Abstract
B7-H3 (CD276) is one of the immune checkpoint molecules at the forefront of cancer biology, plays a diverse role in immune regulation and cancer progression, while its immunosuppressive functions enable tumors to escape immune detection, its contribution to processes such as angiogenesis, metabolic reprogramming and chemoresistance underscores its broader impact on the tumor microenvironment (TME). These properties make B7-H3 an attractive target for cancer therapy. This perspective discusses the immune and non-immune related functions of B7-H3, the challenges in tapping its therapeutic potential.
Introduction
The B7 family is a group of structurally related immune checkpoint molecules which can transduce co-stimulatory and co-inhibitory signals [1]. Family members, such as B7-1 (CD80), B7-2 (CD86), PD-L1 (CD274) and B7-H3 (CD276), modulate T-cell activation and immune regulation. Though some, such as B7-1 and B7-2, are expressed mostly on antigen-presenting cells like dendritic cells and macrophages, others, like B7-H3 and B7-H6, are highly expressed by tumor cells and associated with immune evasion (Table 1) [2], 3]. B7-H3 displays a wide expression profile and performs multifunctional roles in tumor biology that distinguish it from other members of the B7 family, but its receptor is yet to be identified. B7-H3 was reported to be markedly overexpressed in the majority of cancers, including breast cancer, prostate cancer, lung cancer and colorectal cancer with limited expression in normal tissues [4]. It plays a complex role in tumor progression and immune evasion, in that it may stimulate or inhibit immune responses depending on the context. Besides its immunosuppressive action, B7-H3 has been implicated largely in tumor progression through its role in angiogenesis, metastasis, and resistance to chemotherapy [5]. This perspective discusses the molecular bases of B7-H3 functions, the possibility of therapeutic targeting of B7-H3 and their clinical implications regarding the potential impact they may have on the field of oncology.
Overview of B7 Family.
| B7 Molecule |
Alias | Receptor | Function | Expression |
|---|---|---|---|---|
| B7-1 | CD80 | CTLA-4, CD28 | Stimulatory/Inhibitory | DCs, macrophages, activated B cells |
| B7-2 | CD86 | CTLA-4, CD28 | Stimulatory/Inhibitory | DCs, macrophages, monocytes, activated B cells |
| B7-H1 | PD-L1, CD274 | PD-1 | Stimulatory | DCs, macrophages, activated CD4+ T cells, activated CD8+ T cells, tumor cells |
| B7-H2 | ICOS-L, CD275 | ICOS | Inhibitory | DCs, B cells, monocytes, tumor cells |
| B7-DC | PD-L2, CD273 | PD-1 | Inhibitory | DCs, macrophages, lung epithelial cells, tumor cells |
| B7-H3 | CD276 | Not identified | Stimulatory/Inhibitory | DCs, monocytes, activated CD4+ T cells, activated CD8+ T cells, tumor cells, endothelial cells |
| B7-H4 | B7x, B7S1, VTCN1 | BTLA | Inhibitory | DCs, macrophages, tumor-associated fibroblasts, tumor cells |
| B7-H5 | VISTA, Gi24 | PSGL-1 | Inhibitory | Macrophages, monocytes, neutrophils, CD4+ T cells, CD8+ T cells |
| B7-H6 | NCR3LG1 | NKp30 | Stimulatory | NK cells, tumor cells |
| B7-H7 | HHLA2 | CD28H | Stimulatory/Inhibitory | Macrophages, monocytes, activated CD4+ T cells, activated CD8+ T cells, tumor cells, endothelial cells |
-
This table provides a summary of key B7 family molecules, detailing their aliases, known receptors, and primary functions in immune modulation, and expression. DCs = dentritic cells.
