Abstract
Bile acids perform vital functions in the human liver and are the essential component of bile. It is therefore not surprising that the biology of bile acids is extremely complex, regulated on different levels, and involves soluble and membrane receptors as well as transporters. Hereditary disorders of these proteins manifest in different pathophysiological processes that result in liver diseases of varying severity. In this review, we summarize our current knowledge of the physiology and pathophysiology of bile acids with an emphasis on recently established analytical approaches as well as the molecular mechanisms that underlie signaling and transport of bile acids. In this review, we will focus on ABC transporters of the canalicular membrane and their associated diseases. As the G protein-coupled receptor, TGR5, receives increasing attention, we have included aspects of this receptor and its interaction with bile acids.
A general introduction to bile salts
Bile acids (BA) are essential for the absorption of dietary lipids and fat-soluble vitamins and also act as signaling molecules for diverse endocrine and paracrine functions (Keitel et al. 2008). Besides others, they can activate different BA receptors, such as the nuclear hormone farnesoid-X-receptor (FXR, NR1H4) (Makishima et al. 1999; Parks et al. 1999) and are ligands for G-protein coupled receptors such as the sphingosine-1 phosphate receptor 2 (S1PR2) (Studer et al. 2012) and the Takeda G protein-coupled receptor-5 (TGR5, Gpbar1) (Kawamata et al. 2003; Keitel et al. 2019b; Keitel et al. 2020) as well as the adhesion receptor α 5 β 1 integrin (Bonus et al. 2020; Gohlke et al. 2013). Synthesis of BA occurs in the liver through oxidation of cholesterol via two major pathways: the classic and alternative pathways (Figure 1). In the classic pathway, CYP7A1 represents the rate-limiting enzyme that catalyzes the conversion of cholesterol to 7α-hydroxycholesterol, which leads after several additional enzymatic steps to the formation of the primary BA, namely cholic acid (CA) and chenodeoxycholic acid (CDCA) (Sarenac and Mikov 2018). Hydroxylation at position 27 of cholesterol through the first enzyme CYP27A1 in the alternative pathway leads to the formation of 27-hydroxycholesterol, followed by CYP7B1-mediated hydroxylation to produce CDCA finally. The amount of total BA from the alternative pathway under normal human physiological conditions is less than 10%. The up-regulation of the alternative pathway may occur when the main pathway is fully or partially disturbed. Both CA and CDCA undergo conjugation reactions in the liver with taurine and glycine (TCA, GCA, TCDCA, and GCDCA) on their side chains and with sulfate, glucuronic acid, and N-acetylglucosamine on the hydroxyl groups of the cholane scaffold. In bile, the relative proportions of bile acids excreted, e.g., as sulfates or glucuronides are less than 2% (Takikawa et al. 1985). The taurine- and glycine-conjugated BA are secreted into the gallbladder and finally excreted into the small intestine. Three biotransformation reactions may occur to the conjugated bile acids in the intestinal tract through bacterial enzymes (Ridlon et al. 2016). The deconjugation reactions of glycine and taurine, epimerization and dehydroxylation steps generate secondary BA such as deoxycholic acid (DCA) or lithocholic acid (LCA). More than 90% of the BA are re-absorbed across the gut epithelia and transported to the liver via specific transporters. The re-absorbed free BA again undergo conjugations with taurine and glycine. This process is called “the enterohepatic circulation of bile acids” (Hofmann 1984; Keitel et al. 2019a; Ridlon et al. 2006). The most common human BA are CA, CDCA, DCA, LCA, and UDCA (ursodeoxycholic acid), as well as their taurine- and glycine-conjugated forms. UDCA, which accounts for up to 4% of the human BA pool under physiological conditions (Lazaridis et al. 2001), is generated from CDCA through epimerization steps and used as a therapeutic agent, e.g., in cholestatic liver diseases such as primary biliary cholangitis (Paumgartner and Beuers 2002). While, UDCA treatment did not reduce adverse perinatal outcomes in women with intrahepatic cholestasis of pregnancy (Chappell et al. 2019), patients with heterozygous mutations in the phospholipidfloppase ABCB4 (MDR3) treated with UDCA showed a mild disease course with transplant-free survival of 91% (de Vries et al. 2020). The hydrophilicity of the common free and conjugated BA decreases in the order UDCA > CA > CDCA > DCA > LCA, and taurine-conjugated > glycine-conjugated > free species. LCA represents the most lipophilic BA with toxic properties causing severe liver damage and biliary cholestasis. The use of animal models, especially genetically modified mice, plays an important role in studying the relationship between bile acid metabolism and human liver disease. In rodent livers, CDCA is hydroxylated at the 6β-position by CYP2C70 to α-muricholic acid (α-MCA) and ß-muricholic acid (β-MCA), representing further primary BA besides CA and CDCA (Takahashi et al. 2016). Microbial biotransformation including deconjugation, dehydroxylation, and epimerization leads to other secondary BA such as ω-MCA, MDCA (murideoxycholic acid), HCA (hyocholic acid), and HDCA (hyodeoxycholic acid) from α-MCA and ß-MCA (Takahashi et al. 2016). The relatively higher composition of hydrophilic BAs in rodents (muricholic BAs and their taurine conjugates) induces less hepatocyte toxicity than human BAs (Oizumi et al. 2017).
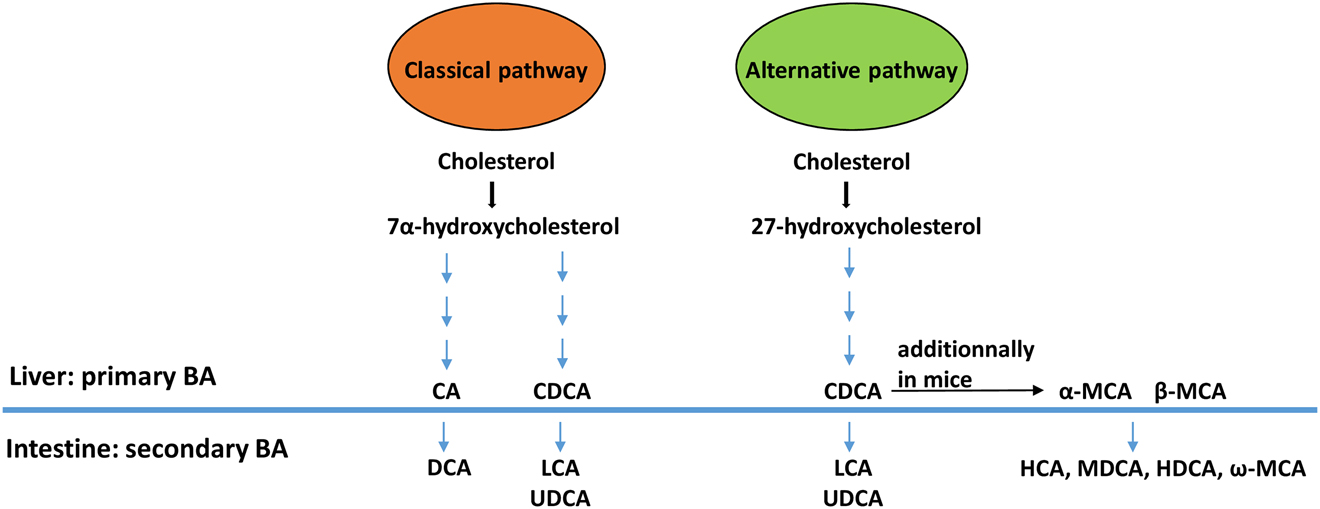
Synthesis of primary and secondary bile acids in the liver and intestine.
BA (bile acids), CA (cholic acid), CDCA (chenodeoxycholic acid), DCA (deoxycholic acid), LCA (lithocholic acid), UDCA (ursodeoxycholic acid), MCA (muricholic acid), HCA (hyocholic acid), HDCA (hyodeoxycholic acid), and MDCA (murideoxycholic acid).
Due to the biological and clinical significance of BA, several bioanalytical methods were established, particularly during the last two decades. These analytical platforms are based on the separation of BA, their identification, and quantification from different biological samples. The most popular techniques used for BA quantitation in clinical laboratories are enzymatic assays, liquid-/gas-chromatography (GC), and mass spectrometry (MS). The application of enzymatic assays offers the possibility for measuring the amount of total bile acids (TBA) in plasma and serum. These assays are simple in use, rapid, suitable for automation, and inexpensive. The enzyme 3-α-hydroxysteroid dehydrogenase (3-α-HSD) converts bile acids to 3-keto steroids and Thio-NADH in the presence of Thio-NAD. The formation of Thio-NADH can be determined spectrophotometrically at 405 nm (Zhang et al. 2005). Another assay is based on the formation of formazan dye and can be performed at 540 nm. Here, the enzyme 3-α-HSD converts bile acids to 3-ketosteroids and NADH in the presence of NAD. The resulting NADH reacts with nitrotetrazolium blue (NBT) to form a formazan dye in the presence of diaphorase enzyme (Mashige et al. 1981). However, measurements of individual BA with these assays, as, e.g., primary and secondary BA or taurine and glycine groups, are not possible. The use of HPLC (high-performance liquid chromatography) for separation of BA compounds in combination with UV-detection can be performed after pre-column derivatization with, e.g., nitrophenacylbromide and detection at a wavelength of 263 nm (Shi et al. 2015). This method is more suitable for very high concentrations of BA in biological samples. GC-MS separation and quantitation of BA is quite sensitive but needs sample derivatization steps and deconjugation reactions due the BAs’ thermal instability and low volatility (Griffiths and Sjovall 2010). In the field of BA profiling in biological matrices, UPLC-MS/MS (ultra-performance liquid chromatography coupled to tandem mass spectrometry) is considered the analytical technique of choice nowadays (Garcia-Canaveras et al. 2014). UPLC has a much higher separation efficiency than HPLC due to < 2 µm particle size technology of the stationary phases and faster elution of the analytes. Identification and quantification of BA, including their derivatives, are conducted with electrospray ionization source in negative ion mode and can be performed in single-ion monitoring (SIM), single-reaction monitoring (SRM), and multiple-reaction monitoring (MRM) modes to increase the selectivity and sensitivity (Yuan et al. 2012). The isobaric masses (same molecular ion/fragment ion) such as TDCA, TCDCA, THDCA, and TUDCA (m/z 498/80) are not distinguishable with tandem MS. Hence, there exists a need for an excellent chromatographic separation with UPLC to assign the proper retention times of each compound. In this way, the combination of UPLC and MS/MS data offers an accurate quantification and confirmation assay. Bile acid profiles can serve as diagnostic markers, e.g., for inborn errors of bile acid metabolism (Herebian and Mayatepek 2011) and different liver or gastrointestinal diseases (Sarafian et al. 2015; Sugita et al. 2015). Therapeutic drug monitoring of UDCA and its derivatives GUDCA/TUDCA in patients with cholestatic diseases is also of clinical significance (Guarino et al. 2013; Lazaridis et al. 2001).
Bile salt transport in the human liver
BA are synthesized de novo from cholesterol (Figure 2) in the cytosol of hepatocytes. However, they perform their function to solubilize hydrophobic compounds such as vitamins in the intestine. Interestingly, approximately 95% of the BA undergo enterohepatic circulation (Hofmann 1984; Keitel et al. 2019a; Ridlon et al. 2006) meaning they are transported back to the liver and re-absorbed by hepatocytes. The main player for the re-uptake of BA across the basolateral membrane of hepatocyte is the sodium taurocholate co-transporting polypeptide (NTCP or SLC10A1), which is a member of the solute carrier family (SLC) and was first cloned and characterized in 1991 (Hagenbuch et al. 1991). Taurocholate (TC)/sodium is transported in a symport fashion and at a stoichiometry of 1:2. However, NCTP not only imports TC, but all BA as well as steroids, thyroid hormones, and certain xenobiotics (Jung et al. 2004). Importantly, NTCP acts as a receptor for the hepatitis B and D viruses and has turned into an important target for drug development (Bogomolov et al. 2016; Ni et al. 2014). Unfortunately, no structural information for NTCP exists, and the bacterial paralogue from N. meningitis, ASBTNM, for which there is a crystal structure (Hu et al. 2011), cannot directly be used as a template for structural modeling since the two interaction sites of NTCP (amino acids 84–87 and 157–165) of the hepatitis B virus are very divergent in sequence in different species (Yan et al. 2013). Nevertheless, the bile salt and sodium ion binding sites of NTCP and ASBTNM are highly conserved (Hu et al. 2011). NTCP is capable of importing all BA. However, an alternative import route is provided by the three OATPs (organic anion transport proteins, OATP1B1, OATP1B3, and OATP2B1) (Konig et al. 2000). Their substrate spectrum is much larger than BA only, and the transport occurs in a Na+-independent manner (Hagenbuch and Meier 2004), but together with bicarbonate (Leuthold et al. 2009).

