Abstract
The second messenger cyclic di-GMP regulates a variety of processes in bacteria, many of which are centered around the decision whether to adopt a sessile or a motile life style. Regulatory circuits include pathogenicity, biofilm formation, and motility in a wide variety of bacteria, and play a key role in cell cycle progression in Caulobacter crescentus. Interestingly, multiple, seemingly independent c-di-GMP pathways have been found in several species, where deletions of individual c-di-GMP synthetases (DGCs) or hydrolases (PDEs) have resulted in distinct phenotypes that would not be expected based on a freely diffusible second messenger. Several recent studies have shown that individual signaling nodes exist, and additionally, that protein/protein interactions between DGCs, PDEs and c-di-GMP receptors play an important role in signaling specificity. Additionally, subcellular clustering has been shown to be employed by bacteria to likely generate local signaling of second messenger, and/or to increase signaling specificity. This review highlights recent findings that reveal how bacteria employ spatial cues to increase the versatility of second messenger signaling.
Introduction: cyclic di-GMP regulates central aspects of bacterial physiology
Bis-(3′,5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) is a second messenger that can be found in all major bacteria phyla (Chan et al. 2004). C-di-GMP was described in 1987 as first cyclic dinucleotide (CDN) by the group of Moshe Benziman as an allosteric activator of cellulose synthase in Komagataeibacter xylinus (Ross et al. 1987). Through work spear headed by Urs Jenal’s group, it has become clear that the signaling networks of this second messenger range from a few proteins per organism to a wide range of players (Galperin et al. 2001), which are involved in a large variety of biological processes and cellular behavior (Hengge 2009; Römling et al. 2013; Simm et al. 2004). For example, signaling plays a role in the coordination of cell growth (Choy et al. 2004) and the replication cycle (Kulasakara et al. 2006; Tischler et al. 2004; Weber et al. 2006), in biofilm formation (Aldridge et al. 2003; Bobrov et al. 2005; Bomchil et al. 2003) or virulence (Hisert et al. 2005) of bacteria. The cytoplasmic level of this second messenger is regulated as an effect on internal and environmental stimuli, by the activity of antagonistic players: diguanylate cyclases (DGCs) and phosphodiesterases (PDEs) (Chan et al. 2004; Römling et al. 2013). Oxygen (Tuckerman et al. 2009), light (Barends et al. 2009), metals (Zähringer et al. 2013), nutrients (Basu Roy et al. 2014; Mills et al. 2015), nitric oxide (Plate et al. 2012) , or surface contact (O’Connor et al. 2012) have been identified as input signals for those enzymes so far.
C-di-GMP is synthesized by a homodimer of DGCs (Figure 1), by an antiparallel arrangement the conserved GGDEF domains of each protomer, each bound to a GTP molecule (Chan et al. 2004). The phosphodiester linkage between the two GTP molecules is achieved by a nucleophilic attack of the deprotonated 3′-OH group from one GTP onto the alpha-phosphate of the other GTP molecule (Jenal et al. 2017). The activity of the DGCs is controlled by the dimerization required for CDN synthesis. Usually, DGCs have an accessory domain that induces the formation of signal-dependent homodimers, as it is the case for PleD from Caulobacter crescentus (C. crescentus) (Jenal et al. 2017) or WspR from Pseudomonas aeruginosa (P. aeruginosa) (Almblad et al. 2015; De et al. 2008). In these organisms, the dimerization of DGCs is facilitated by an amino-terminal receiver domain that dimerizes after phosphorylation. On the other hand, DGC activity is allosterically inhibited by c-di-GMP (Chan et al. 2004; Christen et al. 2005). A majority of the DGCs contain an autoinhibitory site (I-site, RxxD motif; Christen et al. 2005), which is conserved in most GGDEF domains, and has a high affinity for c-di-GMP binding. C-di-GMP binding to the I-site leads to an allosteric product inhibition and therefore has a negative feedback on its synthesis. However, recent studies have shown that the I-site can also take on other functions (Chatterjee et al. 2014; Dahlstrom et al. 2016). In Pseudomonas fluorescence (P. fluorescence), signal transmission between the DGC GcbC and the c-di-GMP effector LapD is controlled by a combination of intracellular c-di-GMP level and I-site-dependent cyclase-effector interaction (Dahlstrom et al. 2015). This observation implies a dual role of this auto-inhibitory site for negative feedback regulation and for protein–protein interactions. GGDEF domains are known to exist independently, or to be associated with multiple sensory and signal transduction domains (phosphorylation or protein binding), PAS domains (Per-ARNT-Sim; Hoffman et al. 1991), GAF domains (named after the first three protein families identified with this domain: mammalian cGMP-dependent phosphodiesterases, Anabaena adenylyl cyclases, and Escherichia coli FhlA (Aravind et al. 1997)), REC domains (CheY-homologous receiver; (Römling et al. 2006)) domains or periplasmatic domains (e.g., solute-binding proteins) (Hurley 2003; Krasteva et al. 2010; Römling et al. 2005; Taylor et al. 1999).
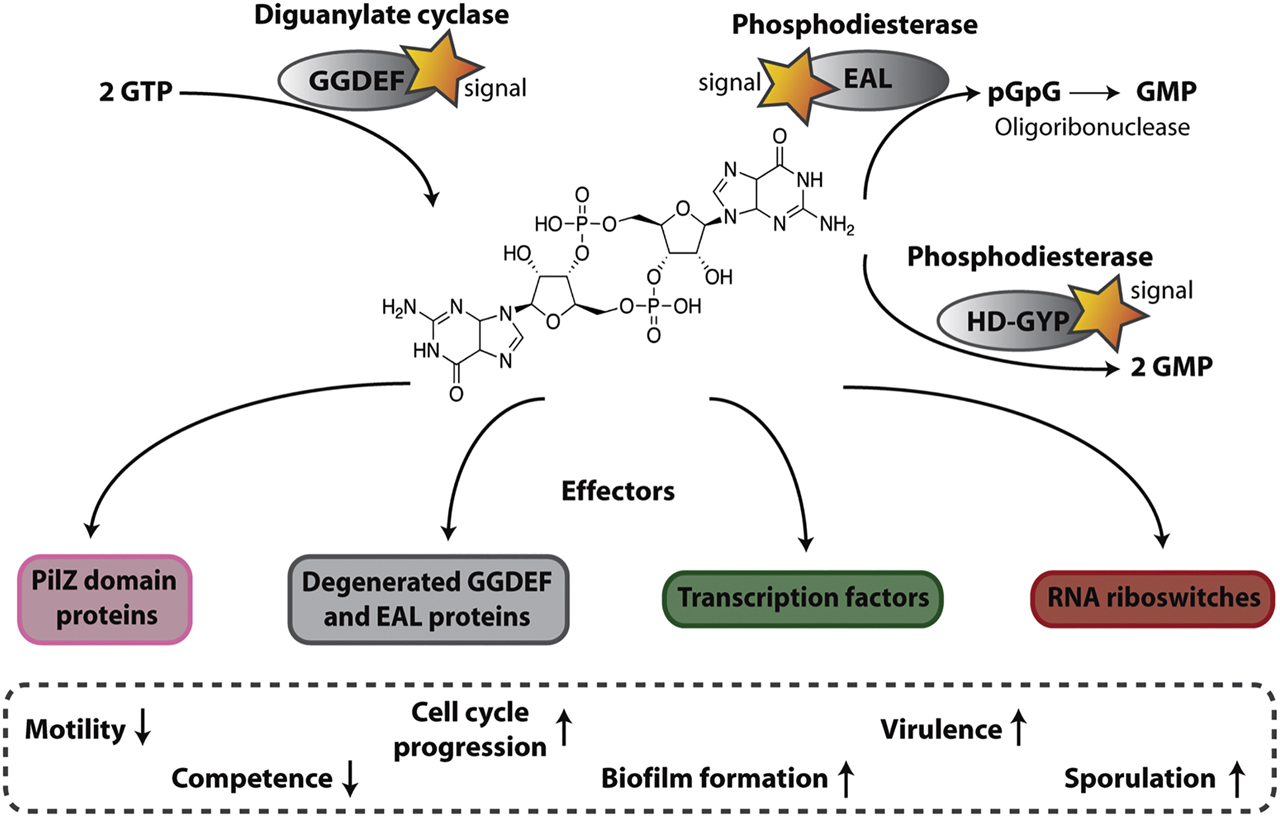
General mechanism of c-di-GMP signaling networks.
See text for details.
On the other hand, c-di-GMP specific phosphodiesterases (PDEs) hydrolyze the second messenger (Chang et al. 2001; Chen et al. 2001; Tal et al. 1998) (Figure 1). EAL domain proteins (Simm et al. 2004; Tischler et al. 2004) are one group of PDEs which degrade c-di-GMP to the linear 5′-phosphoguanylyl-(3′-5′)-guanosine (pGpG) in the presence of Mg2+ or Mn2+(Christen et al. 2005; Tal et al. 1998) (Figure 1). Acting as a dimer (Barends et al. 2009; Sundriyal et al. 2014), crystal structures propose a clam-shell-like opening and closing mechanism for PDE activity regulation. However, the formation of a dimer is not essential for the catalytic activity of the protein (Sundriyal et al. 2014; Winkler et al. 2014). Product inhibition for c-di-GMP hydrolysis is obtained by pGpG binding to its active site (Jenal et al. 2017). The other group of PDEs contains a HD-GYP domain (Ryan et al. 2010), which hydrolyzes c-di-GMP to two GMP molecules in a one-step reaction (Bellini et al. 2014) (Figure 1). This domain is a subgroup of the HD superfamily, which are metal dependent phosphohydrolases and contains an additional GYP motif (Aravind et al. 1998; Dow et al. 2006). As pGpG has a negative feedback control on its PDE, EAL domain proteins can lead to additional complexity in a signaling pathway. The primary enzyme that subsequently degrades pGpG to GMP was identified to be the oligoribonuclease Orn, which hydrolyzes RNAs that are 2–5 nucleotides in length (Cohen et al. 2015; Orr et al. 2015). Organisms that do not encode orn, such as Bacillus subtilis (B. subtilis), have other exoribonucleases: LC-MS/MS studies in a B. subtilis nrnA and nrnB deletion strain revealed that these two potential exoribonucleases might be responsible for pGpG degradation, and might thus be functional homologs of Orn (Orr et al. 2015, 2018).
