Abstract
Somatic embryogenesis in vitro provides an efficient means of plant multiplication, facilitating sunflower improvement and germplasm innovation. In the present study, using interspecific amphiploids (2n=4x=68) between cultivated sunflower and wild perennial Helianthus species as explant donors, somatic embryos were induced directly from the surface of corolla tubes at the late uninucleate or binucleate microspore development stage. Primary somatic embryos (PSEs) were obtained in amphiploids G08/2280 (H. pumilus×P21) and G08/2260 (NMSHA89×H. maximiliani). The PSE induction frequency of G08/2280 on synthesized Medium A and B was 30.27 % and 42.42 %, respectively, while that of G08/2260 was 5.89 % and 12.16 %, respectively. The difference of PSE induction frequency was significant between G08/2280 and G08/2260 (P=0.0058), but was non-significant between induction Medium A and B (P=0.1997). Secondary somatic embryos (SSEs) were rapidly produced from PSEs on subculture Medium 1 with the induction frequency of 100 %. The mean number of SSEs produced from each PSE was 19.2 and 12.2 in G08/2280 and G08/2260 within 30 d of subculture, respectively. Mature SSEs were gradually converted into young shoots on hormone-free subculture Medium 2, with the mean number of small green shoots produced from each PSE of 22.0 and 18.7 in G08/2280 and G08/2260, respectively. Through the additional process of rooting for some shoots without roots on half-strength of MS medium adding 0.25–0.5 mg/l NAA, 0.5 mg–1.0/l IBA, SE-derived shoots without roots gained about 40 % rooting frequency. Regenerated plants acclimated successfully and displayed similar morphological and chromosome number to the amphiploid donors.
Introduction
Sunflower (Helianthus annuus L.) is one of the important oil seed crops supplying healthy oil high in nutritional value for human consumption. Increasing yield, oil quantity and quality, nutritional content and resistance to abiotic and biotic stresses are the main targets of sunflower breeding and germplasm innovation (Hulke and Kleingartner, 2014). Helianthus is known to possess an abundance of unique genes, such as resistance to diseases and insect pests, cytoplasmic male sterility and fertility-restoration, agronomic and seed-oil characteristics, drought tolerance, protein content, and fatty acid composition (Ruso et al., 1996; Seiler and Rieseberg, 1997). However, cultivated sunflower has a relatively narrow genetic base compared to its wild Helianthus species. Thus, the crop wild relatives of Helianthus have been considered an important germplasm resource for cultivated sunflower’s genetic improvement and breeding (Thompson et al., 1981; Gômez-Sânchez and Gonzâlez, 1991). Interspecific hybridizations between cultivated sunflower and wild Helianthus have demonstrated as a useful method for gene transfer and sunflower germplasm development (Laferrière, 1986; Skoric, 1992; Gavrilova et al., 1997; Sukno et al., 1999; Atlagic, 2004), but the transfer of genes is restricted by cross incompatibility and hybrid sterility (Faure et al., 2002). Chromosome doubling has played a key role in improving F1 fertility since the doubled interspecific hybrids can be used as a bridge for interspecific gene transfer (Dewey, 1980). However, low fertility can still affect the utilization of some amphiploids. Alternative conservation and propagation of the interspecific F1 plants and amphiploids through tissue culture has the potential of providing a large number of lines for breeding programs.
Somatic embryogenesis provides an ideal experimental process for investigation of plant differentiation, as well as micro-proliferation for shoots, since the regeneration of plants through somatic embryogenesis is also a preferred method for gene transformation (Litz and Gray, 1992). Hence, in vitro gene-transfer technique can be used for sunflower improvement. A highly-efficient in vitro tissue culture system is essential for providing many plants for studying in gene-transfer experiments (Escandon and Hahne, 1991; Pugliesi et al., 1993a; Dağüstü et al., 2008; Liu et al., 2011), and for selection of genetic variants from somaclonal variation and somatic hybridization (Taski-Ajdukovic et al., 2006; Taski-Ajdukovic et al., 2010), as well as biologically active metabolic substances (Geipel et al., 2014).
Somatic embryogenesis is complex and controlled by a variety of external and internal factors, with the development of embryos from somatic cells regulated by the differential expression pattern of a myriad of genes (Talapatra et al., 2015). It is known that the explant is very important for effective use of totipotency in tissue culture. Since the reports of Greco et al. (1984) and Paterson and Everett (1985) about the regeneration of sunflower, different explants have been used to induce calli or somatic embryos, such as shoot apices, hypocotyls, cotyledons, leaves, protoplasts, and mature embryos. Additionally, immature embryos have been used as explant for inbred lines, interspecific hybrids, and commercial hybrids (Chandler and Beard, 1983; Greco et al., 1984; Paterson and Everett, 1985; Finer, 1987; Wilcox Mccann et al., 1988; Wirtzens et al., 1988; Pugliesi et al., 1991; Yordanov et al., 2002; Vega et al., 2007; El Mostafa et al., 2008; Wang et al., 2011; Zhang and Finer, 2015). However, frequencies of plant regeneration and somatic embryo induction still need further improvement in sunflower. Hence, it is helpful to evaluate new methods for embryogenesis potential in more tissues to develop a more effective tissue culture system in sunflower, especially for somatic embryo induction and regeneration. There are more than a thousand small flowers with tubular corollas in one capitulum of a sunflower head to produce seeds with the corolla tube as an important part of the flower protecting the stamen and pistil. Until now, reports on flower culture in sunflower are still lacking. In this study, using interspecific amphiploids between cultivated sunflower and wild Helianthus species, young flowers at the late uninucleate or binucleate microspore stage of explants, we directly induced somatic embryogenesis from corolla tubes, and obtained secondary somatic embryos and regenerated plants. This will increase the explant types and embryoid induction methods in sunflower.
Materials and methods
Materials
Two interspecific amphiploids (2n=4x=68) between cultivated sunflower and its wild species, G08/2280 (H. pumilus×P21), G08/2260 (NMSHA89×H. maximiliani), and one tetraploid BC1F3 of a hexaploid amphiploid backcrossed with cultivated line, G08/2394 ((H. hirsutus×P21)×HA89) (2n=4x=68), were used as explant donors for in vitro culture. These amphiploids were developed from the interspecific F1 hybrids between cultivated sunflower and its wild relative by chromosomes doubling of the F1 plants (Jan, 1988; Jan et al., 1988; Jan, 1996). The plants were transplanted in 20-cm plastic pots in greenhouse at 25 oC with a 16 h photoperiod. The whole tubular flower, containing corolla tube, anthers and ovary, was used as explants when the microspores were at the late uninucleate or binucleate stage.
