Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
-
Ahmed J. Jasim
, Hilal Ay
, Majid S. Jabir
Abstract
Chrysin (CHR), a dihydroxy flavone, exhibits several bioactivities, i.e., anti-oxidant, anti-inflammatory, and anti-cancer, and is known to possess limited aqueous solubility causing lowered bioavailability, and compromised therapeutic efficacy. Gold nanoparticles (AuNPs) conjugated chrysin (CHR–AuNPs) were prepared and characterized by UV-Vis, Fourier transform infra-red, X-ray diffraction, energy dispersive X-ray (EDX), and zeta potential analyses. The nanoformulated CHR–AuNPs were primarily examined on trial scale for their cytotoxic, anti-oxidant, and anti-microbial activity in comparison to the unformulated CHR. The CHR–AuNPs effectively scavenged the 2,2-diphenyl-1-picrylhydrazyl free radicals, also in comparison to CHR and AuNPs. The CHR–AuNPs also exhibited potential cytotoxic effects in a dose-dependent manner and demonstrated significant reduction (P = 0.05) of the cells proliferation, and growth of the human breast cancer cell lines, AMJ13, which were measured by 3-(4,5-dimethylthiazal-z-yl)-2,5-diphenyltetrazolium, and crystal violet assays, respectively. When compared with the pure CHR and free-AuNPs, the CHR–AuNPs exerted highest anti-microbial bioactivity against Staphylococcus aureus and Escherichia coli. The strong anti-oxidant, anti-microbial, as well as cytotoxic activity of the CHR–AuNPs preparation has the potential for clinical use after considerable appropriate developments.
Graphical abstract
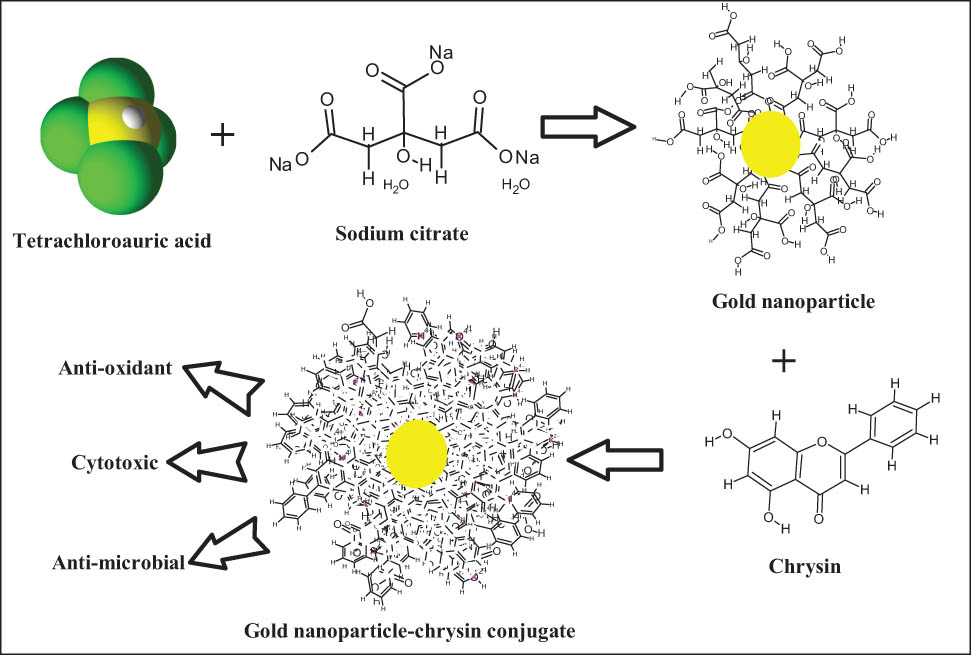
1 Introduction
Cancer, the state of uncontrolled cells proliferation, is a significant health problem all over the world, and more than 11 million people have been diagnosed with various cancer types [1]. It is estimated that by 2025, breast cancer cases will rise to 19 million. In addition, cancer is the most common cause of death, due to factors, such as poor oral absorption and diminished biodistribution of the chemotherapeutic agents. The adverse effects of conventional treatments, i.e., chemotherapy, radiotherapy, and surgery combined co-therapies are insufficiently effective [2]. As a result, new treatment modules with the enhanced oral absorption or facilitated systemic delivery, higher bioavailability, and better biotransport stability are required. Newer strategies for delivering the developed therapeutic modules with site-specificity and appropriate trigger release of the chemotherapeutic agents to tumor cells are a priority [3]. Recently, nanotechnology-based diagnosis and therapy systems have been developed for the majority of cancers treatment approaches [4]. The multifunctional nanosystems are among the most recent developments. Several strategies have been technologically advanced to selectively deliver these anti-cancer drugs to cancer lesions [5]. The majority of these strategies rely on the tumor microenvironment’s distinct biological and physical characteristics, which are manipulated to outreach to the cancer cells. Recently, special emphasis has been placed on the link between inflammation and the development of cancer cells in patients with stabilized (non-metastatic) cancers [6].
Nanomedicine, the nanotechnology branch dealing with the development of drugs and their delivery systems at the nanoscale, is a promising theranostic tool taking care of diagnosis and therapy as well. Recent breakthroughs in the utilization of nanoparticles (NPs) have also offered the renewed potential to dispense anti-cancer drugs to particular tissues with dose accuracy, therapeutic effectiveness, and safety [7,8]. The gold nanoparticles (AuNPs) have shown distinctive properties, and their numerous surface functions have made them one of the choices for a nanoscale particle for use in nanomedicines. These NPs are biocompatible and have a large surface-to-volume ratio which is considered enough for loading higher concentrations of single or multiple drugs for site delivery. The AuNPs have been utilized in a wide range of biotechnological applications, notably in drug delivery systems [9,10]. The AuNPs based therapeutic modules have been reported to have superior outcomes over conventional cancer treatment modalities due to their competitive performance as drug carriers [11].
Epidemiological studies concerning the roles of flavonoids in the healthcare system have confirmed that people with diets high in flavonoid products are less likely to develop cancer and some other chronic diseases [12]. There is also a growing interest in using flavonoid-rich plant-based products, as well as pure flavonoid compounds, as nutritional supplements in the treatment and prophylactics of cancer, metabolic disorders, cardiovascular diseases, and coronary disorders [12]. Flavonoids are poly-hydroxylated compounds with the molecular framework of the three cyclic structures, C6‒C3‒C6 carbons arrangement. The number and distribution of the hydroxyl groups attached to the flavonoid framework seem to play important role in the biological activities elicitations of these flavonoid classes of compounds [13]. Among these, chrysin (CHR) is one of the interesting anti-cancer compounds, which is chemically identified as 5,7-dihydroxyflavone [13]. CHR has been reported to possess several biological activities, and certain therapeutic potentials of this compound are confirmed. The anti-cancer activity of CHR has been established, and its role in the limitation of breast, ovarian, cervical, gastric, bladder, lungs, and prostate cancers have been reported [14]. Unfortunately, the poor bioavailability of CHR associated with its meager aqueous solubility and aqueous dispersion is a reason for its limited clinical applications [15], and this has led to devise suitable delivery systems for CHR-containing formulation [15,16], which needs to be a highly active tissue-targeted drug delivery system that can be synthesized from a wide range of materials, including inorganic NPs [17]. The NP-based therapy and drug delivery systems have the potential to provide a better outcome as a combination therapy. The AuNPs may outperform monotherapy in improving the efficacy and biocompatibility of a cancer therapeutic agent [18]. In this study, CHR was formulated as a gold conjugated nanoparticle (CHR–AuNP) preparation and was investigated as the nanoformulation for anti-oxidant, anti-cancer, and anti-microbial activities in comparison to the pure CHR. The anti-oxidant potential as 2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical scavenging capacity, cytotoxic activity against triple-negative human breast cancer-derived AMJ13 cell lines, and anti-microbial activity against Gram-positive and Gram-negative microbial strains were evaluated.
2 Materials and methods
2.1 Materials and reagents
CHR (structurally 5,7-dihydroxyflavone; purity ∼97%), DPPH, ascorbic acid, ethanol, fetal bovine serum (FBS), and RPMI-1640 medium were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Tetrachloroauric acid trihydrate (HAuCl4·3H2O) was obtained from Strem Chemicals (UK), while sodium citrate dihydrate (C6H5Na3O7·2H2O), dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazal-z-yl)-2,5-diphenyltetrazolium (MTT), and crystal violet were purchased from Sigma-Aldrich Chemie GmBH (Schnelldorf, Germany). Antibiotics such as penicillin and streptomycin were procured from Biosource International (Nivelles, Belgium). Molar Hinton agar was purchased from Merck, Germany. Double distilled water was obtained from Millipore water purification system. All the other chemicals and reagents used in the experiments were of analytical grade.
2.2 Organisms
AMJ13, human triple-negative breast cancer cell lines were provided by the Biotechnology Research Center, Al-Mustansiriyah University, Baghdad, Iraq. The cells were cultured in sterile Falcon flasks (T 25 cm2, USA), and supplemented with RPMI-1640 medium enriched with l-glutamine (2 mM) and FBS (10%, 20 mM) at a condition of 5% of CO2, 37°C of temperature, and 1 atmospheric pressure.
Clinical isolates, Escherichia coli (Gram negative) and Staphylococcus aureus (Gram positive), were used to evaluate the anti-microbial activity of the prepared formulations. The clinical isolates were provided by the Division of Biotechnology, Department of Applied Sciences, University of Technology, Baghdad, Iraq.
2.3 Preparation of AuNPs
AuNPs were prepared by chemical method. In brief, an aqueous solution of HAuCl4·3H2O (MW 393.83 amu, 0.1 mM, 10 mL water) was let to boil on a hot plate at atmospheric pressure under stirring, and at the boiling, trisodium citrate (MW 294 amu, 0.3 mM, 15 mL water) was added in one instance, heating was stopped, and stirring continued for overnight at room temperature (22–25°C) [19].
2.4 CHR–AuNPs conjugation
Pure CHR (10 mg) was dissolved in 5 mL of DMSO and stirred at 1,000 rpm for 15 min under room temperature to obtain a homogeneous solution with complete and clear visible solubility [20]. The CHR suspension was added to the solution of AuNPs (1:9 mL) and stirred for 20 h, through overnight at room temperature. The color of the solution changed to light violet, and the excess CHR was removed by ultracentrifugation (13,000 rpm, 30 min) at the end of the reaction [21]. The percentage of conjugated CHR on the AuNPs were calculated using the following equation:
The CHR in vitro release from CHR–AuNPs was performed as previously reported [20] with minor modification. Briefly, 10 mg of CHR–AuNPs was taken in a release medium constituting PBS and DMSO at 37°C under agitation. The pH was set at 7.4, and CHR is released for different periods from 1 to 15 h, after each hour the measurement of the CHR absorbance was recorded by the UV-Vis spectrophotometer. The concentrations of the released CHR from CHR–AuNPs were calculated using the following equation:
where Wr is the CHR weight in each release and Wt is the weight of CHR–AuNPs taken.
