The role of aspirin, statins, colchicine, and IL-1 inhibitors in prevention of cardiovascular events: a systematic integrative review
-
Vania Arboleda
, Ashley Hackworth
Abstract
Context
Cardiovascular disease (CVD) is the leading cause of death in the United States. As such, an unmet need exists in the primary and secondary prevention of adverse cardiovascular events (CVEs). Specifically, identifying drugs that can reduce the progression of CVD and serious adverse events is much needed. Drugs that work by reducing platelet aggregation, blocking cholesterol formation (3-hydroxy-3-methyl-glutaryl-coenzyme A [HMG-CoA] reductase inhibitors), and/or blocking inflammation pathways (mainly interleukin-1b [IL-1b]) have been linked to preventing adverse CVEs, including acetylsalicylic acid (ASA, aspirin), statins, colchicine, and IL-1 inhibitors (interleukin-1 receptor antagonists). This systematic review aims to provide insight into utilizing these four agents for the primary and/or secondary prevention of CVD.
Objectives
In this systematic review, we opted to review the efficacy of aspirin, statins, colchicine, and IL-1 inhibitors in the primary and secondary prevention of CVE to provide clinical practitioners with evidence-based practice approaches and determine any unmet needs in their utilization.
Methods
Between October 1 and 12, 2021, a search was conducted and completed on five databases: PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, and Biomedical Reference Collection: Comprehensive. A total of 13 researchers (V.A., A.H., S.B., V.G., D.C., C.C., C.B., C.A., S.K., J.H., A.K., S.F., and S.E.) were involved in the search and screening of the articles. Search terms included “aspirin, statins, colchicine, IL-1 inhibitors, and primary, secondary, myocardial infarction (MI).” Inclusion criteria included clinical study design, English language articles, all genders older than 50 years old, and established patient history of CVD, including MI. In addition, articles were excluded if they were animal models, in vitro studies, pharmacokinetic studies, systematic reviews, literature reviews, and studies exploring therapies other than those listed in the inclusion criteria. First, five individuals independently sorted through abstracts or articles based on the inclusion and exclusion criteria. Then, a team of 13 individuals sorted through full-text articles of selected abstracts based on the same criteria. A separate researcher resolved conflicts between the team.
Results
A total of 725 articles were identified from all databases, from which 256 duplicated articles were removed. Thus, a total of 469 articles abstracts were screened, of which 425 articles either did not meet the inclusion criteria or met the exclusion criteria. A total of 42 articles were retrieved and assessed for full-text review, from which 15 articles were retrieved for analysis.
Conclusions
Statins may prevent primary CVEs based on their role in preventing cholesterol formation. Aspirin, canakinumab, and colchicine may be helpful in the secondary prevention of CVEs due to their blocking of various steps in the inflammation pathway leading to CVD. Future research should primarily focus on the use of canakinumab and colchicine in preventing CVD due to the limited number of studies on these drugs.
Cardiovascular disease (CVD) is a leading cause of mortality in the United States [1]. As such, an unmet need exists in the primary and secondary prevention of adverse cardiovascular events (CVEs). Therefore, a better understanding of primary and secondary prevention of CVD strategies is warranted. Currently, CVD prevention involves regular exercise, smoking cessation, healthy eating, and medication compliance [2], [3], [4], [5]. Gaining insight into drugs that can reduce the progression of CVD and serious adverse events is much needed. This review will focus on four drugs that work by reducing platelet aggregation, blocking cholesterol formation (3-hydroxy-3-methyl-glutaryl-coenzyme A [HMG-CoA] reductase inhibitors), and/or blocking inflammation pathways (mainly interleukin-1b [IL-1b]), which have been linked to preventing adverse CVEs, including acetylsalicylic acid (ASA, aspirin), statins, colchicine, and IL-1 inhibitors (interleukin-1 receptor agonists) [6], [7], [8], [9]. For this review, manuscripts were reviewed to determine whether aspirin, colchicine, statins, and IL-1 inhibitor medications were influential in the primary and/or secondary prevention of CVD. In this study, primary prevention of CVD was defined in a patient with no history of angina or myocardial infarction (MI) who undergoes a trial of any one of the four chosen drugs to prevent a first instance of MI. On the other hand, a secondary prevention of CVD was defined in a patient with a history of at least one instance of MI that undergoes a trial of any one of the four chosen drugs to prevent further instances of MI.
Research efforts and clinical practice outcomes have proven that aspirin is an effective anti-inflammatory and anti-platelet drug [6]. Aspirin’s mechanism of action involves irreversibly inhibiting cyclooxygenase (COX), a key enzyme in converting arachidonic acid to prostanoids that reduces thromboxane A2, prostaglandins, and prostacyclin, and leads to a decrease in platelet aggregation, stimulating vasodilation [10]. The effects of aspirin have shown to be beneficial in preventing secondary CVE, but the drug’s effectiveness in preventing primary events remains unclear [6].
Colchicine is an anti-inflammatory drug commonly used to treat gout and autoimmune disorders such as Familial Mediterranean Fever (FMF) [11]. It is thought to be effective in preventing acute coronary syndrome (ACS) due to the drug’s anti-inflammatory properties [8]. Colchicine blocks the NLRP3 inflammasome, a cytosolic multiprotein complex in myeloid cells that include neutrophils, monocytes, and eosinophils involved in producing the pro-inflammatory cytokine IL-1β (and IL-18) and plaque development [12]. Through blockage of NLRP3 inflammasome, colchicine prevents IL-1β production, decreasing endothelial inflammation, inhibiting platelet-leukocyte aggregation, and resulting in atherosclerotic plaque development and destabilization [12]. While effective in reducing CVD complications, colchicine has been associated with gastrointestinal (GI) adverse effects such as diarrhea, nausea, vomiting, and abdominal pain, limiting prevention [8]. Over the past decade, studies investigating colchicine’s beneficial cardiovascular effects in high-risk groups have been conducted. However, few studies have been completed evaluating its effectiveness in the primary prevention of CVD [9].
