Abstract
Triethylamine-promoted domino cyclodimerization reaction of 3-phenacylideneoxindolines with amino ester hydrochlorides in acetonitrile afforded substituted dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines] in good yields. The relative configuration of the major diastereoisomers was determined by the X-ray diffraction analysis of three single crystal structures.
Introduction
The spirooxindole core is one of the most privileged heterocyclic rings, which not only exists in several naturally occurring substances, but is also featured in many medicinally relevant compounds with wide applications as antimicrobial and antitumor agents and inhibitors of the human neurokinin receptor [1], [2], [3], [4], [5]. These properties have prompted many efforts toward the multilateral investigations of various spirooxindoline derivatives [6], [7], [8], [9], [10], [11]. Consequently, many efficient synthetic procedures have been developed for the preparation of the diversely structural spirocyclic oxindoles [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. In recent years, carbocyclic dispirooxindole systems have attracted much attention due to their rich molecular diversity and particularly difficult synthetic methodology [24], [25], [26], [27], [28], [29], [30], [31], [32]. Recently, we found that the base-promoted domino reactions of two molecules of 3-phenacylideneoxindolines with nucleophiles such as alcohol, amine and thiols furnish versatile alkoxy- and amino-substituted and methylene- bridged dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines] in satisfactory yields and with high diastereoselectivity [33], [34]. This finding provides a practical method for the construction of a complex polycyclic spirooxindoline system. Thennarasu has also reported a similar base-catalyzed reaction of 3-phenacylideneoxindolines with 2-aminopyridine or 4-aminopyrimidine to give the corresponding 2-pyridylamino- or 4-pyrimidylamino-substituted dispirocyclopentaneoxindolines [35]. To study the scope of this unique reaction and further demonstrate the synthetic value of this protocol, we wish to report the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indoline] with functionalized amino ester derivatives via a base-promoted cyclodimerization reaction of 3-phenacylideneoxindoles with amino ester hydrochlorides.
Results and discussion
According to our previously established reaction conditions [33], a mixture of 1-benzyl-5-chloro-3-(2-oxo-2-(p-tolyl)ethylidene)indolin-2-one (1.0 mmol) and ethyl glycinate hydrochloride (1.0 mmol) in acetonitrile in the presence of piperidine (1.2 mmol) was stirred at room temperature overnight (Table 1). After workup, the expected amino-ester-substituted dispiro[indoline-3,1′-cyclopentane-3′,3″-indoline] 1a was obtained in good yield. Unfortunately, the 1H NMR spectrum clearly showed that the obtained sample was contaminated with a piperidinyl-substituted dispirocyclopentaneoxindoline, which was obviously derived from the participation of piperidine acting as a competitive nucleophile in the reaction. To avoid this phenomenon, triethylamine was chosen as a scavenger of hydrogen chloride and as the base for the catalysis of the cyclodimerization reaction. The reaction proceeded smoothly to give the desired substituted dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines] 1a–d in good yields (Table 1). Under similar conditions, other amino ester hydrochlorides were employed in the reaction. The desired amino-ester-substituted dispirocyclopentaneoxindolines 1e–l were obtained in satisfactory yields. The results are summarized in Table 1. Analysis of 1H nuclear magnetic resonance spectra revealed that the reaction usually results in the formation of a mixture of diastereoisomers, with molecular ratios in the range from 0.81:0.19 to 0.50:0.50, which cannot be easily separated by column or thin-layer chromatography. To determine the relative configuration of the products, single crystal structures of spiro compounds 1b (Figure 1), 1g (not shown) and 1k (Figure 2) were determined by X-ray diffraction. We were pleased to find that molecules in the three single crystals had the same configuration. As can be seen from Figure 1, the two oxindoline scaffolds at the 1,3-positions are in trans orientation in the newly formed cyclopentyl ring, while the 2-benzoyl group and the 5-aryl group are cis-oriented to each other. The 4-amino ester and 5-hydroxyl group are also in cis orientation. On the basis of 1H NMR spectra and single crystal structures, it can be concluded that all major products 1a–l have this relative configuration. It should be pointed out that this relative configuration of the major disatereoisomer is also the same as that of our previously reported alkoxy- and amino-substituted dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines] [33]. This analysis indicates that the diastereoselectivity of the base-promoted cyclodimerization of 3-phenacylideneoxindole with various nucleophiles is controlled by molecular effects.
Synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines] 1a–j.a

| Compd | R1 | R2 | Ar | R3 | R4 | Yield (%)b |
|---|---|---|---|---|---|---|
| 1a | Cl | Bn | p-CH3C6H4 | H | C2H5 | 64 |
| 1b | Cl | n-Bu | p-CH3OC6H4 | H | C2H5 | 55 |
| 1c | Cl | Bn | p-ClC6H4 | H | C2H5 | 53 |
| 1d | F | Bn | p-CH3C6H4 | H | C2H5 | 62 |
| 1e | Cl | Bn | p-CH3C6H4 | HOCH2 | C2H5 | 65 |
| 1f | F | Bn | p-CH3C6H4 | CH3 | C2H5 | 72 (0.81:0.19) |
| 1g | Cl | Bn | p-CH3C6H4 | CH3 | C2H5 | 70 (0.52:0.48) |
| 1h | Cl | Bn | p-CH3C6H4 | iso-Pr | C2H5 | 63 (0.61:0.39). |
| 1i | Cl | Bn | p-CH3C6H4 | sec-Bu | C2H5 | 61 (0.65:0.35) |
| 1j | Cl | Bn | p-CH3C6H4 | p-HOC6H4CH2 | C2H5 | 67 (0.5:0.5) |
| 1k | Cl | Bn | p-CH3C6H4 | C6H5CH2CH2 | C2H5 | 62 (0.6:0.4) |
| 1l | Cl | Bn | p-CH3C6H4 | 3-indolyl-CH2 | CH3 | 70 (0.61:0.39) |
aReaction conditions: 3-phenacylideneoxindoline (1.0 mmol), amino ester hydrochloride (0.6 mmol) and Et3N (1.2 mmol) in CH3CN (10 mL), rt, 12 h.
bIsolated yields, the ratio of diastereomers was detemined by 1H NMR spectra.

