Abstract
The genus Ajuga, a member of the Lamiaceae family, is comprised of more than 300 species of annual and perennial herbaceous flowering plants mainly distributed throughout the temperate regions of Asia, Europe, Australia, North America and Africa. These plants are used as folk medicines effective for rheumatic fevers, dysentery, malaria, hypertension, diabetes and gastrointestinal disorders, as well as anthelmintic, astringent, febrifuge diuretic, antifungal and anti-inflammatory agents. A variety of constituents has been isolated from these plants. This review summarizes the phytochemical progress of the genus Ajuga and lists the compounds isolated up to 2014.
Introduction
The genus Ajuga, a member of the Lamiaceae family, is comprised of more than 300 species of annual and perennial herbaceous flowering plants mainly distributed throughout the temperate regions of Asia, Europe, Australia, North America and Africa. These species have been used as common house plants and are called bugle or bugleweed. They are mainly characterized by the color and shape of the flower. For example, the flower of Ajuga reptans is somewhat tall and blue, while that of Ajuga decumbens is short and purple. Many of these plants are of medicinal importance and are traditionally used as remedies for rheumatic fevers, dysentery, malaria, hypertension, diabetes and gastrointestinal disorders, as well as anthelmintic, astringent, febrifuge diuretic, antifungal and anti-inflammatory agents [1]. The genus Ajuga has attracted attention since the report in 1976 that Ajuga remota grown in Kenya is not attacked by African armyworms and contains three moderately strong antifeedants [2]. Since then, reports of the isolation of neoclerodanes and phytoecdysteroids, as the insect allelochemicals responsible for antifeedant activity from this genus, have appeared [3]. Several species of this genus have been chemically studied and a series of bioactive metabolites, including phytoecdysteroids, diterpenoids and iridoids have been isolated and characterized. Biological investigations demonstrate that some of these compounds display antibacterial [4], antifungal [5], antiplasmodial [6], cytotoxic, antitumor promoting [7], vasoconstricting [8], insect molting inhibitory, insect antifeeding [9] and enzyme-inhibitory [10] activities. This review summarizes phytochemical progress of the genus Ajuga covering the literature up to 2014. In addition, some biological activities of compounds obtained from this genus are also listed.
Chemical constituents
There have been many phytochemical investigations on the isolation of constituents from the Ajuga genus. This has resulted in the isolation and characterization of a series of secondary metabolites, including phytoecdysteroids, sesquiterpenoids, diterpenoids, triterpenoids, iridoids, withanolides and some other compounds. Phytoecdysteroids, the characteristic components of Ajuga plants, are discussed first.
Steroids
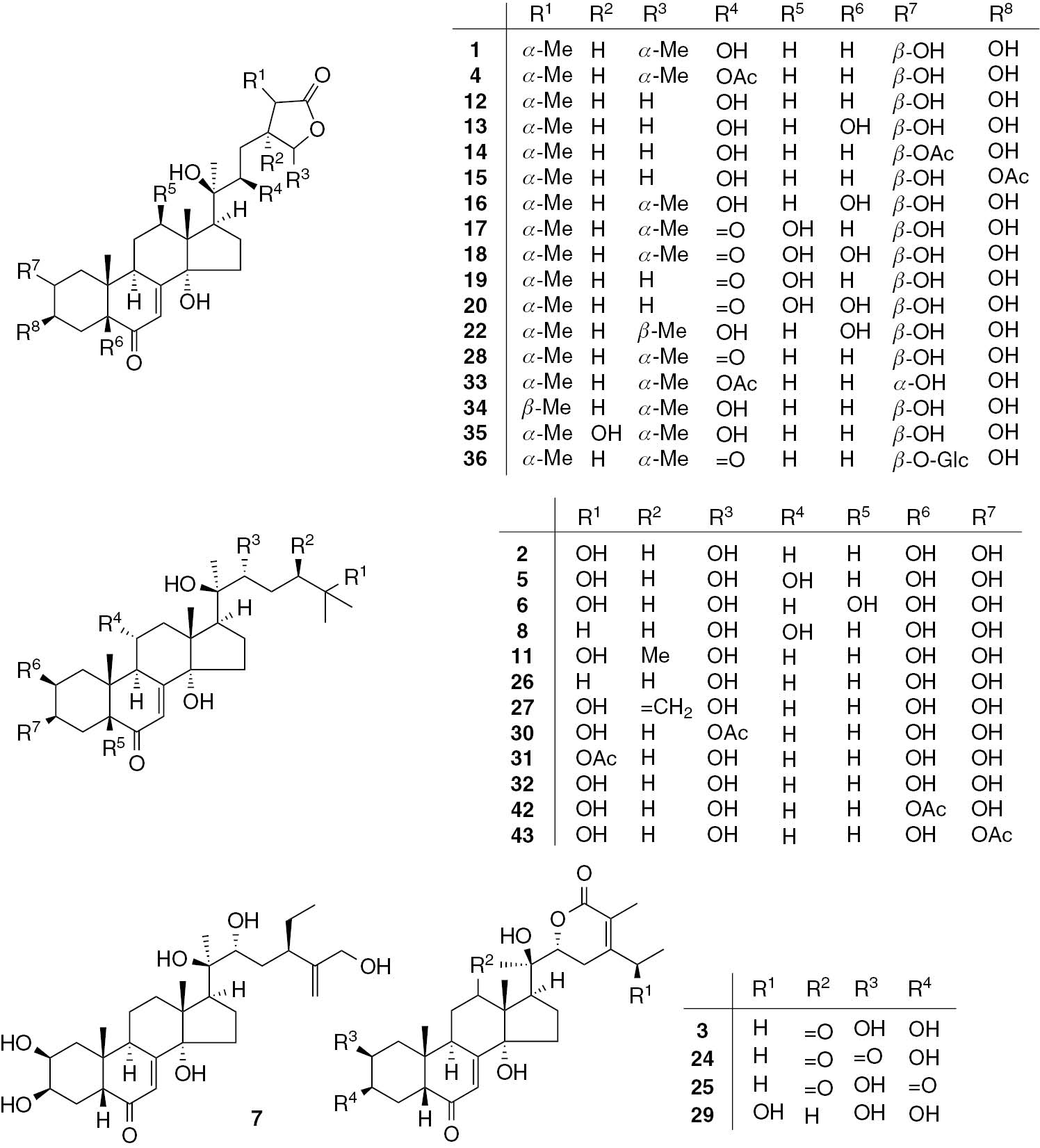
Phytoecdysteroids (Table 1, Figure 1)
Phytoecdysteroids are widespread in the genus Ajuga. These compounds display interesting physiological activities such as insect molting activity and other hormonal functions involving regeneration, metamorphosis, reproduction and differentiation in all arthropods. These compounds play important roles for defense against phytophagous insects. They also display antiulcer, antirheumatic, insulin regulation and diuretic or tonic activities in mammals [34]. Some of the successful applications of plants in folk medicine can be explained by the occurrence of phytoecdysteroids.
Steroids 1: Phytoecdysteroids.
| No. | Name | Source | Part | Ref. |
|---|---|---|---|---|
| 1 | Cyasterone | A. decumbens | whole plant | [11] |
| A. turkestanica | leaf | [12] | ||
| A. iva | aerial part | [13], [14], [15] | ||
| A. chia | leaf, stem | [16] | ||
| A. chamaepitys | whole plant | [17] | ||
| A. multiflora | aerial part | [18] | ||
| A. taiwanensis | whole plant | [19] | ||
| A. nipponensis | aerial part | [20] | ||
| A. macrosperma var. breviflora | root | [21] | ||
| 2 | Ecdysterone | A. decumbens | whole plant | [11] |
| A. nipponensis | whole plant | [11], [22] | ||
| A. turkestanica | leaf | [12] | ||
| A. iva | aerial part | [13], [14], [15] | ||
| A. chamaepitys | whole plant | [17] | ||
| A. multiflora | aerial part | [18] | ||
| A. macrosperma var. breviflora | root | [21] | ||
| A. reptans | whole plant | [23], [24], [25] | ||
| A. remota | leaf, root | [26] | ||
| 3 | Ajugalactone | A. reptans | whole plant | [25] |
| A. turkestanica | root | [27] | ||
| 4 | 22-Acetylcyasterone | A. turkestanica | root | [28] |
| 5 | Turkesterone | A. turkestanica | root | [29] |
| 6 | Ajugasterone A | A. nipponensis | whole plant | [22] |
| A. reptans | whole plant | [23], [25] | ||
| 7 | Ajugasterone B | A. reptans | whole plant | [25] |
| A. turkestanica | root | [29] | ||
| A. iva | whole plant | [30] | ||
| 8 | Ajugasterone C | A. nipponensis | aerial part | [20] |
| A. japonica | leaf | [31] | ||
| 9 | Ajugasterone D | A. nipponensis | whole plant | [22] |
| 10 | Stachysterone D | A. nipponensis | whole plant | [22] |
| 11 | Makisterone A | A. iva | whole plant | [14], [32] |
| A. macrosperma var. breviflora | root | [21] | ||
| 12 | 29-Norcyasterone | A. reptans | whole plant | [23], [24], [25] |
| 13 | 29-Norsengosterone | A. reptans | whole plant | [23], [24], [25] |
| 14 | 2-Acetyl-29-norcyasterone | A. reptans | whole plant | [33] |
| 15 | 3-Acetyl-29-norcyasterone | A. reptans | whole plant | [33] |
| 16 | Sengosterone | A. reptans | whole plant | [25] |
| 17 | 22-Dehydro-12-hydroxycyasterone | A. reptans var. atropurperea | aerial part | [34] |
| 18 | 22-Dehydro-12-hydroxysengosterone | A. reptans var. atropurperea | aerial part | [34] |
| 19 | 22-Dehydro-12-hydroxy-29-nor-cyasterone | A. reptans var. atropurperea | aerial part | [34] |
| 20 | 22-Dehydro-12-hydroxy-29-nor-sengosterone | A. reptans var. atropurperea | aerial part | [34] |
| 21 | Reptansterone | A. reptans var. atropurperea | root | [35] |
| 22 | 28-epi-Sengosterone | A. reptans var. atropurperea | root | [35] |
| 23 | 5,29-Dihydroxycapitasterone | A. reptans var. atropurperea | root | [35] |
| 24 | 2-Dehydroajugalactone | A. reptans var. atropurperea | root | [35] |
| 25 | 3-Dehydroajugalactone | A. reptans var. atropurperea | root | [35] |
| 26 | Ponasterone A | A. remota | leaf, root | [26] |
| 27 | 24,28-Dehydromakisterone A | A. iva | whole plant | [14] |
| 28 | 22-Oxocyasterone | A. iva | whole plant aerial | [14] |
| 22-Dehydrocyasterone | A. nipponensis | part | [20] | |
| 29 | 24,25-Dehydroprecyasterone | A. iva | whole plant | [14] |
| A. reptans var. reptans | whole plant | [36] | ||
| 30 | 20-Hydroxyecdysone 22-acetate | A. nipponensis | aerial part | [20] |
| A. reptans | whole plant | [37] | ||
| 31 | 20-Hydroxyecdysone 25-acetate (Viticosterone E) | A. reptans | whole plant | [37] |
| 32 | Ajusterone | A. pseudoiva | leaf | [38] |
| 33 | Ajugalide-E | A. taiwanensis | whole plant | [19] |
| 34 | Isocyasterone | A. taiwanensis | whole plant | [19] |
| 35 | 24-Hydroxycyasterone | A. iva | whole plant | [30] |
| 36 | 22-Dehydrocyasterone 2-glucopyranoside | A. nipponensis | aerial part | [20] |
| 37 | Ajugacetalsterone A | A. nipponensis | aerial part | [20] |
| 38 | Ajugacetalsterone B | A. nipponensis | aerial part | [20] |
| 39 | Ajugacetalsterone C | A. macrosperma var. breviflora | root | [21] |
| 40 | Ajugacetalsterone D | A. macrosperma var. breviflora | root | [21] |
| 41 | Breviflorasterone | A. macrosperma var. breviflora | root | [21] |
| A. reptans var. reptans | whole plant | [36] | ||
| 42 | 20-Hydroxyecdysone 2-acetate | A. macrosperma var. breviflora | root | [21] |
| 43 | 20-Hydroxyecdysone 3-acetate | A. macrosperma var. breviflora | root | [21] |
| 44 | Reptanslactone A | A. reptans var. reptans | whole plant | [36] |
| 45 | Reptanslactone B | A. reptans var. reptans | whole plant | [36] |
| 46 | Sendreisterone | A. reptans var. reptans | whole plant | [36] |
Steroids 1: Phytoecdysteroids.
