Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
-
Mohamed Ibrahim Ahmed Abdel Maksoud
, Mohamed Mohamady Ghobashy
, Ala’a H. Al-Muhtaseb
and Ahmed H. Ashour
Abstract
Magnetic spinel ferrite nanoparticles (SFNPs) attract high scientific attention from researchers due to their broad area for biomedicine applications, comprising cancer magnetic hyperthermia and targeted drug delivery. Uniquely, its excellent performance, namely, tuning size and surface morphology, excellent magnetism, extraordinary magnetically heat induction, promising biocompatibility, and specific targeting capacity, is essential for their effective utilization in clinical diagnosis and therapeutics of diseases. This review emphasizes the anticancer properties of nanoparticles of spinel ferrites with extra focus on the most recent literature. A critical review is provided on the latest applications of SFNPs in cancer therapy. Based on the results obtained from this review, SFNPs have the indefinite ability in cancer therapy through two mechanisms: (1) hyperthermia, where SFNPs, used as a hyperthermia mediator, elevated the tumor cells heat post-exposure to an external magnetic field and radiosensitizer during cancer radiotherapy; and (2) targeted drug delivery of cytotoxic drugs in tumor treatment. SFNPs induced apoptosis and cell death of cancer cells and prevented cancer cell proliferation.
Graphical abstract
Abbreviations
- CPT
-
camptothecin drug
- DNA
-
deoxyribonucleic acid
- DOX
-
doxorubicin
- FESEM
-
field emission scanning electron microscope
- MRI
-
magnetic resonance imaging
- MTT
-
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium assay
- MWCNT
-
multi-walled carbon nanotubes
- NPs
-
nanoparticles
- RNA
-
ribonucleic acid
- SEM
-
scanning electron microscope
- SAR
-
specific absorption rate
- SF
-
spinel ferrite
- SPM
-
superparamagnetic
- SPMNP
-
superparamagnetic nanoparticle
- TEM
-
transmission electron microscope
- TUNEL
-
terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling
1 Introduction
Cancer is considered one of the most frightening diseases that globally kill millions of people [1]. The universal encumbrance of cancer via the GLOBOCAN 2018 assessments for the cancer incidence and mortality has been given via the International Agency for Research on Cancer, including a geographic variability in 20 world regions (Figure 1) [2]. It was reported that 18.1 million new cancer cases and the cancer mortality were 9.6 million in 2018. These cases are projected to increase to about 24 million by 2035 [3]. Many of the investigations on drug delivery procedures have been carried out in cancer treatment. This investigation has focused on overcoming the principal holdback on cancer treatment via chemotherapy, or, in other words, the damage of healthy (normal) tissue via therapeutic use. To limit the outcome of this issue (the death of the case or the dismissal of the cancerous tumor), delivery procedures that are of a specific size possessing a suit distribution of anticancer agents and a mechanism to make the therapeutic agents release (that causes the agents to assemble at the tumor sites) should be advanced [4,5]. The utilization of nanoparticles (NPs) has grown from the normal function of drug delivery to multifunctional purposes [6]. These merits are diverse such as labeling transports the drug and gene, pathogens and proteins revelation, probes of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA), optical imaging reinforce, design of tissues, biomolecules, and segregation of cells and as a chemotherapeutic agent [3]. Between various types of functional NPs, magnetic spinel ferrite nanoparticles (SFNPs) have a significant focus for their possible utilization such as an opposite to agent enhancer in magnetic resonance imaging (MRI) and an energetic agent in drug delivery [7,8]. SFNPs have several essential biomedical applications [9]. They are used in hyperthermia in cancer therapy, radiosensitizer, drug delivery and release [10,11,12,13], enhancing MRI contrast [14,15,16], and for biomagnetic separation purposes [17]. NPs are of small size, <100 nm, in a minimum of one dimension. As the size diameter decreased to ≤20 nm, the paramagnetic characteristics were lost by SFNPs. In contrast, they were changed to superparamagnetic (SPM) when subjected to an externally applied magnetic field due to thermal effects [18]. The biomedical applications of SFNPs were highly dependent on the synthesis method, shape, size, and types. It is revealed that the ratio of the surface area to volume of NPs was increased as the particle size decreased [19]. Herein, this critical review conducts and evaluates the latest knowledge and exhaustive information about the anticancer activity, hyperthermia, and drug delivery of SFNPs.
Ferrites can be categorized into three categories, viz., spinel, garnet, and hexa-ferrites, depending upon their crystal structures. In this review, we will focus on spinel ferrite (SF) materials. SFs are marked via the nominal formula RFe2O4, where R represents the divalent cations with an ionic radius ranging from 0.6 to 1
![Figure 2
Crystallographic representation of magnesium aluminate (MgAl2O4). Adapted from ref. [21] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_002.jpg)
Crystallographic representation of magnesium aluminate (MgAl2O4). Adapted from ref. [21] with permission from Elsevier™.
Magnetic ferrite NPs have been reported as biomedically significant agents, especially drug delivery vehicles, MRI, and hyperthermia mediators. SFs are favored over other materials due to their broader range of applications, low cost, high sensitivity, where high sensing is required, and selectivity for certain gases. Ferrites find uses in many applications in the areas such as sensors [23], transformer cores [24], chip inductor [25], electromagnetic wave absorber [26], data storage [27], heavy metals removal [28], antibacterial and antibiofilm agents [29], and water remediation [30,31]. Also, ferrites have received huge attention because of their extensive applications, such as power applications [32]. Many continuous efforts are made to control the size, shape, magnetic, and morphological properties of ferrites by different types of synthesis methods.
2 Synthesis methods of SF NPs
The synthesis techniques and conditions exercised through the synthesis route are the prime agents that manage and verify the feature nature for SF NPs [33]. Nanostructured ferrites can be fabricated via several synthesis procedures such as sol–gel [30,34,35,36,37,38,39], co-precipitation [40,41,42,43,44], hydrothermal [45,46,47], thermal decomposition [48,49,50], Polyol [51,52], solvothermal [53,54,55], spray pyrolysis deposition technique [56] and sonochemical techniques [57,58] (see Table 1). The magnetic properties are marked with interdependence on the surface morphology of magnetic NPs and the fabrication processes. These procedures provide excellent control of the crystal’s size, size diffusion, and lattice deficiencies [45].
Synthesis methods of SFs SFNPs
| Synthesis methods | SF | Ref. |
|---|---|---|
| Sol–gel | Co0.5Mg0.25Cd0.25Fe2−x Ce x O4 | [59] |
| Co0.8−x Mn0.2Zn x Fe2O4 | [60] | |
| CoFe2O4 | [61] | |
| NiGd x Fe2−x O4 | [62] | |
| Ni0.8Zn0.2Ce x Fe2−x O4 | [63] | |
| Mn0.85Zn0.15Ni x Fe2O4 | [64] | |
| Co-precipitation | NiGd x Fe2−x O4 | [65] |
| Ni0.5Mg x Zn0.5−x Fe2O4 | [66] | |
| Mn x Zn1–x Fe2O4 | [67] | |
| Zr x Mg0.2−x Co0.8−x Fe2O4 | [68] | |
| Co x Sn1−x Fe2O4 | [69] | |
| ZnFe2O4 | [70] | |
| Hydrothermal | MnFe2O4 | [71] |
| MFe2O4 (M = Co, Ni) | [72] | |
| (Mg,Ni)(Fe,Al)2O4 | [73] | |
| CuFe2O4 | [74] | |
| Mn0.8Zn0.2Fe2O4 | [75] | |
| Thermal decomposition | CoFe2O4 | [76] |
| MnFe2O4 | [77] | |
| NiFe2O4 | [78] | |
| MnFe2O4 | [79] | |
| Polyol | CoFe2O4 | [80] |
| Sr0.3Mg0.7Fe2O4 | [10] | |
| MFe2O4 (M = Mn, Fe, Co, Ni) | [81] | |
| CoFe2O4 | [82] | |
| La0.7Ca0.3−x Ba x MnO3 | [83] | |
| Solvothermal | CoFe2O4 | [84] |
| Mn0.8Zn0.2Fe2O4 | [85] | |
| ZnFe2O4 | [86] | |
| MFe2O4, M = Fe, Co, Ni, Mn, Cu, Zn | [87] | |
| CoFe2O4 | [88] | |
| Spray pyrolysis | Li0.5−x/2Mg x Fe2.5−x/2O4 | [89] |
| Ni1−x Cd x Fe2O4 | [90] | |
| Cu0.1Ni0.3Zn0.6Fe2O4 | [91] | |
| Ni1−x Cu x Fe2O4 | [92] | |
| NiFe2−x Al x O4 | [93] | |
| Sonochemical | Zn1−x Co0.5x Mg0.5x Fe2O4 | [94] |
| Ni0.4Cu0.2Zn0.4Fe2−x Eu x O4 | [95] | |
| Co0.3Ni0.5Mn0.2Eu x Fe2−x O4 | [58] | |
| CoFe2−x Gd x O4 | [96] | |
| Mn1−x Cu x Fe1.85La0.15O4 | [97] |
2.1 Sol–gel method
Metal alkoxide solutions are used as preliminary precursors in the sol–gel fabrication technique, which undergo hydrolysis and condensation polymerization followed by the gel formation stage [59,60]. Besides, more heat strategies are required to exclude any volatile in the obtained gel to get the final crystalline phase [34,61]. The sol–gel technique has some merits such as cost-effectiveness and does not require particular tools; additionally, it can be performed at a moderate temperature. Furthermore, in the sol–gel method, the temperature of the reaction ranges from room temperature up to 199.85°C. This state is suitable to manufacture SF NPs with fine size distribution and manageable form and shape [34,61]. Besides the simplicity in terms of the fabrication methods of SFNPs, these merits make the sol–gel technique so unique [30,34,36,37,38,39]. Furthermore, it is one of the adopted fabrication techniques to manage structure, morphology structure, pureness, and form of SFNPs via altering several factors, such as the sol concentration, the rate of stirring, and cancellation temperature [61,62,63,64].
Abdel Maksoud et al. [30] have used the sol–gel method to synthesize Mn0.5Zn0.5−x Mg x Fe2O4 NPs, which were synthesized in the presence of citric acid (C6H8O7) solution that was added as a fuel and ethylene glycol (C2H6O2) drop by drop to produce the final gel composition. The resulting Mn0.5Zn0.5−x Mg x Fe2O4 solution was dried at 120°C and then the obtained powders were sintered (899.85°) at 1,173 K, as illustrated in Figure 3. They used Mn0.5Zn0.5−x Mg x Fe2O4 NPs as a magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials.
![Figure 3
Schematic diagram of the sol–gel method for the synthesis of Mn0.5Zn0.5−x
Mg
x
Fe2O4 NPs. Adapted with permission from ref. [30], Copyright, 2020, Elsevier.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_003.jpg)
Schematic diagram of the sol–gel method for the synthesis of Mn0.5Zn0.5−x Mg x Fe2O4 NPs. Adapted with permission from ref. [30], Copyright, 2020, Elsevier.
2.2 Co-precipitation
Co-precipitation is among the commonly proficient procedures utilized to fabricate NPs with uniform distribution [65,66,67,68]. In this process related to SFNPs, aqueous solutions comprise the mix up of divalent transition cations and ferric (v/v = 1/2) [69,70]. The synthesis technique demands accurate calibration and checks of pH to obtain SFNPs with excellent features. The pH of the solution is generally maintained using NH4OH solution or NaOH solution [43,71,72]. Then, an energetic stirring of the solution will be used with or without the drying process. Numerous research investigations, which used the co-precipitation approach to fabricate the NPs, have been performed [40,41,42,43,44]. Recently, Gul et al. [73] have reported the fabrication of Al x ZnFe2−x O4, NPs via the co-precipitation technique, where the solutions of the starting chemicals were dissolved in deionized water and stirred at a stoichiometric ratio. The pH of all the solutions was kept at pH = 10 via the dropwise addition of the NH4OH solution. Then, the solution’s color changed from orange to deep brown with coffee-colored precipitates at the ground of the beaker. The precipitates after being dried are crushed and annealed at 600°C for 8 h, as exhibited in Figure 4. The analysis of magnetic properties assumed that the prepared NPs possess excellent magnetic characteristics. Also, the optical investigations of the NPs showed that these SFs additionally possess photocatalytic applications. The photocatalytic degradation of methylene blue via spinel samples has been discussed. The ferrite particles could be separated from contaminated water easily through a magnetic field. Hence, ferrite NPs can be utilized and recycled easily for photocatalytic applications.
![Figure 4
Schematic illustration of the co-precipitation method of Al
x
ZnFe2−x
O4 NPs. Adapted from ref. [73] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_004.jpg)
Schematic illustration of the co-precipitation method of Al x ZnFe2−x O4 NPs. Adapted from ref. [73] with permission from Elsevier™.
2.3 Hydrothermal reactions
The hydrothermal technique uses an aqueous solution as a reaction system in a particular closed reaction vessel to produce a high-temperature, high-pressure reaction via heating the reaction system and pressurizing it [47,74]. In this technique, water serves as a reactant that produces a reaction hydrolysis speedup with improved solubility of related materials in the precursor. This procedure gives more significant mobility due to the lower viscosity of water and further leads to an enhancement in the crystal structure, and accelerated the ability of the NP product [47]. The hydrothermal technique is one of the popular suitable fabrication techniques for the large-scale generation of SFs. The SF synthesized has excellent features with restricted size, and excellent size distribution can be gained by choosing the proper mix of solvents and changing parameters such as temperature, pressure, and reaction time [45]. Ding et al. [75] have synthesized cobalt ferrite through an ethanol-assisted hydrothermal technique. The scanning electron microscopic (SEM) images revealed that the samples possessed a narrow size distribution (Figure 5). The water/ethanol volume ratio was chosen as 0, 5/2, 3/4, and 1/6, and the samples were labeled as sample1, sample2, sample3, and sample4, respectively. A constant increase of the ethanol ratio will appear in the particle extension restraint, and the decrease of particle size is ascribed to the surface passivation effect. It was approved via SEM and transmission electron microscopic (TEM) images of sample3 and sample4, where a speedy reduction in the particle size and crash of agglomerates can be established. It is worth remarking that, with increasing ethanol content in the ethanol–water mixed solution, apparent SPM performance of NPs was seen. The adsorption capability of ferrite NPs for Congo Red (CR) was tested. Improvement of adsorption capability for CR was shown by combining ethanol. Also, the adsorption mechanism was discussed. This examination shows that the composition of ethanol/water mixed solution possesses excellent impacts on the microstructure and magnetic properties and adsorption capacity of the CR dye of CoFe2O4 samples.
![Figure 5
SEM and TEM images and the corresponding particle size distribution obtained from SEM images of samples 1, 2, 3, and 4 represented as (a, e, i), (b, f, j), (c, g, k) and (d, h, l), respectively. Adapted from ref. [75] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_005.jpg)
SEM and TEM images and the corresponding particle size distribution obtained from SEM images of samples 1, 2, 3, and 4 represented as (a, e, i), (b, f, j), (c, g, k) and (d, h, l), respectively. Adapted from ref. [75] with permission from Elsevier™.
2.4 Thermal decomposition
The thermal decomposition approach is among the most simplistic routes for fabricating SFs, including the thermal decomposition of organometallic sources, viz., metal acetylacetonate complexes of carbonyls. Besides, there are suitable solvents for organic and surfactants for the fabrication of SFs [61]. The size and form of SFs have been restrained via altering the temperature, the rate of heat, or the metal source concentration, and it is feasible to achieve SFs having extremely mono dispersion, consistent morphology, and close particle size distribution. Yang et al. [76] have synthesized a nonstoichiometric zinc ferrite (Zn x Fe3−x dO4) NPs with Zn substituent content x = 0–0.5 by the thermal decomposition route via utilizing oleic acid as a surfactant. Alterations in the morphology of the as-synthesized samples could be examined from the SEM images. When the content of the zinc source is about 0.004 mol or smaller, octahedral particles are produced. By increasing the content of the zinc source to 0.006 and 0.008 mol, well-faceted polyhedral crystallites are formed. Meanwhile, using 0.01 mol of zinc source leads to nonuniform particles with huge cubes and some smaller ones. oleic acid acts as both a reducing agent and stabilizer in the fabrication method and the source of the zinc/surfactant ratio is essential for the morphology. Besides, an increase of the zinc precursor will give rise to insufficiency in the surfactant, which it is then challenging to stabilize all nuclei to a uniform shape. This reason for the samples appears unestablished and nonuniform. Size-dependent utilization (radar absorption and hyperthermia) was observed more. Both applications required magnetic NPs with magnetization with extraordinary saturation. In hyperthermia, Zn ferrite NPs (26 nm) coated via the P-mPEG polymer revealed higher biocompatibility and heating efficiency, indicating the possible use in in vivo cancer therapy.
Also, Sharifi et al. [77] have studied the effect of the quantity of solvent on the formation of Fe-substituted ZnFe2O4 NPs through thermal decomposition. Fe0.6Zn0.4Fe2O4 NPs have been synthesized via a thermal decomposition technique by utilizing metal acetylacetonate in a high-temperature boiling point solvent and oleic acid. Figure 6 displays FESEM images of the magnetic NPs. As can be noticed, the sample has approximately homogeneous spherical particles besides possessing narrow size distribution. The particle size was reduced from 39 to 14 nm.
![Figure 6
FESEM micrograph of Fe-substituted ZnFe2O4 NPs with different sizes and three batches including 10 mL (F1), 20 mL (F2,) and 30 mL (F3) of benzyl ether. Adapted from ref. [77] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_006.jpg)
FESEM micrograph of Fe-substituted ZnFe2O4 NPs with different sizes and three batches including 10 mL (F1), 20 mL (F2,) and 30 mL (F3) of benzyl ether. Adapted from ref. [77] with permission from Elsevier™.
2.5 The polyol method
The polyol approach has been lately considered more in the fabrication of SFs. In this process, diethylene glycol acts as both the solvent and reducing agent, viz., CoFe2O4 [78], Co0.50Zn0.50Fe2O4 [79], etc., were recently published. Gaudisson et al. [80] have fabricated a group of nanostructured and compact spinel CoFe2O4 via the polyol method by utilizing three different solvents of polyol category, viz., diethylenglycol, 1,2-dihydroxyethane, and 1,2-propanediol, including different aggregate phases. The obtained CoFe2O4 particles display distinct aggregate states. On utilizing diethylenglycol as a solvent, the CoFe2O4 particles exhibited uniform size (5 nm), were completely nonaggregated, and were in nearly isotropic form (Figure 7a). In the case of utilizing 1,2-propanediol (Figure 7d) and 1,2-ethanediol (Figure 7g) as solvents, the Co-ferrite comprises 10 and 100 nm clusters, respectively. They exhibit well-marked fringes belonging to the crystallographic planes of Co’s spinel lattice, as indicated by the indexation from the corresponding Fourier transform images. Obvious irreversibility is systematically obtained between the zero-field cooling (ZFC) and field-cooling (FC) susceptibility. The ZFC plots exhibit a distinct peak at a critical temperature defined as the blocking temperature, T B, and decrease quickly to zero when the temperature drops below T B, while FC slightly increases. T B displays the threshold temperature above which the magnetic anisotropy barrier was solely defeated through the thermal activation energy, causing NPs to relax from the ferrimagnetic state to the SPM state. These drifts in ZFC and FC were characteristic of superparamagnetism in single magnetic domains.
![Figure 7
TEM images of Co-ferrite obtained with separate polyols: diethylenglycol, 1,2 propanediol, and 1,2 ethanediol (as (a and b), (d and e) and (g and h)). Fourier transform images are presented in every condition ((c), (f), and (i)). Adapted from ref. [80] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_007.jpg)
TEM images of Co-ferrite obtained with separate polyols: diethylenglycol, 1,2 propanediol, and 1,2 ethanediol (as (a and b), (d and e) and (g and h)). Fourier transform images are presented in every condition ((c), (f), and (i)). Adapted from ref. [80] with permission from Elsevier™.
2.6 Solvothermal
In the solvothermal fabrication approach, aqueous or nonaqueous solvents can be utilized to fabricate NPs with accurate restrictions covering the size distribution, form, and phases of the crystals. These physical features have been adjusted via altering specific test factors, viz., the reaction temperature, contact time, type of the solvent, type of used surfactant, and purity of precursors. Numerous SFs and their related composites have been fabricated by utilizing the solvothermal fabrication approach [10,61]. Aparna et al. [81] have synthesized different SFs with nominal composition MFe2O4 (M = Fe, Co, Ni, Mn, Cu, Zn) via the solvothermal method using ethylene glycol as a solvent and polyethylene glycol (PEG) 600 as a co-solvent. The prepared SFs exhibit approximately spherical morphology. The average particle sizes of Fe3O4 (36.1 nm), CoFe2O4 (51.3 nm), NiFe2O4 (41.9 nm), MnFe2O4 (37.6 nm), CuFe2O4 (135.1 nm), and ZnFe2O4 (81.1 nm) are illustrated in Figure 8. The cluster configuration had larger particles produced via the accumulation of tinier particles. The results showed that the change in the solvent during the synthesis generates particles with varying morphologies, and supercapacitive performances were observed. The specific capacitances of Fe3O4, CoFe2O4, NiFe2O4, MnFe2O4, CuFe2O4, and ZnFe2O4 were estimated to be 101, 444.8, 109.26, 190, 250, and 138.95 F/g, respectively. The highest specific capacitance is observed for CoFe2O4 as compared to other metal ferrites. The capacitive behavior of CoFe2O4 was also found to vary with morphology.
![Figure 8
FESEM images of (a) magnetite, (b) CoFe2O4, (c) NiFe2O4, (d) MnFe2O4, (e) CuFe2O4, and (f) ZnFe2O4. Adapted from ref. [81] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_008.jpg)
FESEM images of (a) magnetite, (b) CoFe2O4, (c) NiFe2O4, (d) MnFe2O4, (e) CuFe2O4, and (f) ZnFe2O4. Adapted from ref. [81] with permission from Elsevier™.
