Abstract
The conversion of solar radiation to chemical energy in plants and green algae takes place in the thylakoid membrane. This amphiphilic environment hosts a complex arrangement of light-harvesting pigment-protein complexes that absorb light and transfer the excitation energy to photochemically active reaction centers. This efficient light-harvesting capacity is moreover tightly regulated by a photoprotective mechanism called non-photochemical quenching to avoid the stress-induced destruction of the catalytic reaction center. In this review we provide an overview of single-molecule fluorescence measurements on plant light-harvesting complexes (LHCs) of varying sizes with the aim of bridging the gap between the smallest isolated complexes, which have been well-characterized, and the native photosystem. The smallest complexes contain only a small number (10–20) of interacting chlorophylls, while the native photosystem contains dozens of protein subunits and many hundreds of connected pigments. We discuss the functional significance of conformational dynamics, the lipid environment, and the structural arrangement of this fascinating nano-machinery. The described experimental results can be utilized to build mathematical-physical models in a bottom-up approach, which can then be tested on larger in vivo systems. The results also clearly showcase the general property of biological systems to utilize the same system properties for different purposes. In this case it is the regulated conformational flexibility that allows LHCs to switch between efficient light-harvesting and a photoprotective function.
1 Introduction
Photosynthesis relies, among many other fascinating processes, on the capability of organisms to absorb sunlight and to efficiently transfer the resulting excitation energy to reaction centers (RCs) [1]. The relatively long-lived electronic excitations, in isolation typically decaying in a couple of nanoseconds, are eventually converted into a more stable form of chemical energy. The process spanning light absorption to temporal stabilization of the energy in the form of chemical bonds is often summarized and referred to as the light-dependent reactions of photosynthesis. It involves a complex synergy of ingeniously designed nano-machinery in the form of protein complexes that perform their function despite the high protein density and constantly fluctuating environment of the thylakoid membrane. Single-molecule fluorescence techniques are perfectly suited to study these light-dependent reactions on a wide range of time scales due to their intrinsic strong interaction with visible light. The four main constituents in plant thylakoids are photosystem II (PSII), cytochrome b6f, photosystem I (PSI), and the ATP synthase. Their relative sizes compared to the thylakoid membrane and a model of the main reaction pathways are depicted in Figure 1A. PSI and PSII are large pigment-protein clusters, the structures of which are perfectly adopted to ensure that almost every absorbed photon can be utilized to drive photochemistry [3]. The RCs are surrounded by so-called antenna complexes that increase the effective absorption cross section of individual RCs and provide enough energy for an optimum photochemical turnover rate (see Figure 1B). The smallest complexes contain only a small number (10–20) of interacting pigments, while the native PSII contains dozens of protein subunits and many hundreds of connected pigments [4], [5]. In plants, the major antenna is a trimeric complex called light-harvesting complex II (LHCII). One monomeric subunit contains eight chlorophyll (Chl) a pigments, six Chl b, two luteins (Lut), neoxanthin, and one additional xanthophyll [6], [7] as illustrated in Figure 1C. The state of the latter pigment depends on the external stress level of the plant and is either violaxanthin (no or low stress) or zeaxanthin (high stress) [8]. So-called minor antenna complexes, known as CP24, CP26, and CP29 are the structural link between the LHCII complexes and the core of PSII. Those minor complexes are monomeric and highly homologous to the LHCII subunits.
![Figure 1: The photosynthetic apparatus of higher plants. (A) The thylakoid membrane, with the main photosynthetic supramolecular complexes, photosystems I and II, cytochrome b6f and the ATP synthase. The notations indicate the relevant charge exchange and redox reactions. (B) Top view of the PSII C2S2M2 supercomplex. Depicted are the two core complexes containing the RC, minor LHCs CP24, CP29, and CP26, and the LHCII antenna complexes. Adapted from Ref. [2], Copyright (2013) Springer. (C) Closer view of the LHCII antenna trimer; grey ribbons: protein backbones, green: chl a, blue: chl b chlorine rings (phytol chains are omitted for clarity), yellow: carotenoids.](/document/doi/10.1515/nanoph-2017-0014/asset/graphic/j_nanoph-2017-0014_fig_001.jpg)
The photosynthetic apparatus of higher plants. (A) The thylakoid membrane, with the main photosynthetic supramolecular complexes, photosystems I and II, cytochrome b6f and the ATP synthase. The notations indicate the relevant charge exchange and redox reactions. (B) Top view of the PSII C2S2M2 supercomplex. Depicted are the two core complexes containing the RC, minor LHCs CP24, CP29, and CP26, and the LHCII antenna complexes. Adapted from Ref. [2], Copyright (2013) Springer. (C) Closer view of the LHCII antenna trimer; grey ribbons: protein backbones, green: chl a, blue: chl b chlorine rings (phytol chains are omitted for clarity), yellow: carotenoids.
Additional to the main purpose of effective light-harvesting, a second important function is the down-regulation of the amount of available excitation energy under strong light conditions to avoid the stress-induced destruction of the catalytic RC. This photoprotective mechanism is located in the antenna system and leads to a strong reduction in the fluorescence monitored from leaves, a phenomenon referred to as non-photochemical quenching (NPQ) of Chl a fluorescence [9], [10], [11], [12].
2 The single-molecule approach
The function of the light-harvesting complexes (LHCs) is determined by the structure and conformational flexibility of the protein scaffold. As a result, there are large variations of the LHC properties both from complex to complex and in a single complex over time. In order to investigate their functional role, it is therefore necessary to address the LHCs on an individual basis. The LHCs, by virtue of their function, interact strongly with light, and it is the fate of the light excitation which bears functional significance. Optical spectroscopy is therefore an obvious method of choice.
The first single-molecule spectroscopical observations were done in 1989 by Moerner and Kador by measuring absorption of dye molecules frozen at low temperature in a crystalline matrix [13]. However, the technique quickly moved to fluorescence emission only a year later, following the lead of Orrit and Bernard [14]. The reason is that fluorescence can be detected against a dark background, providing a much better signal-to-noise ratio (SNR) than for absorption measurements. For a first-hand review, see Ref. [15]. For an excellent overview of the concepts of single-molecule spectroscopy (SMS), including application to LHCs, see also Ref. [16]. Using the fluorescence detection approach, the first measurements of single LHCs were performed on light-harvesting complex 2 (LH2) antennas of purple bacteria in the late nineties. The first results were published by Bopp et al. [17], showing the polarization dependence of the fluorescence intensity and lifetime of single LH2 complexes at room temperature. In the following year, van Oijen et al. succeeded in measuring fluorescence excitation spectra of single LH2s frozen in polymer films at 1.2 K [18]. In the same year, Ying and Xie published room-temperature results of individually measured cross-linked allophycocyanin complexes, a derivative of the main LHC of cyanobacteria [19]. Following these successes and additional improvement of experimental instrumentation, various LHCs have been observed and studied by SMS under various conditions.
Most of the information about the structure of the manifold of the excited states of LHCs has been obtained from low-temperature measurements in crystalline-like matrices [18], [20]. On the other hand, experiments at physiological temperature and in environments resembling the native one are more relevant for assessing the biological function. Physiological temperature brings further challenges, though, such as decreased stability and increased mobility of the complexes. The motion of the complexes can be countered in several ways: by immobilizing them by a weak electrostatic interaction on a surface [16], [21], by counteracting their movement by an external electric field [22], or by active particle tracking [23]. The LHC photo-induced instability and eventual denaturation, however, present a limit on the possible measurement time and the excitation light intensity, which in turn determine the achievable SNR and, in effect, also the time resolution.
