A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
-
Mohammadmahdi Akbari Edgahi
, Amirhossein Emamian
Abstract
In this paper, we reviewed the recent advances in nanoscale modifications and evaluated their potential for dental implant applications. Surfaces at the nanoscale provide remarkable features that can be exploited to enhance biological activities. Herein, titanium and its alloys are considered as the main materials due to their background as Ti-based implants, which have been yielding satisfactory results over long-term periods. At first, we discussed the survivability and the general parameters that have high impacts on implant failure and the necessities of nanoscale modification. Afterward, fabrication techniques that can generate nanostructures on the endosseous implant body are categorized as mechanical, chemical, and physical methods. These techniques are followed by biomimetic nanotopographies (e.g., nanopillars, nanoblades, etc.) and their biological mechanisms. Alongside the nanopatterns, the applications of nanoparticles (NPs) including metals, ceramics, polymers, etc., as biofunctional coating or delivery systems are fully explained. Finally, the biophysiochemical impacts of these modifications are discussed as essential parameters for a dental implant to provide satisfactory information for future endeavors.
1 Introduction
Dental implants are the mainstream solution for lost teeth. They are fabricated to mimic the function of teeth and return the lost confidence to the patients. In the early 1980s, a 5 to 10 year follow-up on the survivability of dental prosthesis indicated an 81 and 91% survival rate in maxilla and mandible, respectively [1]. In this term, the definitions of survival rate and success rate are different. The survival rate represents the sustainability of the dental implants but the success rate also includes the patients’ general satisfaction. Therefore, after 40 years, statistics still show complications, especially in patients with diabetes or smoking background, which implies the necessities for the development of dental implants [2,3,4,5].
From the beginning of the 21st century, nanoscale modifications have been of great interest [6,7,8]. Materials at nanoscale display unique properties that can significantly enhance the characteristics of dental implants and further osteogenic responses [9]. These modifications have a key role in controlling essential parameters of an implant. Additionally, remarkable modifications can be obtained by the utilization of different nanoparticles (NPs) or nanopatterns, whereas manifold techniques can produce microscale surfaces, there is only a limited number of commercial techniques that grant nanoscale structures. These techniques, according to their performing conditions, are categorized as mechanical, chemical, and physical methods [10,11,12,13,14,15,16,17,18]. Considering the transgingival nature of dental implants, they form three main interfaces with the host’s biological system that consists of (i) the subgingival hard tissue interface of the endosseous implant body, (ii) the soft tissue transgingival interface at the implant neck and platform, and (iii) the interface to the oral cavity with its salivary environment at the transgingival and the supragingival region [17].
At the implant interface, where most complications are laid on, researchers have been evaluating the responses of bone to various NPs and nanopatterns [19,20,21,22]. Coating the implant surface with nanoscale particles surpassed the restriction of produced residues for some metallic elements (e.g., Cu) or extended their applications. Herein, NPs are classified as (i) metallic-based, (ii) ceramic-based, (iii) polymer-based, (iv) carbon-based, (v) protein-based, and (vi) drug-based [21,22,23,24,25,26,27,28,29,30,31,32,33]. Utilization of these NPs may either provide a suitable environment for biological agents or restrict the harmful agents from disturbing the biological system. Respecting these issues, a long-lasting coating with both promotive and restrictive functions is the optimal requirement for dental implants.
Aside from the inherent function of NPs, by engineering their characteristics, especially ceramic- and polymer-based materials, they can be used as delivery systems for a broad range of biomaterial or biomolecules with biogenic or biocidal activities [23]. Drug delivery in implants mainly occurs under degradation, diffusion, or osmosis mechanisms. Hence, the role of solubility in both the material matrix and payload is highlighted. Designing delivery systems create a suitable environment for these biomolecules to release at a controlled rate and maintain their function over a longer period. Therefore, a desirable drug delivery system can aid us to conquer several bone diseases and secure the success of implantation.
In addition to multifunctional coatings, surface nanotopographies are tremendously useful for biomedical applications that offer a broad spectrum of properties like mechano-bactericidal activity, which leads to the formation of multifunctional modifications. This activity was first found in nature by assessing bugs’ wings like Psaltoda claripennis against Gram-negative bacteria and it was completely related to physical antibacterial mechanisms and not chemical ones [34]. Technically, the mechano-bactericidal function of the surface belongs to the physical geometries of the nanopatterns that increase the stress beyond the elastic tolerance of the membrane [19]. Therefore, the quantity of biomaterials and subsequent adsorption rate are not involved in the elimination of bacteria, which help the surface to maintain its antibacterial function for longer periods than chemical compounds.
In this review, we cover some uses of nanotechnology for the modification of the endosseous implant body. Modifying an implant does not have a gold standard and every method may display acceptable results. However, utilization of state-or-art procedures provides the opportunity to combat the current challenges and guarantees success with higher satisfaction for patients. In the following sections, we provide pieces of information to fulfill the demands for optimizing dental implants.
2 Nanofabrication techniques
To modify the surface morphology of the implant, diverse techniques can be performed (Table 1). The utilization of these techniques, depending on the procedure and performing conditions, results in different surface characteristics. From a broad range of modification techniques, the following ones are frequently used to develop nanoscale surfaces at the endosseous body and can be commercially applicable. Of these techniques, mechanical methods are the initial stage of processing and they target the roughness and grain size of the surface layer. By including the acid etching (AE) from chemical methods, the average roughness (R a) of the surface can be predicted. The second stage is the accompaniment of either chemical or physical methods. Chemical methods refer to the use of chemical solutions, while physical methods are considered as the formation of materials under dry conditions.
A summary of nanofabrication techniques and their advantages for dental implants [101]
| Mechanical methods | ||||
|---|---|---|---|---|
| Technique | Surface morphology | Advantages | Disadvantages | |
| Machining | 0.3–1 µm R a with 10 nm oxide layer | • Enhanced cell adhesion and osteointegration | • Fabricating imperfect surfaces that prolong the healing process | |
| Grinding | Less than 1 µm R a | • Improved osteointegration, hydrophilicity, density, and viscosity | • May cause several damages to the surface | |
| • Difficult operation for dental implants | ||||
| Polishing | ∼ 350 nm R a | • Improved osteointegration and surface quality | • Performing multiple steps | |
| • The potential to use different chemicals within the slurry | ||||
| SB | 0.3–3 µm R a | • Enhanced osteointegration | • Particles remain on the surface | |
| • Controllable surface roughness | ||||
| SP | 25–80 nm grains | • Increased cell adhesion, differentiation, and viability | • Superficial grain refinement | |
| • Higher surface quality | • Limited particle size for dental application | |||
| Attrition | Less than 100 nm grains | • Higher surface quality | • Not applicable for dental implant | |
| • Deeper grain refinement | • Increased hydrophobicity at smaller scales | |||
| Chemical methods | ||||
| Technique | Surface morphology | Advantages | ||
| AE | 0.3–1 µm R a with 10 nm oxide layer | • Increase anchorage of fibrin and osteogenic cells | ||
| • Potential of fabricating nanotopography | ||||
| HPT | Less than 10 nm inner oxide layer | • Improved biocompatibility | ||
| Up to 40 nm outer porous layer | • Ability to form apatite | |||
| AT | ∼1 µm layer of sodium titanate (Na2TiO3) gel | • Improved cell differentiation | ||
| • Ability to form apatite | ||||
| Sol–gel | Less than 10 µm of a thin layer of ceramic | • Wide selectivity of biomaterials | ||
| • Ability to deposit complex compounds | ||||
| • Easy processes | ||||
| • Highly bioactive surface | ||||
| Electrochemical treatment | ∼ 10 nm–10 µm layer of uniform TiO2 | • Enhanced bioactivity and corrosion resistance | ||
| • Potential of fabricating nanotopographies | ||||
| CVD | ∼1 µm layer of TiC, TiN, TiCN, diamond-like carbon, and diamond NPs | • Enhanced biocompatibility | ||
| • Significantly high surface quality | ||||
| SAMs | — | • Highly improved biogenic and/or biocidal activities | ||
| Physical methods | ||||
| Technique | Surface morphology | Advantages | ||
| PS | Less than 100 nm layer of metallic, ceramic, or polymer compounds | • Improved surface quality and biocompatibility | ||
| • Wide selectivity of biomaterials | ||||
| SD | Less than 1 µm layer of TiC, TiN, TiCN, and amorphous carbon | • Enhanced biocompatibility | ||
| • Significantly high surface quality | ||||
| IBAD | ∼10 nm of a modified layer with/without TiN or TiO2 | • Enhanced biocompatibility | ||
| • Significantly high surface quality | ||||
| Lithography | ∼60 nm layer of ultrafine nanotopography | • Potential of fabricating specific nanotopographies | ||
| • Enhanced bioactivity | ||||
| • Improved bone-implant contact | ||||
| Laser treatment | — | • Ability to fabricate multiphase composition at micro- and nanoscale | ||
| • Improved cell adhesion and overall biogenic activities | ||||
Bear in mind that despite the potential of producing the same surface (e.g., HAp-coated Ti) by the same or two different techniques, the ultimate result and performance may be different [35,36]. Hence, nanofabrication techniques and their performing conditions should be wisely selected.
2.1 Mechanical methods
Of the initial manufacturing processes of implants, mechanical methods were commonly used until the 1990s. Gradually, by the development of surface morphology at smaller scales, machining, grinding, polishing, and sandblasting (SB) are employed to modify the surface and enhance the general roughnesses, whereas shot peening (SP) and attrition are used as surface-improving methods to refine the grain size of the top surface.
2.1.1 Machining
Back in the 1990s, machining was an essential step of dental implant treatment and it refers to the lathing, milling, or threading of manufacturing processes. The R a values obtained by machining on the Ti surface were 300 nm to 1 µm with 2–10 nm thickness of the amorphous TiO2 layer [37]. This imperfect surface generated by machining can promote cell adhesion and increase osteointegration but it simultaneously prolongs the healing period [38]. Salou et al. produced a regular array of TiO2 nanotubes with a diameter of 37 nm and thickness of 160 nm by machining and it displayed notable enhancements in osteogenesis and osteointegration [39].
2.1.2 Grinding
Grinding involves diverse methods of abrasive activities to treat metallic surfaces. Generally, there are two frequently used procedures by either utilization of a belt machine with the help of a robotic arm or a grinding wheel with coarse particles to abrade the surface. The belt machine is at disadvantage to producing nanoscale surfaces but the grinding wheel with abrasive grade 60 is able to produce R a values of less than 1 µm [40].
In spite of its simplicity, grinding low thermal conductive metals or alloys like Ti grade-5 could cause issues including thermal damage, surface burn, residual stresses, and grit dislodgement [41]. Therefore, conducting parameters in each procedure should be carefully adjusted. Madarkar et al. abraded Ti grade-5 by using ultrasonic vibration-assisted minimum quantity lubrication (UMQL) and conventional MQL (CMQL) to improve the quality of the surface. They reported that the variation of vegetable oil in UMQL led to different hydrophilicity, density, and viscosity as well as a reduction in cutting forces compared to CMQL. However, resulting roughnesses from UMQL were slightly higher than those of CMQL [42].
2.1.3 Polishing
Alike grinding, polishing is a cost-efficient method that follows similar protocols but with the use of fine abrasive particles. Generally, polishing is conducted through multiple processes using coarse abrasive SiC paper with 50 to 220 grit for the initial step and then finer abrasive SiC papers above 600 grit for the next step to obtain a mirror-like surface [40]. In chemical mechanical polishing, the produced R a values are highly dependent on the slurry. In this case, alumina (Al2O3), silica (SiO2), and diamond slurry are frequently used since they should be chemically inert [43,44,45]. Ozdemir et al. evaluated the influence of pad types to optimize the roughness while forming an oxide layer on the surface. They reported that the lowest roughness, R a of 350 ± 30 nm, was obtained via alumina slurry with 3 wt% hydrogen peroxide (H2O2) [44].
2.1.4 Sandblasting (SB)
SB, also known as grit blasting, is a commonly used method to treat the surface and modify the R a values. Despite machining, surface topography achieved by SB is highly dependent on the particle size [46]. Generally, SB refers to the projection of micro/NPs such as Al2O3, silica, titania (TiO2), and CaP bioceramics through a nozzle onto the surface by compressed air to erode the surface (Figure 1). R a values of blasted Ti were 300 nm to 3 µm [32]. More importantly, the particles used should be chemically stable and not cause further complications [47]. SB is a more favorable technique due to its capability of controlling surface roughness. This advantage became noteworthy as Jamet et al. found that the accumulation of bacteria on a rougher surface is higher than a smoother surface [48]. Schupbash et al. analyzed the surface of seven different dental implant manufacturers. They reported that the presence of particles made by SB on implants is different and not all manufacturers control the remaining particles on their implant surfaces [49].
![Figure 1
Schematic illustration of the SB process [49]. Copyright 2014, Hindawi.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_001.jpg)
Schematic illustration of the SB process [49]. Copyright 2014, Hindawi.
2.1.5 SP
SP, also known as shot blasting, is a surface-improving method that promotes performance and reduces maintenance of the metallic components with a cost-effective solution. SP is a similar action as SB but with sufficient force strikes to create a plastic deformation. Herein, large metallic or ceramic balls with 0.25–1 mm diameter at 20–150 m/s velocity bombard the surface [50]. Technically, peening is employed to refine the grain structure of the surface by inducing residual compressive stress through a projection of high quality and inert particles to remove unfavorable tensile stresses from the surface [51]. With SP, it is possible to obtain 25–80 nm grains on the surface layer [52]. Deng et al. and Ganesh et al. studied the physiobiological effect of SP on Ti surfaces. The results displayed that utilization of different particles can fairly or significantly change the roughness, yet it can always increase hardness and fatigue resistance. Also, a treated surface can enhance cell adhesion, differentiation, and viability [51,53].
2.1.6 Attrition
Surface mechanical attrition treatment (SMAT) is a derived version of the SP method with a higher potential to produce a nanocrystalline surface layer. In SMAT, 2–10 mm diameter particles at 5–15 m/s velocity project multidirectionally to the surface, and consequently, create multidirectional severe plastic deformations. Compared to SP, SMAT has higher kinetic energies that lead to the formation of a thicker nanocrystalline surface layer and deeper residual compressive stress [54]. Jamesh et al. investigated the effects of SMAT on the CP-Ti surface using 8 mm diameter Al2O3 for 900, 1,800, and 2,700 s. They reported that at each time interval, the roughness of the surface increased whereas the level of hydrophilicity decreased. Also, they mentioned that the 900 s projection was not able to form apatite until the 28th day [55].
2.2 Chemical methods
To alter the topography of the implant surface, chemical methods are the best choices. These methods include several techniques that change an inert surface of an implant to a bioactive surface via oxidation, deposition, coating, or immobilization. Chemical solutions and their compounding ratios are the vital parts of the chemical surface treatment that can result in different physiobiological responses [56].
Primarily, chemical methods consist of AE, hydrogen peroxide treatment (HPT), alkali treatment (AT), sol–gel, electrochemical treatment, chemical vapor deposition, and biochemical methods, which form a nanolayer on the implant surface. Moreover, these methods are composed of other diverse techniques like esterification, coupling agent, and surface grafting that grant improvement in the biological activity of the surface, but, herein, they are omitted due to the inability to generate nanostructures [10].
2.2.1 AE
AE is used to clean and remove any oxide contamination from the layer and produce a homogenous surface (Figure 2). Chemical acids such as sulfuric acid (H2SO4), hydrochloric acid (HCl), hydrofluoric acid (HF), and nitric acid (HNO3) are commonly used acids to produce the R a values of 300 nm to 1 µm with approximately 10 nm thickness of the amorphous TiO2 layer [57]. AE is commercially a popular technique and is usually accompanied by another acid as dual AE or another technique (e.g., pre-SB or double AE) to improve osteoconductivity [58].
![Figure 2
Schematic illustration of the AE process [49]. Copyright 2014, Hindawi.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_002.jpg)
Schematic illustration of the AE process [49]. Copyright 2014, Hindawi.
Typically, etching an implant surface changes the R a values that lead to increasing anchorage of fibrin and osteogenic cells [59]. To obtain favorable roughness, diverse parameters including bulk material, material phases, surface structure, surface impurities, acid, temperature, and soaking time must be taken into account. Variola et al. reported that a nanopit network with different diameters ranging from 20 to 100 nm can be fabricated via a combination of strong acids or bases and oxidants on CP-Ti [60]. Lamolle et al. etched Ti implants with HF acid and reported that by controlling the aforementioned parameters and solution contents, it is possible to fabricate micro- and nanoscale topography to increase biocompatibility and provide a suitable substrate for cell growth [61].
2.2.2 HPT
HPT is a technique to enhance the bioactivity of Ti implants by the formation of a gel-like anatase layer on the implant. Anatase permits the bone-like apatite to deposit after the immersion in simulated body fluid (SBF) [62]. The altered surface escalates the osteoblast-like cells and subsequently accelerates osteointegration [63]. Wang et al. fabricated an amorphous layer of TiO2 on the CP-Ti sheets by the combination of HPT with AE, followed by thermally treating up to 800°C (Figure 3). They concluded that the optimal solution contents and heating temperature to obtain the highest bioactivity were H2O2/0.1 M HCl (1 M X/0.1 M Y)-solution and 400–500°C [35]. Khodaei et al. evaluated the addition of F− or Cl− to H2O2 solution and concluded that the addition of different ions to oxidizing ions can affect the phase morphology and wettability [64].
![Figure 3
Scanning electron microscopy (SEM) images of hydroxyapatite deposited on the Ti surface (a and b) using HPT and (c and d) sol–gel techniques. The newly formed apatite layer depending on different conditions, pH, temperature, and time led to (a and c) initial and (b and d) complete stages of HAp formation. [35] Copyright 2002, Elsevier; [36] Copyright 2020, Medico-Legal Update.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_003.jpg)
Scanning electron microscopy (SEM) images of hydroxyapatite deposited on the Ti surface (a and b) using HPT and (c and d) sol–gel techniques. The newly formed apatite layer depending on different conditions, pH, temperature, and time led to (a and c) initial and (b and d) complete stages of HAp formation. [35] Copyright 2002, Elsevier; [36] Copyright 2020, Medico-Legal Update.
2.2.3 AT
AT is the use of basic solutions (e.g., NaOH) to form a bioactive nanostructured layer like sodium titanate (Na2O7Ti3) on the implant surface [65]. Upon immersion in SBF, sodium ions in the layer are exchanged with H3O+ from the adjacent fluid and form Ti–OH groups; these groups interact with Ca2+ ions and produce amorphous calcium titanate (CaTiO3), and ultimately, with phosphate polyatomic ions (e.g., HPO4 2−), they form amorphous bone-like apatite on the implant surface. The formation of the apatite layer on the Ti surface provides a favorable substrate for bone marrow cell differentiation [66].
Pattanayak et al. found that exposing Ti to the strong acid solutions (pH < 1.1) or strong basic solutions (pH > 13.6) can form bone-like apatite on the surface after immersion in SBF within 3 days. Also, they observed immediate apatite formation after the heat treatment process. Generally, the generation of the apatite layer relates to the magnitude of the positive or negative surface charge developed on the Ti surface (Figure 4) [67]. Wang et al. developed an economical surface treatment via a combination of AE and AT techniques to enhance hydrophilicity and osteoconductivity of poly(etheretherketone) (PEEK). They used 98 wt% H2SO4 as an AE solution for 5–90 s and 6 wt% NaOH for 20 s. The best enhancement was observed at 30 s etching, which decreased the contact angle (CA) from 78° to 37° [68].
![Figure 4
FE-SEM images of the Ti surface exposed to solutions with different pH values and subsequently immersed in SBF for 3 days (a) before and (b) after heat treatment [67]. Copyright 2012, The Royal Society.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_004.jpg)
FE-SEM images of the Ti surface exposed to solutions with different pH values and subsequently immersed in SBF for 3 days (a) before and (b) after heat treatment [67]. Copyright 2012, The Royal Society.
2.2.4 Sol–gel
Sol–gel, also called wet chemical deposition, is a widely used technique to enhance the bioactivity of the implant surface (Figure 5). The sol–gel technique is based on colloidal suspensions in a liquid solution that generates a solid layer by deposition of micro/NPs on a substrate. Simply, it is used to coat a thin film of biomimetic compounds (e.g., CaP compounds) to subsequently increase osteointegration [69].
![Figure 5
Schematic illustration of the sol–gel techniques [72]. Copyright 2020, Frontiers.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_005.jpg)
Schematic illustration of the sol–gel techniques [72]. Copyright 2020, Frontiers.
Of the notable benefits of this technique, it is possible to maintain the activity of biomolecules and deposit more complex compounds (e.g., compounds with the incorporation of drugs) on the implant surface [70]. Moreover, sol–gel has the potential to deposit an extended range of metal oxides such as TiO2, TiO2–CaP composite, or silica-based coatings on metallic/nonmetallic surfaces [71]. Esmael et al. successfully coated HAp and chitosan NPs on the Ti surface and immersed it in SBF. They reported that utilization of HAp has a synergetic impact on both the bioactivity and new apatite formation (Figure 3) [36].
2.2.5 Electrochemical treatment
Electrochemical treatment mainly refers to the three techniques, namely, anodic oxidation (AO), macro-arc oxidation, and electrophoretic deposition, which are commonly used in dental implant surface treatments.
2.2.5.1 AO
AO, also known as anodization, is a technique to fabricate nanostructured surfaces via potentiostatic or galvanostatic anodization by controlling several parameters including electrolyte composition, electric current, anode potential, temperature, and distance between the anode and cathode (Figure 6). Herein, implant as the anode is immersed in a homogenous electrolyte containing strong acids such as phosphoric acid (H3PO4), ammonium fluoride (NH4F), H2SO4, HF, or HNO3 or inhomogeneous electrolytes such as NaCl:NH4F [73] and NaHSO4:HF:NaF [74] with the passage of a high current density or voltage.
![Figure 6
Schematic illustration of the AO process [77]. Copyright 2017, Journal Teknologi.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_006.jpg)
Schematic illustration of the AO process [77]. Copyright 2017, Journal Teknologi.
AO enhances the corrosion resistance of metals (e.g., CP-Ti or Ta) by the formation of thick oxide layers. Moreover, the fluoride ions present in the electrolyte results in a nanotubular structure on titania [75]. Fialho et al. compared single and double AO on the Ta surface with the incorporation of Ca2+, PO4 3−, and Mg2+ ions on the surface; after the first anodization, enhancements in roughness and hydrophilicity were observed but after the second one, they also formed an amorphous tricalcium phosphate (TCP) on the surface [76].
2.2.5.2 Micro-arc oxidation (MAO)
MAO, also called plasma electrolytic oxidation (PEO), is the modified version of the AO technique with the assistance of plasma and high voltage to fabricate oxide protective layers on metallic surfaces like Ti, Ta, Mg, Al, and Zr and their alloys [78]. Alike AO, the implant as the anode and the stainless steel as the cathode are immersed in an electrolyte and a high voltage is applied on the electrolyte that gradually coats an oxide layer on the implant surface [79].
In MAO, the electrolyte composition is the key parameter that defines the final composition, porosity, and thickness of the coated layer. Sedelnikova et al. fabricated the Sr–Si–CaP and Ag–CaP incorporated coatings with different ratios of electrolytes and reported that the Ag–CaP coating contained pores and isometric particles of β-TCP uniformly distributed but the Sr–Si–CaP coating had spheroidal elements and open pores on their surfaces [80].
2.2.5.3 Electrophoretic deposition (ED)
ED is a cathode-based electrochemical method. ED refers to the deposition of colloidal particles on the substrate by passing a high voltage in a suspension (Figure 7). This method has the potential to coat HAp NPs with 18 nm thickness on the surface within 30 min [81]. Moreover, the combination of MAO and ED can generate a top layer of phase-pure HAp and an interlayer of anticorrosive TiO2 [82]. Hashim et al. evaluated the syntheses and deposition of TiO2, ZnO, and Al2O3 by rapid breakdown anodization (RBA) and ED. They reported that Ti, TiO2 and Zn, ZnO particles were able to form bone-like apatite using the RBA technique but Al2O3 did not have the potential [83].
![Figure 7
Schematic illustration of the electrophoretic deposition process [84]. Copyright 2015, ACS.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_007.jpg)
Schematic illustration of the electrophoretic deposition process [84]. Copyright 2015, ACS.
2.2.6 Chemical vapor deposition (CVD)
CVD is used to form thin layers on the implant surface by gaseous compounds. CVD involves chemical reactions between gas-phase chemicals and the surface that deposits nonvolatile compounds on the surface (Figure 8). Kania deposited diamond NPs on Ti orthopedic implants and observed notable increases in hardness, toughness, and adhesion [85]. CVD can be performed by many approaches and include atmospheric-pressure CVD (APCVD), low-pressure CVD (LPCVD), plasma-enhanced CVD (PECVD), and laser-enhanced CVD (LECVD). Moreover, a combination of CVD and PVD as a hybrid method was also developed [11].