2.5 Characterization of prepared NPs
For Fourier transform infra-red (FT-IR) analysis, a spectrophotometer (8400S, Shimadzu, Japan) with a spectral range from 4,000 to 400 cm−1 and a resolution of 4 cm−1 was used. An X-ray diffractometer was employed to determine the crystalline state of the prepared samples (XRD-6000, Shimadzu, Japan). The diffraction patterns were obtained using the K copper incident beam (λ = 1.542°) at 2 = 20–60° planes. The X-ray tube had 45 kV voltage and 30 mA current. For field emission scanning electron microscopy (FE-SEM) analysis, MIRA 3 TESCAN was used. To examine the shape and metallic presence, an energy dispersive X-ray analysis (EDX) was performed. The Zeta PALS instrument (UK) was used to measure the zeta potential and particle size [22].
2.6 Cytotoxicity assay
Standard MTT assay was used to evaluate the cytotoxic activity of the AuNPs, CHR, and the CHR–AuNPs nanoformulation against AMJ13 cell lines [23]. The AMJ13 cells were grown and maintained in Falcon® flasks containing RPMI-1640 supplemented with 10% FBS, 100 units/mL penicillin, and 100 µg/mL streptomycin. The cells were passaged twice a week with Trypsin-EDTA, reseeded at 80% confluence, and incubated at 37°C. The cell lines were seeded at a density of 1 × 104 cells/well and treated with AuNPs, CHR, and the CHR–AuNPs formulation at various concentrations after 24 h, or when a confluent monolayer was achieved. After 72 h of treatments, cells viability was assessed by removing the medium, adding 28 µL of 2 mg/mL MTT solution, and incubating the cells for 2.5 h at 37°C. Following the removal of the MTT solution, the crystals in the wells were solubilized by adding 130 µL of DMSO, followed by 15 min of incubation at 37°C with shaking. The absorbance was measured using a microplate reader at 492 nm, and the experiments were repeated thrice. The reduction in cells proliferations (cytotoxicity) was calculated using the following equation:
where Ab control is the absorbance of the control, while Bb is the absorbance obtained for the tested samples.
Crystal violet staining was also used to investigate the viability of the cells in the presence of AuNPs, CHR, and CHR–AuNPs. The cells were seeded into 24-well micro-titration plates at a density of 1 × 105 cells/mL and incubated for 24 h at 37°C to visualize their shape under an inverted microscope [24]. The cells were then treated with the IC50 concentration of AuNPs, CHR, and CHR–AuNPs for 24 h. The plates were stained with crystal violet and incubated for 12 min at 37°C. Then, tap water was used to remove the dye, and the cells were investigated under the microscope at 40× magnification power, where clear images of the cells were captured with a digital camera attached to the microscope [25].
2.7 Anti-oxidant activity
Anti-oxidant activities of AuNPs, CHR, and CHR–AuNPs formulations were measured using DPPH free radicals scavenging method [26]. The tested samples (1 mL in absolute ethanol) at four different concentrations, 1.3, 2.6, 12.5, and 25 μg mL were mixed with 450 µL of DPPH solution. The standard positive control, ascorbic acid (at a concentration of 10 µg/mL), was mixed with DPPH solution in the same manner. The reduction in the DPPH free radicals color in response to the scavenging power of the tested samples and ascorbic acid was measured spectrophotometrically at 517 nm after 30 min of incubation in dark. The anti-oxidant activity of the samples, in terms of their scavenging effects, was calculated from the following equation:
where Ab control and Ab sample are the absorbance of the control and test samples, respectively.
2.8 Anti-microbial activity
The anti-microbial activities of AuNPs, CHR, and CHR–AuNPs were tested against human pathogens: S. aureus and E. coli. The bacteria were extracted from their stock cultures using a sterile wire loop [27]. To investigate the effects of AuNPs, CHR, and CHR–AuNPs, the growth curves of the bacteria were obtained. The microbial strains were cultured on Molar Hinton agar plates at 37°C, with inoculations of 50 mL of nutrient broth on freshly cultured plates [28]. The bacteria grew until the nutrient broth reached an optical density (OD) of 0.1 at 600 nm, which corresponded to a microbial concentration of 108 CFU/mL. The nutrient broth was then supplemented with NPs, and microbial cultures (1 mL) were added and incubated at 37°C for 12 h under slight agitation. Microbial growth was determined using a spectrophotometer by measuring the OD [29].
2.9 Statistical analysis
All the data were analyzed using the unpaired t-test, which compared the studying groups at a significant p-value of <0.05. All experiments were performed in triplicate and data were calculated as the mean ± standard deviation.
3 Results
3.1 NPs preparation and CHR conjugation
The AuNPs were prepared by chemical reduction method utilizing trisodium citrate dehydrate and HAuCl4·3H2O. A solution of HAuCl4·3H2O was reacted with trisodium citrate dihydrate aqueous solution, and the reaction mixture color changed from pale yellow to purple, and finally to red, indicating the preparation of the AuNPs (Figure 1). Based on equation (1), the AuNPs conjugation to CHR was 15.2%, and the release kinetics of CHR in physiological pH of 7.4 was performed using UV-Vis spectroscopy (Figure 2). During the early phase of first hour, it was ∼25% release, in the second hour the release rate was over 50%. In the sixth hour the (%) release rate reached ∼80%. From 6 to 9 h, the release was observed at above 85% and thereafter it stabilized for the rest of the total 15 h of the drug release period during the simulated release experiment. The fast release of the drug was observed from 9 to 15 h at ∼85%.

Samples: (a) AuNPs preparation, (b) CHR solution, and (c) CHR–AuNPs preparation.
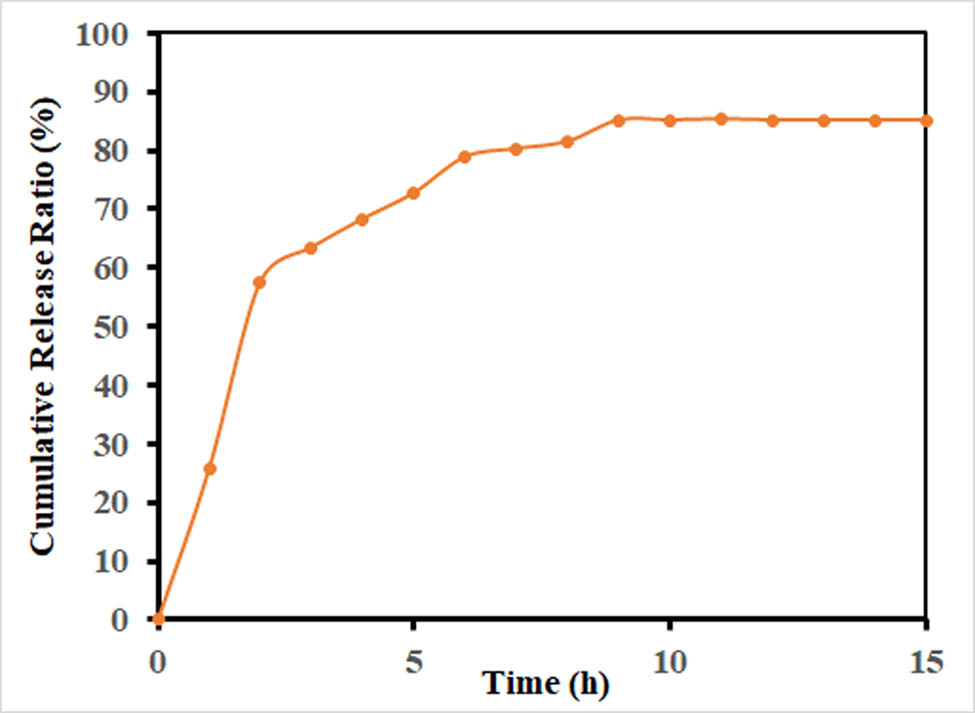
Release profile of CHR from the CHR–AuNPs nanoformulation.
3.2 UV-Vis spectroscopy of prepared NPs
To confirm the formation and binding of AuNPs to CHR, UV-Vis spectrophotometry was used. As seen from Figure 3, the AuNPs’ UV-Vis spectrum showed absorption maxima λ max at 520 nm. While the CHR, a light yellowish solution, showed the UV absorption maxima at λ max 268 nm. The absorption maxima for CHR–AuNPs was exhibited at 260 and 550 nm λ max values.
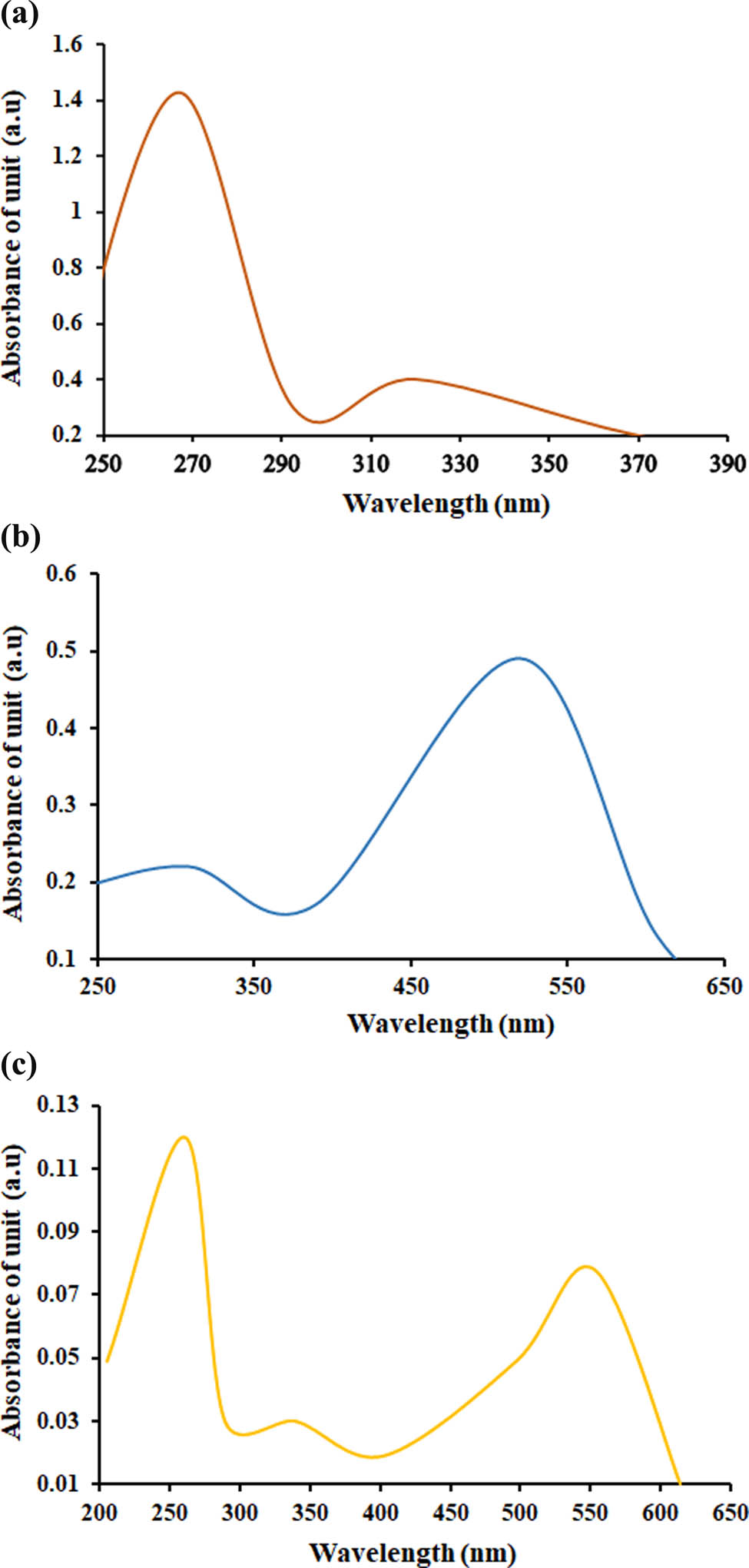
UV-Vis spectroscopic spectra: (a) AuNPs, (b) CHR, and (c) CHR–AuNPs.