2.7 Sonochemical technique
Through ultrasonic radiation, bubbles are generated in the solvent medium and can efficiently compile the diffuse energy of ultrasound; upon breakdown, extraordinary heating energy was discharged to the bubble’s heating treatment [82,83]. This produces a vacating localized hot spot with sufficient temperature inside the bubbles of around 5 × 103 K and 103 bar, respectively. These dominant conditions give rise to various chemical reactions, which are almost always not attainable [84]. Recently, Almessiere et al. [85] have investigated the effect of substitution of terbium ions on the microstructure, dielectric, and microwave properties of Ni–Cu–Zn ferrite synthesized via the sonochemical technique. The terbium-doped Ni–Cu–ZnFe2O4 possesses an average particle size of 21 nm. The distribution is close, which suggests a tight particle size ranging between 5 and 40 nm. The sample with the Tb content (x = 0.06) is in contrast to other samples in the appearance of bimodal size distribution performance, which is seen in Figure 9a–f. The average particle size of the Ni0.4Cu0.2Zn0.4Fe1.94Tb0.06O4 sample reached 57 nm. The substitution ratio revealed a substantial impact on the dielectric characteristics, while the Tb ion replacement had a small but distinguished effect on the AC/DC conductivity variation. The reflection losses as a function of frequency dependences were calculated from S-parameter data in the range of 1–4.5 GHz. The electromagnetic absorption in the frequency interval of 1.85–3.79 GHz was observed. The nonlinear performance of the amplitude-frequency characteristics was changed with Tb ions. It was observed that the microstructural parameters correlate well with the principal absorption characteristics. The decrease of the reflected electromagnetic radiation was explained along with domain-boundary resonance, which well correlates with the microstructure data. The low-dimensional magnetic oxides possessing the domain-boundary resonance have a role in the nature of absorption.
![Figure 9
Particle size distribution of the Tb-doped Ni–Cu–Zn/Fe2O4 (x ≤ 0.1). Adapted from ref. [85] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_009.jpg)
Particle size distribution of the Tb-doped Ni–Cu–Zn/Fe2O4 (x ≤ 0.1). Adapted from ref. [85] with permission from Elsevier™.
2.8 Synthesis of ferrite thin films
The significant problems the researchers face are fabricating ferrite films utilizing easy technology with low-temperature heat treatment and low vacuum. The ferrite films have been fabricated earlier using various methods, viz., RF sputtering, plasma laser deposition, etc. These methods regularly include elaborate and costly apparatus and complicated processes. Besides, the high deposition temperature limits the option of the substrate material as well as restricts various applications of ferrite thin films [56]. It should be remarked that agglomeration could be solved utilizing the synthesis techniques in which the formation and separation of particles occur. These techniques involve spray pyrolysis techniques in which liquid solutions of reagents were sprayed, and the drops of the solution were supplied to a heated reactor or flame. Heat treatment causes the evaporation of the solvent and the formation of a solid phase in the structure of nanocrystalline particles in the absence of aggregation [86]. Pratibha Rao et al. have deposited (Co, Cu, Ni, Zn) ferrite thin films onto the Si (100) and alumina substrates via the spray pyrolysis deposition technique. SEM images of CoFe2O4 and ZnFe2O4 thin films deposited on Si (100) show spherical morphology. The Ni-ferrite thin film reveals a petal-like structure while CuFe2O4 has a cubic morphology with spherical particles inserted in it (Figure 10) [56].
![Figure 10
SEM images of spray deposited and air-annealed (Co, Cu, Ni, Zn) ferrite thin films. Adapted from ref. [56].](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_010.jpg)
SEM images of spray deposited and air-annealed (Co, Cu, Ni, Zn) ferrite thin films. Adapted from ref. [56].
3 Structure and properties of SFs
3.1 Structure of SFs
The SF MFe2O4 possesses a cubic unit cell and comprises eight cubic cells involving 56 ions. These ions were distributed as follows: 32 oxygen (O2−) anions, 8 M2+ cations, and 16 ferric cations. The oxygen ions possess a large radius and settled an almost packed face-centered cubic structure amidst more petite metal cations filling the subsites states belonging to space group Fd3m [87]. As two separate valence cations are possible, two classes of crystallographic sites are in the spinel structure, namely, A sites enclosed via four oxygen ions (tetrahedral) and B sites circled by six oxygen ions (octahedral) [88,89]. Maksoud et al. [90] have investigated the influence of zinc ion substitution on cobalt ferrite NPs prepared using a sol–gel technique. The Rietveld refinements at room temperature for XRD patterns of Co1−x
Zn
x
Fe2O4 NPs are illustrated in Figure 11. The detected reflection peaks correspond to the characteristic Fd3m space group SFs (JCPDS card no. 74-2082) [91,92]. All detected peaks are allowed Bragg 2
![Figure 11
Rietveld refined XRD patterns of Co1−x
Zn
x
Fe2O4 representing the Zn content (x). Adapted from ref. [90] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_011.jpg)
Rietveld refined XRD patterns of Co1−x Zn x Fe2O4 representing the Zn content (x). Adapted from ref. [90] with permission from Elsevier™.
Generally, there are two vibrational bands characteristic for SFs, namely υ1 and υ2 originating from the stretching vibration of A-site groups (tetrahedral) and B-site groups (octahedral), respectively [95]. Massoudi et al. [96] have prepared Ni0.6Zn0.4Fe1.5Al0.5O4 via the sol–gel technique following the annealing process at different temperatures. The Fourier transform infrared spectroscopy spectrum of Ni0.6Zn0.4Fe1.5Al0.5O4 was acquired at room temperature in the range from 400 to 4,000 cm−1. According to Waldron, the prominent peaks at 557–581 cm−1 and 403–410 cm−1 are ascribed to the cation-anion bond stretching vibration in A and B sites, respectively (Figure 12a–b). Consequently, the appearance of these two bands υ1 and υ2 confirmed the formation of the Ni0.6Zn0.4Fe1.5Al0.5O4 SF structure.
![Figure 12
FTIR spectra of Ni0.6Zn0.4Fe1.5Al0.5O4 at different annealing temperatures. Adapted from ref. [96] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_012.jpg)
FTIR spectra of Ni0.6Zn0.4Fe1.5Al0.5O4 at different annealing temperatures. Adapted from ref. [96] with permission from Elsevier™.
Mössbauer spectroscopy is exceptionally sensitive to tiny alterations in electron density at Fe’s nucleus, ascribed to diverse electronic and structural situations. Isomer shift occurs due to the electrostatic interaction within the distribution of nuclear charge and S-electron density, leading to shifting the levels of nuclear energy. The isomer shift relies on the overall nuclear charge and the nuclear radius. Besides, it depends on the densities of the S-electron for both absorber and source. The isomer shift has been utilized to estimate the valence state of iron atoms. The isomer shift values are in the range of 0.1–0.5 mm/s for ferric ions and exceed 0.5 mm/s in the case of ferrous ions [97]. Poudel et al. [98] have studied the Mössbauer spectra of gadolinium-substituted nickel ferrite NiGd x Fe2−x O4. The absorption results from Fe3+ (A Site) adapted a single hyperfine pattern. Hence, the substitution of the Fe3+ ion with a Gd3+ at the B site was not a considerable sufficient change in the whole superexchange interaction to initiate a remarkable diversity in the hyperfine field at the A site. Besides, every iron ion at the B site possessed only six A-site nearest neighbors. Therefore, for each change in the Fe3+ ion at the tetrahedral site via a significant ratio, an altering in the superexchange interaction is observed (Figure 13).
![Figure 13
Room temperature fitted Mössbauer spectra of NiGd
x
Fe2−x
O4. Adapted from ref. [98] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_013.jpg)
Room temperature fitted Mössbauer spectra of NiGd x Fe2−x O4. Adapted from ref. [98] with permission from Elsevier™.
Also, the energy-dispersive X-ray spectroscopy mapping and scanning electron microscopy (SEM) are conducted to affirm the homogeneous distribution of spinel and their elemental mapping photographs. As presented in Figure 14, the homogeneous distribution and elemental mapping photographs of Zn, Cu, and Mn substituted CoFe2O4 are introduced [35].
![Figure 14
Elemental mapping images of (a) CoFe2O4, (b) Zn–Co/Fe2O4, (c) Cu–CoFe2O4, and (d) Mn–Co/Fe2O4. Adapted from ref. [35] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_014.jpg)
Elemental mapping images of (a) CoFe2O4, (b) Zn–Co/Fe2O4, (c) Cu–CoFe2O4, and (d) Mn–Co/Fe2O4. Adapted from ref. [35] with permission from Elsevier™.
3.2 Magnetic properties of SFNPs
Magnetic NPs have exceptional magnetic and structural features in these materials and hence have wide utilization in many applications like MRI, high-density magnetic recording media, magnetically guided drug delivery, and hyperthermia [9]. Moreover, when an SF with magnetic features is exposed to a magnetic field, its magnetization (M) progresses immediately, as shown in Figure 15 [99]. As the magnetic field (H) magnitude improves, the magnetization approaches its highest value, denoted saturation magnetization (M s). When the magnetic field is canceled, the magnetization variations exhibit distinctive performance, in which low magnetization has remained in the SF, denoted residual magnetization (M r). By reducing the magnetic field intensity to negative states, the affected magnetization in SF continuously declines until the field intensity approaches a negative value. The material magnetization fully diminished, which is termed coercivity (H c) [100].
![Figure 15
M-H plot of SF. Adapted from ref. [99] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_015.jpg)
M-H plot of SF. Adapted from ref. [99] with permission from Elsevier™.
4 SFNPs for anticancer applications
Hyperthermia treatment for different types of cancer depends on the increase of the heat from 42 to 46°C in cancer cells by SFNPs [101,102]. It is classified into the whole body, regional, and local hyperthermia upon the position of cancer cells [103,104]. Hyperthermia is considered a targeted differential therapy because cancer cells are more sensitive than normal cells to hyperthermic effects [105]. The supermagnetic SFNPs absorb energy by the alternated external magnetic field and release it as heat by magnetic relaxation mechanism or hysteresis [11]. Hyperthermia therapy is a form of medical treatment that involves raising the temperature of tissues to kill cancer cells or become dysfunctional [106]. Localized temperature increase allows the therapy to be limited to the diseased regions, which is very significant in anticancer therapies. Conventional cancer treatments are frequently linked to the harm they cause to healthy tissues and can result in long-term adverse effects. As a result, one of the primary advances in magnetic hyperthermia treatment is local cell heating. These promising techniques arise from a combination of magnetic oxides with an external oscillating magnetic field, and commonly SF has been used. The unique physiochemical characteristics of SF like low toxicity and high biocompatibility candidate them to various biomedical applications including drug delivery and magnetic hyperthermia treatment [9]. The significant advantages of SFNPs are their high surface-area-to-volume ratios, which make attaching a large number of therapeutic molecules simple, and their magnetic characteristics, which aid in MRI imaging of drug delivery.
The hyperthermia method to treat cancer tumors involved the injection of SF directly into cancer tumors with alternating magnetic fields (AMFs) to produce effective heat. The conditions should be achieved such as (1) the SF particles concentration in the tumor should be higher than that in normal tissues and (2) the SF particles should have a high specific absorption rate (SAR)
where T is the temperature increase during the time interval (t) to give substantial intratumoral doses of heat using AMFs well-tolerated by normal tissues, and c is the specific heat capacity. The SAR is maximized by four parameters to optimize heating in an AMF: (1) dielectric losses in SF has low electrical conductivity; (2) eddy current losses in SF has high electrical conductivity; (3) frictional heating is from the physical rotation of an anisotropic SF particle; and (4) hysteresis losses in an SF. Cancer cells were damaged by the generated heat, based on external field amplitude square, frequency, size, and type of SFNPs [107,108]. The SFNPs of less than 20 nm were releasing heat by Neel relaxation mechanism while Brown relaxation mechanism in case of large NPs size. The frequency and amplitude of the applied external magnetic field would be
Figure 16 shows the SAR dependence on smaller NP size, whose size closer to the single domain is desirable for high SAR than the multidomain [111]. If bulk particles have multiple domains, it is limited to be used in magnetic hyperthermia treatment due to magnetization reversal taking place by the magnetic moments flipping in domains where it is antiparallel to the AMF. It also occurs by domain growth in other domains, which occurs at lower fields. If the applied magnetic fields can fully saturate the magnetization, then the energy losses in multidomain materials depend on coercivity. Jordan et al. [112] investigated at all applied magnetic fields (up to 165 Oe (13.2 kA/m) and found that the power loss in single-domain SFNPs was substantially larger than that in multidomain SFNPs. Thus, for fully saturated magnetic materials, the power loss should decrease with an increase in the domain size. As a result, to minimize the power loss, multidomain particles should be avoided.
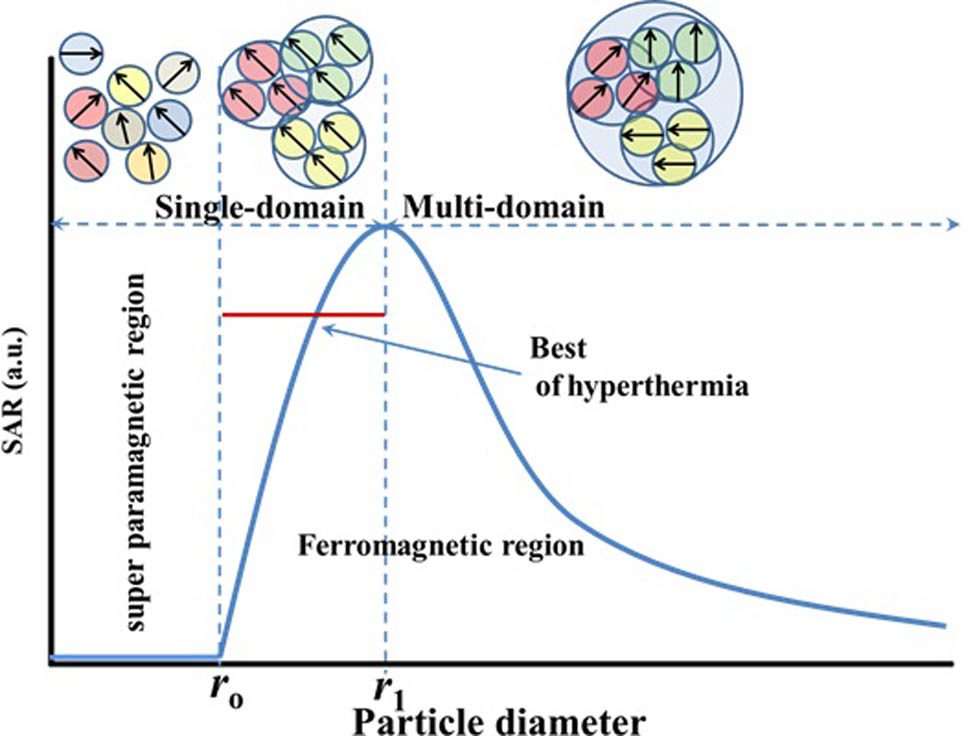
The dependence of SAR on the NP size. The domain magnetic structure of the SF particle would fall into the SPM region (dis-alignment) if their diameter size is smaller than the critical size r o; in contrast, if the size of the SF particle is bigger than r o, the SPM states transform to a single domain (full alignment); and if the size increases to twice the critical size r o, the magnetic structure of SF transforms to the multidomain region. As a result, particles in the r o − 2r o size range are predicted to be used in hyperthermia cancer therapy.
It was also highlighted that SF has the potential to be outstanding contrast agents with respect to MRI image quality contrast, sensitivity, and specificity, as well as potential drug-loaded nanocarrier targets. SF exhibits magnetic anisotropy, which is influenced by the anisotropy of the cations, the symmetry of the interstitial sites, as well as the metal type content and cationic arrangement; some toxic cations such as Sr, La, Y, Mn, Ag, or Al should be avoided. Within the close-packed arrangement of 32 oxygen ions, most SFs contain a cubic unit cell belonging to the Fd3m space group, in which 24 metal ions are arranged in 8 tetrahedral and 16 octahedral positions. The substituted cations such as Ni, Cu, Co, Mn, Mg, and Zn cause O2 displacement due to the substitution of cations at the tetrahedral sites and have to be expanded. On the other hand, the decrease in the lattice constant in SF systems can be explained by several factors: (1) the doping cation has a smaller ionic radius than the excited cation; (2) a potential rearrangement of Fe2+ and Fe3+ ions takes place inside the tetrahedral/octahedral ionic sites, leading to significant changes in magnetic characteristics; (3) Fe3+ is forced to the tetrahedral sites by a proportion of Fe2+ ions occupying the octahedral sites against their structural preferences that affect the optical, electrical, and magnetic properties of SFs [113]. In general, the magnetic behavior of SFs is highly dependent on the cation distribution between tetrahedral and octahedral sites [114]. Because the octahedral site and tetrahedral site spins align antiparallel, one can increase the magnetization in SFs by substituting cations for ferrous and ferric ions. For example, the substitution of Zn2+ by Fe2+ cations in the tetrahedral site gives ZnFe2O4, which exhibits magnetic behavior than Fe3O4 along with high magnetic saturation due to Zn2+ and Fe3+ cations occupying tetrahedral and octahedral sites, respectively. In general, smaller positive ions prefer to occupy the lower coordination site, i.e., the tetrahedral site, due to their relative sizes. The larger positive ions tend to occupy higher-coordination sites, such as octahedral sites [115]. As the Zn fraction increases in the tetrahedral site, the magnetization increases. This approach of cation substitution must be carefully chosen to maximize magnetization in SFs [116].
Furthermore, cationic interactions, surface anisotropy, and shape anisotropy all contribute to increasing total magnetic anisotropy in a single-domain structure [117]. An increase in temperature to about 42°C causes an increase in the tumor blood flow, which may be advantageous for simultaneous administration of chemotherapeutic drugs. This suggests that there is a temperature range in which cancer cells may be killed with little damage on normal cells, which is a key issue when utilizing hyperthermia for treating cancer and increases their anti-tumor effectiveness by loaded drugs. The development of SF has increased the interest of the scientific community in magnetic hyperthermia studies during the last years. Zhang et al. optimized the Curie temperature of 45.7°C and coercivity magnetization of 174 Oe for Cr3+-substituted Co–Zn/Fe2O4 (Zn0.54Co0.46Cr0.6Fe1.4O4) for use in magnetic hyperthermia [118]. Hanini et al. prepared cubic SF of Zn0.9Fe0.1Fe2O4 that exhibited a Curie temperature of 92.8oC under an applied magnetic field of 50 kOe. When Zn0.9Fe0.1Fe2O4 was incubated with glioma cells (U87-MGs) for 4 h at a low dose (0.05 g/L), they exhibit a significant increase of temperature (41.5°C) in a few seconds that is enough to kill malignant cells [101].
Lee et al. [119] loaded doxorubicin (DOX) as an anticancer drug into mesoporous silica-coated magnetite nanocrystals, and their surface was modified with PEG and fluorescence. Both MIR and fluorescence imaging verified in vivo passive targeting and accumulation of NPs at tumor sites. The DOX drug was successfully delivered to the tumor region in mice (the DOX accumulation compared with a control group was analyzed by terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay).
Remarkably, utilizing a hybrid design of a carbon covering of SF for cancer therapy appears to be highly promising. Carbon can solve and limit the main problems resulting in biomedical uses such as the possible toxicological consequences of long-term exposure, as well as low loading capacities and poor dispersibility under physiological conditions. It is known that carbon ions increase the biocompatibility of different biomaterials used and it reduces the immunohistochemical results in the body [120,121]. Gorgizadeh et al. [122] fabricated an SF coated by the carbon ion (NiFe2O4/C) nanocomposite and used it successfully as an effective agent for photoabsorbing in the photothermal therapy toward the melanoma cancer mouse model of C540 (B16/F10) cell line. Because of the excellent thermal conductivities of the carbon coating as well as electrical conductivities, the efficiency of the (NiFe2O4/C) nanocomposite conversion of light to heat is high. Furthermore, the hybrid design of the (NiFe2O4/C) nanocomposite assisted in the creation of a more targeted and definite treatment procedure, resulting in fewer side effects and lower toxicity in normal tissues.
As mentioned above, ZnFe2O4 and CoFe2O4 NPs have been widely studied for electronic applications owing to their self-interaction characteristics and their ability to respond to an external magnetic field. Combining both SF (ZnFe2O4 and CoFe2O4) components through the uniform distribution of MWCNTs show high adsorption of reactive pharmaceutical and biological species because of their large surface areas, leading to their high adsorption to the promoted adsorption of SF/MWCNT nanocomposites on the surface of the target cell.
Furthermore, rather than employing a carbon coating, as can be seen, a number of publications have shown success with alternative coatings, such as hydrogel (chitosan (CS), polyethylene, and phospholipids), which indicates a high efficiency for SF tumor cell adherence [123].
Zhang and Song developed injectable and biodegradable SF-based dual thermo- and magnetic-sensitive poly(organophosphazene) hydrogels for multiple magnetic hyperthermia therapy and MRI contrast [124]. This system is intended to serve as a multipurpose theranostics system due to the following: (1) because of the fast sol–gel transition of the hydrogel after a single injection, SF is retained within the tumor for a long time; (2) a minimally invasive multiple magnetic hyperthermia treatment at a reasonable temperature significantly improves anti-cancer therapeutic results; and (3) acting as a simultaneous long-term MRI contrast to guide and monitor the treatment procedure. Combining the hydrogel with SF loaded by drugs has several advantages, including preventing aggregation, enabling secondary drug functionalization, and providing carrier protection from the body’s immune system, all of which increase circulation duration. Bisht et al. [125] synthesized SF nanocomposites poly(N-isopropyl acrylamide)-ferrite via supercritical CO2-assisted synthesis. Drug loading and release profiles of 10 mL DOX drug were studied by varying the pH of the nanocarrier. The polymer nanocomposites exhibited enhanced drug release activity (20.98–76.54% release efficiency) and better biocompatibility in breast cancer cells (cell viability of 81–93%) as compared to spinal ferrite NPs.
Furthermore, the hydrogel-coated SF has functioned well in both covalent and noncovalent drug-loading processes, allowing access to a variety of drug release mechanisms such as external stimuli control or physiological condition-based adjustment within cancer areas. It selectively targets cancer cells and receptor engagement resulting in particle endocytosis; Figure 17 shows the coated spinal ferrite with polymer and active targeting groups.