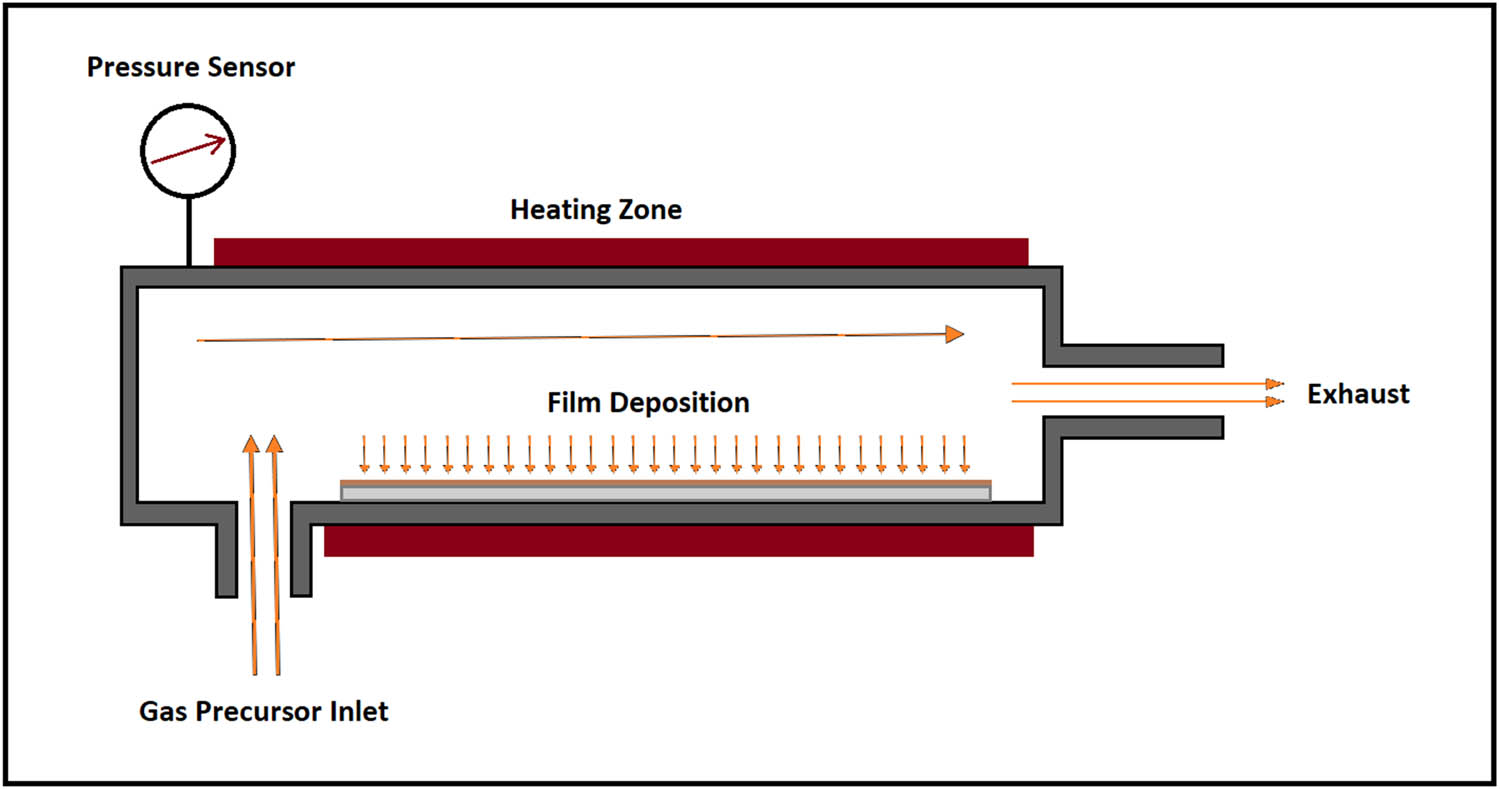
Schematic illustration of a chemical vapor deposition system.
CVD is capable of metalloceramic coatings by which it is possible to form nanocrystalline metallic bonds at the interlayer and hard-ceramic bond on the surface that subsequently overcomes adhesion problems in ceramic hard coatings on metallic substrates [86]. Chen et al. evaluated enhanced fluorine and oxygen mono/dual CVD to produce nanoscale coatings with antibacterial activity on the Ti surface. They found that fluorine deposited surface is able to kill Staphylococcus aureus (S. aureus) bacteria. Also, the presence of F and O elements has synergetic impacts on antibacterial activity, promotion in cell spreading, improvement in corrosion resistance, and biocompatibility [87].
2.2.7 Biochemical methods
To modify the implant surface with superior biological activities, biomolecular cues (BMCs) and antibacterial agents or drugs are the best candidates. The utilization of these large molecules is classified under biochemical methods, which is a subgroup of chemical methods. Hence, sole or incorporation of different biological materials with biomaterials has been seen, like BMCs with osteoinductive effect (e.g., cell adhesive proteins), nano CaP compounds with BMCs or antibacterial drugs, or direct coating of antibacterial agents or drugs. It should be noted that obtaining the aforementioned coatings can also be achieved via methods like sol–gel and magnetron sputtering (MS). However, SAMs are technically used to coat nanoscale biochemical molecules on the implant.
2.2.7.1 Self-assembled monolayers (SAMs)
SAMs can spontaneously coat nanoscale biochemical molecules by exposing specific substrates and functional end groups (Figure 9). SAMs are formed by the adsorption and self-assembly of molecules (e.g., alkane phosphate) and biomolecules [e.g., growth factors like bone morphogenetic protein-2 (BMP-2)] at the interface, which may either accelerate or provide different bioactivities [88,89,90]. Herein, surface roughness has a superior impact on the anchorage of fibroblast cells than wettability [15]. Moreover, surface modification techniques and molecular grafting have a synergetic role to increase the reactiveness between the outward surface and tailored ends of SAM molecules [91].
![Figure 9
Schematic illustration of the SAM process, preparing, and analyzing antibacterial activity of cefotaxime sodium-decorated Ti by coating polydopamine (PDA). The possible chemical structure, as well as the suggested reaction mechanism, is also shown [96]. Copyright 2014, Journal of the Royal Society Interface.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_009.jpg)
Schematic illustration of the SAM process, preparing, and analyzing antibacterial activity of cefotaxime sodium-decorated Ti by coating polydopamine (PDA). The possible chemical structure, as well as the suggested reaction mechanism, is also shown [96]. Copyright 2014, Journal of the Royal Society Interface.
SAMs have the potential to immobilize biocompatible molecules on the surface (linking function) that helps the ECM components (e.g., laminin [92], fibronectin [93], heparin [94], collagen [95], antibiotics [96], and growth factors [97]) to covalently bound onto the Ti surface. Urface et al. self-assembled arginine-glycine-aspartic-cysteine (RGDC) on the gold-coated Ti surface and observed enhancements in cell attachment, spreading, and proliferation [98].
2.3 Physical methods
Physical methods of modification are the transformation of an inert surface to a bioactive one under dry processes. These methods include a variety of single/hybrid procedures which generate a bioactive surface via the formation of layers of material on the surface or decontaminating it. Primarily, physical methods include plasma spray (PS), physical vapor deposition, ion-beam deposition, lithography, and laser treatment.
2.3.1 Plasma spray (PS)
PS is the most widely used technique to coat biomaterials on the implant surface. PS is the process of projection and condensation of high-temperature molten droplets on the surface (Figure 10). The temperature achieved by PS is much higher than similar procedures, which vary its applications of coating an extended group of materials such as Au, Ag, Ti, Zr, as well as other metals, ceramics, and polymers with the thickness of <100 nm [21,66,99]. Wang et al. coated Ti surface with Ta after two-step of AO and reported that Ta/TiO2 nanotubes were fabricated at micro- and nanoscales. Also, numerous enhancements in roughness, wettability, adhesion, differentiation, mineralization, and osteogenesis-related gene were observed [100].
![Figure 10
Schematic illustration of the PS process [72]. Copyright 2020, Frontiers.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_010.jpg)
Schematic illustration of the PS process [72]. Copyright 2020, Frontiers.
2.3.2 Physical vapor deposition (PVD)
PVD is used to produce metal particles that react with reactive gases to form compounds deposited on the implant surface. Technically, high-energy ions are ejected in a vacuum chamber that changes the surface of the substrate and form thin films (Figure 11).
![Figure 11
Schematic illustration of the physical vapor deposition process, sputtering, for deposition of target coatings [103]. Copyright 2021, MDPI.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_011.jpg)
Schematic illustration of the physical vapor deposition process, sputtering, for deposition of target coatings [103]. Copyright 2021, MDPI.
PVD includes three techniques, namely, evaporation, ion plating, and sputtering. Herein, sputtering, also known as sputtering deposition (SD), and derived techniques of ion plating are the most commonly used methods to deposit nanostructured films on the implant surface. Moreover, the development of SD led to more advanced techniques called ion beam sputtering (IBS) and MS (Figure 12) [101]. Huang et al. evaluated the incorporation of antibacterial agents onto the surface of the implant via the MS technique. They observed an acceptable bactericidal effect under optimum processing parameters [102].
![Figure 12
Schematic illustration of the MS process [104]. Copyright 2020, Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_012.jpg)
Schematic illustration of the MS process [104]. Copyright 2020, Royal Society of Chemistry.
2.3.3 Ion-beam-assisted deposition (IBAD)
IBAD is the combination of ion implantation and SD. In the IBAD technique, the implant surface is covered with an elemental cloud that is formed by ion bombarded precursors and targeted by highly energetic gas ions (e.g., inert Ar+ ions or reactive O2+ ions) to collide the gaseous ions to substrate ions and decrease the energy of the precursors in the elemental cloud to form a thin layer of material on the surface (Figure 13).
![Figure 13
Schematic illustration of the IBAD system and process [105]. Copyright 2011, Elsevier™.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_013.jpg)
Schematic illustration of the IBAD system and process [105]. Copyright 2011, Elsevier™.
This technique has several potentials including synthesis of the highly pure layer under ultraclean process and notable adhesion between the deposited layer and implant; also the process does not change the bulk properties of the substrate and it can be performed under controlled conditions [105]. The variables such as time, temperature, and amount of the water vapor present are considered as the main contributors to favorable crystallinity. Usually, this method is accompanied by heat treatment to change the amorphicity of the deposited layer to a crystalline phase. Miralami et al. fabricated ZrO2 and TiO2 coatings on the Ti surface. They found that nanostructures generated by IBAD enhanced bone-associated gene expression at initial cell adhesion, proliferation, and differentiation [106].
2.3.4 Lithography
Lithography is a favorable technique in electronic industries and is used to pattern specific models on a rigid substrate. Lithography consists of both top-down and bottom-up procedures to fabricate nanostructures. In top-down procedures, three widely used techniques including photolithography, electron beam lithography, and colloidal lithography are used and in bottom-up procedures, techniques like polymer phase separation, colloidal lithography, and block copolymer lithography are well-known [20,107]. The concept of patterning is simple, for example in photolithography, a predefined pattern is fabricated on a mask and locates upon the substrate that has been coated by a photoresist layer (e.g., chromium) and after the exposure of ultraviolet (UV) radiations, the pattern will transfer into the surface and the remaining photoresist layer can be chemically etched (Figure 14) [13]. Generating nanostructures with photolithography smaller than 100 nm is restricted by its diffraction limits [108]. However, electron beam lithography has overcome the limitation and has the potential to fabricate nanostructures down to 5–7 nm [109,110].
![Figure 14
Schematic illustration of photolithography [111]. Copyright 2019, Elsevier™.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_014.jpg)
Schematic illustration of photolithography [111]. Copyright 2019, Elsevier™.
2.3.5 Laser treatment
Laser treatment uses high-energy beams to generate 3D structures at the micro/nanoscale. This technique is capable of producing complex selective surface topography with high resolution [112]. Moreover, laser treatment is a rapid and ultraclean nanofabrication technique that has precise, targeted, and guided surface roughening that can be performed under controlled conditions for the selective changes in implants [66]. Chang et al. used 3D laser-printed porous Ti grade-5 and reported that porous dental implant had the active new bone formation and good osteointegration. Also, its biomechanical parameters are significantly higher than the commercially controlled samples [113].
3 Surface nanotopography
Recent advances in implant development have led to nanoscale topographies. Nanotopography is a characteristic of a surface that implies a specific physiobiological interaction between the nanopatterns and the environment. At the nanoscale, better interaction with the cell membrane has been reported [114]. Generally, numerous nanopatterns have been developed for bone regeneration applications (Figure 15). Therefore, biomimetic nanotopographies with mechano-bactericidal activity attracted a lot of attention as they can rupture the cell membrane through physical forces (Figure 16). This group is categorized into two subclasses: (i) nanopillars and (ii) nanoblades and their derivations. The first consists of nanopillars, nanospikes, nanotubes, nanocones, nanospears, nanoneedles, nanowires, and spinules. The latter includes nanoblades and nanocolumns [19,115].
![Figure 15
Schematic illustration of some nanopatterns [121]. Copyright 2016, Elsevier™.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_015.jpg)
Schematic illustration of some nanopatterns [121]. Copyright 2016, Elsevier™.
![Figure 16
Schematic illustration of the mechano-bactericidal function of the surface. (a) Contacts of a bacteria cell with the wing nanopillar surface. (b) Adsorption of the membrane to the surface protrusions leads to stretching of the cell membrane. (c) Gradual progress of adsorption leads to broad stretching at the contact regions and ultimately (d) cell death [122]. Copyright 2013, Elsevier™.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_016.jpg)
Schematic illustration of the mechano-bactericidal function of the surface. (a) Contacts of a bacteria cell with the wing nanopillar surface. (b) Adsorption of the membrane to the surface protrusions leads to stretching of the cell membrane. (c) Gradual progress of adsorption leads to broad stretching at the contact regions and ultimately (d) cell death [122]. Copyright 2013, Elsevier™.
The efficacy of bactericidal activity of nanopillars is dependent on several parameters but only four key geometric parameters including spacing, base diameter, tip diameter, and height are considered for the measurements (Figure 17) [116]. However, obtaining the optimum results requires considering the finite size and shape of bacteria plus the geometry of the nanopattern on the degree of bacterial membrane stretching using parameters such as bacterial stretching modulus, bending modulus, adhesion energy of the cell membrane, as well as radius, height, and spacing of the nanopillars [117].
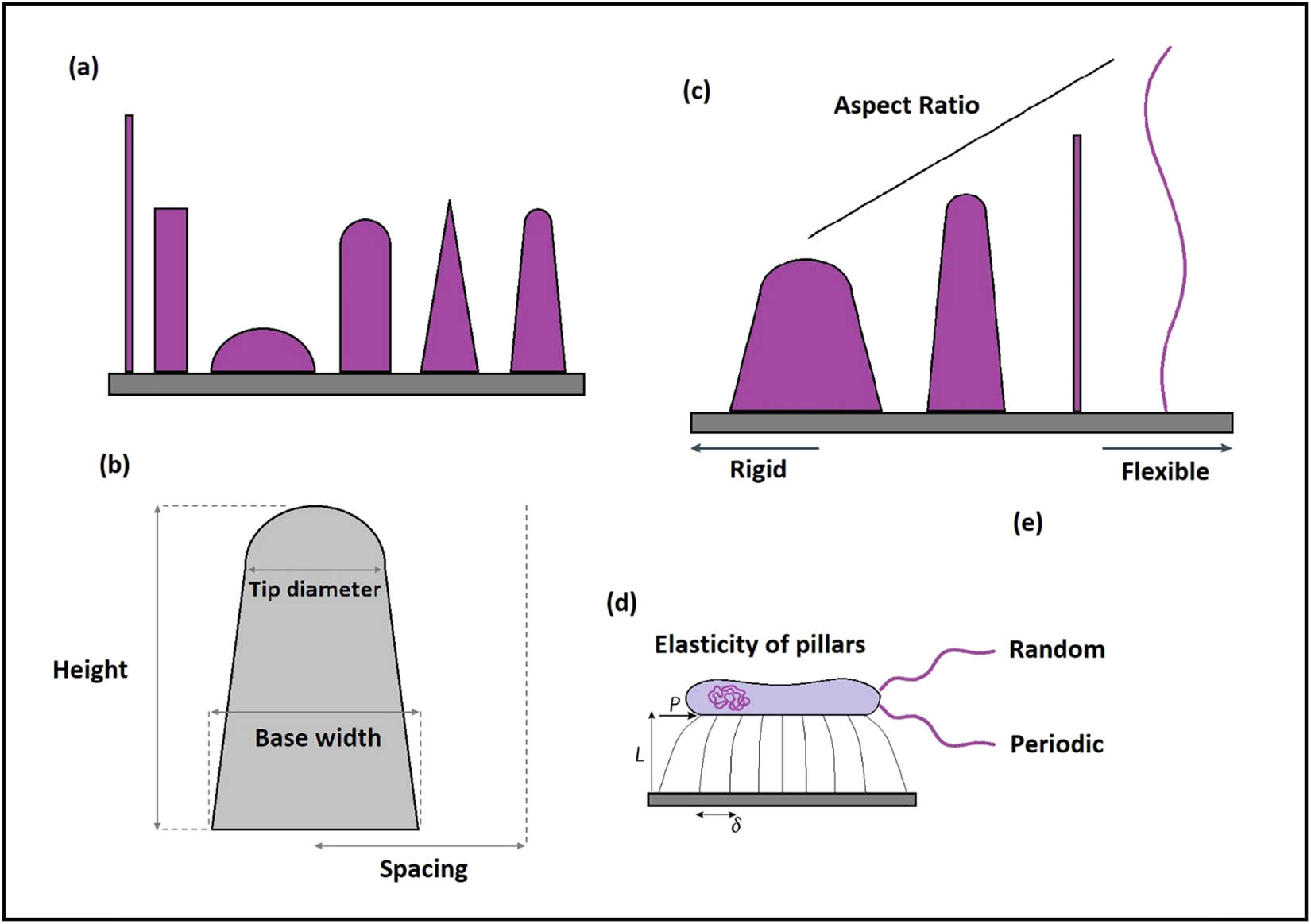
Schematic illustration of the physical parameters affecting the mechano-bactericidal activity of nanopillars and their derivations. (a) Examples of different nanopatterns with varied biocide levels. (b) The geometric characteristics are simplified based on the spacing, base diameter, tip diameter, and height. (c) Increasing the aspect ratio changes the rigid nanopillar to a flexible one, and subsequently, is accompanied by different cellular and antibacterial responses. (d) According to the aforementioned parameters, the strength of each individual nanopattern to impose physical forces on the cell membrane is related to the geometries. (e) Fabrication of nanopatterns can be either in random or periodic arrays. Therefore, the optimized mechano-bactericidal function demands significant attention to the geometric parameters and cautious fabrication.
Normally, nanopatterns are biocompatible yet decreasing in parameters like the tip diameter can reversely affect the osteogenic cells [118]. Therefore, utilization of nanotopographies with no bactericidal potential may be a sensible option as they are still able to chemically interact with bacteria and eliminate them. Of this group, nanopillars, nanotubes, nanofibers, nanogrooves, nanopits, and nanorods demonstrated higher cellular affinity [20,119,120].
3.1 Nanopillars
Nanopillars can be fabricated on the Ti surface by the AO technique with different states in arrangement [123,124]. The mechano-bactericidal activity of this nanotopography was first found from surfaces in nature [34]. This activity occurs by scratching the adsorbed cell wall and rupturing it by putting stress beyond its elastic limit [117,122,125]. Biologically, changes in the height of nanopillars lead to different responses from the cytoskeletal organization and cell morphology. The bactericidal mechanism of nanopillar and its derivations can be augmented by the deflection of elastic nanopatterns that induce additional lateral stress on the bacterial cell wall (Figure 18) [126]. In this case, both carbon nanotubes (CNTs) and high-aspect-ratio silicon nanowires displayed notable promotion in their mechano-bactericidal activity caused by their potential to deflect regarding bacterial attachment.
![Figure 18
SEM images of some (a–d) natural bactericidal nanotopography and (e–h) synthetic bactericidal nanotopography [131]. Copyright 2021, Elsevier™.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_018.jpg)
SEM images of some (a–d) natural bactericidal nanotopography and (e–h) synthetic bactericidal nanotopography [131]. Copyright 2021, Elsevier™.
As shown in Figure 17(d), the amount of lateral tip deflection (δ) caused by the generated forces from bacterial adsorption (P) is controlled by the length (L) of the nanopillars, as well as the attaching position of the bacteria to the cell membrane. Therefore, to obtain equal energy as longer nanopillars, shorter and low-aspect-ratio nanopillars should deflect less. Mcnamara et al. evaluated the cell organization of cytoskeleton cells on different nanopillars’ heights (8, 15, 55, 100 nm pillars) spread to polygonal shapes and observed that cytoskeleton cells were highly organized on 8 and 15 nm pillars, while by a gradual increase in the height, less organization were seen [127,128]. Moreover, planar control can significantly affect the focal adhesions and most of the larger focal adhesion per cell, but it can reversely affect the total number of adhesions per cell [127,128]. Similarly, variation in nanopillars’ height could not affect the osteogenic differentiation but it could enhance its bactericidal activity as it was higher and sharper [123,129]. Also, differences in their shapes can significantly affect osteogenic cell differentiation [130].
3.1.1 Nanospikes
Nanospikes are a derivation of nanopillars with a longer length, sharper tips, and high aspect ratio. Physical parameters of nanospikes were 20–80 nm in diameter and 500 nm in height, which by considering their sharpness, they can also restrict cellular activities. Therefore, Ivanova et al. fabricated the only in vivo biocompatible and mechano-bactericidal nanospikes by using black silicon [118]. Nanospikes are able to induce severe stress on the cell membrane that has high efficacy against both Gram-positive and Gram-negative bacteria, even highly resilient Bacillus subtilis (B. subtilis) endospores were annihilated [132]. Black silicon nanospikes are not able to interact with the biological environment and if the surface sustains unharmed after sterilization, it has the potential for mechano-bactericidal applications.
3.1.2 Nanotubes
Nanotube arrays, also known as nanodarts, on the Ti surface have been achieved by numerous methods. In this nanotopography, physical parameters including the tube diameter, the thickness of the nanotube layer, and the crystalline structure can influence the cellular responses [133,134,135]. Nanotube arrays are of attractive topographies due to their effectiveness to promote osteointegration [136,137]. Moreover, nanotube arrays of Ta on the Ti surface significantly increase the cell morphology and proliferation, and osteogenic differentiation of stem cells [138,139]. Gulati et al. evaluated the influence of dual topography composed of microscale spherical particles and vertically aligned TiO2 nanotubes via 3D printers. This topography could obtain good enhancement in osteogenic gene expression [140].
Generally, when bacteria are adsorbed onto the surface of CNTs, it leads to tip deflection and retraction, which subsequently disturbs the physical membrane of the bacteria and results in cell death [141]. The bactericidal effectiveness of CNTs is different, e.g., short CNTs with an aspect ratio of 100 have higher efficiency than longer CNTs with an aspect ratio of 3,000 (Figure 19). This effect is attributed to the larger storage of elastic energy by short CNTs that can spontaneously pierce the membrane [142]. The mechano-bactericidal of CNTs was discovered when Elimelech et al. observed significant damage to the cell membrane of Escherichia coli (E. coli) as it directly contacts single-walled CNTs (SWCNTs) [143]. SWCNTs with diameters between 1 and 5 nm have less effect on the direct piercing of a phospholipid bilayer and the free energy costs are regarded as the minimum energy for creating a pore in the bilayer [142]. However, hydrophobic SWCNTs demonstrated greater interaction with phospholipid tails and subsequently entrapped the CNT in the bilayer core in parallel orientation [144]. It should be noted that increasing the tension on the membrane facilitates the translocation of nano-objects through the membrane. Moreover, highly hydrophobic CNTs, as well as graphene, are able to create unstable pores in the membrane by rotation of lipid tails to carbon surface and constant extraction of lipids [145].
![Figure 19
Suggested penetration mechanism of hydrophobic ultrashort CNT (USCNT) by rupturing the lipid bilayer of the cell wall. (a) Dioleoylphosphatidyl-choline (DOPC)-capped USCNT interacts with the bacterial cell membrane by exchanging lipids. (b) USCNT becomes wrapped by lipids that results in dysfunctioning the membrane via the formation of pores. To obtain a general perspective of the process, some normal lipids of the bilayer were substituted by fluorescent lipids that can be detected by fluorescence microscopes [145]. Copyright 2018, ACS Publications.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_019.jpg)
Suggested penetration mechanism of hydrophobic ultrashort CNT (USCNT) by rupturing the lipid bilayer of the cell wall. (a) Dioleoylphosphatidyl-choline (DOPC)-capped USCNT interacts with the bacterial cell membrane by exchanging lipids. (b) USCNT becomes wrapped by lipids that results in dysfunctioning the membrane via the formation of pores. To obtain a general perspective of the process, some normal lipids of the bilayer were substituted by fluorescent lipids that can be detected by fluorescence microscopes [145]. Copyright 2018, ACS Publications.