ORTEP drawing of the crystal structure of compound 1b (major).
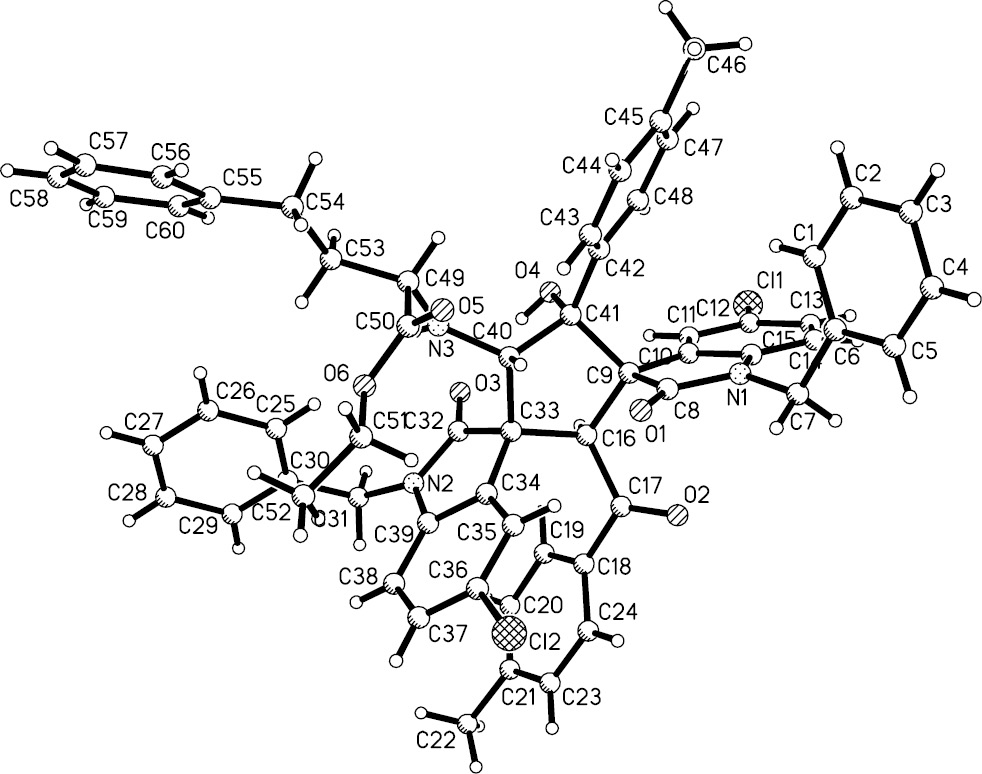
ORTEP drawing of the crystal structure of compound 1k (major).
A plausible reaction mechanism for products 1 is presented in Scheme 1. In the presence of triethylamine, the amino ester hydrochloride is converted into the free amino ester. Then, the Michael addition of the free amino ester to 3-phenacylideneoxindole generates an adduct A, which, in turn, undergoes addition to the second molecule of 3-phenacylideneoxindole to give a double adduct B. Then, the carbanionic center of B undergoes an intramolecular nucleophilic addition to the carbonyl group to afford a cyclic intermediate product C, which is the final precursor to product 1. In the cyclization step, the most stable diastereoisomer would be preferentially formed as the major isomer, as observed.

Proposed mechanism for formation of products 1.
Conclusions
An efficient synthetic protocol for the synthesis of compounds 1 by a base-mediated reaction of two molecules of 3-phenacylideneoxindole with amino ester hydrochloride is described. The reaction uses readily available starting materials, proceeds under mild conditions and the products are obtained in good yields and with high diastereoselectivity.
Experimental
The 1H NMR and 13C NMR spectra were obtained in DMSO-d6 at 600 MHz and 150 MHz, respectively. The IR spectra were obtained using KBr pellets.
General procedure for the preparation of dispirocyclopentanebisoxindolines 1a–l
A solution of 3-phenacylideneoxindoline (1.0 mmol), amino ester hydrochlorides (0.6 mmol) and triethylamine (1.2 mmol) in acetonitrile (10 mL) was stirred at room temperature for 12 h. The resulting precipitate was collected by filtration and washed with cold ethanol to give analytically pure products 1a–l.
Ethyl (1,1″-dibenzyl-5,5″-dichloro-4′-hydroxy-2′-(4-methylbenzoyl)- 2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)glycinate (1a)
White solid; yield 64%; mp 244–246°C; 1H NMR: δ 8.11 (d, J=2.4 Hz, 1H, ArH), 8.00 (d, J=1.8 Hz, 1H, ArH), 7.45–7.44 (m, 2H, ArH), 7.32–7.29 (m, 3H, ArH), 7.21–7.18 (m, 2H, ArH), 7.13–7.11 (m, 2H, ArH), 7.09–7.06 (m, 4H, ArH), 7.05 (brs, 1H, ArH), 6.95 (d, J=8.4 Hz, 2H, ArH), 6.91 (d, J=8.4 Hz, 2H, ArH), 6.61 (d, J=7.2 Hz, 2H, ArH), 6.58 (s, 1H, OH), 6.53 (d, J=8.4 Hz, 1H, ArH), 6.41 (d, J=8.4 Hz, 1H, ArH), 5.55 (d, J=9.0 Hz, 1H, CH), 5.36 (s, 1H, CH), 5.16 (d, J=15.6 Hz, 1H, CH), 4.98 (d, J=16.2 Hz, 1H, CH), 4.50 (d, J=16.2 Hz, 1H, CH), 4.37 (d, J=15.6 Hz, 1H, CH), 3.85 (q, J=7.2 Hz, 2H, CH2), 2.97–2.94 (m, 1H, CH), 2.87–2.84 (m, 1H, CH), 2.36–2.32 (m, 1H, NH), 2.28 (s, 3H, CH3), 2.23 (s, 3H, CH3), 1.00 (t, J=7.2 Hz, 3H, CH3); 13C NMR: δ 196.0, 179.3, 176.2, 170.4, 143.2, 142.2, 141.0, 136.8, 135.4, 135.1, 134.1, 133.7, 132.6, 128.6, 128.5, 128.3, 128.2, 128.1, 128.0, 127.9, 127.6, 127.3, 127.0, 126.8, 126.7, 126.5, 126.3, 125.6, 125.5, 123.5, 110.0, 109.6, 84.7, 69.9, 64.6, 62.3, 60.0, 59.2, 48.8, 43.9, 43.0, 21.1, 20.8, 13.8; IR: υ 3331, 3067, 2922, 1712, 1686, 1608, 1486, 1431, 1375, 1346, 1269, 1179, 1083, 1033, 936, 809, 735, 694 cm−1. HRMS (ESI). Calcd. for C52H45Cl2N3NaO6 ([M+Na]+): m/z 900.2578. Found: m/z 900.2571.
