Abstract
2-Oxoimidazolidine-1,3-disulfonic acid (OImDSA) is a recoverable catalyst for the synthesis of 1,3-thiazolidine-4-ones at room temperature in a one-pot procedure without using any organic solvents. Moreover, the catalyst can be easily recovered and recycled for five runs without significant loss of catalytic activity. The structures of the synthesized 1,3-thiazolidine-4-one compounds were confirmed by 1H NMR, 13C NMR and FTIR spectral data and elemental analysis.
Introduction
Thiazolidine compounds contribute to various pharmacological effects. Thiazolidines are used as anti-seizure, fungicidal, anti-bacterial, anti-tubercular, anti-inflammatory, anti-amoebic, anti-diabetic and local anesthetic agents [1], [2]. Some of these compounds have also shown anti-Parkinsonism [3], anti-oxidant [4], anti-convulsant [5], anti-cancer [6], hypoglycemic [7] and non-narcotic analgesic [8] activities. On the other hand, pyrazoles have shown anti-bacterial, anti-tumor, anti-viral, anti-fungal, anti-tubercular, anti-parasitic, anesthetic, anti-diabetic, anti-inflammatory, analgesic and insecticidal activities [9].
In recent years, silica gel [10], SiCl4 [11], Bi(SCH2COOH)3 [12], ZnCl2 [13], N,N,N′,N′-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate (HBTU) [14], [bmim][PF6] [15], Saccharomyces cerevisiae [16], 1,3-dicyclohexylcarbodiimide (DCC) [17], supported protic acid [18] and nano-Fe3O4@SiO2 supported ionic liquid [19] have been employed for the synthesis of 1,3-thiazolidine-4-ones. To the best of our knowledge, there is no report in the literature on the use of 2-oxoimidazolidine-1,3-disulfonic acid (OImDSA) as a catalyst for the green synthesis of pyrazolyl-1,3-thiazolidine-4-ones. In the search for eco-friendly alternatives to classical synthesis [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22] and in continuation of our studies to synthesize heterocyclic and pharmaceutical compounds under mild and practical protocols [23], [24], [25], [26], [27], we report herein the synthesis of some novel pyrazolyl-1,3-thiazolidine-4-ones using OImDSA (Scheme 1).
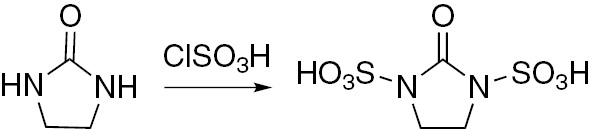
Preparation of 2-oxoimidazolidine-1,3-disulfonic acid (OImDSA).
Results and discussion
Pyrazolecarbaldehyde 1a, 4-methoxyaniline 2a and thioglycolic acid 3 were mixed with 10 mL of H2O in the presence of a catalytic amount of HCl and various solid catalysts, namely montmorillonite K10, ZnCl2, L-proline, nano-Fe3O4, nano-SiO2 and OImDSA (Scheme 2) or under solvent-free condition using 2 mL of an ionic liquid such as [BMIM]Br, [BMIM]OH and [BMIM]SOH. In these experiments, OImDSA was the most efficient catalyst, not only in terms of high yield of pyrazolyl-1,3-thiazolidine-4-one 4a but also high reaction rate (92% yield in 1 h). Moreover, our results showed that 0.1 g of OImDSA per 1 mmol of aldehyde 1a is enough for the synthesis of 4a. The optimal temperature for the synthesis of 4a is room temperature. Increasing the temperature has no effect on the reaction time and yield.

Synthesis of compounds 4a–k.
Various pyrazolecarbaldehydes and anilines can be utilized in this protocol. It has been shown that aldehydes with electron-withdrawing groups react faster than the aldehydes with electron-releasing groups. The yields obtained with substrates having electron-withdrawing groups are also greater. To evaluate the reusability of the OImDSA, the catalyst was separated from the reaction medium by treatment with water. The aqueous solution was fractionally distilled under reduced pressure and the recovered catalyst was reused in subsequent reactions. After five successive runs, OImDSA showed virtually no loss in efficiency regarding reaction time and yield.