Compounds 1 and 2 are usually the most abundant phytoecdysones in the genus Ajuga, and they were reported in A. decumbens, Ajuga incisa, Ajuga turkestanica, Ajuga iva, Ajuga nipponensis, Ajuga chia, Ajuga chamaepitys and Ajuga multiflora [11], [12], [13], [16], [17], [18]. Compound 36 was reported as a 2-O-glucopyranoside [20]. Phytoecdysteroids bearing a γ-lactone ring at various positions, 41, 44 and 45, were also isolated [21], [36]. Derivatives with a δ-lactone ring, 3, 21, 23–25, 29, 37, and 46 were reported as well [14], [25], [27], [35], [36]. In addition, the reduced forms, 37 and 46 with a THP ring, and 38 and 40 with a THF ring, were isolated as acetals or hemiacetals [20], [21], [36]. Two other similar compounds with a THF ring 9 and 10, are biosynthesized from precursors via intramolecular hydration [22]. In addition, ajugacetalsterone C (39) has a rare 6,8-dioxabicyclo[3.2.1]oct-2-ene structure presumably formed by an intramolecular acetalization [21]. Fujimoto and co-workers summarized biosynthesis of ecdysteroids as well as sterols in Ajuga hairy root in detail [39].
Withanolides (Table 2, Figure 2)
Withanolides are characteristic of Solanaceous plants, though there are rare reports on their isolation from other families. In 1999, Khan and co-workers isolated a new withanolide 47 from Ajuga parvifora. This is the first report of naturally occurring withanolides in Lamiaceae [40]. In subsequent studies on the chemical constituents of A. parvifora, the same research group obtained a series of new withanolides 48–56, along with known 57 [41], [42], [43], [44], [45].
Steroids 2: Withanolides.
| No. | Name | Source | Part | Ref. |
|---|---|---|---|---|
| 47 | Ajugin | A. parviflora | whole plant | [40] |
| 48 | Ajugin A | A. parviflora | whole plant | [41] |
| 49 | Ajugin B | A. parviflora | whole plant | [41] |
| 50 | Ajugin C | A. parviflora | whole plant | [42] |
| 51 | Ajugin D | A. parviflora | whole plant | [42] |
| 52 | Ajugin E | A. parviflora | whole plant | [43] |
| 53 | Ajugin F | A. parviflora | whole plant | [43] |
| 54 | 3,14,17,20,28-Pentahydroxy-1-oxo-(20R,22R)-witha-5,24-dienolide | A. parviflora | whole plant | [44] |
| 55 | 3,17,20-Trihydroxy-1-oxo-(20S,22R)-witha-5,14,24-trienolide | A. parviflora | whole plant | [45] |
| 56 | 28-Hydroxy-14,20-epoxy-1-oxo-(22R)-witha-2,5,24-trienolide | A. parviflora | whole plant | [45] |
| 57 | Coagulin-J | A. parviflora | whole plant | [44] |
| 58 | Bracteosin A | A. bracteosa | whole plant | [46] |
| 59 | Bracteosin B | A. bracteosa | whole plant | [46] |
| 60 | Bracteosin C | A. bracteosa | whole plant | [46] |

Steroids 2: Withanolides.
Other steroids (Table 3, Figure 3)
Compounds 61 and 67, two C29 monohydroxy sterols, were isolated from A. reptans and their structures were elucidated by spectral methods [47]. From the aerial parts of Ajuga salicifolia, Akbay and co-workers isolated one new stigmastane-type sterol 72 and eight new sterol glycosides 70, 71, 73–78 [53], [54]. The whole plant of Ajuga relicta afforded two new steroids 79, 80, as well as two known compounds 61, 68 [49]. A steroidal glucopyranoside 64 was isolated from A. chamaepitys ssp. laevigata [51].
Steroids 3: other steroids.
| No. | Name | Source | Part | Ref. |
|---|---|---|---|---|
| 61 | Clerosterol | A. reptans | whole plant | [47] |
| A. pseudoiva | leaf | [48] | ||
| A. relicta | whole plant | [49] | ||
| 62 | Clerosterol 3β-O-(β-D-glucopyranoside) | A. pseudoiva | leaf | [50] |
| 63 | Mighavide (3-O-Butanoylclerosterol) | A. pseudoiva | leaf | [48], [50] |
| 64 | 3-O-β-D-Glucopyranosyl-stigmasta-5,25-diene | A. chamaepitys ssp. laevigata | whole plant | [51] |
| 65 | Stigmasterol | A. taiwanensis | whole plant | [19] |
| 66 | Stigmasterol 3-O-β-D-glucopyranoside | A. taiwanensis | whole plant | [19] |
| 67 | 22,23-Didehydroclerosterol | A. reptans | whole plant | [47] |
| 68 | β-sitosterol | A. relicta | whole plant | [49] |
| A. taiwanensis | whole plant | [19] | ||
| 69 | β-sitosterol 3-O-β-D-glucopyranoside | A. decumbens | whole plant | [52] |
| 70 | Ajugasalicioside A | A. salicifolia | aerial part | [53] |
| 71 | Ajugasalicioside B | A. salicifolia | aerial part | [53] |
| 72 | Ajugasalicigenin | A. salicifolia | aerial part | [54] |
| 73 | Ajugasalicioside C | A. salicifolia | aerial part | [53] |
| 74 | Ajugasalicioside D | A. salicifolia | aerial part | [53] |
| 75 | Ajugasalicioside E | A. salicifolia | aerial part | [53] |
| 76 | Ajugasalicioside F | A. salicifolia | aerial part | [54] |
| 77 | Ajugasalicioside G | A. salicifolia | aerial part | [54] |
| 78 | Ajugasalicioside H | A. salicifolia | aerial part | [54] |
| 79 | (24S)-24-Ethyl-11α-hydroxycholesta-5,25-dien-1-one | A. relicta | whole plant | [49] |
| 80 | (24S)-24-Ethyl-7α-hydroxycholesta-5,25-dien-3-one | A. relicta | whole plant | [49] |
| 81 | Ergosterol 5,8-endoperoxide | A. remota | aerial part | [55] |

Steroids 3: other steroids.
Triterpenoids (Table 4, Figure 4)
In 1997, two lupan triterpenoids 82 and 83 were isolated from the aerial parts of Ajuga macrosperma [56]. Oleananes 84 and 85 were two known triterpenoids isolated from A. relicta [49]. From A. chamaepitys ssp. laevigata, two ursanes and one oleanane 86–88 were isolated [51].
Triterpenoids.
| No. | Name | Source | Part | Ref. |
|---|---|---|---|---|
| 82 | Betulinic acid | A. macrosperma | aerial part | [56] |
| 83 | 3-epi-Betulinic acid | A. macrosperma | aerial part | [56] |
| 84 | Oleanolic acid | A. relicta | whole plant | [49] |
| 85 | 3-O-Acetyloleanolic acid | A. relicta | whole plant | [49] |
| 86 | α-Amyrin | A. chamaepitys ssp. laevigata | whole plant | [51] |
| 87 | β-Amyrin | A. chamaepitys ssp. laevigata | whole plant | [51] |
| 88 | Ursolic acid | A. chamaepitys ssp. laevigata | whole plant | [51] |

Triterpenoids.
Diterpenoids (Table 5, Figure 5)
Ajuga species are rich in diterpenoids. With respect to the carbocyclic skeleton, Ajuga diterpenoids roughly belong to two groups: neoclerodane and abietane types.
Diterpenoids.
| No. | Name | Source | Part | Ref. |
|---|---|---|---|---|
| 89 | Ajugarin I | A. remota | leaf | [2] |
| A. nipponensis | aerial part | [57] | ||
| A. parviflora | aerial part | [58] | ||
| A. decumbens | whole plant | [59] | ||
| 90 | Ajugarin II | A. remota | leaf | [2] |
| A. parviflora | aerial part | [58] | ||
| 91 | Ajugarin III | A. remota | leaf | [2] |
| 92 | Ajugarin IV | A. remota | leaf | [60] |
| A. ciliata var. villosior | aerial part | [61] | ||
| 93 | Ajugarin V | A. remota | leaf | [62] |
| 94 | Clerodin | A. remota | leaf | [63] |
| A. bracteosa | aerial part | [64] | ||
| 95 | Dihydroclerodin | A. parviflora | aerial part | [58] |
| A. bracteosa | whole plant | [46], [64] | ||
| A. remota | aerial part | [65] | ||
| 96 | Ajugareptansin | A. reptans | aerial part | [66], [67], [68] |
| 97 | Ajugareptansone A | A. reptans | whole plant | [69], [70] |
| 98 | Ajugareptansone B | A. reptans | whole plant | [69] |
| 99 | Ivain I | A. iva | whole plant | [71] |
| 100 | Ivain II | A. iva | whole plant | [71] |
| A. bracteosa | aerial part | [64] | ||
| 101 | Ivain III | A. iva | whole plant | [71] |
| 102 | Ivain IV | A. iva | whole plant | [71] |
| 103 | Ajugapitin (Clerodendrin D) | A. chamaepitys | whole plant | [72], [73] |
| A. australis | aerial part | [74] | ||
| A. decumbens | leaf | [75] | ||
| A. remota | aerial part | [65] | ||
| A. turkestanica | aerial part | [76] | ||
| 104 | 14,15-Dihydroajugapitin | A. chamaepitys | whole plant | [72], [73] |
| A. pseudoiva | leaf | [77], [78] | ||
| A. bracteosa | whole plant | [46], [64], [79] | ||
| A. remota | aerial part | [65] | ||
| 105 | Chamaepitin | A. chamaepitys | whole plant | [80] |
| A. turkestanica | aerial part | [76] | ||
| 106 | Ajugamarin | A. nipponensis | leaf | [81], [82], [83] |
| A. decumbens | whole plant | [75], [84], [85], [86] | ||
| A. ciliata | whole plant | [87] | ||
| 107 | Dihydroajugamarin | A. nipponensis | leaf | [81] |
| A. decumbens | leaf | [75] | ||
| 108 | Ajugamarin chlorohydrin | A. nipponensis | leaf | [81] |
| A. ciliata | whole plant | [87] | ||
| 109 | 2-Acetylivain I | A. pseudoiva | whole plant | [77] |
| 110 | Ajugamarin A2 | A. decumbens | whole plant | [88] |
| A. nipponensis | aerial part | [83] | ||
| A. ciliata | whole plant | [87] | ||
| 111 | Ajugamarin B1 | A. ciliata | whole plant | [87] |
| 112 | Ajugamarin B2 | A. nipponensis | aerial part | [57], [83] |
| A. decumbens | whole plant | [88] | ||
| 113 | Ajugamarin B3 | A. nipponensis | aerial part | [57] |
| 114 | Ajugamarin B4 | A. ciliata var. villosior | aerial part | [61] |
| 115 | Ajugamarin B5 | A. ciliata var. villosior | aerial part | [61] |
| 116 | Ajugamarin C1 | A. nipponensis | aerial part | [57] |
| A. taiwanensis | whole plant | [89] | ||
| A. ciliata | whole plant | [90] | ||
| 117 | Ajugamarin D1 | A. nipponensis | aerial part | [57] |
| 118 | Ajugamarin E1 | A. ciliata var. villosior | aerial part | [61] |
| 119 | Ajugamarin E2 | A. ciliata var. villosior | aerial part | [61] |
| 120 | Ajugamarin E3 | A. ciliata var. villosior | aerial part | [61] |
| 121 | Ajugamarin F1 | A. ciliata var. villosior | aerial part | [61] |
| 122 | Ajugamarin F2 | A. ciliata var. villosior | aerial part | [61] |
| 123 | Ajugamarin F3 | A. ciliata var. villosior | aerial part | [61] |
| 124 | Ajugamarin F4 | A. decumbens | whole plant | [86], [88] |
| A. parviflora | aerial part | [58] | ||
| A. nipponensis | aerial part | [83] | ||
| 125 | Ajugamarin G1 | A. decumbens | whole plant | [75], [88] |
| A. ciliata | whole plant | [87] | ||
| 126 | Ajugamarin H1 | A. decumbens | whole plant | [75], [88] |
| A. ciliata | whole plant | [87] | ||
| 127 | Deacetylajugarin IV | A. ciliata var. villosior | aerial part | [61] |
| A. remota | aerial part | [65] | ||
| A. ciliata | whole plant | [91] | ||
