Improving cardiometabolic health in children and adolescents with obesity: a comparison between in-person and virtual supervised training
-
Valeria Calcaterra
, Matteo Vandoni
Abstract
Objectives
Remote physical activity programs have emerged as a promising strategy to improve adherence to exercise guidelines, specifically among pediatric patients with obesity. This study investigated the effectiveness of in-person vs. virtual supervised training in improving cardiometabolic health in children and adolescents with obesity.
Methods
Forty-six children and adolescents (BMI z-score ≥ 2SD; aged 8–16) were enrolled and assigned to either in-person or virtual supervised combined training. All participants underwent a 12-week, thrice-weekly exercise program (36 sessions total), and the metabolic syndrome (MetS) z-score was used to assess metabolic risk.
Results
Both virtual and in-person supervised training programs resulted in improvements in the primary outcome, the MetS z-score, within each group. The virtual group showed a mean reduction of −0.26 (p=0.014), while the in-person group showed a greater reduction of −0.44 (p<0.001). Both interventions also improved waist-to-height ratio and blood pressure (p<0.01). Only the in-person group showed significant reductions in fasting glucose (p<0.001), triglycerides (p<0.001), and BMI z-score (p=0.004). The virtual group showed a greater improvement in diastolic blood pressure (p<0.01).
Conclusions
Both in-person and virtual exercise programs positively impact cardiometabolic health in children with obesity. The research supports the potential of remote exercise programs to enhance adherence to physical activity.
Introduction
Pediatric obesity is a growing global health concern [1], [2], [3]. According to the World Health Organization (WHO), over 340 million children and adolescents aged 5–19 years were affected by overweight or obesity in 2016, and the numbers continue to rise [1]. In industrialized countries, the prevalence of pediatric obesity is estimated to be around 20 %, significantly increasing the risk of developing cardiovascular diseases (CVD), type 2 diabetes (T2D), and metabolic syndrome (MetS) from a young age [2], [3], [4], [5].
It is well established that the pathogenesis of obesity is multifactorial, encompassing genetic, epigenetic, and environmental factors. However, the rising prevalence of pediatric obesity is strongly linked to unhealthy lifestyles, particularly poor dietary habits and insufficient physical activity [6], [7], [8], [9], [10], [11].
As reported by the WHO [12], 81 % of adolescents aged 11 to 17 worldwide did not meet the recommendation of engaging in at least 60 min of moderate-to-vigorous physical activity per day. European Commission data show that 32.4 % of Italian children aged 8–9 years meet the WHO physical activity guidelines, with rates declining to 11.9 % at 11 years and further to 6.8 % by 15 years of age [13].
Low levels of physical activity rank among the top 10 global risk factors for mortality, contributing to approximately 3.2 million deaths annually [12]. Children and adolescents who engage in at least 60 min of moderate-to-vigorous physical activity each day tend to exhibit better cardiorespiratory fitness, greater muscular strength, and enhanced endurance compared to their less active peers [12]. Additionally, regular physical activity plays a crucial role in regulating energy expenditure, making it essential for maintaining energy balance, managing body weight, and preventing obesity [12], 14], 15].
In this context, supervised physical exercise emerges as one of the most effective therapeutic strategies to prevent and combat childhood obesity and related complications [16], 17]. Numerous studies have shown that regular, age-appropriate physical activity reduces visceral fat, improves insulin sensitivity, lipid profiles, and blood pressure, and lowers chronic systemic inflammation and cardiometabolic risk associated with obesity [17], [18], [19], [20], [21], [22]. Recently, Gurka et al. [23] proposed a continuous cardiometabolic risk score (MetS z-score) as a valuable tool for tracking changes over time and detecting variations in risk severity across different clinical profiles. Several studies have linked elevated MetS z-scores to unhealthy lifestyle behaviors and increased cardiovascular risk, as well as to early markers of metabolic dysfunction, such as impaired insulin and glucose levels, in both pediatric and adult populations [24]. Indeed, it has been shown that exercise training, compared to usual care, has the potential to improve the cardiometabolic health in children with obesity [25]. Despite the use of different methods to assess cardiometabolic health, an exercise program efficacy on the MetS z-score is still unknown.
In recent years, driven in part by the COVID-19 pandemic and advances in digital technologies, there has been a significant expansion of virtually delivered supervised exercise programs [26], [27], [28], [29], [30], [31]. Online training offers several advantages: it reduces logistical barriers, increases access to specialized programs for those living in remote areas, and provides greater flexibility in scheduling sessions [32].
As previously reported [33], [34], [35], [36], [37], remote physical activity programs may serve as an effective strategy to enhance adherence to physical activity guidelines, offering an important opportunity for pediatric patients with obesity to maintain their health. However, their effectiveness can vary, largely depending on the degree of professional support provided and the level of personalization within the program [38].
The comparative effectiveness of virtual vs. in-person supervised training, particularly in improving cardiometabolic health outcomes in children and adolescents with obesity, remains limited [39], [40], [41], [42].
We aimed to directly compare these two modalities to identify the most effective approach for improving cardiometabolic health in this vulnerable population. Specifically, we assessed changes in the MetS z-score between in-person and virtually supervised training sessions in children and adolescents. By evaluating both delivery methods, this study provides valuable insights into how virtual training may serve as an effective strategy to reduce sedentary behavior and support obesity management.
Materials and methods
Study design
This study was a parallel-group, non-randomized clinical trial. It was registered and approved by the Lombardia 1 Ethics Committee (protocol number 2020/ST/298). Following an initial visit during which the study evaluators thoroughly explained the protocol, written informed consent was obtained from all participants’ parents or legal guardians. The study adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for randomized controlled trials [43].
Outcome measures
The primary outcome of the study was the change in MetS z-score from baseline to 12 weeks. The MetS z-score reflects the pathophysiological processes of metabolic syndrome, particularly those related to cardiovascular (CV) and type 2 diabetes (T2D) risk [23].
Secondary outcomes included changes in anthropometric and body composition measures (e.g., weight and BMI z-score), as well as specific cardiometabolic markers (e.g., glucose, insulin, HOMA-IR, HDL, triglycerides, and blood pressure).
Participants
We screened 50 children and adolescents referred for obesity to the outpatient clinic of the Pediatric Unit at Buzzi Children’s Hospital and finally enrolled 46 participants (ages 8–16; 36 % female) in the study. Before enrollment, potential participants received clinical and physical examination measurements to ensure inclusion feasibility. Inclusion criteria were age between 8 and 18 years, body mass index (BMI) z-score ≥ 2 (according to the World Health Organization’s guidelines) [44], no significant change in weight in the previous 3 months.
Included participants were required to have the ability to fully understand the study procedures. Written informed consent was obtained from a parent or legal guardian after the study procedures were thoroughly explained.
Exclusion criteria included any known obesity-related comorbidities, being classified as physically active (i.e., engaging in more than 60 min of moderate-to-vigorous physical activity per day), and any absolute contraindications to physical activity.
Eligible participants were recruited and allocated to one of the two groups using a non-probability convenience sampling technique.