Chemical structure of cholesterol (left) and bile salts (right).
The modification present in different bile salts are indicated (position 3 (CH3−), 7 (H or OH), and 12 (H or OH), while the two forms of conjugation (taurine or glycine), which increase solubility, are shown in red.
In the canalicular membrane, BA import is not occurring, rather export into the bile duct takes places and is mediated by ABCB11 (BSEP (bile salt export pump)) or s-Pgp (sister of P-gp (P-glycoprotein, MDR1, ABCB1)), which constitutes the major bile salt export pathway. ABCB11 was first described in 1995 and initially termed sister of P-gp due to the high sequence identity of ABCB1, a classic drug export pump, and ABCB11 (Childs et al. 1995). Only in 1998, its natural function as a bile salt export pump especially for glycine and taurine conjugated BA, although with a preference for taurine-conjugated BA, was demonstrated for rat ABCB11 (Gerloff et al. 1998). A similar preference was observed for mouse and human ABCB11 (Byrne et al. 2002; Hayashi et al. 2005; Noe et al. 2001; Noe et al. 2002). Equally important, mutations of ABCB11 were identified as the molecular reason for progressive familiar intrahepatic cholestasis type 2 (PFIC2) (Strautnieks et al. 1997).
Human ABCB11 represents the rate-limiting step of BA secretion in hepatocytes (Stieger 2009). This makes it an interesting target of functional and structural studies. However, cloning of the ABCB11 gene has been extremely challenging and time-consuming (Byrne et al. 2002; Noe et al. 2002). One of the many problems encountered is the instability and integrity of the gene in E. coli. Therefore, Stindt et al. (Stindt et al. 2011) developed a yeast-based cloning procedure for ABCB11 that completely bypassed E. coli and allowed the subsequent overexpression and purification of ABCB11 in the yeast P. pastoris (Ellinger et al. 2013). P. pastoris had been already used to produce other human ABDC transporters (Chloupkova et al. 2007) and was also successfully used for ABCB11 (Ellinger et al. 2013). In contrast to (over)expression in insect cells (Byrne et al. 2002; Noe et al. 2002), this system allowed to purify ABCB11 based on the speed of cloning and ease of handling and study clinically relevant mutants of ABCB11 in vitro under defined conditions (Ellinger et al. 2017; Stindt et al. 2013). These studies even allowed determination of the minimal number of BA transported by ABCB11, which were eight BA per second and transporter (Ellinger et al. 2017). With a yeast-based expression system at hand, the studies were moved one step further, and the interactome of ABCB11 was determined employing the membrane yeast two-hybrid (MYTH) approach (Stagljar et al. 1998) using a cDNA library of human liver membrane proteins as well as soluble proteins. Positive interaction partners of ABCB11 determined by MYTH were subsequently verified by in vitro interaction studies, which resulted in the identification of 10 interaction partners. This included, e.g., radixin, a protein that cross-links the membrane and the cytoskeleton (Clucas and Valderrama 2014) or the bile acyl-CoA synthetase (BACS or SLC27A5) (Mihalik et al. 2002). Surprisingly, the scaffolding protein NHERF1, a known interaction partner of CFTR/ABCC7, MRP2 (ABCC2), and MRP4 (ABCC4) (Hoque et al. 2009; Li et al. 2010; Short et al. 1998) was not identified in this screen. In summary, the yeast-based overexpression proved to be a valuable tool to rapidly screen clinically relevant mutations, not only of ABCB11, but also of ABCB4 (Dzagania et al. 2012; Kluth et al. 2015). ABCB4 is a lipid-, more precisely, a phosphatidylcholine-specific floppase and will be discussed further at the end of this section. PC next to BA and cholesterol are the major components of bile. Thus, as one has to expect that BA transport by ABCB11 and PC flopping by ABCB4 are coupled, incubation of rat liver vesicles with TC increased PC flopping mediated by ABCB4 (Gerloff et al. 1998).
The single-particle cryo-electron microscopy (EM) structure of ABCB11 (Wang et al. 2020) in the so-called inward-facing conformation, i.e., where the binding site is accessible from the cytosol, was determined in the absence of substrate and nucleotide, respectively (Figure 3). The overall architecture is, as expected, identical to other known structures of the ABCB subfamily (for a recent review see (Locher 2016)). Thus, the transmembrane domains (TMDs) are split into two parts, a domain swapping, which was first observed in the crystal structure of Sav1866 (Dawson and Locher 2006) and connects both nucleotide-binding domains (NBDs) through the so-called coupling helices (CHs; blue and green in Figure 3). In contrast to human ABCB1 (Alam et al. 2019), the N-terminus of ABCB11 is extended. In the structure, this extension adopts a helical fold that protrudes into a cavity that is accessible from the cytosol and harbors the putative ligand binding site (cyan in Figure 3). Such an N-terminal extension was also observed in the ABCB1 orthologue from C. elegans (Jin et al. 2012). However, its functional role remained unclear. Deletion of the N-terminal extension in ABCB11 did not influence function; rather, the expression level was two-fold reduced, pointing to a role in trafficking and/membrane insertion. The structure of ABCB11 (Wang et al. 2020) represents an important achievement. However, additional structures, especially in the presence of BA, are required to understand the transport mechanism of ABCB11.

Structure of ABCB11 in cartoon representation.
The putative position of the membrane is indicated by the two horizontal lines. The individual domains are labeled (ICD: intracellular domain, TMD: transmembrane domain, NBD: nucleotide-binding domain). The N-terminal extension of ABCB11 is highlighted in cyan and the coupling helices of TMD1 in green and TMD2 in blue.
The expression of ABCB11 is regulated in many ways. The most important regulator is the farnesoid X receptor (FXR, NR1H4) (Lefebvre et al. 2009), which regulates not only the expression of ABCB11, but also NTCP and the enzymes of the bile salt synthesis pathway (Eloranta and Kullak-Ublick 2005). Importantly, the regulation of ABCB11 occurs not only on the level of expression. In parallel, a short-term regulation of hepatobiliary transport involves rapid insertion and retrieval of transporters, such as Bsep, Mrp2, and Ntcp into or from the canalicular or sinusoidal membrane, respectively (Anwer et al. 2005; Beuers et al. 2001; Cantore et al. 2011; Kubitz et al. 1997; Kurz et al. 2001; Mayer et al. 2019; Mukhopadhayay et al. 1997; Schmitt et al. 2001; Sommerfeld et al. 2015; Webster et al. 2000). This was extensively studied in isolated perfused rat liver employing hypo- and hyperosmotic perfusion conditions. Shifting the ambient osmolarity from 305 to 225 mosmol/L doubled within minutes the taurocholate excretion capacity into bile, whereas hyperosmolarity (385 mosmol/L) was cholestatic (Häussinger et al. 1992). As shown by immunohistochemistry, these osmolarity shifts were accompanied a rapid and reversible insertion of Abcb11 and Abcc2 (Mrp2) into the canalicular membrane in response to hypoosmotic cell swelling, whereas hyperosmotic hepatocyte shrinkage triggered the endocytosis of these transporters into a subcanalicular vesicular compartment (Cantore et al. 2011; Kubitz et al. 1997; Schmitt et al. 2001). Hyperosmotic hepatocyte shrinkage also induced retrieval of Ntcp from the sinusoidal membrane (Sommerfeld et al. 2015), whereas hypoosmotic hepatocyte swelling stimulated the insertion of Ntcp into the sinusoidal plasma membrane (Webster et al. 2000). Thus, sinusoidal uptake and canalicular excretion of bile acids are coordinately regulated by ambient osmolarity, most likely representing a mechanism to avoid proapoptotic bile acid overload of the hepatocyte. The signal transduction pathways leading to hyperosmotic Ntcp and Abcb11 and Abcc2 retrieval from the sinusoidal and canalicular membrane, respectively, have been identified. Here, activation of the Src family kinase Fyn by NADPH oxidase-derived reactive oxygen species (ROS) is a crucial event, whereas the parallel activation of the Src family kinase member Yes triggers a proapoptotic state in response to hepatocyte shrinkage (for review see Reinehr et al. 2013). Also, oxidative stress exerted by glycochenodeoxycholate (Mayer et al. 2019) or t-butyl hydroperoxide (Schmitt et al. 2000) triggers retrieval of Abcb11 and Abcc2 from the canalicular membrane. On the other hand, hypoosmotic hepatocyte swelling leads to an integrin-dependent c-Src activation, which triggers activation of Erks and p38MAPK (Häussinger et al. 2003) and probably involves the p38α isoform (Schonhoff et al. 2016). Dual activation of these MAP-kinases is required for insertion of Abcb11 and Abcc2 into the canalicular membrane. Interestingly, the hypoosmotic signaling pathway towards choleresis is also activated by tauroursodeoxycholate, which can non-osmotically activate α 5ß1 integrins (Gohlke et al. 2013). Hypoosmotic insertion of Ntcp into the sinusoidal membrane involves activation of PI3 Kinase and protein kinase B (Webster et al. 2000; Webster et al. 2002).
Thus, the vesicular pool of ABCB11, similar to other hepatobiliary transporters, can react fast to external and internal stimuli that require an increase or decrease of the BA levels in the bile duct.
However, ABCB11 is not an isolated entity. Rather, BA secretion by ABCB11 is coupled to cholesterol transport by ABCG5/G8 (Berge et al. 2000) and phosphatidyl (PC) lipid flop from the inner to the outer leaflet of the canalicular membrane (van Helvoort et al. 1996). The latter process is catalyzed by ABCB4 (MDR3) (Prescher et al. 2019). The molecular reason for such a ‘bile triumvirate’ (Kroll et al. 2021) is the harsh detergent action of BA. Thus, the formation of mixed micelles composed mainly of BA, PC lipids, and cholesterol, i.e., bile, diminishes the detergent action of BA and prevents the solubilization of the canalicular membrane and the formation of gallstones (Small 2003). Another factor contributing to the resistance of the canalicular membrane against the detergent action of BA, is the assymetric distribution of lipids between the two leaflets of the lipid bilayer. ATP8B1 flips phosphatidylserine from the outer into the inner leaflet of the membrane, counteracting the action of ABCB4 (Groen et al. 2011). Absence of ATP8B1 reduces the resistance of the canalicular membrane towards BA and impairs BA transport (Paulusma et al. 2006). In humans, mutations in the ATP8B1 gene are associated with hereditary intrahepatic cholestasis ranging from intrahepatic cholestasis of pregnancy (ICP) to progressive familial intrahepatic cholestasis (PFIC) (see below) (Bull et al. 1998; Van Mil et al. 2007; van Mil et al. 2004).
Bile is composed of approximately 70% bile salts, 20% PC lipids, and 8–9% cholesterol in healthy adults (Fisher and Yousef 1973). This cross-communication is also reflected by the fact that bile salts increase the secretion of PC lipids by ABCB4, which requires not only the choline headgroup, but also two acyl chains for proper recognition of the PC lipid (Prescher et al. 2020). A similar observation was also made for ABCG5/G8, which completely lacks ATPase activity in the absence of BA (Johnson et al. 2010). On the other hand, the concentration of cholesterol in the membrane modulates the activity of ABCB11 (Guyot et al. 2014; Kis et al. 2009).
In summary, BA transport is not a simple import by NTCP, diffusion through the cytosol or intracellular lipid membranes and followed by a simple export by ABCB11. Instead, a complex network of interactions and crosstalk between the different transporters is apparently present, which regulates and modulates the amount of bile salts in the hepatocyte and the bile duct. So far, we have only seen the tip of the iceberg. More studies are required to unravel the molecular signals and mechanisms that allow fine-tuned bile salt transport.
Hereditary disorders of bile salt transport
At the canalicular membrane, ABCB11, ABCB4, and the heterodimeric cholesterol transporter ABCG5/8 ensure the coordinated excretion of BA, phospholipids, and cholesterol, respectively, into bile. Within bile, these molecules assemble to form mixed micelles (Admirand and Small 1968), enabling the elimination of cholesterol. Approx. 12–20 g of BA have to be excreted per day into bile to eliminate 500 mg of cholesterol.