Ethyl 1,1″-dibutyl-5,5″-dichloro-4′-hydroxy-2′-(4-methoxybenzoyl)- 4′-(4-methoxyphenyl)-2,2″-dioxodispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)glycinate (1b)
White solid; yield 55%; mp 176–178°C; 1H NMR: δ 8.05 (brs, 1H, ArH), 7.85 (brs, 1H, ArH), 7.31 (dd, J1=7.8 Hz, J2=1.2 Hz, 1H, ArH), 7.15–7.13 (m, 3H, ArH), 6.95 (d, J=8.4 Hz, 2H, ArH), 6.82 (d, J=8.4 Hz, 1H, ArH), 6.73–6.70 (m, 3H, ArH), 6.63 (d, J=8.4 Hz, 2H, ArH), 6.41 (s, 1H, OH), 5.49 (d, J=8.4 Hz, 1H, ArH), 5.16 (s, 1H, CH), 3.88–3.82 (m, 3H, 3CH), 3.72 (s, 3H, OCH3), 3.64 (brs, 4H, CH, OCH3), 3.37–3.35 (m, 1H, CH), 3.22–3.20 (m, 1H, CH), 3.03–2.93 (m, 2H, CH), 2.23–2.20 (m, 1H, NH), 1.57–1.51 (m, 1H, CH), 1.47–1.41 (m, 1H, CH), 1.37–1.30 (m, 2H, CH), 1.02 (t, J=6.6 Hz, 3H, CH3), 0.98–0.95 (m, 1H, CH), 0.92–0.84 (m, 5H, 5CH), 0.80–0.78 (m, 1H, CH), 0.75–0.72 (m, 3H, 3CH); 13C NMR: δ 195.8, 179.5, 176.3, 170.4, 158.8 (d, J=240.0 Hz), 157.8 (d, J=234.8 Hz), 143.2, 139.6, 138.3, 136.7, 135.6, 135.2, 134.1, 133.6, 132.5 (d, J=9.3 Hz), 128.6, 128.5, 128.4, 128.1, 127.9, 127.8, 127.6, 127.0, 126.8, 126.7, 126.5, 114.6 (d, J=19.4 Hz), 114.5 (d, J=19.8 Hz), 114.1 (d, J=25.1 Hz), 113.4 (d, J=26.3 Hz), 109.5, 108.9, 108.8, 84.8, 69.9, 64.7, 62.2, 60.0, 59.4, 48.8, 43.9, 43.1, 21.1, 20.8, 13.8; IR: υ 3370, 3261, 3069, 2958, 1744, 1684, 1604, 1484, 1431, 1351, 1257, 1180, 1115, 1030, 977, 811, 735, 675 cm−1. HRMS (ESI). Calcd. for C46H49Cl2N3NaO8 ([M+Na]+): m/z 864.2789. Found: m/z 864.2795.
Ethyl (1,1″-dibenzyl-5,5″-dichloro-2′-(4-chlorobenzoyl)-4′-(4-chlorophenyl)-4′-hydroxy-2,2″-dioxodispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)glycinate (1c)
White solid; yield 53%; mp 224–226°C; 1H NMR: δ 8.37 (s, 1H, ArH), 8.10 (s, 1H, ArH), 7.40 (d, J=8.4 Hz, 1H, ArH), 7.24–7.23 (m, 7H, ArH), 7.20–7.16 (m, 6H, ArH), 7.06–7.05 (m, 4H, ArH), 6.78 (d, J=7.8 Hz, 1H, ArH), 6.73 (s, 1H, OH), 6.61–6.60 (m, 2H, ArH), 6.41 (d, J=8.4 Hz, 1H, ArH), 5.54 (d, J=12.6 Hz, 1H, CH), 5.31 (s, 1H, CH), 4.86 (d, J=16.2 Hz, 1H, CH), 4.41 (d, J=16.2 Hz, 1H, CH), 4.33 (d, J=15.6 Hz, 1H, CH), 3.98 (d, J=15.6 Hz, 1H, CH), 3.86 (m, 2H, CH), 3.09–3.05 (m, 1H, CH), 2.74 (d, J=15.0 Hz, 1H, CH), 1.86 (brs, 1H, NH), 1.03 (t, J=6.6 Hz, 3H, CH3); 13C NMR: δ 196.4, 177.2, 174.5, 170.6, 143.0, 142.9, 138.1, 138.0, 136.4, 135.8, 135.6, 133.0, 132.1, 130.3, 130.0, 129.2, 129.0, 128.9, 128.7, 128.6, 128.5, 128.1, 127.9, 127.8, 127.5, 127.3, 127.2, 126.9, 125.6, 110.1, 109.2, 84.6, 70.6, 65.1, 62.7, 60.4, 59.0, 48.4, 43.5, 43.3, 14.3; IR: υ 3407, 3072, 2982, 2899, 1733, 1700, 1602, 1486, 1433, 1341, 1268, 1205, 1165, 1092, 1018, 974, 812, 738, 699 cm−1. HRMS (ESI). Calcd. for C50H39Cl4N3NaO6 ([M+Na]+): m/z 940.1485. Found: m/z 940.1476.