| 128 | Ajugachin A | A. chamaepitys | aerial part | [73] |
| A. reptans | aerial part | [68] | ||
| 129 | Ajugachin B | A. chamaepitys | aerial part | [73] |
| A. turkestanica | aerial part | [76] | ||
| 130 | Ajugacumbin A | A. decumbens | whole plant | [59], [85], [92], [93] |
| A. nipponensis | aerial part | [83] | ||
| A. ciliata | whole plant | [87] | ||
| 131 | Ajugacumbin B | A. decumbens | whole plant | [59], [92], [93] |
| A. nipponensis | aerial part | [83], [94] | ||
| A. macrosperma | whole plant | [95] | ||
| A. pantantha | whole plant | [95] | ||
| 132 | Ajugacumbin C | A. decumbens | whole plant | [85], [92] |
| 133 | Ajugacumbin D | A. decumbens | whole plant | [85], [92] |
| 134 | Ajugacumbin E | A. decumbens | whole plant | [84] |
| 135 | Ajugacumbin F | A. decumbens | whole plant | [84] |
| A. ciliata | whole plant | [87] | ||
| 136 | Ajugacumbin G | A. decumbens | whole plant | [93] |
| 137 | Ajugacumbin H | A. decumbens | whole plant | [85] |
| 138 | Ajugacumbin J | A. decumbens | whole plant | [96] |
| 139 | Ajugavensin A | A. genevensis | aerial part | [97] |
| A. reptans | aerial part | [70] | ||
| 140 | Ajugavensin B | A. genevensis | aerial part | [97] |
| 141 | Ajugavensin C | A. genevensis | aerial part | [97] |
| 142 | Ajugamacrin A | A. macrosperma | whole plant | [98] |
| 143 | Ajugamacrin B | A. macrosperma | whole plant | [98] |
| A. taiwanensis | whole plant aerial part | [89] | ||
| A. nipponensis | whole plant aerial part | [83] | ||
| 144 | Ajugamacrin C | A. macrosperma | whole plant | [95] |
| A. pantantha | whole plant | [95] | ||
| 145 | Ajugamacrin D | A. macrosperma | whole plant | [95] |
| A. pantantha | whole plant | [95] | ||
| 146 | Ajugamacrin E | A. macrosperma | whole plant | [95] |
| A. pantantha | whole plant | [95] | ||
| 147 | Ajugapantin A | A. macrosperma | whole plant | [95] |
| A. pantantha | whole plant | [95] | ||
| A. taiwanensis | whole plant | [89] | ||
| A. ciliata | whole plant | [87] | ||
| 148 | Deoxyajugarin-I | A. parviflora | aerial part | [58] |
| 149 | Ajugarin-I chlorohydrin | A. parviflora | aerial part | [58] |
| 150 | 3β-Acetoxyclerodinin C | A. parviflora | aerial part | [58] |
| 151 | Clerodinin A | A. bracteosa | whole plant | [46] |
| 152 | Clerodinin C | A. parviflora | aerial part | [58] |
| 153 | Clerodinin D | A. parviflora | aerial part | [58] |
| 154 | 15-α-Ethoxy-14-hydroajugapitin | A. parviflora | aerial part | [58] |
| 155 | 15-β-Ethoxy-14-hydroajugapitin | A. parviflora | aerial part | [58] |
| 156 | Lupulin A | A. lupulina | whole plant | [99] |
| A. bracteosa | whole plant | [46] | ||
| A. turkestanica A. pseudoiva | aerial part leaf | [76] [78] | ||
| 157 | Lupulin B | A. lupulina | whole plant | [99] |
| 158 | Lupulin C | A. lupulina | whole plant | [99] |
| 159 | Lupulin D | A. lupulina | whole plant | [99] |
| 160 | Lupulin E | A. lupulina | whole plant | [4] |
| 161 | Lupulin F | A. lupulina | whole plant | [4] |
| 162 | 2β-Hydroxy-2-methylbutanoyl-3α-lupulin | A. lupulina | whole plant | [100] |
| 163 | 6-Deacetylajugarin IV | A. lupulina var. major | whole plant | [101] |
| 164 | Ajugorientin (3β-Hydroxyajugavensin B) | A. orientalis A. reptans | aerial part aerial part | [74] [67], [68] |
| 165 | 14,15-Dihydro-15-hydroxyajugapitin | A. australis | aerial part | [74] |
| A. bracteosa | whole plant | [64], [79] | ||
| A. remota | aerial part | [65] | ||
| 166 | Ajugatakasin A | A. decumbens | leaf | [75] |
| A. nipponensis | aerial part | [83] | ||
| A. ciliata | whole plant | [87] | ||
| 167 | Ajugatakasin B | A. decumbens | leaf | [75] |
| A. ciliata | whole plant | [87] | ||
| 168 | 14,15-Dehydroajugareptansin | A. reptans | aerial part | [67] |
| 169 | 3α-Hydroxyajugamarin F4 | A. reptans | aerial part | [67] |
| 170 | Ajugapyrin A | A. pyramidalis | aerial part | [102] |
| 171 | Areptin A | A. reptans | aerial part | [68] |
| 172 | Areptin B | A. reptans | aerial part | [68] |
| 173 | (15R)-14,15-Dihydro-15-hydroxyajugachin A | A. laxmanii | aerial part | [103] |
| 174 | (15S)-14,15-Dihydro-15-hydroxyajugachin A | A. laxmanii | aerial part | [103] |
| 175 | Hativene A | A. pseudoiva | leaf | [78] |
| 176 | Hativene B | A. pseudoiva | leaf | [78] |
| 177 | Hativene C | A. pseudoiva | leaf | [78] |
| 178 | Hativene D | A. pseudoiva | leaf | [104] |
| 179 | Ajugatansin A1 | A. reptans | aerial part | [70] |
| 180 | Ajugatansin B1 | A. reptans | aerial part | [70] |
| 181 | Ajugatansin D1 | A. reptans | aerial part | [70] |
| 182 | Bracteonin-A | A. bracteosa | whole plant | [79] |
| 183 | Ajugareptone | A. reptans | leaf | [105] |
| 184 | Ajugalaevigatic acid | A. chamaepitys ssp. laevigata | whole plant | [51] |
| 185 | (13S)-15-Hydroxylabd-8(17)-en-19-oic acid (Imbricatoloic acid) | A. chamaepitys ssp. laevigata | whole plant | [51] |
| 186 | Ajugalide A | A. taiwanensis | whole plant | [89] |
| 187 | Ajugalide B | A. taiwanensis | whole plant | [89] |
| A. ciliata | whole plant | [87] | ||
| 188 | Ajugalide C | A. taiwanensis | whole plant | [89] |
| A. ciliata | whole plant | [87] | ||
| 189 | Ajugalide D | A. taiwanensis | whole plant | [89] |
| A. ciliata | whole plant | [91] | ||
| 190 | Ajuganipponin A | A. nipponensis | aerial part | [83] |
| A. ciliata | whole plant | [90] | ||
| 191 | Ajuganipponin B | A. nipponensis | aerial part | [83] |
| A. ciliata | whole plant | [87] | ||
| A. decumbens | whole plant | [86] | ||
| 192 | 14-Hydro-15-hydroxyclerodin | A. remota | aerial part | [65] |
| 193 | 14,15-Hihydroajugachin B | A. turkestanica | aerial part | [76] |
| 194 | 14-Hydro-15-methoxyajugachin B | A. turkestanica | aerial part | [76] |
| 195 | 15-epi-Lupulin A | A. decumbens | whole plant | [106] |
| 196 | 15-epi-Lupulin B | A. bracteosa | aerial part | [64] |
| 197 | 6-O-Deacetylajugamarin | A. decumbens | whole plant | [106] |
| 198 | Ajugadecumbenins A | A. decumbens | whole plant | [106] |
| 199 | Ajugadecumbenins B | A. decumbens | whole plant | [106] |
| 200 | Ajubractin A | A. bracteosa | aerial part | [64] |
| 201 | Ajubractin B | A. bracteosa | aerial part | [64] |
| 202 | Ajubractin C | A. bracteosa | aerial part | [64] |
| 203 | Ajubractin D | A. bracteosa | aerial part | [64] |
| 204 | Ajubractin E | A. bracteosa | aerial part | [64] |
| 205 | 3-epi-Caryoptin | A. bracteosa | aerial part | [64] |
| 206 | 3-epi-14,15-Dihydrocaryoptin | A. bracteosa | aerial part | [64] |
| 207 | 15-Hydroxyajubractin C | A. bracteosa | aerial part | [64] |
| 208 | 14-Hydro-15-hydroxyajugachin A | A. bracteosa | aerial part | [64] |
| 209 | Ajugaciliatin A | A. ciliata | whole plant | [87] |
| 210 | Ajugaciliatin B | A. ciliata | whole plant | [87] |
| 211 | Ajugaciliatin C | A. ciliata | whole plant | [87] |
| 212 | Ajugaciliatin D | A. ciliata | whole plant | [87] |
| 213 | Ajugaciliatin E | A. ciliata | whole plant | [87] |
| 214 | Ajugaciliatin F | A. ciliata | whole plant | [87] |
| 215 | Ajugaciliatin G | A. ciliata | whole plant | [87] |
| 216 | Ajugaciliatin H | A. ciliata | whole plant | [87] |
| 217 | Ajugaciliatin I | A. ciliata | whole plant | [87] |
| 218 | Ajugaciliatin J | A. ciliata | whole plant | [87] |
| A. decumbens | whole plant | [59] | ||
| 219 | (12S)-1β,6α,19-Triacetoxy-18-chloro-4α,12-dihydroxyneoclerod-13-en-15,16-olide | A. ciliata | whole plant | [91] |
| 220 | (12S,2′S)-12,19-Diacetoxy-18-chloro-4α,6α-dihydroxy-1β-(2-methylbutanoyloxy)neoclerod-13-en-15,16-olide | A. ciliata | whole plant | [91] |
| 221 | (12S)-6α,18,19-Triacetoxy-4α,12-dihydroxy-1β-tigloyloxyneoclerod-13-en-15,16-olide | A. ciliata | whole plant | [91] |
| 222 | (12S)-6α-Acetoxy-4α,18-epoxy-12-hydroxy-19-tigloyloxyneoclerod-13-en-15,16-olide | A. ciliata | whole plant | [90] |
| 223 | 6α,18-Diacetoxy-4α-hydroxy-19-tigloyloxyneoclerod-13-en-15,16-olide | A. ciliata | whole plant | [90] |
| 224 | Ajugamarin A2 chlorohydrin | A. ciliata | whole plant | [87] |
| 225 | 6α,19-Diacetoxy-4α-hydroxy-1β-tigloyloxyneoclerod-12-en-15-oic acid methyl ester-16-aldehyde | A. decumbens | whole plant | [59] |
| 226 | (12S)-18,19-Diacetoxy-4α,6α,12-trihydroxy-1β-tigloyloxyneoclerod-13-en-15,16-olide | A. decumbens | whole plant | [59] |
| 227 | 4α,6α-Dihydroxy-18-(4′-methoxy-4′-oxobutyryloxy)-19-tigloyloxyneoclerod-13-en-15,16-olide | A. decumbens | whole plant | [59] |
| 228 | (12S)-1α,19-Epoxy-6α,18-diacetoxy-4α,12-dihydroxyneoclerod-13-en-15,16-olide | A. decumbens | whole plant | [86] |
| 229 | (12S)-6α,19-Diacetoxy-18-chloro-4α-hydroxy-12-tigloyloxyneoclerod-13-en-15,16-olide | A. decumbens | whole plant | [86] |
| 230 | (12S,2′′S)-6α,19-Diacetoxy-18-chloro-4α-hydroxy-12-(2-methylbutanoyloxy)neoclerod-13-en-15,16-olide | A. decumbens | whole plant | [86] |
| 231 | Ajugacumbin A chlorohydrin | A. ciliata | whole plant | [87] |
| A. decumbens | whole plant | [59] | ||
| 232 | Ajuforrestin A | A. forrestii | whole plant | [107] |
| A. decumbens | aerial part | [7] | ||
| 233 | Ajuforrestin B | A. forrestii | whole plant | [107] |
| A. decumbens | aerial part | [7] | ||
| 234 | Ajugaside A | A. decumbens | whole plant | [108] |
| 235 | Ajudecumin A | A. decumbens | aerial part | [7] |
| 236 | Ajudecumin B | A. decumbens | aerial part | [7] |
| 237 | Ajudecumin C | A. decumbens | aerial part | [7] |
| 238 | Ajudecumin D | A. decumbens | aerial part | [7] |
| 239 | Carnosol | A. forrestii | whole plant | [109] |
| 240 | Epiisorosmanol | A. forrestii | whole plant | [109] |
| 241 | 2,11,12-Trihydroxy-7,20-epoxy-8,11,13-abietatriene | A. forrestii | whole plant | [109] |
| 242 | Epirosmanol | A. forrestii | whole plant | [109] |
| 243 | 7-Methoxyrosmanol | A. forrestii | whole plant | [109] |
| 244 | 7-Ethoxyrosmanol | A. forrestii | whole plant | [109] |
| 245 | 2α,3β,11,12-Tetrahydroxy-7β,20-epoxy-8,11,13-abietatriene | A. forrestii | whole plant | [109] |

Diterpenoids.