Among HMG-CoA reductase inhibitors, statins, categorized as low-density lipoprotein (LDL)–lowering medications, have been widely used for the treatment of hyperlipidemia and atherosclerotic cardiovascular disease (ASCVD) [13]. Hyperlipidemia refers to disorders that result in high levels of fats circulating in the body, leading to atherosclerotic lipid deposition and other CVD, including MI, stroke, ACS, and peripheral artery disease (PAD) [14]. United States Preventive Services Task Force (USPSTF) guidelines recommend moderate- to low-intensity statin therapy for individuals between the ages of 40 and 70 with CVD risk factors and who are at a 10-year risk of a CVE of 10 % or greater [7]. The 10-year risk of a CVE is determined using a risk calculator that assesses the risk of developing an ASCVD event in 10 years. The results of utilizing the calculator to determine the CVE risk are categorized as less than 5 % (low risk), 5–7.4 % (borderline risk), 7.5–19.9 % (intermediate risk), and greater than 20 % (high risk) [7]. The current 2018 American College of Cardiology and American Heart Association (ACC/AHA) guidelines recommend using appropriate-intensity statin therapy for people with or at risk of ASCVD, with seven Food and Drug Administration (FDA)-approved statins, subcategorized according to their lipid-lowering intensities [13]. Meanwhile, the USPSTF guidelines do not provide conclusive evidence of the effectiveness and risks of treating patients with a statin among those with less than a 10 % risk of developing a CVE in 10 years [7].
Interleukin-1 (IL-1) is a pro-inflammatory cytokine that plays an essential role in the innate immune response, which functions as an initial signal to begin the inflammatory cascade [15] IL-1 has two isoforms, IL-1α and IL-1β, and is a potential drug target in inflammatory conditions [15]. The IL-1 receptor antagonist (IL-1Ra) is a naturally occurring IL-1 antagonist that binds to IL-1α and IL-1β receptors without stimulating the inflammatory cascade [15]. There are currently four IL-1 inhibitors on the market, including canakinumab, anakinra, rilonacept, and gevokizumab [16]. Each of these targets a different isoform. Canakinumab is a human anti-IL-1β monoclonal antibody indicated for several auto-inflammatory syndromes, juvenile arthritis, and cryopyrin-associated periodic syndrome (CAPS) [16]. Anakinra is a recombinant human IL-1Ra indicated for rheumatoid arthritis, juvenile arthritis, gouty arthritis, and CAPS [16]. Rilonacept is a chimeric recombinant IL-1 blocker that acts as a decoy for IL-1α, IL-1β, and IL-1Ra and is indicated for CAPS [16]. Gevokizumab is an experimental humanized IL-1β monoclonal antibody antagonist that is currently being developed as an anticancer drug and that displays residual IL-1 agonist activity in addition to the antagonist activity but has not been evaluated as a preventative treatment in CVD [16].
Methods
Study design
Between October 1 and October 12, 2021, a systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Figure 1) guidelines to review and assess the efficacy of aspirin, statins, colchicine, and IL-1 inhibitors in the primary and secondary prevention of CVE. Ethical and Institutional Review Board (IRB) approval was not required. The systematic review was based exclusively on published literature. The review did not receive funding.
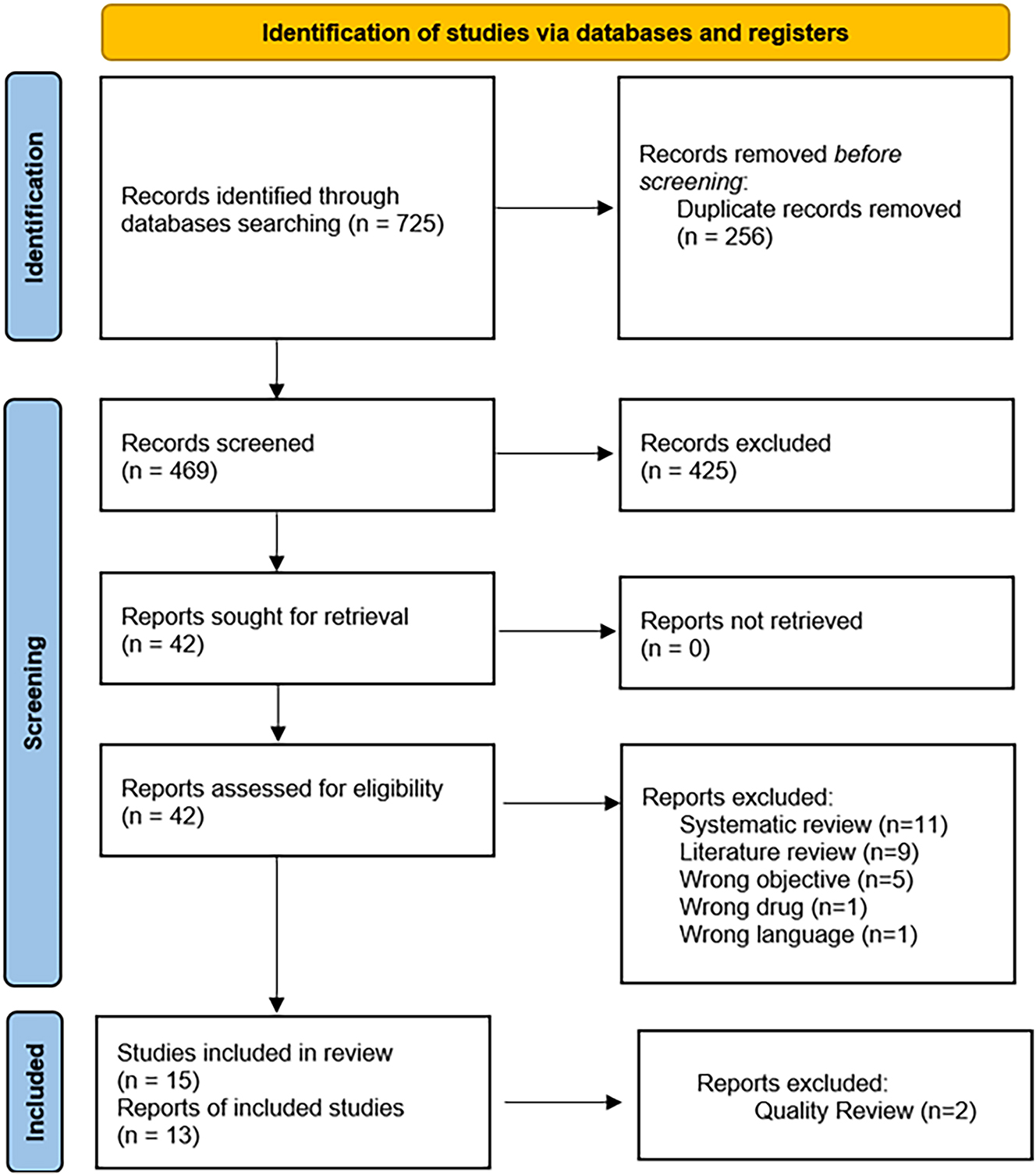
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Inclusion and exclusion criteria
The Population, Intervention, Comparison, and Outcomes (PICO) tool was the framework utilized to determine the inclusion and exclusion criteria for the articles. As for the population, it included clinical study design, all genders older than 50 years of age, established patients with a history of CVD, including MI, and English language articles. On the other hand, animal models, in vitro studies, pharmacokinetic studies, systematic reviews, literature reviews, Spanish articles, studies exploring therapies other than those listed in the inclusion criteria, and articles older than 5 years were excluded from the population. As for intervention, the study included subjects on statins for primary prevention and aspirin, colchicine, and IL-1 inhibitors for secondary CVE prevention, whereas it excluded any other form of therapy. As for comparison, the study aims to investigate the primary and secondary prevention capabilities of previously mentioned medications. Finally, as for outcomes, it included statins for primary prevention and aspirin, colchicine, and IL-1 inhibitors for secondary prevention.
Search strategy and screening
