Abstract
The heterogeneity of each individual oncologic disease can be mirrored by molecular analysis of a simple blood draw in real time. Liquid biopsy testing has been shown useable for cancer detection, proof of minimal residual disease, therapy decision making and monitoring. However, an individual blood analyte does not present a comprehensive picture of the disease. It was recently shown that multi-modal/multi-parametric/multi-analyte liquid biopsy testing has the advantage of generating a high-resolution snapshot of the disease complexity. The different blood analytes such as circulating tumor cells, circulating immune cells, tumor-educated platelets, extracellular vesicles, cell-free DNA, cell-free RNA and circulating proteins complement each other and have additive value for clinical cancer management. We, here, like to review the studies leading to these promising conclusions and like to, at the end, mention that many challenges lie ahead before the translation into the clinic can be accomplished, including issues concerning clinical utility, method standardization, cost reimbursement and data management.
The advantages of liquid biopsy
In analogy to the term “biopsy” – the collection of tissue material, “liquid biopsy” is the collection of body fluids such as blood. Due to the minimal invasive nature of a blood draw, liquid biopsy analysis is repeatable and only carries the risk of a blood draw rather than of the invasive tissue biopsy. In oncologic settings, liquid biopsy is especially relevant in individuals with tumor mass locations difficult to biopsy.
While a small punch biopsy represents locally limited information, blood mirrors the heterogeneity of an entire systemic situation – which occurs as spatial heterogeneity within one tumor mass and between the tumor locations in metastatic cancer patients and as temporal heterogeneity across the treatment lines due to tumor evolution.
In summary, the concept of liquid biopsy is simple – a blood draw can potentially replace – but definitively complement – tissue sampling and its analysis provides more insights into the current state of a disease in real time.
Liquid biopsy analytes
Blood, as the most commonly used body fluid in the liquid biopsy testing, consists of dozens of analytes useable for cancer management. First of all, the tumor cells migrating into the circulation – called circulating tumor cells (CTCs) – and potentially becoming the seeds of metastasis are liquid biopsy analytes with great potential to examine the nature of the tumor disease [1]. The fraction of the cell-free DNA (cfDNA) originating from tumor cells (ctDNA) is also useable to characterize the genomic nature of the disease [2]. Lately, extracellular vesicles (EVs) shed by malignant and non-malignant cells, were found to enable intercellular communication [3] and provide insights into the systemic oncologic setting. Additionally, the ratios and molecular characteristics of the different blood and immune cells might be used to help profiling the entire tumor disease [4] (Figure 1).
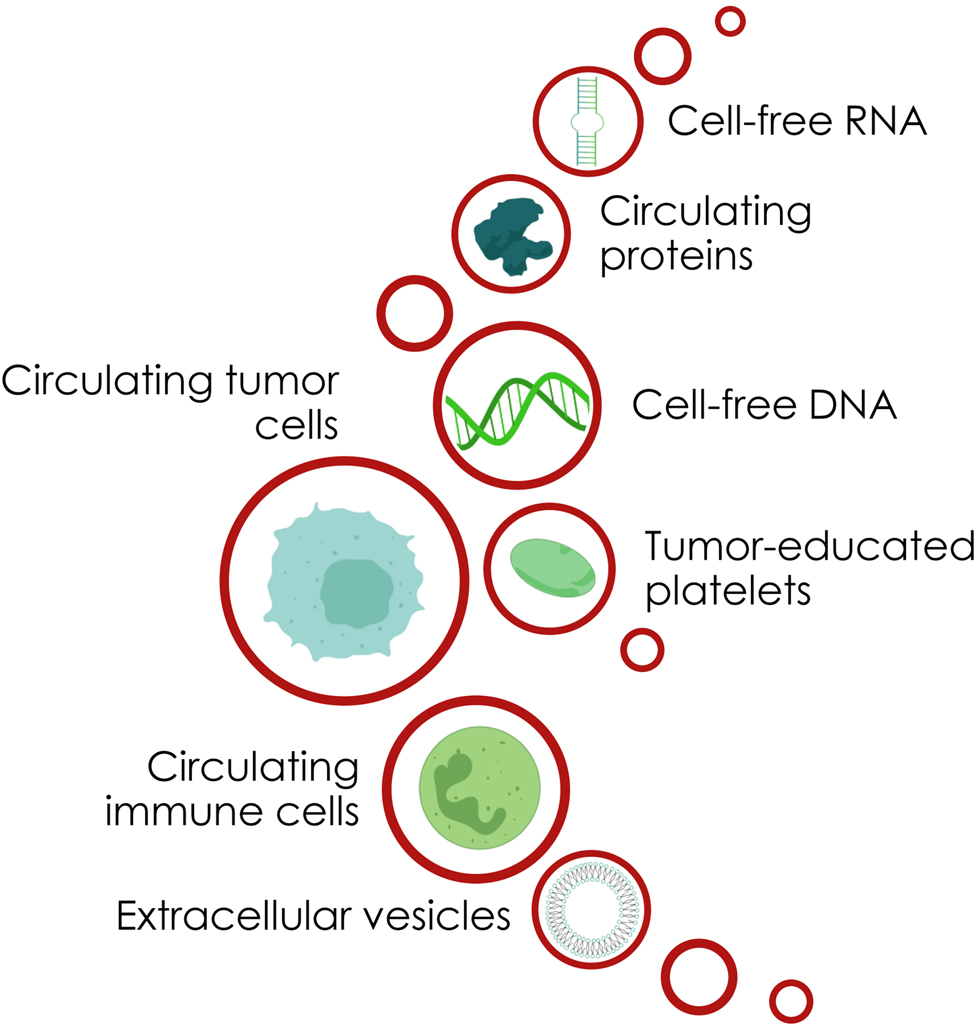
Diversity of liquid biopsy analytes.
Liquid biopsy for therapy management of breast cancer patients
The management of breast cancer (BC), as prominent example of a systemic oncologic disease, can benefit from liquid biopsy in all stages of the disease:
Early cancer detection
The assessment of eight circulating proteins together with aneuploidy and mutational testing in cfDNA can identify cancer patients, including BC patients, with a median sensitivity of 80% and specificity of 99% [5]. In addition, the cfDNA fragmentation pattern can be used to diagnose BC when combined with mutational cfDNA profiling [6].
Prognostication
Regarding the prognostic value of liquid biopsy analytes in BC, the number of CTCs [7, 8] as well as the concentration of ctDNA [9] have been proven to significantly correlate with overall survival (OS) and molecular characterization has identified stemness signatures at a messenger RNA (mRNA) level in EVs to be associated with decreased survival time in metastatic BC (MBC) patients [10].
Therapy decision making
In the era of personalized medicine, therapies should be individually tailored to finally achieve therapeutic success in all patients. For example, the PI3Kα inhibitor Alpelisib was approved for MBC patients with PIK3CA mutations detected in ctDNA [11]. Furthermore, profiling CTCs revealed HER2 overexpression in blood of patients with HER2-negative primary tumors and although clinical effectiveness has not yet been concretely proven, it points towards a possible targeted anti-HER2 therapy based on blood testing [12], [13], [14], [15]. Even in the emerging field of immune therapies in BC, liquid biopsies can become a tool to evaluate the immunogenicity of the tumor [16].
Minimal residual disease detection
After curative intent surgery, the presence of ctDNA correlates with the probability of relapse and the detection of mutations in the blood, detected by personalized assays, has a lead time to the relapse detection by radiologic methods [17], [18], [19]. cfDNA methylation analysis is also suitable to detect minimal residual disease in BC [20, 21].
Treatment success monitoring
Under therapy, detailed characterization of variants occurring in the ctDNA can be used for therapy monitoring, as shown by the use of ESR1 variants [found in cfDNA and occurring during aromatase inhibitor therapy] as indicators of disease progression [22, 23].
Thus, at the moment, different but individual liquid biopsy analytes were observed to provide valuable information for clinical management of BC patients.
Comparison of two liquid biopsy analytes
Regarding the different blood analytes suitable for BC management, the question arose which analyte might be the most advantageous.
