Abstract
Objectives
The current study was undertaken to understand the underlying molecular mechanism(s) by which HIV Transactivator of transcription (Tat) alters oligodendrocyte maturation through the generation of reactive oxygen species (ROS), impairment of lysosomal functioning, and dysregulation of autophagy.
Methods
We exposed primary rat immature oligodendrocytes to HIV Tat and utilized various experimental techniques to assess its effects on oligodendrocytes maturation. We measured ROS levels, assessed lysosomal membrane potential, determined cathepsin D activity, and analyzed the expression of autophagy-related markers. Furthermore, we investigated the potential of ROS scavengers and lysosomal protectants to mitigate the damaging effects of HIV Tat on oligodendrocytes maturation.
Results
Exposure of primary rat immature oligodendrocytes to HIV Tat significantly increased ROS levels, indicating the induction of oxidative stress. This oxidative stress impaired lysosomal functioning, as evidenced by a substantial increase in lysosomal membrane potential and a decrease in cathepsin D activity. Compromised lysosomal function resulted in dysregulated autophagy, which was confirmed by increased expression of SQSTM1. However, the administration of ROS scavengers and lysosomal protectants effectively attenuated the detrimental effects of HIV Tat on oligodendrocytes maturation.
Conclusions
Our findings demonstrate that HIV Tat exposure induces oxidative stress, impairs lysosomal functioning, and dysregulates autophagy in oligodendrocytes. These molecular changes likely contribute to the altered maturation of oligodendrocytes observed in HIV-infected individuals. Understanding these underlying mechanisms provides valuable insights into the pathogenesis of HIV-associated neurocognitive disorders and highlights the potential of therapeutic strategies targeting ROS scavenging and lysosomal protection as adjunctive approaches for managing such complications in HIV +ve individuals.
Introduction
Although combined antiretroviral therapy (cART) has dramatically increased the lifespan of HIV-1 infected individuals, paradoxically, almost 50 % of the infected individuals will go on to develop HIV-1-associated neurocognitive disorders (HAND) ranging from asymptomatic to mild cognitive-motor disorders, which severely impacts the quality of life [1–3]. White matter abnormalities have been linked to neurocognitive dysfunction [4, 5]. Many studies have reported white matter damage in people living with HIV-1, even in the era of cART, and more so with increasing severity in patients with HAND [6–9]. Brain mapping studies using magnetic resonance imaging and diffusion tensor imaging in HIV-1-infected individuals have reported significant white matter damage with disease progression [10–12].
Despite the advances in cART, the ongoing, persistent low-level viral replication in the brain and the simultaneous release and accumulation of viral proteins such as HIV-1 transactivator of transcription (Tat) is known to contribute to HAND pathogenesis [13–17]. Among the nine HIV-1 encoded proteins, HIV-1 Tat is a crucial component for efficient transcription of the HIV-1 genome. HIV-1 Tat is secreted by the infected cells of the CNS [18] and can also be taken up by the recipient cells leading to cytotoxicity [19–23]. Oligodendrocytes are the myelinating cells of the CNS [24–26]. Although HIV-1 Tat-mediated oligodendrocyte and/or myelin injury has been well documented [27–30], the precise molecular mechanism(s) underlying HIV-1 Tat-mediated alterations in oligodendrocyte maturation remain less understood.
Myelination is a complex yet finely regulated process that includes myelin protein synthesis, storage, and transportation [31]. Lysosomes are specialized cellular organelles that play important roles in protein degradation involving various pathways, including autophagy [32, 33]. It has been well reported that lysosomes play a crucial role in myelination, and myelin biogenesis is regulated by transporting one of the major myelin proteins, proteolipid protein (PLP), from the late endosomes/lysosomes to the cell membrane in oligodendrocytes [34–36]. A functional lysosome is necessary for autophagy clearance [37]. Autophagy is essential for oligodendrocyte survival and proper myelination [38, 39]. Dysregulated autophagy in oligodendrocytes has been linked to spinal cord injury [40, 41]. Furthermore, both lysosomal and autophagy dysfunction play critical roles in the pathogenesis of several neurodegenerative diseases [42–47]. However, whether HIV-1 Tat-mediated alterations in oligodendrocyte maturation involve lysosomal dysfunction and defective autophagy is not well explored.
Based on the above, the present study assessed HIV-1 Tat-mediated lysosomal dysfunction and its role in oligodendrocyte maturation. Our findings, for the first time, describe the mechanistic insights underlying HIV-1 Tat-mediated lysosomal dysfunction and possible restoration intervention strategies, such as lysosomal protection, that could be developed as a potential therapeutic target(s) for white matter damage in HIV-infected individuals.
Results
HIV-1 Tat-mediated alteration in primary rat oligodendrocytes maturation
A hallmark of oligodendrocyte maturation is the ability to form elaborated branched processes and myelin protein (PLP and MBP) [48–50]. First, we sought to determine whether exposure of immature oligodendrocytes to HIV-1 Tat could alter their myelin protein. For this, immature oligodendrocytes were exposed to varying doses of HIV-1 Tat (12, 25, 50, and 100 ng/mL) for 24 h, following which the expression levels of myelin proteins such as PLP and MBP were determined using western blotting. As shown in Figure 1(A and B), in the presence of 50 ng/mL of HIV-1 Tat, there was a significant decrease (p<0.05) in the expression of myelin proteins (PLP and MBP) in oligodendrocytes. This concentration (50 ng/mL) of HIV-1 Tat was thus chosen for the subsequent experiments. The rationale for choosing this concentration of HIV-1 Tat was based on the premise that the Tat concentration in the serum and the cerebrospinal fluid of HIV-1-infected individuals ranges from 1 to 40 ng/mL [51, 52] and that this could be higher in the CNS in the vicinity of HIV-1 positive perivascular cells [53]. As expected, exposure of immature oligodendrocytes to heat-inactivated HIV-1 Tat (50 ng/mL) did not significantly affect the expression of myelin proteins (Figure 1(A and B)). Next, we assessed the morphology of the oligodendrocytes exposed to HIV-1 Tat. Cultured oligodendrocytes were stained for actin (Figure 1(C)), which stains the cytoskeleton of the cell. Data showed that cells exposed to HIV-1 Tat exhibited diminished cellular processes (Figure 1(D)) and decreased secondary branches (Figure 1(E)). Next, we sought to assess the distribution of myelin PLP in HIV-1 Tat-exposed oligodendrocytes (Figure 1(F)). Quantification data showed no change in myelin PLP expression in the cell soma (Figure 1(G)); however, there was decreased myelin PLP expression in the cell processes (Figure 1(H)) of HIV-1 Tat exposed oligodendrocytes.

Exposure of immature oligodendrocytes to HIV-1 Tat resulted in decreased myelin proteins and impaired cell process. For this, immature oligodendrocytes were exposed to varying doses of HIV-1 Tat (12, 25, 50, and 100 ng/mL) for 24 h, following which the expression levels of myelin proteins such as PLP and MBP were determined using western blotting. (A and B) HIV-1 Tat dose-dependently downregulated the expression of myelin proteins – PLP (A) and MBP (B) in oligodendrocytes. According to the manufacturer’s instructions, two bands observed near 20 kDa were analyzed for PLP. For MBP, bands between 14 and 21 kDa were analyzed. (C–E) HIV-1 Tat (50 ng/mL)-mediated decreased cellular morphology of oligodendrocytes. (C) Representative fluorescent-microscopic image showing HIV-1 Tat-mediated decreased actin cytoskeleton staining in oligodendrocytes. (D and E) Representative bar graph showing HIV-1 Tat-mediated decreased cell process length and secondary branches in oligodendrocytes. (F–H) HIV-1 Tat (50 ng/mL)-mediated decreased myelin PLP in the cellular process. (F) Representative fluorescent-microscopic image showing HIV-1 Tat-mediated decreased myelin PLP staining in oligodendrocytes. (G and H) The representative bar graph shows myelin PLP in the oligodendrocytes cell soma and cell process. Data are from three independent experiments. Actin served as a protein loading control for western blots. Data are expressed as means ± SEM and were analyzed using student t-test or one-way ANOVA. *p<0.05 vs. control.
HIV-1 Tat-mediated lysosome dysfunction in primary rat oligodendrocytes
Lysosomes are important for myelin protein secretion and sorting in oligodendrocytes [54]. Next, we sought to explore the impact of HIV-1 Tat on lysosomal function in the oligodendrocytes. Immature oligodendrocytes were exposed to HIV-1 Tat (50 ng/mL) for 3–24 h, then the expression of the lysosomal marker, LAMP2 (lysosomal-associated membrane protein 2), was assessed. As shown in Figure 2(A), in oligodendrocytes exposed to HIV-1 Tat, there was significant downregulation of LAMP2 expression starting at 12 h with a continued trend of downregulation up to 24 h. Interestingly, HIV-1 Tat exposure also decreased the expression of mature cathepsin D (mCTSD) in oligodendrocytes (Figure 2(B)). We next examined lysosomal functioning by assessing lysosomal pH and the enzyme activity in HIV-1 Tat exposed oligodendrocytes. As shown in Figure 2(C–E), we found an increase in lysosomal pH (Figure 2(C)) and increased lysosome membrane permeability (LMP) (Figure 2(D)) that was accompanied by a significant decrease in CTSD activity (Figure 2(E)) in oligodendrocytes exposed to HIV-1 Tat.