Between October 1 and 12, 2021, a search was conducted and completed on five databases: PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, and Biomedical Reference Collection: Comprehensive. Relevant search terms were run through electronic databases to extract published articles reporting on the primary and secondary prevention of CVE utilizing aspirin, statins, colchicine, or IL-1 inhibitors. All articles identified were uploaded into EndNote and, subsequently, Rayyan by a group of five researchers (V.A., D.C., S.B., J.H., and S.F.). These researchers (V.A., D.C., S.B., J.H., and S.F.) performed the first round of abstract screening, eliminating duplicate articles and removing abstracts that did not meet the inclusion and exclusion criteria following the PRISMA guidelines. These researchers (V.A., D.C., S.B., J.H., and S.F.) performed the second round of full-text screening, eliminating duplicate articles and removing articles that did not meet the inclusion and exclusion criteria following the PRISMA criteria. All of the researchers (V.A., A.H., S.B., V.G., D.C., C.C., C.B., C.A., S.K., J.H., A.K., S.F., S.E.) performed the qualitative review of the 15 articles, categorizing them as “poor,” “fair,” or “good” quality. The rating of these studies came from a combination of the articles’ relevance to the research question and considered the validity and sources of error present in the studies. “Poor” quality studies were reviewed by additional team members (A.H., S.F., V.A., V.G., S.B., S.E., and S.K.) and excluded by majority vote. A total of 13 studies were included in the final integrated systematic review, which all researchers reviewed. In addition, a separate researcher (M.K.) resolved any conflicts between the team.
Results
Systematic review
This systematic search utilized PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, and Biomedical Reference Collection: Comprehensive. The search resulted in a total of 725 articles, of which 256 duplicates were identified and removed before initiation screening, 469 abstracts from the remaining records were screened, and 425 articles did not meet the inclusion criteria. The resulting 42 records were sought for retrieval and assessed for eligibility. Of the 42 articles, 15 articles were included for qualitative review.
15 articles were categorized as “poor,” “fair,” or “good” quality. The rating of these studies came from a combination of the articles’ relevance to the research question and considered the validity and sources of error present in the studies. “Poor” quality studies were reviewed and excluded by a majority vote. A total of 13 studies were included in the final integrated systematic review, which all researchers reviewed.
Aspirin’s role in primary vs. secondary prevention of CVE
Five of the 13 articles screened for quality review focused on the role that aspirin has on primary and/or secondary CVE (Table 1).
Summary of findings: aspirin for primary vs. secondary prevention of cardiovascular disease [17], [18], [19], [20], [21].
| Study | Study type | Population | Control | Treatment | Outcomes |
|---|---|---|---|---|---|
| Kalantzi et al. [17] | RCT | 1,220 patients enrolled, 1,119 completed 12-month follow-up. Patients with stable CVD or history of ischemic stroke. | Triflusal 300 mg BID or 600 mg daily (n=559) | ASA 100 mg daily (n=560) | Triflusal and aspirin are equivalent in secondary prevention of CVE, but triflusal is more effective at MI prevention and has a better safety profile. |
| Moriarty et al. [18] | Cross-sectional study | 6,618 patients aged≥50 years, Primary prevention: no previous CVD (MI, angina, stroke, TIA), secondary prevention: previous CVD |
No ASA (n=5,186) | ASA (n=1,432) | Prescribing is targeted to those with higher CVD risk, reconsider aspirin as primary prevention (risks likely outweigh benefits). |
| Sim et al. [19] | RCT | 1819 patients treated with clopidogrel or aspirin after DES for acute MI | Clopidogrel (n=534) | ASA (n=1,285) | In patients with acute MI treated with DES and 12-month dual antiplatelet therapy, monotherapy with clopidogrel or aspirin showed similar rates of net adverse clinical events. |
| Batra et al. [20] | Retrospective study | 7,116 patients with Afib, undergoing PCI | Single antiplatelet (n=874) Warfarin (n=111) Dual antiplatelet (n=4,328) |
ASA + warfarin (n=134) vs. clopidogrel + Warfarin (n=516) vs. Triple therapy (aspirin, clopidogrel, and oral anticoagulant) (n=153) | Aspirin or clopidogrel + warfarin were not associated with an increased risk of major bleeds, and when compared to dual antiplatelet therapy, were associated with similar 0–90 days and lower 91–365 days of risk of the composite of mortality, MI, or ischemic stroke. Triple therapy was associated with an almost double risk of major bleeds and did not show a significant lower risk of cardiovascular outcomes. |
| Eriksson [21] | Retrospective study | 549 patients enrolled in stroke or neurology unit between 1986 and 2011 | Men (n=288) Warfarin 18 % Untreated 27 % Unknown 2 % Women (n=261) Warfarin 15 % Untreated 23 % Unknown 2 % |
Men (n=288) ASA 54 % Women (n=261) ASA 60 % |
Both warfarin and aspirin had a beneficial effect on survival after TIA/stroke. Risk of fatal bleed was 0.86 % annually on warfarin vs. 0.17 % on Aspirin. Patients with history of TIA/stroke had higher mortality rate vs. controls, supporting primary and secondary prophylaxis unless contraindications. |
-
Afib, atrial fibrillation; ASA, acetylsalicylic acid (aspirin); BID, twice a day; CVE, cardiovascular events; CVD, cardiovascular disease; DES, drug-eluting stents; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCT, randomized control trial; TIA, transient ischemic attack.