Various families of c-di-GMP effectors have been identified and characterized so far, such as PILZ domain proteins (Amikam et al. 2006; Boehm et al. 2010), proteins containing degenerated GGDEF or EAL domains (Chen et al. 2012; Duerig et al. 2009), transcriptional regulators (Baraquet et al. 2013; Hickman et al. 2008; Krasteva et al. 2010; Leduc et al. 2009; Lee et al. 2007; Tschowri et al. 2014), and mRNA riboswitches (Hengge 2010a) (Figure 1). The first identified class of c-di-GMP receptors, PilZ domain proteins, can bind c-di-GMP via conserved “RxxxR” and “(D/N)x(S/A)xxG” motifs that are located in the disorganized N-terminus of the protein and the neighboring β-barrel domain (Amikam et al. 2006; Habazettl et al. 2011; Ramelot et al. 2007; Römling et al. 2013; Ryjenkov et al. 2006). These domains are stand-alone domains and can be found in various different multidomain proteins, like Alg44 from P. aeruginosa, which is responsible for production of alginate (Merighi et al. 2007), or YcgR, which regulates flagellum-based motility in E. coli (Ryjenkov et al. 2006). It has been shown that these proteins alter their conformation for further signaling. Additional studies revealed that not all c-di-GMP-regulated outputs are dependent on PilZ domain proteins, therefore further c-di-GMP effectors were sought after. As a consequence, another class of c-di-GMP receptors was found: proteins that contain a degenerated GGDEF domain. One instance of this second class of c-di-GMP effectors is the protein PleD from P. aeruginosa, which regulates pellicle formation by binding c-di-GMP to an inhibitory site (Li et al. 2012). After binding of the second messenger it undergoes a structural rearrangement to transmit the signal (Schirmer et al. 2009). Having shown that degenerated GGDEF domains of the c-di-GMP cyclases can serve as receptors, the first EAL domain protein LapD, capable of binding c-di-GMP, was identified in P. fluorescence (Newell et al. 2009). LapD is a dual-domain effector since it harbors a degenerated GGDEF and EAL domain, but it has been shown that it controls biofilm formation trough c-di-GMP binding to its EAL domain (see later) (Navarro et al. 2011; Newell et al. 2009). In recent years additional c-di-GMP effectors have been found. First of all, transcription factors, such as the transcriptional activator CuxR in the plant symbiotic nitrogen-fixing α-proteobacterium Sinorhizobium meliloti (Schäper et al. 2017), which belongs to the AraC-like family. At elevated c-di-GMP levels, the transcription of an exopolymeric substances (EPS) synthesis gene cluster and therefore biofilm formation is activated by binding of the second messenger to CuxR. Interestingly, c-di-GMP binding to this transcription factor is quite similar to that of PilZ domain proteins, because c-di-GMP binds to an N-terminal “RxxxR” motif and a Cupin domain, which leads to a dimerization and DNA binding. Thus, both proteins harbor an “RxxxR” motif and a β-barrel structure, which offers a further c-di-GMP coordination through their amino acids, especially through the (D/N)x(S/a)xxG motif of its outer surface and a conserved arginine residue. It is interesting to note that this similarity in c-di-GMP binding may be due to a convergent evolution of the PilZ domain and of CuxR, where c-di-GMP binding sites with similar topology have developed in two protein families (Schäper et al. 2017). Further examples of transcription factors that can bind c-di-GMP and regulate motility and biofilm formation can be found in Vibrio cholerae, in which the transcription factor VpsT can bind c-di-GMP with a W(F/L/M)(T/S)R motif, thereby causing a conformational change through dimerization (Krasteva et al. 2010), or in P. aeruginosa, in which FleQ can bind c-di-GMP (Hickman et al. 2008). Some organisms have many DGCs and PDEs, but only a few c-di-GMP effectors, so the continued search for effectors brought to light another class of c-di-GMP effectors, mRNA riboswitches that bind c-di-GMP and thus control the transcription and translation of different genes in a concentration-dependent manner (Sudarsan et al. 2008). A highly conserved RNA domain, GEMM, was found upstream of an open reading frame of some DGCs, PDEs and other genes that are controlled by c-di-GMP in V. cholerae. Another class was found in Clostridium difficile that controls the splicing of a self-splicing group I ribozyme after binding of c-di-GMP. Splicing leads to the formation of a ribosomal binding site that activates protein production of the downstream pathogenesis-related gene (Lee et al. 2010).
Modularity and direct protein interactions increase specificity in signaling
C-di-GMP levels control different aspects of bacterial growth and behavior such as motility, biofilm formation, developmental transitions, aggregation behavior, surface adhesion, progression of the cell cycle, sporulation and virulence of animal and plant pathogens (D’Argenio et al. 2004; Dow et al. 2006; Jenal et al. 2006; Römling et al. 2005; Weiss et al. 2019). Generally, low levels of the second messenger are associated with the motility of individual cells, competence and virulence, while high c-di-GMP levels lead to surface attachment as well as loss of motility and thus to biofilm formation, sporulation, or in case of C. crescentus to cell cycle progression (Figure 1) (Abel et al. 2011; Valentini et al. 2016; Weiss et al. 2019). However, it has become clear that the lack of a certain DGC can lead to the loss of output from a specific pathway, but not of many other pathways involving c-di-GMP sensors, or PDEs. E. coli has 29 c-di-GMP signaling proteins (GGDEF/EAL domain proteins) (Sarenko et al. 2017), Vibrio vulnificus has a stunning armada of 100 proteins (Römling et al. 2005). How many pathways run seemingly independently, or independently of most other DGCs, while using a small freely diffusive second messenger has been a major question in the field (Güvener et al. 2007; Hengge 2009; Jenal et al. 2006; Kader et al. 2006; Kulasakara et al. 2006; Ryan et al. 2010; Weber et al. 2006). As the O’Toole group put it: how does a small molecule in a freely diffusible space trigger one cellular output but not another? (Dahlstrom et al. 2015). Interestingly, the binding affinity of c-di-GMP to the different receptors within a cell is varying greatly (Figure 2D). In Salmonella enterica this binding affinity differs more than 40-fold between the two PilZ domain proteins YcgR and BscA that are responsible for motility and cellulose synthesis. The residues next to the RxxxR binding motif seem to cause this difference in binding affinity (Pultz et al. 2012). Therefore, a low level of c-di-GMP leads to the first output, which is inhibition of motility caused by YcgR. Thereafter, the higher concentration of c-di-GMP leads to cellulose synthesis by activation of BcsA (Pultz et al. 2012). Different binding affinities for c-di-GMP were also found in other organisms. In P. aeruginosa for example, a large variety of binding affinities with a difference of more than 140-fold was identified (Pultz et al. 2012). Another way to obtain signal specificity for global c-di-GMP signaling is through temporal separation and activation of the effectors. Different points in time of their expression during cell growth lead to an offset of their activation (Figure 2C). However, even taking into accounts different responses to varying c-di-GMP levels, these can not explain observed signaling specificity.

Mechanisms of c-di-GMP signal specificity for local and global signaling of the second messenger.
(A, B) Local signaling of the second messenger via local c-di-GMP pools. Signal specificity is achieved by (A) close spatial proximity of DGC (yellow), effector (magenta) and PDE (green), where either a direct interaction or no interaction between cyclase and effector occurs The PDE is in close spatial proximity to the DGC and the effector to shield other effectors from the local c-di-GMP pool. (B) Another possibility for signal specificity is the presence of a complex, where all components (DGC, effector and PDE) interact directly. In this case the signal transmission depends on the activation status of the DGC or PDE. In addition, the individual components can influence each other in terms of activity through their interaction. (C, D) Global signaling of the second messenger via global c-di-GMP pools, whereby signal specificity can be controlled via temporal separation (C) or c-di-GMP binding affinity of the effector (D). The PDE thereby regulates the global c-di-GMP pool. In case of temporary separation (C), the separation is obtained through differential expression of several effectors during cell growth, so that the effectors are activated at different times. In (D), different c-di-GMP binding affinities of the effectors activate them at different points in time during cell growth. Effectors with a high binding affinity to the second messenger are activated at lower cellular c-di-GMP levels, whereas effectors with a low binding affinity are only activated at very high c-di-GMP concentrations.