As mentioned above, fluorescence detection of single molecules provides a much higher SNR than absorption detection. The direct time resolution of the detection is given by the photon counting statistics. A typical excitation lifetime, given by both the radiative and non-radiative recombination, is on the order of nanoseconds. When another photon is absorbed by the LHC within the excitation lifetime, singlet-singlet annihilation occurs (the equivalent of Auger recombination known in semiconductor nanocrystals). Thus, at some point further increasing the excitation intensity does not appreciably increase the signal and only leads to a decreased photostability of the complexes. Moreover, the constituent pigments can, when excited, undergo intersystem crossing into a triplet state, which acts as an excitation quencher via singlet-triplet (S-T) annihilation. The detection efficiency depends on the particular setup but typically is on the order of a couple of percent. Overall, the practically achievable fluorescence signal intensity from a non-quenched, continuously excited single LHC is on the order of thousands of counts per second. The major source of noise for such a low signal intensity is the photon-counting shot noise. From the considerations above it follows that the typical minimum integration time is >1 ms. However, higher time resolution can be achieved by utilizing modulated, pulsed excitation. Using an acousto-optical modulator (AOM), microsecond timescales can be accessed [24]. In addition, utilizing a pulsed laser as an excitation source, time-correlated single-photon counting (TCSPC) becomes an option, providing picosecond to nanosecond time resolution. Finally, utilizing coherent or incoherent sequences of pulses, it is possible to follow the excitation dynamics with sub-100 fs resolution [25], [26]. With a combination of these techniques, it is possible to observe, for example, the fluctuations of the ultrafast dynamics on a slow timescale of seconds, dominated by protein conformational dynamics. In Figure 2, the electronic processes of interest can be found, together with their corresponding timescales, covering a range of >15 decades. For each process a method of SMS adequate for their study is given.
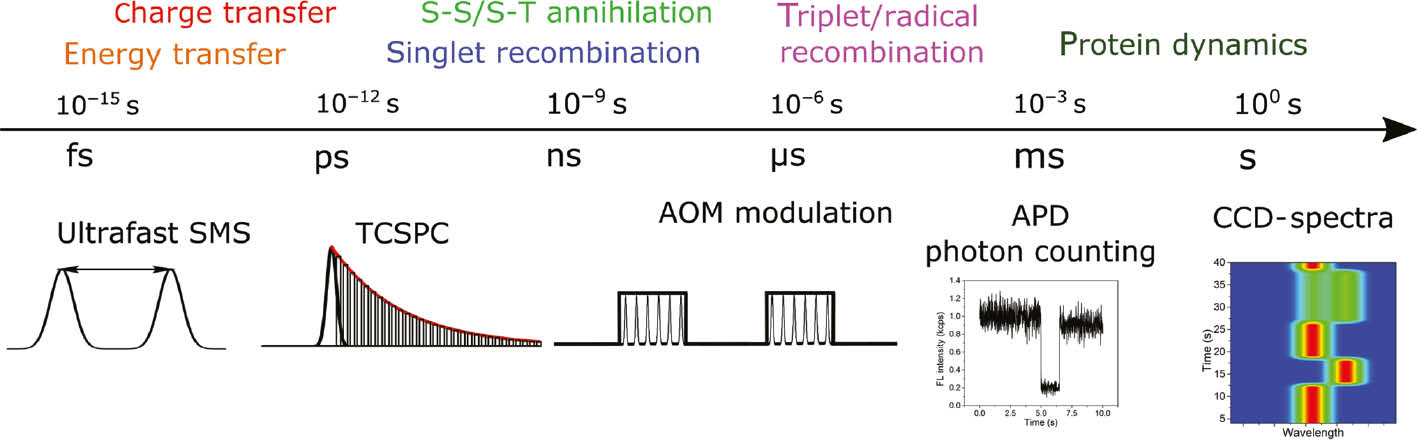
Timescales of photosynthetically relevant electronic processes observable by SMS. Top: processes taking place at the indicated timescale. Bottom: corresponding SMS techniques which can be used for their measurement; see text for further details.
Yet another degree of freedom which can be explored to obtain information about the structure of the complexes is the polarization state of both the excitation and emitted light. This is beautifully illustrated on chlorosomes, the light-harvesting antennas of green sulfur bacteria comprised of up to 200,000 highly ordered bacteriochlorophyll molecules. Circular dichroism [27] and polarization-resolved fluorescence excitation [28] SMS were used to elucidate the pigment ordering in single chlorosomes. Also, the correlation of excitation and emission polarization was used to investigate energy funneling in single LH2 antennas of purple bacteria [29].
3 Characterization of isolated complexes
The main function of LHCs is, as indicated by their name, to harvest solar radiation in the visible frequency range. The protein scaffold contains dozens of pigments that are organized in an ideal way to achieve a high pigment density and efficient energy transfer without introducing unfavorable non-radiative decay channels [30]. In this context, the term “efficiency” should be understood as the ratio of the relevant energy transfer time to the average lifetime of electronic excitations. The resulting electronic excited state manifold is, due to the well-connected pigments, highly sensitive to the presence of energy traps that can potentially quench the whole pigment assembly. The timescale of energy quenching is then determined by the time it takes for an excitation to reach the energy trap (energy diffusion) and the quenching rate of the trap itself [3], [31]. Fluorescence blinking observed in single LHCs is an illustrative example of such a quenching mechanism.
Fluorescence blinking is a phenomenon characterized by abrupt changes between the fluorescent state and a fully dark state in single pigments or quantum dots. It is a characteristic signature of a single quantum emitter and is virtually always present in SMS measurements. In pigment aggregates, like in LHCs, the fluorescence is not fully inhibited but quenched on the characteristic time scale of energy transfer and trapping mentioned above. This effect results in a large dynamic range of partially quenched states [32], [33]. However, fluorescence blinking in pigment aggregates usually closely resembles blinking in single molecules due to the fast energy equilibration and the strong quenching character of the trap, which effectively looks like a dark state of the whole pigment aggregate due to the small number of detected photons. In Figure 3, a typical SMS experiment on single LHCII complexes is demonstrated. A scanned fluorescence image, showing emission from LHCs immobilized on a surface, is shown in Figure 3A. A typical intensity trace of a single LHCII emission exhibiting blinking can then be found in Figure 3B. The intensity correlates with the fluorescence lifetime.
![Figure 3: An illustration of a SMS measurement of individual LHCII trimers. (A) A confocal scan of a 10 μm×10 μm surface area. Bright spots: fluorescence emission from single LHCs immobilized on a poly-l-lysine (PLL) covered surface. The intensity profile is given by the point-spread function. After such a scan the complexes are measured one by one. (B) A fluorescence intensity trace (black line) of a single LHCII trimer that illustrates blinking events both in the millisecond and second time range. The corresponding fluorescence lifetime (without the contribution of S-T annihilation) is depicted on the right axis (red line). Reproduced from Ref. [24] with permission from the physical chemistry chemical physics (PCCP) Owner Societies. (C) Series of recorded spectra of three selected LHCIIs, demonstrating spectral diffusion and state switching. Reprinted from Ref. [34] with permission of Elsevier. (D) Probability distribution of blinking events in LHCII trimers that typically show a power-law behavior for off-times from a few milliseconds to hundreds of seconds. On-times generally follow the same power-law dependence but exhibit an exponential tail at longer dwell times. Off-time data are offset for clarity. Reprinted with permission from Krüger et al. [35]. Copyright (2011) American Chemical Society.](/document/doi/10.1515/nanoph-2017-0014/asset/graphic/j_nanoph-2017-0014_fig_003.jpg)
An illustration of a SMS measurement of individual LHCII trimers. (A) A confocal scan of a 10 μm×10 μm surface area. Bright spots: fluorescence emission from single LHCs immobilized on a poly-l-lysine (PLL) covered surface. The intensity profile is given by the point-spread function. After such a scan the complexes are measured one by one. (B) A fluorescence intensity trace (black line) of a single LHCII trimer that illustrates blinking events both in the millisecond and second time range. The corresponding fluorescence lifetime (without the contribution of S-T annihilation) is depicted on the right axis (red line). Reproduced from Ref. [24] with permission from the physical chemistry chemical physics (PCCP) Owner Societies. (C) Series of recorded spectra of three selected LHCIIs, demonstrating spectral diffusion and state switching. Reprinted from Ref. [34] with permission of Elsevier. (D) Probability distribution of blinking events in LHCII trimers that typically show a power-law behavior for off-times from a few milliseconds to hundreds of seconds. On-times generally follow the same power-law dependence but exhibit an exponential tail at longer dwell times. Off-time data are offset for clarity. Reprinted with permission from Krüger et al. [35]. Copyright (2011) American Chemical Society.