Therefore, the value of parallel CTC enumeration and ctDNA detection was frequently examined in BC patients [9, 24], [25], [26], [27], [28], [29], [30], [31], [32], [33]. The combination of cfDNA and CTC counts improved sensitivity and specificity as a diagnostic tool in non-MBC patients [9, 25]. It was shown in BC patients that cfDNA presented a greater dynamic range and greater correlation to disease burden than CA15-3 and CTCs [27].
However, the molecular characterization of blood analytes, apart from the enumeration of CTCs, EVs or the tumor fraction of cfDNA, holds the greatest potential regarding targeted therapy decision making. Only a few studies have yet been conducted comparing the molecular characteristics of two matched blood analytes in BC patients.
CTCs vs. EVs
The HER2 protein was stained on CTCs and tumor-derived EVs of BC patients and the presence and quantity of HER2+ CTCs and EVs showed similar prognostic value [34]. However, EVs performed better than CTCs in predicting the HER2 status of the primary tissue [35].
CTC mRNA profiling in addition to matched EV mRNA profiling, utilizing blood from HR+ HER2– MBC patients, showed that the ability of an individual analyte to describe the full picture was rather limited [36]. In general, there were great differences in mRNA profiles of EVs and CTCs, with only 5% of positive signals identical in both fractions [36]. In more detail, the gene coding for HER2, namely ERBB2, and ERBB3 detected to be overexpressed in CTCs associated significantly with disease progression in this HER2– cohort, while the correlation became even stronger by the addition of ERBB2 and ERBB3 signals from the EVs [36], revealing the synergistic potential of both analytes. Interestingly, the overexpression prevalence of the same transcript (mTOR) differed significantly between overall responders and overall non-responders in both, CTCs and EVs, but to contrary clinical outcomes [36].
Methylation pattern in matched CTCs and cfDNA
Already in 2013, the SOX17 promotor methylation in CTCs and matched cfDNA has been studied by identical methylation-specific assays and it was concluded that in early BC patients, the methylation of this specific promotor in CTCs and cfDNA correlated significantly [37]. This result was later also validated for MBC patients: the SOX17 methylation in CTCs and cfDNA correlated significantly with OS [38]. The same methylation-specific assay method was used to evaluate the ESR1 methylation in CTCs and cfDNA isolated from the same blood draw [39]. There was an almost perfect agreement in findings for 98.3% patients between CTCs and ctDNA. However, only the ESR1 methylation in CTCs was associated with non-response to an everolimus/exemestane regimen [39].
Mutations in matched CTCs and cfDNA
Variant analysis in CTCs was shown to be able to identify newly emerging resistance mutations in contrast to cfDNA, where resistance mutations might only be detected after apoptosis of the cells harboring new alterations [40]; thereby, highlighting the potential benefit of variant analysis in CTCs over cfDNA. The case study of a HR+ MBC patient with serial liquid biopsy specimens during treatment over four years showed the correlation of single CTC and cfDNA copy number variants and mutations [41]. Interestingly, CNVs within the cfDNA represented a mixture of the individual CNVs of all single CTCs, but only within the single CTC, the subclonal tumor evolution of the CNVs could be resolved [41, 42].
In contrast to the documented advantage of CTCs compared to cfDNA [40], [41], [42], Beije et al. concluded from their matched ddPCR analysis of two ESR1 mutations in CTCs and matched cfDNA that the sensitivity for ESR1 mutation detection was lower in CTCs than in cfDNA [43]. At the time point of progression under endocrine therapy, 42% of patients showed ESR1 cfDNA mutations but only 11% of patients showed ESR1 mutations in CTCs. In accordance to Beije et al., the mutational analysis of CTCs and matched cfDNA in a small cohort of five MBC patients revealed that the cfDNA profiles provided an accurate reflection of mutations seen in individual CTCs [24]. However, in two patients, more mutations were found in cfDNA than in their CTCs, which might be due to the small number of individual CTCs picked [24]. Consequently, in contrast to mutational detection in cfDNA, sensitive mutation detection in (single) CTCs is challenging.
In an approach to use as minimal blood as possible for cfDNA and CTC mutational profiling, blood samples were first used to enrich CTCs (positive immunomagnetic selection) for subsequent CTC genomic DNA (gDNA) analysis. Subsequently, CTC-depleted blood was further taken to isolate cfDNA [44, 45]. 28% of all detected variants were identical in cfDNA and matched CTC gDNA, thus, these analyses complemented each other [46]. In more detail, PIK3CA and ESR1 variants were less common in CTC gDNA than in cfDNA, while ERBB2 variants were only detected in CTC gDNA. The percentage of patients with no detectable cfDNA variants or CTC gDNA variants was 17%/11%, but combined analysis identified variants in 94% of all patients [46]. This comprehensive mutational analysis of cfDNA and CTCs revealed the additive value of these analytes.
Utilizing a more sensitive mutation analysis for ESR1 mutation detection in individual CTCs and cfDNA of MBC patients [47] might be the way to go, although the cohort was too small (n=8) and the prevalence of mutations too low to compare the utility of CTCs and cfDNA. CTC and cfDNA PIK3CA mutational information showed a lack of concordance in early BC samples, but a higher concordance in MBC patients, consolidating the complementary nature of the analytes at specific stages of the disease [48].
On the meta level, it is to conclude that the technical challenges of CTC isolation and mutation analysis of CTC gDNA limit the potential of the CTC analysis in clinical practice, while the technical solutions of sensitive variant calling in cfDNA are more advanced, at the moment [49]. cfDNA can be quantified more easily than CTCs with no need for prior enrichment of ctDNA which contrasts the necessary enrichment of CTCs [50]. While cfDNA mutation and also methylation profiling is very promising for early cancer detection [5, 6, 51], in the advanced oncologic settings, the molecular analysis of the CTCs, as seeds of metastasis, harbours clinical relevance. CTCs are intact cells, thus, present the opportunity to study DNA, RNA, protein and even phenotype, secretome and function. The combined analysis of CTCs and cfDNA was shown to be beneficial [46, 48], as they harbour complementary information and thus, expand the number of patients in whom actionable targets can be identified [46]. Consequently, the hypothesis from Haber and Velculescu in 2014 who stated that ‘CTC and ctDNA technologies are likely to be synergistic rather than strictly competitive’ was recently validated [52]. The complementarity of cfDNA and CTCs might be explained by the different ways both analytes are released into the circulation: CTCs by active migration and epithelial to mesenchymal transition [53] and cfDNA mostly by passive processes like apoptosis and necrosis [54, 55].
Multimodal liquid biopsy
As already mentioned, on DNA level [46, 48], as well as on RNA level [36], different blood analytes have additive value. Concerning the more advanced level of evidence, it is to question, whether a multi-omic approach, integrating information on the DNA, RNA and protein level, might be beneficial. Gene expression analysis and especially protein expression analysis might provide information closer to the disease biology than mutation analysis, but mutation information are more stable and consistent aspects of the disease biology as compared to the highly variable expression.
The diversity of blood analytes is huge. In addition to CTCs, cfDNA and EVs, circulating proteins, cell-free RNAs (microRNAs, small RNAs and long noncoding RNAs), tumor-educated platelets and immune cells influencing the tumor disease and vice versa can be blood analytes of interest in the clinical management of oncologic patients (Figure 1) [56, 57].
One of the most promising paths in the translational space is the combinatorial analysis of more than two liquid biopsy analytes – the multi-analyte approach. It is to speculate, whether the insight hidden in distinctly separated datasets might be even greater than their sum, when properly integrated. For this purpose, we and others established the parallel analysis of more than two liquid biopsy analytes from the same BC blood sample.
The Cancer-ID consortium studied the technical issues of analyzing CTCs, cfDNA, EVs and miRNA from the same blood tube and found that the pre-analytical variables, especially the choice of blood collection tube, is critical for reliable data analysis [58]. In accordance, Gerber et al. found the blood collection tube, blood storage time and sample preparation essential and established a workflow with EDTA or Streck collection tubes for the analysis of cfDNA, CTCs and leukocytes on the DNA and protein level [59].