In addition to the global c-di-GMP signaling hypothesis, local c-di-GMP signaling including direct interactions between DGCs, PDEs and c-di-GMP receptors has been identified in several organisms (Figure 2A and B) such as in B. subtilis (Bedrunka and Graumann 2017a,b; Kunz et al. 2020), P. fluorescens or P. aeruginosa (Dahlstrom et al. 2015; Luo et al. 2015) and E. coli (Lindenberg et al. 2013). A key observation was the identification of the interaction of cyclases and hydrolases by the group of Regine Hengge, playing a central part in a signaling cascade controlling biofilm formation in E. coli (Lindenberg et al. 2013). The DGC DgcE and the PDE PdeH (module I) regulate the activity of the DGC DgcM and the PDE PdeR (module II) by direct interaction. Thereby PdeR connects the two DGC/PDE pairs and acts as a trigger enzyme (Commichau et al. 2008). When it binds and hydrolyzes c-di-GMP, synthesized by module I, it can no longer inhibit the DGC DgcM, which then starts to synthesize c-di-GMP and interacts directly with MlrA to start csgD transcription (Lindenberg et al. 2013). CsgD, which is a key regulator for biofilm formation, enables genes for amyloid curli fibers of the biofilm matrix (Pesavento et al. 2008; Römling et al. 2000). This signaling system shows local signaling as part of macromolecular interactions controlled by the original enzymatic activity of the enzyme. In summary, DGC and PDE can act as bifunctional regulatory trigger enzymes (Commichau et al. 2008).
Another direct interaction of a cyclase and its receptor was demonstrated in P. fluorescens: LapD is a c-di-GMP-responsive inner membrane protein that binds c-di-GMP through its non-canonical EAL domain, which binds, but does not degrade, c-di-GMP (Newell et al. 2009). Upon binding to c-di-GMP, LapD undergoes a conformational change that sequesters the periplasmic protease LapG. This in turn allows adhesin LapA to accumulate on the cell surface, a first step in biofilm formation (Chatterjee et al. 2014; Dahlstrom et al. 2015; Giacalone et al. 2018; Navarro et al. 2011; Newell et al. 2011). The DGC GcbC binds to LapD via alpha helical contacts, which is crucial for signaling efficiency and thereby increases its activity up to eight-fold (Dahlstrom et al. 2015; Giacalone et al. 2018). Also in B. subtilis, two DGCs were shown to bind to two receptor proteins having different c-di-GMP binding domains, depending on c-di-GMP binding for at least one of the pairs (Kunz et al. 2020), which is further detailed below.
A search for protein interactions has identified many potential interactions between a majority of DGCs and PDEs in E. coli, with several enzymes having multiple interactions, and thereby, apparently, setting up an interaction network with several central interaction hubs. Interestingly, deletion of several central DGCs leads to strong colony morphology phenotypes during biofilm formation, but do not have a strong effect on the intracellular c-di-GMP levels, which vary between 40 nM during exponential growth to 40 nM during stationary phase. Conversely, deletion of several more “peripheral” DGCs lowers c-di-GMP levels up to 50%, but does not cause any curli – or EPS related morphology changes (Sarenko et al. 2017). These experiments strongly suggest that c-di-GMP acts to a large degree via local signaling of this second messenger, rather than through global levels.
Thus, specificity in c-di-GMP signaling can be achieved by direct interaction of PDEs and DGCs with their effectors, which are thereby involved in downstream signaling cascades for the control of spatially clustered cellular processes (Dahlstrom et al. 2016; Lindenberg et al. 2013; Tuckerman et al. 2009). In those subcellular nodes, DGCs and PDEs serve, as “c-di-GMP sensors” to regulate those signaling cascades in addition to controlling synthesis and degradation of c-di-GMP (Lindenberg et al. 2013).
Subcellular clustering of c-di-GMP modules
First evidence of compartmentalization of c-di-GMP signaling has been suggested for P. aeuruginosa, since parts of the Wsp system, which is homologous to chemotaxis systems, form subcellular clusters. Both the DGC WspR, when phosphorylated, as well as the receptor WspA form patches within the cell and co-localize in parts. This indicates compartmentalized c-di-GMP synthesis depending on surface growth (Güvener et al. 2007).
C-di-GMP has been shown to act as a transcriptional and posttranslational regulator of machineries producing exopolysaccharides in different bacteria (Liang 2015). Interestingly, PelD from P. aeuruginosa (Lee et al. 2007; Liang 2015) and PssE from Listeria monocytogenes (L. monocytogenes) (Chen et al. 2014; Köseoğlu et al. 2015) are c-di-GMP binding proteins that exert control over genes encoded with in the same operon, PEL exopolysaccharide or Listerial EPS production, respectively. B. subtilis contains a homologous ydaJKLMN operon, also encoding a putative EPS machinery (Bedrunka and Graumann 2017b; Nicolas et al. 2012). YdaK is a membrane protein, encoded by the second gene of the operon, and its GGDEF domain is able to bind but not hydrolyze c-di-GMP, at high concentrations of the second messenger, as shown by isothermal titration calorimetry (Gao et al. 2013). Deletion of genes of this operon in L. monocytogenes leads to loss of cell aggregation and increased motility, as well as to lowered tolerance towards disinfectants or dessication, and also affects invasion of mammalian cells. For non-pathogenic B. subtilis, overexpression of the yda operon leads to an altered biofilm morphology (especially if YdaKLMN are overproduced, but not YdaJ that is thought to act as an EPS hydrolase), clumping of cells in liquid media and an elevated Congo Red staining of biofilms. Interestingly, YdaK forms a single cluster per cell at the cell pole, but also at the lateral side (Figure 3), and co-localizes with the putative glycosyltransferase complex YdaM/YdaN. The presence of YdaK in the membrane (and being able to bind to c-di-GMP) is necessary for the formation of this phenotype, as well as that of the DGC DgcK (or the DGC DgcP upon overproduction) (Bedrunka and Graumann 2017a,b; Kunz et al. 2020). These finding show that clustering of DGCs, their receptors and regulated machineries are employed in c-di-GMP signaling.
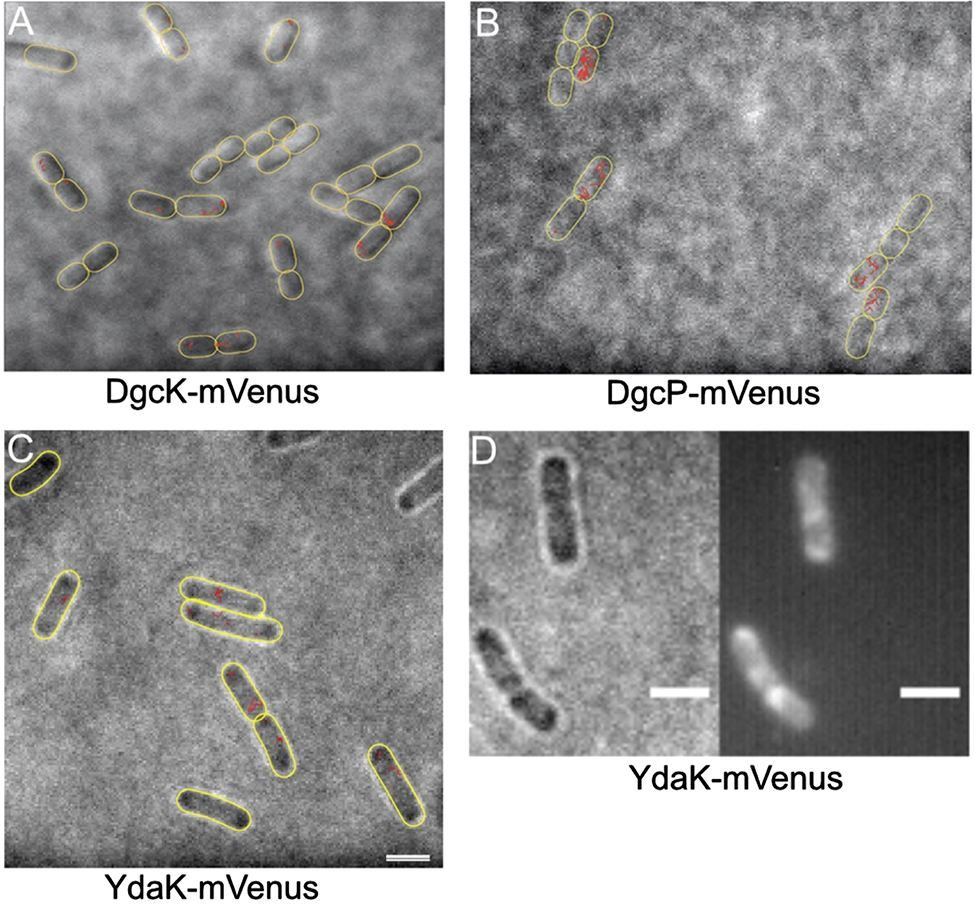
Localization and dynamics of DGCs and c-di-GMP receptor using single molecule tracking (SMT) in Bacillus subtilis.
(A–C) Tracks (marked in red) of single molecules, captured every 15 ms. Note that DgcK and YdaK show small displacements, often at the cell poles, while DgcP moves faster, and throughout the cells. Outlines of cells are indicated by yellow ovals, images overlays of bright field images, and tracks. Note that especially DgcK is present at very low molecule numbers, such that a considerable fraction of cells does not contain any molecules. (D) Sum of 2000 frames of molecules imaged by SMT, note that only statically positioned molecules yield sufficient contrast to be displayed. White bars represent 2 µm.
Bacterial exopolysaccharide machineries or components of these have been reported to localize to the cell poles for several species including Agrobacterium tumefaciens (Xu et al. 2013), E. coli (Le Quéré et al. 2009) and Streptomyces coelicolor (Xu et al. 2008), so subcellular clustering of c-di-GMP receptors and of their targets, as well as of corresponding DGCs could be an efficient means of setting up locally restricted c-di-GMP signaling.