The described quenching phenomena in isolated LHCs will directly influence the efficiency of light-harvesting, which raises the question whether they play a role in native light-harvesting as well, or more precisely, whether this quenching is responsible or involved in NPQ. One explanation for the molecular mechanism governing NPQ is that conformational changes in antenna complexes open up non-radiative decay channels via the carotenoid pigments and lead to excitation energy quenching [36]. Fluorescence blinking observed in all isolated single antenna complexes is likely caused by the same or a very similar mechanism involving conformational changes of the pigment-protein complex. Experimental data on the main LHCII of plants indicate that fluorescence blinking can indeed be influenced and possibly regulated by subtle changes in protein conformation and/or interaction with the environment [33], [37]. It was shown that lowering the pH can shift the equilibrium between highly fluorescent and dark states from the light-harvesting (unquenched) state to quenched configurations. This in vitro experiment mimics the build-up of a pH gradient across the thylakoid membrane in native systems, widely recognized as a main trigger of NPQ [12]. An even stronger quenching response can be obtained by removing detergent and therefore influencing the amphiphilic surrounding and solvation of the pigment-protein complexes. Curiously, fluorescence blinking from the homologous minor peripheral antenna complexes in PSII (CP24, CP26, and CP29) showed a different environmental response when performing a similar pH and detergent dependence. This behavior, which supports the view that the major part of NPQ takes place in LHCII complexes [9], [11], [36], was ascribed to the strong abundance of partially quenched fluorescence states in these complexes.
Studies on single antenna complexes of bacterial systems [38] and cyanobacteria [39] also show photo-activated switching events to quenched states, and another study even suggests a precursor role of fluorescence intermittency in purple bacteria for NPQ in plants [40]. The authors in the latter study further argue that conformational memory, in contrast to purely stochastic conformational switching, is a precondition for functional control of native light-harvesting [41].
Fluorescence blinking in single molecules (e.g. quantum dots) is commonly analyzed with a two-state model to extract blinking statistics and dwell time probability distributions [42], [43]. Such a distribution extracted from the fluorescence blinking of LHCII antennas is given in Figure 3D. This analysis allows to quantitatively describe and investigate changes in the blinking pattern. The resulting probability density distribution of blinking events shows typically a power-law behavior for off-times from a few milliseconds to hundreds of seconds, while on-times generally follow the same power-law dependence, but exhibit an exponential tail at longer dwell times. However, multi-chromophoric systems show substantial intermediate fluorescence intensity levels, and it turns out that accurately resolving individual intensity levels and accounting for shot noise in the data results in a deviation from the pure power-law behavior at short dwell times, as demonstrated for LHCII [35] in Figure 3D. The origin of fluorescence intensity changes and blinking in LHCs is assumed to be subtle changes in protein conformation [44]. Experimental evidence for a direct relationship between the protein flexibility and the number of intensity changes was obtained in a recent study on cyanobacterial LHCs containing different concentrations of cross-linkers [36]. Additional supporting arguments for the important role of protein conformational changes are the quasi-stability of intensity levels, the short decay lifetime of dark states, such as triplets and radicals, and the fact that antenna complexes also show significant spectral diffusion and switching [34]. Spectral traces for three LHCII complexes showing such behavior are demonstrated in Figure 3C.
The spectral diffusion trajectories can be mathematically well described by a random walk [45]. The underlying physical mechanism in LHCs is the protein conformational dynamics on their potential energy surface (PES). Using a combination of normal mode analysis, charge density coupling, and transition charges, it can be theoretically shown that the LHC protein fluctuations predominantly influence the pigment transition energies [46]. Indeed, the changes in the fluorescence spectra of individual complexes have been successfully modeled with a Frenkel-exciton model, including Gaussian disorder of the individual pigment transition energies [21], [34]. Apart from the excited state energies, including the final, fluorescent state, the LHC protein fluctuations also lead to pronounced changes in the energy transfer within the excitonic manifold. Using a pulse sequence for excitation, fluctuations of the intercomplex energy transfer rate in the range of 100 fs were observed in single LH2 complexes of purple bacteria [26]. The same excitonic model that describes the spectral diffusion can be used to model these fluctuations, with the dynamics described by modified Redfield/generalized Förster theory.
Interesting conclusions regarding excitonic states can also be made from the measured and modeled single-molecule fluorescence emission spectra of the mutant LHCII-A2 [47]. This monomeric LHCII mutant lacks two Chls (a611 and a612) of the strongly coupled Chl cluster (a610-a611-a612), which is the lowest-energy site (terminal emitter) in the wild-type complex. The mutant spectra exhibit a significantly increased heterogeneity in fluorescence peak position, and the full width half maximum (FWHM) is increasing with a gradual distribution of red shifted spectra. These results can be explained by the disrupted terminal emitter domain which leads to an increased population, and therefore fluorescence, of the blue shifted Chl cluster a602-a603 and to a varying contribution from Chl a610. The latter is strongly influenced by static disorder (i.e., protein motion) and significantly red shifted due to the larger reorganization energy related with the localized excitation. These findings illustrate the function of the delocalized Chl cluster a610-a611-a612 as a robust terminal emitter domain in the wild type that is less susceptible to the fluctuating environment.
Next to the continuous spectral diffusion, the intensity blinking and spectral switching presents discrete changes (on the milliseconds to seconds timescale of measurement). In the blinking case these can be described by rapid switching between several protein conformational states with a different energy, represented by various PESs [44], [48]. The connection to the excitonic model, which qualitatively describes the spectral diffusion, can be made by assuming that the dark state arises from energy transfer to a short-lived state with fast non-radiative recombination. The protein dynamics then modifies specific parameters of the excitonic model, such as interpigment coupling, which regulate this transfer [49]. In the case of spectral switching, the above-mentioned model for spectral diffusion uses a pure excitonic approach to explain fluorescence spectral shifts of LHCII to within ~15 nm of the ensemble peak position but fails to explain the large variety of spectra that have been observed, including red-shifts of >100 nm [34], [37]. An illuminating result was obtained when individual antenna complexes from PSI were investigated [50]: these complexes, which are structurally homologous to LHCII, typically fluoresce much further into the red but are capable of switching their red emission off completely and reversibly. Their spectra then temporarily resemble those of LHCII complexes of PSII. The antenna complexes of PSI and PSII can therefore be approximated by a single generic protein structure, and their spectra are governed by a particular equilibrium between two or more conformations, each connected to a characteristic spectrum. Furthermore, the red spectra of PSI complexes are known to originate from mixing between charge-transfer and exciton states of a Chl dimer [51], and a similar mechanism is likely the basis for the strongly red-shifted spectra of PSII complexes. Finally, the fraction of LHCII complexes exhibiting red-shifted emission was shown to increase as NPQ is mimicked to a greater extent [37], in agreement with the tradition of using red-shifted emission as a signature for NPQ.