Canalicular secretion is regarded as the rate-limiting step of the vectorial transport of bile acids across hepatocytes (Häussinger et al. 2000; Trauner and Boyer 2003). Disturbance of bile salt transport results in elevated BA concentrations in different compartments and may exert toxic effects due to their detergent and proapoptotic properties (Reinehr et al. 2003). Bile salt retention within hepatocytes is directly or indirectly involved in drug-induced liver injury (DILI) (Morgan et al. 2010; Ogimura et al. 2011). These properties explain why ABCB11 and cooperating canalicular transporter proteins are associated with hereditary liver diseases. These diseases comprise progressive familial intrahepatic cholestasis (PFIC) type 1 to 3, benign recurrent intrahepatic cholestasis (BRIC) type 1 and 2, low phospholipid associated cholelithiasis (LPAC), and intrahepatic cholestasis of pregnancy (ICP) (Figure 4). Here we will focus on ABCB11-associated diseases.

Hereditary cholestatic liver diseases. Within this model of a hepatocyte couplet several proteins are shown, which are associated with distinct cholestatic liver diseases. Severe diseases, called progressive familial intrahepatic cholestasis (PFIC) type 1 to 5, have been identified. PFIC-1 as well as benign recurrent intrahepatic cholestasis (BRIC-1) are linked to the flippase ATP8B1 (FIC1), which translocates phosphatidylserine and phosphatidylethanolamine from the outside to the inner leflet of the membrane bilayer. The ABC transporter ABCB11 (BSEP) and ABCB4 (MDR3) are also related to disaeses of varying severity and symptoms such as intrahepatic cholestasis of pregnancy (ICP) or low phospholipid associated choleslithiasis (LPAC). Certain polymorphisms of ABCB11 (BSEP) are not harmful per se but the liver may be more susceptibable to injury due to certain drugs. More recently, the tight junction protein 2 (TJP2) and the nuclear BA receptor FXR (NR1H4) were identified as disease-relevant.
FIC1, familial intrahepatic cholestasis protein 1; BSEP, bile salt export pump; MDR3, multidrug resistance 3; ABCG5/8, ATP-binding cassette transporter G5/G8; TJP2, tight junction protein 2; FXR, Farnesoid X receptor; PFIC, Progressive familial intrahepatic cholestasis; BRIC, Benign recurrent intrahepatic cholestasis; ICP, Intrahepatic cholestasis of pregnancy; LPAC, phospholipid associated cholelithiasis; DILI, drug induced liver injury.
Progressive familial intrahepatic cholestasis type 2 (PFIC-2)
Progressive familial intrahepatic cholestasis type 2 (PFIC) describes a cholestasis-induced end-stage liver disease in childhood, which is distinct from other neonatal or pediatric cholestatic diseases such as biliary atresia or Alagille syndrome (Ballow et al. 1973). Biochemically, PFIC-2 is characterized by low gamma-glutamyltranspeptidase (gGT) levels and is linked to mutations of ABCB11/BSEP (Bull et al. 1997). In contrast to PFIC-2 (and PFIC-1 due to mutations of FIC1), PFIC-3 is characterized by increased gGT levels and is due to mutations of ABCB4/MDR3. In the case of PFIC-2, normal γGT levels are attributed to the low concentrations of BA in bile and, consequently, reduced damage to cholangiocytes (Jacquemin 2001). PFIC-2 patients present within the first year of life jaundice, itching, growth failure, and occasionally severe hemorrhage due to vitamin K deficiency. Importantly, PFIC-2 patients have a high risk to develop hepatocellular (HCC) (Knisely et al. 2006) or cholangiocellular carcinoma (CCA) (Strautnieks et al. 2008), and an association of the common BSEP polymorphism p.V444A has been described in a study of 172 CCA-patients (Wadsworth et al. 2011).
Reduced or even absent expression of canalicular BSEP depends on the type of genetic variant (Kubitz et al. 2014) and may be utilized for diagnostic purposes (Evason et al. 2011; Keitel et al. 2005; Strautnieks et al. 2008). Nowadays, more than 150 different variants have been associated with a PFIC-2 phenotype (http://www.hgmd.cf.ac.uk) (Droge et al. 2017; Kroll et al. 2021).
The reason for the high rate of absent BSEP in immunohistochemical studies is discussed elsewhere (Kubitz et al. 2014; Strautnieks et al. 2008). Interestingly, BSEP seems to be prone for degradation within the ER-associated degradation (ERAD) pathway as shown in expression studies of cloned BSEP containing the very common polymorphism p.V444A and the common PFIC-2-related variants p.E297G and p.D482G as compared to wildtype BSEP (Hayashi and Sugiyama 2009; Keitel et al. 2009).
Benign recurrent intrahepatic cholestasis type 2 (BRIC-2)
Some ABCB11-mutations are linked to milder forms of cholestasis (Kubitz et al. 2006), which typically resolve spontaneously. It is termed ‘benign recurrent intrahepatic cholestasis type 2’ (BRIC-2) (van Mil et al. 2004) and clinically presents similar to BRIC-1 (Brenard et al. 1989), which is caused by ‘mild’ variants of ATP8B1 (FIC1) (Bull et al. 1998). FIC1 or familial intrahepatic cholestasis 1 protein is encoded by the ATP8B1 gene (Bull et al. 1998) and serves as a flippase, which transports phosphatidylserine or phosphatidylcholine (Groen et al. 2011; Shin and Takatsu 2019).
BRIC-2 patients have a higher incidence of gallstones than BRIC-1 patients (van Mil et al. 2004). The penetrance of single ABCB11 variants in BRIC-2 is variable as shown in siblings with the ABCB11-mutation p.G374S: while the brother developed liver cirrhosis, the sister experienced self-limiting jaundice with pruritus (Stindt et al. 2013).
Intrahepatic cholestasis of pregnancy and bile salt export pump (BSEP)
Intrahepatic cholestasis of pregnancy (ICP) is diagnosed in the absence of any other liver disease and presence of pruritus and elevated serum BA levels (European Association for the Study of the 2009). Typically, ICP occurs during the third trimester, and liver functions tests (AST/GOT and ALT/GPT) are slightly elevated in most cases but may rise up to 50-fold over the upper limit of normal, thereby resembling liver failure (Keitel et al. 2006; Keitel et al. 2016; Lammert et al. 2003; Rioseco et al. 1994; Williamson and Geenes 2014). Jaundice is observed in up to 10% of ICP cases. Increased serum BA concentrations are almost diagnostic for ICP. BA levels above 40 μmol/l are predictive for ICP-related complications, such as preterm birth or asphyxia, while stillbirth risk is increased in women with BA levels above 100 μmol/l, especially after the 35th week of gestation (Glantz et al. 2004; Ovadia et al. 2019). ICP almost invariably resolves spontaneously within 2–4 weeks after delivery; otherwise differential diagnoses have to be taken into account.
Anti-bile salt export pump (BSEP) antibodies and cholestasis
Some PFIC-2 patients may not be sufficiently treated by medical (e.g., UDCA) or surgical (e.g., partial biliary external diversion (PEBD) interventions and require liver transplantation (van Wessel et al. 2020). It has been recognized that 8–33% of PFIC-2 patients develop recurrent cholestasis similar to preceding PFIC-2 following liver transplantation (Droge et al. 2017; Jara et al. 2009; Keitel et al. 2009; Siebold et al. 2010; Stindt et al. 2016). De novo formation of anti-BSEP antibodies, which bind to an extracellular loop of the N-terminal half of BSEP and inhibit BSEP transport activity, have been identified as the underlying cause of antibody-induced BSEP deficiency (AIBD) (Keitel et al. 2009; Stindt et al. 2016). It is likely that the complete absence of ABCB11 expression (e.g., due to Stop-mutation) in the original liver prevents auto-tolerance towards ABCB11 and therefore facilitates antibody-formation after transplantation (Kubitz et al. 2015). Treatment of these children includes changes of the regimen of immunosuppressant, plasmapheresis, immune-adsorption, B-cell depletion by rituximab, or even allogenic haematopoietic stem cell transplantation in order to remove ABCB11-reactive plasma cells (Brinkert et al. 2018; Keitel et al. 2009; Kubitz et al. 2015).
Bile salt signaling in the human liver
While some of the functions of the nuclear BA receptor FXR (NR1H4) for hepatic BA transport were discussed above, we will focus in the following paragraphs on the G-protein coupled BA receptor TGR5.
TGR5 – a bile acid receptor
TGR5 (Kawamata et al. 2003; Keitel and Häussinger 2018; Maruyama et al. 2002), also known as G protein-coupled bile acid receptor 1 (GPBAR1) or membrane-type bile acid receptor (M-BAR), was the first GPCR identified responsive to BA in 2002/2003 (Kawamata et al. 2003; Maruyama et al. 2002). TGR5 mRNA is almost ubiquitously expressed in rodent and human tissues (Keitel and Häussinger 2012, 2018; Maruyama et al. 2002; Maruyama et al. 2006; Reich et al. 2017), with high expression found in organs involved in the enterohepatic circulation and excretion of BAs such as liver, gallbladder, and small and large intestine (Kawamata et al. 2003; Keitel et al. 2010; Keitel et al. 2013; Maruyama et al. 2002; Maruyama et al. 2006; Vassileva et al. 2006). Moreover, TGR5 mRNA was also present in heart, lung, spleen, CD14-positive monocytes, placenta, female and male reproductive organs, adrenal glands, brain, and adipose tissue (Kawamata et al. 2003; Keitel et al. 2010; Keitel et al. 2013; Maruyama et al. 2002; Maruyama et al. 2006; Vassileva et al. 2006). Among other physiological functions, TGR5 has been shown to influence glucose tolerance via GLP-1 secretion and protect bile duct epithelial cells from BA-induced toxicity (Merlen et al. 2020; Reich et al. 2017; Thomas et al. 2009; Velazquez-Villegas et al. 2018).
TGR5 recognizes a broad spectrum of hydrophobic endogenous ligands (Sato et al. 2008), comprising all known BAs and many hydrophobic neurosteroids, such as estradiol, pregnanediol, and allopregnanolone (Keitel et al. 2010; Martin et al. 2013; Sato et al. 2008) (Figure 5). In contrast to other BA-sensing receptors, TGR5 is rather promiscuously sensing BA regardless of their substitution pattern and conjugation state (Sato et al. 2008). This underlines the role of TGR5 as a protective mechanism against the toxicity of BA for gallbladder epithelial cells and cholangiocytes (Beuers et al. 2010; Deutschmann et al. 2018; Reich et al. 2016, 2017). Thus, Tgr5-deficient animals are more susceptible to BA-induced liver damages (e.g., BA feeding, common bile duct ligations (Klindt et al. 2019; Reich et al. 2016)). Conversely, overexpression of Tgr5 ameliorates the activated biliary phenotype characteristic of sclerosing cholangitis induced by Abcb4-deficiency (Reich et al. 2021). Tgr5 expression levels are reduced in cholangiocytes of Abcb4-deficient mice and can be increased/restored by UDCA/nor-UDCA feeding, respectively (Reich et al. 2021). However, overexpression of TGR5 has been observed in human cholangiocarcinoma (Deutschmann et al. 2018; Erice et al. 2018; Reich et al. 2016).

Important TGR5 ligands. The table shows the substitution pattern for selected natural bile acid agonists with their EC50 values (Sato et al. 2008).
In addition, a neurosteroid agonist, pregnanediol, two intestinal agonists, 26a and 15c, three synthetic agonists, 23g, 18, and INT-777, and an antagonist, SBI-115, are shown. Additionally, the cis and trans configurations between the rings of the cholane scaffold of INT-777 are indicated by blue dashed arrows.
TGR5 binding mode model
BA activate TGR5 via the orthosteric ligand binding site (Gertzen et al. 2015). There, BA bridge the transmembrane helices (TM) three and six to activate the receptor, as is common for GPCR agonists (Katritch and Abagyan 2011). Further TMs, particularly TM5, are addressed to increase the efficacy (Gertzen et al. 2015) (Figure 6(A) and (B)). Taurine conjugation increases the size of a BA, which allows forming a likely affinity-increasing salt-bridge interaction to R79 (EL1), while a hydrogen bond to the vital Y240 (TM6) in TGR5 is still possible (Gertzen et al. 2015). All BA employ the 3-hydroxy groups of their cholane scaffold to form a hydrogen bond to Y240, and this interaction is further stabilized by a hydrogen bond to E169 (TM5, Figure 6(A) and (B)) (Gertzen et al. 2015). Agonistic neurosteroids such as pregnanediol also utilize their hydroxy or carbonyl groups to interact with Y240 in TGR5 (Gertzen et al. 2015). Lacking acidic groups, they mainly form additional hydrophobic interactions with Y89 in TM3 to bind to and activate TGR5 at a reasonable EC50 (e.g., pregnanediol EC50 = 0.58 µM (Sato et al. 2008) (Figure 5), allowing them to activate TGR5 in the brain. The epimeric selectivity has been explained by a hydrogen bond formation of CDCA’s 7α-hydroxy group to Y89 in TM3 of TGR5, which is not possible in the ß-configured UDCA. A recently discovered cholic acid 7-sulfate (CA7S, Figure 5) (Chaudhari et al. 2021), in which the 7-hydroxy group is esterified with sulfuric acid, could also form a hydrogen bond.