Clinical evaluation
For all participants, measurements included weight, height and waist circumference (WC) [45] at fasting conditions. Body mass index (BMI) and waist-to-height ratio (WHtR) were also calculated. During measurements, individuals wore lightweight athletic clothing (shorts and a T-shirt) and were barefoot. Body weight was recorded using a digital scale (Seca 864, Seca GmbH and Co., Hamburg, Germany) with 0.1 kg precision. Height was measured with a Harpenden stadiometer (Holtain Ltd., Crymych, UK), featuring a fixed vertical board and a movable headpiece [46]. WC was measured with a flexible tape positioned horizontally at the midpoint between the lowest rib and the iliac crest [46]. From these values, BMI was determined by dividing weight in kilograms by height in meters squared, and BMI z-scores was assessed using the WHO reference data [47]. After anthropometric characteristics evaluation, systolic and diastolic blood pressure were measured using an appropriately sized cuff, according to the American Heart Association (AHA) pediatric guidelines [48].
Biochemical parameters and MetS z-score
Fasting blood samples were collected between 8:30 and 9:00 a.m., following an overnight fast, to assess key metabolic markers: fasting blood glucose, total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, and insulin levels. These samples were processed and analyzed using the Advia XPT system from Siemens Healthcare on the same day.
To assess insulin resistance (IR), the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was assessed by using the following formula: HOMA-IR=(insulin × glucose)/22.5 according to Matthews [49].
The equation to compute the MetS z-score is calculated as follows [23]:
For boys.
MetS z-score=−4.931+0.2804 * BMI z-score – 0.0257 * HDL+0.0189 * SBP+0.6240 * log(Fasting triglycerides)+0.0140 * Fasting glucose.
For girls.
MetS z-score=−4.3757+0.4849 * BMI z-score – 0.0176 * HDL-C+0.0257 * SBP+0.3172 * log(Fasting triglycerides)+0.0083 * Fasting glucose.
Training interventions
Participants were allocated either to a supervised in-person combined training or to a virtual supervised combined training. Both groups followed a 12-week combined exercise program, with training sessions held three times per week, for a total of 36 sessions for each participant. Sessions were conducted face-to-face at a designated facility for the in-person group or online through zoom for the virtual group and led by two qualified kinesiologists. Each 60-min session was composed of a 5–10 min warm-up, 20 min of aerobic interval training, a 20-min strength training circuit, and concluded with a 5–10 min cool-down and stretching routine. Activities included sport-specific movements (e.g., soccer, basketball, rugby, and volleyball), circuit-based aerobic exercises, resistance training, interactive recreational tasks, and playful exercises designed to maintain continuous activity at moderate intensity. No specialized gym equipment was required. Exercise intensity for the aerobic circuit was maintained within 60–75 % of the participant’s maximum heart rate (HRmax, assessed as 220 minus age). During the first week, intensity was maintained to 50–60 % HRmax to support adaptation and ensure participant compliance. As the program progressed, intensity was gradually increased to induce training benefits. Heart rate was monitored throughout each session using Fitbit Charge 6© devices. Trainers made real-time adjustments to maintain the targeted intensity and controlled the intensity using the CERT scale [50]. For both groups, training duration and intensity were consistent throughout the 12 weeks, and participants were encouraged to maintain a healthy diet, though no dietary intervention was imposed.
Statistical analysis
The sample size and power of the study were estimated based on previous studies involving an exercise intervention in children and adolescents with obesity [25]. To detect a medium effect size (F=0.253) in the reduction of MetS z-score as statistically significant using an ANOVA for repeated measure within/between interaction with α=0.05 and a power of 0.95, a correlation among repeated measure equal to 0.7 a minimum of 17 participants for group was required. We accounted for a drop-out rate of 30 % and recruited a total of 46 participants, 23 per group. Data were expressed as mean (95 % confidence interval, CI). A generalized linear mixed for repeated measures was applied to analyze intervention effects on primary and secondary endpoints. This approach was selected to adjust both parametric and non-parametric data, using a Gaussian or a Gamma distribution depending on the nature of the data. Individual changes were modeled as a function of assigned group, assessment time, and their interaction terms. Sex and age were included as covariates. Model-based estimations followed a per protocol analysis using the restricted maximum-likelihood method, assuming that missing values were missing at random. To account for multiple comparisons, we applied Tukey correction. Reported p-values are Tukey-adjusted, with a significance threshold of 0.05 (two-sided). All analyses were conducted using R software, version 4.4 (R Foundation for Statistical Computing), the “zscorer” package was used to compute the BMIz-score, the “lmer” package to compute the generalized linear mixed models and the “emmeans” package to compute the post-hoc analysis.
Results
A total of 50 participants were screened for study eligibility. After the first screening selection, 46 children with obesity were finally enrolled and then assigned to one of the two groups: 23 participants to the virtual training group (16 boys and 7 girls) and 23 to the in-person training (13 boys and 10 girls). However, 2 participants from the in-person training group (1 boy and one girl) dropped out from the intervention. Therefore, a total of 43 participants (aged 12.2 yrs [11.6, 12.9 95%CI]) were included in the per-protocol analysis. The descriptive characteristics at baseline are reported in Table 1, while the study flowchart in Figure 1.
Descriptive characteristics of the sample divided by group.
| Overall (n=44) | Virtual (n=23) | In-person (n=21) | |
|---|---|---|---|
| BMI z-score | 3.07 (2.91, 3.23) | 3.10 (2.88, 3.31) | 3.04 (2.80, 3.28) |
| Waist circumference, cm | 97 (93, 101) | 91 (87, 96) | 104 (99, 108) |
| WtHr | 0.62 (0.61, 0.64) | 0.61 (0.59, 0.63) | 0.64 (0.61, 0.67) |
| Systolic blood pressure, mmHg | 115 (112, 118) | 112 (109, 116) | 118 (114, 123) |
| Diastolic blood pressure, mmHg | 73 (70, 76) | 73 (68, 78) | 72 (69, 75) |
| Fasting glucose, mg/dL | 84 (80, 88) | 90 (87, 93) | 78 (72, 84) |
| Fasting insulin, μU/mL | 18.9 (14.5, 23.3) | 18.3 (14.4, 22.2) | 19.6 (11.3, 27.9) |
| Missinga, n (%) | 2 (4.5 %) | 1 (4.3 %) | 1 (4.8 %) |
| HOMA-IR | 3.8 (3.1, 4.4) | 4.0 (3.2, 4.9) | 3.5 (2.4, 4.5) |
| Missinga, n (%) | 2 (4.5 %) | 1 (4.3 %) | 1 (4.8 %) |
| Tryglicerides, mg/dL | 102 (85, 118) | 114 (90, 138) | 87 (67, 107) |
| Missinga, n (%) | 1 (2.3 %) | 0 (0 %) | 1 (4.8 %) |
| HDL, mg/dL | 46 (43, 50) | 48 (43, 52) | 45 (40, 50) |
| MetS z-score | 1.11 (0.94, 1.27) | 1.12 (0.87, 1.37) | 1.09 (0.87, 1.30) |
| Missinga, n (%) | 1 (2.3 %) | 0 (0 %) | 1 (4.8 %) |
| QUICKI | 0.326 (0.316, 0.335) | 0.319 (0.309, 0.329) | 0.333 (0.316, 0.349) |
| Missinga, n (%) | 2 (4.5 %) | 1 (4.3 %) | 1 (4.8 %) |
-
Data were expressed as mean (95 % confidence interval; CI) unless otherwise stated. BMI, body mass index; WtHr, waist to height ratio; HOMA-IR, homeostatic model assessment for insulin resistance; HDL, high density lipoprotein; MetS, metabolic syndrome; QUICKI, quantitative insulin sensitivity check index. aTechnical problems.