3.3 FT-IR spectroscopic analyses
The FT-IR spectrum (Figure 4) of CHR exhibited absorptions at 3012.79 cm−1 (OH), at 2929.87, 2713.84, and 2630.91 cm−1 (C‒H stretchings), and at 1653.00 cm−1 (α,β-unsaturated carbonyl, C═O). The bands at 1355.96 and 1028.06 cm−1 (C‒O‒C) and 840.96 and 731.02 cm−1 (aromatic character) were observed. The AuNPs-CTT absorptions at 3419.79 cm−1 (O‒H stretching), 2987.74 and 2931.80 cm−1 (C‒H stretchings), at 1583.56 cm−1 (C═O) group, and at 1398.39 cm−1 (ether function) were observed for the citrate-surface AuNPs. The CHR–AuNPs absorption at 3448.72 cm−1 (O‒H), at 2960.73 and 2935.66 cm−1 (CH stretchings), at 1587.42, 1463.97, and 1390.68 cm−1 (aromatic C═C bonds), and 1276.88 cm−1 (C‒O stretchings) were observed.
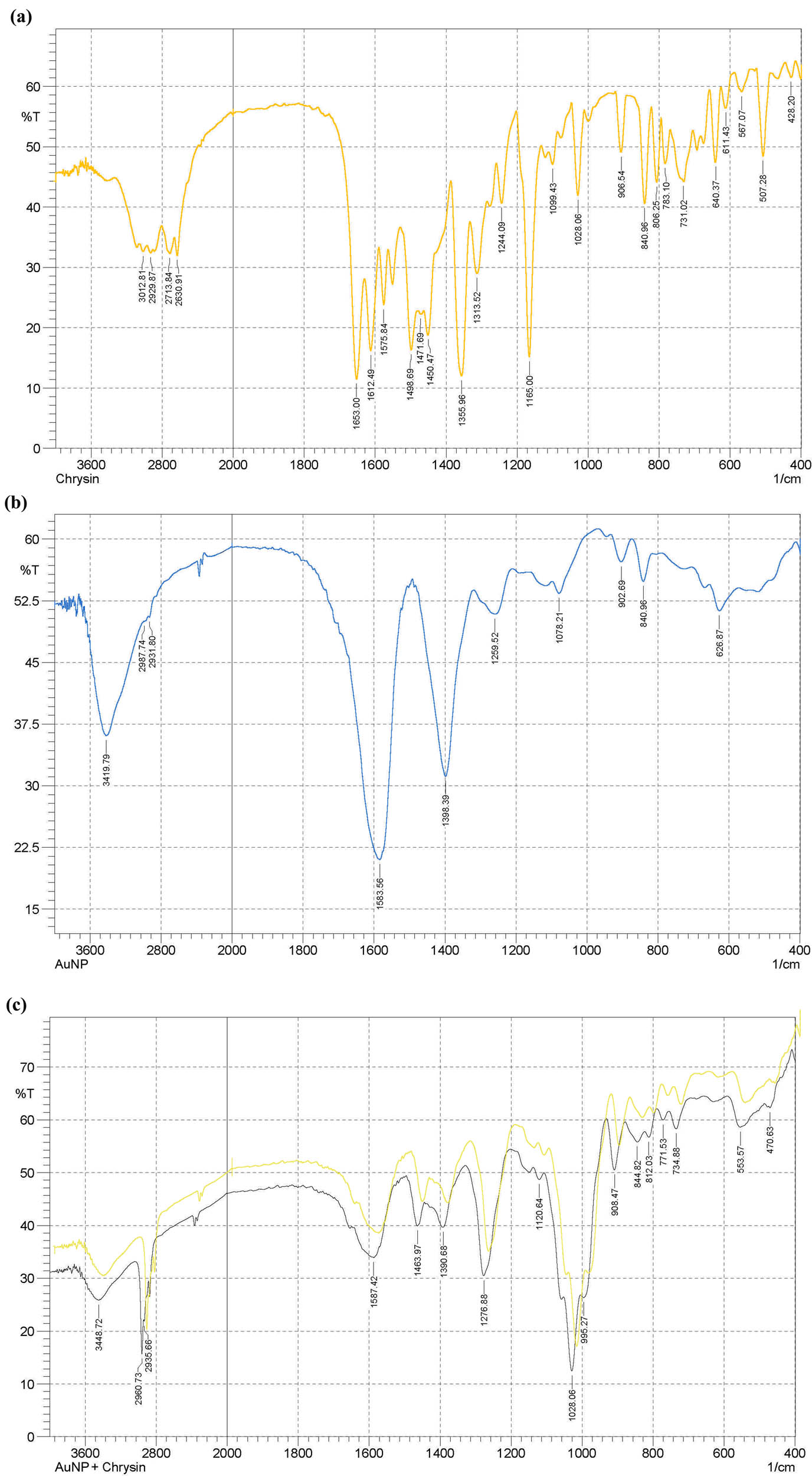
FT-IR spectroscopic analyses: (a) CHR, (b) AuNPs, and (c) CHR–AuNPs.
3.4 X-ray diffraction (XRD) analyses
Crystal structures of CHR, AuNPs, and CHR–AuNPs were confirmed by the XRD analysis (Figure 5). The AuNPs’ diffraction peaks were observed at 30.56°, 38.12°, 44.18°, and 56.35°, which verified the face-centered polycrystalline cubic structure. The crystallinity of AuNPs was compared with the available XRD pattern in the database, JCPDS 00-004-0784 [30]. The CHR showed strong 2θ peaks at 7.18°, 12.60°, 14.92°, 17.75°, 92.14°, 27.34°, and 27.68°, indicating a more crystalline structure. When CHR was loaded onto AuNPs, five peaks were observed, i.e., 23.5°, 27.55°, 38.15°, 44.01°, and 57.45°.
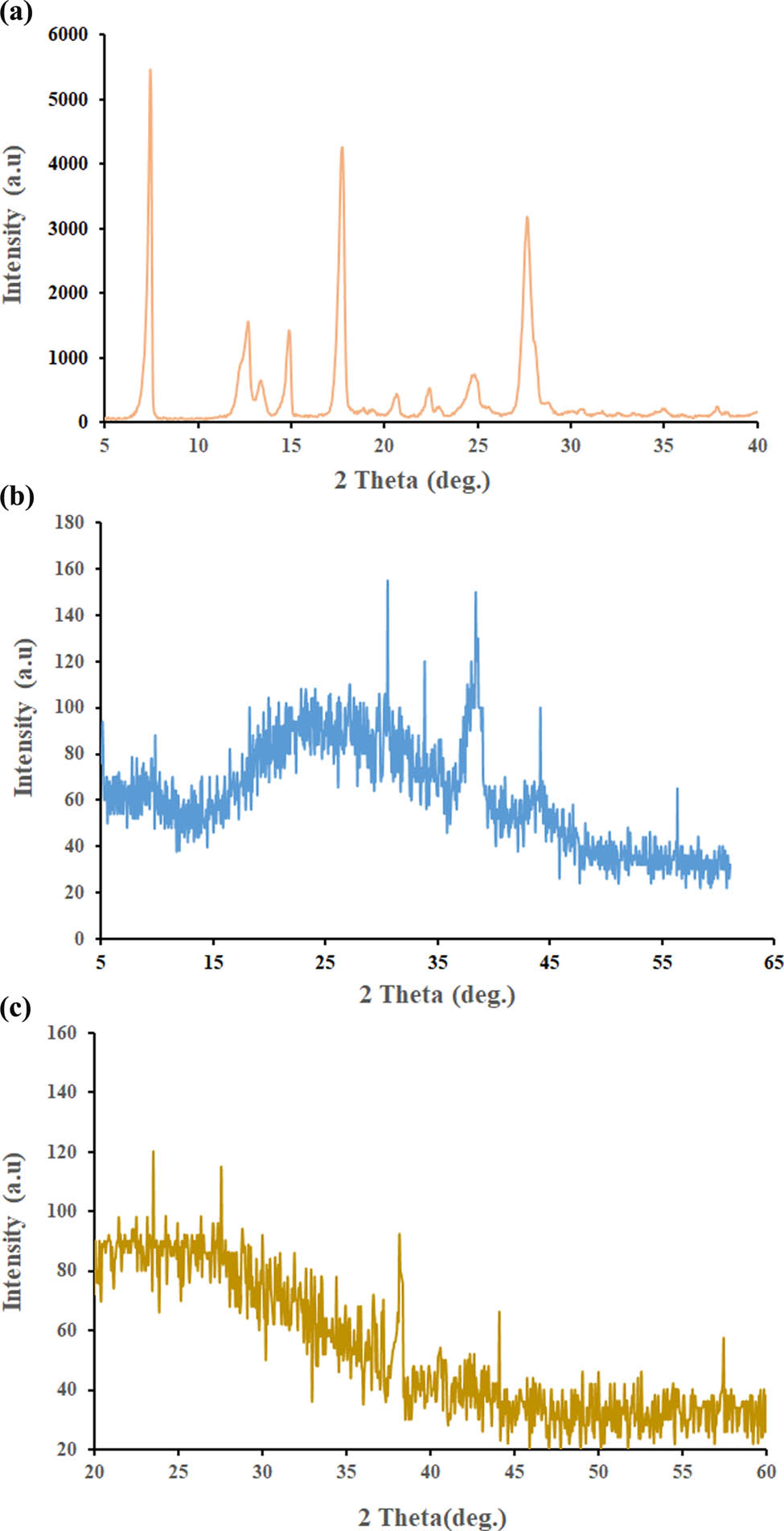
XRD analyses: (a) CHR, (b) AuNPs, and (c) CHR–AuNPs.
3.5 FE-SEM and EDX analyses
FE-SEM images (Figure 6) exhibited relatively spherical shapes of the AuNPs with 17.37–25.90 nm size range. CHR–AuNPs nano-preparation showed spherical, nearly homogenous composition with size ranging between 31.53 and 38.58 nm. The CHR EDX analysis showed the presence of C and O, the AuNPs EDX analysis showed the presence of Au (51.76%, main component), while CHR–AuNPs showed the presence of Au, C, and O elements, as shown in Figure 6; right lane.