Interestingly, cell cycle progression in C. crescentus depends on the interplay of several DGCs and EAL proteins, which may also employ local c-di-GMP signaling. C. crescentus divides in an asymmetric manner, in which the mother cell that is surface attached via a stalk keeps on dividing following cell division, while the daughter cell has been equipped with a single flagellum at the pole opposite to the stalk, and swims away as a swarmer cell having a quiescent cell cycle, which is turned into an active mode once the swarmer cell has differentiated into a stalked mother cell. Intriguingly, formation of the flagellum is initiated by polar c-di-GMP receptor, TipF, whereas hydrolase PdeA is also localized at the flagellar pole, and interacts with essential kinase/phosphatase CckA, whose kinase activity is inactivated by c-di-GMP binding to the active site, while its phosphatase activity upregulated (Jenal et al. 2017). Curiously, CckA needs to be active as a kinase at the flagellated pole, while its phosphatase activity is required at the stalked pole, with c-di-GMP levels being relatively high before cell division, throughout the cells. This has led to the model that PdeA and/or possibly another PDE may shield CckA from c-di-GMP at the flagellated pole, while DGC PleD is localized at the stalk cell pole, becomes activated by phosphorylation in a cross talk with the phosphorelay, and contributes to activation of CckA phosphatase activity required at this pole (Abel et al. 2011). Therefore, local c-di-GMP signaling may contribute to the gradient of kinase/phosphatase activity required to lead to two different cell fates after division.
Spatial specificity of c-di-GMP signaling in B. subtilis
C-di-GMP signaling in B. subtilis and the core components of this second messenger were first established in 2012 by Chen and colleagues (Chen et al. 2012; Gao et al. 2013). Compared to other organisms, B. subtilis harbors a relatively concise c-di-GMP signaling network (Figure 4) (Galperin 2005; Hengge 2010b). The signaling system contains only three DGC, two of which are membrane-bound, DgcK (GGDEF domain) and DgcW (PAS, GGDEF, and EAL domain), as well as a third soluble DGC, DgcP (GGDEF and GAF domains). The only PDE that seems to be active under standard conditions, PdeH (EAL domain), completes the signaling system. Additionally, three c-di-GMP effectors have been described: the PilZ domain protein DgrA, which inhibits motility when bound to c-di-GMP, YdaK, which has a degenerated GGDEF domain and is part of a putative EPS operon as well as YkuI, which has an inactive EAL domain and PAS-like domain with a so far unknown function (Chen et al. 2012; Gao et al. 2013). The physiological concentration of c-di-GMP in B. subtilis has not yet been clarified in detail. In standard rich medium with a detection limit of 50 pg/μl (∼72 nM) of c-di-GMP Gao et al. (2013) could not detect the second messenger, whereas the group of Jörg Stülke identified a c-di-GMP concentration of 35 ng/mg of protein in sporulation medium (Diethmaier et al. 2014). High c-di-GMP concentration in B. subtilis leads to biofilm formation and sporulation while low c-di-GMP level are associated with mobility and competence (Bedrunka and Graumann 2017a,b; Chen et al. 2012; Gao et al. 2013; Kunz et al. 2020; Weiss et al. 2019).

c-di-GMP signaling in B. subtilis.
In B. subtilis, the c-di-GMP signaling system consists of three active diguanylate cyclases DgcK (blue), DgcP (red) and DgcW (not shown). After reaching an unknown signal, these proteins dimerize and start synthesizing c-di-GMP out of two GTP, most likely for local signaling of the second messenger. Furthermore, B. subtilis harbors one active soluble phosphodiesterase, PdeH (yellow) and three effector proteins YdaK (green), DgrA (orange) and YkuI (not shown). The c-di-GMP receptor YdaK is encoded by the second gene of the ydaJKLMN operon, a putative EPS operon, and seems to have an impact on the production of an unknown exoploysaccharide (EPS). The membrane-bound cyclase DgcK interacts with the receptor YdaK so that local c-di-GMP signaling is most likely to occur. After c-di-GMP binding, YdaK transmits a signal to YdaM (pink), which then starts the synthesis of an unknown EPS with the help of YdaN (pale orange), YdaL (pale violet) and YdaJ (mint green). DgrA, a PilZ-protein and homolog of the E. coli YcgR, could be associated with the regulation of motility through interaction with MotA (turquoise). The two DGC DgcK and DgcP interact with the c-di-GMP receptor, synthesize c-di-GMP for a presumably local signaling of the second messenger. Binding of c-di-GMP to its receptor DgrA leads to an interaction with MotA, whereby DgrA acts like a clutch-like protein and thus separates the rotor of the flagellum, the MotA4MotB2 complex (turquoise/light blue) from the stator FliG (beige), and thereby inhibits motility.
The two cyclases, DgcK and DgcP, are present inside the cell with an extremely low copy number. By automated molecule counting using single molecule tracking data analyzed with the SMTracker (Rösch et al. 2018), DgcK was given a molecule count of about six molecules per cell, while DgcP was present with around 25 molecules/cell. Due to the small number of molecules, some cells did not contain a fluorescent signal and therefore most likely no corresponding DGC as they were fused to a single fluorophore molecule, mVenus, at the original locus. Twenty-nine percentage of all cells contained no DgcK-mVenus, whereas 15% of all cells showed no fluorescent signal of DgcP-mVenus molecules, resulting in even more efficient signaling. The c-di-GMP receptor YdaK also has a low copy number and was only found in the presence of ≈12 molecules/cell (Kunz et al. 2020).
An interaction between the DGC DgcK and the c-di-GMP effector YdaK seems to induce EPS production (Bedrunka and Graumann 2017a,b; Kunz et al. 2020). Furthermore, YdaK and the DGC DgcK co-localize in parts which strengthen the local c-di-GMP signaling hypothesis at a single site within the membrane (Bedrunka and Graumann 2017a). Compared to DgcK, DgcP only seems to have an influence on the morphology of the biofilm when it is overproduced (Bedrunka and Graumann 2017a). Since DgcK is present with only a small number of copies within the cell (Kunz et al. 2020), this will lead to heterogeneity in the activity of the putative EPS synthesis, as DgcK is the primary DGC responsible for this activation. Single-molecule tracking of the cyclase DgcK revealed a predominant static position of DgcK molecules in the membrane with increased mobility in the absence of the c-di-GMP receptor YdaK and an even more static behavior in case of overproduction of YdaK. The static behavior of DgcK can most likely indicate binding to the receptor, since YdaK is part of a large nanomachine (putative EPS synthetase), while the mobile population diffuses within the cell membrane. This assumption could be verified by pull down experiments in vitro, which showed a direct interaction between the soluble domains of the DgcK and YdaK, dependent on an intact I-site. These findings strengthen the idea of a local c-di-GMP signaling for the regulation of the synthesis of a so far unknown EPS by a DgcK – YdaJKLMN signaling module (Kunz et al. 2020). Additionally, the presumable physiological c-di-GMP level within the cell strengthens the hypothesis of local c-di-GMP signaling in B. subtilis. As mentioned above, in 2013 Gao et al. tried to investigate the c-di-GMP concentration during vegetative growth in standard rich medium, where the effector usually responds to c-di-GMP. However, the group was unable to detect the second messenger with an detection limit of 50 pg/μl, about 72 nM, of their method (Gao et al. 2013). This allows the assumption that the physiological c-di-GMP concentration is below 72 nM. Considering the Kd value (dissociation constant) of the c-di-GMP effector YdaK, which is about 1.1 µM, the Kd value of YdaK is at least 15 times higher than the detection limit of the physiological c-di-GMP concentration. Since the actual c-di-GMP concentration within the cell is still further below 72 nM, the actual difference is even greater. This strongly supports the idea of local c-di-GMP signaling.
The cyclase DgcK, as well as the DGC DgcP and DgcW, also play a role in the regulation of mobility. It has been also shown that all three DGCs contribute to the inhibition of motility through the c-di-GMP PilZ receptor and E. coli YcgR homolog DgrA (Kunz et al. 2020). By isothermal titration calorimetry it could be shown that DgrA is able to bind c-di-GMP with a Kd of 11 nM in vitro (Gao et al. 2013). Taking into account the number of flagella in B. subtilis, which is more or less 26 (Guttenplan et al. 2013), it makes sense that not only a single cyclase, DgcK, which is present in a very small number of molecules per cell, is solely responsible for influencing mobility, especially since some of the approximately six DgcK molecules additionally interact with the c-di-GMP effector YdaK. It seems rather possible that the other two cyclases, DgcP and DgcW, support DgcK as a convergence of c-di-GMP signal modules. This assumption was reinforced by single molecule tracking of DgcK and DgcP in the present and absence of DgrA. The data showed a reduced mobility of the cyclases in dgrA deletion strains, which means that the two cyclases stop more frequently at certain points on the membrane and possibly interacting with the receptor. Pull-down experiments of DgcK and DgcP with DgrA revealed an interaction in vitro. These findings also indicate a local signaling of c-di-GMP around this receptor and a possible direct hand-over of c-di-GMP for a directed signaling (Kunz et al. 2020).