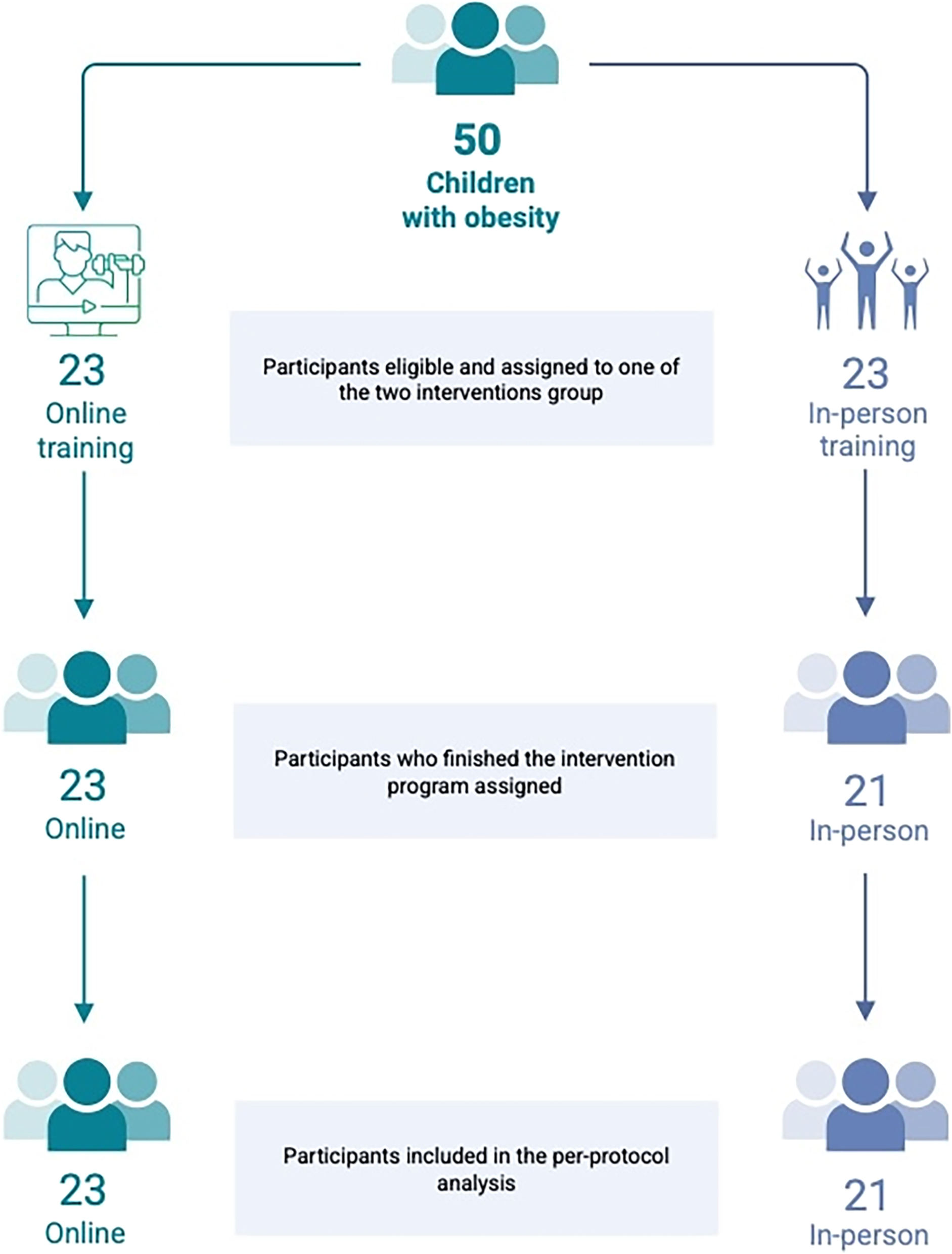
Study design and allocation to group intervention for the participants.
Descriptive characteristics of the two groups and the total sample are reported in Table 1.
Table 2 presents the within- and between-group differences obtained from the generalized linear mixed model. Both virtual and in-person supervised training led to significant improvements in the primary outcome, the MetS z-score, within each group. Specifically, the virtual group showed a mean reduction of −0.26 (95 % CI: −0.48 to −0.04, p=0.014), while the in-person group demonstrated a greater reduction of −0.44 (95 % CI: −0.67 to −0.20, p<0.001). However, no statistically significant difference was observed between the two groups (mean difference=0.17, 95 % CI: −0.07 to 0.42, p=0.155).
Changes between and within groups for primary and secondary outcomes after intervention.
| Virtual (n=23) | In-person (n=21) | Virtual (post-pre) – In-person (post-pre) | |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Anthropometric characteristics | |||
| Waist circumference, cm | −2.13 (−4.41, 0.16) | −4.61 (−7.18, −2.04)a | 2.49 (−0.14, 5.11) |
| WtHr | −0.02 (−0.04, 0.00)a | −0.04 (−0.06, −0.02)a | 0.01 (−0.01, 0.03) |
| BMI z-score | −0.09 (−0.20, 0.01) | −0.15 (−0.26, −0.04)a | 0.06 (−0.06, 0.17) |
| Blood pressure | |||
| Systolic blood pressure, mmHg | −7.16 (−12.17, −2.15)a | −6.66 (−12.00, −1.33)a | −0.50 (−6.10, 5.11) |
| Diastolic blood pressure, mmHg | −4.87 (−6.28, −3.47)a | −3.44 (−5.29, −1.60)a | −1.43 (−2.51, −0.35)b |
| Glycemic profile | |||
| Fasting glucose, mg/dL | −2.05 (−9.64, 5.55) | −12.35 (−18.47, −6.23)a | 10.31 (2.86, 17.75)b |
| Fasting insulin, μU/mL | −0.75 (−4.89, 3.39) | −0.82 (−3.91, 2.27) | 0.07 (−3.87, 4.02) |
| HOMA-IR | −0.20 (−1.16, 0.75) | −0.51 (−1.11, 0.09) | 0.30 (−0.56, 1.16) |
| QUICKI | 0.00 (−0.01, 0.02) | 0.01 (−0.01, 0.02) | −0.01 (−0.02, 0.01) |
| Lipid Profile | |||
| Triglycerides, mg/dL | −0.28 (−0.88, 0.33) | −11.58 (−12.63, −10.52)a | 11.30 (10.57, 12.02)b |
| HDL, mg/dL | 0.88 (−2.10, 3.86) | 1.25 (−1.67, 4.16) | −0.37 (−3.55, 2.81) |
| Primary outcome | |||
| MetS z-score | −0.26 (−0.48, −0.04)a | −0.44 (−0.67, −0.20)a | 0.17 (−0.07, 0.42) |
-
aRepresents significant differences for post-pre changes per groups as determined by the repeated-measures generalized linear mixed-effects models (p<0.05). bRepresents significant between groups differences as determined by the repeated-measures generalized linear mixed-effects models (p<0.05). The p values reported are two-sided and Tukey-adjusted p-values. Primary analysis (MetS z-score) between virtual and in-person (p=0.155). Data are presented as estimated marginal mean differences and 95 % CI, obtained from the repeated-measures generalized linear mixed-effects models. Intervention effects on primary and secondary outcomes after the interventions were assessed based on repeated-measures generalized linear mixed-effects models. Individual measures of change were therefore modeled as the function of assigned group, assessment time and their interaction terms. Sex and age were also considered as covariates. BMI, body mass index; WtHr, waist to height ratio; HOMA-IR, homeostatic model assessment for insulin resistance; HDL, high density lipoprotein; MetS, metabolic syndrome; VAI, visceral adiposity index; QUICKI, quantitative insulin sensitivity check index.