Ethyl (1,1″-dibenzyl-5,5″-difluoro-4′-hydroxy-2′-(4-methylbenzoyl)- 2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)glycinate (1d)
White solid; yield 62%; mp 248–250°C; 1H NMR: δ 7.95 (dd, J1=9.6 Hz, J2=2.4 Hz, 1H, ArH), 7.82 (dd, J1=8.4 Hz, J2=1.8 Hz, 1H, ArH), 7.44–7.43 (m, 2H, ArH), 7.30–7.29 (m, 3H, ArH), 7.20–7.17 (m, 1H, ArH), 7.14–7.09 (m, 4H, ArH), 7.06–7.05 (m, 2H, ArH), 6.99–6.96 (m, 1H, ArH), 6.95–6.92 (m, 4H, ArH), 6.86 (m, 1H, ArH), 6.64 (d, J=7.8 Hz, 2H, ArH), 6.60 (s, 1H, OH), 6.52–6.50 (m, 1H, ArH), 6.40–6.37 (m, 1H, ArH), 5.57 (d, J=8.4 Hz, 1H, CH), 5.33 (s, 1H, CH), 5.18 (d, J=15.6 Hz, 1H, CH), 4.97 (d, J=16.2 Hz, 1H, CH), 4.49 (d, J=16.2 Hz, 1H, CH), 4.34 (d, J=15.6 Hz, 1H, CH), 3.87–3.84 (m, 2H, CH), 2.98–2.95 (m, 1H, CH), 2.86–2.83 (m, 1H, CH), 2.34–2.31 (m, 1H, NH), 2.28 (s, 3H, CH3), 2.24 (s, 3H, CH3), 0.99 (t, J=7.2 Hz, 3H, CH3); 13C NMR: δ 195.8, 179.5, 176.3, 170.4, 159.6, 158.0, 157.0, 143.2, 139.6, 138.3, 136.7, 135.6, 135.2, 134.1, 133.6, 132.5, 132.4, 128.6, 128.5, 128.4, 128.1, 127.9, 127.8, 127.6, 127.0, 126.8, 126.7, 126.5, 114.7, 114.5, 114.4, 114.2, 114.0, 113.5, 113.3, 109.5, 108.9, 108.8, 84.8, 69.9, 64.7, 62.2, 60.0, 59.4, 48.8, 43.9, 43.1, 21.1, 20.8, 13.8; IR: υ 3264, 3063, 2919, 1742, 1692, 1610, 1489, 1452, 1379, 1344, 1266, 1219, 1178, 1029, 984, 940, 898, 810, 743, 693 cm−1. HRMS (ESI). Calcd. for C52H45F2N3NaO6 ([M+Na]+): m/z 868.3169. Found: m/z 868.3167.
Ethyl (1,1″-dibenzyl-5,5″-dichloro-4′-hydroxy-2′-(4-methylbenzoyl)- 2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)serinate (1e)
White solid; yield 65%; mp 239–241°C; 1H NMR: δ 8.17 (s, 1H, ArH), 7.68 (s, 1H, ArH), 7.58 (d, J=7.2 Hz, 2H, ArH), 7.39–7.37 (m, 4H, ArH), 7.33–7.31 (m, 1H, ArH), 7.23–7.21 (m, 1H, ArH), 7.16–7.11 (m, 4H, ArH), 7.03 (dd, J1=7.2 Hz, J2=1.8 Hz, 1H, ArH), 6.96 (d, J=8.4 Hz, 4H, ArH), 6.93 (d, J=8.4 Hz, 1H, ArH), 6.87 (d, J=8.4 Hz, 2H, ArH), 6.61 (d, J=7.2 Hz, 2H, ArH, OH), 6.25 (d, J=7.8 Hz, 1H, ArH), 5.25 (d, J=16.2 Hz, 1H, CH), 5.13 (d, J=16.2 Hz, 1H, CH), 5.06–5.05 (m, 2H, CH, OH), 4.67 (d, J=15.6 Hz, 1H, CH), 3.88 (q, J=7.2 Hz, 2H, CH), 3.83 (d, J=16.2 Hz, 1H, CH), 3.55–3.52 (m, 1H, CH), 3.05–3.03 (m, 2H, CH), 2.77–2.74 (m, 1H, CH), 2.32 (t, J=6.0 Hz, 1H, NH), 2.27 (s, 3H, CH3), 2.16 (s, 3H, CH3), 1.09 (t, J=6.6 Hz, 3H, CH3); 13C NMR: δ 195.5, 180.1, 177.7, 143.2, 141.9, 141.3, 136.7, 135.5, 135.4, 134.8, 133.6, 133.1, 128.8, 128.6, 128.5, 128.2, 128.0, 127.8, 127.7, 127.5, 127.3, 127.2, 127.1, 127.0, 126.6, 125.7, 122.6, 111.1, 109.5, 87.0, 68.0, 65.6, 63.4, 63.0, 60.6, 60.4, 60.1, 43.9, 43.5, 20.9, 20.7, 13.8; IR: υ 3439, 3072, 2989, 1713, 1678, 1606, 1557, 1486, 1432, 1375, 1347, 1244, 1184, 1025, 977, 806, 735, 692 cm−1. HRMS (ESI). Calcd. for C53H48Cl2N3O7 ([M+H]+): m/z 908.2864. Found: m/z 908.2872.