Neoclerodanes
Most of the neoclerodane diterpenoids produced by species of the genus Ajuga contain a substituted decalin with a 4α,18-oxirane ring and two oxygenated substituents bound to C(6) and C(19) [110]. The side chain features several moieties with the most common being: (i) a butenolide function (α-substituted α,β-unsaturated γ-lactone, or 13-en-15,16-olide) as in 89–93 isolated from A. remota [2], [60], [62]; (ii) a tetrahydrofurofuran as in 94 reported as a constituent of A. remota [63]; and (iii) a hexahydrofurofuran as in 95 reported as a component of Ajuga parviflora [58]. In 1983, three new bitter principles, 106–108, were isolated from the leaves of A. nipponensis. The β-hydrin structure of 108 was confirmed by treatment of 106 with methanolic HCl [81], [82]. In addition, by reinvestigation of the aerial parts of A. nipponensis, four new bitter neoclerodanes 112, 113, 116, 117 and a known diterpenoid 89 were isolated [57]. The configuration of methoxy group at C(15) (R5) of lupulin A (156) [99] was revised to be α by Huang and co-workers [106]. Consequently, hativene D (178) [104] is not 15-epi-lupulin (195) but lupulin (156). The structure of 15-epi-lupulin B (196) [64] with a 2β-OH group coincides with lupulin A 156.
Abietanes
A new abietane diglucopyranoside 234 was isolated from the whole plants of A. decumbens [108]. In the course of the search for bioactive metabolites with anticancer effects, Wang et al. isolated four new rearranged abietane hydroquinones 235–238, together with two known abietanes 232 and 233 from the aerial parts of A. decumbens collected in China [7].
Sesquiterpenoids (Table 6, Figure 6)
Only a few other sesquiterpenoids, bisabolene, eudesmanolides and seco-sesquiterpenoids, were reported. Compound 246, a bisabolene sesquiterpenoid, was isolated from the aerial parts of A. decumbens [7]. Research on Ajuga forrestii resulted in the isolation of four eudesmane sesquiterpene lactones 247–250. Among them, compounds 247–249 are new. Compound 249 exhibits weak cytotoxic activity against HepG2 and MCF-7 human cell lines [109]. Four megastigmane derivatives 251–254 and 257 were isolated in 2012 [7], [112]. A new ionone glycoside 255 was also isolated from this plant. This is the first report of the occurrence of ionone glycosides in Ajuga species [111].
Sesquiterpenoids.
| No. | Name | Source | Part | Ref. |
|---|---|---|---|---|
| 246 | Glecholone | A. decumbens | aerial part | [7] |
| 247 | 3α-Acetoxy-1α,8β-dihydroxyeudesm-7(11)-en-8,12-olide | A. forrestii | whole plant | [109] |
| 248 | 3α-Acetoxy-1α-hydroxyeudesm-8,7(11)-dien-8,12-olide | A. forrestii | whole plant | [109] |
| 249 | 1α-Acetoxy-8α-oxyethyl-2-oxoeudesman-3,7(11)-dien-8,12-olide | A. forrestii | whole plant | [109] |
| 250 | 1α-Acetoxy-8α-hydroxy-2-oxoeudesman-3,7(11)-dien-8,12-olide | A. forrestii | whole plant | [109] |
| 251 | (6R,7E,9R)-9-Hydroxy-4,7-megastigmadien-3-one | A. decumbens | aerial part | [7] |
| 252 | (3S,5R,6S,7E)-5,6-Epoxy-3-hydroxy-7-megastigmen-9-one | A. decumbens | aerial part | [7] |
| 253 | (6E,9S)-9-Hydroxy-4,6-megastigmadien-3-one | A. decumbens | aerial part | [7] |
| 254 | 6-Hydroxy-4,7-megastigmadiene-3,9-dione | A. decumbens | aerial part | [7] |
| 255 | 4β-Hydroxy-7,8-dihydro-3-oxo-β-ionol 9-O-β-D-glucopyranoside | A. salicifolia | aerial part | [111] |
| 256 | Corchoionoside C | A. salicifolia | aerial part | [111] |
| 257 | Loliolide | A. decumbens | whole plant | [112] |

Sesquiterpenoids.
Monoterpenoids (Table 7, Figure 7)
Major monoquiterpenoids isolated from the genus Ajuga belong to iridoids. In 1974, Guiso and co-workers isolated three iridoid glucopyranosides 258–260 from A. reptans. Compound 259 is an 8-O-acetyl derivative of 260 and a 6-epimer of 8-O-acetylmioporoside 271 [113], [117]. Four new iridoid glucopyranoside cis- and trans-p-coumaroyl esters 263–266 were isolated from methanol extract of the dried plant of A. decumbens, together with the known compounds 258 and 262 [116]. Compound 270, isolated from the leaves of Ajuga pseudoiva, possesses an unusual 13-membered macrocyclic structure [121].
Iridoids.
| No. | Name | Source | Part | Ref. |
|---|---|---|---|---|
| 258 | Reposide | A. reptans | whole plant | [113], [114], [115] |
| A. decumbens | whole plant | [116] | ||
| 259 | Ajugoside | A. reptans | whole plant | [117] |
| 260 | Ajugol | A. reptans | whole plant | [117] |
| 261 | Jaranidoside | A. spectabilis | whole plant | [118] |
| 262 | 8-O-Acetylharpagide | A. multiflora | whole plant | [119] |
| A. remota | aerial part | [6] | ||
| A. decumbens | whole plant | [116] | ||
| A. iva | aerial part | [120] | ||
| A. reptans | whole plant | [114], [115] | ||
| 263 | Decumbeside A | A. decumbens | whole plant | [116] |
| 264 | Decumbeside B | A. decumbens | whole plant | [116] |
| 265 | Decumbeside C | A. decumbens | whole plant | [116] |
| 266 | Decumbeside D | A. decumbens | whole plant | [116] |
| 267 | Harpagide | A. iva | aerial part | [120] |
| A. reptans | whole plant | [114], [115] | ||
| 268 | 6-Deoxyharpagide | A. iva | aerial part | [120] |
| 269 | Ajureptoside | A. reptans | whole plant | [114] |
| 270 | 7-O-6′-O-Malonylcachinesidic acid | A. pseudoiva | leaf | [121] |
| 271 | 8-O-Acetylmioporoside | A. salicifolia | aerial part | [111] |
| 272 | Galiridoside | A. taiwanensis | whole plant | [19] |
| 273 | Teuhircoside | A. taiwanensis | whole plant | [19] |
| 274 | 6-Keto-8-acetylharpagide | A. remota | aerial part | [122] |
| 275 | 6,7-Dehydro-8-acetylharpagide | A. remota | aerial part | [122] |
| 276 | 7,8-Dehydroharpagide | A. remota | aerial part | [122] |
| 277 | 8-Acetylharpagide 6-O-β-glucopyranoside | A. remota | aerial part | [122] |
| 278 | Harpagide 6-O-β-glucopyranoside | A. remota | aerial part | [122] |
| 279 | 2′,3′-Diacetylharpagide | A. remota | underground part | [123] |
| 280 | 6′-O-Rhamnosylharpagide | A. remota | underground part | [123] |
| 281 | 6′-O-Galloyl-7,8-dehydroharpagide | A. remota | underground part | [123] |
| 282 | 6-O-Xylosylharpagoside-B | A. remota | underground part | [123] |
| 283 | Ajureptaside A | A. reptans | whole plant | [115] |
| 284 | Ajureptaside B | A. reptans | whole plant | [115] |
| 285 | Ajureptaside C | A. reptans | whole plant | [115] |
| 286 | Ajureptaside D | A. reptans | whole plant | [115] |
| 287 | 6-epi-8-O-Acetylharpagide | A. reptans | whole plant | [115] |

Iridoids.
Flavonoids (Table 8, Figure 8)
Flavonoids 288–305 including flavones and flavonols isolated from the genus Ajuga are outlined in Table 8.
Flavonoids.
| No. | Name | Source | Part | Ref. |
|---|---|---|---|---|
| 288 | Luteolin | A. chia | aerial part | [124] |
| A. lupulin | whole plant | [101], [125] | ||
| 289 | Luteolin 7-O-glucopyranoside | A. chia | aerial part | [124] |
| A. lupulina | whole plant | [101], [125] | ||
| 290 | Apigenin | A. chia | aerial part | [124] |
| A. multiflora | aerial part | [126] | ||
| A. forrestii | whole plant | [107] | ||
| 291 | Naringin | A. iva | aerial part | [15] |
| 292 | Apigenin 7-O-neohesperidoside | A. iva | aerial part | [15] |
| 293 | Chrysoriol | A. lupulina | whole plant | [125] |
| 294 | Diosmetin | A. lupulina | whole plant | [125] |
| 295 | Kaempferide | A. lupulina | whole plant | [125] |
| 296 | Quercetin | A. lupulina | whole plant | [125] |
| 297 | Acacetin | A. forrestii | whole plant | [107] |
| 298 | Gnetifolin B | A. forrestii | whole plant | [107] |
| 299 | Apigenin 7-glucuronide | A. multiflora | aerial part | [18] |
| 300 | Kaempferol | A. taiwanensis | whole plant | [19] |
| 301 | Myricetin 3-O-rutinoside 4′-O-rutinoside | A. remota | aerial part | [122] |
| 302 | Myricetin 3-O-rutinoside 3′-O-rutinoside | A. remota | aerial part | [122] |
| 303 | Isorhamnetin 3-O-rutinoside 7-O-rutinoside 4′-O-β-glucopyranoside | A. remota | aerial part | [122] |
| 304 | 3,4′-Dihydroxy-3,6,7-trimethoxyflavone | A. bracteosa | whole plant | [127] |
| 305 | 7-Hydroxy-3,6,3′,4′-tetramethoxyflavone | A. bracteosa | whole plant | [127] |

Flavonoids.
Polyketides and alkaloids (Table 9, Figure 9)
From the leaves of A. iva, three new homologous 1,3-diglycerides 331–333 and compound 339 were obtained. In 1986, Takasaki and co-workers isolated three phenethyl alcohol glycosides 323–325, including a new derivative 323 [108]. A phenylalanine derivative 346 was isolated from this plant recently [96]. In addition, Yu and co-workers isolated a phthalic ester 321 from the aerial parts of A. multiflora [18].
Polyketides and alkaloids.
| No. | Name | Source | Part | Ref. |
|---|---|---|---|---|
| 306 | Ethyl (1-acetoxy-4-oxo-2,5-cyclohexadien-1-yl)acetate | A. parviflora | whole plant | [128] |
| 307 | Methyl (1-acetoxy-4-oxo-2,5-cyclohexadien-1-yl)acetate | A. parviflora | whole plant | [128] |
| 308 | Ethyl (1-hydroxy-4-oxo-2,5-cyclohexadien-1-yl)acetate | A. parviflora | whole plant | [128] |
| 309 | Methyl (1-hydroxy-4-oxo-2,5-cyclohexadien-1-yl)acetate | A. parviflora | whole plant | [128] |
| 310 | (1-Hydroxy-4-oxo-2,5-cyclohexadien-1-yl)acetic acid | A. parviflora | whole plant | [129] |
| 311 | 2-Hydroxy-4β-methyl-4α-(β-D-glucopyranoside)-2,5-cyclohexadien-1-one | A. parviflora | whole plant | [129] |
| 312 | Methyl 2-(2,2-dimethyl-6-oxo-7-dihydro-1,3-benzodioxol-3(6H)-yl)acetate | A. parviflora | whole plant | [129] |
| 313 | 6,7-Dihydroxycoumarin (Esculetin) | A. decumbens | whole plant | [52] |
| 314 | Coumarin | A. laxmanii | aerial part | [103] |
| 315 | Vanillic acid | A. decumbens | whole plant | [112] |
| A. taiwanensis | whole plant | [19] | ||
| 316 | Melilotic acid methyl ester | A. laxmanii | aerial part | [103] |
| 317 | Methyl caffeate | A. decumbens | whole plant | [112] |
| 318 | Methyl (E)-4-acetoxy-3-methoxycinnamate | A. pseudoiva | leaf | [38] |
| 319 | Methyl (E)-4-acetoxycinnamate | A. pseudoiva | leaf | [38] |
| 320 | Ajuganane | A. bracteosa | whole plant | [127] |
| 321 | Bis(2-ethylhexyl) phthalate | A. multiflora | aerial part | [18] |
| 322 | Bis(2S-methylheptyl) phthalate | A. bracteosa | whole plant | [130] |
| 323 | Galactosylmartynoside | A. decumbens | whole plant | [108] |
| 324 | Martynoside | A. decumbens | whole plant | [108] |
| 325 | Darendoside B | A. decumbens | whole plant | [108] |
| 326 | Lavandulifolioside | A. salicifolia | aerial part | [111] |
| 327 | Leonoside A | A. salicifolia | aerial part | [111] |
| 328 | Leonoside B | A. salicifolia | aerial part | [111] |
| 329 | 1-Ethenylhexyl 6-O-β-L-arabinopyranosyl-2-O-β-D–glucopyranosyl-β-D-glucopyranoside | A. decumbens | whole plant | [52] |
| 330 | Butyl β-D-fructopyranoside | A. decumbens | whole plant | [52] |
| 331 | Ivade A | A. iva | leaf | [131] |
| A. pseudoiva | leaf | [48] | ||
| 332 | Ivade B | A. iva | leaf | [131] |
| A. pseudoiva | leaf | [48] | ||
| 333 | Ivade C | A. iva | leaf | [131] |
| A. pseudoiva | leaf | [48] | ||
| 334 | Hizivaide A | A. pseudoiva | leaf | [132] |
| 335 | Hizivaide B | A. pseudoiva | leaf | [132] |
| 336 | Hizivaide C | A. pseudoiva | leaf | [132] |
| 337 | Hizivaide D | A. pseudoiva | leaf | [132] |
| 338 | Hizivaide E | A. pseudoiva | leaf | [132] |
| 339 | Methyl 3-hydroxyhexadecanoate | A. iva | leaf | [133] |
| 340 | (10E,15Z)-9,12,13-Trihydroxyoctadeca-10,15-dienoic acid | A. decumbens | whole plant | [112] |
| 341 | Heptacos-3-en-25-one | A. bracteosa | aerial part | [134] |
| 342 | Bractic acid | A. bracteosa | whole plant | [10] |
| 343 | Ligularinine | A. parviflora | whole plant | [44] |
| 344 | Senecionine | A. parviflora | whole plant | [45] |
| 345 | Integerrimine | A. parviflora | whole plant | [45] |
| 346 | Aurantiamide acetate | A. decumbens | whole plant | [96] |
| 347 | Pheophytin-a | A. taiwanensis | whole plant | [19] |
| 348 | Pheophytin-b | A. taiwanensis | whole plant | [19] |
| 349 | 132-Hydroxy(132-S)pheophytin-a | A. taiwanensis | whole plant | [19] |
| 351 | Nicotinic acid | A. taiwanensis | whole plant | [19] |
| 352 | Bractin A | A. bracteosa | whole plant | [10] |
| 353 | Bractin B | A. bracteosa | whole plant | [10] |
During the search for bioactive metabolites from A. pseudoiva leaves, Ben and co-workers isolated five novel monoglycerides 334–338, two novel cinnamic acids 318, 319 and one new steroid 63, along with five known compounds 61, 62, 331–333. Three compounds 331–333 show significant antifeedant activity, which might be associated with the presence of two β-hydroxyalkanoic moieties in each compound [38], [48], [50], [132]. A phytochemical investigation on A. parviflora resulted in the isolation of quinols 306–312 and pyrrolizidine alkaloids 343–345. Derivatives 306–308 are new compounds. Compound 309, isolated previously from the leaves and branches of Jacaranda species, display cytotoxic and antitumor activities. Three pyrrolizidine alkaloids 343–345 were reported for the first time from this plant [44], [45], [128], [129].