3.1.3 Nanoblades
Graphene sheets, also known as nanoblades, with 10–15 nm edges, are of the second category of mechano-bactericidal nanotopographies that are 2D sheet-like nanomaterials consisting of a single layer of carbon atoms. In most nanoblades, it is essential to be perpendicularly oriented, but in nanosheets, the orientation of 37° demonstrated sufficient bactericidal activity [146,147,148]. The general antibacterial activity of graphene has been achieved through both physical and chemical states [149,150,151,152]. The physical interaction of graphene is shared with its derivations, namely graphene family nanomaterials (GFNs), which due to possessing certain parameters, their bactericidal activities can be predicted [153]. The addition of graphene nanosheets in chemical suspensions (Figure 20) provides sufficient antibacterial activity to rupture both Gram-negative and Gram-positive bacteria by creating pores in their cell membrane and stimulating the alteration of osmotic pressure that resulted in cell death [147].
![Figure 20
Schematic illustration of the antibacterial activity of graphene oxide (GO)-based on mass spectrometry [157]. Copyright 2016, Scientific Reports.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_020.jpg)
Schematic illustration of the antibacterial activity of graphene oxide (GO)-based on mass spectrometry [157]. Copyright 2016, Scientific Reports.
Akhavan et al. evaluated the antibacterial activity of graphene oxide nanowalls (GONWs) on E. coli and S. aureus as Gram-negative and Gram-positive bacteria, respectively. They observed GONWs have stronger antibacterial activities against S. aureus by colony-forming unit (CFU) enumeration and quantification of cytoplasmic RNA leakage [153]. Similarly, Liu et al. confirmed that the bactericidal effect of GFNs against Gram-positive bacteria like E. coli was attributed to their sharp edges that can induce sufficient stress on the cell membrane (Figure 21) [154]. The mechanical disruption of GFNs is bonded to the degree of lipophilicity as the higher lipophilic one is able to facilitate the extraction of lipids from the cell membrane [155,156]. However, the progress of extermination of bacteria consists of complex processes including extraction of the lipids, formation of pores, alternation of osmotic pressure, and ultimately, cell death.
![Figure 21
Schematic illustrations, small angle X-ray scattering (SAXS), and atomic force microscopy (AFM) characterization of GO composite to define its orientation in bulk and on the surface of specimens, respectively. According to the 3D illustration, the X-ray beam is located parallel to the film plane, and the AFM probe is placed above the surface. (a) Composite without GO nanosheets displayed minor scattering intensity in the 2D SAXS pattern and smooth surface in the 3D AFM image. (b) Composite with randomly oriented GO nanosheets displayed a notable increase to a broad, isotropic halo in the 2D SAXS pattern, and a sharp increase in roughness in the 3D AFM image. (c) Composite with vertically GO nanosheets displayed anisotropic equatorial scattering in the 2D SAXS pattern, and vertical alignment in the 3D AFM image. (d) Composite with planar GO nanosheets displayed anisotropicmeridional scattering in the 2D SAXS pattern and a smoother surface in the 3D AFM image [148]. Copyright 2017, PNAS.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_021.jpg)
Schematic illustrations, small angle X-ray scattering (SAXS), and atomic force microscopy (AFM) characterization of GO composite to define its orientation in bulk and on the surface of specimens, respectively. According to the 3D illustration, the X-ray beam is located parallel to the film plane, and the AFM probe is placed above the surface. (a) Composite without GO nanosheets displayed minor scattering intensity in the 2D SAXS pattern and smooth surface in the 3D AFM image. (b) Composite with randomly oriented GO nanosheets displayed a notable increase to a broad, isotropic halo in the 2D SAXS pattern, and a sharp increase in roughness in the 3D AFM image. (c) Composite with vertically GO nanosheets displayed anisotropic equatorial scattering in the 2D SAXS pattern, and vertical alignment in the 3D AFM image. (d) Composite with planar GO nanosheets displayed anisotropicmeridional scattering in the 2D SAXS pattern and a smoother surface in the 3D AFM image [148]. Copyright 2017, PNAS.
3.1.4 Nanofibers
Of the nanotopographies with biological activity, nanofibers are a great choice for bone tissue engineering scaffolds. Nanofibers possess notable properties including high surface-to-volume ratio, well-retained topography, facile control of components, and the capacity to mimic the native ECM that can affect adhesion, proliferation, and differentiation of stem cells [158,159,160]. Xu et al. fabricated a scaffold made of polylactic acid (PLA) and chitosan by the electrospinning technique. To generate a core–shell and island-like structure, they mixed electrospinning with automatic phase separation and crystallization. By culturing preosteoblast cells (MC3T3-E1), they observed a balanced hydrophilic and hydrophobic surface that successfully enhanced mineralization and cell growth, as well as alkaline phosphatase (ALP) activity of MC3T3-E1 cells [161]. The utilization of simple techniques like electrospinning provided a remarkable potential for some bioactive materials or NPs to incorporate into the nanofibrous structured scaffold [162,163,164]. Yao et al. assessed the biological responses of gelatin nanofibrous scaffold with locally immobilized deferoxamine (DFO). It reduced the cytotoxicity of human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (MSCs). Also, it has significantly promoted vascular endothelial growth factor (VEGF) expression in human MSCs and BMP-2 expression in HUVECs [165].
3.1.5 Nanogrooves
Despite no bactericidal activity, nanogrooves are still an interesting option for their potential to promote the migration and proliferation of osteoblast cells [166]. An investigation on cellular affinity on micro- and nanogrooved surfaces displayed a significant reduction of up to 40% of cell-repelling capacity on microgrooved than nanogrooved surfaces [167]. Klymov et al. compared the smooth and grooved CaP-coated surface by culturing osteoblast-like MC3TC cells. They found that not only did nanogrooved surfaces promote the differentiation and mineralization processes but they also organized the morphological deposition of minerals [168].
3.1.6 Nanopits
Nanopits can be generated by PVD techniques with controlled nanopore size. Herein, a few pieces of research have been done to understand the physiobiological function of this nanopattern. Lavenus et al. evaluated the presence of human MSCs on the Ti surface with varied nanopore diameters. They observed that human MSCs exhibited a more branched cell morphology on nanopores with 30 nm pore size [169]. Similarly, Dalby et al. investigated the influence of spatial arrangement of nanopits on osteogenic differentiation on the poly(methyl-methacrylate) (PMMA) surface and cultured osteoprogenitor and MSCs on nanopits with diameter, depth, and center–center spacing of 120, 100, and 300 nm, respectively, as well as with the spatial arrangement of the square array, hexagonal array, disordered square arrays with 20 and 50 nm displacement from their square position (20-DSA and 50-DSA), and randomly positioned. Within the 21st day, observation demonstrated a significant increase in levels of osteopontin (OPN) and osteocalcin (OCN) on 50-DSA surfaces compared to other surfaces. With the same result for MSCs, it was spotted that MSCs have a greater affinity to DSA surfaces than other surfaces [124].
4 Multifunctional coatings
Modifying implants with NPs is a promising procedure to reduce the risk of failure. Implants, depending on the patient’s general condition, may need to be treated with different NPs to guarantee their success (Figure 22). Herein, we classified these NPs according to their nature as (i) metallic-based, (ii) ceramic-based, (iii) polymer-based, (iv) carbon-based, (v) protein-based, and (vi) drug-based coatings (Table 2). In the following sections, the subclasses and their biological applications in modifying the endosseous implant body are explained.
![Figure 22
Applications of nanoparticle coating in dentistry [22]. Copyright 2018, Elsevier™.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_022.jpg)
Applications of nanoparticle coating in dentistry [22]. Copyright 2018, Elsevier™.
A summary of the aforementioned multifunctional coatings and their biogenic mechanisms
| Category | Subcategory | Biogenic and/or biocidal activities | Further advantages | ||||
|---|---|---|---|---|---|---|---|
| Angiogenesis | Osteoinduction | Osteoconduction | Osteogenesis | Antibacterial | |||
| Metal | Tantalum (Ta5+) | × | × | ||||
| Titanium (Ti4+) | × | × | |||||
| Silicon (Si4+) | × | × | × | ||||
| Cerium (Ce4+) | × | × | Anti-inflammatory | ||||
| Gold (Au3+) | × | × | Antifungal and anticancer | ||||
| Iron (Fe3+) | × | Antibiofilm | |||||
| Copper (Cu2+) | × | × | × | Antifungal, antibiofilm, and antimicrobial | |||
| Zinc (Zn2+) | × | × | Antibiofilm | ||||
| Magnesium (Mg2+) | × | × | × | ||||
| Silver (Ag+) | × | Antimicrobial | |||||
| Selenium (Se2−) | × | Viruses and cancer cells | |||||
| Strontium (Sr2+) | × | Osteoclastogenesis | |||||
| Cobalt (Co2+) | × | ||||||
| Ceramic | Zirconium (Zr4+) | × | × | ||||
| Bioactive glass | × | × | × | × | |||
| CaP-Based materials | × | × | |||||
| Polymer | Chitosan | × | Antimicrobial, antiviral, antifungal, antitumor, immunoadjuvant, anti-thrombogenic, and anti-cholesteremic | ||||
| Peptides | × | Antimicrobial | |||||
| Carbon | Carbon family | × | Antibacterial, antimicrobial, antioxidant, and anticarcinogenic | ||||
| Protein | ECM proteins | × | × | × | |||
| Growth factors | × | × | |||||
| Drug | Organic drugs | × | × | ||||
4.1 Metallic-based coatings
4.1.1 Tantalum (Ta5+)
Tantalum (Ta) as both implant material and surface coating obtained considerable attention. Ta is a biocompatible metal with high corrosion resistance and favorable elastic modulus [32]. Ta is able to promote bone ingrowth and osteoconductivity, which are notable parameters as the secondary stability of implants [170]. Alves et al. deposited Ta-derived coatings on the Ti surface. They observed higher Ca:P ratio formation on the surface, which was attributed to the high oxygen content of the coating that increases the affinity for apatite adhesion [171]. Zhang et al. coated tantalum nitride (TaN) on the Ti surface. Results displayed higher antibacterial and corrosion resistance than individual Ti or TiN coatings [172]. Similarly, Zhang et al. coated Ta on the Ti surface to assess its biological activities. Ta-treated Ti could effectively kill Fusobacterium nucleatum (F. nucleatum) and Porphyromonas gingivalis (P. gingivalis) microbes. Also, it promoted osteointegration by activating the secretion of bone-forming proteins [173].
4.1.2 Titanium (Ti4+)
Coating titania (TiO2) NPs have various applications in medicine. Titania possesses unique photocatalytic properties by being exposed to visible or UV light irradiation [174]. TiO2 NPs possess excellent biogenic and biocide properties. The antibacterial mechanism of TiO2 is attributed to the formation of hydroxyl radicals by a photocatalytic reaction in an aqueous environment that targets the peptidoglycan cell membrane and interacts with polyunsaturated phospholipids, ultimately damaging the DNA and resulting in cell death [175]. Chidambaranathan et al. compared the antifungal activity of TiO2, ZrO2, and Al2O3 NPs coated on the Ti surface. After 24, 72 h, and 1-week time intervals, TiO2 demonstrated significant antibacterial activity against Candida albicans (C. albicans) bacteria than the other NPs [176].
On the other hand, the utilization of titania NPs can improve osteogenic activities like increasing the level of osteoblast cells on the surface, which leads to higher bone generation [177,178,179]. However, introducing TiO2 nanotubes attracted considerable attention. Primarily, TiO2 nanotubes were fabricated to increase the biogenic activity and enhance the osteointegration, but, as a biocide agent, they possess antimicrobial function by being accompanied by other antibiotics compounds like vancomycin, gentamicin, silica-gentamicin, gentamicin sulfate, BMP-2, and heparinized-Ti to eliminate Gram-positive bacteria like S. aureus [180,181,182,183,184,185,186].
4.1.3 Silicon (Si4+)
In nanotechnology, silicon (Si) mainly as silica (SiO2) NPs obtained a unique position. The utilization of these NPs enhances osteogenic differentiation of MSCs by increasing the osteoblasts’ adhesive response that consequently leads to higher osteointegration [187]. Moreover, SiO2 NPs depending on their size display different levels of hydrophilicity that can be exploited in drug-releasing applications [188]. Also, their products from degradation generate silicic acid [Si(OH)4], which is a supporting compound to generate connective tissues [185]. Csík et al. coated the CP-Ti surface with calcium silicate (Ca2O4Si) (CaSi) NPs by electrospray deposition (ESD) followed by thermal treatment at 750°C to examine its properties. They observed that CaSi NPs could effectively reduce the E. coli and S. aureus bacteria and promote human MSC activity [189]. Massa et al. assessed the antibacterial activity of SiO2 and the Ag nanocomposite (NSC/AgNPs) coated on Ti grade-5. They reported that not only did NSC/AgNPs kill the Gram-negative bacteria, Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), but it also reduced the biofilm formation up to 70%, compared to the control group [190]. Catauro et al. developed the silica/quercetin hybrid as an antioxidant drug to scavenge the reactive oxygen species (ROS) and reactive nitrogen species (RNS) for dental implant applications. Quercetin is able to maintain the conditions of the general tissues against osteoporosis, pulmonary, and cardiovascular diseases, a particular spectrum of cancer, and premature aging. The results showed that quercetin successfully restricted the dehydrogenases’ activity [191].
4.1.4 Cerium (Ce4+)
Ceria (CeO2) is another NP with strong anti-inflammatory and antibacterial activities. CeO2 NPs possess superoxide dismutase (SOD) and catalase (CAT) enzymatic activities, as well as ROS-scavenging [26]. Li et al. evaluated the biological responses of CeO2 NPs coated on the Ti surface. The results showed that CeO2 NPs have no cytotoxicity to MC3T3-E1 cells and they can improve cell adhesion; also CeO2 coating maintained intracellular antioxidant defense system from H2O2-treated osteoblasts [192]. Qi et al. evaluated the incorporation of CeO2 and calcium silicate (Ca2O4Si). This composite displayed sufficient biocompatibility and antimicrobial activity against Enterococcus faecalis (E. faecalis) [193]. Ceria with varied nanopatterns exhibited different levels of antibacterial activity. Li et al. compared the biocide activity of ceria NPs with different nanotopographies. They fabricated three nanopatterns including nanorod, nanocube, and nano-octahedron to select the one with less presence of Streptococcus sanguinis (S. sanguinis) bacteria. From in vitro and in vivo tests, they concluded that nano-octahedron had stronger anti-inflammatory and antibacterial activities [194].
4.1.5 Gold (Au3+)
In terms of medical application, gold (Au) NPs have been frequently used due to their notable mechanical, chemical, and optical properties. Despite the high price of gold that may be restrictive, still, its utilization is inevitable. In dentistry, Au NPs demonstrated both biogenic and antibacterial activities but within the range of 40–50 nm, they are toxic. Therefore, their applications have been limited to 20–40 nm, which has been found to be the optimum cellular affinity [195]. Au NPs possess antibacterial, antifungal, and anticancer functions [196,197]. The antifungal effects of Au NPs are highly dependent on their geometry [198]. Technically, the antifungal mechanism of Au NPs is based on the prevention of the H+-ATPase action or transmembrane H+ efflux action of Candida bacteria [30]. In general, the bactericidal effects of Au NPs are weaker than Ag on both Gram-positive and Gram-negative bacteria [199,200,201]. The antibacterial mechanism of Au NPs is based on the prevention of ribosome subunit for t-RNA binding or changing the membrane potential and obstructs the ATP synthase, which disturbs both the biological system and results in cell death. Also, the interaction of Au NPs is free of ROS, which implies less toxicity on mammalian cells [202].
Regiel-futyra et al. coated chitosan-based Au composite NPs on Ti implant. This innovative composite displayed remarkable antibacterial function against antibiotic-resistant strains of Pseudomonas aeruginosa (P. aeruginosa) and S. aureus without any sign of cytotoxicity [203]. Wang et al. evaluated the modification of Au NPs with thiol or amine groups to decorate different densities of phenylboronic acid to fabricate Gram-selective antibacterial agents. They observed that modified Au NPs with amine- or thiol-tethered phenylboronic acids can effectively interact with lipopolysaccharide (LPS) and lipoteichoic acid (LTA), as Gram-positive and Gram-negative compounds, respectively. Also, tunable ratios of thiol- and amine-tethered phenylboronic acids lead to different antibacterial activity [204]. Heo et al. assessed the effects of Au NPs on osteoblast differentiation. From in vitro and in vivo tests, they observed an increase in mRNA expression of osteogenic differentiation-specific genes and good bone formation on the Ti surface, respectively [205].
4.1.6 Iron (Fe3+)
Iron oxide (Fe x O x ) has proven its capability as a dental coating. Primarily, magnetite (Fe3O4) and maghemite (Fe2O3, y-Fe2O3) are the two most popular forms of iron oxide NPs without further cytotoxicity [206]. Most Fe x O x -derived NPs in biomedicine possess superparamagnetic properties [207]. For example, Fe2O3-superparamagnetic can improve osteoblast functions and help to eliminate the formed biofilms [208]. Generally, iron oxide NPs are able to interact with exopolymers that are generated by bacteria and are difficult to be eradicated by many antibiotics and immune cells as they are impenetrable [209].
Thukkaram et al. evaluated the efficacy of varying concentrations of iron oxide NPs against biofilm formation on different biomaterials. The optimal effectiveness was obtained at a concentration of 0.15 mg/mL against S. aureus, E. coli, and P. aeruginosa bacteria [210]. In addition to biocide activity, iron oxide NPs possess magnetic properties that displayed higher stability and safety than other commonly used NPs. The cytotoxicity and inflammatory responses of these NPs are highly dependent on concentration rather than size, which could be exploited for designing new drug carriers [211].
4.1.7 Copper (Cu2+)
Copper oxide (Cu x O x ) NPs have unique physical, chemical, and biological properties that expand their medical applications. Similar to Ag, CuO-derived NPs displayed excellent antibacterial activity. However, the utilization of such metallic ions was restricted as their adverse effects were induced by remaining residues [212]. In addition to being antibacterial, Cu x O x NPs possess antifungal, antibiofilm, and antimicrobial activities [21]. The antibacterial mechanism of these NPs is based on passing through nano-mimic pores on the bacteria cell membrane and disturbing their biological activity by damaging the vital enzymes. This progress is highly dependent on the size, stability, and the concentration of NPs in the medium [213].
Likewise, antimicrobial activity is the result of the generation of ROS, which increases the oxidative stress of the cells [214]. Rosenbaum et al. coated CuO-derived NPs on the TiO2 surface and have not found a sign of S. aureus and E. coli bacteria [215,216]. Liu et al. included copper in the Ti alloy composition and observed significant activity against Streptococcus mutans (S. mutans) and P. gingivalis [217]. Khan et al. evaluated the inhibitory effect of Cu x O x NPs within a size of 40 nm to prevent biofilm formation. In vitro results revealed that a concentration of 50 mg/mL can successfully prevent the development of oral bacteria and their polysaccharides [218].
4.1.8 Zinc (Zn2+)
Recently, zinc oxide (ZnO) NPs have become an attractive candidate as a biologically active ion that promote osteointegration and restrict the adhesion of bacteria [219,220]. The general bactericidal mechanism of ZnO NPs consists of a combination of (i) generation of H2O2 and (ii) formation of electrostatic interaction that accumulates ZnO NPs on the bacteria cell membrane, (iii) generation of ROS that leads to the release of Zn2+ ions, and ultimately, dysfunctioning the cell membrane [213]. This progress is applicable against both Gram-positive and Gram-negative bacteria [213,221]. Hu et al. coated ZnO NPs on the TiO2 surface and restricted the growth of S. aureus and E. coli bacteria [222]. Similarly, Luo et al. fabricated ZnO@ZnS nanorod-array and optimized the release rate of Zn2+ ions that demonstrated higher bactericidal activity against S. aureus and E. coli bacteria [223]. Tabrez Khan et al. assessed the inhibitory function of ZnO NPs against biofilm-forming bacteria and colonizers. They observed that ZnO is able to provide significant bactericidal activity on different surfaces [218].
4.1.9 Magnesium (Mg2+)
Magnesium (Mg) is an attractive metal that can be fully adsorbed without acute toxicity. Physically, Mg possesses similar parameters to the human bone but, chemically, it has a limited range of applications that is mainly the result of its high rate of degradation. This high interaction of Mg2+ ion is attributed to the chloride ion (Cl−) available in ECM that forms the MgCl2 compound [224]. In general, the presence of Mg2+ ion promotes proliferation and differentiation of osteoblast cells [225]. Also, magnesium oxide (MgO) possesses good bactericidal activity with the mechanism of disturbing the bacteria cell membrane, which leads to leakage of intracellular contents and ultimately cell death [226,227]. To control the release rate of Mg2+ ions, simply compounding it with other materials cannot increase its corrosion resistance. For example, magnesium phosphates (MgPs) demonstrated higher in vivo adsorption than the calcium phosphate (CaP) compounds [228]. Kishen et al. compared the antimicrobial activity of MgO, sodium hypochlorite (NaOCl), and chitosan NPs. They concluded that both MgO and chitosan NPs have comparable or superior bactericidal activity than NaOCl against E. faecalis bacteria [229].
4.1.10 Silver (Ag+)
Of the most practical antimicrobial coatings, AgNPs have been taking the lead. In retrospect, the high release of Ag+ ions from the implant surface would disturb the normal biological activities, but AgNPs at low doses displayed high biocompatibility and antibacterial activity with no sign of cytotoxicity, genotoxicity as well as side-effects [177,230]. The utilization of Ag+ ions was in the form of silver nitrate (AgNO3), silver sulfadiazine (C10H9AgN4O2S), silver chloride (AgCl), and pure metal that could exterminate a wide spectrum of both Gram-positive and Gram-negative bacteria. However, AgNPs demonstrated higher antimicrobial efficacy than the aforementioned forms [231]. Ag at the nanoscale can facilitate the formation of holes onto the bacteria cell membrane and result in cell death [232]. This mechanism is attributed to the interaction of AgNPs with disulfide or sulfhydryl groups of enzymes [233]. In recent research works, the fabrication of Ag-based composite NPs has been widely seen. Choi et al. fabricated PDA and AgNPs on the Ti surface. They observed lower colonization of S. mutans and P. gingivalis microbes with a coating of these NPs than uncoated Ti [234,235]. Gunputh et al. coated AgNPs on the TiO2 nanotube surface with and without a top coating of HAp NPs to evaluate its biocide activity against S. aureus microbe. In vitro results revealed that AgNPs could effectively reduce the presence of the microbe. Also, the addition of HAp did not improve the biocidal function but it could diminish the release rate of Ag+ ions [236].
4.1.11 Selenium (Se2−)
Selenium (Se) NPs are unique elements with high biocidal potential against bacteria, viruses, and cancer cells. Se2− ions are a vital element in biological processes that control the elimination of ROS and modulation of a specific enzyme that lacks its increased susceptibility to viral infections [237,238]. Nanoscale Se exhibits a reduced risk of toxicity than its other forms like selenomethionine (SeMet) [239]. Therefore, the utilization of SeNPs as chemopreventive and chemotherapeutic coatings with both antibacterial and antiviral activity became attractive [240,241,242,243]. Moreover, SeNPs have proven to possess high bactericidal activity against S. aureus and Staphylococcus epidermidis (S. epidermidis) bacteria, which are the main cause of implant failure [244,245]. Srivastava et al. examined different concentrations of SeNPs to eliminate various bacteria. They found that at concentrations of 100, 100, 100, and 250 µg/mL, these NPs can kill 99% of Pseudomonas aeruginosa (P. aeruginosa), S. aureus, Streptococcus pyogenes (S. pyogenes), and E. coli, respectively [246].
4.2 Ceramic-based coatings
4.2.1 Bioinert ceramics
4.2.1.1 Zirconium (Zr4+)
The utilization of zirconia (ZrO2) NPs in dentistry has been expanding since these NPs displayed great potential in improving the physicochemical properties of different compounds. Zirconia is a bioinert ceramic with low toxicity. Also, it cannot dissolve in water, which causes less adhesion of bacteria [247]. Zirconia-based NPs demonstrated antimicrobial activity against some microorganisms like E. faecalis [248]. In terms of biogenicity, ZrO2 NPs promote the attachment and proliferation of osteoblast and fibroblast cells and release nontoxic ions [249,250,251,252]. However, compared to Ti, ZrO2 NPs are at disadvantage to promote cell viability [253]. Huang et al. coated ZrO2 NPs on the Ti implant via the PS technique. After 2 weeks, the in vivo test showed a higher level of osteoblast cells that was attributed to the theory that adhesion of osteoblast cells can be promoted by higher free-energy surface [136,254].