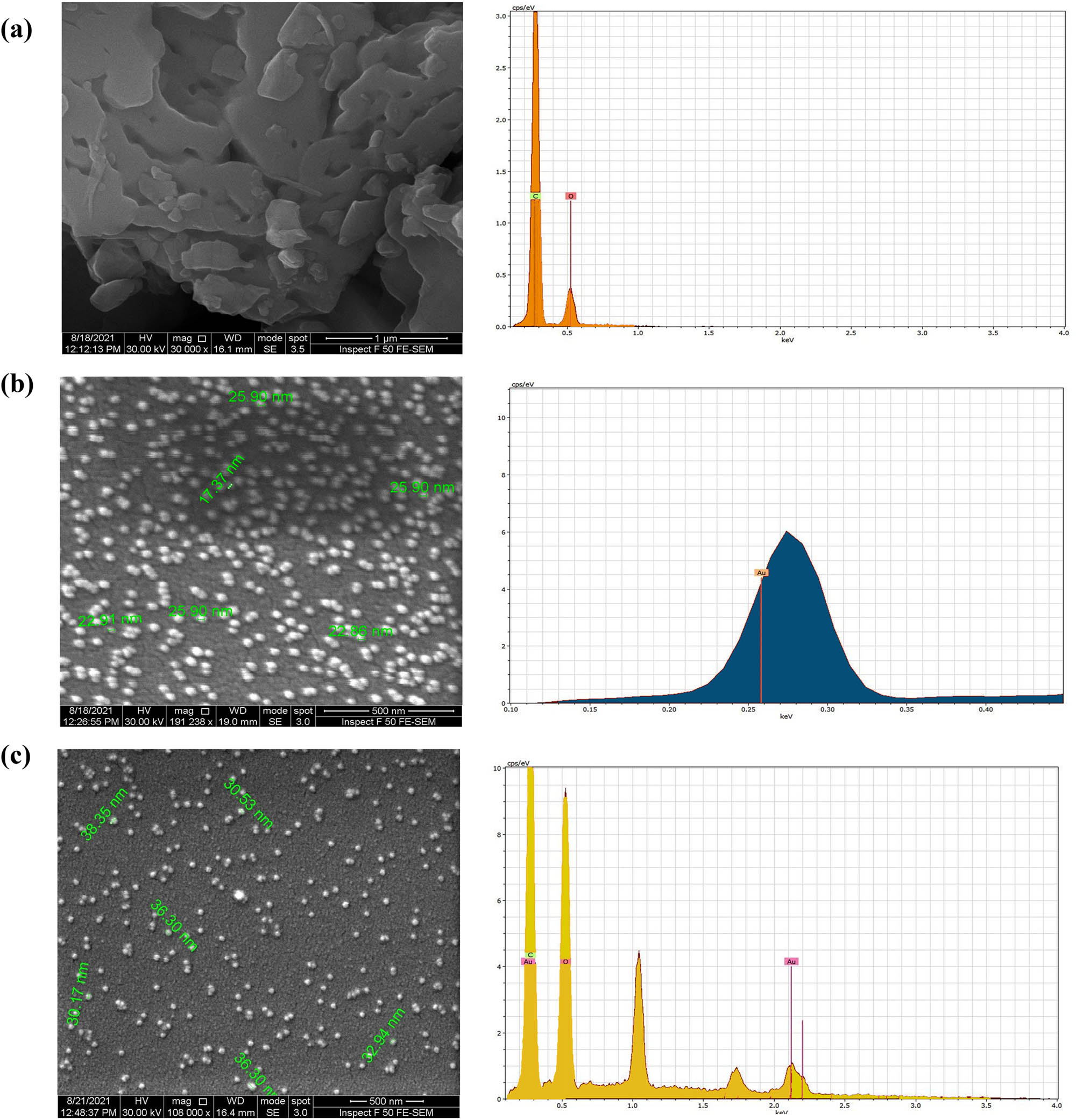
FE-SEM, and energy dispersive analysis (EDX): (a) CHR, (b) AuNPs, and (c) CHR–AuNPs.
3.6 ζ-potential analyses
CHR ζ-potential and mobility were −161.00 and −3.30, respectively. The AuNPs carried −31.54 mV ζ-potential, and the mobility was −2.46, while the CHR–AuNPs carried −48.92 mV ζ-potential, and mobility was −3.82 (Figure 7).

The ζ-potentials: (a) CHR, (b) AuNPs, and (c) CHR–AuNPs.
3.7 Cytotoxicity testing against AMJ13 cell lines
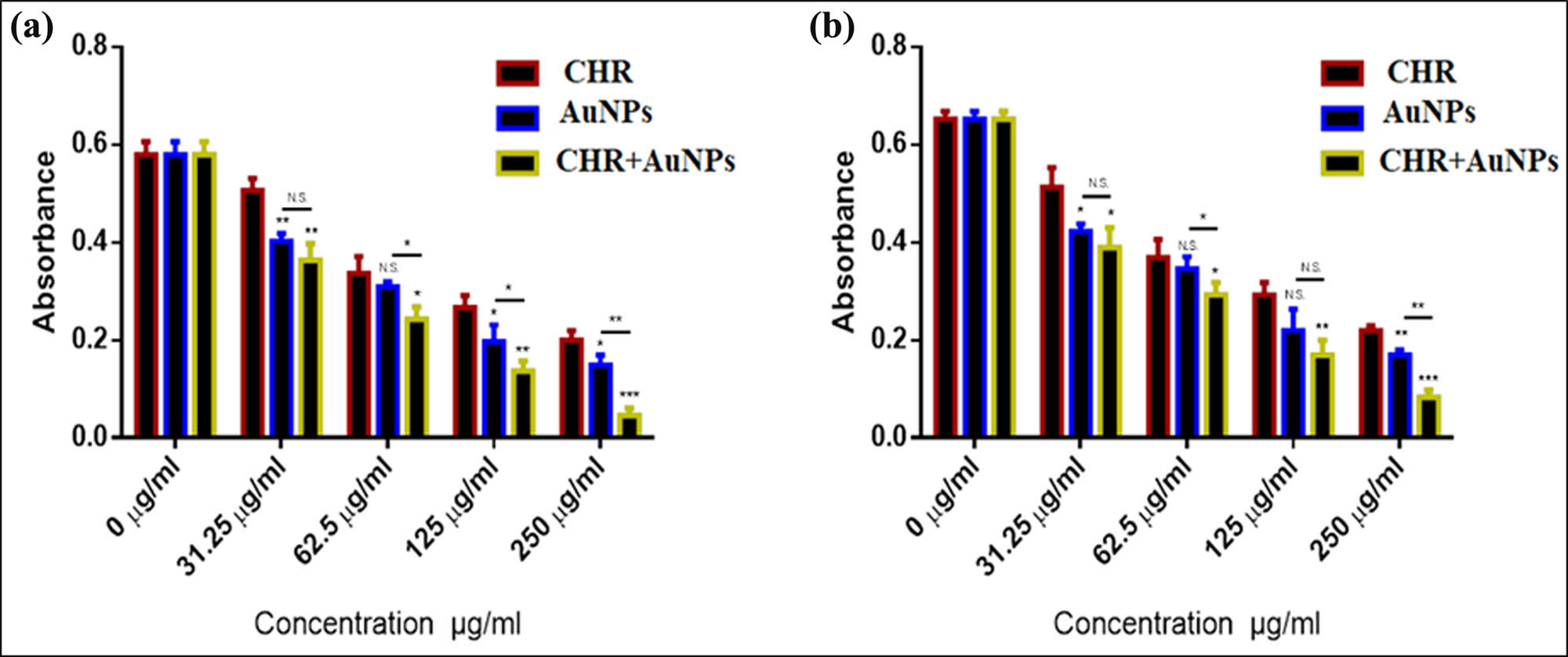
The CHR–AuNPs exhibited higher anti-cancer activity against AMJ13 breast cancer cell lines in comparison to CHR and AuNPs with concentration-dependent cytotoxicity (Figure 8). At 100 µg/mL CHR–AuNPs concentration, highest 89% cytotoxicity was observed, while at 6.25 µg/mL, 17% cytotoxicity was observed for the CHR–AuNPs as compared to the control group. The AuNPs treatment was 73.3% effective at 100 µg/mL, whereas CHR treatment was 41.5% effective. At 6.25 µg/mL, AuNPs were effective at 8.9% and CHR was effective at 4.6%.
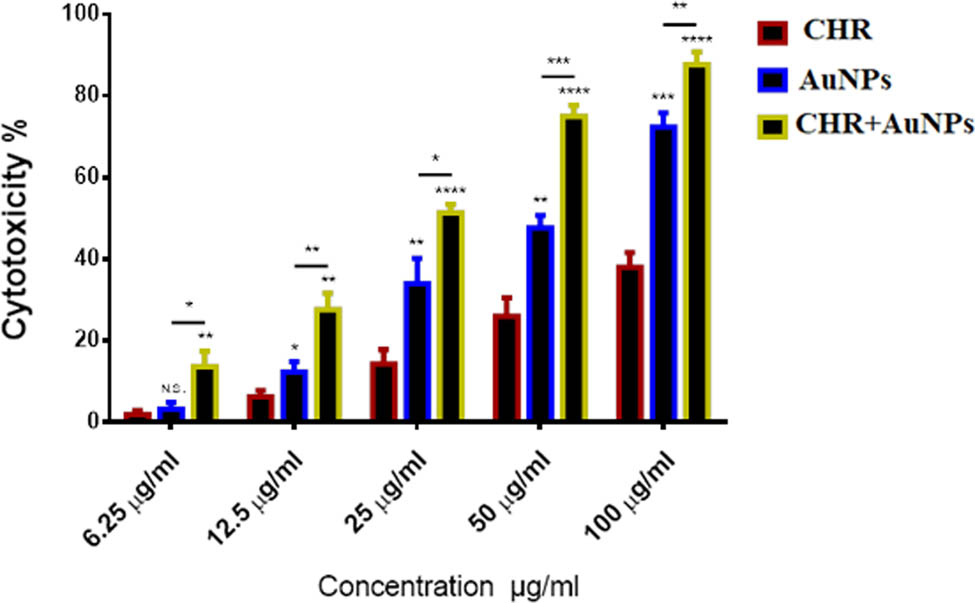
Cytotoxic effects: CHR, AuNPs, and CHR–AuNPs on AMJ13 cell lines; n = 3, N.S., non-significant, *P < 0.05, **P < 0.01, and ***P < 0.001.
Crystal violet staining and microscopic examination (Figure 9) demonstrated stronger cytotoxic impacts of CHR–AuNPs against the AMJ13 cell lines. CHR–AuNPs caused cell shape changes, cell size reduction, cell clustering with reduced cell extensions, and shrinkage of nuclei. These changes were absent in non-treated cells (Figure 9).