Aspirin was found to be effective in the secondary prevention of heart disease, and its use in primary prevention was discouraged due to its adverse effects outweighing its benefits (Table 1). When compared to other drugs, it was found that treatment with aspirin and clopidogrel shared similar rates of efficacy and safety; however, aspirin is still considered a first-line treatment in the secondary prevention of CVE (Table 1). Aspirin used in secondary prevention of CVE was targeted at those with a higher CVD risk. The use of aspirin with warfarin in patients undergoing percutaneous coronary interventions (PCIs) after an MI and atrial fibrillation was associated with a decreased risk of a CVE compared to dual antiplatelet therapy (Table 1). Also, aspirin and triflusal were found to have similar effects in the secondary prevention of CVE in patients with stable CVD. However, triflusal had a safer profile due to a lower risk of bleeding and increased efficacy in preventing MI (Table 1). Lastly, warfarin was more effective than aspirin in preventing stroke and MI in male patients with a previous transient ischemic attack (TIA). However, either treatment was recommended to decrease the risk of vascular death (Table 1).
Statins role in primary vs. secondary prevention of CVE
Six of the 13 articles screened for quality review focused on the role that statins have on primary and/or secondary CVEs (Table 2).
Summary of findings: statins for primary vs. secondary prevention of cardiovascular disease [22], [23], [24], [25], [26], [27].
| Study | Study type | Population | Control | Treatment, s | Outcomes |
|---|---|---|---|---|---|
| Jun et al. [22] | Retrospective cohort analysis | 1,386,765 subjects were enrolled in the analysis, 336,090 subjects were selected for propensity score matched analyses | No statin (n=168,045) | Statin (n=168,045) | Statin treatment was beneficial for primary CVD prevention in patients with type 2 diabetes, graded reduction of cardiovascular risk with a longer duration of statin treatment. |
| Fröhlich et al. [23] | Retrospective cohort analysis | 2,992 patients with nonischemic CHF due to left ventricular systolic dysfunction | No statin (n=1783) | Statin (n=1,209) | Low TC levels were associated with increased mortality compared to high and very high TC levels irrespective of statin treatment. Statins attenuate but do not eliminate the relationship between increased TC and increased survival in nonischemic CHF patients. |
| Kamal et al. [24] | Retrospective cohort analysis | 5,437 patients who underwent CABG between 2010 and 2015 | No statins (n=4,534) | Statin (n=903) | Statins given preoperative to CABG reduced hazard ratio (risk of stroke, MI, or major adverse CVE) in all age groups. Only in elderly patients, results did not show a decreased hazard ratio (especially risk of major adverse CVE) when given high-intensity statins. |
| Tarrant et al. [25] | Retrospective study | 1,166 patients≥65 years with a low-energy hip fracture between 2015 and 2017 | No statin (n=775) | Statin (n=391) | Admission on statins did not reduce rate of inpatient MI or survival after MI, nor was there a dose-dependent relationship between statins and MI. |
| Ovrehus et al. [26] | Retrospective study | 33,552 symptomatic patients with no or nonobstructive CVD undergoing CTA with <50 % coronary stenosis between 2008 and 2017 | No statin (n=17,695) | Statin before coronary CTA (n=7,354) Statins after coronary CTA (n=8,503) |
Statin therapy associated with a reduction of MI and all-cause death across all severities of CVD, but absolute benefit is directly proportional with CVD burden. |
| Jun et al. [27] | Case-control study | 11,017 adults ≥75 years old who developed CVD (MI, stroke, or all-cause death) on statin treatment matched to 55,085 control subjects with no CVD history or events or statin treatment | No statin (n=55,085) | Statin (n=11,017) | Current statin use was associated with a significant reduced risk for composite outcome, stroke, and all-cause death, but not MI. Former statin users had no reduced risk of CVD or all-cause death. Longer durations of statin use led to a significant decreasing trend of composite outcome and individual stroke or all-cause mortality. |
-
CABG, coronary artery bypass graph; CHF, congestive heart failure; CTA, computed tomography angiography; CVD, cardiovascular disease; CVE, cardiovascular event; MI, myocardial infarction; TC, total serum cholesterol.
Statins were found to be mainly effective in the primary prevention of heart disease (Table 2). Reduction in MI, stroke, and major adverse cardiac events were noted when statins were utilized in: patients undergoing coronary artery bypass grafting (CABG), patients above the age of 75 with type 2 diabetes mellitus, and patients with nonobstructive coronary artery disease (Table 2). Long-term use of statins by patients with no previous CVD was associated with a reduced risk of stroke; however, it was not associated with a decreased risk of MI (Table 2). Finally, statin use also benefits patients with congestive heart failure (CHF) and those undergoing hip fracture surgery (Table 2). Little to no evidence was found on the efficacy of statins for the secondary prevention of CVD.