Primary somatic embryo induction
Heads were collected and cold-treated at 4 oC for 7 d before inoculation. Before culturing, heads were surface-sterilized with 70 % (v/v) ethanol for 1 min and rinsed with sterile distilled water once, and subsequently immersed in a 30 % (v/v) commercial bleach solution containing 0.05 ml Tween-20 for 10 min and rinsed 3–4 times in sterile distilled water. Then, the flowers were separated from heads under a sterile stereomicroscope and inoculated in 100×15 mm Petri dishes with 25 ml induction medium under a laminar flow hood. There were 20–30 flowers per Petri dish, with three replications per treatment for every source. All cultures were maintained at 35 oC in the dark for 12 d, followed with 25 oC in the dark. Primary somatic embryo (PSE) induction frequency, defined as the percentage of flowers with globular somatic embryos, was recorded after 30 d of culture.
The induction media were synthesized media A and B. “Medium A” contained half-strength basal salts of MS medium (macro- and micro- elements) (Murashige and Skoog, 1962), vitamins 100.0 mg/l meso-inositol, 1.0 mg/l calcium pantothenate, 1.0 mg/l nicotinic acid, 1.0 mg/l thiamine hydrochloride, 1.0 mg/l pyridoxin hydrochloride, 0.01 mg/l biotin (Morel and Wetmore, 1951), 0.2 mg/l vitamin B-12, a mixture of amino acids (2.0 mg/l L-glycine, 40.0 mg/l L-glutamine, 25.0 mg/l L-serine, 1.0 mg/l L-tryptophan, 2.5 mg/l L-cysteine), 120.0 g/l sucrose, 0.5 mg/l BAP and 0.5 mg/l NAA, and 7.0 g/l phytagel (Sigma Chemical Company, St. Louis, MO, USA). “Medium B” contained MS basal salts (macro- and micro- elements), vitamins of Morel and Wetmore’s, a mixture of amino acids (2.0 mg/l L-glycine, 40.0 mg/l L-glutamine, 25.0 mg/l L-serine, 1.0 mg/l L-tryptophan, 2.5 mg/l L-cysteine), 15.0 g/l sucrose, 1.0 mg/l BAP and 0.5 mg/l NAA, 300.0 mg/l CH (Casein hydrolysate), and 7.0 g/l phytagel. The pH of the media was adjusted to 5.8 by using 1 mol/l NaOH, and 1 mol/l HCl prior to autoclaving at 121 oC for 15 min. Filter-sterilized solutions of vitamins and amino acids were added to the autoclaved medium when the medium temperature dropped to 60 oC. The medium was aliquoted to sterile Petri dishes with 25 ml each.
Secondary embryogenesis and plantlet regeneration
Primary somatic embryos (PSEs) were detached from the explants under a sterile stereomicroscope and transferred to fresh subculture medium after 30 d of culture on induction media. The “subculture Medium 1” for the secondary embryogenesis was MS (Murashige and Skoog, 1962) basal medium containing 20 g/l sucrose, 7 g/l phytagel, 1.0 mg/l BAP and 0.5 mg/l NAA. The PSEs were first cultured on “subculture Medium 1” for 30 d, then some larger secondary somatic embryos (SSEs) with green buds were transferred to “subculture Medium 2” (hormone-free, other ingredients same as “subculture Medium 1”) to differentiate young shoots. All cultures were maintained at 25 oC with 14 h photoperiod. Cultures were evaluated after 30 d for frequency of SSEs and small shoots. Induction frequency of SSE was calculated as the percentage of PSEs producing SSEs with total number of PSEs cultured.
Plantlet rooting, transplanting, chromosome counts and pollen observation
Small shoots more than 2 cm in length were subcultured on “subculture Medium 2” for shoot elongation and root development in conical flask, then plantlets 3–8 cm in length with about four leaves were transferred to rooting medium in test tubes if no roots were visible. The rooting medium contained half-strength MS medium ingredients plus 0.25–0.5 mg/l NAA, 0.5–1.0 mg/l IBA, 20 g/l sucrose and 7.0 g/l phytagel. The growth of root was assessed after 4-weeks of culture. Rooting frequency was calculated as the percentage of rooted shoots with all shoots cultured. Well-developed plantlets with leaves and roots were transplanted from rooting medium to peat pellets and placed in a growth chamber at 23–25 oC for about 7–10 d for acclimation. Afterwards, the young plants were transplanted into 11-cm diameter clay pots in the greenhouse and root tips were sampled for chromosome counts. Two weeks later, plants were transplanted to soil in 20-cm plastic pots under normal greenhouse conditions.
Root-tip preparation and chromosome counts followed the method of Jan (1996). Pollen stainability observation followed Alexander’s procedure (Alexander, 1969). Three somatic embryos (SE)-derived plants of G08/2280 and G08/2260 were randomly selected for examination, respectively. Slides were observed under an Axioplan2 Imaging 7 microscope (Zeiss, Germany). More than 200 pollen grains from each plant were randomly counted. Percentage of the stained pollen grains was used for analysis. Images were captured by a charge-coupled device (CCD) 8 camera (Zeiss AxioCam HRM), and processed using Adobe Photoshop 7.0.
Data analysis
Data were analyzed using Microsoft Excel 2010 and IBM SPSS Statistics 22. A two-factor ANOVA was used to analyze the variance of PSE induction frequency and shoot rooting frequency, and one-factor ANOVA was used to analyze the variance of pollen stainability. The differences between treatment means were tested using Duncan’s multiple range test at P≤0.05 level of probability. Percentage data were transformed by arcsin−1 prior to analysis.
Results
Primary somatic embryo induction
Primary somatic embryos (PSEs) were mainly produced at the upper part of the corolla tubes directly from surface of the corolla tubes of young flowers asynchronously after flowers were cultured on induction medium in the dark for 15 d. PSEs were induced in G08/2280 and G08/2260, but not in G08/2394. The somatic embryos were white and smooth on the surface with compact structure at the globular-shape stage, and some appeared to be in the process of secondary embryogenesis (Figure 1a, b). The somatic embryo induction frequency is shown in Table 1. G08/2280 had the higher induction frequencies of PSEs with 30.27 % and 42.42 % on induction Medium A and B, respectively. In comparison, G08/2260 had 5.89 % and 12.16 %, respectively. The difference was significant between the two lines (P=0.0058), but non-significant between the two media (P=0.1997) (Table 2). When placed under light conditions with continued culturing for another 15 d on the induction medium, white embryos changed to green, and some developed into heart stage, torpedo stage, or cotyledonary stage (Figure 1c).
