A multivariable prediction model for intra-amniotic infection in patients with preterm labor and intact membranes including a point of care system that measures amniotic fluid MMP-8
-
Teresa Cobo
, Silvia Ferrero
Abstract
Objectives
Among patients with preterm labor and intact membranes (PTL), those with intra-amniotic infection (IAI) present the highest risk of adverse perinatal outcomes. Current identification of IAI, based on microbiological cultures and/or polymerase chain reaction amplification of the 16S ribosomal RNA gene, delay diagnosis and, consequently, antenatal management. The aim to of the study was to assess the performance of a multivariable prediction model for diagnosing IAI in patients with PTL below 34.0 weeks using clinical, sonographic and biochemical biomarkers.
Methods
From 2019 to 2022, we prospectively included pregnant patients admitted below 34.0 weeks with diagnosis of PTL and had undergone amniocentesis to rule in/out IAI. The main outcome was IAI, defined by a positive culture and/or 16S ribosomal RNA gene in amniotic fluid. Based on the date of admission, the sample (n=98) was divided into a derivation (2019–2020, n=49) and validation cohort (2021–2022, n=49). Logistic regression models were developed for the outcomes evaluated. As predictive variables we explored ultrasound cervical length measurement at admission, maternal C-reactive protein, gestational age, and amniotic fluid glucose and matrix metalloproteinase-8 (MMP-8) levels. The model was developed in the derivation cohort and applied to the validation cohort and diagnostic performance was evaluated. Clinical management was blinded to the model results.
Results
During the study period, we included 98 patients admitted with a diagnosis of PTL. Of these, 10 % had IAI. The final model included MMP-8 and amniotic fluid glucose levels and showed an area under the receiver operating characteristic curve to predict the risk of IAI of 0.961 (95 % confidence interval: 0.860–0.995) with a sensitivity of 75 %, specificity of 93.3 %, positive likelihood ratio (LR) of 11.3 and negative LR of 0.27 in the validation cohort.
Conclusions
In patients with PTL, a multivariable prediction model including amniotic fluid MMP-8 and glucose levels might help in the clinical management of patients undergoing amniocentesis to rule in/out IAI, providing results within a few minutes.
Introduction
There is wide evidence suggesting that the worst maternal and perinatal outcomes in patients with preterm labor and intact membranes (PTL) occur in those with intra-amniotic infection (IAI) [1], leading to early spontaneous preterm delivery and a high risk of clinical choriamnionitis [1, 2].
The diagnosis of IAI requires the performance of transabdominal amniocentesis. It has been reported that in patients diagnosed with PTL, the presence of IAI may be eradicated with broad-spectrum antibiotics [3]. In addition, this group has the shortest latency to delivery in days and the earliest gestational age at delivery [2] and may therefore benefit from antenatal strategies shown to improve perinatal outcomes in premature infants [4, 5].
The main concern regarding the diagnosis of IAI is that it is based on microbiological results that take 48–72 h, thereby delaying an early-targeted antenatal intervention. Gram staining has shown to be very specific for diagnosing IAI, but its diagnostic sensitivity is limited because it does not identify Ureaplasma spp., the most common microorganism isolated in patients with IAI. And for the other microorganisms usually involved, the sensitivity is lower than culture or molecular methods. In this regard, different authors have proposed biochemical markers of IAI that provide rapid results and can help in clinical decision-making. In this regard, amniotic fluid glucose values have a better sensitivity, although there is no consensus as to the best cut-off for the diagnosis of IAI [6], [7], [8].
In the last decades, several inflammatory amniotic fluid biomarkers for IAI have been studied [9], [10], [11], one of which is amniotic fluid matrix metalloproteinase (MMP)-8. Some authors have described a good diagnostic performance of this biomarker to predict the occurrence of IAI in both patients with PTL and those with preterm prelabor rupture of membranes (PPROM) [10, 11]. However, these studies used non-clinically feasible systems, such as multiplex immunoassay or enzyme-linked immunosorbent assay. Yoon et al. [11] proposed a qualitative point of care procedure based on a immunochromatographic method to measure amniotic fluid MMP-8 concentrations in patients with PTL providing results in 20 min (Yoon’s MMP-8 Check®; OBMed Co., Ltd., Seoul, Republic of Korea). However, to our knowledge, the authors did not validate their findings in a different cohort.
There is increasing evidence suggesting that multivariable models can improve the prediction of different adverse outcomes [12]. The aim of this study was to construct and validate a multivariable prediction model of IAI in patients with PTL using clinical, sonographic and biochemical variables that might be used as point-of-care systems to predict the presence of IAI within a few minutes, allowing early antenatal intervention in patients with PTL.