Conclusions
An efficient protocol for the synthesis of compounds 4a–k using OImDSA as an effective catalyst was developed. To the best of our knowledge, this is the first report on the synthesis of 1,3-thiazolidine-4-ones bearing pyrazole moiety in aqueous media.
Experimental
All commercial chemicals were used as received. Melting points were measured on an Electro-thermal 9100 apparatus and are uncorrected. 1H nuclear magnetic resonance (NMR) (500 MHz) and 13C NMR (125 MHz) spectra were obtained on a Bruker DRX 500 Avance spectrometer using CDCl3 as solvent and TMS as the internal standard. Fourier transform infrared (FT-IR) spectra were recorded as KBr pellets on a Shimadzu FT-IR-8400S spectrometer. Elemental analyses were done on a Carlo-Erba EA1110CNNO-S analyzer.
Synthesis of 2-oxoimidazolidine-1,3-disulfonic acid (OImDSA)
A flask (500 mL) with imidazolidin-2-one (8.6 g, 0.1 mol) was equipped with a constant pressure dropping funnel containing chlorosulfonic acid (23.3 g, 0.2 mol) and a gas outlet tube which was dipped into water to dissolve the generated HCl gas during the reaction. The flask was placed into an ice bath and chlorosulfonic acid was added dropwise over a period of 20 min and the resulting mixture was stirred for an additional 20 min. The temperature of the mixture was brought up to the room temperature and stirring was continued for an additional 60 min. The mixture was triturated with n-hexane (20 mL) and then filtered. The solid residue was washed with n-hexane (20 mL) and dried under reduced pressure to give OImDSA as a white solid: mp >300°C; IR: 984 (S=O symmetric stretch), 1315 (S=O asymmetric stretch), 1661 (C=O stretch), 2963 (C-H aliphatic stretch), 3309 cm−1 (O-H stretch); 1H NMR δ: 3.63 (s, 4H, N-CH2-CH2-N), 13.35 (s, br., 2H, -SO3H); 13C NMR: 51.4 (N-CH2-CH2-N), 152.5 (C=O). Anal. Calcd for C3H6N2O7S2: C, 14.63; H, 2.46; N, 11.38. Found: C, 14.63; H, 2.47; N, 11.37.
General procedure for preparation of 4a–k
A mixture of a pyrazolecarbaldehyde 1 (1 mmol), an aniline 2 (1 mmol), thioglycolic acid 3 (1 mmol), H2O (10 mL) and OImDSA (0.1 g) was stirred at room temperature for 1–2 h. The progress of the reaction was monitored by TLC (EtOAc/petroleum ether, 1:2). After completion of the reaction, the product was extracted with CHCl3 and the aqueous phase containing catalyst OImDSA was fractionally distilled under reduced pressure. Then, the solution in CHCl3 was concentrated under reduced pressure and the residue was purified by column chromatography (EtOAc/petroleum ether, 1:2). The product was crystallized from EtOH.
2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-3-(4-methoxyphenyl)thiazolidin-4-one (4a)
Reaction time 60 min; yield 92%; yellow solid, mp 242–244°C; IR: 1502, 1541, 1600 (C=C aromatic stretch), 1731 (C=O stretch), 2981 (C-H aliphatic stretch), 3126 cm−1 (C-H aromatic stretch); 1H NMR: δ 3.28 (d, J=15.3 Hz, 1H, CH2-S), 3.50 (d, J=15.3 Hz, 1H, CH2-S), 3.52 (s, 3H, CH3O), 5.48 (s, 1H, N-CH-S), 7.32 (t, J=7.4 Hz, 1H, Ar), 7.43–7.49 (m, 4H, Ar), 7.73–7.80 (m, 4H, Ar), 8.22 (s, 1H, N-CH=C); 13C NMR: δ 32.9, 40.4, 60.5, 118.0, 125.5, 127.4, 127.8, 128.4, 128.7, 129.5, 129.8, 133.6, 138.8, 143.1, 168.3 (C=O). Anal. Calcd for C25H20ClN3O2S: C, 65.00; H, 4.36; N, 9.10. Found: C, 65.02; H, 4.35; N, 9.08.
