The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
-
Rabia Usman
, May Nasser Bin-Jumah
, Mohsen Mohammed Al-Qhatani
Abstract
C25H29BF2N2O2, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
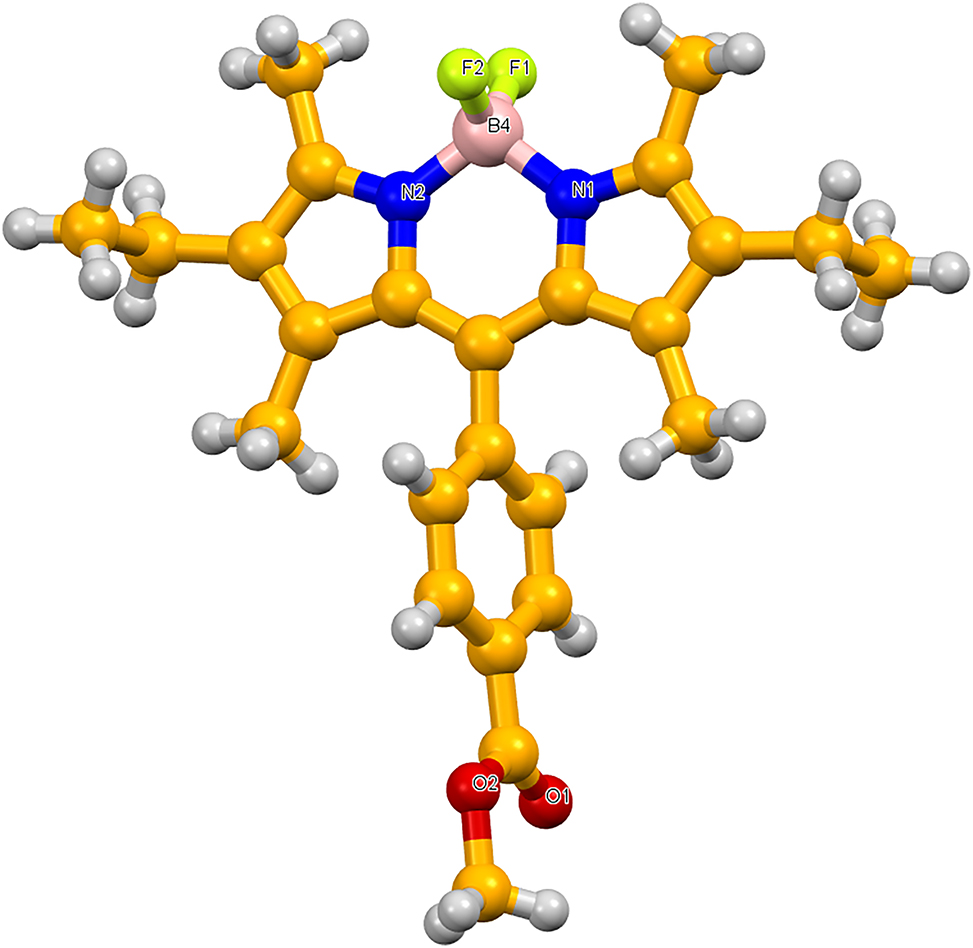
Data collection and handling.