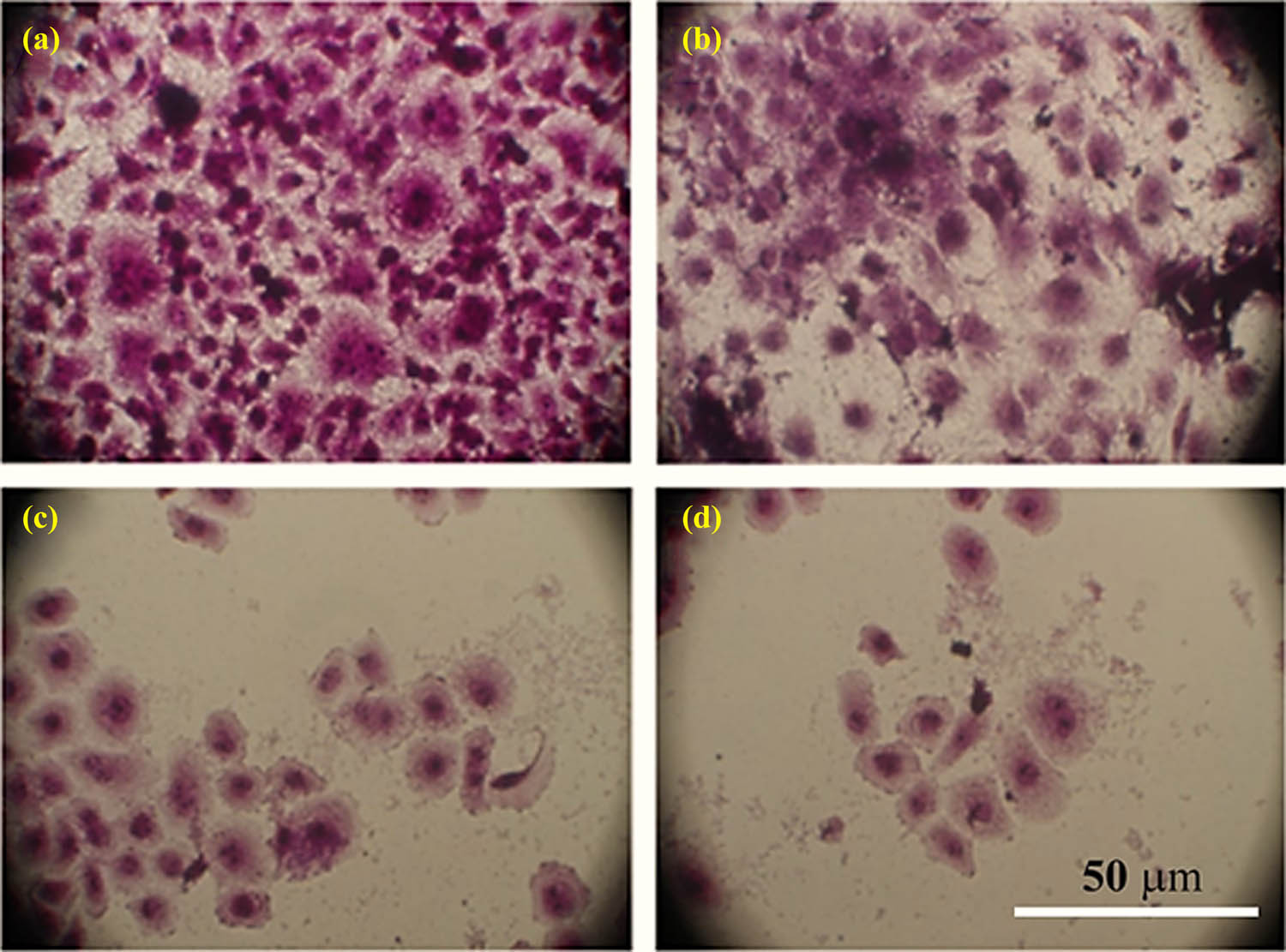
Morphological changes: changes in AMJ13 cell lines stained with crystal violet dye after treatment with CHR, AuNPs, and CHR–AuNPs: (a) non-treated control cells, (b) CHR-treated cells, (c) AuNPs-treated cells, and (d) CHR–AuNPs-treated cells.
3.8 Anti-oxidant activity
The anti-oxidant activity of CHR, AuNPs, and CHR–AuNPs, measured at four different concentrations (Figure 10), showed highest free radicals scavenging at 25 μg/mL for all the preparations and the CHR, as compared to the standard, ascorbic acid. The CHR–AuNPs exhibited 91.33% efficacy, while the AuNPs radical scavenging efficiency was 74.89%, and the CHR showed an efficacy of 52.1%. The lowest concentration, 3.1 µg/mL, showed an efficiency of nearly 15, 25, 30, and 100% for CHR, AuNPs, CHR–AuNPs, and the ascorbic acid, respectively.
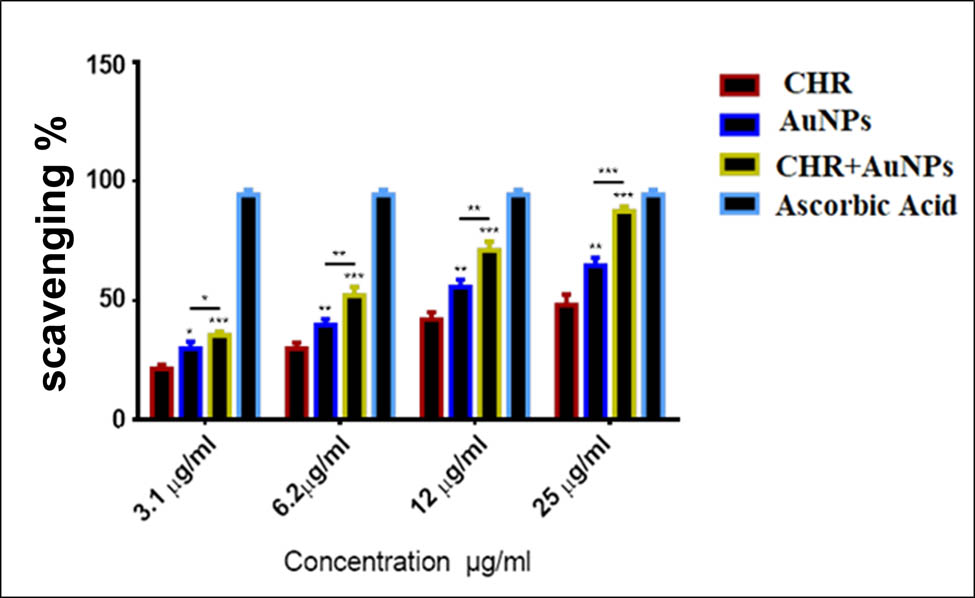
Anti-oxidant activity: CHR–AuNPs, CHR, and AuNPs at different concentrations; n = 3, *P < 0.05, **P < 0.01, and ***P < 0.001.
3.9 Anti-microbial activities
CHR–AuNPs were found the most effective, and showed highest rate of microbial inhibitions against S. aureus and E. coli (Figure 11). The inhibition efficiency was followed by AuNPs and CHR which was concentration dependent for all the three preparations, CHR, AuNPs, and CHR–AuNPs.
Anti-microbial activity: CHR, AuNPs, and CHR–AuNPs anti-microbial activity against (a) S. aureus, (b) E. coli. The values represent mean ± s.d.; n = 3, N.S., non-significant, *P < 0.05, **P < 0.01, and ***P < 0.001.
4 Discussion
CHR, a naturally occurring dihydroxy flavone, is found in passionflower, silver linden, certain geranium species, and is also present in honey and bee propolis [31]. CHR is also used as nutraceutical, and is popular as a supplement for bodybuilding. The compound is used for anxiety, inflammation, gout, HIV/AIDS, erectile dysfunction (ED), and baldness, although no pharmacological evidence has been provided. The compound is also suspected to increase the male hormone, testosterone, levels. The product is found to protect against neurological changes, improve behavioral patterns, and cognitive functions in Parkinson’s disease. It is also anti-inflammatory, anti-neoplastic, anti-oxidant, hepatoprotective, and myosin-light-chain inhibitor in its functions [32,33]. Additionally, CHR has been also reported for its beneficial applications in the treatment of aging, protection against UVA- and UVB-induced skin injuries in experimental animals [34,35].
The need for CHR’s better formulation, controlled delivery, increments in gut absorptions, and enhanced bioavailability was proposed to overcome the aqueous solubility of the compound. Nanoformulation techniques have been adopted to overcome the comparative increased lipophilicity of this flavonoid product which lacks any glycosidal moiety and is devoid of any hydrophilic substitutions, especially hydroxyl groups, commonly present in majority of flavonoids (Figure 12).
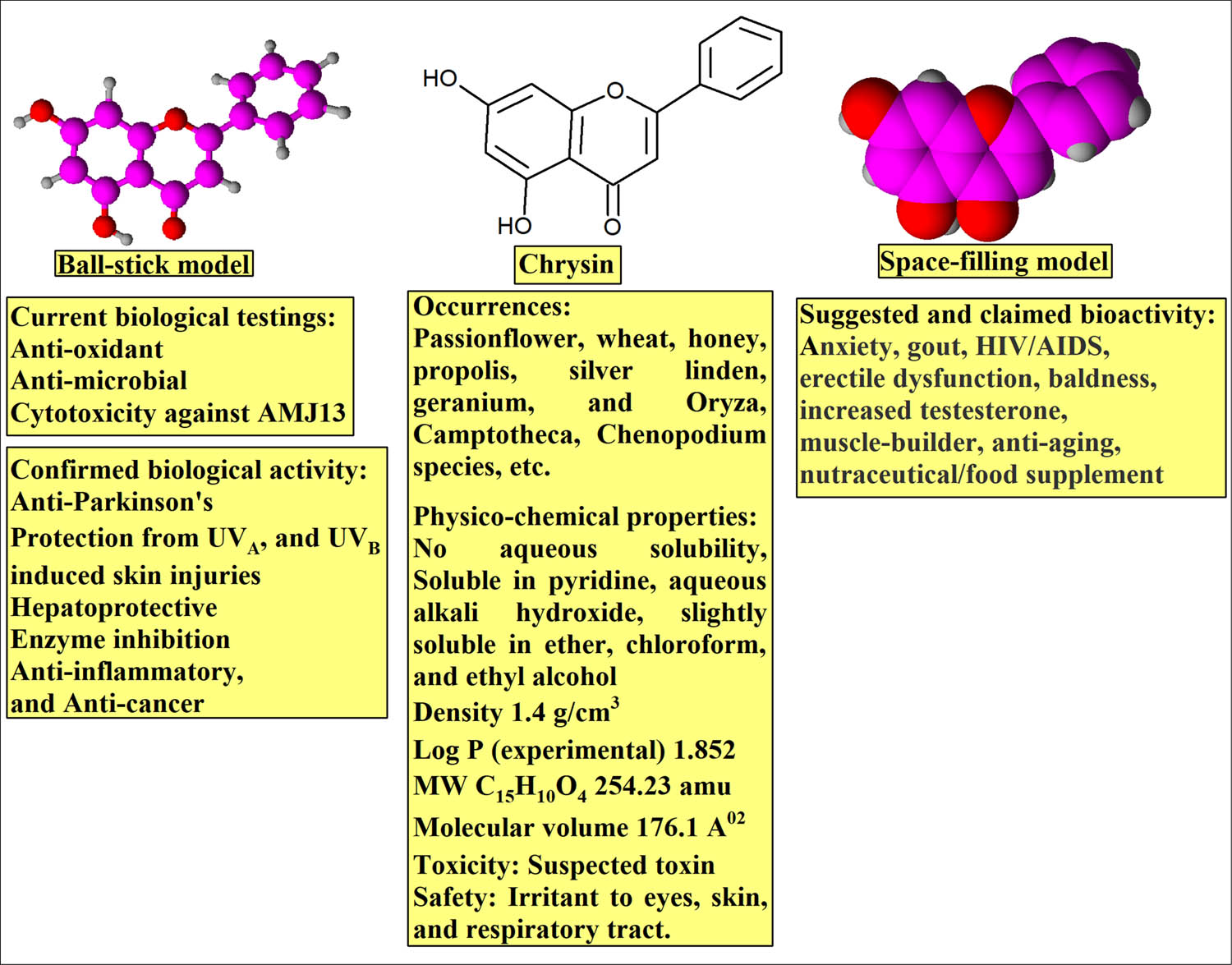
The CHR structure and properties: ball stick, space-filling models, physico-chemical, and biological properties.