Materials and methods
Study design
This was a prospective observational study performed at the Hospital Clinic and Hospital Sant Joan de Déu, Barcelona from May 2019 to April 2022.
We included singleton pregnancies admitted with a diagnosis of PTL between 23.0 and 33.6 weeks, which did not meet exclusion criteria, and with an amniocentesis to rule in/out IAI.
Preterm labor was defined as the presence of regular uterine contractions with a frequency of at least two every 10 min and a cervical length measured by transvaginal ultrasound (US) less than 25 mm in patients with PTL below 28.0 weeks; less than 20 mm between 28.0 and 31.6 weeks and less than 15 mm above 32.0 weeks of gestation [13].
IAI defined as the presence of a positive amniotic fluid culture and/or by specific polymerase chain reaction (PCR) amplification of the 16S ribosomal RNA gene.
We excluded patients less than 18 years of age, patients with multiple gestations, PPROM, clinical chorioamnionitis [14] or Triple I defined by the presence of fever ≥38 °C, fetal tachycardia (>160 beats per minute>10 min) and maternal white blood cells >15,000/mm3 (not justified by the administration of antenatal corticosteroids), cervical dilatation >3 cm, major structural malformations or fetal complications, transvaginal cervical length measurement at admission>fifth centile [13], not feasible to perform amniocentesis and no consent to perform amniocentesis for this indication.
Gestational age was established according to crown-rump length at the first-trimester US scan and was defined as the difference between date of admission and last menstruation period (weeks) [15].
Written informed consent was obtained from all subjects. Patient selection and sampling procedures were performed in accordance with the Declaration of Helsinki and applicable local regulatory requirements after approval from the Institutional Review Boards (HCB/2018/0781; PIC-61-19).
Based on the date of admission, we explored clinical, sonographic and biomarkers as independent predictors of IAI in amniotic fluid and constructed a model in the first half of the cohort of PTL (derivation cohort; 2019–2020) that was validated in the last half of the cohort (validation cohort; 2021–2022).
Clinical management
According to the institutional clinical protocols, patients admitted with diagnosis of PTL before 34.0 weeks were offered amniocentesis to rule in/out IAI.
A complete course of antenatal steroids (betamethasone 12 mg intramuscular injection with two doses given 24 h apart) was administered following our local protocol and FIGO recommendations [16] until 34.6 weeks for fetal lung maturation.
Broad-spectrum antibiotics were initiated in patients with high suspicion of IAI, based on the presence of low amniotic fluid glucose concentrations (<5 mg/dL) and/or with microorganisms identified by Gram staining of amniotic fluid. From May 2019 to December 2019, patients with high suspicion of IAI received parenteral ampicillin 1 g/6 h and gentamycin 80 mg/8 h and a single dose of oral azithromycin 1 g. Beyond December 2019, our local protocol substituted this antibiotic combination to parenteral ceftriaxone 1 g/12 h and ampicillin 2 g/6 h and oral clarithromycin 500 mg/8 h, due to the reported evidence of higher placental penetrance [17]. Antibiotic treatment was discontinued when amniotic fluid cultures were negative. In patients diagnosed with IAI who remained pregnant after microbiological results, we individualized the antibiotic treatment according to the microorganism isolated until 7–10 days. Labor induction was considered only if clinical chorioamnionitis occurred or after 34 weeks in patients with IAI.
Information of amniotic MMP-8 concentrations was not available for clinical decision-making.
Classification of outcomes
The primary outcome was IAI. Both aerobic and anaerobic cultures were performed (Columbia agar, Chocolate agar, Schaedler agar and thioglycollate broth). Moreover, selective media for yeasts (Sabouraud agar) and genital mycoplasma (Mycoplasma IST 2, bioMérieux for Ureaplasma spp. or Mycoplasma hominis) were also used. When there were discrepancies between amniotic fluid glucose concentrations and microbiological culture results (e.g. amniotic fluid glucose <14 mg/dL but negative culture) we performed targeted polymerase chain reaction (PCR) amplification of the 16S ribosomal RNA gene to confirm or not the presence of IAI.
Predictors
Independent predictors of IAI found in the univariate logistic regression analysis were used to develop the multivariable prediction model. We evaluated gestational age at amniocentesis (weeks), US cervical length measurement (mm) at admission, maternal C-reactive protein (CRP) concentrations (mg/L) at admission, amniotic fluid glucose concentrations (mg/dL) and amniotic fluid MMP-8 concentrations (µg/L).
