Abstract
Background
Although it is widely accepted that catecholamines and estrogens influence immunity and have consequences for health, their effect on innate immunity (e.g. monocytes and neutrophils) is still not fully investigated.
Materials and methods
Our study aimed to analyze the production of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, monocyte chemoattractant protein (MCP)-1 and IL-8 by whole blood cells following short-term exposure to epinephrine (Epi) and 17β-estradiol (E2) in the presence or absence of lipopolysaccharide (LPS). We also evaluated the in vitro effect of these hormones on expression of β2 integrin (CD11b/CD18) and L-selectin (CD62L) by circulating neutrophils and monocytes in the blood of healthy subjects.
Results
Epi has shown a potential to modulate the production of pro-inflammatory mediators. Its exposure resulted in significantly increased production of IL-8 in a dose-dependent manner. On the contrary, a dose-dependent suppression of LPS-induced production of IL-1β, IL-8, and MCP-1 by Epi was observed. In neutrophils, a modest rise in CD11b expression was observed after Epi exposure. Simultaneously, Epi suppressed LPS-induced expression of CD11b and CD18. In monocytes, Epi suppressed LPS-induced expression of C11b. E2 inhibited LPS-induced TNF-α production and caused a significant decrease in CD62L expression in both cell populations. No significant changes were observed after double exposure of cells with Epi and E2.
Conclusions
Thus, our results show that Epi and E2 differentially modulate the innate immune response and have a dual effect on cytokine modulation. The findings suggest that the observed immunoregulatory role of Epi and E2 may influence the outcome in endotoxin responses and can be critical in the regulation of inflammatory responses.
Introduction
Hormones are the largest class of cell-cell signaling molecules of the endocrine system that regulate a range of physiological processes from reproductive development to immune homeostasis. Regulation of this well-balanced system occurs through interactions with receptors on immune cells which affect the differentiation and maturation of cytokine-producing immunocytes and ultimately the functioning of immunity. Disturbances within these systems may have adverse effect on a variety of immunological mechanisms and lead to immune activation or suppression, depending on the systems being affected and the nature of the stimuli [1], [2].
There is extensive evidence for gender-based differences in immune responses leading to a sexual dimorphism in the immune response which is mostly mediated by the effects of sex hormones on the nonspecific immune response [3], [4]. Particularly, 17β-estradiol (E2) is the most common circulating form of estrogen with a number of in vivo and in vitro divergent immunomodulatory effects. It is believed that E2 inhibits production and secretion of pro-inflammatory Th1 and Th17 cytokines through the inhibition of NF-κB signaling [5], [6], [7]. However, opposing results have been reported by several groups who have demonstrated the ability of E2 to enhance proinflammatory and Th1 cytokine production [8], [9]. E2 can potentially act on all cellular subsets and the mechanisms involved in the ability of this estrogen to modulate the innate immune response seem to be complex and remain poorly understood.
In addition to sex hormones, the immune system, especially the innate or inflammatory response, is also modulated by stress hormones. Humans can be the subjects of stressful life events, and psychological challenges are capable of modifying various characteristics of the immune response. Stress may have bidirectional effects on immune function, being both immunosuppressive and immunoenhancing [1]. The stress response is characterized by increased secretion of catecholamines such as epinephrine (Epi). Because it is short-term (lasting just minutes to hours) acute stress initially affects cells of the innate immune system such as dendritic cells, neutrophils and macrophages through immunoenhancing effects [10], [11]. Epi affects immune cell function by inducing changes in leukocyte redistribution, cytokine expression, phenotypical surface expression and leukocyte functionality [12], [13].
Although it is widely accepted that both hormones, Epi and E2, influence immunity and have consequences for health, their effect on innate immunity (e.g. monocytes and neutrophils) is still underappreciated and not well investigated. In addition, investigation of E2 and Epi are of particular interest for in vitro estimations of the combined effect of stress- and sex/pregnancy-related hormones, which has not yet been studied. This study was undertaken to analyze the production of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, monocyte chemoattractant protein (MCP)-1 and IL-8 by whole blood cells after Epi and E2 exposure in the presence or absence of lipopolysaccharide (LPS). Simultaneously, we investigated the effects of E2, Epi and LPS on the expression of membrane-associated proteins β2 integrin (subunits CD11b and CD18) and L-selectin (CD62L) by monocytes and neutrophils from the blood of healthy subjects.
