Abstract
Redox-mediated signal transduction depends on the enzymatic production of second messengers such as hydrogen peroxide, nitric oxide and hydrogen sulfite, as well as specific, reversible redox modifications of cysteine-residues in proteins. So-called thiol switches induce for instance conformational changes in specific proteins that regulate cellular pathways e.g., cell metabolism, proliferation, migration, gene expression and inflammation. Reduction, oxidation and disulfide isomerization are controlled by oxidoreductases of the thioredoxin family, including thioredoxins, glutaredoxins, peroxiredoxins and protein dsisulfide isomerases. These proteins are located in different cellular compartments, interact with substrates and catalyze specific reactions. Interestingly, some of these proteins are released by cells. Their extracellular functions and generally extracellular redox control have been widely underestimated. Here, we give an insight into extracellular redox signaling, extracellular thiol switches and their regulation by secreted oxidoreductases and thiol-isomerases, a topic whose importance has been scarcely studied so far, likely due to methodological limitations. We focus on the secreted redox proteins and characterized thiol switches in the ectodomains of membrane proteins, such as integrins and the metalloprotease ADAM17, which are among the best-characterized proteins and discuss their underlying mechanisms and biological implications.
Introduction: redox-mediated signal transduction depends on thiol switches
Cellular functions adapt to changes in the microenvironment, such as changes in oxygen concentration, nutrients, signal molecules as well as immune mediators, pathogenic antigens or factors associated with stress conditions. With respect to oxidizing (such as oxygen) and reducing agents (such as cysteine, glutathione), redox sensing and redox-mediated signal transduction occurs upon physiological conditions. It is known as oxidative eustress, and may be regulated by cells as part of a paracrine signaling. Upon unphysiological/pathological oxidative challenges, termed oxidative distress, redox-mediated signaling is dysregulated and biomolecules are non-specifically and irreversibly oxidized and damaged (Sies and Jones 2020). These changes of the redox milieu are perceived by specific receptor molecules on the cell surface and play a role in cell-cell communication. Indeed, a cell can respond simultaneously to different signals by ligand binding and activation of specific signaling cascades. Rapid and specific reactions are ensured by the regulated production of second messengers such as hydrogen peroxide, nitric oxide and hydrogen sulfite. They control the function of effector proteins by covalent and reversible, posttranslational modifications of cysteine (Cys) residues. These Cys modifications can cause conformational changes within the protein and thus induce a switch between a (more) active and a less active/inactive structure of the protein (Hanschmann et al. 2013; Sies and Jones 2020). Thus, cysteine residues in proteins may function as thiol switches, which regulate interactions, distribution, and biological activity of proteins (Leichert and Dick 2015). Thiol switches are very diverse. Indeed, it has been described that Cys residues are modified in 18 different ways, e.g., by disulfide formation, glutathionylation and nitrosylation (Go et al. 2015). Similarly, to other posttranslational modifications, thiol modifications are enzymatically controlled. Members of the thioredoxin (Trx) family control the oxidation and the reduction of Cys residues. In mammals, this protein family comprises more than 50 members, including Trx, peroxiredoxins (Prx) and protein disulfide isomerases (PDI) as well as glutathione (GSH)-dependent glutaredoxins (Grx), GSH peroxidases (GPx) and GSH-transferases (Chiu et al. 2015; Hanschmann et al. 2013). Trx proteins are ubiquitously expressed and conserved throughout the kingdom of life. They are located in different cellular compartments, and some members were shown to be specifically secreted (Hanschmann et al. 2013) (Figure 1, Table 1). Originally discovered as non-specific antioxidants, they are now esteemed key regulators of redox-mediated signal transduction. They catalyze different reactions (reduction, oxidation and disulfide exchange) and interact with different subgroups of substrates. Interestingly, the substrate specificity is not determined by the redox potential, but is demonstrably based on geometric and electrostatic complementary surfaces within the active sites of the oxidoreductases (Berndt et al. 2015). Underlining the importance of redox regulation in life, the number of cysteine-containing proteins has increased during evolution, along with the appearance of molecular oxygen with its high redox potential and its distinct distribution in different tissues of multicellular organisms (Go et al. 2015). In fact, it was shown that there is at least one redox-regulated element in every signaling pathway (Jones and Go 2011). Moreover, dynamics of the cytoskeleton and organelles such as mitochondria are redox-regulated (Gellert et al. 2015; Wolf et al. 2020).
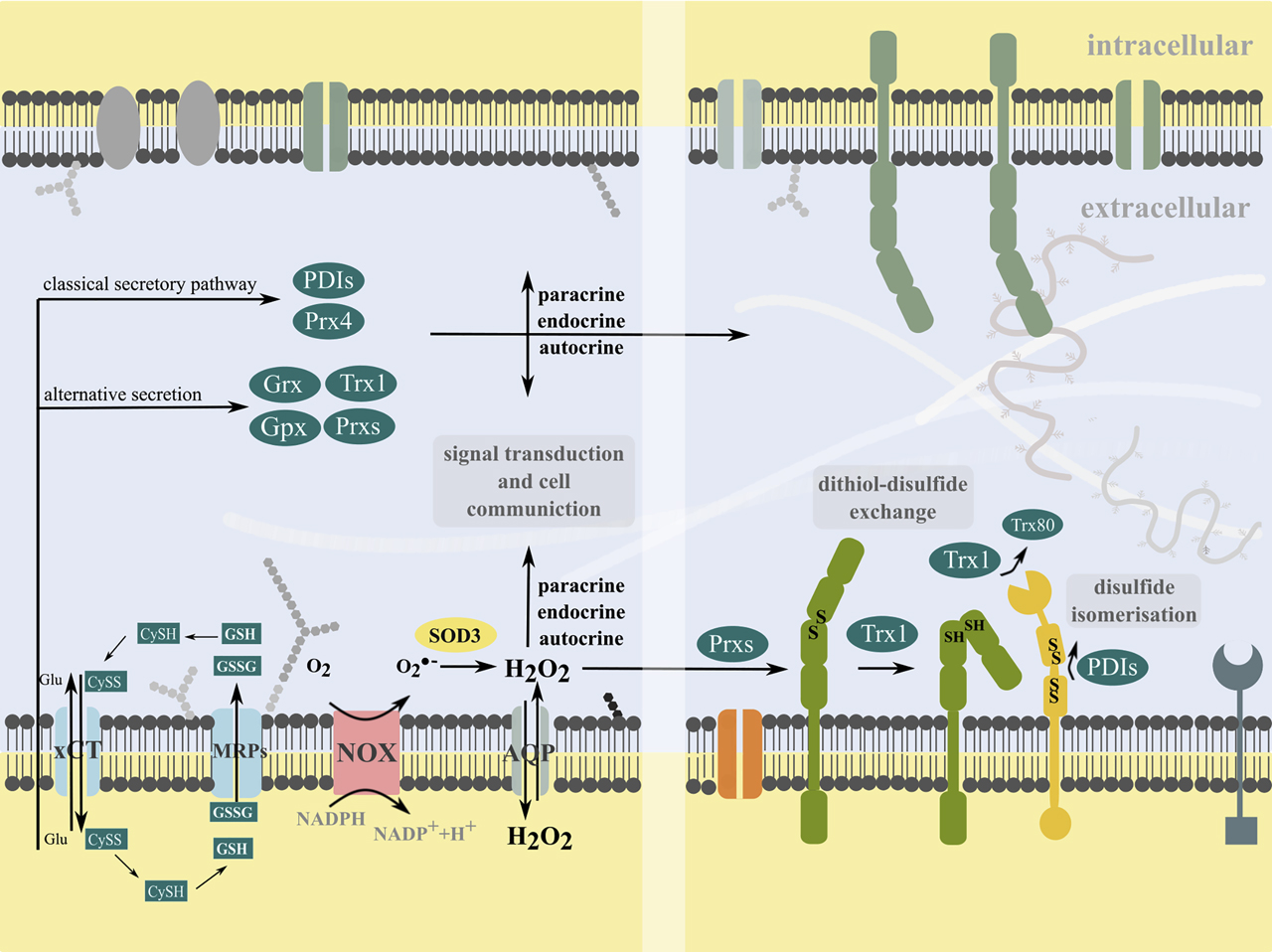
Extracellular redox signaling and cell communication.
Cells can produce superoxide and hydrogen peroxide at the plasma membrane by NADPH oxidases (NOX) and extracellular superoxide dismutase 3 (SOD3). Hydrogen peroxide can bidirectional pass the plasma membrane directly or via aquaporins (AQP), acting in intracellular signal transduction. Moreover, it can participate in extracellular signal transduction by the oxidation of membrane proteins. The xCT transporter mediates the cystine-cysteine shuttle. Cells release various redox active molecules, including glutathione (GSH, GSSG) via multidrug resistance proteins (MRPs) and members of the Thioredoxin (Trx) family of proteins, i.e. Trx1, glutaredoxins (Grx), glutathione peroxidase (GPx), peroxiredoxins (Prx) and protein disulfide isomerases (PDI) via classical and non-classical secretory pathways. Some redoxins can also associate with the membrane (not shown). Trx1 can be cleaved by a membrane-metalloprotease to a truncated form, named Trx80. These redox molecules can participate in transcellular communication and autocrine, endocrine and paracrine signal transduction, e.g. by catalyzing dithiol-disulfide exchange reactions or disulfide isomerization of specific thiol switches in the ectodomains of membrane proteins or independent of their redox activity.
Extracellular redox-modifying enzymes.
| Redoxin | Mode of release | Cell type | Biological function | Reference |
|---|---|---|---|---|
| Grx1 | Unknown, alternative secretory pathway | PBMCs | Unknown | Lundberg et al. (2004) |
| PDIA1,3, 6 | Classical secretory pathway | Platelets | Thrombosis | Araujo et al. (2017), Bartels et al. (2019), Jasuja et al. (2010), Sharda et al. (2015), Thon et al. (2012) |
| Prxs | Prx1,2: Exosomal release | Macrophages, HEK297 | DAMP/PAMP, (membrane-associated) peroxidase, chaperone | Fujii and Ikeda (2002), Mullen et al. (2015), Zhao et al. (2016) |
| Prx4: Classical secretory pathway | ||||
| Trx1 | Leaderless, alternative secretory pathway; extracellular vesicles | Fibroblasts, epithelial cells, immune cells (macrophages, B- and T-cells), hepatocytes, cancer cells | Immunomodulatory functions, signal transduction, proliferation, adhsesion, morphology, regulation of membrane proteins | Bergerhausen et al. (2020), Rubartelli et al. (1992), Rubartelli et al. (1995), Schwertassek et al. (2007), Xu et al. (2008) |
| Truncated Trx1 (Trx80) | Trx1 is cleaved by ADAM10 and ADAM17; released as truncated form Trx80 | PBMCs | Redox-inactive cytokine | Gil-Bea et al. (2012)), Pekkari and Holmgren (2004) |
| TrxR1 | Classical secretory pathway | PBMCs, monocytes, cancer cells | Active in human plasma, reduction of Trx1, potential functions in the immune response and cancer progression | Soderberg et al. (2000) |
Advanced technologies related to specific ex vivo and in vivo redox sensors and redox proteomics broadened our knowledge of thiol switches, their existence and effects on cellular functions and oxidoreductase activity (Lukyanov and Belousov 2014; Staudacher et al. 2018; Yang et al. 2016). So far, many thiol switches have been described in different species such as bacteria, yeast, plants, vertebrates and mammals. These and other thiol switches are assigned to different cellular compartments such as the nucleus, endoplasmic reticulum (ER), mitochondria and also the extracellular space. Even though there are still limitations for redox proteomic approaches, the analysis of membrane proteins and also the analysis of complex biological samples to identify e.g. changes between physiology and pathology is continuously advancing (Duan et al. 2017; Ruiz-May et al. 2019). Here, we focus on extracellular thiol switches in vertebrates, which act as switches in many biological processes, but have hardly been characterized so far.
Extracellular redoxins and thiol switches and their biological functions
We and others have shown that different cell types, such as endothelial cells, specialized cells like immune cells, hepatocytes and pancreatic β cells, as well as cancer cells, secrete redox proteins that can be altered in response to changes in the cellular environment and have been linked to different biological functions (Table 1). Trx1 is the best-studied extracellular oxidoreductase that was shown to be present as a full-length, redox-active and a truncated, redox-inactive form (Trx80) with cytokine- and chemokine like functions. Trx80 contains the Trp-Cys-Gly-Pro-Cys active site motif, exists as a dimer and lacks the C-terminal part that is essential for protein interaction. In fact, Trx80 cannot bind to TrxR and was shown to be redox-inactive in the insulin assay (Pekkari et al. 2000). Interestingly, Trx1 is processed by A Disintegrin And Metalloprotease (ADAM) 17 that is known to cleave other proteins in order to release active signaling molecules such as cytokines (Gil-Bea et al. 2012; Hanschmann et al. 2013; Hanschmann et al. 2020; Tanaka et al. 2020).
Trx1, and also Grx1 that was shown to be secreted as well, lack a signal peptide and are released by an alternative secretory pathway. Grx1 is reduced by GSH. The tripeptide can be released from cells by specific transporters such as multidrug resistance proteins (MRPs). Intracellular GSH-levels are in the millimolar range, whereas the extracellular levels range between 2 and 20 µM (Jones 2002). However, these levels depend on the cell type and can vary in the supernatant of cells and in biological fluids. In fact, the concentrations at the cell membrane and in closeness, proximity to cells could differ depending on the microenvironment of the cell. The same is true for cysteine/cystine. This couple is also present in the extracellular space in concentrations lower than GSH. Extracellular redox molecules are known to affect signal transduction and cellular functions (Jones 2006). They are released by epithelial cells directionally, resulting in different redox environments at their apical and basal cell membranes (Mannery et al. 2010). Moreover, cells can provide glutathione or glutathione precursors for other cells, such as the support of GSH for neurons by astrocytes (Wang and Cynader 2000).
