Abstract
Three tricyclohexyltin aryloxyacetates, p-YC6H4OCH2COOSn(C6H11-c)3 (where Y=H, 1; CHO, 2; CH2OH, 3), have been synthesized and characterized by means of elemental analysis, IR, and NMR (1H, 13C, and 119Sn) spectroscopy. The crystal structures of complexes 1 and 3 are determined by X-ray single-crystal diffraction. The carboxylate in the compounds is monodentate. The tin atom of compound 1 adopts distorted tetrahedral coordination geometry. In the crystal lattice of compound 3, there is a four-coordinated tin and a five-coordinated tin in which the fifth coordination site is occupied by a water molecule, and the molecules are linked by R33(30) and R55(28) hydrogen bonds into a two-dimensional supramolecular network. Bioassay results have shown that the compounds have good in vitro antibacterial activity against Escherichiacoli.
Introduction
Organotin carboxylates have been widely researched because of their novel structures and various applications in the last few decades (Tiekink, 1991, 1994; Davies et al., 2008). Many organotin carboxylates display good activities against tumors, fungi, bacteria, and other microorganisms (Davies et al., 2008; Hadjikakou and Hadjiliadis, 2009; Shang et al., 2011; Amir et al., 2014; Carraher and Roner, 2014; Wang et al., 2014; Mao et al., 2015). The organotin moiety and ligands appear to play an important role in determining their biological activity (Davies et al., 2008; Hadjikakou and Hadjiliadis, 2009; Amir et al., 2014). In general, the toxicity of organotin compounds seems to increase with the chain length of the organic alkyl groups, which are often more active than aryl ones. To design new active tin compounds, it is necessary to balance some factors such as solubility and lipophilicity to achieve efficacy (Hadjikakou and Hadjiliadis, 2009; Arjmand et al., 2014).
Aryloxyacetic acid derivatives possess a wide array of diverse bioactivities such as antimicrobacterial, anti-inflammatory, antibacterial, analgesic, antisickling, antipaemic, antiplatelet, non-prostanoid prostacyclin mimetic, diuretic, and growth regulators (Fracchiolla et al., 2007; Bala et al., 2010; Kumar et al., 2013). The synthesis, structure, and property of some organotin complexes with ligands have been reported, such as dibutyltin bis(2-naphthoxyacetate) (Ma et al., 2004), tris(2-methyl-2-phenylpropyl)tin phenoxyacetate (Bao et al., 1998), tributyltin 2,4-dichlorophenoxyacetate (Yu et al., 2010), and triphenyltin 8-quinolyloxyacetate (Das et al., 1987). To continue to increase the chemistry and therapeutic potential of the organotin complexes of aryloxyacetic acids, we select phenoxyacetic acid with polar substituent groups as ligands (Scheme 1) and synthesized three new tricyclohexyltin complexes, p-YC6H4OCH2COOSn(C6H11-c)3 (where Y=H, CHO, and CH2OH), and determined their in vitro antibacterial activity.

The structure of the ligand.
Results and discussion
Synthesis
The reaction of tricyclohexyltin hydroxide with phenoxyacetic acid or p-substituted phenoxyacetic acid in a 1:1 molar ratio in anhydrous benzene afforded products 1–3, with a yield of 65–81% (Scheme 2).

Synthesis of compounds 1–3.
These compounds are white crystals, air stable, and soluble in benzene and in common polar organic solvents such as methanol, ethanol, dichloromethane, chloroform, acetone, and N,N-dimethylformamide, but insoluble in water and saturated hydrocarbons such as hexane and petroleum ether.
Spectroscopic analysis
In the IR spectra of compounds 1–3, the bands at ~3450 and ~1760 cm-1 assigned to ν (O-H) and ν (C=O), respectively, of free aryloxyacetic acid do not appear, and new strong bands at ~1660 and ~1350 cm-1 are assigned to the asymmetrical, νas(CO2-), and symmetrical stretching vibrations, νs(CO2-), of the aryloxyacetate, respectively. The difference between the νas(CO2-) and the νs(CO2-) bands, Δν(CO2-), is 313 cm-1 for 1, 272 cm-1 for 2, and 331 cm-1 for 3, which is larger than 200 cm-1, suggesting that the carboxylate group is coordinated to tin in the monodentate mode in the solid state (Deacon and Phillips, 1980; Li et al., 2010).
In 1H NMR spectra of 1–3, the single resonances of COOH in the spectra of the free ligands are not observed at ~12 ppm, which further confirms the replacement of the carboxylic acid protons by the tricyclohexyltin moiety on complex formation. The complexes show multiplets in the range of 1.17–1.97 ppm due to the cyclohexyl protons and singlets at 4.43–4.71 ppm assigned to the OCH2 protons. The resonances in the range between 6.76 and 7.82 ppm are assigned to the aryl protons.