Interactions of TGR5 with TLC and TGR5 oligomer formation.
(A) Binding mode model of TLC (light blue sticks) in TGR5 (grey, cartoon), embedded in a lipid bilayer (yellow, lines) (Gertzen et al. 2015). Important interacting residues are shown as green sticks, and position 7 of the cholane scaffold is indicated by an arrow. TLC bridges TM3 and TM6 by interacting with Y89 and Y240, which are important for TGR5 activation. The interaction with E169 is suggested to contribute to the efficacy of TLC. (B) 2D-diagram showing interactions of TLC within TGR5. Hydrogen bonding and salt-bridge interactions are shown as arrows, hydrophobic interactions are shown in green. (C) Schematic of TGR5 oligomerization (Greife et al. 2016; Wäschenbach et al. 2020). TGR5 forms dimers involving TM1 and helix 8 (1–8). Then, oligomerization can occur from dimers via the TM4-TM5 interface (4–5).
Synthetic TGR5 agonists and antagonists
Currently, various TGR5 agonists with a non-steroidal core are available (Cao et al. 2016; Duan et al. 2015) (Figure 5). Here, an acidic or amide moiety connects with three to four variably interconnected aromatic and aliphatic rings. The ring furthest from the acid or amide moiety is heteroaromatic or -aliphatic. Although the binding mode of non-steroidal TGR5 agonists has not been investigated to date, it is possible that the heteroatom mimics the 3-hydroxy group of bile acids and, thus, forms the crucial hydrogen bond to Y240 (TM6) (Spomer et al. 2014). Intestinal specificity can be achieved by either introducing a permanent positive charge via a quaternary ammonia moiety to the synthetic TGR5 agonists (26a, Figure 5) or creating a higher-molar mass agonist via linking two agonists through polyethylene glycol (15c, Figure 5).
As a wide range of hydrophobic substances activates TGR5, it is not trivial to develop a ligand that binds to TGR5 but does not activate it. Hence, only one antagonist has been discovered (SBI-115, Figure 5) (Masyuk et al. 2017) so far. It is composed of two interconnected aromatic rings and an ethyl sulfo substituent and is slightly smaller than the known agonists. Due to the lack of an acid moiety and a steroidal core, the binding mode of this antagonist cannot easily be inferred from the agonist binding modes.
Recently, a cryo-EM structure of TGR5 in complex with INT-777 (6α-ethyl-23(S)-methylcholic acid (S-EMCA), Figure 5), a potent and selective TGR5 agonist with in vivo activity (Pellicciari et al. 2009), has been resolved (PDB-ID: 7CFN) (Yang et al. 2020). In contrast to all other crystallized GPCRs, the conserved tryptophane residue in TM6 (W237), which likely functions as a ligand-dependent trigger for GPCR activation (Katritch et al. 2013), is located one helix turn further down to the cytoplasm, suggesting that the ligand-dependent activation of TGR5 is carried out differently. Unexpectedly, all rings of the cholane scaffold of INT-777 modeled in the complex structure are connected in a trans configuration, although the configuration of the 5β-cholane scaffold of INT-77 is cis-trans-trans (Figure 5) (Pellicciari et al. 2009). As it is not clear to what extent the shown binding mode of INT-77 within TGR5 is impacted by this change, it cannot be used to interpret the epimeric selectivity of TGR5 agonists, particularly between CDCA and UDCA (Sato et al. 2008), and mutational data (Gertzen et al. 2015). Furthermore, note that in Figure 4E of Ref. (Yang et al. 2020), the configurations of chiral centers of the chain and the cholane scaffold are inverted with the exception of C17, and a methyl group has been added to C5. Here, in contrast to the modeled INT-77, the connectivity of the rings is cis-trans-trans.
Regulation of TGR5 through di- and oligomerisation
GPCR oligomerization has been shown to influence various biological functions (Hiller et al. 2013). For TGR5, a combined strategy using cell biology, multiparameter image fluorescence spectroscopy (MFIS) for quantitative FRET analysis, and integrative modeling revealed the oligomerization properties of TGR5, fused to fluorescent proteins, in live cells (Greife et al. 2016). The correlation of FRET parameters with increasing acceptor dye-to-donor dye ratios showed that TGR5 wildtype forms higher-order oligomers. A TGR5 Y111A variant inhibits oligomerization but still allows TGR5 dimerization. From the ratio dependence, it was concluded that higher-order oligomers of TGR5 wildtype are formed from dimers interacting with other dimers. Subsequent integrative modeling considering the energetic preference of dye positions showed that higher-order oligomers are linear arrangements of dimers interacting via TM1 and helix 8, with the oligomerization site involving TM4 and TM5 (Figure 6(C)). Extensive energetic evaluation of GPCR dimerization on the atomistic level showed that the formation of both the 1–8 and 4–5 interface is energetically favorable for TGR5 (Wäschenbach et al. 2020). The formation of the 1–8 interface is favored over that of the 4–5 interface, and differences in lipid-protein interactions contribute to the observed differences in free energies of association. A membrane-proximal, C-terminal helix is structurally required for plasma membrane localization and function of TGR5 (Spomer et al. 2014). Together with the finding that helix 8 is important for TGR5 dimerization, this may imply that dimerization of TGR5 is necessary for its membrane trafficking. Finally, time series MFIS-FRET analysis before, at the time point of, and after stimulation of TGR5 with taurocholate (TCA, Figure 6), a bile acid less cytotoxic than TLCA in live cells, indicated that TCA, and subsequent G protein coupling, does not influence the oligomerization state of TGR5 wildtype and Y111A (Greife et al. 2016), an observation supported by the literature (Patowary et al. 2013).
In summary, TGR5, a GPCR involved in bile duct epithelium protection, among other physiological functions, but which may also contribute to tumor formation, is activated by a wide variety of hydrophobic ligands. By contrast, only one antagonist is known to date. TGR5’s function and membrane localization can be regulated through di- and oligomerization. TM1 and helix 8 as well as TM4 and TM5 constitute the respective interfaces. While recent clinical trials could not establish TGR5 agonists as drugs for diabetes treatment, potent antagonists could be beneficial in treating cholangiocarcinomas.
Summary and concluding remarks
We have witnessed important insights and discoveries in the physiology and pathophysiology of BA in the last decades, which, of course, include different cellular and molecular aspects. Slowly, one starts to appreciate the complex network of interactions that form the basis of these processes. Obviously, the development of new and more sensitive analytical tools was an important milestone and enabled breakthroughs in all areas of BA research. One has also to stress that such methodological improvements are also required in the future to drive research to the next level. Despite all of these discoveries, we only start to see the tip of the iceberg, and the future will for sure hold many surprises in our understanding of function, regulation, and pathophysiology of bile acids.
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: KFO 217
Award Identifier / Grant number: SFB 575
Award Identifier / Grant number: SFB 974
Acknowledgments
L.S. thanks all members of the Institute of Biochemistry and all cooperation partners in and outside of Düsseldorf. V.K., D.H., R.K. thank all members of the experimental hepatology and cooperation partners in and outside of Düsseldorf. H.G. and C.G. thank Lucas Wäschenbach and Lina Zeisler for the fruitful collaborations on TGR5.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Financial support by the DFG through CRC 575, CRC 974 and KFO 217 is highly acknowledged.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Admirand, W.H. and Small, D.M. (1968). The physicochemical basis of cholesterol gallstone formation in man. J. Clin. Invest. 47: 1043–1052. https://doi.org/10.1172/jci105794.Search in Google Scholar
Alam, A., Kowal, J., Broude, E., Roninson, I., and Locher, K.P. (2019). Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science 363: 753–756. https://doi.org/10.1126/science.aav7102.Search in Google Scholar PubMed PubMed Central
Anwer, M.S., Gillin, H., Mukhopadhyay, S., Balasubramaniyan, N., Suchy, F.J., and Ananthanarayanan, M. (2005). Dephosphorylation of Ser-226 facilitates plasma membrane retention of Ntcp. J. Biol. Chem. 280: 33687–33692. https://doi.org/10.1074/jbc.m502151200.Search in Google Scholar
Ballow, M., Margolis, C.Z., Schachtel, B., and Hsia, Y.E. (1973). Progressive familial intrahepatic cholestasis. Pediatrics 51: 998–1007.10.1542/peds.51.6.998Search in Google Scholar
Berge, K.E., Tian, H., Graf, G.A., Yu, L., Grishin, N.V., Schultz, J., Kwiterovich, P., Shan, B., Barnes, R., and Hobbs, H.H. (2000). Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290: 1771–1775. https://doi.org/10.1126/science.290.5497.1771.Search in Google Scholar PubMed
Beuers, U., Bilzer, M., Chittattu, A., Kullak-Ublick, G.A., Keppler, D., Paumgartner, G., and Dombrowski, F. (2001). Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology 33: 1206–1216. https://doi.org/10.1053/jhep.2001.24034.Search in Google Scholar PubMed
Beuers, U., Hohenester, S., de Buy Wenniger, L.J., Kremer, A.E., Jansen, P.L., and Elferink, R.P. (2010). The biliary HCO(3)(-) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 52: 1489–1496. https://doi.org/10.1002/hep.23810.Search in Google Scholar PubMed
Bogomolov, P., Alexandrov, A., Voronkova, N., Macievich, M., Kokina, K., Petrachenkova, M., Lehr, T., Lempp, F.A., Wedemeyer, H., Haag, M., et al. (2016). Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J. Hepatol. 65: 490–498. https://doi.org/10.1016/j.jhep.2016.04.016.Search in Google Scholar PubMed
Bonus, M., Sommerfeld, A., Qvartskhava, N., Gorg, B., Ludwig, B.S., Kessler, H., Gohlke, H., and Häussinger, D. (2020). Evidence for functional selectivity in TUDC- and norUDCA-induced signal transduction via α5β1 integrin towards choleresis. Sci. Rep. 10: 5795. https://doi.org/10.1038/s41598-020-62326-y.Search in Google Scholar PubMed PubMed Central
Brenard, R., Geubel, A.P., and Benhamou, J.P. (1989). Benign recurrent intrahepatic cholestasis. A report of 26 cases. J. Clin. Gastroenterol. 11: 546–551. https://doi.org/10.1097/00004836-198910000-00011.Search in Google Scholar PubMed
Brinkert, F., Pukite, I., Krebs-Schmitt, D., Briem-Richter, A., Stindt, J., Häussinger, D., Keitel, V., Muller, I., and Grabhorn, E. (2018). Allogeneic haematopoietic stem cell transplantation eliminates alloreactive inhibitory antibodies after liver transplantation for bile salt export pump deficiency. J. Hepatol. 69: 961–965. https://doi.org/10.1016/j.jhep.2018.06.003.Search in Google Scholar
Bull, L.N., Carlton, V.E., Stricker, N.L., Baharloo, S., DeYoung, J.A., Freimer, N.B., Magid, M.S., Kahn, E., Markowitz, J., DiCarlo, F.J., et al. (1997). Genetic and morphological findings in progressive familial intrahepatic cholestasis (Byler disease [PFIC-1] and Byler syndrome): evidence for heterogeneity. Hepatology 26: 155–164. https://doi.org/10.1002/hep.510260121.Search in Google Scholar