4.2.2 Bioactive ceramics
4.2.2.1 Bioactive glass
Bioactive glasses (BGs) mainly refer to the mixture of silica, calcium oxide (CaO), sodium oxide (Na2O), and phosphorous pentoxide (P2O5) as silicate-based compounds [255,256]. BGs compared to other bioglasses (e.g., borate-based glass or phosphate-based glass), or ceramic glasses, have a higher potential to merge with the host tissue [257,258,259]. Moreover, manifold elements can be doped with BGs to modify their properties, for example, utilization of Na+, Mg2+, Al3+, Ti4+, or Ta5+ ions can reduce or increase the solubility as well as Ag+, strontium (Sr2+), or Zn2+ for modifying bactericidal activity, cellular viability, or anti-inflammatory responses of BGs, respectively [257,260,261]. Kalantari et al. evaluated the synthesis and osteogenic applications of monticellite for bone tissue engineering and compared its biological activity with HAp. They concluded that monticellite has a higher bone formation than HAp and it also provides minor antibacterial activity due to the presence of Si4+ ions in its components [262,263,264,265,266,267].
4.2.3 Biodegradable ceramics
Biodegradable ceramics refer to calcium-derived compounds (CDCs), mainly calcium phosphate (CaP)-based materials including α/β-TCP, tetracalcium phosphate (TTCP) [Ca4(PO4)2O], and hydroxyapatite (HAp) [Ca10(PO4)6(OH)2], which have above 1.5 Ca:P ratio [21,268]. These CDCs by containing similar components as natural bone demonstrated remarkable cellular affinity that subsequently promotes the osteogenic cells anchorage and higher osteoconductivity and osteointegration [269,270]. Schouten et al. compared the in vivo biological activity of the Ti surface with and without the utilization of CaP coatings. The results showed that the presence of the CaP compound on the implant site increased the bone healing process and led to higher osteointegration [271]. However, the controversial parameter of these CDCs is their low solubility. Okuda et al. evaluated the adsorption of rod-shaped particles of HAp in the rabbit femur. They observed that 24 weeks after implantation, 80% of HAp remained and after 72 weeks, although most of the HAp was resorbed, still the presence of HAp around the implantation site could be detected [272]. Therefore, synthesizing the biphasic CDCs with different combining ratios may be an optimal solution to control their degradation rates [273].
Despite the broad uses of CDCs, still, the challenges of fabricating an ideal compound for bone restorative applications remain. Hence, the utilization of multifunctionalized compounds tends to be a reasonable answer to fulfill the demands for this purpose. Immobilizing growth factors or peptides as a guiding cell behavior have been conducted, yet their fast denaturation is their main drawback [274,275,276,277,278]. Bisphosphonates (BPs), such as alendronate and zoledronate, are other molecules that have been widely used as medicine to treat bone metabolic disorders. BPs are used to prevent osteoclastogenesis, a feature that helps to cure osteoporosis or Paget’s disease [279]. Other interesting molecules that contribute to several transduction pathways are glycosaminoglycans (GAGs). The family of GAGs including hyaluronic acid (HA), heparin, heparan, chondroitin, and keratin sulfates, are ubiquitous molecules that exist in the stem cells niche and ECM, and they have a high affinity for growth factors and proteins [280,281,282,283,284,285,286,287]. GAGs’ family displayed a significant impact on regenerative progress in various cell lineages like the immune system, fibroblast, endothelial, and skeletal cells [280,288,289]. Recent studies have led to the utilization of PDA, a catechol-containing biomimetic molecule. PDA demonstrated a notable impact on bone mineralization via concentrating Ca2+ ions at the interface. Yong et al. deposited PDA-assisted HAp on porous Ti scaffold. In vitro tests revealed that such coating can promote proliferation, attachment, and bioactivity by inducing ALP expression in MC3T3-E1 cells [290]. However, assimilating to osteogenic cells, CDCs also allow bacteria to colonize and increase the risk of failure. To overcome this problem, CDCs are usually accompanied by an antibacterial agent to acquire a biocidal function [291,292].
In addition to CaP-based materials, there are CaP-based salts like calcium sulfate (CaSO4) and calcium carbonate (CaCO3) that have been extensively used in clinical applications. Ohgushi et al. examined the bone-forming responses of Ti surface coated with CaCO3. They concluded that CaCO3 demonstrated considerable potential in bone restorative applications, which can be compared with similar compounds like HAp [293].
4.3 Polymer-based coatings
4.3.1 Chitosan
Chitosan is a natural polymer with vast applications in medicine. Chitosan is a cationic polysaccharide that is derived from the deacetylation of chitin [294]. Chitosan possesses remarkable properties consisting of antimicrobial, antiviral, antifungal, antitumor, immunoadjuvant, anti-thrombogenic, and anti-cholesteremic [295]. The antibacterial mechanism of chitosan NPs is attributed to the principle of electrostatic interaction and result in the dysfunctioning cell membrane, increase in permeability, and ultimately cell death [296]. A combination of chitosan with other materials can overcome different bacteria growth. Divakar et al. fabricated Ag-conjugated chitosan NPs for promoting bioactivity of Ti surfaces. The results demonstrated that NPs successfully restricted the growth, adhesion, and biofilm formation of S.mutans and P.gingivalis bacteria [297,298]. Palla-Rubio et al. coated hybrid silica-chitosan NPs on Ti implants and reported that 5–10% concentration of chitosan is suitable for obtaining sufficient bactericidal efficacy [299].
4.3.2 Peptides
Peptides coating on the implant surface is a recent procedure to increase bioactivity. Antimicrobial peptides (AMPs) with tailored heads are able to be physically adsorbed or chemically bonded to the surface. This coating mechanism is based on the production of AMPs from the host’s proteins and reusing them affords low toxicity and bacterial resistance [300]. These peptides cover a broad spectrum of pathogens, including both Gram-negative and Gram-positive bacteria [301]. Herein, a wide range of AMP families has proven their antimicrobial activity that consists of lactoferrin 1–11 (LF1–11), alpha-defensins (ADs), beta-defensins (BDs), histatin, adrenomedullin, cathelicidins, GL13K, Pac-525, KSL, and LL-37 [302,303,304,305,306,307,308]. Godoy-Gallardo et al. examined the biofunction of hLF1–11 on the Ti surface. In vitro results demonstrated a satisfactory reduction in adherence and biofilm formation of S. sanguinis and Lactobacillus salivarius (L. salivarius) bacteria in the early stage [309,310].
4.4 Carbon-based coatings
Recently, carbon-based coatings (CBCs) have shown remarkable applications in dentistry. These materials with strong C–C bonds demonstrated excellent physical properties such as notable hardness, high thermal conductivity, and optical transparency for the surface. In this term, nanocrystalline diamond (NCD), graphene and its family, and carbon nanotubes are the most frequently used CBCs on endosseous implant bodies. Coating the implant surface with NCD provides a smooth surface with significant corrosion resistance, which is a favorable surface for preventing bacterial colonization [21]. Moreover, these NPs are capable of antioxidant and anticarcinogenic properties [311].
Graphene and GFNs consist of a single layer form of carbon atoms and have a high surface area. Graphene with its honeycomb lattice shape covers the surface of implants and possesses impermeability. This ability can effectively reduce the corrosion rate and biofilm of dental implants [312,313]. Such physiobiological activities can be shared with GFNs like GO and graphene nanoplatelets. In addition to graphene, CNTs provide notable physiochemical characteristics in terms of biocide, biogenic, and drug carrier functions. The hollow cylindrical shape of CNTs is an appropriate surface for osteoblast cells’ anchorage while it can also rupture the bacteria cell membrane. Metzler et al. evaluated the osteointegration of NCD on Ti grade-5 implant. After 5 months, the in vivo results showed sufficient osteointegration [314]. Rago et al. investigated the antibacterial mechanism of graphene nanoplatelets against microorganisms. They found that the strong mechanical bonds between the layers of the graphene nanoplatelet lead to trapping bacteria cells and ultimately result in cell death [315]. Hu et al. evaluated the bactericidal efficacy of GO against certain bacteria. They observed that GO could significantly reduce the level of S. mutans bacteria [316]. However, a few documents regarding CBCs restrict their potential as an independent coating.
4.5 Protein-based coatings
4.5.1 Extracellular matrix proteins
Deposition of ECM proteins on the implant surface is a promising approach to facilitate the adhesion of osteogenic proteins. In general, the immobilization of ECM proteins leads to the promotion of bone healing and osteointegration. Hence, several proteins and biomolecules exist in the ECM and demonstrate biological activities in bone restorative applications that consist of collagens, osteopontin, pectin, cytokine, laminin, elastin, hyaluronan, insulin, fibrinogen, glycans, proteoglycans, poly(amino-acids), sialoprotein, fatty acids, sugars, etc. [317]. Among them, collagens attracted considerable attention as they have the potential to improve the attachment, proliferation, and differentiation of osteoblast and human MSCs [318]. Saadatmand et al. evaluated the collagen-chondroitin sulfate (CCS) and collagen-sulfated hyaluronan (CSH) coatings on a screw-type Ti implant in a minipig model. From in vivo results, they found that the bone formation in both coatings was greater than the uncoated control following 8 weeks [319].
4.5.2 Growth factors
Vascular endothelial growth factor (VEGF) and bone morphogenetic proteins (BMPs) are used in the coating. The first has the signaling function that involves vasculogenesis and angiogenesis. The latter is a family of growth factors, especially in the formation of bone and cartilage. This family can improve the regulation of osteogenic cells and the differentiation of MSCs [320]. Moreover, recombinant human BMPs (rhBMPs) like rhBMP-2 and rhBMP-7 have been used for therapeutic applications [321]. Guang et al. evaluated the biological activities of VEGF on primary rat osteoblast cells. They observed that VEGF can increase the activity of ALP, gene and protein expression of vasculogenesis, and proliferation of osteoblast cells [322]. Faßbender et al. modified the release rate of BMP-2 by optimizing the thickness of gentamicin-loaded poly(d,l-lactide-acid) (PDLLA). They concluded that sustained release was achieved after initial healing with a notable increase in the stiffness and ratio of bone volume to total volume at day 42, and also, higher mineralization was achieved after the 42nd day until the 84th day [323]. Al-Jarsha et al. fabricated BMP7-loaded poly(ethyl-acrylate) (PEA) on Ti surface. The results revealed that a low concentration of BMP-7 is able to optimize osteointegration via establishing a specific delivery system [324].
4.6 Drug-based coatings
Drug-based coatings are of the most practical procedures to combat high infections with multifactorial, complex, and hard-to-manage characteristics. Antibiotic drugs displayed higher efficacy against a broad spectrum of both Gram-positive and Gram-negative bacteria. Coating drugs on the implant surface usually occurs via indirect ways like using drug carriers with different biophysiochemical characteristics and release mechanisms (Figure 23). Herein, the frequently used antibiotic includes amoxicillin, doxycycline (DOX), tetracycline, polydopamine (PDA), cefotaxime, quercitrin, chlorhexidine (CHX), gentamycin, norfloxacin, and vancomycin.
![Figure 23
Schematic illustration of different carrier-based drug-releasing biomaterials [23]. Copyright 2020, MDPI.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_023.jpg)
Schematic illustration of different carrier-based drug-releasing biomaterials [23]. Copyright 2020, MDPI.
Bottino et al. examined the biocidal activity of new antibiotic, tetracycline-containing fibers, for dental applications. Not only did they restrict the biofilm formation and progression of peri-implantitis, but also they reduced the colonization of bacteria including P. gingivalis, F. nucleatum, Prevotella intermedia (P. intermedia), and A. actinomycetemcomitans at different concentrations [325,326]. Gomez-Florit et al. evaluated the anti-inflammatory effect and soft tissue regeneration of quercitrin coated on the Ti surface. For these tests, they used human gingival fibroblasts and interleukin-1-beta (IL-1β) to mimic the situations. The results revealed that quercitrin can increase the attachment of fibroblasts cells and also reduce the level of matrix metalloproteinase-1 (MMP-1), which led to increasing mRNA, and also pro-inflammatory prostaglandin-E2 (PGE2) under both inflammatory and basal conditions [327]. Researchers coated vancomycin-loaded silica films on the Ti surface by the sol–gel technique. The results showed a reduction in the release rate of vancomycin and adherence of S. aureus bacteria [70,328,329]. Ding et al. coated DOX-treated HAp on the Ti surface and from in vivo tests they observed that DOX could reduce the progression of peri-implantitis [330]. Also, by adjusting the environment pH, the release rate of DOX-loaded poly(lactide-co-glycolic-acid) (PLGA) can be controlled [331]. Wood et al. evaluated the inhibitory function of CHX coated on TiO2 surface against Streptococcus gordonii (S. gordonii) bacteria. CHX demonstrated a successful reduction of S. gordonii but it had a high release rate and could only sustain for a short period [230]. Kazek et al. loaded amoxicillin in PLGA for evaluating its antibacterial activity on the Ti surface. Such coatings revealed successful inhibitory activity against S. aureus and S. epidermidis in the first few hours [332]. Rojas-Montoya et al. controlled the release rate of norfloxacin by changing the phases of CaP compounds in the synthesis process. They fabricated a rod-like nanostructure with fast desorption within the first 8 h and gradually reduced it until 32 h [333]. Lucke et al. fabricated PDLLA to reduce bone and soft-tissue infection. They concluded that 10% gentamicin-loaded PDLLA can significantly reduce the level of S. aureus bacteria in rat models [334]. He et al. assessed the effectiveness of cefotaxime sodium immobilized on the PDA-coated Ti surface. The results showed that this coating restricted the growth and adhesion of both E. coli and S. mutans as Gram-negative and Gram-positive bacteria, respectively. The addition of cefotaxime sodium could keep the antibacterial function for a longer period [96].
5 Drug delivery systems (DDSs)
In recent developments, the use of DDSs in implant coatings has attracted the attention of the scientific community. Drug delivery refers to the approaches in transporting and increasing the concentration of therapeutic compounds to the target tissue. Generally, DDS according to the release mechanism is classified as passive or active targeting. Passive targeting refers to the biophysiochemical properties of the delivery system with no affinity for ligands. However, active targeting is the enhanced version of passive targeting in which the release mechanism is determined by an inside or outside inducer. For evaluating a delivery system, three major factors can influence the drug release kinetics that includes the external environment, payload property, and material matrix. In designing a DDS, it should be taken into account that (i) short-term antibacterial drugs are utilized for immediate acute infection, (ii) long-term antibacterial drugs are utilized for preventing bacteria colonization, and (iii) in both cases, drugs should not include alternation in surface materials [32,335].
As mentioned above, drug release kinetics can be determined by the drug release mechanism. The DDS occurs through four main categories and their subcategories including (i) diffusion-controlled as (a) reservoirs and (b) matrices, (ii) chemically controlled as (a) biodegradation and (b) chemical cleavage, (iii) solvent-controlled, and (iv) pH-sensitive. Also, physiochemical and biological mechanisms like diffusion, osmosis, swelling, portioning, dissolution, targeting, and molecular interaction follow these categories [336]. Herein, the challenge is to design a DDS with the selectivity of a therapeutic drug to the target site and an optimal release mechanism in the desired manner. For this purpose, soluble polymers, enzymatically degradable, and pH-responsive DDSs displayed a successful controlled release.
5.1 Material matrix
Of major parameters to obtain the optimal drug release is to design a specific drug system using biomaterials. Generally, the modification of the implant surface occurs via biochemical approaches including physisorption, covalent bonding, or carrier systems [337]. Physisorption refers to the direct coating and adsorption of therapeutic drugs by controlling the surface topography. Covalent binding refers to the immobilization of therapeutic drugs on the implant surface via the utilization of spacers like hydroxyl (–OH) or amine (–NH) groups. Covalent bonding is a suitable approach for coating cell-adhesive proteins including osteopontin, collagen, vitronectin, or fibronectin. Carrier systems refer to the utilization of biomaterials mainly ceramic-based and polymer-based materials for entrapping therapeutic drugs and releasing them over a controlled period. The release of therapeutic drugs in the adjacent environment of an implant using carrier systems occurs via degradation, diffusion, or osmolality mechanisms. Herein, synthetic biodegradable polymers, CaP-based compounds, and bioactive glasses due to their biocompatibility and degradability are the best choices for DDSs [338,339]. Biodegradable polymers are composed of various synthetic and natural polymers with unique characteristics. Polymers depending on their scale provide different advantages and disadvantages. However, it is not possible to use macro- or microsized polymers as DDSs due to their nonbiodegradability or limited cellular uptake. Therefore, restrained applications of large polymers increase the interest in NP-based drug systems. NP-based drug systems as implant coatings are in the form of nanoporous biomaterials, polymeric systems, and hydrogels [340]. The biophysiochemical characteristics of these carriers should be engineered to grant the optimum results for possible therapeutic drugs for bone restorative applications [341].
To design a delivery system, several parameters of drug molecules should be considered. On top of them, solubility is the prior factor as it can influence both material matrix and external environment, and is the main parameter in the release mechanisms of implant coatings. Hence, to obtain the favorable carrier system with a desirable level of hydrophilicity the utilization of copolymerization of different polymers like PLGA, biphasic CaP compounds, and BGs-doped compounds, are highlighted [23].
6 Essential parameters
6.1 Roughness
Surface roughness is one of the key factors in designing an implant. Microroughness was fully explored and confirmed as the better surface for the anchorage of osteoblast cells and primary stability [342,343]. However, most commercial dental implant manufacturers tend to have a smoother surface with a R a of 0.5–1 µm [33,344,345,346]. Selecting this range of roughness is attributed to cell preference, which Yang et al. observed that human bone-marrow MSCs have optimal differentiation on Hap-coated Ti surfaces with R a of 0.77–1.09 µm and mean distances between peaks (R Sm) of 53.9–39.3 µm [347]. By the entering of roughness at the nanoscale, implant surfaces have gone through dramatic changes (Figure 24). Nanoroughness can promote protein adsorption, osteoblast cell attachment, and growth factor incorporation [9]. Moreover, gene expression can be influenced by surface roughness, and several parameters such as the production of growth factors, cytokines, and the response of the adjacent skeletal have a significant impact on the success of an implant [348]. Chen et al. observed that nanoroughness with R a of 71.0 ± 11.0 nm can support more induction of osteogenic genes such as osteopontin (OPN), BMP-2, and runt-related transcription factor (RUNX2). Also, nanoroughness with a R a of 14.3 ± 2.5 nm demonstrated higher expression of other osteogenic genes like collagen type 1 (COL1A1) and osteocalcin (OCN) [349]. Similarly, fibroblast cells adhere more to smooth surfaces. On the contrary, osteoblast cells proliferation and collagen synthesis tend to be higher on moderate roughness but epithelial cells and ECM molecules adsorption have been reported to be greater on rough surfaces [134,350,351,352,353]. The enhancement of protein adsorption is mainly affected by increasing the surface area on nanoroughness, which facilitates cell attachment, and subsequently, promotes osteointegration and mechanical bonding [354,355]. Despite all research done on the behavior of cells, there are no general rules for optimum surface roughness and further investigation is required.
![Figure 24
Schematic illustration of the three levels of interactions between the bone and implant. At the macroscale level, the implant supplies acceptable mechanical stability to the bone. At the micro/ submicroscales, the implant is able to directly interact with osteoblasts and MSCs. At the nanoscale, cell membrane receptors, such as integrins, can recognize proteins adsorbed on the surface, which in turn are modulated by the nanostructures on the surface [342]. Copyright 2014, Elsevier™.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_024.jpg)
Schematic illustration of the three levels of interactions between the bone and implant. At the macroscale level, the implant supplies acceptable mechanical stability to the bone. At the micro/ submicroscales, the implant is able to directly interact with osteoblasts and MSCs. At the nanoscale, cell membrane receptors, such as integrins, can recognize proteins adsorbed on the surface, which in turn are modulated by the nanostructures on the surface [342]. Copyright 2014, Elsevier™.
6.2 Wettability
Wettability refers to the degree of hydrophilicity or hydrophobicity of the surface that is attributed to the result of surface chemistry [356]. To find the wettability, the CA between the interface of the droplet and the horizontal surface is measured. When the measured CA is <90°, the outcome surface is hydrophilic and above 90° it is hydrophobic. Hydrophilic surfaces at nanoscale demonstrated better interactions with the biological environment, which indicates biocompatibility as well as high surface energy (Figure 25) [356,357]. D’Elía et al. examined the wettability of Hap-coated surfaces with macro-, micro-, and nanoroughness under controlled conditions. They used rat osteoblasts and MSCs to test the surfaces and reported different states of physisorbed H2O molecules on their surface [156]. Likewise, Hotchkiss et al. investigated the effect of immune cells on different wettability and micro- and nanoroughness surfaces. After culturing macrophages on a smooth Ti surface, it displayed an increase in the level of interleukins IL-1β, IL-6, and TNF-α, which result in inflammation. However, macrophages on the rough hydrophilic Ti surface increased interleukins IL-4 and IL-10, which activate the anti-inflammatory M2-like state of the macrophage [358]. Thus, there is no standard measurement for wettability to predict cellular behavior and it demands further investigation.
![Figure 25
Schematic illustration of possible interactions at three levels of a (a) hydrophilic and (b) hydrophobic surface. (a) Hydrophilic surface allows direct interaction with biological fluids and subsequent cell membrane receptors. (b) Hydrophobic surface is prone to hydrocarbon contamination that results in the entrapment of air bubbles and interference with the biological environment [356]. Copyright 2014, Elsevier™.](/document/doi/10.1515/ntrev-2022-0037/asset/graphic/j_ntrev-2022-0037_fig_025.jpg)
Schematic illustration of possible interactions at three levels of a (a) hydrophilic and (b) hydrophobic surface. (a) Hydrophilic surface allows direct interaction with biological fluids and subsequent cell membrane receptors. (b) Hydrophobic surface is prone to hydrocarbon contamination that results in the entrapment of air bubbles and interference with the biological environment [356]. Copyright 2014, Elsevier™.
6.3 Charge
Generally, the surface charge indicates the interaction between the implant surface with its environment molecules, which leads to the reduction of bioactivity of the surface. This reduction has a significant impact on wettability, and subsequently, cellular responses that cause fewer bindings with useful ions and proteins [359,360,361]. By positively charging the surface, protein localization and biological responses can be influenced [362,363]. In terms of surface charge, limited research studies have evaluated its necessities but extended studies investigated the concept of decontamination utilizing techniques such as radio frequency glow-discharge (RFGD), photofunctionalization using ultraviolet A (UV-A), and ultraviolet C (UV-C) [360,364,365,366]. Decontaminating the implant results in the generation of a superhydrophilic surface but this reduction in CA is unstable and dramatically increases [367]. However, Rupp et al. evaluated nanocrystalline anatase coated on the Ti surface via the reactive pulse magnetron sputtering (RPMG) technique and fabricated a superhydrophilic surface with CA < 5° in 75 s. They claimed that according to the fast hydrophilization and slow re-hydrophobization, such a surface treatment has adequate potential for clinical applications [368]. Therefore, considering the significant period between production and implantation of an implant, more investigations regarding the sustainability of the surface charge and applicable processes are recommended.
7 Conclusion and outlook
In this review, we organize various aspects of a dental implant from the perspective of nanotechnology. Based on the results, we found that there is no gold standard for modifying an implant because the success rate depends on many parameters such as the patients’ general condition. Nevertheless, the outcomes of cutting-edge technology such as nano-topographies, nanoparticles, and nanodelivery systems demonstrated higher biophysiochemical activities that directly affect the success rate of dental implants. Hence, we provide sufficient pieces of information for researchers to give them a better understanding of nanotechnology in dentistry.
In general, implant failure is associated with inadequate osteointegration that may be exacerbated by microbial infection. Therefore, the combination of long-lasting features such as nanotopographies and NPs may be considered as an ideal modification for the next generation of dental implants. Nanotopography has brought the insight that antibacterial activities cannot be limited to chemical interactions, and nanopatterns with specific geometries can also reduce or abolish bacteria. In this case, dominating the fabrication process is the key factor for producing the optimal geometries. However, a few reports on this issue restrict their potential as an independent modification. Thus, the use of manifold NPs with different biocidal and osteogenic functions as well as engineered delivery systems is more highlighted.