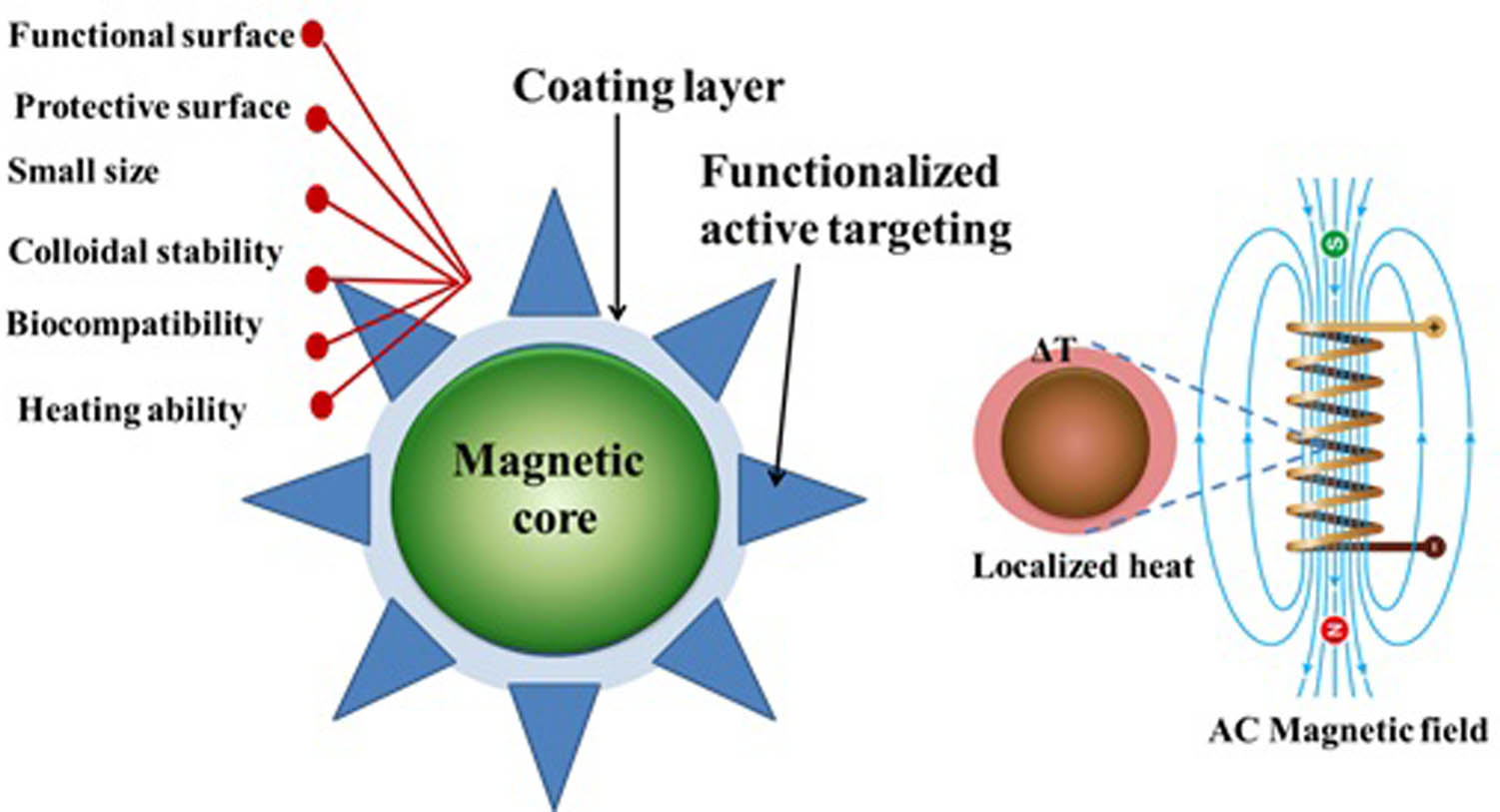
The SF coating with a polymer, the control of SF particle size, shape, biocompatibility, dispersion, and quality. Because of the unique multifunctional characteristics resulting from the wide diversity of functional groups within the polymer structure, it is likely to improve the hyperthermia therapy of SF. This coating layer can also protect SF surfaces against proteins, cell adsorption, and tissue penetration, extending particle circulation duration for in vivo hyperthermia applications.
The physicochemical properties of spinal ferrite are very sensitive to the change in their crystal form and particle size. Almost cubic SF crystal structures exhibit inherent characteristics such as ferromagnetic, antibacterial, and photodegradation activities than sphere-like SF [126]. Complex surface/interface interactions and the crystallite size effects resulting from the breaking of the symmetry of exchange bonds at the particle surface’s boundary are also crucial for determining the magnetic behavior via the formation of spin canting at the particle surface, affecting their different magnetic performances. According to the literature, it seems that the cubic SF crystal structure has only been examined for biomedical applications. The obtained results are extremely impressive, showing great potential in some fields like hyperthermia, drug delivery, and MRI applications. For example, Wang et al. [127] designed hybrid nanocubes of Fe3O4@MoS2 that had a superior SPM with a high surface area of 97.16 m2/g, without aggregation results or the restacking of MoS2 layers. It also demonstrated great magnetic sensitivity and good solution dispersibility, making it suitable for a variety of biological applications; on the hybrid design, to improve blood circulation time and medication accumulation at cancer locations, as well as to make drug loading easier. Xie et al. [128] designed and modified nanocubes of Fe3O4@MoS2 using PEG and 2-deoxy-d-glucose (2-DG) for targeted chemo-photothermal therapy. The obtained Fe3O4@MoS2/PEG/2-DG exhibited a great chemo-photothermal effect with a relaxivity coefficient of T 2 = 48.86/mM s and fast MRI signal detection of tumor sites with high contrast after injection.
The synthesis method can contribute to achieving the desirable properties for targeted drug delivery and hyperthermia applications. Recently, Almessiere et al. [129] have used two different techniques, citrate sol–gel combustion and sonochemical techniques, to synthesize Dy- and Y-codoped MnZn NPs. The principal aim is to compare the synthesis techniques and examine their biological applications by investigating antibacterial and anticancer activities. The different yttrium (Y) and dysprosium (Dy) ions doped Mn-Zn/Fe2O4 showed different magnetic behaviors attributable to the difference between the crystallite sizes of the prepared samples via the two synthesis methods. The prepared SF with a size less than 50 nm exhibited a broad SPM nature. The XRD results revealed that the synthesized samples via the sol–gel technique have a crystallite size of less than 40 nm, whereas the synthesized samples using the sonochemical method showed a crystallite size of less than 10 nm. Also, the magnetic properties at T = 10 K revealed closed hysteresis loops have nonneglected coercivity values ranging between 360 and 610 Oe for synthesized samples via the sol–gel method and from 320 to 695 Oe for the synthesized samples via ultrasonic synthesis. Further, the remanence ranged between 3.2 and 10.5 emu/g for the synthesized samples using the sol–gel method, whereas the remanence ranged between 16.2 and 26.6 emu/g in the ultrasonication route. The SEM images unveiled clusters of small cubic NPs for the samples prepared through the sol–gel method and fine spherical NPs for the samples prepared via the ultrasonication method. Besides, the DyY-MnZn NPs prepared via the ultrasonication method produced better inhibitory action on the cancerous cells as compared to those produced via the sol–gel method. The morphology of cancer cells was investigated by confocal scanning microscopy, and results showed that with the treatment of DyY-MnZn, there was an evident loss of cancer cells prepared via the ultrasonication and sol–gel methods as DAPI staining was detected to be notably reduced in the cancer cells.
Hassanzadeh-Tabrizi et al. [130] produced a cobalt ferrite/hydroxyapatite (HA) nanocomposite using a novel multistep depositional technique for the design of a homogeneous core–shell blend. Controlled drug release trials revealed that the prepared nanocomposite is capable of loading of drug and controlled drug delivery up to 50 h. Furthermore, the quantity of heat produced may be controlled using varying magnetic fields or cobalt ferrite to HA ratios, making it potential for a variety of magnetic-based hyperthermia treatments. The incorporation of hydroxyapatite on the surface of cobalt ferrite NPs greatly promotes cell compatibility while reducing magnetization saturation. The findings show that a multifunctional nanocomposite of cobalt ferrite/HA with a homogeneous structure could be useful in medical applications. Figure 18 shows SEM images. The particle size of the cobalt ferrite generated ranged between 50 and 500 nm, with random and octahedron-like morphologies. After immersing the particles in KH2PO4 solution, their surface shape entirely changes. On the surfaces of the particles, spherical and needle-shaped HA precipitates developed. According to Wijesinghe et al. [131], HA crystals can have a variety of morphologies depending on the process and conditions of synthesis. They found that adding 10% HA to cobalt ferrite lowers the specific surface area. The surface area of the samples was significantly enhanced when a higher amount of HA was added to the samples compared to pure HA. The surface areas of CoFe-30HA and CoFe-50HA samples were found to be quite large. As a result, these specimens were chosen to receive the ibuprofen (IBU) medication. The release of IBU from NPs was considerable in the early stages but it progressively reduced over 72 h. The extremely early release may be due to IBU dissolving rapidly on the surface of NPs. The gradual release was induced by the physical and chemical interactions between the NP surface and IBU, which led IBU to be released from the samples’ mesoporous structure. The initial release rate in this work is lower than several previously produced substances. Ansari et al. [132] studied the quick release of IBU from Cu0.3Zn0.2Mg0.5Fe2O4 in the early hours (65%). Furthermore, the loading capacity in their study was just about 10%. The type of the material surface and its mesoporous structure are two elements that contribute to the variances in the release rate and loading capacity. Static and hydrogen interactions bind IBU molecules and NPs together. As a result, the surface of the produced NPs in the Hassanzadeh-Tabrizi et al. study [130], which comprises hydrogen groups and Ca2+ ions, can form active sites on the NPs’ surfaces, making it more effective to connect with IBU’s carboxylic acid groups. Wu et al. [133] demonstrated that Ca2+ ions might interact with the carboxylic groups of IBU molecules due to their alkalinity. Alkaline earth metals could produce basic sites, according to Khamsehashari et al. [134], leading to improved bonding with IBU’s carboxylic groups. As a consequence of the effective factors for linking IBU molecules and cobalt ferrite/HA, more bonds are created, enabling the drug molecules to bind to samples with greater loading capacity and disperse from the material at a slower pace. When comparing the two samples, it can be observed that the CoFe-50HA sample exhibited a greater drug release. Because materials with bigger pores and higher pore volumes may contain more drug molecules, drug molecules trapped in these pores can naturally be released at a quicker pace. The retention of magnetic properties and the acquisition of optimum magnetization values are critical in drug release applications. The ferromagnetic behavior of the manufactured samples may be seen in their hysteresis loops (Figure 19). CoFe2O4, CoFe-10HA, CoFe-30HA, and CoFe-50HA have saturation magnetization values of around 59.9, 43.4, 18.3, and 11.4 emu/g, respectively. Thus, it can be observed that as HA increased, the saturation magnetization decreased. The CoFe-30HA composite exhibits a greater heating efficiency than samples with larger HA contents, particularly in high field zone, according to magnetic hyperthermia experiments. The magnetic characteristics of ferrites are affected by a variety of parameters, including particle size, shape, composition, defects, ion distributions, and even the synthesis method. For example, increasing the calcination temperature resulted in larger particles, which led to greater saturation magnetization [135]. Because the size of CoFe2O4 particles and the parameters of synthesis were kept fixed throughout the investigation, these variables had no significant impact on the magnetic properties. A decrease in the saturation magnetization of HA-coated Co ferrite NPs could be linked to the spacing of nearby NPs by a diamagnetic coating of HA, resulting in a reduction in the static interaction among them. Based on prior investigations, it was stated that a 10–30 emu/g magnetization is adequate for therapeutic purposes [136]. Furthermore, when the amplitude of the field increases, the temperature increases. By regulating the time and applied AC field, the heating response of samples can be improved.
![Figure 18
SEM images of cobalt ferrite NPs: (a) pure, (b) 50Ca, (c) 10HA, (d) 30HA, (e) 50HA, and (f) HA. Adapted from ref. [130] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_018.jpg)
SEM images of cobalt ferrite NPs: (a) pure, (b) 50Ca, (c) 10HA, (d) 30HA, (e) 50HA, and (f) HA. Adapted from ref. [130] with permission from Elsevier™.
![Figure 19
CoFe, CoFe-10, 30, and 50HA samples hysteresis curve. Adapted from ref. [130] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_019.jpg)
CoFe, CoFe-10, 30, and 50HA samples hysteresis curve. Adapted from ref. [130] with permission from Elsevier™.
Sangeetha et al. [137] developed an intelligent drug delivery system and/or a hyperthermia carrier by creating a potential magnetic nanocomposite of CoFe2O4/HA and loaded a chemotherapy medicine (5-fluorouracil, FU). To achieve it, a microwave-aided wet precipitation approach was used to successfully synthesize a cobalt ferrite/hydroxyapatite nanocomposite, which was then loaded with FU using an adsorption method. With 2.5–8.2 emu/g magnetic saturation, this nanocomposite exhibits ferromagnetic behavior. Using an AMF, they were able to produce hyperthermia in a short amount of time (43°C in 4.5 min) and accelerate the release of encapsulated FU from the composite. These multifunctional carriers show significant proliferative activity against healthy fibroblast cells (L929) and impede the growth of osteosarcoma cells (MG63). As a result, this multifunctional nanoplatform could be a good option for synergistic chemo-hyperthermia therapy, allowing cancer patients to receive chemotherapy and hyperthermia at the same time. TEM analysis showed the core–shell structure of the composites in the calcined sample, and PEG addition enhanced the pore radius and specific surface area of the composites. The saturation magnetization of the composites calcined at 1,100°C was 8.075 emu/g. The produced composites could release FU for 7 days at physiological temperature and could be accelerated at hyperthermia temperature, increasing the proportion of FU released. According to the findings of this study [139], this thermo-responsive nanovehicle offers possibilities as a delivery mechanism for tumor-specific treatment at hyperthermic temperatures.
Talaei et al. [138] produced a magnetic mesoporous CuFe2O4@SiO2 nanocomposite with a core–shell nanostructure by sol–gel combustion and examined it for simultaneous drug release and hyperthermia biomedical applications. Around copper ferrite, TEM images revealed the formation of thin mesoporous silica covering with a thickness of 14 nm (Figure 20). After surface modification of ferrite NPs with mesoporous SiO2 layer, the surface area of the samples increased from 2.59 to 199.2 m2/g. When compared to pure ferrites, the magnetic characteristics of core–shell samples were reduced. The drug IBU was used to test NPs’ ability to store and release the medicine. IBU loading was high and the drug release was regulated in the CuFe2O4@SiO2 system. Following the synthesis of a hybrid core–shell structure, the samples’ storage capacity increased from 4 to 34%. The nanocomposite’s mesoporous structure and increased surface area resulted in these enhancements. The rate of drug release was reduced when the calcination temperature increased but the release mechanism was unaffected. The cytotoxicity of CuFe2O4 NPs was lowered and the drug release characteristics were improved by coating them with mesoporous silica. This coating, however, limited the potential to generate hyperthermia. Although the capacity to heat the samples was reduced when CuFe2O4@SiO2 was synthesized, it increased biocompatibility and drug storage. The results suggested CuFe2O4@SiO2 be a promising option for medicinal applications as a hybrid system that can release medications and create heat at the same time.
![Figure 20
TEM image of the CuFe2O4@SiO2 nanocomposite at 400°C. Adapted from ref. [138] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_020.jpg)
TEM image of the CuFe2O4@SiO2 nanocomposite at 400°C. Adapted from ref. [138] with permission from Elsevier™.
Radmansouri et al. [139] combined titanium oxide NPs with cobalt ferrite NPs via microwave heating, which was subsequently electrospun into CS/cobalt ferrite/titanium oxide composite nanofibers. They tested the impact of DOX hydrochloride-loaded electrospun CS/CoFe2O4/TiO2 nanofibers on melanoma cancer B16F10 cell lines to check whether heat and therapy could be combined. Cobalt ferrite NPs were made via microwave heating. Titanium oxide NPs were mixed with cobalt ferrite to control the temperature increase. The DOX loading efficiency and in vitro drug release of DOX from nanofibers were investigated using an AMF and without a magnetic field under physiological and acidic conditions. As seen in SEM images, the surface of nanofibers was smooth, and no drug crystals were visible on the nanofibers’ surface (Figure 21). As a consequence, DOX molecules were well incorporated into electrospun fibers. The fastest release of DOX from the synthesized magnetic nanofibers was observed at acidic pH by changing the magnetic field. The anticancer effects of the nanofibers generated were also tested on the melanoma cancer B16F10 cell lines. According to the results, DOX-loaded electrospun CS/cobalt ferrite/titanium oxide nanofibers may be used for localized cancer treatment. According to in vitro cell incubation tests, simultaneous loading of DOX and cobalt ferrite/titanium oxide NPs into CS nanofibers following the application of a magnetic field enhanced the cytotoxicity of the nanofibers. The generated cobalt ferrite and cobalt ferrite/titanium oxide NPs had maximum saturation magnetization (M s) values of 90.5 and 81.2 emu/g, respectively. The reduction in M s of cobalt ferrite/titanium oxide NPs may be explained by the addition of titanium oxide NPs. The coercivity values (H c) of NPs composed of cobalt ferrite and cobalt ferrite/titanium oxide NPs were 830 and 640, respectively. The fact that cobalt ferrite NPs are smaller and more homogeneous than the manufactured cobalt ferrite/titanium oxide composites may explain this behavior.
![Figure 21
SEM images of (a) pure CS, (b) CS/CoFe2O4 (10 wt%), (c) CS/CoFe2O4 (20 wt%), (d) CS/CoFe2O4/TiO2 (20 wt%), and (e) CS/CoFe2O4/TiO2/DOX nanofibers. Adapted from ref. [139] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_021.jpg)
SEM images of (a) pure CS, (b) CS/CoFe2O4 (10 wt%), (c) CS/CoFe2O4 (20 wt%), (d) CS/CoFe2O4/TiO2 (20 wt%), and (e) CS/CoFe2O4/TiO2/DOX nanofibers. Adapted from ref. [139] with permission from Elsevier™.
Wang et al. [140] created SPM cobalt ferrite/graphene oxide (CoFe2O4/GO) nanocomposites with MRI and controlled drug delivery through sonochemistry. The method is simple and effective, and GO nanosheets are uniformly coated with CoFe2O4 NPs ranging in size from 5 to 13 nm. The CoFe2O4/GO nanocomposites created have SPM properties, are hydrophilic, and have a low degree of cytotoxicity, indicating that they have a lot of potential in biomedical applications. On CoFe2O4/GO, DOX hydrochloride was loaded as an antitumor model drug. The nanocomposites were shown to be effective in transferring DOX into cancer cells and caused cell death. This nanocarrier had a drug loading capacity of 1.08 mg/mg, and the drug release behavior was delayed and pH-responsive, which is useful for preventing rapid drug release in the neutral circulatory system while promoting drug release at acidic tumor sites or inside cells. Suárez et al. [141] created a composite of CS and polyvinylpyrrolidone (PVP) with CoFe2O4 NPs for use in drug delivery systems and hyperthermia (see Figure 22 for a schematic depiction of the manufacturing process). They examined how the structural, magnetic, and SAR characteristics of Co x Fe3−x O4 (x = 0.25, 0.50, 0.75, and 1.00) as a hyperthermia heat nanomediator were influenced by CS and PVP. At a frequency of 454 kHz and a magnetic field amplitude of 5.5 mT, hyperthermia tests were conducted. At x = 1.00, CS–PVP-coated NPs had a maximum SAR of 386 W/g, compared to 270 W/g for untreated NPs. The coated NPs exhibit higher SAR values than the untreated NPs due to the presence of CS and PVP. The variable mixing of CS and PVP for heating cobalt ferrite NPs improves the biocompatibility and stability of the samples. The impact of changing the Co2+ concentration on the nanocomposite structure may alter magnetic characteristics, increasing hyperthermia SAR, and NPs coated with hydrophilic polymers enhance biocompatibility and SAR efficiency.
![Figure 22
A schematic depiction of the synthesis mechanism of the composite of CS and PVP with CoFe2O4 NPs. Adapted from ref. [141] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_022.jpg)
A schematic depiction of the synthesis mechanism of the composite of CS and PVP with CoFe2O4 NPs. Adapted from ref. [141] with permission from Elsevier™.
Daboin et al. [142] used the thermal decomposition technique to prepare magnetic mixed manganese–cobalt ferrite NPs (Mn1−x Co x Fe2O4); then, they were coated with SiO2 using the Stöber process and adorned with Au@Fe3O4 NPs. TEM images of the undecorated and decorated nanocomposites are shown in Figure 23a–f. These images show that the nanostructured material has a spherical shape and that Au@Fe3O4 NPs were successfully deposited on the silica nanocomposites’ surfaces. They investigated the generated composite as magnetic fluid hyperthermia heat mediators using a hydrogel as a tissue equivalent. The SAR of the nanocomposites increased when they were decorated with Au@Fe3O4 in water. By integrating magnetic NPs, SiO2, and Au@Fe3O4, the magnetic properties of the synthesized nanocomposite system may be fine-tuned to optimize SAR.
![Figure 23
TEM images of the decorated (left) and undecorated (right) nanocomposites with varying Mn2+ contents. Adapted from ref. [142] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_023.jpg)
TEM images of the decorated (left) and undecorated (right) nanocomposites with varying Mn2+ contents. Adapted from ref. [142] with permission from Elsevier™.
Mondal et al. [143] developed a MnFe2O4/ZnS magneto-fluorescent nanocomposite using a simple co-precipitation method. The NPs are near SPM at room temperature, with a small coercivity of 66 G, and saturation magnetization increases significantly after coating ZnS on the MnFe2O4 core surface. MnFe2O4/ZnS core–shell NPs have a magnetic saturation of 1.15 emu/g, which is higher than MnFe2O4 NPs. The Zn2+ ions induce cation rearrangement in the nanocomposites’ interstitial regions, resulting in an increase in saturation magnetization. The heating efficiency of the MnFe2O4/ZnS core–shell nanocomposite is determined using the SAR and intrinsic loss property, which decreases with increasing sample concentration. Hatamie et al. [144] utilized GO/cobalt ferrite NPs to heat-treat the MCF7 breast cancer cell line. The ferrimagnetic NPs were 5 nm in diameter. The NPs were likewise uniformly distributed on the GO nanosheets. The cell survival rate was 58% after 72 h of NP treatment, suggesting that the IC50 had been reached. The vitality of the cells, on the other hand, was decreased by 30% following heat therapy. In addition, BALB/c mice were used in in vivo testing; the findings showed a reduction in tumor growth after 27 days with dosages of 0.001 and 0.002 g/mL and corresponding magnetic frequencies of 400 and 250 kHz for 10 min. The MRI studies revealed the presence of NPs not only inside the tumor but also in adjacent tissues. The MRI validated dark spots as a representation of the presence of NPs and tumor disruption as a consequence of the hyperthermia procedure in contrast to the control group (see Figure 24). The molecular gene expression of the treated tumor showed a higher expression of apoptotic genes. Hematoxylin and eosin (H&E) staining, on the other hand, revealed that NP concentrations of 0.002 g/mL at frequencies of 250 and 350 kHz disrupted the tumor cytoskeleton.