Bull, L.N., van Eijk, M.J., Pawlikowska, L., DeYoung, J.A., Juijn, J.A., Liao, M., Klomp, L.W., Lomri, N., Berger, R., Scharschmidt, B.F., et al. (1998). A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat. Genet. 18: 219–224. https://doi.org/10.1038/ng0398-219.Search in Google Scholar
Byrne, J.A., Strautnieks, S.S., Mieli-Vergani, G., Higgins, C.F., Linton, K.J., and Thompson, R.J. (2002). The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology 123: 1649–1658. https://doi.org/10.1053/gast.2002.36591.Search in Google Scholar
Cantore, M., Reinehr, R., Sommerfeld, A., Becker, M., and Häussinger, D. (2011). The Src family kinase Fyn mediates hyperosmolarity-induced Mrp2 and Bsep retrieval from canalicular membrane. J. Biol. Chem. 286: 45014–45029. https://doi.org/10.1074/jbc.m111.292896.Search in Google Scholar
Cao, H., Chen, Z.X., Wang, K., Ning, M.M., Zou, Q.A., Feng, Y., Ye, Y.L., Leng, Y., and Shen, J.H. (2016). Intestinally-targeted TGR5 agonists equipped with quaternary ammonium have an improved hypoglycemic effect and reduced gallbladder filling effect. Sci. Rep. 6: 28676. https://doi.org/10.1038/srep28676.Search in Google Scholar
Chappell, L.C., Bell, J.L., Smith, A., Linsell, L., Juszczak, E., Dixon, P.H., Chambers, J., Hunter, R., Dorling, J., and Williamson, C., et al., PITCHES Study Group (2019). Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet 394: 849–860, https://doi.org/10.1016/S0140-6736(19)31270-X. 31378395.Search in Google Scholar PubMed
Chaudhari, S.N., Harris, D.A., Aliakbarian, H., Luo, J.N., Henke, M.T., Subramaniam, R., Vernon, A.H., Tavakkoli, A., Sheu, E.G., and Devlin, A.S. (2021). Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat. Chem. Biol. 17: 20–29. https://doi.org/10.1038/s41589-020-0604-z.Search in Google Scholar PubMed PubMed Central
Childs, S., Yeh, R.L., Georges, E., and Ling, V. (1995). Identification of a sister gene to P-glycoprotein. Cancer Res. 55: 2029–2034.Search in Google Scholar
Chloupkova, M., Pickert, A., Lee, J.Y., Souza, S., Trinh, Y.T., Connelly, S.M., Dumont, M.E., Dean, M., and Urbatsch, I.L. (2007). Expression of 25 human ABC transporters in the yeast Pichia pastoris and characterization of the purified ABCC3 ATPase activity. Biochemistry 46: 7992–8003. https://doi.org/10.1021/bi700020m.Search in Google Scholar PubMed
Clucas, J. and Valderrama, F. (2014). ERM proteins in cancer progression. J. Cell Sci. 127: 267–275. https://doi.org/10.1242/jcs.133108.Search in Google Scholar PubMed
Dawson, R.J. and Locher, K.P. (2006). Structure of a bacterial multidrug ABC transporter. Nature 443: 180–185. https://doi.org/10.1038/nature05155.Search in Google Scholar PubMed
de Vries, E., Mazzetti, M., Takkenberg, B., Mostafavi, N., Bikker, H., Marzioni, M., de Veer, R., van der Meer, A., Doukas, M., Verheij, J., et al. (2020). Carriers of ABCB4 gene variants show a mild clinical course, but impaired quality of life and limited risk for cholangiocarcinoma. Liver Int. 40: 3042–3050. https://doi.org/10.1111/liv.14662.Search in Google Scholar PubMed
Deutschmann, K., Reich, M., Klindt, C., Dröge, C., Spomer, L., Häussinger, D., and Keitel, V. (2018). Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim. Biophys. Acta 1864: 1319–1325. https://doi.org/10.1016/j.bbadis.2017.08.021.Search in Google Scholar PubMed
Droge, C., Bonus, M., Baumann, U., Klindt, C., Lainka, E., Kathemann, S., Brinkert, F., Grabhorn, E., Pfister, E.D., Wenning, D., et al. (2017). Sequencing of FIC1, BSEP and MDR3 in a large cohort of patients with cholestasis revealed a high number of different genetic variants. J. Hepatol. 67: 1253–1264. https://doi.org/10.1016/j.jhep.2017.07.004.Search in Google Scholar PubMed
Duan, H., Ning, M., Zou, Q., Ye, Y., Feng, Y., Zhang, L., Leng, Y., and Shen, J. (2015). Discovery of intestinal targeted TGR5 agonists for the treatment of type 2 diabetes. J. Med. Chem. 58: 3315–3328. https://doi.org/10.1021/jm500829b.Search in Google Scholar PubMed
Dzagania, T., Engelmann, G., Häussinger, D., Schmitt, L., Flechtenmacher, C., Rtskhiladze, I., and Kubitz, R. (2012). The histidin-loop is essential for transport activity of human MDR3. A novel mutation of MDR3 in a patient with progressive familial intrahepatic cholestasis type 3. Gene 506: 141–145. https://doi.org/10.1016/j.gene.2012.06.029.Search in Google Scholar PubMed
Ellinger, P., Kluth, M., Stindt, J., Smits, S.H., and Schmitt, L. (2013). Detergent screening and purification of the human liver ABC transporters BSEP (ABCB11) and MDR3 (ABCB4) expressed in the yeast Pichia pastoris. PLoS One 8: e60620. https://doi.org/10.1371/journal.pone.0060620.Search in Google Scholar PubMed PubMed Central
Ellinger, P., Stindt, J., Droge, C., Sattler, K., Stross, C., Kluge, S., Herebian, D., Smits, S.H.J., Burdelski, M., Schulz-Jurgensen, S., et al. (2017). Partial external biliary diversion in bile salt export pump deficiency: association between outcome and mutation. World J. Gastroenterol. 23: 5295–5303. https://doi.org/10.3748/wjg.v23.i29.5295.Search in Google Scholar PubMed PubMed Central
Eloranta, J.J. and Kullak-Ublick, G.A. (2005). Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch. Biochem. Biophys. 433: 397–412. https://doi.org/10.1016/j.abb.2004.09.019.Search in Google Scholar PubMed
Erice, O., Labiano, I., Arbelaiz, A., Santos-Laso, A., Munoz-Garrido, P., Jimenez-Aguero, R., Olaizola, P., Caro-Maldonado, A., Martin-Martin, N., Carracedo, A., et al. (2018). Differential effects of FXR or TGR5 activation in cholangiocarcinoma progression. Biochim. Biophys. Acta 1864: 1335–1344. https://doi.org/10.1016/j.bbadis.2017.08.016.Search in Google Scholar PubMed
European Association for the Study of the, L (2009). EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J. Hepatol. 51: 237–267. https://doi.org/10.1016/j.jhep.2009.04.009.Search in Google Scholar PubMed
Evason, K., Bove, K.E., Finegold, M.J., Knisely, A.S., Rhee, S., Rosenthal, P., Miethke, A.G., Karpen, S.J., Ferrell, L.D., and Kim, G.E. (2011). Morphologic findings in progressive familial intrahepatic cholestasis 2 (PFIC2): correlation with genetic and immunohistochemical studies. Am. J. Surg. Pathol. 35: 687–696. https://doi.org/10.1097/pas.0b013e318212ec87.Search in Google Scholar
Fisher, M.M. and Yousef, I.M. (1973). Sex differences in the bile acid composition of human bile: studies in patients with and without gallstones. Can. Med. Assoc. J. 109: 190–193.Search in Google Scholar
Garcia-Canaveras, J.C., Donato, M.T., and Lahoz, A. (2014). Ultra-performance liquid chromatography-mass spectrometry targeted profiling of bile acids: application to serum, liver tissue, and cultured cells of different species. Methods Mol. Biol. 1198: 233–247. https://doi.org/10.1007/978-1-4939-1258-2_15.Search in Google Scholar PubMed
Gerloff, T., Stieger, B., Hagenbuch, B., Madon, J., Landmann, L., Roth, J., Hofmann, A.F., and Meier, P.J. (1998). The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 273: 10046–10050. https://doi.org/10.1074/jbc.273.16.10046.Search in Google Scholar PubMed
Gertzen, C.G., Spomer, L., Smits, S.H., Häussinger, D., Keitel, V., and Gohlke, H. (2015). Mutational mapping of the transmembrane binding site of the G-protein coupled receptor TGR5 and binding mode prediction of TGR5 agonists. Eur. J. Med. Chem. 104: 57–72. https://doi.org/10.1016/j.ejmech.2015.09.024.Search in Google Scholar PubMed
Glantz, A., Marschall, H.U., and Mattsson, L.A. (2004). Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology 40: 467–474. https://doi.org/10.1002/hep.20336.Search in Google Scholar PubMed
Gohlke, H., Schmitz, B., Sommerfeld, A., Reinehr, R., and Häussinger, D. (2013). alpha5 beta1-integrins are sensors for tauroursodeoxycholic acid in hepatocytes. Hepatology 57: 1117–1129. https://doi.org/10.1002/hep.25992.Search in Google Scholar PubMed
Greife, A., Felekyan, S., Ma, Q., Gertzen, C.G., Spomer, L., Dimura, M., Peulen, T.O., Wohler, C., Häussinger, D., Gohlke, H., et al. (2016). Structural assemblies of the di- and oligomeric G-protein coupled receptor TGR5 in live cells: an MFIS-FRET and integrative modelling study. Sci. Rep. 6: 36792. https://doi.org/10.1038/srep36792.Search in Google Scholar PubMed PubMed Central
Griffiths, W.J. and Sjovall, J. (2010). Bile acids: analysis in biological fluids and tissues. J. Lipid Res. 51: 23–41. https://doi.org/10.1194/jlr.r001941.Search in Google Scholar
Groen, A., Romero, M.R., Kunne, C., Hoosdally, S.J., Dixon, P.H., Wooding, C., Williamson, C., Seppen, J., Van den Oever, K., Mok, K.S., et al. (2011). Complementary functions of the flippase ATP8B1 and the floppase ABCB4 in maintaining canalicular membrane integrity. Gastroenterology 141: 1927–1937, e1921-1924. https://doi.org/10.1053/j.gastro.2011.07.042.Search in Google Scholar PubMed
Guarino, M.P., Cocca, S., Altomare, A., Emerenziani, S., and Cicala, M. (2013). Ursodeoxycholic acid therapy in gallbladder disease, a story not yet completed. World J. Gastroenterol. 19: 5029–5034. https://doi.org/10.3748/wjg.v19.i31.5029.Search in Google Scholar
Guyot, C., Hofstetter, L., and Stieger, B. (2014). Differential effects of membrane cholesterol content on the transport activity of multidrug resistance-associated protein 2 (ABCC2) and of the bile salt export pump (ABCB11). Mol. Pharmacol. 85: 909–920. https://doi.org/10.1124/mol.114.092262.Search in Google Scholar
Hagenbuch, B. and Meier, P.J. (2004). Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflüger’s Arch 447: 653–665. https://doi.org/10.1007/s00424-003-1168-y.Search in Google Scholar
Hagenbuch, B., Stieger, B., Foguet, M., Lubbert, H., and Meier, P.J. (1991). Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc. Natl. Acad. Sci. U.S.A. 88: 10629–10633. https://doi.org/10.1073/pnas.88.23.10629.Search in Google Scholar
Häussinger, D., Hallbrucker, C., Saha, N., Lang, F., and Gerok, W. (1992). Cell volume and bile acid excretion. Biochem. J. 288: 681–689. https://doi.org/10.1042/bj2880681.Search in Google Scholar
Häussinger, D., Kurz, A.K., Wettstein, M., Graf, D., Vom Dahl, S., and Schliess, F. (2003). Involvement of integrins and Src in tauroursodeoxycholate-induced and swelling-induced choleresis. Gastroenterology 124: 1476–1487. https://doi.org/10.1016/s0016-5085(03)00274-9.Search in Google Scholar
Häussinger, D., Schmitt, M., Weiergraber, O., and Kubitz, R. (2000). Short-term regulation of canalicular transport. Semin. Liver Dis. 20: 307–321. https://doi.org/10.1055/s-2000-9386.Search in Google Scholar PubMed
Hayashi, H. and Sugiyama, Y. (2009). Short-chain ubiquitination is associated with the degradation rate of a cell-surface-resident bile salt export pump (BSEP/ABCB11). Mol. Pharmacol. 75: 143–150. https://doi.org/10.1124/mol.108.049288.Search in Google Scholar PubMed
Hayashi, H., Takada, T., Suzuki, H., Onuki, R., Hofmann, A.F., and Sugiyama, Y. (2005). Transport by vesicles of glycine- and taurine-conjugated bile salts and taurolithocholate 3-sulfate: a comparison of human BSEP with rat Bsep. Biochim. Biophys. Acta 1738: 54–62. https://doi.org/10.1016/j.bbalip.2005.10.006.Search in Google Scholar PubMed