Overview of B7-H3-Targeted Therapies.
| Agent | Strategy | Mechanism | Status | Advantages | Challenges |
|---|---|---|---|---|---|
| Enoblituzumab (MGA271) | Monoclonal antibodies | ADCC-mediated tumor cell lysis | Phase II trials | Specific, manageable safety | Limited efficacy as monotherapy |
| B7-H3 × CD28 B7-H3 × PD-L1 | Bispecific antibodies | Activates T cells, blocks immune escape pathways | Preclinical studies | Dual targeting, enhanced specificity | Complex design, off-target effects |
| B7-H3 × 4-1BB | Bispecific antibodies | Stimulates CD8+ TILs, leads to tumor regression | Preclinical studies | Enhances T-cell activation, better antitumor effect | Limited penetration, durability issues |
| TX103 | CAR-T therapy | T Cells targeting B7-H3 | Phase I/II trials | Tumor-specific, minimal toxicity | Tumor penetration, persistence issues |
| B7-H3-targeted NK cells | Adoptive NK cell therapy | Enhances cytotoxicity in non-small cell lung cancer | Preclinical studies | Amplified immune response | Delivery and expansion challenges |
| Ifinatamab deruxtecan (DS-7300) | ADC | Delivers topoisomerase I inhibitor to tumor cells | Phase III trials | Precise delivery, bystander effect, favorable safety | Limited validation in tumor types |
| MGC018 | ADC | Inhibits DNA replication in tumor cells | Phase I/II trials | Bystander effect | Limited in other cancer types |
| B7-H3-conjugated isotopes | Radioimmunotherapy | Delivers targeted radiation to tumor | Preclinical studies | Localized effects, tumor-specific | Radiotoxicity, penetration issues |
| B7-H3/Dox@GNCs | Nanoparticles | pH-responsive drug release in tumor environment | Preclinical studies | Enhanced targeting, reduced toxicity | Clearance by liver macrophages |
-
This table summarizes major therapeutic strategies targeting B7-H3; a description of their mechanisms of action and current clinical/preclinical status, together with advantages and challenges. ADCC = antibody-dependent cellular cytotoxicity; ADCs = antibody-drug conjugates; TILs = tumor-infiltrating lymphocytes; CAR-T = chimeric antigen receptor T cell therapy; NK = natural killer cells; NSCLC = non-small cell lung cancer; GNCs = gold nanocages; 4-1BB = a T-cell co-stimulatory receptor (CD137); PD-L1 = programmed death-ligand 1.
The immuno-suppressive role of B7-H3 in tumor microenvironments
B7-H3 inhibits CD8+ T-cell function by suppressing the production of granzyme B and IFN-γ, as demonstrated in ovarian and colorectal cancer models [6] (Figure 1). Recent studies show that mTORC1 directly upregulates the expression of B7-H3 could enhance its immunosuppressive function on anti-tumor T cells [7]. B7-H3 knockout mice harboring the E.G7 tumor showed a reduction of tumor-infiltrating natural killer (NK) cell activity has indicated by reduced expression of perforin and granzyme B. Anti-B7-H3 antibodies reduced tumor growth by boosting cytotoxic lymphocyte function [8]. In addition, it could also downregulate the STAT3/ULBP2 axis in colon cancer, resulting in reduced expression of ULBP2 and, consequently a reduction in γδ T-cell cytotoxicity [9]. B7-H3 deletion in trans-genic adenocarcinoma of the mouse prostate (TRAMP) mice led to enhanced infiltration and proliferation of FoxP3+ Tregs within tumors, accompanied by higher levels of IL-10 and TGF-β, while the expression of effector cytokines in CD8+ T cells was reduced [10]. B7-H3 increases the polarization of M2 macrophages through the CCL2-CCR2 axis, generating an immunosuppressive tumor microenvironment that increases the secretion of IL-10 and TGF-β [11] (Figure 1).