![Figure 24
MRI scans of mice with (a) NPs and hyperthermia; and (b) NPs without hyperthermia treatment. Adapted from ref. [144] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_024.jpg)
MRI scans of mice with (a) NPs and hyperthermia; and (b) NPs without hyperthermia treatment. Adapted from ref. [144] with permission from Elsevier™.
5 Selected doped SFNPs and their composites
Cobalt ferrite CoFe2O4 has a hyperthermic effect on cancer therapy and drug delivery [145]. Balakrishnan et al. [146] used the cubic-shaped cobalt ferrite NPs (Co–Fe NCs) as magnetic hyperthermia agents and as a cytotoxic agent ascribing to the distinguished cobalt ion toxicity, supporting both heat and cytotoxic impacts from a single platform (Figure 25a). The polymer-coated CoFe2O4 was injected intratumorally (i.t.). NPs were injected when the tumors were ≈80–100 mm3 (day 0), followed by 30 min HT cycles on days 0, 1, and 2, totaling 3× HT cycles on three consecutive days (HT1, HT2, and HT3). The iron oxide cubic-shaped nanoparticles (IONCs) were injected into rats i.t. within the 3× HT (TEM; Figure 25b) or CoFe2O4 (TEM; Figure 25c); the tumor temperature (T Tumor) and the skin tail temperature (T Skin) were detected with an infra-red camera (Figure 25d). They found that ΔT = T Tumor − T Skin were about 6, 3.5, and 3.5°C for IONCs on HT1, HT2, and HT3, respectively; while, CoFe2O4 presented only ΔT of 3°C on HT1, HT2, and HT3. The detected reduction in the temperature for CoFe2O4 and CoFe2O4 in water was because the immobilized nanocubes in the tumor cells’ viscous medium [147,148] caused a decrease in the release of heat from high anisotropy particles [149]. Also, they revealed that monitoring the temperature and cancer growth for 12 days post CoFe2O4 injection showed that the decrease of the growth of the tumor by Co–Fe NCs + hyperthermia, compared to Co–Fe NCs or the ions and the IONCs + hyperthermia, respectively, was nonsignificant (Figure 25f). Further, they compared the obtained IONCs + HT results with those of the previously revealed in vivo study [150]. Kolosnjaj-Tabi et al. [150] found that 18 nm IONCs-based hyperthermia monotherapy did not significantly decrease tumor growth [150]; while Mai et al. revealed that the combination therapy, IONCs hyperthermia + Doxo intravenous injection, enhanced the ions possessing cubic shape-based hyperthermia efficiency [151].
![Figure 25
The efficiency of the ions possessing cubic shape-based hyperthermia and Co–Fe NCs during in vivo study. (a) Plan of the treatment. Particle injection is symbolized by a black arrow (0.7 mg Co–Fe NCs or 0.7 mg IONCs) and the days of HT therapy (3 × hyperthermia) are symbolized by red arrows, using AMF conditions. (b and c) TEM images of the ions possessing the cubic shape of particles (18 nm) (b) and sizes of poly(maleic anhydride-alt-1-octadecene‐coated CoFe2O4 was (17 nm) (c) In vivo studies. Scale bar: 50 nm. (d) Mouse IR images post the ions possessing cubic shape and CoFe2O4 injection during hyperthermia therapy (HT1, HT2, and HT3). Cancer temperature is represented by white arrows, while skin temperature is shown by black arrows, to estimate ΔT values. (e) ΔT graph (ΔT = T
Tumor − T
Skin) plotted for HT1 at day 1, HT2 at day 2, and HT3 at day 3 for (orange bars) the IONCs (in vivo) or (red bars) Co–Fe NCs. (f) Tumor or cancer growth curve represented the marginal decrease in cancer growth for Co–Fe NCs and exposed to 3× hyperthermia (Co–Fe NCs + hyperthermia) compared to Control, the ions possessing cubic shape alone; Co–Fe NCs alone, and the ions possessing cubic shape + hyperthermia; N = 6. Adapted from ref. [146] with permission from John Wiley and Sons.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_025.jpg)
The efficiency of the ions possessing cubic shape-based hyperthermia and Co–Fe NCs during in vivo study. (a) Plan of the treatment. Particle injection is symbolized by a black arrow (0.7 mg Co–Fe NCs or 0.7 mg IONCs) and the days of HT therapy (3 × hyperthermia) are symbolized by red arrows, using AMF conditions. (b and c) TEM images of the ions possessing the cubic shape of particles (18 nm) (b) and sizes of poly(maleic anhydride-alt-1-octadecene‐coated CoFe2O4 was (17 nm) (c) In vivo studies. Scale bar: 50 nm. (d) Mouse IR images post the ions possessing cubic shape and CoFe2O4 injection during hyperthermia therapy (HT1, HT2, and HT3). Cancer temperature is represented by white arrows, while skin temperature is shown by black arrows, to estimate ΔT values. (e) ΔT graph (ΔT = T Tumor − T Skin) plotted for HT1 at day 1, HT2 at day 2, and HT3 at day 3 for (orange bars) the IONCs (in vivo) or (red bars) Co–Fe NCs. (f) Tumor or cancer growth curve represented the marginal decrease in cancer growth for Co–Fe NCs and exposed to 3× hyperthermia (Co–Fe NCs + hyperthermia) compared to Control, the ions possessing cubic shape alone; Co–Fe NCs alone, and the ions possessing cubic shape + hyperthermia; N = 6. Adapted from ref. [146] with permission from John Wiley and Sons.
Figure 26a shows that the Co–Fe NC chains and prolonged cobalt toxicity were present, as confirmed by long‐term in vivo study, resulting in decreased tumor growth and longer survival. It was focused only on Co–Fe NCs with or without hyperthermia efficiency. Also, Balakrishnan et al. [146] monitored the tumor size by digital photographs and showed that the tumor size was significantly reduced in the Co–Fe NCs + HT group than that in the Co–Fe NCs group compared to the control animals group on day 15 (Figure 26b). They revealed that the tumor was completely eradicated on day 30 after injecting Co–Fe NCs + HT i.t. without recurrence up to a post-treatment period of 200 days (Figure 26c). Furthermore, the survival rate was significantly higher in the Co–Fe NCs + hyperthermia group (about 200 days) than in the control group (22 days) and Co–Fe NCs group (30 days) (Figure 26d). As revealed in Figure 26e and f, the long-chain formation was confirmed by transmission electron microscopic images at day 30 for tumor tissues, indicating that strong interactions persisted for a long time. It was also shown that Co–Fe NCs were presented within the tumor at day 30, as indicated by histological Prussian blue staining (Figure 26g and h). The Co–Fe NCs + hyperthermia group showed a whirling movement of nanocubes from the tumor injected point to its peripheral surface, post exposed to an AMF (Figure 26h) [150]. The HT increased the chain length of injected Co–Fe NCs, resulting in mechanical damage to the cancer cells during the whirling movement of Co–Fe NCs. Marangon et al. [152] concluded that one of the important reasons for the tumor drug resistance was the tumor’s outer collagenous peripheral layer. Even the damage or necrosis was induced centrally in the tumor post-treatment; the outer layer was rich in angiogenesis, and viable cells resulted in the uncontrolled growth of cancer cells. Balakrishnan group [146] presented that CoFe2O4 NCs were injected i.t. in rats to avoid nonspecific cobalt toxicity. Chu et al. [153] revealed that the i.t. injection in the case of cancer patients was accepted. The outer layer of the Co–Fe NCs + HT group was rich in collagen (darker pink) (Figure 26h) and cells (pink) in the outer layer had more toxicity at the peripheral region, which led to the destruction of stroma; while in the CoFe2O4 NCs alone group, cancer cells were still viable (Figure 26g). Finally, they found that CoFe2O4 NCs + HT destroyed the outer tumor membrane completely, which led to the prevention of the recurrence or relapse of the tumor (Figure 26c) [146].
![Figure 26
The efficacy of Co–Fe NCs during in vivo HT examination. (a) Diagram of plan therapy. (b) Images of a control animal (at 0 and 15 days) and Co–Fe NCs injected alone, and Co–Fe NCs injected + hyperthermia display the decrease and whole eradication of cancer 30 days after treatment, respectively. The treated cancer is represented by an enlarged image in boxes. (c) The complete eradication of cancer represented in tumor or cancer growth curve and no relapse up to 200 days in case of Co–Fe NCs + hyperthermia (in vivo). (d) A Kaplan–Meier survival graph indicating Co–Fe NCs + hyperthermia enhanced the survival rate up to 200 days after therapy, while in other groups for only one month. (e and f) TEM images proved that cancer cells have a chain shape even at one month after therapy for both Co–Fe NCs alone (e) and Co–Fe NCs + hyperthermia (f). (g and h) Light microscopy images of cancer slices of Co–Fe NCs alone (g) and Co–Fe NCs + hyperthermia (h) display the incidence of NPs and absence of stroma. Blue color representsNPs due to Prussian blue staining; dark-pink stained the collagen and light-pink stained the cells in the case of Fast Red staining. Scale bars in (g) and (h) are 0.5 cm. Adapted from ref. [146], with permission from John Wiley and Sons.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_026.jpg)
The efficacy of Co–Fe NCs during in vivo HT examination. (a) Diagram of plan therapy. (b) Images of a control animal (at 0 and 15 days) and Co–Fe NCs injected alone, and Co–Fe NCs injected + hyperthermia display the decrease and whole eradication of cancer 30 days after treatment, respectively. The treated cancer is represented by an enlarged image in boxes. (c) The complete eradication of cancer represented in tumor or cancer growth curve and no relapse up to 200 days in case of Co–Fe NCs + hyperthermia (in vivo). (d) A Kaplan–Meier survival graph indicating Co–Fe NCs + hyperthermia enhanced the survival rate up to 200 days after therapy, while in other groups for only one month. (e and f) TEM images proved that cancer cells have a chain shape even at one month after therapy for both Co–Fe NCs alone (e) and Co–Fe NCs + hyperthermia (f). (g and h) Light microscopy images of cancer slices of Co–Fe NCs alone (g) and Co–Fe NCs + hyperthermia (h) display the incidence of NPs and absence of stroma. Blue color representsNPs due to Prussian blue staining; dark-pink stained the collagen and light-pink stained the cells in the case of Fast Red staining. Scale bars in (g) and (h) are 0.5 cm. Adapted from ref. [146], with permission from John Wiley and Sons.
It is known that conservative drug delivery suffered from unstable metabolic tissue distribution and was nontargeted to cancer cells, which leads to a decrease in cancer therapy efficiency and increases whole-body toxicity [154]. On the other hand, cytotoxic drugs were efficiently delivered by encapsulating within polymer-SFNPs composite [155,156]. Ponce et al. [157] revealed that antibody-based drugs are bound to specific targets in cancer tissues. However, once the molecular targeted drugs were administered, their target did not change; while the magnetic drug delivery system was guided by a magnet by magnetic field exposure [157,158]. Namiki et al. [159] reported that cytotoxic anticancer drugs were combined with SPM particles [159,160] by micelle emulsification or ionic bond [161]. Gupta and Gupta [162] found that a targeted magnetic drug delivery system was widely used in tissue repair, enhancing contrast for magnetic resonance (MR) imaging, hyperthermic therapy, and cytotoxic anticancer therapy [116].
Especially in hyperthermia therapy, Fe3O4 and γ-Fe2O3 were preferred to Fe3O4 or γ-Fe2O3 NPs due to slower magnetic moment relaxation of CoFe2O4 than that with similar size [163,164]. The hyperthermic efficiency of CoFe2O4 NPs was improved by adding a trace amount of Zn2+ (5–15%) relative to cobalt (Co), while further addition of Zn2+, more than 15% relative to Co, resulted in a reverse effect [165]. Likewise, the anticancer activity of CoFe2O4 NPs against human colon cancer has been enhanced by trace Ce–Nd addition [166].
Besides, the cations of rare earth elements (REs) doped CoFe2O4 were found to be suitable for tumor hyperthermia therapy, specifically NPs of Co0.9Gd0.1Fe2O4 due to their high ability to lose energy [71]. Almessiere et al. [166] reported the anticancer properties of Nd3+ and Ce3+-substituted cubic CoFe2O4 against human colon cancer cells (HCT-116) via MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. After 48 h of treatment with Nd3+ and Ce3+, the substituted CoFe2O4 samples displayed apoptosis of HCT-116 cells. These results showed that Ce–Nd-substituted nano Co-ferrite had anticancer efficiency against colon cancer.
Zhang et al. [167] used leucine-coated cobalt ferrite (CoFe2O4) NPs in drug delivery with a DOX loading capacity of 0.32 mg/mg; the loaded DOX displayed a progressive and sustained release. The MTT assay was used to determine the cytotoxicity on HeLa cells, and the results showed that the Leu-coated CoFe2O4 NPs demonstrated comparatively minimum cytotoxicity compared with that of CoFe2O4 NPs, and the DOX-loaded Leu-coated CoFe2O4 NPs showed an observable cell death for HeLa cells. Iqbal et al. [168] have reported that cobalt ferrite doped with zinc (Zn0.5Co0.5Fe2O4) NPs produced by a novel, simple sol–gel method, resulted in good magnetic amorphous NPs. Neutral red assay (NRA) was used to test the anticancer activity on HepG2 cell lines. The results revealed that 250 μg/mL Zn0.5Co0.5Fe2O4 incubated with HepG2 cell lines at 80 J/cm2 laser fluence resulted in the cell viability loss of about 60−65%. The incubation time increased with increasing the absorbance for Zn0.5Co0.5Fe2O4 NPs (250 μg/mL), which was examined at incubation times 3, 24, and 49 h; the absorbance reached 0.40, 0.51, and 0.65 a.u., respectively. Different studies reported that ferrites have an anticancer cytotoxic effect against malignant cell models in the absence of light/laser [169,170]. Also, they revealed that the cell viability loss significantly differed pre and post laser irradiation, even after post cells exposure to 400 μg of Zn0.5Co0.5Fe2O4 NPs. The cell inhibition was significantly higher (44%) at a laser fluence of 80 J/cm2 than in dark cell inhibition (18%).
McBain et al. [171] reported that CoFe2O4 NPs were promising as drug delivery nanocarriers. Cai et al. [172] reported three potential properties: high effective drug loading; surface area; and varied multifunctional nanocomposites due to feasibility and magnetically targeted controlled through an external magnetic field. The DOX delivery and release were controlled by magnetically driven dandelion pollen-like CoFe2O4 nanostructures. They also revealed a loading capacity of 88.6% of the nanostructure, a loading content of 118 mg of DOX/g of CoFe2O4 microspheres, and drug release of ∼55% under an AMF field at 8 h [173].
Liu et al. [174] reported magnetic affinity and designed mesoporous silica nanotube worm-like with settled CoFe2O4 NPs over the surface that triggered the nanotubes magnetically in biological fluids. They also studied the release-uptake process by using 6-carboxyfluorescein as a drug delivery model in vitro using HeLa cells. The cargo was released within 1 h into HeLa cells, after AMF (0.5 mT, 100 Hz) of the nanotubes.
Iatridi et al. [175] prepared a mixture of MnFe2O4 and CoFe2O4 coated with poly(sodium methacrylate)-co-(dodecyl-methacrylate-co-2,4-diphenyl)-6-(4-vinyl-phenyl quinoline)-g-poly(N,N-dimethylacrylamide-co-N-isopropylacrylamide)(P(MANa-co-DMA-co-SDPQ))-g-(P-(DMAM-co-NIPAM)). It was applied in hyperthermia cancer therapy. Also, Iatridi’s group evaluated the hyperthermic activity of colloidal NPs by exposure to a field of kHz and 250 Oe, which was heated to 44°C within 10 min. It was found that NPs were suitable for luminescence and MR imaging and pH and temperature-controlled drug release along with hyperthermia cancer therapy.
Yang et al. [176] revealed that the CoFe2O4 core and polydopamine and zeolitic imidazolate framework shell (CoFe2O4@PDA@ZIF-8) were used to encapsulate CPT in the ZIF-8 shell and DOX in the CoFe2O4 core where the photothermal NP activity was stimulated by both ZIF-8 and polydopamine. Also, they found that the loading capacities of NPs were 98 and 46% for DOX and CPT, respectively, which were released at pH 5 during 7 h by 75 and 45%, respectively, post 5 min laser irradiation. It was shown that the nanocarrier was heated to 65°C after 10 min exposure to 808 nm laser. Therefore, CoFe2O4 NPs have shown potential results in hyperthermia, diagnosis, and cancer therapy [177].
Magnetite Fe3O4 is one of the oldest and yet one of the most commonly used SFNPs with a unique inverse spinel structure [178]. Fe3O4 NPs were used as therapeutic hyperthermia cancer agents and magnetic-guided drug delivery [178,179] agents. Fe3O4 NPs reached the highest and the lowest hyperthermia by doped Mn2+ and Zn2+, respectively [180,181]. Two types of anticancer drugs, curcumin (Cur) loaded and synthesized SF nanocomposites (SFNCs), were synthesized by using Fe3O4 NPs as the core; one of them (Fe3O4/OCMCS/Cur) was Cur, burdened, and encapsulated with O-carboxy methylchitosan (OCMCS), and another one, (Fe3O4/OCMCS/Cur/Fol) was folate attached to Fe3O4/OCMCS/Cur [182].
The bio-distribution and the effectiveness of SFNCs were investigated by injecting at a dose of 20 μg/kg SFNCs into mice bearing solid tumor Sarcoma-180. The results have shown that Fe3O4/OCMCS/Cur/Fol was distributed uniformly after 5 h than Fe3O4/OCMCS/Cur. It was proved that SFNC was a good curcumin loader in, as well as, cancer-targeted drug delivery; SNFC also showed a synergic action of hyperthermia and chemotherapeutic [183]. It was revealed that CS-based magnetic hybrid NCs with folate-conjugated tetrapeptide composite (CS, CdTe quantum dots QDs (a fluorescent dye), and SPM Fe3O4, camptothecin CPT cytotoxic drug, folate and tetrapeptides) [184].
Manganese ferrite (MnFe2O4) is used for drug delivery and as an enhancer for hyperthermia to increase anticancer efficiency [185]. Five SFNPs were synthesized with a combination of core–shell components or exchanging the order of coating for power loss as follows CoFe2O4@Fe3O4 < CoFe2O4@MnFe2O4 < Fe3O4@CoFe2O4 < MnFe2O4@CoFe2O4 < (Zn0.4Co0.6) Fe2O4@Zn0.4Mn0.6Fe2O4. Lee et al. [185] revealed that the heat efficacy of hyperthermia cancer therapy was enhanced by coupling exchange between a soft shell and a magnetically hard core.
The supermagnetic DOX poly(lactic-co-glycolic acid) (PLGA)@CS-stabilized (CS)@Mn0.9Zn0.1Fe2O4 NPs was synthesized with low M s = 13.2 emu/g as compared to Mn0.9Zn0.1Fe2O4NPs (M s = 56.1) that led to efficient magnetic response during the drug release, which was adjusted by the pH of cancer cells. The DOX-PLGA@CS@Mn0.9Zn0.1Fe2O4NPs revealed drug loading, delivery, and hyperthermic cancer therapeutic effect [186].
Wang et al. [187] synthesized MnFe2O4@GO NC via the sonochemical method within high DOX loading capacity due to π–π interactions and hydrogen bonding between DOX and GO. Hence, the MnFe2O4@GO delivered targeted drug delivery for DOX to cancer cells, and DOX was released due to the acidic tumor microenvironment. Slimani et al. [188] found that HCT-116 growth was significantly inhibited by lanthanide/dysprosium (Ce3+/Dy3+) co-activated co-doped manganese-zinc nanospinel ferrites (CDMZNSFs) (with IC50 values ranging from 0.74 to 2.35 mg/mL for 48 h), whereas the healthy HEK-293 cell growth was not damaged.
Hekmat and Saboury [189] revealed that the antiproliferative and anticancer effects of Co0.3Mn0.2Zn0.5Fe2O4 NPs were determined by MTT assay after the T47D cell line was treated with 6–300 nM Co0.3Mn0.2Zn0.5Fe2O4 NPs 48 h. Also, Hekmat and Saboury found that the IC50 of Co0.3Mn0.2Zn0.5Fe2O4 NPs on the T47D cell line was equal to 70 nM. It was found that Co0.3Mn0.2Zn0.5Fe2O4 NPs were a potential anticancer–antiproliferative agent. Further, Hekmat and Saboury studied the morphological of cancer cells by treating T47D (1 × 105 cells per well) with 70 nM Co0.3Mn0.2Zn0.5Fe2O4 NPs and 1 mg/mL DAPI solution. It is known that DPAI binds to DNA resulting in increased fluorescence by 20-fold based on the displacement of H2O from the minor groove of DNA and DAPI. Furthermore, Hekmat and Saboury showed that the living cells showed regular blue, while the dead cells that were treated with Co0.3Mn0.2Zn0.5Fe2O4 NPs (70 nM) were bright blue doted nuclei, which indicated DNA fragmentation of nuclei and confirmed the previous MTT results [190].
Hekmat et al. found out that flow cytometry was a sensitive and fast apoptotic assay, and could easily differentiate between apoptotic and necrotic cells [98]. Hekmat and Saboury [189] studied the apoptosis after exposure of T47D (1 × 106 cells/well) at 70 nM Co0.3Mn0.2Zn0.5Fe2O4 NPs (24 h) by Annexin V and then evaluated by flow cytometry. It was revealed that the NPs reduced the viable cells and increased the apoptotic cells.