The 13C chemical shifts of the carboxyl and methylene carbon atoms in 1–3 appear at 172.21–173.36 and 65.56–66.41 ppm, respectively. The signals of the cyclohexyl carbon atoms are in the range of 26.97–36.49 ppm, and the 1J(119Sn-13C), 2J(119Sn-13C), and 3J(119Sn-13C) coupling constants are ~330, 20, and 60 Hz, respectively. The coordination number of the tin atom in the organotin compounds has been related to the 1J(119Sn-13C) coupling constants (Nadvornik et al., 1984). The 1J(119Sn-13C) coupling of the compounds is close to that of other four-coordinate tricyclohexyltin carboxylates, such as 2-HOC6H4N=NC6H4COOSn(C6H11-c)3 (335 Hz) (Willem et al., 1998), C4H3SCOOSn(C6H11-c)3 (333 Hz) (Abbas et al., 2013), (c-C6H11)3SnO2CCH2CH2COCH2CH2CO2Sn(C6H11-c)3 (325 Hz) (Chalupa et al., 2006), and (2-C6H5C2HN3)COOSn(C6H11-c)3 (330 Hz) (Tian et al., 2015). Data suggest that the tin atom in these compounds is four-coordinated in CDCl3 solution.
The 119Sn chemical shifts primarily depend on the coordination number and the nature of the donor atom directly bonded to the central tin atom (Davis, 2004). The 119Sn chemical shifts of 1–3 (30.0–33.0 ppm) are in accord with the values of the four-coordinated tricyclohexyltin carboxyl ester in the solution of the non-coordinating solvent (Tian et al., 2005, 2015; Chalupa et al., 2006).
Structure analysis of 1 and 3
The structures of complexes 1 and 3 are shown in Figures 1–3, and the selected geometric parameters are given in Table 1. Compound 1 crystallizes with two independent molecules in the crystallographic asymmetric unit that do not differ from each other significantly. The coordination geometry of the tin atom is a distorted tetrahedron shaped by three carbon atoms from the cyclohexyl groups and one carboxyl O atom [O(1) and O(4)] from the carboxylate ligand. The separation between the other O atom of the carboxylate ligand and Sn atom is Sn(1)···O(2) 3.017(4) Å and Sn(2)···O(5) 2.994(4) Å. The weak interaction distorts the tetrahedral geometry by opening up the C(1)-Sn(1)-C(13) and C(33)-Sn(2)-C(39) to 117.2(4)° and 121.1(3)°, respectively, and reducing the O(1)-Sn(1)-C(7) and O(5)-Sn(2)-C(27) to 94.0(2)° and 95.65(17)°, respectively. The monodentate mode of the carboxylate coordination is expressed in two disparate C-O bond distances (Table 1). The four bond distances around Sn are similar to those found in other reported tricyclohexyltin carboxylates, such as tricyclohexyltin indole-3-acetate (Molloy et al., 1986), 2-(4-chlorophenyl)-3-methylbutyrate (Song et al., 2003), and ferrocene carboxylate (Dong et al., 2014).

The molecular structure of 1.
Hydrogen atoms are omitted for clarity.
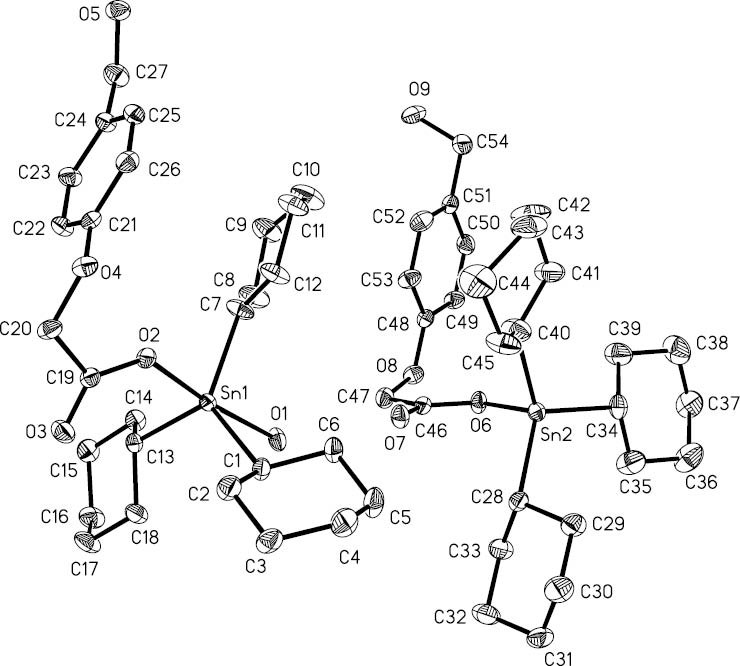
The molecular structure of 3.
Hydrogen atoms are omitted for clarity.

The 2D supramolecular network of 3 formed by the intermolecular O-H···O hydrogen bonds.
Selected bond lengths (Å) and angles (°) for compound 1 and 3.