Ethyl (1,1″-dibenzyl-5,5″-difluoro-4′-hydroxy-2′-(4-methylbenzoyl)- 2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)alaninate (1f)
White solid; yield 72%; mp 224–226°C; 1H NMR: δ (major isomer) 8.25 (s, 1H, ArH), 7.59–7.58 (m, 3H, ArH), 7.35–7.33 (m, 2H, ArH), 7.30–7.28 (m, 1H, ArH), 7.26–7.23 (m, 1H, ArH), 7.22–7.20 (m, 1H, ArH), 7.15–7.09 (m, 5H, ArH), 7.00–6.96 (m, 4H, ArH), 6.94–6.92 (m, 1H, ArH), 6.91–6.88 (m, 2H, ArH), 6.83–6.80 (m, 1H, ArH), 6.59–6.55 (m, 2H, ArH, OH), 6.23–6.19 (m, 1H, ArH), 5.35 (d, J=15.6 Hz, 1H, CH), 5.08 (brs, 1H, CH), 5.03 (d, J=16.2 Hz, 1H, CH), 4.89 (d, J=6.0 Hz, 1H, CH), 4.70–4.66 (m, 1H, CH), 3.87 (q, J=7.2 Hz, 2H, CH2), 3.81–3.78 (m, 1H, CH), 2.95–2.91 (m, 1H, CH), 2.35–2.33 (m, 1H, NH), 2.27 (s, 3H, CH3), 2.17 (s, 3H, CH3), 1.05 (t, J=7.2 Hz, 3H, CH3), 0.63 (t, J=6.6 Hz, 3H, CH3); δ (minor isomer) 8.22 (s, 1H, ArH), 7.66–7.65 (m, 1H, ArH), 5.19 (d, J=16.2 Hz, 1H, CH), 5.12–5.10 (m, 1H, CH), 4.94–4.92 (m, 1H, CH), 3.81–3.78 (m, 1H, CH), 3.75–3.72 (m, 1H, CH), 3.05–3.03 (m, 1H, CH), 2.28 (s, 3H, CH3), 2.00–1.98 (m, 1H, NH), 1.01 (t, J=7.2 Hz, 3H, CH3), 0.86 (t, J=6.6 Hz, 3H, CH3); ratio of isomers=0.81:0.19; 13C NMR: δ 195.4, 190.4, 180.5, 177.8, 173.4, 166.8, 159.7, 158.1, 156.8, 145.0, 143.2, 141.1, 139.2, 138.7, 136.7, 135.8, 135.6, 135.5, 135.0, 134.4, 133.6, 129.7, 128.8, 128.7, 128.5, 128.4, 128.1, 127.7, 127.6, 127.5, 127.3, 127.2, 127.1, 127.0, 126.9, 126.6, 123.4, 120.4, 118.9, 118.7, 115.6, 115.4, 115.1, 115.0, 114.5, 114.4, 113.6, 113.4, 110.6, 110.5, 110.4, 108.9, 108.8, 86.9, 69.2, 65.8, 62.7, 61.1, 60.2, 59.9, 54.4, 49.4, 43.7, 43.6, 43.0, 21.3, 20.9, 20.7, 19.8, 13.7; IR: υ 3438, 3175, 2977, 2926, 1704, 1678, 1614, 1492, 1451, 1375, 1350, 1265, 1180, 1032, 983, 814, 741, 695 cm−1. HRMS (ESI). Calcd. for C53H48F2N3NaO6 ([M+Na]+): m/z 860.3506. Found: m/z 860.3537.
Ethyl (1,1″-dibenzyl-5,5″-dichloro-4′-hydroxy-2′-(4-methylbenzoyl)- 2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)alaninate (1g)
White solid; yield 70%; mp 236–238°C; 1H NMR: δ (major isomer) 8.06 (s, 1H, ArH), 7.47 (brs, 2H, ArH), 7.31 (brs, 3H, ArH), 7.18–7.05 (m, 10H, ArH), 6.97–6.92 (m, 5H, ArH), 6.65–6.61 (m, 3H, ArH, OH), 6.39 (t, J=9.0, 1H, ArH), 5.58–5.54 (m, 1H, CH), 5.34 (s, 1H, CH), 5.06 (d, J=15.6 Hz, 1H, CH), 4.98 (d, J=16.2 Hz, 1H, CH), 4.55–4.49 (m, 1H, CH), 4.45 (d, J=16.2 Hz, 1H, CH), 3.85–3.78 (m, 2H, CH), 3.61–3.56 (m, 1H, CH), 2.32–2.31 (m, 1H, NH), 2.27 (s, 3H, CH3), 2.24 (s, 2H, CH), 2.20 (s, 1H, CH3), 1.02–1.00 (m, 1H, CH), 0.93–0.91 (m, 2H, CH), 0.85 (d, J=5.4 Hz, 2H, CH), 0.56 (d, J=6.0 Hz, 1H, CH); δ (minor isomer) 8.14 (s, 1H, ArH), 8.01–7.99 (m, 2H, ArH), 6.85–6.84 (m, 2H, ArH), 6.65–6.61 (m, 1H, ArH), 6.50–6.49 (m, 1H, ArH), 5.14 (d, J=15.0 Hz, 1H, CH), 4.40 (d, J=15.6 Hz, 1H, CH), 3.70–3.67 (m, 1H, CH), 3.04–3.03 (m, 1H, CH), 2.80–2.78 (m, 1H, NH), 2.37–2.35 (m, 1H, CH), 2.20 (s, 1H, CH), 1.02–1.00 (m, 1H, CH), 0.56 (d, J=6.0 Hz, 1H, CH); ratio of isomers=0.52:0.48; 13C NMR: δ 196.0, 195.9, 179.3, 179.0, 176.4, 176.1, 173.1, 173.0, 143.2, 143.1, 142.1, 142.0, 141.4, 141.0, 136.9, 136.8, 135.3, 135.2, 135.0, 134.2, 133.7, 133.6, 133.5, 132.4, 132.3, 128.6, 128.5, 128.4, 128.2, 128.1, 128.0, 127.9, 127.8, 127.7, 127.4, 127.3, 127.0, 126.9, 126.8, 126.6, 126.5, 126.4, 126.3, 126.2, 125.6, 125.5, 125.4, 110.1, 109.8, 109.6, 85.0, 84.7, 69.1, 68.3, 64.6, 64.5, 62.4, 61.9, 60.2, 60.0, 59.5, 59.1, 54.4, 53.5, 44.0, 43.7, 43.0, 21.1, 21.0, 20.8, 20.7, 18.9, 17.9, 13.7, 13.6; IR: υ 3278, 3069, 2979, 1711, 1698, 1608, 1484, 1435, 1358, 1237, 1180, 1088, 1030, 988, 944, 807, 732, cm−1. HRMS (ESI). Calcd. for C53H47Cl2N3NaO6 ([M+Na]+): m/z 914.2734. Found: m/z 914.2740.