| Crystal: | Orange irregular |
| Size: | 0.17 × 0.12 × 0.08 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.76 mm−1 |
| Diffractometer, scan mode: | Xcalibur, ω |
| θ max, completeness: | 66.9°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 6,506, 6,506 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 5,852 |
| N(param)refined: | 297 |
| Programs: | CrysAlis PRO, 1 Shelx, 2 , 3 Olex2 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| F1 | 0.5391 (3) | 0.6594 (3) | 0.9315 (2) | 0.0399 (6) |
| F2 | 0.4016 (3) | 0.8967 (3) | 0.7903 (2) | 0.0405 (6) |
| O1 | 0.5544 (5) | −0.0501 (4) | 0.2263 (3) | 0.0438 (7) |
| O2 | 0.4647 (4) | 0.2126 (4) | 0.0854 (3) | 0.0345 (6) |
| N1 | 0.3431 (4) | 0.5901 (4) | 0.7983 (3) | 0.0292 (7) |
| N2 | 0.6427 (4) | 0.7121 (4) | 0.6897 (3) | 0.0278 (7) |
| C1 | 0.1927 (5) | 0.4140 (5) | 0.7163 (4) | 0.0319 (8) |
| C2 | 0.0923 (5) | 0.4390 (6) | 0.8379 (4) | 0.0351 (9) |
| C3 | 0.1875 (5) | 0.5475 (5) | 0.8856 (4) | 0.0324 (8) |
| C5 | 0.7966 (5) | 0.7952 (5) | 0.6660 (4) | 0.0302 (8) |
| C6 | 0.9059 (5) | 0.7688 (5) | 0.5462 (4) | 0.0316 (8) |
| C7 | 0.8142 (5) | 0.6629 (5) | 0.4948 (4) | 0.0303 (8) |
| C8 | 0.5043 (5) | 0.5251 (5) | 0.5895 (4) | 0.0270 (8) |
| C9 | 0.3535 (5) | 0.5077 (5) | 0.6926 (4) | 0.0281 (8) |
| C10 | 0.6475 (5) | 0.6268 (5) | 0.5862 (4) | 0.0276 (8) |
| C11 | 0.1342 (6) | 0.3099 (6) | 0.6326 (4) | 0.0366 (9) |
| H11A | 0.1876 | 0.1861 | 0.6543 | 0.055* |
| H11B | 0.1723 | 0.3674 | 0.5382 | 0.055* |
| H11C | 0.0049 | 0.3090 | 0.6512 | 0.055* |
| C12 | −0.0797 (6) | 0.3529 (6) | 0.9117 (5) | 0.0431 (10) |
| H12A | −0.1553 | 0.3527 | 0.8465 | 0.052* |
| H12B | −0.1451 | 0.4254 | 0.9715 | 0.052* |
| C13 | −0.0418 (6) | 0.1567 (7) | 0.9948 (5) | 0.0499 (12) |
| H13A | 0.0301 | 0.0868 | 0.9369 | 0.075* |
| H13B | −0.1540 | 0.1020 | 1.0343 | 0.075* |
| H13C | 0.0224 | 0.1577 | 1.0655 | 0.075* |
| C14 | 0.1347 (6) | 0.6081 (7) | 1.0132 (4) | 0.0413 (10) |
| H14A | 0.1835 | 0.5190 | 1.0855 | 0.062* |
| H14B | 0.0053 | 0.6200 | 1.0360 | 0.062* |
| H14C | 0.1814 | 0.7245 | 1.0001 | 0.062* |
| C15 | 0.8397 (6) | 0.8963 (6) | 0.7588 (4) | 0.0361 (9) |
| H15A | 0.7799 | 1.0175 | 0.7399 | 0.054* |
| H15B | 0.9679 | 0.9044 | 0.7447 | 0.054* |
| H15C | 0.7988 | 0.8318 | 0.8513 | 0.054* |
| C16 | 1.0806 (5) | 0.8544 (6) | 0.4829 (4) | 0.0389 (10) |
| H16A | 1.1569 | 0.7774 | 0.4309 | 0.047* |
| H16B | 1.1432 | 0.8623 | 0.5537 | 0.047* |
| C17 | 1.0483 (7) | 1.0477 (8) | 0.3892 (6) | 0.0574 (13) |
| H17A | 0.9845 | 1.0403 | 0.3199 | 0.086* |
| H17B | 1.1624 | 1.0975 | 0.3478 | 0.086* |
| H17C | 0.9778 | 1.1258 | 0.4414 | 0.086* |
| C18 | 0.8839 (5) | 0.6043 (6) | 0.3675 (4) | 0.0360 (9) |
| H18A | 0.8205 | 0.6770 | 0.2966 | 0.054* |
| H18B | 0.8652 | 0.4772 | 0.3831 | 0.054* |
| H18C | 1.0107 | 0.6211 | 0.3411 | 0.054* |
| C19 | 0.5140 (5) | 0.4246 (5) | 0.4844 (3) | 0.0261 (8) |
| C20 | 0.5436 (5) | 0.2340 (5) | 0.5209 (4) | 0.0301 (8) |
| H20 | 0.5614 | 0.1730 | 0.6088 | 0.036* |
| C21 | 0.5466 (6) | 0.1342 (5) | 0.4278 (4) | 0.0303 (8) |
| H21 | 0.5671 | 0.0071 | 0.4528 | 0.036* |
| C22 | 0.5189 (5) | 0.2260 (5) | 0.2965 (4) | 0.0254 (7) |