The plant of Ajuga bracteosa afforded several new compounds including unsaturated ketone 341, phthalic ester 322, phenolic compound 320, two sphingolipids 352, 353 and a long-chain polyhydroxy acid 342 [10], [130], [134].

Polyketides and alkaloids.
Biological activity
Antifeedant and larvicidal activity
A neoclerodane 103 was isolated from the leaves of A. decumbens as a feeding stimulant for Athalia rosae ruficornis [75]. Three new neoclerodanes 164, 168, 169 were isolated from the aerial parts of A. reptans cv. catlins giant. Insect antifeedant testing revealed that 168 has significant activity against sixth stadium larvae of Spodoptera littoralis [67]. A series of active clerodanes 104, 156, 175–177 were isolated from the acetone extract of A. pseudoiva leaves by bioassay-guided chromatography. The behavioral responses of Spodoptera littoralis larvae to all clerodanes showed strong antifeedant activity at 100 to 1 mg/L. In addition, this study also indicated that a methoxy group at C(15), either in the α- or β-position, might decrease antifeedant activity [9]. Manguro and co-workers tested larvicidal activity of the extracts of A. remota using second instar Aedes aegypti larvae [123]. The ethyl acetate extract is toxic with LC50 value of 5.30 μg/L, while the methanol extract displays weak toxicity with an LC50 of 65.94 μg/L. Compound 81, obtained from the ethyl acetate extract, is the active component with an LC50 value of 4.40 μg/L.
Antimicrobial activity
Compounds 156–161 are six new neoclerodanes isolated from Ajuga lupulina. The diterpenoids 156 and 160 show strong activity against Pseudomonas aeruginosa and Escherichia coli (inhibitory zones are 3–5 mm and 3.5–4.5 mm, respectively, at a concentration of 0.02 mg/mL). In addition, 156 displays weak activity against Staphylococcus aureus (1.5 mm). The antibacterial activity of 161 against P. aeruginosa (2.1 mm) and E. coli (2.0 mm) is poor compared to 156 and 160. Compound 157 exhibits weak antibacterial activity against S. aureus and E. coli (1.2 mm) [4], [99]. In 2001, Kariba tested the extracts of A. remota for in vitro antifungal activity. The petroleum ether and methanol extracts exhibit antifungal activity against the dermatophytic fungi Trichophyton mentagrophytes and Microsporum gypseum [5]. Ergosterol 5,8-endoperoxide 81, isolated from the methanol extract of A. remota, shows activity against Mycobacterium tuberculosis [55].
Antimalarial activity
Ajuga remota is commonly used as medicinal herb for malaria treatment in Kenya. Three isolates, 81, 89 and 262, were tested for their in vitro antiplasmodial activity. Compound 89 is moderately active against a chloroquine-sensitive (FCA 20/GHA) strain of Plasmodium falciparum, with an IC50 of 23.0 μm, compared to a 0.041 μm IC50 for chloroquine. Compared to 89, compound 262 is approximately 3 times as potent. Compound 262 is also equally potent towards chloroquine-sensitive (FCA 20/GHA) and chloroquine-resistant (W2) strains [6]. An excellent review article summarizes antimalarial activity of compounds contained in A. remota and A. bracteosa [135].
Anti-inflammatory activity
Gautam et al. tested a 70% ethanol extract of A. bracteosa whole plants in a mice acute inflammation model based on topical application of TPA. The result showed that the extract exhibits a remarkable and dose-dependent anti-inflammatory activity at 0.5 and 1.0 mg/ear. In addition, it showed a significant in vitro COX-1 and COX-2 inhibitory activity at 25 and 50 μg/mL. Among the isolates from the bioactive extract, compound 156 exhibited the highest inhibition of COX-1, and compound 268 displayed the highest inhibition of COX-2 [136]. The compounds 342, 352, 353 exhibited remarkable inhibition of lipoxygenase. Compound 342 was more active than baicalein (IC50=22.4 μm) with an IC50 of 10.0 μm [10].
Hypoglycemic activity
Ajuga iva has been used as traditional medicine to control diabetes mellitus for many centuries. In 2002, a study to examine the hypoglycemic effect of A. iva was carried out, and the results demonstrated that A. iva aqueous extract exhibits strong hypoglycemic activity. Lyophilized aqueous extract of A. iva whole plant was found to decrease plasma glucopyranose levels of streptozotocin-induced diabetic rats from 337 to 102.2 mg/dL after 6 h of oral administration. Furthermore, repeated oral administration significantly reduced plasma glucopyranose levels after 1 week of treatment (112 mg/dL at 1 week vs. 337 mg/dL at the baseline values) [137].
Cytotoxic activity
Compounds 70–75 are five new sterol glycosides isolated from a methanol extract of the aerial parts of A. salicifolia. Their cytotoxicity against HeLa cells (KB), human T cell leukemia (Jurkat), and peripheral mononuclear blood cells (PMBC) have been evaluated. Compounds 70–74 significantly inhibit the viability and growth of Jurkat T cells at concentrations below 10 μm. Compound 73 is the most active substance with an IC50 values of 3 μm, followed by 70 (IC50=6 μm). An additional glucopyranose substituent leads to weaker cytotoxicity against Jurkat T cells, as observed for 71 (IC50=10 μm) and 74 (IC50=8 μm). Compound 70 induces cell-cell contacts in a Jurkat T cell population, and remarkably up-regulated mRNA levels of the cell-cycle regulator cyclin D1, which might be an indication for cell differentiation [53]. In 2003, Akbay and co-workers investigated the cytotoxicity of sterols obtained from A. salicifolia against KB (HeLa) and Jurkat T cancer cells. This study demonstrated that compound 72 is active against KB cells with an IC50 of 1 μg/mL, while the corresponding 3-O-β-glucopyranoside, compound 76, is less potent (IC50=13 μg/mL) [54]. Four new A. decumbens abietane diterpenoids, 235–238, were evaluated for in vitro inhibition of cell proliferation. The diterpenoids 235 and 237 exhibit moderate cytotoxic activities against MCF-7 cells (human breast cancer), with IC50 values of 19.4 and 12.5 μm, respectively [7].
Cholinesterase inhibitory activity
Compounds 95, 104, 151, 156, 342, 352 and 353 were obtained from A. bracteosa, and their enzyme-inhibitory potential was evaluated. The diterpenoids 95, 104, 151 and 156 display inhibitory activity against cholinesterase (AChE and BChE) with IC50 values in the range of 14.0–35.2 μm for AChE and 10.0–19.0 μm for BChE, respectively. Compound 104 is the most active against cholinesterase while 156 is comparatively less active, indicating that the presence of a MeO group at C(15) increases the cholinesterase inhibitory activity [10].
Antioxidative activity
Bouderbala and co-workers studied the effect of A. iva aqueous extract on lipid peroxidation and antioxidant enzyme activity in hypercholesterolemic rats. The results showed that A. iva extract is more effective at improving RBC antioxidant capacity relative to that of tissues. In addition, A. iva aqueous extract can reduce oxidative stress, which may prevent lipid peroxidation in hypercholesterolemic models by increasing antioxidant enzyme activity [138].
Vasorelaxant activity
El-Hilaly and co-workers investigated vascular activity of A. iva aqueous extract in normotensive Wistar rats. The aqueous extract displayed NO-mediated and NO-independent vasorelaxing properties in vitro. The A. iva extract contains more than one active compound. One of these compounds is responsible for inhibition of noradrenaline evoked contraction. Another compound was identified in vitro as a transient NO-dependent relaxation [8].
Conclusions
The plants of the genus Ajuga are widely distributed globally and many of these plants are used as traditional herbal medicines. The compounds isolated from this genus exert a broad spectrum of biological and pharmacological activities, however, our review indicates that phytochemical investigation has mainly focused on a few species. Further studies on the remaining species, their constituents and biological activities, should be carried out.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81302664
Award Identifier / Grant number: 81241101
Funding statement: We are grateful for the financial supports from the National Natural Science Foundation of China (81302664, 81241101), and the Scientific Research Foundation of Hebei Province (C2010000489). We also wish to extend our sincere thanks for financial support from Syngenta Ltd. (20013-Hebei Medical University-Syngenta-04) and JSPS KAKENHI (Grant Numbers 19580120, 22560112, 25450144, 17K07772, 26•04392).