Point-of-care system to measure amniotic fluid MMP-8
MMP-8 concentrations were measured using a point-of-care system (Actim Oy) that employs monoclonal antibodies specific for active MMP-8. The procedure is an immunochromatographic, lateral flow method that provides quantitative results. The reference method in developing the point-of-care method has been an in-house ELISA. The analytical sensitivity, i.e. limit of detection, LoD, of the MMP-8 point-of-care system was 4.6 μg/L. The clinical performance of the point-of-care system has been evaluated using 101 amniotic fluid specimens, obtained from pregnant subjects with and without infection. Using a threshold 50 μg/L, the clinical sensitivity was 95.8 % and clinical specificity 94.8 %, respectively. Clinical management was blinded to the point-of-care system results.
Amniotic fluid glucose methodology
To measure glucose concentrations in amniotic fluid, the GLUC Dimension Ref DF40 procedure of Siemens Healthineers was used. This procedure is based on the hexokinase measurement principle and is automated in the Dimension® EXL analyzer, also from Siemens. Glucose concentrations range from 1–500 mg/dL. The detection level was 1 mg/dL with a coefficient of variation for inter-assay imprecision of 1.6 %. For calibration, the CHEM 1 Calibrator Dimension was used, which is traceable to NIST SRM 917. The method was evaluated for interference from hemolysis (up to 1,000 mg/dL of hemoglobin), icterus (20 mg/dL of bilirubin), and lipemia (400 mg/dL of intralipid®) according to CLSI/NCCLS EP7-P. The method did not show interference up to the concentrations studied.
Statistical analysis
Statistical analysis was performed using Stata 14.2 for Mac (StatCorp, LP). The Shapiro Wilk test was initially used to assess continuous data for normality. Continuous variables were compared using a non-parametric Mann–Whitney U test presented as median with a 95 % confidence interval (CI). Categorical variables were compared using the Chi-squared or Fisher exact test. Differences were considered statistically significant with a p <0.05 with two-sided alternative hypotheses.
Multivariable logistic regression analysis was used to identify independent factors associated with the outcomes (prediction model development). The model that could best predict IAI was constructed based on the final regression model and the direction of effects. Goodness-of-fit models were assessed by calculating Nagelkerke’s R2.
From the probabilistic output of each model, the diagnostic performance was calculated using receiver operating characteristic (ROC) curves. The area under the curve was reported and then the optimal cut-off threshold was selected as that maximizing accuracy and was used to compute sensitivity, specificity, the positive likelihood ratio (LR+), and the negative likelihood ratio (LR−) for IAI in the derivation and the validation cohorts.
Results
During the study period (2019–2022), 98 patients diagnosed with PTL were included (Figure 1). Of these, 10/98 (10 %) had IAI.
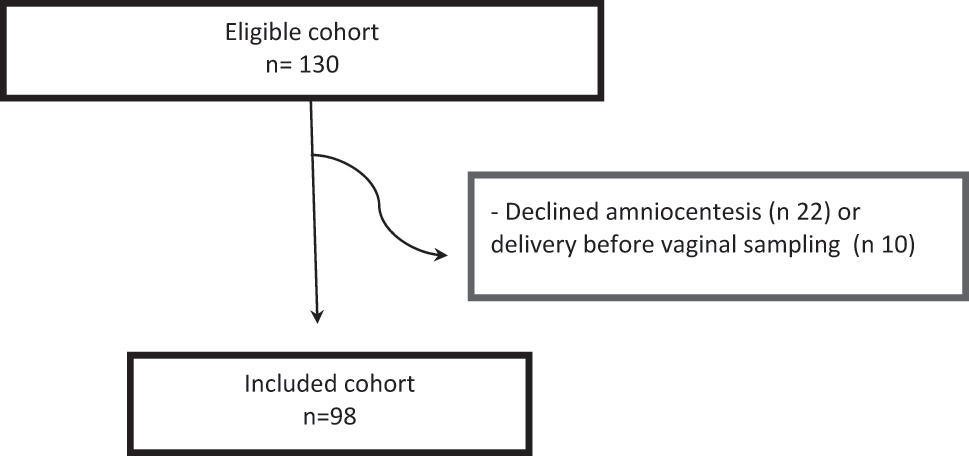
Flow-chart of entire study population.
Median (25th percentile; 75th percentile) of maternal age was 32.3 (26.9; 35.9) years; gestational age at amniocentesis was 29.5 (27.4; 31.1) weeks; US cervical length was 16 (12; 22) mm; gestational age at delivery was 34.9 (32.2; 38.2) weeks and latency to delivery was 35.5 (9; 63.5) days.
According to gestational age at admission, 36 patients were admitted below 28.0 weeks and 62 above.
From the entire study group, 10/98 (10 %) had IAI: four patients below 28.0 weeks and the remaining six between 28.0 and 31.6 weeks. All patients with IAI showed high levels of amniotic fluid IL-6 (according to the cut-off proposed by our group ≥13.4 ng/mL [18]).