| C23 | 0.4937 (5) | 0.4163 (5) | 0.2583 (3) | 0.0282 (8) |
| H23 | 0.4788 | 0.4774 | 0.1698 | 0.034* |
| C24 | 0.4907 (5) | 0.5152 (5) | 0.3523 (3) | 0.0279 (8) |
| H24 | 0.4731 | 0.6426 | 0.3267 | 0.033* |
| C25 | 0.5156 (5) | 0.1132 (5) | 0.2014 (4) | 0.0286 (8) |
| C26 | 0.4590 (6) | 0.1106 (6) | −0.0125 (4) | 0.0413 (10) |
| H26A | 0.4132 | 0.1912 | −0.0898 | 0.062* |
| H26B | 0.3814 | 0.0124 | 0.0287 | 0.062* |
| H26C | 0.5787 | 0.0608 | −0.0409 | 0.062* |
| B4 | 0.4806 (6) | 0.7180 (6) | 0.8069 (4) | 0.0308 (9) |
1 Source of materials
The target compound was synthesized according to the procedure reported in ref 5. Good quality orange single-crystals were obtained by the slow evaporation of the ethyl acetate solution.
2 Experimental details
The structure of the as title compound was solved using Shelxt 2 and refined by Shelxl program 3 through the Olex2 interface. 4 All hydrogen atoms were positioned at calculated coordinates and refined isotropically. The crystal was refined as a two-component twin. The diffraction intensities were integrated according to a non-merohedral twin law: component two is rotated by 179.9507° around [0.00 0.00 1.00] in reciprocal space or [−0.20 −0.33 0.92] in direct space with respect to component one.
3 Comment
Boron dipyrromethene (BODIPY) dyes are extensively employed as organic fluorophores, owing to their outstanding photochemical stability, tunable photophysical properties, and adaptability to structural modifications. 6 , 7 , 8 BODIPY derivatives have been applied in various fields, including optoelectronic materials, subcellular-targeting bioimaging, and as theranostic reagents. 9 , 10 , 11 , 12 , 13 , 14 A successful strategy to achieve a red-shifted spectrum for BODIPY dyes is the incorporation of aromatic moieties at the periphery of the BODIPY scaffold. 15 , 16 , 17 , 18
Single X-ray analysis indicate the expected tetrahedral geometry of the boron center with N,N-chelation and the two fluoro ligands [N–B–N angle (107.2°) F–B–F angle (109.5°)]. Formally, the boron center is coordinated to one of the N atoms via an ionic bond and to the other via a coordination covalent bond to form a slightly strained-six membered ring that is perpendicular to the plane of the F–B–F group. No C–H⋯π or π–π stacking connections were detected in the complex lattice. Bond lengths are in the expected ranges. 19
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R325), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2015.Search in Google Scholar
2. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. A Short History of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Shipalova, M.; Bobrov, A.; Usoltsev, S.; Marfin, Y. S.; Rumyantsev, E. Influence of Structure and Solvatation on Photophysical Characteristics of Meso-Substituted Boron Dipyrrins in Solution and Bulk Hybrid Materials. J. Mol. Liq. 2019, 283, 688–694; https://doi.org/10.1016/j.molliq.2019.03.099.Search in Google Scholar
6. Loudet, A.; Burgess, K. BODIPY Dyes and their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932; https://doi.org/10.1021/cr078381n.Search in Google Scholar PubMed