Ethyl (1,1″-dibenzyl-5,5″-dichloro-4′-hydroxy-2′-(4-methylbenzoyl)- 2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)valinate (1h)
White solid; yield 63%; mp 254–256°C 1H NMR: δ (major isomer) 8.09 (brs, 1H, ArH), 8.02–8.00 (m, 1H, ArH), 7.53–7.49 (m, 2H, ArH), 7.39–7.35 (m, 3H, ArH), 7.21–7.13 (m, 4H, ArH), 7.07–7.03 (m, 5H, ArH), 6.96 (d, J=7.8 Hz, 2H, ArH), 6.75–6.69 (m, 4H, ArH), 6.67–6.66 (m, 1H, ArH), 6.61–6.60 (m, 1H, OH), 6.38 (d, J=8.4 Hz, 1H, ArH), 5.53 (d, J=10.2 Hz, 1H, CH), 5.32–5.30 (m, 1H, CH), 4.98–4.91 (m, 2H, CH), 4.63–4.52 (m, 2H, CH), 3.70–3.52 (m, 2H, CH), 2.78–2.76 (m, 1H, CH), 2.27–2.23 (m, 4H, NH, CH3), 2.16 (s, 3H, CH3), 1.60–1.54 (m, 1H, CH), 0.97–0.87 (m, 3H, 3CH), 0.50 (d, J=6.6 Hz, 3H, CH3), 0.41 (d, J=7.2 Hz, 3H, CH3); δ (minor isomer) 7.21–7.13 (m, 2H, ArH), 7.10–7.09 (m, 2H, ArH), 6.91 (d, J=7.8 Hz, 2H, ArH), 5.49 (d, J=9.0 Hz, 1H, CH), 2.66–2.64 (m, 1H, CH), 2.17 (s, 3H, CH3), 1.34–1.29 (m, 1H, CH), 0.36 (d, J=6.6 Hz, 3H, CH3), 0.29 (d, J=7.2 Hz, 3H, CH3); ratio of isomers=3:2; 13C NMR: δ 196.4, 180.2, 177.0, 173.2, 172.9, 143.5, 142.6, 142.1, 137.2, 135.9, 135.5, 134.2, 134.1, 132.8, 129.2, 129.1, 129.0, 128.9, 128.8, 128.7, 128.6, 128.5, 128.3, 128.2, 128.1, 127.8, 127.5, 127.4, 127.3, 127.1, 127.0, 126.8, 126.1, 125.7, 110.7, 110.1, 85.5, 85.3, 70.2, 65.7, 65.5, 65.4, 65.2, 62.3, 60.4, 60.3, 59.9, 59.8, 44.8, 43.6, 32.2, 30.7, 21.5, 21.2, 18.8, 18.4, 18.2, 18.1, 14.3, 14.2; IR: υ 3273, 2965, 1711, 1684, 1609, 1485, 1432, 1354, 1260, 1180, 1081, 1029, 979, 810, 735, 696 cm−1. HRMS (ESI). Calcd. for C55H51Cl2N3NaO6 ([M+Na]+): m/z 942.3047. Found: m/z 942.3054.
Ethyl 2-((1,1″-dibenzyl-5,5″-dichloro-4′-hydroxy-2′-(4-methylbenzoyl)-2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)amino)-3-methylpentanoate (1i)
White solid, 61%; mp 246–248°C; 1H NMR: δ (major isomer) 8.10–8.09 (m, 1H, ArH), 8.03–8.02 (m, 1H, ArH), 7.48 (brs, 2H, ArH), 7.39–7.35 (m, 3H, ArH), 7.21–7.16 (m, 2H, ArH), 7.14–7.09 (m, 3H, ArH), 7.04–7.02 (m, 4H, ArH), 6.96–6.95 (m, 2H, ArH), 6.74–6.71 (m, 3H, ArH), 6.68–6.66 (m, 2H, ArH), 6.62 (d, J=8.4 Hz, 1H, ArH), 6.39–6.37 (m, 1H, OH), 5.55 (d, J=10.2 Hz, 1H, CH), 5.33 (s, 1H, CH), 4.99–4.92 (m, 2H, CH), 4.62–4.52 (m, 2H, CH), 3.73–3.69 (m, 1H, CH), 3.68–3.62 (m, 1H, CH), 2.86–2.85 (m, 1H, CH), 2.32–2.29 (m, 1H, NH), 2.27 (s, 3H, CH3), 2.17 (s, 3H, CH3), 1.42–1.38 (m, 1H, CH), 0.94 (t, J=7.2 Hz, 3H, CH3), 0.84 (t, J=7.2 Hz, 2H, CH), 0.49–0.46 (m, 3H, CH3), 0.41 (t, J=7.2 Hz, 3H, CH3); δ (minor isomer) 8.06–8.05 (m, 1H, ArH), 8.00 (brs, 1H, ArH), 7.55–7.54 (m, 2H, ArH), 6.93–6.91 (m, 2H, ArH), 6.76 (d, J=8.4 Hz, 1H, ArH), 6.68–6.66 (m, 2H, ArH), 5.45–5.43 (m, 1H, CH), 5.30 (s, 1H, CH), 3.59–3.54 (m, 1H, CH), 2.74–2.72 (m, 1H, CH), 2.26 (s, 3H, CH3), 2.16 (s, 3H, CH3), 0.94 (t, J=7.2 Hz, 2H, CH), 0.63–0.58 (m, 1H, CH), 0.28 (d, J=6.6 Hz, 3H, CH3); ratio of isomers=0.65:0.35; 13C NMR: δ 195.9, 195.8, 179.6, 176.4, 176.3, 172.3, 172.2, 143.0, 142.1, 141.5, 136.8, 135.4, 135.0, 133.8, 133.7, 133.6, 133.4, 132.4, 128.9, 128.7, 128.6, 128.5, 128.4, 128.2, 128.1, 128.0, 127.8, 127.7, 127.6, 127.5, 127.4, 126.9, 126.8, 126.7, 126.6, 126.5, 126.3, 125.7, 125.6, 125.3, 110.2, 110.1, 109.6, 84.9, 71.2, 68.9, 64.9, 64.6, 64.3, 62.3, 62.0, 61.8, 59.9, 59.8, 59.5, 59.3, 44.3, 43.1, 36.4, 25.1, 24.6, 21.0, 20.7, 14.5, 14.2, 13.8, 13.7, 11.3, 10.5; IR: υ 3436, 3072, 2968, 1710, 1685, 1610, 1486, 1432, 1371, 1236, 1180, 1080, 1029, 980, 811, 735, 693 cm−1. HRMS (ESI). Calcd. for C56H54Cl2N3O6 ([M+H]+): m/z 934.3384. Found: m/z 934.3396.