Materials and methods
Sampling
A group of healthy volunteers were recruited for the study and signed informed consent was obtained from all subjects who provided blood samples. The study was approved by the Ethical Committee of the Institute of Molecular Biology of the NAS RA (IRB IORG0003427). Venous blood samples were obtained from 22 healthy subjects: ten men (mean age of 29 ± 5.1 years) and 12 women (mean age of 33 ± 3.4 years). Selection criteria for the subjects were as follows: (1) studied subjects were not taking any medication at the time of the experiments; (2) none of the individuals suffered from acute or chronic diseases; (3) not smokers; (4) women included in the study had regular menstrual cycles and did not use oral contraception; (5) blood of selected women was collected in the mid follicular phase to minimize diversity of estrogen levels in the blood of studied women. In vitro experiments with E2 were conducted only with the female blood.
In vitro stimulation of whole blood with EPI, E2 and LPS
To investigate the in vitro effect of Epi and E2 in the presence or absence of LPS, peripheral blood was collected in EDTA tubes. Aliquots of whole blood were diluted 1:10 with RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal calf serum (FCS) and 2 mM L-glutamine (Sigma-Aldrich, St. Louis, MO, USA). The blood was distributed in 24-well plates and stimulated with Epi (10, 100, and 1000 µM), E2 (100 µM) in the presence or absence of LPS (100 ng/mL), or Epi (10, 100, and 1000 µM) + E2 (100 µM) for 4 h at 37 °C. Four hour time period has been used for a variety of reasons, including cell viability being 100% during this period, making it optimal for measurement of cells positive for IL-1, TNF-α, and IL-8. To determine the dose-effect curves for E2, kinetic studies were performed (data not shown). Following culturing, the supernatants were collected and frozen at −70 °C until determination of cytokines concentrations, and the cells were taken for the cytometric analyses.
Cytokine measurements
Cytokine release in supernatants was measured with specific immunoassays according to the manufacturer’s instructions. Cytokine production was analyzed using Human IL-1β, IL-8, TNF-α and MCP-1 ELISA MAX Deluxe kits (BioLegend, Inc., London, UK). The samples were read at 450 nm in a 96-well plate reader (HumaReader HS, Human Diagnostics Worldwide, Germany). Results were calibrated with serial dilutions of known quantities of recombinant cytokines.
Antibodies used for flow cytometry
APC/Cy7 anti-human CD14 and PerCP anti-human CD16 (BioLegend, Inc., London, UK) were used to discriminate neutrophils from monocytes in whole blood staining. FITC anti-human CD62L, PE anti-human CD18, and APC anti-human CD11b (BioLegend, Inc., London, UK) were used to detect surface expression of adhesion molecules. Irrelevant antibodies of the same isotypes were used as negative controls.
Flow cytometric analysis
At the end of stimulations, cells were washed and harvested. Afterwards, the cells were incubated with fluorescent mAb toward CD14, CD16, CD11b, CD18, and CD62L for 30 min. Labeled cells, after further washing, were resuspended in PBS supplemented with 1% BSA. Antigen expression was analyzed on a Partec CyFlow Space (Partec, Germany). Ten thousand events were collected from each sample. The neutrophil population in peripheral blood was distinguished by side scatter, CD16bright positivity and CD14 negativity. The monocyte population was determined as CD14++/CD16- cells. Gating and determination the mean fluorescence intensity (MFI) were done by FlowJo software (FlowJo, Ashland, OR, USA).
Statistics
Statistical analyses were carried out using the Statsoft Statistica package (http://www.statsoft.com). All values are given as means ± standard errors of the means. Normal distribution was checked visually from distributions and with the Shapiro-Wilk’s W test. For continuous variables, groups were compared using the paired Student’s t-test. p-Values ≤ 0.05 were considered as significant.
Results
Effect of Epi, E2, and LPS on surface expression of CD11b, CD18 and CD62L
To examine the changes in the receptor expression on neutrophil and monocyte cell surface after Epi (100 µM), E2 (100 µM) and LPS (100 ng/mL) exposure, we measured the relative expression of the molecules CD11b, CD18, and CD62L by flow cytometry. All studied surface markers were constitutively present on the surface of neutrophils and monocytes (data now shown). Overall the changes in expression of surface markers in neutrophils mostly overlap with those expressed in monocytes (Figure 1).