For the secondary outcomes, participants in both the virtual and in-person training groups showed significant improvements in waist-to-height ratio (virtual p=0.009; in-person p<0.001), systolic blood pressure (virtual p=0.001; in-person p=0.007), and diastolic blood pressure (virtual p<0.001; in-person p<0.001). However, no significant changes were observed in either group for fasting insulin, HOMA-IR (virtual p=0.947; in-person p=0.130), QUICKI (virtual p=0.978; in-person p=0.614), or HDL levels (virtual p=0.873; in-person p=0.690).
Within-group analysis revealed that only the in-person training group experienced significant reductions in fasting glucose (p<0.001), triglycerides (p<0.001), and BMI z-score (p=0.004). Additionally, a greater reduction in diastolic blood pressure was observed in the virtual training group compared to the in-person group (p=0.009). To facilitate the visualization of the results, we provided graphical comparisons from Figures 2–5.

The left panels show changes in MetS z-score from baseline to post-intervention for the virtual and in-person training groups. The right panels display the individual changes (Δ) in MetS z-score. In the box plots, the ends of the boxes represent the first and third quartiles, while the black line within each box indicates the median. Whiskers extend to the most extreme data points within 1.5 interquartile ranges (IQRs) from the first and third quartiles. The parallel line plots display one vertical line per participant, connecting their baseline measurement to the post-intervention value. A downward slope indicates improvement in the measured outcome. Baseline values are ordered in descending sequence for the control group and ascending sequence for the intervention group.

Post-pre differences between the two exercise programs in cardiometabolic risk outcomes.

Individual changes among the virtual supervised groups after the 12-week intervention. The light blue bars represent participants who experienced a reduction. The dark blue bars represent participants who experienced an improvement for the outcome analyzed (e.g., reduction for BMI z-score, increase in HDL-C).

Individual changes among the in-person supervised groups after the 12-week intervention. The light blue bars represent participants who experienced a reduction. The dark orange bars represent participants who experienced an improvement for the outcome analyzed (e.g., reduction for BMI z-score, increase in HDL-C).
Discussion
Our study demonstrated that both exercise programs positively impacted MetS z-scores in children with obesity, with no significant difference between the two groups. However, the interventions yielded distinct effects on specific cardiometabolic markers: while both improved WHtR and blood pressure, only the in-person group achieved significant reductions in fasting glucose, triglycerides, and BMI z-score. In contrast, the virtual group showed a greater improvement in diastolic blood pressure. These findings support the potential of virtual training as an effective complementary strategy to reduce sedentary behavior and aid in obesity management.
Childhood obesity is a multifaceted condition that affects various systems and is associated with numerous health complications, potentially leading to early mortality in adulthood [2], 51]. Regular physical activity is widely recognized as a key non-pharmacological strategy to address excess weight and obesity, as well as their associated disorders, beginning at an early age [16]. Numerous studies have shown that reducing BMI z-scores, WC, and WHtR in children with obesity can help lower cardiovascular risk and improve overall health [52], [53], [54], [55], [56], [57]. In addition to these benefits, regular physical activity is linked to significant improvements in several health markers, including blood pressure, body composition, insulin sensitivity, and fasting levels of triglycerides and glucose [17], [58], [59], [60], [61]. Specifically, interventions that combine aerobic and resistance training have been shown to enhance metabolic profiles, reduce systemic inflammation, and play a crucial role in the long-term prevention of chronic diseases such as type 2 diabetes and cardiovascular conditions [25], 62], 63]. Building on this evidence and addressing the limited information on the topic, our study aimed to explore how a structured exercise program, combining aerobic and resistance training, delivered online might influence the cardiometabolic profile of children with obesity.
In our study, to evaluate the impact of exercise on the cardiometabolic profile, we analyzed changes in the MetS z-score, a continuous cardiometabolic risk score proposed as an alternative to the traditional categorical diagnosis of metabolic syndrome [23]. This score combines multiple components, WC, blood pressure, fasting glucose, triglycerides, and HDL cholesterol, into a single standardized variable adjusted for age and sex, providing a more individualized and accurate assessment of cardiometabolic risk, particularly in pediatric populations. We noted that MetS z-score revealed beneficial effects from both in-person and virtually supervised training programs, confirming that structured physical activity, regardless of the delivery mode, can positively influence cardiometabolic health [34], 36], 37]. Specifically, both groups experienced significant reductions in WHtR and blood pressure, highlighting the general efficacy of physical exercise in addressing central adiposity and cardiovascular strain [64], [65], [66]. Interestingly, diastolic blood pressure improved to a greater extent in the virtual group, which may reflect the flexibility and comfort of home-based training, potentially reducing anxiety or exertional stress in some participants [67], 68]. In contrast, the in-person training group exhibited significant reductions in fasting glucose, triglyceride levels, and BMI z-score, suggesting a more pronounced effect on metabolic regulation and overall adiposity. These differences could be also attributed to factors such as more consistent supervision, real-time feedback, higher training intensity, or increased peer motivation during in-person sessions. Our results are in accordance with previous studies and meta-analyses that demonstrated the effectiveness of interval moderate intensity aerobic training combined with resistance training on cardiometabolic health in children with obesity [25], 62], 63].
In recent years, online exercise programs have emerged as a promising alternative or complement to traditional in-person interventions [33], [34], [35], [36], [37, [69], [70], [71], [72]. Their flexibility, accessibility, and potential for individual customization make them particularly valuable, especially in situations where access to gyms or supervised programs is limited. While the absence of face-to-face interaction may present challenges, well-designed virtual interventions have demonstrated the ability to maintain high adherence levels and encourage the adoption of active lifestyles [73].