References
[1] Israili, Z. H.; Lyoussi, B. Ethnopharmacology of the plants of genus Ajuga. Pak. J. Pharm. Sci.2009, 22, 425–462.Search in Google Scholar
[2] Kubo, I.; Lee, Y.-W.; Balogh-Nair, V.; Nakanishi, K.; Chapya, A. Structure of ajugarins. J. Chem. Soc., Chem. Commun.1976, 949–950.10.1039/c39760000949Search in Google Scholar
[3] Camps, F.; Coll, J. Insect allelochemicals from Ajuga plants. Phytochemistry1993, 32, 1361–1370.10.1016/0031-9422(93)85139-ISearch in Google Scholar
[4] Chen, H.; Liu, D. Q.; Li, X. Z.; Xia, Z. H.; Li, Y.; Liu, Z. L.; Tan, R. X. Two new clerodane diterpenes with antibacterial activity from Ajuga lupulina. Indian J. Chem. B1999, 38, 743–745.Search in Google Scholar
[5] Kariba, R. M. Antifungal activity of Ajuga remota. Fitoterapia2001, 72, 177–178.10.1016/S0367-326X(00)00280-XSearch in Google Scholar
[6] Kuria, K. A. M.; Chepkwony, H.; Govaerts, C.; Roets, E.; Busson, R.; Witte, P. D.; Zupko, I.; Hoornaert, G.; Quirynen, L.; Maes, L.; et al. The antiplasmodial activity of isolates from Ajuga remota. J. Nat. Prod.2002, 65, 789–793.10.1021/np0104626Search in Google Scholar
[7] Wang, B.; Wang, X. N.; Shen, T.; Wang, S. Q.; Guo, D. X.; Lou, H. X. Rearranged abietane diterpenoid hydroquinones from aerial parts of Ajuga decumbens Thunb. Phytochem. Lett.2012, 5, 271–275.10.1016/j.phytol.2012.01.010Search in Google Scholar
[8] El-Hilaly, J.; Lyoussi, B.; Wibo, M.; Morel, N. Vasorelaxant effect of the aqueous extract of Ajuga iva in rat aorta. J. Ethnopharmacol.2004, 93, 69–74.10.1016/j.jep.2004.03.020Search in Google Scholar
[9] Jannet, H. B.; Harzallah-Skhiri, F.; Mighri, Z.; Simmonds, M. S. J.; Blaney, W. M. Responses of Spodoptera littoralis larvae to Tunisian plant extracts and to neo-clerodane diterpenoids isolated from Ajuga pseudoiva leaves. Fitoterapia2000, 71, 105–112.10.1016/S0367-326X(99)00146-XSearch in Google Scholar
[10] Riaz, N.; Nawaz, S. A.; Mukhtar, N.; Malik, A.; Afza, N.; Ali, S.; Ullah, S.; Muhammad, P.; Choudhary, M. I. Isolation and enzyme-inhibition studies of the chemical constituents from Ajuga bracteosa. Chem. Biodivers.2007, 4, 72–83.10.1002/cbdv.200790008Search in Google Scholar PubMed
[11] Imai, S.; Toyosato, T.; Sakai, M.; Sato, Y.; Fujioka, S.; Murata, E.; Goto, M. Isolation of cyasterone and ecdysterone from plant materials. Chem. Pharm. Bull.1969, 17, 340–342.10.1248/cpb.17.340Search in Google Scholar
[12] Usmanov, B. Z.; Gorovits, M. B.; Abubakirov, N. K. Phytoecdysones from Ajuga turkestanica. Khim. Prir. Soedin.1971, 7, 535–536.10.1007/BF00564772Search in Google Scholar
[13] Ikan, R.; Ravid, U. The isolation and identification of ecdysterone from Ajuga iva. Planta Med.1971, 20, 33–35.10.1055/s-0028-1099661Search in Google Scholar PubMed
[14] Wessner, M.; Champion, B.; Girault, J. P.; Kaouadji, N.; Saidi, B.; LaFont, R. Ecdysteroids from Ajuga iva. Phytochemistry1992, 31, 3785–3788.10.1016/S0031-9422(00)97527-7Search in Google Scholar
[15] Ghedira, K.; Chemli, R.; Richard, B.; Zeches, M.; Men-Olivier, L. L. Contribution to the study of the traditional Tunisian pharmacopoeia: study of aerial parts of Ajuga iva (L.) Schreb. Plant. Med. Phytother.1991, 25, 100–111.Search in Google Scholar
[16] Ikan, R.; Ravid, U. The isolation and identification of cyasterone from Ajuga chia (labiatae). Phytochemistry1971, 10, 1659–1661.10.1016/0031-9422(71)85043-4Search in Google Scholar
[17] Camps, F.; Coll, J.; Dargallo, O. Phytoecdysteroids from Ajuga chamaepitys. An. Quim., Ser. C.1985, 81, 74–75.Search in Google Scholar
[18] Yu, Y. J.; Do, J. C.; Kwon, S. Y.; Son, K. H. Studies on the constituents from the herbs of Ajuga multiflora (II). Saengyak Hakhoechi1998, 29, 318–322.Search in Google Scholar
[19] Chan, Y.-Y.; Wu, T.-S.; Kuoh, C. S.; Damu, A. G. A new phytoecdysteroid from Ajuga taiwanensis. Chem. Pharm. Bull.2005, 53, 836–838.10.1248/cpb.53.836Search in Google Scholar PubMed
[20] Coll, J.; Tandron, Y. A.; Zeng, X. New phytoecdysteroids from cultured plants of Ajuga nipponensis Makino. Steroids2007, 72, 270–277.10.1016/j.steroids.2006.11.017Search in Google Scholar PubMed
[21] Castro, A.; Coll, J.; Tandron, Y. A.; Pant, A. K.; Mathela, C. S. Phytoecdysteroids from Ajuga macrosperma var. breviflora Roots. J. Nat. Prod.2008, 71, 1294–1296.10.1021/np800131fSearch in Google Scholar PubMed
[22] Sinica, I. H. Isolation and identification of phytoecdysones from Ajuga nipponensis Makino. Huaxue Xuebao1981, 39, 466–470.Search in Google Scholar
[23] Camps, F.; Coll, J.; Cortel, A. Allelochemicals on insects isolated from Ajuga reptans (Labiatae). Rev. Latinoam. Quim.1981, 12, 81–88.Search in Google Scholar
[24] Camps, F.; Coll, J.; Cortel, A. 29-Norsengosterone and 29-norcyasterone, new C-28 phytoecdysteroids from Ajuga reptans (Labiatae). Chem. Lett.1982, 1313–1316.10.1246/cl.1982.1313Search in Google Scholar
[25] Alekseeva, L. I.; Lafont, R.; Volodin, V. V.; Luksha, V. G. Ecdysteroids from Ajuga reptans. Russ. J. Plant Physiol.1998, 45, 316–321.Search in Google Scholar
[26] Kubo, I.; Klocke, J. A. Plant resistance to insects. ACS Symp. Ser.1983, 208, 329–346.10.1021/bk-1983-0208.ch019Search in Google Scholar
[27] Saatov, Z.; Usmanov, B. Z.; Abubakirov, N. K. Phytoecdysones of Ajuga turkestanica. IV. Khim. Prir. Soedin.1977, 422.10.1007/BF00573570Search in Google Scholar
[28] Usmanov, B. Z.; Rashkes, Y. V.; Abubakirov, N. K. Phytoecdysones of Ajuga turkestanica. VI. 22-Acetylcyasterone. Khim. Prir. Soedin.1978, 215–219.10.1007/BF01134622Search in Google Scholar
[29] Usmanov, B. Z.; Saatov, Z.; Abubakirov, N. K. Phytoecdysones of Ajuga turkestanica. V. Khim. Prir. Soedin.1977, 710.10.1007/BF00569612Search in Google Scholar
[30] Coll, J. New minor ecdysteroids from Ajuga iva (Labiatae) and complete 1H-NMR assignment of cyasterone. Afinidad2007, 64, 242–250.Search in Google Scholar
[31] Imai, S.; Murata, E.; Fujioka, S.; Koreeda, M.; Nakanishi, K. Structure of ajugasterone C, a phytoecdysone with an 11-hydroxy-group. J. Chem. Soc. D.1969, 546–547.10.1039/c29690000546Search in Google Scholar
[32] Khafagy, S. M.; Sabri, N. N.; El-Sebakhy, N.; Blessington, B.; Asaad, A. A C-28 ecdysone-like substance from Ajuga iva. Planta Med.1979, 35, 184–185.10.1055/s-0028-1097201Search in Google Scholar
[33] Camps, F.; Coll, J.; Cortel, A.; Molins, E.; Miravitlles, C. 2-Acetyl- and 3-acetyl-29-norcyasterone, new minor phytoecdysteroids from Ajuga reptans (Labiatae). J. Chem. Res (S).1985, 14–15.Search in Google Scholar
[34] Calcagno, M. P.; Camps, F.; Coll, J.; Mele, E.; Sanchez-Baeza, F. A new family of phytoecdysteroids isolated from aerial part of Ajuga reptans var. atropurpurea. Tetrahedron1995, 51, 12119–12126.10.1016/0040-4020(95)00767-3Search in Google Scholar
[35] Calcagno, M. P.; Camps, F.; Coll, J.; Mele, E.; Sanchez-Baeza, F. New phytoecdysteroids from roots of Ajuga reptans varieties. Tetrahedron1996, 52, 10137–10146.10.1016/0040-4020(96)00536-4Search in Google Scholar
[36] Vanyolos, A.; Simon, A.; Toth, G.; Polgar, L.; Kele, Z.; Ilku, A.; Matyus, P.; Bathori, M. C-29 Ecdysteroids from Ajuga reptans var. reptans. J. Nat. Prod.2009, 72, 929–932.10.1021/np800708gSearch in Google Scholar
[37] Alekseeva, L. I.; Volodin, V. V.; Luksha, V. G.; Lafont, R. Ecdysteroid acetates from Ajuga reptans. Chem. Nat. Compd.1999, 35, 532–534.10.1007/BF02323288Search in Google Scholar
[38] Chaari, A.; Jannet, H. B.; Mighri, Z. Isolement et élucidation structurale d’un nouveau phytoecdystéroïde antibactérien et de deux dérivés de l’acide cinnamique des feuilles de la plante Ajuga pseudo-iva. J. Soc. Chim. Tunis.2000, 4, 789–799.Search in Google Scholar
[39] Fujimoto, Y.; Ohyama, K.; Nomura, K.; Hyodo, R.; Takahashi, K.; Yamada, J.; Morisaki, M. Biosynthesis of sterols and ecdysteroids in Ajuga hairy roots. Lipids2000, 35, 279–288.10.1007/s11745-000-0524-zSearch in Google Scholar
[40] Khan, P. M.; Ahmad, S.; Rubnawaz, H.; Malik, A. The first report of a withanolide from the family Labiatae. Phytochemistry1999, 51, 669–671.10.1016/S0031-9422(99)00045-XSearch in Google Scholar
[41] Khan, P. M.; Malik, A.; Ahmad, S.; Nawaz, H. R. Withanolides from Ajuga parviflora. J. Nat. Prod.1999, 62, 1290–1292.10.1021/np990029kSearch in Google Scholar
[42] Khan, P. M.; Nawaz, H. R.; Ahmad, S.; Malik, A. Ajugins C and D, new withanolides from Ajuga parviflora. Helv. Chim. Acta1999, 82, 1423–1426.10.1002/(SICI)1522-2675(19990908)82:9<1423::AID-HLCA1423>3.0.CO;2-FSearch in Google Scholar
[43] Nawaz, H. R.; Malik, A.; Khan, P. M.; Ahmed, S. Ajugin E and F: two withanolides from Ajuga parviflora. Phytochemistry1999, 52, 1357–1360.10.1016/S0031-9422(99)00345-3Search in Google Scholar
[44] Nawaz, H. R.; Riaz, M.; Malik, A.; Khan, P. M.; Ullah, N. Withanolides and alkaloid from Ajuga parviflora. J. Chem. Soc. Pak.2000, 22, 138–141.Search in Google Scholar
[45] Nawaz, H. R.; Malik, A.; Muhammad, P.; Ahmed, S.; Riaz, M. Chemical constituents of Ajuga parviflora. Z. Naturforsch. B.2000, 55, 100–103.10.1515/znb-2000-0116Search in Google Scholar
[46] Riaz, N.; Malik, A.; Aziz ur, R.; Nawaz, S. A.; Muhammad, P.; Choudhary, M. I. Cholinesterase-inhibiting withanolides from Ajuga bracteosa. Chem. Biodivers.2004, 1, 1289–1295.10.1002/cbdv.200490091Search in Google Scholar
[47] Camps, F.; Coll, J.; Cortel, A. Sterols from Ajuga reptans (Labiatae). An. Quim., Ser. C.1983, 79, 228–229.Search in Google Scholar
[48] Jannet, H. B.; H-Skhiri, F.; Mighri, Z.; Simmonds, M. S. J.; Blaney, W. M. Antifeedant activity of plant extracts and of new natural diglyceride compounds isolated from Ajuga pseudoiva leaves against Spodoptera littoralis larvae. Ind. Crops Prod.2001, 14, 213–222.10.1016/S0926-6690(01)00086-3Search in Google Scholar
[49] Kokdil, G.; Topcu, G.; Goren, A. C.; Voelter, W. Steroids and terpenoids from Ajuga relicta. Z. Naturforsch. B.2002, 57, 957–960.10.1515/znb-2002-0817Search in Google Scholar
[50] Chaari, A.; Jannet, H. B.; Martin, M. T.; Mighri, Z. Identification of a new steroid and a biological active heteroside in Ajuga pseudoiva leaves. J. Soc. Chim. Tunis.1998, 4, 257–266.Search in Google Scholar