The nanoscale polymeric conjugation, nanoencapsulation, micellar loading, nanoemulsion, aqueous polymeric nanodispersions, cyclodextrin inclusion, nanoliposomes, nanoscale moiety-tagging, and nanometal carriers have been among the important methods to modulate the delivery of the many of the lipophilic products to achieve the therapeutic goals through increased bioavailability, notwithstanding the oral delivery route [36]. The metal NP tagging of drugs has provided specific drug targeting, chosen on-site delivery, reduction in toxicity, increased therapeutic efficacy, enhanced safety, biocompatibility, reduced toxicity, and faster development of safe formulations [37]. In this context, the use of gold nanoscale platforms, including gold nanorods, gold nanoclusters, AuNPs, colloidal gold tagging of drugs, and bio-based entities for therapy and diagnosis have been developed [38]. The AuNPs have proved to be inert, safe, least toxic, tunable, mostly monodisperse nature with synthetic protocols well-developed to produce size and shape differentiated nanoentities, and flexible to surface modifications. The AuNPs tagged drugs are known to resist enzymatic degradation, and have been used to improve poor pharmacokinetics of drugs, its solubility, cancer tissue uptake of the drugs, and can simultaneously engage to adjacent and multiple receptors [39]. Nonetheless, the approach to AuNPs synthesis including the biogenetic, biomimetic, green, environmentally-sustainable, microbial, physical, light amplification by stimulated emission of radiation (LASER), and chemical reduction methods have been developed. Some of these methods have made available the synthesis of AuNPs defining the size, shape, and surface coatings through facile, robust, and quick procedures [40].
The citrate reduction method for producing AuNPs is preferred owing to the ease with which AuNPs can be dispersed in water, and the negative charges of the citrate ions found on the AuNPs surface provide the needed stability to the monodispersed aqueous solution. The reduction of tetrachloroauric acid (HAuCl4) by citric acid was introduced by Turkevich et al. [41] and later modified by Frens [42]. The citrate ions act both as reducing agent and a surface capping agent. The reaction goes without the concern for pH and the particle size decreases with the increase in ratio of the citric acid to the auric acid up to 3.5:1.
The prepared AuNPs also confirmed the surface-plasmonic resonance of the gold metallic NPs and the color-based observations during the preparation of the AuNPs which exhibited distinct color changes (Figure 1). Moreover, for the cancer cell uptake, NPs under 50 nm have been reported to be allowed access to the cancer cells, where they bind to the cellular sites. Because of their ability to bind to the cell membrane, the AuNPs provide better drug delivery options. The negatively charged citrate coated AuNPs, and the aqueous solution of free chrysin, as examples of gold nanoparticles and molecular entity, are being suggested to provide ionic and hydrophobic interactions, respectively, while the prepared final formulation, gold nanoparticles-conjugated-chrysin, CHR-AuNPs, apparently facilitated the biochemical interactions against the cancer cell lines to produce the cytotoxic effects [43,44]. The present findings indicated that the NPs were stable in solution, as described by the literature [45]. The zeta potential varies depending on the surface charge of dispersion, and higher values imply greater physical stability. At least ±30 mV are required to prevent flocculation and maintain stable dispersion, more than 60 mV confer excellent stability, although value as higher as 100 mV can be obtained. A value of 20 mV confers short-term stability, whereas those ranging between −5 and 5 mV demonstrate fast aggregation [45]. Zeta potential value can also be employed to indicate if the encapsulation of an active, charged material occurs within the center of the NP or on its surface [21].
The UV absorption λ max change from the AuNPs values at 520 nm, to the CHR–AuNPs at 570 nm demonstrate the ability of the AuNPs to bind to the CHR molecules, wherein the UV absorption λ max value for the CHR was observed at 268 nm which falls in the UV range, while the absorption change from 520 to 560 nm, of the CHR–AuNPs, falls in the visible range of the spectrum, again demonstrating the plasmon resonance phenomenon, and changes in the optical properties of the CHR–AuNPs, showed the presence of strong CHR, λ max at 268, and the conjugated AuNPs with UV-Vis absorption λ max value at 560 nm (Figure 3). However, several other factors, including particle size, morphology, and probable aggregation determines the absorption maxima [46]. Furthermore, towards establishing the identity of the CHR–AuNPs preparation, the FT-IR spectrum (Figure 4) of CHR, AuNPs, and CHR–AuNPs exhibited characteristic infra-red vibrational absorptions of the constituent functional groups. The OH stretching vibration frequencies of the CHR were found centered at 3012.79 cm−1 indicating the presence of alcoholic functions in the flavonoid structure, while the stretchings at 2929.87, 2713.84, and 2630.91 cm−1 indicated the presence of the aromatic CH bonds, while the α,β-unsaturated carbonyl (C═O) appeared at 1653.00 cm−1. The FT-IR spectrum also indicated the characteristics of an aromatic product with needle-sharp peaks and peaks observed at 840.96 and 731.02 cm−1, the typical aromatic character’s absorption peaks. The IR absorptions observed at 3419.79 cm−1 indicated the presence of O‒H stretching vibrations, while absorptions at 2987.74 and 2931.80 cm−1 indicated the CH stretching vibrations. The absorption peak at 1583.56, confirmed the presence of C═O group, while the absorption at 1398.39 cm−1 was confirmed for ethereal functionality of the citrate surface AuNPs. The spectrum also displayed non-aromatic characteristics of the product where no aromatic system related absorptions were observed together with non-sharp, somewhat rounded-tip, and lesser peaks. The FT-IR spectrum of CHR–AuNPs showed absorption peak at 3448.72 cm−1 belonging the hydroxyl group (O‒H), while the CH stretchings were reflected by the absorptions bands observed at 2960.73 and 2935.66 cm−1. The absorption at 1587.42 cm−1 for the metal conjugated C═O, at 1463.97 and 1390.68 were attributed to aromatic C‒C bonds, and the aromatic C–O stretchings were observed at 1276.88 cm−1. All the CHR, AuNPs, and CHR–AuNPs specific peaks have been observed which exhibited the plausible hydrogen bond, as well as the electrostatic and protonated carbonyl groups’ strong associations with the anionic AuNPs [47]. The functional groups IR confirmation, together with the XRD and EDX analyses provided the final conformity to the prepared structure. According to the XRD analysis results obtained, the final product was confirmed to be molecularly dispersed, and in an amorphous state. Since the CHR coupling interaction did not affect the size of the mineral core, the changes in the NP size, and the observed differences were attributed to the organic envelope of the NP structure [48]. The percentage of carbon and oxygen compositions in the drug-loaded AuNPs was high, thereby confirming that CHR was conjugated with the AuNPs. The anionic nature of the preparations and their size determinations were also obtained from the ζ-potential and the SEM analyses. The high, −30 mV, ζ-potential value confirmed the stable nature of the product (Figure 7) [49]. The surface charge of the NPs also determines the drug’s affinity for NPs [50]. The high ζ-potential is also used to interpret the surface attachments of the NPs [51]. The ζ-potential values are also a driving factor for biodistribution [52]. It is a design guide for the rational nanomedicine for providing maximum therapeutic efficacy and bioaccumulation predictability in vivo, where particle size and surface charge controls are critical [53].
The CHR–AuNPs exhibited higher anti-cancer activity against AMJ13 breast cancer cell lines in comparison to CHR and AuNPs with concentration-dependent cytotoxicity (Figure 8). At 100 µg/mL CHR–AuNPs concentration, highest 89% cytotoxicity was observed, while at 6.25 µg/mL, 17% cytotoxicity was observed for the CHR–AuNPs as compared to the control group. The AuNPs treatment was 73.3% effective at 100 μg/mL, whereas CHR treatment was 41.5% effective. At 6.25 μg/mL, AuNPs were effective at 8.9% and CHR was effective at 4.6%. CHR–AuNPs were much more cytotoxic than free CHR, indicating that the anti-cancer efficacy of CHR has improved since it was functionalized with AuNPs. An overall increase of nearly 18× (85%) was obtained through nanoformulation as compared to the pure CHR.
The concentration of synthetic CHR–AuNPs used in this study was significantly lower than that of CHR. Cell viability decreases as NP concentrations rise, implying that more NPs can be accumulated inside cells, resulting in increased stress, and eventually cell death. The size, shape, surface area, and surface actuations affect NPs biokinetics and toxicity. The CHR–AuNPs inhibited cell proliferation and growth in human breast cancer cell lines, AMJ13, in a concentration-dependent manner (P < 0.05). The CHR–AuNPs showed stronger cytotoxic effects than CHR, indicating that nano-based CHR is better bioavailable, more outreached to the cancer cell lines, and co-activated in conjugation with the AuNPs. Although the concentration of CHR–AuNPs used in this study was much lower than that of the pure CHR, the decreased cell viability observed as the CHR–AuNPs concentration increased, indicated that more NPs were able to accumulate within cells, and thereby putting stress to the cells, and consequently killing them. The cell death rates were dose-dependent, which could be because of the apoptosis, or necrotic activity, and that would be interesting to investigate the mechanism of cancer cells death.
The cytotoxic effects of CHR, AuNPs, and CHR–AuNPs as shown in AMJ13 cancer cell lines were also proved by the crystal violet staining and the microscopic examination after 24 h of the dose-delivery (Figure 9). The CHR–AuNPs demonstrated stronger cytotoxic impacts against the AMJ13 cell lines. The results prove that CHR–AuNPs caused several impacts, such as changes in the cells shape, reduction in cells sizes, clustering of the cancer cells with a reduced number of cell extensions, suggestive suppressed inter-cellular communications, and shrinking of the nuclei. However, these impacts were absent in non-treated cells (Figure 9). The observations confirned the cytotoxic activity of the CHR–AuNPs conclusively against human breast cancer cell lines AMJ13.