Within the last five years, we established a workflow for analysis of CTCs, EVs and ctDNA from as little as 20 mL blood [60]. The cohort consisted of 26 HR+/HER2– MBC patients of whom blood was drawn at the time point of disease progression. The resulting project was called ELIMA: Evaluation of multiple Liquid biopsy analytes In Metastatic breast cancer patients All from one blood sample (Figure 2). To gain comprehensive insights from a limited sample, we employed a multi-omic approach to simultaneously analyze the transcriptional and genomic complexity. In detail, CTC gDNA, CTC mRNA, cfDNA and EV mRNA were isolated from 20 mL blood and mRNA expression and variant profiling was conducted via a qPCR panel and a targeted NGS panel. The final integration showed an increased number of patients with actionable signals, that can be used for personalized therapy decision, when considering all four blood analytes, in comparison to the assessment of only a single analyte [60]. Regarding prognostication, the ‘ELIMA.score’, that is the combination of clustering results of all four analytes, resulted in a significant correlation with OS with decreased p-value when compared to the clustering of each individual analyte alone. We have shown that some of the information within the four analytes overlapped, while all of the analytes added information to the global multi-modal dataset. CTC gDNA showed the highest sensitivity, the highest number of signals per patient, contained the greatest amount of information about the other analytes and was dominating the list of the most influential parameters. However, using CTC gDNA alone, does not seem to be sufficient for a comprehensive picture of each individual disease [60]. In conclusion, we demonstrated that CTC gDNA, CTC mRNA, EV mRNA, and ctDNA are complementary because the information of one analyte was not entirely conveyed by another one, but information was unique in each individual analyte. This multi-omic, multi-modal liquid biopsy approach deconvolutes the genomic and transcriptomic complexity, enables the generation of a high-resolution snapshot and maximizes the information available for deliberated cancer therapy management.

Blood analytes and logo of the ELIMA study.
© 2019 by Nadine Vostatek – designable. All rights reserved.
A longitudinal approach using CTC mRNA, EV mRNA and ctDNA isolated from blood drawn at three time points during therapy of HR+/HER2– MBC patients underscored the additional value of these analytes and their own unique features for disease monitoring [61]. Usage of one analyte might be favored over usage of other liquid biopsy analytes depending on the clinical question. For example, the detection of ESR1 and PIK3CA variants for therapy decisions was more frequent in cfDNA, than in CTC gDNA, whereas somatic BRCA2 variants were more frequently found in CTC gDNA [46].
Multi-modal liquid biopsy testing unveils an excellent potential for early detection because a combination of complementary analytes improves the sensitivity [2]. A sensitivity of 80% and specificity of 99% was reached for cancer identification by usage of circulating proteins and CNV and SNV testing of cfDNA [5].
Challenges to be overcome
The promise of multi-analyte liquid biopsy testing, sometimes also called total liquid biopsy testing [40], is undisputable (Figure 3). However, most of the described studies are in the translational space. Liquid biopsy testing of individual analytes was recently shown in interventional trials to harbour some benefit for therapy decision making in BC [11, 62, 63], but multi-modal liquid biopsy testing with more than two analytes has not found the way into prospective interventional clinical trials yet, which has already been demanded four years ago [50]. Only prospective, interventional, randomized, multicenter, well-powered clinical trials with large cohorts, rigorous pre-analytical conditions, standardized wet-lab and data evaluation protocols (ensuring sensitive and specific analyte characterization) with long time follow-up (if cancer detection is the aim), clearly reporting the results, can provide evidence of the clinical utility of multimodal testing [62, 64]. In the era of biomarker-guided clinical trials, the possibilities for study design increased with designs more flexible, more rigorous, with higher statistical power or with multiple agents or biomarkers to be tested simultaneously [65], [66], [67].
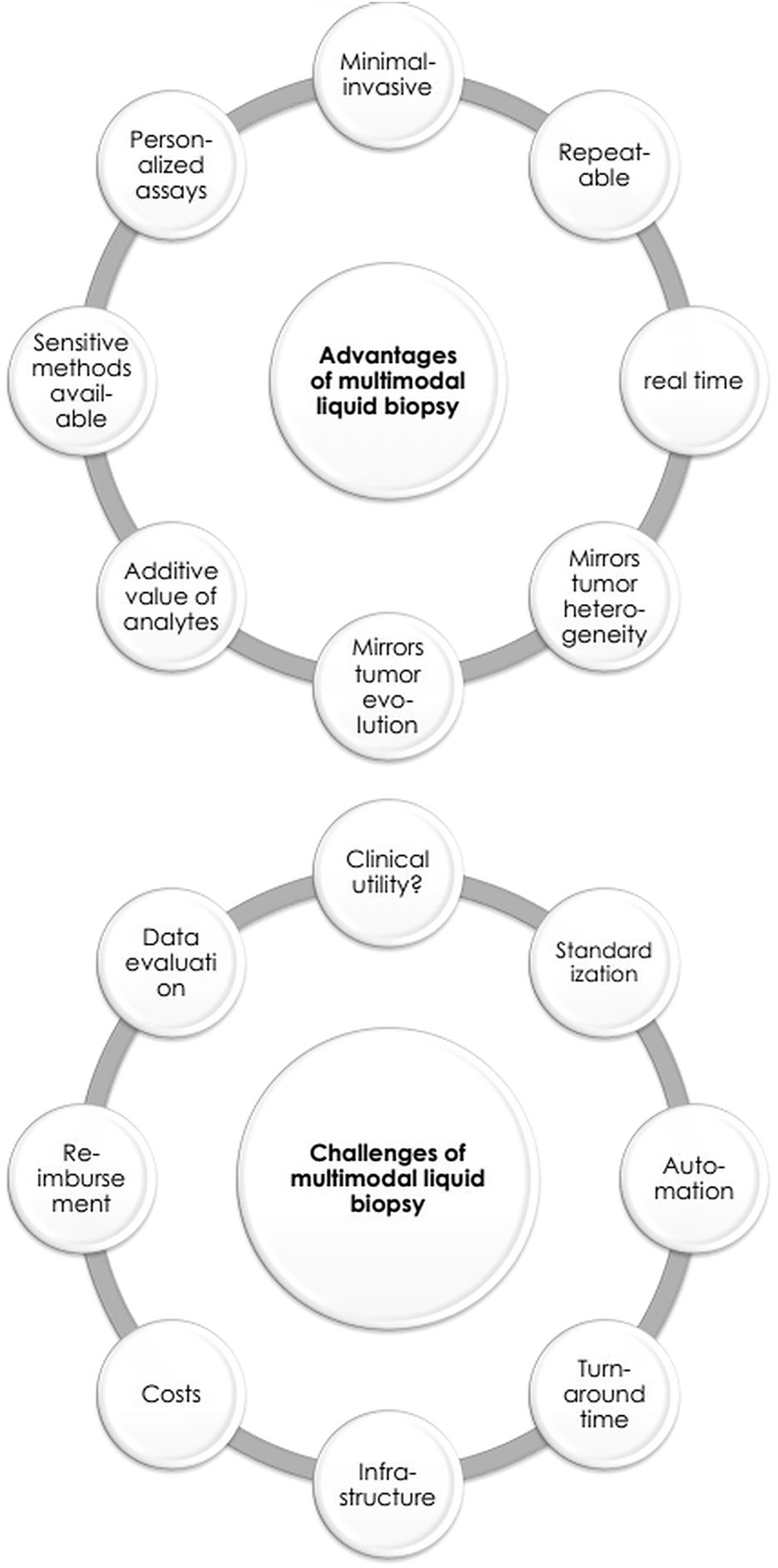
Advantages and challenges of multi-modal liquid biopsy testing.
