Abstract
The rearrangement reaction of 1-alkyl-2-(benzoylmethyl)pyridinium iodides 3a–d, 6 in the presence of various nucleophiles leads to 2-alkylaminobenzophenone derivatives 4a–c. Best results were achieved with alkylammonium sulfites as recyclization reagents.
Introduction
2-Aminobenzophenones are usually obtained from N-methylaniline derivatives [1], [2], [3]. Fadda and coworkers [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] studied the recyclization/isomerization of pyridinium salts to anilines in the presence of aqueous alkali, alkylamine or alkylammonium sulphite and observed that the nature and position of substituents in the pyridine ring have a considerable effect on this reaction [7], [8]. The present work was undertaken to study the effect of substituents of picolinium salts 3 on the course of the recyclization reaction.
Results and discussion
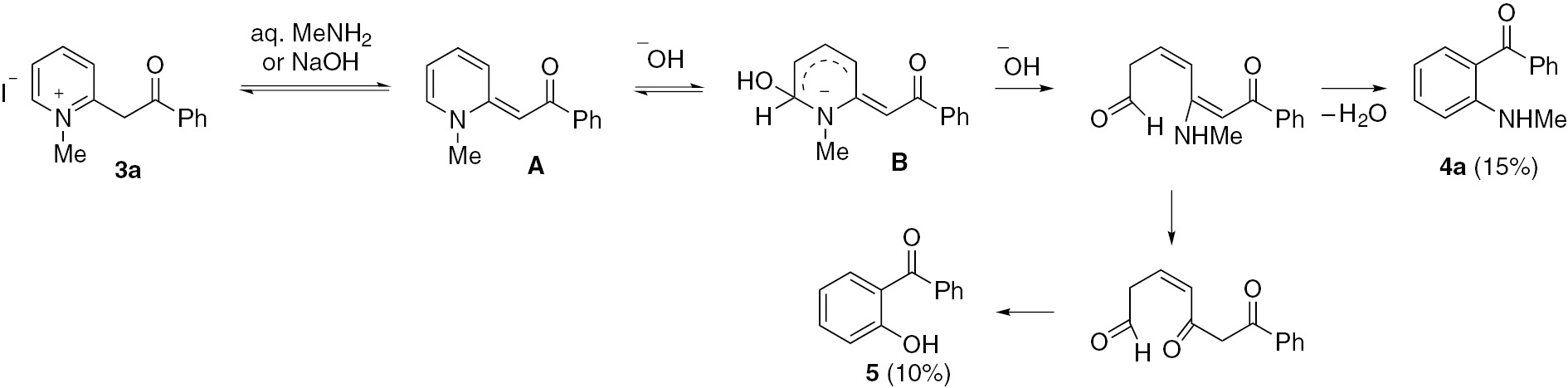
2-Pyridylacetophenones 2 were obtained according to the reported method [14]. Compounds 2 were methylated (Scheme 1) to compounds 3 (Schemes 2 and 3 ), which served as direct precursors to the desired products 4 and 5 with an expected broad spectrum of biological effects [15], [16]. Thus, treatment of compound 3a with methylamine furnished products 4a and 5 in low yields (Scheme 2). It can be suggested that the initially formed anhydrobase A undergoes addition of a hydroxide anion to generate the anionic σ-complex B. After ring opening, the recyclization into a benzene ring takes place. As simple α-picolinium salts do not undergo enamine rearrangement in aqueous ethanolic alkali or aqueous alkylamine [17], the formation of 4a and 5 in the present study indicates that the benzoyl group activates the α-methylene group to undergo the rearrangement. However, in the presence of alkylammonium sulphite as a recyclization reagent, the 2-picolinium iodide 3a undergoes rearrangement to give 2-methylaminobenzophenone (4a) in a much-improved yield of 78% (Scheme 3). The mechanism of 3 is consistent with many reported similar cases [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [18]. The sulphite ion being a weak nucleophile undergoes addition to the softest position C-4 in the pyridine ring to generate a 1,4-dihydropyridine of the type A, which then undergoes ring opening under hydrolytic conditions followed by recyclization with elimination of the reagent, forming the product 4a.