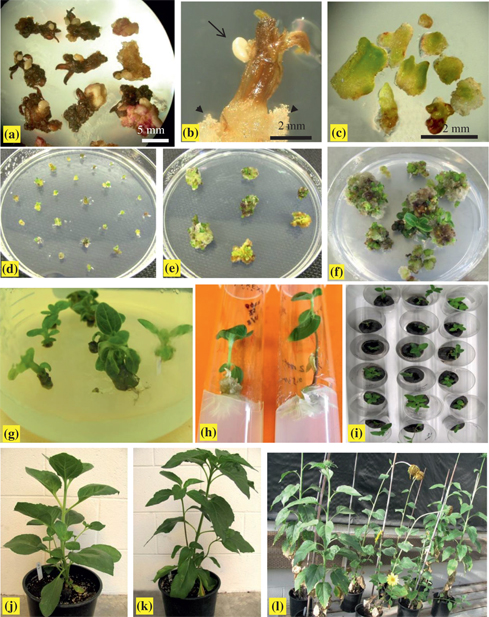
Somatic embryogenesis, plant regeneration and acclimation of interspecific amphiploids. (a, b) Primary somatic embryos were produced directly from the surface of corolla tubes on induction “Medium A” after 15 d of culture in the dark. (a) Asynchronous development of embryos. (b) The enlargement of a flower with a somatic embryo at the globular stage (long arrow), callus formed only at the wounded edge of the flower (arrow head). (c) Primary somatic embryos at different development stages after 15 d of culture under light condition on induction Medium A. (d) PSEs proliferation on subculture Medium 1 after 15 d of culture under light condition, showing white embryos gradually turned green ones and began to proliferate. (e) Asynchronous development occurred in secondary embryogenesis, showing some secondary embryos produced and some that begun their shoot conversion on subculture Medium 1 after 30 d of culture. (f) Germination of SSEs on subculture Medium 2 without plant growth regulators, showing young shoots produced from green buds after 30 d of culture. (g) Shoots more than 2 cm in length with about two leaves continued to subculture on subculture Medium 2 for shoot elongation in conical flask. (h) Plantlets with about 4 leaves transferred onto rooting medium. (i) Plantlets with well-developed roots when transplanted to peat pellet for acclimation. (j, k) Somatic embryo-derived plantlets of G08/2280 (j) and G08/2260 (k) at the vegetative stage. (l) Flowering of some somatic embryo-derived plants.
Primary somatic embryo (PSE) induction frequency and secondary somatic embryo (SSE) production.
| Lines | Induction medium | Mean frequency of PSEs induction (%) * | SSE producing medium | Mean No. SSEs per PSE | Percent of SSEs producing (%) | Mean No. shoots regenerated from per PSE |
|---|---|---|---|---|---|---|
| G08/2280 | A | 30.27±0.25 a | Subculture medium 1 | 19.2 | 100 | 22.0 |
| B | 42.42±0.08 a | |||||
| G08/2260 | A | 5.89±0.03 b | Subculture medium 1 | 12.2 | 100 | 18.7 |
| B | 12.16±0.07 b | |||||
| G08/2394 | A | 0 | – | – | – | – |
| B | 0 | – | – | – | – |
*Values were mean±standard error. Numbers followed by a different letter within the column for different lines and medium were significantly different at P≤0.05.
ANOVA of PSE induction frequency.
| Source of variation | SS | df | MS | F | P-value |
|---|---|---|---|---|---|
| Lines | 1149.83 | 1 | 1149.83 | 13.88 | 0.0058 |
| Medium | 161.90 | 1 | 161.90 | 1.95 | 0.1997 |
| Interaction | 3.05 | 1 | 3.05 | 0.04 | 0.8525 |
| Error | 662.67 | 8 | 82.83 | ||
| Total | 1977.46 | 11 |
Secondary embryogenesis and plant development
PSEs proliferated rapidly on “subculture Medium 1” and showed asynchronous development, that is, a number of globular secondary embryos were produced from PSEs, and meanwhile some PSEs germinated and directly developed into small green buds (Figure 1d, e). Induction frequency of secondary somatic embryos (SSEs) was 100 % in the two lines, and the mean number of SSEs produced per PSE was 19.2 and 12.2 for G08/2280 and G08/2260 within 30 d of subculture. Many young shoots gradually developed after transferring SSEs and small green buds to the hormone-free “subculture Medium 2” (Figure 1f). The mean number of small green shoots produced per PSE was 22.0 and 18.7, respectively, for G08/2280 and G08/2260. It was observed that new SSEs continuously developed from PSEs, suggesting a higher propagation capacity of both secondary embryogenesis and shoot regeneration.
During the propagation process, shoots longer than 2 cm in length with about two leaves continued to be subcultured on “subculture Medium 2” for shoot elongation in conical flask (Figure 1g), with approximately 30 % of the shoots developing roots during this process. Young shoots with about four leaves, but no roots were transferred to rooting medium (Figure 1h). The results in Tables 3 and 4 suggested non-significant differences for rooting frequency (P=0.0970) between the two lines. Rooting “Media C” and “Media D” had the same ingredients of half-strength of MS medium basic elements, 20 g/l sucrose and 7.0 g/l phytagel; the difference was that “Media C” was supplemented with 0.5 mg/l NAA and 0.5 mg/l IBA, and “Media D” was supplemented with 0.25 mg/l NAA and 0.5 mg/l IBA. Both media induced rooting, however, rooting frequency of “Media C” was significantly higher than that of “Media D” (P=0.0030), implying that rooting ability of SSE-derived shoots were affected by both genotype and medium, with more effects from the medium.
ANOVA of rooting frequency of SSE-derived shoots.
| Source of variation | SS | df | MS | F | P-value |
|---|---|---|---|---|---|
| Lines | 89.82 | 1 | 89.82 | 3.53 | 0.0970 |
| Medium | 446.64 | 1 | 446.64 | 17.56 | 0.0030 |
| Interaction | 9.56 | 1 | 9.56 | 0.38 | 0.5567 |
| Error | 203.44 | 8 | 25.43 | ||
| Total | 749.46 | 11 |
Multiple comparisons of shoot rooting frequency and root numbers.
| Lines derived SSE-plants | Medium | Rooting frequency % * |
|---|---|---|
| G08/2280 | C | 59.72±8.67 a |
| D | 35.92±11.03 b | |
| G08/2260 | C | 47.22±8.61 a |
| D | 29.70±4.27 b |
*Values were mean±standard error. Numbers followed by a different letter in each “Line” showing medium effects significant at P≤0.05.
Acclimation and characterization of plantlets
Plants with well-developed roots were transplanted to peat pellets and placed in a growth chamber at 25 oC for about 7–10 d for acclimation. All plants survived and grew normally (Figure 1i), and afterwards, the young plants were transplanted into 11-cm diameter clay pots and placed in the greenhouse for two weeks. Subsequently, plants were transplanted in soil in 20-cm plastic pots, and continued to grow well during the vegetative and flowering stages under normal greenhouse condition (Figure 1j, k, l). The SE-derived plants displayed high similarity in plant type with the donor plants of G08/2280 and G08/2260, such as in stem diameter, height, disc flower diameter, and branching (Figure 2a, b). We counted chromosome numbers of root-tip cells of more than 50 regenerated plants; all of them had 2n=68 (Figure 2c), the same as the parental amphiploids.