The non-immune role of B7-H3 in tumor microenvironments
Besides regulating immune cell function, B7-H3 contributes to tumor development by promoting epithelial-mesenchymal transition (EMT), metastasis, metabolic reprogramming and cell proliferation [12] (Figure 1). B7-H3 activates NF-κB and the mTOR pathway, promoting angiogenesis via vascular endothelial growth factor A (VEGF-A), which facilitates tumor survival and proliferation [7]. Soluble B7-H3 was identified in the plasma of healthy individuals and elevated in cancer patients [13]. Exosome B7-H3 activates the NF-κB pathway, increasing VEGFA expression and angiogenesis in colorectal cancer. B7-H3 knockdown reduced p-p65 phosphorylation, and NF-κB inhibition with BAY11–7082 decreased VEGFA expression and micro-vessel density [14]. B7-H3 regulates lipogenesis in lung cancer through the SREBP1/FASN pathway, with silencing B7-H3 leading to decreased SREBP1 and FASN levels, thus inhibiting lipid synthesis [15]. Additionally, B7-H3 stabilizes HIF-1α by increasing ROS, promoting glycolysis through HIF-1α targets like LDHA and PDK1 [16]. Thus, it supports tumor growth, acidification of TME, and immune impairment. Moreover, B7-H3 mediates chemotherapy resistance through the STAT3 and PI3K/Akt pathway and enhances anti-apoptotic protein expression like Bcl-2 and Mcl-1 [17].
Emerging therapeutic strategies targeting B7-H3 in cancer treatment
B7-H3 has emerged as a promising therapeutic target due to its overexpression in multiple cancer types and its low expression in normal tissues. Its role in immune evasion and tumor progression has led to several therapeutic strategies, including CAR-T cells, bispecific antibodies (BsAbs), antibody-drug conjugates (ADCs) and nanoparticle. However, while these approaches demonstrate potential, their efficacy often remains constrained by TME complexity (Table 2).
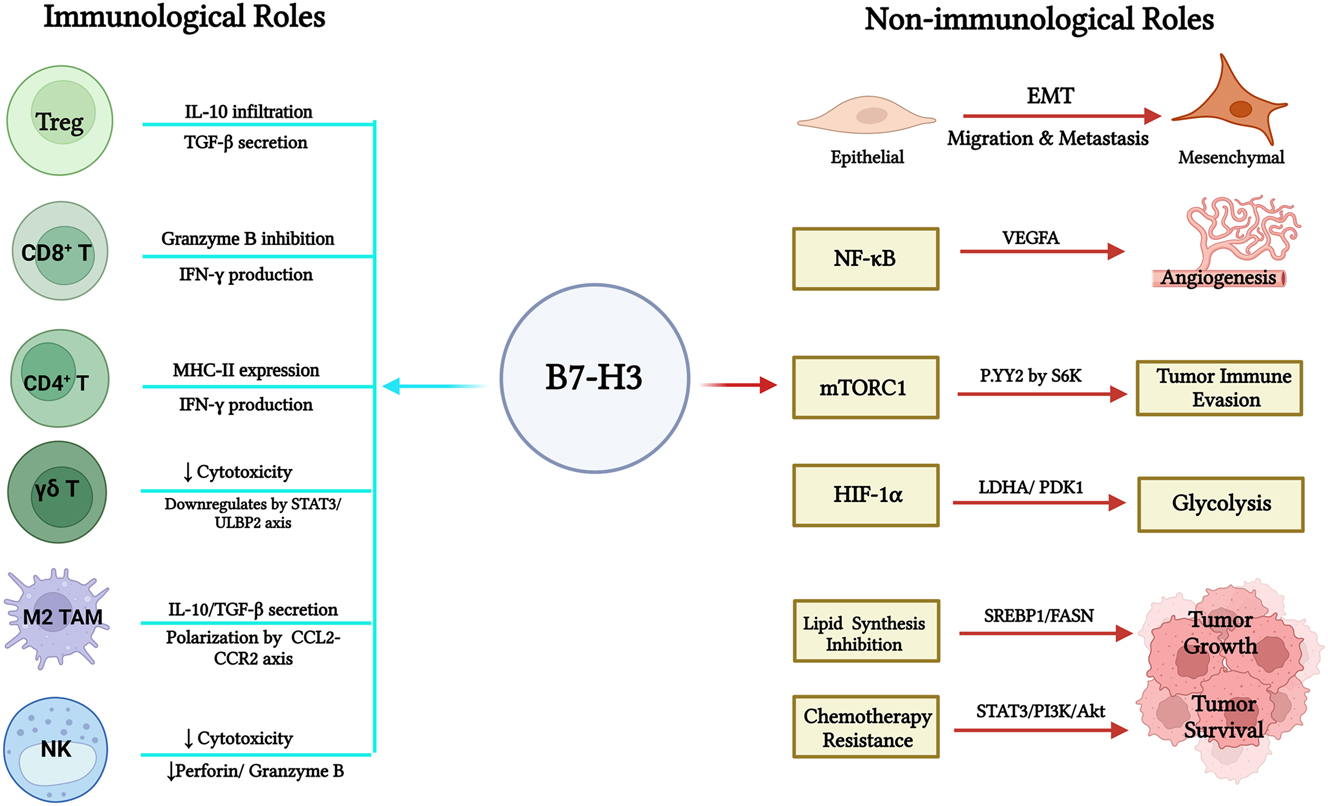
Roles of B7-H3 in tumor immunosuppression and progression. This figure shows the immunological and non-immunological roles of B7-H3 in tumor microenvironment. The left-hand side represents how B7-H3 exerts its immunosuppressive functions by interacting with T cells, marcophages and NK cells, hence promoting immune evasion. The right-hand side shows B7-H3 promotes tumor development through EMT, metabolic reprogramming, and activation of the NF-κB and mTOR pathways, enhancing angiogenesis, cell survival, and chemoresistance. EMT: Epithelial-mesenchymal transition.