In the case of the c-di-GMP effector DgrA, the c-di-GMP binding affinity is much higher compared to the other c-di-GMP effector YdaK. The dissociation constant of this effector is 11 nM in vitro (Gao et al. 2013), which indicates an extremely high c-di-GMP binding affinity. As the precise physiological c-di-GMP level within the cell is not known yet, it can only be assumed that the concentration of the second messenger is below the detection limit of 72 nM (Gao et al. 2013), which opens up the possibility that the physiological c-di-GMP level is above the Kd value of DgrA. This would imply that DgrA is possibly controlled by a global c-di-GMP pool. Furthermore, the different binding affinities of the two effectors, YdaK and DgrA, which have a 90-fold difference c-di-GMP binding affinity, might also indicate a global c-di-GMP signaling resulting in signal specificity due to their different effector binding affinity. However, these assumptions raise the question why the effector interacts directly with the two cyclases DgcK and DgcP, although DgrA would respond to a global c-di-GMP level. One can only speculate that these interactions may provide a kinetic advantage. On the other hand, there is also the option that the intracellular c-di-GMP concentration is below the Kd value of DgrA, which would support the idea of local c-di-GMP signaling. Considering that the physiological c-di-GMP levels inside the cell are not precisely known yet, it is more likely that these cyclase – effector interactions lead to a local c-di-GMP signaling for DgrA.
The DGC DgcK plays a dual role in c-di-GMP signaling by regulation of putative EPS synthesis and motility, which is understandable since biofilm formation is associated with reduced mobility (Kunz et al. 2020). After binding of the second messenger, DgrA interacts with MotA and acts as a molecular clutch that disengages the flagellum stator MotA4MotB2 from the cytoplasmic C ring rotor of the flagellum FliG and thereby inhibits motility (Subramanian et al. 2017).
The interactions between DgcK and YdaK, as well as DgcK or DgcP and DgrA, show that divergent as well as convergent direct connections between cyclases and their effector proteins exist in B. subtilis. Due to the additional low number of molecules of the cyclases, DgcK and DgcP, as well as YdaK within the cell, the signaling specificity is further increased.
The third c-di-GMP effector, YkuI, is able to bind c-di-GMP, but does not seem to hydrolyze the second messenger in a pdeH deletion strain (Minasov et al. 2009). Its apo-and c-di-GMP-bound states have already been characterized by crystal structure analysis (Minasov et al. 2009), but its function is still unknown. Because an inactivation of ykuI and the fla-che operon lead to resistance to high Zink (Zn2+) concentrations, YkuI might control zinc homeostasis (Chandrangsu et al. 2016). The YkuI ortholog CdgJ of B. cereus positively influences biofilm formation, so that it can be speculated that also in B. subtilis, increased ECM production – activated by YkuI – might inhibit Zn2+ entry into the cell (Chandrangsu et al. 2016). Interestingly, CdgJ seems to play a role in sporulation, since overexpression of cdhJ leads to an earlier entry into sporulation (Fagerlund et al. 2016). It will be interesting if YkuI also controls sporulation in B. subtilis. Clearly, this organism makes use of spatial organisation and direct protein/protein interactions to take advantage of a minimalistic c-di-GMP signaling network.
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: SFB987
Acknowledgments
This work was supported by funding from the Deutsche Forschungsgemeinschaft, SFB987.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: The research was funded by the Deutsche Forschungsgemeinschaft, SFB987.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Abel, S., Chien, P., Wassmann, P., Schirmer, T., Kaever, V., Laub, M.T., Baker, T.A., and Jenal, U. (2011). Regulatory cohesion of cell cycle and cell differentiation through interlinked phosphorylation and second messenger networks. Mol. Cell 43: 550–560, https://doi.org/10.1111/j.1365-2958.2006.05123.x.Suche in Google Scholar
Aldridge, P., Paul, R., Goymer, P., Rainey, P., and Jenal, U. (2003). Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus . Mol. Microbiol. 47: 1695–1708. https://doi.org/10.1046/j.1365-2958.2003.03401.x.Suche in Google Scholar
Almblad, H., Harrison, J.J., Rybtke, M., Groizeleau, J., Givskov, M., Parsek, M.R., and Tolker-Nielsen, T. (2015). The cyclic AMP-Vfr signaling pathway in Pseudomonas aeruginosa is inhibited by cyclic di-GMP. J. Bacteriol. 197: 2190–2200, https://doi.org/10.1126/science.1181185.Suche in Google Scholar
Amikam, D. and Galperin, M.Y. (2006). PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 22: 3–6, https://doi.org/10.1073/pnas.0601679103.Suche in Google Scholar
Aravind, L. and Koonin, E.V. (1998). The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem. Sci. 23: 469–472, https://doi.org/10.1128/mbio.03122-19.Suche in Google Scholar
Aravind, L. and Ponting, C.P. (1997). The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem. Sci. 12: 458–459, https://doi.org/10.1111/j.1365-2958.2009.06678.x.Suche in Google Scholar
Baraquet, C. and Harwood, C.S. (2013). Cyclic diguanosine monophosphate represses bacterial flagella synthesis by interacting with the Walker A motif of the enhancer-binding protein FleQ. Proc. Natl. Acad. Sci. U.S.A. 110: 18478–18483, https://doi.org/10.1128/jb.00845-09.Suche in Google Scholar
Barends, T.R., Hartmann, E., Griese, J.J., Beitlich, T., Kirienko, N.V., Ryjenkov, D.A., Reinstein, J., Shoeman, R.L., Gomelsky, M., and Schlichting, I. (2009). Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature 459: 1015–1018, https://doi.org/10.1126/science.1190713.Suche in Google Scholar
Basu Roy, A. and Sauer, K. (2014). Diguanylate cyclase NicD‐based signalling mechanism of nutrient‐induced dispersion by Pseudomonas aeruginosa . Mol. Microbiol. 94: 771–793, https://doi.org/10.1111/j.1365-2958.2007.05879.x.Suche in Google Scholar
Bedrunka, P. and Graumann, P.L. (2017a). New functions and subcellular localization patterns of c-di-GMP components (GGDEF domain proteins) in B. subtilis . Front. Microbiol. 8: 794, https://doi.org/10.1074/jbc.m112.378273.Suche in Google Scholar
Bedrunka, P. and Graumann, P.L. (2017b). Subcellular clustering of a putative c-di-GMP-dependent exopolysaccharide machinery affecting macro colony architecture in Bacillus subtilis . Environ Microbiol. Rep. 9: 211–222, https://doi.org/10.1039/c4np00086b.10.1111/1758-2229.12496Suche in Google Scholar PubMed
Bellini, D., Caly, D.L., McCarthy, Y., Bumann, M., An, S.Q., Dow, J.M., Ryan, R.P., and Walsh, M.A. (2014). Crystal structure of an HD‐GYP domain cyclic‐di‐GMP phosphodiesterase reveals an enzyme with a novel trinuclear catalytic iron centre. Mol. Microbiol. 91: 26–38, https://doi.org/10.1038/emboj.2013.120.Suche in Google Scholar
Bobrov, A.G., Kirillina, O., and Perry, R.D. (2005). The phosphodiesterase activity of the HmsP EAL domain is required for negative regulation of biofilm formation in Yersinia pestis . FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 247: 123–130, https://doi.org/10.1128/mbio.02456-14.Suche in Google Scholar
Boehm, A., Kaiser, M., Li, H., Spangler, C., Kasper, C.A., Ackermann, M., Kaever, V., Sourjik, V., Roth, V., and Jenal, U. (2010). Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141: 107–116, https://doi.org/10.1111/j.1365-2958.2007.05817.x.Suche in Google Scholar
Bomchil, N., Watnick, P., and Kolter, R. (2003). Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J. Bacteriol. 185: 1384–1390, https://doi.org/10.1126/scisignal.aaa1796.Suche in Google Scholar
Chan, C., Paul, R., Samoray, D., Amiot, N.C., Giese, B., Jenal, U., and Schirmer, T. (2004). Structural basis of activity and allosteric control of diguanylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 101: 17084–17089, https://doi.org/10.1074/jbc.m808221200.Suche in Google Scholar
Chandrangsu, P. and Helmann, J.D. (2016). Intracellular Zn (II) intoxication leads to dysregulation of the PerR regulon resulting in heme toxicity in Bacillus subtilis . PLoS Genet. 12: e1006515, https://doi.org/10.1371/journal.pbio.1000588.Suche in Google Scholar
Chang, A.L., Tuckerman, J.R., Gonzalez, G., Mayer, R., Weinhouse, H., Volman, G., Amikam, D., Benziman, M., and Gilles-Gonzalez, M.-A. (2001). Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor. Biochemistry 40: 3420–3426, https://doi.org/10.1371/journal.pbio.1000587.Suche in Google Scholar