Plants at the flowering stage and chromosomes of root-tip cell. (a) Plant of G08/2280. (b) SE-derived plant of G08/2280. (c) Mitotic metaphase chromosome of a root-tip cell of SE-derived plant of G08/2280, 2n=4x=68. Bar=5 µm in the picture of c.
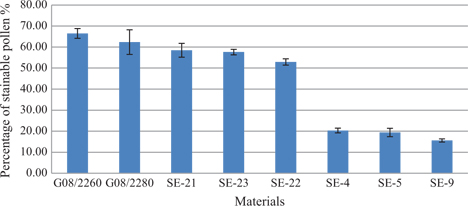
Pollen stainability of donors and six randomly selected SE-derived plants are shown in Figures 3 and 4. Pollen stainability of G08/2260 and G08/2280 was 66.48 % and 62.36 %, respectively. Three SE-derived plants of G08/2260, SE21, SE22 and SE23, had pollen stainability of 58.46 %, 52.90 % and 57.60 %, respectively; while another three SE-derived plants of G08/2280, SE4, SE5 and SE9, had pollen stainability of 20.31 %, 19.38 % and 15.59 %, respectively. The differences of pollen stainability among the eight plants were significant (Table 5), but the difference between the donor plants G08/2280 and G08/2260 was non-significant (P=0.5453); SE21, SE22 and SE23 had significantly lower pollen stainability than their donor G08/2260, and SE4, SE5 and SE9 were significantly lower than G08/2280. In general, the SE-derived plants from G08/2260 had the semi-sterility characteristic of the tetraploid amphiploids, but the SE-derived plants from G08/2280 had much reduced pollen stainability compared to its parental amphiploids.
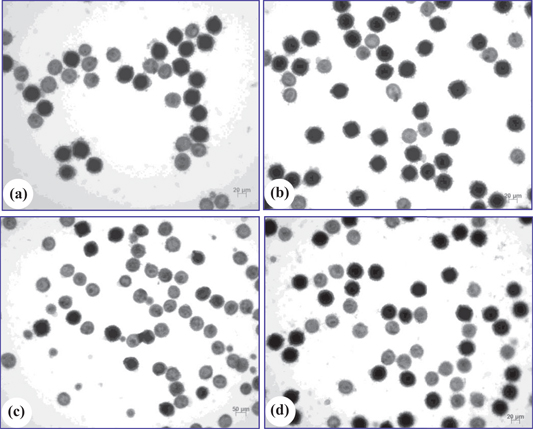
Mature pollen grains of SE-derived plants and the donor plants. (a) Pollen grains of G08/2280. (b) Pollen grains of G08/2260. (c) Pollen grains of SE-9. (d) Pollen grains of SE-23. Dark stained pollen grains were fertile, light stained ones were sterile. Bars=20 µm in pictures a, b and d, Bar=50 µm in the picture of c.
Pollen stainability of SE-derived plants and the donor plants.
ANOVA of pollen stainability.
| Source of variation | SS | df | MS | F | P-value |
|---|---|---|---|---|---|
| Lines | 3701.51 | 7 | 528.79 | 70.18 | 0.0000 |
| Error | 120.55 | 16 | 7.53 | ||
| Total | 3822.05 | 23 |
Discussion
Sunflower is reported to be recalcitrant to manipulations in vitro (Sujatha et al., 2012). Of the reports on sunflower tissue culture to date, regardless of the explant used, organogenesis through callus differentiation, somatic embryogenesis, and direct organogenesis were the basic pathways to regenerate plants in vitro. However, more reports were focused on direct organogenesis and callus-mediated organogenesis (Wirtzens et al., 1988; Espinasse and Lay, 1989; Espinasse et al., 1989; Knittel et al., 1991; Ceriani et al., 1992; Chraîbi et al., 1992; Pugliesi et al., 1993b). A recent example of direct organogenesis from Sujatha et al. (2012) indicated a high frequency (93.86 %) of adventitious shoots was obtained directly from cotyledons of mature sunflower seeds within 2 weeks of culture on MS medium supplemented with 9.84 µm 2-iP, 2.85 µm IAA and 0.45 µm TDZ. It also revealed significant effects of explant orientation, age of seedlings, and genotype on adventitious organogenesis. Somatic embryogenesis directly from zygotic embryos (Finer, 1987; Freyssinet and Freyssinet, 1988; Espinasse et al., 1989; Knittel et al., 1991; Jeannin et al., 1995) and seedling tissues such as leaf and hypocotyl (Pélissier et al., 1990; Fambrini et al., 1996; Carola Fiore et al., 1997; Laparra et al., 1997; Zhang and Finer, 2015), with the explant donors including inbred lines and their hybrids, wild species, and interspecific hybrids have been reported. Indirect somatic embryogenesis from callus was also observed by Paterson and Everett (1985), Wilcox Mccann et al. (1988) and Pugliesi et al. (1993b).
In our study, direct somatic embryogenesis was observed using flower corolla tubes of sunflower from interspecific amphiploids. Primary somatic embryos produced directly from the surface of corolla tubes, and subsequently secondary embryogenesis occurred and secondary embryos were continuously produced from PSEs. The three amphiploid donors from different wild perennial species and cultivars showed a significant difference in PSE induction frequency, that is, G08/2280 from the cross of H. pumilus and P21 had significant higher PSE induction ability than G08/2260 from the cross of NMS HA89 and H. maximiliani, as well as G08/2394 from H. hirsutus, P21 and HA89, indicating that the genotype was a main factor affecting the somatic embryogenesis, although shoot regeneration and rooting frequency were non-significant among the genotypes. In fact, genotype dependence has been reported in sunflower tissue culture and other plants, for example, in sunflower, the genotype HA89 was classified as recalcitrant (Knittel et al., 1991; Nestares et al., 1996; Vega et al., 2006) or comparatively lower response in shoot regeneration (Zhang and Finer, 2015), and the genotype HA300 exhibited a high morphogenic response (Power, 1987; Wirtzens et al., 1988; Pélissier et al., 1990; Knittel et al., 1991; Ceriani et al., 1992; Alibert et al., 1994; Burrus et al., 1996; Baker et al., 1999; Muller et al., 2001; Sujatha et al., 2012).
