Abstract
This review deals with the evolution of bio-based packaging and the emergence of various nanotechnologies for primary food packaging. The end-of life issues of packaging is discussed and particularly the environmental problems associated with microplastics in the marine environment, which serve as a vector for the assimilation of persistent organic pollutants in the oceans and are transported into the food chain via marine and wild life. The use of biodegradable polymers has been a primary route to alleviate these environmental problems, but for various reasons the market has not developed at a sufficient pace that would cope with the mentioned environmental issues. Currently, the biodegradable plastics only constitute a small fraction of the fossil-based plastic market. Fossil-based plastics are, however, indispensable for food safety and minimization of food waste, and are not only cheap, but has generally more suitable mechanical and barrier properties compared to biodegradable polymers. More recently, various nanotechnologies such as the use of nanoclays, nanocellulose, layer-by-layer technologies and polyelectrolyte complexes have emerged as viable technologies to make oxygen and water vapor barriers suitable for food packaging. These technological developments are highlighted as well as issues like biodegradation, recycling, legislation issues and safety and toxicity of these nanotechnologies.
Introduction
Synthetic polymer plastic materials are manufactured and designed to various needs to mankind in the global economy. The largest use segment of synthetic plastics is in various packaging applications. Whereas sustainable paper and board materials are indispensable for secondary and tertiary packaging, synthetic polymers are needed in food and electronic packaging because the necessity to create barriers to oxygen and water vapor. Such barriers are necessary for food packaging both to get fresh food and avoid infections and various diseases and to delay food spoilage and, hence, avoid food waste. Total yearly world production of synthetic polymers 2016 was currently 335 million tonnes and these polymers are predominantly based on fossil resources. Thus, there is a great challenge to replace fossil resources with renewable materials. Synthetic polymers are primarily designed to meet performance and durability and not for recyclability and degradability, which has resulted in a strong growth of disposed polymers on both land and in marine environments. The current waste disposable systems are landfilling, incineration, composting and mechanical recycling. The vast majority of plastic waste (40 %) finds its way into landfills, 25 % is incinerated, while another 22 % is unmanaged dumps and rejected in recycling (Degan and Shinde 2019). Landfilling will destroy the soil quality and is a disaster for the marine fauna, whereas incineration of used plastic requires extensive cleaning of flue gases. Mechanical recycling has been a temporary recovery but the volumes are generally too small. Composting is a viable technology, ignoring the material waste and economics, but is also depending on the efficiency of the biodegradation. Hence, the major strategy during the past decades has been to use biodegradable polymers such as poly(lactide), polybutylene succinate, polyalkanoates etc. and various natural biopolymers from plants, wooden materials, seaweeds etc.
There is a rich number of scientific approaches to develop biodegradable materials and many excellent reviews in this area, to which a few reviews such as Delidovich et al. (2016), Galbis et al. (2016), Gandini et al. (2016), Schneiderman and Hillmyer (2017) can be suggested.
The substitution of fossil plastics with biodegradable materials in all different areas of food packaging remains difficult given the broad spectrum of functionality offered by fossil-based polymers. In addition to their high water sensitivity, the properties of biodegradable polymers make them economically uncompetitive in comparison with those of conventional plastic polymers, particularly regarding their limited barrier properties. This remains an ultimate technical challenge in this field.
Apart from the developments of biodegradable polymers and natural biopolymers there is the demand of the circular economy approach to sustainable polymers namely the chemical recyclable polymers (Hong and Chen 2017, MacArthur and McKinsey 2017, 2019).
An interesting recent paper has also discussed such a circular economy approach to poly(lactide) (Payne et al. 2019).
This contribution intends to review the most available biodegradable polymers and discuss the most popular approaches to develop food packaging materials during the past decades. It is also recognized that, there are extensive general reviews of bio-based packaging that has recently been published, e. g., Helanto et al. (2019). Whereas, there is a long story of biodegradable polymers, particularly in the food packaging segment of edible packaging, the market for bioplastics (except edible packaging) is still very small compared to fossil-based plastics. During the latest two decades, there has also been a focus on various nanotechnologies, like nanocellulose and nanoclay composites, and layer-by layer (Lbl) technologies together with polyelectrolyte complexes, which have added a niche of interesting developments, albeit the fact that nanotechnology has its own various issues such as toxicity, legislation issues and customer suspicion.
Our ambition has been to provide a comprehensive view of major bioplastics and nanotechnologies and also including the field of plastic problems in the marine environment, end-of life issues of plastics (recycling, legislative issues, safety issues) under current focus today.
The choice of selecting food packaging is that this is the field of packaging that has an immense complexity and is the largest field of packaging materials and also the most need of developments because of the need of sustainability packaging and the current environmental issues.
Biodegradation of plastics in compost, soil and marine environments
Composting is a process in which the organic matter is converted to
The degradation of fossil based plastics results in very little mineralization (conversion into biomass,
Soil environments contain a vast number of microorganisms, which enable degradation of plastics to be more feasible than in other environments like water and air. Generally, soils are very different depending on their composition (microorganisms, pH, temperature, moisture etc.), but it is expected that lower temperatures, a higher crystallinity of the polymer will decrease the biodegradation rate.
Poly(lactide) is a major bioplastic and has several packaging application areas, has high crystallinity and has desirable properties for the packaging segment, but higher temperatures are required for biodegradation as in industrial composting. Whereas, microorganisms and enzymes are known to degrade polylactic acid (PLA) non-enzymatic hydrolysis is the major degradation mechanism for PLA. It has been observed (Narancic and O’Connor 2019) that there is a very slow degradation, taking over three decades, for PLA in soils. By using certain blends between PLA and other biocompostable polymers, and using a smart design, possibilities could open up for faster biodegradation. It can be concluded that biodegradable plastics must be managed, and biodegradable plastics cannot be seen as a solution for the current plastic pollution problems (Narancic and O’Connor 2019, Narancic et al. 2018, Scaffaro et al. 2019, Yin and Yang 2020).
There has been a major focus in society for the end-of-life management of biodegradable plastics using industrial composting, but another option is anaerobic gasification, and this option have also been investigated with various bioplastics with successful results (Narancic et al. 2018).
Plastic makes up about 80 to 85 % of marine litter (Auta et al. 2017) and this constitutes a major problem.
In general, the plastic waste is consigned to landfills. However, large amounts end up as marine debris as a result of insufficient treatment capacity, accidental inputs, littering, illegal dumping and coastal human activities. It’s calculated that 275 million metric tons (MT) of plastic waste was generated in 192 coastal countries in 2010, with 4.8–12.7 million MT entering the ocean, which is approximately 60–80 % of the marine litter (Jambeck et al. 2015).
Although the defined size of these microparticles has varied between different studies over the past decade, the National Oceanic and Atmospheric Administration (NOAA) now defines the term “microplastic” as fragments smaller than 5 mm in diameter (Horton et al. 2017). In the marine environment, microplastics can be generated from primary sources and enter directly into the environment as plastic pellets that were used as raw material in the plastic industry and/or in hygiene and personal care products (Cole et al. 2011). They may also enter indirectly from secondary sources (e. g. food packaging), such as fragments and fibres obtained from the fragmentation of larger plastic debris.
Microplastics are easily taken for zooplankton, a common food source for the marine environment, and therefore end up in stomachs of various marine organisms. Hence, through direct and indirect ingestion of microplastics, it ends up in the marine food chain. This is critical as hydrophobic plastics tend to adsorb hydrophobic organic pollutants such as polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs) to a very high extent (Tanaka et al. 2020, Yeo et al. 2019). Hence, microplastics can serve as a vector for the assimilation of persistent organic pollutants and heavy metals by marine organisms in the environment.
As indicated before, the degradation of plastics in marine environments, is different and there are, at least, three important degradation mechanism for plastics in oceans (Min et al. 2020). Firstly, bacteria colonize the surface (depending on the surface energy) of plastics and have a propensity for biofilm formation and this provides an opportunity for biodegradation in the form of mass loss via surface erosion. There are many bacteria and microbial enzymes that facilitate surface erosion. Typical rates for these processes decrease as follows:
Secondly, abiotic hydrolysis of functional groups, like esters, which reduce the molecular weight (
Finally, the exposure of ultraviolet (UV) radiation and oxygen causes photodegradation and results in a decrease in
This is the theory, but practical experiments regarding the fate of biodegradable polymers in freshwater and seawater has concluded that even after more than a year, there is very little degradation of polymers classified as biodegradable polymers. The authors tested these biodegradable polymers: Polycaprolactone (PCL), polylactic acid (PLA), Poly(3-hydroxybutyrate) (PHB) and poly(lactic-co-glycolic acid) (PLGA) and it was only PLGA that was degraded within a year. Neither was there any difference between the degradation in freshwater and seawater (Bagheri et al. 2017). This fact should be considered in the following text, where we follow the standard classification of biodegradable polymers.
Recycling
A wide range of technologies are currently used for waste pre-treatment and sorting. These range from manual dismantling and picking to automated processes such as shredding, sieving, air or liquid density separation, magnetic separation, and highly sophisticated spectrophotometric sorting technologies such as UV/visible spectroscopy (VIS), near infrared (NIR), Laser, etc. (www.plasticseurope.org).
Plastic fractions contained in complex waste streams may not be easily enough sortable. In such cases, alternative recovery solutions exist, such as feedstock/chemical recycling or recovery of the calorific value of the plastic waste to substitute conventional fuels.
Mechanical recycling of plastics refers to the processing of plastics waste into secondary raw material or products without significantly changing the chemical structure of the material. The difficulty of plastic sorting logistics, washing and drying before the secondary melt processing also leads to thermomechanical degradation due to high operating temperatures resulting in deterioration of the polymer properties.
These problems associated with the recycling and degradation of synthetic plastics also motivates the development of biobased, degradable materials.
The concept of a circular economy suggests that chemical recovery may be a viable route. An end-of-life (EOL) analysis suggests that chemical recovery has several interesting options (Hong and Chen 2017, McKeown et al. 2019, Payne et al. 2019). For instance, the conversion of PLA to LA has by the US Department of Energy been identified as a future platform chemical with its plethora of commodity chemicals.
Legislative actions
The first comprehensive piece of EU legislation on packaging waste entered into force in 1994 with Directive 94/62 EC on Packaging and Packaging Waste (PPWD). The directive deals with the problems of packaging waste and setting targets for recovery and recycling (eur-lex.europa.eu, document 31994L0062). Recently the EU Green Deal Policy (European commission, Brussels 11.12.2019) was taken. Strategies for inter alia plastics waste published are the Circular Economy 2.0 (March 11, 2020) and the Industrial Strategy (March 10, 2020). The circular economy addresses all plastics packaging placed on the EU market, either as reusable, or to be recycled in a cost-efficient way by 2030. Further, more than 50 % of the plastic waste is to be recycled and 55 % recycled content is required in plastic packagings. Microplastics are specifically addressed with restrictions, developing labelling, standardisation and regulatory measures on unintentional release. The Commission will address sustainability challenges on sourcing, labelling and use of bio-based plastics and the use of biodegradable plastics (eur-lex.europa.eu, document 52020DC0098).
The industrial strategy taken is directed towards requirements for sustainable products, obligation to sustainable environmental claims through standard approaches. Essential is also to foster new markets for climate-neutral and circular products, having a transparency towards consumers about carbon and environmental footprints (ec.europa.eu March 10, 2020).
The single use plastic (SUP) directive was proposed 2018 which is a part of the EU plastic strategy in a circular economy. The main objectives are to phase out “unnecessary” single use plastics and reduce consumption, production and introduce incentives towards reusable systems. The SUP directive will be effective earliest mid-2021. The SUP directive is taken into account in many European countries by plastic bags bans. In the proposal it is voluntary to determine reduction targets and offer alternative products within each country, which has given rise to differences in enforcement.
Also, world-wide actions to ban plastic bags usage have been introduced in 74 countries with varying degrees of enforcement and in 37 countries a charge per bag has been taken. Expanded polystyrene (EPS) is also banned in many countries (The Swedish government official investigations 2018:84).