Chatterjee, D., Cooley, R.B., Boyd, C.D., Mehl, R.A., O’Toole, G.A., and Sondermann, H. (2014). Mechanistic insight into the conserved allosteric regulation of periplasmic proteolysis by the signaling molecule cyclic-di-GMP. eLife 3: e03650, https://doi.org/10.1073/pnas.0808933106.Suche in Google Scholar
Chen, H., Luo, Z., and Tahara, H. (2001). Formal solutions of nonlinear first order totally characteristic type PDE with irregular singularity. Ann. Inst. Fourier, 51: 1599–1620. https://doi.org/10.1126/science.1206848.Suche in Google Scholar
Chen, Y., Chai, Y., Guo, J.-h., and Losick, R. (2012). Evidence for cyclic di-GMP-mediated signaling in Bacillus subtilis . J. Bacteriol. 194: 5080–5090, https://doi.org/10.1073/pnas.1507245112.Suche in Google Scholar
Chen, L.-H., Köseoğlu, V.K., Güvener, Z.T., Myers-Morales, T., Reed, J.M., D’Orazio, S.E., Miller, K.W., and Gomelsky, M. (2014). Cyclic di-GMP-dependent signaling pathways in the pathogenic Firmicute Listeria monocytogenes . PLoS Pathog. 10, https://doi.org/10.1111/mmi.12013.Suche in Google Scholar
Choy, W.-K., Zhou, L., Syn, C.K.-C., Zhang, L.-H., and Swarup, S. (2004). MorA defines a new class of regulators affecting flagellar development and biofilm formation in diverse Pseudomonas species. J. Bacteriol. 186: 7221–7228, https://doi.org/10.1128/jb.00300-18.Suche in Google Scholar
Christen, M., Christen, B., Folcher, M., Schauerte, A., and Jenal, U. (2005). Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 280: 30829–30837, https://doi.org/10.1101/gad.475808.Suche in Google Scholar
Cohen, D., Mechold, U., Nevenzal, H., Yarmiyhu, Y., Randall, T.E., Bay, D.C., Rich, J.D., Parsek, M.R., Kaever, V., and Harrison, J.J. (2015). Oligoribonuclease is a central feature of cyclic diguanylate signaling in Pseudomonas aeruginosa . Proc. Natl. Acad. Sci. U.S.A. 112: 11359–11364, https://doi.org/10.1016/j.molcel.2012.03.023.Suche in Google Scholar
Commichau, F.M. and Stülke, J. (2008). Trigger enzymes: bifunctional proteins active in metabolism and in controlling gene expression. Mol. Microbiol. 67: 692–702, https://doi.org/10.1111/mmi.12066.Suche in Google Scholar
D’Argenio, D.A. and Miller, S.I. (2004). Cyclic di-GMP as a bacterial second messenger. Microbiology 150: 2497–2502. https://doi.org/10.1099/mic.0.27099-0.Suche in Google Scholar
Dahlstrom, K.M., Giglio, K.M., Collins, A.J., Sondermann, H., and O’Toole, G.A. (2015). Contribution of physical interactions to signaling specificity between a diguanylate cyclase and its effector. mBio 6: e01915–e01978, https://doi.org/10.1016/j.mib.2006.02.010.Suche in Google Scholar
Dahlstrom, K.M., Giglio, K.M., Sondermann, H., and O’Toole, G.A. (2016). The inhibitory site of a diguanylate cyclase is a necessary element for interaction and signaling with an effector protein. J. Bacteriol. 198: 1595–1603, https://doi.org/10.1128/mmbr.00043-12.Suche in Google Scholar
De, N., Pirruccello, M., Krasteva, P.V., Bae, N., Raghavan, R.V., and Sondermann, H. (2008). Phosphorylation-independent regulation of the diguanylate cyclase WspR. PLoS Biol. 6, https://doi.org/10.1111/j.1365-2958.2005.04697.x.Suche in Google Scholar
Diethmaier, C., Newman, J.A., Kovács, Á.T., Kaever, V., Herzberg, C., Rodrigues, C., Boonstra, M., Kuipers, O.P., Lewis, R.J., and Stülke, J. (2014). The YmdB phosphodiesterase is a global regulator of late adaptive responses in Bacillus subtilis . J. Bacteriol. 196: 265–275, https://doi.org/10.1046/j.1365-2958.2000.01822.x.Suche in Google Scholar
Dow, J.M., Fouhy, Y., Lucey, J.F., and Ryan, R.P. (2006). The HD-GYP domain, cyclic di-GMP signaling, and bacterial virulence to plants. Mol. Plant Microbe Interact. 19: 1378–1384, https://doi.org/10.1038/s41598-018-33842-9.Suche in Google Scholar
Duerig, A., Abel, S., Folcher, M., Nicollier, M., Schwede, T., Amiot, N., Giese, B., and Jenal, U. (2009). Second messenger-mediated spatiotemporal control of protein degradation regulates bacterial cell cycle progression. Gene Dev. 23: 93–104, https://doi.org/10.1038/325279a0.Suche in Google Scholar
Fagerlund, A., Smith, V., Røhr, Å.K., Lindbäck, T., Parmer, M.P., Andersson, K.K., Reubsaet, L., and Økstad, O.A. (2016). Cyclic diguanylate regulation of Bacillus cereus group biofilm formation. Mol. Microbiol. 101: 471–494, https://doi.org/10.1073/pnas.0912839107.Suche in Google Scholar
Galperin, M.Y. (2005). A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 5: 35, https://doi.org/10.1074/jbc.c600179200.Suche in Google Scholar
Galperin, M.Y., Nikolskaya, A.N., and Koonin, E.V. (2001). Novel domains of the prokaryotic two-component signal transduction systems. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 203: 11–21, https://doi.org/10.1128/mbio.01639-17.Suche in Google Scholar
Gao, X., Mukherjee, S., Matthews, P.M., Hammad, L.A., Kearns, D.B., and Dann, C.E.3rd (2013). Functional characterization of core components of the Bacillus subtilis cyclic-di-GMP signaling pathway. J. Bacteriol. 195: 4782–4792, https://doi.org/10.1073/pnas.1702435114.Suche in Google Scholar
Giacalone, D., Smith, T.J., Collins, A.J., Sondermann, H., Koziol, L.J., and O’Toole, G.A. (2018). Ligand-mediated biofilm formation via enhanced physical interaction between a diguanylate cyclase and its receptor. mBio 9: e01254–e01218, https://doi.org/10.1038/nrmicro2203.Suche in Google Scholar
Guttenplan, S.B., Shaw, S., and Kearns, D.B. (2013). The cell biology of peritrichous flagella in Bacillus subtilis . Mol. Microbiol. 87: 211–229, https://doi.org/10.1111/j.1365-2958.2004.04206.x.Suche in Google Scholar
Güvener, Z.T. and Harwood, C.S. (2007). Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic‐di‐GMP in response to growth on surfaces. Mol. Microbiol. 66: 1459–1473, https://doi.org/10.1073/pnas.1716231114.Suche in Google Scholar
Habazettl, J., Allan, M.G., Jenal, U., and Grzesiek, S. (2011). Solution structure of the PilZ domain protein PA4608 complex with cyclic di-GMP identifies charge clustering as molecular readout. J. Biol. Chem. 286: 14304–14314, https://doi.org/10.1126/science.1159519.Suche in Google Scholar
Hengge, R. (2009). Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7: 263–273. https://doi.org/10.1038/nrmicro2109.Suche in Google Scholar
Hengge, R. (2010a). Cyclic-di-GMP reaches out into the bacterial RNA world. Sci. Signal. 3: pe44, https://doi.org/10.1074/jbc.m113.516195.Suche in Google Scholar
Hengge, R. (2010b). Role of cyclic di-GMP in the regulatory networks of Escherichia coli . In: The second messenger cyclic di-GMP. American Society of Microbiology, Washington, D.C. pp. 230–252.10.1128/9781555816667.ch16Suche in Google Scholar
Hickman, J.W. and Harwood, C.S. (2008). Identification of FleQ from Pseudomonas aeruginosa as ac‐di‐GMP‐responsive transcription factor. Mol. Microbiol. 69: 376–389, https://doi.org/10.1111/j.1365-2958.2004.04155.x.Suche in Google Scholar
Hisert, K.B., MacCoss, M., Shiloh, M.U., Darwin, K.H., Singh, S., Jones, R.A., Ehrt, S., Zhang, Z., Gaffney, B.L., and Gandotra, S. (2005). A glutamate‐alanine‐leucine (EAL) domain protein of Salmonella controls bacterial survival in mice, antioxidant defence and killing of macrophages: role of cyclic diGMP. Mol. Microbiol. 56: 1234–1245, https://doi.org/10.1016/j.cell.2014.07.022.Suche in Google Scholar
Hoffman, E.C., Reyes, H., Chu, F.-F., Sander, F., Conley, L.H., Brooks, B.A., and Hankinson, O. (1991). Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 252: 954–958, https://doi.org/10.1021/bi901409g.Suche in Google Scholar
Hurley, J.H. (2003). GAF domains: cyclic nucleotides come full circle. Sci. Signal. 2003: pe1, https://doi.org/10.1074/jbc.r115.711507.Suche in Google Scholar
Jenal, U. and Malone, J. (2006). Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40: 385–407, https://doi.org/10.1111/j.1365-2958.2006.05440.x.Suche in Google Scholar
Jenal, U., Reinders, A., and Lori, C. (2017). Cyclic di-GMP: second messenger extraordinaire. Nat. Rev. Microbiol. 15: 271–284, https://doi.org/10.1128/jb.00247-19.Suche in Google Scholar
Kader, A., Simm, R., Gerstel, U., Morr, M., and Römling, U. (2006). Hierarchical involvement of various GGDEF domain proteins in development of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 60: 602–616, https://doi.org/10.1111/j.1365-2958.2006.05123.x.Suche in Google Scholar