The microorganisms isolated in the amniotic fluid in patients with PTL were: Ureaplasma spp. (n=5), Fusobacterium nucleatum (n=2) and Haemophilus influenzae (n=1). A polymicrobial infection was found in two patients: Peptosteptococcus anaerobius and Candida albicans (n=1) and Bacteroides caccae and Bacteroides vulgatus (n=1).
The maternal characteristics and perinatal outcomes according to the presence of IAI in patients with PTL are shown in Table 1. There were no complications related to the invasive procedure.
Maternal characteristics and perinatal outcomes in patients with preterm labor and intact membranes (n 98).
| IAI (n=10) | No IAI (n=88) | p | |
|---|---|---|---|
| Maternal age at admission, years | 36.5 (32.5; 39.7) | 31.8 (26.5;34.8) | 0.0557 |
| BMI | 21.6 (19.9;23.4) | 23.9 (20.5; 28.6) | 0.1615 |
| Caucasian ethnicity | 5 (50) | 63 (72) | 0.013 |
| Nulliparity | 4 (40) | 48 (55) | 0.382 |
| Smoking | 0 | 15 (17) | 0.156 |
| Prior invasive procedure | 0 | 3 (3) | 0.553 |
| Gestational age at admission, weeks | 29.2 (26.8; 30.8) | 29.5 (27.4; 31.2) | 0.5299 |
| US cervical length, mm | 19 (15;25) | 16 (12;21) | 0.5338 |
| Maternal CRP, mg/dL | 0.95 (0.3; 1.77) | 0.52 (0.28; 1.41) | 0.3100 |
| Maternal WBC, ×109 | 13,915 (10,360; 18,120) | 12,625 (10,960;15,925) | 0.6097 |
| Gestational age at amniocentesis, week | 29.2 (26.8; 30.8) | 29.5 (27.4; 31.2) | 0.5338 |
| AF glucose, mg/dL | 4 (2; 11) | 38.5 (30; 49.5) | <0.0001 |
| AF MMP-8, µg/L | 566.5 (305; 992) | 5.45 (0; 25.85) | 0.0001 |
| Gestational age at delivery, weeks | 30.6 (27.7; 32.1) | 35.4 (32.8; 38.4) | 0.0008 |
| Latency from admission to delivery, days | 6.5 (0;9) | 40.5 (13;68) | 0.0009 |
| Spontaneous onset of labor | 2 (20) | 75/82 (91) | 0.249 |
| Vaginal delivery | 5 (50) | 58/82 (71) | 0.150 |
| Neonatal weight, g | 1,510 (1,000; 1,560) | 2,410 (1,880; 2,980) | 0.0005 |
| 5 min Apgar<7 | 1 (10) | 1 (1) | 0.060 |
| UA pH<7.1 | 2 (20) | 4 (5) | 0.053 |
| Clinical chorioamnionitis | 5 (50) | 5/80 (6) | <0.001 |
-
BMI, body mass index; US, ultrasound; CRP, C-reactive protein; WBC, white blood cells; AF, amniotic fluid; UA, umbilical artery; MMP-8, matrix metalloproteinase-8; IAI, intra-amniotic infection. Continuous variables were compared using a nonparametric Mann Whitney U test presented as medians (25th percentile; 75 % percentile). Categorical variables were compared using Chi-square or Fisher exact tests and presented as number (%).
The derivation cohort included 49 patients (6 with IAI (12 %)) and the validation cohort 49 patients (8 with IAI (8 %)). Maternal characteristics and perinatal outcomes in the derivation and the validation cohorts are shown in Table 2. Multivariable analysis indicated that amniotic fluid glucose and MMP-8 were independent predictors for IAI (Table 3). The best regression formula for predicting IAI was: 1.6488–0.1835*amniotic fluid glucose−0.0000588*amniotic fluid MMP-8. R2 55.71 %.
Maternal characteristics and perinatal outcomes in the derivation and the validation cohorts.