Our findings support the notion that, although online programs may not entirely replace in-person sessions, they can offer an effective alternative in situations where access to sports facilities is limited or maintaining adherence to exercise routines is challenging. For healthcare professionals, particularly those managing pediatric obesity, integrating online exercise programs into a multimodal approach to treatment and the prevention of metabolic complications appears to be a valuable and necessary strategy [34], 36], 37], 74], 75]. These results further highlight the potential of flexible, hybrid models tailored to individual needs and logistical constraints, helping to expand the reach of effective obesity interventions in pediatric populations [74], 75].
Furthermore, it is vital to develop personalized interventions that consider the unique preferences and needs of children and their families. This approach can maximize long-term effectiveness and support the adoption of sustainable healthy lifestyles [76], 77].
While our study provides valuable insights into the effectiveness of both in-person and virtual exercise programs, several limitations should be acknowledged. First, the relatively short duration of the intervention may not fully capture the long-term effects of exercise on cardiometabolic health; a longer follow-up period would be necessary to assess the sustainability of the observed benefits. Second, a larger sample size would have enabled sex-specific analyses, which were not feasible in the current study. Moreover, the higher proportion of boys compared to girls in our sample may have influenced the results. Lastly, the study did not control for potential confounding factors such as dietary habits or other lifestyle interventions, which could have impacted the cardiometabolic outcomes.
In conclusion, our study highlights the beneficial impact of both in-person and virtual exercise programs on the cardiometabolic health of children with obesity. Both intervention types resulted in improvements in key cardiometabolic markers, with in-person programs yielding more pronounced effects on metabolic regulation, while the virtual group showed greater improvements in blood pressure. These findings suggest that online interventions can be a valuable adjunct when in-person training is not feasible. However, further robust, comparative trials are needed to validate their long-term efficacy. Online programs may play an important role in enhancing adherence and promoting physical activity, particularly in contexts where remote delivery is necessary to ensure children’s access to structured exercise. Future research should investigate the long-term outcomes and work toward refining personalized approaches to optimize the effectiveness of these interventions in managing pediatric obesity.
-
Research ethics: The study was registered and approved by the Ethics committee Lombardia 1 (protocol number 2020/ST/298).
-
Informed consent: All participants’ parents or legal guardians, after a first visit in which study’s evaluators explained extensively the study protocol, provided written informed consent.
-
Author contributions: V.C., M.V., A.G., and G.Z. conceived and designed the study. A.G., V.C.P., V.R., C.C., and A.P. were responsible for data collection and the literature review. A.G. performed the data analysis. V.C., M.V., A.G., D.L., and G.Z. contributed to data interpretation. All authors were involved in writing the manuscript and approved the final version. The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. World Health Organization (WHO). Obesity and overweight [Internet] 2024 [cited 2025 May 2]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:∼:text=The%20prevalence%20of%20overweight%20(including,21%25%20of%20boys%20were%20overweight.Search in Google Scholar
2. The Lancet Diabetes & Endocrinology. Childhood obesity: a growing pandemic. Lancet Diabetes Endocrinol 2022;10:1. https://doi.org/10.1016/s2213-8587-21-00314-4.Search in Google Scholar
3. Kerr, JA, Patton, GC, Cini, KI, Abate, YH, Abbas, N, Abd Al Magied, AHA, et al.. Global, regional, and national prevalence of child and adolescent overweight and obesity, 1990–2021, with forecasts to 2050: a forecasting study for the global burden of disease study 2021. Lancet 2025;405:785–812. https://doi.org/10.1016/s0140-6736-25-00397-6.Search in Google Scholar
4. Reisinger, C, Nkeh-Chungag, BN, Fredriksen, PM, Goswami, N. The prevalence of pediatric metabolic syndrome—A critical look on the discrepancies between definitions and its clinical importance. Int J Obes 2021;45:12–24. https://doi.org/10.1038/s41366-020-00713-1.Search in Google Scholar PubMed PubMed Central
5. Ball, GDC, Merdad, R, Birken, CS, Cohen, TR, Goodman, B, Hadjiyannakis, S, et al.. Managing obesity in children: a clinical practice guideline. CMAJ (Can Med Assoc J) 2025;197:E372–89. https://doi.org/10.1503/cmaj.241456.Search in Google Scholar PubMed PubMed Central
6. Panera, N, Mandato, C, Crudele, A, Bertrando, S, Vajro, P, Alisi, A. Genetics, epigenetics and transgenerational transmission of obesity in children. Front Endocrinol 2022;13:1006008. https://doi.org/10.3389/fendo.2022.1006008.Search in Google Scholar PubMed PubMed Central
7. Verma, M, Kapoor, N, Senapati, S, Singh, O, Bhadoria, AS, Khetarpal, P, et al.. Comprehending the epidemiology and aetiology of childhood obesity: integrating life course approaches for prevention and intervention. Diabetes Ther [Internet] 2025. https://doi.org/10.1007/s13300-025-01734-7 [cited 2025 May 2]; Available from: https://link.springer.com/10.1007/s13300-025-01734-7.Search in Google Scholar PubMed PubMed Central
8. Górczyńska-Kosiorz, S, Kosiorz, M, Dzięgielewska-Gęsiak, S. Exploring the interplay of genetics and nutrition in the rising epidemic of obesity and metabolic diseases. Nutrients 2024;16:3562. https://doi.org/10.3390/nu16203562.Search in Google Scholar PubMed PubMed Central
9. Sivakumar, S, Lama, D, Rabhi, N. Childhood obesity from the genes to the epigenome. Front Endocrinol 2024;15:1393250. https://doi.org/10.3389/fendo.2024.1393250.Search in Google Scholar PubMed PubMed Central
10. Minabe, S, Sutoh, Y, Otsuka-Yamasaki, Y, Komaki, S, Nakao, M, Ohmomo, H, et al.. Risk factors and prediction for pediatric obesity: current status and future perspectives. Endocr J 2025;EJ24-0724. https://doi.org/10.1507/endocrj.ej24-0724.Search in Google Scholar PubMed PubMed Central