| 1 | |||||
|---|---|---|---|---|---|
| Sn(1)-O(1) | 2.052(3) | Sn(2)-O(4) | 2.062(3) | C(19)-O(2) | 1.206(6) |
| Sn(1)-C(1) | 2.122(6) | Sn(1)-C(27) | 2.151(5) | C(19)-O(1) | 1.294(6) |
| Sn(1)-C(7) | 2.137(7) | Sn(1)-C(33) | 2.160(5) | C(45)-O(5) | 1.210(7) |
| Sn(1)-C(13) | 2.143(7) | Sn(1)-C(39) | 2.139(6) | C(45)-O(4) | 1.282(6) |
| O(1)-Sn(1)-C(1) | 107.8(2) | C(1)-Sn(1)-C(13) | 117.2(4) | O(4)-Sn(2)-C(33) | 106.43(19) |
| O(1)-Sn(1)-C(7) | 94.0(2) | C(7)-Sn(1)-C(13) | 116.7(3) | C(39)-Sn(2)-C(27) | 110.1(3) |
| O(1)-Sn(1)-C(13) | 107.0(2) | O(4)-Sn(2)-C(39) | 107.0(2) | C(39)-Sn(2)-C(33) | 121.1(3) |
| C(1)-Sn(1)-C(7) | 111.1(3) | O(4)-Sn(2)-C(27) | 95.65(17) | C(27)-Sn(2)-C(33) | 113.2(2) |
| 3 | |||||
| Sn(1)-O(1) | 2.432(4) | Sn(2)-O(6) | 2.081(5) | C(19)-O(2) | 1.287(7) |
| Sn(1)-O(2) | 2.175(4) | Sn(2)-C(28) | 2.148(6) | C(19)-O(3) | 1.222(7) |
| Sn(1)-C(1) | 2.159(6) | Sn(2)-C(34) | 2.127(6) | C(46)-O(6) | 1.282(7) |
| Sn(1)-C(7) | 2.132(5) | Sn(2)-C(40) | 2.126(7) | C(46)-O(7) | 1.221(7) |
| Sn(1)-C(13) | 2.143(5) | ||||
| C(7)-Sn(1)-C(13) | 119.2(2) | C(7)-Sn(1)-O(1) | 87.4(2) | O(6)-Sn(2)-C(34) | 97.3(2) |
| C(7)-Sn(1)-C(1) | 123.0(3) | C(13)-Sn(1)-O(1) | 85.81(18) | C(40)-Sn(2)-C(34) | 114.5(3) |
| C(13)-Sn(1)-C(1) | 116.2(2) | C(1)-Sn(1)-O(1) | 84.3(2) | O(6)-Sn(2)-C(28) | 107.8(2) |
| C(7)-Sn(1)-O(2) | 87.4(2) | O(2)-Sn(1)-O(1) | 174.52(15) | C(40)-Sn(2)-C(28) | 116.9(3) |
| C(13)-Sn(1)-O(2) | 98.34(19) | O(6)-Sn(2)-C(40) | 102.1(3) | C(34)-Sn(2)-C(28) | 115.0(3) |
| C(1)-Sn(1)-O(2) | 96.9(2) | ||||
Compound 3 crystallizes in the monoclinic space group P21, and the crystal structure reveals that there are two crystallographically non-equivalent molecules in the crystallographic asymmetric unit. The tin atom Sn(1) of one molecule (A) is five-coordinated, and the tin atom Sn(2) of the other molecule (B) is four-coordinated. In A, the coordination geometry of the tin center displays a distorted trans-O2SnC3 trigonal bipyramid with three carbon atoms [C(1), C(7), and C(13)] of cyclohexyl groups defining the trigonal plane and a unidentate carboxylate O(2) and a O(1) atom from the water molecule occupying the axial positions. The C-Sn-C angles were in the range of 116.2(2)–123.0(3)°. The O(1)-Sn(1)-O(2) angle is 174.52(15)°, which is similar to that observed in related tricyclohexyltin analogues, such as 3-C5H4NCO2Sn(C6H11-c)3(H2O) [176.11(11)°] (Teoh et al., 1999), (4-MeC6H4)3GeCH(C6H4OMe-4)CH2CO2Sn(C6H11-c)3(H2O) [170.35(6)°] (Din et al., 2003), and 2-Cl-3-C5H4NCO2Sn(C6H11-c)3(H2O) [176.40(11)°] (Yu et al., 2012). The bond length of Sn(1)-O(1) [2.432(4) Å] is significantly longer than that of Sn(1)-O(2) [2.175(4)Å], so that the Sn(1) atom is displaced out of the C3 trigonal plane of the trans-C3SnO2 trigonal bipyramidal polyhedron in the direction of O(2) by 0.155(2) Å.
The structure of molecule B is similar to compound 1, and Sn(2) has also a distorted tetrahedral geometry with the angles range of 97.3(2)–116.9(3)°. As expected, the C-O bond distances [C(19)-O(3) 1.222(7) Å and C(46)-O(7) 1.221(7) Å] associated with the non-coordinating carbonyl O atoms are shorter than the coordinating C-O bond distances [C(19)-O(2) 1.287(7) Å and C(46)-O(6) 1.282(7) Å]. Although not involved in coordination to tin, the O(3) and O(7) atoms form significant intermolecular contacts in the crystal lattice. Through the coordinated water molecules and the hydroxyl groups of ligands, the monomeric structures (A and B) get into contact with each other via hydrogen bonds (Table 2), and a two-dimensional (2D) network containing R33(30) and R55(28) hydrogen bonding patterns is formed (Figure 3).
Hydrogen bonds in compound 3.