2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-3-(4-methoxyphenyl)thiazolidin-4-one (4b)
Reaction time 70 min, yield 90%; yellow solid; mp 231–233°C; IR: 1288 (C-O aromatic stretch), 1500, 1541, 1596 (C=C aromatic stretch), 1737 (C=O stretch), 2977 (C-H aliphatic stretch), 3058 cm−1 (C-H aromatic stretch); 1H NMR: δ 3.29 (d, J=15.2 Hz, 1H, CH2-S), 3.49 (d, J=15.2 Hz, 1H, CH2-S), 3.58 (s, 3H, CH3O), 5.49 (s, 1H, N-CH-S), 7.30 (td, J=7.9, 0.7 Hz, 2H, CH=C- OCH3), 7.37–7.41 (m, 2H, Ar), 7.45–7.48 (m, 6H, Ar), 7.76 (dd, J=8.4 Hz and 0.6 Hz, 2H, Ar), 7.80 (d, J=8.4 Hz, 2H, CH=C-Cl), 8.09 (s, 1H, N-CH=C); 13C NMR δ: 32.9, 43.5, 60.5, 118.0, 125.7, 127.1, 127.3, 127.4, 127.5, 127.6, 128.4, 131.3, 138.6, 150.0, 168.8 (C=O). Anal. Calcd for C25H21N3O2S: C, 70.24; H, 4.95; N, 9.83. Found: C, 70.26; H, 4.97; N, 9.81.
2-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-(2-methyl-4-nitrophenyl)thiazolidin-4-one (4c)
Reaction time 75 min; yield 86% of yellow solid; mp 237–239°C; IR: 1363, 1541 (NO2 stretch), 1450, 1500, 1596 (C=C aromatic stretch), 1730 (C=O stretch), 2981 (C-H aliphatic stretch), 3126 cm−1 (C-H aromatic stretch); 1H NMR: δ 3.30 (d, J=15.2 Hz, 1H, CH2-S), 3.50 (d, J=15.2 Hz, 1H, CH2-S), 3.58 (s, 3H, CH3), 5.50 (s, 1H, N-CH-S), 7.31 (t, J=7.4 Hz, 1H, Ar), 7.40–7.42 (m, 1H, Ar), 7.45–7.49 (m, 7H, Ar), 7.75–7.78 (m, 2H, Ar), 7.80–7.82 (m, 2H, Ar), 8.23 (s, 1H, N-CH=C); 13C NMR: δ 30.9, 40.4, 43.5, 60.5, 60.6, 118.0, 125.7, 127.1, 127.3, 127.4, 127.5, 127.6, 128.4, 131.3, 138.6, 150.0, 168.8 (C=O). Anal. Calcd for C25H20N4O3S: C, 65.77; H, 4.42; N, 12.27. Found: C, 65.75; H, 4.39; N, 12.29.
2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-3-(2-methyl-4-nitrophenyl)thiazolidin-4-one (4d)
Reaction time 60 min; yield 87% of yellow solid; mp 240–242°C; IR: 1400, 1539 (NO2 stretch), 1454, 1500, 1598 (C=C aromatic stretch), 1731 (C=O stretch), 2981 (C-H aliphatic stretch), 3128 cm−1 (C-H aromatic stretch); 1H NMR: δ 3.24 (d, J=15.2 Hz, 1H, CH2-S), 3.49 (d, J=15.2 Hz, 1H, CH2-S), 3.55 (s, 1H, CH3), 5.45 (s, 1H, N-CH-S),7.26–7.30 (m, 2H, Ar),7.40–7.45 (m, 6H, Ar), 7.71–7.73 (m, 2H, Ar), 7.75–7.78 (m, 2H, CH=C-Cl), 8.20 (s, 1H, N-CH=C); 13C NMR: δ 32.7, 40.3, 43.2, 60.4, 60.5, 117.8, 117.9, 125.7, 127.3, 127.6, 128.3, 128.6, 129.7, 133.1, 138.4, 148.7, 168.1 (C=O). Anal. Calcd for C25H19ClN4O3S, %: C, 61.16; H, 3.90; N, 11.41. Found: C, 61.14; H, 3.87; N, 11.39.