In addition to the importance of a carefully chosen trial design, considerations about the biological and life style factors of each patient, potentially influencing the liquid biopsy results, should be included. Until now, some groups examined the effect of biological and life style factors on cfDNA concentration [68]. However, most of the studies examining a specific factor still ended up with conflicting results, partly due to methodological inconsistencies, i.e. the effect of smoking, body mass index, hypertension, circadian rhythm, biological sex, age [68]. While other studies consistently showed no effect of menstruation and alcohol consumption [69], [70], [71], a clear increase in cfDNA under psychological stress [72, 73] and during/after physical activity and exercise was proven [74], [75], [76]. Physical activity and exercise was also shown to increase the concentration of EVs [77, 78]. Nutrition [79], allergic sensitization [80], social stress [81] and smoking [82] were shown to alter the concentration, surface-markers or enclosed nucleic acids of EVs. While EVs are discussed as functional component in the circadian rhythm [83], the number of CTCs and other circulating cells were described to be influenced by the circadian rhythm [84]. Evidence about the influence of other biological and lifestyle factors on CTCs is still missing. In general, applicable for all liquid biopsy analytes, insights into the biological mechanisms of release and degradation of the analytes are mostly lacking and thus, impedes the analytical robustness [85]. For now, controlling for physical activity, smoking, stress and time of the day before/at blood draw should be included in the study design – with many more factors such as sex, age, diet, obesity and hypertension to be considered as well. In the future, there will be an urgent need to further investigate the biological and functional relationships of the analyses used for liquid biopsy testing.
Despite the development of workflows to use a reduced input amount (blood volume), it will be critical for implementation to standardize all pre-/post- and analytical processes. At the moment, different blood collection tubes, different storage and transport conditions, a huge number of plasma, EV and cell isolation methods and diverse analyses exist, making a direct comparison and even reproducibility challenging. While standardization might be achieved for specific analytes (cfDNA) and distinct analyses (qPCR, ddPCR or NGS) in the near future [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], an entire multi-modal workflow will be elaborate to standardize and to achieve consensus in the entire scientific field. Automated workflows might be more suitable to avoid human mistakes and standardize the large number of sequential processes. The turnaround-time and also the infrastructure (establishment of decentralized testing strategies) also have to be considered [96].
In addition, the costs increase dramatically in multi-parametric workflows and reimbursement of the tests has to be clarified before multi-analyte testing can be rolled out in clinical practice. It is to repeat that liquid biopsy testing as companion diagnostic for specific treatment regimens or as monitoring tool is economically profitable for the health care systems, because unsuccessful expensive treatment is avoided and elaborate cancer management in late-stage cancer patients can be reduced by early diagnosis, early detection of minimal residual disease and early therapy failure identification [97, 98].
Another hurdle of multi-modal workflows is the optimal storage and integration of the large amount of orthogonal data [2]. While in the last years, data storage systems were quickly developed to process and store huge quantities of data, data integration might benefit from artificial neuronal networks and deep learning approaches in the future. Furthermore, data integration should be followed by data interpretation considering the clinical goals. Consortia had already discussed the level of evidence of specific test results [99] and companies implemented data interpretation tools, but it was shown that different tools differed in their final clinical recommendation [100]. Finally, the results should be presented in a user-friendly way for the clinicians and should clearly point out recommendations for clinical practice.
Only after having solved these challenges (Figure 3), a shift can be accomplished from merely trusting the tissue analysis to translating liquid biopsy testing for real time molecular characterization of the individual oncological disease into clinical routine.
Conclusions
Liquid biopsy testing presents undisputable potential for clinical management of cancer patients. The advantage of the liquid biopsy repertoire is to mirror the individual tumoral heterogeneity in real time by repeatable analysis. In BC, liquid biopsy analysis has already been shown to be used for cancer detection, minimal residual disease detection and therapy decision making. The diversity of analytes in blood rose the question whether it is worth characterizing multiple analytes from one blood sample. Two analytes have already been compared in some studies using blood from BC patients. In summary, variant analysis in cfDNA and CTCgDNA was shown to have additive value, partly because of the different sensitivity for variant analysis and the different biological origins of the analytes and corresponding state of the disease. Only a very few studies molecularly characterized more than two blood analytes in samples of BC patients via multi-modal testing. While mutational analysis of CTC gDNA was shown to mirror the disease evolution in the individual patients, some of the most influential parameters were detected in CTC gDNA. However, the CTC gDNA analysis does not suffice for a comprehensive picture of the disease. In this regard, it was clearly shown that all analytes complement each other and thus, have additive value for early diagnosis, prognostication and therapy decision making. Multi-parametric liquid biopsy approaches enable the generation of a high-resolution snapshot of the disease complexity.
To move these findings into clinical practice, many challenges still have to be overcome. Clinical utility has to be shown by meticulous clinical trials, methods should be standardized and automated, the testing infrastructure must be improved, costs have to be reduced and reimbursement ensured, while data storage, data evaluation, data interpretation and data readability has to be enhanced. After having solved these issues, the promising multi-modal liquid biopsy testing can revolutionize clinical management of oncological patients.
-
Research funding: None declared.
-
Author contributions: Conceptualization: C.K.; Investigation: C.K.; Writing – Original Draft: C.K.; Writing – Review & Editing: S.K.B.; Visualization: C.K.; Funding acquisition: R.K., S.K.B. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: C.K. received support for travel expenses from QIAGEN, Hilden, Germany. R.K. has received honoraria from Tesaro and Astra-Zeneca in the last 3 years, is part of the advisory board from Medtronic and council of IGCS, president of SERGS and proctored and presented for Intuitive Surgical. S.K.B. is a consultant for QIAGEN, Hilden, Germany.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
References
1. Keller, L, Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer 2019;19:553–67. https://doi.org/10.1038/s41568-019-0180-2.Search in Google Scholar
2. Heitzer, E, Haque, IS, Roberts, CES, Speicher, MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet 2019;20:71–88. https://doi.org/10.1038/s41576-018-0071-5.Search in Google Scholar