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | D-H···A (°) | Symmetry code |
|---|---|---|---|---|---|
| O(1)-H(1A)···O(7) | 0.85 | 1.987 | 2.744(6) | 147.8 | |
| O(5)-H(5)···O(3)#1 | 0.82 | 1.874 | 2.655(7) | 158.7 | #1: x-1, y, z |
| O(1)-H(1B)···O(9)#2 | 0.85 | 1.817 | 2.656(7) | 169.0 | #2: x+1, y, z |
| O(9)-H(9)···O(5)#3 | 0.82 | 1.963 | 2.708(7) | 150.8 | #3: -x, y-1/2, -z+2 |
Antibacterial activity
The antibacterial activities of the compounds and the reference drug (penicillin sodium and cefazolin sodium) are listed in Table 3. Results show that complexes 1–3 is active against Escherichia coli, which is comparable with the reported tricyclohexyltin 2-phenyl-1,2,3-triazole-4-carboxylate (MIC=13.50 μg/mL) (Tian et al., 2005) and ferrocenecarboxylate (MIC=23.98 μg/mL) (Dong et al., 2014). The activity of the three compounds against E. coli decreased in the order 3>2>1 under experimental conditions. However, the activity is lower than that of the reference drugs.
Antibacterial activity (MIC, μg/mL) of the synthesized compounds.
| Compound | Penicillin sodium | Cefazolin sodium | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| E. coli | 25.22 | 16.50 | 14.78 | 8.03 | 2.01 |
Conclusion
Three tricyclohexyltin aryloxyacetates have been synthesized from triorganotin hydroxide and aryloxyacetic acid and characterized. In both the solid state and the CDCl3 solution, the carboxylate moiety coordinates the tin center in a monodentate fashion. The tin atom of compound 1 possesses a distorted tetrahedral geometry. In the crystal lattice of compound 3, there is a four-coordinated tin and a five-coordinated tin possessing a trigonal bipyramidal environment with the axial positions occupied by a carboxylate oxygen atom and an oxygen atom of a water molecule and the 2D supramolecular network is formed by R33(30) and R55(28) hydrogen bonds. In the CDCl3 solution, the tin atoms of the compounds are all four-coordinated. The compounds have activity against E. coli and can be considered as antibacterial compounds to further study.
Experimental
General
All chemicals used in the syntheses were of analytical grade and were purchased from commercial sources (Sinopharm Chemical Reagent Company, Shanghai, China) and used as received. Carbon and hydrogen analyses were determined using a Perkin Elmer 2400 Series II elemental analyzer (Perkin Elmer, Waltham, MA, USA). The IR spectra were recorded on a Nicolet Nexus 470 FT-IR spectrophotometer using KBr discs in the range of 4000–400 cm-1 (Thermo Nicolet, Madison, WI, USA). 1H and 13C NMR spectral data were collected using a Bruker Avance DPX300 NMR spectrometer (Bruker, Fallanden, Switzerland) with CD3SOCD3 or CDCl3 as solvent and TMS as internal standard. 119Sn NMR spectra were recorded in CDCl3 on a Varian Mercury Vx300 spectrometer. Me4Sn was used as external reference (Varian, Palo Alto, USA).
Synthesis of ligands
Synthesis of p-formylphenoxyacetic acid:
The ligand was prepared by a modified literature procedure (Liu et al., 2005). To a stirred solution of p-hydroxybenzaldehyde (1.22 g, 10 mmol) and bromoacetic acid (1.67 g, 12 mmol) in water (20 mL) was dropwise added 20 mL water solution of sodium hydroxide (0.96 g, 24 mmol) within 30 min at room temperature. Stirring of the reaction mixture continued for 1.5 h under reflux and then cooled to room temperature. The solution was acidified with concentrated hydrochloric acid to pH 1–2, and the resulting solid was filtered and recrystallized from 95% alcohol to give p-formylphenoxyacetyl acid as a white solid (1.40 g, 78%); m.p. 200–201°C. Anal. calcd. for C9H8O4 (%): C, 60.00; H, 4.48. Found: C, 59.94; H, 4.42. IR (KBr): 3460 (br, OH), 1755 (COOH), 1718 (CHO), 1226 (Ar-O) cm-1. 1H NMR (CD3SOCD3) δ 12.46 (s, 1H, COOH), 9.85 (s, 1H, CHO), 7.80 (d, J=8.4 Hz, 2H, m-H-C6H4), 7.08 (d, J=8.4 Hz, 2H, o-H-C6H4), 4.81 (s, 2H, OCH2) ppm.
Synthesis of p-(hydroxymethyl)phenoxyacetic acid:
To a solution of p-formylphenoxyacetic acid (1.80 g, 10 mmol) in water (15 mL) containing KOH (0.56 g, 10 mmol) was slowly added an excess of sodium borohydride (0.46 g, 12 mmol) in water (10 mL) containing a few drops of sodium hydroxide solution within 15 min in an ice bath. The reactive mixture was stirred for 1 h at room temperature and then acidified with concentrated HCl to a pH of 2–3. The resulting white solid was filtered off, washed with cold water, dried, and was recrystallized from ethyl acetate/n-hexane (1:1, v/v). Yield 1.56 g (86%); m.p. 116–117°C. Anal. calcd. for C9H10O4 (%): C, 59.34; H, 5.53. Found: C, 59.36; H, 5.56. IR (KBr): 3500 (br, OH), 1761 (COOH), 1235 (Ar-O) cm-1. 1H NMR (CD3SOCD3) δ 12.31 ((s, 1H, COOH), 7.22 (d, J=8.0 Hz, 2H, m-H-C6H4), 6.82 (d, J=8.0 Hz, 2H, o-H-C6H4), 4.85 (s, 1H, OH), 4.50 (s, 2H, OCH2), 4.42 (s, 2H, OCH2Ar) ppm.