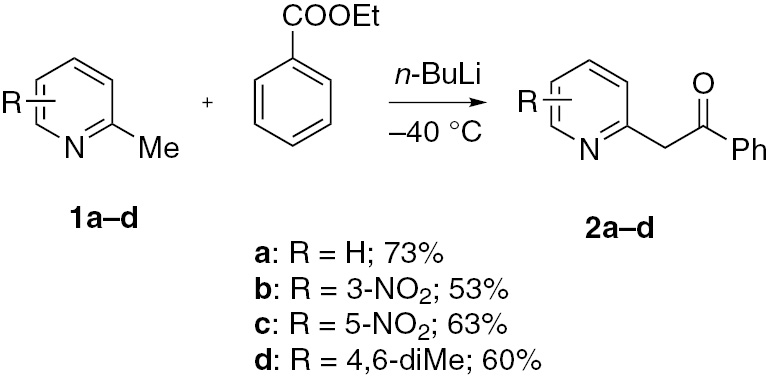

The reaction of N-methylpicolinium iodides 3b and 3c with methylammonium sulphite furnished the expected rearrangement products 4b and 4c in 71% and 68% yields, respectively (Scheme 3).
However, the attempted reaction of compound 3d failed to give 2-methylaminobenzophenone, and instead, the parent 4,6-dimethyl-2-pyridylacetophenone (2d) was obtained, apparently, by direct attack of the nucleophile to the CH3-N bond (SN2 mechanism) (Scheme 3). Alkyl groups R reduce the electron deficiency of the ring and give rise to steric difficulties for the sulphite ion addition. Accordingly, the yield of R-susbstituted recyclization products decrease, particularly with a 4,6-dimethylpyridine derivative.
3-Substituted pyridines are less susceptible to this effect. This suggests a predominant addition of the sulfite ion to the C-4 atom, so that the initial products are the 1,4-dihydropyridines A, which recyclize into aromatic compounds by elimination of the reagent.
The recyclization of unquaternized 2-pyridylacetophenone 2a was also studied [9]. Upon treatment of 2a with aqueous methylammonium sulphite, the amine fragment was exchanged for a methylamine fragment in the open chain intermediate and the product of the recyclization reaction was 4a obtained in a 35% yield (Scheme 4). However, the bulk of the compound 2a was recovered unchanged. There is no evidence for the formation of 2-aminobenzophenone as a product of the recyclization reaction by spectroscopic data. All attempts to obtain the latter compound by treatment of compound 2a with aqueous ammonium sulphite failed.

When zinc chloride was used as a catalyst [8] in the reaction of 2a, the yield of product 4a increased to 51% (Scheme 4). It can be noted that the yield of 4a was invariably less in the presence of aqueous alkylamine than in the presence of methylammonium sulphite as a recyclizing agent. It was also demonstrated that the treatment of N-ethyl-2-picolinium iodide (6, analog of 2a, not shown) with methylammonium sulphite led to the formation of a mixture of 2-methylaminobenzophenone (4a) (60%), 2-hydroxybenzophenone (5) (12%) and 2-pyridylacetophenone (2a) (10%).
Experimental
Melting points are uncorrected. The infrared (IR) spectra were recorded in KBr disks on a Mattson 5000 FTIR spectrometer. The 1H-NMR spectra were determined on a Bruker WPSY 200 MHz spectrometer in DMSO-d6 with tetramethylsilane (TMS) as an internal standard. The electron ionization (EI) mass spectra were recorded at 70 eV with Varian MAT 311. Elemental analyses (C, H and N) were carried out at the Faculty of Science, Cairo University, Egypt. Compounds 2a [14], 2b [14], [19], 2c [18], [20], 2d [21], [22], 4a [23], 4c [24], 5 [25] and 6 [19] were synthesized as previously reported.
General procedure for synthesis of pyridinium salts 3a–d
A mixture of 2-pyridylacetophenone 2a–d (0.02 mol) and methyl iodide (0.03 mol) was heated in a pressure tube at 100°C for 15 h. The resulting solid material was crystallized from ethanol-ether (3:1) as yellow crystals.
1-Methyl-2-(2-oxo-2-phenylethyl)pyridin-1-ium iodide (3a)
Yield 85%; mp 171–173°C; IR: ν 1710 cm−1; 1H-NMR: δ 3.80 (s, 2H, CH2), 4.10 (s, 3H, N-CH3), 7.51–8.63 (m, 9H, Ar-H); MS: m/z 340 (M++1, 40), 339 (M+, 100%). Anal. Calcd for C14H14INO (339.18): C, 49.60; H, 4.20; N, 4.10. Found: C, 49.58; H, 4.20; N, 4.08.
1-Methyl-3-nitro-2-(2-oxo-2-phenylethyl)pyridin-1-ium iodide (3b)
Yield 90%; mp 165–167°C; IR: ν 1695, 1530, 1345 cm−1; 1H-NMR: δ 3.80 (s, 2H, CH2), 4.38 (s, 3H, N-CH3), 7.55–8.61 (m, 8H, Ar-H); MS: m/z 385 (M++1, 15), 384 (M+, 100%). Anal. Calcd for C14H13IN2O3 (384.17): C, 43.77; H, 3.41; N, 7.29. Found: C, 43.71; H, 3.39; N, 7.26.