Food packaging demands
Different foods have different demands on packaging. Table 1 shows the maximum gain of oxygen to maintain the freshness and the maximum water gain or loss. There are many quality factors for foods such as colour, oxidation, microbiology, structure, flavour, enzymatic degradation, photooxidation and chemical changes such as hydrolysis, protein denaturation, cross-linking etc. This means that food packaging has a very high complexity in developing the optimum packaging strategy for various foods. Certain examples are given below to show the complexity for the demand of various foods, see also a very good early review by Petersen et al. (1999). Exposure to oxygen can cause deterioration of colours, aromas, vitamins and induce oxidation. These foods benefit from packaging that can maintain a vacuum/nitrogen that will provide a barrier to oxygen. Fresh meat, poultry, pasta products benefit from environments that can maintain either a vacuum or a targeted concentration of oxygen and high concentration of carbon dioxide to prevent oxidation or microbial growth. Fresh meat can be the subject of enzymatic, microbial and physical changes and demand a high oxygen concentration by using modified atmospheric packaging (MAP) with oxygen contents of 70–80 % O2 and 20–30 %
Seafood is subjected to autolysis caused by intrinsic enzymes, metabolic activity by microorganisms and oxidation. High fat fish demands low oxygen contents in order to avoid oxidation and a moderate water vapor barrier is also demanded. Breads, cakes, crackers and chocolate demand a high moisture barrier and a low oxygen barrier to avoid oxidation of fatty products. Fruits and vegetables are living organisms requiring O2 and produce
Approximate critical amounts of oxygen and moisture that foods can stand in order to maintain fresh. Adapted from Robertson (2006).
| Food or beverage | Maximum oxygen gain (ppm) | Maximum water gain or loss |
| Canned milk, vegetables and fresh foods | 1–5 | 3 % loss |
| Wines and beer | 1–5 | 3 % loss |
| Instant coffee | 1–5 | 2 % gain |
| Canned fruits | 5–15 | 3 % loss |
| Dried foods | 5–15 | 1 % gain |
| Nuts and snacks | 5–15 | 5 % gain |
| Fruit juices and carbonated drinks | 10–40 | 3 % loss |
| Oils and salad dressings | 50–200 | 10 % gain |
| Jellies, syrups, pickles, olives and vinegar | 50–200 | 3 % loss |
| Liquors | 50–200 | 3 % loss |
| Condiments | 50–200 | 1 % gain |
| Peanut butter | 50–200 | 10 % gain |
The water activity affects the food quality in a number of different ways. Figure 1 shows the relative reaction rate of lipid oxidation, non-enzymatic browning, hydrolytic reactions, enzyme activity, mould, yeast and bacterial growth. Interestingly, a too low water activity allows an increased oxidation of lipids, whereas in zone II oxidation decreases, but tend to increase in the bulk zone III. In conclusion, the barrier properties (the oxygen and water vapour permeability) are the key properties in the design of food packaging materials. There are many reviews on applications of bioplastics for food packaging, e. g., Jiménez et al. (2012), Lagarón et al. (2016), Peelman et al. (2013).
Food packaging materials
A comparison of bio-based polymers relatively to synthetic polymers with respect to their oxygen transmission rate and water vapour transmittance is given in Figure 2. From Figure 2, the following conclusions can be made:
The superior performance of ethylene vinyl alcohol (EVOH) in the oxygen transmission rate (OTR) cannot be met by PLA or polyhydroxyalkanoates (PHA).
As far as the water vapor transmittance (WVT) is concerned, the PHA is close to low-density polyethene (LDPE).
Neither PLA or PHA are good gas barriers.
Polyvinylidene chloride is the only polymer with both an excellent gas barrier and water vapour barrier.

Barrier properties of some bio-based materials versus foil-based packaging materials. a) oxygen transmission rate; b) water vapour transmittance (Rastogi and Samyn 2015).

Material coordinate system of bioplastics: EVOH: ethylene vinyl alcohol; PA: polyamide; PBAT: polybuthylene adipate terephthalate; PE: polyethylene; PE-HD: high density polyethylene; PE-LD: low density polyethylene; PET: poly(ethylene terephthalate); PHA: polyhydroxyalkanoate; PHB: polyhydroxybutyrate; PLA: polylactic acid; PP: polypropylene; PS: polystyrene; PTT: polytrimethylene terephthalate; PVA: polyvinyl alcohol; PVC: polyvinyl chloride; TPS: thermoplastic starch. Adopted from Rujnić-Sokele and Pilipović (2017).
It should be considered that there are many nanocoatings, that were developed in the mid 1980s, such as thin glass-like
Bioplastics
The term bioplastics was coined by the European Bioplastics 2016 and there are some confusions about of the world “bioplastic”. Bioplastics are defined as biodegradable, biobased or both. A material coordinate for bioplastics is given in Figure 3. Hence there are four different groups of plastics:
One group that refer to classical plastics that are not biodegradable and made from fossil based polymers, such as polyethylene, polystyrene etc.
A second group is plastics made from biomass feedstock and are biodegradable. Examples in this group are cellulose, starches and polyesters such as PLA and PHA.
A third group are biodegradable plastics from fossil resources. Examples in this group are polycaprolactone (PCL), polybutylene succinate (PBS) and poly(butylene adipate-co-terephthalate) (PBAT).
The fourth group is non-biodegradable plastics that are produced from biomass. Example are bio-polyethylene (PE), produced from bioethanol fuel produced from sugar cane.

The projected global production of bioplastics 2024 (European Plastics).
Poly (lactic acid)
PLA is a well-known biobased plastic that has attracted immense attention over the past few decades (Auras et al. 2011, Castro-Aguirre et al. 2016, Garlotta 2002, Rabnawaz et al. 2017) and has enjoyed many reviews. PLA is commercially synthesized via ring opening polymerization via lactides in a two-step process. In nature, lactides predominantly exist as the L-lactide isomer along with a few % of its D-lactide isomer. Some selected properties of PLA are shown in Table 2 together with the properties of PHA polypropylene (PP) and poly (ethylene terephthalate) (PET).
Commercial PLA for the packaging industry generally has a L-lactide content in excess of 92 %. The higher the L-lactide content, the higher is the crystallinity and at a higher content of D-lactide, PLA becomes amorphous and, hence, deteriorating the barrier properties. Interestingly, in contrast to proteins and polysaccharides the oxygen permeability of PLA decreases with a higher relative humidity, possibly because water blocks the pathway through which oxygen diffuses through the plastic. PLA has, however, a poor barrier for water vapor. Compared to PP, the water barrier performance of PLA is only 10 % of the barrier performance of PP. PLA has 2–3 times higher modulus than PP and the tensile strength is higher than for PHAs. Manufacturing of PLA-plastic does not create any problems and blow moulding, extrusion and thermoforming can be conducted similar to other thermoplastics. Typical applications of PLA are in compost bags, food packaging, and disposable tableware.
There are numerous manufacturers of PLA, see, e. g., lists in Armentano et al. (2013), Castro-Aguirre et al. (2016), Scaffaro et al. (2016). There is an extensive number of PLA/polysaccharide composites reported, see, e. g., Scaffaro et al. (2016) as well as reviews on nanostructured PLA and polyester materials, see, e. g., Armentano et al. (2013), Bordes et al. (2009).
Selected properties of various polyesters and polypropylene (PP). Abbreviations: polyhydroxyalkanoate (PHA), polylactic acid(PLA), poly (ethylene terephthalate) (PET), Water vapour transmitance (WVTR). Adapted from Rabnawaz et al. (2017).
| Property (units) | PHAs | PLA | PP | PET |
| 2 | 66 | −10 | 73–80 | |
| 160–175 | 141 | 160–175 | 245–265 | |
| Modulus (GPa) | 1–2 | 2.3–2.8 | 1.1–1.5 | 2.7–4.1 |
| Tensile strength (MPa) | 15–40 | 74–82 | 31–42 | 48.2–72.3 |
| Elongation at break (%) | 1–15 | 78–97 | 400 | 30–3000 |
| 27.3 | 28.9 | 1.2–6.9 | 4.5–5.9 | |
| 0.75–1.9 | 2–2.9 | 23.8 | 0.2–0.49 |
Polyhydroxyalkanoate
PHAs are promising polyesters produced by bacteria through aerobic fermentation of various carbon sources. (Anjum et al. 2016, Rabnawaz et al. 2017, Samori et al. 2015). PHAs can also be prepared synthetically. The simplest member of the PHA-family is poly(hydroxybutanolate) (PHB), which is prepared via a fermentative approach. Bacteria-based PHB is isotactic (repeating conformic), whereas PHBs that are prepared from butyrolactone are atactic (non conformic) with a lower melting point.
Important is that the large majority of current biodegradable polymers can only biodegrade under very specific conditions of constant high temperature and humidity in industrial composting installations and are not fit for home composting nor do they compost in reasonable time when littered. PHAs are completely biodegradable in both soil and marine conditions in contrast to PLA polymers (Guillard et al. 2018).
The oxygen barrier properties are good, and twice as good as the oxygen barrier of PLA. The reasonable barrier properties along with the biodegradable nature of PHAs have facilitated the development of many commercial products based on this family of polymers (Bugnicourt et al. 2014). Yet, PHAs have limited applications in the packaging industry because of the current high production costs of these polymers.
In the 80s, Imperial Chemical industries developed poly (3-hydroxybutyrate-co-3-hydroxyvalerate) obtained via fermentation under the trade name of Biopol and was distributed in the US by Monsanto and later by Metabolix.
Polybutylene succinate
Polybutylene succinate (Gigli et al. 2016, Xu and Guo 2010) is a biodegradable aliphatic polyester with similar properties to those of PET. PBS is produced by condensation of 1,4-butandiol and succinic acid. Conventional processes for the production of 1,4-butandiol use fossil feedstocks. The bio-based process involves the use of glucose to produce succinic acid followed by chemical reduction to produce 1,4-butandiol.
PBS is a semi-crystalline polymer with a higher melting point than polylactic acid. In comparison with PLA, PBS is tougher but with lower rigidity and Young’s modulus, but by changing the monomer composition mechanical properties can be tuned to suit applications. PBS decomposes naturally in water (Xu and Guo 2010) and has therefore poor water resistance and limited gas barrier properties, but better than PLA (Genovese et al. 2016). PBS can be processed into films, bags, or boxes for food packaging and has also other industrial applications, see, e. g., Babu et al. (2013).
The Japanese company Showa High Polymer started production of PBS 1993, and sold PBS under the trade name Bionolle, but terminated their production 2016. Other manufacturers include Mitsubishi Chemicals, and Chinese producers Hexing Chemicals and Xinfu Pharmaceutical and IRE Chemical in Korea (Babu et al. 2013).
Poly(butylene adipate terephthalate)
PBAT is synthesized (Zhao et al. 2010) from the polymer of 1,4-butanediol and adipic acid and the polymer of dimethyl terephthalate (DMT) with 1,4-butanediol. The polymer is a fully biodegradable polymer. The polymer has very limited water vapor resistance (Xie et al. 2016) and has a higher oxygen permeance than PLA. As with most biodegradable polymers, there are an extensive number of composite formulations that have been investigated with PBAT (Ferreira et al. 2019). Highlighted applications by manufacturers are cling wrap for food packaging, compostable plastic bags and water resistant coatings for various materials.
PBAT is produced commercially by many companies such as BASF under the name Ecoflex® and in a blend with poly(lactic acid) called Ecovio, by Novamont as Origo-Bi® and in a blend with starch called Mater-Bi®, by Zhuhai Wango Chemical Co Ltd under the name Wango®, by JinHui Zhaolong as Ecoworld® and in a blend with starch called Ecowill®, and by Eastman Chemical as Eastar Bio® etc.
Polysaccharides
Polysaccharides are long-chain polymers formed from mono- or disaccharide repeating units joined together by glycosidic bonds. As a result of the large number of hydroxyl groups, there is extensive hydrogen bonding, which are important for film formation and for the characteristics of the films.
A variety of polysaccharides and their derivatives have been tested for potential use as edible packaging because they are abundant, low cost, and easy to handle.
Polysaccharides have good film forming properties and exhibit good mechanical and gas barrier properties and are efficient barriers against oil and lipids but offer little resistance to water migration.
Polysaccharides can easily be modified to improve their physiochemical properties by salt addition, solvent changes, heat gelatinization, pH changes, chemical modification of hydroxyl groups and cross-linking of polysaccharides, hydrolysis of polysaccharides, and employing various nanotechnology options (Nesic et al. 2019).
Starch
Starch is composed of two polymers, amylose and amylopectin. There are many reviews in this field such as Cazón et al. (2017), Jiménez et al. (2012), Thakur et al. (2019). Native starch molecules arrange themselves in the form of starch granules in which amylose and amylopectin are structured by hydrogen bonding in a semi-crystalline network.
The manufacturing of starch films is obtained by two techniques: the dry process and the wet process. The dry process is based on extrusion of thermoplastic starch (TPS). In this method the starch is plasticized and heated above its glass transition temperature in conditions of low water contents. In the wet process the starch is gelatinized, whereupon a film is formed and dried. The wet process is preferred to form edible preformed films, or to apply coatings by dipping, brushing or spraying. Nevertheless, the dry method is preferred for industrial applications.
Starch films have a good oxygen barrier at a low RH, but high water vapor permeability. It’s common to use lipids in combination with starch and the most popular plasticizers are glycerol and sorbitol. The hydrophilic nature of starch and retrogradation phenomena limit their usefulness. Edible starch films and coatings are used in bakery, fresh fruits, confectionary and meat products.
Cellulose, hemicelluloses and its derivatives
Cellulose is composed of (1-4)-α-D-glucopyranosyl units and is insoluble in water and paper materials do not constitute any significant barrier properties, with the exception micro/nanobased cellulosic materials, discussed separately below. On the other hand, there has been extensive research activities over many years in order to combine bioplastics with paper/paperboard materials, e. g., Andersson (2008), Aulin and Lindström (2011), Khwaldia et al. (2010), Rastogi and Samyn (2015), Su et al. (2018). Water soluble derivatives can be formed by etherification and the commonly commercial cellulose ethers are: methyl cellulose, carboxymethyl cellulose (Paunonen 2013), hydroxypropyl cellulose, hydroxypropyl methyl cellulose (Ghadermazi et al. 2019), which all have good film forming properties. Coatings of these derivatives have been applied to various foods to provide barriers to moisture, oxygen and oil resistance (Janjarasskul and Krochta 2010), but none of these derivatives have gas barrier properties at the high end of the scale.
There have also been extensive activities using hemicellulose barriers, e. g., review by Ibn Yaich et al. (2015).