Enoblituzumab (MGA271) is the first developed humanized IgG1 monoclonal antibody targeting against B7-H3. It induces antibody-dependent cellular cytotoxicity (ADCC) in B7-H3-positive tumors and has demonstrated encouraging safety and efficacy in clinical trials, Ifinatamab deruxtecan (DS-7300), a B7-H3-targeted ADC conjugated to a topoisomerase I inhibitor, exhibits potent antitumor activity against lung and breast cancer models while minimizing systemic toxicity [18]. While MGC018 is another duocarmycin-based ADC, uses a vc-seco-DUBA payload to inhibit DNA replication in tumor cells [19]. DS-7300 has shown promising results in small-cell lung cancer (SCLC), whereas MGC018 is more effective in breast and ovarian cancers. CAR-T therapies, such as TX103, show tumor infiltration and regression in glioblastoma and neuroblastoma but face challenges with stromal barriers and persistence [20]. There is no related death in TX103-CAR-T therapy. However, some adverse events and treatment-related AEs (AE) like cytokine release syndrome, increased intracranial pressure were experienced in patients [20]. Furthermore, adoptive transfer chimeric antigen receptor-modified NK cells targeting B7-H3 showed amplified cytotoxic effect against B7-H3 in non-small lung cancer cells, hence further indicating the vital role for B7-H3 in immune evading [21]. Bispecific antibodies targeting B7-H3 × CD28 enhance T-cell co-stimulation, improving immune responses, while other bi-specific antibodies targeting B7-H3 × PD-L1 show superior antitumor activity in head and neck squamous cell carcinoma (HNSCC) and laryngeal squamous cell carcinoma (LSCC) [22]. For B7-H3 × 4-1BB bispecific antibodies, stimulating CD8+ TILs leads to tumor regression in colorectal cancer, melanoma, and breast cancer models, with even greater antitumor effects when combined with PD-1 blockade [23]. Radioimmunotherapy conjugates radioactive isotopes to B7-H3 antibodies, achieving localized glioblastoma ablation but requiring better delivery systems to reduce radiotoxicity [24].