Trx is reduced by TrxR, which is also present in the extracellular space. TrxR is released by human monocytes via the classical secretory pathway. TrxR release can be induced by different stimuli, including sodium selenite, LPS and cytokines (Soderberg et al. 2000). Since TrxR is a NADPH-dependent enzyme, it is not known, if its enzymatic function can be recovered in the extracellular space. However, there is evidence for redox systems that can deliver reducing equivalents across the plasma membrane to the cell surface (Ly et al. 2003). In addition, endothelial microvesicles have the capacity to produce NADPH (Bodega et al. 2018). The cargo of extracellular vesicles contains various redox proteins. Thus, Prx1 and 2 are released by macrophages via exosomes (Mullen et al. 2015). Extracellular functions include their general functions as peroxidases and chaperones, as well as the specific function as damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) by binding and activating the TLR4 receptor, independent of their redox activity. The same is true for Prx4 (Zhao et al. 2016). Prx4 and also PDIs contain a signal peptide and are released via the classical secretory pathway. Various PDIs, PDIA1, PDIA3 and PDIA6, are transported constitutively to the cell surface via the KDEL-receptor 1 (KDELR1) (Bartels et al. 2019). Unlike KDELR2, 3a and 3b, the KDELR1 prefers the KDEL sequence over the HDEL sequence (Raykhel et al. 2007). The different selectivity likely correlates with the distinct function of the receptors. The function of KDELR1 is to transport cargo proteins bearing a KDEL motif, such as the above mentioned PDIs, to the cell surface with a higher preference than ER-resident proteins carrying an HDEL sequence. Besides the constitutive transport, the anterograde transport of KDELR1 can be induced in different physiological and pathophysiological situations by src-dependent signaling (Giannotta et al. 2012; Pulvirenti et al. 2008) In addition to the Golgi-passing KDELR1-dependent route, PDIs may leave the cell via ER in a Golgi-independent manner (Araujo et al. 2017). The selected secretion path may depend on whether, in a localized response, PDIs remain associated with the cell surface, or whether secretion of the PDIs is required to achieve a broader response. Other members of the Trx family reportedly associate to the membrane, such as Trx, TrxR, Prxs and GPx3. Interestingly, the latter two were predicted to function as membrane-associated peroxidases (Fujii and Ikeda 2002; Sasagawa et al. 2001; Soderberg et al. 2000; Szabo-Taylor et al. 2012).
Although extracellular redox regulation has been underestimated, there is increasing evidence that secreted members of the Trx family of proteins are involved in the specific regulation of extracellular thiol switches, functioning in autocrine, paracrine and endocrine signaling (Figure 1). Potential extracellular substrates, which serve as targets of a redox modification, are soluble pericellular proteins and ectodomains of transmembrane proteins. Redox changes in soluble proteins alter their conformation and function in biological processes such as cell signaling, adhesion, migration, coagulation and inflammation. Examples include albumin (Anraku et al. 2013; Matsuyama et al. 2009), β defensins (Jaeger et al. 2013; Schroeder et al. 2011; Taylor et al. 2008), β2 glycoprotein I (Ioannou et al. 2010; Passam et al. 2010), HMGB1 (Hoppe et al. 2006; Janko et al. 2014), MIF (Son et al. 2009) and transglutaminase (Eckert et al. 2014; Iversen et al. 2014; Kiraly et al. 2011; Stamnaes et al. 2010). Remarkably, also secreted proteins are regulated by oxidative modifications. HMGB1, for instance, contains a regulatory thiol switch that controls translocation and release (Kwak et al. 2019). Moreover, it was shown that macrophages secrete oxidized and glutathionylated proteins in the presence of LPS, including Prx1 and 2. Mutating the catalytic Cys residues of Prxs inhibits their release (Checconi et al. 2015). In addition, ectodomains of certain membrane proteins also possess regulatory cysteine residues, which are the focus of this review article (Table 2).
Examples of transmembrane proteins with an identified thiol switch in the ectodomain.
| Transmembrane protein | Thiol switch/ modification | Redox protein | Biological effect | Reference |
|---|---|---|---|---|
| ADAM17 | Disulfide isomerisation | PDIA1, 3, 6 | Inactivation of shedding activity | Düsterhöft et al. (2013), Krossa et al. (2018), Willems et al. (2010) |
| Integrins: α4β1, α4β7, α7β1; αIIbβ3 | Disulfide bridge formation/cleavage | Trx1, PDIA6 | ECM-ligand binding, binding force, adhesion and migration | Bergerhausen et al. (2020), Laragione et al. (2003), Mor-Cohen (2016), Passam et al. (2018), Zhang et al. (2013) |
| MHC class I polypeptide–related sequence A/B | Mixed disulfide | PDIA6 | Accessibility for shedding by the metalloprotease ADAM17 and its close relative ADAM10, protection of tumor cells from cytolytic cell death | Boutet et al. (2009)), Huergo-Zapico et al. (2012), Kaiser et al. (2007), Kohga et al. (2009), Waldhauer et al. (2008) |
| TRP channel proteins: e.g. TRPC5 | Cysteines 553 and 558 form intramolecular disulfide and can be nitrosylated (in TRPC5, TRPC1 and possibly TRPC4. | Trx1 | Sensor for changes in the redox environment (e.g. NO sensors), regulation of ion flux and related cellular functions, signal transduction and protein secretion example: Reduction of intramolecular disulfides within TRPC5 homomultimers- or TRPC5-TRPC1 heteromultimers activates the channel. Nitrosylation of Cys553 and 558 activates the channels. | Takahashi and Mori (2011)), Xu et al. (2008), Yoshida et al. (2006) |
| Tumor necrosis factor receptor superfamily member 8 | Disulfide exchange | Trx1 | Conformational change affects the CD30 receptor–ligand interaction; Trx1-mediated reduction prevents ligand binding and therefore interrupts signal transduction | Schwertassek et al. (2007) |
Thiol switches in ectodomains of membrane proteins
Transmembrane proteins contain specific thiol switches in their ectodomains, which are part of signal transduction and the cellular response to specific signals and changes in the cellular microenvironment. Albeit described in integral membrane proteins, thiol switches most likely are relevant also for peripheral membrane proteins. However, the number of identified transmembrane proteins with a thiol switch in their ectodomains is so far limited (Table 2). Various membrane proteins such as ion channels and enzymes were shown to be sensitive to oxidation. Here, we will focus on the best characterized examples and describe how these thiol switches in the ectodomains of transmembrane proteins mechanistically rule signal transduction and biological function.
Examples include transient receptor potential (TRP) channels which are homo- or hetero-tetramers of putative six-transmembrane subunits. The mammalian TRP channels comprise six subfamilies of proteins: TRPC, TRPV, TRPM, TRPA, TRPP and TRPML. TRP channels are sensitive to changes in microenvironment including heat, osmotic pressure, mechanical force and changes in the redox environment of cells. They control signal transduction and cellular functions by regulated transport of divalent cations such as Ca2+. As many other ion channels, TRP channels have been shown to be sensitive to oxidation by hydrogen peroxide and nitric oxide (Sakaguchi and Mori. 2020). A conserved pair of cysteines (Cys 554 and 558) within an extracellular loop next to the ion-selectivity filter was found in TRPC1, 4 and 5 which forms a disulfide bridge. Upon reduction of the disulfide by extracellular Trx1, TRPC5 homotetramers or heterotetramers with TRPC1 are activated. Interestingly, these Cys residues were also shown to undergo nitrosylation (Sakaguchi and Mori. 2020; Xu et al. 2008).
The tumor necrosis factor receptor superfamily member 8 (TNFRSF8/CD30) is regulated by disulfide formation/ reduction. The mechanistic thiol switch in the transmembrane protein determines its conformation and function. Even though the involved Cys residues were not identified, so far, the authors show that the regulatory thiol switch is located in the ectodomain of the receptor. Following reduction by extracellular Trx1, TNFRSF8 cannot bind to its ligand CD30L or agonistic antibodies, any longer. This thiol switch regulates signal transduction and cell communication and is important in the inflammatory response (Schwertassek et al. 2007).
Extracellular PDIs promote viral infections and toxicity
Already in the 1990s, inhibitory PDI antibodies and PDI inhibitors revealed the influence of extracellular PDI on the entry and cytotoxicity of diphtheria toxin (DT) and immunodeficiency virus (HIV) (Mandel et al. 1993; Ryser et al. 1994). The reductive catalytic activity of the PDI is required to separate the disulfide bound heterodimer of DT and reduce disulfide bridges in the viral protein gp120, both of which are prerequisites for the entry of the respective pathogen (Fenouillet et al. 2001). Due to the interaction of gp120 with the T-cell receptor CD4, which interacts with the PDI, there is a spatial proximity of gp120 and the PDI. The reduction of gp120 induces a structural change that leads to the fusiogenic activity of the viral membrane protein gp41(Gallina et al. 2002; Mandel et al. 1993; Markovic et al. 2004; Ryser et al. 2005; Ryser et al. 1994). To date, the influence of extracellular PDIs on the toxicity and entry of viruses is well known. The reduction of disulfide bridges between the A and B units of DT serves to separate them and is a prerequisite for the entry of the A subunit into the cytosol. Therefore, no actual thiol switch is suspected in this process. This is in contrast to the reduction of the two disulfide bridges in gp120 ectodomain (Barbouche et al. 2003). Here, a rearrangement and the associated formation of new disulfide bridges are to be assumed. In this case, no free or less than four thiol groups per gp120 ectodomain should be detectable after the thiol switch. This assumption requires further validation and detailed structural analysis. Interestingly, pathogens stimulate the translocation of PDIs, such as PDIA1 and PDIA3, from the ER to the cell surface to promote penetration (Lasecka and Baron 2014; Wan et al. 2012). Dengue virus infection accumulates PDIA1 in lipid raft regions, resulting in an increase of active β1 and β3 integrin. This complex is associated with a viral protein that helps the virus enter the cell (Wan et al. 2012). Both dengue virus and HIV infection induce increased secretion of lectin-galectin-9 (Chagan-Yasutan et al. 2013; Dapat et al. 2017; Hsu et al. 2015; Tandon et al. 2014), which promotes cell surface association of PDIA1, PDIA3 and PDIA6, thereby increasing viral entry and activation of β3 integrin (Bi et al. 2011). Being a lectin, exogenous galectin-9 promotes the cell surface association of PDIA1 through its O-glycosylation (Schaefer et al. 2017).
Integrins are regulated by specific thiol switches and dithiol-disulfide exchange
Integrins are the principal class of cell adhesion receptors that mediate interaction between the cell and the extracellular matrix (ECM) or between different cells (Zheng and Leftheris 2020). They not only anchor the cell to their extracellular ligand, but also convey environmental signals into cells (Humphries et al. 2019). Integrins consist of two non-covalently associated, glycoconjugated subunits, α and β each with characteristic domains (Figure 2A). Their molecular structure and their conformation was solved by protein crystallography, high resolution electron microscopy, and conformation-dependent antibodies (Adair et al. 2013; Byron et al. 2009; Xie et al. 2010; Xiong et al. 2009; Zhu et al. 2008). Both integrin ectodomains are rich in cysteine-residues (Calvete et al. 1991; Krokhin et al. 2003) with characteristic intradomain disulfide patterns, e.g., within the EGF-domains of β subunit leg domain (Mor-Cohen 2016), but also a long-range disulfide bridge within the upper leg domains of the β subunit (Calvete et al. 1991; Krokhin et al. 2003; Mor-Cohen 2016). However, the redox status of some cysteine residues and pairs of cysteine-residues have not been defined yet (Zhu et al. 2008).
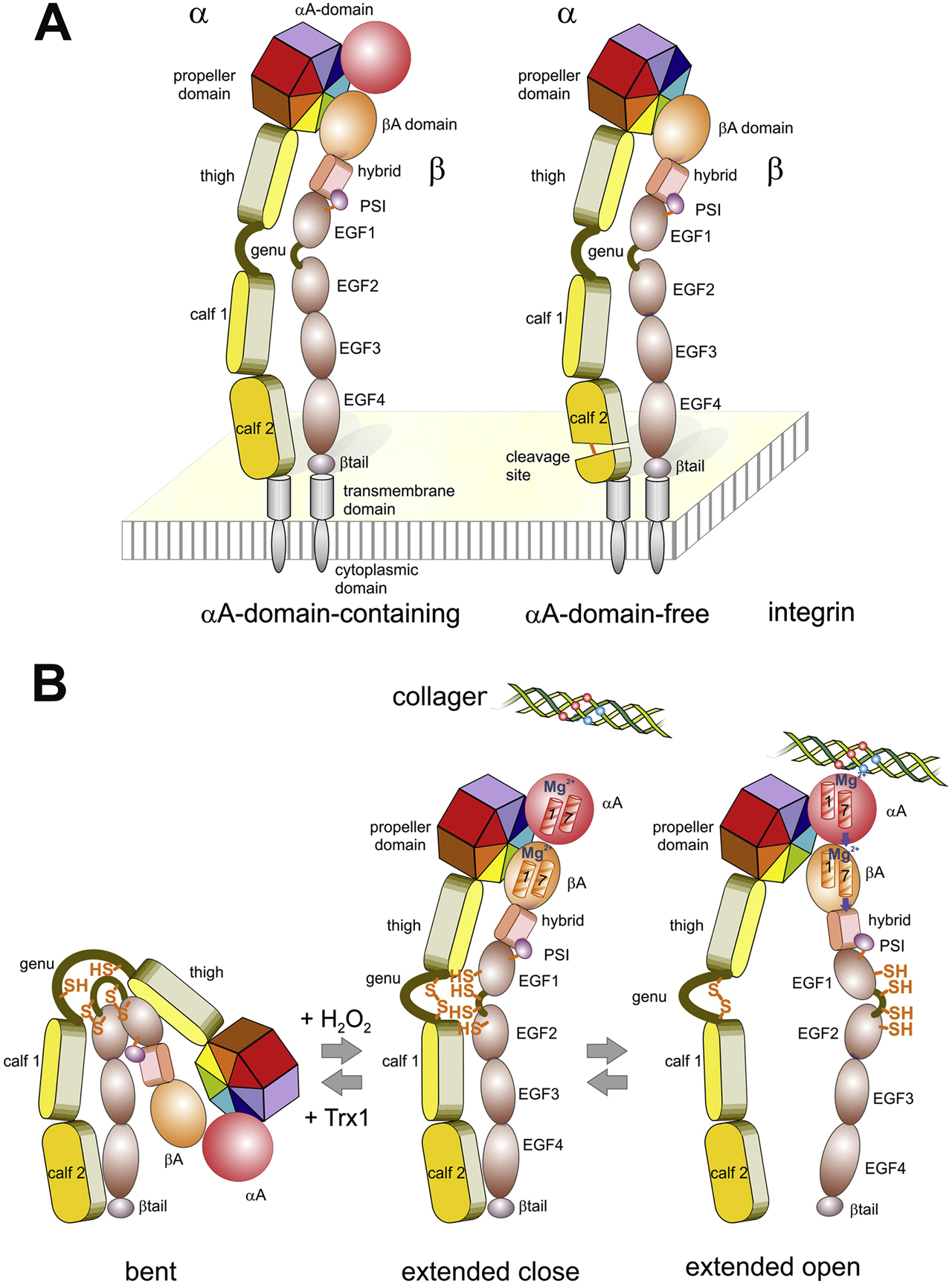
Domain structure (A) and conformation changes within the ectodomains (B) of integrins. (A) Integrins consists of two non-covalently associated glycoprotein subunits, α and β. In both subunits, several different domains form the ectodomain, which is anchored by a single span α-helical transmembrane domain with a C-terminally adjacent cytoplasmic domain. Depending on the presence of an additional A-domain within the α-subunit, they are divided into subsets of αA domain-containing (left) and αA domain-free (right) integrins. The latter are proteolytically cleaved within the calf 2 domain of the α-chain. (B) The ectodomains undergoes changes between a bent, extended close and extended open conformation, which influences ligand binding. Vice versa, the conformational changes are regulated by ligand binding, intracellular signaling molecules, divalent cations and redox-modifiable pairs of cysteines. These potential thiol switches are indicated within the α hinge domain and within the EFG1 and EGF2-domain of the β-chain. Oxidation/closing of the α hinge thiol switch with H2O2 is connected with ectodomains extension, while Trx1 reduces the disulfide and converts the integrin from the extended to bent conformation (Bergerhausen et al. 2020). The effect of oxidation/reduction of the thiol switches of the β-chains seem to depend on the respective disulfide bridge (Zhang et al. 2013). During the conversion from the extended close to the extended open conformation, the legs of both subunits segregate. This conformational change is triggered by a ligand-induced shift of α helices 1 and 7 within the α-subunit A-domain (αA), and subsequently within the βA domain, which make the hybrid-PSI and EGF1-domain swing out. In αA-domain-free integrins, the ECM ligand directly interacts with the βA-domain and triggers a similar movement of its α helices 1 and 7.