An enhanced knowledge of the genetic mechanisms and controlling genes would lay a foundation for radically improving the effectiveness and efficiency in plant somatic embryogenesis and organogenesis. However, little is known about the gene regulations and genetic pathways leading to somatic embryogenesis in many dicots, and only a very few limited number of preliminary reports exist for sunflower. Bolandi et al. (2000) crossed three sunflower cytoplasmic male-sterile and five restorer inbred lines, and used the F1 hybrids and their parents to study the heredity of their embryogenetic ability from the epidermal layers of hypocotyls. General combining ability and specific combining ability showed significant effects for the investigated traits, including the number of embryogenic explants per 100 explants plated, and the number of embryos per ten embryogenic explants. Parental female line ‘CMS-PET1B9’ was selected as the promising parent in crossing programs for the enhancement of somatic embryogenesis. Flores Berrios et al. (2000a) used 74 recombinant inbred lines (RILs) from a cross between two cultivars to detect QTLs for two traits; four QTLs for the number of embryogenic explants per 40 explants plated, and seven QTLs for the number of embryos per 40 explants were detected, which explained 48 % and 89 % of the phenotypic variation for the two traits, respectively. It revealed that several chromosome regions were related to in vitro somatic embryogenesis in sunflower RILs. Using the same RILs, Flores Berrios et al. (2000b) also detected 13 QTLs related to the in vitro cotyledon organogenesis traits of the mean number of shoots per explant and the mean number of shoots per regenerating explants, which were distributed on four chromosome regions in the RILs. The above reports further validated a genetic influence in sunflower tissue cultures, and were very helpful for precisely mapping the dominant genes and in the selection of organogenesis-responsive genotypes and the transfer of regeneration ability to genotypes that respond poorly.
To date, modification and selection of effective media were mostly focused on factors for induction and regeneration of somatic embryos in sunflower, and a genotype- independent regeneration system applicable to a wide range of genotypes is very important for overcoming a major bottleneck in producing transgenic plants and rapid shoot in vitro propagation in sunflower (Chraîbi et al., 1992). A variety of combinations of plant-growth-regulator types (auxin and cytokinin) and the concentration ratios have been studied, but the selected unique medium with different ingredients in each report was still mainly specific for the genotypes used in their studies. In our study, two primary somatic embryo induction media were used, the main differences of the ingredients were in basal salts (“Medium A” contained half-strength MS basal salts, “Medium B” contained full-strength MS basal salts), sucrose concentration (“Medium A” added as high as 120 g/l sucrose, “Medium B” added as low as 15.0 g/l sucrose), auxin and cytokinin ratio (0.5 mg/l BAP and 0.5 mg/l NAA in “Medium A”, 1.0 mg/l BAP and 0.5 mg/l NAA in “Medium B”), but the PSE induction frequency was non-significant between the two media. While in two SSE-derived shoot rooting media, the difference was only in auxin and cytokinin ratio, that is, “Media C” supplemented with 0.5 mg/l NAA and 0.5 mg/l IBA, and “Media D” with 0.25 mg/l NAA and 0.5 mg/l IBA. Although the two media both obtained good rooting results, “Media C” showed significantly higher rooting frequency than “Media D”. It showed the complexity of media used for somatic embryo induction and shoot development. However, recently, Sujatha et al. (2012) reported that through assessing about 169 media combinations comprising 12 different growth regulator combinations in various concentrations, they have developed an efficient protocol for shoot regeneration via direct adventitious shoot organogenesis from cotyledons of mature seeds of sunflower, which was tested on 42 genotypes and found to be applicable to a wide range of genotypes. However, this system still should be further verified in sunflower tissue culture by other researchers.
Phenotypes of SE-derived plants in our study showed great similarity in plant types with the donor plants of G08/2280 and G08/2260, such as thin stem, tall plant height, small disc flower, and branching. Although pollen stainability of the newly SE-derived plants (meaning the first generation of the regenerated plants from somatic embryos) was significantly lower than that of the relative donor plant, it revealed the obvious abnormal fertility maybe mainly caused by the instability of chromosomes in the newly regenerated plants from SSE carrying different genomes of cultivated sunflower and wild Helianthus species. In fact, chromosome stability and infertility of neoallopolyploids and autopolyploids is common in many plant species, probably due to different stability of parental genome and disordered meiotic pairing, and will need a natural process of diploidization, that is, several generations of self-crossing or asexual propagation by tillering, the fertility could be increased to a high and stable level (Chen et al., 2007; Chester et al., 2012; Geng et al., 2013; Cheng et al., 2016), the mechanism of diploidization is the under-studied topic today (Hollister, 2015).
Conclusions
Somatic embryogenesis occurred directly in young flower corolla tubes of sunflower interspecific amphiploids with somatic embryos developed on the surface of corolla tube at the late uninucleate or binucleate microspore developmental stage. Systems for secondary somatic embryogenesis from primary somatic embryos and shoots regeneration were developed. Regenerated plants acclimated successfully and displayed similar morphological and chromosome number to the amphiploid donors. Continued validation and optimization of this system with a larger number of sunflower genotypes should be carried out in the future.
Funding statement: This research was funded by a grant from a consortium of sunflower seed companies through the National Sunflower Association, Mandan, ND, USA. Mention of trade names or commercial products in this report is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The USDA is an equal opportunity provider and employer.
Acknowledgments
The authors thank Dr. Richard Horsley for his guidance during the course of this study, Lisa Brown for technical assistance, Drs. Jayma Moore and Zhaohui Liu for their help with photographs, Drs. Zhao Liu, Zahirul Talukder and Leonard Cook for their help in conducting this study, and Dr. Steven Xu for his critical review of the manuscript.