Effect of Epi (100 µM), E2 (100 µM) and LPS (100 ng/mL) on expression CD11b, CD62L, and CD18 surface markers in neutrophils and monocytes from lyzed whole blood.
Data represented as mean fluorescence intensity (MFI) ± SEM. Neutrophils were identified according to FSC/SSC gate and showing CD16bright and CD14− staining. Monocytees were identified according to FSC/SSC gate and showing CD14+ staining. *p < 0.05, **p < 0.01 compared with the untreated control group (Ctl), #p < 0.05 compared with the LPS exposure.
In neutrophils, LPS triggered a significant upregulation of CD11b and CD18 membrane expression (p < 0.01), while CD62L expression was significantly downregulated (p < 0.05). Double exposure of the cells with Epi and E2 did not significantly influence the expressions of surface molecules (Figure 1). A modest rise in CD11b expression (p < 0.05) was observed after Epi exposure, as well as a decreased expression of CD62L after E2 exposure (p < 0.05). E2 did not alter LPS-induced expression of surface markers, while Epi suppressed expression of CD11b and CD18 when cells were cultured with LPS and Epi together compared to LPS exposure alone (p < 0.05).
In monocytes, the effects of hormones and LPS were less profound, albeit similar. LPS exposure resulted in upregulated expression of CD11b (p < 0.01). As in neutrophils, LPS suppressed expression of CD62L in monocytes, however, this effect did not reach statistical significance (p > 0.05). Epi and E2 separately and together did not influence the expressions of CD11b and CD18, while CD62L expression was significantly suppressed by these two hormones (p < 0.05). In monocytes, only Epi was able to suppress LPS-induced expression of C11b (p < 0.05). CD18 expression was not affected by the exposure of Epi, E2 and LPS on gated monocytes (Figure 1).
Cytokine production by whole blood cells in the presence of Epi, E2 and LPS
To determine the effect of Epi and E2 on the production of IL-1β, IL-8, TNF-α and MCP-1, peripheral blood cells were incubated for 4 h with different concentrations of Epi (10, 100, and 1000 µM) or E2 (100 µM) in the absence or presence of LPS (100 ng/mL).
A low level of constitutive IL-1β, IL-8, TNF-α and MCP-1 was detected in supernatants from unstimulated control cells. As expected, endotoxin induced a substantial increase in the concentrations of all tested cytokines in cultured whole blood supernatants in comparison to unstimulated controls (Figure 2and Figure 3). Incubation of whole blood in the presence of different concentrations of Epi did not significantly influence supernatant levels