Herebian, D. and Mayatepek, E. (2011). Inborn errors of bile acid metabolism and their diagnostic confirmation by means of mass spectrometry. J. Pediatr. Sci. 3: 1–11.Search in Google Scholar
Hiller, C., Kuhhorn, J., and Gmeiner, P. (2013). Class A G-protein-coupled receptor (GPCR) dimers and bivalent ligands. J. Med. Chem. 56: 6542–6559. https://doi.org/10.1021/jm4004335.Search in Google Scholar PubMed
Hofmann, A.F. (1984). Chemistry and enterohepatic circulation of bile acids. Hepatology 4: 4S–14S. https://doi.org/10.1002/hep.1840040803.Search in Google Scholar PubMed
Hoque, M.T., Conseil, G., and Cole, S.P. (2009). Involvement of NHERF1 in apical membrane localization of MRP4 in polarized kidney cells. Biochem. Biophys. Res. Commun. 379: 60–64. https://doi.org/10.1016/j.bbrc.2008.12.014.Search in Google Scholar PubMed
Hu, N.J., Iwata, S., Cameron, A.D., and Drew, D. (2011). Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature 478: 408–411. https://doi.org/10.1038/nature10450.Search in Google Scholar PubMed PubMed Central
Jacquemin, E. (2001). Role of multidrug resistance 3 deficiency in pediatric and adult liver disease: one gene for three diseases. Semin. Liver Dis. 21: 551–562. https://doi.org/10.1055/s-2001-19033.Search in Google Scholar PubMed
Jara, P., Hierro, L., Martinez-Fernandez, P., Alvarez-Doforno, R., Yanez, F., Diaz, M.C., Camarena, C., De la Vega, A., Frauca, E., Munoz-Bartolo, G., et al. (2009). Recurrence of bile salt export pump deficiency after liver transplantation. N. Engl. J. Med. 361: 1359–1367. https://doi.org/10.1056/nejmoa0901075.Search in Google Scholar PubMed
Jin, M.S., Oldham, M.L., Zhang, Q., and Chen, J. (2012). Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature 490: 566–569, doi:https://doi.org/10.2210/pdb4f4c/pdb.Search in Google Scholar
Johnson, B.J., Lee, J.Y., Pickert, A., and Urbatsch, I.L. (2010). Bile acids stimulate ATP hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry 49: 3403–3411. https://doi.org/10.1021/bi902064g.Search in Google Scholar PubMed
Jung, D., Hagenbuch, B., Fried, M., Meier, P.J., and Kullak-Ublick, G.A. (2004). Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am. J. Physiol. Gastrointest. Liver Physiol. 286: G752–G761. https://doi.org/10.1152/ajpgi.00456.2003.Search in Google Scholar PubMed
Katritch, V. and Abagyan, R. (2011). GPCR agonist binding revealed by modeling and crystallography. Trends Pharmacol. Sci. 32: 637–643. https://doi.org/10.1016/j.tips.2011.08.001.Search in Google Scholar PubMed PubMed Central
Katritch, V., Cherezov, V., and Stevens, R.C. (2013). Structure-function of the G-protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 53: 531. https://doi.org/10.1146/annurev-pharmtox-032112-135923.Search in Google Scholar PubMed PubMed Central
Kawamata, Y., Fujii, R., Hosoya, M., Harada, M., Yoshida, H., Miwa, M., Fukusumi, S., Habata, Y., Itoh, T., Shintani, Y., et al. (2003). A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278: 9435–9440. https://doi.org/10.1074/jbc.m209706200.Search in Google Scholar
Keitel, V., Burdelski, M., Vojnisek, Z., Schmitt, L., Häussinger, D., and Kubitz, R. (2009). De novo bile salt transporter antibodies as a possible cause of recurrent graft failure after liver transplantation: a novel mechanism of cholestasis. Hepatology 50: 510–517. https://doi.org/10.1002/hep.23083.Search in Google Scholar PubMed
Keitel, V., Burdelski, M., Warskulat, U., Kuhlkamp, T., Keppler, D., Häussinger, D., and Kubitz, R. (2005). Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology 41: 1160–1172. https://doi.org/10.1002/hep.20682.Search in Google Scholar PubMed
Keitel, V., Droge, C., and Häussinger, D. (2019a). Targeting FXR in cholestasis. Handb. Exp. Pharmacol. 256: 299–324. https://doi.org/10.1007/164_2019_231.Search in Google Scholar PubMed
Keitel, V., Droge, C., Stepanow, S., Fehm, T., Mayatepek, E., Kohrer, K., and Häussinger, D. (2016). Intrahepatic cholestasis of pregnancy (ICP): case report and review of the literature. Z. Gastroenterol. 54: 1327–1333. https://doi.org/10.1055/s-0042-118388.Search in Google Scholar PubMed
Keitel, V., Gertzen, C.G.W., Schäfer, S., Klindt, C., Wöhler, C., Deutschmann, K., Reich, M., Gohlke, H., and Häussinger, D. (2020). Bile acids and TGR5 (Gpbar1) signaling. In: Rozman, R., and Gebhardt, G. (Eds.), Mammalian sterols. Springer Nature, Switzerland.10.1007/978-3-030-39684-8_4Search in Google Scholar
Keitel, V., Görg, B., Bidmon, H.J., Zemtsova, I., Spomer, L., Zilles, K., and Häussinger, D. (2010). The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58: 1794–1805. https://doi.org/10.1002/glia.21049.Search in Google Scholar PubMed
Keitel, V. and Häussinger, D. (2012). Perspective: TGR5 (Gpbar-1) in liver physiology and disease. Clin Res Hepatol Gastroenterol 36: 412–419. https://doi.org/10.1016/j.clinre.2012.03.008.Search in Google Scholar PubMed
Keitel, V., and Häussinger, D. (2018). Role of TGR5 (GPBAR1) in liver disease. Semin. Liver Dis. 333–339, doi:https://doi.org/10.1055/s-0038-1669940.Search in Google Scholar PubMed
Keitel, V., Kubitz, R., and Häussinger, D. (2008). Endocrine and paracrine role of bile acids. World J. Gastroenterol. 14: 5620–5629. https://doi.org/10.3748/wjg.14.5620.Search in Google Scholar PubMed PubMed Central
Keitel, V., Spomer, L., Marin, J.J., Williamson, C., Geenes, V., Kubitz, R., Häussinger, D., and Macias, R.I. (2013). Effect of maternal cholestasis on TGR5 expression in human and rat placenta at term. Placenta 34: 810–816. https://doi.org/10.1016/j.placenta.2013.06.302.Search in Google Scholar PubMed
Keitel, V., Stindt, J., and Häussinger, D. (2019b). Bile acid-activated receptors: GPBAR1 (TGR5) and other G protein-coupled receptors. Handb. Exp. Pharmacol. 256: 19–49. https://doi.org/10.1007/164_2019_230.Search in Google Scholar PubMed
Keitel, V., Vogt, C., Häussinger, D., and Kubitz, R. (2006). Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology 131: 624–629. https://doi.org/10.1053/j.gastro.2006.05.003.Search in Google Scholar PubMed
Kis, E., Ioja, E., Nagy, T., Szente, L., Heredi-Szabo, K., and Krajcsi, P. (2009). Effect of membrane cholesterol on BSEP/Bsep activity: species specificity studies for substrates and inhibitors. Drug Metab. Dispos. 37: 1878–1886. https://doi.org/10.1124/dmd.108.024778.Search in Google Scholar PubMed
Klindt, C., Reich, M., Hellwig, B., Stindt, J., Rahnenfuhrer, J., Hengstler, J.G., Kohrer, K., Schoonjans, K., Häussinger, D., and Keitel, V. (2019). The G protein-coupled bile acid receptor TGR5 (Gpbar1) modulates endothelin-1 signaling in liver. Cells 8. https://doi.org/10.3390/cells8111467.Search in Google Scholar PubMed PubMed Central
Kluth, M., Stindt, J., Droge, C., Linnemann, D., Kubitz, R., and Schmitt, L. (2015). A mutation within the extended X loop abolished substrate-induced ATPase activity of the human liver ATP-binding cassette (ABC) transporter MDR3. J. Biol. Chem. 290: 4896–4907. https://doi.org/10.1074/jbc.m114.588566.Search in Google Scholar
Knisely, A.S., Strautnieks, S.S., Meier, Y., Stieger, B., Byrne, J.A., Portmann, B.C., Bull, L.N., Pawlikowska, L., Bilezikci, B., Ozcay, F., et al. (2006). Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44: 478–486. https://doi.org/10.1002/hep.21287.Search in Google Scholar PubMed
Konig, J., Cui, Y., Nies, A.T., and Keppler, D. (2000). Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J. Biol. Chem. 275: 23161–23168. https://doi.org/10.1074/jbc.m001448200.Search in Google Scholar PubMed
Kubitz, R., D’Urso, D., Keppler, D., and Häussinger, D. (1997). Osmodependent dynamic localization of the multidrug resistance protein 2 in the rat hepatocyte canalicular membrane. Gastroenterology 113: 1438–1442. https://doi.org/10.1053/gast.1997.v113.pm9352844.Search in Google Scholar PubMed
Kroll, T., Prescher, M., Smits, S.H.J., and Schmitt, L. (2021). Structure and function of hepatobiliary ATP binding cassette transporters. Chem. Rev. 121: 5240–5288, doi:https://doi.org/10.1021/acs.chemrev.0c00659.Search in Google Scholar PubMed
Kubitz, R., Droge, C., Kluge, S., Stindt, J., and Häussinger, D. (2014). Genetic variations of bile salt transporters. Drug Discov. Today Technol. 12: e55–67. https://doi.org/10.1016/j.ddtec.2014.03.006.Search in Google Scholar
Kubitz, R., Droge, C., Kluge, S., Stross, C., Walter, N., Keitel, V., Häussinger, D., and Stindt, J. (2015). Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin. Rev. Allergy Immunol. 48: 273–284. https://doi.org/10.1007/s12016-014-8457-4.Search in Google Scholar
Kubitz, R., Keitel, V., Scheuring, S., Kohrer, K., and Häussinger, D. (2006). Benign recurrent intrahepatic cholestasis associated with mutations of the bile salt export pump. J. Clin. Gastroenterol. 40: 171–175. https://doi.org/10.1097/01.mcg.0000196406.15110.60.Search in Google Scholar
Kurz, A.K., Graf, D., Schmitt, M., Vom Dahl, S., and Häussinger, D. (2001). Tauroursodesoxycholate-induced choleresis involves p38(MAPK) activation and translocation of the bile salt export pump in rats. Gastroenterology 121: 407–419. https://doi.org/10.1053/gast.2001.26262.Search in Google Scholar
Lammert, F., Marschall, H.U., and Matern, S. (2003). Intrahepatic cholestasis of pregnancy. Curr. Treat. Options Gastroenterol. 6: 123–132. https://doi.org/10.1007/s11938-003-0013-x.Search in Google Scholar
Lazaridis, K.N., Gores, G.J., and Lindor, K.D. (2001). Ursodeoxycholic acid ’mechanisms of action and clinical use in hepatobiliary disorders. J. Hepatol. 35: 134–146. https://doi.org/10.1016/s0168-8278(01)00092-7.Search in Google Scholar
Lefebvre, P., Cariou, B., Lien, F., Kuipers, F., and Staels, B. (2009). Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 89: 147–191. https://doi.org/10.1152/physrev.00010.2008.Search in Google Scholar PubMed
Leuthold, S., Hagenbuch, B., Mohebbi, N., Wagner, C.A., Meier, P.J., and Stieger, B. (2009). Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am. J. Physiol. Cell Physiol. 296: C570–C582. https://doi.org/10.1152/ajpcell.00436.2008.Search in Google Scholar PubMed
Li, M., Wang, W., Soroka, C.J., Mennone, A., Harry, K., Weinman, E.J., and Boyer, J.L. (2010). NHERF-1 binds to Mrp2 and regulates hepatic Mrp2 expression and function. J. Biol. Chem. 285: 19299–19307. https://doi.org/10.1074/jbc.m109.096081.Search in Google Scholar
Locher, K.P. (2016). Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 23: 487–493. https://doi.org/10.1038/nsmb.3216.Search in Google Scholar PubMed
Makishima, M., Okamoto, A.Y., Repa, J.J., Tu, H., Learned, R.M., Luk, A., Hull, M.V., Lustig, K.D., Mangelsdorf, D.J., and Shan, B. (1999). Identification of a nuclear receptor for bile acids. Science 284: 1362–1365. https://doi.org/10.1126/science.284.5418.1362.Search in Google Scholar
Martin, R.E., Bissantz, C., Gavelle, O., Kuratli, C., Dehmlow, H., Richter, H.G., Obst Sander, U., Erickson, S.D., Kim, K., Pietranico-Cole, S.L., et al. (2013). 2-Phenoxy-nicotinamides are potent agonists at the bile acid receptor GPBAR1 (TGR5). ChemMedChem 8: 569–576. https://doi.org/10.1002/cmdc.201200474.Search in Google Scholar
Maruyama, T., Miyamoto, Y., Nakamura, T., Tamai, Y., Okada, H., Sugiyama, E., Nakamura, T., Itadani, H., and Tanaka, K. (2002). Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 298: 714–719. https://doi.org/10.1016/s0006-291x(02)02550-0.Search in Google Scholar