Integrins occur in three major conformations, i.e., bent, extended close and extended open, which are interconvertible in response to different factors and correlate with ligand binding activity and signal transduction (Arnaout et al. 2005; Shattil et al. 2010). Two distinct molecular movement within the integrin heterodimer underlie the conformational equilibria. First, the entire ectodomains extends like a swing-blade knife, as the globular head with the upper leg portions pivots around the hinge regions against the lower leg portions, which are anchored in the cell membrane (Moore et al. 2018; Xie et al. 2004). Secondly, the conversion from the extended close to the extended open conformation separates the two legs as the upper leg portion of the β subunit swings out from the compact, extended close conformation (Luo and Springer 2006) (Figure 2B). The latter conformational movements are mechanistically triggered by binding of an ECM ligand to the A-domain of the α-subunit within the globular head of integrins, which ensues a piston movement of two α-helices against each other within the α-subunit A domain (Emsley et al. 2000). In those integrins lacking an A-domain within their α-subunits (αA-domain free integrins in Figure 2), the ECM ligand directly binds to the A-domain of the β-subunit (βA-domain), thereby eliciting a similar piston movement of two α-helices against each other (Alonso et al. 2002; Yang et al. 2004). Thus, either directly or indirectly via the α-subunit A-domain, the information of ECM ligand binding is conformationally passed through the entire protein and eventually induces separation of the legs (Gupta et al. 2007; Li et al. 2017a; Zhu et al. 2013) and of the transmembrane and cytosolic tails (Arnaout et al. 2005; Shattil et al. 2010). The conformational transition from bent via extended close to extended open is an energy-demanding process (Li et al. 2017a). It goes along with an increase in ligand binding affinities (Li et al. 2017b; Zhu et al. 2013). Moreover, via these conformational changes, integrins convey a signal into the cell, which is accompanied by corralling of several activated integrins and by recruitment of adaptor proteins into a highly organized cell organelle termed adhesome (Zaidel-Bar et al. 2010). Conformational changes of integrins are caused by different factors, such as extracellular and intracellular ligands, mechanical forces, divalent cations and redox-active compounds (Li et al. 2017a; Sun et al. 2019; Zhang et al. 2012). One of the most recently discovered effectors of integrin binding activity are hydrogen peroxide and likely other reactive oxygen species (Eble and de Rezende 2014; Sies et al. 2020). With respect to cell adhesion and migration, vascular smooth muscle cells transiently produce a pulse of hydrogen peroxide locally and thus form sites of high redox potential and of local oxidative protein modifications, termed redox hot spots, at sites of adhesion (de Rezende et al. 2012). NADPH oxidase (Nox) isoforms, preferentially Nox4, are recruited to these local cellular protrusions, where also integrins gather to form adhesive contacts with the ECM (Figure 3). Due to their abundant number in integrins, cysteine residues were studied as potential targets of oxidation. In fact, several cysteine residues were identified to be oxidized to sulfenic acid residues or disulfide bridges by hydrogen peroxide (de Rezende et al. 2012). Moreover, several disulfide bonds between cysteine pairs, especially between vicinal cysteines (Manickam et al. 2011), were pinpointed for redox regulation of integrins (Bergerhausen et al. 2020; Hu and Luo 2018; Mor-Cohen et al. 2012; Passam et al. 2018). Disulfide bridges within integrins may serve either structural or regulatory functions. Whereas the structure-stabilizing cysteine bonds, such as the long range disulfide bridge within the β-subunit, are less accessible and buried within the protein fold, regulatory disulfide bridges are more accessible to redox enzymes and may reversibly split and re-form by reduction and oxidation, respectively (Mor-Cohen 2016). These redox-modifiable disulfide bridges can act as thiol switches, which alter the conformation and ligand binding activity of integrins (Bergerhausen et al. 2020; Metcalfe et al. 2011).
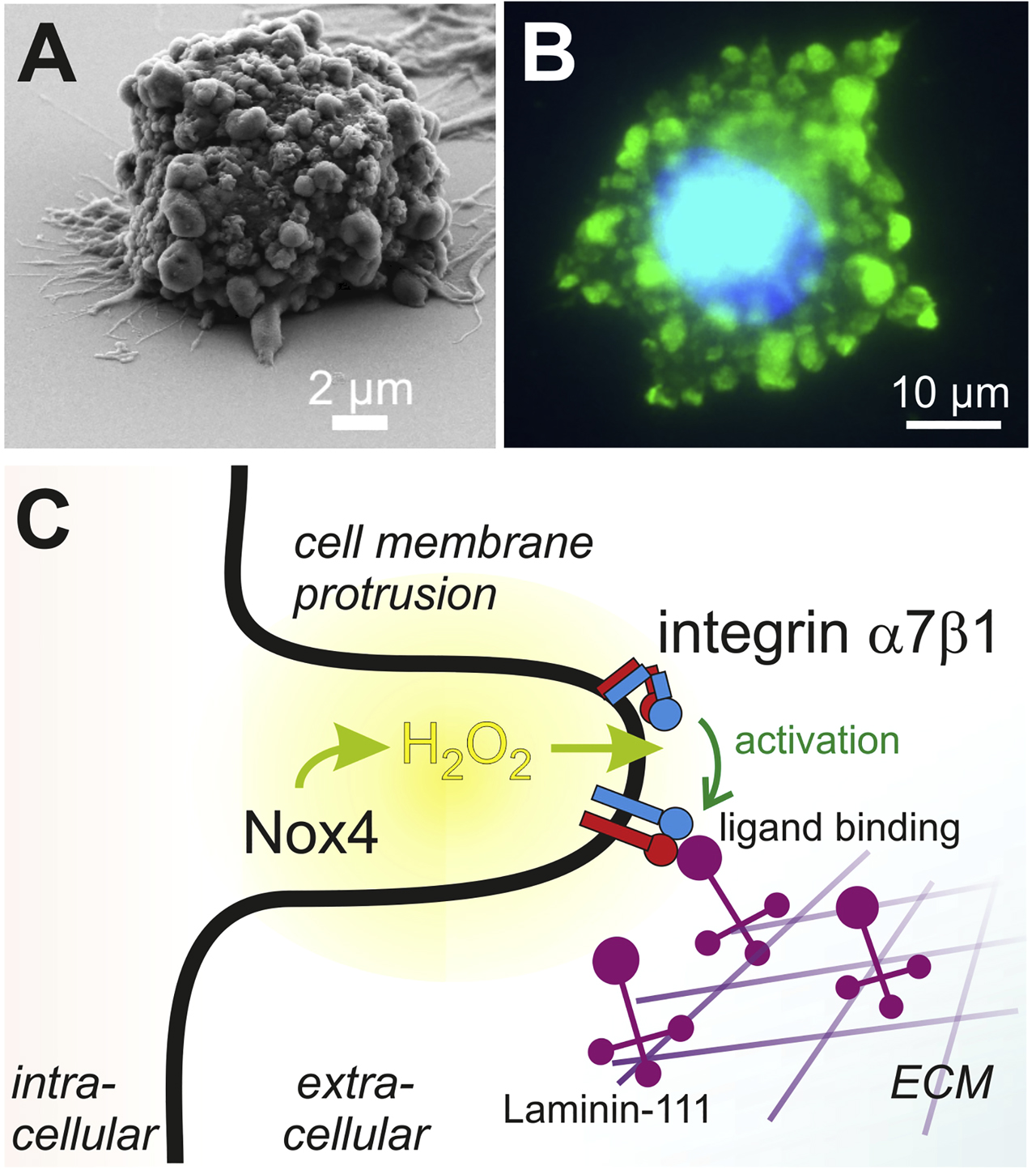
During cell adhesion to the ECM, cells form membrane protrusions in which ROS are produced, thereby redox-regulating integrin-mediating cell adhesion. (A) During adhesion to a laminin-111-coated surface, vascular smooth muscle cells form cell protrusion to get in contact to the substratum, as visualized by scanning electron microscopy. (B) At the site of cellular protrusions, proteins are reversibly and transiently oxidized at their cysteines into cysteine sulfenic acid, which after modification with dimedon is detected by immunofluorescence with a green fluorophore. The nucleus is stained in blue. These locally concentrated sites of higher redox potential were called redox hot spots. (C) Scheme illustrating the underlying potential mechanism of redox hot spots in cell protrusions and promoting integrin-mediated cell attachment to the ECM. Upon initial contact of cells to the ECM, cell protrusions are formed and ROS-generating molecules, such as the H2O2-producing Nox4 is recruited. Being cell membrane-permeable, H2O2 oxidizes the α hinge thiol switch and thus activates α7β1 integrin by conversion into its extended conformation. This rearrangement increases integrin binding activity towards its ECM ligand, laminin-111 and reinforces cell attachment, required for adhesion and migration. This figure is adapted from (de Rezende et al. 2012).
The integrins, α4β1, αMβ2, and αIIbβ3, were among the first redox-regulated integrins to be discovered, with consequences on leukocyte integrin activation and platelet aggregation (Blouin et al. 1999; Laragione et al. 2003; Liu et al. 2008; Murphy et al. 2014; Yan and Smith 2000). Many of these studies revealed re-shuffling as well as reducing and reforming of disulfide bonds within the EGF domains of the cysteine-rich integrin β chain (Hu and Luo 2018; Mor-Cohen et al. 2012). The corresponding cysteine-residues are surface-exposed and thus accessible (Mor-Cohen 2016). The disulfide pattern of the PSI domain was even hypothesized to act as PDI (Zhu et al. 2017). However, it neither shares the characteristic Trx-fold, nor does it contain the characteristic Cys-X-X-Cys active site motif (Weichsel et al. 1996). Passam and coworkers identified the first cysteine pair within the βA-domain of αIIbβ3 as a thiol switch, which is redox-regulated in a binding force-dependent manner (Passam et al. 2018). Thereafter, this cysteine pair is accessible to and modified by PDIA6 only if the integrin binds to fibrin and transmits mechanical forces. Then, however, ligand binding strength of the integrin is reduced. In contrast to the integrin β subunits, the α chains have been mapped less intensely for redox-modifiable cysteine residues, likely due to their lower number of Cys residues. However, a redox proteomics study on α7β1 integrin revealed that hydrogen peroxide selectively oxidized 6 Cys-residues of the α7 subunit: two in the α hinge domain and four cysteine-residues in the calf 2 domain (de Rezende et al. 2012). The cysteine pair within the α hinge region is accessible (Mor-Cohen 2016) and is in closeness, proximity to a divalent cation binding site, which reportedly mediates the extension of the bent to the extended conformation of the integrin (Xie et al. 2004). This thiol switch within the hinge region of the α subunit seems to be the dominant thiol switch that upon oxidation into a disulfide bridge results in conformational extension and hence activation of the integrin (Bergerhausen et al. 2020). In addition, the two cysteine pairs flanking the hinge region of the β subunit, the most C-terminal disulfide bridge of the EGF1 domain (Hu and Luo 2018) and the most N-terminal disulfide bridge of the EGF2 domain (Mor-Cohen et al. 2012; Zhang et al. 2013) (Figure 2B), also influence ligand binding, albeit with an activating effect if mutated into non-oxidizable residues (Zhang et al. 2013). Thus, integrins are potential substrates of extracellular redox-modifying enzymes.
Enzymes that redox-modify integrins are PDIs and redoxins, as described for α2β1 (Lahav et al. 2003) and αIIbβ3 integrin (Essex and Wu. 2018) on platelets, for α11β1 on fibroblasts (Popielarski et al. 2018), and for αMβ2 integrin on neutrophil granulocytes (Hahm et al. 2013). Each of the different PDIs plays a non-compensatible role (Essex and Wu. 2018), physically interacts with the respective integrin (Essex and Wu. 2018; Popielarski et al. 2018), and influences integrin ligand binding positively (Essex and Wu. 2018; Hahm et al. 2013) or negatively (Passam et al. 2018). Another redox-modifying enzyme, Trx1, was also shown to act as extracellular effector on α7β1-mediated cell migration by cleaving the disulfide within the α-subunit hinge region (Bergerhausen et al. 2020). As Trx1 reverts the oxidated thiol switch within the α hinge, which represents the activate integrin conformation, it is conceivable that integrins via their cysteine pairs are targets also for other redox-modifying enzymes on the cell surface and the pericellular space (Hanschmann et al. 2013; Tanaka et al. 2020). Being part of a regulatory redox circuit, integrins and their related cellular functions are redox-regulated by cells via the extracellular ratio of relevant reducing and oxidizing agents and the presence of the respective redox-enzymes.