Chitosan
Chitin is a β-1,4-linked linear polymer of 2-acetamido-2-deoxy-D-glucopyranosyl residues. It is made by treating the chitin shells of shrimp and other crustaceans with an alkaline substance, like NaOH. The cationic amino groups of chitosan confers opportunities for chemical modification because cationic groups can react with negatively charged groups on polysaccharides such as carboxymethyl cellulose, alginate, pectins and various proteins, extracts like beeswax and synthetic polymers, and therefore there has been extensive investigations of chitin films recently, see reviews (Dufresne et al. 2013, Elsabee and Abdou 2013, Wang et al. 2018). Another advantage of chitosan is the antibacterial properties of this polymer. The cationicity has also been investigated with nanoclays, layer-by layer films as well as polyelectrolyte complexes for food packaging. A drawback is that chitosan is expensive, so new technologies are still needed for mass-production of chitosan-based films to meet packaging applications.
Pectin
Pectins are water-soluble anionic polymers composed mainly of (1 → 4)-α-D-galactopyranosyluronic acid units. Pectins with a degree of esterification (DE) above 50 % are labeled high-methoxyl pectin (HMP) and those below 50 % are termed low-methoxyl pectin (LMP). The differences in methyl ester content and DE affect solubility and gelation properties of pectin. The main industrial sources for pectins are apple pomace and citrus peels. In general, edible films like pectin do not pretend to replace traditional food packaging materials, but is used for specific food related functions like antimicrobial functions, such as anti-browning, texture enhancers, probiotics, flavors etc. The manufacture of films is done by using the casting method or extrusion (Espitia et al. 2014, Janjarasskul and Krochta 2010).
Alginate
Alginate is a naturally occurring polysaccharide and is mainly derived from brown algae species. Alginate is a linear (1 → 4) linked polyuronic acid molecule containing three types of block structures. These highly anionic polymers have the ability to form strong gel structures by reacting with multivalent cations.
Monovalent salts of alginates are water soluble, but the solubility is limited at low pH-values. Alginate films can be formed from evaporating solvent from alginate gel or by a two-step procedure that involves drying of alginate solution followed by treatment with a calcium salt solution to induce cross-linking at the interface. Film strength and permeability can be altered by the concentration and type of polyvalent cations.
The rate of its addition and time of exposure, pH, temperature, and the presence of composite constituents are important. Owing to their good O2 barrier properties, alginate coatings can protect foods against oxidation but has a high water vapor permeability (Janjarasskul and Krochta 2010, Kester and Fennema 1986, Senturk Parreidt et al. 2018).
Carrageenan
Carrageenan is a complex mixture of several polysaccharides. Three principal carrageenan fractions, kappa (κ), iota (ι), and lambda (λ), differ in sulfate ester and 3,6-anhydro-α-D-galactopyranosyl content, which results in various degrees of negative charge and solubility in water. Thermo-reversible carrageenan gels can be used as food coatings to retard moisture loss from an enrobed food by acting as a sacrificing agent. Carrageenan has been applied to a variety of foods to carry antimicrobials and to reduce moisture loss, oxidation, or disintegration (Janjarasskul and Krochta 2010, Kester and Fennema 1986).
Proteins
Proteins are made up of many different amino acids linked together. Amino acids can be categorized into various groups on the type of interaction they create, such as covalent bonding, ionic, disulfide cross-linking or hydrogen bonding. There are twenty different types of these amino acid building blocks commonly found in plants and animals.
Only few of the many proteins are widely commercially available for packaging applications, and high costs and unfavorable technical properties make many proteins less interesting for packaging. The intrinsic hydrophilicity of proteins results in good adhesion to polar surfaces such as paper materials and they provide good gas barriers but have limited water vapor resistance. Cross-linking plays a fundamental role for proteins in order to enhance their water vapor barriers. Procedures such as thermal crosslinking, enzymes, such as transglutaminase can crosslink proteins and disulfide crosslinking, or chemical crosslinking using, e. g., glutaraldehyde, formaldehyde and glycol are also common methods. Plasticizers are usually required in the development of protein films in order to acquire the desired physiochemical properties such as material flexibility.
There are two major processes for making protein films: a wet process based on dispersion and solubilization of proteins and a dry process based on the thermoplastic properties of proteins, when they are extruded.
It will be obvious that the proteins have many similarities regarding their barrier properties. The oxygen permeability of proteins is very good at low RH-conditions because of their hydrophilicity, but it also results in a high water vapor permeability. Therefore, developments focus often on multilayering or use of nanocomposites, and the cost for that may be offset if the proteins are cheap byproducts from other manufacturing uses of proteins.
Most proteins have been subjected to various bionano-composite materials and there are many recent reviews on these investigations, e. g., Coltelli et al. (2015), Zink et al. (2016), Zubair and Ullah (2020).
Casein and caseinates
Casein is the principal protein fraction in cow milk, which accounts for 80 % of its total protein content and is used ubiquitously by the food industry. Casein appears as micelles with 50–300 nm micelles and is relatively heat stable. In contrast to whey proteins, caseins lack sulfhydryl-groups, hence they do not show the typical denaturation behavior of proteins.
Caseins are precipitated from the milk by adjusting the pH to the isoelectric point. Casein tends to aggregate once dried but the functionality of the protein can be restored by re-neutralization using alkali. The Na-caseinate (NaCAS) can be dissolved in water and forms easy to films. Like most proteins NaCAS shows a high water vapor permeability, but shows a higher oxygen barrier than non-ionic polysaccharides, and has high cohesive energy density and a low free volume (Miller and Krochta 1997). It has been reported that carnauba wax and glycerol had a strong effect on the water vapor barrier. Whereas glycerol had increased the permeability, the carnauba waxes strongly decreased the permeability. There have been many efforts to crosslink casein in various ways, but the effects of an enhanced water vapor barrier have not been confirmed. On the other hand, the water vapor permeability can be enhanced by molecular weight and developed crystallinity (Coltelli et al. 2015, Khwaldia et al. 2010, Zink et al. 2016).
Whey proteins
Whey protein is the soluble part of the milk and constitutes about 20 % of the total amount of the proteins. As whey protein is a by-product of cheese and casein manufacturing, it’s highly available but still underused due to its high water content and high processing and transportation costs.
In order to manufacture whey proteins, they are processed by membrane and ion exchange chromatography techniques, followed by spray drying to create purified whey protein isolate powders (WPI). WPI contains above 90 % protein based on dry matter and comprise α-lactalbumin and β-lactoglobulin in a ratio of 1:4 when manufactured using the membrane process and somewhat less α-lactalbumin when employing the chromatography process. ß-lactoglobulin contains disulphide bonds and a free thiol group, which can form disulphide bridges once the molecule is unfolded. Due to the crosslinking and intermolecular hydrogen bonds, whey proteins provide excellent oxygen barrier properties and at low RH, the oxygen permeability is in the range of EVOH polymers and is therefore useful to improve the oxygen barrier properties of food packaging. Whey proteins are also useful for oil resistance (Coltelli et al. 2015, Khwaldia et al. 2010, Zink et al. 2016).
Gelatin
Gelatin refers to a purified and modified collagen protein. Collagen is the primary component in animal connective tissue such as cartilages, skins and bones and appears in a fibrous triple helical structure consisting of three proteins.
There are two types (A and B) that are synthesized by partial hydrolysis of collagen proteins. The type A is extracted by acid hydrolysis, whereas the B-type is prepared by solubilization with alkali at a temperature between 60–90 °C. Gelatin is a heterogeneous mixture comprising glycine, 4-hydroxyproline and proline contents. Gelatin has suitable film forming properties with good adhesiveness and good barrier properties against oxygen and aroma but is brittle and plasticizers, e. g., polyvinyl alcohol (PVA) and sorbitol, are necessary for most applications. The best treatments for improving the water vapor barrier has been found to be enzymatic treatments, because of the fact that the polymer is partly hydrophobic. Cross-linking is not significant for the oxygen barrier properties (Coltelli et al. 2015, Khwaldia et al. 2010, Zink et al. 2016).
Wheat gluten
Wheat gluten is known to form elastic networks for a wide range of bakery and other food products since centuries. Gluten is a generic term for more than 50 different electrolyte insoluble wheat flour proteins of different classes representing up to 85 % of all proteins. Gluten contains glutelin, prolamin fractions, referred as glutenin and gliadin, respectively. Although insoluble in most natural waters, wheat gluten can be dissolved in aqueous solutions at high or low pH-values (avoiding the isoelectric point of proteins) at low ionic strength.
The ability of glutenin to form covalent disulphide bonds and covalent bonds with tyrosine side chains account for the elastic properties of gluten networks.
The manufacture takes place by shearing wheat flour with water, which enables the separation and purification of aggregated wheat gluten in industrial scale, while separating the starch, fibres and other components in the water.
Gluten films are typically prepared from aqueous alcoholic protein solutions. The manufacturing of gluten films takes place by solvent coating, aided by mechanical mixing, heating as well as adjustments to acid and alkaline conditions. The breaking of native disulphide bonds takes place during heating, resulting in free thiol groups, which can form new bonds during drying.
As with other hydrophilic proteins, gluten films have good oxygen barriers at low RH while the water vapor barriers are low. Films prepared from the glutenin fraction show higher water vapor permeability than those produced by the gliadin fraction (Coltelli et al. 2015, Khwaldia et al. 2010, Zink et al. 2016).
Soy protein
Proteins from soy consist of two principal components and about 20 % stems from water soluble albumins, whereas the major fraction exists of large globular storage proteins. In soy, these proteins are named ß-conglycinin and glycinin. The aqueous extraction procedure to manufacture soy protein isolates (SPI) is based on alternating increases and decreases in the pH, akin to other protein extraction procedures. The soy protein isolates are usually a by-product from the soybean oil industry. Soybean films show good-film-forming properties and is useful for edible films and coatings and are often produced by aqueous casting. The plasticizer mainly used is glycerol and by using plasticizers, dry processes like extrusion can also be possible. Like other protein films the oxygen permeability is reasonably good, but the water permeability is high, but it has been observed that thermally induced cross-linking of the barriers can decrease the water vapor permeability (Coltelli et al. 2015, Khwaldia et al. 2010, Zink et al. 2016).
Corn Zein
Corn contains all the Osborne fractions of proteins (albumins, globulins, prolamin and glutelin). The prolamin fraction is named corn zein or corn gluten and is the principal component in corn, where it represents about 50 % of the protein followed by glutelin. The protein composition varies, however, widely depending on the corn variety. Zein consists of a polypeptide with a molecular weight of 85 kDa or higher and zein dissolves in 70–80 % aqueous ethanol or pure propanol and is insoluble in water at room temperature. The commercial manufacture of zein is based on products extracted with aqueous-alcoholic and contain a purity greater than 90 %. The manufacturing of zein protein isolates (ZPI) is more sophisticated than proteins from other plants and therefore zein isolates are quite expensive. The barrier properties suffer similar disadvantages as other proteins, so to produce efficient barriers, the developments of multilayering products are considered similar to the above proteins (Coltelli et al. 2015, Khwaldia et al. 2010, Zink et al. 2016).
Lipids, waxes, and resins
Many lipids, waxes and other resin materials have been utilized as protective coatings against moisture transfer. There are disadvantages of employing these materials in packaging materials, such as their waxy taste and texture, greasy surface, and potential rancidity. The lipid component in formulations reduce the water transmission and the hydrocolloid component serve as a gas barrier and provide some strength and structural integrity.
Waxes are esters with a long-chain fatty acid with a long-chain alcohol. Waxes are more resistant to diffusion of water than lipids owing to their low level of polar groups. Typical waxes are carnauba wax, candelilla wax, rice bran wax, beeswax and synthetic waxes such as paraffin wax and petroleum wax and have been used as protective coatings alone or as components with other ingredients. Edible resins such as shellac, terpene and wood resin have also been used in various formulation (Aulin and Ström 2013, Janjarasskul and Krochta 2010, Kester and Fennema 1986).
Nanotechnologies and food packaging
From the very beginning of the emergence of various nanotechnologies, such technologies were considered to have a high potential for food packaging. Nanotechnologies have, however, failed to have the large expected use, mainly because of uncertainties regarding safety issues, life-cycle consideration, recycling etc. which have limited legal and consumer acceptance. Regulatory and toxicity issues, migration issues, as well as environmental considerations and various nanotechnology applications for packaging have been given extensive reviews recently (Wyser et al. 2016, Din et al. 2020).
The range of nanomaterials and nanotechnologies are extremely broad and span into the fields of active and intelligent packaging in various forms such as oxygen scavengers, antimicrobial packaging, freshness indicators, interactive packaging etc, and are not considered in this context.
During the last decades, a number of different routes to develop new types of packaging materials, such as the use of nanocellulose, nanoclays, LbL technology and polyelectrolyte complexes. Apart from these technologies, a general trend in the literature is the focus on different blends of materials, see, e. g., Din et al. (2020).
There is also the field of nano-inspired oxygen barrier coatings, which dates back to the end of the last century, when the Ormocer® coating technology was developed at the Fraunhofer institute (Haas et al. 1999). This was probably one of the first relevant examples for food packaging applications using nanocoatings (Rovera et al. 2020). It should be mentioned that there is a large field of advanced hybrid organic-inorganic nanomaterials with numerous different applications (Sanchez et al. 2011).