Maruyama, T., Tanaka, K., Suzuki, J., Miyoshi, H., Harada, N., Nakamura, T., Miyamoto, Y., Kanatani, A., and Tamai, Y. (2006). Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J. Endocrinol. 191: 197–205. https://doi.org/10.1677/joe.1.06546.Search in Google Scholar PubMed
Mashige, F., Tanaka, N., Maki, A., Kamei, S., and Yamanaka, M. (1981). Direct spectrophotometry of total bile acids in serum. Clin. Chem. 27: 1352–1356. https://doi.org/10.1093/clinchem/27.8.1352.Search in Google Scholar
Masyuk, T.V., Masyuk, A.I., Lorenzo Pisarello, M., Howard, B.N., Huang, B.Q., Lee, P.Y., Fung, X., Sergienko, E., Ardecky, R.J., Chung, T.D.Y., et al. (2017). TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Galphas signaling. Hepatology 66: 1197–1218. https://doi.org/10.1002/hep.29284.Search in Google Scholar PubMed PubMed Central
Mayer, P.G.K., Qvartskhava, N., Sommerfeld, A., Gorg, B., and Häussinger, D. (2019). Regulation of plasma membrane localization of the Na(+)-Taurocholate Co-transporting polypeptide by glycochenodeoxycholate and tauroursodeoxycholate. Cell. Physiol. Biochem. 52: 1427–1445. https://doi.org/10.33594/000000100.Search in Google Scholar PubMed
Merlen, G., Kahale, N., Ursic-Bedoya, J., Bidault-Jourdainne, V., Simerabet, H., Doignon, I., Tanfin, Z., Garcin, I., Pean, N., Gautherot, J., et al. (2020). TGR5-dependent hepatoprotection through the regulation of biliary epithelium barrier function. Gut 69: 146–157. https://doi.org/10.1136/gutjnl-2018-316975.Search in Google Scholar PubMed
Mihalik, S.J., Steinberg, S.J., Pei, Z., Park, J., Kim, D.G., Heinzer, A.K., Dacremont, G., Wanders, R.J., Cuebas, D.A., Smith, K.D., et al. (2002). Participation of two members of the very long-chain acyl-CoA synthetase family in bile acid synthesis and recycling. J. Biol. Chem. 277: 24771–24779. https://doi.org/10.1074/jbc.m203295200.Search in Google Scholar
Morgan, R.E., Trauner, M., van Staden, C.J., Lee, P.H., Ramachandran, B., Eschenberg, M., Afshari, C.A., Qualls, C.W.Jr., Lightfoot-Dunn, R., and Hamadeh, H.K. (2010). Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol. Sci. 118: 485–500. https://doi.org/10.1093/toxsci/kfq269.Search in Google Scholar PubMed
Mukhopadhayay, S., Ananthanarayanan, M., Stieger, B., Meier, P.J., Suchy, F.J., and Anwer, M.S. (1997). cAMP increases liver Na+-taurocholate cotransport by translocating transporter to plasma membranes. Am. J. Physiol. 273: G842–G848. https://doi.org/10.1152/ajpgi.1997.273.4.g842.Search in Google Scholar
Ni, Y., Lempp, F.A., Mehrle, S., Nkongolo, S., Kaufman, C., Falth, M., Stindt, J., Koniger, C., Nassal, M., Kubitz, R., et al. (2014). Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 146: 1070–1083. https://doi.org/10.1053/j.gastro.2013.12.024.Search in Google Scholar
Noe, J., Hagenbuch, B., Meier, P.J., and St-Pierre, M.V. (2001). Characterization of the mouse bile salt export pump overexpressed in the baculovirus system. Hepatology 33: 1223–1231. https://doi.org/10.1053/jhep.2001.24171.Search in Google Scholar
Noe, J., Stieger, B., and Meier, P.J. (2002). Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology 123: 1659–1666. https://doi.org/10.1053/gast.2002.36587.Search in Google Scholar
Ogimura, E., Sekine, S., and Horie, T. (2011). Bile salt export pump inhibitors are associated with bile acid-dependent drug-induced toxicity in sandwich-cultured hepatocytes. Biochem. Biophys. Res. Commun. 416: 313–317. https://doi.org/10.1016/j.bbrc.2011.11.032.Search in Google Scholar
Oizumi, K., Sekine, S., Fukagai, M., Susukida, T., and Ito, K. (2017). Identification of bile acids responsible for inhibiting the bile salt export pump, leading to bile acid accumulation and cell toxicity in rat hepatocytes. J. Pharm. Sci. 106: 2412–2419, https://doi.org/10.1016/j.xphs.2017.05.017. 28552691.Search in Google Scholar PubMed
Ovadia, C., Seed, P.T., Sklavounos, A., Geenes, V., Di Ilio, C., Chambers, J., Kohari, K., Bacq, Y., Bozkurt, N., Brun-Furrer, R., et al. (2019). Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet 393: 899–909. https://doi.org/10.1016/s0140-6736(18)31877-4.Search in Google Scholar
Parks, D.J., Blanchard, S.G., Bledsoe, R.K., Chandra, G., Consler, T.G., Kliewer, S.A., Stimmel, J.B., Willson, T.M., Zavacki, A.M., Moore, D.D., et al. (1999). Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368. https://doi.org/10.1126/science.284.5418.1365.Search in Google Scholar PubMed
Patowary, S., Alvarez-Curto, E., Xu, T.R., Holz, J.D., Oliver, J.A., Milligan, G., and Raicu, V. (2013). The muscarinic M3 acetylcholine receptor exists as two differently sized complexes at the plasma membrane. Biochem. J. 452: 303–312. https://doi.org/10.1042/bj20121902.Search in Google Scholar PubMed
Paulusma, C.C., Groen, A., Kunne, C., Ho-Mok, K.S., Spijkerboer, A.L., Rudi de Waart, D., Hoek, F.J., Vreeling, H., Hoeben, K.A., van Marle, J., et al. (2006). Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology 44: 195–204. https://doi.org/10.1002/hep.21212.Search in Google Scholar PubMed
Paumgartner, G. and Beuers, U. (2002). Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology 36: 525–531. https://doi.org/10.1053/jhep.2002.36088.Search in Google Scholar
Pellicciari, R., Gioiello, A., Macchiarulo, A., Thomas, C., Rosatelli, E., Natalini, B., Sardella, R., Pruzanski, M., Roda, A., Pastorini, E., et al. (2009). Discovery of 6α-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 52: 7958–7961. https://doi.org/10.1021/jm901390p.Search in Google Scholar
Prescher, M., Kroll, T., and Schmitt, L. (2019). ABCB4/MDR3 in health and disease - at the crossroads of biochemistry and medicine. Biol. Chem. 400: 1245–1259. https://doi.org/10.1515/hsz-2018-0441.Search in Google Scholar
Prescher, M., Smits, S.H.J., and Schmitt, L. (2020). Stimulation of ABCB4/MDR3 ATPase activity requires an intact phosphatidylcholine lipid. J. Lipid Res. 61: 1605–1616. https://doi.org/10.1194/jlr.ra120000889.Search in Google Scholar
Reich, M., Deutschmann, K., Sommerfeld, A., Klindt, C., Kluge, S., Kubitz, R., Ullmer, C., Knoefel, W.T., Herebian, D., Mayatepek, E., et al. (2016). TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut 65: 487–501. https://doi.org/10.1136/gutjnl-2015-309458.Search in Google Scholar
Reich, M., Klindt, C., Deutschmann, K., Spomer, L., Häussinger, D., and Keitel, V. (2017). Role of the G protein-coupled bile acid receptor TGR5 in liver damage. Dig. Dis. 35: 235–240. https://doi.org/10.1159/000450917.Search in Google Scholar
Reich, M., Spomer, L., Klindt, C., Fuchs, K., Stindt, J., Deutschmann, K., Hohne, J., Liaskou, E., Hov, J.R., Karlsen, T.H., et al. (2021). Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J. Hepatol. https://doi.org/10.1016/j.jhep.2021.03.029.Search in Google Scholar
Reinehr, R., Graf, D., and Häussinger, D. (2003). Bile salt-induced hepatocyte apoptosis involves epidermal growth factor receptor-dependent CD95 tyrosine phosphorylation. Gastroenterology 125: 839–853. https://doi.org/10.1016/s0016-5085(03)01055-2.Search in Google Scholar
Reinehr, R., Sommerfeld, A., and Häussinger, D. (2013). The Src family kinases: distinct functions of c-Src, Yes, and Fyn in the liver. Biomol. Concepts 4: 129–142. https://doi.org/10.1515/bmc-2012-0047.Search in Google Scholar PubMed
Ridlon, J.M., Harris, S.C., Bhowmik, S., Kang, D.J., and Hylemon, P.B. (2016). Consequences of bile salt biotransformations by intestinal bacteria. Gut Microb. 7: 22–39. https://doi.org/10.1080/19490976.2015.1127483.Search in Google Scholar PubMed PubMed Central
Ridlon, J.M., Kang, D.J., and Hylemon, P.B. (2006). Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47: 241–259. https://doi.org/10.1194/jlr.r500013-jlr200.Search in Google Scholar
Rioseco, A.J., Ivankovic, M.B., Manzur, A., Hamed, F., Kato, S.R., Parer, J.T., and Germain, A.M. (1994). Intrahepatic cholestasis of pregnancy: a retrospective case-control study of perinatal outcome. Am. J. Obstet. Gynecol. 170: 890–895. https://doi.org/10.1016/s0002-9378(94)70304-3.Search in Google Scholar
Sarafian, M.H., Lewis, M.R., Pechlivanis, A., Ralphs, S., McPhail, M.J., Patel, V.C., Dumas, M.E., Holmes, E., and Nicholson, J.K. (2015). Bile acid profiling and quantification in biofluids using ultra-performance liquid chromatography tandem mass spectrometry. Anal. Chem. 87: 9662–9670. https://doi.org/10.1021/acs.analchem.5b01556.Search in Google Scholar PubMed
Sarenac, T.M. and Mikov, M. (2018). Bile acid synthesis: from nature to the chemical modification and synthesis and their applications as drugs and nutrients. Front. Pharmacol. 9: 939.10.3389/fphar.2018.00939Search in Google Scholar PubMed PubMed Central
Sato, H., Macchiarulo, A., Thomas, C., Gioiello, A., Une, M., Hofmann, A.F., Saladin, R., Schoonjans, K., Pellicciari, R., and Auwerx, J. (2008). Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J. Med. Chem. 51: 1831–1841. https://doi.org/10.1021/jm7015864.Search in Google Scholar PubMed
Schmitt, M., Kubitz, R., Lizun, S., Wettstein, M., and Häussinger, D. (2001). Regulation of the dynamic localization of the rat Bsep gene-encoded bile salt export pump by anisoosmolarity. Hepatology 33: 509–518. https://doi.org/10.1053/jhep.2001.22648.Search in Google Scholar PubMed
Schmitt, M., Kubitz, R., Wettstein, M., vom Dahl, S., and Häussinger, D. (2000). Retrieval of the mrp2 gene encoded conjugate export pump from the canalicular membrane contributes to cholestasis induced by tert-butyl hydroperoxide and chloro-dinitrobenzene. Biol. Chem. 381: 487–495. https://doi.org/10.1515/bc.2000.063.Search in Google Scholar PubMed
Schonhoff, C.M., Park, S.W., Webster, C.R., and Anwer, M.S. (2016). p38 MAPK alpha and beta isoforms differentially regulate plasma membrane localization of MRP2. Am. J. Physiol. Gastrointest. Liver Physiol. 310: G999–G1005. https://doi.org/10.1152/ajpgi.00005.2016.Search in Google Scholar PubMed PubMed Central
Shi, Y., Xiong, J., Sun, D., Liu, W., Wei, F., Ma, S., and Lin, R. (2015). Simultaneous quantification of the major bile acids in artificial Calculus bovis by high-performance liquid chromatography with precolumn derivatization and its application in quality control. J. Separ. Sci. 38: 2753–2762. https://doi.org/10.1002/jssc.201500139.Search in Google Scholar PubMed
Shin, H.W. and Takatsu, H. (2019). Substrates of P4-ATPases: beyond aminophospholipids (phosphatidylserine and phosphatidylethanolamine). Faseb. J. 33: 3087–3096. https://doi.org/10.1096/fj.201801873r.Search in Google Scholar PubMed
Short, D.B., Trotter, K.W., Reczek, D., Kreda, S.M., Bretscher, A., Boucher, R.C., Stutts, M.J., and Milgram, S.L. (1998). An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 273: 19797–19801. https://doi.org/10.1074/jbc.273.31.19797.Search in Google Scholar PubMed
Siebold, L., Dick, A.A., Thompson, R., Maggiore, G., Jacquemin, E., Jaffe, R., Strautnieks, S., Grammatikopoulos, T., Horslen, S., Whitington, P.F., et al. (2010). Recurrent low gamma-glutamyl transpeptidase cholestasis following liver transplantation for bile salt export pump (BSEP) disease (posttransplant recurrent BSEP disease). Liver Transplant. 16: 856–863. https://doi.org/10.1002/lt.22074.Search in Google Scholar PubMed