The thiol switch in a disintegrin and metalloprotease 17 regulates protein conformation and activity
ADAM17 is a type I transmembrane protein with a proteolytically active ectodomain, catalyzing pericellular proteolysis and shedding of membrane proteins. Due to its broad substrate spectrum, ADAM17 is crucial for numerous biological processes such as development, regeneration and immune defence. The increased, non-physiological release of its substrates such as tumour necrosis factor (TNFα) and soluble interleukin-6 receptor (sIL-6R) or epidermal growth factor receptor (EGFR) ligands through erroneous activation of ADAM17 is associated with autoimmune diseases, chronic inflammation, and cancer development and progression (Black et al. 1997; Düsterhöft et al. 2019; Ford et al. 1990; Moss et al. 1997; Müllberg et al. 1993; Peschon et al. 1998). EGFR ligands are expressed as inactive transmembrane precursors and must be cleaved from the membrane to become soluble and active growth factors (Blobel 2005; Peschon et al. 1998). The release of TNFα and sIL-6R converts membrane-bound molecules into pro-inflammatory mediators. Being inactive on resting cells, activation of ADAM17 is a prerequisite for its shedding activity, and is part of complex regulation processes, including redox-dependent disulfide bridge reshuffling. A detailed description of its structure, control and activation is excellently presented in previous review articles (Düsterhöft et al. 2019; Grötzinger et al. 2017; Rose-John 2012). Therefore, this article mainly describes the structural changes of ADAM17 that led to its activation and inactivation, as this is controlled by a thiol switch. These changes occur in its ectodomain, which consist at the cell surface of its metalloprotease domain, a disintegrin domain, a membrane-proximal domain (MPD) and a highly conserved adjacent helical stem region, called Conserved ADAM 17 Interaction Sequence (CANDIS). The MPD and CANDIS together act as the key control unit of ADAM17 and are involved in substrate recognition, membrane interaction and inactivation (Düsterhöft et al. 2014; Düsterhöft et al. 2013; Düsterhöft et al. 2015; Lorenzen et al. 2012; Lorenzen et al. 2011; Reddy et al. 2000; Sommer et al. 2016; Veit et al. 2019). The disintegrin domain orients the ectodomain into a C-like structure, which is essential for the cleavage of substrates by the N-terminal metalloprotease domain adjacent to the membrane (Janes et al. 2005). In dormant cells ADAM17 is inactive. The metalloprotease domain of ADAM17 is too far away from the plasma membrane (Düsterhöft et al. 2020). Activation and structural rearrangement can be achieved by a variety of molecules such as thrombin, histamine or even growth factors (D’Alessio et al. 2012; Le Gall et al. 2010; Prenzel et al. 1999). As a result, phosphatidylserine (PS) is flipped from the inner leaflet to the outer leaflet of the plasma membrane (Figure 4A). The head groups of PS interact with the MPD (Sommer et al. 2016). This interaction makes the extracellular part point downwards and localizes the catalytic domain in closeness, proximity to the plasma membrane where the cleavage sites of their substrates are located (Düsterhöft et al. 2020; Grötzinger et al. 2017), thereby enabling shedding. To cancel the activity of ADAM17 and prevent the pathological release of its substrates, the protease could be degraded endocytotically (Dombernowsky et al. 2015; Lorenzen et al. 2016) and/or a thiol switch could cancel the substrate recognition and membrane interaction. The latter is mechanistically implemented by the isomerization of two disulfide bridges within the MPD and catalyzed by PDI (Figure 4) (Bennett et al. 2000; Düsterhöft et al. 2013; Wang et al. 2009; Willems et al. 2010). MPD, and thus the entire extracellular part, exist in two conformations that control the activity of the protease (Düsterhöft et al. 2013). In the activatable conformation, the MPD is flexible and open (opMPD) and consists of a linear arrangement of two disulfide bridges (Figure 4C) (C600–C630 and C635–C640). This structure allows interaction with substrates and with the PS head groups in the plasma membrane, which is essential for activation (Düsterhöft et al. 2014; Sommer et al. 2016). During inactivation, PDIs isomerize these disulfide bridges into an overlapping connection order (C600–C635 and C630–C640). The resulting structural change leads to a tight, compact structure that cancels the interaction with the membrane and substrates and thus the activity of ADAM17 (Figure 4).
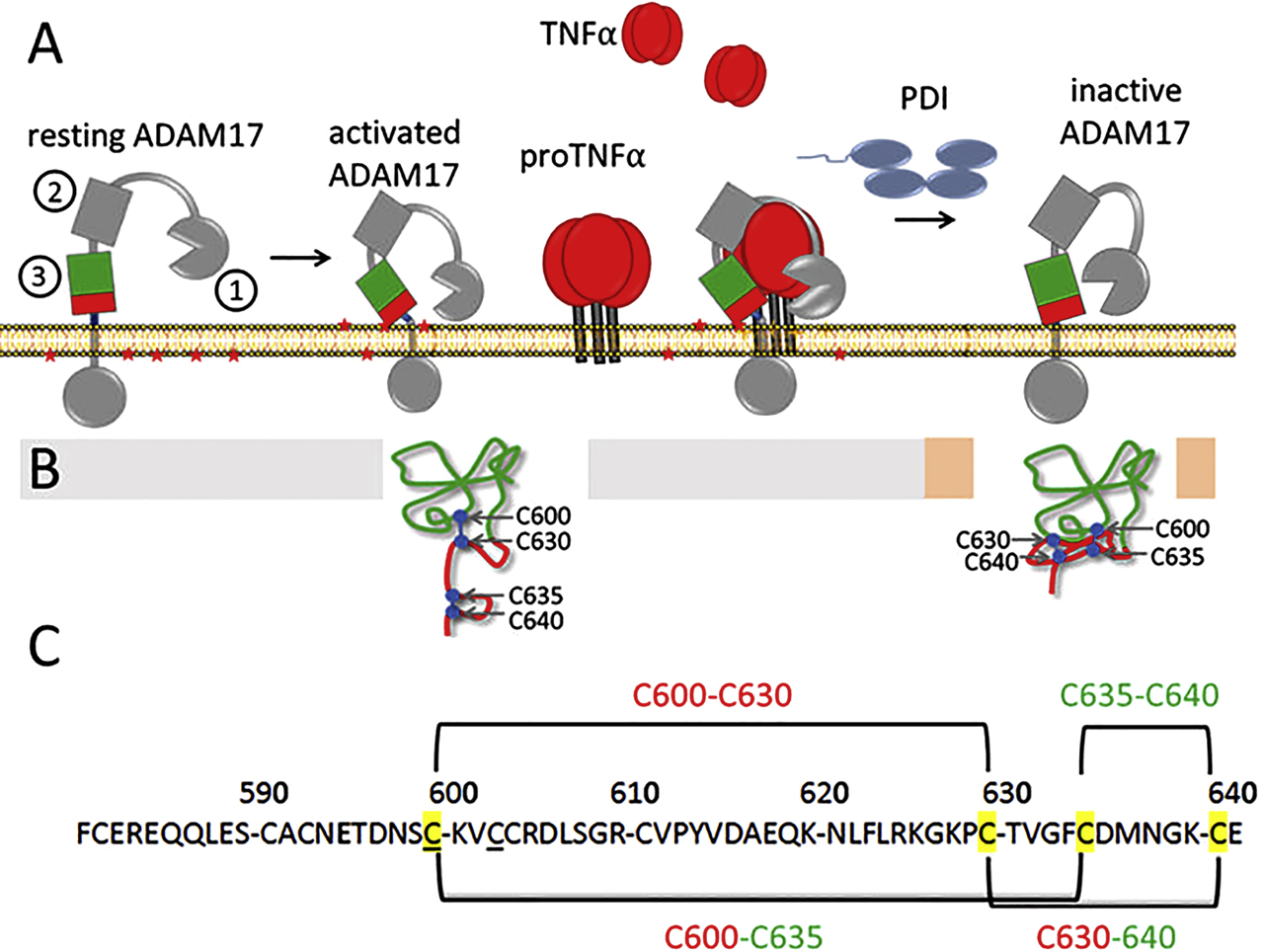
The activity of ADAM17 depends on its disulfide pattern. (A) ADAM17 is inactive in resting cells. Activation leads to exposure of phosphatidylserine (PS) head groups (red stars) that interact with the membrane-proximal domain (MPD) of ADAM17, The extracellular domains of ADAM17 on the cell surface are marked as follows: 1: Metalloprotease domain, 2: Disintegrin domain, 3: MPD, CANDIS is stained blue and is located between the MPD and the plasma membrane. This interaction moves the catalytic domain towards the membrane where the cleavage sites of their substrates are located. ADAM17 is regulated via two disulfide bonds. PDIs catalyze the thiol switch, resulting in a structural change that cancels the interaction with PS and substrates and inactivates the metalloprotease. (B) Structure of MPD in its open conformation existing in dormant activatable and catalytically active ADAM17 and the structure of closed MPD of inactivated ADAM17. (C) Shown is the pattern of the two disulfide bridges constituting the thiol switch. In the open MPD, the two disulfide bridges are arranged in a linear pattern, so that the C-terminal part is flexible and can interact with PS and substrates. The thiol switch leads to isomerization in a superimposed arrangement, resulting in a compact structure with a hidden PS binding motif.
Several PDIs, such as PDIA1, PDIA3, PDIA4 and PDIA6, are located on the cell surface, and PDIA1 and PDIA6 can catalyze the extracellular thiol switch in ADAM17 (Kim et al. 2017; Willems et al. 2010). Recombinant PDIA1, PDIA3 and PDIA6 do not show any catalytic preference for the thiol switch in ADAM17 and have comparable affinities to MPD and the entire extracellular part of ADAM17 (Krossa et al. 2018). Interestingly, the dissociation constants KD of 1–8 µM are comparable to those of PDIA1 and β3-integrin (KD 1 µM) and PDIA6 and β3-integrin (KD 21 µM) (Cho et al. 2012; Passam et al. 2015). In the presence of integrin-activating Mn2+ ions, higher affinities were found for PDIA1 and αII3 integrin with a KD of 0.1–0.018 µM, which are comparable to those of PDIA1 and αIIβ3 integrin (Swiatkowska et al. 2008; Wang et al. 2019). This finding implies that the activation of integrins increases their affinities to PDIs, probably due to the formation of new interaction sites (Swiatkowska et al. 2008; Wang et al. 2019). Conspicuously, the affinities towards integrins do not differ between the two conformations, opMPD and clMPD, of the tested PDIs, suggesting that the thiol switch in ADAM17 neither blocked the interaction nor created new interaction sites for PDIs. The affinities are in the range of isomerization processes during protein folding of KD values between 1 and 10 µM (Irvine et al. 2014) and are not as strong as a receptor-ligand interaction, therefore only a temporary interaction between PDIs and ADAM17 can be expected.
Significance and future perspective
Many types of transmembrane proteins were shown to be sensitive to oxidation, including receptors, ion channels and enzymes. The thiol switches in integrins and ADAM17 are among the most extensively described so far, both functionally and structurally. As described above, both proteins undergo a drastic conformational change as a consequence of the reversible oxidation, reduction, and reshuffling of their respective thiol switches catalyzed by members of the Trx family. While PDIs disable ADAM17, their role on the activation of integrins is largely unknown to date.
Many questions regarding regulation of thiol switches, their biological consequences and their impact on autocrine, endocrine and paracrine signal transduction are still open. In the future, additional extracellular thiol switches will be identified and characterized at the molecular and functional level which is needed to gain a comprehensive understanding of cell biological processes and the impact of extracellular redox control. Innovative new technologies are indispensable for this purpose, including better chemical agents to detect more selectively even metastable redox modifications, such as dime done derivatives and click-based detection methods, in combination with redox proteomics and/or redox sensors. Also, many messenger agents, such as hydrogen peroxide, are difficult to monitor as their lifetime and the length of the diffusion path are limited (Sies et al. 2020). Moreover, typical for a messenger compounds, hydrogen peroxide is produced spatially and temporally limited in certain cell areas, the so-called redox hotspots, in a regulated manner (de Rezende et al. 2012). Hence, it will be a huge challenge to determine the hydrogen peroxide content reliably, sensitively and spatially accurately enough to gain a comprehensive and deep insight into its physiological role in redox signaling.
The complexity of how thiol switches are coordinated is far from clear. Deciphering the underlying mechanisms of the regulation of redox-modifying enzymes is still in its beginnings. This includes the coordination of their secretion, their specific localization and thus their recruitment to redox-active sites as in the pericellular space. The complexity is further increased by the regulation through protein-protein interactions in redox-regulated networks. Consequently, not only the localization of the respective proteins is important, but also their accessibility to regulatory events that influence their activity and probably also their stability. It has been shown that this is not only true for substrate-enzyme interactions, as is the case with integrins and Trx1 or ADAM17 and PDIs, but also for the interaction of redox targets with each other, such as integrins and ADAM17. These proteins are part of a redox-dependent regulatory network that influences their accessibility to redox-regulating enzymes and can be specifically modulated by their respective secretion.
The inhibitory integrin-ADAM17-complex, which can increase cell-cell association dissociates when transmembrane proteins are activated (Bax et al. 2004; Gooz et al. 2012; Machado-Pineda et al. 2018; Saha et al. 2010; Trad et al. 2013). Both proteins are translated into the ER. Without a protective protein complex, they would be directly accessible to the PDIs, which are very abundant in these compartments. For ADAM17, protection from the PDIs through interaction with the ER-resident chaperone GRP78 has already been described (Schäfer et al. 2017). Accordingly, a protective protein complex for integrins, during transport through the secretory pathway to the cell surface but also at the cell surface is well conceivable and could be a common principle. In contrast to ADAM17, a redox-mediated activation of α7β1 integrin by Trx1 can be reversed, indicating the principle reversibility of extracellular thiol switches (Bergerhausen et al. 2020). Both targets, as well as other membrane proteins, are remarkably rich in Cys residues, and one of ADAM17’s two Cys-X-X-Cys motifs is part of the thiol switch (Düsterhöft et al. 2013). This motif occurs repeatedly in β integrins. Accordingly, one or more of these motifs in β integrins could also be the target of specific PDIs.
Specific thiol switches in the ectodomains of transmembrane proteins, catalyzed by extracellular redox enzymes, influence a variety of essential biological processes such as the cell-ECM contacts, migration, immune response, coagulation, and wound healing and signal transduction. Thiol switches allow a quick response to changes of the microenvironments. Moreover, cells and tissues of multicellular organisms are able to change their redox milieu substantially. The local concentration of oxygen as major oxidizing agent, its consumption and its conversion into specific reactive species with their even higher redox potential and faster kinetics, as well as the extracellular concentration of reducing agents, such as cysteine, glutathione, and extracellular and/or membrane-bound redoxins constitute parameters that can be regulated by cells. Although not fully understood yet, the secretion of redox-relevant enzymes and redox-relevant agents may allow a subtler regulation than by other known extracellular parameters, such as concentrations and composition of divalent cations, or of the pH. Thus, cells may actively regulate and react to changes in the pericellular milieu with changes in adhesion, migration, anchorage-dependent growth and survival. Interestingly, it was shown that cells respond differently to redox changes in their microenvironment. Extracellular Trx1 for instance was shown to have different/opposite immunomodulatory functions. Moreover, the administration of Trx1 for instance can inhibit or induce the proliferation of different cell types (Rubartelli et al. 1995). As an aberrant secretion of various redox proteins is associated with a number of diseases (Hanschmann et al. 2013), understanding extracellular redox regulation and the influence of specific redox enzymes is of key interest in medicine to obtain a comprehensive overview of physiological and pathophysiological processes.