The emerging picture is that of a highly dynamic protein structure that significantly influences the electronic properties of the constituting pigments on timescales ranging from 100 fs to seconds. The environment of a pigment protein is therefore an important parameter in this intrinsic flexibility and crucial to understand its functional relevance.
4 Lipid environment
The native environment of LHCs is the lipid membrane, which creates an almost two-dimensional scaffold. The lateral binding and interaction of embedded LHCs with other protein complexes is regulated by the structural arrangement of thylakoid structures. The isolation of well-defined photosystem mega-complexes (e.g. PSII C2S2M2 supercomplexes as illustrated in Figure 1B) nicely demonstrates this lateral ordering. The hydrophobic core of the lipid bilayer shields the inner pigments of photosynthetic complexes from detrimental chemical reactions and uncontrolled electrostatic interactions. In order to study individual complexes, photosynthetic pigment-protein complexes are commonly solubilized and isolated in detergent micelles that mimic this hydrophobic interface. Detergent solubilized LHCs of plants exhibit a fluorescence lifetime of more than 3.5 ns, which is characteristic for their light-harvesting capacity as antennas when compared to the overall trapping times of PSII on the order of about 300 ps. The term “trapping time” describes the time it takes until an electronic excitation results in a stable charge separated state in the RC. Removing the detergent from solubilized LHCs results in protein aggregation and significant fluorescence quenching. Furthermore, the removal of detergent also leads to quenching in individual, immobilized complexes that do not form aggregates with other complexes [37], [52]. This quenching effect has been thoroughly studied and often been linked to and discussed with regards to its role in NPQ [53], [54]. The direct link to native system remains, however, elusive.
One approach to investigate the underlying molecular mechanism of fluorescence quenching and its functional relevance for native photoprotection is to disentangle the interaction of complexes with detergent or lipids from protein-protein interactions. It is furthermore crucial to control the number of interacting complexes in order to better understand crowding effects. The size of detergent deprived LHC aggregates is, for example, hard to determine in typical ensemble experiments.
Although the dual function of LHC complexes (light-harvesting and photoprotection) has been extensively studied in detergent micelles, ensemble experiments have indicated that the properties of LHC complexes can differ in a lipid environment. Detergent-isolated complexes can be incorporated back into a lipid environment to investigate this dependence on the environment. A variety of ensemble studies have been performed on LHC complexes incorporated into liposomes, a spherical vesicle consisting of a lipid bilayer shell around an aqueous core [55], [56], [57], [58], [59]. Those experiments also contain the effect of protein-protein interaction of multiple complexes within a single liposome which makes it difficult to extract purely the influence of the lipid environment [60]. Alternatively, LHCII complexes have recently been isolated from their native thylakoid environment by using styrene maleic acid (SMA) [32]. This SMA copolymer solubilizes lipid nanodisk particles that contain a single protein complex.
SMS is an ideal method to investigate these research questions as it provides quantitative data on the size of the sample and allows to investigate the dynamic and time-dependent behavior of individual complexes. The fluorescence intensity of LHCII complexes in single nanodisks together with the average fluorescence lifetime allows estimating their size based on the calculated relative absorption compared to detergent-isolated complexes [32]. The amount and kinetics of S-T annihilation, a non-linear quenching mechanism observed for multiple excitations in one complex, are the same in SMA nanodisks and detergent-isolated LHC trimers. The obtained results therefore demonstrate the successful isolation of trimeric LHCII complexes in SMA nanodisks in their native trimeric structure. The observed unquenched fluorescence lifetime of 3.5 ns for single LHCII complexes in SMA nanodisks coincides with detergent-isolated complexes and notably differs from 2 ns typically found from ensemble-average experiments on native thylakoid structures [61]. A distribution of the fluorescence lifetime and intensity in LHCII complexes measured in detergent and in SMA nanodisks is shown in Figure 4A and B. Combining bulk and single molecule measurements, the fluorescence characteristics of LHCs incorporated in liposomes can be investigated with respect to the number of LHCs per liposome [60]. The same unquenched fluorescence lifetime of up to 3.5 ns has been found for liposomes that contain only one or a very small number of complexes, therefore avoiding protein-protein interactions and protein crowding. This experiment revealed that the properties of individual LHCs remain similar to those in detergent, in line with the results from LHCs in lipid nanodisks [32], [62]. However, protein interactions of multiple units of LHCs and protein clustering result in a reduced fluorescence yield. Finally, ensemble experiments on LHCs incorporated into membrane scaffolding protein nanodisks have shown the same result [62]. These studies show conclusively that a lipid environment per se is likely not the reason for the decreased fluorescence lifetime of antenna complexes found in thylakoid structures. It does not, however, exclude the possibility that specific protein-lipid interactions or structural changes of the lipid bilayer (e.g. thickness or curvature) have an indirect influence on the excited-state lifetime via protein conformation or electrostatic interactions with charged head-groups. Indeed, lipids do play a crucial role for the structural assembly and functionality of photosynthetic complexes [63], [64], [65].
![Figure 4: Comparison of the fluorescence lifetime of about 150 LHCII trimers in detergent (A) and in the lipid environment of SMA nanodisks (B). The color code represents the overall dwell-time probability of complexes within an intensity bin of 50 cps and a lifetime bin of 100 ps. The two graphs depict the fluorescence lifetime without the contribution of S-T annihilation, while the measured fluorescence intensity is saturating slightly at larger lifetime values due to the effect of annihilation. Reproduced from Ref. [32]. Copyright (2016) Society of Photo Optical Instrumentation Engineers.](/document/doi/10.1515/nanoph-2017-0014/asset/graphic/j_nanoph-2017-0014_fig_004.jpg)
Comparison of the fluorescence lifetime of about 150 LHCII trimers in detergent (A) and in the lipid environment of SMA nanodisks (B). The color code represents the overall dwell-time probability of complexes within an intensity bin of 50 cps and a lifetime bin of 100 ps. The two graphs depict the fluorescence lifetime without the contribution of S-T annihilation, while the measured fluorescence intensity is saturating slightly at larger lifetime values due to the effect of annihilation. Reproduced from Ref. [32]. Copyright (2016) Society of Photo Optical Instrumentation Engineers.
The significant decrease of the fluorescence lifetime for liposomes that contain many protein complexes points towards the hypothesis that protein-protein interactions and protein clustering play a major role in the decrease of the observed fluorescence lifetime [60]. This is consistent with the strong quenching effect observed in detergent-deprived protein aggregation studies. It is also consistent with an increased influence of protein-protein interactions within the native thylakoid membrane. Contrary to lateral aggregation in lipid bilayer structures, the aggregated structures in solution could be even less ordered due to a higher degree of freedom in three dimensions. That would influence the conformational state of individual proteins within the aggregate and therefore change the excitonic manifold of strongly and weakly coupled pigments. The discrepancy could also be explained by the large dynamic range of fluorescence intensities exhibited by complexes in SMS experiments, where the highest intensities correspond to 3.5 ns lifetimes, while 2 ns reflects the average intensity. In other words, the conformational and energetic flexibility of these complexes gives rise to a large range of partially quenched complexes both in detergent micelles and lipid nanodisks [32], [33], [50]. This highly flexible protein conformation landscape has recently also been shown by molecular dynamics simulations on a monomeric LHCII complex [66].
5 Fully assembled PSII supercomplexes
A complementary experimental approach to investigate the functional significance of fluorescence intermittency and to get closer to the native structure of the thylakoid membrane is to measure at room temperature individual PSII particles that contain multiple antenna complexes as well as two RCs.
