Abstract
Nitrogen-containing chiral heterocycles represent important structural elements with wide occurrence in natural and unnatural compounds exhibiting important biological activities. Herein, we describe our recent endeavors directed toward the Brønsted acid-catalyzed 1,3-dipolar cycloaddition and alkylation reactions, together with the metal/Brønsted acid relay catalytic cascade reactions to access some typical nitrogenous heterocyclic structures and natural alkaloids, including (+)-folicanthine and (+)-gliocladin C.
Introduction
Optically pure nitrogenous heterocyclic compounds have long been receiving a great deal of attention due to their frequent occurrence in natural and unnatural products with a diverse range of pharmaceutical activities [1]. For example (Fig. 1), chiral pyrrolidines, spirooxindoles, tetrahydroquinolines, and related heterocyclic compounds not only constitute core structural elements in the medicinally relevant compounds, but also serve as key synthetic intermediates for the total synthesis of alkaloids. In particular, as highlighted in Fig. 1, L-689560 (4), a potent N-methyl-D-aspartate, is a promising drug candidate for the treatment of Alzheimer’s disease and for reducing ischemic brain damage [2]. The alkaloid, gliocladin C (8) exhibited cytotoxic activity against murine P388 lymphocytic leukemia cells [3]. Thus, the development of novel synthetic protocols to readily access these targets in excellent levels of stereoselectivity and highly structural diversity is undoubtedly appealing in organic and medicinal chemistry as well as other related fields.
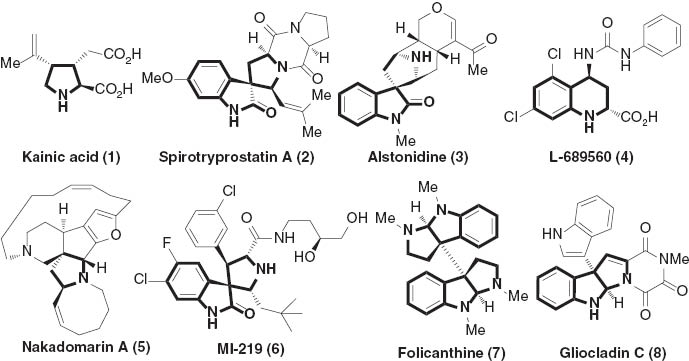
The structural significance of five and six membered nitrogenous heterocycles.
Among numerous facile synthetic pathways to access nitrogenous heterocycles, chiral Brønsted acid-catalyzed asymmetric multicomponent reactions have emerged as robust and privileged tools for building up enantioenriched heterocyclic skeletons with high structural diversity and complexity [4]. As shown in Scheme 1, if a substrate bearing both nucleo- and electrophilic functionalities, it may undergo an enantioselective addition (path a) to an imine generated from an aldehyde and primary amine to give rise to an intermediate I, which then undergoes cyclization reaction to furnish heterocyclic compounds. Because either of the reaction components is structurally tunable, such a multicomponent protocol is principally able to provide a general approach to building up collections of optically active nitrogenous heterocycles (Scheme 1, A) [5]. On the other hand, if one of the reaction components contains both an electrophile (E) and a functional group (FG), and the amine bears a nucleophilic moiety, the chiral Brønsted acid should promote an asymmetric cyclization reaction via intermediate II generated from the path b. In addition to the strategy shown in Scheme 1, we have recently proposed an asymmetric relay catalysis (ARC) comprising binary chiral Brønsted acids and metal complexes, which have enabled the discovery of unprecedented enantioselective cascade transformations with high enantioselectivities. We envisaged that the functionalized amines (9) are basically able to undergo a coupling reaction under the catalysis of either a metal or an organocatalyst to give an intermediate III, which would presumably participate in the cyclization reaction under the promotion of either organo- or metal catalyst to generate heterocycles (Scheme 1, B) [6].
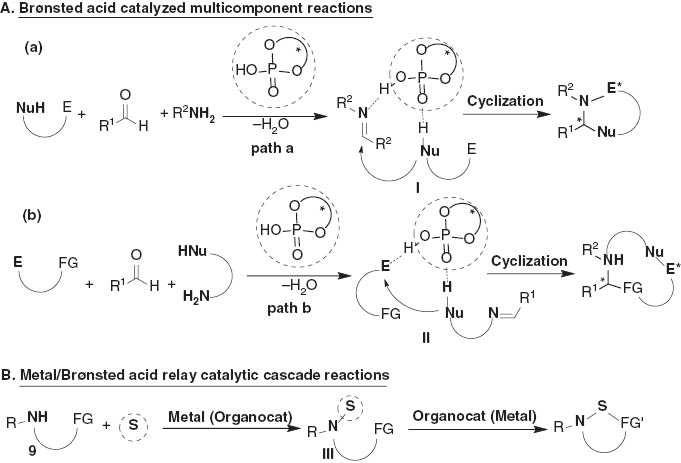
General strategy to access nitrogenous heterocycles.
In this article, we will describe our recent efforts directing at the discovery of asymmetric reactions to access these nitrogenous structures basically using the strategies described in Scheme 1. In addition, the organocatalytic enantioselective synthesis of (+)-folicanthine (7) and (+)-gliocladin C (8) will also be reported.
Results and discussion
Asymmetric 1,3-dipolar cycloaddition catalyzed by bisphosphoric acid
The 1,3-dipolar cycloaddition of azomethine ylides with olefins gives rise to pyrrolidines 13 and spiro[pyrrolidin-3,3’-oxindole] derivatives 17, which represent two of the important structural elements, frequently found in natural products and artificial biologically active molecules [7]. In recent decades, elegant processes have been described in this field using either chiral metal complex [8] or organocatalyst [9]. We have found that chiral binol-derived phosphoric acids were good catalysts for three-component 1,3-dipolar cycloaddition reactions [10].