References
Alexander, M.P., 1969. Differential staining of aborted and non-aborted pollen. Stain Technology 44: 117–122.10.3109/10520296909063335Search in Google Scholar PubMed
Alibert, G., Aslane-Chanabe, J.C., Burrus, M., 1994. Sunflower tissue and cell cultures and their use in biotechnology. Plant Physiology a Nd Biochemistry 32: 31–44.Search in Google Scholar
Atlagic, J., 2004. Roles of interspecific hybridization and cytogenetic studies in sunflower breeding. Helia 27(41): 1–24.10.2298/HEL0441001ASearch in Google Scholar
Baker, C.M., Munoz-Fernandez, N., Carter, C.D., 1999. Improved shoot development and rooting from mature cotyledons of sunflower. Plant Cell Tissue Organ Culture 58: 39–49.10.1023/A:1006306111905Search in Google Scholar
Bolandi, A.R., Branchard, M., Alibert, G., Gentzbittel, L., Berville, A., Sarrafi, A., 2000. Combining ability analysis of somatic embryogenesis from epidermic layers in sunflower (Helianthus annuus L.). Theoretical and Applied Genetics 100: 621–624.10.1007/s001220050082Search in Google Scholar
Burrus, M., Molinier, J., Himber, C., Hunold, R., Bronner, R., Rousselin, P., Hahne, G., 1996. Agrobacterium-mediated transformation of sunflower (Helianthus annuus L.) shoot apices: Transformation patterns. Molecular Breeding 2: 329–338.10.1007/BF00437911Search in Google Scholar
Carola Fiore, M., Trabace, T., Sunseri, F., 1997. High frequency of plant regeneration in sunflower from cotyledon via somatic embryogenesis. Plant Cell Reports 16: 295–298.10.1007/BF01088284Search in Google Scholar PubMed
Ceriani, M.F., Hopp, H.E., Hahne, G., Escandon, A.S., 1992. Cotyledons: An explant for routine regeneration of sunflower plants. Plant Cell Physiology 33: 157–164.Search in Google Scholar
Chandler, J.H., Beard, B.H., 1983. Embryo culture of Helianthus hybrids. Crop Science 23: 1004–1006.10.2135/cropsci1983.0011183X002300050046xSearch in Google Scholar
Chen, L.Z., Lou, Q.F., Zhuang, Y., Chen, J.F., Zhang, X.Q., Woluka, J.N., 2007. Cytological diploidization and rapid genome changes of the newly synthesized allotetraploids Cucumis ╳ hytivus. Planta 225: 603–614.10.1007/s00425-006-0381-2Search in Google Scholar PubMed
Cheng, M.J., Zheng, M.M., Yang, S.P., Li, Y., Dong, X.C., Li, J., Sun, R.L., Li, H.X., Zhou, S.F., Wu, Y.Q., Rong, T.Z., Tang, Q.L., 2016. The effect of different genome and cytoplasm on meiotic pairing in maize newly synthetic polyploids. Euphytica 207: 593–603.10.1007/s10681-015-1552-7Search in Google Scholar
Chester, M., Gallagher, J.P., Vaughan Symonds, V., Veruska Cruz Da Silva, A., Mavrodiev, E.V., Leitch, A.R., Soltis, P.S., Soltis, D.E., 2012. Extensive chromosomal variation in a recently formed natural allopolyploid species, Tragopogon miscellus. Proceedings of the National Academy of Sciences of the United States of America 109: 1176–1181.10.1073/pnas.1112041109Search in Google Scholar PubMed PubMed Central
Chraîbi, M.B., Castelle, J.C., Latche, A., Roustan, J.P., Fallot, J., 1992. A genotype-independent system of regeneration from cotyledons of sunflower (Helianthus annuus L.). The role of ethylene. Plant Science 89: 215–221.10.1016/0168-9452(92)90167-KSearch in Google Scholar
Dağüstü, N., Fraser, P., Enfıssi, E., Bramley, P., 2008. Screening for high callus induction and agrobacterium- mediated transformation of sunflower (Helianthus Annuus L.). Biotechnology and Biotechnological Equipment 22: 933–937.10.1080/13102818.2008.10817582Search in Google Scholar
Dewey, D.R., 1980. Some applications and misapplications of induced polyploidy to plant breeding. In: Lewis, W.H. (ed.) Polyploid: Biological Relevance. Plenum Publishing Corporation, New York, pp. 445–470.10.1007/978-1-4613-3069-1_23Search in Google Scholar
El Mostafa, N., Fakiri, M., Benchekroun, M., Amzil, J., El Arbaoui, A., Hilali, S., 2008. Effect of plant growth regulators on somatic embryogenesis from leaf in vitro cultures of Helianthus tuberosus L. Journal of Food Agriculture and Environment 6: 213–216.Search in Google Scholar
Escandon, A.S., Hahne, G., 1991. Genotype and composition of culture medium are factors important in the selection for transformed sunflower (Helianthus annum) callus. Physiologia Plantarum 81: 367–376.10.1111/j.1399-3054.1991.tb08745.xSearch in Google Scholar
Espinasse, A., Lay, C., 1989. Shoot regeneration of callus derived from globular to torpedo embryos from 59 sunflower genotypes. Crop Science 29: 201–205.10.2135/cropsci1989.0011183X002900010043xSearch in Google Scholar
Espinasse, A., Lay, C., Volin, J., 1989. Effects of growth regulator concentrations and explant size on shoot organogenesis from callus derived from zygotic embryos of sunflower (Helianthus annuus L.). Plant Cell Tissue Organ Culture 17: 171–181.10.1007/BF00046865Search in Google Scholar
Fambrini, M., Cionini, G., Pugliese, C., 1996. Development of somatic embryos from morphogenetic cells of the interspecific hybrid Helianthus annuus ╳ Helianthus tuberosus. Plant Science 114: 205–214.10.1016/0168-9452(95)04320-9Search in Google Scholar
Faure, N., Serieys, H., Bervillé, A., Kaan, E.C., 2002. Occurrence of partial hybrids in wide crosses between sunflower (Helianthus annuus) and perennial species H. mollis and H. orgyalis. Theoretical and Applied Genetics 104: 652–660.10.1007/s001220100746Search in Google Scholar PubMed
Finer, J.J., 1987. Direct somatic embryogenesis and plant regeneration from immature embryos of hybrid sunflower (Helianthus annuus L.) on a high sucrose-containing medium. Plant Cell Reports 6: 372–374.10.1007/BF00269564Search in Google Scholar PubMed
Flores Berrios, E., Gentzbittel, L., Kayyal, H., Alibert, G., Sarrafi, A., 2000b. AFLP Mapping of QTLs for in vitro organogenesis traits using recombinant inbred lines in sunflower (Helianthus annuus L.). Theoretical and Applied Genetics 101: 1299–1306.10.1007/s001220051610Search in Google Scholar
Flores Berrios, E., Sarrafi, A., Fabre, F., Alibert, G., Gentzbittel, L., 2000a. Genotypic variation and chromosomal location of QTLs for somatic embryogenesis revealed by epidermal layers culture of recombinant inbred lines in the sunflower (Helianthus annuus L.). Theoretical and Applied Genetics 101: 1307–1312.10.1007/s001220051611Search in Google Scholar