Synthesis of the complexes
Tricyclohexyltin phenoxyacetate (1):
To a suspension of tricyclohexyltin hydroxide (0.77 g, 2 mmol) in 50 mL of benzene was added phenoxyacetic acid (0.30 g, 2 mmol). Under magnetic stirring, the reaction mixture heated at reflux for 5 h with a Dean-Stark separator and then allowed to cool to room temperature. The solution was filtered, and the solvent was removed under reduced pressure by a rotary evaporator. The resulting white solid was recrystallized from methanol and dried in a vacuum dryer for 24 h to afford colorless crystal of 1 (0.81 g, 78%); m.p. 70–71°C. Anal. calcd. for C26H40O3Sn (%): C, 60.13; H, 7.76. Found: C, 59.94; H, 7.52. IR (KBr): 1665 [ν(COO-)as], 1352 [ν(COO-)s] cm-1. 1H NMR (CDCl3) δ 7.27 (t, 2H, m-H-C6H5), 7.10–6.89 (m, 3H, o-H-, p-H-C6H5), 4.56 (s, 2H, OCH2), 1.21–1.90 (m, 33H, c-C6H11) ppm. 13C NMR (CDCl3) δ 173.36 (COOSn), 158.17 (i-C-C6H5), 129.26 (m-C-C6H5), 121.04 (p-C-C6H5), 114.56 (o-C-C6H5), 65.56 (OCH2), 34.04 [1J(119Sn-13C)=332 Hz, C-α], 31.01 [2J(119Sn-13C)=20 Hz, C-β], 28.86 [3J(119Sn-13C)=62 Hz, C-γ], 26.97 (C-δ) ppm. 119Sn NMR (CDCl3) δ 30.0 ppm.
Tricyclohexyltin p-formylphenoxyacetate (2):
This compound was prepared in the same way as 1 by the reaction of tricyclohexyltin hydroxide (0.77 g, 2 mmol) with p-formylphenoxyacetic acid (0.36 g, 2 mmol). Yield 0.71 g (65%); m.p. 88–89°C. Anal. calcd for C27H40O4Sn: C, 59.25; H, 7.37. Found: C, 59.19; H, 7.25. IR (KBr): 1720 (CHO), 1668 [ν(COO-)as], 1396 [ν(COO-)s] cm-1, 1H NMR (CDCl3) δ 9.86 (1H, s, CHO), 7.82 (d, J=8.2 Hz, 2H, m-H-C6H4), 7.01 (d, J=8.2 Hz, 2H, o-H-C6H4), 4.71 (s, 2H, OCH2), 1.30–1.97 (m, 33H, c-C6H11). 13C NMR (CDCl3) δ 191.76 (CH=O), 172.21 (COOSn), 164.87 (i-C-C6H4), 131.92 (m-C-C6H4), 129.93 (p-C-C6H4), 114.48 (o-C-C6H4), 65.76 (OCH2), 34.12 [1J(119Sn-13C)=320 Hz, C-α], 30.92 [2J(119Sn-13C)=20 Hz, C-β], 28.83 [3J(119Sn-13C)=62 Hz, C-γ], 27.54 (C-δ) ppm. 119Sn NMR (CDCl3) δ 33.0 ppm.
Tricyclohexyltin p-(hydroxymethyl)phenoxyacetate (3):
Complex 3 was prepared by the same procedure as 1 by the reaction of tricyclohexyltin hydroxide (0.77 g, 2 mmol) with p-(hydroxymethyl)phenoxyacetic acid (0.36 g, 2 mmol). Yield 0.89 g (81%); m.p. 91–92°C. Anal. calcd. for C27H42O4Sn (%): C, 59.03; H, 7.71. Found: C, 58.77; H, 7.64. IR (KBr): 3452 (br, OH), 1656 [ν(COO-)as], 1325 [ν(COO-)s] cm-1. 1H NMR (CDCl3) δ 7.14 (d, J=8.0 Hz, 2H, m-H-C6H4), 6.76 (d, J=8.0 Hz, 2H, o-H-C6H4), 5.01 (s, 1H, OH), 4.43 (s, 2H, OCH2COO), 4.36 (s, 2H, OCH2Ar), 1.17–1.83 (m, 33H, c-C6H11). 13C NMR (CDCl3) δ 172.37 (COOSn), 157.57 (i-C-C6H4), 134.78 (m-C-C6H4), 127.90 (p-C-C6H4), 114.41 (o-C-C6H4), 66.14 (OCH2), 62.98 (HOCH2), 36.49 [1J(119Sn-13C)=332 Hz, C-α], 30.88 [2J(119Sn-13C)=20 Hz, C-β], 29.17 [3J(119Sn-13C)=64 Hz, C-γ], 27.07 (C-δ) ppm. 119Sn NMR (CDCl3) δ 30.8 ppm.