The examination of BINOL-based phosphoric acids revealed that the bisphosphoric acid 14 was the best catalyst for the three-component 1,3-dipolar cycloaddition with a wide range of aldehydes 10 and aminomalonates as well as α-arylglycine esters and phenylalanine esters, which allowed the formation of multiple substituted pyrrolidine derivatives in high yields and excellent enantioselectivities [10a]. DFT calculations performed on the transition states suggested that one phosphate of the bisphosphoric acid 14 acts as the Lewis base to activate the 1,3-dipole and the two hydroxyl groups of the catalyst may form two hydrogen-bonds with the two ester groups of maleate, respectively (TS-1), to make it more electron-deficient as a much stronger dipolarophile to conduct a concerted 1,3-dipolar cycloaddition with azomethine ylide IV (Fig. 2) [10b].
![Fig. 2 Transition states of [3+2] cycloaddition catalyzed by bisphosphoric acid 14.](/document/doi/10.1515/pac-2013-1208/asset/graphic/pac-2013-1208_fig2.jpg)
Transition states of [3+2] cycloaddition catalyzed by bisphosphoric acid 14.
A diverse spectrum of dipolarophiles, including maleates 12 [10a,b], methyleneindolinones 16 [10c], N-aryl imines 18 [10d], allenoates 19 [10e], quinones 20 [10f] and ynones 21 [10g], were able to react with azomethine ylides IV derived from aldehydes 10 by using either the bisphosphoric acid 14 or chiral phosphoric acids 15 as a chiral catalyst, to give several collections of pyrrolidine derivatives in high levels of enantioselectivity and structural diversity (Scheme 2).
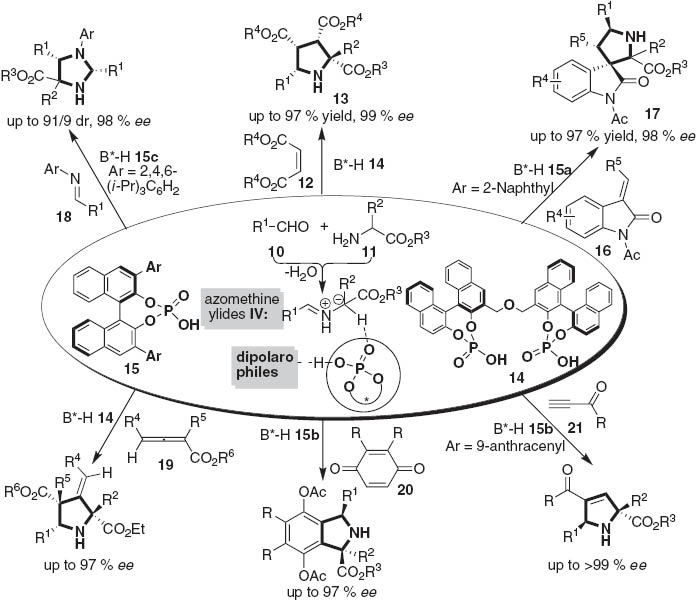
Asymmetric catalytic 1,3-dipolar cycloaddition reactions.
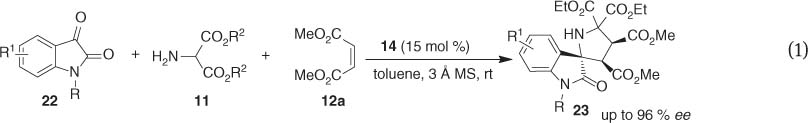
Very recently, a spiro[pyrrolidin-3,2′-oxindole] core 23, similar to spiro[pyrrolidin-3,3′-oxindole] derivatives 17, is also a privileged heterocyclic ring system which is featured in a large family of medicinally relevant compounds [11]. Encouraged by our previous achievements on the 1,3-dipolar cycloaddition reactions and in view of the absence of enantioselective version of the cycloaddition reactions involving azomethine ylides generated from unsymmetrical cyclic ketones, an asymmetric 1,3-dipolar cycloaddition of isatin-based azomethine ylides efficiently catalyzed by the bisphosphoric acid 14 was subsequently developed, affording the spiro[pyrrolidin-3, 2′-oxindole] scaffolds 23 with contiguous quaternary stereogenic centers in excellent stereoselectivities (eq. 1) [12]. This protocol represents the first example of catalytic 1,3-dipolar cycloaddition involving azomethine ylides generated in situ from unsymmetrical cyclic ketones. Moreover, the preliminary bioassay revealed that some of these spiro[pyrrolidin-3,2′-oxindole] derivatives have shown moderate cytotoxicity to SW116 cells.
Organocatalytic enantioselective total synthesis of cyclotryptamine alkaloids
Enantioselective total synthesis of (+)-folicanthine from organocatalytic alkylation of 3-hydroxyoxindoles
The fascinating cyclotryptamine alkaloids (Fig. 3), which contain an octahydro-3a,3a′-bispyrrolo[2,3-b]indole subunit (Fig. 3, core structure 24) with vicinal all-carbon quaternary stereogenic centers, exhibit important biological activities [13] and have therefore led to a great demand for enatioselective and efficient synthetic methods. As a consequence, important contributions have been directed to the development of novel protocols for accessing hexahydropyrroloindole skeletons [14]. Overman has reported enantioselective syntheses of optically pure chimonanthines [15], wherein the related quaternary stereocenters were built up from a naturally occurring chiral pool, a tartaric acid derivative. Starting from the bromocyclization of naturally available tryptophan and tryptamine derivatives, Movassaghi and coworkers established a radical coupling reaction to directly access hexahydropyrroloindoles. This synthetic strategy has allowed efficient total synthesis of several members of cyclotryptamine alkaloids [16].