1-Methyl-5-nitro-2-(2-oxo-2-phenylethyl)pyridin-1-ium iodide (3c)
Yield 80%; mp 148–150°C; IR: ν 1710, 1530, 1345 cm−1; 1H-NMR: δ 3.83 (s, 2H, CH2), 4.20 (s, 3H, N-CH3), 7.45–8.55 (m, 8H, Ar-H); MS: m/z 385 (M++1, 15), 384 (M+, 100%). Anal. Calcd for C14H13IN2O3 (384.17): C, 43.77; H, 3.41; N, 7.29. Found: C, 43.71; H, 3.39; N, 7.26.
1,2,4-Trimethyl-6-(2-oxo-2-phenylethyl)pyridin-1-ium iodide (3d)
Yield 76%; mp 156–158°C; IR: ν 1710 cm−1; 1H-NMR: δ 2.45 (s, 3H, CH3), 2.91 (s, 3H, CH3), 3.81 (s, 2H, CH2), 4.39 (s, 3H, N-CH3), 7.55–8.00 (m, 5H, Ar-H), 7.63 (s, 2H, Ar-H); MS: m/z 378 (M++1, 40), 367 (M+, 100%). Anal. Calcd for C16H18INO (367.23): C, 52.33; H, 4.49; N, 3.81. Found: C, 52.23; H, 4.36; N, 3.70.
Synthesis of 2-methylaminobenzophenone (4a)
Aqueous methylamine (25 mL, 35%) was added to 3a (0.15 mol) and the mixture was heated in a pressure tube at 150°C for 15 h. The products were extracted with ether and the extract was dried (MgSO4), concentrated and subjected to silica gel chromatography, eluting with benzene to give 4a (15%), 5 (10%) and 2a (50%).
Reaction of 3a–d with methylammonium sulphite
Aqueous methylammonium sulphite (33%, 35 mL) was added to the pyridinium salts 3a–d (0.02 mol) and the mixture was heated in a pressure tube at 150°C for 20–30 h. The product was extracted with ether, the extract was dried (MgSO4), concentrated and chromatographed over silica gel using benzene as eluent. Concentration under reduced pressure furnished 4a–c. Product 4a was obtained in a 78% yield.
2-Methylamino-3-nitrobenzophenone (4b)
This compound was obtained from 3b in a 71% yield; mp 115°C; IR: ν 1705, 1530, 1345 cm−1; 1H-NMR: δ 2.64 (s, 3H, N-CH3), 6.72 (s, 1H, NH), 7.31–7.75 (m, 8H, Ar-H); MS: m/z 256 (M+, 100%). Anal. Calcd for C14H12N2O3 (256.22): C, 65.62; H, 4.72; N, 10.93. Found: C, 65.50; H, 4.60; N, 10.80.
2-Methylamino-5-nitrobenzophenone (4c)
This compound was obtained from pyridinium salt 3c in a 68% yield; mp 163°C (Lit. [26] mp 165–166°C); IR: ν 1700, 1530, 1345 cm−1 ; 1H-NMR δ 2.60 (s, 3H, N-CH3), 6.62 (s, 1H, NH), 7.31–7.75 (m, 8H, Ar-H); MS: m/z 256 (M+, 100%).
Ring opening and recyclization of 2a
Method A
Compound 2a (1.98 g, 0.01 mol) was treated with methylammonium sulphite (33%, 25 mL) and the mixture heated in a pressure tube for 60 h at 150°C. After cooling, the mixture was extracted with ether. The extract was dried (MgSO4) and concentrated and the residue was chromatographed over silica gel using benzene-ethyl acetate (4:1) as eluent to give compounds 4a (35%), 5 (15%) and the unreacted 2a (45%).
Method B
A mixture consisting of 2a (0.01 mol), aqueous methylammonium sulphite (33%, 25 mL) and ZnCl2 (2g) was heated in a pressure tube at 150°C for 60 h. Analysis of the products showed that the presence of 4a (51%), 5 (15%) and 2a (30%).
Acknowledgments
The author thanks Prof. Dr. Ahmed A. Fadda, Faculty of Science, Mansoura University, Egypt, for guidance, help and valuable discussion.