| Derivation cohort (n=49) | Validation cohort (n=49) | p | |
|---|---|---|---|
| Maternal age at admission, years | 32.5 (27.3; 36.6) | 32.1 (26.1; 34.6) | 0.744 |
| BMI | 24.7 (21.6; 27.5) | 22.1 (20.3; 28.7) | 0.319 |
| Caucasian ethnicity | 29 (59) | 39 (80) | 0.171 |
| Nulliparity | 30 (61) | 22 (45) | 0.105 |
| Smoking | 9 (18) | 6 (12) | 0.400 |
| Prior invasive procedure | 0 | 3 (6) | 0.079 |
| Gestational age at admission, weeks | 29.6 (27.4; 31.1) | 29 (27.1; 31.3) | 0.678 |
| US cervical length, mm | 16 (10; 23) | 16 (13; 21) | 0.719 |
| Maternal CRP, mg/dL | 0.51 (0.35; 1.28) | 0.58 (0.25; 1.77) | 0.551 |
| Maternal WBC, ×109 | 13,870 (11,110; 16,900) | 12,590 (10,360; 14,290) | 0.112 |
| Gestational age at amniocentesis, weeks | 29.6 (27.6; 31.1) | 29.1 (27.1; 31.3) | 0.672 |
| AF glucose, mg/dL | 35 (25; 44) | 39 (29; 49) | 0.287 |
| AF MMP-8, µg/L | 10 (0; 31.3) | 10 (0; 31.9) | 0.903 |
| Gestational age at delivery, weeks | 34.8 (32.3; 38.1) | 35.1 (32.3; 38,3) | 0.937 |
| Latency from admission to delivery, days | 39 (5; 68) | 35 (12; 59) | 0.749 |
| Spontaneous onset of labor | 46/47 (98) | 37/45 (82) | 0.012 |
| Vaginal delivery | 33/47 (70) | 30/45 (67) | 0.349 |
| Neonatal weight, g | 2,280 (1,750; 2,950) | 2,248 (1,711; 2,953) | 0.930 |
| 5 min Apgar<7 | 1/47 (2) | 1/45 (2) | 1.000 |
| UA pH<7.1 | 3/47 (6) | 3/45 (7) | 1.000 |
| Clinical chorioamnionitis at labor | 9/46 (20) | 1/44 (2) | 0.009 |
-
PTL, preterm labor and intact membranes; BMI, body mass index; US, ultrasound; CRP, C-reactive protein; WBC, white blood cells; AF, amniotic fluid; UA, umbilical artery; MMP-8, matrix metalloproteinase-8. Continuous variables were compared using a nonparametric Mann Whitney U test presented as medians (25th percentile; 75 % percentile). Categorical variables were compared using Chi-square or Fisher exact tests and presented as number (%).
Independence of different variables to predict intra-amniotic infection.
| Variables | OR | 95 % CI |
|---|---|---|
| AF MMP-8, µg/L | 1.004 | 1.002–1.006 |
| Maternal age, years | 1.122 | 0.986–1.276 |
| GA at amniocentesis, weeks | 0.979 | 0.804–1.194 |
| US cervical length, mm | 1.061 | 0.974–1.155 |
| Maternal CRP, mg/dL | 1.071 | 0.799–1.436 |
| AF glucose, mg/dL | 0.832 | 0.752–0.919 |
-
OR, odds ratio; CI, confidence interval; AF, amniotic fluid; MMP-8, matrix metalloproteinase-8; GA, gestational age; CRP, C-reactive protein; US, ultrasound.
The diagnostic performance of the model for predicting IAI was assessed using ROC curves. The area under the ROC curve (AUROC) of the model proposed was 0.969 (95 % CI: 0.905–1.000) in the derivation cohort and 0.961 (95 % CI: 0.860–0.995) in the validation cohort (Figure 2). In the validation cohort, the sensitivity to predict IAI was 75 %, with a specificity of 93.3 %, a LR+ of 11.3 and a LR− of 0.27.

Diagnostic performance of the model for predicting IAI using ROC curve in the validation cohort.
Discussion
Main finding
We proposed a multivariable prediction model for IAI including amniotic fluid MMP-8 and amniotic fluid glucose concentrations in patients with PTL, showing good diagnostic performance and providing results within a few minutes.
Results in the context of “What is Known”
Similar to previous reports [1, 2, 19], we found IAI in 10 % of patients with PTL below 34 weeks. As expected, and similar to the results of other authors, IAI was related to early spontaneous preterm delivery and a short latency to delivery.
In line with previous reports [11] we found amniotic fluid MMP-8 to be an independent predictor of IAI. However, other authors did not validate their findings or did not use a point-of-care system that provides result within a few minutes as in our study.
Similarly, several authors have proposed different cutoffs of amniotic fluid glucose concentrations to predict IAI [6], [7], [8], all with a low false-positive rate. However, there is no consensus about what cutoff to use to diagnose IAI.
We proposed a prediction model integrating amniotic fluid glucose (mg/dL) and MMP-8 (µg/L) concentrations, improving the diagnostic performance of both biomarkers used separately.
Clinical implications
Yoon et al. [3] has shown that IAI in some patients with PTL might be eradicated using a combination of broad-spectrum antibiotics. The performance of amniocentesis to diagnose IAI targets a group with high risk of worse perinatal outcomes [1, 2] who might benefit of an early antibiotic treatment before definitive microbiological results are available.