3-(2-Methyl-4-nitrophenyl)-2-(3-(3-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)thiazolidin-4-one (4e)
Reaction time 70 min; yield 91% of yellow solid; mp 251–253°C; IR: 1353, 1533 (NO2 stretch) 1456, 1595 (C=C aromatic stretch), 1731 (C=O stretch), 2981 (C-H aliphatic stretch), 3128 cm−1 (C-H aromatic stretch); 1H NMR: δ 3.28 (d, J=15.1 Hz, 1H, CH2-S), 3.34 (d, J=15.1 Hz, 1H, CH2-S), 3.56 (s, 3H, CH3), 5.52 (s, 1H, N-CH-S), 7.31–7.35 (m, 1H, Ar), 7.45–7.48 (m, 3H, Ar), 7.64–7.66 (m, 1H, Ar), 7.66–7.76 (m, 3H, Ar), 8.21 (d, J=2.0 Hz, 1H, Ar), 8.23 (d, J=2.0 Hz, 1H, CH3-CH-CH=C-NO2), 8.25 (s, 1H, N-CH=C), 8.72 (t, J=1.8 Hz, 1H, Pyrazolyl-CH=C-NO2); 13C NMR δ: 32.8, 40.3, 60.4, 118.0, 118.3, 121.8, 122.3, 126.1, 127.7, 128.4, 128.5, 128.6, 133.1, 133.3, 138.3, 147.3, 147.4, 168.2 (C=O). Anal. Calcd for C25H19N5O5S: C, 59.87; H, 3.82; N, 13.96. Found: C, 59.85; H, 3.84; N, 13.95.
2-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-phenylthiazolidin-4-one (4f)
Reaction time 60 min; yield 89% of yellow solid; mp 221–223°C, IR: 1498, 1539, 1596 (C=C aromatic stretch), 1733 (C=O stretch), 2981 (C-H aliphatic stretch), 3058 cm−1 (C-H aromatic stretch); 1H NMR: δ 3.29 (d, J=15.1 Hz, 1H, CH2-S), 3.52 (d, J=15.1 Hz, 1H, CH2-S), 5.53 (s, 1H, N-CH-S), 7.18–7.30 (m, 2H, Ar), 7.40–7.44 (m, 2H, Ar) 7.46–7.52 (m, 2H, Ar), 7.77 (d, J=8.2, 2.4 Hz, 2H, Ar), 7.83 (dd, J=8.2 Hz and 2.4 Hz, 2H, Ar), 8.26 (s, 1H, N-CH=C); 13C NMR: δ 43.5, 44.5, 119.0, 119.1, 126.7, 128.2, 128.3, 128.4, 128.6, 129.4, 132.4, 139.7, 151.1, 169.8 (C=O). Anal. Calcd for C24H19N3OS: C, 72.52; H, 4.82; N, 10.57. Found: C, 72.50; H, 4.79; N, 10.59.
2-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-(4-nitrophenyl)thiazolidin-4-one (4g)
Reaction time 75 min; yield 83% of yellow solid; mp 224–226°C; IR 1361 (NO2 symmetric stretch), 1542 (NO2 asymmetric stretch), 1730 (C=O stretch), 2981 (C-H aliphatic stretch), 3060 cm−1 (C-H aromatic stretch); 1H NMR: δ 3.29 (d, J=15.2 Hz, 1H, CH2-S), 3.50 (d, J=15.2 Hz, 1H, CH2-S), 5.50 (s, 1H, N-CH-S), 7.28–7.32 (m, 2H, Ar), 7.39–7.42 (m, 2H, Ar), 7.44–7.49 (m, 6H, Ar), 7.76 (d, J=7.9 Hz, 2H, CH-CH=C-NO2), 7.82 (dd, J=8.5 Hz and 1.4 Hz, 2H, CH=C-NO2), 8.23 (s, 1H, N-CH=C); 13C NMR δ: 32.9, 43.4, 117.3, 117.9, 118.0, 125.6, 126.7, 127.1, 127.2, 127.4, 127.5, 127.6, 127.7, 128.4, 131.2, 138.6, 150.0, 168.7 (C=O). Anal. Calcd for C24H18N4O3S: C, 65.14; H, 4.10; N, 12.66. Found: C, 65.12; H, 4.09; N, 12.63.