A very pure sample of so-called C2S2 PSII supercomplexes can be isolated biochemically [67], and the sample homogeneity can in SMS experiments again be confirmed by calculating the relative absorption cross section of individual complexes [24]. These C2S2 PSII supercomplexes are comprised of the dimeric core (C2), two strongly bound LHCII trimers (S-trimer), and two copies of CP26 and CP29 each. The measurements further revealed that larger C2S2M2 supercomplexes frequently lost some of their weaker bound LHCII trimers (M-trimer), which clearly show up as isolated LHCII complexes. An example of a fluctuating fluorescence intensity and lifetime trace of a C2S2 supercomplex is in Figure 5A. In the inset the comparison of the 3 ns lifetime of an isolated LHCII is contrasted with a shorter, 100–150 ps lifetime of the C2S2. This measured average excited state lifetime of C2S2 supercomplexes is at a first glance almost identical to the fluorescence decay of photochemically active PSII complexes measured at 104 times smaller excitation intensities in solution [68]. The cause for the fast lifetime in these SMS experiments is, however, not photochemical quenching but another competing quenching mechanism. The used excitation intensity in SMS experiments fully inhibits photochemical quenching because the first couple of excitation cycles will result in a charge separated state and subsequently in a negatively charged quinone QA. This situation is usually referred to as a “closed” RC because the rate of charge separation is reduced significantly and the fluorescence lifetime is determined by the excited state lifetime of the surrounding antenna complexes [61], [69]. Triplet states on the carotenoid pigments with an excited state lifetime of a few microseconds cannot be the main reason because control experiments show neither accumulation nor decay of a quencher in the microsecond time range. A quenching effect due to low detergent concentrations was ruled out by control experiments on LHCII complexes that do not show any sign of quenching under identical conditions. The most likely explanation for the quenched fluorescence lifetime of PSII supercomplexes in the described SMS experiments is instead multiple quenched monomeric LHCs, which is consistent with the frequent blinking events on individual isolated antennas. Modulation of the excitation intensity with a periodic on and off pattern on the millisecond time scale furthermore shows the reversible and light-induced switching from an unquenched fluorescence lifetime of 3.5 ns to the quenched situation of about 100–150 ps. The fluorescence decay traces for various modulation cycles are depicted in Figure 5B, showing the decay of the quencher on a millisecond time scale. This outcome supports the hypothesis that the RCs are closed (detection of a 3 ns fluorescence lifetime) but the supercomplexes are most of the time quenched by trap states on individual LHC monomers. Detailed Monte Carlo simulations that are based on a coarse-grained model of C2S2 supercomplexes show similar fluorescence and confirm the feasibility of this interpretation. This is a direct illustration of how environmental control (induced by strong excitation intensity in this case) over a fluctuating antenna can regulate light-harvesting in plant photosynthesis.
![Figure 5: (A) Fluorescence intensity traces of a C2S2 supercomplex (blue line) with intensity variations on a second time scale that resemble fluorescence blinking events exhibited by isolated antenna complexes. The quenched average lifetime (red line) is shown on the right y axis. The corresponding fluorescence decay histograms of the C2S2 supercomplex (blue line in A) and the LHCII complex (black line in Figure 1A), accumulated over 60 s, are shown in the inset of (A) on a semi-logarithmic scale with the same respective colors. The instrument response function with a FWHM of 38 ps is depicted in green. (B) The light-induced fluorescence intensity kinetics of an exemplary single C2S2 complex for three different excitation modulation settings. Periodic modulation of the excitation intensity (i.e. switching the laser illumination on and off) and histogramming the fluorescence photon arrival times into one such modulation cycle allows one to directly visualize the accumulation and decay of a quencher on the millisecond time scale. The dashed lines indicate a fitted single-exponential function of the form F(t)=F0+F1·e−kt. The decay kinetics were normalized to F0=1 (identical steady state conditions due to the same excitation intensity for all modulation settings). Reproduced from Ref. [24]. With permission from the PCCP Owner Societies.](/document/doi/10.1515/nanoph-2017-0014/asset/graphic/j_nanoph-2017-0014_fig_005.jpg)
(A) Fluorescence intensity traces of a C2S2 supercomplex (blue line) with intensity variations on a second time scale that resemble fluorescence blinking events exhibited by isolated antenna complexes. The quenched average lifetime (red line) is shown on the right y axis. The corresponding fluorescence decay histograms of the C2S2 supercomplex (blue line in A) and the LHCII complex (black line in Figure 1A), accumulated over 60 s, are shown in the inset of (A) on a semi-logarithmic scale with the same respective colors. The instrument response function with a FWHM of 38 ps is depicted in green. (B) The light-induced fluorescence intensity kinetics of an exemplary single C2S2 complex for three different excitation modulation settings. Periodic modulation of the excitation intensity (i.e. switching the laser illumination on and off) and histogramming the fluorescence photon arrival times into one such modulation cycle allows one to directly visualize the accumulation and decay of a quencher on the millisecond time scale. The dashed lines indicate a fitted single-exponential function of the form F(t)=F0+F1·e−kt. The decay kinetics were normalized to F0=1 (identical steady state conditions due to the same excitation intensity for all modulation settings). Reproduced from Ref. [24]. With permission from the PCCP Owner Societies.
From a theoretical point of view, there are two types of approaches to simulate the excitation dynamics in the supercomplexes. One is a bottom-up approach, keeping the (essentially quantum) description of the individual LHCs, and putting them together, attempting a large-scale quantum-correct description. Such a calculation was performed for PSII, reproducing the experimental fluorescence decay curves [70] and demonstrating the robustness of energy transfer [71]. However, when keeping the full description, it is hard to reduce the number of parameters in order to deduce the key physical principles. The latter aim is fulfilled in the second approach, which employs a coarse-grained description of the supercomplex. This method is able to correctly describe NPQ in PSII, based on annihilation measurements [72], and fluorescence decay in LHCII aggregates [73]. Recently, a combination of these two approaches, making a “cut” on the level of LHCs, was presented, describing NPQ in PSII probed by fluorescence decay and pulse-amplitude modulation [74].
The described research clearly showcases the general property of biological systems to use the same effect for different purposes. LHCs are ingeniously designed nanoparticles that maximize the absorption of sunlight and efficiently transfer the excitation energy further to the RC. The key properties are a long lifetime of electronic excitations on densely packed pigments in a protein environment and their efficient energy transfer without significant losses. At the same time, their conformational flexibility opens up the possibility to induce energy dissipation channels for photoprotection on a fast timescale and in an environmentally controlled manner.
Acknowledgments
J.M.G., P.M., and R.v.G. were supported by the VU University and by an Advanced Investigator grant from the European Research Council (no. 267333, PHOTPROT) to R.v.G. R.v.G. was further supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Council of Chemical Sciences (NWO-CW) via a TOP-grant (700.58.305), and by the EU FP7 project PAPETS (GA 323901). R.v.G. gratefully acknowledges his Academy Professor grant from the Netherlands Royal Academy of Sciences (KNAW). T.P.J.K. was supported by the University of Pretoria’s Research Development Program (Grant no. A0W679) and the Thuthuka Program of the National Research Foundation (NRF) of South Africa (Grant no. 94107). Any opinion, findings, and conclusions or recommendations expressed in this article are those of the authors, and therefore the NRF does not accept liability in regards thereto.