While there have been promising therapeutic developments, there are still important areas for improvement related to the complex role of B7-H3 in cancer progression. For example, Targeting B7-H3 alone does not completely eradicate tumor, one reason could be upregulation of IFN-γ-induced genes which have been shown to be involved in immunotherapy resistance [7]. The combination of B7-H3 inhibitors with drugs that target interconnected pathways like PD-1 or CTLA-4 may therefore show synergistic effects, but patient stratification and biomarker-driven approaches should be used to maximize such benefit. Secondly, many strategies focus on advanced-stage cancers, leaving early-stage applications underexplored. Enhancing real-time monitoring with delivery systems such as MMP-2-sensitive nanoparticles carrying anti-B7-H3 × CD3 bispecific antibodies and dEGCG (S-biAb/dEGCG@NPs) could enhance the precision of treatments and avoid unnecessary toxicities [25]. By addressing these challenges and further developing novel strategies, B7-H3 can transform its role from an emerging target to a cornerstone of cancer therapy.
Future directions
Identifying receptors is still a top priority for understanding B7-H3’s mechanism of action. Combination therapies that target B7-H3, along with other immune checkpoint inhibitors, metabolic modulators, or angiogenesis inhibitors, may achieve greater therapeutic efficacy and potentially overcome certain therapeutic resistance. Assessing relevant biomarker responses could help predict outcomes and guide treatment strategies. Artificial intelligence and big data analytics can also be further used to optimize patient stratification and make B7-H3-targeting therapies much more precise and effective.
B7-H3 exemplifies complex immune checkpoint biology, demonstrating both cell-autonomous and non-cell-autonomous functions. While challenges are being acknowledged in identifying its receptors and resistance to therapy, given its wide expression pattern, B7-H3 still represents an intriguing cancer therapy target.
-
Research ethics: This study did not involve human subjects, and ethical approval was not required for animal studies.
-
Informed consent: Not applicable as this study did not involve human participants.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. HLF and HJL contributed to manuscript writing. YQL and GGW contributed to manuscript editing.
-
Use of Large Language Models, AI and Machine Learning Tools: Not applicable.
-
Conflict of interest: No conflict of interest
-
Research funding: Not applicable.
-
Data availability: Not applicable.
References
1. Chen, L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004;4:336–47. https://doi.org/10.1038/nri1349.Search in Google Scholar PubMed
2. Suh, WK, Gajewska, BU, Okada, H, Gronski, MA, Bertram, EM, Dawicki, W, et al.. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol 2003;4:899–906. https://doi.org/10.1038/ni967.Search in Google Scholar PubMed
3. Greenwald, RJ, Freeman, GJ, Sharpe, AH. The B7 family revisited. Annu Rev Immunol 2005;23:515–48. https://doi.org/10.1146/annurev.immunol.23.021704.115611.Search in Google Scholar PubMed
4. Koumprentziotis, IA, Theocharopoulos, C, Foteinou, D, Angeli, E, Anastasopoulou, A, Gogas, H, et al.. New emerging targets in cancer immunotherapy: the role of B7-H3. Vaccines 2024;12:54. https://doi.org/10.3390/vaccines12010054.Search in Google Scholar PubMed PubMed Central