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: SPP1710: EB 177/14-1SPP1710: HA 8334/2-2SPP1710: LO 1722/1-2
Acknowledgments
The authors want to thank the DFG for the financial support in the framework of the SPP1710 “Dynamics of Thiol-based Redox Switches in Cellular Physiology” under the grant numbers: LO 1722/1–2 to I.L, EB 177/14–1 to J.A.E, and HA 8334/2-2 to E-M.H.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This work was financed by the Deutsche Forschungsgemeinschaft (DFG) under the grant numbers: LO 1722/1-2 to I.L, EB 177/14-1 to J.A.E, and HA 8334/2-2 to E-M.H within the framework of the SPP1710 “Dynamics of Thiol-based Redox Switches in Cellular Physiology”.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Adair, B.D., Xiong, J.P., Alonso, J.L., Hyman, B.T., and Arnaout, M.A. (2013). EM structure of the ectodomain of integrin CD11b/CD18 and localization of its ligand-binding site relative to the plasma membrane. PloS One 8, https://doi.org/10.1371/journal.pone.0057951, e57951.Search in Google Scholar
Alonso, J.L., Essafi, M., Xiong, J.P., Stehle, T., and Arnaout, M.A. (2002). Does the integrin αA domain act as a ligand for its βA domain?. Curr. Biol. 12: R340–342, https://doi.org/10.1016/s0960-9822(02)00852-7.Search in Google Scholar
Anraku, M., Chuang, V.T., Maruyama, T., and Otagiri, M. (2013). Redox properties of serum albumin. Biochim. Biophys. Acta 1830: 5465–5472, https://doi.org/10.1016/j.bbagen.2013.04.036.Search in Google Scholar PubMed
Araujo, T.L.S., Fernandes, C.G., and Laurindo, F.R.M. (2017). Golgi-independent routes support protein disulfide isomerase externalization in vascular smooth muscle cells. Redox Biol 12: 1004–1010, https://doi.org/10.1016/j.redox.2017.04.034.Search in Google Scholar PubMed PubMed Central
Arnaout, M.A., Mahalingam, B., and Xiong, J.P. (2005). Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 21: 381–410, https://doi.org/10.1146/annurev.cellbio.21.090704.151217.Search in Google Scholar PubMed
Barbouche, R., Miquelis, R., Jones, I.M., and Fenouillet, E. (2003). Protein-disulfide isomerase-mediated reduction of two disulfide bonds of HIV envelope glycoprotein 120 occurs post-CXCR4 binding and is required for fusion. J. Biol. Chem. 278: 3131–3136, https://doi.org/10.1074/jbc.m205467200.Search in Google Scholar PubMed
Bartels, A.K., Gottert, S., Desel, C., Schafer, M., Krossa, S., Scheidig, A.J., Grötzinger, J., and Lorenzen, I. (2019). KDEL receptor 1 contributes to cell surface association of protein disulfide isomerases. Cell. Physiol. Biochem. 52: 850–868, https://doi.org/10.33594/000000059.Search in Google Scholar PubMed
Bax, D.V., Messent, A.J., Tart, J., van Hoang, M., Kott, J., Maciewicz, R.A., and Humphries, M.J. (2004). Integrin α5β1 and ADAM-17 interact in vitro and co-localize in migrating HeLa cells. J. Biol. Chem. 279: 22377–22386, https://doi.org/10.1074/jbc.m400180200.Search in Google Scholar
Bennett, T.A., Edwards, B.S., Sklar, L.A., and Rogelj, S. (2000). Sulfhydryl regulation of L-selectin shedding: phenylarsine oxide promotes activation-independent L-selectin shedding from leukocytes. J. Immunol. 164: 4120–4129, https://doi.org/10.4049/jimmunol.164.8.4120.Search in Google Scholar PubMed
Bergerhausen, L., Grosche, J., Meissner, J., Hecker, C., Caliandro, M.F., Westerhausen, C., Kamenac, A., Rezaei, M., Morgelin, M., Poschmann, G., et al. (2020). Extracellular redox regulation of α7β1 integrin-mediated cell migration is signaled via a dominant thiol-switch. Antioxidants 9, https://doi.org/10.3390/antiox9030227.Search in Google Scholar PubMed PubMed Central
Berndt, C., Schwenn, J.D., and Lillig, C.H. (2015). The specificity of thioredoxins and glutaredoxins is determined by electrostatic and geometric complementarity. Chem. Sci. 6: 7049–7058, https://doi.org/10.1039/c5sc01501d.Search in Google Scholar
Bi, S., Hong, P.W., Lee, B., and Baum, L.G. (2011). Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc. Natl. Acad. Sci. U.S.A. 108: 10650–10655, https://doi.org/10.1073/pnas.1017954108.Search in Google Scholar
Black, R.A., Rauch, C.T., Kozlosky, C.J., Peschon, J.J., Slack, J.L., Wolfson, M.F., Castner, B.J., Stocking, K.L., Reddy, P., Srinivasan, S., et al. (1997). A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385: 729–733, https://doi.org/10.1038/385729a0.Search in Google Scholar
Blobel, C.P. (2005). ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 6: 32–43, https://doi.org/10.1038/nrm1548.Search in Google Scholar
Blouin, E., Halbwachs-Mecarelli, L., and Rieu, P. (1999). Redox regulation of β2-integrin CD11b/CD18 activation. Eur. J. Immunol. 29: 3419–3431, https://doi.org/10.1002/(sici)1521-4141(199911)29:11<3419::aid-immu3419>3.0.co;2-1.10.1002/(SICI)1521-4141(199911)29:11<3419::AID-IMMU3419>3.0.CO;2-1Search in Google Scholar
Bodega, G., Alique, M., Bohorquez, L., Moran, M., Magro, L., Puebla, L., Ciordia, S., Mena, M.C., Arza, E., and Ramirez, M.R. (2018). Young and especially senescent endothelial microvesicles produce NADPH: the fuel for their antioxidant machinery. Oxid Med Cell Longev 2018: 3183794, https://doi.org/10.1155/2018/3183794.Search in Google Scholar
Boutet, P., Aguera-Gonzalez, S., Atkinson, S., Pennington, C.J., Edwards, D.R., Murphy, G., Reyburn, H.T., and Vales-Gomez, M. (2009). Cutting edge: the metalloproteinase ADAM17/TNF-alpha-converting enzyme regulates proteolytic shedding of the MHC class I-related chain B protein. J. Immunol. 182: 49–53, https://doi.org/10.4049/jimmunol.182.1.49.Search in Google Scholar
Byron, A., Humphries, J.D., Askari, J.A., Craig, S.E., Mould, A.P., and Humphries, M.J. (2009). Anti-integrin monoclonal antibodies. J. Cell Sci. 122: 4009–4011, https://doi.org/10.1242/jcs.056770.Search in Google Scholar
Calvete, J.J., Henschen, A., and Gonzalez-Rodriguez, J. (1991). Assignment of disulphide bonds in human platelet GPIIIa. A disulphide pattern for the beta-subunits of the integrin family. Biochem. J. 274: 63–71, https://doi.org/10.1042/bj2740063.Pt 1.Search in Google Scholar
Chagan-Yasutan, H., Ndhlovu, L.C., Lacuesta, T.L., Kubo, T., Leano, P.S., Niki, T., Oguma, S., Morita, K., Chew, G.M., Barbour, J.D., et al. (2013). Galectin-9 plasma levels reflect adverse hematological and immunological features in acute dengue virus infection. J. Clin. Virol. 58: 635–640, https://doi.org/10.1016/j.jcv.2013.10.022.Search in Google Scholar
Checconi, P., Salzano, S., Bowler, L., Mullen, L., Mengozzi, M., Hanschmann, E.M., Lillig, C.H., Sgarbanti, R., Panella, S., Nencioni, L., et al. (2015). Redox proteomics of the inflammatory secretome identifies a common set of redoxins and other glutathionylated proteins released in inflammation, influenza virus infection and oxidative stress. PloS One 10, https://doi.org/10.1371/journal.pone.0127086, e0127086.Search in Google Scholar PubMed PubMed Central
Chiu, J., Passam, F., Butera, D., and Hogg, P.J. (2015). Protein disulfide isomerase in thrombosis. Semin. Thromb. Hemost. 41: 765–773, https://doi.org/10.1055/s-0035-1564047.Search in Google Scholar PubMed
Cho, J., Kennedy, D.R., Lin, L., Huang, M., Merrill-Skoloff, G., Furie, B.C., and Furie, B. (2012). Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of β3 integrins. Blood 120: 647–655, https://doi.org/10.1182/blood-2011-08-372532.Search in Google Scholar PubMed PubMed Central
D’Alessio, A., Esposito, B., Giampietri, C., Ziparo, E., Pober, J.S., and Filippini, A. (2012). Plasma membrane microdomains regulate TACE-dependent TNFR1 shedding in human endothelial cells. J. Cell Mol. Med. 16: 627–636.10.1111/j.1582-4934.2011.01353.xSearch in Google Scholar
Dapat, I.C., Pascapurnama, D.N., Iwasaki, H., Labayo, H.K., Chagan-Yasutan, H., Egawa, S., and Hattori, T. (2017). Secretion of galectin-9 as a DAMP during dengue virus infection in THP-1 cells. Int. J. Mol. Sci. 18, https://doi.org/10.3390/ijms18081644.Search in Google Scholar PubMed PubMed Central
de Rezende, F.F., Martins Lima, A., Niland, S., Wittig, I., Heide, H., Schroder, K., and Eble, J.A. (2012). Integrin α7β1 is a redox-regulated target of hydrogen peroxide in vascular smooth muscle cell adhesion. Free Radic. Biol. Med. 53: 521–531, https://doi.org/10.1016/j.freeradbiomed.2012.05.032.Search in Google Scholar PubMed
Dombernowsky, S.L., Samsoe-Petersen, J., Petersen, C.H., Instrell, R., Hedegaard, A.M., Thomas, L., Atkins, K.M., Auclair, S., Albrechtsen, R., Mygind, K.J., et al. (2015). The sorting protein PACS-2 promotes ErbB signalling by regulating recycling of the metalloproteinase ADAM17. Nat. Commun. 6: 7518, https://doi.org/10.1038/ncomms8518.Search in Google Scholar PubMed PubMed Central
Duan, J., Gaffrey, M.J., and Qian, W.J. (2017). Quantitative proteomic characterization of redox-dependent post-translational modifications on protein cysteines. Mol. Biosyst. 13: 816–829, https://doi.org/10.1039/c6mb00861e.Search in Google Scholar PubMed PubMed Central
Düsterhöft, S., Bartels, A.K., Koudelka, T., Lilienthal, E., Schäfer, M., Garbers, C., Tholey, A., Grötzinger, J., and Lorenzen, I. (2020). Distance dependent shedding of IL-6R. Biochem. Biophys. Res. Commun. 526: 355–360, https://doi.org/10.1016/j.bbrc.2020.03.093.Search in Google Scholar PubMed
Düsterhöft, S., Hobel, K., Oldefest, M., Lokau, J., Waetzig, G.H., Chalaris, A., Garbers, C., Scheller, J., Rose-John, S., Lorenzen, I., et al. (2014). A disintegrin and metalloprotease 17 dynamic interaction sequence, the sweet tooth for the human interleukin 6 receptor. J. Biol. Chem. 289: 16336–16348, https://doi.org/10.1074/jbc.m114.557322.Search in Google Scholar PubMed PubMed Central
Düsterhöft, S., Jung, S., Hung, C.W., Tholey, A., Sonnichsen, F.D., Grötzinger, J., and Lorenzen, I. (2013). Membrane-proximal domain of a disintegrin and metalloprotease-17 represents the putative molecular switch of its shedding activity operated by protein-disulfide isomerase. J. Am. Chem. Soc. 135: 5776–5781, https://doi.org/10.1021/ja400340u.Search in Google Scholar PubMed
Düsterhöft, S., Lokau, J., and Garbers, C. (2019). The metalloprotease ADAM17 in inflammation and cancer. Pathol. Res. Pract. 215: 152410, https://doi.org/10.1016/j.prp.2019.04.002.Search in Google Scholar
Düsterhöft, S., Michalek, M., Kordowski, F., Oldefest, M., Sommer, A., Roseler, J., Reiss, K., Grötzinger, J., and Lorenzen, I. (2015). Extracellular juxtamembrane segment of ADAM17 interacts with membranes and is essential for its shedding activity. Biochemistry 54: 5791–5801, https://doi.org/10.1021/acs.biochem.5b00497.Search in Google Scholar
Eble, J.A., and de Rezende, F.F. (2014). Redox-relevant aspects of the extracellular matrix and its cellular contacts via integrins. Antioxidants Redox Signal. 20: 1977–1993, https://doi.org/10.1089/ars.2013.5294.Search in Google Scholar
Eckert, R.L., Kaartinen, M.T., Nurminskaya, M., Belkin, A.M., Colak, G., Johnson, G.V., and Mehta, K. (2014). Transglutaminase regulation of cell function. Physiol. Rev. 94: 383–417, https://doi.org/10.1152/physrev.00019.2013.Search in Google Scholar
Emsley, J., Knight, C.G., Farndale, R.W., Barnes, M.J., and Liddington, R.C. (2000). Structural basis of collagen recognition by integrin alpha2beta1. Cell 101: 47–56, https://doi.org/10.1016/s0092-8674(00)80622-4.Search in Google Scholar
Essex, D.W. and Wu, Y. (2018). Multiple protein disulfide isomerases support thrombosis. Curr. Opin. Hematol. 25: 395–402, https://doi.org/10.1097/moh.0000000000000449.Search in Google Scholar
Fenouillet, E., Barbouche, R., Courageot, J., and Miquelis, R. (2001). The catalytic activity of protein disulfide isomerase is involved in human immunodeficiency virus envelope-mediated membrane fusion after CD4 cell binding. J. Infect. Dis. 183: 744–752, https://doi.org/10.1086/318823.Search in Google Scholar PubMed
Ford, L., Kaluzny, A.D., and Sondik, E. (1990). Diffusion and adoption of state-of-the-art therapy. Semin. Oncol. 17: 485–494.Search in Google Scholar
Fujii, J. and Ikeda, Y. (2002). Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 7: 123–130, https://doi.org/10.1179/135100002125000352.Search in Google Scholar PubMed
Gallina, A., Hanley, T.M., Mandel, R., Trahey, M., Broder, C.C., Viglianti, G.A., and Ryser, H.J. (2002). Inhibitors of protein-disulfide isomerase prevent cleavage of disulfide bonds in receptor-bound glycoprotein 120 and prevent HIV-1 entry. J. Biol. Chem. 277: 50579–50588, https://doi.org/10.1074/jbc.m204547200.Search in Google Scholar PubMed
Gellert, M., Hanschmann, E.M., Lepka, K., Berndt, C., and Lillig, C.H. (2015). Redox regulation of cytoskeletal dynamics during differentiation and de-differentiation. Biochim. Biophys. Acta 1850: 1575–1587, https://doi.org/10.1016/j.bbagen.2014.10.030.Search in Google Scholar PubMed