[51] Topcu, G.; Kokdil, G.; Turkmen, Z.; Voelter, W.; Adou, E.; Kingston, D. G. I. A new clerodane diterpene and other constituents from Ajuga chamaepitys ssp. laevigata. Z. Naturforsch. B.2004, 59, 584–588.10.1002/chin.200437183Search in Google Scholar
[52] Guo, X.; Huang, Z.; Bao, Y.; An, L.; Ma, L.; Gu, L. Isolation and structure identification of chemical constituents in Ajuga decumbens. Zhongcaoyao2005, 36, 646–648.Search in Google Scholar
[53] Akbay, P.; Gertsch, J.; Cahs, I.; Heilmann, J.; Zerbe, O.; Sticher, O. Novel antileukemic sterol glycosides from Ajuga salicifolia. Helv. Chim. Acta2002, 85, 1930–1942.10.1002/1522-2675(200207)85:7<1930::AID-HLCA1930>3.0.CO;2-CSearch in Google Scholar
[54] Akbay, P.; Calis, I.; Heilmann, J.; Sticher, O. New stigmastane sterols from Ajuga salicifolia. J. Nat. Prod.2003, 66, 461–465.10.1021/np020427eSearch in Google Scholar
[55] Cantrell, C. L.; Rajab, M. S.; Franzblau, S. G.; Fronczek, F. R.; Fischer, N. H. Antimycobacterial ergosterol-5, 8-endoperoxide from Ajuga remota. Planta Med.1999, 65, 732–734.10.1055/s-1999-14053Search in Google Scholar
[56] Dinda, B.; Banerjee, J.; Guha, S. Triterpenes from Ajuga macrosperma Wall. J. Indian Chem. Soc.1997, 74, 424.Search in Google Scholar
[57] Shimomura, H.; Sashida, Y.; Ogawa, K. Neo-clerodane diterpenes from Ajuga nipponensis. Chem. Pharm. Bull.1989, 37, 354–357.10.1248/cpb.37.354Search in Google Scholar
[58] Beauchamp, P. S.; Botini, A. T.; Caselles, M. C.; Dev, V.; Hope, H.; Larter, M.; Lee, G.; Mathela, C. S.; Melkani, A. B.; Millar, P. D.; et al. Neo-clerodane diterpenoids from Ajuga parviflora. Phytochemistry1996, 43, 827–834.10.1016/0031-9422(96)00207-5Search in Google Scholar
[59] Sun, Z.; Li, Y.; Jin, D.-Q.; Guo, P.; Xu, J.; Guo, Y.; Zhang, L. Structure elucidation and Inhibitory effects on NO production of clerodane diterpenes from Ajuga decumbens. Planta Med.2012, 78, 1579–1593.10.1055/s-0032-1315215Search in Google Scholar
[60] Kubo, I.; Klocke, J. A.; Miura, I.; Fukuyama, Y. Structure of ajugarin-IV. J. Chem. Soc., Chem. Commun.1982, 618–619.10.1039/c39820000618Search in Google Scholar
[61] Shimomura, H.; Sashida, Y.; Ogawa, K. Neo-clerodane diterpenes from Ajuga ciliata var. villosior. Chem. Pharm. Bull.1989, 37, 988–992.10.1248/cpb.37.988Search in Google Scholar
[62] Kubo, I.; Fukuyama, Y.; Chapya, A. Structure of ajugarin W. Chem. Lett.1983, 223–234.10.1246/cl.1983.223Search in Google Scholar
[63] Kubo, I.; Kido, M.; Fukuyama, Y. X-Ray crystal structure of 12-bromoajugarin-I and conclusion on the absolute configuration of ajugarins. J. Chem. Soc., Chem. Commun.1980, 897–898.10.1039/c39800000897Search in Google Scholar
[64] Castro, A.; Coll, J.; Arfan, M. Neo-Clerodane diterpenoids from Ajuga bracteosa. J. Nat. Prod.2011, 74, 1036–1041.10.1021/np100929uSearch in Google Scholar
[65] Coll, J.; Tandron, Y. Isolation and identification of neo-clerodane diterpenes from Ajuga remota by high-performance liquid chromatography. Phytochem. Anal.2005, 16, 61–67.10.1002/pca.812Search in Google Scholar
[66] Camps, F.; Coll, J.; Cortel, A.; Messeguer, A. Ajugareptansin, a new diterpenoid from Ajuga reptans (L.). Tetrahedron Lett.1979, 20, 1709–1712.10.1016/S0040-4039(01)93631-7Search in Google Scholar
[67] Bremner, P. D.; Simmonds, M. S. J.; Blaney, W. M.; Veitch, N. C. Neo-clerodane diterpenoid insect antifeedants from Ajuga reptans cv Catlins Giant. Phytochemistry1998, 47, 1227–1232.10.1016/S0031-9422(97)00706-1Search in Google Scholar
[68] Malakov, P. Y.; Papanov, G. Y. Areptins A and B two new neo-clerodane diterpenoids from Ajuga reptans. Phytochemistry1998, 49, 2443–2447.10.1016/S0031-9422(98)00445-2Search in Google Scholar
[69] Camps, F.; Coll, J.; Cortel, A. Two new clerodane diterpenids from Ajuga reptans (Labiatae). Chem. Lett.1981, 1093–1096.10.1246/cl.1981.1093Search in Google Scholar
[70] Carbonell, P.; Coll, J. Ajugatansins, neo-clerodane diterpenes from Ajuga reptans. Phytochem. Anal.2001, 12, 73–78.10.1002/1099-1565(200101/02)12:1<73::AID-PCA561>3.0.CO;2-8Search in Google Scholar
[71] Camps, F.; Coll, J.; Cortel, A. New clerodane diterpenoids from Ajuga iva. Chem. Lett.1982, 1053–1056.10.1246/cl.1982.1053Search in Google Scholar
[72] Hernandez, A.; Pascual, C.; Sanz, J.; Rodriguez, B. Diterpenoids from Ajuga chamaepitys: Two neo-clerodane derivatives. Phytochemistry1982, 21, 2909–2911.10.1016/0031-9422(80)85066-7Search in Google Scholar
[73] Boneva, I.; Mikhova, B.; Malakov, P.; Papanov, G.; Duddeck, H.; Spasov, S. Neo-clerodane diterpenoids from Ajuga chamaepitys. Phytochemistry1990, 29, 2931–2933.10.1016/0031-9422(90)87108-7Search in Google Scholar
[74] Torre, M. C. D. L.; Rodriguez, B.; Bruno, M.; Piozzi, F.; Vassallo, N.; Bondi, M. L.; Servettaz, O. Neo-clerodane diterpenoids from Ajuga australis and A. orientalis. Phytochemistry1997, 45, 121–123.10.1016/S0031-9422(96)00850-3Search in Google Scholar
[75] Amano, T.; Nishida, R.; Kuwahara, Y. Ajugatakasins A and B, new diterpenoids from Ajuga decumbens, and feeding stimulative activity of related neoclerodane analogs toward the turnip sawfly. Biosci. Biotechnol. Biochem.1997, 61, 1518–1522.10.1271/bbb.61.1518Search in Google Scholar
[76] Grace, M. H.; Cheng, D. M.; Raskin, I.; Lila, M. A. Neo-clerodane diterpenes from Ajuga turkestanica. Phytochem. Lett.2008, 1, 81–84.10.1016/j.phytol.2008.03.004Search in Google Scholar
[77] Camps, F.; Coll, J.; Dargallo, O. Neo-clerodane diterpenoids from Ajuga pseudoiva. Phytochemistry1984, 23, 387–389.10.1016/S0031-9422(00)80337-4Search in Google Scholar
[78] Jannet, H. B.; Chaari, A.; Mighri, Z.; Martin, M. T.; Loukaci, A. Neo-clerodane diterpenoids from Ajuga pseudoiva leaves. Phytochemistry1999, 52, 1541–1545.10.1016/S0031-9422(99)00337-4Search in Google Scholar
[79] Verma, V. H. K.; Mahmood, U.; Singh, B. Clerodane diterpenoids from Ajuga bracteosa Wall. Nat. Prod. Lett.2002, 16, 255–259.10.1080/10575630290020578Search in Google Scholar
[80] Camps, F.; Coll, J.; Dargallo, O.; Rius, J.; Miravitlles, C. Clerodane diterpenoids from Teucrium and Ajuga plants. Phytochemistry1987, 26, 1475–1479.10.1016/S0031-9422(00)81838-5Search in Google Scholar
[81] Shimomura, H.; Sashida, Y.; Ogawa, K.; Iitaka, Y. The chemical constituents of Ajuga plants. I. neo-Clerodanes from the leaves of Ajuga nipponensis MAKINO. Chem. Pharm. Bull.1983, 31, 2192–2199.10.1248/cpb.31.2192Search in Google Scholar
[82] Shimomura, H.; Sashida, Y.; Ogawa, K.; Iitaka, Y. Ajugamarin, a new bitter diterpene from Ajuga nipponensis Makino. Tetrahedron Lett.1981, 22, 1367–1368.10.1016/S0040-4039(01)90321-1Search in Google Scholar
[83] Coll, J.; Tandron, Y. A. Isolation and identification of neo-clerodane diterpenes from Ajuga nipponensis Makino. Nat. Prod. Commun.2006, 1, 183–189.10.1177/1934578X0600100302Search in Google Scholar
[84] Min, Z.; Mizuno, M.; Wang, S.; Iinuma, M.; Tanaka, T. Two new neo-clerodane diterpenes in Ajuga decumbens. Chem. Pharm. Bull.1990, 38, 3167–3168.10.1248/cpb.38.3167Search in Google Scholar
[85] Sang, Y.-S.; Huang, Z.-H.; Min, Z.-D. A new neoclerodane diterpene isolated from Ajuga decumbens. Zhongguo Tianran Yaowu2005, 3, 284–286.Search in Google Scholar
[86] Sun, Z.; Li, Y.; Jin, D.-Q.; Guo, P.; Song, H.; Xu, J.; Guo, Y.; Zhang, L. neo-Clerodane diterpenes from Ajuga decumbens and their inhibitory activities on LPS-induced NO production. Fitoterapia2012, 83, 1409–1414.10.1016/j.fitote.2012.08.003Search in Google Scholar PubMed
[87] Guo, P.; Li, Y.; Xu, J.; Liu, C.; Ma, Y.; Guo, Y. Bioactive neo-clerodane diterpenoids from the whole plants of Ajuga ciliata Bunge. J. Nat. Prod.2011, 74, 1575–1583.10.1021/np2001557Search in Google Scholar PubMed
[88] Shimomura, H.; Sashida, Y.; Ogawa, K. Neo-clerodane diterpenes from Ajuga decumbens. Chem. Pharm. Bull.1989, 37, 996–998.10.1248/cpb.37.996Search in Google Scholar
[89] Chan, Y.-Y. Neoclerodane diterpenoids from Ajuga taiwanensis. Chem. Pharm. Bull.2005, 53, 164–167.10.1248/cpb.53.164Search in Google Scholar PubMed
[90] Guo, P.; Li, Y.; Jin, D.-Q.; Xu, J.; He, Y.; Zhang, L.; Guo, Y. Neo-Clerodane diterpenes from Ajuga ciliata and their inhibitory activities on LPS-induced NO production. Phytochem. Lett.2012, 5, 563–566.10.1016/j.phytol.2012.05.014Search in Google Scholar
[91] Guo, P.; Li, Y.; Xu, J.; Guo, Y.; Jin, D.-Q.; Gao, J.; Hou, W.; Zhang, T. Neo-Clerodane diterpenes from Ajuga ciliata Bunge and their neuroprotective activities. Fitoterapia2011, 82, 1123–1127.10.1016/j.fitote.2011.07.010Search in Google Scholar
[92] Min, Z.-D.; Wang, S.-Q.; Zheng, Q.-T.; Wu, B.; Mizuno, M.; Tanaka, T.; Inuma, M. Four new insect antifeedant neo-clerodane diterpenoids, ajugacumbins A, B, C and D, from Ajuga decumbens. Chem. Pharm. Bull.1989, 37, 2505–2508.10.1248/cpb.37.2505Search in Google Scholar
[93] Chen, H.-M.; Min, Z.-D.; Iinuma, M.; Tanaka, T. Clerodane diterpenoids from Ajuga decumbens. Chem. Pharm. Bull.1995, 43, 2253–2255.10.1248/cpb.43.2253Search in Google Scholar
[94] Liu, Z.; Li, Z. Q.; Zhang, C. X.; Chen, R. Y.; Zhang, Y. G.; Qiu, Y. T.; Zhao, S. H. A new neo-clerodane diterpene from Ajuga nipponensis. Chin. Chem. Lett.1995, 6, 581–582.Search in Google Scholar
[95] Shen, X.; Isogai, A.; Furihata, K.; Sun, H.; Suzuki, A. Neo-clerodane diterpenoids from Ajuga macrosperma and Ajuga pantantha. Phytochemistry1993, 34, 1091–1094.10.1016/S0031-9422(00)90721-0Search in Google Scholar
[96] Lv, H.; Luo, J.; Kong, L. A new neo-clerodane diterpene from Ajuga decumbens. Nat. Prod. Res.2014, 28, 196–200.10.1080/14786419.2013.866114Search in Google Scholar
[97] Malakov, P. Y.; Papanov, G. Y.; Torre, M. C. D. l.; Rodriguez, B. Neo-clerodane diterpenoids from Ajuga genevensis. Phytochemistry1991, 30, 4083–4085.10.1016/0031-9422(91)83472-WSearch in Google Scholar
[98] Shen, X.; Isogai, A.; Furihata, K.; Sun, H.; Suzuki, A. Two neo-clerodane diterpenoids from Ajuga macrosperma. Phytochemistry1993, 33, 887–889.10.1016/0031-9422(93)85297-5Search in Google Scholar
[99] Chen, H.; Tan, R.-X.; Liu, Z.-L.; Zhang, Y.; Yang, L. Antibacterial neoclerodane diterpenoids from Ajuga lupulina. J. Nat. Prod.1996, 59, 668–670.10.1021/np960385sSearch in Google Scholar PubMed