-
Funding information: The authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Adell R , Lekholm U , Rockler B , Branemark P . A I5-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surgery. 1981;10(6):387–416. 10.1016/S0300-9785(81)80077-4.Suche in Google Scholar
[2] Adler L , Buhlin K , Jansson L . Survival and complications: a 9 ‐ to 15 ‐ year retrospective follow ‐ up of dental implant therapy. J Oral Rehabilitation. 2020 Jan;47:67–77. 10.1111/joor.12866.Suche in Google Scholar PubMed
[3] Oh S , Shiau HJ , Sc D , Reynolds MA . Survival of dental implants at sites after implant failure: a systematic review. J Prosthet Dent. 2020 Jan;123:1–7. 10.1016/j.prosdent.2018.11.007.Suche in Google Scholar PubMed
[4] Güven SŞ , Cabbar DF , Güler N . Local and systemic factors associated with marginal bone loss around dental implants: a retrospective clinical study. Quintessence Int. 2020;51:128–41. 10.3290/j.qi.a42950.Suche in Google Scholar PubMed
[5] Bazli L , Nargesi khoramabadi H , Chahardehi AM , Arsad H , Malekpouri B , Asgari Jazi M , et al. Factors influencing the failure of dental implants: a systematic review. J Compos Compd. 2020;2:18–25.10.29252/jcc.2.1.3Suche in Google Scholar
[6] Naghib SM , Zare Y , Rhee KY . A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites. Nanotechnol Rev. 2020;9:53–60. 10.1515/ntrev-2020-0005.Suche in Google Scholar
[7] Naghib SM , Behzad F , Rahmanian M , Zare Y , Rhee KY . A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination. Nanotechnol Rev. 2020;9:760–7. 10.1515/ntrev-2020-0061.Suche in Google Scholar
[8] Sartipzadeh O , Naghib SM , Shokati F , Rahmanian M , Majidzadeh-A K , Zare Y , et al. Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications. Nanotechnol Rev. 2020;9:1397–407. 10.1515/ntrev-2020-0097.Suche in Google Scholar
[9] Mendonça G , Mendonça DBS , Araga FJL . Advancing dental implant surface technology – From micron- to nanotopography. Biomaterials. 2008;29:3822–35. 10.1016/j.biomaterials.2008.05.012.Suche in Google Scholar PubMed
[10] Xia L . Importance of nanostructured surfaces. Amsterdam: Elsevier Ltd; 2021. 10.1016/b978-0-08-102999-2.00002-8.Suche in Google Scholar
[11] Rajendran YSKIN . Surface modification methods for titanium and its alloys and their corrosion behavior in biological environment: a review. J Bio- Tribo-Corrosion. 2019. 10.1007/s40735-019-0229-5.Suche in Google Scholar
[12] Rasouli R , Barhoum A , Uludag H . A review of nanostructured surfaces and materials for dental implants: surface coating, patterning and functionalization for improved performance. Biomater Sci. 2018;6:1312–38. 10.1039/c8bm00021b.Suche in Google Scholar PubMed
[13] Fox KE , Tran NL , Nguyen TA , Nguyen TT , Tran PA . Surface modification of medical devices at nanoscale – recent development and translational perspectives. Amsterdam: Elsevier Inc.; 2019. 10.1016/B978-0-12-813477-1.00008-6.Suche in Google Scholar
[14] Kurup A , Dhatrak P , Khasnis N . Materials today: proceedings surface modification techniques of titanium and titanium alloys for biomedical dental applications: a review. Mater Today Proc. 2020;39:6. 10.1016/j.matpr.2020.06.163.Suche in Google Scholar
[15] Subramani K , Mathew RT , Pachauri P . Titanium surface modification techniques for dental implants-From microscale to nanoscale. Second edn. Amsterdam: Elsevier Inc.; 2018. 10.1016/B978-0-12-812291-4.00006-6.Suche in Google Scholar
[16] Cavalu S , Antoniac IV , Mohan A , Bodog F , Doicin C , Mates I , et al. Nanoparticles and nanostructured surface fabrication for innovative cranial and maxillofacial surgery. Mater (Basel). 2020;13:1–23. 10.3390/ma13235391.Suche in Google Scholar PubMed PubMed Central
[17] Rupp F , Liang L , Geis-Gerstorfer J , Scheideler L , Hüttig F . Surface characteristics of dental implants: a review. Dent Mater. 2018;34:40–57. 10.1016/j.dental.2017.09.007.Suche in Google Scholar PubMed
[18] Article R . Implant surface microtopography – a review. Asian Pac J Health Sci. 2020;7:48–53. 10.21276/apjhs.2020.7.2.12.Suche in Google Scholar
[19] Linklater DP , Baulin VA , Juodkazis S , Crawford RJ , Stoodley P , Ivanova EP . Mechano-bactericidal actions of nanostructured surfaces. Nat Rev Microbiol. 2021;19:8–22. 10.1038/s41579-020-0414-z.Suche in Google Scholar PubMed
[20] Chen Z , Bachhuka A , Wei F , Wang X , Liu G , Vasilev K , et al. Manipulation of osteoimmunomodulation in bone. Nanoscale. 2017;9:18129–52. 10.1039/c7nr05913b.Suche in Google Scholar PubMed
[21] Parnia F , Yazdani J , Javaherzadeh V , Maleki Dizaj S . Overview of nanoparticle coating of dental implants for enhanced osseointegration and antimicrobial purposes. J Pharm Pharm Sci. 2017;20:148–60. 10.18433/J3GP6G.Suche in Google Scholar PubMed
[22] Priyadarsini S , Mukherjee S , Mishra M . Nanoparticles used in dentistry: a review. J Oral Biol Craniofacial Res. 2018;8:58–67. 10.1016/j.jobcr.2017.12.004.Suche in Google Scholar PubMed PubMed Central
[23] Latif M , Habib SR , Khurshid Z . Customized therapeutic surface coatings for dental implants. Fish Shellfish Immunol. 2020;104:1–37.Suche in Google Scholar
[24] Bapat RA , Joshi CP , Bapat P , Chaubal TV , Pandurangappa R , Jnanendrappa N , et al. The use of nanoparticles as biomaterials in dentistry. Drug Discov Today. 2019;24:85–98. 10.1016/j.drudis.2018.08.012.Suche in Google Scholar PubMed
[25] Hosnedlova B , Kepinska M , Skalickova S , Fernandez C , Ruttkay-Nedecky B , Peng Q , et al. Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomed. 2018;13:2107–28. 10.2147/IJN.S157541.Suche in Google Scholar PubMed PubMed Central
[26] Dong H , Liu H , Zhou N , Li Q , Yang G , Chen L . Surface modified techniques and emerging functional coating of dental implants. Coating. 2020;10:1–25. 10.3390/coatings10111012.Suche in Google Scholar
[27] Das S , Kumar S , Samal SK , Mohanty S , Nayak SK . Review a review on superhydrophobic polymer nanocoatings: recent development, application. Ind Eng Chem Res. 2018;57:2727–45. 10.1021/acs.iecr.7b04887.Suche in Google Scholar
[28] Devgan S , Sidhu SS . Evolution of surface modification trends in bone related biomaterials: a review. Mater Chem Phys. 2019;233:68–78. 10.1016/j.matchemphys.2019.05.039.Suche in Google Scholar
[29] Raura N , Garg A , Arora A , Roma M . Nanoparticle technology and its implications in endodontics: a review. Biomater Res. 2020;24:1–8. 10.1186/s40824-020-00198-z.Suche in Google Scholar PubMed PubMed Central
[30] Bapat RA , Chaubal TV , Dharmadhikari S , Abdulla AM , Bapat P , Alexander A , et al. Recent advances of gold nanoparticles as biomaterial in dentistry. Int J Pharm. 2020;586:119596. 10.1016/j.ijpharm.2020.119596.Suche in Google Scholar PubMed
[31] Koopaie M . Nanoengineered biomaterials for advanced drug delivery, nanoparticulate syst dental drug delivery. Amsterdam: Elsevier Ltd.; 2020. p. 525–59. 10.1016/B978-0-08-102985-5.00022-X.Suche in Google Scholar
[32] Mandracci P , Mussano F , Rivolo P , Carossa S . Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings. 2016;6:6. 10.3390/coatings6010007.Suche in Google Scholar
[33] Tobin EJ . AC NU SC. Adv Drug Deliv Rev. 2017;112:88–100. 10.1016/j.addr.2017.01.007.Suche in Google Scholar
[34] Ivanova EP , Hasan J , Webb HK , Truong VK , Watson GS , Watson JA , et al. natural bactericidal surfaces: mechanical rupture of pseudomonas aeruginosa cells by cicada wings. Small (Weinh an der Bergstrasse, Ger). 2012;8:72–4. 10.1002/smll.201200528.Suche in Google Scholar
[35] Wang XX , Hayakawa S , Tsuru K , Osaka A . Bioactive titania gel layers formed by chemical treatment of Ti substrate with a H2O2/HCl solution. Biomaterials. 2002;23:1353–7. 10.1016/S0142-9612(01)00254-X.Suche in Google Scholar
[36] Esmael SK , Jassim RK , Al-hiloh SA . In vitro study for nano hydroxyl apatite and chitosan coated surface after immersion in simulated body fluid. Med -Leg Update. 2020;20:1919–26.Suche in Google Scholar
[37] Ballo AM , Omar O , Xia W , Palmquist A . Dental implant surfaces – physicochemical properties, biological performance, and trends. Implant Dent – A Rapidly Evol Pract. 2011;6:209–27. 10.5772/17512.Suche in Google Scholar
[38] Anil S , Anand PS , Alghamdi H , Janse JA . Dental implant surface enhancement and osseointegration. Implant Dent – A Rapidly Evol Pract. 2011;15:80–7. 10.5772/16475.Suche in Google Scholar
[39] Salou L , Hoornaert A , Louarn G , Layrolle P . Enhanced osseointegration of titanium implants with nanostructured surfaces: an experimental study in rabbits. Acta Biomater. 2015;11:494–502. 10.1016/j.actbio.2014.10.017.Suche in Google Scholar PubMed
[40] Alla RK , Ginjupalli K , Upadhya N , Shammas M , Krishna R , Sekhar R . Surface roughness of implants: a review. 2011;25:112–8.Suche in Google Scholar
[41] Zhong Z . Advanced polishing, grinding and finishing processes for various manufacturing applications: a review. Mater Manuf Process. 2020;35:1–25. 10.1080/10426914.2020.1772481.Suche in Google Scholar
[42] Madarkar R , Agarwal S , Attar P , Ghosh S , Rao P . V, Application of ultrasonic vibration assisted MQL in grinding of Ti – 6Al – 4V. Mater Manuf Process. 2017;33:1–8. 10.1080/10426914.2017.1415451.Suche in Google Scholar
[43] Okawa S , Watanabe K . Chemical mechanical polishing of titanium with colloidal silica containing hydrogen peroxide – mirror polishing and surface properties. Dental Mater J. 2009;28:68–74.10.4012/dmj.28.68Suche in Google Scholar PubMed
[44] Ozdemir Z , Ozdemir A , Basim GB . Application of chemical mechanical polishing process SC. Mater Sci Eng C. 2016;68:383–96. 10.1016/j.msec.2016.06.002.Suche in Google Scholar PubMed
[45] Lee J , Kim S , Han J , Yeo IL , Yoon H , Lee J . Effects of ultrasonic scaling on the optical properties and surface characteristics of highly translucent CAD/CAM ceramic restorative materials: an in vitro study. Ceram Int. 2019;45:14594–601. 10.1016/j.ceramint.2019.04.177.Suche in Google Scholar
[46] Shemtov-yona K , Rittel D , Dorogoy A . Mechanical assessment of grit blasting surface treatments of dental implants. J Mech Behav Biomed Mater. 2014;39:375–90. 10.1016/j.jmbbm.2014.07.027.Suche in Google Scholar PubMed
[47] Le Guéhennec L , Soueidan A , Layrolle P , Amouriq Y . Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23:844–54. 10.1016/j.dental.2006.06.025.Suche in Google Scholar PubMed
[48] Jemat A , Ghazali MJ , Razali M , Otsuka Y . Surface modifications and their effects on titanium dental implants. Biomed Res Int. 2015;2015:1–11. 10.1155/2015/791725.Suche in Google Scholar PubMed PubMed Central
[49] Schupbach P , Glauser R , Bauer S . Al2O3 particles on titanium dental implant systems following sandblasting and acid-etching process. Int J Biomater. 2019;2019:9–12.10.1155/2019/6318429Suche in Google Scholar PubMed PubMed Central
[50] Prof A , Prof A . Surface modification of titanium and titanium alloys: technologies, developments and future interests. 10.1002/adem.201901258.Suche in Google Scholar
[51] Ganesh BKC , Sha W , Ramanaiah N , Krishnaiah A . Effect of shotpeening on sliding wear and tensile behavior of titanium implant alloys. J Mater. 2014;56:480–6. 10.1016/j.matdes.2013.11.052.Suche in Google Scholar
[52] Unal O , Karaoglanli AC , Varol R , Kobayashi A . Microstructure evolution and mechanical behavior of severe shot peened commercially pure titanium. Vaccum. 2014;110:1–5. 10.1016/j.vacuum.2014.08.004.Suche in Google Scholar
[53] Deng Z , Yin B , Li W , Liu J , Yang J , Zheng T , et al. Surface characteristics of and in vitro behavior of osteoblast-like cells on titanium with nanotopography prepared by high-energy shot peening. Int J Nanomed. 2014;9:5565–73.10.2147/IJN.S71625Suche in Google Scholar PubMed PubMed Central
[54] Jelliti S , Richard C , Retraint D , Roland T , Chemkhi M , Demangel C . Effect of surface nanocrystallization on the corrosion behavior of Ti – 6Al – 4V titanium alloy. Surf Coat Technol. 2013;224:82–7. 10.1016/j.surfcoat.2013.02.052.Suche in Google Scholar
[55] Jamesh M . Effect of surface mechanical attrition treatment of titanium using alumina balls: surface roughness, contact angle and apatite forming ability. Front Mater Sci. 2013;7:285–94. 10.1007/s11706-013-0208-6.Suche in Google Scholar
[56] Jain S , Williamson RS , Janorkar AV , Griggs JA , Roach MD . Osteoblast response to nanostructured and phosphorus-enhanced titanium anodization surfaces. J Biomater Appl. 2019;34:419–30. 10.1177/0885328219852741.Suche in Google Scholar PubMed
[57] Palmquist A , Omar OM , Esposito M , Lausmaa J , Thomsen P . Titanium oral implants: surface characteristics, interface biology and clinical outcome. J R Soc Interface. 2010;7 Suppl 5:7–27. 10.1098/rsif.2010.0118.focus.Suche in Google Scholar
[58] Nicolas-Silvente AI , Velasco-Ortega E , Ortiz-Garcia I , Monsalve-Guil L , Gil J , Jimenez-Guerra A . Influence of the titanium implant surface treatment on the surface roughness and chemical composition. Mater (Basel). 2020;13:13. 10.3390/ma13020314.Suche in Google Scholar
[59] Orsini G , Assenza B , Scarano A , Piattelli M , Piattelli A . Surface analysis of machined versus sandblasted and acid-etched titanium implants. Int J Oral Maxillofac Implant. 2000;15:779–84.Suche in Google Scholar
[60] Variola F , Zalzal SF , Leduc A , Barbeau J , Nanci A . Oxidative nanopatterning of titanium generates mesoporous surfaces with antimicrobial properties. Int J Nanomed. 2014;9:2319–25. 10.2147/IJN.S61333.Suche in Google Scholar
[61] Lamolle F , Monjo M , Rubert M , Haugen HJ , Lyngstadaas SP , Ellingsen JE . The effect of hydrofluoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells. Biomaterials. 2009;30:736–42. 10.1016/j.biomaterials.2008.10.052.Suche in Google Scholar
[62] Khodaei M , Amini K , Valanezhad A , Watanabe I . Surface treatment of titanium dental implant with H2O2 solution. Int J Min Metall Mater. 2020;27:1281–6. 10.1007/s12613-020-2016-1.Suche in Google Scholar
[63] Pan J , Liao H , Leygraf C , Thierry D , Li J . Variation of oxide films on titanium induced by osteoblast-like cell culture and the influence of an H2O2 pretreatment. J Biomed Mater Res. 1998;40:244–56. 10.1002/(SICI)1097-4636(199805)40:2<244:AID-JBM9>3.0.CO;2-L.Suche in Google Scholar
[64] Khodaei M , Kelishadi HS . US CR. Surf Coat Technol. 2018. 10.1016/j.surfcoat.2018.08.037.Suche in Google Scholar
[65] Zhou J , Chang C , Zhang R , Zhang L . Hydrogels prepared from unsubstituted cellulose in NaOH/urea aqueous solution. Macromol Biosci. 2007;7:804–9. 10.1002/mabi.200700007.Suche in Google Scholar
[66] Pachauri P , Bathala LR , Sangur R . Techniques for dental implant nanosurface modifications. J Adv Prosthodont. 2014;6:498–504. 10.4047/jap.2014.6.6.498.Suche in Google Scholar
[67] Pattanayak DK , Yamaguchi S , Matsushita T , Nakamura T , Kokubo T . Apatite-forming ability of titanium in terms of pH of the exposed solution. J R Soc Interface. 2012;9:2145–55. 10.1098/rsif.2012.0107.Suche in Google Scholar
[68] Wang W , Luo CJ , Huang J , Edirisinghe M . PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications. J R Society Interface. 2019;16:20180955.10.1098/rsif.2018.0955Suche in Google Scholar
[69] Alcázar JCB , Lemos RMJ , Conde MCM , Chisini LA , Salas MMS , Noremberg BS , et al. Progress in Organic Coatings Preparation, characterization, and biocompatibility of di ff erent metal oxide/PEG-based hybrid coating synthesized by sol–gel dip coating method for surface modification of titanium. Prog Org Coat. 2019;130:206–13. 10.1016/j.porgcoat.2019.02.007.Suche in Google Scholar
[70] Adams Jr CS , Antoci V , Harrison G , Patal P , Freeman TA , Shapiro IM , et al. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J Orthopaedic Res: Off Publ Orthopaedic Res Soc. 2009;27:701–9. 10.1002/jor.20815.Suche in Google Scholar
[71] Peltola T , Pätsi M , Rahiala H , Kangasniemi I , Yli-Urpo A . Calcium phosphate induction by sol-gel-derived titania coatings on titanium substrates in vitro . J Biomed Mater Res, 1998. 1998;41:504–10. 10.1002/(SICI)1097-4636(19980905)41:3<504:AID-JBM22>3.0.CO;2-G.Suche in Google Scholar
[72] Liu S , Liu J , Tang Y . Surface modification techniques of titanium and its alloys to functionally optimize their biomedical properties: thematic review. Front Bioeng Biotechnol. 2020;8:1–19. 10.3389/fbioe.2020.603072.Suche in Google Scholar
[73] Hsu HC , Hsu SK , Wu SC , Hung YH , Ho WF . Surface modification of nanotubular anodized Ti–7.5Mo alloy using NaOH treatment for biomedical application. Thin Solid Films. 2020;710:138273. 10.1016/j.tsf.2020.138273.Suche in Google Scholar
[74] Qiao X , Yang J , Shang Y , Deng S , Yao S , Wang Z , et al. Magnesium-doped nanostructured titanium surface modulates macrophage-mediated inflammatory response for ameliorative osseointegration. Int J Nanomed. 2020;15:7185–98. 10.2147/IJN.S239550.Suche in Google Scholar
[75] Indira K , Ningshen S , Mudali UK , Rajendran N . Effect of anodization parameters on the structural morphology of titanium in fluoride containing electrolytes. Mater Charact. 2012;71:58–65. 10.1016/j.matchar.2012.06.005.Suche in Google Scholar
[76] Fialho L , Carvalho S . Surface engineering of nanostructured Ta surface with incorporation of osteoconductive elements by anodization. Appl Surf Sci. 2019;495:143573. 10.1016/j.apsusc.2019.143573.Suche in Google Scholar
[77] Ismail S , Mamat S , Azam MA . Effect of voltage on Tio2 nanotubes formation in ethylene glycol solution. J Teknologi. 2017;79:2–5. 10.11113/jt.v79.11294.Suche in Google Scholar
[78] Rameshbabu N , Ravisankar B , Saikiran A , Parfenov EV , Valiev RZ . Surface modification of CP-Ti metallic implant material by plasma electrolytic oxidation. IOP Conf Ser Mater Sci Eng. 2019;672:672. 10.1088/1757-899X/672/1/012012.Suche in Google Scholar
[79] Dehnavi V , Binns WJ , Noël JJ , Shoesmith DW , Luan BL . Growth behaviour of low-energy plasma electrolytic oxidation coatings on a magnesium alloy. J Magnes Alloy. 2018;6:229–37. 10.1016/j.jma.2018.05.008.Suche in Google Scholar
[80] Sedelnikova MB , Komarova EG , Sharkeev YP , Ugodchikova AV , Tolkacheva TV , Rau JV , et al. Modification of titanium surface via Ag-, Sr- and Si-containing micro-arc calcium phosphate coating. Bioact Mater. 2019;4:224–35. 10.1016/j.bioactmat.2019.07.001.Suche in Google Scholar
[81] Narayanan R , Kim SY , Kwon TY , Kim KH . Nanocrystalline hydroxyapatite coatings from ultrasonated electrolyte: preparation, characterization, and osteoblast responses. J Biomed Mater Res – Part A. 2008;87:1053–60. 10.1002/jbm.a.31852.Suche in Google Scholar
[82] Nie X , Leyland A , Matthews A . Deposition of layered bioceramic hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis. Surf Coat Technol. 2000;125:407–14. 10.1016/S0257-8972(99)00612-X.Suche in Google Scholar
[83] Hashim MS , Khaleel RS . The bioactivities of prepared Ti, Zn, TiO2, ZnO and Al2O nanoparticles by rapid breakdown anodization technique. Surf Interfaces. 2020;20:100640. 10.1016/j.surfin.2020.100640.Suche in Google Scholar
[84] Khanaki A , Abdizadeh H , Golobostanfard MR . Electrophoretic deposition of CuIn1-xGaxSe2 thin films using solvothermal synthesized nanoparticles for solar cell application. J Phys Chem C. 2015;119:23250–8. 10.1021/acs.jpcc.5b07300.Suche in Google Scholar
[85] Kania DR . Biocompatibility of chemical-vapour- deposited diamond. Biomaterials. 1995;16:483–8.10.1016/0142-9612(95)98822-VSuche in Google Scholar
[86] Catledge SA , Fries MD , Vohra YK , Lacefield WR , Lemons JE , Woodard S , et al. Nanostructured ceramics for biomedical implants review. J Nanosci Nanotechnol. 2002;2:293–312. 10.1166/jnn.2002.116.Suche in Google Scholar PubMed
[87] Chen M , Li H , Wang X , Qin G , Zhang E . Improvement in antibacterial properties and cytocompatibility of titanium by fluorine and oxygen dual plasma-based surface modification. Appl Surf Sci. 2019;463:261–74. 10.1016/j.apsusc.2018.08.194.Suche in Google Scholar
[88] Hofer R , Textor M , Spencer ND . Alkyl phosphate monolayers, self-assembled from aqueous solution onto metal oxide surfaces. Langmuir. 2001;17(13):4014–20.10.1021/la001756eSuche in Google Scholar
[89] Hybrid chitosan-β-1,3-glucan matrix of bone scaffold enhances osteoblast adhesion, spreading and proliferation via promotion of serum protein adsorption.pdf, n.d. 2016.Suche in Google Scholar
[90] Gawalt ES , Avaltroni MJ , Koch N , Schwartz J . Self-assembly and bonding of alkanephosphonic acids on the native oxide surface of titanium. Langmuir. 2001;17(19):5736–8.10.1021/la010649xSuche in Google Scholar
[91] Scotchford CA , Gilmore CP , Cooper E , Leggett GJ , Downes S . Protein adsorption and human osteoblast-like cell attachment and growth on alkylthiol on gold self-assembled monolayers. Hoboken, New Jersey: Wiley Online Library; 2001.10.1002/jbm.1220Suche in Google Scholar
[92] Min SK , Kang HK , Jang DH , Jung SY , Kim OB , Min BM , et al. Titanium surface coating with a laminin-derived functional peptide promotes bone cell adhesion. BioMed Res Int. 2013;2013:1–8.10.1155/2013/638348Suche in Google Scholar PubMed PubMed Central
[93] Kennedy SB , Washburn NR , George Jr CS , Amis EJ . Combinatorial screen of the effect of surface energy on fibronectin-mediated osteoblast adhesion, spreading and proliferation. Biomaterials. 2006;27:3817–24. 10.1016/j.biomaterials.2006.02.044.Suche in Google Scholar PubMed
[94] Kim SE , Yun Y , Lee JY , Shim J , Park K , Huh J . Co-delivery of platelet-derived growth factor ( PDGF- BB) and bone morphogenic protein (BMP-2) coated onto heparinized titanium for improving osteoblast function and osteointegration. Hoboken, New Jersey: Wiley Online Library; 2013. 10.1002/term.Suche in Google Scholar