Giannotta, M., Ruggiero, C., Grossi, M., Cancino, J., Capitani, M., Pulvirenti, T., Consoli, G.M., Geraci, C., Fanelli, F., Luini, A., et al. (2012). The KDEL receptor couples to Gαq/11 to activate Src kinases and regulate transport through the Golgi. EMBO J. 31: 2869–2881, https://doi.org/10.1038/emboj.2012.134.Search in Google Scholar PubMed PubMed Central
Gil-Bea, F., Akterin, S., Persson, T., Mateos, L., Sandebring, A., Avila-Carino, J., Gutierrez-Rodriguez, A., Sundstrom, E., Holmgren, A., Winblad, B., et al. (2012). Thioredoxin-80 is a product of α-secretase cleavage that inhibits amyloid-β aggregation and is decreased in Alzheimer’s disease brain. EMBO Mol. Med. 4: 1097–1111.10.1002/emmm.201201462Search in Google Scholar PubMed PubMed Central
Go, Y.M., Chandler, J.D., and Jones, D.P. (2015). The cysteine proteome. Free Radical Biol. Med. 84: 227–245, https://doi.org/10.1016/j.freeradbiomed.2015.03.022.Search in Google Scholar PubMed PubMed Central
Gooz, P., Dang, Y., Higashiyama, S., Twal, W.O., Haycraft, C.J., and Gooz, M. (2012). A disintegrin and metalloenzyme (ADAM) 17 activation is regulated by α5β1 integrin in kidney mesangial cells. PloS One 7, https://doi.org/10.1371/journal.pone.0033350, e33350.Search in Google Scholar PubMed PubMed Central
Grötzinger, J., Lorenzen, I., and Düsterhöft, S. (2017). Molecular insights into the multilayered regulation of ADAM17: the role of the extracellular region. Biochim. Biophys. Acta Mol. Cell Res. 1864: 2088–2095, https://doi.org/10.1016/j.bbamcr.2017.05.024.Search in Google Scholar PubMed
Gupta, V., Gylling, A., Alonso, J.L., Sugimori, T., Ianakiev, P., Xiong, J.P., and Arnaout, M.A. (2007). The β-tail domain (βTD) regulates physiologic ligand binding to integrin CD11b/CD18. Blood 109: 3513–3520, https://doi.org/10.1182/blood-2005-11-056689.Search in Google Scholar PubMed PubMed Central
Hahm, E., Li, J., Kim, K., Huh, S., Rogelj, S., and Cho, J. (2013). Extracellular protein disulfide isomerase regulates ligand-binding activity of αMβ2 integrin and neutrophil recruitment during vascular inflammation. Blood 121: 3789–3800, https://doi.org/10.1182/blood-2012-11-467985, S3781-3715.Search in Google Scholar PubMed PubMed Central
Hanschmann, E.M., Godoy, J.R., Berndt, C., Hudemann, C., and Lillig, C.H. (2013). Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxidants Redox Signal. 19: 1539–1605, https://doi.org/10.1089/ars.2012.4599.Search in Google Scholar PubMed PubMed Central
Hanschmann, E.M., Petry, S.F., Eitner, S., Maresch, C.C., Lingwal, N., Lillig, C.H., and Linn, T. (2020). Paracrine regulation and improvement of β-cell function by thioredoxin. Redox Biol 34: 101570, https://doi.org/10.1016/j.redox.2020.101570.Search in Google Scholar PubMed PubMed Central
Hoppe, G., Talcott, K.E., Bhattacharya, S.K., Crabb, J.W., and Sears, J.E. (2006). Molecular basis for the redox control of nuclear transport of the structural chromatin protein HMGB1. Exp. Cell Res. 312: 3526–3538, https://doi.org/10.1016/j.yexcr.2006.07.020.Search in Google Scholar PubMed
Hsu, Y.L., Wang, M.Y., Ho, L.J., Huang, C.Y., and Lai, J.H. (2015). Up-regulation of galectin-9 induces cell migration in human dendritic cells infected with dengue virus. J. Cell Mol. Med. 19: 1065–1076, https://doi.org/10.1111/jcmm.12500.Search in Google Scholar PubMed PubMed Central
Hu, P. and Luo, B.H. (2018). The interface between the EGF1 and EGF2 domains is critical in integrin affinity regulation. J. Cell. Biochem. 119: 7264–7273, https://doi.org/10.1002/jcb.26921.Search in Google Scholar PubMed
Huergo-Zapico, L., Gonzalez-Rodriguez, A.P., Contesti, J., Gonzalez, E., Lopez-Soto, A., Fernandez-Guizan, A., Acebes-Huerta, A., de Los Toyos, J.R., Lopez-Larrea, C., Groh, V., et al. (2012). Expression of ERp5 and GRP78 on the membrane of chronic lymphocytic leukemia cells: association with soluble MICA shedding. Cancer Immunol. Immunother. 61: 1201–1210, https://doi.org/10.1007/s00262-011-1195-z.Search in Google Scholar PubMed
Humphries, J.D., Chastney, M.R., Askari, J.A., and Humphries, M.J. (2019). Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 56: 14–21, https://doi.org/10.1016/j.ceb.2018.08.004.Search in Google Scholar PubMed
Ioannou, Y., Zhang, J.Y., Passam, F.H., Rahgozar, S., Qi, J.C., Giannakopoulos, B., Qi, M., Yu, P., Yu, D.M., Hogg, P.J., et al. (2010). Naturally occurring free thiols within β 2-glycoprotein I in vivo: nitrosylation, redox modification by endothelial cells, and regulation of oxidative stress-induced cell injury. Blood 116: 1961–1970, https://doi.org/10.1182/blood-2009-04-215335.Search in Google Scholar PubMed
Irvine, A.G., Wallis, A.K., Sanghera, N., Rowe, M.L., Ruddock, L.W., Howard, M.J., Williamson, R.A., Blindauer, C.A., and Freedman, R.B. (2014). Protein disulfide-isomerase interacts with a substrate protein at all stages along its folding pathway. PloS One 9, https://doi.org/10.1371/journal.pone.0082511, e82511.Search in Google Scholar PubMed PubMed Central
Iversen, R., Mysling, S., Hnida, K., Jorgensen, T.J., and Sollid, L.M. (2014). Activity-regulating structural changes and autoantibody epitopes in transglutaminase 2 assessed by hydrogen/deuterium exchange. Proc. Natl. Acad. Sci. U. S. A. 111: 17146–17151, https://doi.org/10.1073/pnas.1407457111.Search in Google Scholar PubMed PubMed Central
Jaeger, S.U., Schroeder, B.O., Meyer-Hoffert, U., Courth, L., Fehr, S.N., Gersemann, M., Stange, E.F., and Wehkamp, J. (2013). Cell-mediated reduction of human β-defensin 1: a major role for mucosal thioredoxin. Mucosal Immunol. 6: 1179–1190, https://doi.org/10.1038/mi.2013.17.Search in Google Scholar PubMed PubMed Central
Janes, P.W., Saha, N., Barton, W.A., Kolev, M.V., Wimmer-Kleikamp, S.H., Nievergall, E., Blobel, C.P., Himanen, J.P., Lackmann, M., and Nikolov, D.B. (2005). Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 123: 291–304, https://doi.org/10.1016/j.cell.2005.08.014.Search in Google Scholar PubMed
Janko, C., Filipovic, M., Munoz, L.E., Schorn, C., Schett, G., Ivanovic-Burmazovic, I., and Herrmann, M. (2014). Redox modulation of HMGB1-related signaling. Antioxidants Redox Signal. 20: 1075–1085, https://doi.org/10.1089/ars.2013.5179.Search in Google Scholar PubMed PubMed Central
Jasuja, R., Furie, B., and Furie, B.C. (2010). Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood 116: 4665–4674, https://doi.org/10.1182/blood-2010-04-278184.Search in Google Scholar
Jones, D.P. (2002). Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 348: 93–112, https://doi.org/10.1016/s0076-6879(02)48630-2.Search in Google Scholar
Jones, D.P. (2006). Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res. 9: 169–181, https://doi.org/10.1089/rej.2006.9.169.Search in Google Scholar PubMed
Jones, D.P. and Go, Y.M. (2011). Mapping the cysteine proteome: analysis of redox-sensing thiols. Curr. Opin. Chem. Biol. 15: 103–112, https://doi.org/10.1016/j.cbpa.2010.12.014.Search in Google Scholar PubMed
Kaiser, B.K., Yim, D., Chow, I.T., Gonzalez, S., Dai, Z., Mann, H.H., Strong, R.K., Groh, V., and Spies, T. (2007). Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature 447: 482–486, https://doi.org/10.1038/nature05768.Search in Google Scholar PubMed
Kim, T.W., Ryu, H.H., Li, S.Y., Li, C.H., Lim, S.H., Jang, W.Y., and Jung, S. (2017). PDIA6 regulation of ADAM17 shedding activity and EGFR-mediated migration and invasion of glioblastoma cells. J. Neurosurg. 126: 1829–1838, https://doi.org/10.3171/2016.5.JNS152831.Search in Google Scholar PubMed
Kiraly, R., Demeny, M., and Fesus, L. (2011). Protein transamidation by transglutaminase 2 in cells: a disputed Ca2+-dependent action of a multifunctional protein. FEBS J. 278: 4717–4739, https://doi.org/10.1111/j.1742-4658.2011.08345.x.Search in Google Scholar PubMed
Kohga, K., Takehara, T., Tatsumi, T., Miyagi, T., Ishida, H., Ohkawa, K., Kanto, T., Hiramatsu, N., and Hayashi, N. (2009). Anticancer chemotherapy inhibits MHC class I-related chain a ectodomain shedding by downregulating ADAM10 expression in hepatocellular carcinoma. Canc. Res. 69: 8050–8057, https://doi.org/10.1158/0008-5472.can-09-0789.Search in Google Scholar PubMed
Krokhin, O.V., Cheng, K., Sousa, S.L., Ens, W., Standing, K.G., and Wilkins, J.A. (2003). Mass spectrometric based mapping of the disulfide bonding patterns of integrin α chains. Biochemistry 42: 12950–12959, https://doi.org/10.1021/bi034726u.Search in Google Scholar PubMed
Krossa, S., Scheidig, A.J., Grötzinger, J., and Lorenzen, I. (2018). Redundancy of protein disulfide isomerases in the catalysis of the inactivating disulfide switch in A Disintegrin and Metalloprotease 17. Sci. Rep. 8: 1103, https://doi.org/10.1038/s41598-018-19429-4.Search in Google Scholar PubMed PubMed Central
Kwak, M.S., Kim, H.S., Lkhamsuren, K., Kim, Y.H., Han, M.G., Shin, J.M., Park, I.H., Rhee, W.J., Lee, S.K., Rhee, S.G., et al. (2019). Peroxiredoxin-mediated disulfide bond formation is required for nucleocytoplasmic translocation and secretion of HMGB1 in response to inflammatory stimuli. Redox Biol 24: 101203, https://doi.org/10.1016/j.redox.2019.101203.Search in Google Scholar PubMed PubMed Central
Lahav, J., Wijnen, E.M., Hess, O., Hamaia, S.W., Griffiths, D., Makris, M., Knight, C.G., Essex, D.W., and Farndale, R.W. (2003). Enzymatically catalyzed disulfide exchange is required for platelet adhesion to collagen via integrin α2β1. Blood 102: 2085–2092, https://doi.org/10.1182/blood-2002-06-1646.Search in Google Scholar PubMed
Laragione, T., Bonetto, V., Casoni, F., Massignan, T., Bianchi, G., Gianazza, E., and Ghezzi, P. (2003). Redox regulation of surface protein thiols: identification of integrin α4 as a molecular target by using redox proteomics. Proc. Natl. Acad. Sci. U.S.A. 100: 14737–14741, https://doi.org/10.1073/pnas.2434516100.Search in Google Scholar PubMed PubMed Central
Lasecka, L. and Baron, M.D. (2014). The nairovirus nairobi sheep disease virus/ganjam virus induces the translocation of protein disulphide isomerase-like oxidoreductases from the endoplasmic reticulum to the cell surface and the extracellular space. PloS One 9, https://doi.org/10.1371/journal.pone.0094656, e94656.Search in Google Scholar PubMed PubMed Central
Le Gall, S.M., Maretzky, T., Issuree, P.D., Niu, X.D., Reiss, K., Saftig, P., Khokha, R., Lundell, D., and Blobel, C.P. (2010). ADAM17 is regulated by a rapid and reversible mechanism that controls access to its catalytic site. J. Cell Sci. 123: 3913–3922, https://doi.org/10.1242/jcs.069997.Search in Google Scholar PubMed PubMed Central
Leichert, L.I. and Dick, T.P. (2015). Incidence and physiological relevance of protein thiol switches. Biol. Chem. 396: 389–399, https://doi.org/10.1515/hsz-2014-0314.Search in Google Scholar PubMed
Li, J. and Springer, T.A. (2017a). Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc. Natl. Acad. Sci. U.S.A. 114: 4685–4690, https://doi.org/10.1073/pnas.1704171114.Search in Google Scholar PubMed PubMed Central
Li, J., Su, Y., Xia, W., Qin, Y., Humphries, M.J., Vestweber, D., Cabanas, C., Lu, C., and Springer, T.A. (2017b). Conformational equilibria and intrinsic affinities define integrin activation. EMBO J. 36: 629–645, https://doi.org/10.15252/embj.201695803.Search in Google Scholar PubMed PubMed Central
Liu, J., Gurpur, P.B., and Kaufman, S.J. (2008). Genetically determined proteolytic cleavage modulates α7β1 integrin function. J. Biol. Chem. 283: 35668–35678, https://doi.org/10.1074/jbc.m804661200.Search in Google Scholar PubMed PubMed Central
Lorenzen, I., Lokau, J., Düsterhöft, S., Trad, A., Garbers, C., Scheller, J., Rose-John, S., and Grötzinger, J. (2012). The membrane-proximal domain of A Disintegrin and Metalloprotease 17 (ADAM17) is responsible for recognition of the interleukin-6 receptor and interleukin-1 receptor II. FEBS Lett. 586: 1093–1100, https://doi.org/10.1016/j.febslet.2012.03.012.Search in Google Scholar PubMed
Lorenzen, I., Lokau, J., Korpys, Y., Oldefest, M., Flynn, C.M., Kunzel, U., Garbers, C., Freeman, M., Grötzinger, J., and Düsterhöft, S. (2016). Control of ADAM17 activity by regulation of its cellular localisation. Sci. Rep. 6: 35067, https://doi.org/10.1038/srep37364.Search in Google Scholar PubMed PubMed Central
Lorenzen, I., Trad, A., and Grötzinger, J. (2011). Multimerisation of A disintegrin and metalloprotease protein-17 (ADAM17) is mediated by its EGF-like domain. Biochem. Biophys. Res. Commun. 415: 330–336, https://doi.org/10.1016/j.bbrc.2011.10.056.Search in Google Scholar PubMed
Lukyanov, K.A. and Belousov, V.V. (2014). Genetically encoded fluorescent redox sensors. Biochim. Biophys. Acta 1840: 745–756, https://doi.org/10.1016/j.bbagen.2013.05.030.Search in Google Scholar PubMed