11. Stroes, ASR, Vos, M, Benninga, MA, Koot, BGP. Pediatric MASLD: current understanding and practical approach. Eur J Pediatr 2024;184:29. https://doi.org/10.1007/s00431-024-05848-1.Search in Google Scholar PubMed
12. World Health Organization. Global status report on noncommunicable diseases 2014. Geneva: World Health Organization; 2014 [Internet] [cited 2025 May 2]. 280 p. Available from: https://iris.who.int/handle/10665/148114.Search in Google Scholar
13. World Health Organization (WHO), European Commission. Italy physical activity factsheet [Internet]. [cited 2025 May 2]. Available from: https://ec.europa.eu/assets/eac/sport/library/factsheets/italy-factsheet_en.pdf.Search in Google Scholar
14. Dericioglu, D, Methven, L, Clegg, ME. Does physical activity level and total energy expenditure relate to food intake, appetite, and body composition in healthy older adults? A cross-sectional study. Eur J Nutr 2025;64:71. https://doi.org/10.1007/s00394-024-03571-z.Search in Google Scholar
15. Pontzer, H, Durazo-Arvizu, R, Dugas, LR, Plange-Rhule, J, Bovet, P, Forrester, TE, et al.. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr Biol 2016;26:410–7. https://doi.org/10.1016/j.cub.2015.12.046.Search in Google Scholar PubMed PubMed Central
16. Calcaterra, V, Zuccotti, G. Physical exercise as a non-pharmacological intervention for attenuating obesity-related complications in children and adolescents. IJERPH 2022;19:5046. https://doi.org/10.3390/ijerph19095046.Search in Google Scholar PubMed PubMed Central
17. García-Hermoso, A, Ceballos-Ceballos, RJM, Poblete-Aro, CE, Hackney, AC, Mota, J, Ramírez-Vélez, R. Exercise, adipokines and pediatric obesity: a meta-analysis of randomized controlled trials. Int J Obes 2017;41:475–82. https://doi.org/10.1038/ijo.2016.230.Search in Google Scholar PubMed PubMed Central
18. Chomiuk, T, Niezgoda, N, Mamcarz, A, Śliż, D. Physical activity in metabolic syndrome. Front Physiol 2024;15:1365761. https://doi.org/10.3389/fphys.2024.1365761.Search in Google Scholar PubMed PubMed Central
19. Venkataraman, V, Turaga, P, Lehrer, N, Baran, M, Rikakis, T, Wolf, S, et al.. Decision support for stroke rehabilitation therapy via describable attribute-based decision trees. In 2014. p. 3154–9, https://doi.org/10.1109/embc.2014.6944292.Search in Google Scholar
20. Calcaterra, V, Magenes, VC, Bianchi, A, Rossi, V, Gatti, A, Marin, L, et al.. How can promoting skeletal muscle health and exercise in children and adolescents prevent insulin resistance and type 2 diabetes? Life 2024;14:1198. https://doi.org/10.3390/life14091198.Search in Google Scholar PubMed PubMed Central
21. Popescu, C, Matei, D, Amzolini, AM, Trăistaru, MR. Inflammation and physical performance in overweight and obese schoolchildren. Life 2024;14:1583. https://doi.org/10.3390/life14121583.Search in Google Scholar PubMed PubMed Central
22. Powell-Wiley, TM, Poirier, P, Burke, LE, Després, JP, Gordon-Larsen, P, Lavie, CJ, et al.. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation [Internet] 2021;143. https://doi.org/10.1161/cir.0000000000000973 [cited 2025 May 2] Available from https://www.ahajournals.org/doi/10.1161/CIR.0000000000000973.Search in Google Scholar PubMed PubMed Central
23. Gurka, MJ, Ice, CL, Sun, SS, DeBoer, MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol 2012;11:128. https://doi.org/10.1186/1475-2840-11-128.Search in Google Scholar PubMed PubMed Central
24. DeBoer, MD, Gurka, MJ, Woo, JG, Morrison, JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the princeton lipid research cohort study. Diabetologia 2015;58:2745–52. https://doi.org/10.1007/s00125-015-3759-5.Search in Google Scholar PubMed PubMed Central
25. Migueles, JH, Cadenas-Sanchez, C, Lubans, DR, Henriksson, P, Torres-Lopez, LV, Rodriguez-Ayllon, M, et al.. Effects of an exercise program on cardiometabolic and mental health in children with overweight or obesity: a secondary analysis of a randomized clinical trial. JAMA Netw Open 2023;6:e2324839. https://doi.org/10.1001/jamanetworkopen.2023.24839.Search in Google Scholar PubMed PubMed Central
26. Anastasiadou, O, Tsipouras, M, Mpogiatzidis, P, Angelidis, P. Digital healthcare innovative services in times of crisis: a literature review. Healthcare 2025;13:889. https://doi.org/10.3390/healthcare13080889.Search in Google Scholar PubMed PubMed Central
27. Wallraf, S, Dierks, ML, John, C, Lander, J. Patient organizations’ digital responses to the COVID-19 pandemic: scoping review. J Med Internet Res 2024;26:e58566. https://doi.org/10.2196/58566.Search in Google Scholar PubMed PubMed Central
28. Krotkiewicz, M, Szynkaruk, A, Stachyra, A. Digital transformation in healthcare management: from artificial intelligence to blockchain. Wiadomości Lek 2025:578–83. https://doi.org/10.36740/wlek/202445.Search in Google Scholar PubMed
29. Kracht, CL, Tovar, A, Gans, KM, Lee, RE, Tandon, PS, Von Ash, T, et al.. How to integrate and leverage digital health modalities for health promotion in early childhood education: opportunities to improve intervention access and engagement. Transl Behav Med 2025;15:ibaf006. https://doi.org/10.1093/tbm/ibaf006.Search in Google Scholar PubMed PubMed Central
30. Jiménez-Zarco, A, Mateos, SC, Bosque-Prous, M, Espelt, A, Torrent-Sellens, J, Adib, K, et al.. Impact of the COVID-19 pandemic on mHealth adoption: identification of the main barriers through an international comparative analysis. Int J Med Inf 2025;195:105779. https://doi.org/10.1016/j.ijmedinf.2024.105779.Search in Google Scholar PubMed PubMed Central
31. Denny, A, Ndemera, I, Chirwa, K, Wu, JTS, Chirambo, GB, Yosefe, S, et al.. Evaluation of the development, implementation, maintenance, and impact of 3 digital surveillance tools deployed in Malawi during the COVID-19 pandemic: protocol for a modified Delphi expert consensus study. JMIR Res Protoc 2024;13:e58389. https://doi.org/10.2196/58389.Search in Google Scholar PubMed PubMed Central
32. Milne-Ives, M, Burns, L, Swancutt, D, Calitri, R, Ananthakrishnan, A, Davis, H, et al.. The effectiveness and usability of online, group-based interventions for people with severe obesity: a systematic review and meta-analysis. Int J Obes 2025;49:564–77. https://doi.org/10.1038/s41366-024-01669-2.Search in Google Scholar PubMed PubMed Central
33. Giuriato, M, Gatti, A, Pellino, VC, Bianchi, A, Zanelli, S, Pirazzi, A, et al.. A tele‐coaching pilot study: an innovative approach to enhance motor skills in adolescents with Down syndrome. Research Intellect Disabil 2025;38:e70036. https://doi.org/10.1111/jar.70036.Search in Google Scholar PubMed PubMed Central