Ethyl (1,1″-dibenzyl-5,5″-dichloro-4′-hydroxy-2′-(4-methylbenzoyl)-2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)tyrosinate (1j)
White solid; yield 67%, mp 259–261°C; 1H NMR: δ (major isomer) 9.23–9.11 (m, 1H, OH), 8.02–7.97 (m, 2H, ArH), 7.56 (brs, 1H, ArH), 7.52 (brs, 1H, ArH), 7.38–7.35 (m, 3H, ArH), 7.19–7.11 (m, 5H, ArH), 7.04 (brs, 3H, ArH), 6.99 (m, 2H, ArH), 6.92 (brs, 1H, ArH), 6.78 (brs, 1H, ArH), 6.71–6.67 (m, 3H, ArH), 6.62 (brs, 2H, ArH), 6.44 (brs, 2H, ArH), 6.38–6.35 (m, 3H, ArH, OH), 5.57–5.54 (m, 1H, CH), 5.33–5.29 (m, 1H, CH), 5.01–4.93 (m, 2H, CH), 4.57–4.49 (m, 2H, CH), 3.64–3.55 (m, 1H, CH), 3.25 (brs, 1H, CH), 3.04–2.98 (m, 1H, CH), 2.80 (brs, 1H, CH), 2.43–2.37 (m, 1H, NH), 2.30–2.27 (m, 3H, 3CH), 2.17 (brs, 3H, 3CH), 1.14 (brs, 1H, CH), 0.83–0.78 (m, 3H, 3CH); δ (minor isomer) 8.10 (brs, 1H, ArH), 6.52 (brs, 1H, ArH), 2.75–2.74 (m, 1H, CH); ratio of isomers=1:1. 13C NMR: δ 195.9, 195.8, 179.6, 179.5, 176.4, 176.2, 172.4, 172.3, 162.3, 155.7, 143.0, 142.2, 142.0, 141.6, 141.2, 136.9, 136.8, 135.4, 135.3, 134.9, 133.8, 133.6, 133.5, 132.4, 132.2, 129.6, 129.4, 128.9, 128.7, 128.5, 128.4, 128.3, 128.1, 128.0, 127.9, 127.8, 127.5, 127.4, 127.2, 127.0, 126.9, 126.8, 126.6, 126.5, 126.3, 126.2, 126.1, 125.6, 125.3, 114.9, 114.7, 110.2, 110.0, 109.6, 85.0, 84.8, 70.0, 69.5, 64.8, 64.7, 62.1, 61.6, 61.4, 61.1, 59.9, 59.8, 59.6, 59.1, 45.5, 44.4, 43.8, 43.0, 37.4, 35.7, 21.0, 13.6, 13.5; IR: υ 3433, 3208, 2933, 1708, 1682, 1608, 1513, 1485, 1434, 1363, 1333, 1244, 1181, 1108, 1032, 978, 885, 811, 737, 692 cm−1. HRMS (ESI). Calcd. for C59H52Cl2N3O7 ([M+H]+): m/z 984.3177. Found: m/z 984.3184.