A selected number of general reviews are suggested to mirror these developments (Amass et al. 1998, Duncan 2011).
Nanocellulose and food packaging
The original inventors of microfibrillar cellulose (Herrick et al. 1983, Turbak et al. 1983), later called nanofibrillar cellulose or cellulose nanofibrils (CNF) in the late 90s were simply produced by delamination of fibres through high-pressure homogenizers, but later it was understood that chemical/enzymatic treatment and hydrolysis pre-treatments of pulps could decrease the energy consumption during delamination to a large extent and could also produce materials with a higher content of the elementary fibrils.
There are three major families of nanocellulosic materials: CNF (width: 5–60 nm; length over a micrometer), cellulose nanocrystals (CNC) (width 5–70 nm; length 100–250 nm) and bacterial nanocellulose (width 20–100 nm; length exceeding micrometers) (Klemm et al. 2011) and there are numerous reviews on the manufacture and properties of CNF materials in the field, e. g., Klemm et al. (2011), Moon et al. (2011), Isogai et al. (2011), Siró and Plackett (2010) to which the reader is referred to regarding their manufacturing processes and properties. In the context of food packaging materials, the CNF materials are in focus. Apart from the mechanical properties of CNF films and composite materials, their oxygen and moisture permeability together with grease and oil resistance is in focus. It should be noted that CNF materials have been found to be a better gas barrier than CNC materials (Belbekhouche et al. 2011). There is an extensive number of publications and many excellent reviews on the use of CNF materials in the field of packaging, e. g., Abdul Khalil et al. (2016), Bharimalla et al. (2019), de Azeredo et al. (2017), Ferrer et al. (2017), Hubbe et al. (2017), Lavoine et al. (2012), Lavoine et al. (2014), Scaffaro et al. (2016). When it comes to the oxygen barrier properties, it was early recognized that highly charged films produced higher density films compared to non-treated fibres or enzymatically treated films (Aulin et al. 2010a, 2010b, Fujisawa et al. 2011, Minelli et al. 2010, Syverud and Stenius 2009). Basically, the higher the charge density, the higher is the film density and the oxygen permeability decreases the higher the carboxyl group content as illustrated in Table 3.
Another very important factor is the effects of the counterion. It has been found that if the sodium salt is exchanged to a cation of higher valency, e. g.,
Characteristics of sodium carboxylated CNF films on 50 micrometers PET-films at 0 % RH. Adapted from Wang et al. (2017).
| Oxygen permeability ( | Coating (mic.) | Carboxylate (mmol/g) | Dp | Density ( |
| 0.17 | 1 | 1.74 | 1440 | 1.47 |
| 4 | 1,5 | 1.5 | 550 | 1.43 |
| 4 | 1 | 1.4 | 400 | 1.45 |
| 10 | 1 | 1.2 | 550 | 1.11 |
| 107 | 2 | 0.8 | 690 | 1.10 |
| 1293 | 5 | 0.3 | 920 | 1.13 |

Oxygen permeability of the original TOCN-COONa and ion-exchanged TOCN-COOM films under various conditions (Shimizu et al. 2016).
Because of a high crystallinity and a high cohesive energy density such films have a superior oxygen barrier compared to other polymers (Figure 6) but only at a low relative humidity. At a high relative humidity the oxygen barrier is destroyed and the permeability increases with several orders of magnitude (Figure 7). This is by no means surprising as the moisture content swells the films and open up the films for gas transfer. All biodegradable materials sorb moisture so a low oxygen barrier is akin to all biopolymers.
The films have a density close to the density of cellulose, indicating little porosity, but only at higher basis weights than
When it comes to the moisture barrier properties of CNF-films they are inherently inferior because of the moisture uptake. The use of waxes, such as paraffin, candelilla, carnauba, beeswax to decrease the moisture and water barrier properties of paper has been known to papermakers for more than 100 years, see, e. g., Minelli et al. (2010), Donhowe and Fennema (1993) but has also been investigated for nanocellulose, e. g., Spence et al. (2011) and alkyd resins (Aulin and Ström 2013, Dufresne 2018) and beeswax (Hult et al. 2010).
At this point it should also be remembered that nanocellulosic materials have been extensively investigated as green nano reinforcements for polymer nanocomposites (Dufresne 2018).
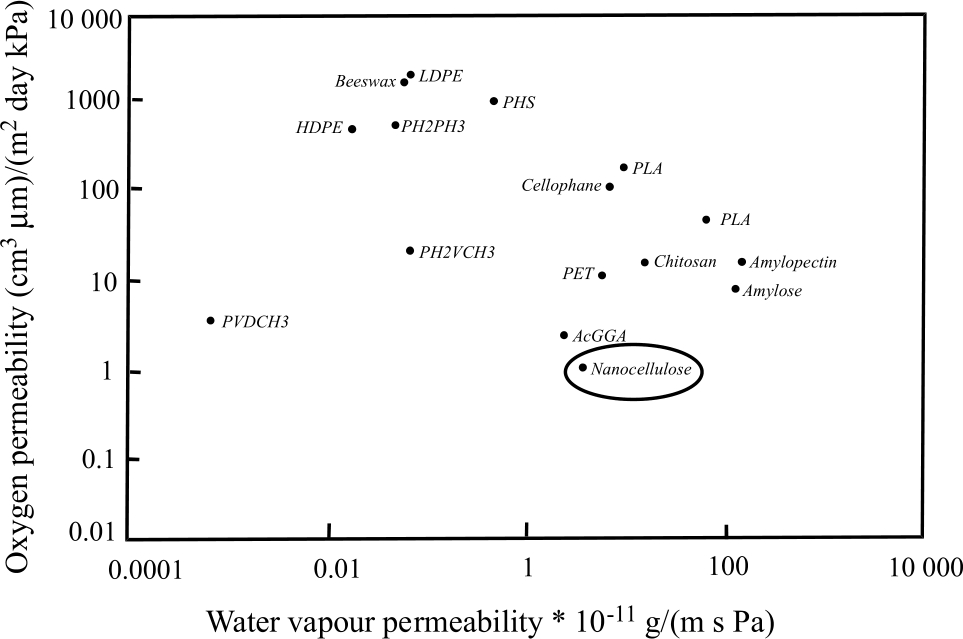
Oxygen permeability and water vapour permeability of some polymers at 50 % RH (Aulin et al. 2010a).

Oxygen barrier properties for a carboxymethylated CNF at different relative humidities (Aulin et al. 2010a).
Nanoclays in food packaging

Illustration of the tortuous pathway created by incorporation of exfoliated nanoclay in a polymer matrix film (Duncan 2011).

Three types of composites when layered clays are incorporated with a polymer: a) tactoid, phase separated microcomposite; b) intercalated nanocomposte; c) exfoliated polymer-clay nanocomposite (Duncan 2011).
The first steps toward the fabrication of polymer-clay hybrid materials goes back the developments Toyota Central Research and Development Laboratory in the mid 80s. The technology was demonstrated with a nanometer sized composite of nylon-6 and a nanometer thick exfoliated aluminosilicate (Kojima et al. 1993). This constituted a new type of composite material that could exhibit large improvements in mechanical, barrier properties and high transparency by means of nanofiller geometry and a high surface area exceeding higher than
The nanoclays could have a thickness in the order of a few nm and flakes with a width up to 100 nm. The nanoclays investigated for this purpose are mostly from the group of 2:1 phyllosilicates, including minerals such as montmorillonite, hectorite, saponite, vermiculite. Montmorillonites (
The phyllosilicates mainly present three different multi-scale structures as shown in Figure 9:
Aggregated particles (tactoids), where the size of the particles is from 0.1 micron to 10 microns (microcomposite structures).
Aggregated particles with a thickness of 10 nm. Intercalated structures are obtained by a moderate expansion of the clay interlayer.
Disc or a platelet having a width from 10 nm up to 1 micrometer and a thickness of 1 nm. (Exfoliated structure). The clay should be exfoliated into a singlet platelet and distributed homogeneously throughout the polymer matrix to take full advantage of the high surface area of the clays.
In situ polymerization. In this method layered silicates are swollen into a monomer solution, but this can obviously not be the case for polysaccharides, as they are synthesized during growth.
Solvent intercalation. This process is based on a solvent system that can swell the silicate layers (aqueous systems) and this is the natural way for polysaccharides, which can be melt processed due to thermal degradation.
Melt intercalation process.
Generally, many matrix materials are hydrophobic and it is important to reduce the interfacial energy or reducing the cohesive force between the clay and the matrix. For hydrophobic matrix materials the most used method is to use ammonium cations attached to long chain alkyl chains (C14–C18).
The use of nanohybrid composites for food packaging started some 15 tears ago, e. g., Chivrac et al. (2009), Duncan (2011), Ray et al. (2002), Sorrentino et al. (2007), Yang et al. (2007) and there are also several recent reviews in the field (Attaran et al. 2017, Kuswandi 2017, Youssef and El-Sayed 2018).
The tortuous diffusion of gas has also been modelled by Nielsen (1967) assuming the that the platelets are evenly dispersed throughout the matrix and supposes the tortuosity is the only factor influencing the gas diffusion. In this model the gas permeability is given by the following equation:
where the K-values represent the permeabilities of the composite structure and of the matrix in absence of the platelets and ∅, the volume fraction of the platelets and α, the aspect ratio of the of the platelets. Assuming a 10 % volume fraction and an aspect ratio of 100, the gas diffusion rate should decrease 12.5 % of the gas diffusion in the polymer matrix. The Nielsen formula has also been verified by Choudalakis and Gotsis (2009).
An investigation of the gas permeation through nanohybrid materials (Attaran et al. 2017, Choudalakis and Gotsis 2009, Rhim et al. 2013) also reveal that the gas permeation (oxygen and moisture permeation) decreases less than an order of magnitude, which is helpful but requires additional measures to combat an inferior gas permeation. Figure 10 displays, for instance, typical data on the effects of different montmorillonite on the oxygen permeability of PLA-clay composites.

The effects of various types of montmorillonites on the carbon dioxide, oxygen and nitrogen permeability of PLA-clay composites (Rhim et al. 2013).
Biodegradability of bio-nanocomposites is one of the most interesting, but also controversial issues. Since the purpose for using biopolymers in nanocomposites materials is to utilize the biodegradability, it is important to secure that these composites are biodegradable. Most investigations have been made on PLA-nanocomposites and indeed it has been found that the biodegradability is enhanced in the nanocomposite when compared with the PLA polymer, see, e. g., reviews (Duncan 2015, Kumar et al. 2009, Rhim et al. 2013). The enhanced biodegradability may be explained by the high hydrophilicity of the clay, allowing an easier permeability of water into the polymer matrix and activating the degradation process.
Most investigations on nanocomposites have been executed on thermoplastic fossil-based polymers and there are only a smaller number of investigations on bio-nanocomposites, but still a considerable number of reviews, e. g., de Azeredo (2009), Ghanbarzadeh et al. (2015), Rhim et al. (2013), Reddy et al. (2013), Tang et al. (2012).
There is another related area to montmorillonite, namely Layered Double Hydroxides (LDH’s), which is a class of lamellar anionic clays. The first was termed hydrotalcite, it was found in a Norwegian geological specimen. The LDH’s aspire to replace aluminium packaging to enter the area of high barrier food packaging (Yu et al. 2019). The synthesis of LDH nanosheets is, however, extremely demanding because the very high in-plane charge density need to be over-comed requiring demanding exfoliation procedures, but has created orders of decreased oxygen and water vapor transmission rates.
Layer-by Layer assembly to improve barrier properties
Lbl-assembly is a technology to build multifunctional thin films through alternating exposure of a substrate to aqueous cationic and anionic polymers, see Figure 11. Instead of utilizing charged polymers the assembly can be built using nanoclays like montmorillonites, vermiculites etc. and various nanocellulosic substrates. The thickness of such films depends on the number of layers and the adsorption thickness. The adsorption thickness is depending on a number of many variables such as
This method is a very elegant way to build multilayers though the method has its disadvantages with the upscaling (multiple deposition technology) of the technology and is not applicable for many kinds of non-amphiphilic materials (Ariga et al. 2007). By alternatively depositing oppositely charged materials conformal and ultra-thin coatings can be prepared by the Lbl- technology from aqueous environments (Li et al. 2020, Richardson et al. 2015). Lbl assembly is a nanocoating technology for gas barrier and gas separation coating (Qin et al. 2019a, 2019b, Song et al. 2018).
Interestingly, Lbl manufactured from PEI and CMC or CNF outperforms CNF-films with respect to oxygen permeability (Aulin et al. 2013). It was early found that Lbl manufactured from PEI and montmorillonite clay had such a low oxygen transmission 0.013 cc (
Despite excellent oxygen barriers polyelectrolyte multilayers (PEM) (Song et al. 2018) coatings have very limited moisture barrier coatings, loosened chain packing and higher chain mobility (Qin et al. 2019a). There are, however, approaches that may alleviate this problem. Hence, it has been shown that

Outline of LbL assembly through electrostatic interaction (Duncan 2011).

Oxygen permeability values for “brick wall” films of various bilayer numbers formed from branched poly(ethylene imine) (PEI) and sodium montmorillonite at pH 10 (Priolo et al. 2010).