X-ray crystallography
The colorless single crystals of 1 and 3 were obtained from methanol by slow evaporation at room temperature. Diffractions measurements were performed on a Bruker Smart Apex imaging plate area detector fitted with graphite monochromatized Mo-Kα radiation (0.71073 Å) using the φ and ω scan technique at 295(2) K. The structures were solved by direct methods and refined by a full-matrix least-squares procedure based on F2 using SHELXL-97 (Sheldrick, 2008). The non-hydrogen atoms were refined anisotropically, and the hydrogen atoms were placed at calculated positions in the riding model approximation, with C-H=0.93 Å for aromatic and formyl H atoms, C-H=0.97 Å for methylene H atoms, C-H=0.98 Å for methine H atoms, O-H=0.82 Å for hydroxy H atoms, and O-H=0.85(1) Å for water H atoms. In complex 1, two cyclohexyl groups are disordered over two positions, and their site occupancies were refined to 0.54(6):0.46(6) for C(13)-C(18) and 0.70(2):0.30(2) for C(39)-C(44). In complex 3, the site occupancies of the cyclohexyls were refined to 0.802(10):0.198(10) for C(7)-C(12) and 0.65(2):0.35(2) for C(40)-C(45), respectively. In refinements, the C-C bonds and 1,3-distances of the disorderly cyclohexyl groups were restrained to 1.52(1) and 2.50(2) Å, respectively. Crystal data, collection procedures, and refinement results are shown in Table 4. The crystallographic data of compounds 1 and 3 were deposited at the Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC 1014268 and 1043087.
Crystallographic data and structure refinement for 1 and 3.
| Compound | ||
|---|---|---|
| 1 | 3 | |
| Empirical formula | C26H40O3Sn | C54H86O9Sn2 |
| Formula weight | 519.27 | 1116.61 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21 |
| a (Å) | 9.7697(12) | 10.6609(14) |
| b (Å) | 28.546(4) | 18.274(2) |
| c (Å) | 18.427(2) | 14.1107(18) |
| α (°) | 90 | 90 |
| β (°) | 92.028(2) | 90.016(2) |
| γ (°) | 90 | 90 |
| Volume (Å3) | 5135.9(11) | 2749.1(6) |
| Z | 8 | 2 |
| Dc (g/cm3) | 1.343 | 1.349 |
| μ (mm-1) | 1.017 | 0.959 |
| F(000) | 2160 | 1164 |
| θ range | 1.32–26.00 | 1.44–25.50 |
| Crystal size (mm) | 0.20×0.07×0.05 | 0.20×0.18×0.10 |
| Unique reflections | 10,020 (Rint=0.045) | 10,186 (Rint=0.031) |
| Reflections [I>2σ(I)] | 6422 | 8986 |
| Goodness of fit on F2 | 1.024 | 1.028 |
| R indices [I>2σ(I)] | R=0.050, wR=0.126 | R=0.044, wR=0.098 |
| R indices (all data) | R=0.087, wR=0.147 | R=0.052, wR=0.102 |
| Δρmax, Δρmin (e/Å-3) | 0.710, -0.414 | 0.803, -0.392 |
Antibacterial activity
The antibacterial activity of compounds 1–3 against E. coli was determined by the microcalorimetric method according to the literature (Zhang et al., 2004). A 2277 Thermal Activity Monitor (Thermometric AB, Jarfalla, Sweden) was used to determine the power-time curves of bacterial growth at 310 K. After a stable baseline was obtained, the bacterial sample, a beef-extract-soluble medium (pH=7.2–7.4) containing NaCl (1 g), peptone (2 g), beef extract (1 g), and different concentrations of organotin medicine in each 200 mL were pumped into the flow cell system and the monitor began to record the power-time curves of continuous growth for bacteria. When the recording pen returned to the baseline, the process of bacterial growth was completed. Based on the data of power-time curves and theoretical model, the growth rate constants were calculated (Zhang et al., 2004). The relationship between the growth rate constants (μ) and the concentration (C) of the organotin medicine was fitted using a computer (μ=aC+b). When the growth rate constant is 0, the minimum inhibitory concentration (MIC) was confirmed.
Acknowledgments
This work was supported by Shandong Provincial Natural Science Foundation, China (ZR2013BM007), and the National Natural Science Foundation of China (21302110).