of IL-1β, TNF-α, and MCP-1. However, Epi exposure resulted in significantly increased levels of the chemokine IL-8 in a dose-dependent manner (Figure 2and Figure 4). A more profound effect of Epi was detected in LPS-exposed cells. Epi resulted in suppression of LPS-induced production of IL-1β, IL-8, and MCP-1 (Figure 2). The modulatory effect of Epi on TNF-α production in response to LPS was not statistically significant (p > 0.05).
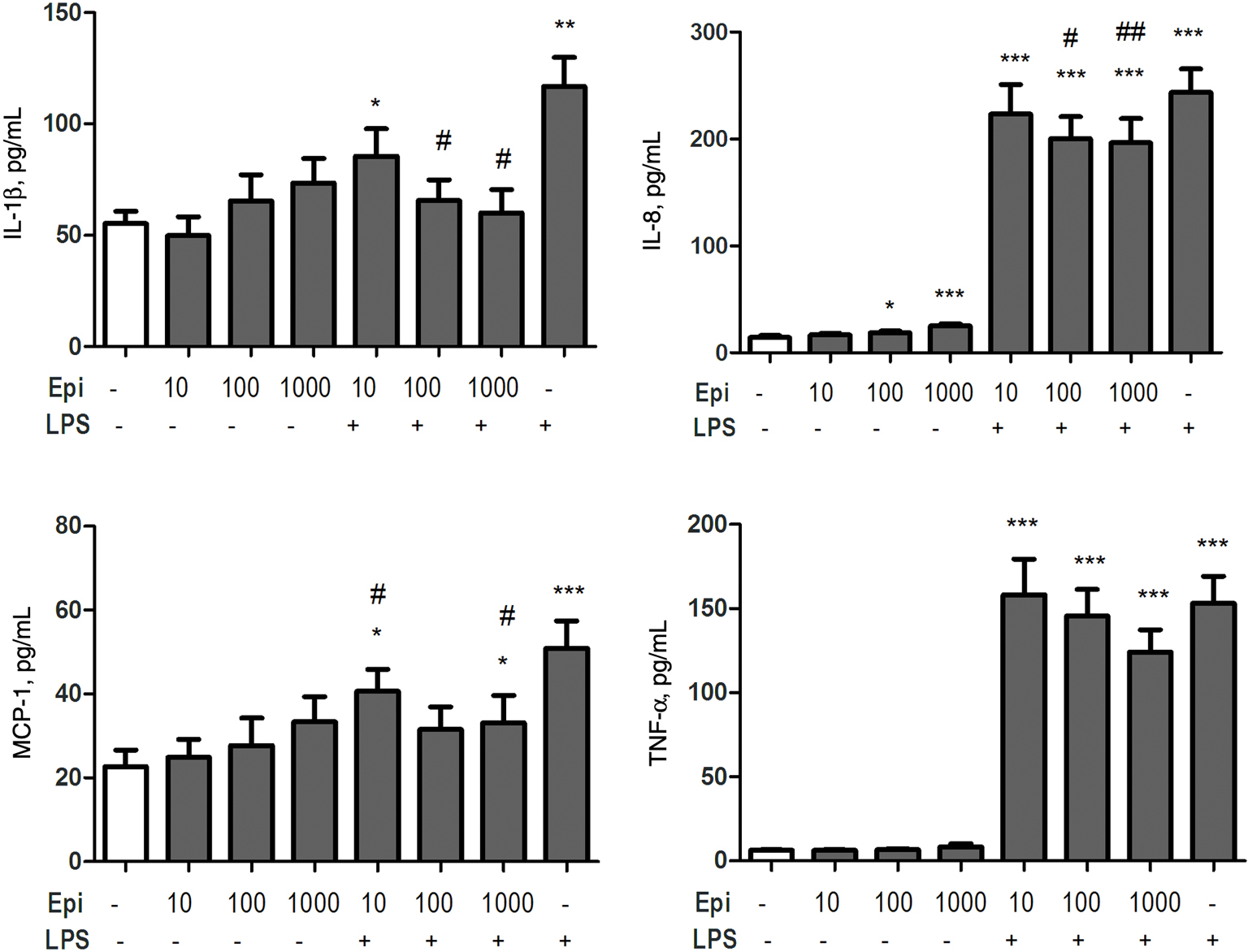
Effect of Epi (10, 100, and 1000 µM) and LPS (100 ng/mL) on the production of IL-1β, IL-8, TNF-α and MCP-1.
Data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the untreated control group (Ctl), #p < 0.05, ##p < 0.01 compared with the LPS exposure.

Effect of E2 (100 µM) and LPS (100 ng/mL) on the production of IL-1β, IL-8, TNF-α and MCP-1.
Data are presented as means ± SEM. **p < 0.01, ***p < 0.001 compared with the untreated control group (Ctl), #p < 0.05 compared with the LPS exposure.

Effect of double exposure by Epi (10, 100, and 1000 µM) and E2 (100 µM) of the whole blood cells.
Data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the untreated control group (Ctl).
Less profound effects were detected when whole blood cells were cultured with E2. E2 exposure had no significant effect on production of all studied cytokines. The presence of E2 had an inhibitory effect on LPS-induced production of TNF-α (Figure 3). The production of IL-8, IL-1β and MCP-1 was not affected by the presence E2 in LPS-treated cells.
We also aimed to study the effect of double exposure of cells with Epi and E2 and measure production of pro-inflammatory mediators by whole blood cells. E2-stimulated production of IL-1β, IL-8, TNF-α, and MCP-1 was not different as compared to the cultures with both E2 and different concentrations of Epi. Only IL-8 concentrations were affected by the presence of both hormones which were significantly higher than levels of control cultures (Figure 4). However, it is likely that this effect is mediated by the presence of Epi, as the effect of Epi exposure alone was similar to that obtained in double exposure. No significant difference was observed between males and females for all studied cytokines, independent of the inducer used (LPS and Epi) (data not shown).
Discussion
In the current study we have investigated the immunoregulatory role of hormones, focusing on Epi and E2, as well as their role in an LPS-induced immune response. Unfortunately, despite numerous studies demonstrating modulatory effects of Epi and E2, data have been variable and sometimes controversial.