2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-3-(4-nitrophenyl)thiazolidin-4-one (4h)
Reaction time 80 min; yield 79% of yellow solid, mp 239–241°C; IR: 1380 (NO2 symmetric stretch), 1544 (NO2 asymmetric stretch), 1731 (C=O stretch), 2981 cm−1 (C-H aliphatic stretch); 1H NMR: δ 3.31 (d, J=15.2 Hz, 1H, CH2-S), 3.53 (d, J=15.2 Hz, 1H, CH2-S), 5.51 (s, 1H, N-CH-S), 7.36–7.37 (m, 1H, Ar), 7.46–7.50 (m, 5H, Ar), 7.52 (dd, J=5.6 Hz and 3.2 Hz, 1H, Ar), 7.75–7.85 (m, 6H, Ar), 8.26 (s, 1H, N-CH=C); 13C NMR δ: 32.7, 44.0, 114.8, 118.2, 119.7, 123.9, 125.8, 128.4, 129.4, 129.8, 138.7, 150.3, 151.3, 170.3 (C=O). Anal. Calcd for C24H17ClN4O3S: C, 60.44; H, 3.59; N, 11.75. Found: C, 60.45; H, 3.61; N, 11.72.
3-(4-Nitrophenyl)-2-(3-(3-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)thiazolidin-4-one (4i)
Reaction time 90 min; yield 84% of yellow solid, mp 263–265°C: IR: 1350 (NO2 symmetric stretch), 1533 (NO2 asymmetric stretch), 1598, 1627 (C=C aromatic stretch), 1731 (C=O stretch), 2923 cm−1 (C-H aliphatic stretch); 1H NMR: δ 3.30 (d, J=15.3 Hz, 1H, CH2-S), 3.53 (d, J=15.3 Hz, 1H, CH2-S), 5.54 (s, 1H, N-CH-S), 7.35 (t, J=7.4 Hz, 2H, Ar), 7.48–7.52 (m, 3H, Ar), 7.67 (t, J=8.0 Hz, 2H, Ar), 7.76–7.80 (m, 3H, Ar), 8.24–8.76 (m, 4H, Ar); 13C NMR: δ 43.3, 60.6, 118.1, 121.9, 122.4, 126.2, 127.8, 128.5, 128.6, 133.4, 168.7 (C=O). Anal. Calcd for C24H17N5O5S: C, 59.13; H, 3.51; N, 14.37. Found: C, 59.15; H, 3.49; N, 14.39.
2-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-p-tolylthiazolidin-4-one (4j)
Reaction time 90 min; yield 84% of yellow solid; mp 246–248°C; IR: 1350 (NO2 symmetric stretch), 1533 (NO2 asymmetric stretch), 1598, 1627 (C=C aromatic stretch), 1731 (C=O stretch), 2923 cm−1 (C-H aliphatic stretch); 1H NMR δ: 3.32 (d, J=15.2 Hz, 1H, CH2-S), 3.54 (d, J=15.2 Hz, 1H, CH2-S), 3.63 (s, 3H, CH3), 5.54 (s, 1H, N-CH-S), 7.34 (t, J=7.6 Hz, 1H, Ar), 7.63 (t, J=7.6 Hz, 1H, Ar), 7.48–7.55 (m, 6H, Ar), 7.80 (d, J=7.6 Hz, 2H, Ar), 7.85 (dd, J=7.6 Hz and 1.2 Hz, 2H, Ar), 8.27 (s, 1H, N-CH=C); 13C NMR: δ 34.0, 41.48, 44.6, 119.0, 126.7, 128.2, 128.3, 128.5, 128.6, 129.0, 129.5, 129.7, 132.4, 139.7, 151.1, 169.8 (C=O). Anal. Calcd for C25H21N3OS: C, 72.97; H, 5.14; N, 10.21. Found: C, 72.95; H, 5.15; N, 10.19.