The main concern in the diagnosis of IAI is that it is based on microbiological cultures that delay an early and targeted intervention. We proposed a multivariable prediction model including biomarkers that can provide a rapid result in a few minutes. Discriminating high and low-risk groups of IAI allows efficient use of resources and targeted use of antibiotics, which when used indiscriminately show no improvement in the short- and long-term outcomes of newborns and may negatively affect long-term neurodevelopment [20, 21].
Strengths and limitations
One of the main strengths of this study was the validation of the model in an independent cohort. Finally, another strength of this study was its prospective design and the use of fresh amniotic fluid samples in which amniotic fluid MMP-8 concentrations were determined using point-of-care systems.
However, as limitations of this study, we acknowledge that the prevalence of IAI in patients with PTL might not justify the indiscriminate performance of an invasive procedure to diagnose IAI. However, in patients with symptoms of PTL at early gestational ages (e.g. less than 28 weeks) and/or with a short US cervical length [18], tertiary centers such ours might consider the performance of amniocentesis to rule out an infectious origin. We also acknowledge as limitation we did not perform PCR targeting the 16S ribosomal RNA gene sequence in all patients and this might under-diagnose the prevalence of intra-amniotic infection in our population. In addition, the study was not designed to predict adverse outcomes other than IAI. Finally, to our knowledge, the amniotic fluid MMP-8 point-of-care system provided by Actim Oy is not yet commercialized.
Research implications
Future studies are required to prospectively evaluate the influence of the model in improving clinical management and the potential benefits of early antibiotic treatment in patients with a high-predicted risk of IAI.
Conclusions
The present study shows that in patients suspected of having IAI, a prediction model including amniotic fluid glucose and MMP-8 concentrations may be useful to individualize the antenatal intervention since this is the group with the highest risk of imminent delivery within the following days.
Funding source: Actim Oy
Funding source: Instituto Carlos Slim de la Salud
Award Identifier / Grant number: PI19/00580
Award Identifier / Grant number: PI22/00669
Acknowledgments
The authors would like to acknowledge the antenatal clinic at the maternal and fetal medicine department of Hospital Clinic and Hospital Sant Joan de Déu, Barcelona, Spain, for their help with recruitment and sampling.
-
Research ethics: Patient selection and sampling procedures were performed in accordance with the Declaration of Helsinki and applicable local regulatory requirements after approval from the Institutional Review Boards (HCB/2018/0781; PIC-61-19).
-
Informed consent: Written informed consent was obtained from all subjects.
-
Author contributions: Conceptualization: TC, MP; methodology: ABS, JB, AG, BG, SS; investigation: SF, CM, CR, JP, DB; funding acquisition: TC, MP; supervision: TC, MP; writing – original draft: TC; writing – review & editing: TC, MP. The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: AH and PL are employed by Actim Oy. The remaining authors report no conflict of interest.
-
Research funding: This Project has been funded by Actim Oy and by Instituto de Salud Carlos III through the projects PI19/00580, PI22/00669 and co-funded by the European Union.
-
Data availability: Data generated or analyzed during this study are provided in full within the published article. The raw data can be obtained on request from the corresponding author.
References
1. Combs, CA, Gravett, M, Garite, TJ, Hickok, DE, Lapidus, J, Porreco, R, et al.. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125 e1–15, https://doi.org/10.1016/j.ajog.2013.11.032.Search in Google Scholar PubMed
2. Cobo, T, Vives, I, Rodríguez-Trujillo, A, Murillo, C, Ángeles, MA, Bosch, J, et al.. Impact of microbial invasion of amniotic cavity and the type of microorganisms on short-term neonatal outcome in women with preterm labor and intact membranes. Acta Obstet Gynecol Scand 2017;96:570–9, https://doi.org/10.1111/aogs.13095.Search in Google Scholar PubMed
3. Yoon, BH, Romero, R, Park, JY, Oh, KJ, Lee, J, Conde-agudelo, A, et al.. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol 2019;221:142.e1–22, https://doi.org/10.1016/j.ajog.2019.03.018.Search in Google Scholar PubMed PubMed Central
4. Roberts, D, Brown, J, Medley, N, Dalziel, SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;3:CD004454, https://doi.org/10.1002/14651858.cd004454.pub3.Search in Google Scholar PubMed PubMed Central