5. Lee, YH, Martin-Orozco, N, Zheng, P, Li, J, Zhang, P, Tan, H, et al.. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res 2017;27:1034–45. https://doi.org/10.1038/cr.2017.90.Search in Google Scholar PubMed PubMed Central
6. Lu, H, Shi, T, Wang, M, Li, X, Gu, Y, Zhang, X, et al.. B7-H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. OncoImmunology 2020;9:1748991. https://doi.org/10.1080/2162402x.2020.1748991.Search in Google Scholar PubMed PubMed Central
7. Liu, HJ, Du, H, Khabibullin, D, Zarei, M, Wei, K, Freeman, GJ, et al.. mTORC1 upregulates B7-H3/CD276 to inhibit antitumor T cells and drive tumor immune evasion. Nat Commun 2023;14:1214. https://doi.org/10.1038/s41467-023-36881-7.Search in Google Scholar PubMed PubMed Central
8. Tyagi, A, Ly, S, El-Dana, F, Yuan, B, Grimm, S, Bühring, HJ, et al.. Anti-B7-H3 antibody (T-1A5) blocks immunomodulatory function of B7-H3 and enhances NK cell-mediated cytotoxicity against acute myeloid leukemia cells. Blood 2021;138(Supplement 1):3336. https://doi.org/10.1182/blood-2021-151535.Search in Google Scholar
9. Lu, H, Ma, Y, Wang, M, Shen, J, Wu, H, Li, J, et al.. B7-H3 confers resistance to Vγ9Vδ2 T cell-mediated cytotoxicity in human colon cancer cells via the STAT3/ULBP2 axis. Cancer Immunol Immunother 2021;70:1213–26. https://doi.org/10.1007/s00262-020-02771-w.Search in Google Scholar PubMed PubMed Central
10. Delfino, T, Wang, Y, Schartner, J, Rutz, S. Targeted ablation of FoxP3+ T cells activates peripheral and tumor-infiltrating cytotoxic CD8+ T cells in multiple syngeneic mouse tumor models. J Immunol 2019;202(1_Supplement):134.18. https://doi.org/10.4049/jimmunol.202.supp.134.18.Search in Google Scholar
11. Miyamoto, T, Murakami, R, Hamanishi, J, Tanigaki, K, Hosoe, Y, Mise, N, et al.. B7-H3 suppresses antitumor immunity via the CCL2-CCR2-M2 macrophage Axis and contributes to ovarian cancer progression. Cancer Immunol Res 2022;10:56–69. https://doi.org/10.1158/2326-6066.cir-21-0407.Search in Google Scholar
12. Feng, R, Chen, Y, Liu, Y, Zhou, Q, Zhang, W. The role of B7-H3 in tumors and its potential in clinical application. Int Immunopharmacol 2021;101:108153. https://doi.org/10.1016/j.intimp.2021.108153.Search in Google Scholar PubMed
13. Zhao, L, Xie, C, Liu, D, Li, T, Zhang, Y, Wan, C. Early detection of hepatocellular carcinoma in patients with hepatocirrhosis by soluble B7-H3. J Gastrointest Surg 2017;21:807–12. https://doi.org/10.1007/s11605-017-3386-1.Search in Google Scholar PubMed
14. Wu, R, Zhang, Y, Xu, X, You, Q, Yu, C, Wang, W, et al.. Exosomal B7-H3 facilitates colorectal cancer angiogenesis and metastasis through AKT1/mTOR/VEGFA pathway. Cell Signal 2023;109:110737. https://doi.org/10.1016/j.cellsig.2023.110737.Search in Google Scholar PubMed
15. Luo, D, Xiao, H, Dong, J, Li, Y, Feng, G, Cui, M, et al.. B7-H3 regulates lipid metabolism of lung cancer through SREBP1-mediated expression of FASN. Biochem Biophys Res Commun 2017;482:1246–51. https://doi.org/10.1016/j.bbrc.2016.12.021.Search in Google Scholar PubMed
16. Lim, S, Liu, H, Madeira Da Silva, L, Arora, R, Liu, Z, Phillips, JB, et al.. Immunoregulatory protein B7-H3 reprograms glucose metabolism in cancer cells by ROS-mediated stabilization of HIF1α. Cancer Res 2016;76:2231–42. https://doi.org/10.1158/0008-5472.CAN-15-1538.Search in Google Scholar PubMed PubMed Central
17. Chen, G, Park, D, Magis, AT, Behera, M, Ramalingam, SS, Owonikoko, TK, et al.. Mcl-1 interacts with akt to promote lung cancer progression. Cancer Res 2019;79:6126–38. https://doi.org/10.1158/0008-5472.can-19-0950.Search in Google Scholar