References
[1] Blankenship RE. Molecular mechanisms of photosynthesis, 2nd edition. Chichester, West Sussex, UK, Wiley-Blackwell, 2014.Suche in Google Scholar
[2] Van Amerongen H, Croce R. Light harvesting in photosystem II. Photosynth Res 2013;116:251–63.10.1007/s11120-013-9824-3Suche in Google Scholar PubMed
[3] Van Grondelle R, Dekker JP, Gillbro T, Sundstrom V. Energy transfer and trapping in photosynthesis. Biochim Biophys Acta 1994;1187:1–65.10.1016/0005-2728(94)90166-XSuche in Google Scholar
[4] Wei X, Su X, Cao P, et al. Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution. Nature 2016;534:69–74.10.1038/nature18020Suche in Google Scholar PubMed
[5] Boekema EJ, Hankamer B, Bald D, et al. Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc Natl Acad Sci USA 1995;92:175–79.10.1073/pnas.92.1.175Suche in Google Scholar PubMed PubMed Central
[6] Liu Z, Yan H, Wang K, et al. Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 2004;428:287–92.10.1038/nature02373Suche in Google Scholar PubMed
[7] Standfuss J, Terwisscha van Scheltinga AC, Lamborghini M, Kühl-brandt W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J 2005;24:919–28.10.1038/sj.emboj.7600585Suche in Google Scholar PubMed PubMed Central
[8] Ruban AV, Lee PJ, Wentworth M, Young AJ, Horton P. Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. J Biol Chem 1999;274:10458–65.10.1074/jbc.274.15.10458Suche in Google Scholar PubMed
[9] Ruban AV, Johnson MP, Duffy CDPP. The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 2012;1817:167–81.10.1016/j.bbabio.2011.04.007Suche in Google Scholar PubMed
[10] Niyogi KK, Truong TB. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol 2013;16:307–14.10.1016/j.pbi.2013.03.011Suche in Google Scholar PubMed
[11] Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 1996;47:655–84.10.1146/annurev.arplant.47.1.655Suche in Google Scholar PubMed
[12] Muller P, Li X, Niyogi K. Non-photochemical quenching. A response to excess light energy. Plant Physiol 2001;125:1558–66.10.1104/pp.125.4.1558Suche in Google Scholar PubMed PubMed Central
[13] Moerner W, Kador L. Optical detection and spectroscopy of single molecules in a solid. Phys Rev Lett 1989;62:2535–8.10.1103/PhysRevLett.62.2535Suche in Google Scholar PubMed
[14] Orrit M, Bernard J. Single pentacene molecules detected by fluorescence excitation in a p-terphenyl crystal. Phys Rev Lett 1990;65:2716.10.1103/PhysRevLett.65.2716Suche in Google Scholar PubMed
[15] Moerner WE, Orrit M. Illuminating single molecules in condensed matter. Science 1999;283:1670–6.10.1126/science.283.5408.1670Suche in Google Scholar PubMed
[16] Rigler R, Orrit M, Basche T, eds. Single molecule spectroscopy. Berlin, Heidelberg, Springer, 2001.10.1007/978-3-642-56544-1Suche in Google Scholar
[17] Bopp MA, Jia Y, Li LQ, Cogdell RJ, Hochstrasser RM. Fluorescence and photobleaching dynamics of single light-harvesting complexes. Proc Natl Acad Sci USA 1997;94:10630–5.10.1073/pnas.94.20.10630Suche in Google Scholar PubMed PubMed Central
[18] Van Oijen AM, Ketelaars M, Köhler J, Aartsma TJ, Schmidt J. Spectroscopy of single light-harvesting complexes from purple photosynthetic bacteria at 1.2 K. J Phys Chem B 1998;102:9363–6.10.1021/jp9830629Suche in Google Scholar
[19] Ying L, Xie XS. Fluorescence spectroscopy, exciton dynamics, and photochemistry of single allophycocyanin trimers. J Phys Chem B 1998;102:10399–409.10.1021/jp983227dSuche in Google Scholar
[20] Aartsma TJ, Köhler J. Optical spectroscopy of individual light-harvesting complexes. In: Aartsma TJ, Matysik J, eds. Biophysical Techniques in Photosynthesis. Advances in Photosynthesis and Respiration. Vol. 26, Dordrecht, Springer, 2008.10.1007/978-1-4020-8250-4Suche in Google Scholar
[21] Rutkauskas D, Novoderezkhin V, Cogdell RJ, Van Grondelle R. Fluorescence spectral fluctuations of single LH2 complexes from Rhodopseudomonas acidophila strain 10050. Biochemistry 2004;43:4431–8.10.1021/bi0497648Suche in Google Scholar PubMed
[22] Cohen AE, Moerner WE. Method for trapping and manipulating nanoscale objects in solution. Appl Phys Lett 2005;86:93109.10.1063/1.1872220Suche in Google Scholar
[23] Schmidt T, Schütz GJ, Baumgartner W, Gruber HJ, Schindler H. Imaging of single molecule diffusion. Proc Natl Acad Sci USA 1996;93:2926.10.1073/pnas.93.7.2926Suche in Google Scholar
[24] Gruber JM, Xu P, Chmeliov J, et al. Dynamic quenching in single photosystem II supercomplexes. Phys Chem Chem Phys 2016;18:25852–60.10.1039/C6CP05493ESuche in Google Scholar PubMed
[25] Hildner R, Brinks D, Nieder JB, Cogdell RJ, van Hulst NF. Quantum coherent energy transfer over varying pathways in single light-harvesting complexes. Science 2013;340:1448–51.10.1126/science.1235820Suche in Google Scholar PubMed
[26] Malý P, Gruber JM, Cogdell RJ, Mančal T, van Grondelle R. Ultrafast energy relaxation in single light-harvesting complexes. Proc Natl Acad Sci USA 2016;113:2934–9.10.1073/pnas.1522265113Suche in Google Scholar
[27] Furumaki S, Yabiku Y, Habuchi S, et al. Circular dichroism measured on single chlorosomal light-harvesting complexes of green photosynthetic bacteria. J Phys Chem Lett 2012;3:3545–49.10.1021/jz301671pSuche in Google Scholar PubMed
[28] Jendrny M, Aartsma TJ, Köhler J. Insights into the excitonic states of individual chlorosomes from chlorobaculum tepidum. Biophys J 2014;106:1921–7.10.1016/j.bpj.2014.03.020Suche in Google Scholar PubMed
[29] Camacho R, Tubasum S, Southall J, et al. Fluorescence polarization measures energy funneling in single light-harvesting antennas-LH2 vs conjugated polymers. Sci Rep 2015;5:15080.10.1038/srep15080Suche in Google Scholar PubMed
[30] Beddard GS, Porter G. Concentration quenching in chlorophyll. Nature 1976;260:366–367.10.1038/260366a0Suche in Google Scholar
[31] Van Grondelle R. Excitation energy transfer, trapping and annihilation in photosynthetic systems. Biochim Biophys Acta 1985;811:147–95.10.1016/0304-4173(85)90017-5Suche in Google Scholar
[32] Gruber JM, Scheidelaar S, van Roon H, et al. Photophysics in single light-harvesting complexes II: from micelle to native nanodisks. In: Enderlein J, Gregor I, Gryczynski ZK, Erdmann R, Koberling F, eds. San Francisco, California, United States, SPIE BiOS, International Society for Optics and Photonics. 2016, 97140A.10.1117/12.2211588Suche in Google Scholar
[33] Schlau-Cohen GS, Yang HY, Krüger TPJ, et al.Single-molecule identification of quenched and unquenched states of LHCII. J Phys Chem Lett 2015;6:860–7.10.1021/acs.jpclett.5b00034Suche in Google Scholar PubMed
[34] Krüger TPJ, Novoderezkhin VI, Ilioaia C, van Grondelle R. Fluorescence spectral dynamics of single LHCII trimers. Biophys J 2010;98:3093–101.10.1016/j.bpj.2010.03.028Suche in Google Scholar PubMed PubMed Central
[35] Krüger TPJ, Ilioaia C, van Grondelle R. Fluorescence intermittency from the main plant light-harvesting complex: resolving shifts between intensity levels. J Phys Chem B 2011;115:5071–82.10.1021/jp201609cSuche in Google Scholar PubMed