5. Huusom, LD, Wolf, HT. Antenatal magnesium sulfate treatment for women at risk of preterm birth is safe and might decrease the risk of cerebral palsy. BMJ Evid Based Med 2018;23:195–6, https://doi.org/10.1136/bmjebm-2018-110897.Search in Google Scholar PubMed
6. Greig, PC, Ernest, JM, Teot, L. Low amniotic fluid glucose concentrations are a specific but not a sensitive marker for subclinical intrauterine infections in patients in preterm labor with intact membranes. Am J Obstet Gynecol 1994;171:365–70. https://doi.org/10.1016/s0002-9378(94)70036-2; discussion 70-1.Search in Google Scholar PubMed
7. Romero, R, Jimenez, C, Lohda, AK, Nores, J, Hanaoka, S, Avila, C, et al.. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–74, https://doi.org/10.1016/0002-9378(90)91106-m.Search in Google Scholar PubMed
8. Hussey, MJ, Levy, ES, Pombar, X, Meyer, P, Strassner, HT. Evaluating rapid diagnostic tests of intra-amniotic infection: Gram stain, amniotic fluid glucose level, and amniotic fluid to serum glucose level ratio. Am J Obstet Gynecol 1998;179:650–6, https://doi.org/10.1016/s0002-9378(98)70059-6.Search in Google Scholar PubMed
9. Chaemsaithong, P, Romero, R, Korzeniewski, SJ, Martínez-Varea, A, Dong, Z, Yoon, BH, et al.. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med 2016;29:349–59, https://doi.org/10.3109/14767058.2015.1006620.Search in Google Scholar PubMed PubMed Central
10. Chaemsaithong, P, Romero, R, Docheva, N, Chaiyasit, N, Bhatti, G, Pacora, P, et al.. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2018;31:228–44, https://doi.org/10.1080/14767058.2017.1281904.Search in Google Scholar PubMed PubMed Central
11. Nien, JK, Yoon, BH, Espinoza, J, Kusanovic, JP, Erez, O, Soto, E, et al.. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol 2006;195:1025–30, https://doi.org/10.1016/j.ajog.2006.06.054.Search in Google Scholar PubMed
12. Celik, E, To, M, Gajewska, K, Smith, GC, Nicolaides, KH, Fetal Medicine Foundation Second Trimester Screening Group. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol 2008;31:549–54, https://doi.org/10.1002/uog.5333.Search in Google Scholar PubMed
13. Palacio, M, Sanin-Blair, J, Sánchez, M, Crispi, F, Gómez, O, Carreras, E, et al.. The use of a variable cut-off value of cervical length in women admitted for preterm labor before and after 32 weeks. Ultrasound Obstet Gynecol 2007;29:421–6, https://doi.org/10.1002/uog.3950.Search in Google Scholar PubMed
14. Peng, CC, Chang, JH, Lin, HY, Cheng, PJ, Su, BH. Intrauterine inflammation, infection, or both (Triple I): a new concept for chorioamnionitis. Pediatr Neonatol 2018;59:231–7, https://doi.org/10.1016/j.pedneo.2017.09.001.Search in Google Scholar PubMed
15. Robinson, HP. Sonar measurement of fetal crown-rump length as means of assessing maturity in first trimester of pregnancy. Br Med J 1973;4:28–31, https://doi.org/10.1136/bmj.4.5883.28.Search in Google Scholar PubMed PubMed Central
16. Di Renzo, GC, Gratacós, E, Kurstser, M, Malone, F, Nambiar, S, Sierra, N, et al.. Good clinical practice advice: antenatal corticosteroids for fetal lung maturation. Int J Obstet Gynecol 2019;144:352–5, https://doi.org/10.1002/ijgo.12746.Search in Google Scholar PubMed
17. Johnson, CT, Adami, RR, Farzn, A. Antibiotic therapy for chorioamnionitis to reduce the global burden of associated disease. Front Pharmacol 2017;8:97, https://doi.org/10.3389/fphar.2017.00097.Search in Google Scholar PubMed PubMed Central
18. Palacio, M, Cobo, T, Bosch, J, Filella, X, Navarro-Sastre, A, Ribes, A, et al.. Cervical length and gestational age at admission as predictors of intra-amniotic inflammation in preterm labor with intact membranes. Ultrasound Obstet Gynecol 2009;34:441–7, https://doi.org/10.1002/uog.6437.Search in Google Scholar PubMed
19. Romero, R, Miranda, J, Chaiworapongsa, T, Korzeniewski, SJ, Chaemsaithong, P, Gotsch, F, et al.. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–74, https://doi.org/10.1111/aji.12296.Search in Google Scholar PubMed PubMed Central
20. Kenyon, SL, Taylor, DJ, Tarnow-Mordi, W. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. ORACLE Collaborative Group. Lancet 2001;357:989–94, https://doi.org/10.1016/s0140-6736(00)04234-3.Search in Google Scholar PubMed
21. Kenyon, S, Pike, K, Jones, DR, Brocklehurst, P, Marlow, N, Salt, A, et al.. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet 2008;372:1319–27, https://doi.org/10.1016/s0140-6736(08)61203-9.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Mini Review
- Artificial intelligence in the NICU to predict extubation success in prematurely born infants
- Original Articles – Obstetrics
- Amniotic fluid embolism: a reappraisal
- A multivariable prediction model for intra-amniotic infection in patients with preterm labor and intact membranes including a point of care system that measures amniotic fluid MMP-8