34. Calcaterra, V, Bernardelli, G, Malacarne, M, Vandoni, M, Mannarino, S, Pellino, VC, et al.. Effects of endurance exercise intensities on autonomic and metabolic controls in children with obesity: a feasibility study employing online exercise training. Nutrients 2023;15:1054. https://doi.org/10.3390/nu15041054.Search in Google Scholar PubMed PubMed Central
35. Mannarino, S, Santacesaria, S, Raso, I, Garbin, M, Pipolo, A, Ghiglia, S, et al.. Benefits in cardiac function from a remote exercise program in children with obesity. IJERPH 2023;20:1544. https://doi.org/10.3390/ijerph20021544.Search in Google Scholar PubMed PubMed Central
36. Vandoni, M, Carnevale Pellino, V, Gatti, A, Lucini, D, Mannarino, S, Larizza, C, et al.. Effects of an online supervised exercise training in children with obesity during the COVID-19 pandemic. IJERPH 2022;19:9421. https://doi.org/10.3390/ijerph19159421.Search in Google Scholar PubMed PubMed Central
37. Vandoni, M, Codella, R, Pippi, R, Carnevale Pellino, V, Lovecchio, N, Marin, L, et al.. Combatting sedentary behaviors by delivering remote physical exercise in children and adolescents with obesity in the COVID-19 era: a narrative review. Nutrients 2021;13:4459. https://doi.org/10.3390/nu13124459.Search in Google Scholar PubMed PubMed Central
38. Meneses, C, Lopes, A, Fonseca, F, Eusébio, A, Peixoto, R, Vilaça-Alves, J, et al.. Effects of a multidisciplinary program with physical exercise in pediatric obesity. Eur J Publ Health 2024;34:ckae144–1155. https://doi.org/10.1093/eurpub/ckae144.1155.Search in Google Scholar
39. Thorén, A, Filipsson, T, Englund, E, Sandström, O, Janson, A, Silfverdal, SA. Significant effects of childhood obesity treatment with a web‐based component in a randomised controlled study (Web‐COP). Acta Paediatr 2024;113:276–85. https://doi.org/10.1111/apa.17000.Search in Google Scholar PubMed
40. Azevedo, LB, Stephenson, J, Ells, L, Adu‐Ntiamoah, S, DeSmet, A, Giles, EL, et al.. The effectiveness of e‐health interventions for the treatment of overweight or obesity in children and adolescents: a systematic review and meta‐analysis. Obes Rev 2022;23:e13373. https://doi.org/10.1111/obr.13373.Search in Google Scholar PubMed
41. Kouvari, M, Karipidou, M, Tsiampalis, T, Mamalaki, E, Poulimeneas, D, Bathrellou, E, et al.. Digital health interventions for weight management in children and adolescents: systematic review and meta-analysis. J Med Internet Res 2022;24:e30675. https://doi.org/10.2196/30675.Search in Google Scholar PubMed PubMed Central
42. Yien, JM, Wang, HH, Wang, RH, Chou, FH, Chen, KH, Tsai, FS. Effect of Mobile health technology on weight control in adolescents and preteens: a systematic review and meta-analysis. Front Public Health 2021;9:708321. https://doi.org/10.3389/fpubh.2021.708321.Search in Google Scholar PubMed PubMed Central
43. Hopewell, S, Chan, AW, Collins, GS, Hróbjartsson, A, Moher, D, Schulz, KF, et al.. CONSORT 2025 statement: updated guideline for reporting randomised trials. BMJ 2025;389:e081123. https://doi.org/10.1136/bmj-2024-081123.Search in Google Scholar
44. World Health Organization (WHO). Child growth standards [Internet]. [cited 2023 Jan 12]. Available from: https://www.who.int/tools/child-growth-standards.Search in Google Scholar
45. Marshall, WA, Tanner, JM. Variations in pattern of pubertal changes in girls. Archives Dis Child 1969;44:291. https://doi.org/10.1136/adc.44.235.291.Search in Google Scholar PubMed PubMed Central
46. Calcaterra, V, Giuseppe, RD, Biino, G, Mantelli, M, Marchini, S, Bendotti, G, et al.. Relation between circulating oxidized-LDL and metabolic syndrome in children with obesity: the role of hypertriglyceridemic waist phenotype. J Pediatr Endocrinol Metabol 2017;30:1257–63. https://doi.org/10.1515/jpem-2017-0239.Search in Google Scholar PubMed
47. De Onis, M. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–7. https://doi.org/10.2471/blt.07.043497.Search in Google Scholar PubMed PubMed Central
48. Flynn, JT, Urbina, EM, Brady, TM, Baker-Smith, C, Daniels, SR, Hayman, LL, et al.. Ambulatory blood pressure monitoring in children and adolescents: 2022 update: a scientific statement from the American heart association. Hypertension 2022;79:e114–24. https://doi.org/10.1161/hyp.0000000000000215.Search in Google Scholar PubMed PubMed Central
49. Matthews, DR, Hosker, JP, Rudenski, AS, Naylor, BA, Treacher, DF, Turner, RC. Homeostasis model assessment: insulin resistance and ? cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. https://doi.org/10.1007/bf00280883.Search in Google Scholar
50. Williams, JG, Eston, R, Furlong, B. CERT: a perceived exertion scale for young children. Percept Mot Ski 1994;79:1451–8. https://doi.org/10.2466/pms.1994.79.3f.1451.Search in Google Scholar PubMed
51. Headid, IRJ, Park, SY. The impacts of exercise on pediatric obesity. Clin Exp Pediatr 2021;64:196–207. https://doi.org/10.3345/cep.2020.00997.Search in Google Scholar PubMed PubMed Central
52. Kolsgaard, MLP, Joner, G, Brunborg, C, Anderssen, SA, Tonstad, S, Andersen, LF. Reduction in BMI z-score and improvement in cardiometabolic risk factors in Obese children and adolescents. The Oslo adiposity intervention study - a hospital/public health nurse combined treatment. BMC Pediatr 2011;11:47. https://doi.org/10.1186/1471-2431-11-47.Search in Google Scholar PubMed PubMed Central
53. Kolsgaard, MLP, Joner, G, Brunborg, C, Anderssen, SA, Tonstad, S, Andersen, LF. Erratum to: “reduction in BMI z-score and improvement in cardiometabolic risk factors in obese children and adolescents. The Oslo adiposity intervention study - a hospital/public health nurse combined treatment.”. BMC Pediatr 2012;12:77. https://doi.org/10.1186/1471-2431-12-77.Search in Google Scholar PubMed PubMed Central
54. Muñoz-Hernando, J, Escribano, J, Ferré, N, Closa-Monasterolo, R, Grote, V, Koletzko, B, et al.. Usefulness of the waist-to-height ratio for predicting cardiometabolic risk in children and its suggested boundary values. Clin Nutr 2022;41:508–16. https://doi.org/10.1016/j.clnu.2021.12.008.Search in Google Scholar PubMed
55. Martin-Calvo, N, Moreno-Galarraga, L, Martinez-Gonzalez, M. Association between body mass index, waist-to-height ratio and adiposity in children: a systematic review and meta-analysis. Nutrients 2016;8:512. https://doi.org/10.3390/nu8080512.Search in Google Scholar PubMed PubMed Central
56. Valerio, G, Di Bonito, P, Calcaterra, V, Cherubini, V, Corica, D, De Sanctis, L, et al.. Cardiometabolic risk in children and adolescents with obesity: a position paper of the Italian society for pediatric endocrinology and diabetology. Ital J Pediatr 2024;50:205. https://doi.org/10.1186/s13052-024-01767-x.Search in Google Scholar PubMed PubMed Central
