractal formation and chaotic soliton phenomena in nonlinear conformable Heisenberg ferromagnetic spin chain equation
- Single-step fabrication of Mn(iv) oxide-Mn(ii) sulfide/poly-2-mercaptoaniline porous network nanocomposite for pseudo-supercapacitors and charge storage
- Novel constructed dynamical analytical solutions and conserved quantities of the new (2+1)-dimensional KdV model describing acoustic wave propagation
- Tavis–Cummings model in the presence of a deformed field and time-dependent coupling
- Spinning dynamics of stress-dependent viscosity of generalized Cross-nonlinear materials affected by gravitationally swirling disk
- Design and prediction of high optical density photovoltaic polymers using machine learning-DFT studies
- Robust control and preservation of quantum steering, nonlocality, and coherence in open atomic systems
- Coating thickness and process efficiency of reverse roll coating using a magnetized hybrid nanomaterial flow
- Dynamic analysis, circuit realization, and its synchronization of a new chaotic hyperjerk system
- Decoherence of steerability and coherence dynamics induced by nonlinear qubit–cavity interactions
- Finite element analysis of turbulent thermal enhancement in grooved channels with flat- and plus-shaped fins
- Modulational instability and associated ion-acoustic modulated envelope solitons in a quantum plasma having ion beams
- Statistical inference of constant-stress partially accelerated life tests under type II generalized hybrid censored data from Burr III distribution
- On solutions of the Dirac equation for 1D hydrogenic atoms or ions
- Entropy optimization for chemically reactive magnetized unsteady thin film hybrid nanofluid flow on inclined surface subject to nonlinear mixed convection and variable temperature
- Stability analysis, circuit simulation, and color image encryption of a novel four-dimensional hyperchaotic model with hidden and self-excited attractors
- A high-accuracy exponential time integration scheme for the Darcy–Forchheimer Williamson fluid flow with temperature-dependent conductivity
- Novel analysis of fractional regularized long-wave equation in plasma dynamics
- Development of a photoelectrode based on a bismuth(iii) oxyiodide/intercalated iodide-poly(1H-pyrrole) rough spherical nanocomposite for green hydrogen generation
- Investigation of solar radiation effects on the energy performance of the (Al2O3–CuO–Cu)/H2O ternary nanofluidic system through a convectively heated cylinder
- Quantum resources for a system of two atoms interacting with a deformed field in the presence of intensity-dependent coupling
- Studying bifurcations and chaotic dynamics in the generalized hyperelastic-rod wave equation through Hamiltonian mechanics
- A new numerical technique for the solution of time-fractional nonlinear Klein–Gordon equation involving Atangana–Baleanu derivative using cubic B-spline functions
- Interaction solutions of high-order breathers and lumps for a (3+1)-dimensional conformable fractional potential-YTSF-like model
- Hydraulic fracturing radioactive source tracing technology based on hydraulic fracturing tracing mechanics model
- Numerical solution and stability analysis of non-Newtonian hybrid nanofluid flow subject to exponential heat source/sink over a Riga sheet
- Numerical investigation of mixed convection and viscous dissipation in couple stress nanofluid flow: A merged Adomian decomposition method and Mohand transform
- Effectual quintic B-spline functions for solving the time fractional coupled Boussinesq–Burgers equation arising in shallow water waves
- Analysis of MHD hybrid nanofluid flow over cone and wedge with exponential and thermal heat source and activation energy
- Solitons and travelling waves structure for M-fractional Kairat-II equation using three explicit methods
- Impact of nanoparticle shapes on the heat transfer properties of Cu and CuO nanofluids flowing over a stretching surface with slip effects: A computational study
- Computational simulation of heat transfer and nanofluid flow for two-sided lid-driven square cavity under the influence of magnetic field
- Irreversibility analysis of a bioconvective two-phase nanofluid in a Maxwell (non-Newtonian) flow induced by a rotating disk with thermal radiation