References
[1] Katritzky, A. R.; Fan, W.-Q.; Akutagawa, K. Carbon dioxide: a reagent for the simultaneous protection of nucleophilic centers and the activation of alternative locations to electrophilic attack. Part III. A new synthetic method for the ortho-substitution of N-monoalkylanilines. Tetrahedron1986, 42, 4027–4034.10.1016/S0040-4020(01)87558-XSearch in Google Scholar
[2] Wu, Y.; Feng, L.-J.; Lu, X.; Kwong, F.; Luo, H. B. Palladium-catalyzed oxidative C–H bond acylation of N-nitrosoanilines with toluene derivatives: a traceless approach to synthesize N-alkyl-2-aminobenzophenones. Chem. Commun.2014, 50, 15352–15354.10.1039/C4CC07440HSearch in Google Scholar PubMed
[3] Patent; Shionogi and Co., Ltd.; 454160784, 1979, (A1).Search in Google Scholar
[4] Fadda, A. A.; Kost, A. N.; Sagitullin, R. S.; Gromov, S. P. Recyclization of pyridinium salts to anilines. Reaktsionnaya Sposobnost`Azinov SSSR Novosibirsk. 1979, 76–79.Search in Google Scholar
[5] Fadda, A. A.; Kost, A. N.; Sagitullin, R. S. New synthesis of 2-aminobiphenyls: part III. Org. Prep. Proc. Int. 1981, 13, 203–207.10.1080/00304948109356127Search in Google Scholar
[6] Fadda, A. A.; Kost, A. N.; Sagitullin, R. S. New synthesis of 2-aminobiphenyls: part II. Chem. Heterocycl. Compd.1981, 125–127; Chem. Abstr. 1981, 95, 61643v.Search in Google Scholar
[7] Kost, A. N.; Fadda, A. A.; Sagitullin, R. S.; Gromov, S. P.; Petrunina, T. I.; Sharbatyan, P. A. Recyclization of 2-benzylpyrimidine salts to 2-aminobiphenyls. Chem. Heterocycl. Compd.1983, 9, 970–976.10.1007/BF00506883Search in Google Scholar
[8] Fadda, A. A.; Sagitullin, R. S. Enamine rearrangement of 2-benzylpyridinium salts to 2-aminobiphenyls. Indian J. Chem. Sec. B1985, 24B, 707–710.Search in Google Scholar
[9] Fadda, A. A.; Afsah, E. M. Ring opening and reamination of 2-benzylpyridinium salts to 2-aminobiphenyls. Indian J. Chem. Sec. B1985, 24B, 970–971.10.1002/chin.198607178Search in Google Scholar
[10] Fadda, A. A.; El-Morsy, S. S. A new route for the synthesis of 2-aminobiphenyls involving enamine rearrangement of pyridinium salts. Indian J. Chem. Sec. B1988, 27B, 361–363.10.1002/chin.198839150Search in Google Scholar
[11] Fadda, A. A.; Abdelrazek, F. M.; El-Habbal, M. M. Ring opening and transamination of pyridinium salts. Indian J. Chem. Sec. B1986, 25B, 194–195.Search in Google Scholar
[12] Fadda, A. A.; Khalil, A. M.; El-Morsy, S. S.; El-Habbal, M. M. Enamine rearrangement of pyridinium salts: part viii-effect of nucleophilic reagent on pyridinium salts. Indian J. Chem. Sec. B1988, 27B, 266–268.10.1002/chin.198837106Search in Google Scholar
[13] Fadda, A. A.; Hossan, A. Enamine rearrangement of pyridinium salts to indole ring: a combined experimental and molecular modeling study. J. Heterocycl. Chem.2013, 50, 638–644.10.1002/jhet.1629Search in Google Scholar
[14] Muir, C. W.; Kennedy, A. R.; Redmond, J. M.; Watson, A. J. B. Synthesis of functionalised 4H-quinolizin-4-ones via tandem Horner-Wadsworth-Emmons olefination/cyclisation. Org. Biomol. Chem.2013, 11, 3337–3340.10.1039/c3ob40578hSearch in Google Scholar PubMed
[15] Singh, R. K.; Devi, S.; Prasad, D. N. Synthesis, physiocochemical and biological evaluation of 2-amino-5-chlorobenzophenone derivatives as potent skeletal muscle relaxants. Arabian J. Chem.2015, 8, 307–312.10.1016/j.arabjc.2011.11.013Search in Google Scholar
[16] Cortez-Maya, S.; Cortes, C. E.; Hernandez-Ortega, S.; Ramirez, A. T.; Martinez-Garcia, M. Synthesis of 2-aminobenzophenone derivatives and their anticancer activity. Synth. Commun.2012, 42, 46–54.10.1080/00397911.2010.521435Search in Google Scholar
[17] Kost, A. N.; Gromov, S. P.; Sagitullin, R. S. Recyclization of pyridinium salts to anilines. Dokl Akad Nauk SSSR1977, 236, 634–636; Chem. Abstr. 1977, 86, 89519x.Search in Google Scholar