Over recent years, the essential role of catecholamines, particularly Epi, in the regulation of cytokine production during inflammatory response has become increasingly apparent. The effect of Epi is highly dependent on the concentration and duration of exposure. The results of the present study demonstrate that cells of the innate immune system, under a short exposure of Epi, show evidence of activation, as measured by the increased expression of CD11b in neutrophils, decreased CD62L in monocytes, and increased production of IL-8 in whole blood. The reduced expression of the surface molecule CD62L may be attributed to the shedding effect which occurs after cell activation [14], [15]. This is followed by tight adhesion and migration of leukocytes mediated by β2 integrins, such as CD11b and CD18 [16]. The findings of a significantly lower expression of L-selectin and higher CD11b expression suggest an activated phenotype of innate immune cells after short exposure by Epi, however whether the modulated expression of these receptors is implicit in the process of inflammation or it reflects migratory processes initiated by Epi is unknown. Our data confirm the evidence that these molecules are involved in the stress response [17] which in terms of physiological stress can promote redistribution of immune cells [18]. Stress-induced cell redistribution represents an important tool to increase trafficking of immune cells to potential compartments within the body. Stress is proposed to play a role in the etiology of many pathologies, however, in certain conditions, such as short acute exposure, stress may enhance immune function and prepare the immune system for challenge.
The modulatory role of Epi is further supported by our observations that Epi exhibits anti-inflammatory activity in whole blood cells induced by LPS. Epi differentially modulated the LPS-induced production of IL-1β, IL-8, and MCP-1, as well as the expression of integrin CD11b/CD18 by downregulating their levels (Figure 1and Figure 2). Several studies have demonstrated that Epi is able to inhibit LPS-induced responses, but the data have been variable. Our findings are consistent with studies showing that Epi inhibits endotoxin-induced IL-1β production in whole blood [19]. In contrast, other groups have showed that Epi potentiates LPS-induced IL-8 production [20] and inhibits the production of TNF-α stimulated by LPS [21]. We have also detected a tendency towards downregulation of LPS-induced production of TNF-α after Epi exposure, however, this effect did not reach statistical significance (p > 0.05). The discrepancy between the results may be due to the different in vitro models employed. For example, in a study by Van der Poll et al. LPS-induced IL-8 production by human whole blood was achieved by using very low concentrations of LPS (5 ng/mL) and shorter period of incubation (2 h) [19]. These factors may lead to a completely different immune responses elicited by Epi.
Pro- and anti-inflammatory effects of Epi are mediated through specific adrenergic receptor (AR) subtypes. β1-AR activation has been shown to potentiate production of IL-1β in LPS stimulated THP-1 cells [22], while earlier investigations demonstrated β2-AR-mediated inhibition of pro-inflammatory IL-18 in LPS-stimulated human PBMCs and TNF-α in unstimulated THP-1 cells [23], [24]. These results suggest that Epi has a dual effect on cytokine modulation depending on different experimental methodologies or different in vitro conditions which may have led to these conflicting results. In confirmation of the dual effect of Epi is the synergism between IL-8, MCP-1 and β2-integrin in response to Epi (Figure 1and Figure 2). The trafficking of leukocytes from the blood to the surrounding tissue is crucial for immune reactions and is tightly regulated by cell adhesion molecules on immune and endothelial cells [16]. IL-8 and MCP-1 contribute to neutrophil and monocyte recruitment and migration by activating integrins such as β2-integrin [25], [26]. Taken together, our findings confirm the functional involvement of these molecules in the development of immune responses towards Epi.