Köseoğlu, V.K., Heiss, C., Azadi, P., Topchiy, E., Güvener, Z.T., Lehmann, T.E., Miller, K.W., and Gomelsky, M. (2015). Listeria monocytogenes exopolysaccharide: origin, structure, biosynthetic machinery and c‐di‐GMP‐dependent regulation. Mol. Microbiol. 96: 728–743. https://doi.org/10.1111/mmi.12966.Suche in Google Scholar
Krasteva, P.V., Fong, J.C., Shikuma, N.J., Beyhan, S., Navarro, M.V., Yildiz, F.H., and Sondermann, H. (2010). Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327: 866–868, https://doi.org/10.1126/science.1181185.Suche in Google Scholar
Kulasakara, H., Lee, V., Brencic, A., Liberati, N., Urbach, J., Miyata, S., Lee, D.G., Neely, A.N., Hyodo, M. and Hayakawa, Y. (2006). Correction for Kulasakara et al., analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence . Proc. Natl. Acad. Sci. U.S.A. 103: 6411, https://doi.org/10.1073/pnas.0601679103.Suche in Google Scholar
Kunz, S., Tribensky, A., Steinchen, W., Oviedo-Bocanegra, L., Bedrunka, P., and Graumann, P.L. (2020). Cyclic di-GMP signaling in Bacillus subtilis is governed by direct interactions of diguanylate cyclases and cognate receptors. mBio 11, https://doi.org/10.1128/mbio.03122-19.Suche in Google Scholar
Le Quéré, B. and Ghigo, J.M. (2009). BcsQ is an essential component of the Escherichia coli cellulose biosynthesis apparatus that localizes at the bacterial cell pole. Mol. Microbiol. 72: 724–740, https://doi.org/10.1111/j.1365-2958.2009.06678.x.Suche in Google Scholar
Leduc, J.L. and Roberts, G.P. (2009). Cyclic di-GMP allosterically inhibits the CRP-like protein (Clp) of Xanthomonas axonopodis pv. citri. J. Bacteriol. 191: 7121–7122, https://doi.org/10.1128/jb.00845-09.Suche in Google Scholar
Lee, V.T., Matewish, J.M., Kessler, J.L., Hyodo, M., Hayakawa, Y., and Lory, S. (2007). A cyclic‐di‐GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 65: 1474–1484, https://doi.org/10.1111/j.1365-2958.2007.05879.x.Suche in Google Scholar
Lee, E.R., Baker, J.L., Weinberg, Z., Sudarsan, N., and Breaker, R.R. (2010). An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science 329: 845–848, https://doi.org/10.1126/science.1190713.Suche in Google Scholar
Li, Z., Chen, J.-H., Hao, Y., and Nair, S.K. (2012). Structures of the PelD cyclic diguanylate effector involved in pellicle formation in Pseudomonas aeruginosa PAO1. J. Biol. Chem. 287: 30191–30204, https://doi.org/10.1074/jbc.m112.378273.Suche in Google Scholar
Liang, Z.-X. (2015). The expanding roles of c-di-GMP in the biosynthesis of exopolysaccharides and secondary metabolites. Nat. Prod. Rep. 32: 663–683, https://doi.org/10.1039/c4np00086b.Suche in Google Scholar
Lindenberg, S., Klauck, G., Pesavento, C., Klauck, E., and Hengge, R. (2013). The EAL domain protein YciR acts as a trigger enzyme in a c-di-GMP signalling cascade in E. coli biofilm control. EMBO J. 32: 2001–2014, https://doi.org/10.1038/emboj.2013.120.Suche in Google Scholar
Luo, Y., Zhao, K., Baker, A.E., Kuchma, S.L., Coggan, K.A., Wolfgang, M.C., Wong, G.C., and O’Toole, G.A. (2015). A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. mBio 6: e02414–e02456, https://doi.org/10.1128/mbio.02456-14.Suche in Google Scholar
Merighi, M., Lee, V.T., Hyodo, M., Hayakawa, Y., and Lory, S. (2007). The second messenger bis‐(3′‐5′)‐cyclic‐GMP and its PilZ domain‐containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa . Mol. Microbiol. 65: 876–895, https://doi.org/10.1111/j.1365-2958.2007.05817.x.Suche in Google Scholar
Mills, E., Petersen, E., Kulasekara, B.R., and Miller, S.I. (2015). A direct screen for c-di-GMP modulators reveals a Salmonella typhimurium periplasmic ʟ-arginine–sensing pathway. Sci. Signal. 8: ra57. https://doi.org/10.1126/scisignal.aaa1796.Suche in Google Scholar
Minasov, G., Padavattan, S., Shuvalova, L., Brunzelle, J.S., Miller, D.J., Baslé, A., Massa, C., Collart, F.R., Schirmer, T., and Anderson, W.F. (2009). Crystal structures of YkuI and its complex with second messenger cyclic di-GMP suggest catalytic mechanism of phosphodiester bond cleavage by EAL domains. J. Biol. Chem. 284: 13174–13184, https://doi.org/10.1074/jbc.m808221200.Suche in Google Scholar
Navarro, M.V., Newell, P.D., Krasteva, P.V., Chatterjee, D., Madden, D.R., O’Toole, G.A., and Sondermann, H. (2011). Structural basis for c-di-GMP-mediated inside-out signaling controlling periplasmic proteolysis. PLoS Biol. 9: e1000588, https://doi.org/10.1371/journal.pbio.1000588.Suche in Google Scholar
Newell, P.D., Boyd, C.D., Sondermann, H., and O’Toole, G.A. (2011). A c-di-GMP effector system controls cell adhesion by inside-out signaling and surface protein cleavage. PLoS Biol. 9, https://doi.org/10.1371/journal.pbio.1000587.Suche in Google Scholar
Newell, P.D., Monds, R.D. and O’Toole, G.A. (2009). LapD is a bis-(3′,5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0–1 . Proc. Natl. Acad. Sci. U.S.A. 106, 3461–3466. https://doi.org/10.1073/pnas.0808933106.Suche in Google Scholar
Nicolas, P., Mäder, U., Dervyn, E., Rochat, T., Leduc, A., Pigeonneau, N., Bidnenko, E., Marchadier, E., Hoebeke, M., and Aymerich, S. (2012). Condition-dependent reveals high-level regulatory architecture in Bacillus subtilis . Science 335: 1103–1106, https://doi.org/10.1126/science.1206848.Suche in Google Scholar
O’Connor, J.R., Kuwada, N.J., Huangyutitham, V., Wiggins, P.A., and Harwood, C.S. (2012). Surface sensing and lateral subcellular localization of WspA, the receptor in a chemosensory‐like system leading to c‐di‐GMP production. Mol. Microbiol. 86: 720–729, https://doi.org/10.1111/mmi.12013.Suche in Google Scholar
Orr, M.W., Donaldson, G.P., Severin, G.B., Wang, J., Sintim, H.O., Waters, C.M. and Lee, V.T. (2015). Oligoribonuclease is the primary degradative enzyme for pGpG in Pseudomonas aeruginosa that is required for cyclic-di-GMP turnover . Proc. Natl. Acad. Sci. U.S.A. 112, E5048–E5057, https://doi.org/10.1073/pnas.1507245112.Suche in Google Scholar
Orr, M.W., Weiss, C.A., Severin, G.B., Turdiev, H., Kim, S.-K., Turdiev, A., Liu, K., Tu, B.P., Waters, C.M., and Winkler, W.C. (2018). A subset of exoribonucleases serve as degradative enzymes for pGpG in c-di-GMP signaling. J. Bacteriol. 200: e00300–e00318, https://doi.org/10.1128/jb.00300-18.Suche in Google Scholar
Pesavento, C., Becker, G., Sommerfeldt, N., Possling, A., Tschowri, N., Mehlis, A. and Hengge, R. (2008). Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli . Genes & Development 22, 2434–2446, https://doi.org/10.1101/gad.475808.Suche in Google Scholar
Plate, L. and Marletta, M.A. (2012). Nitric oxide modulates bacterial biofilm formation through a multicomponent cyclic-di-GMP signaling network. Mol. Cell 46: 449–460, https://doi.org/10.1016/j.molcel.2012.03.023.Suche in Google Scholar
Pultz, I.S., Christen, M., Kulasekara, H.D., Kennard, A., Kulasekara, B., and Miller, S.I. (2012). The response threshold of Salmonella PilZ domain proteins is determined by their binding affinities for c‐di‐GMP. Mol. Microbiol. 86: 1424–1440, https://doi.org/10.1111/mmi.12066.Suche in Google Scholar
Ramelot, T.A., Yee, A., Cort, J.R., Semesi, A., Arrowsmith, C.H., and Kennedy, M.A. (2007). NMR structure and binding studies confirm that PA4608 from Pseudomonas aeruginosa is a PilZ domain and ac‐di‐GMP binding protein. Proteins: Struct. Funct. Bioinform. 66: 266–271. https://doi.org/10.1002/prot.21199.Suche in Google Scholar
Römling, U. and Amikam, D. (2006). Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 9: 218–228, https://doi.org/10.1016/j.mib.2006.02.010.Suche in Google Scholar
Römling, U., Galperin, M.Y., and Gomelsky, M. (2013). Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 77: 1–52, https://doi.org/10.1128/mmbr.00043-12.Suche in Google Scholar
Römling, U., Gomelsky, M., and Galperin, M.Y. (2005). C‐di‐GMP: the dawning of a novel bacterial signalling system. Mol. Microbiol. 57: 629–639, https://doi.org/10.1111/j.1365-2958.2005.04697.x.Suche in Google Scholar
Römling, U., Rohde, M., Olsén, A., Normark, S., and Reinköster, J. (2000). AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 36: 10–23, https://doi.org/10.1046/j.1365-2958.2000.01822.x.Suche in Google Scholar
Rösch, T.C., Oviedo-Bocanegra, L.M., Fritz, G., and Graumann, P.L. (2018). SMTracker: a tool for quantitative analysis, exploration and visualization of single-molecule tracking data reveals highly dynamic binding of B. subtilis global repressor AbrB throughout the genome. Sci. Rep. 8: 1–12, https://doi.org/10.1038/s41598-018-33842-9.Suche in Google Scholar