2-(3-(4-Hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)-3-(4-nitrophenyl)thiazolidin-4-one (4k)
Reaction time 75 min, 87% of yellow solid, mp 254–256°C; IR: 1276 (C-O aromatic), 1502 (NO2 symmetric stretch), 1533 (NO2 asymmetric stretch), 1602 (C=C aromatic stretch), 1726 (C=O stretch), 2923 (C-H aliphatic stretch), 3425 cm−1 (O-H aliphatic stretch); 1H NMR: δ 3.32 (d, J=15.3 Hz, 1H, CH2-S), 3.54 (d, J=15.3 Hz, 1H, CH2-S), 5.48 (s, 1H, N-CH-S), 6.91 (d, J=8.4 Hz, 2H, CH=C-OH), 7.29–7.35 (m, 2H, Ar), 7.49–7.51 (m, 4H, Ar), 7.68 (d, J=8.2 Hz, 2H, Ar), 7.75–7.78 (m, 3H, Ar), 8.22 (s, 1H, N-CH=C); 13C NMR: δ 33.9, 44.6, 115.6, 118.7, 119.1, 124.4, 126.7, 128.1, 129.5, 129.9, 139.6, 151.1, 156.3, 170.0 (C=O). Anal. Calcd for C24H18N4O4S: C, 62.87; H, 3.96; N, 12.22. Found: C, 62.86; H, 3.97; N, 12.20.
Acknowledgments
We gratefully acknowledge the financial support from the Islamic Azad University, Rasht Branch, Iran.
References
[1] Lesyk, R. B.; Zimenkovsky, B. S.; Kaminskyy, D. V.; Kryshchyshyn, A. P.; Havryluk, D. Y.; Atamanyuk, D. V.; Subtelna, I. Y.; Khyluk, D. V. Thiazolidinone motif in anticancer drug discovery. Experience of DH LNMU medicinal chemistry scientific group. Biopolym. Cell2011, 27, 107–117.10.7124/bc.000089Search in Google Scholar
[2] Lesyk, R. B.; Zimenkovsky B. S. 4-Thiazolidones: centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem.2004, 8, 1547–1577.10.2174/1385272043369773Search in Google Scholar
[3] Amr, A. E. E.; Maigali, S. S.; Abdulla, M. M. Synthesis and analgesic and antiparkinsonian activities of thiopyrimidine, pyrane, pyrazoline and thiazolopyrimidine derivatives from 2-chloro-6-ethoxy-4-acetylpyridine. Monat. für Chemie2008, 139, 1409–1415.10.1007/s00706-008-0937-xSearch in Google Scholar
[4] Soni, B. K.; Singh, T.; Bhalgat, C. M. In-vitro antioxidant studies of some 1, 2, 3-thiadiazole derivatives. Int. J. Res. Phar. Biomed. Sci.2011, 24, 1590–1592.Search in Google Scholar
[5] Swinyard, E. A.; Brown, W. C.; Goodman, L. S. The anticonvulsant effect of benzhydryl piperazines on pentylenatetrazol-induced in mice. J. Pharmacol. Exp. Ther.1952, 106, 319–330.Search in Google Scholar
[6] Colomboa, A.; Fernàndez, J. C.; Fernández-Forner, D.; de la Figuera, N.; Albericio, F.; Fornsa, P. Stereomeric studies on the oxidation and alkylation of 4-thiazolidinones. Tetrahedron Lett.2008, 49, 1569–1572.10.1016/j.tetlet.2008.01.038Search in Google Scholar
[7] Gaikwad, N. J.; Gaikwad, N. S. Synthesized mannich reaction products of 5- benzylidine-4- thiazolidinone and evaluated for their hypoglycemic activity. Indian J. Heterocycl. Chem.2002, 12, 101–102.Search in Google Scholar
[8] Woolfe, G.; MacDonald, A. D. The potentiation of a non–narcotic analgesic. Dipyrone by cholinomimetic drug. J. Pharm. Exp. Ther. 1944, 80, 300–307.Search in Google Scholar
[9] Sharshira, E. M.; Hamada, N. M. M. Synthesis and antimicrobial evaluation of some pyrazole derivatives. Molecules2012, 17, 4962–4971.10.3390/molecules17054962Search in Google Scholar PubMed PubMed Central
[10] Thakare, M. P.; Kumar, P.; Kumar, N.; Pandey, S. K. Silica gel promoted environment-friendly synthesis of 2,3-disubstituted 4-thiazolidinones. Tetrahedron Lett. 2014, 55, 2463–2466.10.1016/j.tetlet.2014.03.007Search in Google Scholar