Estrogenic hormones mediate its physiological effects thought various signaling pathways. Classical “genomic” action of estrogens is mediated by nuclear receptors (ERα and ERβ) and leads to epigenetic modifications of chromatin as well as transcription initiation [27]. Estrogens can also elicit nonnuclear (“nongenomic”) effects through the activation of membrane-associated signaling to initiate rapid (minutes) responses [28]. Studies that explored the possible crosstalk between this signaling cascades indicate that modifications induced by membrane localized ER could be required for further transcriptional activity of nuclear receptors [29], [30]. In our study, short exposure of the cells with E2 has lead to the less prominent effect compared to Epi. E2 itself did not induce cytokine production in the whole blood model, but significantly inhibited LPS-induced TNF-α production. These findings are in agreement with the studies showing the same effect in cardiomyocytes [31] and human macrophages [32] which was mediated through the modulation of NF-κB activation. Conflicting data concerning estradiol’s effect on endotoxin-induced cells have been also published, varying from inhibition to no effect or even stimulation of IL-1β production [33], [34], [35]. The same conflicting results are available for IL-12 and IL-8 [8], [36], [37], [38]. Despite these discrepancies, it is believed that E2 reduces the chemotaxis of neutrophils and monocytes [39], [40] through suppressing cytokine-mediated expression of adhesion molecules such as E-selectin and ICAM-1 [40], [41], [42], [43]. In our study, E2 caused a significant decrease in CD62L expression in both cell populations without affecting the expression of β2 integrin (CD11b/CD18), while LPS-induced expression of these molecules was not affected. The reason for CD62L shedding caused by this estrogen is unknown. Taken together, our results suggest that under non-inflamed conditions, E2 leads to the shedding of CD62L with no modulation of CD11b, while under inflamed conditions (LPS exposure) E2 was able to exhibit protective anti-inflammatory response by reducing TNF-α production. The E2 concentrations used in our study were equivalent to those seen in pregnancy. The immunological shift toward anti-inflammatory response might be crucial for successful pregnancy outcome. The described suppression of LPS-induced production of inflammatory TNF-α by high doses of E2 might contribute significantly to the outcome of infection during pregnancy. Of note, simultaneous combined exposure of the cells with both hormones did not exacerbate or suppress the effect of another.
In conclusion, the findings of this work extend our knowledge of the ability of stress and sex hormones to modulate the innate immune response. The balance between cytokines with pro-inflammatory and anti-inflammatory properties is important in the control of the intensity and duration of an immune response. Our findings suggest that the observed immunomodulatory role of Epi and E2 may influence the outcome in endotoxin responses and can be critical in the regulation of inflammatory responses. Contradictory results on immunomodulatory effects highlight the complexity of interactions between innate immune cells and hormones. Despite this complexity, our results have shown a differential and synergetic response of neutrophils and monocytes towards hormonal stimuli which highlights their cooperative work in terms of hormonal imbalance.
Acknowledgments
This study was supported by State Committee Science MES RA, in frame of the research project no. SCS 15T-1F213.
Author Statement
Research funding: Authors state no funding involved.
Conflict of interest: Authors state no conflict of interest.
Informed consent: Informed consent has been obtained from all individuals.
Ethical approval: The research related to human use complied with all the relevant national regulations and institutional policies and was performed in accordance to the tenets of the Helsinki Declaration and has been approved by the author’s institutional review board or equivalent committee.
1. Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–17.10.1159/000216188Search in Google Scholar
2. Straub RH. Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis Res Ther. 2014;16:203.10.1186/ar4484Search in Google Scholar
3. Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34:177–92.10.1385/IR:34:3:177Search in Google Scholar
4. Ahmed SA, Karpuzoglu E, Khan D. Effects of sex steroids on innate and adaptive immunity. In: Klein SL, Roberts CW, editors. Sex hormones and immunity to infection. Berlin: Springer-Verlag, 2010:19–52.10.1007/978-3-642-02155-8_2Search in Google Scholar