Nickel ferrite NiFe2O4 NPs were found to be a potent differentiated hyperthermia agent for cancer therapy [191]. The new nanoformulation β-cyclodextrin-dextran layered on NF NPs (Ni1.04Fe1.96O4) was synthesized in about 6 nm. It was found that camptothecin CPT was loaded by 88% in the polymer-coated NPs. Moreover, the nanocarrier sustained the CPT release profile; on the other hand, a decrease in pH by 1.4 leads to faster release of CPT. The CPT-loaded β-cyclodextrin-dextran coated on magnetic nickel ferrite nanocomposite displays cell growth inhibition of HeLa human cervical, breast MDA-MB-231, and lung A549 cancer cell lines with cytotoxicity IC50 values of 1.49, 1.35, and 1.41 µg/mL, respectively. It was found that the anticancer efficacy of CPT-loaded CD-Dx MNP nanoformulation was greater than 5-fluorouracil and cisplatin against different cancer cell lines [191].
Alahmari et al. [192] synthesized Ni0.5Co0.5−x Cd x Fe1.78Nd0.02O4 (x ≤ 0.25) nanofibers (NFs). They evaluated the cytotoxic anticancer effect of NFs by MTT assay on HEK-293 and HCT-116 cells [193,194]. The cells were treated with a range of dosages from 2 to 25 μg/mL for 48 h. They found that NFs profound a significant cell growth inhibition in HCT-116 cancer cells but not in HEK-293. Finally, they concluded that NFs were highly specific and targeted to colon cancer cells.
Tombuloglu et al. [195] revealed that synthesized nanocomposites showed promising results for drug delivery and cancer therapy. They synthesized Co0.5Ni0.5Nb x Fe2−x O4 by the hydrothermal combination of Co, Ni, Nb, and Fe2O4. They examined the effect of the prepared nanocomposite by incubating different concentrations (0.3, 1.5, 3.8, 6.1, and 12.2 mg/mL) at 48 h with HCT-116 and HEK-293 using MTT assay and stained with DAPI (microscopic). The prepared nanocomposites led to a dose-dependent decrease in the proliferation and viability of cancer cells. The 0.3 mg/mL dose of the nanocomposite resulted in 98.2, 95.2, 93.2, 92.2, 91.2, and 90.2% cell viability versus Nb(x) of 0.00, 0.02, 0.04, 0.06, 0.08, and 0.1, respectively; while 1.52 mg/mL MNP led to decreased 68.0, 59.9, 59.1, 56.9, 57.6, and 56.5% cell viability. The 3.85 mg/mL dose of the nanocomposite repressed cell viability to 66.2, 59.1 58.5, 55.9, 57.9, and 55.9%, respectively. Increasing the nanocomposites concentration to 6.1 and 12.2 mg/mL led to decrease in cell viability (65.3, 58.1, 57.7, 54.7, 55.7, and 54.7%;and 64.3, 57.9, 57.0, 54.0, 54.0, and 54.0%), respectively. It was found that the use of Co0.5Ni0.5Nb x Fe2−x O4 did not cause cytotoxicity on HEK-293 cells [195].
Zinc ferrite (ZnFe2O4) has been used as an essential radiosensitizer in human prostate tumor radiotherapy (RT) [164]. It was reported that 100 μg/mL ZnFe2O4 enhanced the γ-irradiation efficiency 17 times greater than only γ-irradiation via 53% cancer cell growth inhibition. Gamma irradiation induces a dose-dependent alteration of structural integrity [196,197,198,199,200]. Also, gamma irradiation offers advantages in economy and convenience over autoclaving and has been used in sterilization and preparation of different materials [201,202,203,204,205]. The SPM NPs synthesized should possess high Ms and small particle size to be suitable and safe for cancer patients during hyperthermia [101,102,108] and RT [33]. The enhancement of hyperthermia cancer therapy by manganese-doped zinc SFNP (Zn0.4Mn0.6) Fe2O4 was due to their high values of M s (175 emu/g) and specific loss power [206]. Almessiere et al. [129] synthesized DyY-MnZn NSFs by ultrasonic irradiation and citrate sol–gel combustion methods; a chain of Mn0.5Zn0.5Fe2−2x (Dy x Y x )O4 (0.0 ≤ x ≤ 0.05) was formed. They found out that the pure phase of SF was produced by ultrasonic irradiation, while a mixture of hematite and SFs was produced by the citrate sol–gel method. It was also shown that both the mixture or pure DyY-MnZn NSFs inhibited cell growth of the colon cancer cell line (HCT-116), but did not inhibit the healthy cell line growth (HEK-293). The ultrasonicated DyY-MnZn NSFs were better than the sol–gel DyY-MnZn NSFs in inhibition of HCT-116 cell growth.
Al-Qubaisi et al. [207] studied the cytotoxicity of NiZn ferrite NPs against HT29 (human colon cancer), MCF7 (Michigan Cancer Foundation7; breast cancer), and HepG2 (liver cancer) cells by MTT assay. The NiZn ferrite NPs were used in different concentrations (15.6–1,000 μg/mL; 72 h) and the cancer cell growth inhibition showed a dose-dependent behavior. The DNA fragmentation and caspase-3 and -9 activities were assayed to confirm the apoptotic effect of NiZn ferrite NPs on different cancer cell lines. The cytotoxic effects of HT29 or MCF7 cells were less sensitive than that of HepG2 to NiZn ferrite NPs (72 h). Also, Al-Qubaisi et al. reported considerable morphological variations in MCF7, HT29 and HepG2 cells after incubation with NiZn ferrite NPs (72 h). More apparent morphological changes (membrane blebbing, cytoplasmic shrinking, cells dispersion, and cell shrinking) were found in HepG2 than the other cell lines. Besides, Al-Qubaisi et al. revealed that NiZn ferrite NPs selectively killed HepG2 cells via induction of apoptosis and suppression of proliferation. The apoptotic effect of NiZn ferrite NPs in cancer cells was due to to the significant activation of caspase-3 and caspase-9, which was responsible for the induction of DNA fragmentation in HT29, MCF7, and HepG2 cell lines.
Lartigue et al. [208] presented that cells treated with NiZn ferrite NPs induced apoptosis and were associated with morphological changes. The magnetic NiZn ferrite NPs were speedily dispersed in epithelial tissues that were firmly bound to albumin. Mitamura et al. [209] revealed that the potential candidate in apoptosis was the activated endogenous nuclease enzymes, which led to DNA fragmentation and was mediated by caspase-3 as a nuclease activator. Al-Qubaisi et al. [207] found that NiZn ferrite NPs induced apoptosis in cancer cells with the highest effective dose of ∼100 μg/mL in HepG2 cells and 1,000 μg/mL for HT29 and MCF7 cells after 12 h. Ahamed et al. [210] reported that nickel ferrite NPs induced apoptosis in the human alveolar adenocarcinoma A549 cell line at 100 μg/mL. Hathaway et al. [211] found that the magnetic NPs had two essential characteristics as an effective anticancer drug, cancer specificity, and minimum cytotoxicity to normal cells. In conclusion, Ni–Zn ferrite had potential apoptotic anticancer activity against cancer cells.
Sarala et al. [212] revealed that ZnFe2O4 NPs were synthesized with Lawsonia inermis leaf extract as a surfactant by the green method. The MTT method was used to detect mitochondria’s cell activity and that reflected the viable cells [213]. The ferrite NPs induced cytotoxicity by the generation of ROS. ROS such as superoxide anions, hydroxyl radicals, hydrogen peroxide, and alkoxy radicals denatured biological macromolecules (DNA, carbohydrates, proteins, lipids, etc.). MTT assay was used to determine the anticancer effect of ZnFe2O4 NPs; MCF-7 cells were incubated with 25, 50, 100, 250, and 300 μg per well ZnFe2O4 NPs for 24 h. The cell viability decreased by 60, 53, 44, 32, and 24%, respectively.
The cytotoxicity mechanism of ZnFe2O4 NPs based on ROS generation due to intracellular Zn2+ resulted in cellular redox machinery failure [214]. ROS enhanced mitochondrial respiration and apoptosis in mitochondria, cellular redox disequilibrium, and lipid peroxidation in the cell membranes [215]. The direct interaction of ZnFe2O4 NPs with cell walls led to the damage and denaturation of the membrane. ROS generated on the surface of the ZnFe2O4 NPs and particle disbanding and free Zn2+ ions release resulted in the ROS production within the cells [212].
Magnesium ferrite MgFe2O4 NPs were selected due to their easy breakdown in the human body and their application in biomedical fields, such as biocompatibility, magnetic hyperthermia therapy, as a contrasting agent in MRI, and drug delivery [216,217,218]. Selvam et al. [219] revealed the cytotoxicity on the healthy human embryonic kidney cell line (HEK293) for poly(vinyl alcohol) cross-linked β-cyclodextrin polymer coating of magnesium ferrite NPs (PVA-CD-MNPs) and camptothecin-loaded PVA-CD-MNP nanocomposite (PVA-CDMNPs@ CPT), where IC50 values were found to be 18.96 and 5.104 μg/mL, respectively. The anticancer efficacy was studied on colorectal cancer HCT-15 cell lines, and the IC50 values were 28.89 and 66.17 μg/mL for PVA-CD-MNPs@CPT and the PVA-CD-MNPs, respectively. The HEK 293 cells are more sensitive to PVA-CDMNPs@ CPT. Together with the host polymer, the magnetic NPs combined with an excellent antineoplastic nanocomposite when loaded with the topoisomerase inhibitor (camptothecin), a promising drug delivery vehicle for cancer therapy. The nanocomposite vehicle accommodated any drug of suitable size that fits into the cyclodextrin cavity. It does not rely upon the open-on covalent linking of the drug to the polymer through complex methods of synthesis and the requirement to cleave the drug-polymer bond at the target site for drug release. Therefore, the nanoformulation vehicle can be loaded and released by any anticancer drug.
RT was considered to be one of the effective cancer therapy methods [220]. Furthermore, the efficacy of the RT therapeutic effect can be enhanced by NPs [216]. The anticancer therapeutic effect of magnesium ferrite NPs MgFe2O4 on the human breast cancer cell line MCF-7 [216,217,218] was investigated. Meidanchi and Motamed [221] revealed that MgFe2O4 was successfully synthesized in a single-step reaction hydrothermally with an average particle size of 10.5 nm and sample crystallite size of 9 nm. The SPM MgFe2O4 behavior was revealed at room temperature. Additionally, the MgFe2O4 as X-ray irradiation radiosensitizers was applied in MCF-7 RT. It was found that (a) the concentration of MgFe2O4 up to 10 μg/mL had no significant cytotoxicity on MCF-7 and cytotoxicity was evident only at 100 μg/mL; and (b) significant cell damage was found in the presence of 10 μg/mL MgFe2O4 concentration under 2 and 4 Gy X-ray irradiation. Therefore, no significant cytotoxicity and significant cell damage were observed at 10 μg/mL MgFe2O4, indicating that MgFe2O4 could be used as a radiosensitizer for cancer cell therapy in comparison with RT alone. MgFe2O4 was considered as a radiosensitizer due to the production of Auger low-energy electrons due to its photoelectric effect under X-ray irradiation.
Copper ferrite CuFe2O4 NPs and their composites are also used in cancer therapy. Jermy et al. [3] investigated the anticancer cytotoxic effect of CS enclosed CuFe2O4/HYPS. Cell inhibition was detected by MTT assay. They studied CS over spherical hydrophilic silica (HYPS) NPs at two concentrations (0.06 and 0.6) wt% and varied pH values (5, 6, and 6.5). Also, they used the impregnation method for the synthesis of four nanocomposites of SF/silica. It was found out that their magnetization order was CoFe2O4/HYPS (14.15 emu/g) > NiFe2O4/HYPS (7.73 emu/g) ∼ CuFe2O4/HYPS (7.65 emu/g) > MnFe2O4/HYPS (1.49 emu/g).
The SPM effect was observed in MnFe2O4/HYPS, whereas the ferromagnetic effect was observed in CoFe2O4/HYPS. Both Cu Fe2O4/HYPS and Ni Fe2O4/HYPS presented similar paramagnetic effects. Jermy et al. revealed a physicochemical method (method I) for 6–12 nm CuFe2O4, CS, and cis-Pt over HYPS. It was verified that CS was essential for the restricted release of cisplatin (cis-Pt) over CuFe2O4/HYPS by pH adjustment of CS (method I). In the same context, they proved that nanoformulation of cis-Pt/CuFe2O4/HYPS/CS (method I) had an insignificant cell viability effect. However, the CS/CuFe2O4/HYPS/cis-Pt nanocomposite synthesized via the impregnation method (method II) by CS prewrapping and preloading cis-pt(0.15 mmol/g cis-pt). Also, they showed that 64% cis-pt was released during 72 h from the nanocomposite and had a significant cytotoxic effect. Jermy et al. studied the cell viability of CS/CuFe2O4/HYPS/cis-Pt on the healthy human embryonic kidney (HEK293) and MCF-7 cells. Besides, they found that CuFe2O4/HYPS was noncytotoxic, and cis-Pt, cis-Pt/CuFe2O4/HYPS, and CS/CuFe2O4/HYPS/cis-Pt significantly induced cell inhibition in both HEK293 and MCF7 dose-dependently. The MCF7 cell viability at the lowest concentration was as follows: cis-Pt, 58.17%; cis-Pt/CuFe2O4/HYPS, 63.36%; and CS/CuFe2O4/HYPS/cis-Pt, 70.73%. However HEK293 cell viability was 73.47% for cis-Pt, 80.24% for cis-Pt/CuFe2O4/HYPS, and 95.07% for CS/CuFe2O4/HYPS/cis-Pt. Finally, Jermy et al. showed that CS/CuFe2O4/HYPS/cis-Pt method II was the best nanoformulation due to the biocompatibility advantage of CS, avoiding the premature cis-pt release during the pH adsorption of CS (Figure 27) and effectively targeted tumor cells with a similar pure cis-pt efficiency; thus making it a novel magnetically targeted drug delivery system.
![Figure 27
The CS/CuFe2O4/HYPS/cis-Pt nanocomposite optimized route. Adapted from ref. [3] with permission from Elsevier™.](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_027.jpg)
The CS/CuFe2O4/HYPS/cis-Pt nanocomposite optimized route. Adapted from ref. [3] with permission from Elsevier™.
Meidanchi [222] used the hydrothermal process in the synthesis of Mg(1−x)Cu x Fe2O4 SPMNPs (0.2 ≤ x ≤ 0.8). Meidanchi studied the anticancer effect (MTT assay) of Mg1−x Cu x Fe2O4 SPMNPs at various concentrations (0.1, 1, 10, and 100 μg/mL) on the MCF-7 cell line before and after 2 Gy X-ray RT. Also, Meidanchi showed that X-ray irradiation at a high concentration of Cu and Mg(1−x)Cu x Fe2O4 SPMNPs resulted in significant inhibition of the growth of MCF-7 cells. However, nonsignificant cell growth inhibition of Mg(1−x)Cu x Fe2O4 SPMNPs was observed at lower concentrations (0.1–10 μg/mL). Further, Meidanchi concluded that Mg(1−x)Cu x Fe2O4 SPMNPs (x = 0.2 (10 μg/mL) and x = 0.6 (1 μg/mL)) was considered as a nano-radiosensitizer due to synergistic therapeutic effect without cytotoxicity on the MCF-7 cells.
6 Challenges and perspectives
Diverse properties of SFNPs, such as the shape, size, charge of the surface, and surface adjustment, should be identified when composites-incorporated SFNPs were formed to reduce the elimination of SFNPs from circulation and maximize their influence on the targeted tissues. The size of the composites-incorporatedSFNPs is significant for controlling the rate of their internalization through target cells and magnetic properties. The suitable size range was reported to be in the range of 10-100 nm to allow them to pass through tiny capillaries and restrict SFNPs’ elimination from circulation. The decrease in the size is of importance due to the tendency to aggregate. The second significant factor was the surface charge or zeta potential of SFNPs incorporated with composites. The best zeta potential was found between 10 and 30 mV or between −10 and −30 mV to obtain a stable suspension of SFNPs with minimum aggregation. The surface charge of the SFNPs was influenced by surface modification. The incorporation of composites on the SFNP surface led to a decrease in their magnetization, resulting in weak magnetic targeting. So, thickness and type of material incorporated significantly impact variations in the magnetic characteristics of SFNPs. Consequently, the beneficial characteristics of magnetic composites for drug delivery would be traded off with the increase in the particle size and the decreased magnetic properties. In contrast to hyperthermic applications where heat damage was initiated via exposing diseased tissues to high temperatures for a period of time, controlled heating (4–10°C) in short periods of time is in demand in drug delivery purposes via the heat-responsive polymer-incorporated SFNPs. This is a challenging problem because if the polymer is subjected to any excessive heating, its structure can be degraded, and hence, its reversible phase transition may be destroyed. Overall, complex synthesis methods are usually needed for the successful configuration and purpose of nanomaterials [82,83,205,223,224,225], and remarkable unclear aspects concerning their magnetic response are still lingering.
7 Bibliometric mapping analysis
Search methodology for bibliometric mapping: (anticancer SF OR drug delivery SF or cancer therapy SF) OR TOPIC: (anticancer Manganese ferrite) OR TOPIC: (anticancer Nickel ferrite) OR TOPIC: (anticancer Zinc ferrite) OR TOPIC: (anticancer Magnesium ferrite) OR TOPIC: (anticancer Copper ferrite) OR TOPIC: (anticancer Lithium ferrite) OR TOPIC: (“anticancer NPs”) OR TOPIC: (drug delivery Manganese ferrite) OR TOPIC: (drug delivery Nickel ferrite) OR TOPIC: (drug delivery Zinc ferrite) AND TOPIC: (drug delivery Magnesium ferrite) AND TOPIC: (drug delivery Copper ferrite) AND TOPIC: (drug delivery Lithium ferrite) OR TOPIC: (hyperthermia SF)
Timespan: All years. Indexes: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI.
The bibliometric mapping analysis is shown in with the network visualization and density visualization, respectively. The bibliometric mapping analysis showed 402 results using the search methodology mentioned above from the Web of Science (WOS). There is a strong relationship in the literature between hyperthermia and iron oxide NPs and different types of SF NPs, as shown in Figure 28. Many investigation studies in the literature on parameters are linked to the hyperthermia phenomenon such as the temperature, particle size, cation distribution, and facile synthesis methods. The hyperthermia phenomenon also showed large co-occurrence with anticancer NPs and drug delivery for magnetic materials and SF. On the other hand, Figure 28 shows a significant gap in the literature regarding in vivo, photothermal therapy, heating efficiency, toxicity, theranostics, and controlled release. Figure 29 shows prominent keywords in this field as shown from the density visualization map such as magnetic properties, hyperthermia, NP size, and drug delivery.
The bibliometric network mapping of SF NPs as anticancer and drug delivery research field in all years in WOS.
The bibliometric density visualization mapping of SF NPs as anticancer and drug delivery research field in all years in WOS.
8 Conclusion
One of the essential applications of SF NP SFNPs is cancer therapy, hyperthermia, drug targeted delivery, and release. In this review, the synthesis methods used to prepare SFNPs are presented in detail. Also, the structural and magnetic properties of SFNPs are discussed. Further, the relation between the surface morphology and different properties of SFNPs is revealed. The unique physicochemical characteristics of SF include low toxicity and high biocompatibility for use in various biomedical applications, including drug delivery and magnetic hyperthermia treatment. The frequency and amplitude of the applied external magnetic field, and NP sizes are the main factors for safe hyperthermia cancer therapy. The anticancer efficiency of SFNPs depends upon their synthesis and cytotoxicity methodology, which was evaluated by MTT assay on normal cell lines and different cancer cell lines. The results showed acceptable cell viability on normal cell lines and cytotoxicity and cancer cell growth inhibition. Generally, the surface of SFNPs coated with nontoxic chemicals enhanced cell viability and biocompatibility with normal cells. The hyperthermia effect of SFNPs is considered a targeted differential therapy due to cancer cells being more sensitive to the hyperthermia effect than normal cells. Cancer cells were damaged by the heat generated based on the external field amplitude square, frequency, size, and type of SFNPs. It is known that the conservative drug delivery suffered from unstable metabolic tissue distribution and was nontargeted to cancer cells, which leads to a decrease in cancer therapy efficiency and increased whole-body toxicity. On the other hand, cytotoxic drugs were efficiently delivered by encapsulating within polymer-SFNPs composite.