7. Kolemen, S.; Akkaya, E. U. Reaction-Based BODIPY Probes for Selective Bio-Imaging. Coord. Chem. Rev. 2018, 354, 121–134; https://doi.org/10.1016/j.ccr.2017.06.021.Search in Google Scholar
8. Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201; https://doi.org/10.1002/anie.200702070.Search in Google Scholar PubMed
9. Ziegler, C. J.; Chanawanno, K.; Hasheminsasab, A.; Zatsikha, Y. V.; Maligaspe, E.; Nemykin, V. N. Synthesis, Redox Properties, and Electronic Coupling in the Diferrocene Aza-Dipyrromethene and azaBODIPY Donor–Acceptor Dyad with Direct Ferrocene-α-Pyrrole Bond. Inorg. Chem. 2014, 53, 4751–4755; https://doi.org/10.1021/ic500526k.Search in Google Scholar PubMed
10. Zhang, G.; Wang, M.; Fronczek, F. R.; Smith, K. M.; Vicente, M. G. H. Lewis-Acid-Catalyzed BODIPY Boron Functionalization Using Trimethylsilyl Nucleophiles. Inorg. Chem. 2018, 57, 14493–14496; https://doi.org/10.1021/acs.inorgchem.8b02775.Search in Google Scholar PubMed
11. Wang, M.; Zhang, G.; Bobadova-Parvanova, P.; Merriweather, A. N.; Odom, L.; Barbosa, D.; Fronczek, F. R.; Smith, K. M.; Vicente, M. G. H. Synthesis and Investigation of Linker-Free BODIPY-Gly Conjugates Substituted at the Boron Atom. Inorg. Chem. 2019, 58, 11614–11621; https://doi.org/10.1021/acs.inorgchem.9b01474.Search in Google Scholar PubMed
12. Ziessel, R.; Ulrich, G.; Haefele, A.; Harriman, A. An Artificial Light-Harvesting Array Constructed from Multiple Bodipy Dyes. J. Am. Chem. Soc. 2013, 135, 11330–11344; https://doi.org/10.1021/ja4049306.Search in Google Scholar PubMed
13. Bernhard, C.; Goze, C.; Rousselin, Y.; Denat, F. First Bodipy-DOTA Derivatives as Probes for Bimodal Imaging. Chem. Commun. 2010, 46, 8267–8269; https://doi.org/10.1039/c0cc02749a.Search in Google Scholar PubMed
14. Brizet, B.; Goncalves, V.; Bernhard, C.; Harvey, P. D.; Denat, F.; Goze, C. DMAP-BODIPY Alkynes: A Convenient Tool for Labeling Biomolecules for Bimodal PET-Optical Imaging. Chem. Eur. J. 2014, 20, 12933–12944; https://doi.org/10.1002/chem.201402379.Search in Google Scholar PubMed
15. Xiao, X.; Tian, W.; Imran, M.; Cao, H.; Zhao, J. Controlling the Triplet States and their Application in External Stimuli-Responsive Triplet–Triplet-Annihilation Photon Upconversion: From the Perspective of Excited State Photochemistry. Chem. Soc. Rev. 2021, 50, 9686–9714; https://doi.org/10.1039/d1cs00162k.Search in Google Scholar PubMed
16. Ramu, V.; Gautam, S.; Kondaiah, P.; Chakravarty, A. R. Diplatinum(II) Catecholate of Photoactive Boron-Dipyrromethene for Lysosome-Targeted Photodynamic Therapy in Red Light. Inorg. Chem. 2019, 58, 9067–9075; https://doi.org/10.1021/acs.inorgchem.9b00567.Search in Google Scholar PubMed
17. Zatsikha, Y. V.; Maligaspe, E.; Purchel, A. A.; Didukh, N. O.; Wang, Y.; Kovtun, Y. P.; Blank, D. A.; Nemykin, V. N. Tuning Electronic Structure, Redox, and Photophysical Properties in Asymmetric NIR-Absorbing Organometallic BODIPYs. Inorg. Chem. 2015, 54, 7915–7928; https://doi.org/10.1021/acs.inorgchem.5b00992.Search in Google Scholar PubMed