Multilayer micro and nanolayer co-extrusion
Whereas, there has been extensive work on Lbl using dip coating, spraying and spin-coating, the upscaling of these technologies is often not suitable for large scale applications with the solvent manipulation. Multilayer (micro-nanolayer) coextrusion is such a technology (Li et al. 2020, Zhang et al. 2019), were layered composites can be manufactured with up to thousands of layers. This technology is not new but was developed by the Dow Chemical company in the 70s (Ponting et al. 2010). The uniqueness of this coextrusion technology is the combination of conventional coextrusion of two or more polymers in a layered feed-block with additional layer multiplication accomplished through a series of multiplayer dies. This design creates a highly flexible and novel process for production of polymer film with tens to thousands of layers, see Figure 13.
There are very few investigations dealing with this high barrier technology using biodegradable polymers, but this melt processing technology has been demonstrated at MIT using polyvinyl alcohol and polyhydroxyalkanoates (Thellen 2010).

(Left) Two component multilayer system comprised of extruders, pumps, feedblock, multiplying dies, surface layer extruders, and exit die. (Right) Below: Layering structure of the composites (Li et al. 2020).
Polyelectrolyte complexes (PEC)
The discussed Lbl technology has many advantages, but from a technological point, the many processing steps to make Lbl-films is an inherent disadvantage. It has, however, been shown that the sedimentation of PEC has been shown to produce coalesced and uniform thin films (Ball et al. 2013, Cain et al. 2014). Thin PEC-films have also been manufactured by spraying oppositely charged polyelectrolytes onto a substrate but in fewer steps similar to Lbl-films (Lefort et al. 2010, Porcel et al. 2005).
The formation of polyelectrolyte complexes is driven by the entropy gain, when the counterions are released from the polyelectrolytes and an interesting array of interesting morphologies are formed and there is an extensive number of publications to make PEC-films from oppositely charged biopolymers for food-packaging applications, see, e. g., Chi and Catchmark (2018a, 2018b), Farris et al. (2009), Ibn Yaich et al. (2015). These blends have been shown to have desirable gas-barrier properties when deposited onto substrates and has opened up opportunities for engineers to make novel food packaging systems. Nonetheless, the gas and water barrier properties of biopolymers are impaired at high relative humidities, which limits their applications in food packaging applications.
It should, however, be pointed out that the very thin films of PEC materials using non-biobased materials may not be a problem with regard to microplastic if non-biobased PEC-films are deposited onto biobased plastics. Such systems constitute a viable route to efficient food packaging material also at high relative humidities. It has, for instance, been shown that a PEC-system of poly (diallyldimethylammonium chloride) and poly(acrylic acid), albeit not on a biobased substrate, but on a poly (ethylene terephtalate) film, decreased the oxygen transmission two orders of magnitude (Smith et al. 2018).
It should be pointed out that there are numerous parameters that must be controlled making PEC-systems: Apart from the choice of polymers the
Finally, there is an emerging area of PEC with nanocellulosic materials that are promising, see, e. g., Chi and Catchmark (2018a).
Safety issues, and toxicity of nanomaterials in food packaging
Whereas, nanoclays like montmorillonites are considered as generally recognized as safe (GRAS) organomodified clays may not be safe. In food packaging, using hydrophobic barrier materials, the nanoclays are organically modified to allow for better exfoliation within the polymer matrix. The organic modification generally occurs through an ion-exchange reaction with a positively charged ion present between the nanoclay platelets. Examples of organomodified nanoclays used in food packaging are modifications like aminopropyltriethoxysilane and octadecylamine (Nanomer I.31PS), which have raised some concern, because they show some toxicity (Wagner et al. 2017).
This concern relates to the release of such nanoclays during manufacturing and disposal of food packaging materials, which may pose health concerns if the exposure is via inhalation and or ingestion routes.
The conclusion, however, is that the probability of migration into foods is very low as long as the nanoclays are completely embedded in the polymer matrix. However, due to mechanical impact, nanoparticles may come in contact with the food. Therefore, it is important to investigate migration of nanoparticles before and after flexing of food packaging materials (Bandyopadhyay and Ray 2019).
For nanocellulose there have been an overwhelming number of cytotoxic studies on nanocellulosic materials, basically concluding that there are no cytotoxic effects as defined by ISO Standard 109993-5, i. e. it does not reduce the cell viability by more than 30 %, see, e. g., Camarero-Espinosa et al. (2016), Shatkin and Kim (2015).
There is, however, a concern over occupational inhalation exposure with handling of nanocellulose nanomaterials as dry powder and this has been the highest priority.
Basically, particles in the size of 5–50 nm can be stuck in the Alveolar part of the respiratory tract. Such particles are dangerous for the human health, because there are no enzymes breaking down cellulose in the lung tract and their bioduration will cause concern. Even though wood cellulose pulp, powdered cellulose, and microcrystalline cellulose are considered as safe materials, wood and cotton dust may contain cellulose nanoparticles among other constituents of wood and such dust has been shown to be cancerogenic and a well-known example is cotton dust in cotton factories. The current state of the risk analysis of nanocellulose materials by inhalation is that the quality of the available studies is generally inadequate for risk assessment (Ede et al. 2019). There are, however, numerous different nanocellulose materials, treated in different ways, having widely different morphologies and size distribution, and moreover they are difficult to characterize, which was a reason that a number of scientists recently published an extensive review and characterization issues (Foster et al. 2018, Shatkin 2020)
In conclusion, it’s the occupational air dust exposure for both nanoclays and nanocellulose, which are of concern and appropriate engineering approaches must therefore be taken into account to assure the safety measures (Shatkin 2020).
Outlook
It is clear that nonbiodegradable plastics poses a major problem to the environment. Hydrophobic microplastics in the marine environment serve as a vector for the assimilation of persistent organic pollutants in the oceans and are transported into the food chain via marine life and animals in general. This constitutes a challenging threat to wild-life and also to the safety of foods in general. On the other hand, fossil-based plastics have a tremendous role for the preservation of foods and their security aspects and it’s obvious that a single solution to secure food safety and these large environmental problems are not at hand. Historically, the use of biodegradable polymers has been considered as a solution to these environmental problems, but the evolution of biodegradable plastics has been retarded not only by economics due but to their inferior barrier properties, but also because that all biodegradable polymers must be susceptible to water sorption, which spoils the barrier properties at high humidities.
The European Commission has recently published a Green deal with strategies for a circular economy and for an industrial strategy. Requirements are published for an increased recycling and an inmixing of recycled plastics in new products as well as replacement of fossil based plastics. Microplastics are of special concern with restrictions of unintentional usage as well as promotion of biobased plastics.
The survey addresses food packaging demands and requirements for a safe food contact. Oxygen and humid barriers are essential properties of food packaging, which need to be addressed for alternative bioplastics. The survey describes some different materials to replace the traditional food safety plastics. A problem is the non-existence of a single replacement material, which will fulfill these requirements. There is a need for a combination of several materials in sandwich constructions and layer-by-layer techniques are identified options to achieve both oxygen and humidity barriers suitable for food packaging.
The evolution of biodegradable plastics points in several different directions: Firstly, the biodegradable polymers have not been enjoying large upscaling and its associated economic advantages. Secondly the use of blends between different biodegradable polymers to cope with economics (e. g., use of starch blends) and optimizing barrier performance and other properties and finally the emerging field of various nanotechnological solutions discussed in this review. Nanotechnology is of great interest with promising barrier properties. There is, however, the uncertainty regarding safety, toxicity and life-cycle considerations of nanomaterials, public skepticism and a legal acceptance needs to be taken into account.
It is obvious that there is not one solution like composting or incineration and other measures that will be the key to the alleviation of the environmental hazards, but a broad palette of technical and political solutions (legislative) that must be managed and will constitute a major challenge for mankind.
Funding statement: Authors state no funding involved.
Acknowledgments
The authors acknowledge prof. Gunnar Henriksson, KTH for the rewriting of Figure 6.
Conflict of interest: The authors declare no conflicts of interest.
References
Abdul Khalil, H.P.S., Davoudpour, Y., Saurabh, C.K., Hossain, M.S., Adnan, A.S., Dungani, R., Paridah, M.T., Islam Sarker, M.Z., Fazita, M.R.N., Syakir, M.I., Haafiz, M.K.M. (2016) A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 64:823–836.10.1016/j.rser.2016.06.072Search in Google Scholar
Alexandre, M., Dubois, P. (2000) Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mater. Sci. Eng., R Rep. 28(1-2):1–63.10.1016/S0927-796X(00)00012-7Search in Google Scholar
Amass, W., Amass, A., Tighe, B. (1998) A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 47:89–144.10.1002/(SICI)1097-0126(1998100)47:2<89::AID-PI86>3.0.CO;2-FSearch in Google Scholar
Andersson, C. (2008) New ways to enhance the functionality of paperboard by surface treatment – a review. Packag. Technol. Sci. 21(6):339–373.10.1002/pts.823Search in Google Scholar
Anjum, A., Zuber, M., Zia, K.M., Noreen, A., Anjum, M.N., Tabasum, S. (2016) Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 89:161–174.10.1016/j.ijbiomac.2016.04.069Search in Google Scholar
Ariga, K., Hill, J.P., Ji, Q. (2007) Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. Phys. Chem. Chem. Phys. 9(19):2319–2340.10.1039/b700410aSearch in Google Scholar
Armentano, I., Bitinis, N., Fortunati, E., Mattioli, S., Rescignano, N., Verdejo, R., Lopez-Manchado, M.A., Kenny, J.M. (2013) Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 38(10-11):1720–1747.10.1016/j.progpolymsci.2013.05.010Search in Google Scholar
Attaran, S.A., Hassan, A., Wahit, M.U. (2017) Materials for food packaging applications based on bio-based polymer nanocomposites. J. Thermoplast. Compos. Mater. 30(2):143–173.10.1177/0892705715588801Search in Google Scholar
Aulin, C., Gällstedt, M., Lindström, T. (2010a) Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 17:559–574.10.1007/s10570-009-9393-ySearch in Google Scholar
Aulin, C., Karabulut, E., Tran, A., Wågberg, L., Lindström, T. (2013) Transparent nanocellulosic multilayer thin films on polylactic acid with tunable gas barrier properties. ACS Appl. Mater. Interfaces 5(15):7352–7359.10.1021/am401700nSearch in Google Scholar
Aulin, C., Lindström, T. (2011) Biopolymer coatings for paper and paperboard. In: Biopolymers – New Materials for Sustainable Films and Coatings. Eds. Plackett, D.. John Wiley & Sons Ltd, Chichester, West Sussex, UK.10.1002/9781119994312.ch12Search in Google Scholar