Colchicine and IL-1 inhibitors role in primary vs. secondary prevention of CVE
One of the 13 articles screened for a quality review focused on the role that colchicine has on primary and/or secondary CVE (Table 3), and one of 13 articles screened for a quality review focused on the role that canakinumab has on primary and/or secondary prevention on CVE (Table 3).
Summary of findings: colchicine [28] and canakinumab [29] for primary vs. secondary prevention of cardiovascular disease.
| Study | Study type | Population | Control | Treatment, s | Outcomes |
|---|---|---|---|---|---|
| Hennessy et al. [28] | RCT | 237 adults (>18 yo) who sustained a type I acute MI within the previous 7 days | Placebo (n=118) | Colchicine (n=119) | Low-dose colchicine was not associated with significantly increased likelihood of achieving CRP level ≤2 mg/L 30 days after an acute MI when compared to placebo. However, it was safe and well tolerated. |
| Everett et al. [29] | Retrospective study | 10,061 patients with a history of MI and hsCRP | Placebo (n=3,343) | Canakinumab (n=6,717 | Therapy with canakinumab led to significant reductions in rates of serious CVEs in men and women with prior MI and evidence of residual inflammatory risk. |
-
CVE, cardiovascular events; CRP, C-reactive protein; CVE, cardiovascular events; hsCRP, high-sensitivity C-reactive protein; MI, myocardial infarction; RCT, randomized controlled trial.
Colchicine may benefit patients in secondary prevention of CVD if utilized with other secondary-prevention medications (Table 3). However, GI side effects of colchicine may be severe enough to necessitate treatment cessation. Studies demonstrated that treatment with low-dose colchicine was not associated with a significant reduction of C-reactive protein (CRP) 30 days after acute MI (Table 3). However, CRP was slightly reduced in treatment vs. placebo (Table 3). Treatment with canakinumab (IL-1 inhibitor) was associated with a significant decrease in serious CVEs in patients with a history of MI and high CRP (Table 3). In addition, this study demonstrated greater efficacy in the secondary prevention of CVD vs. the primary prevention of CVD (Table 3).
In summary, Kalantzi et al. [17] and Moriarty et al. [18] showed that the adverse effects of aspirin outweighed the benefits when used in the primary prevention of CVD (Table 4). However, Sim et al. [19], Batra et al. [20], and Eriksson [21] described the beneficial effects of utilizing aspirin in the secondary prevention of CVD (Table 4). Statins were found beneficial in the primary prevention of CVD, but no benefits of statins were found in the secondary prevention [22], [23], [24], [25], [26], [27] (Table 4). Colchicine was beneficial in the secondary prevention of CVD [28] (Table 4). Further, canakinumab was beneficial in the secondary prevention of CVD [29] (Table 4). The use of both colchicine and canakinumab together in the primary prevention of CVD was not explored [28, 29] (Table 4).
Summary of findings for aspirin, statins, colchicine, and canakinumab and their role in primary vs. secondary prevention of CVD [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29].
| Medications | Primary prevention of CVD | Secondary prevention of CVD |
|---|---|---|
| Aspirin | Adverse effects outweigh benefits [17, 18] | Beneficial [19], [20], [21] |
| Statins | Beneficial [22], [23], [24], [25], [26], [27] | No benefit [25] |
| Colchicine | Not explored | Beneficial [28] |
| Canakinumab | Not explored | Beneficial [29] |
-
CVD, cardiovascular disease.
Discussion
This manuscript utilized an integrated systematic review methodology to investigate the effectiveness of aspirin, statins, colchicine, and IL-1 inhibitor (canakinumab) medications for the primary and secondary prevention of CVE in 13 articles.
Statin use in primary prevention was associated with a decreased risk of stroke, all-cause mortality, and CVD in type 2 diabetes patients. Longer duration of statin use was associated with greater risk reduction. However, the use of statins did not decrease the risk of MI after hip fracture surgery [22, 26, 27]. Statin use seemed to attenuate the risk of elevated total cholesterol on survival of nonischemic CHF. However, low total cholesterol was associated with increased mortality irrespective of statin treatment [23]. Colchicine may benefit such patients in combination with other secondary prevention medications, although the GI side effects of colchicine may be severe enough to stop treatment [28]. Future research should focus on the long-term efficacy of combination drugs for the optimal secondary prevention of CVE. Canakinumab (IL-1 inhibitor) could benefit patients with residual inflammatory risk following MI, supporting its secondary prevention efficacy in this population [29]. The literature showed that aspirin might not be efficacious for the primary prevention of CVE but may be effective in the secondary prevention of CVE [18]. Finally, it is suggested that treatment with a combination of aspirin or clopidogrel with warfarin after PCI decreases the composite risk of MI compared to dual-platelet therapy and is not associated with an increased risk of bleeding [20]. Clopidogrel and aspirin had similar rates of net adverse events for patients with acute MI after drug-eluting stent therapy [19].
The findings of this review generally fit with previously conducted research and current protocols regarding the investigated drug classes. The USPSTF recently advised against initiating low-dose aspirin for primary prevention of CVD in adults aged 60 years and older. It is also recommended that the decision to initiate low-dose aspirin use for the primary prevention of CVD in adults ages 40–59 years who have a 10 % or greater 10-year CVD risk should be an individual one [6]. The findings of this review regarding statins also fit with the current research and guidelines. The USPSTF recommends that clinicians prescribe a statin for the primary prevention of CVD for adults aged 40–75 years who have one or more CVD risk factors and have an estimated 10-year risk of a CVE of 10 % or greater [7]. Colchicine has been associated with decreased revascularization rates, stroke, and major cardiac events [8]. However, no study has directly focused on colchicine and its role in primary prevention. One study did find that adding low-dose colchicine to standard medical therapy reduces the incidence of major CVEs, except cardiovascular mortality, when compared with standard medical therapy alone [30]. The Colchicine Cardiovascular Outcomes Trial (COLCOT) demonstrated that colchicine effectively prevented major adverse cardiac events after MI compared with placebo [31]. Primarily, it reduced the incidence of stroke and urgent hospitalization for unstable angina leading to coronary revascularization. The benefits of colchicine studied in COLCOT were at least as significant as those of canakinumab in Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) [32]. Besides the CANTOS trial, there is very little published research investigating canakinumab alone in either primary or secondary prevention. Further research is certainly needed with both colchicine and canakinumab.