18. Xu, H.; Yao, S.; Chen, Y.; Zhang, C.; Zhang, S.; Yuan, H.; Chen, Z.; Bai, Y.; Yang, T.; Guo, Z.; He, W. Tracking Labile Copper Fluctuation In Vivo/Ex Vivo: Design and Application of a Ratiometric Near-Infrared Fluorophore Derived from 4-Aminostyrene-Conjugated Boron Dipyrromethene. Inorg. Chem. 2021, 60, 18567–18574; https://doi.org/10.1021/acs.inorgchem.1c01779.Search in Google Scholar PubMed
19. Al-Hazmi, G. H.; Refat, M. S.; Usman, R.; Khan, A. A. The Crystal Structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2. Z. Kristallogr. - N. Cryst. Struct. 2024, 239, 477–479; https://doi.org/10.1515/ncrs-2024-0053.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of the co-crystal 2,4,6-triamino-1,3,5-triazine-1,3-dioxide — acetic acid (1/2) C7H14N6O6
- Crystal structure of the dinuclear mercury(II) complex bis(μ2-bromido)-dibromido-bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-ethyl-5-methyl-imidazol)-κ1 N} dimercury(II), C26H30N10Hg2Br4
- Crystal structure of poly[hexaqua-pentakis(μ4-2,2′-bipyridine-4,4′-dicarboxylato-κ4 O:O′:O″:O‴)-(μ2-2,2′-bipyridine-4,4′-dicarboxylato-κ2 O:O)tetraytterbium(III)] hydrate, C36H26N6O16Yb2
- Hydrothermal synthesis and crystal structure of catena-poly[(1,10-phenanthroline-κ 2 N,N′)-bis(μ 2-nitroisophthalato-κ 3 O,O′:O″)nickel(II)], C20H13NiN3O7
- Crystal structure of 72,73,75,76-tetrafluoro-25,44-dimethyl-31,33,36,38-tetraoxo-31,32,33,36,37,38-hexahydro-3(2,7)-benzo[lmn][3,8]phenanthrolina-1,5(4,1)-dipyridin-1-iuma-2,4(1,2),7(1,4)-tribenzenacyclooctaphane-11,51-diium hexafluoridophosphate, [C46H28F4N4O4][PF6]2, a dicationic cyclophane
- Crystal structure of (E)-2-(4-(1H-imidazol-1-yl)benzylidene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C20H15FN2O
- The salt crystal structure of etoricoxib hydrochloride, C18H16Cl2N2O2S
- The structure of t-butyl 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate, C37H43FN2O5
- The crystal structure of (μ4-oxo)-tri(μ4-2,2′-bipyridine-6,6′-bis(olato)-κ5 O,O′:N:N′:O″)tetrazinc(II) – methylformamide (1/1), C33H25N7O8Zn4
- The co-crystal structure of 4-chlorobenzophenone–salicylhydrazide(1/1), C20H17ClN2O3
- Crystal structure of 9-fluoro-4-(6-methoxypyridin-2-yl)-5,6-dihydrobenzo[h]quinazolin-2-amine, C18H15FN4O
- The crystal structure of the co-crystal composed of benzhydrazide and 5-aminoisophthalic acid, C8H7NO4⋅C7H8N2O
- The cocrystal structure of praziquantel-hesperetin (1/1), C35H38N2O8
- Crystal structure of new barium manganese fluorides dihydrates, Ba10Mn2F25·2H2O
- The crystal structure of bis[μ2-(3-(3-(pyridin-2-yl)-1H-pyrazol-1-yl)propanoate-κ2O:N)-bis(2,2′-bipyridine-κ2 N, N′)dicopper(II)]dinitrate, C42H36Cu2N12O10
- Crystal structure of (3,6-di(2-pyridyl)-4-phenylaminopyridazine-κ2N,N′)-bis(2-(p-toluene)pyridinyl-κ2C,N)-iridium(III) hexafluorophosphate –dichloromethane (1/1), C45H37Cl2F6IrN7P
- The crystal structure of 2-(2′-carboxybenzyl)benzoic acid, C15H12O5
- The crystal structure of dichlorido-[(E)-N′,N″-bis((2E,3E)-3-(hydroxyimino)butan-2-ylidene)-2-((E)-3-(hydroxyimino)butan-2-ylidene)hydrazine-1-carbohydrazonhydrazide-κ 4 N 4]cobalt(II), C13H22N9O3Cl2Co
- Crystal structure of (−)-flavesine H, C15H22N2O2
- Crystal structure of 3-methoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C22H22O4