The anti-oxidant potentials of the CHR, AuNPs, and CHR–AuNPs were observed at 1.3, 2.6, 12.5, and 25 μg/mL, which revealed that the CHR significantly reduced the levels of DPPH free radicals in a concentration-dependent manner, with 25 μg/mL concentration performing significantly better than the other concentrations (Figure 10). The CHR–AuNPs were found to have higher, 91.33%, ability to quench the DPPH radicals than the CHR and AuNPs which showed 51.2 and 74.89% anti-oxidant activity as compared to the control ascorbic acid, which can be attributed to the increased surface energy and the catalytic potential of the CHR–AuNPs’. The anti-oxidants can donate electrons to the reactive radicals, and convert them to stable, non-reactive forms [54]. The anti-oxidant potential also resumed importance owing to the observations that the free radicals biology has been known to involve in the pathogenesis of several diseases, including cancers. The high anti-oxidant potential of the CHR–AuNPs preparation is in accordance with the increased cytotoxic effects of the preparation in comparison to the anti-oxidant potentials of the CHR and the AuNPs, and their cytotoxic effects.
The nano-CHR, CHR–AuNPs, was also more effective as an anti-microbial preparation, and showed higher rates of microbial inhibitions against microbial isolates, S. aureus and E. coli, which were observed after treatment with different concentrations, 25.31, 62.5, 125, and 250 μg/mL, of the preparation of CHR–AuNPs (Figure 11), and the anti-microbial activity was found to be concentration -dependent. The CHR–AuNPs exhibited highest anti-microbial inhibition rate, much higher than the AuNPs and pure CHR, thereby indicating that the pure CHR improved its anti-microbial efficacy when conjugated with the AuNPs. In addition, the AuNPs showed moderate anti-microbial properties, while the flavonoid CHR showed lesser anti-microbial activity.
The anti-microbial activity against the Gram-positive and Gram-negative bacteria, S. aureus and E. coli, respectively, followed similar patterns of the dose-dependent activity elicitations. The concentration of CHR on the AuNPs surface may have increased their ability to inhibit the bacteria. The mechanisms of microbial inhibitions have been reported to include several pathways, and activities including inhibition of DNA synthesis, alteration of cytoplasmic membrane functions, inhibition of energy metabolism, ligand reductions of the cells, formation of biofilms, inhibition of purines on the cell membrane, alteration of membrane permeability, and damage to the cytoplasmic membrane. The anti-microbial activity of NPs has been studied against Bacillus subtilis, S. aureus, Pseudomonas aeruginosa, Campylobacter jejuni, and E. coli. The Gram-negative microbial strains have thin peptidoglycan layer, and an outer lipopolysaccharide membrane, which is a barrier to cell entry of the negatively charged reactive oxygen species (ROS) [55], while the Gram-positive bacteria allowed entry of negatively charged ROS. Moreover, the anti-microbial activity of NPs is dependent on the particle size. It was demonstrated that decreasing the particle size of the NPs resulted in enhancing the anti-microbial activity [56]. Jones et al. [57] compared the activity MgO, TiO2, Al2O3, CuO, CeO2, and ZnO NPs against S. aureus. Their results demonstrated that the ZnO NPs showed significant anti-microbial activity. The activity was size dependent and differently-sized ZnO NPs were used in the ranges of >1 μm, 8 nm, and 50–70 nm. Their results confirmed that the small-sized, 8 nm of ZnO NPs was the best in terms of anti-microbial activity. In addition, some studies also referred to the toxicity mechanism of the metal oxide NPs against microbial strains which was also related to their size, morphology, and electrostatic attractions [58]. However, another study reported by Jiang et al. [59] suggested that the NPs affinity to aggregate and attach to the microbial surface may also contribute to NPs toxicity. Additionally, Aruoja et al. [60] suggested that the bactericidal effects of NPs might be specific to the type of metal oxide NPs, and it is a well-known fact that the metallic ions NPs have high affinity for electron-rich molecules, such as, the genetic material DNA. Moreover, Jose et al. [61] demonstrated that the NPs could interact with the isolated DNA molecules, and also cause a dose-dependent degradation.
5 Conclusions
The CHR–AuNPs were prepared through simple and efficient chemical method of AuNPs synthesis, and the AuNPs conjugation to the CHR molecules was carried out. The products’ physico-chemical characterizations and preliminary trial of biological studies were performed on an initial pilot test scale which included the anti-oxidant, anti-microbial, and cytotoxic activity. It was found that the CHR–AuNPs preparation possessed strong cytotoxicity against human breast cancer cell lines, AMJ13, as well as showed strong anti-oxidant potential and significant anti-microbial activity against Gram-positive and Gram-negative microbial strains, S. aureus and E. coli. The CHR–AuNPs has the potential to be used as an effective therapy as well as a co-therapy agent as part of multiple formulations-based treatments to control and combat the breast cancer. However, more experimentations in this regard are suggested.
Acknowledgments
The researchers would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
-
Funding information: This research received no external funding.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33:315–8.10.1016/j.canep.2009.10.003Suche in Google Scholar PubMed
[2] Salavati H, Debbaut C, Pullens P, Ceelen W. Resistance to intraperitoneal drug delivery and heterogeneity of peritoneal metastasis: the role of hydraulic conductivity. Proceedings of the 2nd Congress of the International Society for the Study of Pleura and Peritoneum; 2021.Suche in Google Scholar
[3] Talib WH, Alsayed AR, Barakat M, Abu-Taha MI, Mahmod AI. Targeting drug chemo-resistance in cancer using natural products. Biomedicines. 2021;9:1353.10.3390/biomedicines9101353Suche in Google Scholar PubMed PubMed Central
[4] Barani M, Mukhtar M, Rahdar A, Sargazi S, Pandey S, Kang M. Recent advances in nanotechnology-based diagnosis and treatments of human osteosarcoma. Biosensors. 2021;11:55.10.3390/bios11020055Suche in Google Scholar PubMed PubMed Central
[5] Manisekaran R, García-Contreras R, Rasu Chettiar A-D, Serrano-Díaz P, Lopez-Ayuso CA, Arenas-Arrocena MC, et al. 2D nanosheets – a new class of therapeutic formulations against cancer. Pharmaceutics. 2021;13:1803.10.3390/pharmaceutics13111803Suche in Google Scholar PubMed PubMed Central
[6] Storti G, Scioli MG, Kim B-S, Terriaca S, Fiorelli E, Orlandi A, et al. Mesenchymal stem cells in adipose tissue and extracellular vesicles in ovarian cancer patients: a bridge toward metastatic diffusion or a new therapeutic opportunity? Cells. 2021;10:2117.10.3390/cells10082117Suche in Google Scholar PubMed PubMed Central
[7] Aboubakr EM, Mohammed HA, Hassan AS, Mohamed HB, El Dosoky MI, Ahmad AM. Glutathione-loaded non-ionic surfactant niosomes: a new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione. Nanotechnol Rev. 2022;11:117–37.10.1515/ntrev-2022-0010Suche in Google Scholar
[8] Wahab S, Alshahrani MY, Ahmad MF, Abbas H. Current trends and future perspectives of nanomedicine for the management of colon cancer. Eur J Pharmacol. 2021;910:174464.10.1016/j.ejphar.2021.174464Suche in Google Scholar PubMed
[9] Al-Omar MS, Jabir M, Karsh E, Kadhim R, Sulaiman GM, Taqi ZJ, et al. Gold nanoparticles and graphene oxide flakes enhance cancer cells’ phagocytosis through granzyme-perforin-dependent biomechanism. Nanomaterials. 2021;11:1382.10.3390/nano11061382Suche in Google Scholar PubMed PubMed Central
[10] Campora S, Ghersi G. Smart nanoparticles in biomedicine: an overview of recent developments and applications; 2021.10.20944/preprints202102.0619.v1Suche in Google Scholar
[11] Kong F-Y, Zhang J-W, Li R-F, Wang Z-X, Wang W-J, Wang W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22:1445.10.3390/molecules22091445Suche in Google Scholar PubMed PubMed Central
[12] Kozlowska A, Szostak-Wegierek D. Flavonoids-food sources and health benefits. Rocz Państwowego Zakładu Hig. 2014;65:79–85.Suche in Google Scholar
[13] Mohammed H. Natural and synthetic flavonoid derivatives with potential antioxidant and anticancer activities. 2009.Suche in Google Scholar
[14] Moghadam ER, Ang HL, Asnaf SE, Zabolian A, Saleki H, Yavari M, et al. Broad-spectrum preclinical antitumor activity of chrysin: current trends and future perspectives. Biomolecules. 2020;10:1374.10.3390/biom10101374Suche in Google Scholar PubMed PubMed Central
[15] Baidya D, Kushwaha J, Mahadik K, Patil S. Chrysin-loaded folate conjugated PF127-F68 mixed micelles with enhanced oral bioavailability and anticancer activity against human breast cancer cells. Drug Dev Ind Pharm. 2019;45:852–60.10.1080/03639045.2019.1576726Suche in Google Scholar PubMed
[16] Gnanasekar S, Palanisamy P, Jha PK, Murugaraj J, Kandasamy M, Mohamed Hussain AMK, et al. Natural honeycomb flavone chrysin (5,7-dihydroxyflavone)-reduced graphene oxide nanosheets fabrication for improved bactericidal and skin regeneration. ACS Sustain Chem Eng. 2018;6:349–63.10.1021/acssuschemeng.7b02603Suche in Google Scholar
[17] Andleeb A, Andleeb A, Asghar S, Zaman G, Tariq M, Mehmood A, et al. A systematic review of biosynthesized metallic nanoparticles as a promising anti-cancer-strategy. Cancers (Basel). 2021;13:2818.10.3390/cancers13112818Suche in Google Scholar PubMed PubMed Central
[18] Azeeze MSTA, Bhupathi SS, Mohammad EB, Kaliannan D, Balasubramanian B, Meyyanathan SN. Biologically synthesized plant-derived nanomedicines and their in vitro–in vivo toxicity studies in various cancer therapeutics: regulatory perspectives. Cancer Nanotheranostics. 2021;2:217–60.10.1007/978-3-030-76263-6_9Suche in Google Scholar