[95] 238 – Collagen fiber orientation around machined titanium and zirconia dental implant necks- an animal study.pdf, n.d. 2009.Suche in Google Scholar
[96] He S , Zhou P , Wang L , Xiong X , Zhang Y , Deng Y , et al. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J R Society, Interface. 2014;11:20140169.10.1098/rsif.2014.0169Suche in Google Scholar PubMed PubMed Central
[97] Tahriri M , Rasoulianboroujeni M , Bader R , Vashaee D , Tayebi L . 13. Growth factors for oral and maxillofacial regeneration applications. Amsterdam: Elsevier Ltd; 2017. 10.1016/B978-0-08-100961-1.00013-X.Suche in Google Scholar
[98] Urface IMS , Huang H . E o f rgd i s, n.d. p. 73–9.Suche in Google Scholar
[99] Liu X , Chu PK , Ding C . Surface nano-functionalization of biomaterials. Mater Sci Eng R. 2010;70:275–302. 10.1016/j.mser.2010.06.013.Suche in Google Scholar
[100] Pre-proof J , Page C . ur l P of. Surf Coat Technol. 2019;382:125161. 10.1016/j.surfcoat.2019.125161.Suche in Google Scholar
[101] Liu X , Chu PK , Ding C . Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng R Rep. 2005;47:49–121. 10.1016/j.mser.2004.11.001.Suche in Google Scholar
[102] Huang X , Wang Y , Tang B , Zhao L , Chu K . Ac ce pt cr t. Appl Surf Sci. 2015;355:32–44. 10.1016/j.apsusc.2015.07.064.Suche in Google Scholar
[103] Safavi MS , Walsh FC , Surmeneva MA , Surmenev RA , Allafi JK . Electrodeposited hydroxyapatite ‐ based biocoatings: recent progress and future challenges. Coatings. 2021;11:110.10.3390/coatings11010110Suche in Google Scholar
[104] Ul-Hamid A . The effect of deposition conditions on the properties of Zr-carbide, Zr-nitride and Zr-carbonitride coatings – a review. Mater Adv. 2020;1:988–1011. 10.1039/d0ma00232a.Suche in Google Scholar
[105] Rautray TR , Narayanan R , Kim K . Ion implantation of titanium based biomaterials. Prog Mater Sci. 2011;56:1137–77. 10.1016/j.pmatsci.2011.03.002.Suche in Google Scholar
[106] Miralami R , Haider H , Sharp JG , Namavar F , Hartman CW , Garvin KL , et al. Surface nano-modification by ion beam – assisted deposition alters the expression of osteogenic genes in osteoblasts. Eng Med. 2019;233:921–30. 10.1177/0954411919858018.Suche in Google Scholar PubMed
[107] Mas-moruno C , Su B , Dalby MJ . Multifunctional coatings and nanotopographies: toward cell instructive and antibacterial implants. Adv Healthc Mater. 2018;8(1):1801103. 10.1002/adhm.201801103.Suche in Google Scholar PubMed
[108] Biswas A , Bayer IS , Biris AS , Wang T , Dervishi E , Faupel F . Advances in top – down and bottom – up surface nanofabrication: techniques, applications & future prospects. Adv Colloid Interface Sci. 2012;170:2–27. 10.1016/j.cis.2011.11.001.Suche in Google Scholar PubMed
[109] Cumming DRS , Thoms S , Beaumont SP , Weaver JMR . Fabrication of 3 nm wires using 100 keV electron beam lithography and poly (methyl methacrylate) resist and poly (methyl methacrylate) resist. Hospital pediatrics. 1996;322:18–21. 10.1063/1.116073.Suche in Google Scholar
[110] Vieu C , Carcenac F , Pepin A , Chen Y , Mejias M , Lebib A , et al. Electron beam lithography: resolution limits and applications. Appl Surf Sci. 2000;164:111–7.10.1016/S0169-4332(00)00352-4Suche in Google Scholar
[111] Yilbas BS , Al-Sharafi A , Ali H . Self-cleaning of surfaces and water droplet mobility. Amsterdam: Elsevier; 2019. p. 45–98. 10.1016/b978-0-12-814776-4.00003-3.Suche in Google Scholar
[112] Aly HM . Lett Editor. 2016;7658:236–8.Suche in Google Scholar
[113] Chang C , Tsai P , Chen S , Kuo MY , Sun J , Chang JZ . ScienceDirect 3D laser-printed porous Ti6Al4V dental implants for compromised bone support. J Formos Med Assoc. 2019;119:420–9. 10.1016/j.jfma.2019.07.023.Suche in Google Scholar PubMed
[114] Gittens RA , McLachlan T , Olivares-Navarrete R , Cai Y , Berner S , Tannenbaum R , et al. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011;32:3395–403.10.1016/j.biomaterials.2011.01.029Suche in Google Scholar PubMed PubMed Central
[115] Lin N , Berton P , Moraes C , Rogers RD , Tufenkji N . Nanodarts, nanoblades, and nanospikes: mechano-bactericidal nanostructures and where to find them. Adv Colloid Interface Sci. 2018;252:55–68. 10.1016/j.cis.2017.12.007.Suche in Google Scholar PubMed
[116] Kelleher SM , Habimana O , Lawler J , O' Reilly B , Daniels S , Casey E , et al. Cicada wing surface topography: an investigation into the bactericidal properties of nanostructural features. ACS Appl Mater & interfaces. 2015;8:14966–74. 10.1021/acsami.5b08309.Suche in Google Scholar PubMed
[117] Chem P , Phys C . Bactericidal mechanism of nanopatterned surfaces. Phys Chem Chem Phys. 2015;18:1311–6. 10.1039/C5CP05646B.Suche in Google Scholar PubMed
[118] Pham VT , Truong VK , Orlowska A , Ghanaati S , Barbeck M , Booms P , et al. “Race for the surface”: eukaryotic cells can win. ACS Appl Mater Interfaces. 2016;8:22025–31. 10.1021/acsami.6b06415.Suche in Google Scholar PubMed
[119] Wang Q , Huang Y , Qian Z . Nanostructured surface modification to bone implants for bone regeneration. J Biomed Nanotechnol. 2018;14:628–48. 10.1166/jbn.2018.2516.Suche in Google Scholar PubMed
[120] Kim HN , Jiao A , Hwang NS , Kim MS , Kang DH , Kim DH , et al. Nanotopography-guided tissue engineering and regenerative medicine. Adv Drug Deliv Rev. 2012;65:536–58. 10.1016/j.addr.2012.07.014.Suche in Google Scholar PubMed PubMed Central
[121] Kalaskar DM , Alshomer F . In situ tissue regeneration, micro-nanotopographical cues guiding biomater host response. Amsterdam: Elsevier Inc; 2016. p. 137–63. 10.1016/B978-0-12-802225-2.00008-8. Suche in Google Scholar
[122] Pogodin S , Hasan J , Baulin VA , Webb HK , Truong VK , Phong Nguyen TH , et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys J. 2013;104:835–40. 10.1016/j.bpj.2012.12.046.Suche in Google Scholar PubMed PubMed Central
[123] Sjo T , Dalby MJ , Hart A , Tare R , Oreffo ROC , Su B . Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009;5:1433–41. 10.1016/j.actbio.2009.01.007.Suche in Google Scholar PubMed
[124] Dalby MJ , Gadegaard N , Tare R , Andar A , Riehle MO , Herzyk P , et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. 10.1038/nmat2013.Suche in Google Scholar PubMed
[125] Xue F , Liu J , Guo L , Zhang L , Li Q . Theoretical study on the bactericidal nature of nanopatterned surfaces. J Theor Biol. 2015;385:1–7. 10.1016/j.jtbi.2015.08.011.Suche in Google Scholar PubMed
[126] Ivanova EP , Linklater DP , Werner M , Baulin VA , Xu X , Vrancken N , et al. The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. Proc Natl Acad Sci U S A. 2020;117:12598–605. 10.1073/pnas.1916680117.Suche in Google Scholar PubMed PubMed Central
[127] Sjöström T , Mcnamara LE , Meek RMD , Dalby MJ , Su B . 2D and 3D nanopatterning of titanium for enhancing osteoinduction of stem cells at implant surfaces. Adv Healthc Mater. 2013;2:1–9. 10.1002/adhm.201200353.Suche in Google Scholar PubMed
[128] Mcnamara LE , Sjöström T , Burgess KE , Kim JJ , Liu E , Gordonov S , et al. Skeletal stem cell physiology on functionally distinct titania nanotopographies. Biomaterials. 2011;32:32–10. 10.1016/j.biomaterials.2011.06.063.Suche in Google Scholar PubMed
[129] Kantawong F , Burgess KE , Jayawardena K , Hart A , Burchmore RJ , Gadegaard N , et al. Whole proteome analysis of osteoprogenitor differentiation induced by disordered nanotopography and mediated by ERK signalling. Biomaterials. 2009;30:4723–31. 10.1016/j.biomaterials.2009.05.040.Suche in Google Scholar PubMed
[130] Guvendik S , Trabzon L , Ramazanoglu M . The effect of Si nano-columns in 2-D and 3-D on cellular behaviour: nanotopography-induced CaP deposition from differentiating mesenchymal stem cells. J Nanosci Nanotechnol. 2011;11:8896–902. 10.1166/jnn.2011.3449.Suche in Google Scholar PubMed
[131] Zhou C , Koshani R , O’Brien B , Ronholm J , Cao X , Wang Y . Bio-inspired mechano-bactericidal nanostructures: a promising strategy for eliminating surface foodborne bacteria. Curr Opin Food Sci. 2021;39:110–9. 10.1016/j.cofs.2020.12.021.Suche in Google Scholar
[132] Ivanova EP , Hasan J , Webb HK , Gervinskas G , Juodkazis S , Truong VK , et al. Bactericidal activity of black silicon. Nat Commun. 2013;4:1–7. 10.1038/ncomms3838.Suche in Google Scholar PubMed PubMed Central
[133] Park J , Bauer S , Schlegel KA , Neukam FW , Von Der MK , Schmuki P . TiO2 nanotube surfaces: 15 nm – an optimal length scale of surface topography for cell adhesion and differentiation. Small (Weinh an der Bergstrasse, Ger). 2009;5:666–71. 10.1002/smll.200801476.Suche in Google Scholar PubMed
[134] 350 – Nanosize and vitality – TiO2 nanotube diameter directs cell fate.pdf. 2007.Suche in Google Scholar
[135] Minagar S , Wang J , Berndt CC , Ivanova EP , Wen C . Cell response of anodized nanotubes on titanium and titanium alloys. J Biomed Mater Res Part A. 2013;101(9):2726–39. 10.1002/jbm.a.34575.Suche in Google Scholar PubMed
[136] Press D . Adhesion of osteoblasts to a nanorough titanium implant surface. Int J Nanomed. 2011;6:1801.10.2147/IJN.S21755Suche in Google Scholar
[137] Feller L , Jadwat Y , Khammissa RAG , Meyerov R , Schechter I , Lemmer J . Cellular responses evoked by different surface characteristics of intraosseous titanium implants. BioMed Res Int. 2015;2015:1–8.10.1155/2015/171945Suche in Google Scholar PubMed PubMed Central
[138] Deng Y , Peng S . Boron nitride nanotubes reinforce tricalcium phosphate scaffolds and promote the osteogenic differentiation of mesenchymal stem cells. J Biomed Nanotechnol. 2016;12:934–47. 10.1166/jbn.2016.2224.Suche in Google Scholar PubMed
[139] Kulkarni M , Mazare A , Gongadze E , Perutkova Š , Kralj-igli V . Titanium nanostructures for biomedical applications. Nanotechnology. 2015 Feb 13;26:062002. 10.1088/0957-4484/26/6/062002.Suche in Google Scholar PubMed
[140] Gulati K , Prideaux M , Kogawa M , Lima-marques L , Atkins GJ , Findlay DM , et al. Anodized 3D – printed titanium implants with dual micro- and nano-scale topography promote interaction with human osteoblasts and osteocyte-like cells. Hoboken, New Jersey: Wiley Online Library; 2016. 10.1002/term.Suche in Google Scholar
[141] Linklater DP , De Volder M , Baulin VA , Werner M , Jessl S , Golozar M , et al. High aspect ratio nanostructures kill bacteria via storage and release of mechanical energy. ACS Nano. 2018;12:6657–67. 10.1021/acsnano.8b01665.Suche in Google Scholar PubMed
[142] Skandani AA , Zeineldin R . Effect of chirality and length on the penetrability of single-walled carbon nanotubes into lipid bilayer cell membranes. Langmuir ACS J Surf colloids. 2012;28:7872–9.10.1021/la3011162Suche in Google Scholar PubMed
[143] Kang S , Pinault M , Pfefferle LD , Elimelech M , Engineering C . Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir : ACS J Surf colloids. 2007;23:8670–3.10.1021/la701067rSuche in Google Scholar PubMed
[144] Werner M , Sommer J , Baulin VA . Homo-polymers with balanced hydrophobicity translocate through lipid bilayers and enhance local solvent permeability. Soft Matter. 2012;8:11714. 10.1039/c2sm26008e.Suche in Google Scholar
[145] Guo Y , Werner M , Seemann R , Baulin VA , Fleury J . Tension-induced translocation of ultra-short carbon nanotube through a phospholipid bilayer. ACS Nano. 2018;12:12042–9. 10.1021/acsnano.8b04657.Suche in Google Scholar PubMed
[146] Pandit S , Gaska K , Mokkapati VRSS , Celauro E , Derouiche A , Forsberg S , et al. Precontrolled alignment of graphite nanoplatelets in polymeric composites prevents bacterial attachment. Small (Weinh an der Bergstrasse, Ger). 2020;1904756:1–11. 10.1002/smll.201904756.Suche in Google Scholar PubMed
[147] Pham VTH , Truong VK , Quinn MDJ , Notley SM , Guo Y . Graphene induces formation of pores killing spherical and rod-shaped bacteria. Washington, D.C.: ACS Publications; 2015. p. 1–33.Suche in Google Scholar
[148] Lu X , Feng X , Werber JR , Chu C , Zucker I , Kim JH , et al. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc Natl Acad Sci U S Am. 2017;114:9793. 10.1073/pnas.1710996114.Suche in Google Scholar PubMed PubMed Central
[149] Wang X , Lyu C , Wu S , Ben Y , Li X , Ge Z , et al. Electrophoresis deposited mesoporous graphitic carbon nitride surfaces with efficient bactericidal properties. ACS Appl bio Mater. 2020;3:2255–62. 10.1021/acsabm.0c00061.Suche in Google Scholar PubMed
[150] B J.M.C. Ctbf D.O.I. Mater Chem B. 2019;7:4424–31. 10.1039/C9TB00102F.Suche in Google Scholar
[151] Bhadra CM , Truong VK , Pham VTH , Kobaisi MAl , Seniutinas G , Wang JY , et al. Antibacterial titanium nano- patterned arrays inspired by dragonfly wings. Nat Publ Gr.; p. 1–12. 10.1038/srep16817.Suche in Google Scholar PubMed PubMed Central
[152] Reed JH , Gonsalves AE , Román JK , Oh J , Cha H , Dana CE , et al. Ultra-scalable multifunctional nanoengineered copper and aluminum for anti-adhesion and bactericidal applications Ultra-scalable multifunctional nanoengineered copper and aluminum for anti- adhesion and bactericidal applications. ACS Appl Bio Mater. 2019;2:2726–37. 10.1021/acsabm.8b00765.Suche in Google Scholar PubMed
[153] Akhavan O , Ghaderi E . Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4:5731–6.10.1021/nn101390xSuche in Google Scholar PubMed
[154] Liu S , Zeng TH , Hofmann M , Burcombe E , Wei J , Jiang R . Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano. 2011;5:6971–80.10.1021/nn202451xSuche in Google Scholar PubMed
[155] Tan KH , Sattari S , Beyranvand S , Faghani A , Ludwig K , Schwibbert K , et al. Biological and environmental phenomena at the interface thermoresponsive amphiphilic functionalization of thermally reduced graphene oxide to study graphene/bacteria hydrophobic interactions. Langmuir: ACS J Surf colloids. 2019;35:4736–46. 10.1021/acs.langmuir.8b03660.Suche in Google Scholar PubMed
[156] Manuscript A . Cell interaction with graphene microsheets: near-orthogonal cutting versus parallel attachment. Nanoscale. 2015;7:5457–67. 10.1039/C4NR06170E.Suche in Google Scholar
[157] Zhang N , Hou J , Chen S , Xiong C , Liu H , Jin Y . Rapidly probing antibacterial activity of graphene oxide by mass spectrometry-based metabolite fingerprinting. New York City: Nat Publ Gr.; 2016. p. 1–10. 10.1038/srep28045.Suche in Google Scholar PubMed PubMed Central
[158] Liu SP , Lin CH , Lin SJ , Fu RH , Huang YC , Chen SY , et al. Electrospun polyacrylonitrile-based nanofibers maintain embryonic stem cell stemness via TGF-beta signaling. J Biomed Nanotechnol. 2016;12:732–42. 10.1166/jbn.2016.2201.Suche in Google Scholar PubMed
[159] Zhang C , Yuan H , Liu H , Chen X , Lu P . Well-aligned chitosan-based ultrafine fibers committed teno-lineage differentiation of human induced pluripotent stem cells for Achilles tendon regeneration. Biomaterials. 2015;53:716–30. 10.1016/j.biomaterials.2015.02.051.Suche in Google Scholar PubMed
[160] Wang S , Hu F , Li J , Zhang S , Shen M , Huang M , et al. PT state key laboratory for modification of chemical fibers and polymer materials. Coll of Elsevier Inc. 2016;14:2505–20. 10.1016/j.nano.2016.12.024.Suche in Google Scholar PubMed
[161] Xu T , Yang H , Yang D , Yu Z . Polylactic acid nanofibers scaffold decorated with chitosan island-like topography for bone tissue engineering. Sci Rep. 2017;7:4794.10.1038/s41598-017-05184-5Suche in Google Scholar PubMed PubMed Central
[162] Lee YM , Yun HM , Lee HY , Lim HC , Lee HH , Kim HW , et al. Xerogel interfaced nanofibers stimulate bone regeneration through the activation of integrin and bone morphogenetic protein pathways. J Biomed Nanotechnol. 2017;13:180–91. 10.1166/jbn.2017.2329.Suche in Google Scholar PubMed
[163] Thrivikraman G , Lee PS , Hess R , Haenchen V , Basu B , Scharnweber D . Interplay of substrate conductivity, cellular microenvironment, and pulsatile electrical stimulation toward osteogenesis of human mesenchymal stem cells in vitro . ACS Appl Mater Interfaces. 2015;7:23015–28. 10.1021/acsami.5b06390.Suche in Google Scholar PubMed
[164] Polycaprolactone C , Leszczak V , Baskett DA , Popat KC . Endothelial cell growth and differentiation on. J Biomed Nanotechnol. 2021;11:1080–92. 10.1166/jbn.2015.2021.Suche in Google Scholar PubMed
[165] Yao Q , Liu Y , Tao J , Baumgarten KM , Sun H . Promote endogenous bone regeneration Hypoxia-mimicking nanofibrous scaffolds promote endogenous bone regeneration. Washington, D.C.: The University of South Dakota; 2016. 10.1021/acsami.6b10538.Suche in Google Scholar PubMed PubMed Central
[166] Matsuura Y , Hirano T , Sakai K . Friction torque reduction by ultrasonic vibration and its application to electromagnetically spinning viscometer. Jpn J Appl Phys. 2014;53(7S):07KC12.10.7567/JJAP.53.07KC12Suche in Google Scholar
[167] Klymov A , Bronkhorst EM , Jansen JA , Walboomers XF . Bone marrow-derived mesenchymal cells feature selective migration behavior on submicro- and nano-dimensional multi-patterned substrates. Acta Biomater. 2015;16:117–25. 10.1016/j.actbio.2015.01.016.Suche in Google Scholar PubMed
[168] Klymov A , Song J , Cai X , Leeuwenburgh S , Jansen JA , Walboomers XF . Increased acellular and cellular surface mineralization induced by nanogrooves in combination with a calcium-phosphate coating. Acta Biomater. 2016;31:368–77. 10.1016/j.actbio.2015.11.061.Suche in Google Scholar PubMed
[169] Lavenus S , Berreur M , Trichet V , Pilet P , Louarn G , Layrolle P . Adhesion and osteogenic differentiation of human mesenchymal. Eur Cell & Mater. 2011;22:84–96.10.22203/eCM.v022a07Suche in Google Scholar PubMed
[170] Liu Y , Bao C , Wismeijer D , Wu G . The physicochemical/biological properties of porous tantalum and the potential surface modification techniques to improve its clinical application in dental implantology. Mater Sci Eng C. 2015;49:323–9. 10.1016/j.msec.2015.01.007.Suche in Google Scholar PubMed
[171] Alves CFA , Cavaleiro A , Carvalho S . Bioactivity response of Ta1−x O x coatings deposited by reactive DC magnetron sputtering. Mater Sci Eng C. 2015;58:110–8. 10.1016/j.msec.2015.08.017.Suche in Google Scholar PubMed
[172] Zhang Y , Zheng Y , Li Y , Wang L , Bai Y , Zhao Q . Tantalum nitride-decorated titanium with enhanced resistance to microbiologically induced corrosion and mechanical property for dental application. PLoS One. 2015;10:1–22. 10.1371/journal.pone.0130774.Suche in Google Scholar PubMed PubMed Central
[173] Zhang XM , Li Y , Gu YX , Zhang CN , Lai HC , Shi JY . Ta-coated titanium surface with superior bacteriostasis and osseointegration. Int J Nanomed. 2019;14:8693–706. 10.2147/IJN.S218640.Suche in Google Scholar PubMed PubMed Central
[174] Wu XF , Song HY , Yoon JM , Yu YT , Chen YF . Synthesis of core-shell Au@TiO2 nanopartides with truncated wedge-shaped morphology and their photocatalytic properties. Langmuir. 2009;25:6438–47. 10.1021/la900035a.Suche in Google Scholar PubMed
[175] Besinis A , De Peralta T , Handy RD . The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology. 2014;8:1–16. 10.3109/17435390.2012.742935.Suche in Google Scholar PubMed PubMed Central
[176] Chidambaranathan AS , Mohandoss K , Balasubramaniam MK . Comparative evaluation of antifungal effect of titanium, zirconium and aluminium nanoparticles coated titanium plates against C. albicans. J Clin Diagn Res. 2016;10:ZC56-9. 10.7860/JCDR/2016/15473.7114.Suche in Google Scholar PubMed PubMed Central
[177] Zhang P , Zhang Z , Li W . Antibacterial TiO2 coating incorporating silver nanoparticles by microarc oxidation and ion implantation. J Nanomater. 2013;124:2013–7. 10.1155/2013/542878.Suche in Google Scholar
[178] Zhang L , Pornpattananangkul D , Hu C-M , Huang C-M . Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem. 2010;17:585–94. 10.2174/092986710790416290.Suche in Google Scholar PubMed
[179] Zhao L , Chu PK , Zhang Y , Wu Z . Antibacterial coatings on titanium implants. J Biomed Mater Res – Part B Appl Biomater. 2009;91:470–80. 10.1002/jbm.b.31463.Suche in Google Scholar PubMed
[180] Raphel J , Holodniy M , Goodman SB , Heilshorn SC . Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Vol. 84, Amsterdam: Elsevier Ltd; 2016. 10.1016/j.biomaterials.2016.01.016.Suche in Google Scholar PubMed PubMed Central
[181] Hickok NJ , Shapiro IM . Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64:1165–76. 10.1016/j.addr.2012.03.015.Suche in Google Scholar PubMed PubMed Central
[182] Lee DW , Yun YP , Park K , Kim SE . Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone. 2012;50:974–82. 10.1016/j.bone.2012.01.007.Suche in Google Scholar PubMed
[183] Almeida MAN , Servulo EFC , de FPF . Bacterial colonization of metallic surfaces on industrial cooling systems. International Corrosion Congress Front Corrosion Science Technology, 15th; 2002. p. 594/1–/5.Suche in Google Scholar
[184] Mydin RBSMN , Hazan R , FaridWajidi MF , Sreekantan S . Titanium dioxide nanotube arrays for biomedical implant materials and nanomedicine applications. Titanium dioxide – material for a sustainable environment. London, UK: IntechOpen; 2018. 10.5772/intechopen.73060.Suche in Google Scholar
[185] Wang J , Wu G , Liu X , Sun G , Li D , Wei H . A decomposable silica-based antibacterial coating for percutaneous titanium implant. Int J Nanomed. 2017;12:371–9. 10.2147/IJN.S123622.Suche in Google Scholar PubMed PubMed Central
[186] Yang Y , Ao HY , Yang SB , Wang YG , Lin WT , Yu ZF , et al. In vivo evaluation of the anti-infection potential of gentamicin-loaded nanotubes on titania implants. Int J Nanomed. 2016;11:2223–34. 10.2147/IJN.S102752.Suche in Google Scholar PubMed PubMed Central