[11] Jyotirling, R. M.; Umesh, R. P.; Prashant, D. N.; Ramrao, A. M. An efficient synthetic route for quinazolinyl 4-thiazolidinones. Tetrahedron Lett.2009, 50, 5025–5027.10.1016/j.tetlet.2009.06.086Search in Google Scholar
[12] Foroughifar, N.; Ebrahimi, S. One-pot synthesis of 1,3-thiazolidin-4-one using Bi(SCH2COOH)3 as catalyst. Chin. Chem. Lett. 2013, 24, 389–391.10.1016/j.cclet.2013.03.019Search in Google Scholar
[13] Srivastava, S. K.; Srivastava, S. L.; Srivastava, S. D. Synthesis of 5-arylidene-2-aryl-3-(2-chlorophenothiazinoacetamidyl)-1, 3-thiazolidin-4-ones as antifungal and anticonvulsant agents. J. Ind. Chem. Soc.2000, 77, 104–105.10.1002/chin.200117133Search in Google Scholar
[14] Rawal, R. K.; Srivastava, T.; Haq, W.; Katti, S. B. An expeditious synthesis of thiazolidinones and tetathiazanones. J. Chem. Res. 2004, 5, 368–369.10.3184/0308234041639746Search in Google Scholar
[15] Yadav, A. K.; Kumar, M.; Yadav, T.; Jain, R. An ionic liquid mediated one-pot synthesis of substituted thiazolidinones and benzimidazoles. Tetrahedron Lett. 2009, 50, 5031–5034.10.1016/j.tetlet.2009.06.091Search in Google Scholar
[16] Umesh, R. P.; Dhanaji, V. J.; Manisha, R. B.; Ramrao, A. M. Saccharomyces cerevisiae catalyzed one-pot three component synthesis of 2,3-diaryl-4-thiazolidinones. Tetrahedron Lett. 2011, 52, 1689–1691.10.1016/j.tetlet.2011.01.143Search in Google Scholar
[17] Srivastava, T.; Haq, W.; Katti, S. B. Carbodiimide mediated synthesis of 4-thiazolidinones by one-pot three-component condensation. Tetrahedron2002, 58, 7619–7624.10.1016/S0040-4020(02)00866-9Search in Google Scholar
[18] Kumar, D.; Sonawane, M.; Pujala, B.; Jain, V. K.; Bhagat, S.; Chakraborti, A. K. Supported protic acid-catalyzed synthesis of 2,3-disubstituted thiazolidin-4-ones: enhancement of the catalytic potential of protic acid by adsorption on solid supports. Green Chem.2013, 15, 2872–2884.10.1039/c3gc41218kSearch in Google Scholar
[19] Azgomi, N.; Mokhtary, M. Nano-Fe3O4@SiO2 supported ionic liquid as an efficient catalyst for the synthesis of 1, 3-thiazolidin-4-ones under solvent-free conditions. J. Mol. Cat. A: Chem.2015, 398, 58–64.10.1016/j.molcata.2014.11.018Search in Google Scholar
[20] Kiasat, A. R.; Zayadi, M. Polyethylene glycol immobilized on silica gel as a new solid-liquid phase-transfer catalyst for regioselective azidolysis of epoxides in water: an efficient route to 1, 2-azido alcohols. Catal. Commun. 2008, 9, 2063–2067.10.1016/j.catcom.2008.03.053Search in Google Scholar
[21] Kiasat, A. R.; Badri, R.; Zargar, B.; Sayyahi, S. Poly (ethylene glycol) grafted onto dowex resin: an efficient, recyclable and mild polymer-supported phase transfer catalyst for the regioselective azidolysis of epoxides in water. J. Org. Chem. 2008, 73, 8382–8385.10.1021/jo801356ySearch in Google Scholar PubMed
[22] Kiasat, A. R.; Mehrjardi, M. F. PEG-SO3H as eco-friendly polymeric catalyst for regioselective ring opening of epoxides using thiocyanate anion in water: an efficient route to synthesis of β-hydroxy thiocyanate. Catal. Commun. 2008, 9, 1497–1500.10.1016/j.catcom.2007.12.019Search in Google Scholar