[18] Kost, A. N.; Gromov, S. P.; Sagitullin, R. S. Alkylamino group exchange upon recyclization of pyridinium salts into anilines. Tetrahedron1978, 34, 2213–2216.10.1016/0040-4020(78)89030-9Search in Google Scholar
[19] Kost, A. N.; Sagitullin, R. S.; Gromov, S. P. Recyclization of the pyridine ring under the influence of nucleophiles. Chem. Heterocycl. Compd. 1979, 15, 87–91.10.1007/BF00471207Search in Google Scholar
[20] Melton, T.; Wibberley, D. G. Indolizines. Part IV. Syntheses from 2-acylmethylene-1-benzyl-1,2-dihydropyridine, phenyl-2-picolyl sulphone, and related compounds. J. Chem. Soc. (Section C)1967, 983–988.10.1039/j39670000983Search in Google Scholar
[21] Graser, M.; Kopacka, H.; Wurst, K.; Mueller, T. Structurally diverse pyridyl or quinolyl enolato/enamido metal complexes of Li, Zr, Fe, Co, Ni, Cu and Zn. Inorg. Chem. Acta2013, 401, 38–49.10.1016/j.ica.2013.02.040Search in Google Scholar
[22] Compagnon, P.-L.; Gasquez, F.; Kimny, T. Pyridines. XIV. Acylation de pyridyl-lithioènamidines. Synthèse de Pyridyl-N-Acylènamidines et de Pyridylpyrimidones-4. Bull. Soc. Chim. Belg.1986, 95, 57–64.10.1002/bscb.19860950107Search in Google Scholar
[23] Sugasawa, T.; Toyoda, T.; Adachi, M.; Sasakura, K. Aminohaloborane in organic synthesis. 1. Specific ortho substitution reaction of anilines. J. Am. Chem. Soc.1978, 100, 4842–4852.10.1021/ja00483a034Search in Google Scholar
[24] Sugasawa, T.; Adachi, M.; Toyoda, T.; Sasakura, K. A new simple synthesis of 1,4-benzodiazepines. J. Heterocycl. Chem.1979, 16, 445–448.10.1002/jhet.5570160306Search in Google Scholar
[25] Weng, F.; Wang, C.; Xu, B. Direct C–H bond arylation of 2-hydroxybenzaldehydes with arylboronic acids via ligand-free palladium catalysis. Tetrahedron Lett. 2010, 51, 2593–2596.10.1016/j.tetlet.2010.02.166Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Chemical and pharmacological research on the plants from genus Ajuga
- Research Articles
- One-pot synthesis of annulated 1,8-naphthyridines
- Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone
- Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids
- Regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole
- A simple one-pot synthesis of 2,4-diaryl- 9H-pyrido[2,3-b]indoles under solvent-free conditions
- Cyclodimerization of 3-phenacylideneoxindolines with amino esters for the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines]
- An efficient approach to the synthesis of coumarin-fused dihydropyridinones
- Halogenoheterocyclization of terminally substituted 2-allylthio(seleno)quinolin- 3-carbaldehydes
- A new synthetic route to benzophenone derivatives
- Design, synthesis, docking and in vitro antifungal study of 1,2,4-triazole hybrids of 2-(aryloxy)quinolines
- Synthesis, antimicrobial activity and anti-biofilm activity of novel tetrazole derivatives
Articles in the same Issue
- Frontmatter
- Review
- Chemical and pharmacological research on the plants from genus Ajuga
- Research Articles
- One-pot synthesis of annulated 1,8-naphthyridines
- Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone
- Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids
- Regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole
- A simple one-pot synthesis of 2,4-diaryl- 9H-pyrido[2,3-b]indoles under solvent-free conditions
- Cyclodimerization of 3-phenacylideneoxindolines with amino esters for the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines]
- An efficient approach to the synthesis of coumarin-fused dihydropyridinones
- Halogenoheterocyclization of terminally substituted 2-allylthio(seleno)quinolin- 3-carbaldehydes
- A new synthetic route to benzophenone derivatives
- Design, synthesis, docking and in vitro antifungal study of 1,2,4-triazole hybrids of 2-(aryloxy)quinolines
- Synthesis, antimicrobial activity and anti-biofilm activity of novel tetrazole derivatives