Exposure of immature oligodendrocytes to HIV-1 Tat resulted in lysosomal dysfunction. Immature oligodendrocytes were exposed to HIV-1 Tat (50 ng/mL) for 3–24 h, then the expression of the lysosomal marker, LAMP2 (lysosomal-associated membrane protein 2), and mature cathepsin D (mCTSD) were assessed. (A and B) HIV-1 Tat (50 ng/mL) time-dependently downregulated the expression of lysosome markers – LAMP2 (A) and mCTSD (B) in oligodendrocytes. (C–D) HIV-1 Tat (50 ng/mL) dysregulates functions of lysosome in oligodendrocytes. (C) Representative bar graph showing HIV-1 Tat-mediated increased lysosomal pH in oligodendrocytes. (D) Representative bar graph showing HIV-1 Tat-mediated increased LMP in oligodendrocytes. (E) Representative bar graph showing HIV-1 Tat-mediated decreased CTSD activity in oligodendrocytes. Data are from three independent experiments. Actin served as a protein loading control for western blots. Data are expressed as means ± SEM and were analyzed using student t-test or one-way ANOVA. *p<0.05 vs. control.
HIV-1 Tat-mediated autophagy dysregulation in rat primary oligodendrocytes
During the autophagy process, lysosomes fuse with autophagosomes and form autolysosomes. The formation of autolysosomes ensures complete clearance of misfolded proteins. Also, studies have shown that autophagy plays key role in oligodendrocyte maturation [38, 55]. We thus rationalized that impaired lysosomal functioning could block the autophagic flux. We thus sought to assess the effects of HIV-1 Tat on autophagy mediators. We assessed the expression of multiple autophagy-related proteins, including BECN1/Beclin1 (beclin 1), MAP1LC3B/LC3B (microtubule-associated protein 1 light chain 3 beta), and SQSTM1/p62 (sequestosome 1) in oligodendrocytes treated with HIV-1 Tat (50 ng/mL) for varying times ranging from 3 to 24 h. As shown in Figure 3, expression of BECN1 (Figure 3(A)), MAP1LC3B (Figure 3(B)), and SQSTM1 (Figure 3(C)) were significantly upregulated in a time-dependent manner in the oligodendrocytes treated with HIV-1 Tat (50 ng/mL). To confirm whether the increased expression of MAP1LC3B was a result of enhanced autophagosome synthesis or reduced autophagosome turnover (due to delayed trafficking or reduced fusion with the lysosomes), MAP1LC3B turnover and SQSTM1 degradation assays in the presence of bafilomycin A1 (BAF- a known fusion inhibitor of autophagosome and lysosome) alone, HIV-1 Tat alone and combination of BAF (400 nM) with HIV-1 Tat (50 ng/mL) were performed. As shown in Figure 3(D) and (E), western blot analysis showed no significant difference in the accumulation of MAP1LC3B-II and SQSTM1 in oligodendrocytes exposed to HIV-1 Tat in the presence or absence of BAF, further indicating the involvement of defective autophagy flux in HIV-1 Tat exposed oligodendrocytes. MAP1LC3B turnover and the SQSTM1 degradation assays determine the autophagy flux. Accumulation of MAP1LC3B-II and SQSTM1 in HIV-1 Tat exposed oligodendrocytes indicated blockade of the autophagic flux. Adding BAF (autophagosome-lysosome fusion inhibitor) to HIV-1 Tat-treated oligodendrocytes failed to cause a further increase in the expression of MAP1LC3B-II and SQSTM1, thereby confirming maximal accumulation of MAP1LC3B-II and SQSTM1 in HIV-1 Tat exposed oligodendrocytes. Studies have shown that the accumulation of MAP1LC3B-II and SQSTM1 protein levels directly correlates with the number of autophagosomes and impaired autophagic vesicle degradation [56, 57].

Exposure of immature oligodendrocytes to HIV-1 Tat resulted in autophagy dysregulation in oligodendrocytes. We assessed the expression of multiple autophagy-related proteins, including BECN1/Beclin1 (beclin 1), MAP1LC3B/LC3B (microtubule-associated protein 1 light chain 3 beta), and SQSTM1/p62 (sequestosome 1) in oligodendrocytes treated with HIV-1 Tat (50 ng/mL) for varying times ranging from 3 to 24 h. (A–C) HIV-1 Tat (50 ng/mL) time-dependently increased the expression of autophagy markers – BECN1 (A), MAPLC3BII (B), and (C) SQSTM1 in oligodendrocytes. Two bands were observed for LC3B; LC3BI and LC3BII. For analysis, LC3BII was evaluated and compared to a loading control to assess autophagy in all the experiments. (D and E) HIV-1 Tat (50 ng/mL)-mediated decreased autophagic flux in oligodendrocytes. Representative western blots showing the expression of MAP1LC3B-II (D) and SQSTM1 (E) in oligodendrocytes exposed to HIV-1 Tat (50 ng/mL) for 24 h followed by treatment with 400 nM BAF, added in the last 4 h of the 24 h treatment period. Data is from three independent experiments. Actin served as a protein loading control for western blots. Data are expressed as means ± SEM and were analyzed using student t-test or one-way ANOVA. *p<0.05 vs. control; N.S., non-significant.
HIV-1 Tat-mediated ROS generation in primary rat oligodendrocytes
Having established that HIV-1 Tat dysregulated both lysosomal function and autophagy flux while blocking oligodendrocyte maturation, we next sought to explore the mechanism(s) underlying these processes. It is well-known that oxidative stress is one of the critical mediators responsible for the dysregulation of antioxidant enzymes leading, in turn, to cognitive impairment in HIV-1 patients [58]. Intriguingly, exacerbated generation of ROS via dysregulated antioxidant enzymes has also been demonstrated in the monocytes and CSF of HIV-1 infected individuals [59]. We next sought to determine the possible involvement of ROS in HIV-1 Tat-mediated lysosomal dysfunction and autophagy dysregulation. Immature oligodendrocytes exposed to HIV-1 Tat (50 ng/mL) for various time points (0–4 h) were monitored for ROS production using the DCFH-DA assay. As shown in Figure 4(A), exposure of oligodendrocytes to HIV-1 Tat resulted in significant induction of ROS within 1 h (Figure 4(A)). Next, we inhibited ROS generation by pretreating the oligodendrocytes with various ROS scavengers such as TEMPOL (20 μM) and N-acetyl cysteine (NAC) (5 mM) for 1 h followed by HIV-1 Tat (50 ng/mL). As shown in Figure 4(B), pretreatment of the cells with these ROS scavengers significantly abrogated HIV-1 Tat-mediated induction of ROS.

HIV-1 Tat-mediated ROS generation in oligodendrocytes. (A) Immature oligodendrocytes exposed to HIV-1 Tat (50 ng/mL) for various time points (0–4 h) were monitored for ROS production using the DCFH-DA assay. Representative graph showing increased ROS levels in oligodendrocytes exposed to HIV-1 Tat (50 ng/mL) for different time points. (B) Representative bar graph showing effects of ROS scavengers TEMPOL and NAC on HIV-1 Tat-mediated upregulation of ROS. Data are from three independent experiments and is represented as means ± SEM using one-way ANOVA and Student’s t-test. *p<0.05 vs. control; #p<0.05 vs. HIV-1 Tat.
NAC abrogated HIV-1 Tat-mediated defects in primary rat oligodendrocytes
Next, we examined whether HIV-1 Tat-mediated induction of ROS played a role in lysosomal dysfunction, autophagy dysregulation, and decreased oligodendrocyte maturation. To validate this, immature oligodendrocytes were pretreated with NAC (5 mM) for 1 h followed by exposure of cells to HIV-1 Tat (50 ng/mL) for 24 h, following which the protein expression of lysosome markers (LAMP2 and mCTSD), autophagy markers (MAP1LC3B and SQSTM1) and myelin markers (PLP and MBP) was assessed by western blotting. Interestingly, pretreatment of oligodendrocytes with NAC abrogated HIV-1 Tat-mediated downregulation of LAMP2 (Figure 5(A)) and mCTSD (Figure 5(B)). We next examined the protective effects of NAC on HIV-1 Tat-mediated dysregulation of autophagy. As expected, and as shown in Figure 5(C and D), pretreatment of oligodendrocytes with NAC notably blocked HIV-1 Tat-mediated upregulation of MAP1LC3B and SQSTM1, thereby implying increased autophagosome-lysosome fusion. Next, we examined whether pretreatment with NAC could also block HIV-1 Tat-mediated altered oligodendrocyte maturation. As shown in Figure 5(E and F), pretreatment of oligodendrocytes to NAC significantly blocked HIV-1 Tat-mediated downregulation of myelin proteins such as MBP and PLP, thereby indicating the protective role of NAC in HIV-1 Tat-exposed oligodendrocytes.