- Hydrodynamic and sensitivity analysis of a polymeric calendering process for non-Newtonian fluids with temperature-dependent viscosity
- Exploring the peakon solitons molecules and solitary wave structure to the nonlinear damped Kortewege–de Vries equation through efficient technique
- Modeling and heat transfer analysis of magnetized hybrid micropolar blood-based nanofluid flow in Darcy–Forchheimer porous stenosis narrow arteries
- Activation energy and cross-diffusion effects on 3D rotating nanofluid flow in a Darcy–Forchheimer porous medium with radiation and convective heating
- Insights into chemical reactions occurring in generalized nanomaterials due to spinning surface with melting constraints
- Influence of a magnetic field on double-porosity photo-thermoelastic materials under Lord–Shulman theory
- Soliton-like solutions for a nonlinear doubly dispersive equation in an elastic Murnaghan's rod via Hirota's bilinear method
- Analytical and numerical investigation of exact wave patterns and chaotic dynamics in the extended improved Boussinesq equation
- Nonclassical correlation dynamics of Heisenberg XYZ states with (x, y)-spin--orbit interaction, x-magnetic field, and intrinsic decoherence effects
- Exact traveling wave and soliton solutions for chemotaxis model and (3+1)-dimensional Boiti–Leon–Manna–Pempinelli equation
- Unveiling the transformative role of samarium in ZnO: Exploring structural and optical modifications for advanced functional applications
- On the derivation of solitary wave solutions for the time-fractional Rosenau equation through two analytical techniques
- Analyzing the role of length and radius of MWCNTs in a nanofluid flow influenced by variable thermal conductivity and viscosity considering Marangoni convection
- Advanced mathematical analysis of heat and mass transfer in oscillatory micropolar bio-nanofluid flows via peristaltic waves and electroosmotic effects
- Exact bound state solutions of the radial Schrödinger equation for the Coulomb potential by conformable Nikiforov–Uvarov approach
- Some anisotropic and perfect fluid plane symmetric solutions of Einstein's field equations using killing symmetries
- Nonlinear dynamics of the dissipative ion-acoustic solitary waves in anisotropic rotating magnetoplasmas
- Curves in multiplicative equiaffine plane
- Exact solution of the three-dimensional (3D) Z2 lattice gauge theory
- Propagation properties of Airyprime pulses in relaxing nonlinear media
- Symbolic computation: Analytical solutions and dynamics of a shallow water wave equation in coastal engineering
- Wave propagation in nonlocal piezo-photo-hygrothermoelastic semiconductors subjected to heat and moisture flux
- Comparative reaction dynamics in rotating nanofluid systems: Quartic and cubic kinetics under MHD influence
- Laplace transform technique and probabilistic analysis-based hypothesis testing in medical and engineering applications
- Physical properties of ternary chloro-perovskites KTCl3 (T = Ge, Al) for optoelectronic applications
- Gravitational length stretching: Curvature-induced modulation of quantum probability densities
- Review Article
- Examination of the gamma radiation shielding properties of different clay and sand materials in the Adrar region
- Special Issue on Fundamental Physics from Atoms to Cosmos - Part II
- Possible explanation for the neutron lifetime puzzle
- Special Issue on Nanomaterial utilization and structural optimization - Part III
- Numerical investigation on fluid-thermal-electric performance of a thermoelectric-integrated helically coiled tube heat exchanger for coal mine air cooling
- Special Issue on Nonlinear Dynamics and Chaos in Physical Systems
- Analysis of the fractional relativistic isothermal gas sphere with application to neutron stars
- Abundant wave symmetries in the (3+1)-dimensional Chafee–Infante equation through the Hirota bilinear transformation technique
- Successive midpoint method for fractional differential equations with nonlocal kernels: Error analysis, stability, and applications
- Novel exact solitons to the fractional modified mixed-Korteweg--de Vries model with a stability analysis
Articles in the same Issue
- Research Articles
- Single-step fabrication of Ag2S/poly-2-mercaptoaniline nanoribbon photocathodes for green hydrogen generation from artificial and natural red-sea water