57. Iafusco, D, Franceschi, R, Maguolo, A, Guercio Nuzio, S, Crinò, A, Delvecchio, M, et al.. From metabolic syndrome to type 2 diabetes in youth. Children 2023;10:516. https://doi.org/10.3390/children10030516.Search in Google Scholar PubMed PubMed Central
58. Bird, SR, Hawley, JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med 2016;2:e000143. https://doi.org/10.1136/bmjsem-2016-000143.Search in Google Scholar PubMed PubMed Central
59. García-Hermoso, A, López-Gil, JF, Izquierdo, M, Ramírez-Vélez, R, Ezzatvar, Y. Exercise and insulin resistance markers in children and adolescents with excess weight: a systematic review and network meta-analysis. JAMA Pediatr 2023;177:1276. https://doi.org/10.1001/jamapediatrics.2023.4038.Search in Google Scholar PubMed PubMed Central
60. Whooten, R, Kerem, L, Stanley, T. Physical activity in adolescents and children and relationship to metabolic health. Curr Opin Endocrinol Diabetes Obes 2019;26:25–31. https://doi.org/10.1097/med.0000000000000455.Search in Google Scholar PubMed PubMed Central
61. Casas, R, Ruiz-León, AM, Argente, J, Alasalvar, C, Bajoub, A, Bertomeu, I, et al.. A new mediterranean lifestyle pyramid for children and youth: a critical lifestyle tool for preventing obesity and associated cardiometabolic diseases in a sustainable context. Adv Nutr 2025;16:100381. https://doi.org/10.1016/j.advnut.2025.100381.Search in Google Scholar PubMed PubMed Central
62. Davis, CL, Litwin, SE, Pollock, NK, Waller, JL, Zhu, H, Dong, Y, et al.. Exercise effects on arterial stiffness and heart health in children with excess weight: the SMART RCT. Int J Obes 2020;44:1152–63. https://doi.org/10.1038/s41366-019-0482-1.Search in Google Scholar PubMed PubMed Central
63. García-Hermoso, A, Ramírez-Vélez, R, Ramírez-Campillo, R, Peterson, MD, Martínez-Vizcaíno, V. Concurrent aerobic plus resistance exercise versus aerobic exercise alone to improve health outcomes in paediatric obesity: a systematic review and meta-analysis. Br J Sports Med 2018;52:161–6. https://doi.org/10.1136/bjsports-2016-096605.Search in Google Scholar PubMed
64. Pinckard, K, Baskin, KK, Stanford, KI. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med 2019;6:69. https://doi.org/10.3389/fcvm.2019.00069.Search in Google Scholar PubMed PubMed Central
65. Zhang, Y, Wang, R, Liu, T, Wang, R. Exercise as a therapeutic strategy for obesity: central and peripheral mechanisms. Metabolites 2024;14:589. https://doi.org/10.3390/metabo14110589.Search in Google Scholar PubMed PubMed Central
66. Ferrulli, A, Terruzzi, I, Senesi, P, Succi, M, Cannavaro, D, Luzi, L. Turning the clock forward: new pharmacological and non pharmacological targets for the treatment of obesity. Nutr Metabol Cardiovasc Dis 2022;32:1320–34. https://doi.org/10.1016/j.numecd.2022.02.016.Search in Google Scholar PubMed
67. Babak, A, Motamedi, N, Mousavi, SZ, Darestani, NG. Effects of mindfulness-based stress reduction on blood pressure, mental health, and quality of life in hypertensive adult women: a randomized clinical trial study. JTHC [Internet] 2022. https://doi.org/10.18502/jthc.v17i3.10845 [cited 2025 May 2]; Available from: https://publish.kne-publishing.com/index.php/JTHC/article/view/10845.Search in Google Scholar PubMed PubMed Central
68. Rodrigues, GD, Lima, LS, Da Silva, NCS, Telles, PGL, Da Mota Silva Rocha, TM, De Aragão Porto, VQ, et al.. Are home-based exercises effective to reduce blood pressure in hypertensive adults? A systematic review. Clin Hypertens 2022;28:28. https://doi.org/10.1186/s40885-022-00211-8.Search in Google Scholar PubMed PubMed Central
69. Montón-Martínez, R, Ballester-Ferrer, JA, Baladzhaeva, S, Sempere-Ruiz, N, Casanova-Lizón, A, Roldan, A, et al.. Exploring the impact of web-based vs. In-Person exercise training on benefits and adherence in substance use disorder interventions: a pilot study. Healthcare 2024;12:684. https://doi.org/10.3390/healthcare12060684.Search in Google Scholar PubMed PubMed Central
70. Oginni, J, Otinwa, G, Gao, Z. Physical impact of traditional and virtual physical exercise programs on health outcomes among corporate employees. JCM. 2024;13(3):694, https://doi.org/10.3390/jcm13030694.Search in Google Scholar PubMed PubMed Central
71. Kardan, M, Jung, A, Iqbal, M, Keshtkar, S, Geidl, W, Pfeifer, K. Efficacy of digital interventions on physical activity promotion in individuals with noncommunicable diseases: an overview of systematic reviews. BMC Digit Health 2024;2:40. https://doi.org/10.1186/s44247-024-00097-6.Search in Google Scholar
72. Fabbrizio, A, Fucarino, A, Cantoia, M, De Giorgio, A, Garrido, ND, Iuliano, E, et al.. Smart devices for health and wellness applied to tele-exercise: an overview of new trends and technologies such as IoT and AI. Healthcare 2023;11:1805. https://doi.org/10.3390/healthcare11121805.Search in Google Scholar PubMed PubMed Central
73. World Health Organization (WHO). Recommendations on digital interventions for health system strengthening [Internet]. [cited 2025 May 2]. Available from: https://iris.who.int/bitstream/handle/10665/311941/9789241550505-eng.pdf.Search in Google Scholar
74. Smith, JD, Berkel, C, Carroll, AJ, Fu, E, Grimm, KJ, Mauricio, AM, et al.. Health behaviour outcomes of a family based intervention for paediatric obesity in primary care: a randomized type II hybrid effectiveness‐implementation trial. Pediatr Obes 2021;16:e12780. https://doi.org/10.1111/ijpo.12780.Search in Google Scholar PubMed
75. Hayes, CB, O’Shea, MP, Foley-Nolan, C, McCarthy, M, Harrington, JM. Barriers and facilitators to adoption, implementation and sustainment of obesity prevention interventions in Schoolchildren– a DEDIPAC case study. BMC Public Health 2019;19:198. https://doi.org/10.1186/s12889-018-6368-7.Search in Google Scholar PubMed PubMed Central
76. Chatterjee, A, Prinz, A, Gerdes, M, Martinez, S. Digital interventions on healthy lifestyle management: systematic review. J Med Internet Res 2021;23:e26931. https://doi.org/10.2196/26931.Search in Google Scholar PubMed PubMed Central
77. Vos, M, Deforche, B, Van Kerckhove, A, Michels, N, Geuens, M, Van Lippevelde, W. Intervention strategies to promote healthy and sustainable food choices among parents with lower and higher socioeconomic status. BMC Public Health 2022;22:2378. https://doi.org/10.1186/s12889-022-14817-y.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