3. Ludwig, A-K, Giebel, B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol 2012;44:11–5. https://doi.org/10.1016/j.biocel.2011.10.005.Search in Google Scholar
4. Corbeau, I, Jacot, W, Guiu, S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in breast cancer patients: a systematic review. Cancers 2020;12:958. https://doi.org/10.3390/cancers12040958.Search in Google Scholar
5. Douville, C, Cohen, JD, Ptak, J, Popoli, M, Schaefer, J, Silliman, N, et al.. Assessing aneuploidy with repetitive element sequencing. Proc Natl Acad Sci U S A 2020;117:4858–63. https://doi.org/10.1073/pnas.1910041117.Search in Google Scholar
6. Cristiano, S, Leal, A, Phallen, J, Fiksel, J, Adleff, V, Bruhm, DC, et al.. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 2019;570:385–9. https://doi.org/10.1038/s41586-019-1272-6.Search in Google Scholar
7. Cristofanilli, M, Budd, GT, Ellis, MJ, Stopeck, A, Matera, J, Miller, MC, et al.. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781–91. https://doi.org/10.1056/nejmoa040766.Search in Google Scholar
8. Bidard, F-C, Peeters, DJ, Fehm, T, Nolé, F, Gisbert-Criado, R, Mavroudis, D, et al.. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15:406–14. https://doi.org/10.1016/s1470-2045(14)70069-5.Search in Google Scholar
9. Rossi, G, Mu, Z, Rademaker, AW, Austin, LK, Strickland, KS, Costa, RLB, et al.. Cell-free DNA and circulating tumor cells: comprehensive liquid biopsy analysis in advanced breast cancer. Clin Cancer Res 2018;24:560–8. https://doi.org/10.1158/1078-0432.ccr-17-2092.Search in Google Scholar PubMed
10. Rodríguez, M, Silva, J, Herrera, A, Herrera, M, Peña, C, Martín, P, et al.. Exosomes enriched in stemness/metastatic-related mRNAS promote oncogenic potential in breast cancer. Oncotarget 2015;6:40575–87. https://doi.org/10.18632/oncotarget.5818.Search in Google Scholar PubMed PubMed Central
11. André, F, Ciruelos, E, Rubovszky, G, Campone, M, Loibl, S, Rugo, HS, et al.. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019;380:1929–40. https://doi.org/10.1056/NEJMoa1813904.Search in Google Scholar PubMed
12. Georgoulias, V, Bozionelou, V, Agelaki, S, Perraki, M, Apostolaki, S, Kallergi, G, et al.. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol 2012;23:1744–50. https://doi.org/10.1093/annonc/mds020.Search in Google Scholar PubMed
13. Cabel, L, Proudhon, C, Gortais, H, Loirat, D, Coussy, F, Pierga, J-Y, et al.. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol 2017;22:421–30. https://doi.org/10.1007/s10147-017-1105-2.Search in Google Scholar PubMed
14. Wang, C, Mu, Z, Ye, Z, Zhang, Z, Abu-Khalaf, MM, Silver, DP, et al.. Prognostic value of HER2 status on circulating tumor cells in advanced-stage breast cancer patients with HER2-negative tumors. Breast Cancer Res Treat 2020;181:679–89. https://doi.org/10.1007/s10549-020-05662-x.Search in Google Scholar PubMed PubMed Central
15. Fehm, T, Mueller, V, Banys-Paluchowski, M, Fasching, PA, Friedl, TWP, Hartkopf, A, et al.. Abstract PD3-12: efficacy of the tyrosine kinase inhibitor lapatinib in the treatment of patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells - results from the randomized phase III DETECT III trial. Cancer Res 2021;81(4 Suppl):PD3-12. https://doi.org/10.1158/1538-7445.sabcs20-pd3-12.Search in Google Scholar
16. Keup, C, Kimmig, R, Kasimir-Bauer, S. Liquid biopsies to evaluate immunogenicity of gynecological/breast tumors: on the way to blood-based biomarkers for immunotherapies. Breast Care 2020;15:470–80. https://doi.org/10.1159/000510509.Search in Google Scholar PubMed PubMed Central
17. Garcia-Murillas, I, Schiavon, G, Weigelt, B, Ng, C, Hrebien, S, Cutts, RJ, et al.. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015;7:302ra133. https://doi.org/10.1126/scitranslmed.aab0021.Search in Google Scholar PubMed
18. Coombes, RC, Page, K, Salari, R, Hastings, RK, Armstrong, A, Ahmed, S, et al.. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res 2019;25:4255–63. https://doi.org/10.1158/1078-0432.ccr-18-3663.Search in Google Scholar
19. Parsons, HA, Rhoades, J, Reed, SC, Gydush, G, Ram, P, Exman, P, et al.. Sensitive detection of minimal residual disease in patients treated for early-stage breast cancer. Clin Cancer Res 2020;26:2556–64. https://doi.org/10.1158/1078-0432.ccr-19-3005.Search in Google Scholar PubMed PubMed Central
20. Shen, SY, Singhania, R, Fehringer, G, Chakravarthy, A, Roehrl, MHA, Chadwick, D, et al.. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 2018;563:579–83. https://doi.org/10.1038/s41586-018-0703-0.Search in Google Scholar PubMed
21. Liu, MC, Oxnard, GR, Klein, EA, Swanton, C, Seiden, MV, Cummings, SR, et al.. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol 2020;31:745–59. https://doi.org/10.1016/j.annonc.2020.02.011.Search in Google Scholar PubMed PubMed Central
22. Takeshita, T, Yamamoto, Y, Yamamoto-Ibusuki, M, Tomiguchi, M, Sueta, A, Murakami, K, et al.. Analysis of ESR1 and PIK3CA mutations in plasma cell-free DNA from ER-positive breast cancer patients. Oncotarget 2017;8:52142–55. https://doi.org/10.18632/oncotarget.18479.Search in Google Scholar PubMed PubMed Central
23. Clatot, F, Perdrix, A, Beaussire, L, Lequesne, J, Lévy, C, Emile, G, et al.. Risk of early progression according to circulating ESR1 mutation, CA-15.3 and cfDNA increases under first-line anti-aromatase treatment in metastatic breast cancer. Breast Cancer Res 2020;22:56. https://doi.org/10.1186/s13058-020-01290-x.Search in Google Scholar PubMed PubMed Central
24. Shaw, JA, Guttery, DS, Hills, A, Fernandez-Garcia, D, Page, K, Rosales, BM, et al.. Mutation analysis of cell-free DNA and single circulating tumor cells in metastatic breast cancer patients with high circulating tumor cell counts. Clin Cancer Res 2017;23:88–96. https://doi.org/10.1158/1078-0432.ccr-16-0825.Search in Google Scholar
25. Wang, W, Liang, M, Ma, G, Li, L, Zhou, W, Xia, T, et al.. Plasma cell-free DNA integrity plus circulating tumor cells: a potential biomarker of no distant metastasis breast cancer. Neoplasma 2017;64:611–8. https://doi.org/10.4149/neo_2017_417.Search in Google Scholar PubMed
26. Pierga, J-Y, Silveira, A, Tredan, O, Tanguy, M-L, Lorgis, V, Dubot, C, et al.. Multimodality liquid biopsy for early monitoring and outcome prediction in first-line metastatic HER2-negative breast cancer: final results of the prospective cohort from the French Breast Cancer InterGroup Unicancer (UCBG)— COMET study. J Clin Oncol 2019;37:3019. https://doi.org/10.1200/jco.2019.37.15_suppl.3019.Search in Google Scholar
27. Dawson, S-J, Tsui, DWY, Murtaza, M, Biggs, H, Rueda, OM, Chin, S-F, et al.. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368:1199–209. https://doi.org/10.1056/nejmoa1213261.Search in Google Scholar
28. Guttery, DS, Page, K, Hills, A, Woodley, L, Marchese, SD, Rghebi, B, et al.. Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen receptor-positive metastatic breast cancer. Clin Chem 2015;61:974–82. https://doi.org/10.1373/clinchem.2015.238717.Search in Google Scholar PubMed
29. Fernandez-Garcia, D, Hills, A, Page, K, Hastings, RK, Toghill, B, Goddard, KS, et al.. Plasma cell-free DNA (cfDNA) as a predictive and prognostic marker in patients with metastatic breast cancer. Breast Cancer Res 2019;21:149. https://doi.org/10.1186/s13058-019-1235-8.Search in Google Scholar PubMed PubMed Central
30. Page, K, Guttery, DS, Fernandez-Garcia, D, Hills, A, Hastings, RK, Luo, J, et al.. Next generation sequencing of circulating cell-free DNA for evaluating mutations and gene amplification in metastatic breast cancer. Clin Chem 2017;63:532–41. https://doi.org/10.1373/clinchem.2016.261834.Search in Google Scholar PubMed PubMed Central