[36] Duffy CDP, Ruban AV. Dissipative pathways in the photosystem-II antenna in plants. J Photochem Photobiol B 2015;152:215–26.10.1016/j.jphotobiol.2015.09.011Suche in Google Scholar PubMed
[37] Krüger TPJ, Ilioaia C, Johnson MP, et al.Controlled disorder in plant light-harvesting complex II explains its photoprotective role. Biophys J 2012;102:2669–76.10.1016/j.bpj.2012.04.044Suche in Google Scholar PubMed PubMed Central
[38] Schlau-Cohen GS, Wang Q, Southall J, Cogdell RJ, Moerner WE. Single-molecule spectroscopy reveals photosynthetic LH2 complexes switch between emissive states. Proc Natl Acad Sci USA 2013;110:10899–903.10.1073/pnas.1310222110Suche in Google Scholar PubMed PubMed Central
[39] Gwizdala M, Berera R, Kirilovsky D, van Grondelle R, Krüger TPJ. Controlling light harvesting with light. J Am Chem Soc 2016;138:11616–22.10.1021/jacs.6b04811Suche in Google Scholar PubMed
[40] Schörner M, Beyer SR, Southall J, Cogdell RJ, Köhler J. Multi-level, multi time-scale fluorescence intermittency of photosynthetic LH2 complexes: a precursor of non-photochemical quenching? J Phys Chem B 2015;119:13958–63.10.1021/acs.jpcb.5b06979Suche in Google Scholar PubMed
[41] Schörner M, Beyer SR, Southall J, Cogdell RJ, Köhler J. Conformational memory of a protein revealed by single-molecule spectroscopy. J Phys Chem B 2015;119:13964–70.10.1021/acs.jpcb.5b07494Suche in Google Scholar PubMed
[42] Cichos F, von Borczyskowski C, Orrit M. Power-law intermittency of single emitters. Curr Opin Colloid Interface Sci 2007;12:272–84.10.1016/j.cocis.2007.07.012Suche in Google Scholar
[43] Barkai E, Jung Y, Silbey R. Theory of single-molecule spectroscopy: beyond the ensemble average. Annu Rev Phys Chem 2004;55:457–507.10.1146/annurev.physchem.55.111803.143246Suche in Google Scholar PubMed
[44] Valkunas L, Chmeliov J, Krüger TPJ, Ilioaia C, van Grondelle R. How photosynthetic proteins switch. J Phys Chem Lett 2012;3:2779–84.10.1021/jz300983rSuche in Google Scholar
[45] Šanda F, Mukamel S. Anomalous continuous-time random-walk spectral diffusion in coherent third-order optical response. Phys Rev E 2006;73:11103.10.1103/PhysRevE.73.011103Suche in Google Scholar PubMed
[46] Renger T, Klinger A, Steinecker F, et al. Normal mode analysis of the spectral density of the Fenna–Matthews–Olson light-harvesting protein: how the protein dissipates the excess energy of excitons. J Phys Chem B 2012;116:14565–80.10.1021/jp3094935Suche in Google Scholar PubMed PubMed Central
[47] Ramanan C, Gruber JM, Malý P, et al. The role of exciton delocalization in the major photosynthetic light-harvesting antenna of plants. Biophys J 2015;108:1047–56.10.1016/j.bpj.2015.01.019Suche in Google Scholar PubMed PubMed Central
[48] Kondo T, Chen WJ, Schlau-Cohen GS. Single-molecule fluorescence spectroscopy of photosynthetic systems. Chem Rev 2017;117:860–98.10.1021/acs.chemrev.6b00195Suche in Google Scholar PubMed
[49] Malý P, Gruber JM, van Grondelle R, Mančal T. Single molecule spectroscopy of monomeric LHCII: experiment and theory. Sci Rep 2016;6:26230.10.1038/srep26230Suche in Google Scholar PubMed PubMed Central
[50] Krüger TPJ, Wientjes E, Croce R, van Grondelle R. Conformational switching explains the intrinsic multifunctionality of plant light-harvesting complexes. Proc Natl Acad Sci USA 2011;108:13516–21.10.1073/pnas.1105411108Suche in Google Scholar PubMed PubMed Central
[51] Romero E, Mozzo M, van Stokkum IHM, et al. The origin of the low-energy form of photosystem I light-harvesting complex Lhca4: mixing of the lowest exciton with a charge-transfer state. Biophys J 2009;96:L35–7.10.1016/j.bpj.2008.11.043Suche in Google Scholar PubMed PubMed Central
[52] Ilioaia C, Johnson MP, Horton P, Ruban AV. Induction of efficient energy dissipation in the isolated light-harvesting complex of photosystem II in the absence of protein aggregation. J Biol Chem 2008;283:29505–12.10.1074/jbc.M802438200Suche in Google Scholar PubMed PubMed Central
[53] Horton P, Wentworth M, Ruban A. Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photo-chemical quenching. FEBS Lett 2005;579:4201–6.10.1016/j.febslet.2005.07.003Suche in Google Scholar
[54] Kirchhoff H, Hinz HJ, Rösgen J. Aggregation and fluorescence quenching of chlorophyll a of the light-harvesting complex II from spinach in vitro. Biochim Biophys Acta 2003;1606: 105–16.10.1016/S0005-2728(03)00105-1Suche in Google Scholar PubMed
[55] Zhou F, Liu S, Hu Z, et al. Effect of monogalactosyldiacylglycerol on the interaction between photosystem II core complex and its antenna complexes in liposomes of thylakoid lipids. Photosynth Res 2009;99:185–93.10.1007/s11120-008-9388-9Suche in Google Scholar PubMed
[56] Yang C, Boggasch S, Haase W, Paulsen H. Thermal stability of trimeric light-harvesting chlorophyll a/b complex (LHCIIb) in liposomes of thylakoid lipids. Biochim Biophys Acta 2006;1757:1642–8.10.1016/j.bbabio.2006.08.010Suche in Google Scholar PubMed
[57] Wardak A, Brodowski R, Krupa Z, Gruszecki WI. Effect of light-harvesting complex II on ion transport across model lipid membranes. J Photochem Photobiol B 2000;56:12–8.10.1016/S1011-1344(00)00050-6Suche in Google Scholar PubMed
[58] Liu C, Gao Z, Liu K, et al. Simultaneous refolding of denatured PsbS and reconstitution with LHCII into liposomes of thylakoid lipids. Photosynth Res 2015;127:109–16.10.1007/s11120-015-0176-zSuche in Google Scholar PubMed
[59] Wilk L, Grunwald M, Liao PN, Walla PJ, Kuhlbrandt W. Direct interaction of the major light-harvesting complex II and PsbS in nonphotochemical quenching. Proc Natl Acad Sci USA 2013;110:5452–6.10.1073/pnas.1205561110Suche in Google Scholar PubMed PubMed Central
[60] Natali A, Gruber JM, Dietzel L, et al. Light-harvesting complexes (LHCs) cluster spontaneously in membrane environment leading to shortening of their excited state lifetimes. J Biol Chem 2016;291:16730–9.10.1074/jbc.M116.730101Suche in Google Scholar PubMed PubMed Central
[61] Belgio E, Johnson MP, Jurić S, Ruban AV. Higher plant photosystem II light-harvesting antenna, not the reaction center, determines the excited-state lifetime – both the maximum and the nonphotochemically quenched. Biophys J 2012;102:2761–71.10.1016/j.bpj.2012.05.004Suche in Google Scholar PubMed PubMed Central
[62] Pandit A, Shirzad-Wasei N, Wlodarczyk LM, et al. Assembly of the major light-harvesting complex II in lipid nanodiscs. Biophys J 2011;101:2507–15.10.1016/j.bpj.2011.09.055Suche in Google Scholar PubMed PubMed Central
[63] Mizusawa N, Wada H. The role of lipids in photosystem II. Biochim Biophys Acta 2012;1817:194–208.10.1016/j.bbabio.2011.04.008Suche in Google Scholar PubMed
[64] Jones MR. Lipids in photosynthetic reaction centres: structural roles and functional holes. Prog Lipid Res 2007;46:56–87.10.1016/j.plipres.2006.06.001Suche in Google Scholar PubMed
[65] Nußberger S, Dörr K, Wang DN, Kühlbrandt W. Lipid-protein interactions in crystals of plant light-harvesting complex. J Mol Biol 1993;234:347–56.10.1006/jmbi.1993.1591Suche in Google Scholar PubMed