-
Funding information: The authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Feng Q, Tong R. Anticancer nanoparticulate polymer-drug conjugate. Bioeng Transl Med. 2016;1(3):277–96.10.1002/btm2.10033Search in Google Scholar PubMed PubMed Central
[2] Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer J Clinicians. 2018;68(6):394–424.10.3322/caac.21492Search in Google Scholar PubMed
[3] Jermy R, Ravinayagam V, Alamoudi W, Almohazey D, Elanthikkal S, Dafalla H, et al. Tuning pH sensitive chitosan and cisplatin over spinel ferrite/silica nanocomposite for anticancer activity in MCF-7 cell line. J Drug Delivery Sci Technol. 2020;57:101711.10.1016/j.jddst.2020.101711Search in Google Scholar
[4] Pon-On W, Tithito T, Maneeprakorn W, Phenrat T, Tang IM. Investigation of magnetic silica with thermoresponsive chitosan coating for drug controlled release and magnetic hyperthermia application. Mater Sci Eng C. 2019;97:23–30.10.1016/j.msec.2018.11.076Search in Google Scholar PubMed
[5] Angelova A, Garamus VM, Angelov B, Tian Z, Li Y, Zou A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv Colloid Interface Sci. 2017;249:331–45.10.1016/j.cis.2017.04.006Search in Google Scholar PubMed
[6] Singh TA, Das J, Sil PC. Zinc oxide nanoparticles: a comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv Colloid Interface Sci. 2020;286:102317.10.1016/j.cis.2020.102317Search in Google Scholar PubMed
[7] Mohapatra S, Rout SR, Maiti S, Maiti TK, Panda AB. Monodisperse mesoporous cobalt ferrite nanoparticles: synthesis and application in targeted delivery of antitumor drugs. J Mater Chem. 2011;21(25):9185–93.10.1039/c1jm10732aSearch in Google Scholar
[8] Algarou NA, Slimani Y, Almessiere MA, Rehman S, Younas M, Unal B, et al. Developing the magnetic, dielectric and anticandidal characteristics of SrFe12O19/(Mg0.5Cd0.5Dy0.03Fe1.97O4)x hard/soft ferrite nanocomposites. J Taiwan Inst Chem Eng. 2020;113:344–62.10.1016/j.jtice.2020.07.022Search in Google Scholar
[9] Amiri M, Salavati-Niasari M, Akbari A. Magnetic nanocarriers: evolution of spinel ferrites for medical applications. Adv Colloid Interface Sci. 2019;265:29–44.10.1016/j.cis.2019.01.003Search in Google Scholar PubMed
[10] Wu W, Wu Z, Yu T, Jiang C, Kim W-S. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater. 2015;16(2):23501.10.1088/1468-6996/16/2/023501Search in Google Scholar PubMed PubMed Central
[11] Obaidat IM, Issa B, Haik Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials. 2015;5(1):63–89.10.3390/nano5010063Search in Google Scholar PubMed PubMed Central
[12] Meidanchi A, Akhavan O, Khoei S, Shokri AA, Hajikarimi Z, Khansari N. ZnFe2O4 nanoparticles as radiosensitizers in radiotherapy of human prostate cancer cells. Mater Sci Eng C. 2015;46:394–9.10.1016/j.msec.2014.10.062Search in Google Scholar PubMed
[13] Spirou SV, Basini M, Lascialfari A, Sangregorio C, Innocenti C. Magnetic hyperthermia and radiation therapy: radiobiological principles and current practice. Nanomaterials (Basel, Switzerland). 2018;8(6):401.10.3390/nano8060401Search in Google Scholar PubMed PubMed Central
[14] Sohn C-H, Park SP, Choi SH, Park S-H, Kim S, Xu L, et al. MRI molecular imaging using GLUT1 antibody-Fe3O4 nanoparticles in the hemangioma animal model for differentiating infantile hemangioma from vascular malformation. Nanomed Nanotechnol Biol Med. 2015;11(1):127–35.10.1016/j.nano.2014.08.003Search in Google Scholar PubMed
[15] Yang M, Gao L, Liu K, Luo C, Wang Y, Yu L, et al. Characterization of Fe3O4/SiO2/Gd2O(CO3)2 core/shell/shell nanoparticles as T1 and T2 dual mode MRI contrast agent. Talanta. 2015;131:661–5.10.1016/j.talanta.2014.08.042Search in Google Scholar PubMed
[16] Lee H, Shin T-H, Cheon J, Weissleder R. Recent developments in magnetic diagnostic systems. Chem Rev. 2015;115(19):10690–724.10.1021/cr500698dSearch in Google Scholar PubMed PubMed Central
[17] Plouffe BD, Murthy SK, Lewis LH. Fundamentals and application of magnetic particles in cell isolation and enrichment: a review. Rep Prog Phys Phys Soc (Gt Br). 2015;78(1):16601.10.1088/0034-4885/78/1/016601Search in Google Scholar PubMed PubMed Central
[18] Ling D, Lee N, Hyeon T. Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications. Acc Chem Res. 2015;48(5):1276–85.10.1021/acs.accounts.5b00038Search in Google Scholar PubMed
[19] Issa B, Obaidat IM, Albiss BA, Haik Y. Magnetic nanoparticles: surface effects and properties related to biomedicine applications. Int J Mol Sci. 2013;14(11):21266–305.10.3390/ijms141121266Search in Google Scholar PubMed PubMed Central
[20] Cao W, Wu R, Cao G, He Z. A comprehensive review of computer-aided diagnosis of pulmonary nodules based on computed tomography scans. IEEE Access. 2020;8:154007–23.10.1109/ACCESS.2020.3018666Search in Google Scholar
[21] Ma Y, Liu X. Kinetics and thermodynamics of Mg–Al disorder in MgAl2O4-spinel: a review. Molecules. 2019;24(9):1704.10.3390/molecules24091704Search in Google Scholar
[22] Ashour A, Hemeda O, Heiba Z, Al-Zahrani S. Electrical and thermal behavior of PS/ferrite composite. J Magn Magn Mater. 2014;369:260–7.10.1016/j.jmmm.2014.06.005Search in Google Scholar
[23] Srivastava R, Yadav BC. Ferrite materials: introduction, synthesis techniques, and applications as sensors. Int J Green Nanotechnol. 2012;4(2):141–54.10.1080/19430892.2012.676918Search in Google Scholar
[24] Arshak K, Ajina A, Egan D. Development of screen-printed polymer thick film planner transformer using Mn–Zn ferrite as core material. Microelectron J. 2001;32(2):113–6.10.1016/S0026-2692(00)00122-1Search in Google Scholar
[25] Tsay C, Liu K, Lin T, Lin I. Microwave sintering of NiCuZn ferrites and multilayer chip inductors. J Magn Magn Mater. 2000;209(1–3):189–92.10.1016/S0304-8853(99)00684-8Search in Google Scholar
[26] Nakamura T, Miyamoto T, Yamada Y. Complex permeability spectra of polycrystalline Li–Zn ferrite and application to EM-wave absorber. J Magn Magn Mater. 2003;256(1–3):340–7.10.1016/S0304-8853(02)00698-4Search in Google Scholar
[27] Harasawa T, Suzuki R, Shimizu O, Olcer S, Eleftheriou E. Barium-ferrite particulate media for high-recording-density tape storage systems. IEEE Trans Magn. 2010;46(6):1894–7.10.1109/TMAG.2010.2042286Search in Google Scholar
[28] Abdel Maksoud MIA, Sami NM, Hassan HS, Bekhit M, Ashour AH. Novel adsorbent based on carbon-modified zirconia/spinel ferrite nanostructures: evaluation for the removal of cobalt and europium radionuclides from aqueous solutions. J Colloid Interface Sci. 2021;607:111–124.10.1016/j.jcis.2021.08.166Search in Google Scholar PubMed
[29] Abdel Maksoud MIA, El-Sayyad GS, El-Bastawisy HS, Fathy RM. Antibacterial and antibiofilm activities of silver-decorated zinc ferrite nanoparticles synthesized by a gamma irradiation-coupled sol–gel method against some pathogenic bacteria from medical operating room surfaces. RSC Adv. 2021;11(45):28361–74.10.1039/D1RA04785JSearch in Google Scholar PubMed PubMed Central
[30] Abdel Maksoud MIA, El-Sayyad GS, El-Khawaga AM, Abd Elkodous M, Abokhadra A, Elsayed MA, et al. MANanostructured Mg substituted Mn–Zn ferrites: a magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials. J Hazard Mater. 2020;399:123000.10.1016/j.jhazmat.2020.123000Search in Google Scholar PubMed
[31] Hassan HS, Abdel Maksoud MIA, Attia LA. Assessment of zinc ferrite nanocrystals for removal of 134Cs and 152 + 154Eu radionuclides from nitric acid solution. J Mater Sci: Mater Electron. 2020;31(2):1616–33.10.1007/s10854-019-02678-ySearch in Google Scholar
[32] Verma A, Alam M, Chatterjee R, Goel T, Mendiratta R. Development of a new soft ferrite core for power applications. J Magn Magn Mater. 2006;300(2):500–5.10.1016/j.jmmm.2005.05.040Search in Google Scholar
[33] Kefeni KK, Msagati TAM, Nkambule TTI, Mamba BB. Spinel ferrite nanoparticles and nanocomposites for biomedical applications and their toxicity. Mater Sci Eng C. 2020;107:110314.10.1016/j.msec.2019.110314Search in Google Scholar PubMed
[34] Masthoff IC, Kraken M, Mauch D, Menzel D, Munevar JA, Baggio Saitovitch E, et al. Study of the growth process of magnetic nanoparticles obtained via the non-aqueous sol–gel method. J Mater Sci. 2014;49(14):4705–14.10.1007/s10853-014-8160-0Search in Google Scholar
[35] Ashour AH, El-Batal AI, Maksoud MIAA, El-Sayyad GS, Labib S, Abdeltwab E, et al. Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology. 2018;40:141–51.10.1016/j.partic.2017.12.001Search in Google Scholar
[36] Maksoud MIAA, El-Sayyad GS, Ashour AH, El-Batal AI, Elsayed MA, Gobara M, et al. Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microb Pathogenesis. 2019;127:144–58.10.1016/j.micpath.2018.11.045Search in Google Scholar PubMed
[37] Abdel Maksoud MIA, El-Sayyad GS, Ashour AH, El-Batal AI, Abd-Elmonem MS, Hendawy HAM, et al. Synthesis and characterization of metals-substituted cobalt ferrite [Mx Co(1−x) Fe2O4; (M = Zn, Cu and Mn; x = 0 and 0.5)] nanoparticles as antimicrobial agents and sensors for Anagrelide determination in biological samples. Mater Sci Eng C. 2018;92:644–56.10.1016/j.msec.2018.07.007Search in Google Scholar PubMed
[38] Maksoud MIAA, El-ghandour A, El-Sayyad GS, Awed AS, Fahim RA, Atta MM, et al. Tunable structures of copper substituted cobalt nanoferrites with prospective electrical and magnetic applications. J Mater Sci Mater Electron. 2019;30(5):4908–19.10.1007/s10854-019-00785-4Search in Google Scholar
[39] Abdel Maksoud MIA, El-ghandour A, El-Sayyad GS, Awed AS, Ashour AH, El-Batal AI, et al. Incorporation of Mn2+ into cobalt ferrite via sol–gel method: insights on induced changes in the structural, thermal, dielectric, and magnetic properties. J Sol–Gel Sci Technol. 2019;90(3):631–42.10.1007/s10971-019-04964-xSearch in Google Scholar
[40] Kefeni KK, Msagati TM, Mamba BB. Synthesis and characterization of magnetic nanoparticles and study their removal capacity of metals from acid mine drainage. Chem Eng J. 2015;276:222–31.10.1016/j.cej.2015.04.066Search in Google Scholar
[41] Thakur S, Rai R, Sharma S. Structural characterization and magnetic study of NiFexO4 synthesized by co-precipitation method. Mater Lett. 2015;139:368–72.10.1016/j.matlet.2014.10.014Search in Google Scholar
[42] Zi Z, Sun Y, Zhu X, Yang Z, Dai J, Song W. Synthesis and magnetic properties of CoFe2O4 ferrite nanoparticles. J Magn Magn Mater. 2009;321(9):1251–5.10.1016/j.jmmm.2008.11.004Search in Google Scholar
[43] Xing Y, Jin Y-Y, Si J-C, Peng M-L, Wang X-F, Chen C, et al. Controllable synthesis and characterization of Fe3O4/Au composite nanoparticles. J Magn Magn Mater. 2015;380:150–6.10.1016/j.jmmm.2014.09.060Search in Google Scholar
[44] Nabiyouni G, Julaee M, Ghanbari D, Aliabadi PC, Safaie N. Room temperature synthesis and magnetic property studies of Fe3O4 nanoparticles prepared by a simple precipitation method. J Ind Eng Chem. 2015;21:599–603.10.1016/j.jiec.2014.03.025Search in Google Scholar
[45] Sharifi I, Shokrollahi H, Amiri S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J Magn Magn Mater. 2012;324(6):903–15.10.1016/j.jmmm.2011.10.017Search in Google Scholar
[46] Wang J, White WB, Adair JH. Optical properties of hydrothermally synthesized hematite particulate pigments. J Am Ceram Soc. 2005;88(12):3449–54.10.1111/j.1551-2916.2005.00643.xSearch in Google Scholar
[47] Zhang J, Song J-M, Niu H-L, Mao C-J, Zhang S-Y, Shen Y-H. ZnFe2O4 nanoparticles: Synthesis, characterization, and enhanced gas sensing property for acetone. Sens Actuators B Chem. 2015;221:55–62.10.1016/j.snb.2015.06.040Search in Google Scholar
[48] Asogekar PA, Verenkar VMS. Structural and magnetic properties of nanosized CoxZn(1−x)Fe2O4 (x = 0.0, 0.5, 1.0) synthesized via autocatalytic thermal decomposition of hydrazinated cobalt zinc ferrous succinate. Ceram Int. 2019;45(17, Part A):21793–803.10.1016/j.ceramint.2019.07.182Search in Google Scholar
[49] Zakiyah LB, Saion E, Al-Hada NM, Gharibshahi E, Salem A, Soltani N, et al. Up-scalable synthesis of size-controlled copper ferrite nanocrystals by thermal treatment method. Mater Sci Semiconductor Process. 2015;40:564–9.10.1016/j.mssp.2015.07.027Search in Google Scholar
[50] Sartori K, Choueikani F, Gloter A, Begin-Colin S, Taverna D, Pichon BP. Room temperature blocked magnetic nanoparticles based on ferrite promoted by a three-step thermal decomposition process. J Am Chem Soc. 2019;141(25):9783–7.10.1021/jacs.9b03965Search in Google Scholar PubMed
[51] Flores-Arias Y, Vázquez-Victorio G, Ortega-Zempoalteca R, Acevedo-Salas U, Ammar S, Valenzuela R. Magnetic phase transitions in ferrite nanoparticles characterized by electron spin resonance. J Appl Phys. 2015;117(17):17A503.10.1063/1.4916935Search in Google Scholar
[52] Gaudisson T, Beji Z, Herbst F, Nowak S, Ammar S, Valenzuela R. Ultrafine grained high density manganese zinc ferrite produced using polyol process assisted by spark plasma sintering. J Magn Magn Mater. 2015;387:90–5.10.1016/j.jmmm.2015.03.045Search in Google Scholar
[53] Tian Y, Yu B, Li X, Li K. Facile solvothermal synthesis of monodisperse Fe3O4 nanocrystals with precise size control of one nanometre as potential MRI contrast agents. J Mater Chem. 2011;21(8):2476–81.10.1039/c0jm02913kSearch in Google Scholar
[54] Ameer S, Gul IH, Mujahid M. Ultra low permittivity/loss CoFe2O4 and CoFe2O4–rGO nanohybrids by novel 1-hexanol assisted solvothermal process. J Alloy Compd. 2015;642:78–82.10.1016/j.jallcom.2015.04.101Search in Google Scholar
[55] Shen CQ, Ji HN, Wu J, Zhu N, Niu JQ, Li HD, et al. editors. Synthesis and characterization of MnZn ferrite nanoparticles for biomedical applications. In 2018 IEEE International Conference on Applied Superconductivity and Electromagnetic Devices (ASEMD). Tianjin, China: IEEE; 2018.10.1109/ASEMD.2018.8559592Search in Google Scholar
[56] Rao P, Godbole RV, Phase DM, Chikate RC, Bhagwat S. Ferrite thin films: synthesis, characterization and gas sensing properties towards LPG. Mater Chem Phys. 2015;149–150:333–8.10.1016/j.matchemphys.2014.10.025Search in Google Scholar
[57] Singh Yadav R, Kuřitka I, Vilcakova J, Jamatia T, Machovsky M, Skoda D, et al. Impact of sonochemical synthesis condition on the structural and physical properties of MnFe2O4 spinel ferrite nanoparticles. Ultrason Sonochem. 2020;61:104839.10.1016/j.ultsonch.2019.104839Search in Google Scholar PubMed
[58] Almessiere MA, Slimani Y, Guner S, Sertkol M, Demir Korkmaz A, Shirsath SE, et al. Sonochemical synthesis and physical properties of Co0.3Ni0.5Mn0.2EuxFe2−xO4 nano-spinel ferrites. Ultrason Sonochem. 2019;58:104654.10.1016/j.ultsonch.2019.104654Search in Google Scholar PubMed
[59] Jalili NA, Muscarello M, Gaharwar AK. Nanoengineered thermoresponsive magnetic hydrogels for biomedical applications. Bioeng Transl Med. 2016;1(3):297–305.10.1002/btm2.10034Search in Google Scholar PubMed PubMed Central
[60] Sung B, Kim M-H, Abelmann L. Magnetic microgels and nanogels: physical mechanisms and biomedical applications. Bioeng Transl Med. 2021;6(1):e10190.10.1002/btm2.10190Search in Google Scholar PubMed PubMed Central
[61] Kefeni KK, Msagati TAM, Mamba BB. Ferrite nanoparticles: synthesis, characterisation and applications in electronic device. Mater Sci Eng B. 2017;215:37–55.10.1016/j.mseb.2016.11.002Search in Google Scholar
[62] Reda SM. Synthesis of ZnO and Fe2O3 nanoparticles by sol–gel method and their application in dye-sensitized solar cells. Mater Sci Semiconductor Process. 2010;13(5):417–25.10.1016/j.mssp.2011.09.007Search in Google Scholar
[63] Alshahrani B, ElSaeedy HI, fares S, Korna AH, Yakout HA, Maksoud MIAA, et al. The effect of Ce3+ doping on structural, optical, ferromagnetic resonance, and magnetic properties of ZnFe2O4 nanoparticles. Sci: Mater Electron. 2020;32(1):780–97.10.1007/s10854-020-04856-9Search in Google Scholar
[64] Maksoud MA, El-Sayyad GS, Abokhadra A, Soliman L, El-Bahnasawy H, Ashour A. Influence of Mg2+ substitution on structural, optical, magnetic, and antimicrobial properties of Mn–Zn ferrite nanoparticles. J Mater Sci Mater Electron. 2020;31(3):2598–616.10.1007/s10854-019-02799-4Search in Google Scholar
[65] Osman AI, Skillen NC, Robertson PKJ, Rooney DW, Morgan K. Exploring the photocatalytic hydrogen production potential of titania doped with alumina derived from foil waste. Int J Hydrog Energy. 2020;45(59):34494–502.10.1016/j.ijhydene.2020.02.065Search in Google Scholar
[66] Osman AI, Abu-Dahrieh JK, McLaren M, Laffir F, Nockemann P, Rooney D. A facile green synthetic route for the preparation of highly active gamma-Al2O3 from aluminum foil waste. Sci Rep. 2017;7(1):3593.10.1038/s41598-017-03839-xSearch in Google Scholar PubMed PubMed Central
[67] Osman AI, Abu-Dahrieh JK. Kinetic Investigation of η-Al2O3 catalyst for dimethyl ether production. Catal Lett. 2018;148(4):1236–45.10.1007/s10562-018-2319-2Search in Google Scholar
[68] Osman AI, Meudal J, Laffir F, Thompson J, Rooney D. Enhanced catalytic activity of Ni on η-Al2O3 and ZSM-5 on addition of ceria zirconia for the partial oxidation of methane. Appl Catal B Environ. 2017;212:68–79.10.1016/j.apcatb.2016.12.058Search in Google Scholar
[69] Rahimi R, Maleki A, Maleki S, Morsali A, Rahimi MJ. Synthesis and characterization of magnetic dichromate hybrid nanomaterials with triphenylphosphine surface modified iron oxide nanoparticles (Fe3O4@SiO2@PPh3@Cr2O72−). Solid State Sci. 2014;28:9–13.10.1016/j.solidstatesciences.2013.11.013Search in Google Scholar
[70] El Ghandoor H, Zidan H, Khalil MM, Ismail M. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int J Electrochem Sci. 2012;7(6):5734–45.10.1016/S1452-3981(23)19655-6Search in Google Scholar
[71] Amiri S, Shokrollahi H. Magnetic and structural properties of RE doped Co-ferrite (REåNd, Eu, and Gd) nano-particles synthesized by co-precipitation. J Magn Magn Mater. 2013;345:18–23.10.1016/j.jmmm.2013.05.030Search in Google Scholar
[72] Li H, Qin L, Feng Y, Hu L, Zhou C. Preparation and characterization of highly water-soluble magnetic Fe3O4 nanoparticles via surface double-layered self-assembly method of sodium alpha-olefin sulfonate. J Magn Magn Mater. 2015;384:213–8.10.1016/j.jmmm.2015.01.065Search in Google Scholar
[73] Gul S, Yousuf MA, Anwar A, Warsi MF, Agboola PO, Shakir I, et al. Al-substituted zinc spinel ferrite nanoparticles: preparation and evaluation of structural, electrical, magnetic and photocatalytic properties. Ceram Int. 2020;46(9):14195–205.10.1016/j.ceramint.2020.02.228Search in Google Scholar
[74] Zhang H, Zhu G. One-step hydrothermal synthesis of magnetic Fe3O4 nanoparticles immobilized on polyamide fabric. Appl Surf Sci. 2012;258(11):4952–9.10.1016/j.apsusc.2012.01.127Search in Google Scholar
[75] Ding Z, Wang W, Zhang Y, Li F, Liu JP. Synthesis, characterization and adsorption capability for Congo red of CoFe2O4 ferrite nanoparticles. J Alloy Compd. 2015;640:362–70.10.1016/j.jallcom.2015.04.020Search in Google Scholar
[76] Yang Y, Liu X, Yang Y, Xiao W, Li Z, Xue D, et al. Synthesis of nonstoichiometric zinc ferrite nanoparticles with extraordinary room temperature magnetism and their diverse applications. J Mater Chem C. 2013;1(16):2875–85.10.1039/c3tc00790aSearch in Google Scholar
[77] Sharifi I, Zamanian A, Behnamghader A. Synthesis and characterization of Fe0.6Zn0.4Fe2O4 ferrite magnetic nanoclusters using simple thermal decomposition method. J Magn Magn Mater. 2016;412:107–13.10.1016/j.jmmm.2016.03.091Search in Google Scholar