Aulin, C., Netraval, J., Wågberg, L., Lindström, T. (2010b) Aerogels from nanofibrillated cellulose with tunable oleophobicity. Soft Matter 6:3298–3305.10.1039/c001939aSearch in Google Scholar
Aulin, C., Ström, G. (2013) Multilayered alkyd resin/nanocellulose coatings for use in renewable packaging solutions with a high level of moisture resistance. Ind. Eng. Chem. Res. 52(7):2582–2589.10.1021/ie301785aSearch in Google Scholar
Auras, R.A., Lim, L.-T., Selke, S.E., Tsuji, H. Poly (Lactic Acid): Synthesis, Structures, Properties, Processing and Applications. John Wiley & Sons, 2011.10.1002/9780470649848Search in Google Scholar
Auta, H.S., Emenike, C.U., Fauziah, S.H. (2017) Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 102:165–176.10.1016/j.envint.2017.02.013Search in Google Scholar PubMed
Babu, R.P., O’Connor, K., Seeram, R. (2013) Current progress on bio-based polymers and their future trends. Prog. Biomater. 2:8–14.10.1186/2194-0517-2-8Search in Google Scholar PubMed PubMed Central
Bagheri, A.R., Laforsch, C., Greiner, A., Agarwal, S. (2017) Fate of so-called biodegradable polymers in seawater and freshwater. Glob. Chall.. 1(4):1700048.10.1002/gch2.201700048Search in Google Scholar PubMed PubMed Central
Ball, V., Michel, M., Toniazzo, V., Ruch, D. (2013) The possibility of obtaining films by single sedimentation of polyelectrolyte complexes. Ind. Eng. Chem. Res. 52(16):5691–5699.10.1021/ie303535sSearch in Google Scholar
Bandyopadhyay, J., Ray, S.S. (2019) Are nanoclay-containing polymer composites safe for food packaging applications? – An overview. J. Appl. Polym. Sci. 136:12.10.1002/app.47214Search in Google Scholar
Belbekhouche, S., Bras, J., Siqueira, G., Chappey, C., Lebrun, L., Khelifi, B., Marais, S., Dufresne, A. (2011) Water sorption behavior and gas barrier properties of cellulose whiskers and microfibrillar films. Carbohydr. Polym.. 83(4):1740–1748.10.1016/j.carbpol.2010.10.036Search in Google Scholar
Bharimalla, A.K., Patil, P.G., Mukherjee, S., Yadav, V., Prasad, V. (2019) Nanocellulose-polymer composites: novel materials for food packaging applications. In: Polymers for Agri-Food Applications. Eds. Guitiérrez, T. J. Springer Nature Switzerland AG.10.1007/978-3-030-19416-1_27Search in Google Scholar
Bordes, P., Pollet, E., Averous, L. (2009) Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 34(2):125–155.10.1016/j.progpolymsci.2008.10.002Search in Google Scholar
Bugnicourt, E., Cinelli, P., Lazzeri, A., Alvarez, V.A. (2014) Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. eXPRESS Polym. Lett. 8:791–808.10.3144/expresspolymlett.2014.82Search in Google Scholar
Bumbudsanpharoke, N., Ko, S. (2015) Nano-food packaging: an overview of market, migration research, and safety regulations. J. Food Sci. 80(5):R910–R923.10.1111/1750-3841.12861Search in Google Scholar PubMed
Cain, A.A., Murray, S., Holder, K.M., Nolen, C.R., Grunlan, J.C. (2014) Intumescent nanocoating extinguishes flame on fabric using aqueous polyelectrolyte complex deposited in single step. Macromol. Mater. Eng. 299(10):1180–1187.10.1002/mame.201400022Search in Google Scholar
Camarero-Espinosa, S., Endes, C., Mueller, S., Petri-Fink, A., Rothen-Rutishauser, B., Weder, C., Clift, M., Foster, E. (2016) Elucidating the potential biological impact of cellulose nanocrystals. Fibers 4(4):21.10.3390/fib4030021Search in Google Scholar
Castro-Aguirre, E., Iniguez-Franco, F., Samsudin, H., Fang, X., Auras, R. (2016) Poly(lactic acid)-Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 107:333–366.10.1016/j.addr.2016.03.010Search in Google Scholar PubMed
Cazón, P., Velazquez, G., Ramírez, J.A., Vázquez, M. (2017) Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 68:136–148.10.1016/j.foodhyd.2016.09.009Search in Google Scholar
Chi, K., Catchmark, J.M. (2018a) Improved eco-friendly barrier materials based on crystalline nanocellulose/chitosan/carboxymethyl cellulose polyelectrolyte complexes. Food Hydrocoll. 80:195–205.10.1016/j.foodhyd.2018.02.003Search in Google Scholar
Chi, K., Catchmark, J.M. (2018b) Green Polymer Chemistry: New Products Processes, and Applications, Eds. Cheng, H. N., Gross, R. A., Smith, P. B. ACS Symposium Series. American Chemical Society, Washington DC, pp. 109–123.10.1021/bk-2018-1310.ch008Search in Google Scholar
Chivrac, F., Pollet, E., Avérous, L. (2009) Progress in nano-biocomposites based on polysaccharides and nanoclays. Mater. Sci. Eng., R Rep. 67(1):1–17.10.1016/j.mser.2009.09.002Search in Google Scholar
Choudalakis, G., Gotsis, A.D. (2009) Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 45(4):967–984.10.1016/j.eurpolymj.2009.01.027Search in Google Scholar
Cole, M., Lindeque, P., Halsband, C., Galloway, T.S. (2011) Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62(12):2588–2597.10.1016/j.marpolbul.2011.09.025Search in Google Scholar PubMed
Coltelli, M.-B., Wild, F., Bugnicourt, E., Cinelli, P., Lindner, M., Schmid, M., Weckel, V., Müller, K., Rodriguez, P., Staebler, A., Rodríguez-Turienzo, L., Lazzeri, A. (2015) State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 6(1):1.10.3390/coatings6010001Search in Google Scholar
de Azeredo, H.M.C. (2009) Nanocomposites for food packaging applications. Food Res. Int. 42(9):1240–1253.10.1016/j.foodres.2009.03.019Search in Google Scholar
de Azeredo, H.M.C., Rosa, M.F., Mattoso, L.H.C. (2017) Nanocellulose in bio-based food packaging applications. Ind. Crop. Prod. 97:664–671.10.1016/j.indcrop.2016.03.013Search in Google Scholar
Decher, G. (1997) Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 277:1232–1237.10.1126/science.277.5330.1232Search in Google Scholar
Decher, G., Schlenoff, J.B. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials. Wiley-VCH, New York, NY, 2003.10.1002/3527600574Search in Google Scholar
Degan, T., Shinde, S.L. (2019) Waste-plastic processing provides global challenges and opportunities. Mater. Res. Soc. Bull. 44(6):436–437.10.1557/mrs.2019.133Search in Google Scholar
Delidovich, I., Hausoul, P.J., Deng, L., Pfutzenreuter, R., Rose, M., Palkovits, R. (2016) Alternative monomers based on lignocellulose and their use for polymer production. Chem. Rev. 116(3):1540–1599.10.1021/acs.chemrev.5b00354Search in Google Scholar PubMed
Din, M.I., Ghaffar, T., Najeeb, J., Hussain, Z., Khalid, R., Zahid, H. (2020) Potential perspectives of biodegradable plastics for food packaging application – review of properties and recent developments. Food Add. Contam. Part A 37(4):665–680.10.1080/19440049.2020.1718219Search in Google Scholar PubMed
Donhowe, I.G., Fennema, O.R. (1993) Water vapor and oxygen permeability of wax films. J. Am. Oil Chem. Soc. 70(9):867–873.10.1007/BF02545345Search in Google Scholar
Dufresne, A. (2018) Cellulose nanomaterials as green nanoreinforcements for polymer nanocomposites. Philos. Trans. R. Soc. Lond. Ser. A, Math. Phys. Sci. 376:2112.10.1098/rsta.2017.0040Search in Google Scholar PubMed PubMed Central
Dufresne, A., Thomas, S., Pothan, L.A. Biopolymer Nanocomposites: Processing, Properties, and Applications. Springer, John Wiley & Sons, Inc., Hoboken, New Jersey, 2013.10.1002/9781118609958Search in Google Scholar
Duncan, T.V. (2011) Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 363(1):1–24.10.1016/j.jcis.2011.07.017Search in Google Scholar PubMed PubMed Central
Duncan, T.V. (2015) Release of engineered nanomaterials from polymer nanocomposites: the effect of matrix degradation. ACS Appl. Mater. Interfaces 7(1):20–39.10.1021/am5062757Search in Google Scholar PubMed
Ede, J.D., Ong, K.J., Goergen, M., Rudie, A., Pomeroy-Carter, C.A., Shatkin, J.A. (2019) Risk analysis of cellulose nanomaterials by inhalation: Current state of science. Nanomaterials 9:337.10.3390/nano9030337Search in Google Scholar PubMed PubMed Central
Elsabee, M.Z., Abdou, E.S. (2013) Chitosan based edible films and coatings: a review. Mater. Sci. Eng. C 33(4):1819–1841.10.1016/j.msec.2013.01.010Search in Google Scholar PubMed
Emadian, S.M., Onay, T.T., Demirel, B. (2017) Biodegradation of bioplastics in natural environments. Waste Manag. 59:526–536.10.1016/j.wasman.2016.10.006Search in Google Scholar PubMed
Espitia, P.J.P., Du, W.-X., Avena-Bustillos, R. d. J., Soares, N.d.F.F., McHugh, T.H. (2014) Edible films from pectin: Physical-mechanical and antimicrobial properties – a review. Food Hydrocoll. 35:287–296.10.1016/j.foodhyd.2013.06.005Search in Google Scholar
Fares, H.M., Schlenoff, J.B. (2017) Equilibrium overcompensation in polyelectrolyte complexes. Macromolecules 50(10):3968–3978.10.1021/acs.macromol.7b00665Search in Google Scholar
Farris, S., Schaich, K.M., Liu, L., Piergiovanni, L., Yam, K.L. (2009) Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: a review. Trends Food Sci. Technol. 20(8):316–332.10.1016/j.tifs.2009.04.003Search in Google Scholar
Ferreira, F.V., Cividanes, L.S., Gouveia, R.F., Lona, L.M.F. (2019) An overview on properties and applications of poly(butylene adipate-co-terephthalate)-PBAT based composites. Polym. Eng. Sci. 59(s2):E7–E15.10.1002/pen.24770Search in Google Scholar
Ferrer, A., Pal, L., Hubbe, M. (2017) Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crop. Prod. 95:574–582.10.1016/j.indcrop.2016.11.012Search in Google Scholar
Foster, E.J., Moon, R.J., Agarwal, U.P., Bortner, M.J., Bras, J., Camarero Espinosa, S., Chan, K.J., Clift, M.J.D., Cranston, E.D., Eichhorn, S.J., Fox, D.M., Hamad, W.Y., Heux, L., Jean, B., Korey, M., Nieh, W., Ong, K.J., Reid, M.S., Renneckar, S., Roberts, R., Shatkin, J.A., Simonsen, J., Stinson-Bagby, K., Wanasekara, N., Youngblood, J. (2018) Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 47:2511–3006.10.1039/C6CS00895JSearch in Google Scholar
Fujisawa, S., Okita, Y., Fukuzumi, H., Saito, T., Isogai, A. (2011) Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr. Polym. 84(1):579–583.10.1016/j.carbpol.2010.12.029Search in Google Scholar
Fukuzumi, H., Saito, T., Iwamoto, S., Kumamoto, Y., Ohdaira, T., Suzuki, R., Isogai, A. (2011) Pore size distribution of TEMPO-oxidized cellulose nanofibril films by positron annihilation lifetime spectroscopy. Biomacromolecules 12:4057–4062.10.1021/bm201079nSearch in Google Scholar PubMed
Galbis, J.A., Garcia-Martin Mde, G., de Paz, M.V., Galbis, E. (2016) Synthetic polymers from sugar-based monomers. Chem. Rev. 116(3):1600–1636.10.1021/acs.chemrev.5b00242Search in Google Scholar PubMed
Gandini, A., Lacerda, T.M., Carvalho, A.J., Trovatti, E. (2016) Progress of polymers from renewable resources: furans, vegetable oils, and polysaccharides. Chem. Rev. 116(3):1637–1669.10.1021/acs.chemrev.5b00264Search in Google Scholar PubMed
Garlotta, D. (2002) A literature review of poly(lactic acid). J. Polym. Environ. 9(2):63–84.10.1023/A:1020200822435Search in Google Scholar
Genovese, L., Lotti, N., Gazzano, M., Siracusa, V., Dalla Rosa, M., Munari, A. (2016) Novel biodegradable aliphatic copolyesters based on poly(butylene succinate) containing thioether-linkages for sustainable food packaging applications. Polym. Degrad. Stab. 132:191–201.10.1016/j.polymdegradstab.2016.02.022Search in Google Scholar
Ghadermazi, R., Hamdipour, S., Sadeghi, K., Ghadermazi, R., Khosrowshahi Asl, A. (2019) Effect of various additives on the properties of the films and coatings derived from hydroxypropyl methylcellulose – A review. Food Sci. Nutr. 7(11):3363–3377.10.1002/fsn3.1206Search in Google Scholar
Ghanbarzadeh, B., Oleyaei, S.A., Almasi, H. (2015) Nanostructured materials utilized in biopolymer-based plastics for food packaging applications. Crit. Rev. Food Sci. Nutr. 55(12):1699–1723.10.1080/10408398.2012.731023Search in Google Scholar
Giannelis, E.P., Krishnamoorti, R., Manias, E. (1999) In: Adv. in Polym. Sci. Ed. Granick, S. Springer. pp. 107–148.10.1007/3-540-69711-X_3Search in Google Scholar
Gigli, M., Fabbri, M., Lotti, N., Gamberini, R., Rimini, B., Munari, A. (2016) Poly(butylene succinate)-based polyesters for biomedical applications: A review. Eur. Polym. J. 75:431–460.10.1016/j.eurpolymj.2016.01.016Search in Google Scholar
Guillard, V., Gaucel, S., Fornaciari, C., Angellier-Coussy, H., Buche, P., Gontard, N. (2018) The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 5:121.10.3389/fnut.2018.00121Search in Google Scholar
Haas, K.-H., Amberg-Schwab, S., Rose, K., Schottner, G. (1999) Functionalized coatings based on inorganic–organic polymers (ORMOCERS) and their combination with vapor deposited inorganic thin films. Surf. Coat. Technol. 111:72–79.10.1016/S0257-8972(98)00711-7Search in Google Scholar