- Analysis of gastric fluid in preterm newborns supports the view that the amniotic cavity is sterile before the onset of parturition: a retrospective cohort study
- The effect of uterine closure technique on cesarean scar niche development after multiple cesarean deliveries
- The association between obesity and the success of trial of labor after cesarean delivery (TOLAC) in women with past vaginal delivery
- Validation of an automated software (Smartpelvic™) in assessing hiatal area from three dimensional transperineal pelvic volumes of pregnant women: comparison with manual analysis
- Pathogenic recurrent copy number variants in 7,078 pregnancies via chromosomal microarray analysis
- Obstetric pulmonary embolism and long-term cardiovascular symptoms: a cross-sectional study in Western Mexico
- Clinical characteristics and outcomes of women with adenomyosis pain during pregnancy: a retrospective study
- Disparities in preconception health indicators in U.S. women: a cross-sectional analysis of the behavioral risk factor surveillance system 2019
- Vertical transmission of SARS-CoV-2 – data from the German COVID-19 related obstetric and neonatal outcome study (CRONOS)
- Effects of sildenafil on Doppler parameters, maternal and neonatal outcomes in the active labor phase of low-risk pregnancies: a randomized clinical trial
- Pregnancy and neonatal outcomes of SARS-CoV-2 infection discovered at the time of delivery: a tertiary center experience in North Italy
- The impact of the COVID-19 pandemic on antenatal care provision and associated mental health, obstetric and neonatal outcomes
- Original Articles – Fetus
- Left atrial strain in fetal echocardiography – could it be introduced to everyday clinical practice?
- The evaluation of fetal interventricular septum with M-mode and spectral tissue Doppler imaging in gestational diabetes mellitus: a case-control study
- Letters to the Editor
- ChatGPT, artificial intelligence and the Journal of Perinatal Medicine: correspondence
- Re: to the Letter to the Editor: “ChatGPT and artificial intelligence in the Journal of Perinatal Medicine”
- Retraction
- Clinical potential of human amniotic fluid stem cells
Articles in the same Issue
- Frontmatter
- Mini Review
- Artificial intelligence in the NICU to predict extubation success in prematurely born infants
- Original Articles – Obstetrics
- Amniotic fluid embolism: a reappraisal
- A multivariable prediction model for intra-amniotic infection in patients with preterm labor and intact membranes including a point of care system that measures amniotic fluid MMP-8
- Analysis of gastric fluid in preterm newborns supports the view that the amniotic cavity is sterile before the onset of parturition: a retrospective cohort study
- The effect of uterine closure technique on cesarean scar niche development after multiple cesarean deliveries
- The association between obesity and the success of trial of labor after cesarean delivery (TOLAC) in women with past vaginal delivery
- Validation of an automated software (Smartpelvic™) in assessing hiatal area from three dimensional transperineal pelvic volumes of pregnant women: comparison with manual analysis
- Pathogenic recurrent copy number variants in 7,078 pregnancies via chromosomal microarray analysis
- Obstetric pulmonary embolism and long-term cardiovascular symptoms: a cross-sectional study in Western Mexico
- Clinical characteristics and outcomes of women with adenomyosis pain during pregnancy: a retrospective study
- Disparities in preconception health indicators in U.S. women: a cross-sectional analysis of the behavioral risk factor surveillance system 2019
- Vertical transmission of SARS-CoV-2 – data from the German COVID-19 related obstetric and neonatal outcome study (CRONOS)
- Effects of sildenafil on Doppler parameters, maternal and neonatal outcomes in the active labor phase of low-risk pregnancies: a randomized clinical trial
- Pregnancy and neonatal outcomes of SARS-CoV-2 infection discovered at the time of delivery: a tertiary center experience in North Italy
- The impact of the COVID-19 pandemic on antenatal care provision and associated mental health, obstetric and neonatal outcomes
- Original Articles – Fetus
- Left atrial strain in fetal echocardiography – could it be introduced to everyday clinical practice?
- The evaluation of fetal interventricular septum with M-mode and spectral tissue Doppler imaging in gestational diabetes mellitus: a case-control study
- Letters to the Editor
- ChatGPT, artificial intelligence and the Journal of Perinatal Medicine: correspondence
- Re: to the Letter to the Editor: “ChatGPT and artificial intelligence in the Journal of Perinatal Medicine”
- Retraction
- Clinical potential of human amniotic fluid stem cells