[23] Nikpassand, M.; Pirdelzendeh, D. Green synthesis of novel azo-linked 2-phenyl benzimidazoles using ionic liquid [BDBDMIm]Br. Dyes Pigm.2016, 130, 314–318.10.1016/j.dyepig.2016.03.038Search in Google Scholar
[24] Zare Fekri, L.; Nikpassand, M.; Hassanpour, K. Green aqueous synthesis of mono, bis and tris dihydropyridines using using nano Fe3O4 under ultrasound irradiation. Curr. Org. Chem.2015, 12, 76–79.10.2174/1570179411666140806005614Search in Google Scholar
[25] Nikpassand, M.; Zare Fekri, L.; Sanagou, S. One-pot synthesis of 2-hydrazonyl-4-phenylthiazoles via [PDBMDIm]Br-catalyzed reaction under solvent-free conditions. Heterocycl. Commun. 2016, 22, 243–246.10.1515/hc-2016-0024Search in Google Scholar
[26] Nikpassand, M.; Mamaghani, M.; Shirini, F.; Tabatabaeian, K. A convenient ultrasound-promoted regioselective synthesis of fused polycyclic 4-aryl-3-methyl-4,7-dihydro-1H-pyrazolo[3,4-b]pyridines. Ultrason. Sonochem.2010, 17, 301–305.10.1016/j.ultsonch.2009.08.001Search in Google Scholar PubMed
[27] Nikpassand, M.; Zare Fekri, L.; Farokhian, P. An efficient and green synthesis of novel benzoxazole under ultrasound irradiation. Ultrason. Sonochem.2016, 28, 341–345.10.1016/j.ultsonch.2015.08.014Search in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- One-pot synthesis of benzopyrans catalyzed by silica supported dual acidic ionic liquid under solvent-free conditions
- An efficient synthesis of 5-halo-6-trifluoromethylpyridine-3-carbonitriles and carboxylic acids
- Research Articles
- Novel 2H-1,3-benzoxazine ring formation by intramolecular heterocyclization of N-(α-aryloxyalkyl)imidoyl chlorides
- Green synthesis of novel 2-pyrazolyl-1,3-thiazolidine-4-ones using 2-oxoimidazolidine-1,3-disulfonic acid
- A facile synthesis of (E)-2-(aryl/hetaryl)vinyl-4-phenylquinoline-3-carboxylic acids
- Efficient synthesis of new pyrano[3,2-b]pyran derivatives via Fe3O4@SiO2-IL-Fc catalyzed three-component reaction
- An improved and scalable synthesis of zolpidem via a CuI/BINOL-mediated tandem reaction of imine and alkyne
- Scalable synthesis and properties of 7-methyl- 4-azaindole
- Synthesis and insecticidal activities of novel 1H-pyrazole-5-carboxylic acid derivatives
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- One-pot synthesis of benzopyrans catalyzed by silica supported dual acidic ionic liquid under solvent-free conditions
- An efficient synthesis of 5-halo-6-trifluoromethylpyridine-3-carbonitriles and carboxylic acids
- Research Articles
- Novel 2H-1,3-benzoxazine ring formation by intramolecular heterocyclization of N-(α-aryloxyalkyl)imidoyl chlorides
- Green synthesis of novel 2-pyrazolyl-1,3-thiazolidine-4-ones using 2-oxoimidazolidine-1,3-disulfonic acid
- A facile synthesis of (E)-2-(aryl/hetaryl)vinyl-4-phenylquinoline-3-carboxylic acids
- Efficient synthesis of new pyrano[3,2-b]pyran derivatives via Fe3O4@SiO2-IL-Fc catalyzed three-component reaction
- An improved and scalable synthesis of zolpidem via a CuI/BINOL-mediated tandem reaction of imine and alkyne
- Scalable synthesis and properties of 7-methyl- 4-azaindole
- Synthesis and insecticidal activities of novel 1H-pyrazole-5-carboxylic acid derivatives