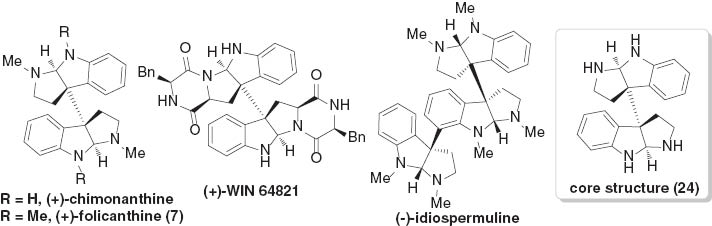
Representative cyclotryptamine alkaloids.
In spite of these achievements, an enantioselective catalytic method to access a chiral intermediate for the synthesis of 3a,3a′-bispyrrolo[2,3-b]indoline skeleton still remains elusive. As indicated in Scheme 3, the core structure 24 could be prepared from diamide 25 via reductive cyclization. The intermediate 25 was planned to be accessed by classical transformations from 26, which is principally able to be prepared from 27 by Beckmann rearrangement or sequential Baeyer–Villiger oxidation/amidation reaction. Obviously, the development of asymmetric catalytic reaction to generate compound 27 is the key point of the total synthesis [17].
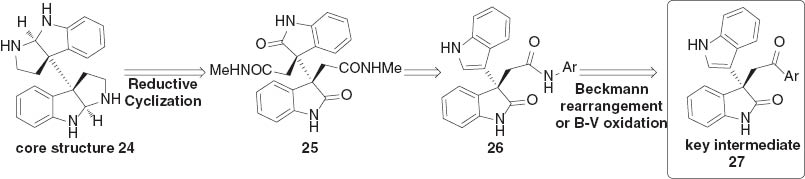
Retrosynthetic analysis of core structure 24.
Thus, an asymmetric enantioselective substitution reaction of 3-hydroxyoxindoles 28 with enamide 29 was first investigated and found that chiral phosphoric acid 15a turned out to be the best catalyst. The protocol was very general for 3-hydroxyoxindoles 28 with enamide 29, giving a diverse spectrum of 3,3′-disubstituted oxindoles 27 in high yields with excellent enantioselectivities (Scheme 4).
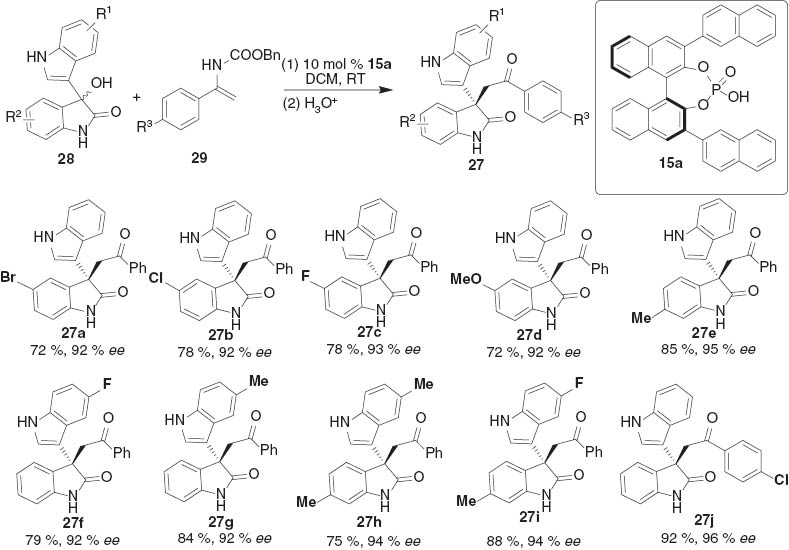
The scope of asymmetric alkylation of 3-hydroxyoxindoles.
Then, we started to synthesize (+)-folicanthine 7 by using this asymmetric alkylation to build up the stereogenic centers (Scheme 5). The enantioselective substitution reaction of 28a and enamide 29a generated 27k in 90 % ee. The subsequent dimethylation of 27k furnish 30 in 76 % yield. Since the Baeyer–Villiger oxidation of 30 failed to give desired products, we turned our attention to the Beckmann rearrangement, a reliable reaction to transform ketones into amides. However, the treatment of 30 with NH2OH·HCl in EtOH gave an isomeric mixture of ketoximes 31a and 31b (31a/31b = 1/2.1). Fortunately, the ketoxime 31a could be completely converted into ketoxime 31b in the presence of p-toluenesulfonic acid. A clean Beckmann rearrangement of 31b promoted by HgCl2 was achieved to give rise to amide 32 in 90 % yield [18]. After the amide nitrogen was methylated, the resultant crude product was directly oxidized with DMSO/HCl, to produce bisoxindole 33 in high yield. The exposure of bisoxindole 33 to BrCH2COOEt under basic conditions allowed an alkylation to afford 34 in 66 % yield. The removal of the p-methoxyphenyl group by means of ceric ammonium nitrate (CAN) furnished the compound 35. The amidation of 35 with methyl amine afforded the intermediate 36 in 77 % yield. Following the procedure reported by Rodrigo [19], compound 36 was transformed into (+)-folicanthine 7 in 26 % overall yield.