- Crystal structure of dicarbonyl(2-oxopyridin-1(2H)-olato-κ 2 O,O)iridium(I), C7H4IrNO4
- The crystal structure of 4-(3-(triphenylphosphonio)propyl)piperazin-1-ium dibromide trihydrate, C25H37Br2N2O3P
- The crystal structure of ethyl 5,6-dihydroxybenzofuran-3-carboxylate, C11H10O5
- Crystal structure of 14-(R)-(2′-cyano-phenoxy)-3,19-diacetyl andrographolide, C31H37NO7
- The twinned crystal structure of 10-(4-methyl benzoate)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-di-pyrrolo[1,2-c:2′,1′-f] [1,3,2]diazaborinin-4-ium-5-uide, C25H29BF2N2O2
- The crystal structure of (9H-thioxanthen-9- ylidene)hydrazine monohydrate, C13H11N2SO0.5
- The crystal structure of pyridinium diaqua-{1,2-phenylenebis((carboxylatocarbonyl)amido-κ4 N,N′,O,O′)manganese(III), C15H14MnN3O8
- Crystal structure of the hydrogen storage active high entropy phase Tb0.82Sm0.18Ni0.83Co0.17Mg
- Crystal structure of diaqua-bis[5-methyl-1-(1H-pyrazol-3-yl)-1H-1,2,3-triazole-4-carboxylato-κ 2 N,O)]manganese(II), C14H16MnN10O6
- Crystal structures of diiodido-3-((pyridin-2-ylmethylene)amino)-2-(pyridin-3-yl)-2,3-dihydroquinazolin-4(1H)-one-cadmium(II)
- Synthesis and crystal structure of methyl 4-(2-ethoxy-2-oxoethoxy)-3,5-dimethoxybenzoate, C14H18O7
- Crystal structure of isoxazolo[4,5-b]pyridin-3-amine, C6H5N3O
- Crystal structure of 4-chloro-1-isobutyl-1H-imidazo, C14H14ClN3
- The crystal structure of 1,1,1,2,2,2-hexakis(2-methyl-2-phenylpropyl)distannane,C60H78Sn2
- The crystal structure of (2,7-dimethoxynaphthalene-1,8-diyl)bis((3-nitrophenyl)methanone), C26H18N2O8
- Crystal structure of diaqua-tetra((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)zinc(II) dinitrate dihydrate, C60H76Cl8N14O14Zn
- The crystal structure of diphenyl bis(2-((diphenoxyphosphoryl)amino)ethyl)phosphoramidate monohydrate C40H42N3O10P3
- Crystal structure of 4,4′-bis(dibromomethyl)-1,1′-biphenyl, C14H10Br4
- Crystal structure of CaPtZn
- Crystal structure of 3-chloro-5-(trifluoromethyl)pyridine-2-carboxylic acid, C7H3ClF3NO2
- The crystal structure of (3′-(2-bromophenyl)-2-phenyl-[2,2′-bioxiran]-3-yl)(phenyl)methanone, C92H68O12Br4
- Crystal structure of ethyl 4-(4-benzylpiperazin-1-yl)benzoate, C20H24N2O2
- The crystal structure of bis(selenocyanato-κ1 N)-bis(methanol)-bis((1E,2E)-1,2-bis (1-(pyridin-4-yl)ethylidene)-hydrazine)iron(II) methanol solvate, C34H44FeN10O4Se2
- Crystal structure of (E)-1-(5-bromo-2-hydroxyphenyl)-3-(5-(4-methoxyphenoxy)-3-methyl-1-phenyl-1H-pyrazol-4-yl)prop-2-en-1-one, C26H21BrN2O4
- The crystal structure of methyl 4-(4-(methylsulfonyl)phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C19H21NO5S
- Crystal structure of 1′,3′-dihydro-2,2′-spirobi[indene]-1,3-dione, C17H12O2
- Crystal structure of (E)-2,2′,3,3′-tetrahydro-[1,1′-biindenylidene]-4,4′-diol, C18H16O2
- Crystal structure of di-glycylglycinium squarate dihydrate, C12H22N4O12, at 105 K
- Crystal structure of {[(4-fluorophenyl)methyl]triphenylphosphonium}dibromocopper(I), [C25H21FP]+[CuBr2]−
- Crystal structure of poly[diaqua-bis(μ2-5-((pyridin-4-yl-methyl)amino)benzene-1,3-dicarboxylato-κ 2 N:O)cadmium(II)], C28H26CdN4O10