[100] Chen, H.; Tan, R.-X.; Liu, Z.-L.; Zhao, C.-Y.; Sun, J. A clerodane diterpene with antibacterial activity from Ajuga lupulina. Acta Crystallogr., Sect. C: Cryst. Struct. Commun.1997, 53, 814–816.10.1107/S0108270196013637Search in Google Scholar
[101] Li, B.; Zheng, C.; Lin, Z.; Sun, H. Chemical constituents of Ajuga lupulina var. Major. Tianran Chanwu Yanjiu Yu Kaifa2000, 12, 45.Search in Google Scholar
[102] Boneva, I. M.; Malakov, P. Y.; Papanov, G. Y. Ajugapyrin A, a neo-clerodane diterpene from Ajuga pyramidalis. Phytochemistry1997, 47, 303–305.10.1016/S0031-9422(97)00558-XSearch in Google Scholar
[103] Malakov, P. Y.; Papanov, G. Y.; Torre, M. C. D. L.; Rodriguez, B. Constituents of Ajuga laxmanii. Fitoterapia1998, 69, 552–555.Search in Google Scholar
[104] Jannet, H. B.; Oueslati, M. H.; Chaari, A.; Martin, M. T.; Loukaci, A.; Simmonds, M. J.; Mighri, Z. Structure of a new neo-clerodane diterpenoid from Ajuga pseudoiva leaves and its insect antifeedant and antibacterial activities. J. Soc. Chim. Tunis.2002, 4, 1545–1549.Search in Google Scholar
[105] Nishida, R.; Kawai, K.; Amano, T.; Kuwahara, Y. Pharmacophagous feeding stimulant activity of neo-clerodane diterpenoids for the turnip sawfly, Athalia rosae ruficornis. Biochem. System. Ecol.2004, 32, 15–25.10.1016/S0305-1978(03)00160-1Search in Google Scholar
[106] Huang, X.-C.; Qin, S.; Guo, Y.-W.; Krohn, K. Four new neoclerodane diterpenoids from Ajuga decumbens. Helv. Chim. Acta2008, 91, 628–634.10.1002/hlca.200890066Search in Google Scholar
[107] Wang, A. G.; Lu, Y.; Feng, X. Z. Chemical constituents of Ajuga forrestii Diels. Yaoxue Xuebao1994, 29, 899–904.Search in Google Scholar
[108] Takasaki, M.; Yamauchi, I.; Haruna, M.; Konoshima, T. New glycosides from Ajuga decumbens. J. Nat. Prod.1998, 61, 1105–1109.10.1021/np980148kSearch in Google Scholar
[109] Xiong, Y.; Qu, W.; Sun, J.-B.; Wang, M.-T.; Liang, J.-Y. Eudesmane sesquiterpenoid lactones and abietane diterpenoids from Ajuga forrestii Diels. Phytochem. Lett.2013, 6, 457–460.10.1016/j.phytol.2013.05.017Search in Google Scholar
[110] Coll, J. NMR shift data of neo-clerodane diterpenes from the genus Ajuga. Phytochem. Anal.2002, 13, 372–380.10.1002/pca.671Search in Google Scholar
[111] Akbay, P.; Calis, I.; Heilmann, J.; Sticher, O. Ionone, iridoid and phenylethanoid glycosides from Ajuga salicifolia. Z. Naturforsch. C.2003, 58, 177–180.10.1515/znc-2003-3-406Search in Google Scholar
[112] Sun, Z.-P.; Gui, L.-P.; Guo, Y.-Q.; Xu, J.; Li, Y.-S. Isolation and identification of chemical constituents from whole plants of Ajuga decumbens. Shenyang Yaoke Daxue Xuebao2012, 29, 758–764.Search in Google Scholar
[113] Guiso, M.; Agosini, A.; Marini-Bettolo, R. Reptoside: structure and configuration. Gazz. Chim. Ital.1974, 104, 403–407.Search in Google Scholar
[114] Shoji, N.; Umeyama, A.; Sunahara, N.; Arihara, S. Ajureptoside, a novel C9 iridoid glucoside from Ajuga reptans. J. Nat. Prod.1992, 55, 1004–1006.10.1021/np50085a030Search in Google Scholar
[115] Ono, M.; Furusawa, C.; Ozono, T.; Oda, K.; Yasuda, S.; Okawa, M.; Kinjo, J.; Ikeda, T.; Miyashita, H.; Yoshimitsu, H.; et al. Four new iridoid glucosides from Ajuga reptans. Chem. Pharm. Bull.2011, 59, 1065–1068.10.1248/cpb.59.1065Search in Google Scholar
[116] Takeda, Y.; Tsuchida, S.; Fujita, T. Four new iridoid glucoside p-coumaroyl esters from Ajuga decumbens. Phytochemistry1987, 26, 2303–2306.10.1016/S0031-9422(00)84707-XSearch in Google Scholar
[117] Guiso, M.; Marini-Bettolo, R.; Agostini, A. Ajugoside and ajugol: structure and configuration. Gazz. Chim. Ital.1974, 104, 25–33.Search in Google Scholar
[118] Chung, B.-S.; Lee, H.-K.; Kim, J.-W. Iridoid glycoside. I. Studies on the iridoid glycoside of Ajuga spectabilis Nakai. Saengyak Hakhoechi1980, 11, 15–23.Search in Google Scholar
[119] Chung, B. S.; Yoo, W. K. Iridoid glycoside (VI). Studies on the iridoid glycoside of Ajuga multiflora Bunge. Saengyak Hakhoechi1985, 16, 160–164.Search in Google Scholar
[120] Assaad, A. M.; Lahloub, M. F. Irdoid glycosides of Ajuga iva (L.) Schreb. (Lamiaceae). Alex. J. Pharm. Sci.1988, 2, 132–135.Search in Google Scholar
[121] Chaari, A.; Jannet, H. B.; Mighri, Z.; Lallemand, M.-C.; Kunesch, N. 7-O-6′-O-Malonylcachinesidic acid, a new macrocyclic iridoid ester of malonic acid from the Tunisian plant Ajuga pseudoiva. J. Nat. Prod.2002, 65, 618–620.10.1021/np010453xSearch in Google Scholar
[122] Manguro, L. O. A.; Ogur, J. A.; Okora, D. M.; Wagai, S. O.; Lemmen, P. Further flavonol and iridoid glycosides from Ajuga remota aerial parts. J. Asian Nat. Prod. Res.2007, 9, 617–629.10.1080/10286020600979480Search in Google Scholar
[123] Manguro, L. O. A.; Lemmen, P.; Hao, P. Iridoid glycosides from underground parts of Ajuga remota. Rec. Nat. Prod.2011, 5, 147–157.Search in Google Scholar
[124] Prokopenko, S. A. Chemical composition of the forb Ajuga chia. Khim. Prir. Soedin.1986, 514.10.1007/BF00579839Search in Google Scholar
[125] Wang, M.; Zhang, H.; Chen, Y. Studies on the chemical constituents of Ajuga lupulina Maxim. Lanzhou Daxue Xuebao, Ziran Kexueban1991, 27, 93–96.Search in Google Scholar
[126] Yu, Y. J.; Do, J. C.; Jung, K. Y.; Kwon, S. Y.; Son, K. H. Studies on the constituents of the herb Ajuga multiflora (I). Saengyak Hakhoechi1998, 29, 75–78.Search in Google Scholar
[127] Hussain, J.; Begum, N.; Hussain, H.; Khan, F. U.; Rehman, N. U.; Al-Harrasi, A.; Ali, L. Ajuganane: a new phenolic compound from Ajuga bracteosa. Nat. Prod. Commun.2012, 7, 615–616.10.1177/1934578X1200700518Search in Google Scholar
[128] Muhammad, P.; Ahmad, S.; Nawaz, H. R.; Ullah, N.; Malik, A. New acetylated quinols from Ajuga parviflora. Fitoterapia1999, 70, 229–232.10.1016/S0367-326X(99)00019-2Search in Google Scholar
[129] Akbar, E.; Nawaz, H. R.; Malik, A. Dihydroquinol and quinol derivatives from Ajuga parviflora. Z. Naturforsch. B.2001, 56, 842–846.10.1515/znb-2001-0819Search in Google Scholar
[130] Singh, N.; Mahmood, U.; Kaul, V. K.; Jirovetz, L. A new phthalic acid ester from Ajuga bracteosa. Nat. Prod. Res.2006, 20, 593–597.10.1080/14786410500185550Search in Google Scholar PubMed
[131] Jannet, H. B.; Mighri, Z.; Serani, L.; Laprevote, O.; Jullian, J. C.; Roblot, F. Isolation and structure elucidation of three new diglyceride compounds from Ajuga iva leaves. Nat. Prod. Lett.1997, 10, 157–164.10.1080/10575639708043732Search in Google Scholar
[132] Jannet, H. B.; Chaari, A.; Martin, M. T.; Mighri, Z. Structure of five new monoglyceride compounds isolated from Ajuga pseudoiva. J. Soc. Chim. Tunis.1999, 4, 483–488.Search in Google Scholar
[133] Jannet, H. B.; Chaari, A.; Martin, M. T.; Mighri, Z. Clerosterol and 3-hydroxyhexadecanoic acid methyl ester from Ajuga iva leaves. J. Soc. Chim. Tunis.1998, 4, 179–187.Search in Google Scholar
[134] Bhakuni, R. S.; Shukla, Y. N.; Thakur, R. S. Heptacos-3-en-25-one. A new unsaturated ketone from Ajuga bracteosa. J. Indian Chem. Soc.1990, 67, 355.Search in Google Scholar
[135] Cocquyt, L.; Cos, P.; Herdewijn, P.; Maes, L.; Van den Steen, P. E. Laekeman, Ajuga remota Benth.: From ethnopharmacology to phytomedical perspective in the treatment of malaria. Phytomedicine2011, 18, 1229–1237.10.1016/j.phymed.2011.08.063Search in Google Scholar PubMed
[136] Gautam, R.; Jachak, S. M.; Saklani, A. Anti-inflammatory effect of Ajuga bracteosa Wall Ex Benth. mediated through cyclooxygenase (COX) inhibition. J. Ethnopharmacol.2011, 133, 928–930.10.1016/j.jep.2010.11.003Search in Google Scholar PubMed
[137] El, H. J.; Lyoussi, B. Hypoglycaemic effect of the lyophilised aqueous extract of Ajuga iva in normal and streptozotocin diabetic rats. J. Ethnopharmacol.2002, 80, 109–113.10.1016/S0378-8741(01)00407-XSearch in Google Scholar
[138] Bouderbala, S.; Lamri-Senhadji, M.; Prost, J.; Lacaille-Dubois, M. A.; Bouchenak, M. Changes in antioxydant defense status in hypercholesterolemic rats treated with Ajuga iva. Phytomedicine2008, 15, 453–461.10.1016/j.phymed.2007.10.001Search in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Chemical and pharmacological research on the plants from genus Ajuga
- Research Articles
- One-pot synthesis of annulated 1,8-naphthyridines
- Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone
- Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids
- Regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole
- A simple one-pot synthesis of 2,4-diaryl- 9H-pyrido[2,3-b]indoles under solvent-free conditions
- Cyclodimerization of 3-phenacylideneoxindolines with amino esters for the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines]
- An efficient approach to the synthesis of coumarin-fused dihydropyridinones
- Halogenoheterocyclization of terminally substituted 2-allylthio(seleno)quinolin- 3-carbaldehydes
- A new synthetic route to benzophenone derivatives
- Design, synthesis, docking and in vitro antifungal study of 1,2,4-triazole hybrids of 2-(aryloxy)quinolines
- Synthesis, antimicrobial activity and anti-biofilm activity of novel tetrazole derivatives
Articles in the same Issue
- Frontmatter
- Review
- Chemical and pharmacological research on the plants from genus Ajuga
- Research Articles
- One-pot synthesis of annulated 1,8-naphthyridines
- Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone
- Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids
- Regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole
- A simple one-pot synthesis of 2,4-diaryl- 9H-pyrido[2,3-b]indoles under solvent-free conditions
- Cyclodimerization of 3-phenacylideneoxindolines with amino esters for the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines]
- An efficient approach to the synthesis of coumarin-fused dihydropyridinones
- Halogenoheterocyclization of terminally substituted 2-allylthio(seleno)quinolin- 3-carbaldehydes
- A new synthetic route to benzophenone derivatives
- Design, synthesis, docking and in vitro antifungal study of 1,2,4-triazole hybrids of 2-(aryloxy)quinolines
- Synthesis, antimicrobial activity and anti-biofilm activity of novel tetrazole derivatives