31. Davis, AA, Zhang, Q, Gerratana, L, Shah, AN, Zhan, Y, Qiang, W, et al.. Association of a novel circulating tumor DNA next-generating sequencing platform with circulating tumor cells (CTCs) and CTC clusters in metastatic breast cancer. Breast Cancer Res 2019;21:137. https://doi.org/10.1186/s13058-019-1229-6.Search in Google Scholar PubMed PubMed Central
32. Gerratana, L, Davis, AA, Zhang, Q, Basile, D, Rossi, G, Strickland, K, et al.. Longitudinal dynamics of circulating tumor cells and circulating tumor DNA for treatment monitoring in metastatic breast cancer. JCO Precis Oncol 2021;5:943–52. https://doi.org/10.1200/po.20.00345.Search in Google Scholar PubMed PubMed Central
33. Bortolini Silveira, A, Bidard, F-C, Tanguy, M-L, Girard, E, Trédan, O, Dubot, C, et al.. Multimodal liquid biopsy for early monitoring and outcome prediction of chemotherapy in metastatic breast cancer. NPJ Breast Cancer 2021;7:115. https://doi.org/10.1038/s41523-021-00319-4.Search in Google Scholar PubMed PubMed Central
34. Nanou, A, Miller, MC, Zeune, LL, de Wit, S, Punt, CJA, Groen, HJM, et al.. Tumour-derived extracellular vesicles in blood of metastatic cancer patients associate with overall survival. Br J Cancer 2020;122:801–11. https://doi.org/10.1038/s41416-019-0726-9.Search in Google Scholar PubMed PubMed Central
35. Nanou, A, Zeune, LL, Bidard, F-C, Pierga, J-Y, Terstappen, LWMM. HER2 expression on tumor-derived extracellular vesicles and circulating tumor cells in metastatic breast cancer. Breast Cancer Res 2020;22:86. https://doi.org/10.1186/s13058-020-01323-5.Search in Google Scholar PubMed PubMed Central
36. Keup, C, Mach, P, Aktas, B, Tewes, M, Kolberg, H-C, Hauch, S, et al.. RNA profiles of circulating tumor cells and extracellular vesicles for therapy stratification of metastatic breast cancer patients. Clin Chem 2018;64:1054–62. https://doi.org/10.1373/clinchem.2017.283531.Search in Google Scholar PubMed
37. Chimonidou, M, Strati, A, Malamos, N, Georgoulias, V, Lianidou, ES. SOX17 promoter methylation in circulating tumor cells and matched cell-free DNA isolated from plasma of patients with breast cancer. Clin Chem 2013;59:270–9. https://doi.org/10.1373/clinchem.2012.191551.Search in Google Scholar PubMed
38. Chimonidou, M, Strati, A, Malamos, N, Kouneli, S, Georgoulias, V, Lianidou, E. Direct comparison study of DNA methylation markers in EpCAM-positive circulating tumour cells, corresponding circulating tumour DNA, and paired primary tumours in breast cancer. Oncotarget 2017;8:72054–68. https://doi.org/10.18632/oncotarget.18679.Search in Google Scholar PubMed PubMed Central
39. Mastoraki, S, Strati, A, Tzanikou, E, Chimonidou, M, Politaki, E, Voutsina, A, et al.. ESR1 methylation: a liquid biopsy-based epigenetic assay for the follow-up of patients with metastatic breast cancer receiving endocrine treatment. Clin Cancer Res 2018;24:1500–10. https://doi.org/10.1158/1078-0432.ccr-17-1181.Search in Google Scholar PubMed
40. Liu, HE, Vuppalapaty, M, Wilkerson, C, Renier, C, Chiu, M, Lemaire, C, et al.. Detection of EGFR mutations in cfDNA and CTCs, and comparison to tumor tissue in non-small-cell-lung-cancer (NSCLC) patients. Front Oncol 2020;10:572895. https://doi.org/10.3389/fonc.2020.572895.Search in Google Scholar PubMed PubMed Central
41. Welter, L, Xu, L, McKinley, D, Dago, AE, Prabakar, RK, Restrepo-Vassalli, S, et al.. Treatment response and tumor evolution: lessons from an extended series of multianalyte liquid biopsies in a metastatic breast cancer patient. Cold Spring Harb Mol Case Stud 2020;6:a005819. https://doi.org/10.1101/mcs.a005819.Search in Google Scholar PubMed PubMed Central
42. Shishido, SN, Masson, R, Xu, L, Welter, L, Prabakar, RK, D’ Souza, A, et al.. Disease characterization in liquid biopsy from HER2-mutated, non-amplified metastatic breast cancer patients treated with neratinib. NPJ Breast Cancer 2022;8:22. https://doi.org/10.1038/s41523-022-00390-5.Search in Google Scholar PubMed PubMed Central
43. Beije, N, Sieuwerts, AM, Kraan, J, Van, NM, Onstenk, W, Vitale, SR, et al.. Estrogen receptor mutations and splice variants determined in liquid biopsies from metastatic breast cancer patients. Mol Oncol 2018;12:48–57. https://doi.org/10.1002/1878-0261.12147.Search in Google Scholar PubMed PubMed Central
44. Keup, C, Storbeck, M, Hauch, S, Hahn, P, Sprenger-Haussels, M, Tewes, M, et al.. Cell-free DNA variant sequencing using CTC-depleted blood for comprehensive liquid biopsy testing in metastatic breast cancer. Cancers 2019;11:238. https://doi.org/10.3390/cancers11020238.Search in Google Scholar PubMed PubMed Central
45. Keup, C, Benyaa, K, Hauch, S, Sprenger-Haussels, M, Tewes, M, Mach, P, et al.. Targeted deep sequencing revealed variants in cell-free DNA of hormone receptor-positive metastatic breast cancer patients. Cell Mol Life Sci 2019;77:497–509. https://doi.org/10.1007/s00018-019-03189-z.Search in Google Scholar PubMed PubMed Central
46. Keup, C, Storbeck, M, Hauch, S, Hahn, P, Sprenger-Haussels, M, Hoffmann, O, et al.. Multimodal targeted deep sequencing of circulating tumor cells and matched cell-free DNA provides a more comprehensive tool to identify therapeutic targets in metastatic breast cancer patients. Cancers 2020;12:1084. https://doi.org/10.3390/cancers12051084.Search in Google Scholar PubMed PubMed Central
47. Stergiopoulou, D, Markou, A, Tzanikou, E, Ladas, I, Makrigiorgos, GM, Georgoulias, V, et al.. ESR1 NAPA assay: development and analytical validation of a highly sensitive and specific blood-based assay for the detection of ESR1 mutations in liquid biopsies. Cancers 2021;13:556. https://doi.org/10.3390/cancers13030556.Search in Google Scholar PubMed PubMed Central
48. Tzanikou, E, Markou, A, Politaki, E, Koutsopoulos, A, Psyrri, A, Mavroudis, D, et al.. PIK3CA hotspot mutations in circulating tumor cells and paired circulating tumor DNA in breast cancer: a direct comparison study. Mol Oncol 2019;13:2515–30. https://doi.org/10.1002/1878-0261.12540.Search in Google Scholar PubMed PubMed Central
49. Appierto, V, Di Cosimo, S, Reduzzi, C, Pala, V, Cappelletti, V, Daidone, MG. How to study and overcome tumor heterogeneity with circulating biomarkers: the breast cancer case. Semin Cancer Biol 2017;44:106–16. https://doi.org/10.1016/j.semcancer.2017.04.007.Search in Google Scholar PubMed
50. Lianidou, E, Pantel, K. Liquid biopsies. Genes Chromosomes Cancer 2019;58:219–32. https://doi.org/10.1002/gcc.22695.Search in Google Scholar PubMed
51. Cohen, JD, Li, L, Wang, Y, Thoburn, C, Afsari, B, Danilova, L, et al.. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018;359:926–30. https://doi.org/10.1126/science.aar3247.Search in Google Scholar PubMed PubMed Central
52. Haber, DA, Velculescu, VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov 2014;4:650–61. https://doi.org/10.1158/2159-8290.CD-13-1014.Search in Google Scholar PubMed PubMed Central
53. Lambert, AW, Weinberg, RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer 2021;21:325–38. https://doi.org/10.1038/s41568-021-00332-6.Search in Google Scholar PubMed
54. Wan, JCM, Massie, C, Garcia-Corbacho, J, Mouliere, F, Brenton, JD, Caldas, C, et al.. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017;17:223–38. https://doi.org/10.1038/nrc.2017.7.Search in Google Scholar PubMed
55. Bronkhorst, AJ, Ungerer, V, Holdenrieder, S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol Detect Quantif 2019;17:100087. https://doi.org/10.1016/j.bdq.2019.100087.Search in Google Scholar PubMed PubMed Central