[78] Leonel LV, Barbosa JBS, Miquita DR, Oliveira FP, Fernandez-Outon LE, Oliveira EF, et al. Facile polyol synthesis of ultrasmall water-soluble cobalt ferrite nanoparticles. Solid State Sci. 2018;86:45–52.10.1016/j.solidstatesciences.2018.09.011Search in Google Scholar
[79] Hanini A, Massoudi ME, Gavard J, Kacem K, Ammar S, Souilem O. Nanotoxicological study of polyol-made cobalt-zinc ferrite nanoparticles in rabbit. Environ Toxicol Pharmacol. 2016;45:321–7.10.1016/j.etap.2016.06.010Search in Google Scholar PubMed
[80] Gaudisson T, Artus M, Acevedo U, Herbst F, Nowak S, Valenzuela R, et al. On the microstructural and magnetic properties of fine-grained CoFe2O4 ceramics produced by combining polyol process and spark plasma sintering. J Magn Magn Mater. 2014;370:87–95.10.1016/j.jmmm.2014.06.014Search in Google Scholar
[81] Aparna ML, Grace AN, Sathyanarayanan P, Sahu NK. A comparative study on the supercapacitive behaviour of solvothermally prepared metal ferrite (MFe2O4, M═ Fe, Co, Ni, Mn, Cu, Zn) nanoassemblies. J Alloy Compd. 2018;745:385–95.10.1016/j.jallcom.2018.02.127Search in Google Scholar
[82] Ghobashy MM. In-situ core-shell polymerization of magnetic polymer nanocomposite (PAAc/Fe3O4) particles via gamma radiation. Nanocomposites. 2017;3(1):42–6.10.1080/20550324.2017.1316600Search in Google Scholar
[83] Ghobashy MM. Combined ultrasonic and gamma-irradiation to prepare TiO2@ PET-g-PAAc fabric composite for self-cleaning application. Ultrason Sonochem. 2017;37:529–35.10.1016/j.ultsonch.2017.02.014Search in Google Scholar PubMed
[84] Bang JH, Suslick KS. Applications of ultrasound to the synthesis of nanostructured materials. Adv Mater. 2010;22(10):1039–59.10.1002/adma.200904093Search in Google Scholar PubMed
[85] Almessiere MA, Slimani Y, Unal B, Zubar TI, Sadaqat A, Trukhanov AV, et al. Microstructure, dielectric and microwave features of [Ni0.4Cu0.2Zn0.4](Fe2−xTbx)O4(x ≤ 0.1) nanospinel ferrites. J Mater Res Technol. 2020;9(5):10608–23.10.1016/j.jmrt.2020.07.094Search in Google Scholar
[86] Minin R, Itin V, Zhuravlev V, Svetlichnyi V, editors. Synthesis of cubic ferrite CoFe2O4 by spray pyrolysis. In Journal of Physics: Conference Series. IOP Publishing; 2018. p. 04201110.1088/1742-6596/1115/4/042011Search in Google Scholar
[87] Abdel Maksoud MIA, Elgarahy AM, Farrell C, Al-Muhtaseb AH, Rooney DW, Osman AI. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord Chem Rev. 2020;403:213096.10.1016/j.ccr.2019.213096Search in Google Scholar
[88] Shah MS, Ali K, Ali I, Mahmood A, Ramay SM, Farid MT. Structural and magnetic properties of praseodymium substituted barium-based spinel ferrites. Mater Res Bull. 2018;98:77–82.10.1016/j.materresbull.2017.09.063Search in Google Scholar
[89] Yadav RS, Kuřitka I, Havlica J, Hnatko M, Alexander C, Masilko J, et al. Structural, magnetic, elastic, dielectric and electrical properties of hot-press sintered Co1−xZnxFe2O4 (x = 0.0, 0.5) spinel ferrite nanoparticles. J Magn Magn Mater. 2018;447:48–57.10.1016/j.jmmm.2017.09.033Search in Google Scholar
[90] Abdel Maksoud MIA, El-Ghandour A, El-Sayyad GS, Fahim RA, El-Hanbaly AH, Bekhit M, et al. Unveiling the effect of Zn2+ substitution in enrichment of structural, magnetic, and dielectric properties of Cobalt Ferrite. J Inorg Organomet Polym Mater. 2020;30(9):3709–21.10.1007/s10904-020-01523-8Search in Google Scholar
[91] Reyes-Rodríguez PY, Cortés-Hernández DA, Escobedo-Bocardo JC, Almanza-Robles JM, Sánchez-Fuentes HJ, Jasso-Terán A, et al. Structural and magnetic properties of Mg–Zn ferrites (Mg1−xZnxFe2O4) prepared by sol–gel method. J Magn Magn Mater. 2017;427:268–71.10.1016/j.jmmm.2016.10.078Search in Google Scholar
[92] Ghodake U, Kambale RC, Suryavanshi S. Effect of Mn2+ substitution on structural, electrical transport and dielectric properties of Mg–Zn ferrites. Ceram Int. 2017;43(1):1129–34.10.1016/j.ceramint.2016.10.053Search in Google Scholar
[93] McCusker LB, Von Dreele RB, Cox DE, Louer D, Scardi P. Rietveld refinement guidelines. J Appl Crystal. 1999;32(1):36–50.10.1107/S0021889898009856Search in Google Scholar
[94] Gao Y, Wang Z, Pei J, Zhang H. Structural, elastic, thermal and soft magnetic properties of Ni–Zn–Li ferrites. J Alloy Compd. 2019;774:1233–42.10.1016/j.jallcom.2018.09.394Search in Google Scholar
[95] Ashour A, El-Batal AI, Maksoud MA, El-Sayyad GS, Labib S, Abdeltwab E, et al. Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology. 2018;40:141–51.10.1016/j.partic.2017.12.001Search in Google Scholar
[96] Massoudi J, Smari M, Nouri K, Dhahri E, Khirouni K, Bertaina S, et al. Magnetic and spectroscopic properties of Ni–Zn–Al ferrite spinel: from the nanoscale to microscale. RSC Adv. 2020;10(57):34556–80.10.1039/D0RA05522KSearch in Google Scholar
[97] Ghosh MP, Mukherjee S. Tuning the microstructural, magnetic and optical properties of Cr substituted nanocrystalline copper-nickel ferrites. J Magn Magn Mater. 2020;498:166185.10.1016/j.jmmm.2019.166185Search in Google Scholar
[98] Poudel TP, Rai BK, Yoon S, Guragain D, Neupane D, Mishra SR. The effect of gadolinium substitution in inverse spinel nickel ferrite: structural, magnetic, and Mössbauer study. J Alloy Compd. 2019;802:609–19.10.1016/j.jallcom.2019.06.201Search in Google Scholar
[99] Jadhav VV, Shirsat SD, Tumberphale UB, Mane RS. Chapter 3 – Properties of ferrites. In Spinel ferrite nanostructures for energy storage devices. Oxford, Oxfordshire, United Kingdom: Elsevier; 2020. p. 35–50.10.1016/B978-0-12-819237-5.00003-1Search in Google Scholar
[100] Sharifianjazi F, Moradi M, Parvin N, Nemati A, Jafari Rad A, Sheysi N, et al. Magnetic CoFe2O4 nanoparticles doped with metal ions: a review. Ceram Int. 2020;46(11, Part B):18391–412.10.1016/j.ceramint.2020.04.202Search in Google Scholar
[101] Hanini A, Lartigue L, Gavard J, Kacem K, Wilhelm C, Gazeau F, et al. Zinc substituted ferrite nanoparticles with Zn0.9Fe2.1O4 formula used as heating agents for in vitro hyperthermia assay on glioma cells. J Magn Magn Mater. 2016;416:315–20.10.1016/j.jmmm.2016.05.016Search in Google Scholar
[102] Zargar T, Kermanpur A. Effects of hydrothermal process parameters on the physical, magnetic and thermal properties of Zn0.3Fe2.7O4 nanoparticles for magnetic hyperthermia applications. Ceram Int. 2017;43(7):5794–804.10.1016/j.ceramint.2017.01.127Search in Google Scholar
[103] Krishnan KM. Biomedical nanomagnetics: a spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans Magn. 2010;46(7):2523–58.10.1109/TMAG.2010.2046907Search in Google Scholar PubMed PubMed Central
[104] Kumar CSSR, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Delivery Rev. 2011;63(9):789–808.10.1016/j.addr.2011.03.008Search in Google Scholar PubMed PubMed Central
[105] Tran N, Webster TJ. Magnetic nanoparticles: biomedical applications and challenges. J Mater Chem. 2010;20(40):8760–7.10.1039/c0jm00994fSearch in Google Scholar
[106] Di Corato R, Béalle G, Kolosnjaj-Tabi J, Espinosa A, Clement O, Silva AKA, et al. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano. 2015;9(3):2904–16.10.1021/nn506949tSearch in Google Scholar PubMed
[107] Psimadas D, Baldi G, Ravagli C, Comes Franchini M, Locatelli E, Innocenti C, et al. Comparison of the magnetic, radiolabeling, hyperthermic and biodistribution properties of hybrid nanoparticles bearing CoFe2O4and Fe3O4 metal cores. Nanotechnology. 2013;25(2):25101.10.1088/0957-4484/25/2/025101Search in Google Scholar PubMed
[108] Hasirci C, Karaagac O, Köçkar H. Superparamagnetic zinc ferrite: a correlation between high magnetizations and nanoparticle sizes as a function of reaction time via hydrothermal process. J Magn Magn Mater. 2019;474:282–6.10.1016/j.jmmm.2018.11.037Search in Google Scholar
[109] Guardia P, Di Corato R, Lartigue L, Wilhelm C, Espinosa A, Garcia-Hernandez M, et al. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano. 2012;6(4):3080–91.10.1021/nn2048137Search in Google Scholar PubMed
[110] Sasikala ARK, Unnithan AR, Yun Y-H, Park CH, Kim CS. An implantable smart magnetic nanofiber device for endoscopic hyperthermia treatment and tumor-triggered controlled drug release. Acta Biomater. 2016;31:122–33.10.1016/j.actbio.2015.12.015Search in Google Scholar PubMed
[111] Giustini AJ, Petryk AA, Cassim SM, Tate JA, Baker I, Hoopes PJ. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life. 2010;1(1–2):17–32.10.1142/S1793984410000067Search in Google Scholar PubMed PubMed Central
[112] Jordan A, Wust P, Scholz R, Faeling H, Krause J, Felix R. Scientific and clinical applications of magnetic carriers. In: Häfeli W, Schütt J, editors. Springer, Boston, MA: Springer Nature; 1997Search in Google Scholar
[113] Manikandan A, Vijaya JJ, Kennedy LJ, Bououdina M. Structural, optical and magnetic properties of Zn1−xCuxFe2O4 nanoparticles prepared by microwave combustion method. J Mol Structure. 2013;1035:332–40.10.1016/j.molstruc.2012.11.007Search in Google Scholar
[114] Singh SB, Srinivas C, Tirupanyam BV, Prajapat CL, Singh MR, Meena SS, et al. Structural, thermal and magnetic studies of MgxZn1−xFe2O4 nanoferrites: study of exchange interactions on magnetic anisotropy. Ceram Int. 2016;42(16):19179–86.10.1016/j.ceramint.2016.09.081Search in Google Scholar
[115] Sagayaraj R, Aravazhi S, Praveen P, Chandrasekaran G. Structural, morphological and magnetic characters of PVP coated ZnFe2O4 nanoparticles. J Mater Sci Mater Electron. 2018;29(3):2151–8.10.1007/s10854-017-8127-4Search in Google Scholar
[116] Pawar RA, Patange SM, Shitre AR, Gore SK, Jadhav SS, Shirsath SE. Crystal chemistry and single-phase synthesis of Gd3+ substituted Co–Zn ferrite nanoparticles for enhanced magnetic properties. RSC Adv. 2018;8(44):25258–67.10.1039/C8RA04282ASearch in Google Scholar PubMed PubMed Central
[117] Da Silva FG, Depeyrot J, Campos AF, Aquino R, Fiorani D, Peddis D. Structural and magnetic properties of spinel ferrite nanoparticles. J Nanosci Nanotechnol. 2019;19(8):4888–902.10.1166/jnn.2019.16877Search in Google Scholar PubMed
[118] Zhang W, Zuo X, Niu Y, Wu C, Wang S, Guan S, et al. Novel nanoparticles with Cr3+ substituted ferrite for self-regulating temperature hyperthermia. Nanoscale. 2017;9(37):13929–37.10.1039/C7NR02356ASearch in Google Scholar
[119] Lee JE, Lee N, Kim H, Kim J, Choi SH, Kim JH, et al. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J Am Chem Soc. 2010;132(2):552–7.10.1021/ja905793qSearch in Google Scholar PubMed
[120] Zhou X, Chen X, Mao TC, Li X, Shi XH, Fan DL, et al. Carbon ion implantation: a good method to enhance the biocompatibility of silicone rubber. Plastic Reconstruct Surg. 2016;137(4):690e–9e.10.1097/PRS.0000000000002022Search in Google Scholar PubMed
[121] Ghobashy MM, El-Sawy NM, Kodous AS. Nanocomposite of cosubstituted carbonated hydroxyapatite fabricated inside poly(sodium hyaluronate-acrylamide) hydrogel template prepared by gamma radiation for osteoblast cell regeneration. Radiat Phys Chem. 2021;183:109408.10.1016/j.radphyschem.2021.109408Search in Google Scholar
[122] Gorgizadeh M, Azarpira N, Sattarahmady N. In vitro and in vivo tumor annihilation by near-infrared photothermal effect of a NiFe2O4/C nanocomposite. Colloids Surf B: Biointerfaces. 2018;170:393–400.10.1016/j.colsurfb.2018.06.034Search in Google Scholar PubMed
[123] Vilas-Boas V, Carvalho F, Espiña B. Magnetic hyperthermia for cancer treatment: main parameters affecting the outcome of in vitro and in vivo studies. Molecules. 2020;25(12):2874.10.3390/molecules25122874Search in Google Scholar PubMed PubMed Central
[124] Zhang Z-Q, Song S-C. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials. 2016;106:13–23.10.1016/j.biomaterials.2016.08.015Search in Google Scholar PubMed
[125] Bisht G, Zaidi MGH, Rayamajhi S. Supercritical carbon dioxide–assisted synthesis of stimuli-responsive magnetic poly (N-isopropylacrylamide)–ferrite biocompatible nanocomposites for targeted and controlled drug delivery. Int J Polymeric Mater Polymeric Biomater. 2017;66(14):708–16.10.1080/00914037.2016.1263949Search in Google Scholar
[126] Li Q, Kartikowati CW, Horie S, Ogi T, Iwaki T, Okuyama K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci Rep. 2017;7(1):1–7.10.1038/s41598-017-09897-5Search in Google Scholar PubMed PubMed Central
[127] Wang G, Zhou F, Du T, Lu Z, Ma Y, Mu J, et al. Controlled assembly of MnFe2O4 nanoparticles on MoS2 nanosheets by a facile sonochemical method. J Magn Magn Mater. 2019;476:453–8.10.1016/j.jmmm.2019.01.019Search in Google Scholar
[128] Xie W, Gao Q, Wang D, Guo Z, Gao F, Wang X, et al. Doxorubicin-loaded Fe3O4@MoS2-PEG-2DG nanocubes as a theranostic platform for magnetic resonance imaging-guided chemo-photothermal therapy of breast cancer. Nano Res. 2018;11(5):2470–87.10.1007/s12274-017-1871-1Search in Google Scholar
[129] Almessiere MA, Slimani Y, Rehman S, Khan FA, Polat EG, Sadaqat A, et al. Synthesis of Dy-Y co-substituted manganese-zinc spinel nanoferrites induced anti-bacterial and anti-cancer activities: Comparison between sonochemical and sol–gel auto-combustion methods. Mater Sci Eng C. 2020;116:111186.10.1016/j.msec.2020.111186Search in Google Scholar PubMed
[130] Hassanzadeh-Tabrizi S, Norbakhsh H, Pournajaf R, Tayebi M. Synthesis of mesoporous cobalt ferrite/hydroxyapatite core-shell nanocomposite for magnetic hyperthermia and drug release applications. Ceram Int. 2021;47(13):18167–76.10.1016/j.ceramint.2021.03.135Search in Google Scholar
[131] Wijesinghe W, Mantilaka M, Premalal E, Herath H, Mahalingam S, Edirisinghe M, et al. Facile synthesis of both needle-like and spherical hydroxyapatite nanoparticles: effect of synthetic temperature and calcination on morphology, crystallite size and crystallinity. Mater Sci Eng: C. 2014;42:83–90.10.1016/j.msec.2014.05.032Search in Google Scholar PubMed
[132] Ansari M, Bigham A, Hassanzadeh-Tabrizi S, Ahangar HA. Synthesis and characterization of Cu0.3Zn0.5Mg0.2Fe2O4 nanoparticles as a magnetic drug delivery system. J Magn Magn Mater. 2017;439:67–75.10.1016/j.jmmm.2017.04.084Search in Google Scholar
[133] Wu J, Zhu YJ, Cao SW, Chen F. Hierachically nanostructured mesoporous spheres of calcium silicate hydrate: surfactant-free sonochemical synthesis and drug-delivery system with ultrahigh drug-loading capacity. Adv Mater. 2010;22(6):749–53.10.1002/adma.200903020Search in Google Scholar PubMed
[134] Khamsehashari N, Hassanzadeh-Tabrizi S, Bigham A. Effects of strontium adding on the drug delivery behavior of silica nanoparticles synthesized by P123-assisted sol–gel method. Mater Chem Phys. 2018;205:283–91.10.1016/j.matchemphys.2017.11.034Search in Google Scholar
[135] Gharibshahian M, Mirzaee O, Nourbakhsh M. Evaluation of superparamagnetic and biocompatible properties of mesoporous silica coated cobalt ferrite nanoparticles synthesized via microwave modified Pechini method. J Magn Magn Mater. 2017;425:48–56.10.1016/j.jmmm.2016.10.116Search in Google Scholar
[136] Aval NA, Islamian JP, Hatamian M, Arabfirouzjaei M, Javadpour J, Rashidi M-R. Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment. Int J Pharm. 2016;509(1–2):159–67.10.1016/j.ijpharm.2016.05.046Search in Google Scholar PubMed
[137] Sangeetha K, Ashok M, Girija E. Development of multifunctional cobalt ferrite/hydroxyapatite nanocomposites by microwave assisted wet precipitation method: a promising platform for synergistic chemo-hyperthermia therapy. Ceram Int. 2019;45(10):12860–9.10.1016/j.ceramint.2019.03.209Search in Google Scholar
[138] Talaei M, Hassanzadeh-Tabrizi S, Saffar-Teluri A. Synthesis of mesoporous CuFe2O4@ SiO2 core–shell nanocomposite for simultaneous drug release and hyperthermia applications. Ceram Int. 2021;47:30287–97.10.1016/j.ceramint.2021.07.209Search in Google Scholar
[139] Radmansouri M, Bahmani E, Sarikhani E, Rahmani K, Sharifianjazi F, Irani M. Doxorubicin hydrochloride-loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int J Biol Macromol. 2018;116:378–84.10.1016/j.ijbiomac.2018.04.161Search in Google Scholar PubMed
[140] Wang G, Ma Y, Wei Z, Qi M. Development of multifunctional cobalt ferrite/graphene oxide nanocomposites for magnetic resonance imaging and controlled drug delivery. Chem Eng J. 2016;289:150–60.10.1016/j.cej.2015.12.072Search in Google Scholar
[141] Suárez J, Daboin V, González G, Briceño S. Chitosan-polyvinylpyrrolidone CoxFe3−xO4(0.25 ≤ x ≤ 1) nanoparticles for hyperthermia applications. Int J Biol Macromol. 2020;164:3403–10.10.1016/j.ijbiomac.2020.08.043Search in Google Scholar PubMed
[142] Daboin V, Briceño S, Suárez J, Carrizales-Silva L, Alcalá O, Silva P, et al. Magnetic SiO2-Mn1−xCoxFe2O4 nanocomposites decorated with Au@Fe3O4 nanoparticles for hyperthermia. J Magn Magn Mater. 2019;479:91–8.10.1016/j.jmmm.2019.02.002Search in Google Scholar
[143] Mondal D, Borgohain C, Paul N, Borah J. Improved heating efficiency of bifunctional MnFe2O4/ZnS nanocomposite for magnetic hyperthermia application. Phys B Condens Matter. 2019;567:122–8.10.1016/j.physb.2018.11.068Search in Google Scholar
[144] Hatamie S, Balasi ZM, Ahadian MM, Mortezazadeh T, Shams F, Hosseinzadeh S. Hyperthermia of breast cancer tumor using graphene oxide-cobalt ferrite magnetic nanoparticles in mice. J Drug Delivery Sci Technol. 2021;65:102680.10.1016/j.jddst.2021.102680Search in Google Scholar
[145] Lin Q, Xu J, Yang F, Lin J, Yang H, He Y. Magnetic and Mössbauer spectroscopy studies of zinc-substituted cobalt ferrites prepared by the sol–gel method. Materials (Basel, Switzerland). 2018;11(10):1799.10.3390/ma11101799Search in Google Scholar PubMed PubMed Central
[146] Balakrishnan PB, Silvestri N, Fernandez-Cabada T, Marinaro F, Fernandes S, Fiorito S, et al. Exploiting unique alignment of cobalt Ferrite nanoparticles, mild hyperthermia, and controlled intrinsic cobalt toxicity for cancer therapy. Adv Mater. 2020;32(45):2003712.10.1002/adma.202003712Search in Google Scholar PubMed
[147] Di Corato R, Espinosa A, Lartigue L, Tharaud M, Chat S, Pellegrino T, et al. Magnetic hyperthermia efficiency in the cellular environment for different nanoparticle designs. Biomaterials. 2014;35(24):6400–11.10.1016/j.biomaterials.2014.04.036Search in Google Scholar PubMed
[148] Zyuzin MV, Cassani M, Barthel MJ, Gavilan H, Silvestri N, Escudero A, et al. Confining iron oxide nanocubes inside submicrometric cavities as a key strategy to preserve magnetic heat losses in an intracellular environment. ACS Appl Mater Interfaces. 2019;11(45):41957–71.10.1021/acsami.9b15501Search in Google Scholar PubMed
[149] Cabrera D, Lak A, Yoshida T, Materia ME, Ortega D, Ludwig F, et al. Unraveling viscosity effects on the hysteresis losses of magnetic nanocubes. Nanoscale. 2017;9(16):5094–101.10.1039/C7NR00810DSearch in Google Scholar
[150] Kolosnjaj-Tabi J, Di Corato R, Lartigue L, Marangon I, Guardia P, Silva AKA, et al. Heat-generating iron oxide nanocubes: subtle “Destructurators” of the tumoral microenvironment. ACS Nano. 2014;8(5):4268–83.10.1021/nn405356rSearch in Google Scholar PubMed
[151] Mai BT, Balakrishnan PB, Barthel MJ, Piccardi F, Niculaes D, Marinaro F, et al. Thermoresponsive iron oxide nanocubes for an effective clinical translation of magnetic hyperthermia and heat-mediated chemotherapy. ACS Appl Mater Interfaces. 2019;11(6):5727–39.10.1021/acsami.8b16226Search in Google Scholar PubMed PubMed Central