The 15 studies that met the inclusion/exclusion criteria underwent a quality review and were labeled as poor, fair, or good quality. The rating of these studies came from a combination of the studies’ relevance to our research question, and considered the validity and sources of error present in the articles. “Poor”-quality studies were reviewed by additional team members and excluded by majority vote. Although only one article was identified for colchicine and canakinumab, both articles were considered “good”-quality articles and useful to include when comparing the effectiveness of all drugs in the prevention of secondary CVEs. Although most articles about statins or aspirin were considered “fair quality,” there was still a sufficient number of total articles [19, 25] to draw strong conclusions. Specifically, among the articles labeled “fair,” the most common concern was that either the primary or secondary prevention of CVEs only applied to a specific etiology [8, 17, 18, 24, 26] or a specific medical intervention [18, 30]. Thus, this limited the applications of the review’s findings to a broad prevention of primary and secondary CVEs.
Some studies investigated specific patient demographics and current health status. Given the nature of CVD, it is difficult to find patients at that stage of life who are not on other medications or have comorbid diseases. Specific studies investigated the acute post-CVE time frame. It is possible that the mechanism that brought on these CVE events may influence the body’s reaction to the care administered. The age ranges of the studies also varied, which could have implications on the age that affects the body’s natural ability to heal itself. Three studies also investigated the results following invasive procedures: CABG, PCI, and hip fracture repair. The stress that these procedures can put on the body may also directly affect the potential of CVEs [20, 24, 25].
In the context of osteopathic principles, this review highlights how continued research on primary or secondary prevention of CVEs can shine a light on new options for early-on care or diverse add-on management to target a broader and more varied patient population. This systematic review reinforces statins’ benefits in primary prevention, particularly for type 2 diabetes patients, with longer-term use yielding a more significant risk reduction of CVEs [22, 26, 27]. Meanwhile, when combined with other therapies, colchicine’s potential in secondary prevention can be an answer for possible add-on options. In June of 2023, the US FDA approved the use of low-dose colchicine to reduce the risk of myocardial infarction in adults with established atherosclerotic disease or with multiple risk factors for CVD [33]. Further, canakinumab’s prospect of addressing residual inflammatory risk post-MI highlights the importance of targeting all pathology aspects [32]. While cautious with its side effects, the enduring efficacy of aspirin in secondary prevention reinforces the idea that the preventive medicine community should continue exploring the topic. Considering the holistic approach, this review underscores the importance of study quality, patient profile, and procedural context to advance cardiovascular care. This study provides insight into optimizing cardiovascular prevention strategies to provide care to a broader patient population.
In summary, aspirin, colchicine, and canakinumab may be useful in the secondary prevention of CVE, while statin therapy benefits the primary prevention of CVE. Aspirin remains the first-line treatment for secondary prevention of CVE [17], [18], [19], [20], [21]. Aspirin had beneficial effects in the secondary prevention of CVE in patients with stable CVD and those with a high risk for CVD. However, triflusal, compared to aspirin for chronic treatment, in individuals with stable CVD and a history of noncardioembolic ischemic stroke, was more preventative of myocardial infarction due to a safer profile [17]. Further research is needed to determine the effectiveness of triflusal chronic CVD treatment in a large population. Colchicine’s role in secondary prevention of major CVE, except cardiovascular mortality, was mainly an add-on therapy to the standard medical therapy alone. Likewise, canakinumab reduced CVE in individuals with prior MI due to inflammation inhibition [18]. Further research should focus on conducting a large-scale analysis of the effects of utilizing canakinumab and colchicine in lower-risk populations.
The role of statins in the primary prevention of CVEs is focused on reducing atherosclerotic plaque development. Utilizing statins attenuates the inverse relationship between total serum cholesterol and mortality; however, the exact mechanism of action remains unknown [23]. Statins are primarily used on patients undergoing CABG, patients above 75 with type 2 diabetes mellitus, patients with nonobstructive CVD, and patients with no previous CVD. Beneficial effects of statins were not observed in individuals under the age of 40 years of age with type 2 diabetes for primary prevention [22]. Because statins appear ineffective in individuals in this age range, further research should focus on analyzing the cardioprotective effects of other medications for primary prevention. A current option for patients who do not respond to or tolerate statins is a trial of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors [34]. PCSK9 inhibitors are superior at lowering LDL and similar at lowering the risk of CVE compared to statins, while statins remain superior at reducing all-cause death and CVE-related death. Research on the role of PSCK9 inhibitors in the primary prevention of CVE in patients for whom statins are ineffective or not tolerated should be considered [34].
Limitations
The most relevant strength of the review process was having a team of 13 members to eliminate the bias that may occur with a smaller research team. The primary limitation of the review process was that only one study investigated the use of canakinumab and only one study investigated the use of colchicine in the prevention of CVD. This may be due to the narrow definition of what is considered primary and secondary prevention of CVE as defined by the study team. Additionally, the search terms utilized to identify articles in electronic data based on the exclusion of non-English articles may have led to a lack of studies identified for these drugs.