5. Nadkarni S, McArthur S. Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol. 2004;13:576–81.10.1016/j.coph.2013.05.007Search in Google Scholar PubMed
6. Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER) alpha and ERbeta ligand treatment. Proc Natl Acad Sci USA. 2007;104:14813–8.10.1073/pnas.0703783104Search in Google Scholar PubMed PubMed Central
7. Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS One. 2012;7:44552.10.1371/journal.pone.0044552Search in Google Scholar PubMed PubMed Central
8. Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104:1404–10.10.1182/blood-2003-10-3380Search in Google Scholar PubMed
9. Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lélu K, Krust A, Pipy B, Bayard F, Arnal JF, Guéry JC, Gourdy P. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–76.10.4049/jimmunol.0902383Search in Google Scholar PubMed
10. Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30.10.1037/0033-2909.130.4.601Search in Google Scholar PubMed PubMed Central
11. Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. Int Immunol. 2005;17:1059–69.10.1093/intimm/dxh286Search in Google Scholar PubMed
12. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51.10.1038/nri1571Search in Google Scholar PubMed
13. Chrousos GP. Stress and sex versus immunity and inflammation. Sci Signal. 2010;3:pe36.10.1126/scisignal.3143pe36Search in Google Scholar PubMed
14. Chen A, Engel P, Tedder TF. Structural requirements regulate endoproteolytic release of the L-selectin (CD62L) adhesion receptor from the cell surface of leukocytes. J Exp Med. 1995;182:519–30.10.1084/jem.182.2.519Search in Google Scholar PubMed PubMed Central
15. Zhao LC, Edgar JB, Dailey MO. Characterization of the rapid proteolytic shedding of murine L-selectin. Dev Immunol. 2001;8:267–77.10.1155/2001/91831Search in Google Scholar PubMed PubMed Central
16. Muller WA. Getting leukocytes to the site of inflammation. Vet Pathol. 2013;50:7–22.10.1177/0300985812469883Search in Google Scholar PubMed PubMed Central
17. Redwine L, Snow S, Mills P, Irwin M. Acute psychological stress: effects on chemotaxis and cellular adhesion molecule expression. Psychosom Med. 2003;65:598–603.10.1097/01.PSY.0000079377.86193.A8Search in Google Scholar
18. Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells – from barracks to boulevards to battlefields: a tale of three hormones – Curt Richter Award winner. Psychoneuroendocrinology. 2012;37:1345–68.10.1016/j.psyneuen.2012.05.008Search in Google Scholar PubMed PubMed Central
19. Van der Poll T, Lowry SF. Lipopolysaccharide-induced interleukin 8 production by human whole blood is enhanced by epinephrine and inhibited by hydrocortisone. Infect Immun. 1997;65:2378–81.10.1128/iai.65.6.2378-2381.1997Search in Google Scholar PubMed PubMed Central
20. Van der Poll T, Lowry SF. Epinephrine inhibits endotoxin-induced IL-1 beta production: roles of tumor necrosis factor-alpha and IL-10. Am J Physiol. 1996;273:1885–90.10.1152/ajpregu.1997.273.6.R1885Search in Google Scholar
21. Van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97:713–9.10.1172/JCI118469Search in Google Scholar PubMed PubMed Central
22. Grisanti LA, Evanson J, Marchus E, Jorissen H, Woster AP, DeKrey W, Sauter ER, Combs CK, Porter JE. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol Immunol. 2010;47:1244–54.10.1016/j.molimm.2009.12.013Search in Google Scholar
23. Mizuno K, Takahashi HK, Iwagaki H, Katsuno G, Kamurul HA, Ohtani S, Mori S, Yoshino T, Nishibori M, Tanaka N. β2-Adrenergic receptor stimulation inhibits LPS-induced IL-18 and IL-12 production in monocytes. Immunol Lett. 2005;101:168–72.10.1016/j.imlet.2005.05.008Search in Google Scholar
24. Talmadge J, Scott R, Castelli P, Newman-Tarr T, Lee J. Molecular pharmacology of the β-adrenergic receptor on THP-1 cells. Int J Immunopharmacol. 1993;15:219–28.10.1016/0192-0561(93)90098-JSearch in Google Scholar
25. Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–47.10.1161/CIRCRESAHA.107.151860bSearch in Google Scholar
26. Onuffer JJ, Horuk R. Chemokines, chemokine receptors and small molecule antagonists: recent developments. Trends Pharmacol Sci. 2002;23:459–67.10.1016/S0165-6147(02)02064-3Search in Google Scholar
27. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–31.10.1152/physrev.00026.2006Search in Google Scholar PubMed
28. Ueda K, Karas RH. Emerging evidence of the importance of rapid, non-nuclear estrogen receptor signaling in the cardiovascular system. Steroids. 2013;78:589–96.10.1016/j.steroids.2012.12.006Search in Google Scholar PubMed
29. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294:63–9.10.1016/j.cellimm.2015.01.018Search in Google Scholar PubMed PubMed Central
30. La Rosa P, Pesiri V, Leclercq G, Marino M, Acconcia F. Palmitoylation regulates 17beta-estradiol-induced estrogen receptor-alpha degradation and transcriptional activity. Mol Endocrinol. 2012;26:762–74.10.1210/me.2011-1208Search in Google Scholar PubMed PubMed Central
31. Liu CJ, Lo JF, Kuo CH, Chu CH, Chen LM, Tsai FJ, Tsai CH, Tzang BS, Kuo WW, Huang CY. Akt mediates 17beta-estradiol and/or estrogen receptor-alpha inhibition of LPS-induced tumor necresis factor-alpha expression and myocardial cell apoptosis by suppressing the JNK1/2-NFkappaB pathway. J Cell Mol Med. 2009;13:3655–67.10.1111/j.1582-4934.2009.00669.xSearch in Google Scholar PubMed PubMed Central
32. Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol. 2010;184:5029–37.10.4049/jimmunol.0903463Search in Google Scholar
33. Pioli PA, Weaver LK, Schaefer TM, Wright JA, Wira CR, Guyre PM. Lipopolysaccharide-induced IL-1 beta production by human uterine macrophages up-regulates uterine epithelial cell expression of human beta-defensin 2. J Immunol. 2006;176:6647–55.10.4049/jimmunol.176.11.6647Search in Google Scholar
34. Bouman A, Schipper M, Heineman MJ, Faas M. 17beta-estradiol and progesterone do not influence the production of cytokines from lipopolysaccharide-stimulated monocytes in humans. Fertil Steril. 2004;82:1212–9.10.1016/j.fertnstert.2004.05.072Search in Google Scholar
35. Morishita M, Miyagi M, Iwamoto Y. Effects of sex hormones on production of interleukin-1 by human peripheral monocytes. J Periodontol. 1999;70:757–60.10.1902/jop.1999.70.7.757Search in Google Scholar
36. Elenkov IJ, Wilder RL, Bakalov VK, Link AA, Dimitrov MA, Fisher S, Crane M, Kanik KS, Chrousos GP. IL-12, TNF-alpha, and hormonal changes during late pregnancy and early postpartum: implications for autoimmune disease activity during these times. J Clin Endocrinol Metab. 2001;86:4933–8.10.1210/jcem.86.10.7905Search in Google Scholar
37. Matalka KZ. The effect of estradiol, but not progesterone, on the production of cytokines in stimulated whole blood, is concentration-dependent. Neuro Endocrinol Lett. 2003;24:185–91.Search in Google Scholar
38. Pioli PA, Jensen AL, Weaver LK, Amiel E, Shen Z, Shen L, Wira CR, Guyre PM. Estradiol attenuates lipopolysaccharide-induced CXC chemokine ligand 8 production by human peripheral blood monocytes. J Immunol. 2007;179:6284–90.10.4049/jimmunol.179.9.6284Search in Google Scholar
39. Yamada K, Hayashi T, Kuzuya M, Naito M, Asai K, Iguchi A. Physiological concentration of 17 beta-estradiol inhibits chemotaxis of human monocytes in response to monocyte chemotactic protein 1. Artery. 1996;22:24–35.Search in Google Scholar
40. Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–9.10.1161/01.CIR.0000142050.19488.C7Search in Google Scholar
41. Stork S, von Schacky C, Angerer P. The effect of 17β-estradiol on endothelial and inflammatory markers in postmenopausal women: a randomized, controlled trial. Atherosclerosis. 2002;165:301–7.10.1016/S0021-9150(02)00242-3Search in Google Scholar
42. Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17β-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996;98:36–42.10.1172/JCI118774Search in Google Scholar PubMed PubMed Central
43. Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol. 2006;79:963–70.10.1189/jlb.1005596Search in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Long-term dutasteride therapy in men with benign prostatic hyperplasia alters glucose and lipid profiles and increases severity of erectile dysfunction
- Differential modulation of innate immune response by epinephrine and estradiol
- Association of transient hyperthyroidism and severity of hyperemesis gravidarum
- Effects of interleukin-1 receptor antagonist (IL-1Ra) gene 86 bp VNTR polymorphism on recurrent pregnancy loss: a case-control study
- Morning sickness of pregnancy: more than meets the eye
Articles in the same Issue
- Long-term dutasteride therapy in men with benign prostatic hyperplasia alters glucose and lipid profiles and increases severity of erectile dysfunction
- Differential modulation of innate immune response by epinephrine and estradiol
- Association of transient hyperthyroidism and severity of hyperemesis gravidarum
- Effects of interleukin-1 receptor antagonist (IL-1Ra) gene 86 bp VNTR polymorphism on recurrent pregnancy loss: a case-control study
- Morning sickness of pregnancy: more than meets the eye