[152] Marangon I, Silva AAK, Guilbert T, Kolosnjaj-Tabi J, Marchiol C, Natkhunarajah S, et al. Tumor stiffening, a key determinant of tumor progression, is reversed by nanomaterial-induced photothermal therapy. Theranostics. 2017;7(2):329–43.10.7150/thno.17574Search in Google Scholar PubMed PubMed Central
[153] Chu X-Y, Huang W, Wang Y-L, Meng L-W, Chen L-Q, Jin M-J, et al. Improving antitumor outcomes for palliative intratumoral injection therapy through lecithin- chitosan nanoparticles loading paclitaxel- cholesterol complex. Int J Nanomed. 2019;14:689–705.10.2147/IJN.S188667Search in Google Scholar
[154] Lee N, Yoo D, Ling D, Cho MH, Hyeon T, Cheon J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem Rev. 2015;115(19):10637–89.10.1021/acs.chemrev.5b00112Search in Google Scholar
[155] Chen Y, Nan J, Lu Y, Wang C, Chu F, Gu Z. Hybrid Fe3O4-poly(acrylic acid) nanogels for theranostic cancer treatment. J Biomed Nanotechnol. 2015;11(5):771–9.10.1166/jbn.2015.2001Search in Google Scholar
[156] Yang Y, Li Y, Sun Q-y. Archaeal and bacterial communities in acid mine drainage from metal-rich abandoned tailing ponds, Tongling, China. Trans Nonferrous Met Soc China. 2014;24(10):3332–42.10.1016/S1003-6326(14)63474-9Search in Google Scholar
[157] Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J, et al. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J Natl Cancer Inst. 2007;99(1):53–63.10.1093/jnci/djk005Search in Google Scholar PubMed
[158] Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–64.10.1038/nrclinonc.2010.139Search in Google Scholar PubMed PubMed Central
[159] Namiki Y, Namiki T, Yoshida H, Ishii Y, Tsubota A, Koido S, et al. A novel magnetic crystal–lipid nanostructure for magnetically guided in vivo gene delivery. Nat Nanotechnol. 2009;4(9):598–606.10.1038/nnano.2009.202Search in Google Scholar PubMed
[160] Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, et al. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000;60(23):6641.Search in Google Scholar
[161] Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discovery. 2005;4(2):145–60.10.1038/nrd1632Search in Google Scholar PubMed
[162] Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021.10.1016/j.biomaterials.2004.10.012Search in Google Scholar PubMed
[163] Balakrishnan S, Bonder MJ, Hadjipanayis GC. Particle size effect on phase and magnetic properties of polymer-coated magnetic nanoparticles. J Magn Magn Mater. 2009;321(2):117–22.10.1016/j.jmmm.2008.08.055Search in Google Scholar
[164] Karimi Z, Karimi L, Shokrollahi H. Nano-magnetic particles used in biomedicine: Core and coating materials. Mater Sci Eng C. 2013;33(5):2465–75.10.1016/j.msec.2013.01.045Search in Google Scholar PubMed
[165] Albino M, Fantechi E, Innocenti C, López-Ortega A, Bonanni V, Campo G, et al. Role of Zn2+ substitution on the magnetic, hyperthermic, and relaxometric properties of cobalt ferrite nanoparticles. J Phys Chem C. 2019;123(10):6148–57.10.1021/acs.jpcc.8b10998Search in Google Scholar
[166] Almessiere MA, Slimani Y, Sertkol M, Khan FA, Nawaz M, Tombuloglu H, et al. Ce–Nd Co-substituted nanospinel cobalt ferrites: an investigation of their structural, magnetic, optical, and apoptotic properties. Ceram Int. 2019;45(13):16147–56.10.1016/j.ceramint.2019.05.133Search in Google Scholar
[167] Zhang H, Wang J, Zeng Y, Wang G, Han S, Yang Z, et al. Leucine-coated cobalt ferrite nanoparticles: Synthesis, characterization and potential biomedical applications for drug delivery. Phys Lett A. 2020;384(24):126600.10.1016/j.physleta.2020.126600Search in Google Scholar
[168] Iqbal S, Fakhar-e-Alam M, Atif M, Amin N, Alimgeer KS, Ali A, et al. Structural, morphological, antimicrobial, and in vitro photodynamic therapeutic assessments of novel Zn+2-substituted cobalt ferrite nanoparticles. Results Phys. 2019;15:102529.10.1016/j.rinp.2019.102529Search in Google Scholar
[169] Ahamed M, Akhtar MJ, Alhadlaq HA, Alshamsan A. Copper ferrite nanoparticle-induced cytotoxicity and oxidative stress in human breast cancer MCF-7 cells. Colloids Surf B Biointerfaces. 2016;142:46–54.10.1016/j.colsurfb.2016.02.043Search in Google Scholar PubMed
[170] Atif M, Fakhar-e-Alam M, AlSalhi MS. Role of sensitivity of zinc oxide nanorods (ZnO-NRs) based photosensitizers in hepatocellular site of biological tissue. Laser Phys. 2011;21(11):1950–61.10.1134/S1054660X11190029Search in Google Scholar
[171] McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomed. 2008;3(2):169–80.10.2147/IJN.S1608Search in Google Scholar
[172] Cai B, Zhao M, Ma Y, Ye Z, Huang J. Bioinspired formation of 3D hierarchical CoFe2O4 porous microspheres for magnetic-controlled drug release. ACS Appl Mater Interfaces. 2015;7(2):1327–33.10.1021/am507689aSearch in Google Scholar PubMed
[173] Srinivasan SY, Paknikar KM, Bodas D, Gajbhiye V. Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine. 2018;13(10):1221–38.10.2217/nnm-2017-0379Search in Google Scholar PubMed
[174] Liu M, Pan L, Piao H, Sun H, Huang X, Peng C, et al. Magnetically actuated wormlike nanomotors for controlled cargo release. ACS Appl Mater Interfaces. 2015;7(47):26017–21.10.1021/acsami.5b08946Search in Google Scholar PubMed
[175] Iatridi Z, Vamvakidis K, Tsougos I, Vassiou K, Dendrinou-Samara C, Bokias G. Multifunctional polymeric platform of magnetic ferrite colloidal superparticles for luminescence, imaging, and hyperthermia applications. ACS Appl Mater Interfaces. 2016;8(51):35059–70.10.1021/acsami.6b13161Search in Google Scholar PubMed
[176] Yang J-C, Chen Y, Li Y-H, Yin X-B. Magnetic resonance imaging-guided multi-drug chemotherapy and photothermal synergistic therapy with pH and NIR-stimulation release. ACS Appl Mater Interfaces. 2017;9(27):22278–88.10.1021/acsami.7b06105Search in Google Scholar PubMed
[177] Fantechi E, Innocenti C, Zanardelli M, Fittipaldi M, Falvo E, Carbo M, et al. A smart platform for hyperthermia application in cancer treatment: cobalt-doped ferrite nanoparticles mineralized in human ferritin cages. ACS Nano. 2014;8(5):4705–19.10.1021/nn500454nSearch in Google Scholar PubMed
[178] Ebrahimi Fard A, Zarepour A, Zarrabi A, Shanei A, Salehi H. Synergistic effect of the combination of triethylene-glycol modified Fe3O4 nanoparticles and ultrasound wave on MCF-7 cells. J Magn Magn Mater. 2015;394:44–9.10.1016/j.jmmm.2015.06.040Search in Google Scholar
[179] Vallabani NVS, Singh S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech. 2018;8(6):279.10.1007/s13205-018-1286-zSearch in Google Scholar PubMed PubMed Central
[180] Saha P, Rakshit R, Mandal K. Enhanced magnetic properties of Zn doped Fe3O4 nano hollow spheres for better bio-medical applications. J Magn Magn Mater. 2019;475:130–6.10.1016/j.jmmm.2018.11.061Search in Google Scholar
[181] de Mello LB, Varanda LC, Sigoli FA, Mazali IO. Co-precipitation synthesis of (Zn-Mn)-co-doped magnetite nanoparticles and their application in magnetic hyperthermia. J Alloy Compd. 2019;779:698–705.10.1016/j.jallcom.2018.11.280Search in Google Scholar
[182] Thu Huong LT, Nam NH, Doan DH, My Nhung HT, Quang BT, Nam PH, et al. Folate attached, curcumin loaded Fe3O4 nanoparticles: a novel multifunctional drug delivery system for cancer treatment. Mater Chem Phys. 2016;172:98–104.10.1016/j.matchemphys.2015.12.065Search in Google Scholar
[183] Tietze R, Zaloga J, Unterweger H, Lyer S, Friedrich RP, Janko C, et al. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem Biophys Res Commun. 2015;468(3):463–70.10.1016/j.bbrc.2015.08.022Search in Google Scholar PubMed
[184] Shen J-M, Guan X-M, Liu X-Y, Lan J-F, Cheng T, Zhang H-X. Luminescent/magnetic hybrid nanoparticles with folate-conjugated peptide composites for tumor-targeted drug delivery. Bioconjugate Chem. 2012;23(5):1010–21.10.1021/bc300008kSearch in Google Scholar PubMed
[185] Lee JH, Jang JT, Choi JS, Moon SH, Noh SH, Kim JW, et al. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat Nanotechnol. 2011;6(7):418–22.10.1038/nnano.2011.95Search in Google Scholar PubMed
[186] Montha W, Maneeprakorn W, Buatong N, Tang IM, Pon-On W. Synthesis of doxorubicin-PLGA loaded chitosan stabilized (Mn, Zn)Fe2O4 nanoparticles: biological activity and pH-responsive drug release. Mater Sci Eng C. 2016;59:235–40.10.1016/j.msec.2015.09.098Search in Google Scholar PubMed
[187] Wang G, Ma Y, Zhang L, Mu J, Zhang Z, Zhang X, et al. Facile synthesis of manganese ferrite/graphene oxide nanocomposites for controlled targeted drug delivery. J Magn Magn Mater. 2016;401:647–50.10.1016/j.jmmm.2015.10.096Search in Google Scholar
[188] Almessiere MA, Slimani Y, Rehman S, Khan FA, Güngüneş ÇD, Güner S, et al. Magnetic properties, anticancer and antibacterial effectiveness of sonochemically produced Ce3+/Dy3+ co-activated Mn–Zn nanospinel ferrites. Arab J Chem. 2020;13(10):7403–17.10.1016/j.arabjc.2020.08.017Search in Google Scholar
[189] Hekmat A, Saboury AA. Structural effects of the syntheticcobalt–manganese-zinc ferrite nanoparticles (Co0.3Mn0.2Zn0.5Fe2O4 NPs) on DNA and its antiproliferative effect on T47Dcells. BioNanoScience. 2019;9(4):821–32.10.1007/s12668-019-00657-5Search in Google Scholar
[190] Jin KL, Park JY, Noh EJ, Hoe KL, Lee JH, Kim JH, et al. The effect of combined treatment with cisplatin and histone deacetylase inhibitors on HeLa cells. J Gynecol Oncol. 2010;21(4):262–8.10.3802/jgo.2010.21.4.262Search in Google Scholar PubMed PubMed Central
[191] Ramasamy S, Enoch IVMV, Rex Jeya Rajkumar S. Polymeric cyclodextrin-dextran spooled nickel ferrite nanoparticles: expanded anticancer efficacy of loaded camptothecin. Mater Lett. 2020;261:127114.10.1016/j.matlet.2019.127114Search in Google Scholar
[192] Alahmari F, Rehman S, Almessiere M, Khan FA, Slimani Y, Baykal A. Synthesis of Ni0.5Co0.5−xCdxFe1.78Nd0.02O4 (x ≤ 0.25) nanofibers by using electrospinning technique induce anti-cancer and anti-bacterial activities. J Biomol Struct Dyn. 2021;39(16):1–8.10.1080/07391102.2020.1761880Search in Google Scholar
[193] Khan FA, Akhtar S, Almohazey D, Alomari M, Almofty SA, Eliassari A. Fluorescent magnetic submicronic polymer (FMSP) nanoparticles induce cell death in human colorectal carcinoma cells. Artif Cells Nanomed Biotechnol. 2018;46(Sup 3):S247–53.10.1080/21691401.2018.1491476Search in Google Scholar PubMed
[194] Khan FA, Akhtar S, Almohazey D, Alomari M, Almofty SA, Badr I, et al. Targeted delivery of poly (methyl methacrylate) particles in colon cancer cells selectively attenuates cancer cell proliferation. Artif Cell Nanomed Biotechnol. 2019;47(1):1533–42.10.1080/21691401.2019.1577886Search in Google Scholar PubMed
[195] Tombuloglu H, Khan FA, Almessiere MA, Aldakheel S, Baykal A. Synthesis of niobium substituted cobalt-nickel nano-ferrite (Co0.5Ni0.5NbxFe2−xO4 (x ≤ 0.1) by hydrothermal approach show strong anti-colon cancer activities. J Biomol Struct Dyn. 2021;39(6):1–9.10.1080/07391102.2020.1748719Search in Google Scholar PubMed
[196] Ghobashy MM, El‐Sattar NEAA. Radiation synthesis of rapidly self-healing hydrogel derived from poly (acrylic acid) with good mechanical strength. Macromol Chem Phys. 2020;221(19):2000218.10.1002/macp.202000218Search in Google Scholar
[197] Ghobashy MM, Alshangiti DM, Alkhursani SA, Al-Gahtany SA, Shokr FS, Madani M. Improvement of in vitro dissolution of the poor water-soluble amlodipine drug by solid dispersion with irradiated polyvinylpyrrolidone. ACS Omega. 2020;5(34):21476–87.10.1021/acsomega.0c01910Search in Google Scholar PubMed PubMed Central
[198] Elhady MA, Ghobashy MM, Mahmoud MA. Effect of gamma irradiation on the adhesive property and antibacterial activity of blend polymer (abietic acid-EVA). Polym Polym Compos. 2020;29:138–47.10.1177/0967391120904619Search in Google Scholar
[199] Alshangiti DM, Ghobashy MM, Alkhursani SA, Shokr FS, Al-Gahtany SA, Madani MM. Semi-permeable membrane fabricated from organoclay/PS/EVA irradiated by ɣ-rays for water purification from dyes. J Mater Res Technol. 2019;8(6):6134–45.10.1016/j.jmrt.2019.10.008Search in Google Scholar
[200] Ghobashy MM, Alkhursani SA, Madani M. Radiation-induced nucleation and pH-controlled nanostructure shape of polyaniline dispersed in DMF. Polym Bull. 2018;75(12):5477–92.10.1007/s00289-018-2336-8Search in Google Scholar
[201] Ghobashy MM, Elhady MA. pH-sensitive wax emulsion copolymerization with acrylamide hydrogel using gamma irradiation for dye removal. Radiat Phys Chem. 2017;134:47–55.10.1016/j.radphyschem.2017.01.021Search in Google Scholar
[202] Ghobashy MM, El-Sawy NM, Kodous AS. Nanocomposite of cosubstituted carbonated hydroxyapatite fabricated inside poly (sodium hyaluronate-acrylamide) hydrogel template prepared by gamma radiation for osteoblast cell regeneration. Radiat Phys Chem. 2021;183:109408.10.1016/j.radphyschem.2021.109408Search in Google Scholar
[203] Ghobashy MM, El-Damhougy BK, El-Wahab HA, Madani M, Amin MA, Naser AEM, et al. Controlling radiation degradation of a CMC solution to optimize the swelling of acrylic acid hydrogel as water and fertilizer carriers. Polym Adv Technol. 2021;32(2):514–24.10.1002/pat.5105Search in Google Scholar
[204] Younis SA, Ghobashy MM, Bassioni G, Gupta AK. Tailored functionalized polymer nanoparticles using gamma radiation for selected adsorption of barium and strontium in oilfield wastewater. Arab J Chem. 2020;13(2):3762–74.10.1016/j.arabjc.2018.12.010Search in Google Scholar
[205] Mohamady Ghobashy M, Sayed WAA, El-Helaly A. Impact of silver nanoparticles synthesized by irradiated polyvinylpyrrolidone on spodoptera littoralis nucleopolyhedrosis virus activity. J Polym Environ. 2021;29:3364–74.10.1007/s10924-021-02116-3Search in Google Scholar
[206] Jang JT, Nah H, Lee JH, Moon SH, Kim MG, Cheon J. Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew Chem Int Ed Engl. 2009;48(7):1234–8.10.1002/anie.200805149Search in Google Scholar PubMed
[207] Al-Qubaisi MS, Rasedee A, Flaifel MH, Ahmad SH, Hussein-Al-Ali S, Hussein MZ, et al. Cytotoxicity of nickel zinc ferrite nanoparticles on cancer cells of epithelial origin. Int J Nanomed. 2013;8:2497–508.10.2147/IJN.S42367Search in Google Scholar PubMed PubMed Central
[208] Lartigue L, Wilhelm C, Servais J, Factor C, Dencausse A, Bacri J-C, et al. Nanomagnetic sensing of blood plasma protein interactions with iron oxide nanoparticles: impact on macrophage uptake. ACS Nano. 2012;6(3):2665–78.10.1021/nn300060uSearch in Google Scholar PubMed
[209] Mitamura S, Ikawa H, Mizuno N, Kaziro Y, Itoh H. Cytosolic nuclease activated by caspase-3 and inhibited by DFF-45. Biochem Biophys Res Commun. 1998;243(2):480–4.10.1006/bbrc.1998.8122Search in Google Scholar PubMed
[210] Ahamed M, Akhtar MJ, Siddiqui MA, Ahmad J, Musarrat J, Al-Khedhairy AA, et al. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology. 2011;283(2–3):101–8.10.1016/j.tox.2011.02.010Search in Google Scholar PubMed
[211] Hathaway HJ, Butler KS, Adolphi NL, Lovato DM, Belfon R, Fegan D, et al. Detection of breast cancer cells using targeted magnetic nanoparticles and ultra-sensitive magnetic field sensors. Breast Cancer Res. 2011;13(5):R108.10.1186/bcr3050Search in Google Scholar PubMed PubMed Central
[212] Sarala E, Madhukara Naik M, Vinuth M, Rami Reddy YV, Sujatha HR. Green synthesis of Lawsonia inermis-mediated zinc ferrite nanoparticles for magnetic studies and anticancer activity against breast cancer (MCF-7) cell lines. J Mater Sci Mater Electron. 2020;31(11):8589–96.10.1007/s10854-020-03394-8Search in Google Scholar
[213] Karthik K, Dhanuskodi S, Gobinath C, Prabukumar S, Sivaramakrishnan S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J Photochem Photobiol B Biol. 2019;190:8–20.10.1016/j.jphotobiol.2018.11.001Search in Google Scholar PubMed
[214] Kanagesan S, Hashim M, Aziz AB, Ismail S, Tamilselvan I, Alitheen SNB, et al. Evaluation of antioxidant and cytotoxicity activities of copper ferrite (CuFe2O4) and zinc ferrite (ZnFe2O4) nanoparticles synthesized by sol–gel self-combustion method. Appl Sci. 2016;6(9):184.10.3390/app6090184Search in Google Scholar
[215] Karthik K, Shashank M, Revathi V, Tatarchuk T. Facile microwave-assisted green synthesis of NiO nanoparticles from Andrographis paniculata leaf extract and evaluation of their photocatalytic and anticancer activities. Mol Cryst Liq Cryst. 2018;673(1):70–80.10.1080/15421406.2019.1578495Search in Google Scholar
[216] Foroughi F, Hassanzadeh-Tabrizi SA, Bigham A. In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposite as a magnetic drug delivery system. Mater Sci Eng C. 2016;68:774–9.10.1016/j.msec.2016.07.028Search in Google Scholar PubMed
[217] John Ł, Janeta M, Szafert S. Designing of macroporous magnetic bioscaffold based on functionalized methacrylate network covered by hydroxyapatites and doped with nano-MgFe2O4 for potential cancer hyperthermia therapy. Mater Sci Eng C. 2017;78:901–11.10.1016/j.msec.2017.04.133Search in Google Scholar PubMed
[218] Manohar A, Krishnamoorthi C. Photocatalytic study and superparamagnetic nature of Zn-doped MgFe2O4 colloidal size nanocrystals prepared by solvothermal reflux method. J Photochem Photobiol B Biol. 2017;173:456–65.10.1016/j.jphotobiol.2017.06.025Search in Google Scholar PubMed
[219] Selvam R, Ramasamy S, Mohiyuddin S, Enoch IVMV, Gopinath P, Filimonov D. Molecular encapsulator–appended poly(vinyl alcohol) shroud on ferrite nanoparticles. Augmented cancer–drug loading and anticancer property. Mater Sci Eng C. 2018;93:125–33.10.1016/j.msec.2018.07.058Search in Google Scholar PubMed
[220] Her S, Jaffray DA, Allen C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv Drug Deliv Rev. 2017;109:84–101.10.1016/j.addr.2015.12.012Search in Google Scholar PubMed
[221] Meidanchi A, Motamed A. Preparation, characterization and in vitro evaluation of magnesium ferrite superparamagnetic nanoparticles as a novel radiosensitizer of breast cancer cells. Ceram Int. 2020;46(11, Part A):17577–83.10.1016/j.ceramint.2020.04.057Search in Google Scholar
[222] Meidanchi A. Mg(1−x)CuxFe2O4 superparamagnetic nanoparticles as nano-radiosensitizer agents in radiotherapy of MCF-7 human breast cancer cells. Nanotechnology. 2020;31(32):325706.10.1088/1361-6528/ab8cf2Search in Google Scholar PubMed
[223] Ghobashy MM, Mohamed TM. Radiation preparation of conducting nanocomposite membrane based on (copper/polyacrylic acid/poly vinyl alcohol) for rapid colorimetric sensor of mercury and silver ions. J Inorg Organomet Polym Mater. 2018;28(6):2297–305.10.1007/s10904-018-0882-zSearch in Google Scholar
[224] Abdel-Fattah AA, Soliman YS, Ghobashy MM. Synthesis and characterization of conjugated and nanostructured poly (propargyl alcohol) polymers. J Polym Res. 2018;25(4):1–13.10.1007/s10965-018-1496-4Search in Google Scholar
[225] Ghobashy MM, Abd Elkodous M, Shabaka SH, Younis SA, Alshangiti DM, Madani M, et al. An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment. Nanotechnol Rev. 2021;10(1):954–77.10.1515/ntrev-2021-0066Search in Google Scholar
© 2022 Mohamed Ibrahim Ahmed Abdel Maksoud et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption


![Figure 1
Global cancer statistics of prevalence and death worldwide [2,20].](/document/doi/10.1515/ntrev-2022-0027/asset/graphic/j_ntrev-2022-0027_fig_001.jpg)