Conclusions
In conclusion, statins may prevent primary CVEs based on their role in preventing cholesterol formation. Aspirin and canakinumab may be helpful in the secondary prevention of CVEs due to their blocking of various steps in the inflammation pathway leading to CVD. Continued monitoring of colchicine’s role in the secondary prevention of CVEs should focus on side effects in the target population. Future research should also focus on the use of canakinumab in preventing CVD due to the limited number of systematic studies on this drug.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: None declared.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Ahmad, FB, Cisewski, JA, Anderson, RN. Provisional mortality data — United States, 2021. MMWR Morb Mortal Wkly Rep 2022;71:597–600. https://doi.org/10.15585/mmwr.mm7117e1.Search in Google Scholar PubMed PubMed Central
2. Lavie, CJ, Ozemek, C, Carbone, S, Katzmarzyk, PT, Blair, SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res 2019;124:799–815. https://doi.org/10.1161/circresaha.118.312669.Search in Google Scholar
3. Duncan, MS, Freiberg, MS, Greevy, RAJr, Kundu, S, Vasan, RS, Tindle, HA. Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA 2019;322:642–50. https://doi.org/10.1001/jama.2019.10298.Search in Google Scholar PubMed PubMed Central
4. Arnett, DK, Blumenthal, RS, Albert, MA, Buroker, AB, Goldberger, ZD, Hahn, EJ, et al.. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American heart association Task Force on clinical practice guidelines. Circulation 2019;140:e563–95. https://doi.org/10.1161/CIR.0000000000000677.Search in Google Scholar PubMed PubMed Central
5. Feng, Y, Zhao, Y, Yang, X, Li, Y, Han, M, Qie, R, et al.. Adherence to antihypertensive medication and cardiovascular disease events in hypertensive patients: a dose-response meta-analysis of 2 769 700 participants in cohort study. QJM 2022;115:279–86. https://doi.org/10.1093/qjmed/hcaa349.Search in Google Scholar PubMed
6. US Preventive Services Task Force, Davidson, KW, Barry, MJ, Mangione, CM, Cabana, M, Chelmow, D, Coker, TR, et al.. Aspirin use to prevent cardiovascular disease: US preventive services task force recommendation statement. JAMA 2022;327:1577–84. https://doi.org/10.1001/jama.2022.4983.Search in Google Scholar PubMed
7. US Preventive Services Task Force, Mangione, CM, Barry, MJ, Nicholson, WK, Cabana, M, Chelmow, D, Coker, TR, et al.. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA 2022;328:746–53. https://doi.org/10.1001/jama.2022.13044.Search in Google Scholar PubMed
8. Wudexi, I, Shokri, E, Abo-Aly, M, Shindo, K, Abdel-Latif, A. Comparative effectiveness of anti-inflammatory drug treatments in coronary heart disease patients: a systematic review and network meta-analysis. Mediat Inflamm 2021;2021:5160728. https://doi.org/10.1155/2021/5160728.Search in Google Scholar PubMed PubMed Central
9. Ridker, PM, Everett, BM, Thuren, T, MacFadyen, JG, Chang, WH, Ballantyne, C, et al.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. https://doi.org/10.1056/nejmoa1707914.Search in Google Scholar
10. Vane, JR, Botting, RM. The mechanism of action of Aspirin. Thromb Res 2003;110:255–8. https://doi.org/10.1016/s0049-3848(03)00379-7.Search in Google Scholar PubMed
11. Leung, YY, Yao Hui, LL, Kraus, VB. Colchicine – Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015;45:341–50. https://doi.org/10.1016/j.semarthrit.2015.06.013.Search in Google Scholar PubMed PubMed Central
12. Grebe, A, Hoss, F, Latz, E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res 2018;122:1722–40. https://doi.org/10.1161/CIRCRESAHA.118.311362.Search in Google Scholar PubMed
13. Grundy, SM, Stone, NJ, Bailey, AL, Beam, C, Birtcher, KK, Blumenthal, RS, et al.. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–209. https://doi.org/10.1016/j.jacc.2018.11.002.Search in Google Scholar PubMed
14. Nelson, RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 2013;40:195–211. https://doi.org/10.1016/j.pop.2012.11.003.Search in Google Scholar PubMed PubMed Central
15. Libby, P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol 2017;70:2278–89. https://doi.org/10.1016/j.jacc.2017.09.028.Search in Google Scholar PubMed PubMed Central
16. Arnold, DD, Yalamanoglu, A, Boyman, O. Systematic review of safety and efficacy of IL-1-targeted biologics in treating immune-mediated disorders. Front Immunol 2022;13:888392. https://doi.org/10.3389/fimmu.2022.888392.Search in Google Scholar PubMed PubMed Central
17. Kalantzi, KI, Ntalas, IV, Chantzichristos, VG, Tsoumani, ME, Adamopoulos, D, Asimakopoulos, C, et al.. Comparison of triflusal with aspirin in the secondary prevention of atherothrombotic events; Α randomised clinical trial. Curr Vasc Pharmacol 2019;17:635–43. https://doi.org/10.2174/1570161116666180605090520.Search in Google Scholar PubMed
18. Moriarty, F, Barry, A, Kenny, RA, Fahey, T. Aspirin prescribing for cardiovascular disease in middle-aged and older adults in Ireland: findings from the Irish Longitudinal Study on Ageing. Prev Med 2021;147:106504. https://doi.org/10.1016/j.ypmed.2021.106504.Search in Google Scholar PubMed
19. Sim, DS, Jeong, MH, Kim, HS, Gwon, HC, Seung, KB, Rha, SW, et al.. Clopidogrel versus aspirin after dual antiplatelet therapy in acute myocardial infarction patients undergoing drug-eluting stenting. Kor Circ J 2020;50:120. https://doi.org/10.4070/kcj.2019.0166.Search in Google Scholar PubMed PubMed Central
20. Batra, G, Friberg, L, Erlinge, D, James, S, Jernberg, T, Svennblad, B, et al.. Antithrombotic therapy after myocardial infarction in patients with atrial fibrillation undergoing percutaneous coronary intervention. Eur Heart J – Cardiovasc Pharmacother 2017:4;36–45. https://doi.org/10.1093/ehjcvp/pvx033.Search in Google Scholar PubMed