- Abundant new interaction solutions and nonlinear dynamics for the (3+1)-dimensional Hirota–Satsuma–Ito-like equation
- A novel gold and SiO2 material based planar 5-element high HPBW end-fire antenna array for 300 GHz applications
- Explicit exact solutions and bifurcation analysis for the mZK equation with truncated M-fractional derivatives utilizing two reliable methods
- Optical and laser damage resistance: Role of periodic cylindrical surfaces
- Numerical study of flow and heat transfer in the air-side metal foam partially filled channels of panel-type radiator under forced convection
- Water-based hybrid nanofluid flow containing CNT nanoparticles over an extending surface with velocity slips, thermal convective, and zero-mass flux conditions
- Dynamical wave structures for some diffusion--reaction equations with quadratic and quartic nonlinearities
- Solving an isotropic grey matter tumour model via a heat transfer equation
- Study on the penetration protection of a fiber-reinforced composite structure with CNTs/GFP clip STF/3DKevlar
- Influence of Hall current and acoustic pressure on nanostructured DPL thermoelastic plates under ramp heating in a double-temperature model
- Applications of the Belousov–Zhabotinsky reaction–diffusion system: Analytical and numerical approaches
- AC electroosmotic flow of Maxwell fluid in a pH-regulated parallel-plate silica nanochannel
- Interpreting optical effects with relativistic transformations adopting one-way synchronization to conserve simultaneity and space–time continuity
- Modeling and analysis of quantum communication channel in airborne platforms with boundary layer effects
- Theoretical and numerical investigation of a memristor system with a piecewise memductance under fractal–fractional derivatives
- Tuning the structure and electro-optical properties of α-Cr2O3 films by heat treatment/La doping for optoelectronic applications
- High-speed multi-spectral explosion temperature measurement using golden-section accelerated Pearson correlation algorithm
- Dynamic behavior and modulation instability of the generalized coupled fractional nonlinear Helmholtz equation with cubic–quintic term
- Study on the duration of laser-induced air plasma flash near thin film surface
- Exploring the dynamics of fractional-order nonlinear dispersive wave system through homotopy technique
- The mechanism of carbon monoxide fluorescence inside a femtosecond laser-induced plasma
- Numerical solution of a nonconstant coefficient advection diffusion equation in an irregular domain and analyses of numerical dispersion and dissipation
- Numerical examination of the chemically reactive MHD flow of hybrid nanofluids over a two-dimensional stretching surface with the Cattaneo–Christov model and slip conditions
- Impacts of sinusoidal heat flux and embraced heated rectangular cavity on natural convection within a square enclosure partially filled with porous medium and Casson-hybrid nanofluid
- Stability analysis of unsteady ternary nanofluid flow past a stretching/shrinking wedge
- Solitonic wave solutions of a Hamiltonian nonlinear atom chain model through the Hirota bilinear transformation method
- Bilinear form and soltion solutions for (3+1)-dimensional negative-order KdV-CBS equation
- Solitary chirp pulses and soliton control for variable coefficients cubic–quintic nonlinear Schrödinger equation in nonuniform management system
- Influence of decaying heat source and temperature-dependent thermal conductivity on photo-hydro-elasto semiconductor media
- Dissipative disorder optimization in the radiative thin film flow of partially ionized non-Newtonian hybrid nanofluid with second-order slip condition
- Bifurcation, chaotic behavior, and traveling wave solutions for the fractional (4+1)-dimensional Davey–Stewartson–Kadomtsev–Petviashvili model
- New investigation on soliton solutions of two nonlinear PDEs in mathematical physics with a dynamical property: Bifurcation analysis
- Mathematical analysis of nanoparticle type and volume fraction on heat transfer efficiency of nanofluids
- Creation of single-wing Lorenz-like attractors via a ten-ninths-degree term
- Optical soliton solutions, bifurcation analysis, chaotic behaviors of nonlinear Schrödinger equation and modulation instability in optical fiber