References
Abbas, S.; Hussain, M.; Ali, S.; Parvez, M.; Raza, A.; Haider, A.; Iqbal, J. Structural, enzyme inhibition, antibacterial and DNA protection studies of organotin(IV) derivatives of thiophene-2-carboxylic acid. J. Organomet. Chem.2013, 724, 255–261.Search in Google Scholar
Amir, M. K.; Khan, S.; Rehman, Z.; Shah, A; Butler, I. S. Anticancer activity of organotin carboxylates. Inorg. Chim. Acta2014, 423, 14–25.Search in Google Scholar
Arjmand, F.; Parveen, S.; Tabassum, S.; Pettinari, C. Organo-tin antitumor compounds: their present status in drug development and future perspectives. Inorg. Chim. Acta2014, 423, 26–37.Search in Google Scholar
Bala, V.; Chhonker, Y. S.; Hashim, S. R. Synthesis and antimicrobial activity of Schiff bases derived from 2-formylphenoxyacetic acid. Asian J. Chem.2010, 22, 3447–3452.Search in Google Scholar
Bao, M.; He, Q.; Liu, B.; Xing, Y.; Lin, Y. Synthesis and crystal structure of tris(2-methyl-2-phenylpropyl)tin phenoxyacetates. Chinese J. Inorg. Chem.1998, 14, 114–117.Search in Google Scholar
Carraher, C. E.; Roner, M. R. Organotin polymers as anticancer and antiviral agents. J. Organomet. Chem.2014, 751, 67–82.Search in Google Scholar
Chalupa, J.; Handlir, K.; Cisarova, I.; Jirasko, R.; Brus, J.; Lycka, A.; Ruzicka, A.; Holecek, J. Structural study of bis(triorganotin) esters of 4-ketopimelic acid. J. Organomet. Chem.2006, 691, 2631–2640.Search in Google Scholar
Das, G. K.; Wei, C.; Ng, S. W.; Mak, T. C. W. Triphenyltin 8-quinolyloxyacetate hydrate, an organotin ester derivative built of hydrogen-bonded helical chains. J. Organomet. Chem.1987, 322, 33–47.Search in Google Scholar
Davies, A. G.; Gielen, M.; Pannell, K. H.; Tiekink, E. R. T. Tin Chemistry: Fundamentals, Frontiers, and Applications. John Wiley & Sons: Chichester, UK, 2008.10.1002/9780470758090Search in Google Scholar
Davis, A. G. Organotin Chemistry, 2nd ed. Wiley-VCH: Weinheim, 2004, pp 8–23.Search in Google Scholar
Deacon, G. B.; Phillips, R. J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev.1980, 33, 227–250.Search in Google Scholar
Din, I. U.; Molloy, K. C.; Mazhar, M.; Dastgir, S.; Ali, S.; Mahon, M. F. Some tricyclohexyltin carboxylates containing germanium: synthesis, spectral and crystallographic characterization. Appl. Organomet. Chem.2003, 17, 781–787.Search in Google Scholar
Dong, Y.; Yu, Y.; Tian, L. Synthesis, structural characterization and antibacterial activity of triorganotin ferrocenecarboxylates. Main Group Met. Chem.2014, 37, 91–95.Search in Google Scholar
Fracchiolla, G.; Laghezza, A.; Piemontese, L.; Carbonara, G.; Lavecchia, A.; Tortorella, P.; Crestani, M.; Novellino, E.; Loiodice, F. Synthesis, biological evaluation, and molecular modeling investigation of chiral phenoxyacetic acid analogues with PPARα and PPARγ agonist activity. Chem. Med. Chem.2007, 2, 641–654.Search in Google Scholar
Hadjikakou, S. K.; Hadjiliadis, N. Antiproliferative and anti-tumor activity of organotin compounds. Coord. Chem. Rev.2009, 253, 235–249.Search in Google Scholar
Kumar G. S. S.; Seethalakshmi P. G.; Bhuvanesh N.; Kumaresan, S. Cocrystals of caffeine with formylphenoxyaliphatic acids: syntheses, structural characterization, and biological activity. J. Mol. Struct.2013, 1034, 302–309.Search in Google Scholar
Li, W.; Du, D.; Liu, S.; Zhu, C.; Sakho, A. M.; Zhu, D.; Xu, L. Self-assembly of a novel 2D network polymer: syntheses, characterization, crystal structure and properties of a four-tin-nuclear 36-membered diorganotin(IV) macrocyclic carboxylate. J. Organomet. Chem.2010, 695, 2153–2159.Search in Google Scholar
Liu, H.; Shao, X.-B; Jia, M.-X.; Jiang, X.-K.; Lia, Z.-T.; Chen, G.-J. Selective recognition of sodium cyanide and potassium cyanide by diaza-crown ether-capped Zn-porphyrin receptors in polar solvents. Tetrahedron2005, 61, 8095–8100.Search in Google Scholar
Ma, C.; Han, Y.; Zhang, R. Synthesis, characterizations and crystal structures of dibutyltin (IV) complexes with heteroatomic (N, O or S) acid. J. Organomet. Chem. 2004, 689, 1675–1683.Search in Google Scholar
Mao, W.; Bao, K.; Feng, Y.; Wang, Q.; Li, J.; Fan, Z. Synthesis, crystal structure, and fungicidal activity of triorganotin1-methyl-1H-imidazole-4-carboxylates. Main Group Met. Chem. 2015, 38, 27–30.Search in Google Scholar
Molloy, K. C.; Purcell, T. G.; Hahn, E.; Schumann H.; Zuckerman, J. J. Organotin biocides. Crystal and molecular structure of tricyclohexylstannyl 3-indolylacetate, incorporating the first monodentate carboxylate group bonded to a triorganotin. Organometallics.1986, 5, 85–89.Search in Google Scholar
Nadvornik, M.; Holecek, J.; Handlir, K.; Lycka, A. The 13C and 119Sn NMR spectra of some four- and five-coordinate tri-n-butyltin(IV) compounds. J. Organomet. Chem.1984, 275, 43–51.Search in Google Scholar