21. Eriksson, S-E. Secondary prophylactic treatment and long-term prognosis after TIA and different subtypes of stroke. A 25-year follow-up hospital-based observational study. Brain Behav 2016;7:e00603. https://doi.org/10.1002/brb3.603.Search in Google Scholar PubMed PubMed Central
22. Jun, JE, Jeong, I-K, Ahn, KJ, Chung, HY, Hwang, Y-C. Statin use for primary prevention in patients with type 2 diabetes: can it benefit all ages? – a nationwide propensity-matched cohort study. Diabetes Res Clin Pract 2021;180:109044. https://doi.org/10.1016/j.diabres.2021.109044.Search in Google Scholar PubMed
23. Fröhlich, H, Raman, N, Täger, T, Schellberg, D, Goode, KM, Kazmi, S, et al.. Statins attenuate but do not eliminate the reverse epidemiology of total serum cholesterol in patients with non-ischemic chronic heart failure. Int J Cardiol 2017;238:97–104. https://doi.org/10.1016/j.ijcard.2017.03.028.Search in Google Scholar PubMed
24. Kamal, Y, Abdel-Gaber, S, Amr, M, Al-Elwany, S. Cardio-protective effects of Statin therapy in elder patients undergoing coronary artery bypass grafting. J Gerontol Geriatr 2018;66:21–9.Search in Google Scholar
25. Tarrant, SM, Kim, RG, McDonogh, JM, Clapham, M, Palazzi, K, Attia, J, et al.. Preadmission statin prescription and inpatient myocardial infarction in geriatric hip fracture. J Clin Med 2021;10:2441. https://doi.org/10.3390/jcm10112441.Search in Google Scholar PubMed PubMed Central
26. Øvrehus, KA, Diederichsen, A, Grove, EL, Steffensen, FH, Mortensen, MB, Jensen, JM, et al.. Reduction of myocardial infarction and all-cause mortality associated to statins in patients without obstructive CAD. JACC (J Am Coll Cardiol): Cardiovascular Imag 2021;14:2400–10. https://doi.org/10.1016/j.jcmg.2021.05.022.Search in Google Scholar PubMed
27. Jun, JE, Cho, I-J, Han, K, Jeong, I-K, Ahn, KJ, Chung, HY, et al.. Statins for primary prevention in adults aged 75 years and older: a nationwide population-based case-control study. Atherosclerosis 2019;283:28–34. https://doi.org/10.1016/j.atherosclerosis.2019.01.030.Search in Google Scholar PubMed
28. Hennessy, T, Soh, L, Bowman, M, Kurup, R, Schultz, C, Patel, S, et al.. The Low Dose Colchicine after Myocardial Infarction (LoDoCo-MI) study: a pilot randomized placebo controlled trial of Colchicine following acute myocardial infarction. Am Heart J 2019;215:62–9. https://doi.org/10.1016/j.ahj.2019.06.003.Search in Google Scholar PubMed
29. Everett, BM, MacFadyen, JG, Thuren, T, Libby, P, Glynn, RJ, Ridker, PM. Inhibition of interleukin-1β and reduction in atherothrombotic cardiovascular events in the CANTOS trial. J Am Coll Cardiol 2020;76:1660–70. https://doi.org/10.1016/j.jacc.2020.08.011.Search in Google Scholar PubMed
30. Samuel, M, Tardif, JC, Bouabdallaoui, N, Khairy, P, Dubé, MP, Blondeau, L, et al.. Colchicine for secondary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Can J Cardiol 2021;37:776–85. https://doi.org/10.1016/j.cjca.2020.10.006.Search in Google Scholar PubMed
31. Akrami, M, Izadpanah, P, Bazrafshan, M, Hatamipour, U, Nouraein, N, Drissi, HB, et al.. Effects of Colchicine on major adverse cardiac events in next 6-month period after acute coronary syndrome occurrence; a randomized placebo-control trial. BMC Cardiovasc Disord 2021;21:583. https://doi.org/10.1186/s12872-021-02393-9.Search in Google Scholar PubMed PubMed Central
32. Tardif, JC, Kouz, S, Waters, DD, Bertrand, OF, Diaz, R, Maggioni, AP, et al.. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–505. https://doi.org/10.1056/NEJMoa1912388.Search in Google Scholar PubMed
33. Nelson, K, Fuster, V, Ridker, PM. Low-dose colchicine for secondary prevention of coronary artery disease: JACC review topic of the week. J Am Coll Cardiol 2023;82:648–60. https://doi.org/10.1016/j.jacc.2023.05.055.Search in Google Scholar PubMed
34. Zhao, Z, Du, S, Shen, S, Luo, P, Ding, S, Wang, G, et al.. Comparative efficacy and safety of lipid-lowering agents in patients with hypercholesterolemia: a frequentist network meta-analysis. Medicine 2019;98:e14400. https://doi.org/10.1097/MD.0000000000014400.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Cardiopulmonary Medicine
- Review Article
- The role of aspirin, statins, colchicine, and IL-1 inhibitors in prevention of cardiovascular events: a systematic integrative review
- Medical Education
- Original Article
- Teaching ultrasound in osteopathic medical schools
- Brief Report
- Expansion of osteopathic medicine practitioner education on substance use disorders
- Musculoskeletal Medicine and Pain
- Original Article
- Increased circulating microRNA-21 level as a potential indicator for predicting a higher risk of incident fragility fractures
- Public Health and Primary Care
- Commentary
- The evolution of type 2 diabetes management: glycemic control and beyond with SGLT-2 inhibitors and GLP-1 receptor agonists
- Clinical Image
- Green nail syndrome in a teenager
- Letter to the Editor
- Challenges to address prior to considering performing musculoskeletal point-of-care ultrasound
Articles in the same Issue
- Frontmatter
- Cardiopulmonary Medicine
- Review Article
- The role of aspirin, statins, colchicine, and IL-1 inhibitors in prevention of cardiovascular events: a systematic integrative review
- Medical Education
- Original Article
- Teaching ultrasound in osteopathic medical schools
- Brief Report
- Expansion of osteopathic medicine practitioner education on substance use disorders
- Musculoskeletal Medicine and Pain
- Original Article
- Increased circulating microRNA-21 level as a potential indicator for predicting a higher risk of incident fragility fractures
- Public Health and Primary Care
- Commentary
- The evolution of type 2 diabetes management: glycemic control and beyond with SGLT-2 inhibitors and GLP-1 receptor agonists
- Clinical Image
- Green nail syndrome in a teenager
- Letter to the Editor
- Challenges to address prior to considering performing musculoskeletal point-of-care ultrasound