Freyssinet, M., Freyssinet, G., 1988. Fertile plant regeneration from sunflower (Helianthus annuus L.) immature embryos. Plant Science 56: 177–181.10.1016/0168-9452(88)90032-5Search in Google Scholar
Gavrilova, V.A., Tolstaya, T.T., Rozhkova, V.T., 1997. Analysis of interspecific hybrids resulting from crosses between perennial wild Helianthus species and the cultivated sunflower. In: FAO Progress Report 1995–1996, March 20–22, 1997, Giessen, Germany, FAO, Rome, Italy, pp. 75–80.Search in Google Scholar
Geipel, K., Song, X., Socher, M.L., Kümmritz, S., Püschel, J., Bley, T., Ludwig-Müller, J., Steingroewer, J., 2014. Induction of a photomixotrophic plant cell culture of Helianthus annuus and optimization of culture conditions for improved α-tocopherol production. Applied Microbiology Biotechnology 98: 2029–2040.10.1007/s00253-013-5431-7Search in Google Scholar
Geng, X.X., Chen, S., Astarini, I.A., Yan, G.J., Tian, E., Meng, J., Li, Z.Y., Ge, X.H., Nelson, M.N., Mason, A.S., Pradhan, A., Zhou, W.J., Cowling, W.A., 2013. Doubled haploids of novel trigenomic Brassica derived from various interspecific crosses. Plant Cell Tissue Organ Culture 113: 501–511.10.1007/s11240-013-0292-4Search in Google Scholar
Gômez-Sânchez, D., Gonzâlez, S., 1991. Exploration and collection of wild species from the genus Helianthus from Northern Mexico. Helia 14: 49–54.Search in Google Scholar
Greco, B., Tanzorella, O.A., Carrozzo, G., Bianco, A., 1984. Callus induction and shoot regeneration in sunflower (Helianthus annuus L.). Plant Science Letters 36: 73–77.10.1016/0304-4211(84)90278-5Search in Google Scholar
Hollister, J.D., 2015. Polyploidy: Adaptation to the genomic environment. New Phytologist 205: 1034–1039.10.1111/nph.12939Search in Google Scholar
Hulke, B.S., Kleingartner, L.W., 2014. Sunflower. In: Smith, S., Diers, B., Specht, J., Carver, B. (eds.) Yield Gains in Major US Field Crops, Volume 33, CSSA Special Publications, Madison433–457.10.2135/cssaspecpub33.c15Search in Google Scholar
Jan, C.C., 1988. Chromosome doubling of wild × cultivated sunflower interspecific hybrids and its direct effect on backcross success. In Proceedings of 12th International Sunflower Conference, Novi Sad, Yugoslavia, July 25–29, 1988. International Sunflower Association, Paris, France287–292.Search in Google Scholar
Jan, C.C., 1996. Developing unique interspecific germplasm for sunflower improvement. In Proceedings of 14th International Sunflower Conference, Beijing/Shenyang, PR China, June 12–20, 1996. International Sunflower Association, Paris, France1111–1116.Search in Google Scholar
Jan, C.C., Chandler, J.M., Wagner, S.A., 1988. Induced tetraploid and trisomic production of Helianthus annuus L. Genome 30: 647–651.10.1139/g88-109Search in Google Scholar
Jeannin, G., Bronner, R., Hahne, G., 1995. Somatic embryogenesis and organogenesis induced on the immature zygotic embryo of sunflower (Helianthus annuus L.) cultivated in vitro: Role of the sugar. Plant Cell Reports 15: 200–204.10.1007/BF00193720Search in Google Scholar
Knittel, N., Escandon, A.S., Hahne, G., 1991. Plant regeneration at high frequency from mature sunflower cotyledons. Plant Science 73: 219–226.10.1016/0168-9452(91)90031-3Search in Google Scholar
Laferrière, J.E., 1986. Interspecific hybridization in sunflowers: An illustration of the importance of wild genetic resources in plant breeding. Outlook on Agriculture 15: 104–129.10.1177/003072708601500301Search in Google Scholar
Laparra, H., Bronner, R., Hahne, G., 1997. Histological analysis of somatic embryogenesis induced in leaf explants of Helianthus smithii Heiser. Protoplasma 196: 1–11.10.1007/BF01281053Search in Google Scholar
Litz, R.E., Gray, D.J., 1992. Organogenesis and somatic embryogenesis. In Hammerschlag, F.A., Litz, R.E. (eds.) Biotechnology of Perennial Fruit Crops. CAB International, Wallingford, UK3–34.Search in Google Scholar
Liu, H.X., Xie, X.D., Sun, S.J., Zhu, W.B., Ji, J., Wang, G., 2011. Optimization of Agrobacterium-mediated transformation of sunflower (Helianthus annuus L.) immature embryos. Australian Journal of Crop Science 5: 1616–1621.Search in Google Scholar
Morel, G.M., Wetmore, R.H., 1951. Tissue culture of monocotyledons. American Journal of Botany 38: 138–140.10.1002/j.1537-2197.1951.tb14803.xSearch in Google Scholar
Muller, A., Iser, M., Hess, D., 2001. Stable transformation of sunflower (Helianthus annuus L.) using a non-meristematic regeneration protocol and green fluorescent protein as a vital marker. Transgenic Research 10: 435–444.10.1023/A:1012029032572Search in Google Scholar
Murashige, T., Skoog, F., 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 495–497.10.1111/j.1399-3054.1962.tb08052.xSearch in Google Scholar
Nestares, G., Zorzoli, R., Mroginski, L., Picardi, L., 1996. Plant regeneration from cotyledons derived from mature sunflower seeds. Helia 19: 107–112.Search in Google Scholar
Paterson, K.E., Everett, N.P., 1985. Regeneration of Helianthus annuus inbred plants from callus. Plant Science 42: 125–132.10.1016/0168-9452(85)90152-9Search in Google Scholar
Pélissier, B., Bouchefra, O., Pépin, R., Freyssinet, G., 1990. Production of isolated somatic embryos from sunflower thin cell layer. Plant Cell Reports 9: 47–50.10.1007/BF00232134Search in Google Scholar
Power, C.J., 1987. Organogenesis from Helianthus annuus inbreds and hybrids from the cotyledons of zygotic embryos. American Journal of Botany 74: 497–503.10.1002/j.1537-2197.1987.tb08669.xSearch in Google Scholar
Pugliesi, C., Biasini, M.G., Fambrini, M., Baroncelli, S., 1993a. Genetic transformation by Agrobacterium tumefaciens in the interspecific hybrid Helianthus annuus × Helianthus tuberosus. Plant Science 93: 105–115.10.1016/0168-9452(93)90039-3Search in Google Scholar
Pugliesi, C., Cecconi, F., Mandolfo, A., Baroncelli, S., 1991. Plant regeneration and genetic variability from tissue cultures of sunflower (Helianthus annuus L,). Plant Breeding 106: 114–121.10.1111/j.1439-0523.1991.tb00489.xSearch in Google Scholar