Helanto, K., Martikainen, L., Talja, R., Rojas, O.J. (2019) Bio-based polymers for sustainable packaging and biobarriers: A critical review. BioResources 14(2):4902–4951.10.15376/biores.14.2.HelantoSearch in Google Scholar
Herrick, F.W., Casebier, R.L., Hamilton, J.K., Sandberg, K.R. (1983) Microfibrillated cellulose: morphology and accessibility. J. Appl. Polym. Sci., Appl. Polym. Symp. 37:797–813.Search in Google Scholar
Hong, M., Chen, E.Y.X. (2017) Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 19(16):3692–3706.10.1039/C7GC01496ASearch in Google Scholar
Horton, A.A., Walton, A., Spurgeon, D.J., Lahive, E., Svendsen, C. (2017) Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 586:127–141.10.1016/j.scitotenv.2017.01.190Search in Google Scholar PubMed
Hubbe, M.A., Ferrer, A., Tyagi, P., Yin, Y., Salas, C., Pal, L., Rojas, J. (2017) Nanocellulose in thin films, coatings, and plies for packaging applications. BioResources 12(1):2143–2233.10.15376/biores.12.1.2143-2233Search in Google Scholar
Hult, E.-L., Iotti, M., Lenes, M. (2010) Efficient approach to high barrier packaging using microfibrillar cellulose and shellac. Cellulose 17(3):575–586.10.1007/s10570-010-9408-8Search in Google Scholar
Ibn Yaich, A., Edlund, U., Albertsson, A.-C. (2015) Barriers from wood hydrolysate/quaternized cellulose polyelectrolyte complexes. Cellulose 22(3):1977–1991.10.1007/s10570-015-0621-3Search in Google Scholar
Iler, R.K. (1966) Multilayers of colloidal particles. J. Colloid Interface Sci. 21:569–594.10.1016/0095-8522(66)90018-3Search in Google Scholar
Isogai, A., Saito, T., Fukuzumi, H. (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3(1):71–85.10.1039/C0NR00583ESearch in Google Scholar PubMed
Jambeck, J.R., Geyer, R., Wilcox, C., Siegler, T.R., Perryman, M., Andrady, R., Narayan, R., Law, K.L. (2015) Plastic waste inputs from land into the ocean. Science 347(6223):768–771.10.1126/science.1260352Search in Google Scholar PubMed
Janjarasskul, T., Krochta, J.M. (2010) Edible packaging materials. Annu. Rev. Food Sci. Technol. 1:415–448.10.1146/annurev.food.080708.100836Search in Google Scholar PubMed
Jiménez, A., Fabra, M.J., Talens, P., Chiralt, A. (2012) Edible and biodegradable starch films: A review. Food Bioprocess Technol. 5(6):2058–2076.10.1007/s11947-012-0835-4Search in Google Scholar
Kale, G., Kijchavengkul, T., Auras, R., Rubino, M., Seike, S.E., Singh, S.P. (2007) Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 7:255–277.10.1002/mabi.200600168Search in Google Scholar PubMed
Kester, J.J., Fennema, O.R. (1986) Edible films and coatings: A review. J. Food Technol. 40(12):47–59.Search in Google Scholar
Khwaldia, K., Arab-Therany, E., Desobry, S. (2010) Biopolymer coatings on paper packaging materials. Compr. Rev. Food Sci. Food Saf. 9:82–91.10.1111/j.1541-4337.2009.00095.xSearch in Google Scholar PubMed
Klemm, D., Kramer, F., Moritz, S., Lindström, T., Ankerfors, M., Gray, D., Dorris, A. (2011) Nanocelluloses: A new family of nature-based materials. Angew. Chem., Int. Ed. Engl. 50(24):5438–5466.10.1002/anie.201001273Search in Google Scholar PubMed
Kojima, Y., Usuki, A., Kawasumi, M., Okada, A., Fukushima, Y., Kurauchi, T., Kamigaito, O. (1993) Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 8(5):1185–1189.10.1557/JMR.1993.1185Search in Google Scholar
Koros, W.J. (1990) Barrier polymers and structures: Overview. ACS Symp. Ser. 423:1–21.10.1021/bk-1990-0423.ch001Search in Google Scholar
Krochta, J.M. In: Handbook of Food Engineering. Eds. Heldman, D.R., Lund, D.B., Sabliov, C. CRC Press, Boca Raton, 2006. p. 847.Search in Google Scholar
Kumar, A.P., Depan, D., Singh Tomer, N., Singh, R.P. (2009) Nanoscale particles for polymer degradation and stabilization – trends and future perspectives. Prog. Polym. Sci. 34(6):479–515.10.1016/j.progpolymsci.2009.01.002Search in Google Scholar
Kuswandi, B. (2017) Environmental friendly food nano-packaging. Environ. Chem. Lett. 15(2):205–221.10.1007/s10311-017-0613-7Search in Google Scholar
Lagarón, J.M., Catala, R., Gavara, R. (2004) Structural characteristics defining high barrier properties in polymeric materials. Mater. Sci. Technol. 20:1–7.10.1179/026708304225010442Search in Google Scholar
Lagarón, J.M., López-Rubio, A., José Fabra, M. (2016) Bio-based packaging. J. Appl. Polym. Sci. 133(2):42165. (1 of 11).10.1002/app.42971Search in Google Scholar
Lange, J., Wyser, Y. (2003) Recent innovations in barrier technologies for plastic packaging – a review. Packag. Technol. Sci. 16(4):149–158.10.1002/pts.621Search in Google Scholar
Lavoine, N., Desloges, I., Dufresne, A., Bras, J. (2012) Microfibrillated cellulose – its barrier properties and applications in cellulosic materials: a review. Carbohydr. Polym. 90(2):735–764.10.1016/j.carbpol.2012.05.026Search in Google Scholar PubMed
Lavoine, N., Desloges, I., Khelifi, B., Bras, J. (2014) Impact of different coating processes of microfibrillated cellulose on the mechanical and barrier properties of paper. J. Mater. Sci. 49(7):2879–2893.10.1007/s10853-013-7995-0Search in Google Scholar
Lefort, M., Popa, G., Seyrek, E., Szamocki, R., Felix, O., Hemmerle, J., Vidal, L., Voegel, J.C., Boulmedais, F., Decher, G., Schaaf, P. (2010) Spray-on organic/inorganic films: a general method for the formation of functional nano- to microscale coatings. Angew. Chem., Int. Ed. Engl. 49(52):10110–10113.10.1002/anie.201002729Search in Google Scholar PubMed
Leja, K., Lewandowicz, G. (2010) Polymer biodegradation and biodegradable polymers – a review. Pol. J. Environ. Stud. 19(2):255–266.Search in Google Scholar
Li, Z., Olah, A., Baer, E. (2020) Micro- and nano-layered processing of new polymeric systems. Prog. Polym. Sci. 102:101210.10.1016/j.progpolymsci.2020.101210Search in Google Scholar
MacArthur, E., McKinsey. The New Plastic Economy: Rethinking the Future of Plastic & Catalyzing Action. Ellen MacArthur Foundation and McKinsey, 2017.Search in Google Scholar
MacArthur, E., McKinsey. Reuse-Rethinking Packaging, World Economic Forum. Ellen MacArthur Foundation and McKinsey, 2019.Search in Google Scholar
McKeown, P., Roman-Ramirez, L.A., Bates, S., Wood, J., Jones, M.D. (2019) Zinc complexes for PLA formation and chemical recycling: towards a circular economy. ChemSusChem 12(24):5233–5238.10.1002/cssc.201902755Search in Google Scholar
Miller, K.S., Krochta, J.M. (1997) Oxygen and aroma barrier properties of edible films: A review. Trends Food Sci. Technol. 8:228–238.10.1016/S0924-2244(97)01051-0Search in Google Scholar
Min, K., Cuiffi, J.D., Mathers, R.T. (2020) Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 1:1–11.10.1038/s41467-020-14538-zSearch in Google Scholar PubMed PubMed Central
Minelli, M., Baschetti, M.G., Doghieri, F., Ankerfors, M., Lindström, T., Siro, I., Plackett, D. (2010) Investigation of mass transport properties of microfibrillated cellulose (MFC) films. J. Membr. Sci. 358:67–75.10.1016/j.memsci.2010.04.030Search in Google Scholar
Moon, R.J., Martini, A., Nairn, J., Simonsen, J., Youngblood, J. (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 401:3941–3994.10.1039/c0cs00108bSearch in Google Scholar PubMed
Narancic, T., O’Connor, K.E. (2019) Plastic waste as a global challenge: are biodegradable plastics the answer to the plastic waste problem? Microbiology 165(2):129–137.10.1099/mic.0.000749Search in Google Scholar PubMed
Narancic, T., Verstichel, S., Reddy Chaganti, S., Morales-Gamez, L., Kenny, S.T., De Wilde, B., Babu Padamati, R., O’Connor, K.E. (2018) Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 52(18):10441–10452.10.1021/acs.est.8b02963Search in Google Scholar PubMed
Nesic, A., Cabrera-Barjas, G., Dimitrijevic-Brankovic, S., Davidovic, S., Radovanovic, N., Delattre, C. (2019) Prospect of polysaccharide-based materials as advanced food packaging. Molecules 25(1):135.10.3390/molecules25010135Search in Google Scholar
Nielsen, L.E. (1967) Models for the permeability of filled polymer systems. J. Macromol. Sci. Part A, Chem. 1(5):929–942.10.1080/10601326708053745Search in Google Scholar
Paunonen, S. (2013) Strength and barrier enhancements of composites and packaging boards by nanocelluloses – A literature review. Nord. Pulp Pap. Res. J. 28(2):165–181.10.3183/npprj-2013-28-02-p165-181Search in Google Scholar
Payne, J., McKeown, P., Jones, M.D. (2019) A circular economy approach to plastic waste. Polym. Degrad. Stab. 165:170–181.10.1016/j.polymdegradstab.2019.05.014Search in Google Scholar
Peelman, N., Ragaert, P., De Meulenaer, B., Adons, D., Peeters, R., Cardon, L., Van Impe, F., Devlieghere, F. (2013) Application of bioplastics for food packaging. Trends Food Sci. Technol. 32(2):128–141.10.1016/j.tifs.2013.06.003Search in Google Scholar
Petersen, K., Nielsen, P.V., Bertelsen, G., Lawther, M., Olsen, M.B., Nilsson, N.H., Mortensen, G. (1999) Potential of biobased materials in food packaging. Trends Food Sci. Technol. 10:52–68.10.1016/S0924-2244(99)00019-9Search in Google Scholar
Ponting, M., Hiltner, A., Baer, E. (2010) Polymer nanostructures by forced assembly: Process, structure, and properties. Macromol. Symp. 294(1):19–32.10.1002/masy.201050803Search in Google Scholar
Porcel, C.H., Izquierdo, A., Ball, V., Decher, G., Voegel, J.-C., Schaaf, P. (2005) Ultrathin coatings and (poly(glutamic acid)/polyallylamine) films deposited by continuous and simultaneous spraying. Langmuir 21:800–802.10.1021/la047570nSearch in Google Scholar PubMed
Priolo, M.A., Gamboa, D., Holder, K.M., Grunlan, J.C. (2010) Super gas barrier of transparent polymer-clay multilayer ultrathin films. Nano Lett. 10(12):4970–4974.10.1021/nl103047kSearch in Google Scholar PubMed
Qin, S., Pour, M.G., Lazar, S., Köklükaya, O., Gerringer, J., Song, Y., Wågberg, L., Grunlan, J.C. (2019a) Super gas barrier and fire resistance of nanoplatelet/nanofibril multilayer thin films. Adv. Mater. Interfaces 6(2):1801424.10.1002/admi.201801424Search in Google Scholar
Qin, S., Xiang, S., Eberle, B., Xie, K., Grunlan, J.C. (2019b) High moisture barrier with synergistic combination of SiOx and polyelectrolyte nanolayers. Adv. Mater. Interfaces 6(16):1900740.10.1002/admi.201900740Search in Google Scholar
Rabnawaz, M., Wyman, I., Auras, R., Cheng, S. (2017) A roadmap towards green packaging: the current status and future outlook for polyesters in the packaging industry. Green Chem. 19(20):4737–4753.10.1039/C7GC02521ASearch in Google Scholar
Rastogi, V., Samyn, P. (2015) Bio-based coatings for paper applications. Coatings 5(4):887–930.10.3390/coatings5040887Search in Google Scholar
Ray, S.S., Yamada, K., Okamoto, M., Ueda, K. (2002) Polylactide-layered silicate nanocomposite: A novel biodegradable material. Nano Lett. 2:1093–1096.10.1021/nl0202152Search in Google Scholar
Reddy, M.M., Vivekanandhan, S., Misra, M., Bhatia, S.K., Mohanthy, A.K. (2013) Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 38:1653–1689.10.1016/j.progpolymsci.2013.05.006Search in Google Scholar
Rhim, J.-W., Park, H.-M., Ha, C.-S. (2013) Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 38(10-11):1629–1652.10.1016/j.progpolymsci.2013.05.008Search in Google Scholar
Rhim, J.W., Ng, P.K. (2007) Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. Nutr. 47(4):411–433.10.1080/10408390600846366Search in Google Scholar PubMed
Richardson, J.J., Bjornmalm, M., Caruso, F. (2015) Multilayer assembly. Technology-driven layer-by-layer assembly of nanofilms. Science 348(6233):aaa2491.10.1126/science.aaa2491Search in Google Scholar PubMed
Robertson, G.L. Food Packaging. Principles and Practice, 2 Ed. CRC and Taylor & Francis Group, Boca Raton, London, New York, 2006.Search in Google Scholar
Robertson, G.L. Food Packaging and Shelf Life: A Practical Guide. CRC Press and Taylor & Francis Group, Boca Raton, London, New York, 2009.10.1201/9781420078459Search in Google Scholar
Rossi, M., Passeri, D., Sinibaldi, A., Angjellari, M., Tamburri, E., Sorbo, A., Carata, E., Dini, L. (2017) Nanotechnology for food packaging and food quality assessment. Adv. Food. Nutr. Res. 82:149–204.10.1016/bs.afnr.2017.01.002Search in Google Scholar PubMed
Rovera, C., Ghaani, M., Farris, S. (2020) Nano-inspired oxygen barrier coatings for food packaging applications: An overview. Trends Food Sci. Technol. 97:210–220.10.1016/j.tifs.2020.01.024Search in Google Scholar
Rujnić-Sokele, M., Pilipović, A. (2017) Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 35(2):132–140.10.1177/0734242X16683272Search in Google Scholar PubMed
Samori, C., Abbondanzi, F., Galletti, P., Giorgini, L., Mazzocchetti, L., Torri, C., Tagliavini, E. (2015) Extraction of polyhydroxyalkanoates from mixed microbial cultures: Impact on polymer quality and recovery. Bioresour. Technol. 189:195–202.10.1016/j.biortech.2015.03.062Search in Google Scholar PubMed
Sanchez, C., Belleville, P., Popall, M., Nicole, L. (2011) Applications of advanced hybrid organic–inorganic nanomaterials: from laboratory to market. Chem. Soc. Rev. 40:696–753.10.1039/c0cs00136hSearch in Google Scholar PubMed
Scaffaro, R., Botta, L., Lopresti, F., Maio, A., Sutera, F. (2016) Polysaccharide nanocrystals as fillers for PLA based nanocomposites. Cellulose 24(2):447–478.10.1007/s10570-016-1143-3Search in Google Scholar
Scaffaro, R., Maio, A., Sutera, F., Gulino, E.F., Morreale, M. (2019) Degradation and recycling of films based on biodegradable polymers: A short review. Polymers 11(4):651.10.3390/polym11040651Search in Google Scholar PubMed PubMed Central
Schneiderman, D.K., Hillmyer, M.A. (2017) 50th anniversary perspective: There is a great future in sustainable polymers. Macromolecules 50(10):3733–3749.10.1021/acs.macromol.7b00293Search in Google Scholar
Senturk Parreidt, T., Muller, K., Schmid, M. (2018) Alginate-based edible films and coatings for food packaging applications. Foods 7(10):170.10.3390/foods7100170Search in Google Scholar PubMed PubMed Central
Shatkin, J.A. (2020) The future in nanosafety. Nano Lett. 20(3):1479–1480.10.1021/acs.nanolett.0c00432Search in Google Scholar PubMed
Shatkin, J.A., Kim, B. (2015) Cellulose nanomaterials: life cycle risk assessment, and environmental health and safety roadmap. Environ. Sci. Nano 2(5):477–499.10.1039/C5EN00059ASearch in Google Scholar
Shimizu, M., Saito, T., Isogai, A. (2016) Water-resistant and high oxygen-barrier nanocellulose films with interfibrillar cross-linkages formed through multivalent metal ions. J. Membr. Sci. 500:1–7.10.1016/j.memsci.2015.11.002Search in Google Scholar
Sinha Ray, S., Okamoto, M. (2003) Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog. Polym. Sci. 28:1539–1641.10.1016/j.progpolymsci.2003.08.002Search in Google Scholar
Sinha Ray, S.S., Yamada, K., Okamoto, M., Ueda, K. (2002) Polylactide-layered silicate nanocomposite: A novel biodegradable material. Nano Lett. 2(10):1093–1096.10.1021/nl0202152Search in Google Scholar
Siró, I., Plackett, D. (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17(3):459–494.10.1007/s10570-010-9405-ySearch in Google Scholar
Smith, R.J., Long, C.T., Grunlan, J.C. (2018) Transparent polyelectrolyte complex. Thin films with ultralow oxygen transmission rate. Langmuir 34(37):11086–11091.10.1021/acs.langmuir.8b02391Search in Google Scholar PubMed
Song, Y., Gerringer, J., Qin, S., Grunlan, J.C. (2018) High oxygen barrier thin film from aqueous polymer/clay slurry. Ind. Eng. Chem. Res. 57(20):6904–6909.10.1021/acs.iecr.8b01077Search in Google Scholar
Sorrentino, A., Gorrasi, G., Vittoria, V. (2007) Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 18(2):84–95.10.1016/j.tifs.2006.09.004Search in Google Scholar
Spence, K.L., Venditti, R.A., Rojas, O.J., Pawlak, J.J., Hubbe, M.A. (2011) Water vapor barrier properties of coated and filled microfibrillated cellulose composite films. BioResources 6(4):4370–4388.10.15376/biores.6.4.4370-4388Search in Google Scholar
Su, Y., Yang, B., Liu, J., Sun, B., Cao, C., Zou, X., Lutes, R., He, Z. (2018) Prospects for replacement of some plastics in packaging with lignocellulose materials: A brief review. BioResources 13(2):4550–4576.10.15376/biores.13.2.SuSearch in Google Scholar
Syverud, K., Stenius, P. (2009) Strength and barrier properties of MFC films. Cellulose 16(1):75–85.10.1007/s10570-008-9244-2Search in Google Scholar
Sæther, H.V., Holme, H.K., Maurstad, G., Smidsrød, O., Stokke, B.T. (2008) Polyelectrolyte complex formation using alginate and chitosan. Carbohydr. Polym. 74(4):813–821.10.1016/j.carbpol.2008.04.048Search in Google Scholar
Tanaka, K., Watanuki, Y., Takada, H., Ishizuka, M., Yamashita, R., Kazama, M., Hiki, N., Kashiwada, F., Mizukawa, K., Mizukawa, H., Hyrenbach, D., Hester, M., Ikenaka, Y., Nakayama, S.M.M. (2020) In vivo accumulation of plastic-derived chemicals into seabird tissues. Curr. Biol. 30(4):723–728.10.1016/j.cub.2019.12.037Search in Google Scholar PubMed
Tang, X.Z., Kumar, P., Alavi, S., Sandeep, K.P. (2012) Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 52(5):426–442.10.1080/10408398.2010.500508Search in Google Scholar PubMed
Thakur, R., Pristijono, P., Scarlett, C.J., Bowyer, M., Singh, S.P., Vuong, Q.V. (2019) Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 132:1079–1089.10.1016/j.ijbiomac.2019.03.190Search in Google Scholar PubMed
Thellen, C.T. High barrier multilayer packaging by the coextrusion method: The effect of nanocomposites and biodegradable polymers on flexible film properties. Univ. of Massachusetts, Lowell, Massachusetts, USA, 2010.Search in Google Scholar
Turbak, A.F., Snyder, F.W., Sandberg, K.R. (1983) Microfibrillated cellulose, a new cellulose product: Properties, uses and commercial potential. J. Appl. Polym. Sci., Appl. Polym. Symp. 37:815–827.Search in Google Scholar
Wagner, A., White, A.P., Stueckle, T.A., Banerjee, D., Sierros, K.A., Rojanasakul, Y., Agarwal, S., Gupta, R.K., Dinu, C.Z. (2017) Early assessment and correlations of nanoclay’s toxicity to their physical and chemical properties. ACS Appl. Mater. Interfaces 9(37):32323–32335.10.1021/acsami.7b06657Search in Google Scholar PubMed PubMed Central
Wang, H., Qian, J., Ding, F. (2018) Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 66(2):395–413.10.1021/acs.jafc.7b04528Search in Google Scholar PubMed
Wang, J., Gardner, D.J., Stark, N.M., Bousfield, D.W., Tajvidi, M., Cai, Z. (2017) Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 6(1):49–70.10.1021/acssuschemeng.7b03523Search in Google Scholar
Wei, R., Zimmermann, W. (2017) Microbial enzymes for the recycling of recalcitrant petroleum based plastics: How far are we? Microb. Biotechnol. 10:1308–1322.10.1111/1751-7915.12710Search in Google Scholar PubMed PubMed Central
Wyser, Y., Adams, M., Avella, M., Carlander, D., Garcia, L., Pieper, G., Rennen, M., Schuermans, J., Weiss, J. (2016) Outlook and challenges of nanotechnologies for food packaging. Packag. Technol. Sci. 29(12):615–648.10.1002/pts.2221Search in Google Scholar
Xie, J., Zhang, K., Wu, J., Ren, G., Chen, H., Xu, J. (2016) Bio-nanocomposite films reinforced with organo-modified layered double hydroxides: Preparation, morphology and properties. Appl. Clay Sci. 126:72–80.10.1016/j.clay.2016.02.025Search in Google Scholar
Xu, J., Guo, B.H. (2010) Poly(butylene succinate) and its copolymers: research, development and industrialization. Biotechnol. J. 5(11):1149–1163.10.1002/biot.201000136Search in Google Scholar PubMed
Yang, K.K., Wang, X.-L., Wang, Y.-Z. (2007) Progress in nanocomposite of biodegradable polymer. J. Ind. Eng. Chem. 13(4):485–500.Search in Google Scholar
Yeo, B.G., Takada, H., Yamashita, R., Okazaki, Y., Uchida, K., Tokai, T., Tanaka, K., Trenholm, N. (2019) PCBs and PBDEs in microplastic particles and zooplankton in open water in the Pacific Ocean and around the coast of Japan. Mar. Pollut. Bull. 151:110806.10.1016/j.marpolbul.2019.110806Search in Google Scholar PubMed
Yin, G.-Z., Yang, X.-M. (2020) Biodegradable polymers: a cure for the planet, but a long way to go. J. Polym. Res. 27(2):38.10.1007/s10965-020-2004-1Search in Google Scholar
Youssef, A.M., El-Sayed, S.M. (2018) Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 193:19–27.10.1016/j.carbpol.2018.03.088Search in Google Scholar PubMed
Yu, J., Ruengkajorn, K., Crivoi, D.G., Chen, C., Buffet, J.C., O’Hare, D. (2019) High gas barrier coating using non-toxic nanosheet dispersions for flexible food packaging film. Nat. Commun. 10(1):2398.10.1038/s41467-019-10362-2Search in Google Scholar PubMed PubMed Central
Zeng, S.H., Duan, P.P., Shen, M.X., Xue, Y.J., Wang, Z.Y. (2016) Preparation and degradation mechanisms of biodegradable polymer: a review. IOP Conf. Ser., Mater. Sci. Eng. 137:012003.10.1088/1757-899X/137/1/012003Search in Google Scholar
Zhang, X., Xu, Y., Zhang, X., Wu, H., Shen, J., Chen, R., Xiong, Y., Li, J., Guo, S. (2019) Progress on the layer-by-layer assembly of multilayered polymer composites: Strategy, structural control and applications. Prog. Polym. Sci. 89:76–107.10.1016/j.progpolymsci.2018.10.002Search in Google Scholar
Zhao, P., Liu, W., Wu, Q., Ren, J. (2010) Preparation, mechanical, and thermal properties of biodegradable polyesters/poly (lactic acid) blends. J. Nanomater. 2010:1–8.10.1155/2010/287082Search in Google Scholar
Zink, J., Wyrobnik, T., Prinz, T., Schmid, M. (2016) Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 17(9):1376.10.3390/ijms17091376Search in Google Scholar PubMed PubMed Central
Zubair, M., Ullah, A. (2020) Recent advances in protein derived bionanocomposites for food packaging applications. Crit. Rev. Food Sci. Nutr. 60(3):406–434.10.1080/10408398.2018.1534800Search in Google Scholar PubMed
© 2020 Lindström and Österberg, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Evolution of biobased and nanotechnology packaging – a review
- Chemical pulping
- Evaluation of sodium salt scaling in black liquor evaporators using existing process data
- Assessing the value of a diversified by-product portfolio to allow for increased production flexibility in pulp mills
- Bleaching
- Effect of introducing ozone in elemental chlorine free bleaching of pulp on generation of chlorophenolic compounds
- Chlorine dioxide bleaching of nineteen non-wood plant pulps
- A solid-phase extraction method that eliminates matrix effects of complex pulp mill effluents for the analysis of lipophilic wood extractives
- Mechanical pulping
- Development of fibre properties in mill scale high- and low consistency refining of thermomechanical pulp (Part 1)
- Measurement and interpretation of spatially registered bar-forces in LC refining
- Paper technology
- Production of a fine fraction using micro-perforated screens
- The effect of Plantago psyllium seed husk flour on the properties of cellulose sheet
- Comprehensive evaluation of the industrial processing effects on the fiber properties of the pulps from wood residues
- Paper chemistry
- Application of CS-CHO-g-PMMA emulsion in paper reinforcement and protection
- Effects of metal ions and wood pitch on retention and physical properties of TMP
- Coating
- Effect of the glass-transition temperature of latexes on drying-stress development of latex films and inkjet coating layers
- Nanotechnology
- Study of LCNF and CNF from pine and eucalyptus pulps
- Miscellaneous
- The component composition of planted pine wood cultivated in the boreal zone
Articles in the same Issue
- Frontmatter
- Review
- Evolution of biobased and nanotechnology packaging – a review
- Chemical pulping
- Evaluation of sodium salt scaling in black liquor evaporators using existing process data
- Assessing the value of a diversified by-product portfolio to allow for increased production flexibility in pulp mills
- Bleaching
- Effect of introducing ozone in elemental chlorine free bleaching of pulp on generation of chlorophenolic compounds
- Chlorine dioxide bleaching of nineteen non-wood plant pulps
- A solid-phase extraction method that eliminates matrix effects of complex pulp mill effluents for the analysis of lipophilic wood extractives
- Mechanical pulping
- Development of fibre properties in mill scale high- and low consistency refining of thermomechanical pulp (Part 1)
- Measurement and interpretation of spatially registered bar-forces in LC refining
- Paper technology
- Production of a fine fraction using micro-perforated screens
- The effect of Plantago psyllium seed husk flour on the properties of cellulose sheet
- Comprehensive evaluation of the industrial processing effects on the fiber properties of the pulps from wood residues
- Paper chemistry
- Application of CS-CHO-g-PMMA emulsion in paper reinforcement and protection
- Effects of metal ions and wood pitch on retention and physical properties of TMP
- Coating
- Effect of the glass-transition temperature of latexes on drying-stress development of latex films and inkjet coating layers
- Nanotechnology
- Study of LCNF and CNF from pine and eucalyptus pulps
- Miscellaneous
- The component composition of planted pine wood cultivated in the boreal zone