[187] Inzunza D , Covarrubias C , Von Marttens A , Leighton Y , Carvajal JC , Valenzuela F , et al. Synthesis of nanostructured porous silica coatings on titanium and their cell adhesive and osteogenic differentiation properties. J Biomed Mater Res Part A. 2013;102:1–12. 10.1002/jbm.a.34673.Suche in Google Scholar PubMed
[188] Kim I , Joachim E , Choi H , Kevin KK . Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed Nanotechnol, Biol Med. 2015;11:1–10. 10.1016/j.nano.2015.03.004.Suche in Google Scholar PubMed
[189] Csík A , Heged C . Electrosprayed calcium silicate nanoparticle-coated titanium implant with improved antibacterial activity and osteogenesis. Colloids Surf B: Biointerfaces. 2021;202:202. 10.1016/j.colsurfb.2021.111699.Suche in Google Scholar PubMed
[190] Massa MA , Covarrubias C , Bittner M , Fuentevilla IA , Capetillo P , Von Marttens A , et al. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater Sci Eng C. 2014;45:146–53. 10.1016/j.msec.2014.08.057.Suche in Google Scholar PubMed
[191] Catauro M , Papale F , Bollino F . Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci Technol Adv Mater. 2015 Jun;035001:035001. 10.1088/1468-6996/16/3/035001.Suche in Google Scholar PubMed PubMed Central
[192] Li K , Xie Y , You M , Huang L , Zheng X . Plasma sprayed cerium oxide coating inhibits H2O2-induced oxidative stress and supports cell viability. J Mater Sci Mater Med. 2016;27:1–10. 10.1007/s10856-016-5710-9.Suche in Google Scholar PubMed
[193] Qi S , Wu J , Xu Y , Zhang Y , Wang R , Li K , et al. Chemical stability and antimicrobial activity of plasma-sprayed cerium oxide-incorporated calcium silicate coating in dental implants. Implant Dent. 2019;28:564–70. 10.1097/ID.0000000000000937.Suche in Google Scholar PubMed
[194] Li X , Qi M , Sun X , Weir MD , Tay FR , Oates TW , et al. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019;94:627–43. 10.1016/j.actbio.2019.06.023.Suche in Google Scholar PubMed
[195] Gold and Silver Nanoparticles in Sensing and Imaging- Sensitivity of Plasmon Response to Size, Shape, and Metal Composition.pdf, n.d. 2006.Suche in Google Scholar
[196] Kesharwani P , Choudhury H , Meher JG , Pandey M , Gorain B . Dendrimer-entrapped gold nanoparticles as promising nanocarriers for anticancer therapeutics and imaging. Prog Mater Sci. 2019;103:484–508. 10.1016/j.pmatsci.2019.03.003.Suche in Google Scholar
[197] Zaki M , Akhter S , Rahman Z , Akhter S , Anwar M , Pradesh H . Nanometric gold in cancer nanotechnology: current status and future prospect. J Pharm pharmacology. 2013;65:634–51. 10.1111/jphp.12017.Suche in Google Scholar PubMed
[198] Wani IA , Ahmad T . Size and shape dependant antifungal activity of gold nanoparticles: a case study of Candida. Colloids Surf B Biointerfaces. 2013;101:162–70. 10.1016/j.colsurfb.2012.06.005.Suche in Google Scholar PubMed
[199] Nautiyal CS . Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour Technol. 2014;166:235–42. 10.1016/j.biortech.2014.04.085.Suche in Google Scholar PubMed
[200] Tp SD , Zhang Y , Yu H . Antibacterial activity and cytotoxicity of Gold(i) and (iii) ions and gold. Biochem Pharmacol: Open Access. 2015;4:4. 10.4172/2167-0501.1000199.Suche in Google Scholar PubMed PubMed Central
[201] Padmos JD , Langman M , Macdonald K , Comeau P , Yang Z , Filiaggi M , et al. Correlating the atomic structure of bimetallic silver − gold nanoparticles to their antibacterial and cytotoxic activities. J Phys Chem C. 2015;119:7472–82. 10.1021/acs.jpcc.5b00145.Suche in Google Scholar
[202] Cui Y , Zhao Y , Tian Y , Zhang W , Lü X , Jiang X . The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli q. Biomaterials. 2012;33:2327–33. 10.1016/j.biomaterials.2011.11.057.Suche in Google Scholar PubMed
[203] Regiel-Futyra A , Kus-Liśkiewicz M , Sebastian V , Irusta S , Arruebo M , Stochel G , et al. Development of noncytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl Mater Interfaces. 2015;7:1087–99. 10.1021/am508094e. Suche in Google Scholar
[204] Wang L , Li S , Yin J , Yang J , Li Q , Zheng W , et al. The density of surface coating can contribute to different antibacterial activities of gold nanoparticles. Nano Lett. 2020;20:5036–42. 10.1021/acs.nanolett.0c01196.Suche in Google Scholar PubMed
[205] Heo DN , Ko WK , Lee HR , Lee SJ , Lee D , Um SH , et al. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J Colloid Interface Sci. 2016;469:129–37. 10.1016/j.jcis.2016.02.022.Suche in Google Scholar PubMed
[206] Laurent S , Forge D , Port M , Roch A , Robic C , Vander Elst L , et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem Rev. 2008;108:2064–110. 10.1021/cr068445e.Suche in Google Scholar PubMed
[207] Motte L . What are the current advances regarding iron oxide nanoparticles for nanomedicine? J Bioanal Biomed. 2012;4:e110. 10.4172/1948-593X.1000e110.Suche in Google Scholar
[208] Taylor EN , Webster TJ . The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int J Nanomed. 2009;4:145–52. 10.2147/ijn.s5976.Suche in Google Scholar
[209] Sathyanarayanan MB , Balachandranath R , Genji Srinivasulu Y , Kannaiyan SK , Subbiahdoss G . The effect of gold and iron-oxide nanoparticles on biofilm-forming pathogens. ISRN Microbiol. 2013;2013:1–5. 10.1155/2013/272086.Suche in Google Scholar PubMed PubMed Central
[210] Thukkaram M , Sitaram S , Kannaiyan SK , Subbiahdoss G . Antibacterial efficacy of iron-oxide nanoparticles against biofilms on different biomaterial surfaces. Int J Biomater. 2014;2014:2014. 10.1155/2014/716080.Suche in Google Scholar PubMed PubMed Central
[211] Injumpa W , Ritprajak P , Insin N . Size-dependent cytotoxicity and inflammatory responses of PEGylated silica-iron oxide nanocomposite size series. J Magn Magn Mater. 2017;427:60–6. 10.1016/j.jmmm.2016.11.015.Suche in Google Scholar
[212] Ruparelia JP , Chatterjee AK , Duttagupta SP , Mukherji S . Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4:707–16. 10.1016/j.actbio.2007.11.006.Suche in Google Scholar PubMed
[213] Maleki Dizaj S , Barzegar-Jalali M , Zarrintan MH , Adibkia K , Lotfipour F . Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin Drug Deliv. 2015;12:1649–60. 10.1517/17425247.2015.1049530.Suche in Google Scholar PubMed
[214] Akhavan O , Ghaderi E . Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf Coat Technol. 2010;205:219–23. 10.1016/j.surfcoat.2010.06.036.Suche in Google Scholar
[215] Wang X , Dong H , Liu J , Qin G , Chen D , Zhang E . In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater Sci Eng C. 2019;100:38–47. 10.1016/j.msec.2019.02.084.Suche in Google Scholar PubMed
[216] Rosenbaum J , Versace DL , Abbad-Andallousi S , Pires R , Azevedo C , Cénédese P , et al. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater Sci. 2017;5:455–62. 10.1039/c6bm00868b.Suche in Google Scholar PubMed
[217] Liu R , Memarzadeh K , Chang B , Zhang Y , Ma Z , Allaker RP , et al. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci Rep. 2016;6:1–10. 10.1038/srep29985.Suche in Google Scholar PubMed PubMed Central
[218] Tabrez Khan S , Ahamed M , Al-Khedhairy A , Musarrat J . Biocidal effect of copper and zinc oxide nanoparticles on human oral microbiome and biofilm formation. Mater Lett. 2013;97:67–70. 10.1016/j.matlet.2013.01.085.Suche in Google Scholar
[219] Shen X , Hu Y , Xu G , Chen W , Xu K , Ran Q , et al. Regulation of the biological functions of osteoblasts and bone formation by Zn-incorporated coating on microrough titanium. ACS Appl Mater Interfaces. 2014;6:16426–40. 10.1021/am5049338.Suche in Google Scholar PubMed
[220] Chou AHK , LeGeros RZ , Chen Z , Li Y . Antibacterial effect of zinc phosphate mineralized guided bone regeneration membranes. Implant Dent. 2007;16:89–100. 10.1097/ID.0b013e318031224a.Suche in Google Scholar PubMed
[221] Mehdipour M , Taghavi Zenouz A , Bahramian A , Yazdani J , Pouralibaba F , Sadr K . Comparison of the effect of mouthwashes with and without zinc and fluocinolone on the healing process of erosive oral lichen planus. J Dent Res Dent Clin Dent Prospect. 2010;4:25–8. 10.5681/joddd.2010.007.Suche in Google Scholar PubMed PubMed Central
[222] Hu H , Zhang W , Qiao Y , Jiang X , Liu X , Ding C . Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012;8:904–15. 10.1016/j.actbio.2011.09.031.Suche in Google Scholar PubMed
[223] Luo Q , Cao H , Wang L , Ma X , Liu X . ZnO@ZnS nanorod-array coated titanium: good to fibroblasts but bad to bacteria. J Colloid Interface Sci. 2020;579:50–60. 10.1016/j.jcis.2020.06.055.Suche in Google Scholar PubMed
[224] Park J , Park M , Seo H , Han HS , Lee JY , Koo D , et al. A new corrosion-inhibiting strategy for biodegradable magnesium: reduced nicotinamide adenine dinucleotide (NADH). Sci Rep. 2018;8:1–10. 10.1038/s41598-018-36240-3.Suche in Google Scholar PubMed PubMed Central
[225] Nabiyouni M , Ren Y , Bhaduri SB . Magnesium substitution in the structure of orthopedic nanoparticles: a comparison between amorphous magnesium phosphates, calcium magnesium phosphates, and hydroxyapatites. Mater Sci Eng C. 2015;52:11–7. 10.1016/j.msec.2015.03.032.Suche in Google Scholar PubMed
[226] Yamamoto O , Ohira T , Alvarez K , Fukuda M . Antibacterial characteristics of CaCO3-MgO composites. Mater Sci Eng B Solid-State Mater Adv Technol. 2010;173:208–12. 10.1016/j.mseb.2009.12.007.Suche in Google Scholar
[227] Jin T , He Y . Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J Nanopart Res. 2011;13:6877–85. 10.1007/s11051-011-0595-5.Suche in Google Scholar
[228] Ewald A , Kreczy D , Brückner T , Gbureck U , Bengel M , Hoess A , et al. Development and bone regeneration capacity of premixed magnesium phosphate cement pastes. Mater (Basel). 2019;12:12. 10.3390/ma12132119.Suche in Google Scholar PubMed PubMed Central
[229] Kishen A , Shi Z , Shrestha A , Neoh KG . An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod. 2008;34:1515–20. 10.1016/j.joen.2008.08.035.Suche in Google Scholar PubMed
[230] Wood NJ , Jenkinson HF , Davis SA , Mann S , O’Sullivan DJ , Barbour ME . Chlorhexidine hexametaphosphate nanoparticles as a novel antimicrobial coating for dental implants. J Mater Sci Mater Med. 2015;26:26. 10.1007/s10856-015-5532-1.Suche in Google Scholar PubMed PubMed Central
[231] Hamouda IM . Current perspectives of nanoparticles in medical and dental biomaterials. J Biomed Res. 2012;26:143–51. 10.7555/JBR.26.20120027.Suche in Google Scholar PubMed PubMed Central
[232] Zhao IS , Mei ML . The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomed. 2020;15:2555–62.10.2147/IJN.S246764Suche in Google Scholar PubMed PubMed Central
[233] Maleki Dizaj S . Preparation and study of vitamin A palmitate microemulsion drug delivery system and investigation of co-surfactant effect. J Nanostruct Chem. 2013;3:3. 10.1186/2193-8865-3-59.Suche in Google Scholar
[234] Li M , Liu Q , Jia Z , Xu X , Shi Y , Cheng Y , et al. Polydopamine-induced nanocomposite Ag/CaP coatings on the surface of titania nanotubes for antibacterial and osteointegration functions. J Mater Chem B. 2015;3:8796–805. 10.1039/c5tb01597a.Suche in Google Scholar PubMed
[235] Choi S , Jang Y , Jang J , Lee S , Lee M . Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J Appl Biomater Funct Mater. 2019;17:2280800019847067. 10.1177/2280800019847067.Suche in Google Scholar
[236] Gunputh UF , Le H , Lawton K , Besinis A , Tredwin C , Handy RD . Antibacterial properties of silver nanoparticles grown in situ and anchored to titanium dioxide nanotubes on titanium implant against Staphylococcus aureus. Nanotoxicology. 2020;14:97–110. 10.1080/17435390.2019.1665727.Suche in Google Scholar
[237] Li Y , Lin Z , Zhao M , Xu T , Wang C , Xia H , et al. Multifunctional selenium nanoparticles as carriers of HSP70 siRNAto induce apoptosis of HepG2 cells. Int J Nanomed. 2016;11:3065–76. 10.2147/IJN.S109822.Suche in Google Scholar
[238] Li Y , Li X , Zheng W , Fan C , Zhang Y , Chen T . Functionalized selenium nanoparticles with nephroprotective activity, the important roles of ROS-mediated signaling pathways. J Mater Chem B. 2013;1:6365–72. 10.1039/c3tb21168a.Suche in Google Scholar
[239] Wang H , Zhang J , Yu H . Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med. 2007;42:1524–33. 10.1016/j.freeradbiomed.2007.02.013.Suche in Google Scholar
[240] Hiraoka K , Komiya S , Hamada T , Zenmyo M , Inoue A . Osteosarcoma cell apoptosis induced by selenium. J Orthop Res. 2001;19:809–14. 10.1016/S0736-0266(00)00079-6.Suche in Google Scholar
[241] Tran PA , Sarin L , Hurt RH , Webster TJ . Titanium surfaces with adherent selenium nanoclusters as a novel anticancer orthopedic material. J Biomed Mater Res – Part A. 2010;93:1417–28. 10.1002/jbm.a.32631.Suche in Google Scholar PubMed
[242] Wei W , Abnet CC , Qiao Y , Dawsey SM , Dong Z , Sun X , et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2018;79(1):80–5.10.1093/ajcn/79.1.80Suche in Google Scholar PubMed
[243] Perla V , Webster TJ . Better osteoblast adhesion on nanoparticulate selenium – A promising orthopedic implant material. J Biomed Mater Res – Part A. 2005;75:356–64. 10.1002/jbm.a.30423.Suche in Google Scholar PubMed
[244] Stevanović M , Filipović N , Djurdjević J , Lukić M , Milenković M , Boccaccini A . 45S5Bioglass®-based scaffolds coated with selenium nanoparticles or with poly(lactide-co-glycolide)/selenium particles: processing, evaluation and antibacterial activity. Colloids Surf B Biointerfaces. 2015;132:208–15. 10.1016/j.colsurfb.2015.05.024.Suche in Google Scholar PubMed
[245] Webster TJ . Ijn-6-1553.Pdf. 2011.10.2147/IJN.S21729Suche in Google Scholar
[246] Srivastava N , Mukhopadhyay M . Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst Eng. 2015;38:38. 10.1007/s00449-015-1413-8.Suche in Google Scholar PubMed
[247] Dion I , Bordenave L , Lefebvre F , Bareille R , Baquey C , Monties JR , et al. Physico-chemistry and cytotoxicity of ceramics – Part II Cytotoxicity of ceramics. J Mater Sci Mater Med. 1994;5:18–24. 10.1007/BF00121148.Suche in Google Scholar
[248] Guerreiro-Tanomaru JM , Trindade-Junior A , Costa BC , Da Silva GF , Drullis Cifali L , Basso Bernardi MI , et al. Effect of zirconium oxide and zinc oxide nanoparticles on physicochemical properties and antibiofilm activity of a calcium silicate-based material. Sci World J. 2014;2014:975213. 10.1155/2014/975213.Suche in Google Scholar
[249] Marunick M , Gordon S . Prosthodontic treatment during active osteonecrosis related to radiation and bisphosphonate therapy: a clinical report. J Prosthet Dent. 2006;96:7–12. 10.1016/j.prosdent.2006.05.008.Suche in Google Scholar
[250] Größner-Schreiber B , Herzog M , Hedderich J , Dück A , Hannig M , Griepentrog M . Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: an in vitro study. Clin Oral Implant Res. 2006;17:736–45. 10.1111/j.1600-0501.2006.01277.x.Suche in Google Scholar
[251] Depprich R , Zipprich H , Ommerborn M , Naujoks C , Wiesmann HP , Kiattavorncharoen S , et al. Osseointegration of zirconia implants compared with titanium: an in vivo study. Head Face Med. 2008;4:1–8. 10.1186/1746-160X-4-30.Suche in Google Scholar
[252] Degidi M , Artese L , Scarano A , Perrotti V , Gehrke P , Piattelli A . Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J Periodontol. 2006;77:73–80. 10.1902/jop.2006.77.1.73.Suche in Google Scholar
[253] Bächle M , Butz F , Hübner U , Bakalinis E , Kohal RJ . Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin Oral Implant Res. 2007;18:53–9. 10.1111/j.1600-0501.2006.01292.x.Suche in Google Scholar
[254] Huang Z , Wang Z , Li C , Yin K , Hao D , Lan J . Application of implants: study in implants plasma-sprayed zirconia coating in dental. J Oral Implantol. 2018;44:102–8. 10.1563/aaid-joi-D-17-00020.Suche in Google Scholar
[255] Gou Z , Chang J . Synthesis and in vitro bioactivity of dicalcium silicate powders. J European Ceramic Soc. 2004;24:93–9. 10.1016/S0955-2219(03)00320-0.Suche in Google Scholar
[256] Zhao W , Chang J . Sol–gel synthesis and in vitro bioactivity of tricalcium silicate powders. Mater Lett. 2004;58:2350–3. 10.1016/j.matlet.2004.02.045.Suche in Google Scholar
[257] Hoppe A , Güldal NS , Boccaccini AR . A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–74. 10.1016/j.biomaterials.2011.01.004.Suche in Google Scholar PubMed
[258] Askari E , Rasouli M , Darghiasi SF , Naghib SM , Zare Y , Rhee KY . Reduced graphene oxide-grafted bovine serum albumin/bredigite nanocomposites with high mechanical properties and excellent osteogenic bioactivity for bone tissue engineering. Bio-Design Manuf. 2021;4:243–57. 10.1007/s42242-020-00113-4.Suche in Google Scholar
[259] Naghib SM , Pedram A , Ansari M , Moztarzadeh F . Development and characterization of 316 L stainless steel coated by melt-derived and sol-gel derived 45S5 bioglass for orthopedic applications. Ceram – Silikaty. 2012;56:89–93.Suche in Google Scholar
[260] Jones JR . Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9:4457–86. 10.1016/j.actbio.2012.08.023.Suche in Google Scholar PubMed
[261] Gross UM . The anchoring of glass ceramics of different solubility in the femur of the rat. J of Biomed Mater Res. 2000;14:607–18.10.1002/jbm.820140507Suche in Google Scholar PubMed
[262] Kalantari E , Morteza S , Reza NM , Mozafari M . Green solvent-based sol–gel synthesis of monticellite nanoparticles: a rapid and ef fi cient approach. J Sol–Gel Sci Technol. 2017;84:87–95. 10.1007/s10971-017-4461-5.Suche in Google Scholar
[263] Kalantari E , Naghib SM . PT SC. Mater Sci Eng C. 2018;98:1087–96. 10.1016/j.msec.2018.12.140.Suche in Google Scholar PubMed
[264] Nanostructured monticellite for tissue engineering applications – Part II- Molecular and biological characteristics.pdf, n.d. 2018.Suche in Google Scholar
[265] Kalantari E , Morteza S , Naimi-jamal MR , Esmaeili R . ScienceDirect nanostructured monticellite: an emerging player in tissue engineering. Mater Today Proc. 2018;5:15744–53. 10.1016/j.matpr.2018.04.187.Suche in Google Scholar
[266] Kalantari E , Naghib SM , Iravani NJ , Esmaeili R , Naimi-jamal MR , Mozafari M . Biocomposites based on hydroxyapatite matrix reinforced with nanostructured monticellite (CaMgSiO4) for biomedical application: synthesis, characterization, and biological studies. PT, Mater Sci Eng C. 2019;105:109912. 10.1016/j.msec.2019.109912.Suche in Google Scholar PubMed
[267] Kalantari E , Naghib SM , Reza M , Aliahmadi A , Jafarbeik N , Mozafari M . Author’ s accepted manuscript. Ceram Int. 2018;44:12731–38. 10.1016/j.ceramint.2018.04.076.Suche in Google Scholar
[268] Dorozhkin SV . Calcium orthophosphates in nature. Biol Med. 2009;2:399–498. 10.3390/ma2020399.Suche in Google Scholar
[269] Lin X , de Groot K , Wang D , Hu Q , Wismeijer D , Liu Y . A review paper on biomimetic calcium phosphate coatings. Open Biomed Eng J. 2015;9:56–64. 10.2174/1874120701509010056.Suche in Google Scholar PubMed PubMed Central
[270] Jing W , Zhang M , Jin L , Zhao J , Gao Q , Ren M , et al. Assessment of osteoinduction using a porous hydroxyapatite coating prepared by micro-arc oxidation on a new titanium alloy. Int J Surg. 2015;24:51–6. 10.1016/j.ijsu.2015.08.030.Suche in Google Scholar PubMed
[271] Schouten C , Meijer GJ , Van Den Beucken JJ , Leeuwenburgh SC , De Jonge LT , Wolke JG , et al. In vivo bone response and mechanical evaluation of electrosprayed CaP nanoparticle coatings using the iliac crest of goats as an implantation model. Acta Biomater. 2010;6:2227–36. 10.1016/j.actbio.2009.11.030.Suche in Google Scholar PubMed
[272] Okuda T , Ioku K , Yonezawa I , Minagi H , Gonda Y , Kawachi G , et al. The slow resorption with replacement by bone of a hydrothermally synthesized pure calcium-deficient hydroxyapatite. Biomaterials. 2008;29:2719–28. 10.1016/j.biomaterials.2008.03.028.Suche in Google Scholar PubMed
[273] Daculsi G . Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res. 1989;23:883–94.10.1002/jbm.820230806Suche in Google Scholar PubMed
[274] Mas-Moruno C. Surface functionalization of biomaterials for bone tissue regeneration and repair. Amsterdam: Elsevier Ltd; 2018. 10.1016/B978-0-08-100803-4.00003-6.Suche in Google Scholar
[275] Verron E , Bouler JM , Guicheux J . Controlling the biological function of calcium phosphate bone substitutes with drugs. Acta Biomater. 2012;8:3541–51. 10.1016/j.actbio.2012.06.022.Suche in Google Scholar PubMed
[276] Balasundaram G , Sato M , Webster TJ . Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2020;27:2798–805. 10.1016/j.biomaterials.2005.12.008.Suche in Google Scholar PubMed
[277] Schumacher M , Reither L , Thomas J , Kampschulte M , Gbureck U , Lode A , et al. Bioactive glass composites for controlled growth factor delivery. Biomater Sci. 2017;5:578–88. 10.1039/c6bm00903d.Suche in Google Scholar PubMed
[278] 200 – Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and β-tricalcium phosphate.pdf, n.d. 2005.Suche in Google Scholar
[279] Bigi A , Boanini E . Functionalized biomimetic calcium phosphates for bone tissue repair. J Appl Biomater Funct Mater. 2017;15:313–25. 10.5301/jabfm.5000367.Suche in Google Scholar PubMed
[280] Wang Z , Flax LA , Kemp MM , Linhardt RJ , Baron MJ . Host and pathogen glycosaminoglycan-binding proteins modulate antimicrobial peptide responses in Drosophila melanogaster. Infect Immun. 2011;79:606–16. 10.1128/IAI.00254-10.Suche in Google Scholar PubMed PubMed Central
[281] Salbach J , Kliemt S , Rauner M , Rachner TD , Goettsch C , Kalkhof S , et al. The effect of the degree of sulfation of glycosaminoglycans on osteoclast function and signaling pathways. Biomaterials. 2012;33:8418–29. 10.1016/j.biomaterials.2012.08.028.Suche in Google Scholar PubMed
[282] David G , Bernfield M . The emerging rotes of cett surface heparan sutfate proteogtycans. Amsterdam: Elsevier; 1992.Suche in Google Scholar