- Chaotic dynamics and some solutions for the (n + 1)-dimensional modified Zakharov–Kuznetsov equation in plasma physics
- Fractal formation and chaotic soliton phenomena in nonlinear conformable Heisenberg ferromagnetic spin chain equation
- Single-step fabrication of Mn(iv) oxide-Mn(ii) sulfide/poly-2-mercaptoaniline porous network nanocomposite for pseudo-supercapacitors and charge storage
- Novel constructed dynamical analytical solutions and conserved quantities of the new (2+1)-dimensional KdV model describing acoustic wave propagation
- Tavis–Cummings model in the presence of a deformed field and time-dependent coupling
- Spinning dynamics of stress-dependent viscosity of generalized Cross-nonlinear materials affected by gravitationally swirling disk
- Design and prediction of high optical density photovoltaic polymers using machine learning-DFT studies
- Robust control and preservation of quantum steering, nonlocality, and coherence in open atomic systems
- Coating thickness and process efficiency of reverse roll coating using a magnetized hybrid nanomaterial flow
- Dynamic analysis, circuit realization, and its synchronization of a new chaotic hyperjerk system
- Decoherence of steerability and coherence dynamics induced by nonlinear qubit–cavity interactions
- Finite element analysis of turbulent thermal enhancement in grooved channels with flat- and plus-shaped fins
- Modulational instability and associated ion-acoustic modulated envelope solitons in a quantum plasma having ion beams
- Statistical inference of constant-stress partially accelerated life tests under type II generalized hybrid censored data from Burr III distribution
- On solutions of the Dirac equation for 1D hydrogenic atoms or ions
- Entropy optimization for chemically reactive magnetized unsteady thin film hybrid nanofluid flow on inclined surface subject to nonlinear mixed convection and variable temperature
- Stability analysis, circuit simulation, and color image encryption of a novel four-dimensional hyperchaotic model with hidden and self-excited attractors
- A high-accuracy exponential time integration scheme for the Darcy–Forchheimer Williamson fluid flow with temperature-dependent conductivity
- Novel analysis of fractional regularized long-wave equation in plasma dynamics
- Development of a photoelectrode based on a bismuth(iii) oxyiodide/intercalated iodide-poly(1H-pyrrole) rough spherical nanocomposite for green hydrogen generation
- Investigation of solar radiation effects on the energy performance of the (Al2O3–CuO–Cu)/H2O ternary nanofluidic system through a convectively heated cylinder
- Quantum resources for a system of two atoms interacting with a deformed field in the presence of intensity-dependent coupling
- Studying bifurcations and chaotic dynamics in the generalized hyperelastic-rod wave equation through Hamiltonian mechanics
- A new numerical technique for the solution of time-fractional nonlinear Klein–Gordon equation involving Atangana–Baleanu derivative using cubic B-spline functions
- Interaction solutions of high-order breathers and lumps for a (3+1)-dimensional conformable fractional potential-YTSF-like model
- Hydraulic fracturing radioactive source tracing technology based on hydraulic fracturing tracing mechanics model
- Numerical solution and stability analysis of non-Newtonian hybrid nanofluid flow subject to exponential heat source/sink over a Riga sheet
- Numerical investigation of mixed convection and viscous dissipation in couple stress nanofluid flow: A merged Adomian decomposition method and Mohand transform
- Effectual quintic B-spline functions for solving the time fractional coupled Boussinesq–Burgers equation arising in shallow water waves
- Analysis of MHD hybrid nanofluid flow over cone and wedge with exponential and thermal heat source and activation energy
- Solitons and travelling waves structure for M-fractional Kairat-II equation using three explicit methods
- Impact of nanoparticle shapes on the heat transfer properties of Cu and CuO nanofluids flowing over a stretching surface with slip effects: A computational study
- Computational simulation of heat transfer and nanofluid flow for two-sided lid-driven square cavity under the influence of magnetic field
- Irreversibility analysis of a bioconvective two-phase nanofluid in a Maxwell (non-Newtonian) flow induced by a rotating disk with thermal radiation
- Hydrodynamic and sensitivity analysis of a polymeric calendering process for non-Newtonian fluids with temperature-dependent viscosity
- Exploring the peakon solitons molecules and solitary wave structure to the nonlinear damped Kortewege–de Vries equation through efficient technique
- Modeling and heat transfer analysis of magnetized hybrid micropolar blood-based nanofluid flow in Darcy–Forchheimer porous stenosis narrow arteries
- Activation energy and cross-diffusion effects on 3D rotating nanofluid flow in a Darcy–Forchheimer porous medium with radiation and convective heating
- Insights into chemical reactions occurring in generalized nanomaterials due to spinning surface with melting constraints
- Influence of a magnetic field on double-porosity photo-thermoelastic materials under Lord–Shulman theory
- Soliton-like solutions for a nonlinear doubly dispersive equation in an elastic Murnaghan's rod via Hirota's bilinear method
- Analytical and numerical investigation of exact wave patterns and chaotic dynamics in the extended improved Boussinesq equation
- Nonclassical correlation dynamics of Heisenberg XYZ states with (x, y)-spin--orbit interaction, x-magnetic field, and intrinsic decoherence effects
- Exact traveling wave and soliton solutions for chemotaxis model and (3+1)-dimensional Boiti–Leon–Manna–Pempinelli equation
- Unveiling the transformative role of samarium in ZnO: Exploring structural and optical modifications for advanced functional applications
- On the derivation of solitary wave solutions for the time-fractional Rosenau equation through two analytical techniques
- Analyzing the role of length and radius of MWCNTs in a nanofluid flow influenced by variable thermal conductivity and viscosity considering Marangoni convection
- Advanced mathematical analysis of heat and mass transfer in oscillatory micropolar bio-nanofluid flows via peristaltic waves and electroosmotic effects
- Exact bound state solutions of the radial Schrödinger equation for the Coulomb potential by conformable Nikiforov–Uvarov approach
- Some anisotropic and perfect fluid plane symmetric solutions of Einstein's field equations using killing symmetries
- Nonlinear dynamics of the dissipative ion-acoustic solitary waves in anisotropic rotating magnetoplasmas
- Curves in multiplicative equiaffine plane
- Exact solution of the three-dimensional (3D) Z2 lattice gauge theory
- Propagation properties of Airyprime pulses in relaxing nonlinear media
- Symbolic computation: Analytical solutions and dynamics of a shallow water wave equation in coastal engineering
- Wave propagation in nonlocal piezo-photo-hygrothermoelastic semiconductors subjected to heat and moisture flux
- Comparative reaction dynamics in rotating nanofluid systems: Quartic and cubic kinetics under MHD influence
- Laplace transform technique and probabilistic analysis-based hypothesis testing in medical and engineering applications
- Physical properties of ternary chloro-perovskites KTCl3 (T = Ge, Al) for optoelectronic applications
- Gravitational length stretching: Curvature-induced modulation of quantum probability densities
- Review Article
- Examination of the gamma radiation shielding properties of different clay and sand materials in the Adrar region
- Special Issue on Fundamental Physics from Atoms to Cosmos - Part II
- Possible explanation for the neutron lifetime puzzle
- Special Issue on Nanomaterial utilization and structural optimization - Part III
- Numerical investigation on fluid-thermal-electric performance of a thermoelectric-integrated helically coiled tube heat exchanger for coal mine air cooling
- Special Issue on Nonlinear Dynamics and Chaos in Physical Systems
- Analysis of the fractional relativistic isothermal gas sphere with application to neutron stars
- Abundant wave symmetries in the (3+1)-dimensional Chafee–Infante equation through the Hirota bilinear transformation technique
- Successive midpoint method for fractional differential equations with nonlocal kernels: Error analysis, stability, and applications
- Novel exact solitons to the fractional modified mixed-Korteweg--de Vries model with a stability analysis