56. van der Pol, Y, Mouliere, F. Toward the early detection of cancer by decoding the epigenetic and environmental fingerprints of cell-free DNA. Cancer Cell 2019;36:350–68. https://doi.org/10.1016/j.ccell.2019.09.003.Search in Google Scholar PubMed
57. Keup, C, Kimmig, R, Kasimir-Bauer, S. Combinatorial power of cfDNA, CTCs and EVs in oncology. Diagnostics 2022;12:870. https://doi.org/10.3390/diagnostics12040870.Search in Google Scholar PubMed PubMed Central
58. Schneegans, S, Lück, L, Besler, K, Bluhm, L, Stadler, J-C, Staub, J, et al.. Pre-analytical factors affecting the establishment of a single tube assay for multiparameter liquid biopsy detection in melanoma patients. Mol Oncol 2020;14:1001–15. https://doi.org/10.1002/1878-0261.12669.Search in Google Scholar PubMed PubMed Central
59. Gerber, T, Taschner-Mandl, S, Saloberger-Sindhöringer, L, Popitsch, N, Heitzer, E, Witt, V, et al.. Assessment of pre-analytical sample handling conditions for comprehensive liquid biopsy analyses. J Mol Diagn 2020;22:1070–86. https://doi.org/10.1016/j.jmoldx.2020.05.006.Search in Google Scholar PubMed
60. Keup, C, Suryaprakash, V, Hauch, S, Storbeck, M, Hahn, P, Sprenger-Haussels, M, et al.. Integrative statistical analyses of multiple liquid biopsy analytes in metastatic breast cancer. Genome Med 2021;13:85. https://doi.org/10.1186/s13073-021-00902-1.Search in Google Scholar PubMed PubMed Central
61. Keup, C, Suryaprakash, V, Storbeck, M, Hoffmann, O, Kimmig, R, Kasimir-Bauer, S. Longitudinal multi-parametric liquid biopsy approach identifies unique features of circulating tumor cell, extracellular vesicle, and cell-free DNA characterization for disease monitoring in metastatic breast cancer patients. Cells 2021;10:212. https://doi.org/10.3390/cells10020212.Search in Google Scholar PubMed PubMed Central
62. Cisneros-Villanueva, M, Hidalgo-Pérez, L, Rios-Romero, M, Cedro-Tanda, A, Ruiz-Villavicencio, CA, Page, K, et al.. Cell-free DNA analysis in current cancer clinical trials: a review. Br J Cancer 2022;126:391–400. https://doi.org/10.1038/s41416-021-01696-0.Search in Google Scholar PubMed PubMed Central
63. Bidard, FC, Hardy-bessard, AC, Bachelot, T, Pierga, J-Y, Canon, JL, Clatot, F, et al.. editors. Fulvestrant-palbociclib vs continuing aromatase inhibitor-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2- metastatic breast cancer patients: Results of PADA-1, a UCBG-GINECO randomized phase 3 trial: Abstract GS3-05. Cancer Res 2022;82(4 Suppl):GS3-05. https://doi.org/10.1158/1538-7445.SABCS21-GS3-05.Search in Google Scholar
64. Rolfo, C, Cardona, AF, Cristofanilli, M, Paz-Ares, L, Diaz Mochon, JJ, Duran, I, et al.. Challenges and opportunities of cfDNA analysis implementation in clinical practice: perspective of the International Society of Liquid Biopsy (ISLB). Crit Rev Oncol Hematol 2020;151:102978.10.1016/j.critrevonc.2020.102978Search in Google Scholar PubMed
65. Antoniou, M, Jorgensen, AL, Kolamunnage-Dona, R. Biomarker-guided adaptive trial designs in phase II and phase III: a methodological review. PLoS One 2016;11:e0149803. https://doi.org/10.1371/journal.pone.0149803.Search in Google Scholar PubMed PubMed Central
66. Wulfkuhle, JD, Spira, A, Edmiston, KH, Petricoin, EF. Innovations in clinical trial design in the era of molecular profiling. Methods Mol Biol 2017;1606:19–36. https://doi.org/10.1007/978-1-4939-6990-6_2.Search in Google Scholar PubMed
67. Freidlin, B, Korn, EL. Biomarker enrichment strategies: matching trial design to biomarker credentials. Nat Rev Clin Oncol 2014;11:81–90. https://doi.org/10.1038/nrclinonc.2013.218.Search in Google Scholar PubMed
68. Yuwono, NL, Warton, K, Ford, CE. The influence of biological and lifestyle factors on circulating cell-free DNA in blood plasma. Elife 2021;10:e69679. https://doi.org/10.7554/eLife.69679.Search in Google Scholar PubMed PubMed Central
69. Yuwono, NL, Henry, CE, Ford, CE, Warton, K. Total and endothelial cell-derived cell-free DNA in blood plasma does not change during menstruation. PLoS One 2021;16:e0250561. https://doi.org/10.1371/journal.pone.0250561.Search in Google Scholar PubMed PubMed Central
70. Hsieh, C-C, Hsu, H-S, Chang, S-C, Chen, Y-J. Circulating cell-free DNA levels could predict oncological outcomes of patients undergoing esophagectomy for esophageal squamous cell carcinoma. Int J Mol Sci 2016;17:2131. https://doi.org/10.3390/ijms17122131.Search in Google Scholar PubMed PubMed Central
71. Kim, K, Shin, DG, Park, MK, Baik, SH, Kim, TH, Kim, S, et al.. Circulating cell-free DNA as a promising biomarker in patients with gastric cancer: diagnostic validity and significant reduction of cfDNA after surgical resection. Ann Surg Treat Res 2014;86:136–42. https://doi.org/10.4174/astr.2014.86.3.136.Search in Google Scholar PubMed PubMed Central
72. Hummel, EM, Hessas, E, Müller, S, Beiter, T, Fisch, M, Eibl, A, et al.. Cell-free DNA release under psychosocial and physical stress conditions. Transl Psychiatry 2018;8:236. https://doi.org/10.1038/s41398-018-0264-x.Search in Google Scholar PubMed PubMed Central
73. Czamanski-Cohen, J, Sarid, O, Cwikel, J, Levitas, E, Lunenfeld, E, Douvdevani, A, et al.. Decrease in cell free DNA levels following participation in stress reduction techniques among women undergoing infertility treatment. Arch Womens Ment Health 2014;17:251–3. https://doi.org/10.1007/s00737-013-0407-2.Search in Google Scholar PubMed
74. Tug, S, Mehdorn, M, Helmig, S, Breitbach, S, Ehlert, T, Simon, P. Exploring the potential of cell-free-DNA measurements after an exhaustive cycle-ergometer test as a marker for performance-related parameters. Int J Sports Physiol Perform 2017;12:597–604. https://doi.org/10.1123/ijspp.2016-0157.Search in Google Scholar PubMed
75. Ohlsson, L, Hall, A, Lindahl, H, Danielsson, R, Gustafsson, A, Lavant, E, et al.. Increased level of circulating cell-free mitochondrial DNA due to a single bout of strenuous physical exercise. Eur J Appl Physiol 2020;120:897–905. https://doi.org/10.1007/s00421-020-04330-8.Search in Google Scholar PubMed PubMed Central
76. Mavropalias, G, Calapre, L, Morici, M, Koeda, T, Poon, WCK, Barley, OR, et al.. Changes in plasma hydroxyproline and plasma cell-free DNA concentrations after higher- versus lower-intensity eccentric cycling. Eur J Appl Physiol 2021;121:1087–97. https://doi.org/10.1007/s00421-020-04593-1.Search in Google Scholar PubMed
77. Frühbeis, C, Helmig, S, Tug, S, Simon, P, Krämer-Albers, E-M. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles 2015;4:28239. https://doi.org/10.3402/jev.v4.28239.Search in Google Scholar PubMed PubMed Central
78. Brahmer, A, Neuberger, E, Esch-Heisser, L, Haller, N, Jorgensen, MM, Baek, R, et al.. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J Extracell Vesicles 2019;8:1615820. https://doi.org/10.1080/20013078.2019.1615820.Search in Google Scholar PubMed PubMed Central
79. Chiva-Blanch, G, Laake, K, Myhre, P, Bratseth, V, Arnesen, H, Solheim, S, et al.. High adherence to the nordic diet is associated with lower levels of total and platelet-derived circulating microvesicles in a Norwegian population. Nutrients 2019;11:1114. https://doi.org/10.3390/nu11051114.Search in Google Scholar PubMed PubMed Central
80. Torregrosa Paredes, P, Gutzeit, C, Johansson, S, Admyre, C, Stenius, F, Alm, J, et al.. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy 2014;69:463–71. https://doi.org/10.1111/all.12357.Search in Google Scholar PubMed
81. Bogeska, R. Resilience to social stress: is it in the blood? FEBS Open Bio 2021;11:2675–7. https://doi.org/10.1002/2211-5463.13291.Search in Google Scholar PubMed PubMed Central
82. Wu, F, Yin, Z, Yang, L, Fan, J, Xu, J, Jin, Y, et al.. Smoking induced extracellular vesicles release and their distinct properties in non-small cell lung cancer. J Cancer 2019;10:3435–43. https://doi.org/10.7150/jca.30425.Search in Google Scholar PubMed PubMed Central