[283] Salbach-Hirsch J , Ziegler N , Thiele S , Moeller S , Schnabelrauch M , Hintze V , et al. Sulfated glycosaminoglycans support osteoblast functions and concurrently suppress osteoclasts. Cell Biochem. 2014;1111:1101–11. 10.1002/jcb.24750.Suche in Google Scholar PubMed
[284] Rudd TR , Skidmore MA , Guerrini M , Hricovini M , Powell AK , Siligardi G , et al. The conformation and structure of GAGs: recent progress and perspectives. Curr Opin Struct Biol. 2010;20:567–74. 10.1016/j.sbi.2010.08.004.Suche in Google Scholar PubMed
[285] Korn P , Schulz MC , Hintze V , Range U , Mai R , Eckelt U , et al. Sulfated glycosaminoglycans exploit the conformational plasticity of bone morphogenetic protein‑2 (BMP-2) and alter the interaction profile with its receptor. J Biomed Mater Res Part A. 2014;102:2–44.Suche in Google Scholar
[286] 216 – Sulfated hyaluronan and chondroitin sulfate derivatives interact differently with human transforming growth factor-β1 (TGF-β1).pdf, n.d. 2011.Suche in Google Scholar
[287] Hempel U , Preissler C , Vogel S , Möller S , Hintze V , Becher J , et al. Artificial extracellular matrices with oversulfated glycosaminoglycan derivatives promote the differentiation of osteoblast-precursor cells and premature osteoblasts. BioMed Res Int. 2014;2014:938368.10.1155/2014/938368Suche in Google Scholar PubMed PubMed Central
[288] Baud'huin M , Ruiz-velasco C , Jego G , Charrier C , Gasiunas N , Gallagher J , et al. Glycosaminoglycans inhibit the adherence and the spreading of osteoclasts and their precursors: role in osteoclastogenesis and bone resorption. Eur J Cell Biol. 2011;90:49–57. 10.1016/j.ejcb.2010.08.001.Suche in Google Scholar PubMed
[289] Irie A , Takami M , Kubo H , Sekino-suzuki N , Kasahara K , Sanai Y . Heparin enhances osteoclastic bone resorption by inhibiting osteoprotegerin activity. Bone. 2007;41:165–74. 10.1016/j.bone.2007.04.190.Suche in Google Scholar PubMed
[290] Li Y , Yang W , Li X , Zhang X , Wang C , Meng X , et al. Improving osteointegration and osteogenesis of three-dimensional porous Ti6Al4V scaffolds by polydopamine-assisted biomimetic hydroxyapatite coating. ACS Appl Mater Interfaces. 2015;7:5715–24. 10.1021/acsami.5b00331.Suche in Google Scholar PubMed
[291] Saran N , Zhang R , Turcotte RE . Osteogenic protein-1 delivered by hydroxyapatite-coated implants improves bone ingrowth in extracortical bone bridging. Clin Orthop Relat Res. 2011;469:1470–8. 10.1007/s11999-010-1573-4.Suche in Google Scholar PubMed PubMed Central
[292] He J , Huang T , Gan L , Zhou Z , Jiang B , Wu Y , et al. Collagen-infiltrated porous hydroxyapatite coating and its osteogenic properties: in vitro and in vivo study. J Biomed Mater Res – Part A. 2012;100 A:1706–15. 10.1002/jbm.a.34121.Suche in Google Scholar PubMed
[293] Ohgushi H , Okumura M , Yoshikawa T , Inoue K , Senpuku N , Tamai S , et al. Bone formation processin porous calcium carbonate and hydroxyapatite. J Biomed Mater Res. 1992;26:885–95. 10.1002/jbm.820260705.Suche in Google Scholar PubMed
[294] Xu A , Zhou L , Deng Y , Chen X , Xiong X , Deng F , et al. A carboxymethyl chitosan and peptide-decorated polyetheretherketone ternary biocomposite with enhanced antibacterial activity and osseointegration as orthopedic/dental implants. J Mater Chem B. 2016;4:1878–90. 10.1039/c5tb02782a.Suche in Google Scholar PubMed
[295] Younes I , Rinaudo M . Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13:1133–74. 10.3390/md13031133.Suche in Google Scholar PubMed PubMed Central
[296] Shrestha A , Zhilong S , Gee NK , Kishen A . Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J Endod. 2010;36:1030–5. 10.1016/j.joen.2010.02.008.Suche in Google Scholar PubMed
[297] Li W , Yang Y , Zhang H , Xu Z , Zhao L , Wang J , et al. Improvements on biological and antimicrobial properties of titanium modified by AgNPs-loaded chitosan-heparin polyelectrolyte multilayers. J Mater Sci Mater Med. 2019;30:30. 10.1007/s10856-019-6250-x.Suche in Google Scholar PubMed
[298] Divakar DD , Jastaniyah NT , Altamimi HG , Alnakhli YO , Muzaheed , Alkheraif AA , et al. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int J Biol Macromol. 2018;108:790–7. 10.1016/j.ijbiomac.2017.10.166.Suche in Google Scholar PubMed
[299] Palla-Rubio B , Araújo-Gomes N , Fernández-Gutiérrez M , Rojo L , Suay J , Gurruchaga M , et al. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr Polym. 2019;203:331–41. 10.1016/j.carbpol.2018.09.064.Suche in Google Scholar PubMed
[300] Townsend L , Williams RL , Anuforom O , Berwick MR , Halstead F , Hughes E , et al. Antimicrobial peptide coatings for hydroxyapatite: electrostatic and covalent attachment of antimicrobial peptides to surfaces. J R Soc Interface. 2017;14:14. 10.1098/rsif.2016.0657.Suche in Google Scholar PubMed PubMed Central
[301] Gao Q , Feng T , Huang D , Liu P , Lin P , Wu Y , et al. Antibacterial and hydroxyapatite-forming coating for biomedical implants based on polypeptide-functionalized titania nanospikes. Biomater Sci. 2020;8:278–89. 10.1039/c9bm01396b.Suche in Google Scholar PubMed
[302] He Y , Zhang Y , Shen X , Tao B , Liu J , Yuan Z , et al. The fabrication and in vitro properties of antibacterial polydopamine-LL-37-POPC coatings on micro-arc oxidized titanium. Colloids Surf B Biointerfaces. 2018;170:54–63. 10.1016/j.colsurfb.2018.05.070.Suche in Google Scholar PubMed
[303] Warnke PH , Voss E , Russo PA , Stephens S , Kleine M , Terheyden H , et al. Antimicrobial peptide coating of dental implants: biocompatibility assessment of recombinant human beta defensin-2 for human cells. Int J Oral Maxillofac Implant. 2013;28:982–8. 10.11607/jomi.2594.Suche in Google Scholar PubMed
[304] Li JY , Wang XJ , Wang LN , Ying XX , Ren X , Liu HY , et al. High in vitro antibacterial activity of Pac-525 against Porphyromonas gingivalis biofilms cultured on titanium. Biomed Res Int. 2015;2015:2015. 10.1155/2015/909870.Suche in Google Scholar PubMed PubMed Central
[305] Gorr SU , Abdolhosseini M , Shelar A , Sotsky J . Dual host-defence functions of SPLUNC2/PSP and synthetic peptides derived from the protein. Biochem Soc Trans. 2011;39:1028–32. 10.1042/BST0391028.Suche in Google Scholar PubMed PubMed Central
[306] Chen X , Hirt H , Li Y , Gorr SU , Aparicio C . Antimicrobial GL13K peptide coatings killed and ruptured the wall of streptococcus gordonii and prevented formation and growth of biofilms. PLoS One. 2014;9:9. 10.1371/journal.pone.0111579.Suche in Google Scholar PubMed PubMed Central
[307] Holmberg KV , Abdolhosseini M , Li Y , Chen X , Gorr SU , Aparicio C . Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013;9:8224–31. 10.1016/j.actbio.2013.06.017.Suche in Google Scholar PubMed PubMed Central
[308] Khurshid Z , Naseem M , Sheikh Z , Najeeb S , Shahab S , Zafar MS . Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm J. 2016;24:515–24. 10.1016/j.jsps.2015.02.015.Suche in Google Scholar PubMed PubMed Central
[309] Godoy-Gallardo M , Mas-Moruno C , Yu K , Manero JM , Gil FJ , Kizhakkedathu JN , et al. Antibacterial properties of hLf1-11 peptide onto titanium surfaces: a comparison study between silanization and surface initiated polymerization. Biomacromolecules. 2015;16:483–96. 10.1021/bm501528x.Suche in Google Scholar PubMed
[310] Godoy-Gallardo M , Mas-Moruno C , Fernández-Calderón MC , Pérez-Giraldo C , Manero JM , Albericio F , et al. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014;10:3522–34. 10.1016/j.actbio.2014.03.026.Suche in Google Scholar PubMed
[311] Leoncini E , Ricciardi W , Cadoni G , Arzani D , Petrelli L , Paludetti G , et al. Adult height and head and neck cancer: a pooled analysis within the INHANCE consortium. Head Neck. 2014;36:1391–48. 10.1002/HED.Suche in Google Scholar
[312] Mejías Carpio IE , Santos CM , Wei X , Rodrigues DF . Toxicity of a polymer-graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale. 2012;4:4746–56. 10.1039/c2nr30774j.Suche in Google Scholar PubMed
[313] Malhotra R , Han YM , Morin JLP , Luong-Van EK , Chew RJJ , Castro Neto AH , et al. Inhibiting corrosion of biomedical-grade Ti-6Al-4V alloys with graphene nanocoating. J Dent Res. 2020;99:285–92. 10.1177/0022034519897003.Suche in Google Scholar PubMed
[314] Metzler P , Von Wilmowsky C , Stadlinger B , Zemann W , Schlegel KA , Rosiwal S , et al. Nano-crystalline diamond-coated titanium dental implants – A histomorphometric study in adult domestic pigs. J Cranio-Maxillofacial Surg. 2013;41:532–8. 10.1016/j.jcms.2012.11.020.Suche in Google Scholar PubMed
[315] Rago I , Bregnocchi A , Zanni E , D’Aloia AG , De Angelis F , Bossu M , et al. Antimicrobial activity of graphene nanoplatelets against Streptococcus mutans. IEEE-NANO 2015 – 15th International Conference on Nanotechnology; 2015, p. 9–12. 10.1109/NANO.2015.7388945.Suche in Google Scholar
[316] Hu W , Peng C , Lv M , Li X , Zhang Y , Chen N , et al. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano. 2011;5:3693–700. 10.1021/nn200021j.Suche in Google Scholar PubMed
[317] Terheyden H , Lang NP , Bierbaum S , Stadlinger B . Osseointegration – communication of cells. Clin Oral Implant Res. 2012;23:1127–35. 10.1111/j.1600-0501.2011.02327.x.Suche in Google Scholar PubMed
[318] Zhang BGX , Myers DE , Wallace GG , Brandt M , Choong PFM . Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int J Mol Sci. 2014;15:11878–921. 10.3390/ijms150711878.Suche in Google Scholar PubMed PubMed Central
[319] Saadatmand S , Vos JR , Hooning MJ , Oosterwijk JC , Koppert LB , Bock GHDe , et al. This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the version of record. Laryngoscope. 2014;102:2–31.Suche in Google Scholar
[320] Katagiri T , Watabe T . Bone morphogenetic proteins. Cold Spring Harb Perspect Biol. 2016;8:1–28. 10.1101/cshperspect.a021899.Suche in Google Scholar PubMed PubMed Central
[321] Kim JE , Kang SS , Choi KH , Shim JS , Jeong CM , Shin SW , et al. The effect of anodized implants coated with combined rhBMP-2 and recombinant human vascular endothelial growth factors on vertical bone regeneration in the marginal portion of the peri-implant. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e24–31. 10.1016/j.oooo.2011.10.040.Suche in Google Scholar PubMed
[322] Guang M , Huang B , Yao Y , Zhang L , Yang B , Gong P . Effects of vascular endothelial growth factor on osteoblasts around dental implants in vitro and in vivo . J Oral Sci. 2017;59:215–23. 10.2334/josnusd.16-0406.Suche in Google Scholar PubMed
[323] Faßbender M , Minkwitz S , Strobel C , Schmidmaier G , Wildemann B . Stimulation of bone healing by sustained bone morphogenetic protein 2 (BMP-2) delivery. Int J Mol Sci. 2014;15:8539–52. 10.3390/ijms15058539.Suche in Google Scholar PubMed PubMed Central
[324] Al-Jarsha M , Moulisová V , Leal-Egaña A , Connell A , Naudi KB , Ayoub AF , et al. Engineered coatings for titanium implants to present ultralow doses of BMP-7. ACS Biomater Sci Eng. 2018;4:1812–9. 10.1021/acsbiomaterials.7b01037.Suche in Google Scholar PubMed PubMed Central
[325] Bottino MC , Münchow EA , Albuquerque MTP , Kamocki K , Shahi R , Gregory RL , et al. Tetracycline-incorporated polymer nanofibers as a potential dental implant surface modifier. J Biomed Mater Res – Part B Appl Biomater. 2017;105:2085–92. 10.1002/jbm.b.33743.Suche in Google Scholar PubMed PubMed Central
[326] Shahi RG , Albuquerque MTP , Münchow EA , Blanchard SB , Gregory RL , Bottino MC . Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology. 2017;105:354–63. 10.1007/s10266-016-0268-z.Suche in Google Scholar PubMed PubMed Central
[327] Gomez-Florit M , Pacha-Olivenza MA , Fernández-Calderón MC , Córdoba A , González-Martín ML , Monjo M , et al. Quercitrin-nanocoated titanium surfaces favour gingival cells against oral bacteria. Sci Rep. 2016;6:1–9. 10.1038/srep22444.Suche in Google Scholar PubMed PubMed Central
[328] Butler RJ , Marchesi S , Royer T , Davis IS . The effect of a subject-specific amount of lateral wedge on knee. J Orthop Res. Sept., 2007;25:1121–7. 10.1002/jor.Suche in Google Scholar
[329] Radin S , Ducheyne P . Controlled release of vancomycin from thin sol-gel films on titanium alloy fracture plate material. Biomaterials. 2007;28:1721–9. 10.1016/j.biomaterials.2006.11.035.Suche in Google Scholar PubMed
[330] Ding L , Zhang P , Wang X , Kasugai S . A doxycycline-treated hydroxyapatite implant surface attenuates the progression of peri-implantitis: a radiographic and histological study in mice. Clin Implant Dent Relat Res. 2019;21:154–9. 10.1111/cid.12695.Suche in Google Scholar PubMed
[331] Alécio ABW , Ferreira CF , Babu J , Shokuhfar T , Jo S , Magini R , et al. Doxycycline release of dental implants with nanotube surface, coated with poly lactic-co-glycolic acid for extended pH-controlled drug delivery. J Oral Implantol. 2019;45:267–73. 10.1563/aaid-joi-D-18-00069.Suche in Google Scholar PubMed
[332] Kazek-k A , Nosol A , Joanna P , Monika Ś , Go M , Brzychczy-w M . PLGA-amoxicillin-loaded layer formed on anodized Ti alloy as a hybrid material for dental implant applications. Mater Sci & Eng C. 2019;94:998–1008. 10.1016/j.msec.2018.10.049.Suche in Google Scholar PubMed
[333] Rojas-Montoya ID , Fosado-Esquivel P , Henao-Holguín LV , Esperanza-Villegas AE , Bernad-Bernad MJ , Gracia-Mora J . Adsorption/desorption studies of norfloxacin on brushite nanoparticles from reverse microemulsions. Adsorption. 2020;26:825–34. 10.1007/s10450-019-00138-x.Suche in Google Scholar
[334] Lucke M , Schmidmaier G , Sadoni S , Wildemann B , Schiller R , Haas NP , et al. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32:521–31. 10.1016/S8756-3282(03)00050-4.Suche in Google Scholar
[335] de Avila ED , Castro AGB , Tagit O , Krom BP , Löwik D , van Well AA , et al. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl Surf Sci. 2019;488:194–204. 10.1016/j.apsusc.2019.05.154.Suche in Google Scholar
[336] Kamaly N , Yameen B , Wu J , Farokhzad OC . Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116:2602–63. 10.1021/acs.chemrev.5b00346.Suche in Google Scholar PubMed PubMed Central
[337] Bruschi M , Steinmüller-Nethl D , Goriwoda W , Rasse M . Composition and modifications of dental implant surfaces. J Oral Implant. 2015;2015:1–14. 10.1155/2015/527426.Suche in Google Scholar
[338] Askari E , Khoshghadam-Pireyousefan M , Naghib SM , Akbari H , Khosravani B , Zali A , et al. A hybrid approach for in-situ synthesis of bioceramic nanocomposites to adjust the physicochemical and biological characteristics. J Mater Res Technol. 2021;14:464–74. 10.1016/j.jmrt.2021.06.063.Suche in Google Scholar
[339] Rahmanian M , seyfoori A , Dehghan MM , Eini L , Naghib SM , Gholami H , et al. Multifunctional gelatin–tricalcium phosphate porous nanocomposite scaffolds for tissue engineering and local drug delivery: in vitro and in vivo studies. J Taiwan Inst Chem Eng. 2019;101:214–20. 10.1016/j.jtice.2019.04.028.Suche in Google Scholar
[340] Mauri E , Rossi F , Sacchetti A . Tunable drug delivery using chemoselective functionalization of hydrogels. Mater Sci Eng C. 2016;61:851–7. 10.1016/j.msec.2016.01.022.Suche in Google Scholar PubMed
[341] Askari E , Naghib SM , Zahedi A , Seyfoori A , Zare Y , Rhee KY . Local delivery of chemotherapeutic agent in tissue engineering based on gelatin/graphene hydrogel. J Mater Res Technol. 2021;12:412–22. 10.1016/j.jmrt.2021.02.084.Suche in Google Scholar
[342] Gittens RA , Olivares-navarrete R , Schwartz Z , Boyan BD . Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. ACTA Biomater. 2014;10:3363–71. 10.1016/j.actbio.2014.03.037.Suche in Google Scholar PubMed PubMed Central
[343] Nelson C , Assis F , Lucia A , Guilherme P . Influence of implant shape, surface morphology, surgical technique and bone quality on the primary stability of dental implants. J Mech Behav Biomed Mater. 2012;16:169–80. 10.1016/j.jmbbm.2012.10.010.Suche in Google Scholar
[344] Marinucci L , Balloni S , Becchetti BE , Belcastro S , Guerra M , Calvitti M , et al. Effect of titanium surface roughness on human osteoblast proliferation and gene expression in vitro . Int J Oral Maxillofac Implants. 2005;21:719–25.Suche in Google Scholar
[345] Gil FJ , Manzanares N , Badet A , Aparicio C , Ginebra M . Biomimetic treatment on dental implants for short-term bone regeneration. Clin oral investigations. 2013;18:59–66. 10.1007/s00784-013-0953-z.Suche in Google Scholar
[346] Ronold HJ , Lyngstadaas SP , Ellingsen JE . Analysing the optimal value for titanium implant roughness in bone attachment using a tensile test. Biomaterials. 2003;24:4559–64. 10.1016/S0142-9612(03)00256-4.Suche in Google Scholar
[347] Yang W , Han W , He W , Li J , Wang J , Feng H , et al. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater Sci Eng C. 2016;60:45–53. 10.1016/j.msec.2015.11.012.Suche in Google Scholar PubMed
[348] Boyan BD , Sylvia VL , Liu Y , Sagun R , Cochran DL , Lohmann CH , et al. Surface roughness mediates its e! ects on osteoblasts via protein kinase A and phospholipase A. Biomaterials. 1999;20:2305–10.10.1016/S0142-9612(99)00159-3Suche in Google Scholar
[349] Chen H , Huang X , Zhang M , Damanik F , Baker MB , Leferink A , et al. Tailoring surface nanoroughness of electrospun scaffolds for skeletal tissue engineering. Acta Biomater. 2017;59:82–93. 10.1016/j.actbio.2017.07.003.Suche in Google Scholar
[350] 349 – Bioactive nanofibers- synergistic effects of nanotopography and chemical signaling on cell guidance.pdf, n.d. 2007.Suche in Google Scholar
[351] Lopez BS . Determining optimal surface roughness of TiO2 blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin Oral Implants Res. 2001;12:515–25.10.1034/j.1600-0501.2001.120513.xSuche in Google Scholar
[352] Cochran DL , Schenk RK , Lussi A , Higginbottom FL , Buser D . Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. Jpn Soc Biomater Aust Soc Biomater. 1997;40:1–11.10.1002/(SICI)1097-4636(199804)40:1<1::AID-JBM1>3.0.CO;2-QSuche in Google Scholar
[353] Wennerberg ANN . The importance of surface roughness for implant. Int. J Mach Tools Manuf. 1998;38:657–62.10.1016/S0890-6955(97)00114-4Suche in Google Scholar
[354] Lim JY , Liu X , Vogler EA , Donahue HJ . Systematic variation in osteoblast adhesion and phenotype with substratum surface characteristics. Hoboken, New Jersey: Wiley Online Library; 2003.10.1002/jbm.a.20087Suche in Google Scholar
[355] Jayaraman M , Meyer U , Martin B , Joos U , Wiesmann H . Influence of titanium surfaces on attachment of osteoblast-like cells in vitro . Biomaterials. 2004;25:625–31. 10.1016/S0142-9612(03)00571-4.Suche in Google Scholar
[356] Gittens RA , Scheideler L , Rupp F , Hyzy SL , Geis-gerstorfer J , Schwartz Z , et al. A review on the wettability of dental implant surfaces II: biological and clinical aspects. ACTA Biomater. 2014;10:2907–18. 10.1016/j.actbio.2014.03.032.Suche in Google Scholar
[357] Elias CN . Factors affecting the success of dental implants. London, UK: IntechOpen; 1990.Suche in Google Scholar
[358] Hotchkiss KM , Reddy GB , Hyzy SL , Schwartz Z , Boyan BD , Olivares-navarrete R . Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016;31:425–34. 10.1016/j.actbio.2015.12.003.Suche in Google Scholar
[359] Dobrovolskaia MA , Neil SEM . Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–78.10.1142/9789814287005_0029Suche in Google Scholar
[360] Att W , Hori N , Iwasa F , Yamada M , Ueno T , Ogawa T . The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium – cobalt alloys. Biomaterials. 2009;30:4268–76. 10.1016/j.biomaterials.2009.04.048.Suche in Google Scholar
[361] Ohgaki M , Kizuki T , Katsura M , Yamashita K . Manipulation of selective cell adhesion and growth by surface charges of electrically polarized hydroxyapatite. J Biomed Mater Res. 2001;57:2–3.10.1002/1097-4636(20011205)57:3<366::AID-JBM1179>3.0.CO;2-XSuche in Google Scholar
[362] Zheng H , Mortensen LJ , Ravichandran S , Bentley K , Delouise LA , Carolina S . Effect of nanoparticle surface coating on cell toxicity and mitochondria uptake of. J Biomed Nanotechnol. 2017;13:155–66. 10.1166/jbn.2017.2337.Suche in Google Scholar
[363] Chen L , Mccrate JM , Lee JC , Li H . The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology. 2011 Mar 11;105708:105708. 10.1088/0957-4484/22/10/105708.Suche in Google Scholar
[364] Roy M , Pompella A , Kubacki J , Szade J , Roy RA . Photofunctionalization of titanium: an alternative explanation of its chemical- physical mechanism. PLoS One. 2016;11:1–11. 10.1371/journal.pone.0157481.Suche in Google Scholar
[365] Li S , Ni J , Liu X , Zhang X , Yin S , Rong M . Surface characteristics and biocompatibility of sandblasted and acid-etched titanium surface modified by ultraviolet irradiation: an in vitro study. J Biomed Mater Res Part B, Appl Biomater. 2012;100:1587–98. 10.1002/jbm.b.32727.Suche in Google Scholar
[366] Baier E , Cornell F , York N . Investigation of three-surface properties of several metals and their relation to blood compatibility Hoboken, New Jersey: Wiley Online Library; 1972.Suche in Google Scholar
[367] Dini C , Nagay BE , Cordeiro JM , Nilson C , Rangel EC . UV-photofunctionalization of a biomimetic coating for dental implants application. Mater Sci Eng C. 2020;110:110657. 10.1016/j.msec.2020.110657.Suche in Google Scholar
[368] Rupp F , Haupt M , Klostermann H , Kim HS , Eichler M , Peetsch A , et al. Multifunctional nature of UV-irradiated nanocrystalline anatase thin films for biomedical applications. Acta Biomater. 2010;6:4566–77. 10.1016/j.actbio.2010.06.021.Suche in Google Scholar PubMed
© 2022 Mohammadmahdi Akbari Edgahi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption