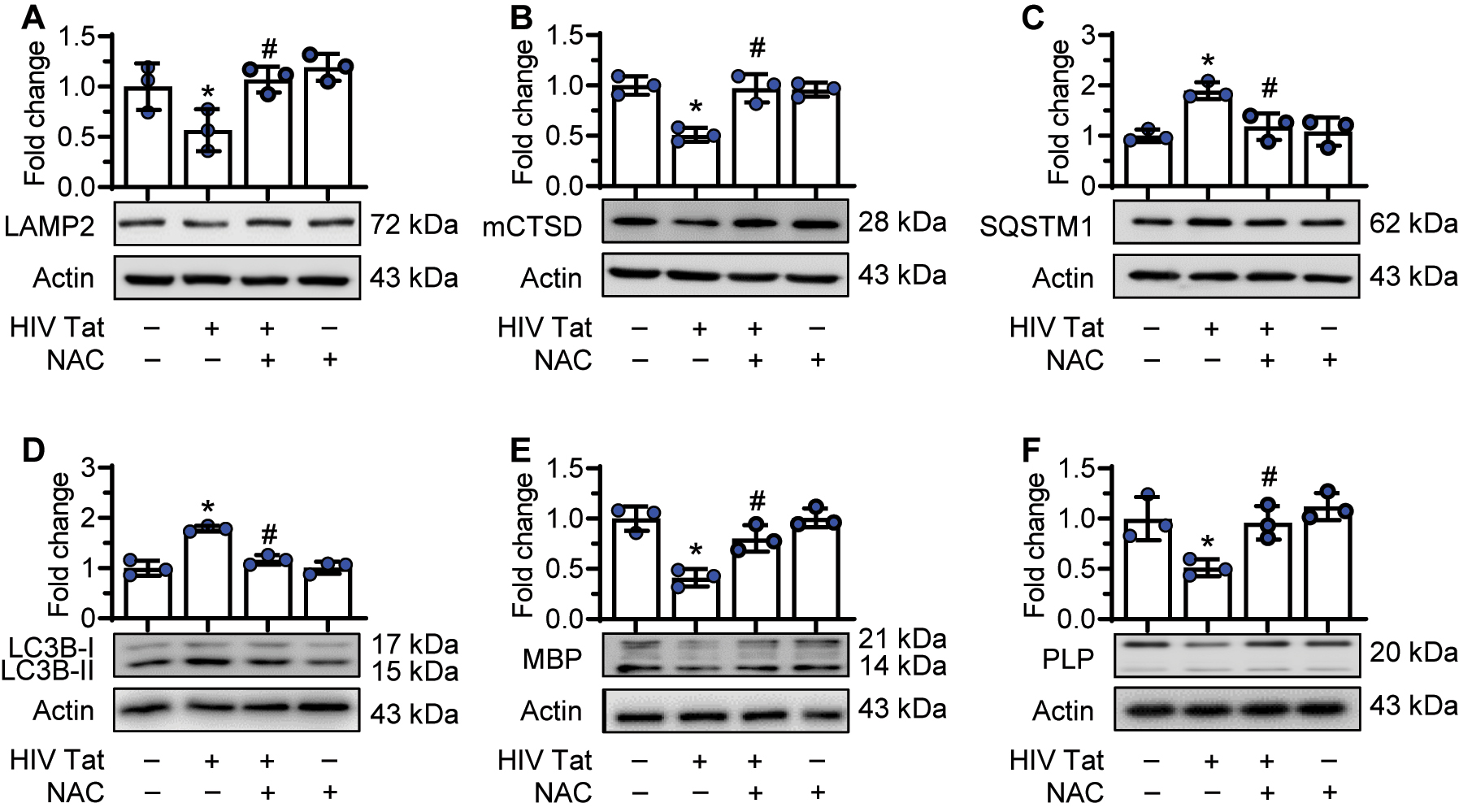
NAC reversed HIV-1 Tat-mediated defects in oligodendrocytes. Immature oligodendrocytes were pretreated with NAC (5 mM) for 1 h, followed by exposure of cells to HIV-1 Tat (50 ng/mL) for 24 h, following which the protein expression of lysosome markers (LAMP2 and mCTSD), autophagy markers (MAP1LC3B and SQSTM1) and myelin markers (PLP and MBP) were assessed by western blotting. (A and B) Representative western blots showing pretreatment of oligodendrocytes to NAC reversed HIV-1 Tat-mediated downregulation of LAMP2 (A) and mCTSD expression levels (B). (C and D) Representative western blots showing pretreatment of oligodendrocytes to NAC reversed HIV-1 Tat-mediated upregulation of SQSTM1 (C) and MAP1LC3BII expression levels (D). (E and F) Representative western blots showing pretreatment of oligodendrocytes to NAC reversed HIV-1 Tat-mediated downregulation of myelin MBP (E) and myelin PLP expression levels (F). Data is from three independent experiments and is represented as means ± SEM using one-way ANOVA. *p<0.05 vs. control; #p<0.05 vs. HIV-1 Tat.
Hsp70 overexpression abrogated HIV-1 Tat-mediated defects in primary rat oligodendrocytes
Having confirmed the importance of LMP in HIV-1 Tat-mediated dysfunctioning of oligodendrocytes, we next sought to determine the protective role of Heat shock protein 70 (Hsp70) in blocking LMP. Hsp70 has been shown to stabilize lysosomes by blocking LMP [60, 61]. Overexpression of Hsp70 protected immature oligodendrocytes against HIV-1 Tat-mediated defects. As shown in Figure 6, Hsp70 overexpression reversed HIV-1 Tat-mediated downregulation of LAMP2 (Figure 6(A)), upregulation of SQSTM1 (Figure 6(B)), and downregulation of myelin protein, MBP (Figure 6(C)). Additionally, Hsp70 overexpression abrogated HIV-1 Tat-mediated increased lysosomal pH (Figure 6(D)) and decreased CTSD activity (Figure 6(E)). To further validate the findings on HIV-1 Tat-mediated lysosome and myelin defects, we performed immunostaining to demonstrate the LAMP2 puncta and myelin PLP intensity in oligodendrocytes (Figure 6(F)). Quantification data showed that Hsp70 overexpression significantly blocked HIV-1 Tat-mediated downregulation of LAMP2 puncta (Figure 6(G)) and PLP intensity (Figure 6(H)).

Hsp70 overexpression reversed HIV-1 Tat-mediated defects in oligodendrocytes. (A–C) Representative western blots and bar graphs showing overexpressing Hsp70 in oligodendrocytes reversed HIV-1 Tat-mediated downregulation of LAMP2 (A), upregulation of SQSTM1 (B), and downregulation of MBP (C) expression levels. (D) Representative bar graph showing Hsp70 overexpression in oligodendrocytes reversed HIV-1 Tat-mediated increased lysosome pH. (E) Representative bar graph showing Hsp70 overexpression in oligodendrocytes reversed HIV-1 Tat-mediated decreased cathepsin D activity. (F) Representative fluorescent-microscopic image showing Hsp70 overexpression reversed HIV-1 Tat-mediated downregulation of myelin PLP and LAMP2 in oligodendrocytes. (G) Representative bar graph showing Hsp70 overexpression in oligodendrocytes reversed HIV-1 Tat-mediated downregulation of LAMP2 puncta. (H) Representative bar graph showing Hsp70 overexpression in oligodendrocytes reversed HIV-1 Tat-mediated downregulation of PLP intensity. Data is from three independent experiments. ACTB served as a protein loading control for western blots. Data is from three independent experiments and is represented as means ± SEM using one-way ANOVA. *p<0.05 vs. control; #p<0.05 vs. HIV-1 Tat.
Discussion
In the cART era, white matter abnormalities are frequently observed in HIV + individuals and with increased severity in those afflicted with HAND [6–9]. HIV-1 Tat continues to be detectable in the CSF of HIV-1-infected patients with well-controlled viremia [62]. HIV-1 Tat protein has been implicated in the pathophysiology of the neurocognitive deficits associated with HIV infection [20]. HIV +ve individuals suffer from cognitive, behavioral, or motor abnormalities, currently classified as HIV-associated neurocognitive disorders (HAND) [63]. Oligodendrocyte damage can cause axonal demyelination and neuronal injury, leading to neurological disorders, and demyelination also cause cognitive impairment in a variety of neurological disorders, including HAND [64]. While HIV-1 Tat-mediated oligodendrocyte and/or myelin injury has been well documented [27–30], the precise molecular mechanism(s) underlying these processes remain less understood. Our findings outline a molecular pathway underlying HIV-1 Tat-mediated impaired oligodendrocyte maturation that involves lysosomal dysfunction and autophagy dysregulation. While the current study focused on investigating the effects of a specific dose of HIV Tat, future research will explore the impact of HIV Tat at different doses and its relevance to other diseases or conditions.
Lysosomes are specialized cellular organelles crucial for the degradation of misfolded proteins and macromolecules/damaged organelles. Myelin abnormalities are a common feature in lysosomal disorders [65]. Lysosomes are well-known targets of cytotoxic HIV proteins, with dysfunction of lysosomes being inherently implicated in pathogenesis [66]. Reports indicate HIV-1 Tat-mediated disruption of endolysosomal function, leading to the accumulation of amyloid beta in rat hippocampal neurons, ultimately culminating in neurotoxicity [67]. Lysosomes contain several proteases, including cathepsins. Inactive pro-cathepsins (pCTSD) can be proteolytically converted to active mature cathepsins (mCTSD) in an acidic environment (low pH) of the lysosomes [68–70]. Our findings suggest increased lysosomal pH, decreased mCTSD expression, and decreased CTSD activity in the oligodendrocytes exposed to HIV-1 Tat.
LMP is the primary cause of elevated lysosomal pH. Protons leak through the destabilized membrane, resulting in a loss of the pH gradient [71, 72]. LAMP, a glycosylated transmembrane protein, protects the lysosomal membrane from its enzymes. Paraquat-induced oxidative stress has been shown to permeabilize the lysosomal membrane and causes lysosomal alkalinization in astrocytes [73]. Our results showed HIV-1 Tat-mediated downregulation of LAMP2 expression with increased LMP. Overall, HIV-1 Tat-mediated LMP resulted in elevated lysosomal pH and decreased CTSD activity.
Lysosomes are critical for the maturation of the autophagy process. Lysosomes fuse with autolysosomes to form autophagosomes, which is critical for protein degradation [74, 75]. Autophagy dysregulation has been implicated as one of the hallmark features of HIV neuropathogenesis [76, 77]. Our findings demonstrated HIV-1 Tat-mediated dysregulation of autophagy, assessed by increased expression of autophagy markers such as BECN1, MAP1LC3B, and SQSTM1. MAP1LC3B turnover and the SQSTM1 degradation assays determine the autophagy flux. Accumulation of MAP1LC3B-II and SQSTM1 in HIV-1 Tat exposed oligodendrocytes indicated blockade of the autophagic flux. Adding BAF (autophagosome-lysosome fusion inhibitor) to HIV-1 Tat-treated oligodendrocytes failed to cause a further increase in the expression of MAP1LC3B-II and SQSTM1, thereby confirming maximal accumulation of MAP1LC3B-II and SQSTM1 in HIV-1 Tat exposed oligodendrocytes.
HIV-1 Tat-mediated upregulation of ROS has been reported in various CNS cells [78, 79]. Furthermore, it has also been shown that induction of ROS can lead to LMP [72, 80]. ROS can quickly diffuse into lysosomes and interact with free intralysosomal iron to generate highly reactive hydroxyl radicals in a Fenton-type reaction. Such a hydroxyl radical can induce LMP by causing lipid peroxidation of lysosomal membranes and damaging lysosomal membrane proteins [81–83].
We thus explored the role of ROS scavengers, such as NAC and Hsp70, in abrogating HIV-1 Tat-mediated lysosomal dysfunction and autophagy dysregulation. Our findings demonstrated HIV-1 Tat-mediated upregulation of ROS generation within 1 h of exposure in oligodendrocytes. Further, pretreatment of cells with ROS scavenger or lysosomal protector NAC significantly abrogated HIV-1 Tat-mediated lysosomal dysfunction and autophagy dysregulation in oligodendrocytes. Pretreatment with NAC reversed lysosomal pH and CTSD activity while also blocking HIV-1 Tat-mediated upregulated expression of autophagy markers (BECN1, MAP1LC3B-II, and SQSTM1). NAC also abrogated HIV-1 Tat-mediated downregulation of myelin proteins (MBP and PLP) in oligodendrocytes.
Having confirmed the role of LMP in HIV-1 Tat-mediated lysosomal dysfunction in oligodendrocytes, we next sought to understand the role of lysosomal membrane protection in reversing HIV-1 Tat-mediated defects in autophagy and oligodendrocyte maturation. Heat shock protein 70 (Hsp70) has been shown to localize to the luminal side of the endosomal-lysosomal system and stabilizes the lysosomes by blocking LMP [60, 61]. Interestingly, our findings demonstrated that overexpression of Hsp70 in oligodendrocytes significantly abrogated HIV-1 Tat-mediated lysosomal dysfunction (evidenced by analysis of LAMP2 expression, lysosomal pH, and CTSD activity), autophagy dysregulation (evidenced by analysis of LC3BII and SQSTM1 protein expression), and oligodendrocyte maturation (evidenced by analysis of myelin proteins; MBP and PLP).
These observations implicate ROS as an upstream regulator of the lysosomal and autophagic processes. NAC can thus be considered an ideal candidate for scavenging cellular ROS and protecting lysosomal functions, which, in turn, can ameliorate myelin damage in HIV + individuals. Also, lysosome membrane protection by Hsp70 overexpression reversed HIV-Tat-mediated effects. Our findings implicate that lysosomal dysfunction is central in HIV-1 Tat-mediated autophagy dysfunction and myelin defects (Figure 7). Lysosomal protective agents could thus be developed as future adjunctive treatment options for dampening white matter damage in HIV-infected individuals.

Schematic depicting the mechanism(s) involved in HIV-Tat-mediated lysosomal dysfunction in oligodendrocytes. Exposure of immature oligodendrocytes to HIV-Tat increases ROS generation and LMP, leading, in turn, to lysosomal dysfunction and autophagy dysregulation, ultimately leading to decreased oligodendrocyte maturation. The maturation of oligodendrocytes is important for increased myelination. ROS scavenger N-acetylcysteine (NAC) and LMP protector heat shock protein 70 (Hsp70) reversed these deleterious effects of HIV-1 Tat. (CTSD, cathepsin D; LMP, lysosomal membrane permeabilization; MBP, myelin basic protein; PLP, myelin proteolipid protein).
Materials and methods
Reagents
HIV-1 Tat protein (1032–10, recombinant endotoxin-free; ImmunoDX, MA, USA). N-Acetyl Cysteine (NAC) (A7250), and bafilomycin A1 (B1793) were purchased from Sigma-Aldrich. TEMPOL (sc-200825) was purchased from Santa Cruz. Antibody resources: BECN1 (sc-11427) and were purchased from Santa Cruz Biotechnology. LAMP2 (NB300-591) and MAP1LC3B (NB100-2220) were purchased from Novus Biological Company. CTSD (ab75852), myelin PLP (ab9311) and myelin MBP (ab62631) were purchased from Abcam. SQSTM1 (PM045) was purchased from MBL International. Peroxidase-AffiniPure Goat Anti-Rabbit IgG (H+L) [111035-003]; Goat Anti-Mouse IgG (H+L) [115-035-003] used at 1:1000 dilution; from Jackson ImmunoResearch Inc.
Primary oligodendrocyte isolation
Oligodendrocyte progenitor cells (OPCs) were cultured by a previously published method [84, 85]. Briefly, OPCs were isolated from postnatal day 0–2 rat cortices. Dissociated cells were plated on poly-L-lysine (P4707, Millipore Sigma) -coated T75 cm2 flasks with high-glucose Dulbecco’s modified Eagle’s medium (11995-065, Thermo Fisher) containing 10 % fetal bovine serum (16000-044, Thermo Fisher) and with penicillin-streptomycin (P0781, Millipore Sigma) then incubated at 37 °C with 5 % CO2. The culture medium was changed every third day for 10–12 days to obtain mixed glial cultures containing OPCs on the astrocytic monolayer bed. Purification of OPCs was done by shaking (18–20 h) on an orbital shaker at 37 °C, followed by differential adhesion for 1 h on the non-tissue culture plastic petri dish (351006, Fisher Scientific). Isolated OPCs were plated in Sato medium [DMEM, 1X Insulin-Transferrin-Selenium (ITS-G, 41400045, Thermo Fisher) 30 nM triiodothyronine (T6397, Millipore Sigma), penicillin-streptomycin (P0781, Millipore Sigma), 1X GlutaMAX™ Supplement (A1286001, Thermo Fisher)) for 2 days to reach immature status before further experiments [29, 84]. The purity of oligodendrocyte cultures were consistently >95 % oligodendrocytes with <1 % astrocyte contamination [84, 86, 87].
Treatments
At day 2, immature oligodendrocytes [27, 29] were treated with HIV-1 Tat protein with varying doses (12, 25, 50, and 100 ng/mL) for 24 h. The rationale for choosing HIV-1 Tat concentration was based on the premise that the HIV-1 Tat concentration in the serum and the cerebrospinal fluid of HIV-1-infected individuals ranges from 1 to 40 ng/mL [51, 52] and that this could be higher in the CNS in the vicinity of HIV-1 positive perivascular cells [53]. Immature oligodendrocytes were pretreated with ROS scavengers such as TEMPOL (20 μM) and N-acetyl cysteine (NAC) (5 mM) for 1 h followed by HIV-1 Tat.
Western blotting
Cells were lysed using a Lysis kit (Sigma, MCL1-1KT). After protein quantification, samples were electrophoresed in a sodium dodecyl sulfate-polyacrylamide gel under reducing conditions and then transferred to PVDF membranes (Millipore, IPVH00010). After the transfer, PVDF membranes were blocked with 5 % nonfat dry milk in 1× TTBS buffer (1.21 g Tris [Fisher Scientific, BP152-5], 8.77 g NaCl [Fisher Scientific, BP358-212], 500 μL Tween-20 [Fisher Scientific, BP337-500], pH 7.6 for 1 L). Membranes were then probed with antibodies targeting proteins of interest. Beta-actin (ACTB; A5441; Sigma-Aldrich) was used to normalize the loading protein. Goat anti-mouse/rabbit IgG secondary antibodies were HRP conjugated.
Immunocytochemistry
After the treatment, cells were fixed with 4 % paraformaldehyde for 15 min at room temperature, followed by permeabilization with 0.1 % Triton X-100 (Fisher Scientific, BP151-500) in phosphate-buffered saline (PBS; Hyclone Laboratories, SH3025801). 10 % normal goat serum (31872, Thermo Fisher) in PBS was used as blocking for 1 h at room temperature. Primary antibodies against the protein of interest were added and incubated overnight at 4 °C. After 3 washes with buffer, the secondary antibody was added (2 h at room temperature). Cells were washed 3 times in buffer and mounted with Prolong gold antifade reagent with 4,6-diamidino-2-phenylindole (DAPI; Molecular Probes, P36935). Fluorescence images were taken with a Zeiss Observer using a Z1 inverted microscope, and the acquired images were analyzed using the Axio vs. 40 Version 4.8.0.0 software.
Morphological analyses
For the morphological analyses, the visualization of oligodendrocyte cellular process length and branching involved several steps. First, the maximum-intensity confocal images were converted to binary images and then skeletonized using ImageJ software. The tracings of cellular processes were performed using the NeuronJ plugin within ImageJ. The secondary branches of each cellular process were individually traced and counted, with each tracing labeled as primary or secondary. A primary process was defined as any branch emerging from the soma, while a secondary process referred to any branch emerging from a primary cellular process. The number of cell process endpoints and process lengths was measured and normalized with the number of cell somas using the ImageJ software. To establish the pixel-to-µm ratio, the micrometer scale bar from each reconstruction was utilized, enabling the construction of a scale bar in ImageJ. The total length of the cellular process was then summarized for statistical comparisons, employing the Analyze Skeleton plugin within ImageJ software. In addition, for the cell soma analysis, the area around the cell body was delineated using ImageJ selection tools. Subsequently, the region of interest was measured using the ROI Manager in ImageJ, and the values were exported to an Excel file. The percent area and mean fluorescence intensity were multiplied to calculate the total fluorescence intensity for each threshold image [88]. The experiment was repeated three times, with five images per group analyzed. For statistical analysis, a minimum of 25 cells per group were included.
Quantification of LAMP2 puncta
Zeiss Observer using a Z1 inverted microscope (Carl Zeiss, Thornwood, NY, USA) was used to take fluorescence images. Image J software was used to analyze the images. The region of interest was chosen using the polygon selection tool. Fluorescence is converted to black pixels over a white background. The regions of interest to be measured were then analyzed by the Measure Particles algorithm to record puncta number, area, and size, and the results were transferred to an excel spreadsheet using the functions of ImageJ.
ROS detection
ROS detection was performed as per the manufacturer’s protocol (Life Technologies, D-339) protocol using Image-iT™ LIVE Green Reactive Oxygen Species (ROS) Detection Kit obtained from Invitrogen (136007). Briefly, cells grown in 96 well-plates were washed with HBSS, supplemented with 25 μM carboxy-H2DCFDA working solution, and incubated for 30 min at 37 °C. The change in fluorescence was measured using a spectrofluorometer (485 nm excitation and 530 nm emission).
CTSD activity determination
CTSD Activity Assay Kit (Fluorometric) from Abcam (ab65302) was used to analyze CTSD activity. The oligodendrocytes lysates were incubated with reaction buffer for 1 h at 37 °C then the fluorescence was measured at Ex/Em=328/460 nm. Protease activities were determined by comparing the relative fluorescence units (RFUs) against the levels of the controls and represented as fold change.
Lysosomal pH Measurement
Lysosomal pH was measured using LysoSensor (Yellow/Blue DND-160) from Thermo Fisher Scientific (Waltham, MA, USA). LysoSensor is a dual ratio-metric indicator dye specifically designed for measuring the pH of acidic organelles such as lysosomes. In our experimental procedure, cells were incubated with 2 µM LysoSensor for 5 min at 37 °C. The fluorescence intensity was recorded at wavelengths of 340 and 380 nm. The obtained 340/380 nm ratios were then converted to pH units using a calibration curve established with 20 mM MES (+120 mM KCl, 20 mM NaCl, 10 µM Monensin, 20 µM Nigericin). The pH was adjusted using either HCl or NaOH to cover a pH range of 3.0–7.0.
Lysosomal membrane permeability assay
Acridine orange, a fluorescent dye, reversibly accumulates into the acidified membrane-bound compartments of the cell. Acridine orange shows fluorescence emission in a concentration-dependent manner. At high concentrations (e.g., in lysosomes), it shows red fluorescence; at low concentrations (e.g., in the cytosol) shows green fluorescence and yellow fluorescence as intermediate (e.g., upon trapping in nucleoli), thus monitoring lysosomal leakage or change in lysosomal pH. Cells were cultured in 96-well culture plates and firstly exposed with acridine orange (5 μg/mL) at 37 °C for 15 min, then rinsed and incubated in HBSS with or without HIV-1 Tat and NAC for the different time points as indicated. Fluorescence of the cells was examined at 1 h intervals using a Synergy™ Mx Monochromator-Based Multi-Mode Microplate Reader (BioTek Instruments) with excitation at 485 nm and emission at 530 and 620 nm.
MAP1LC3B turnover and SQSTM1 degradation assays
MAP1LC3B turnover and SQSTM1 degradation assays were already published [89]. Briefly, oligodendrocytes were seeded at a density of 5 × 105 cells/well in a 6-well plate at 5 % CO2 incubator at 37 °C for attachment. Cells were starved overnight in the serum-free culture medium. Oligodendrocytes were treated with HIV-1 Tat (50 ng/mL) for 24 h alone, or the cells were exposed to 400 nM BAF in the last 4 h of the 24 h HIV-1 Tat treatment. At the end of the experiment, cells were harvested, and protein samples were prepared for western blotting analysis.
Plasmids transfection
For plasmid transfection, immature oligodendrocytes were maintained in the SATO medium. Upon 70 % confluency, the culture medium was replaced with 250 µl of Opti-MEM® I Reduced Serum Medium. Using Lipofectamine® 3000 Reagent (manufacturer’s protocol), cells were transfected with pEGFP Hsp70 plasmid (a gift from Lois Greene; Addgene, 15215) for 12 h. After that medium was replaced with the fresh medium for another 24 h. The transfection efficiency of the immature oligodendrocytes was 60–65 %. Hsp70 plasmid (a gift from Lois Greene; Addgene, 15215) is tagged with EGFP, a green fluorescent protein that exhibits bright green fluorescence when exposed to light in the blue to ultraviolet range. After transfection, green fluorescent cells were counted, taking the ratio with the total cell by counting DAPI staining. Then, cells were treated with various reagents for the indicated time and processed for further analysis.
Statistical analysis
The results are presented as means ± SEM and were evaluated using a one-way analysis of variance followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means. Probability levels of <0.05 were considered statistically significant.
Funding source: Nebraska Center for Substance Abuse Research
-
Research funding: This work was supported by the Nebraska Center for Substance Abuse Research (NCSAR).
-
Author contributions: Investigation, formal analysis: A.T., P.P. and M.L.G.; conceptualization, and writing: A.T., P.P., and S.B. All authors have read and have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical Approval: Animals were housed and maintained in accordance with the National Institutes of Health institutional guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center.
-
Disclosure statement: No potential conflict of interest was reported by the authors.
References
1. Saloner, R, Cysique, LA. HIV-associated neurocognitive disorders: a global perspective. J Int Neuropsychol Soc 2017;23:860–9. https://doi.org/10.1017/s1355617717001102.Search in Google Scholar PubMed PubMed Central
2. Farhadian, S, Patel, P, Spudich, S. Neurological complications of HIV infection. Curr Infect Dis Rep 2017;19:50. https://doi.org/10.1007/s11908-017-0606-5.Search in Google Scholar PubMed PubMed Central
3. Ru, W, Tang, SJ. HIV-associated synaptic degeneration. Mol Brain 2017;10:40. https://doi.org/10.1186/s13041-017-0321-z.Search in Google Scholar PubMed PubMed Central
4. Alkonyi, B, Govindan, RM, Chugani, HT, Behen, ME, Jeong, JW, Juhasz, C. Focal white matter abnormalities related to neurocognitive dysfunction: an objective diffusion tensor imaging study of children with Sturge-Weber syndrome. Pediatr Res 2011;69:74–9. https://doi.org/10.1203/pdr.0b013e3181fcb285.Search in Google Scholar PubMed PubMed Central
5. Kochunov, P, Coyle, TR, Rowland, LM, Jahanshad, N, Thompson, PM, Kelly, S, et al.. Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatr 2017;74:958–66. https://doi.org/10.1001/jamapsychiatry.2017.2228.Search in Google Scholar PubMed PubMed Central
6. Su, T, Caan, MW, Wit, FW, Schouten, J, Geurtsen, GJ, Cole, JH, et al.. White matter structure alterations in HIV-1-infected men with sustained suppression of viraemia on treatment. AIDS 2016;30:311–22. https://doi.org/10.1097/qad.0000000000000945.Search in Google Scholar PubMed
7. Underwood, J, Cole, JH, Caan, M, De Francesco, D, Leech, R, van Zoest, RA, et al.. Gray and white matter abnormalities in treated human immunodeficiency virus disease and their relationship to cognitive function. Clin Infect Dis 2017;65:422–32. https://doi.org/10.1093/cid/cix301.Search in Google Scholar PubMed PubMed Central
8. Alakkas, A, Ellis, RJ, Watson, CW, Umlauf, A, Heaton, RK, Letendre, S, et al.. White matter damage, neuroinflammation, and neuronal integrity in HAND. J Neurovirol 2019;25:32–41. https://doi.org/10.1007/s13365-018-0682-9.Search in Google Scholar PubMed PubMed Central
9. Oh, SW, Shin, NY, Choi, JY, Lee, SK, Bang, MR. Altered white matter integrity in human immunodeficiency virus-associated neurocognitive disorder: a tract-based spatial statistics study. Korean J Radiol 2018;19:431–42. https://doi.org/10.3348/kjr.2018.19.3.431.Search in Google Scholar PubMed PubMed Central
10. Chen, Y, An, H, Zhu, H, Stone, T, Smith, JK, Hall, C, et al.. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage 2009;47:1154–62. https://doi.org/10.1016/j.neuroimage.2009.04.030.Search in Google Scholar PubMed PubMed Central
11. Ragin, AB, Storey, P, Cohen, BA, Edelman, RR, Epstein, LG. Disease burden in HIV-associated cognitive impairment: a study of whole-brain imaging measures. Neurology 2004;63:2293–7. https://doi.org/10.1212/01.wnl.0000147477.44791.bd.Search in Google Scholar PubMed PubMed Central
12. Stubbe-Drger, B, Deppe, M, Mohammadi, S, Keller, SS, Kugel, H, Gregor, N, et al.. Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC Neurol 2012;12:23. https://doi.org/10.1186/1471-2377-12-23.Search in Google Scholar PubMed PubMed Central
13. Ene, L. Human immunodeficiency virus in the brain-culprit or facilitator? Infect Disord 2018;11:1178633717752687. https://doi.org/10.1177/1178633717752687.Search in Google Scholar PubMed PubMed Central
14. Marban, C, Forouzanfar, F, Ait-Ammar, A, Fahmi, F, El Mekdad, H, Daouad, F, et al.. Targeting the brain reservoirs: toward an HIV cure. Front Immunol 2016;7:397. https://doi.org/10.3389/fimmu.2016.00397.Search in Google Scholar PubMed PubMed Central
15. Rochat, MA, Schlaepfer, E, Kuster, SP, Li, D, Audige, A, Ivic, S, et al.. Monitoring HIV DNA and cellular activation markers in HIV-infected humanized mice under cART. Virol J 2018;15:191. https://doi.org/10.1186/s12985-018-1101-9.Search in Google Scholar PubMed PubMed Central
16. Jayadev, S, Garden, GA. Host and viral factors influencing the pathogenesis of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol 2009;4:175–89. https://doi.org/10.1007/s11481-009-9154-6.Search in Google Scholar PubMed PubMed Central
17. Johnson, TP, Nath, A. New insights into immune reconstitution inflammatory syndrome of the central nervous system. Curr Opin HIV AIDS 2014;9:572–8. https://doi.org/10.1097/coh.0000000000000107.Search in Google Scholar PubMed PubMed Central
18. Ensoli, B, Buonaguro, L, Barillari, G, Fiorelli, V, Gendelman, R, Morgan, RA, et al.. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol 1993;67:277–87. https://doi.org/10.1128/jvi.67.1.277-287.1993.Search in Google Scholar PubMed PubMed Central
19. Nath, A, Conant, K, Chen, P, Scott, C, Major, EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem 1999;274:17098–102. https://doi.org/10.1074/jbc.274.24.17098.Search in Google Scholar PubMed
20. Li, W, Li, G, Steiner, J, Nath, A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res 2009;16:205–20. https://doi.org/10.1007/s12640-009-9047-8.Search in Google Scholar PubMed
21. Bagashev, A, Sawaya, BE. Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J 2013;10:358. https://doi.org/10.1186/1743-422x-10-358.Search in Google Scholar PubMed PubMed Central
22. Paris, JJ, Singh, HD, Carey, AN, McLaughlin, JP. Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res 2015;291:209–18. https://doi.org/10.1016/j.bbr.2015.05.021.Search in Google Scholar PubMed PubMed Central
23. Hahn, YK, Podhaizer, EM, Farris, SP, Miles, MF, Hauser, KF, Knapp, PE. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct 2015;220:605–23. https://doi.org/10.1007/s00429-013-0676-6.Search in Google Scholar PubMed PubMed Central
24. Simons, M, Nave, KA. Oligodendrocytes: myelination and axonal support. Cold Spring Harbor Perspect Biol 2015;8:a020479. https://doi.org/10.1101/cshperspect.a020479.Search in Google Scholar PubMed PubMed Central
25. Parikh, ZS, Tripathi, A, Pillai, PP. Differential regulation of MeCP2 phosphorylation by laminin in oligodendrocytes. J Mol Neurosci 2017;62:309–17. https://doi.org/10.1007/s12031-017-0939-4.Search in Google Scholar PubMed
26. Emery, B. Regulation of oligodendrocyte differentiation and myelination. Science 2010;330:779–82. https://doi.org/10.1126/science.1190927.Search in Google Scholar PubMed
27. Zou, S, Balinang, JM, Paris, JJ, Hauser, KF, Fuss, B, Knapp, PE. Effects of HIV-1 Tat on oligodendrocyte viability are mediated by CaMKIIbeta-GSK3beta interactions. J Neurochem 2019;149:98–110. https://doi.org/10.1111/jnc.14668.Search in Google Scholar PubMed PubMed Central
28. Liu, H, Liu, J, Xu, E, Tu, G, Guo, M, Liang, S, et al.. Human immunodeficiency virus protein Tat induces oligodendrocyte injury by enhancing outward K(+) current conducted by KV1.3. Neurobiol Dis 2017;97:1–10. https://doi.org/10.1016/j.nbd.2016.10.007.Search in Google Scholar PubMed PubMed Central
29. Wheeler, NA, Fuss, B, Knapp, PE, Zou, S. HIV-1 Tat inhibits autotaxin lysophospholipase D activity and modulates oligodendrocyte differentiation. ASN Neuro 2016;8:1759091416669618. https://doi.org/10.1177/1759091416669618.Search in Google Scholar PubMed PubMed Central
30. Zou, S, Fuss, B, Fitting, S, Hahn, YK, Hauser, KF, Knapp, PE. Oligodendrocytes are targets of HIV-1 Tat: NMDA and AMPA receptor-mediated effects on survival and development. J Neurosci 2015;35:11384–98. https://doi.org/10.1523/jneurosci.4740-14.2015.Search in Google Scholar PubMed PubMed Central
31. Ozgen, H, Baron, W, Hoekstra, D, Kahya, N. Oligodendroglial membrane dynamics in relation to myelin biogenesis. Cell Mol Life Sci 2016;73:3291–310. https://doi.org/10.1007/s00018-016-2228-8.Search in Google Scholar PubMed PubMed Central
32. Platt, FM, Boland, B, van der Spoel, AC. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J Cell Biol 2012;199:723–34. https://doi.org/10.1083/jcb.201208152.Search in Google Scholar PubMed PubMed Central
33. Blott, EJ, Griffiths, GM. Secretory lysosomes. Nat Rev Mol Cell Biol 2002;3:122–31. https://doi.org/10.1038/nrm732.Search in Google Scholar PubMed
34. Shen, YT, Gu, Y, Su, WF, Zhong, JF, Jin, ZH, Gu, XS, et al.. Rab27b is involved in lysosomal exocytosis and proteolipid protein trafficking in oligodendrocytes. Neurosci Bull 2016;32:331–40. https://doi.org/10.1007/s12264-016-0045-6.Search in Google Scholar PubMed PubMed Central
35. Garcia-Mateo, N, Pascua-Maestro, R, Perez-Castellanos, A, Lillo, C, Sanchez, D, Ganfornina, MD. Myelin extracellular leaflet compaction requires apolipoprotein D membrane management to optimize lysosomal-dependent recycling and glycocalyx removal. Glia 2018;66:670–87. https://doi.org/10.1002/glia.23274.Search in Google Scholar PubMed
36. Feldmann, A, Amphornrat, J, Schonherr, M, Winterstein, C, Mobius, W, Ruhwedel, T, et al.. Transport of the major myelin proteolipid protein is directed by VAMP3 and VAMP7. J Neurosci 2011;31:5659–72. https://doi.org/10.1523/jneurosci.6638-10.2011.Search in Google Scholar PubMed PubMed Central
37. Kundu, M, Thompson, CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol 2008;3:427–55. https://doi.org/10.1146/annurev.pathmechdis.2.010506.091842.Search in Google Scholar PubMed
38. Bankston, AN, Forston, MD, Howard, RM, Andres, KR, Smith, AE, Ohri, SS, et al.. Autophagy is essential for oligodendrocyte differentiation, survival, and proper myelination. Glia 2019;67:1745–59. https://doi.org/10.1002/glia.23646.Search in Google Scholar PubMed
39. Strohm, L, Behrends, C. Glia-specific autophagy dysfunction in ALS. Semin Cell Dev Biol 2020;99:172–82. https://doi.org/10.1016/j.semcdb.2019.05.024.Search in Google Scholar PubMed
40. Mei, X, Wang, H, Zhang, H, Liu, C, Guo, Z, Wang, Y, et al.. Blockade of receptor for advanced glycation end products promotes oligodendrocyte autophagy in spinal cord injury. Neurosci Lett 2019;698:198–203. https://doi.org/10.1016/j.neulet.2019.01.030.Search in Google Scholar PubMed
41. Saraswat Ohri, S, Bankston, AN, Mullins, SA, Liu, Y, Andres, KR, Beare, JE, et al.. Blocking autophagy in oligodendrocytes limits functional recovery after spinal cord injury. J Neurosci 2018;38:5900–12. https://doi.org/10.1523/jneurosci.0679-17.2018.Search in Google Scholar PubMed PubMed Central
42. Koh, JY, Kim, HN, Hwang, JJ, Kim, YH, Park, SE. Lysosomal dysfunction in proteinopathic neurodegenerative disorders: possible therapeutic roles of cAMP and zinc. Mol Brain 2019;12:18. https://doi.org/10.1186/s13041-019-0439-2.Search in Google Scholar PubMed PubMed Central
43. Lie, PPY, Nixon, RA. Lysosome trafficking and signaling in health and neurodegenerative diseases. Neurobiol Dis 2019;122:94–105. https://doi.org/10.1016/j.nbd.2018.05.015.Search in Google Scholar PubMed PubMed Central
44. Fraldi, A, Klein, AD, Medina, DL, Settembre, C. Brain disorders due to lysosomal dysfunction. Annu Rev Neurosci 2016;39:277–95. https://doi.org/10.1146/annurev-neuro-070815-014031.Search in Google Scholar PubMed
45. Fujikake, N, Shin, M, Shimizu, S. Association between autophagy and neurodegenerative diseases. Front Neurosci 2018;12:255. https://doi.org/10.3389/fnins.2018.00255.Search in Google Scholar PubMed PubMed Central
46. Guo, F, Liu, X, Cai, H, Le, W. Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol 2018;28:3–13. https://doi.org/10.1111/bpa.12545.Search in Google Scholar PubMed PubMed Central
47. Frake, RA, Ricketts, T, Menzies, FM, Rubinsztein, DC. Autophagy and neurodegeneration. J Clin Invest 2015;125:65–74. https://doi.org/10.1172/jci73944.Search in Google Scholar PubMed PubMed Central
48. Sanchez-Gomez, MV, Serrano, MP, Alberdi, E, Perez-Cerda, F, Matute, C. Isolation, expansion, and maturation of oligodendrocyte lineage cells obtained from rat neonatal brain and optic nerve. Methods Mol Biol 2018;1791:95–113. https://doi.org/10.1007/978-1-4939-7862-5_8.Search in Google Scholar PubMed
49. Marton, RM, Miura, Y, Sloan, SA, Li, Q, Revah, O, Levy, RJ, et al.. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci 2019;22:484–91. https://doi.org/10.1038/s41593-018-0316-9.Search in Google Scholar PubMed PubMed Central
50. Schuster, KH, Zalon, AJ, Zhang, H, DiFranco, DM, Stec, NR, Haque, Z, et al.. Impaired oligodendrocyte maturation is an early feature in SCA3 disease pathogenesis. J Neurosci 2022;42:1604–17. https://doi.org/10.1523/jneurosci.1954-20.2021.Search in Google Scholar
51. Westendorp, MO, Frank, R, Ochsenbauer, C, Stricker, K, Dhein, J, Walczak, H, et al.. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 1995;375:497–500. https://doi.org/10.1038/375497a0.Search in Google Scholar PubMed
52. Xiao, H, Neuveut, C, Tiffany, HL, Benkirane, M, Rich, EA, Murphy, PM, et al.. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A 2000;97:11466–71. https://doi.org/10.1073/pnas.97.21.11466.Search in Google Scholar PubMed PubMed Central
53. Hayashi, K, Pu, H, Andras, IE, Eum, SY, Yamauchi, A, Hennig, B, et al.. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J Cereb Blood Flow Metab 2006;26:1052–65. https://doi.org/10.1038/sj.jcbfm.9600254.Search in Google Scholar PubMed
54. Kreher, C, Favret, J, Maulik, M, Shin, D. Lysosomal functions in glia associated with neurodegeneration. Biomolecules 2021;11:400. https://doi.org/10.3390/biom11030400.Search in Google Scholar PubMed PubMed Central
55. Belgrad, J, De Pace, R, Fields, RD. Autophagy in myelinating glia. J Neurosci 2020;40:256–66. https://doi.org/10.1523/jneurosci.1066-19.2019.Search in Google Scholar
56. Yamamoto, A, Tagawa, Y, Yoshimori, T, Moriyama, Y, Masaki, R, Tashiro, Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 1998;23:33–42. https://doi.org/10.1247/csf.23.33.Search in Google Scholar PubMed
57. Zhou, C, Zhong, W, Zhou, J, Sheng, F, Fang, Z, Wei, Y, et al.. Monitoring autophagic flux by an improved tandem fluorescent-tagged LC3 (mTagRFP-mWasabi-LC3) reveals that high-dose rapamycin impairs autophagic flux in cancer cells. Autophagy 2012;8:1215–26. https://doi.org/10.4161/auto.20284.Search in Google Scholar PubMed
58. Velazquez, I, Plaud, M, Wojna, V, Skolasky, R, Laspiur, JP, Melendez, LM. Antioxidant enzyme dysfunction in monocytes and CSF of Hispanic women with HIV-associated cognitive impairment. J Neuroimmunol 2009;206:106–11. https://doi.org/10.1016/j.jneuroim.2008.10.013.Search in Google Scholar PubMed PubMed Central
59. Sharma, B. Oxidative stress in HIV patients receiving antiretroviral therapy. Curr HIV Res 2014;12:13–21. https://doi.org/10.2174/1570162x12666140402100959.Search in Google Scholar PubMed
60. Kirkegaard, T, Roth, AG, Petersen, NH, Mahalka, AK, Olsen, OD, Moilanen, I, et al.. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 2010;463:549–53. https://doi.org/10.1038/nature08710.Search in Google Scholar PubMed
61. Nylandsted, J, Gyrd-Hansen, M, Danielewicz, A, Fehrenbacher, N, Lademann, U, Hoyer-Hansen, M, et al.. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med 2004;200:425–35. https://doi.org/10.1084/jem.20040531.Search in Google Scholar PubMed PubMed Central
62. Johnson, TP, Patel, K, Johnson, KR, Maric, D, Calabresi, PA, Hasbun, R, et al.. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A 2013;110:13588–93. https://doi.org/10.1073/pnas.1308673110.Search in Google Scholar PubMed PubMed Central
63. Jensen, BK, Roth, LM, Grinspan, JB, Jordan-Sciutto, KL. White matter loss and oligodendrocyte dysfunction in HIV: a consequence of the infection, the antiretroviral therapy or both? Brain Res 2019;1724:146397. https://doi.org/10.1016/j.brainres.2019.146397.Search in Google Scholar PubMed PubMed Central
64. Liu, H, Xu, E, Liu, J, Xiong, H. Oligodendrocyte injury and pathogenesis of HIV-1-Associated neurocognitive disorders. Brain Sci 2016;6:23. https://doi.org/10.3390/brainsci6030023.Search in Google Scholar PubMed PubMed Central