Pugliesi, C., Megale, P., Cecconi, F., Baroncelli, S., 1993b. Organogenesis and embryogenesis in Helianthus tuberosus and in the interspecific hybrid Helianthus annuus × Helianthus tuberosus. Plant Cell Tissue Organ Culture 33: 187–193.10.1007/BF01983233Search in Google Scholar
Ruso, J., Sukno, S., Domínguez-Giménez, J., Melero-Vara, J.M., Fernández-Martínez, J.M., 1996. Screening of wild Helianthus species and derived lines for resistance to several populations of Orobanche cernua. Plant Disease 80: 1165–1169.10.1094/PD-80-1165Search in Google Scholar
Seiler, G.J., Rieseberg, L.H., 1997. Systematics, origin and germplasm resources of the wild and domesticated sunflower. In Schneiter, A. (ed.) Sunflower Technology and Production. American Society of Agronomy, Madison, Wisconsin21–26.10.2134/agronmonogr35.c2Search in Google Scholar
Skoric, D., 1992. Achievements and future directions of sunflower breeding. Field Crops Research 30: 231–270.10.1016/0378-4290(92)90003-RSearch in Google Scholar
Sujatha, M., Vijay, S., Vasavi, S., Sivaraj, N., Rao, S.C., 2012. Combination of thidiazuron and 2-isopentenyladenine promotes highly efficient adventitious shoot regeneration from cotyledons of mature sunflower (Helianthus annuus L.) seeds. Plant Cell Tissue Organ Culture 111: 359–372.10.1007/s11240-012-0202-1Search in Google Scholar
Sukno, S., Ruso, J., Jan, C.C., Melero-Vara, J.M., Fernández-Martínez, J.M., 1999. Interspecific hybridization between sunflower and wild perennial Helianthus species via embryo rescue. Euphytica 106: 69–78.10.1023/A:1003524822284Search in Google Scholar
Talapatra, S., Goswami, P., Das, S., Raychaudhuri, S.S., 2015. Role of SERK during somatic embryogenesis and its interaction with Brassinosteroids. In Mujib, A. (ed.) Somatic Embryogenesis in Ornamentals and Its Applications. Springer (India) Pvt. Ltd., 141–154.10.1007/978-81-322-2683-3_9Search in Google Scholar
Taski-Ajdukovic, K., Nagl, N., Miladinovic, D., 2010. Towards reducing genotype specificity in regeneration protocols after somatic hybridization between cultivated sunflower and wild Helianthus species. Acta Biologica Hungarica 61: 214–223.10.1556/ABiol.61.2010.2.9Search in Google Scholar
Taski-Ajdukovic, K., Vasic, D., Nagl, N., 2006. Regeneration of interspecific somatic hybrids between Helianthus annuus L. and Helianthus maximiliani (Schrader) via protoplast electrofusion. Plant Cell Reports 25: 698–704.10.1007/s00299-006-0134-5Search in Google Scholar
Thompson, T., Zimmerman, D., Rogers, C., 1981. Wild Helianthus as a genetic resource. Field Crops Research 4: 333–343.10.1016/0378-4290(81)90083-6Search in Google Scholar
Vega, T.A., Nestares, G.M., Pratta, G., Zorzoli, R., Gattuso, S., Picardi, L., 2007. Biochemical and histological changes associated with in vitro responses in sunflower cotyledonary explants. In Vitro Cellular and Developmental Biology-Plant 43: 415–422.10.1007/s11627-007-9087-9Search in Google Scholar
Vega, T.A., Nestares, G.M., Zorzoli, R., Picardi, L., 2006. Responsive regions for direct organogenesis in sunflower cotyledons. Acta Physiologiae Plantarum 28: 427–432.10.1007/BF02706625Search in Google Scholar
Wang, Y., Li, C., Zhang, Y., Chen, Y., Zhao, L., Yue, P., Teng, X., Wang, N., 2011. Establishing the regeneration system of sunflower. Sheng Wu Gong Cheng Xue Bao (Chinese Journal of Biotechnology) 27: 1379–1389. [Article in Chinese with English abstract].Search in Google Scholar
Wilcox Mccann, A., Cooly, G., Van Dresser, J., 1988. A system for routine plantlet regeneration of sunflower (Helianthus annuus L.) from immature embryo-derived callus. Plant Cell Tissue Organ Culture 14: 103–110.10.1007/BF00041183Search in Google Scholar
Wirtzens, B., Scowcroft, W.R., Downes, R.W., Larkin, P.J., 1988. Tissue culture and plant regeneration from sunflower (Helianthus annuus) and interspecific hybrids (H. tuberosus × H. annuus). Plant Cell Tissue Organ Culture 13: 61–76.10.1007/BF00043047Search in Google Scholar
Yordanov, Y., Yordanova, E., Atanassov, A., 2002. Plant regeneration from interspecific hybrid and backcross progeny of Helianthus eggertii × Helianthus annuus. Plant Cell Tissue Organ Culture 71: 7–14.10.1023/A:1016510109911Search in Google Scholar
Zhang, Z.F., Finer, J.J., 2015. Sunflower (Helianthus annuus L.) organogenesis from primary leaves of young seedlings preconditioned by cytokinin. Plant Cell Tissue Organ Culture 123: 645–655.10.1007/s11240-015-0867-3Search in Google Scholar
© 2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Somatic Embryogenesis from Corolla Tubes of Interspecific Amphiploids between Cultivated Sunflower (Helianthus annuus L.) and Its Wild Species
- Inheritance of Leaf Tip Shape and Fringed Leaf Margin in Sunflower
- Morphological Characterization of Sunflower Under Organic Fertilization and Seed Oil Content and Yield Pie
- Molecular Characterization of Broomrape Populations from Republic of Moldova using SSR Markers
- Line × Tester Analysis for Duration of Flowering, Yield Components and Seed Yield in Sunflower (Helianthus Annuus L.)
- Effect of Alien Cytoplasm on Combining Ability for Earliness and Seed Yield in Sunflower under Irrigation and Drought Stress
- Genetic Analysis of Half Diallel Matting with Different Methods and their Comparisons for Yield and its Associated Traits in Sunflower under Saline Soil Stress Conditions
Articles in the same Issue
- Frontmatter
- Somatic Embryogenesis from Corolla Tubes of Interspecific Amphiploids between Cultivated Sunflower (Helianthus annuus L.) and Its Wild Species
- Inheritance of Leaf Tip Shape and Fringed Leaf Margin in Sunflower
- Morphological Characterization of Sunflower Under Organic Fertilization and Seed Oil Content and Yield Pie
- Molecular Characterization of Broomrape Populations from Republic of Moldova using SSR Markers
- Line × Tester Analysis for Duration of Flowering, Yield Components and Seed Yield in Sunflower (Helianthus Annuus L.)
- Effect of Alien Cytoplasm on Combining Ability for Earliness and Seed Yield in Sunflower under Irrigation and Drought Stress
- Genetic Analysis of Half Diallel Matting with Different Methods and their Comparisons for Yield and its Associated Traits in Sunflower under Saline Soil Stress Conditions