[19] Zhang J, Li Z, Zheng K, Li G. Synthesis and characterization of size-controlled atomically precise gold clusters. Phys Sci Rev. 2018;3–268.10.1515/9783110345100-005Suche in Google Scholar
[20] Sulaiman GM, Jabir MS, Hameed AH. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artif Cells Nanomed Biotechnol. 2018;46:708–20.10.1080/21691401.2018.1434661Suche in Google Scholar PubMed
[21] Sulaiman GM, Waheeb HM, Jabir MS, Khazaal SH, Dewir YH, Naidoo Y. hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci Rep. 2020;10:10.10.1038/s41598-020-66419-6Suche in Google Scholar PubMed PubMed Central
[22] Khanna PK, Gaikwad S, Adhyapak PV, Singh N, Marimuthu R. Synthesis and characterization of copper nanoparticles. Mater Lett. 2007;61:4711–4.10.1016/j.matlet.2007.03.014Suche in Google Scholar
[23] Mohammed HA, Al-Omar MS, El-Readi MZ, Alhowail AH, Aldubayan MA, Abdellatif AAH. Formulation of ethyl cellulose microparticles incorporated pheophytin a isolated from suaeda vermiculata for antioxidant and cytotoxic activities. Molecules. 2019;24:24.10.3390/molecules24081501Suche in Google Scholar PubMed PubMed Central
[24] Sagheer OM, Mohammed MH, Ibraheem ZO, Wadi JS, Tawfeeq MF. Synthesis of gamma biguanides butyric acid analogues as HDAC inhibitors and studying their cytotoxic activity. Mater Today Proc. 2021;47:5983–91.10.1016/j.matpr.2021.04.539Suche in Google Scholar
[25] Robson A-L, Dastoor PC, Flynn J, Palmer W, Martin A, Smith DW, et al. Advantages and limitations of current imaging techniques for characterizing liposome morphology. Front Pharmacol. 2018;9:80.10.3389/fphar.2018.00080Suche in Google Scholar
[26] Reddy GR, Morais AB, Gandhi NN. 2,2-Diphenyl-1-picrylhydrazyl free radical scavenging assay and bacterial toxicity of protein capped silver nanoparticles for antioxidant and antibacterial applications. Asian J Chem. 2013;25:9249–54.10.14233/ajchem.2013.15215Suche in Google Scholar
[27] Nalubega R, Kabasa JD, Olila D, Kateregga J. Evaluation of antibacterial activity of selected ethnomedicinal plants for poultry in Masaka district, Uganda. Res J Pharmacol. 2011;5:18–21.10.3923/rjpharm.2011.18.21Suche in Google Scholar
[28] Asghar M, Habib S, Zaman W, Hussain S, Ali H, Saqib S. Synthesis and characterization of microbial mediated cadmium oxide nanoparticles. Microsc Res Tech. 2020;83:1574–84.10.1002/jemt.23553Suche in Google Scholar
[29] Applerot G, Perkas N, Amirian G, Girshevitz O, Gedanken A. Coating of glass with ZnO via ultrasonic irradiation and a study of its antibacterial properties. Appl Surf Sci. 2009;256:S3–S8.10.1016/j.apsusc.2009.04.198Suche in Google Scholar
[30] Xin Lee K, Shameli K, Miyake M, Kuwano N, Bt Ahmad Khairudin NB, Bt Mohamad SE, et al. Green synthesis of gold nanoparticles using aqueous extract of Garcinia mangostana fruit peels. J Nanomater. 2016;2016.10.1155/2016/8489094Suche in Google Scholar
[31] Siddiqui A, Badruddeen, Akhtar J, Uddin MSS, Khan MI, Khalid M, et al. A naturally occurring flavone (chrysin): chemistry, occurrence, pharmacokinetic, toxicity, molecular targets and medicinal properties. J Biol Act Prod Nat. 2018;8:208–27.10.1080/22311866.2018.1498750Suche in Google Scholar
[32] Mohammed HA, Ba LA, Burkholz T, Schumann E, Diesel B, Zapp J, et al. Facile synthesis of chrysin-derivatives with promising activities as aromatase inhibitors. Nat Prod Commun. 2011;6(1):31–4.10.1177/1934578X1100600108Suche in Google Scholar
[33] Cho H, Yun C-W, Park W-K, Kong J-Y, Kim KS, Park Y, et al. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol Res. 2004;49:37–43.10.1016/S1043-6618(03)00248-2Suche in Google Scholar
[34] Shin EK, Kwon H-S, Kim YH, Shin H-K, Kim J-K. Chrysin, a natural flavone, improves murine inflammatory bowel diseases. Biochem Biophys Res Commun. 2009;381:502–7.10.1016/j.bbrc.2009.02.071Suche in Google Scholar PubMed
[35] Wu N-L, Fang J-Y, Chen M, Wu C-J, Huang C-C, Hung C-F. Chrysin protects epidermal keratinocytes from UVA-and UVB-induced damage. J Agric Food Chem. 2011;59:8391–400.10.1021/jf200931tSuche in Google Scholar PubMed
[36] Jeevanandam J, San Chan Y, Danquah MK. Nano-formulations of drugs: recent developments, impact and challenges. Biochimie. 2016;128:99–112.10.1016/j.biochi.2016.07.008Suche in Google Scholar PubMed
[37] Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 2022;1–23.10.1007/s42247-021-00335-xSuche in Google Scholar PubMed PubMed Central
[38] Koushki K, Keshavarz Shahbaz S, Keshavarz M, Bezsonov EE, Sathyapalan T, Sahebkar A. Gold nanoparticles: multifaceted roles in the management of autoimmune disorders. Biomolecules. 2021;11:1289.10.3390/biom11091289Suche in Google Scholar PubMed PubMed Central
[39] Chiu C-F, Fu R-H, Hsu S, Yu Y-HA, Yang S-F, Tsao TC-Y, et al. Delivery capacity and anticancer ability of the berberine-loaded gold nanoparticles to promote the apoptosis effect in breast cancer. Cancers (Basel). 2021;13:5317.10.3390/cancers13215317Suche in Google Scholar PubMed PubMed Central
[40] Hammami I, Alabdallah NM. Gold nanoparticles: synthesis properties and applications. J King Saud Univ. 2021;33:101560.10.1016/j.jksus.2021.101560Suche in Google Scholar
[41] Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75.10.1039/df9511100055Suche in Google Scholar
[42] Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20–2.10.1038/physci241020a0Suche in Google Scholar
[43] Jazayeri MH, Amani H, Pourfatollah AA, Pazoki-Toroudi H, Sedighimoghaddam B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens Bio-sensing Res. 2016;9:17–22.10.1016/j.sbsr.2016.04.002Suche in Google Scholar
[44] Gao R, Hao Y, Zhang L, Cui X, Liu D, Zhang M, et al. A facile method for protein imprinting on directly carboxyl-functionalized magnetic nanoparticles using non-covalent template immobilization strategy. Chem Eng J. 2016;284:139–48.10.1016/j.cej.2015.08.123Suche in Google Scholar
[45] Nakach M, Authelin J-R, Tadros T, Galet L, Chamayou A. Engineering of nano-crystalline drug suspensions: employing a physico-chemistry based stabilizer selection methodology or approach. Int J Pharm. 2014;476:277–88.10.1016/j.ijpharm.2014.09.048Suche in Google Scholar PubMed
[46] Shamaila S, Zafar N, Riaz S, Sharif R, Nazir J, Naseem S. Gold nanoparticles: an efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials. 2016;6:71.10.3390/nano6040071Suche in Google Scholar PubMed PubMed Central
[47] Park J-W, Shumaker-Parry JS. Structural study of citrate layers on gold nanoparticles: role of intermolecular interactions in stabilizing nanoparticles. J Am Chem Soc. 2014;136:1907–21.10.1021/ja4097384Suche in Google Scholar PubMed
[48] Fitzpatrick AWP, Debelouchina GT, Bayro MJ, Clare DK, Caporini MA, Bajaj VS, et al. Atomic structure and hierarchical assembly of a cross-β amyloid fibril. Proc Natl Acad Sci. 2013;110:5468–73.10.1073/pnas.1219476110Suche in Google Scholar PubMed PubMed Central
[49] Kumar A, Dixit CK. Methods for characterization of nanoparticles. Advances in nanomedicine for the delivery of therapeutic nucleic acids. Amsterdam, Netherlands: Elsevier; 2017. p. 43–58.10.1016/B978-0-08-100557-6.00003-1Suche in Google Scholar
[50] Nandhakumar S, Dhanaraju MD, Sundar VD, Heera B. Influence of surface charge on the in vitro protein adsorption and cell cytotoxicity of paclitaxel loaded poly (ε-caprolactone) nanoparticles. Bull Fac Pharmacy, Cairo Univ. 2017;55:249–58.10.1016/j.bfopcu.2017.06.003Suche in Google Scholar
[51] Nejat H, Rabiee M, Varshochian R, Tahriri M, Jazayeri HE, Rajadas J, et al. Preparation and characterization of cardamom extract-loaded gelatin nanoparticles as effective targeted drug delivery system to treat glioblastoma. React Funct Polym. 2017;120:46–56.10.1016/j.reactfunctpolym.2017.09.008Suche in Google Scholar
[52] Lagaly G, Dékány I. Colloid clay science. Developments in clay science. Amsterdam, Netherlands: Elsevier; 2013. p. 243–345. ISBN 1572-4352.10.1016/B978-0-08-098258-8.00010-9Suche in Google Scholar
[53] Liu Y, Tan J, Thomas A, Ou-Yang D, Muzykantov VR. The shape of things to come: importance of design in nanotechnology for drug delivery. Ther Deliv. 2012;3:181–94.10.4155/tde.11.156Suche in Google Scholar PubMed PubMed Central
[54] Engwa GA. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochem Source Antioxidants Role Dis Prev BoD–Books Demand. 2018;7:49–74.10.5772/intechopen.76719Suche in Google Scholar
[55] Russell AD. Similarities and differences in the responses of microorganisms to biocides. J Antimicrob Chemother. 2003;52:750–63.10.1093/jac/dkg422Suche in Google Scholar PubMed
[56] Abebe B, Murthy HCA, Zerefa E, Adimasu Y. PVA assisted ZnO based mesoporous ternary metal oxides nanomaterials: synthesis, optimization, and evaluation of antibacterial activity. Mater Res Exp. 2020;7:45011.10.1088/2053-1591/ab87d5Suche in Google Scholar
[57] Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008;279:71–6.10.1111/j.1574-6968.2007.01012.xSuche in Google Scholar PubMed
[58] Heinlaan M, Ivask A, Blinova I, Dubourguier H-C, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria vibrio fischeri and crustaceans daphnia magna and thamnocephalus platyurus. Chemosphere. 2008;71:1308–16.10.1016/j.chemosphere.2007.11.047Suche in Google Scholar PubMed
[59] Jiang W, Mashayekhi H, Xing B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ Pollut. 2009;157:1619–25.10.1016/j.envpol.2008.12.025Suche in Google Scholar PubMed
[60] Aruoja V, Dubourguier H-C, Kasemets K, Kahru A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae pseudokirchneriella subcapitata. Sci Total Environ. 2009;407:1461–8.10.1016/j.scitotenv.2008.10.053Suche in Google Scholar PubMed
[61] Jose GP, Santra S, Mandal SK, Sengupta TK. Singlet oxygen mediated DNA degradation by copper nanoparticles: potential towards cytotoxic effect on cancer cells. J Nanobiotechnol. 2011;9:1–8.10.1186/1477-3155-9-9Suche in Google Scholar PubMed PubMed Central
© 2022 Ahmed J. Jasim et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption