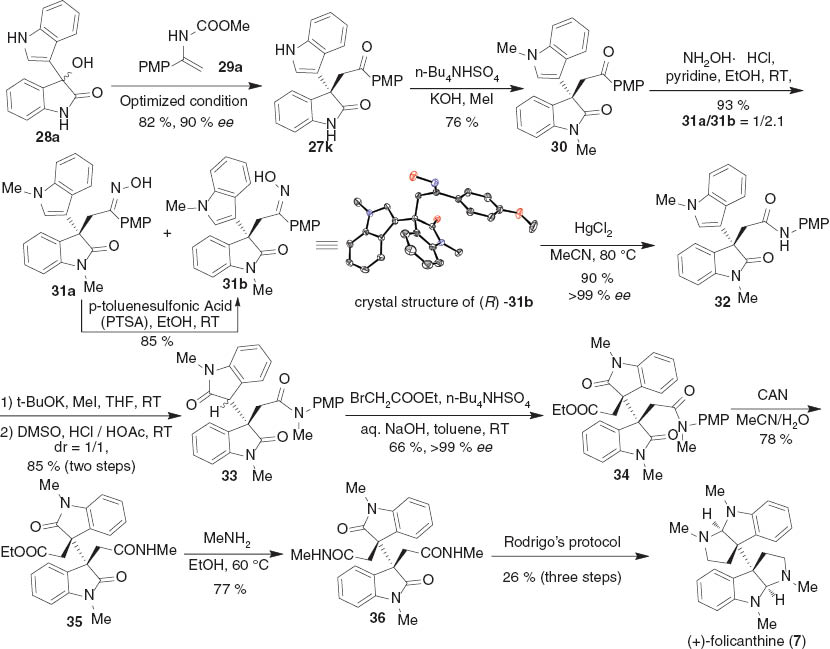
Enantioselective total synthesis of (+)-folicanthine 7.
Total synthesis of (+)-gliocladin C via organocatalytic α-alkylation of aldehydes
Owing to the significant biological activity of (+)-gliocladin C (8) [3], Overman and Shin established the first total synthesis of this molecule and confirm its stereochemical assignment in 2007 [20a]. Subsequently, Overman et al., developed a concise and elegant enantioselective synthesis of (+)-gliocladin C (8) and illustrated the importance of gliocladin C as a key intermediate for the construction of other C3-C3′ bisindole alkaloids [20b]. Stephenson and co-workers also completed the total synthesis of (+)-gliocladin C (8) by using photoredox catalysis to introduce an indolyl substructure as a key step [20c]. The most recent synthesis of (+)-gliocladin C (8), reported by Movassaghi et al., used a Friedel–Crafts-based coupling strategy to access the C3-(3′-indolyl)hexahydropyrroloindole substructure [20d].
As illustrated in Scheme 6, the core skeleton of (+)-gliocladin C (8) could be obtained from the intermediates 37 and 38. The indoline aldehyde 38 (Overman intermediate) [20b] could be easily synthesized from biindolin-1,2-diol 39, which would be accessed by reduction of the aldehyde 40. Thus, we developed an enantioselective alkylation reaction of 3-hydroxindoles 28 with enalizable aldehydes 41 for the preparation of the key building block 40 [21].
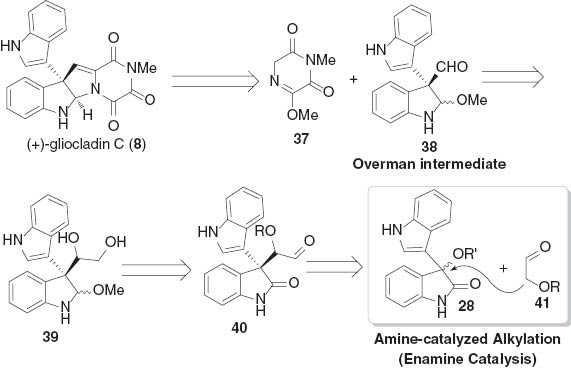
Retrosynthetic analysis of (+)-gliocladin C.
The cinchona-based primary amines have been proved to be successful in either iminium or enamine catalyzed asymmetric transformations [22]. Inspired by these achievements, we screened several families of chiral organocatalysts for the alkylation of 3-hydroxindoles 28a with 2-alkyloxy-acetaldehyde 41 and found that the combined use of cinchona alkaloid-based primary amine 42 and chiral phosphoric acid 15 provided the highest levels of stereoselectivity [23]. The desired key intermediate 40a was obtained in 80 % yield with 8:1 dr and 94 % ee under the optimized conditions. The expansion of the reaction conditions to other 3-hydroxindole derivatives was also successful to generate alkylation products in high yields and excellent stereoselectivities (Scheme 7).
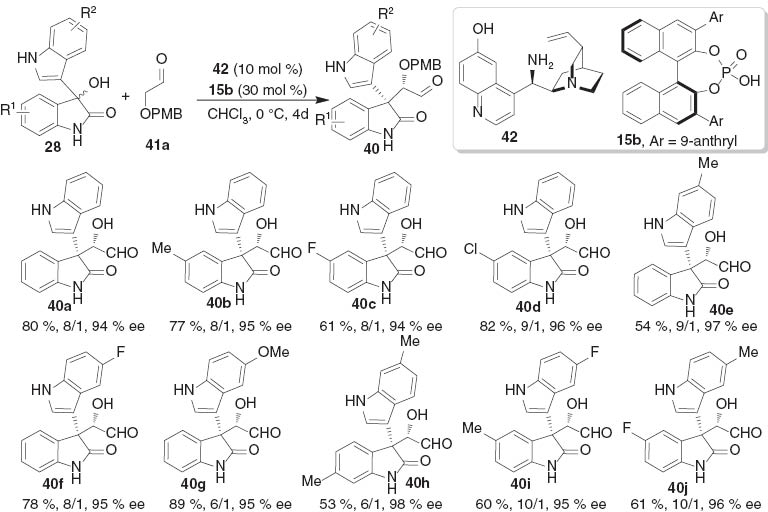
The scope of asymmetric alkylation of 3-hydroxyoxindoles with 2-alkyloxy-acetaldehyde.
Then we applied this organocatalytic reaction to the enantioselective total synthesis of (+)-gliocladin C (8) (Scheme 8). The reduction of the chiral aldehyde 40a to the corresponding alcohol, followed by protection with TBS group afforded 43 in 90 % yield. After the introduction of two Boc groups to the nitrogen atoms of 43 furnished 44 in 95 % yield, the reduction of oxindole carbonyl group with NaBH4 afforded 45 in 5:1 dr. The treatment of 45 with trimethyl orthoformate in the presence of pyridinium p-toluenesulfonate (PPTS) led to the formation of 46 in 89 % yield. The O-protecting groups in 46 were then cleanly removed by treatment with TBAF and subsequently subjecting to hydrogenolysis catalyzed by Pd(OH)2/C, provided diol 47 in 90 % yield. The indoline aldehyde 48 (Overman intermediate) was successfully accessed by the oxidative cleavage of the diol 47 with NaIO4. Following Overman’s procedure [20b], (+)-gliocladin C 8 finally was obtained from 48 in 53 % yield over 3 steps. The highly enantioselective total synthesis of (+)-gliocladin C 8 was presented within 12 steps from 3-hydroxyoxindole 28a in an overall 19 % yield.
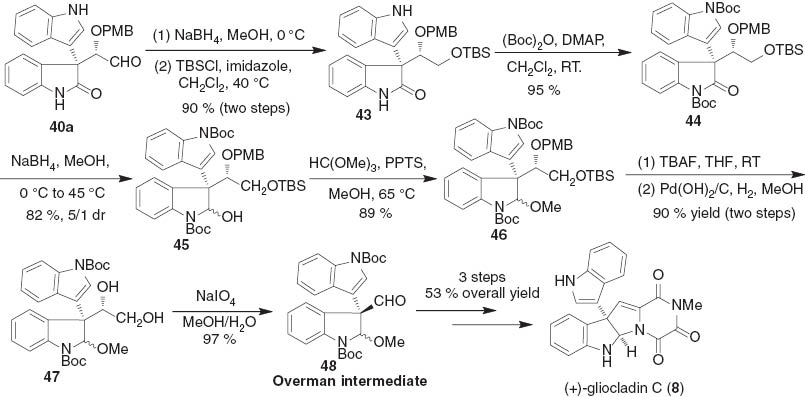
Total synthesis of (+)-gliocladin C.
Asymmetric relay catalytic Friedländer condensation/transfer hydrogenation
Recently, the combination of metal complexes and organic molecules in relay and cooperative catalysis has gained increasing attention, because it could combine multiple transformations in one synthetic operation [6]. More importantly, asymmetric relay catalysis (ARC) holds great potential in creation of new step economy transformations wherein either type of catalyst failed to afford alone. The combination of transition metal and organocatalysts has enabled a diverse range of unprecedented transformations, providing efficient methods to access optically active heterocycles [6, 24].
We found that Lewis acids, such as Mg(OTf)2, and chiral phosphoric acid 15c were highly compatible and were able to catalyze a cascade Friedländer condensation [25]/asymmetric hydrogen transfer reaction (Scheme 9) [26]. Starting from commercially available 2-aminobenzaldehyde or 2-amino phenyl ketones 49 and β-ketoester 50, this step-economical protocol provides an efficient approach to highly substituted tetrahydroquinolines 51 in excellent levels of stereoselectivity. The kinetic experiments suggested that the asymmetric hydrogen transfer process was solely catalyzed by chiral phosphoric acid, while Lewis and Brønsted acids cooperatively catalyzed the Friedländer condensation. The compatibility and synergism of the Lewis acid and chiral Brønsted acid may provide a new reaction mode potentially amenable to disclosing other relay catalytic asymmetric transformations.
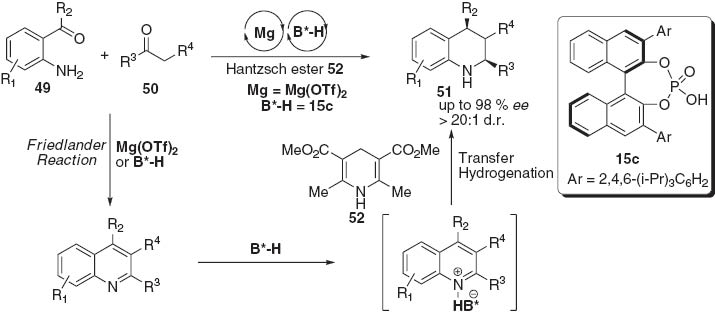
Step-economical synthesis of tetrahydroquinolines by asymmetric relay catalytic Friedländer condensation/transfer hydrogenation.
Conclusions
In the field of organic synthesis, the development of new asymmetric transformations leading to novel scaffolds with unprecedented chemical and biological activities always hold great importance, but still represents a formidable challenge. Our recent development in the synthesis of nitrogenous heterocyclic structures via Brønsted acid catalyzed [3 + 2] cycloaddition reactions or metal/Brønsted acid catalytic relay cascade reactions provides a diverse range of protocols for the preparation of nitrogeneous five- or six-membered heterocycles in highly structural diversity and excellent stereoselectivities. Remarkably, the organocatalytic alkylation of 3-hydroxyoxindoles has been established to synthesize the building blocks for total synthesis of cyclotryptamine alkaloids, including (+)-folicanthine and (+)-gliocladin C.
Article note: A collection of invited papers based on presentations at the 24th International Congress on Heterocyclic Chemistry (ICHC-24), Shanghai, China, 8–13 September 2013.
Acknowledgments
The authors thank MOST (2010CB833300) and NSFC (21232007) for supporting this work.
References
[1] Modern Alkaloids: Structure, Isolation, Synthesis and Biology. E. Fattorusso, O. Taglialatela-Scafati (Eds.) Wiley-VCH, Weinheim, Germany (2008).Search in Google Scholar
[2] K. S. Atwal, B. N. Swanson, S. E. Unger, D. M. Floyd, S. Moreland, A. Hedberg, B. C. O’Reilly. J. Med. Chem. 34, 806 (1991).Search in Google Scholar
[3] Y. Usami, J. Yamaguchi, A. Numata. Heterocycles. 63, 1123 (2004).Search in Google Scholar
[4] For leading literatures of chiral phosphoric acid catalysis: (a) T. Akiyama, J. Itoh, K. Yokota, K. Fuchibe. Angew. Chem. Int. Ed. 43, 1566 (2004); (b) D. Uraguchi, M. Terada. J. Am. Chem. Soc. 126, 5356 (2004); for recent reviews, see: (c) T. Akiyama. Chem. Rev. 107, 5744 (2007); (d) A. Zamfir, S. Schenker, M. Freund, S. B. Tsogoeva. Org. Biomol. Chem. 8, 5262 (2010); (e) M. Terada. Synthesis. 1929 (2010).Search in Google Scholar
[5] J. Yu, F. Shi, L. -Z. Gong. Acc. Chem. Res. 44, 1156 (2011).Search in Google Scholar
[6] For reviews, see: (a) Z. Shao, H. Zhang. Chem. Soc. Rev. 38, 2745 (2009); (b) J. Zhou. Chem. Asian J. 5, 422 (2010). (c) C. Zhong, X. Shi. Eur. J. Org. Chem. 2999 (2010); (d) A. S. K. Hashmi, C. Hubbert. Angew. Chem. Int. Ed. 49, 1010 (2010); for first example illustrating the compatibility: (e) K. Sorimachi, M. Terada. J. Am. Chem. Soc. 130, 14452 (2008).Search in Google Scholar
[7] For some reviews, see: (a) I. Coldham, R. Hufton. Chem. Rev. 105, 2765 (2005); (b) G. Pandey, P. Banerjee, S. R. Gadre. Chem. Rev. 106, 4484 (2006).Search in Google Scholar
[8] For early reports, see: (a) J. M. Longmire, B. Wang, X. Zhang. J. Am. Chem. Soc. 124, 13400 (2002); (b) A. S. Gothelf, K. V. Gothelf, R. G. Hazell, K. A. Jørgensen. Angew. Chem. Int. Ed. 41, 4236 (2002); for reviews, see: (c) C. Nájera, J. M. Sansano. Angew. Chem. Int. Ed. 44, 6272 (2005); (d) L. M. Stanley, M. P. Sibi. Chem. Rev. 108, 2887 (2008); (e) J. Adrio, J. C. Carretero. Chem. Commun. 47, 6784 (2011) and references cited therein.Search in Google Scholar
[9] (a) J. L. Vicario, S. Reboredo, D. Badía, L. Carrillo. Angew. Chem. Int. Ed. 46, 5168 (2007); (b) I. Ibrahem, R. Rios, J. Vesely, A. Córdova. Tetrahedron Lett. 48, 6252 (2007); (c) M. -X. Xue, X. -M. Zhang, L. -Z. Gong. Synlett 691 (2008); (d) Y. -K. Liu, H. Liu, W. Du, L. Yue, Y. -C. Chen. Chem. Eur. J. 14, 9873 (2008).Search in Google Scholar
[10] (a) X. -H. Chen, W. -Q. Zhang, L. -Z. Gong. J. Am. Chem. Soc. 130, 5652 (2008); (b) L. He, X. -H. Chen, D. -N. Wang, S. -W. Luo, W. -Q. Zhang, J. Yu, L. Ren, L. -Z. Gong. J. Am. Chem. Soc. 133, 13504 (2011); (c) X. -H. Chen, Q. Wei, S. -W. Luo, H. Xiao, L. -Z. Gong. J. Am. Chem. Soc. 131, 13819 (2009); (d) W. -J. Liu, X. -H. Chen, L. -Z. Gong. Org. Lett. 10, 5357 (2008); (e) J. Yu, L. He, X. -H. Chen, J. Song, W. -J. Chen, L. -Z. Gong. Org. Lett. 11, 4946 (2009); (f) C. Wang, X. -H. Chen, L. -Z. Gong. Chem. Commun. 1275 (2010); (g) F. Shi, S. -W. Luo, Z. -L. Tao, L. He, J. Yu, S. -J. Tu, L.-Z. Gong. Org. Lett. 13, 4680 (2011).Search in Google Scholar