Ross, P., Weinhouse, H., Aloni, Y., Michaeli, D., Weinberger-Ohana, P., Mayer, R., Braun, S., De Vroom, E., Van der Marel, G., and Van Boom, J. (1987). Regulation of cellulose synthesis in Acetobacter by cyclic acid. Nature 325: 279–281, https://doi.org/10.1038/325279a0.Suche in Google Scholar
Ryan, R.P., McCarthy, Y., Andrade, M., Farah, C.S., Armitage, J.P., and Dow, J.M. (2010). Cell–cell signal-dependent dynamic interactions between HD-GYP and GGDEF domain proteins mediate virulence in Xanthomonas campestris . Proc. Natl. Acad. Sci. U.S.A. 107, 5989–5994, https://doi.org/10.1073/pnas.0912839107.Suche in Google Scholar
Ryjenkov, D.A., Simm, R., Römling, U., and Gomelsky, M. (2006). The PilZ domain is a receptor for the second messenger c-di-GMP the PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281: 30310–30314, https://doi.org/10.1074/jbc.c600179200.Suche in Google Scholar
Sarenko, O., Klauck, G., Wilke, F.M., Pfiffer, V., Richter, A.M., Herbst, S., Kaever, V., and Hengge, R. (2017). More than enzymes that make or break cyclic di-GMP—local signaling in the interactome of GGDEF/EAL domain proteins of Escherichia coli . mBio 8: e01617–e01639, https://doi.org/10.1128/mbio.01639-17.Suche in Google Scholar
Schäper, S., Steinchen, W., Krol, E., Altegoer, F., Skotnicka, D., Søgaard-Andersen, L., Bange, G. and Becker, A. (2017). AraC-like transcriptional activator CuxR binds c-di-GMP by a PilZ-like mechanism to regulate extracellular polysaccharide production . Proc. Natl. Acad. Sci. U.S.A. 114: E4822–E4831. https://doi.org/10.1073/pnas.1702435114.Suche in Google Scholar
Schirmer, T. and Jenal, U. (2009). Structural and mechanistic determinants of c-di-GMP signalling. Nat. Rev. Microbiol. 7: 724–735, https://doi.org/10.1038/nrmicro2203.Suche in Google Scholar
Simm, R., Morr, M., Kader, A., Nimtz, M., and Römling, U. (2004). GGDEF and EAL domains inversely regulate cyclic di‐GMP levels and transition from to motility. Mol. Microbiol. 53: 1123–1134, https://doi.org/10.1111/j.1365-2958.2004.04206.x.Suche in Google Scholar
Subramanian, S., Gao, X., Dann, C.E.3rd and Kearns, D.B. (2017). MotI (DgrA) acts as a molecular clutch on the flagellar stator protein MotA in Bacillus subtilis . Proc. Natl. Acad. Sci. U.S.A. 114: 13537–13542, https://doi.org/10.1073/pnas.1716231114.Suche in Google Scholar
Sudarsan, N., Lee, E., Weinberg, Z., Moy, R., Kim, J., Link, K., and Breaker, R. (2008). Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 321: 411–413, https://doi.org/10.1126/science.1159519.Suche in Google Scholar
Sundriyal, A., Massa, C., Samoray, D., Zehender, F., Sharpe, T., Jenal, U., and Schirmer, T. (2014). Inherent regulation of EAL domain-catalyzed hydrolysis of second messenger cyclic di-GMP. J. Biol. Chem. 289: 6978–6990, https://doi.org/10.1074/jbc.m113.516195.Suche in Google Scholar
Tal, R., Gelfand, D.H., Calhoon, R.D., Ben-Bassat, A., Benziman, M., and Wong, H.C. (1998). Cyclic di-guanylate metabolic enzymes. United States Patent No 5,759,828.Suche in Google Scholar
Taylor, B.L. and Zhulin, I.B. (1999). PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63: 479–506, https://doi.org/10.1128/mmbr.63.2.479-506.1999.Suche in Google Scholar
Tischler, A.D. and Camilli, A. (2004). Cyclic diguanylate (c‐di‐GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 53: 857–869, https://doi.org/10.1111/j.1365-2958.2004.04155.x.Suche in Google Scholar
Tschowri, N., Schumacher, M.A., Schlimpert, S., babu Chinnam, N., Findlay, K.C., Brennan, R.G., and Buttner, M.J. (2014). Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development. Cell 158: 1136–1147, https://doi.org/10.1016/j.cell.2014.07.022.Suche in Google Scholar
Tuckerman, J.R., Gonzalez, G., Sousa, E.H., Wan, X., Saito, J.A., Alam, M., and Gilles-Gonzalez, M.-A. (2009). An oxygen-sensing diguanylate cyclase and phosphodiesterase couple for c-di-GMP control . Biochemistry 48: 9764–9774. https://doi.org/10.1021/bi901409g.Suche in Google Scholar
Valentini, M. and Filloux, A. (2016). Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 291: 12547–12555, https://doi.org/10.1074/jbc.r115.711507.Suche in Google Scholar
Weber, H., Pesavento, C., Possling, A., Tischendorf, G., and Hengge, R. (2006). Cyclic‐di‐GMP‐mediated signalling within the σS network of Escherichia coli . Mol. Microbiol. 62: 1014–1034, https://doi.org/10.1111/j.1365-2958.2006.05440.x.Suche in Google Scholar
Weiss, C.A., Hoberg, J.A., Liu, K., Tu, B.P., and Winkler, W.C. (2019). Single-cell microscopy reveals that levels of cyclic di-GMP vary among Bacillus subtilis subpopulations. J. Bacteriol. 201: e00219–e00247, https://doi.org/10.1128/jb.00247-19.Suche in Google Scholar
Winkler, A., Udvarhelyi, A., Hartmann, E., Reinstein, J., Menzel, A., Shoeman, R.L., and Schlichting, I. (2014). Characterization of elements involved in allosteric light regulation of phosphodiesterase activity by comparison of different functional BlrP1 states. J. Mol. Biol. 426: 853–868, https://doi.org/10.1016/j.jmb.2013.11.018.Suche in Google Scholar
Xu, H., Chater, K.F., Deng, Z., and Tao, M. (2008). A cellulose synthase-like protein involved in hyphal tip growth and morphological differentiation in Streptomyces . J. Bacteriol. 190: 4971–4978, https://doi.org/10.1128/jb.01849-07.Suche in Google Scholar
Xu, J., Kim, J., Koestler, B.J., Choi, J.H., Waters, C.M., and Fuqua, C. (2013). Genetic analysis of Agrobacterium tumefaciens unipolar polysaccharide production reveals complex integrated control of the motile‐to‐sessile switch. Mol. Microbiol. 89: 929–948, https://doi.org/10.1111/mmi.12321.Suche in Google Scholar
Zähringer, F., Lacanna, E., Jenal, U., Schirmer, T., and Boehm, A. (2013). Structure and signaling mechanism of a zinc-sensory diguanylate cyclase. Structure 21: 1149–1157, https://doi.org/10.1016/j.str.2013.04.026.Suche in Google Scholar
© 2020 Sandra Kunz and Peter L. Graumann, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Highlight: How Microorganisms View and Respond to Their World
- Masters of change
- Physiology of guanosine-based second messenger signaling in Bacillus subtilis
- Spatial organization enhances versatility and specificity in cyclic di-GMP signaling
- Cyclic di-GMP signaling controlling the free-living lifestyle of alpha-proteobacterial rhizobia
- Generating asymmetry in a changing environment: cell cycle regulation in dimorphic alphaproteobacteria
- Multicellular and unicellular responses of microbial biofilms to stress
- Biosynthesis and function of cell-surface polysaccharides in the social bacterium Myxococcus xanthus
- Diversity of GPI-anchored fungal adhesins
- Glutaredoxins and iron-sulfur protein biogenesis at the interface of redox biology and iron metabolism
- Biochemical unity revisited: microbial central carbon metabolism holds new discoveries, multi-tasking pathways, and redundancies with a reason
- The ups and downs of ectoine: structural enzymology of a major microbial stress protectant and versatile nutrient
- Unexpected metabolic versatility among type II methanotrophs in the Alphaproteobacteria
- Metabolism of non-growing bacteria
- DASH-type cryptochromes – solved and open questions
- Specific acclimations to phosphorus limitation in the marine diatom Phaeodactylum tricornutum
Artikel in diesem Heft
- Frontmatter
- Highlight: How Microorganisms View and Respond to Their World
- Masters of change
- Physiology of guanosine-based second messenger signaling in Bacillus subtilis
- Spatial organization enhances versatility and specificity in cyclic di-GMP signaling
- Cyclic di-GMP signaling controlling the free-living lifestyle of alpha-proteobacterial rhizobia
- Generating asymmetry in a changing environment: cell cycle regulation in dimorphic alphaproteobacteria
- Multicellular and unicellular responses of microbial biofilms to stress
- Biosynthesis and function of cell-surface polysaccharides in the social bacterium Myxococcus xanthus
- Diversity of GPI-anchored fungal adhesins
- Glutaredoxins and iron-sulfur protein biogenesis at the interface of redox biology and iron metabolism
- Biochemical unity revisited: microbial central carbon metabolism holds new discoveries, multi-tasking pathways, and redundancies with a reason
- The ups and downs of ectoine: structural enzymology of a major microbial stress protectant and versatile nutrient
- Unexpected metabolic versatility among type II methanotrophs in the Alphaproteobacteria
- Metabolism of non-growing bacteria
- DASH-type cryptochromes – solved and open questions
- Specific acclimations to phosphorus limitation in the marine diatom Phaeodactylum tricornutum