Small, D.M. (2003). Role of ABC transporters in secretion of cholesterol from liver into bile. Proc. Natl. Acad. Sci. U.S.A. 100: 4–6. https://doi.org/10.1073/pnas.0237205100.Search in Google Scholar PubMed PubMed Central
Sommerfeld, A., Mayer, P.G., Cantore, M., and Häussinger, D. (2015). Regulation of plasma membrane localization of the Na+-taurocholate cotransporting polypeptide (Ntcp) by hyperosmolarity and tauroursodeoxycholate. J. Biol. Chem. 290: 24237–24254. https://doi.org/10.1074/jbc.m115.666883.Search in Google Scholar PubMed PubMed Central
Spomer, L., Gertzen, C.G., Schmitz, B., Häussinger, D., Gohlke, H., and Keitel, V. (2014). A membrane-proximal, C-terminal alpha-helix is required for plasma membrane localization and function of the G Protein-coupled receptor (GPCR) TGR5. J. Biol. Chem. 289: 3689–3702. https://doi.org/10.1074/jbc.m113.502344.Search in Google Scholar PubMed PubMed Central
Stagljar, I., Korostensky, C., Johnsson, N., and te Heesen, S. (1998). A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc. Natl. Acad. Sci. U.S.A. 95: 5187–5192. https://doi.org/10.1073/pnas.95.9.5187.Search in Google Scholar PubMed PubMed Central
Stieger, B. (2009). Recent insights into the function and regulation of the bile salt export pump (ABCB11). Curr. Opin. Lipidol. 20: 176–181. https://doi.org/10.1097/mol.0b013e32832b677c.Search in Google Scholar
Stindt, J., Ellinger, P., Stross, C., Keitel, V., Häussinger, D., Smits, S.H., Kubitz, R., and Schmitt, L. (2011). Heterologous overexpression and mutagenesis of the human bile salt export pump (ABCB11) using DREAM (Directed REcombination-Assisted Mutagenesis). PLoS One 6: e20562. https://doi.org/10.1371/journal.pone.0020562.Search in Google Scholar PubMed PubMed Central
Stindt, J., Ellinger, P., Weissenberger, K., Droge, C., Herebian, D., Mayatepek, E., Homey, B., Braun, S., Schulte am Esch, J., Horacek, M., et al. (2013). A novel mutation within a transmembrane helix of the bile salt export pump (BSEP, ABCB11) with delayed development of cirrhosis. Liver Int. 33: 1527–1535. https://doi.org/10.1111/liv.12217.Search in Google Scholar PubMed
Stindt, J., Kluge, S., Droge, C., Keitel, V., Stross, C., Baumann, U., Brinkert, F., Dhawan, A., Engelmann, G., Ganschow, R., et al. (2016). Bile salt export pump-reactive antibodies form a polyclonal, multi-inhibitory response in antibody-induced bile salt export pump deficiency. Hepatology 63: 524–537. https://doi.org/10.1002/hep.28311.Search in Google Scholar PubMed
Strautnieks, S.S., Byrne, J.A., Pawlikowska, L., Cebecauerova, D., Rayner, A., Dutton, L., Meier, Y., Antoniou, A., Stieger, B., Arnell, H., et al. (2008). Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology 134: 1203–1214. https://doi.org/10.1053/j.gastro.2008.01.038.Search in Google Scholar
Strautnieks, S.S., Kagalwalla, A.F., Tanner, M.S., Knisely, A.S., Bull, L., Freimer, N., Kocoshis, S.A., Gardiner, R.M., and Thompson, R.J. (1997). Identification of a locus for progressive familial intrahepatic cholestasis PFIC2 on chromosome 2q24. Am. J. Hum. Genet. 61: 630–633. https://doi.org/10.1086/515501.Search in Google Scholar
Studer, E., Zhou, X., Zhao, R., Wang, Y., Takabe, K., Nagahashi, M., Pandak, W.M., Dent, P., Spiegel, S., Shi, R., et al. (2012). Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 55: 267–276. https://doi.org/10.1002/hep.24681.Search in Google Scholar
Sugita, T., Amano, K., Nakano, M., Masubuchi, N., Sugihara, M., and Matsuura, T. (2015). Analysis of the serum bile Acid composition for differential diagnosis in patients with liver disease. Gastroenterol Res Pract 2015: 717431. https://doi.org/10.1155/2015/717431.Search in Google Scholar
Takahashi, S., Fukami, T., Masuo, Y., Brocker, C.N., Xie, C., Krausz, K.W., Wolf, C.R., Henderson, C.J., and Gonzalez, F.J. (2016). Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid Res. 57: 2130–2137. https://doi.org/10.1194/jlr.m071183.Search in Google Scholar
Takikawa, H., Beppu, T., and Seyama, Y. (1985). Profiles of bile acids and their glucuronide and sulphate conjugates in the serum, urine and bile from patients undergoing bile drainage. Gut 26: 38–42, https://doi.org/10.1136/gut.26.1.38. 3965365.Search in Google Scholar PubMed
Thomas, C., Gioiello, A., Noriega, L., Strehle, A., Oury, J., Rizzo, G., Macchiarulo, A., Yamamoto, H., Mataki, C., Pruzanski, M., et al. (2009). TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metabol. 10: 167–177. https://doi.org/10.1016/j.cmet.2009.08.001.Search in Google Scholar
Trauner, M. and Boyer, J.L. (2003). Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83: 633–671. https://doi.org/10.1152/physrev.00027.2002.Search in Google Scholar
van Helvoort, A., Smith, A.J., Sprong, H., Fritsche, I., Schinkel, A.H., Borst, P., and van Meer, G. (1996). MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 87: 507–517. https://doi.org/10.1016/s0092-8674(00)81370-7.Search in Google Scholar
Van Mil, S.W., Milona, A., Dixon, P.H., Mullenbach, R., Geenes, V.L., Chambers, J., Shevchuk, V., Moore, G.E., Lammert, F., Glantz, A.G., et al. (2007). Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology 133: 507–516. https://doi.org/10.1053/j.gastro.2007.05.015.Search in Google Scholar PubMed
van Mil, S.W., van der Woerd, W.L., van der Brugge, G., Sturm, E., Jansen, P.L., Bull, L.N., van den Berg, I.E., Berger, R., Houwen, R.H., and Klomp, L.W. (2004). Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology 127: 379–384. https://doi.org/10.1053/j.gastro.2004.04.065.Search in Google Scholar PubMed
van Wessel, D.B.E., Thompson, R.J., Gonzales, E., Jankowska, I., Sokal, E., Grammatikopoulos, T., Kadaristiana, A., Jacquemin, E., Spraul, A., Lipinski, P., et al. (2020). Genotype correlates with the natural history of severe bile salt export pump deficiency. J. Hepatol. 73: 84–93. https://doi.org/10.1016/j.jhep.2020.02.007.Search in Google Scholar PubMed
Vassileva, G., Golovko, A., Markowitz, L., Abbondanzo, S.J., Zeng, M., Yang, S., Hoos, L., Tetzloff, G., Levitan, D., Murgolo, N.J., et al. (2006). Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem. J. 398: 423–430. https://doi.org/10.1042/bj20060537.Search in Google Scholar PubMed PubMed Central
Velazquez-Villegas, L.A., Perino, A., Lemos, V., Zietak, M., Nomura, M., Pols, T.W.H., and Schoonjans, K. (2018). TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 9: 245. https://doi.org/10.1038/s41467-017-02068-0.Search in Google Scholar PubMed PubMed Central
Wadsworth, C.A., Dixon, P.H., Wong, J.H., Chapman, M.H., McKay, S.C., Sharif, A., Spalding, D.R., Pereira, S.P., Thomas, H.C., Taylor-Robinson, S.D., et al. (2011). Genetic factors in the pathogenesis of cholangiocarcinoma. Dig. Dis. 29: 93–97. https://doi.org/10.1159/000324688.Search in Google Scholar PubMed PubMed Central
Wang, L., Hou, W.T., Chen, L., Jiang, Y.L., Xu, D., Sun, L., Zhou, C.Z., and Chen, Y. (2020). Cryo-EM structure of human bile salts exporter ABCB11. Cell Res. 30: 623–625, doi:https://doi.org/10.2210/pdb6lr0/pdb.Search in Google Scholar
Wäschenbach, L., Gertzen, C.G., Keitel, V., and Gohlke, H. (2020). Dimerization energetics of the G‐protein coupled bile acid receptor TGR5 from all‐atom simulations. J. Comput. Chem. 41: 874–884. https://doi.org/10.1002/jcc.26135.Search in Google Scholar PubMed
Webster, C.R., Blanch, C.J., Phillips, J., and Anwer, M.S. (2000). Cell swelling-induced translocation of rat liver Na+/taurocholate cotransport polypeptide is mediated via the phosphoinositide 3-kinase signaling pathway. J. Biol. Chem. 275: 29754–29760. https://doi.org/10.1074/jbc.m002831200.Search in Google Scholar PubMed
Webster, C.R., Srinivasulu, U., Ananthanarayanan, M., Suchy, F.J., and Anwer, M.S. (2002). Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J. Biol. Chem. 277: 28578–28583. https://doi.org/10.1074/jbc.m201937200.Search in Google Scholar
Williamson, C. and Geenes, V. (2014). Intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 124: 120–133. https://doi.org/10.1097/aog.0000000000000346.Search in Google Scholar PubMed
Yan, H., Peng, B., He, W., Zhong, G., Qi, Y., Ren, B., Gao, Z., Jing, Z., Song, M., Xu, G., et al. (2013). Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J. Virol. 87: 7977–7991. https://doi.org/10.1128/jvi.03540-12.Search in Google Scholar
Yang, F., Mao, C., Guo, L., Lin, J., Ming, Q., Xiao, P., Wu, X., Shen, Q., Guo, S., Shen, D.-D., et al. (2020). Structural basis of GPBAR activation and bile acid recognition. Nature 587: 499–504. https://doi.org/10.1038/s41586-020-2569-1.Search in Google Scholar PubMed
Yuan, M., Breitkopf, S.B., Yang, X., and Asara, J.M. (2012). A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 7: 872–881. https://doi.org/10.1038/nprot.2012.024.Search in Google Scholar PubMed PubMed Central
Zhang, G.H., Cong, A.R., Xu, G.B., Li, C.B., Yang, R.F., and Xia, T.A. (2005). An enzymatic cycling method for the determination of serum total bile acids with recombinant 3α-hydroxysteroid dehydrogenase. Biochem. Biophys. Res. Commun. 326: 87–92. https://doi.org/10.1016/j.bbrc.2004.11.005.Search in Google Scholar PubMed
© 2021 Christoph G.W. Gertzen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Highlight: Liver Regeneration / Experimental Hepatology
- Liver damage and regeneration – 20 years of liver research in the Collaborative Research Centers 575 and 974
- Role of vasodilation in liver regeneration and health
- Hepatic stellate cells: current state and open questions
- Liver cell hydration and integrin signaling
- The many facets of bile acids in the physiology and pathophysiology of the human liver
- Glutamine synthetase as a central element in hepatic glutamine and ammonia metabolism: novel aspects
- Characterization of the scavenger cell proteome in mouse and rat liver
- Pathomechanisms in hepatic encephalopathy
- Rapid metabolic and bioenergetic adaptations of astrocytes under hyperammonemia – a novel perspective on hepatic encephalopathy
- The role of ADAM17 during liver damage
- Acute-phase protein synthesis: a key feature of innate immune functions of the liver
- Lymphotoxin-β-receptor (LTβR) signaling on hepatocytes is required for liver regeneration after partial hepatectomy
Articles in the same Issue
- Frontmatter
- Highlight: Liver Regeneration / Experimental Hepatology
- Liver damage and regeneration – 20 years of liver research in the Collaborative Research Centers 575 and 974
- Role of vasodilation in liver regeneration and health
- Hepatic stellate cells: current state and open questions
- Liver cell hydration and integrin signaling
- The many facets of bile acids in the physiology and pathophysiology of the human liver
- Glutamine synthetase as a central element in hepatic glutamine and ammonia metabolism: novel aspects
- Characterization of the scavenger cell proteome in mouse and rat liver
- Pathomechanisms in hepatic encephalopathy
- Rapid metabolic and bioenergetic adaptations of astrocytes under hyperammonemia – a novel perspective on hepatic encephalopathy
- The role of ADAM17 during liver damage
- Acute-phase protein synthesis: a key feature of innate immune functions of the liver
- Lymphotoxin-β-receptor (LTβR) signaling on hepatocytes is required for liver regeneration after partial hepatectomy