[11] For examples, see: (a) A. S. Girgis. Eur. J. Med. Chem. 44, 91 (2009); (b) R. Murugan, S. Anbazhagan, S. S. Narayanan. Eur. J. Med. Chem. 44, 3272 (2009); (c) S. U. Maheswari, K. Balamurugan, S. Perumal, P. Yogeeswari, D. Sriram. Bioorg. Med. Chem. Lett. 20, 7278 (2010); (d) R. S. Kumar, S. M. Rajesh, S. Perumal, D. Banerjee, P. Yogeeswari, D. Sriram. Eur. J. Med. Chem. 45, 411 (2010); (e) A. Thangamani. Eur. J. Med. Chem. 45, 6120 (2010); (f) P. Prasanna, K. Balamurugan, S. Perumal, P. Yogeeswari, D. Sriram. Eur. J. Med. Chem. 45, 5653 (2010); (g) M. A. Ali, R. Ismail, T. S. Choon, Y. K. Yoon, A. C. Wei, S. Pandian, R. S. Kumar, H. Osman, E. Manogaran. Bioorg. Med. Chem. Lett. 20, 7064 (2010).Search in Google Scholar
[12] F. Shi, Z. -L. Tao, S. -W. Luo, S. -J. Tu, L. -Z. Gong. Chem. Eur. J. 18, 6885 (2012).Search in Google Scholar
[13] (a) “Bisindole Alkaloids”: G. A. Cordell, J. E. Saxton in The Alkaloids: Chemistry and Physiology, R. H. F. Manske, R. G. A. Rodrigo (Eds.) Academic Press, New York, Vol. 20, p 3–294 (1981); (b) “Chemistry and Reactions of Cyclic Tautomers of Tryptamines and Tryptophans”: T. Hino, M. Nakagawa in The Alkaloids: Chemistry and Pharmacology, A. Brossi (Ed.) Academic Press, New York, Vol. 34, p 1–75 (1989); (c) “Alkaloids from the Medicinal Plants of New Caledonia”: T. Sévenet, J. Pusset in The Alkaloids: Chemistry and Pharmacology, G. A. Cordell (Ed.) Academic Press, New York, Vol. 48, p 58–59 (1996); (d) “Naturally Occurring Cyclotryptophans and Cyclotryptamines”: U. Anthoni, C. Christophersen, P. H. Nielsen in Alkaloids: Chemical & Biological Perspectivese, S.W. Pelletier (Ed.) Wiley, New York, Vol. 13, p 163–236 (1999); <softenter;(e) D. Crich,; A. Banerjee, Acc. Chem. Res. 40, 151 (2007).Search in Google Scholar
[14] A. Steven, L. E. Overman. Angew. Chem. Int. Ed. 46, 5488 (2007).Search in Google Scholar
[15] (a) L. E. Overman, D. V. Paone, B. A. Stearns. J. Am. Chem. Soc. 121, 7702 (1999); (b) L. E. Overman, J. F. Larrow, B. A. Stearns, J. M. Vance. Angew. Chem. Int. Ed. 39, 213 (2000).Search in Google Scholar
[16] (a) M. Movassaghi, M. A. Schmidt. Angew. Chem. Int. Ed. 46, 3725 (2007); (b) M. Movassaghi, M. A. Schmidt, J. A. Ashehurst. Angew. Chem. Int. Ed. 47, 1485 (2008); (c) J. Kim, J. A. Ashenhurst, M. Movassaghi. Science. 324, 238 (2009); (d) J. Kim, M. Movassaghi. J. Am. Chem. Soc. 132, 14376 (2010).Search in Google Scholar
[17] C. Guo, J, Song, J. -Z. Huang, P. -H. Chen, S. -W. Luo, L. -Z. Gong. Angew. Chem. Int. Ed. 51, 1046 (2012).Search in Google Scholar
[18] C. Ramalingan, Y. -T. Park. J. Org. Chem. 72, 4536 (2007).Search in Google Scholar
[19] C. -L. Fang, S. Horne, N. Taylor, R. Rodrigo. J. Am. Chem. Soc. 116, 9480 (1994).Search in Google Scholar
[20] (a) L. E. Overman, Y. Shin. Org. Lett. 9, 339 (2007); (b) J. E. Delorbe, S. Y. Jabri, S. M. Mennen, L. E. Overman, F. Zhang. J. Am. Chem. Soc. 133, 6549 (2011); (c) L. Furst, J. M. R. Narayanam, C. R. J. Stephenson. Angew. Chem. Int. Ed. 50, 9655 (2011); (d) N. Boyer, M. Movassaghi. Chem. Sci. 3, 1798 (2012).Search in Google Scholar
[21] J. Song, C. Guo, A. Adele, H. Yin, L. -Z. Gong. Chem. Eur. J. 19, 3319 (2013).Search in Google Scholar
[22] For a review on primary amine catalysis: (a) L. -W. Xu, J. Luo, Y. Lu. Chem. Commun. 1807 (2009); for reviews focusing on the reasons that have brought about the development of cinchona-based primary amines, see: (b) L. Jiang, Y. -C. Chen. Catal. Sci. Technol. 1, 354 (2011); (c) G. Bartoli, P. Melchiorre. Synlett 1759 (2008); (d) Y. -C. Chen. Synlett 1919 (2008); (e) P. Melchiorre. Angew. Chem. Int. Ed. 51, 9748 (2012).Search in Google Scholar
[23] (a) J. -W. Xie, X. Huang, L. -P. Fan, D. -C. Xu, X. -S. Li, H. Su, Y. -H. Wen. Adv. Synth. Catal. 351, 3077 (2009); (b) X. Tian, C. Cassani, Y. Liu, A. Moran, A. Urakawa, P. Galzerano, E. Arceo, P. Melchiorre. J. Am. Chem. Soc. 133, 17934 (2011); (c) G. Bergonzini, S. Vera, P. Melchiorre. Angew. Chem. Int. Ed. 49, 9685 (2010); (d) O. Lifchits, C. M. Reisinger, B. List. J. Am. Chem. Soc. 132, 10227 (2010).Search in Google Scholar