65. Faust, PL, Kaye, EM, Powers, JM. Myelin lesions associated with lysosomal and peroxisomal disorders. Expert Rev Neurother 2010;10:1449–66. https://doi.org/10.1586/ern.10.127.Search in Google Scholar PubMed
66. Hui, L, Chen, X, Haughey, NJ, Geiger, JD. Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro 2012;4:243–52. https://doi.org/10.1042/an20120017.Search in Google Scholar
67. Chen, X, Hui, L, Geiger, NH, Haughey, NJ, Geiger, JD. Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol Aging 2013;34:2370–8. https://doi.org/10.1016/j.neurobiolaging.2013.04.015.Search in Google Scholar PubMed PubMed Central
68. Turk, V, Stoka, V, Vasiljeva, O, Renko, M, Sun, T, Turk, B, et al.. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta 2012;1824:68–88. https://doi.org/10.1016/j.bbapap.2011.10.002.Search in Google Scholar PubMed PubMed Central
69. Ishidoh, K, Kominami, E. Processing and activation of lysosomal proteinases. Biol Chem 2002;383:1827–31. https://doi.org/10.1515/bc.2002.206.Search in Google Scholar
70. Erickson, AH. Biosynthesis of lysosomal endopeptidases. J Cell Biochem 1989;40:31–41. https://doi.org/10.1002/jcb.240400104.Search in Google Scholar PubMed
71. Repnik, U, Cesen, MH, Turk, B. The use of lysosomotropic dyes to exclude lysosomal membrane permeabilization. Cold Spring Harb Protoc 2016;2016. https://doi.org/10.1101/pdb.prot087106.Search in Google Scholar PubMed
72. Wang, F, Gomez-Sintes, R, Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018;19:918–31. https://doi.org/10.1111/tra.12613.Search in Google Scholar PubMed
73. Pascua-Maestro, R, Diez-Hermano, S, Lillo, C, Ganfornina, MD, Sanchez, D. Protecting cells by protecting their vulnerable lysosomes: identification of a new mechanism for preserving lysosomal functional integrity upon oxidative stress. PLoS Genet 2017;13:e1006603. https://doi.org/10.1371/journal.pgen.1006603.Search in Google Scholar PubMed PubMed Central
74. Zhong, Z, Sanchez-Lopez, E, Karin, M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 2016;166:288–98. https://doi.org/10.1016/j.cell.2016.05.051.Search in Google Scholar PubMed PubMed Central
75. Green, DR, Levine, B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell 2014;157:65–75. https://doi.org/10.1016/j.cell.2014.02.049.Search in Google Scholar PubMed PubMed Central
76. Leymarie, O, Lepont, L, Berlioz-Torrent, C. Canonical and non-canonical autophagy in HIV-1 replication cycle. Viruses 2017;9:270. https://doi.org/10.3390/v9100270.Search in Google Scholar PubMed PubMed Central
77. Liu, Z, Xiao, Y, Torresilla, C, Rassart, E, Barbeau, B. Implication of different HIV-1 genes in the modulation of autophagy. Viruses 2017;9:389. https://doi.org/10.3390/v9120389.Search in Google Scholar PubMed PubMed Central
78. Louboutin, JP, Strayer, D. Role of oxidative stress in HIV-1-Associated neurocognitive disorder and protection by gene delivery of antioxidant enzymes. Antioxidants 2014;3:770–97. https://doi.org/10.3390/antiox3040770.Search in Google Scholar PubMed PubMed Central
79. Louboutin, JP, Agrawal, L, Reyes, BA, Van Bockstaele, EJ, Strayer, DS. Oxidative stress is associated with neuroinflammation in animal models of HIV-1 Tat neurotoxicity. Antioxidants 2014;3:414–38. https://doi.org/10.3390/antiox3020414.Search in Google Scholar PubMed PubMed Central
80. Cai, X, Liu, Y, Hu, Y, Liu, X, Jiang, H, Yang, S, et al.. ROS-mediated lysosomal membrane permeabilization is involved in bupivacaine-induced death of rabbit intervertebral disc cells. Redox Biol 2018;18:65–76. https://doi.org/10.1016/j.redox.2018.06.010.Search in Google Scholar PubMed PubMed Central
81. Aits, S, Jaattela, M. Lysosomal cell death at a glance. J Cell Sci 2013;126:1905–12. https://doi.org/10.1242/jcs.091181.Search in Google Scholar PubMed
82. Cesen, MH, Pegan, K, Spes, A, Turk, B. Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res 2012;318:1245–51. https://doi.org/10.1016/j.yexcr.2012.03.005.Search in Google Scholar PubMed
83. Serrano-Puebla, A, Boya, P. Lysosomal membrane permeabilization in cell death: new evidence and implications for health and disease. Ann N Y Acad Sci 2016;1371:30–44. https://doi.org/10.1111/nyas.12966.Search in Google Scholar PubMed
84. Chen, Y, Balasubramaniyan, V, Peng, J, Hurlock, EC, Tallquist, M, Li, J, et al.. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc 2007;2:1044–51. https://doi.org/10.1038/nprot.2007.149.Search in Google Scholar PubMed
85. Tripathi, A, Parikh, ZS, Vora, P, Frost, EE, Pillai, PP. pERK1/2 peripheral recruitment and filopodia protrusion augment oligodendrocyte progenitor cell migration: combined effects of PDGF-A and fibronectin. Cell Mol Neurobiol 2017;37:183–94. https://doi.org/10.1007/s10571-016-0359-y.Search in Google Scholar PubMed
86. Li, J, Lin, JC, Wang, H, Peterson, JW, Furie, BC, Furie, B, et al.. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci 2003;23:5816–26. https://doi.org/10.1523/jneurosci.23-13-05816.2003.Search in Google Scholar
87. Rosen, CL, Bunge, RP, Ard, MD, Wood, PM. Type 1 astrocytes inhibit myelination by adult rat oligodendrocytes in vitro. J Neurosci 1989;9:3371–9. https://doi.org/10.1523/jneurosci.09-10-03371.1989.Search in Google Scholar PubMed PubMed Central
88. Holmes, JR, Berkowitz, A. Dendritic orientation and branching distinguish a class of multifunctional turtle spinal interneurons. Front Neural Circuits 2014;8:136. https://doi.org/10.3389/fncir.2014.00136.Search in Google Scholar PubMed PubMed Central
89. Tripathi, A, Thangaraj, A, Chivero, ET, Periyasamy, P, Callen, S, Burkovetskaya, ME, et al.. Antiretroviral-mediated microglial activation involves dysregulated autophagy and lysosomal dysfunction. Cells 2019;8:1168. https://doi.org/10.3390/cells8101168.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Brief Reports
- Ongoing oxidative stress in individuals with post-acute sequelae of COVID-19
- Quantifying the neuropsychiatric symptoms in post-acute sequelae of COVID-19 (PASC) using the NIH Toolbox ® and PROMIS
- Review Article
- Early-career research education mentoring: a successful program in NeuroHIV and mental health (TRNAMH)
- Research Articles
- PurA sensitizes cells to toxicity induced by oxidative stress
- Poor subjective sleep reported by people living with HIV is associated with impaired working memory
- HIV Tat-mediated altered oligodendrocyte maturation involves autophagy-lysosomal dysfunction
- Constitutive expression of HIV-1 viral proteins induces progressive synaptodendritic alterations in medium spiny neurons: implications for substance use disorders
- Review Article
- The role of tunneling nanotubes during early stages of HIV infection and reactivation: implications in HIV cure
- Brief Report
- The 27th Scientific Conference of the Society on NeuroImmune Pharmacology: New Delhi, India, March 15–18, 2023
Articles in the same Issue
- Frontmatter
- Brief Reports
- Ongoing oxidative stress in individuals with post-acute sequelae of COVID-19
- Quantifying the neuropsychiatric symptoms in post-acute sequelae of COVID-19 (PASC) using the NIH Toolbox ® and PROMIS
- Review Article
- Early-career research education mentoring: a successful program in NeuroHIV and mental health (TRNAMH)
- Research Articles
- PurA sensitizes cells to toxicity induced by oxidative stress
- Poor subjective sleep reported by people living with HIV is associated with impaired working memory
- HIV Tat-mediated altered oligodendrocyte maturation involves autophagy-lysosomal dysfunction
- Constitutive expression of HIV-1 viral proteins induces progressive synaptodendritic alterations in medium spiny neurons: implications for substance use disorders
- Review Article
- The role of tunneling nanotubes during early stages of HIV infection and reactivation: implications in HIV cure
- Brief Report
- The 27th Scientific Conference of the Society on NeuroImmune Pharmacology: New Delhi, India, March 15–18, 2023