Shang, X.; Meng, X.; Alegria, E. C. B. A.; Li, Q.; Guedes Da Silva, M. F. C.; Kuznetsov, M. L.; Pombeiro, A. J. L. Syntheses, molecular structures, electrochemical behavior, theoretical study, and antitumor activities of organotin complexes containing 1-(4-chlorophenyl)-1-cyclopentanecarboxylato ligands. Inorg. Chem.2011, 50, 8158–8167.Search in Google Scholar
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr.2008, A64, 112–122.Search in Google Scholar
Song, X.; Cahill, C.; Eng, G. Crystallographic report: triorganotin 2-(p-chlorophenyl)- 3-methylbutyrates. Appl. Organomet. Chem.2003, 17, 743–744.Search in Google Scholar
Teoh, S.-G.; Tan, D.-S.; Yeap, G.-Y.; Fun, H.-K. Tricyclohexyltin complexes with picolinic, nicotinic and 5-bromonicotinic acids. J. Coord. Chem.1999, 48, 53–61.Search in Google Scholar
Tian, L.; Sun, Y.; Li, H.; Zheng, X.; Cheng, Y.; Liu, X.; Qian, B. Synthesis, characterization and biological activity of triorganotin 2-phenyl-1,2,3-triazole- 4-carboxylates. J. Inorg. Biochem. 2005, 99, 1646–1652.Search in Google Scholar
Tian, L.; Kong, L.; Zhang, C. Synthesis, structure and in vitro cytotoxic activity of two organotin complexes of 2-phenyl-1,2,3-triazole-4-carboxylic acid. Main Group Met. Chem.2015, 38, 83–91.Search in Google Scholar
Tiekink, E. R. T. Structural chemistry of organotin carboxylates: a review of the crystallographic literature. Appl. Organomet. Chem. 1991, 5, 1–23.Search in Google Scholar
Tiekink, E. R. T. The rich diversity in tin carboxylate structures. Trends Organomet. Chem.1994, 1, 71–116.Search in Google Scholar
Wang, X.; Liu, X.; Tian, L. Synthesis, characterization and in vitro cytotoxic activity of bis(triorganotin) 2,6-pyridinedicarboxylates. Main Group Met. Chem.2014, 37, 143–147.Search in Google Scholar
Willem, R.; Verbruggen, I.; Gielen, M.; Biesemans, M.; Mahieu, B.; Baul, T. S. B. Tiekink, E. R. T. Correlating Mössbauer and solution- and solid-state 117Sn NMR data with X-ray diffraction structural data of triorganotin 2-[(E)-2-(2-hydroxy-5-methylphenyl)-1-diazenyl]benzoates. Organometallics1998, 17, 5758–5766.Search in Google Scholar
Yu, J.-X.; Kuang, D.-Z.; Yin, D.-L.; Feng, Y.-L.; Zhang, F.-X.; Wang, J.-Q.; Xu, Z.-F. Synthesis, crystal structure and electrochemical properties of the one-dimensional chain tributyltin 2,4-dichlorophenoxyacetate. Chinese J. Inorg. Chem.2010, 26, 1507–1510.Search in Google Scholar
Yu, J.-X.; Feng, Y.-L.; Kuang, D.-Z.; Yin, D.-L.; Zhang, F.-X.; Wang, J.-Q. Synthesis, crystal structure and quantum chemistry of two tricyclohexyltin pyridinecarboxylate complexes. Chinese J. Inorg. Chem.2012, 28, 279–284.Search in Google Scholar
Zhang, H.; Yu, X.; Li, X.; Pan, X. A study of promotive and fungistatic actions of steroidal saponin by microcalorimetric method. Thermochim. Acta2004, 416, 71–74.Search in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Two structurally related, hydrogen-bonded polymorphs of the zwitterionic complex trichlorido- ((dimethylphosphoryl)methanaminium-κO)zinc(II)
- CuO/polypyrrole nanocomposites as a marker of toxic lead ions for ecological remediation in contrast with CuO and polypyrrole
- Template synthesis, characterization, and antimicrobial activity of a new lead (II) Schiff base complex
- Synthesis of novel penta and hexa coordinated monobutyltin(IV) derivatives based on oximes and N-protected amino acids
- Synthesis, structural characterization, and antibacterial activity of tricyclohexyltin aryloxyacetates
- Short Communications
- Synthesis and crystal structures of extremely bulky phosphinoamido and phosphinoamino germanium(II) chloride complexes
- [4-tBu-2,6-{P(O)(OiPr)2}2C6H2Sn(PPh3)Cr(CO)5]ClO4 – a salt containing a cationic triphenylphosphane-stabilized organostannylene transition metal complex
Articles in the same Issue
- Frontmatter
- Research Articles
- Two structurally related, hydrogen-bonded polymorphs of the zwitterionic complex trichlorido- ((dimethylphosphoryl)methanaminium-κO)zinc(II)
- CuO/polypyrrole nanocomposites as a marker of toxic lead ions for ecological remediation in contrast with CuO and polypyrrole
- Template synthesis, characterization, and antimicrobial activity of a new lead (II) Schiff base complex
- Synthesis of novel penta and hexa coordinated monobutyltin(IV) derivatives based on oximes and N-protected amino acids
- Synthesis, structural characterization, and antibacterial activity of tricyclohexyltin aryloxyacetates
- Short Communications
- Synthesis and crystal structures of extremely bulky phosphinoamido and phosphinoamino germanium(II) chloride complexes
- [4-tBu-2,6-{P(O)(OiPr)2}2C6H2Sn(PPh3)Cr(CO)5]ClO4 – a salt containing a cationic triphenylphosphane-stabilized organostannylene transition metal complex