[66] Liguori N, Periole X, Marrink SJ, Croce R. From light-harvesting to photoprotection: structural basis of the dynamic switch of the major antenna complex of plants (LHCII). Sci Rep 2015;5:15661.10.1038/srep15661Suche in Google Scholar PubMed PubMed Central
[67] Caffarri S, Kouřil R, Kereïche S, Boekema EJ, Croce R. Functional architecture of higher plant photosystem II supercomplexes. EMBO J 2009;28:3052–63.10.1038/emboj.2009.232Suche in Google Scholar PubMed PubMed Central
[68] Caffarri S, Broess K, Croce R, van Amerongen H, van Amerongen H. Excitation energy transfer and trapping in higher plant photosystem II complexes with different antenna sizes. Biophys J 2011;100:2094–103.10.1016/j.bpj.2011.03.049Suche in Google Scholar PubMed PubMed Central
[69] Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 2008;59:89–113.10.1146/annurev.arplant.59.032607.092759Suche in Google Scholar PubMed
[70] Bennett DIG, Amarnath K, Fleming GR. A structure-based model of energy transfer reveals the principles of light harvesting in photosystem II supercomplexes. J Am Chem Soc 2013;135:9164–73.10.1021/ja403685aSuche in Google Scholar PubMed
[71] Roden JJJ, Bennett DIG, Whaley KB. Long-range energy transport in photosystem II. J Chem Phys 2016;144:245101.10.1063/1.4953243Suche in Google Scholar PubMed
[72] Valkunas L, Chmeliov J, Trinkunas G, et al. Excitation migration, quenching, and regulation of photosynthetic light harvesting in photosystem II. J Phys Chem B 2011;115:9252–60.10.1021/jp2014385Suche in Google Scholar PubMed
[73] Chmeliov J, Gelzinis A, Songaila E, et al. The nature of self-regulation in photosynthetic light-harvesting antenna. Nat Plants 2016;2:16045.10.1038/nplants.2016.45Suche in Google Scholar PubMed
[74] Amarnath K, Bennett DIG, Schneider AR, Fleming GR. Multiscale model of light harvesting by photosystem II in plants. Proc Natl Acad Sci USA 2016;113:1156–61.10.1073/pnas.1524999113Suche in Google Scholar PubMed PubMed Central
©2017, J. Michael Gruber, et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Review articles
- Advances in optoplasmonic sensors – combining optical nano/microcavities and photonic crystals with plasmonic nanostructures and nanoparticles
- Periodic array-based substrates for surface-enhanced infrared spectroscopy
- From isolated light-harvesting complexes to the thylakoid membrane: a single-molecule perspective
- Theory and applications of toroidal moments in electrodynamics: their emergence, characteristics, and technological relevance
- Multiple exciton generation in quantum dot-based solar cells
- All-integrated terahertz modulators
- Exciton-plasmon coupling interactions: from principle to applications
- Emerging technologies for high performance infrared detectors
- Research articles
- Magneto-optical response in bimetallic metamaterials
- Saturated evanescent-wave absorption of few-layer graphene-covered side-polished single-mode fiber for all-optical switching
- Reversible thermochromic response based on photonic crystal structure in butterfly wing
- Selection rule engineering of forbidden transitions of a hydrogen atom near a nanogap
- Heterogeneous terahertz quantum cascade lasers exceeding 1.9 THz spectral bandwidth and featuring dual comb operation
- Effect of temperature on the structural, linear, and nonlinear optical properties of MgO-doped graphene oxide nanocomposites
- Engineering light emission of two-dimensional materials in both the weak and strong coupling regimes
- Anisotropic excitation of surface plasmon polaritons on a metal film by a scattering-type scanning near-field microscope with a non-rotationally-symmetric probe tip
- Achieving pattern uniformity in plasmonic lithography by spatial frequency selection
- Controllable all-fiber generation/conversion of circularly polarized orbital angular momentum beams using long period fiber gratings
- High-efficiency/CRI/color stability warm white organic light-emitting diodes by incorporating ultrathin phosphorescence layers in a blue fluorescence layer
- Relative merits of phononics vs. plasmonics: the energy balance approach
- Efficiency enhancement of InGaN amber MQWs using nanopillar structures
- Color display and encryption with a plasmonic polarizing metamirror
- Experimental demonstration of an optical Feynman gate for reversible logic operation using silicon micro-ring resonators
- Specialized directional beaming through a metalens and a typical application
- Anomalous extinction in index-matched terahertz nanogaps
Artikel in diesem Heft
- Review articles
- Advances in optoplasmonic sensors – combining optical nano/microcavities and photonic crystals with plasmonic nanostructures and nanoparticles
- Periodic array-based substrates for surface-enhanced infrared spectroscopy
- From isolated light-harvesting complexes to the thylakoid membrane: a single-molecule perspective
- Theory and applications of toroidal moments in electrodynamics: their emergence, characteristics, and technological relevance
- Multiple exciton generation in quantum dot-based solar cells
- All-integrated terahertz modulators
- Exciton-plasmon coupling interactions: from principle to applications
- Emerging technologies for high performance infrared detectors
- Research articles
- Magneto-optical response in bimetallic metamaterials
- Saturated evanescent-wave absorption of few-layer graphene-covered side-polished single-mode fiber for all-optical switching
- Reversible thermochromic response based on photonic crystal structure in butterfly wing
- Selection rule engineering of forbidden transitions of a hydrogen atom near a nanogap
- Heterogeneous terahertz quantum cascade lasers exceeding 1.9 THz spectral bandwidth and featuring dual comb operation
- Effect of temperature on the structural, linear, and nonlinear optical properties of MgO-doped graphene oxide nanocomposites
- Engineering light emission of two-dimensional materials in both the weak and strong coupling regimes
- Anisotropic excitation of surface plasmon polaritons on a metal film by a scattering-type scanning near-field microscope with a non-rotationally-symmetric probe tip
- Achieving pattern uniformity in plasmonic lithography by spatial frequency selection
- Controllable all-fiber generation/conversion of circularly polarized orbital angular momentum beams using long period fiber gratings
- High-efficiency/CRI/color stability warm white organic light-emitting diodes by incorporating ultrathin phosphorescence layers in a blue fluorescence layer
- Relative merits of phononics vs. plasmonics: the energy balance approach
- Efficiency enhancement of InGaN amber MQWs using nanopillar structures
- Color display and encryption with a plasmonic polarizing metamirror
- Experimental demonstration of an optical Feynman gate for reversible logic operation using silicon micro-ring resonators
- Specialized directional beaming through a metalens and a typical application
- Anomalous extinction in index-matched terahertz nanogaps