Lundberg, M., Fernandes, A.P., Kumar, S., and Holmgren, A. (2004). Cellular and plasma levels of human glutaredoxin 1 and 2 detected by sensitive ELISA systems. Biochem. Biophys. Res. Commun. 319: 801–809, https://doi.org/10.1016/j.bbrc.2004.04.199.Search in Google Scholar PubMed
Luo, B.H. and Springer, T.A. (2006). Integrin structures and conformational signaling. Curr. Opin. Cell Biol. 18: 579–586, https://doi.org/10.1016/j.ceb.2006.08.005.Search in Google Scholar PubMed PubMed Central
Ly, J.D. and Lawen, A. (2003). Transplasma membrane electron transport: enzymes involved and biological function. Redox Rep. 8: 3–21, https://doi.org/10.1179/135100003125001198.Search in Google Scholar PubMed
Machado-Pineda, Y., Cardenes, B., Reyes, R., Lopez-Martin, S., Toribio, V., Sanchez-Organero, P., Suarez, H., Grötzinger, J., Lorenzen, I., Yanez-Mo, M., et al. (2018). CD9 Controls integrin α5β1-mediated cell adhesion by modulating its association with the metalloproteinase ADAM17. Front. Immunol. 9: 2474, https://doi.org/10.3389/fimmu.2018.02474.Search in Google Scholar PubMed PubMed Central
Mandel, R., Ryser, H.J., Ghani, F., Wu, M., and Peak, D. (1993). Inhibition of a reductive function of the plasma membrane by bacitracin and antibodies against protein disulfide-isomerase. Proc. Natl. Acad. Sci. U.S.A. 90: 4112–4116, https://doi.org/10.1073/pnas.90.9.4112.Search in Google Scholar PubMed PubMed Central
Manickam, N., Ahmad, S.S., and Essex, D.W. (2011). Vicinal thiols are required for activation of the αIIbβ3 platelet integrin. J. Thromb. Haemostasis 9: 1207–1215, https://doi.org/10.1111/j.1538-7836.2011.04266.x.Search in Google Scholar PubMed
Mannery, Y.O., Ziegler, T.R., Hao, L., Shyntum, Y., and Jones, D.P. (2010). Characterization of apical and basal thiol-disulfide redox regulation in human colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G523–530, https://doi.org/10.1152/ajpgi.00359.2009.Search in Google Scholar PubMed PubMed Central
Markovic, I., Stantchev, T.S., Fields, K.H., Tiffany, L.J., Tomic, M., Weiss, C.D., Broder, C.C., Strebel, K., and Clouse, K.A. (2004). Thiol/disulfide exchange is a prerequisite for CXCR4-tropic HIV-1 envelope-mediated T-cell fusion during viral entry. Blood 103: 1586–1594, https://doi.org/10.1182/blood-2003-05-1390.Search in Google Scholar
Matsuyama, Y., Hayashi, T., Terawaki, H., Negawa, T., Terada, T., Okano, Y., and Era, S. (2009). Human astrocytes and aortic endothelial cells actively convert the oxidized form of albumin to the reduced form: reduced albumin might participate in redox regulation of nerve and blood vessel systems. J. Physiol. Sci. 59: 207–215, https://doi.org/10.1007/s12576-009-0028-8.Search in Google Scholar
Metcalfe, C., Cresswell, P., Ciaccia, L., Thomas, B., and Barclay, A.N. (2011). Labile disulfide bonds are common at the leukocyte cell surface. Open Biol 1: 110010, https://doi.org/10.1098/rsob.110010.Search in Google Scholar
Moore, T.I., Aaron, J., Chew, T.L., and Springer, T.A. (2018). Measuring integrin conformational change on the cell surface with super-resolution microscopy. Cell Rep. 22: 1903–1912, https://doi.org/10.1016/j.celrep.2018.01.062.Search in Google Scholar
Mor-Cohen, R. (2016). Disulfide bonds as regulators of integrin function in thrombosis and hemostasis. Antioxidants Redox Signal. 24: 16–31, https://doi.org/10.1089/ars.2014.6149.Search in Google Scholar
Mor-Cohen, R., Rosenberg, N., Einav, Y., Zelzion, E., Landau, M., Mansour, W., Averbukh, Y., and Seligsohn, U. (2012). Unique disulfide bonds in epidermal growth factor (EGF) domains of β3 affect structure and function of αIIbβ3 and αvβ3 integrins in different manner. J. Biol. Chem. 287: 8879–8891, https://doi.org/10.1074/jbc.m111.311043.Search in Google Scholar
Moss, M.L., Jin, S.L., Milla, M.E., Bickett, D.M., Burkhart, W., Carter, H.L., Chen, W.J., Clay, W.C., Didsbury, J.R., Hassler, D., et al. (1997). Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature 385: 733–736, https://doi.org/10.1038/385733a0.Search in Google Scholar
Müllberg, J., Dittrich, E., Graeve, L., Gerhartz, C., Yasukawa, K., Taga, T., Kishimoto, T., Heinrich, P.C., and Rose-John, S. (1993). Differential shedding of the two subunits of the interleukin-6 receptor. FEBS Lett. 332: 174–178, https://doi.org/10.1016/0014-5793(93)80507-q.Search in Google Scholar
Mullen, L., Hanschmann, E.M., Lillig, C.H., Herzenberg, L.A., and Ghezzi, P. (2015). Cysteine oxidation targets peroxiredoxins 1 and 2 for exosomal release through a novel mechanism of redox-dependent secretion. Mol. Med. 21: 98–108, https://doi.org/10.2119/molmed.2015.00033.Search in Google Scholar PubMed PubMed Central
Murphy, D.D., Reddy, E.C., Moran, N., and O’Neill, S. (2014). Regulation of platelet activity in a changing redox environment. Antioxidants Redox Signal. 20: 2074–2089, https://doi.org/10.1089/ars.2013.5698.Search in Google Scholar PubMed
Passam, F., Chiu, J., Ju, L., Pijning, A., Jahan, Z., Mor-Cohen, R., Yeheskel, A., Kolsek, K., Tharichen, L., Aponte-Santamaria, C., et al. (2018). Mechano-redox control of integrin de-adhesion. Elife 7, https://doi.org/10.7554/elife.34843.Search in Google Scholar PubMed PubMed Central
Passam, F.H., Lin, L., Gopal, S., Stopa, J.D., Bellido-Martin, L., Huang, M., Furie, B.C., and Furie, B. (2015). Both platelet- and endothelial cell-derived ERp5 support thrombus formation in a laser-induced mouse model of thrombosis. Blood 125: 2276–2285, https://doi.org/10.1182/blood-2013-12-547208.Search in Google Scholar PubMed PubMed Central
Passam, F.H., Rahgozar, S., Qi, M., Raftery, M.J., Wong, J.W., Tanaka, K., Ioannou, Y., Zhang, J.Y., Gemmell, R., Qi, J.C., et al. (2010). Redox control of β2-glycoprotein I-von Willebrand factor interaction by thioredoxin-1. J. Thromb. Haemostasis 8: 1754–1762, https://doi.org/10.1111/j.1538-7836.2010.03944.x.Search in Google Scholar PubMed PubMed Central
Pekkari, K., Gurunath, R., Arner, E.S., and Holmgren, A. (2000). Truncated thioredoxin is a mitogenic cytokine for resting human peripheral blood mononuclear cells and is present in human plasma. J. Biol. Chem. 275: 37474–37480, https://doi.org/10.1074/jbc.m001012200.Search in Google Scholar PubMed
Pekkari, K. and Holmgren, A. (2004). Truncated thioredoxin: physiological functions and mechanism. Antioxidants Redox Signal. 6: 53–61, https://doi.org/10.1089/152308604771978345.Search in Google Scholar PubMed
Peschon, J.J., Slack, J.L., Reddy, P., Stocking, K.L., Sunnarborg, S.W., Lee, D.C., Russell, W.E., Castner, B.J., Johnson, R.S., Fitzner, J.N., et al. (1998). An essential role for ectodomain shedding in mammalian development. Science 282: 1281–1284, https://doi.org/10.1126/science.282.5392.1281.Search in Google Scholar PubMed
Popielarski, M., Ponamarczuk, H., Stasiak, M., Michalec, L., Bednarek, R., Studzian, M., Pulaski, L., and Swiatkowska, M. (2018). The role of protein disulfide isomerase and thiol bonds modifications in activation of integrin subunit α11. Biochem. Biophys. Res. Commun. 495: 1635–1641, https://doi.org/10.1016/j.bbrc.2017.11.186.Search in Google Scholar PubMed
Prenzel, N., Zwick, E., Daub, H., Leserer, M., Abraham, R., Wallasch, C., and Ullrich, A. (1999). EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402: 884–888, https://doi.org/10.1038/47260.Search in Google Scholar PubMed
Pulvirenti, T., Giannotta, M., Capestrano, M., Capitani, M., Pisanu, A., Polishchuk, R.S., San Pietro, E., Beznoussenko, G.V., Mironov, A.A., Turacchio, G., et al. (2008). A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat. Cell Biol. 10: 912–922, https://doi.org/10.1038/ncb1751.Search in Google Scholar PubMed
Raykhel, I., Alanen, H., Salo, K., Jurvansuu, J., Nguyen, V.D., Latva-Ranta, M., and Ruddock, L. (2007). A molecular specificity code for the three mammalian KDEL receptors. J. Cell Biol. 179: 1193–1204, https://doi.org/10.1083/jcb.200705180.Search in Google Scholar PubMed PubMed Central
Reddy, P., Slack, J.L., Davis, R., Cerretti, D.P., Kozlosky, C.J., Blanton, R.A., Shows, D., Peschon, J.J., and Black, R.A. (2000). Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J. Biol. Chem. 275: 14608–14614, https://doi.org/10.1074/jbc.275.19.14608.Search in Google Scholar
Rose-John, S. (2012). IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 8: 1237–1247, https://doi.org/10.7150/ijbs.4989.Search in Google Scholar
Rubartelli, A., Bajetto, A., Allavena, G., Wollman, E., and Sitia, R. (1992). Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 267: 24161–24164.10.1016/S0021-9258(18)35742-9Search in Google Scholar
Rubartelli, A., Bonifaci, N., and Sitia, R. (1995). High rates of thioredoxin secretion correlate with growth arrest in hepatoma cells. Canc. Res. 55: 675–680.Search in Google Scholar
Ruiz-May, E., Segura-Cabrera, A., Elizalde-Contreras, J.M., Shannon, L.M., and Loyola-Vargas, V.M. (2019). A recent advance in the intracellular and extracellular redox post-translational modification of proteins in plants. J. Mol. Recogn. 32, e2754, https://doi.org/10.1002/jmr.2754.Search in Google Scholar
Ryser, H.J. and Fluckiger, R. (2005). Progress in targeting HIV-1 entry. Drug Discov. Today 10: 1085–1094, https://doi.org/10.1016/s1359-6446(05)03550-6.Search in Google Scholar
Ryser, H.J., Levy, E.M., Mandel, R., and DiSciullo, G.J. (1994). Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction. Proc. Natl. Acad. Sci. U.S.A. 91: 4559–4563, https://doi.org/10.1073/pnas.91.10.4559.Search in Google Scholar PubMed PubMed Central
Saha, A., Backert, S., Hammond, C.E., Gooz, M., and Smolka, A.J. (2010). Helicobacter pylori CagL activates ADAM17 to induce repression of the gastric H, K-ATPase α subunit. Gastroenterology 139: 239–248, https://doi.org/10.1053/j.gastro.2010.03.036.Search in Google Scholar PubMed PubMed Central
Sakaguchi, R. and Mori, Y. (2020). Transient receptor potential (TRP) channels: biosensors for redox environmental stimuli and cellular status. Free Radical Biol. Med. 146: 36–44, https://doi.org/10.1016/j.freeradbiomed.2019.10.415.Search in Google Scholar PubMed
Sasagawa, I., Matsuki, S., Suzuki, Y., Iuchi, Y., Tohya, K., Kimura, M., Nakada, T., and Fujii, J. (2001). Possible involvement of the membrane-bound form of peroxiredoxin 4 in acrosome formation during spermiogenesis of rats. Eur. J. Biochem. 268: 3053–3061, https://doi.org/10.1046/j.1432-1327.2001.02200.x.Search in Google Scholar PubMed
Schaefer, K., Webb, N.E., Pang, M., Hernandez-Davies, J.E., Lee, K.P., Gonzalez, P., Douglass, M.V., Lee, B., and Baum, L.G. (2017). Galectin-9 binds to O-glycans on protein disulfide isomerase. Glycobiology 27: 878–887, https://doi.org/10.1093/glycob/cwx065.Search in Google Scholar PubMed PubMed Central
Schäfer, M., Granato, D.C., Krossa, S., Bartels, A.K., Yokoo, S., Düsterhöft, S., Koudelka, T., Scheidig, A.J., Tholey, A., Paes Leme, A.F., et al. (2017). GRP78 protects a disintegrin and metalloprotease 17 against protein-disulfide isomerase A6 catalyzed inactivation. FEBS Lett. 591: 3567–3587, https://doi.org/10.1002/1873-3468.12858.Search in Google Scholar PubMed
Schroeder, B.O., Wu, Z., Nuding, S., Groscurth, S., Marcinowski, M., Beisner, J., Buchner, J., Schaller, M., Stange, E.F., and Wehkamp, J. (2011). Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469: 419–423, https://doi.org/10.1038/nature09674.Search in Google Scholar PubMed
Schwertassek, U., Balmer, Y., Gutscher, M., Weingarten, L., Preuss, M., Engelhard, J., Winkler, M., and Dick, T.P. (2007). Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 26: 3086–3097, https://doi.org/10.1038/sj.emboj.7601746.Search in Google Scholar PubMed PubMed Central
Sharda, A., Kim, S.H., Jasuja, R., Gopal, S., Flaumenhaft, R., Furie, B.C., and Furie, B. (2015). Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome. Blood 125: 1633–1642, https://doi.org/10.1182/blood-2014-08-597419.Search in Google Scholar PubMed PubMed Central
Shattil, S.J., Kim, C., and Ginsberg, M.H. (2010). The final steps in integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 11: 288–300, https://doi.org/10.1038/nrm2871.Search in Google Scholar PubMed PubMed Central
Sies, H. and Jones, D.P. (2020). Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-020-0230-3.Search in Google Scholar PubMed
Soderberg, A., Sahaf, B. and Rosen, A. (2000). Thioredoxin reductase, a redox-active selenoprotein, is secreted by normal and neoplastic cells: presence in human plasma. Canc. Res. 60: 2281–2289.Search in Google Scholar
Sommer, A., Kordowski, F., Buch, J., Maretzky, T., Evers, A., Andra, J., Düsterhöft, S., Michalek, M., Lorenzen, I., Somasundaram, P., et al. (2016). Phosphatidylserine exposure is required for ADAM17 sheddase function. Nat. Commun. 7: 11523, https://doi.org/10.1038/ncomms11523.Search in Google Scholar PubMed PubMed Central