18. Aggarwal, C, Prawira, A, Antonia, S, Rahma, O, Tolcher, A, Cohen, RB, et al.. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer 2022;10. https://doi.org/10.1136/jitc-2021-004424.Search in Google Scholar PubMed PubMed Central
19. Scribner, JA, Brown, JG, Son, T, Chiechi, M, Li, P, Sharma, S, et al.. Preclinical development of MGC018, a duocarmycin-based antibody-drug conjugate targeting B7-H3 for solid cancer. Mol Cancer Therapeut 2020;19:2235–44. https://doi.org/10.1158/1535-7163.mct-20-0116.Search in Google Scholar
20. Zhang, Y, Feng, R, Chi, X, Xian, N, Chen, X, Huang, N, et al.. Safety and efficacy of B7-H3 targeting CAR-T cell therapy for patients with recurrent GBM. J Clin Oncol 2024;42(16):2062. https://doi.org/10.1200/jco.2024.42.16_suppl.2062.Search in Google Scholar
21. Yang, S, Cao, B, Zhou, G, Zhu, L, Wang, L, Zhang, L, et al.. Targeting B7-H3 immune checkpoint with chimeric antigen receptor-engineered natural killer cells exhibits potent cytotoxicity against non-small cell lung cancer. Front Pharmacol 2020;11:1089. https://doi.org/10.3389/fphar.2020.01089.Search in Google Scholar PubMed PubMed Central
22. Dong, Y, Zhang, Z, Luan, S, Zheng, M, Wang, Z, Chen, Y, et al.. Novel bispecific antibody-drug conjugate targeting PD-L1 and B7-H3 enhances antitumor efficacy and promotes immune-mediated antitumor responses. J Immunother Cancer 2024;12. https://doi.org/10.1136/jitc-2024-009710.Search in Google Scholar PubMed PubMed Central
23. You, G, Lee, Y, Kang, YW, Park, HW, Park, K, Kim, H, et al.. B7-H3×4-1BB bispecific antibody augments antitumor immunity by enhancing terminally differentiated CD8(+) tumor-infiltrating lymphocytes. Sci Adv 2021;7:eaax3160. https://doi.org/10.1126/sciadv.aax3160.Search in Google Scholar PubMed PubMed Central
24. Zheng, M, Wang, Y, Fu, F, Zhang, K, Zhao, S, Liu, Q, et al.. Radioimmunotherapy Targeting B7-H3 in situ glioma models enhanced antitumor efficacy by Reconstructing the tumor microenvironment. Int J Biol Sci 2023;19:4278–90. https://doi.org/10.7150/ijbs.87763.Search in Google Scholar PubMed PubMed Central
25. Fan, R, Chen, C, Mu, M, Chuan, D, Liu, H, Hou, H, et al.. Engineering MMP-2 activated nanoparticles carrying B7-H3 bispecific antibodies for ferroptosis-enhanced glioblastoma immunotherapy. ACS Nano 2023;17:9126–39. https://doi.org/10.1021/acsnano.2c12217.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Immune cells in Alzheimer’s disease: insights into pathogenesis and potential therapeutic targets
- Selenium compounds for cancer prevention and therapy – human clinical trial considerations
- Ventricular ion channels and arrhythmias: an overview of physiology, pathophysiology and pharmacology
- Yoga and chronic diseases: an umbrella review of systematic reviews and meta-analyses
- Perspectives
- Histone lactylation as a driver of metabolic reprogramming and immune evasion
- From tumor immunity to precision medicine: the next step in B7-H3/CD276 research
Articles in the same Issue
- Frontmatter
- Reviews
- Immune cells in Alzheimer’s disease: insights into pathogenesis and potential therapeutic targets
- Selenium compounds for cancer prevention and therapy – human clinical trial considerations
- Ventricular ion channels and arrhythmias: an overview of physiology, pathophysiology and pharmacology
- Yoga and chronic diseases: an umbrella review of systematic reviews and meta-analyses
- Perspectives
- Histone lactylation as a driver of metabolic reprogramming and immune evasion
- From tumor immunity to precision medicine: the next step in B7-H3/CD276 research