[24] For our recent contributions on relay catalysis, see: (a) Z. Y. Han, H. Xiao, X. H. Chen, L. Z. Gong. J. Am. Chem. Soc. 131, 9182 (2009); (b) C. Wang, Z. Y. Han, H. W. Luo, L. Z. Gong. Org. Lett. 12, 2266 (2010); (c) Z. -Y. Han, R. Guo, P. -S. Wang, D. -F. Chen, H. Xiao, L. -Z. Gong. Tetrahedron Lett. 52, 5963 (2011); (d) H. Wu, Y. -P. He, L. -Z. Gong. Adv. Syn. Catal. 354, 975 (2012); (e) Z. -Y. Han, D. -F. Chen, Y. -Y. Wang, L. -Z. Gong. J. Am. Chem. Soc. 134, 6532 (2012); (f) H. Wu, Y. -P. He, L. -Z. Gong. Org. Lett. 15, 460 (2013); (g) Y. -P. He, H. Wu, D. -F. Chen, J. Yu, L. -Z. Gong. Chem. Eur. J. 19, 5232 (2013); (h) P. -S. Wang, K. -N. Li, X. -L. Zhou, X. Wu, Z. -Y. Han, R. Guo, L. -Z. Gong. Chem. Eur. J. 19, 6234 (2013); (i) D. -F. Chen, P. -Y. Wu, L. -Z. Gong. Org. Lett. 15, 3958 (2013).Search in Google Scholar
[25] (a) P. Friedländer. Berichte 15, 2572 (1882); (b) C. C. Cheng, S. J. Yan. Org. React. 28, 37 (1982); (c) J. M. Contelles, E. P. Mayoral, A. Samadi, M. C. Carreiras, E. Soriano. Chem. Rev. 109, 2652 (2009).Search in Google Scholar
[26] L. Ren, T. Lei, J. -X. Ye, L. -Z. Gong. Angew. Chem. Int. Ed. 51, 771 (2012).Search in Google Scholar
©2014 IUPAC & De Gruyter Berlin/Boston
Articles in the same Issue
- Frontmatter
- Congress-44 Environmental Chemistry
- Preface
- 44th IUPAC Congress: Environmental Chemistry
- Conference papers
- Estimating the bioavailability of trace metals/metalloids and persistent organic substances in terrestrial environments: challenges and need for multidisciplinary approaches
- Chemical speciation in fresh, saline and hyper-saline waters
- Uranium toxicity and chelation therapy
- Analysis of trace elements in surface sediments, mussels, seagrass and seawater along the southeastern Adriatic coast – a chemometric approach
- Advances in understanding the transformation of engineered nanoparticles in the environment
- Novel Fe-Pd/SiO2 catalytic materials for degradation of chlorinated organic compounds in water
- Nanotechnology management for a safer work environment
- Bibliometric analysis of research on secondary organic aerosols: Update
- Surface-functionalized silica gel adsorbents for efficient remediation of cationic dyes
- Experimental study of cadmium bioaccumulation in three Mediterranean marine bivalve species: correlation with selected biomarkers
- Water scarcity, water reuse, and environmental safety
- ICHC-24
- Preface
- The 24th International Society of Heterocyclic Chemistry Congress (ICHC-24)
- Conference papers
- Organocatalytic asymmetric synthesis of chiral nitrogenous heterocycles and natural products
- Efficient asymmetric syntheses of alkaloids and medicinally relevant molecules based on heterocyclic chiral building blocks
- Naphthalimides for labeling and sensing applications
- Metal-catalyzed synthesis of heterocycles bearing a trifluoromethyl group
Articles in the same Issue
- Frontmatter
- Congress-44 Environmental Chemistry
- Preface
- 44th IUPAC Congress: Environmental Chemistry
- Conference papers
- Estimating the bioavailability of trace metals/metalloids and persistent organic substances in terrestrial environments: challenges and need for multidisciplinary approaches
- Chemical speciation in fresh, saline and hyper-saline waters
- Uranium toxicity and chelation therapy
- Analysis of trace elements in surface sediments, mussels, seagrass and seawater along the southeastern Adriatic coast – a chemometric approach
- Advances in understanding the transformation of engineered nanoparticles in the environment
- Novel Fe-Pd/SiO2 catalytic materials for degradation of chlorinated organic compounds in water
- Nanotechnology management for a safer work environment
- Bibliometric analysis of research on secondary organic aerosols: Update
- Surface-functionalized silica gel adsorbents for efficient remediation of cationic dyes
- Experimental study of cadmium bioaccumulation in three Mediterranean marine bivalve species: correlation with selected biomarkers
- Water scarcity, water reuse, and environmental safety
- ICHC-24
- Preface
- The 24th International Society of Heterocyclic Chemistry Congress (ICHC-24)
- Conference papers
- Organocatalytic asymmetric synthesis of chiral nitrogenous heterocycles and natural products
- Efficient asymmetric syntheses of alkaloids and medicinally relevant molecules based on heterocyclic chiral building blocks
- Naphthalimides for labeling and sensing applications
- Metal-catalyzed synthesis of heterocycles bearing a trifluoromethyl group