Son, A., Kato, N., Horibe, T., Matsuo, Y., Mochizuki, M., Mitsui, A., Kawakami, K., Nakamura, H., and Yodoi, J. (2009). Direct association of thioredoxin-1 (TRX) with macrophage migration inhibitory factor (MIF): regulatory role of TRX on MIF internalization and signaling. Antioxidants Redox Signal. 11: 2595–2605, https://doi.org/10.1089/ars.2009.2522.Search in Google Scholar PubMed
Stamnaes, J., Pinkas, D.M., Fleckenstein, B., Khosla, C., and Sollid, L.M. (2010). Redox regulation of transglutaminase 2 activity. J. Biol. Chem. 285: 25402–25409, https://doi.org/10.1074/jbc.m109.097162.Search in Google Scholar PubMed PubMed Central
Staudacher, V., Trujillo, M., Diederichs, T., Dick, T.P., Radi, R., Morgan, B., and Deponte, M. (2018). Redox-sensitive GFP fusions for monitoring the catalytic mechanism and inactivation of peroxiredoxins in living cells. Redox Biol 14: 549–556, https://doi.org/10.1016/j.redox.2017.10.017.Search in Google Scholar PubMed PubMed Central
Sun, Z., Costell, M., and Fassler, R. (2019). Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 21: 25–31, https://doi.org/10.1038/s41556-018-0234-9.Search in Google Scholar PubMed
Swiatkowska, M., Szymanski, J., Padula, G., and Cierniewski, C.S. (2008). Interaction and functional association of protein disulfide isomerase with αVβ3 integrin on endothelial cells. FEBS J. 275: 1813–1823, https://doi.org/10.1111/j.1742-4658.2008.06339.x.Search in Google Scholar PubMed
Szabo-Taylor, K.E., Eggleton, P., Turner, C.A., Faro, M.L., Tarr, J.M., Toth, S., Whiteman, M., Haigh, R.C., Littlechild, J.A., and Winyard, P.G. (2012). Lymphocytes from rheumatoid arthritis patients have elevated levels of intracellular peroxiredoxin 2, and a greater frequency of cells with exofacial peroxiredoxin 2, compared with healthy human lymphocytes. Int. J. Biochem. Cell Biol. 44: 1223–1231, https://doi.org/10.1016/j.biocel.2012.04.016.Search in Google Scholar PubMed PubMed Central
Takahashi, N., and Mori, Y. (2011). TRP channels as sensors and signal integrators of redox status changes. Front. Pharmacol. 2: 58, https://doi.org/10.3389/fphar.2011.00058.Search in Google Scholar PubMed PubMed Central
Tanaka, L.Y., Oliveira, P.V.S., and Laurindo, F.R.M. (2020). Peri/epicellular thiol oxidoreductases as mediators of extracellular redox signaling. Antioxidants Redox Signal. https://doi.org/10.1089/ars.2019.8012.Search in Google Scholar PubMed
Tandon, R., Chew, G.M., Byron, M.M., Borrow, P., Niki, T., Hirashima, M., Barbour, J.D., Norris, P.J., Lanteri, M.C., Martin, J.N., et al. (2014). Galectin-9 is rapidly released during acute HIV-1 infection and remains sustained at high levels despite viral suppression even in elite controllers. AIDS Res. Hum. Retrovir. 30: 654–664, https://doi.org/10.1089/aid.2014.0004.Search in Google Scholar
Taylor, K., Barran, P.E., and Dorin, J.R. (2008). Structure-activity relationships in β-defensin peptides. Biopolymers 90: 1–7, https://doi.org/10.1002/bip.20900.Search in Google Scholar PubMed
Thon, J.N., Peters, C.G., Machlus, K.R., Aslam, R., Rowley, J., Macleod, H., Devine, M.T., Fuchs, T.A., Weyrich, A.S., Semple, J.W., et al. (2012). T granules in human platelets function in TLR9 organization and signaling. J. Cell Biol. 198: 561–574, https://doi.org/10.1083/jcb.201111136.Search in Google Scholar PubMed PubMed Central
Trad, A., Riese, M., Shomali, M., Hedeman, N., Effenberger, T., Grötzinger, J., and Lorenzen, I. (2013). The disintegrin domain of ADAM17 antagonises fibroblastcarcinoma cell interactions. Int. J. Oncol. 42: 1793–1800, https://doi.org/10.3892/ijo.2013.1864.Search in Google Scholar PubMed
Veit, M., Ahrens, B., Seidel, J., Sommer, A., Bhakdi, S., and Reiss, K. (2019). Mutagenesis of the ADAM17-phosphatidylserine-binding motif leads to embryonic lethality in mice. Life Sci Alliance 2, https://doi.org/10.26508/lsa.201900430.Search in Google Scholar PubMed PubMed Central
Waldhauer, I., Goehlsdorf, D., Gieseke, F., Weinschenk, T., Wittenbrink, M., Ludwig, A., Stevanovic, S., Rammensee, H.G., and Steinle, A. (2008). Tumor-associated MICA is shed by ADAM proteases. Canc. Res. 68: 6368–6376, https://doi.org/10.1158/0008-5472.can-07-6768.Search in Google Scholar
Wan, S.W., Lin, C.F., Lu, Y.T., Lei, H.Y., Anderson, R., and Lin, Y.S. (2012). Endothelial cell surface expression of protein disulfide isomerase activates β1 and β3 integrins and facilitates dengue virus infection. J. Cell. Biochem. 113: 1681–1691, htpps://doi.org/10.1002/jcb.24037.10.1002/jcb.24037Search in Google Scholar
Wang, L., Zhou, J., Wang, L., Wang, C.C., and Essex, D.W. (2019). The b’ domain of protein disulfide isomerase cooperates with the a and a’ domains to functionally interact with platelets. J. Thromb. Haemostasis 17: 371–382, https://doi.org/10.1111/jth.14366.Search in Google Scholar
Wang, X.F. and Cynader, M.S. (2000). Astrocytes provide cysteine to neurons by releasing glutathione. J. Neurochem. 74: 1434–1442, https://doi.org/10.1046/j.1471-4159.2000.0741434.x.Search in Google Scholar
Wang, Y., Herrera, A.H., Li, Y., Belani, K.K., and Walcheck, B. (2009). Regulation of mature ADAM17 by redox agents for L-selectin shedding. J. Immunol. 182: 2449–2457, https://doi.org/10.4049/jimmunol.0802770.Search in Google Scholar
Weichsel, A., Gasdaska, J.R., Powis, G., and Montfort, W.R. (1996). Crystal structures of reduced, oxidized, and mutated human thioredoxins: evidence for a regulatory homodimer. Structure 4: 735–751, https://doi.org/10.1016/s0969-2126(96)00079-2.Search in Google Scholar
Willems, S.H., Tape, C.J., Stanley, P.L., Taylor, N.A., Mills, I.G., Neal, D.E., McCafferty, J., and Murphy, G. (2010). Thiol isomerases negatively regulate the cellular shedding activity of ADAM17. Biochem. J. 428: 439–450, https://doi.org/10.1042/bj20100179.Search in Google Scholar
Wolf, C., Lopez Del Amo, V., Arndt, S., Bueno, D., Tenzer, S., Hanschmann, E.M., Berndt, C., and Methner, A. (2020). Redox modifications of proteins of the mitochondrial fusion and fission machinery. Cells 9, https://doi.org/10.3390/cells9040815.Search in Google Scholar PubMed PubMed Central
Xie, C., Shimaoka, M., Xiao, T., Schwab, P., Klickstein, L.B., and Springer, T.A. (2004). The integrin α-subunit leg extends at a Ca2+-dependent epitope in the thigh/genu interface upon activation. Proc. Natl. Acad. Sci. U.S.A. 101: 15422–15427, https://doi.org/10.1073/pnas.0406680101.Search in Google Scholar PubMed PubMed Central
Xie, C., Zhu, J., Chen, X., Mi, L., Nishida, N., and Springer, T.A. (2010). Structure of an integrin with an αI domain, complement receptor type 4. EMBO J. 29: 666–679, https://doi.org/10.1038/emboj.2009.367.Search in Google Scholar PubMed PubMed Central
Xiong, J.P., Mahalingham, B., Alonso, J.L., Borrelli, L.A., Rui, X., Anand, S., Hyman, B.T., Rysiok, T., Muller-Pompalla, D., Goodman, S.L., et al. (2009). Crystal structure of the complete integrin αVβ3 ectodomain plus an α/β transmembrane fragment. J. Cell Biol. 186: 589–600, https://doi.org/10.1083/jcb.200905085.Search in Google Scholar PubMed PubMed Central
Xu, S.Z., Sukumar, P., Zeng, F., Li, J., Jairaman, A., English, A., Naylor, J., Ciurtin, C., Majeed, Y., Milligan, C.J., et al. (2008). TRPC channel activation by extracellular thioredoxin. Nature 451: 69–72, https://doi.org/10.1038/nature06414.Search in Google Scholar PubMed PubMed Central
Yan, B. and Smith, J.W. (2000). A redox site involved in integrin activation. J. Biol. Chem. 275: 39964–39972, https://doi.org/10.1074/jbc.m007041200.Search in Google Scholar
Yang, J., Carroll, K.S., and Liebler, D.C. (2016). The expanding landscape of the thiol redox proteome. Mol. Cell. Proteomics 15: 1–11, https://doi.org/10.1074/mcp.o115.056051.Search in Google Scholar
Yang, W., Shimaoka, M., Salas, A., Takagi, J., and Springer, T.A. (2004). Intersubunit signal transmission in integrins by a receptor-like interaction with a pull spring. Proc. Natl. Acad. Sci. U.S.A. 101: 2906–2911, https://doi.org/10.1073/pnas.0307340101.Search in Google Scholar PubMed PubMed Central
Yoshida, T., Inoue, R., Morii, T., Takahashi, N., Yamamoto, S., Hara, Y., Tominaga, M., Shimizu, S., Sato, Y., and Mori, Y. (2006). Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2: 596–607, https://doi.org/10.1038/nchembio821.Search in Google Scholar PubMed
Zaidel-Bar, R. and Geiger, B. (2010). The switchable integrin adhesome. J. Cell Sci. 123: 1385–1388, https://doi.org/10.1242/jcs.066183.Search in Google Scholar PubMed PubMed Central
Zhang, K. and Chen, J. (2012). The regulation of integrin function by divalent cations. Cell Adhes. Migrat. 6: 20–29, https://doi.org/10.4161/cam.18702.Search in Google Scholar PubMed PubMed Central
Zhang, K., Pan, Y., Qi, J., Yue, J., Zhang, M., Xu, C., Li, G., and Chen, J. (2013). Disruption of disulfide restriction at integrin knees induces activation and ligand-independent signaling of α4β7. J. Cell Sci. 126: 5030–5041, https://doi.org/10.1242/jcs.134528.Search in Google Scholar PubMed
Zhao, L.X., Du, J.R., Zhou, H.J., Liu, D.L., Gu, M.X., and Long, F.Y. (2016). Differences in proinflammatory property of six subtypes of peroxiredoxins and anti-inflammatory effect of ligustilide in macrophages. PloS One 11, https://doi.org/10.1371/journal.pone.0164586, e0164586.Search in Google Scholar PubMed PubMed Central
Zheng, Y. and Leftheris, K. (2020). Insights into protein-ligand interactions in integrin complexes: advances in structure determinations. J. Med. Chem. 63: 5675–5696, https://doi.org/10.1021/acs.jmedchem.9b01869.Search in Google Scholar PubMed
Zhu, G., Zhang, Q., Reddy, E.C., Carrim, N., Chen, Y., Xu, X.R., Xu, M., Wang, Y., Hou, Y., Ma, L., et al. (2017). The integrin PSI domain has an endogenous thiol isomerase function and is a novel target for antiplatelet therapy. Blood 129: 1840–1854, https://doi.org/10.1182/blood-2016-07-729400.Search in Google Scholar PubMed PubMed Central
Zhu, J., Luo, B.H., Xiao, T., Zhang, C., Nishida, N., and Springer, T.A. (2008). Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell. 32: 849–861, https://doi.org/10.1016/j.molcel.2008.11.018.Search in Google Scholar PubMed PubMed Central
Zhu, J., Zhu, J., and Springer, T.A. (2013). Complete integrin headpiece opening in eight steps. J. Cell Biol. 201: 1053–1068, https://doi.org/10.1083/jcb.201212037.Search in Google Scholar PubMed PubMed Central
© 2020 Inken Lorenzen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Dynamics of thiol-based redox switches: redox at its peak!
- Highlight: Dynamics of Thiol-Based Redox Switches
- 3-Mercaptopyruvate sulfurtransferase: an enzyme at the crossroads of sulfane sulfur trafficking
- Thiol-based switching mechanisms of stress-sensing chaperones
- Thiol switches in membrane proteins - Extracellular redox regulation in cell biology
- Emerging roles for non-selenium containing ER-resident glutathione peroxidases in cell signaling and disease
- Apoptosis inducing factor and mitochondrial NADH dehydrogenases: redox-controlled gear boxes to switch between mitochondrial biogenesis and cell death
- Redox regulation in host-pathogen interactions: thiol switches and beyond
- Targeting spectrin redox switches to regulate the mechanoproperties of red blood cells
- Thiol-based redox switches in the major pathogen Staphylococcus aureus
- Genetically encoded thiol redox-sensors in the zebrafish model: lessons for embryonic development and regeneration
- Thioredoxin-dependent control balances the metabolic activities of tetrapyrrole biosynthesis
- Shifting paradigms and novel players in Cys-based redox regulation and ROS signaling in plants - and where to go next
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Dynamics of thiol-based redox switches: redox at its peak!
- Highlight: Dynamics of Thiol-Based Redox Switches
- 3-Mercaptopyruvate sulfurtransferase: an enzyme at the crossroads of sulfane sulfur trafficking
- Thiol-based switching mechanisms of stress-sensing chaperones
- Thiol switches in membrane proteins - Extracellular redox regulation in cell biology
- Emerging roles for non-selenium containing ER-resident glutathione peroxidases in cell signaling and disease
- Apoptosis inducing factor and mitochondrial NADH dehydrogenases: redox-controlled gear boxes to switch between mitochondrial biogenesis and cell death
- Redox regulation in host-pathogen interactions: thiol switches and beyond
- Targeting spectrin redox switches to regulate the mechanoproperties of red blood cells
- Thiol-based redox switches in the major pathogen Staphylococcus aureus
- Genetically encoded thiol redox-sensors in the zebrafish model: lessons for embryonic development and regeneration
- Thioredoxin-dependent control balances the metabolic activities of tetrapyrrole biosynthesis
- Shifting paradigms and novel players in Cys-based redox regulation and ROS signaling in plants - and where to go next