83. Tao, S-C, Guo, S-C. Extracellular vesicles: potential participants in circadian rhythm synchronization. Int J Biol Sci 2018;14:1610–20. https://doi.org/10.7150/ijbs.26518.Search in Google Scholar PubMed PubMed Central
84. Cortés-Hernández, LE, Eslami-S, Z, Dujon, AM, Giraudeau, M, Ujvari, B, Thomas, F, et al.. Do malignant cells sleep at night? Genome Biol 2020;21:276. https://doi.org/10.1186/s13059-020-02179-w.Search in Google Scholar PubMed PubMed Central
85. Ungerer, V, Bronkhorst, AJ, Holdenrieder, S. Preanalytical variables that affect the outcome of cell-free DNA measurements. Crit Rev Clin Lab Sci 2020;57:484–507. https://doi.org/10.1080/10408363.2020.1750558.Search in Google Scholar PubMed
86. Stenzinger, A, Allen, JD, Maas, J, Stewart, MD, Merino, DM, Wempe, MM, et al.. Tumor mutational burden standardization initiatives: recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosomes Cancer 2019;58:578–88. https://doi.org/10.1002/gcc.22733.Search in Google Scholar PubMed PubMed Central
87. Roy, S, Coldren, C, Karunamurthy, A, Kip, NS, Klee, EW, Lincoln, SE, et al.. Standards and guidelines for validating next-generation sequencing bioinformatics pipelines: a joint recommendation of the Association for Molecular Pathology and the College of American Pathologists. J Mol Diagn 2018;20:4–27. https://doi.org/10.1016/j.jmoldx.2017.11.003.Search in Google Scholar PubMed
88. Richards, S, Aziz, N, Bale, S, Bick, D, Das, S, Gastier-Foster, J, et al.. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. https://doi.org/10.1038/gim.2015.30.Search in Google Scholar PubMed PubMed Central
89. Li, MM, Datto, M, Duncavage, EJ, Kulkarni, S, Lindeman, NI, Roy, S, et al.. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn 2017;19:4–23. https://doi.org/10.1016/j.jmoldx.2016.10.002.Search in Google Scholar PubMed PubMed Central
90. Jennings, LJ, Arcila, ME, Corless, C, Kamel-Reid, S, Lubin, IM, Pfeifer, J, et al.. Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn 2017;19:341–65. https://doi.org/10.1016/j.jmoldx.2017.01.011.Search in Google Scholar PubMed PubMed Central
91. Haselmann, V, Geilenkeuser, WJ, Helfert, S, Eichner, R, Hetjens, S, Neumaier, M, et al.. Thirteen years of an international external quality assessment scheme for genotyping: results and recommendations. Clin Chem 2016;62:1084–95. https://doi.org/10.1373/clinchem.2016.254482.Search in Google Scholar PubMed
92. Godsey, JH, Silvestro, A, Barrett, JC, Bramlett, K, Chudova, D, Deras, I, et al.. Generic protocols for the analytical validation of next-generation sequencing-based ctDNA assays: a joint consensus recommendation of the BloodPAC’s Analytical Variables Working Group. Clin Chem 2020;66:1156–66. https://doi.org/10.1093/clinchem/hvaa164.Search in Google Scholar PubMed PubMed Central
93. Merino, DM, McShane, LM, Fabrizio, D, Funari, V, Chen, S-J, White, JR, et al.. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer 2020;8:e000147. https://doi.org/10.1136/jitc-2019-000147.Search in Google Scholar PubMed PubMed Central
94. Théry, C, Witwer, KW, Aikawa, E, Alcaraz, MJ, Anderson, JD, Andriantsitohaina, R, et al.. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. https://doi.org/10.1080/20013078.2018.1535750.Search in Google Scholar PubMed PubMed Central
95. Bustin, SA, Benes, V, Garson, JA, Hellemans, J, Huggett, J, Kubista, M, et al.. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009;55:611–22. https://doi.org/10.1373/clinchem.2008.112797.Search in Google Scholar PubMed
96. Stewart, CM, Kothari, PD, Mouliere, F, Mair, R, Somnay, S, Benayed, R, et al.. The value of cell-free DNA for molecular pathology. J Pathol 2018;244:616–27. https://doi.org/10.1002/path.5048.Search in Google Scholar PubMed PubMed Central
97. Sánchez-Calderón, D, Pedraza, A, Mancera Urrego, C, Mejía-Mejía, A, Montealegre-Páez, AL, Perdomo, S. Analysis of the cost-effectiveness of liquid biopsy to determine treatment change in patients with Her2-positive advanced breast cancer in Colombia. Clinicoecon Outcomes Res 2020;12:115–22.10.2147/CEOR.S220726Search in Google Scholar PubMed PubMed Central
98. Aravanis, AM, Lee, M, Klausner, RD. Next-generation sequencing of circulating tumor DNA for early cCancer detection. Cell 2017;168:571–4. https://doi.org/10.1016/j.cell.2017.01.030.Search in Google Scholar PubMed
99. Mateo, J, Chakravarty, D, Dienstmann, R, Jezdic, S, Gonzalez-Perez, A, Lopez-Bigas, N, et al.. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol 2018;29:1895–902. https://doi.org/10.1093/annonc/mdy263.Search in Google Scholar PubMed PubMed Central
100. Perakis, SO, Weber, S, Zhou, Q, Graf, R, Hojas, S, Riedl, JM, et al.. Comparison of three commercial decision support platforms for matching of next-generation sequencing results with therapies in patients with cancer. ESMO Open 2020;5:e000872. https://doi.org/10.1136/esmoopen-2020-000872.Search in Google Scholar PubMed PubMed Central
© 2022 Corinna Keup et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Liquid profiling – circulating nucleic acid diagnostics gains momentum
- Articles
- The rising tide of cell-free DNA profiling: from snapshot to temporal genome analysis
- Liquid profiling for cancer patient stratification in precision medicine – current status and challenges for successful implementation in standard care
- Status of liquid profiling in precision oncology – the need for integrative diagnostics for successful implementation into standard care
- Pan-cancer screening by circulating tumor DNA (ctDNA) – recent breakthroughs and chronic pitfalls
- Multimodality in liquid biopsy: does a combination uncover insights undetectable in individual blood analytes?
- Circulating cell-free DNA and its clinical utility in cancer
- Are extracellular vesicles ready for the clinical laboratory?
- Profiling disease and tissue-specific epigenetic signatures in cell-free DNA
- Cell-free DNA in sports medicine: implications for clinical laboratory medicine
- Clonal hematopoiesis of indeterminate potential: clinical relevance of an incidental finding in liquid profiling
- Non-invasive prenatal screening tests – update 2022
- The DKTK EXLIQUID consortium – exploiting liquid biopsies to advance cancer precision medicine for molecular tumor board patients
Articles in the same Issue
- Frontmatter
- Editorial
- Liquid profiling – circulating nucleic acid diagnostics gains momentum
- Articles
- The rising tide of cell-free DNA profiling: from snapshot to temporal genome analysis
- Liquid profiling for cancer patient stratification in precision medicine – current status and challenges for successful implementation in standard care
- Status of liquid profiling in precision oncology – the need for integrative diagnostics for successful implementation into standard care
- Pan-cancer screening by circulating tumor DNA (ctDNA) – recent breakthroughs and chronic pitfalls
- Multimodality in liquid biopsy: does a combination uncover insights undetectable in individual blood analytes?
- Circulating cell-free DNA and its clinical utility in cancer
- Are extracellular vesicles ready for the clinical laboratory?
- Profiling disease and tissue-specific epigenetic signatures in cell-free DNA
- Cell-free DNA in sports medicine: implications for clinical laboratory medicine
- Clonal hematopoiesis of indeterminate potential: clinical relevance of an incidental finding in liquid profiling
- Non-invasive prenatal screening tests – update 2022
- The DKTK EXLIQUID consortium – exploiting liquid biopsies to advance cancer precision medicine for molecular tumor board patients