Ethyl 2-((1,1″-dibenzyl-5,5″-dichloro-4′-hydroxy-2′-(4-methylbenzoyl)-2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)amino)-4-phenylbutanoate (1k)
White solid; yield 62%; mp 216–218°C; 1H NMR: δ (major isomer) 8.10 (brs, 1H, ArH), 8.00 (brs, 1H, ArH), 7.51–7.47 (m, 2H, ArH), 7.32–7.26 (m, 3H, ArH), 7.21–7.16 (m, 3H, ArH), 7.14–7.09 (m, 5H, ArH), 7.07–7.05 (m, 4H, ArH), 6.93–6.90 (m, 2H, ArH), 6.87–6.85 (m, 2H, ArH), 6.79–6.78 (m, 2H, ArH), 6.69–6.64 (m, 3H, ArH), 6.53 (d, J=8.4 Hz, 1H, ArH), 6.38–6.37 (m, 1H, OH), 5.56 (d, J=10.2 Hz, 1H, CH), 5.34 (s, 1H, CH), 5.01–4.94 (m, 2H, CH), 4.55–4.51 (m, 2H, CH), 3.69–3.63 (m, 1H, CH), 3.60–3.54 (m, 1H, CH), 3.06 (brs, 1H, CH), 2.42–2.40 (m, 1H, NH), 2.27 (s, 3H, CH3), 2.18 (s, 3H, CH3), 2.16–2.12 (m, 1H, CH), 2.08–2.04 (m, 1H, CH), 1.60–1.54 (m, 1H, CH), 1.45–1.38 (m, 1H, CH), 0.92 (t, J=7.2 Hz, 3H, CH3); δ (minor isomer) 8.17 (brs, 1H, ArH), 7.03–7.02 (m, 2H, ArH), 6.62–6.61 (m, 2H, ArH), 6.57 (d, J=8.4 Hz, 1H, ArH), 5.49 (d, J=9.6 Hz, 1H, CH), 5.07–5.05 (m, 1H, CH), 4.44–4.42 (m, 1H, CH), 3.83–3.78 (m, 1H, CH), 2.92–2.89 (m, 1H, CH), 2.59–2.57 (m, 1H, NH), 2.24 (s, 3H, CH3), 2.22 (s, 3H, CH3), 1.90–1.87 (m, 2H, CH), 1.31–1.28 (m, 1H, CH), 0.99 (t, J=7.2 Hz, 3H, CH3); ratio of isomers=0.6:0.4; 13C NMR: δ 196.5, 180.1, 179.8, 176.9, 173.3, 173.2, 143.7, 143.6, 142.6, 142.1, 141.9, 141.3, 137.3, 137.2, 135.9, 135.5, 134.5, 134.2, 134.1, 132.9, 132.8, 129.2, 129.1, 129.0, 128.9, 128.8, 128.7, 128.6, 128.5, 128.4, 128.3, 128.2, 128.0, 127.9, 127.7, 127.6, 127.5, 127.4, 127.2, 127.1, 127.0, 126.8, 126.2, 126.1, 125.9, 110.4, 110.1, 85.4, 85.1, 70.3, 70.1, 65.6, 65.2, 62.3, 62.2, 60.8, 60.6, 60.1, 60.0, 59.8, 58.7, 44.5, 43.5, 35.3, 34.5, 31.3, 30.6, 21.5, 21.3, 21.2, 14.6, 14.2; IR: υ 3423, 3278, 3066, 2920, 2852, 1711, 1683, 1609, 1486, 1432, 1353, 1235, 1180, 1080, 1033, 980, 942, 811, 732, 694 cm−1. HRMS (ESI). Calcd. for C60H54Cl2N3O6 ([M+H]+): m/z 982.3384. Found: m/z 982.3379.
Methyl (1,1″-dibenzyl-5,5″-dichloro-4′-hydroxy-2′-(4-methylbenzoyl)-2,2″-dioxo-4′-(p-tolyl)dispiro[indoline-3,1′-cyclopentane-3′,3″-indolin]-5′-yl)tryptophanate (1l)
White solid; yield 70%; mp 254–256°C; 1H NMR: δ (major isomer) 10.65 (brs, 1H, NH), 8.11 (s, 1H, ArH), 8.03–7.97 (m, 1H, ArH), 7.53–7.46 (m, 2H, ArH), 7.39–7.31 (m, 3H, ArH), 7.21–7.14 (m, 7H, ArH), 7.06 (brs, 3H, ArH), 6.98–6.89 (m, 4H, ArH), 6.79 (brs, 3H, ArH), 6.71–6.68 (m, 3H, ArH), 6.51–6.47 (m, 2H, ArH, CH), 6.38 (s, 1H, OH), 5.58 (brs, 1H, CH), 5.35–5.31 (m, 1H, CH), 5.03–4.94 (m, 2H, CH), 4.61–4.44 (m, 2H, CH), 3.46 (s, 1H, CH), 3.17 (s, 1H, CH), 2.98 (s, 2H, CH), 2.66 (brs, 1H, CH), 2.46 (brs, 1H, CH), 2.37 (brs, 1H, CH), 2.30 (s, 3H, CH3), 2.18 (s, 3H, CH3); δ minor isomer 8.03–7.97 (m, 1H, ArH), 6.98–6.89 (m, 1H, ArH), 6.71–6.68 (m, 1H, ArH), 6.63 (brs, 2H, ArH, CH), 4.61–4.44 (m, 1H, CH), 2.66 (brs, 1H, CH), 2.26 (s, 3H, CH3), 2.14 (s, 3H, CH3); ratio of isomers=0.60:0.40; 13C NMR: δ 196.0, 179.6, 176.5, 173.0, 143.0, 142.0, 141.6, 136.9, 135.9, 135.4, 135.0, 133.7, 133.6, 132.3, 128.8, 128.6, 128.4, 128.1, 127.8, 126.8, 126.6, 123.1, 122.8, 120.8, 118.2, 118.1, 117.7, 111.2, 110.0, 109.6, 108.9, 108.5, 85.1, 84.9, 70.3, 64.8, 64.6, 61.6, 60.9, 60.8, 59.7, 59.3, 51.1, 51.0, 43.8, 43.0, 30.1, 28.5, 21.0, 20.8, 20.7; IR: υ 3443, 3271, 2921, 2852, 1706, 1684, 1608, 1485, 1432, 1351, 1180, 1082, 1025, 977, 938, 816, 741, 691 cm−1. HRMS (ESI). Calcd. for C60H50Cl2N4NaO6 ([M+Na]+): m/z 1015.3000. Found: m/z 1015.2990.
Supporting information
Crystallographic data for 1b (CCDC 1480217), 1g (CCDC 1480218) and 1k (CCDC1480219) have been deposited at the Cambridge Crystallographic Database Centre.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant 21272200) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We thank the Analysis and Test Center of Yangzhou University for providing analytical instruments.
References
[1] Ashimori, A.; Bachand, B.; Overman, L. E.; Poon, D. J. Catalytic asymmetric synthesis of quaternary carbon centers. Exploratory investigations of intramolecular Heck reactions of (E)-α,β-unsaturated 2-haloanilides and analogues to form enantioenriched spirocyclic products. J. Am. Chem. Soc.1998, 120, 6477–6487.10.1021/ja980786pSearch in Google Scholar
[2] Sebahar, P. R.; Williams, R. M. The asymmetric total synthesis of (+)- and (−)-spirotryprostatin B. J. Am. Chem. Soc.2000, 122, 5666–5667.10.1021/ja001133nSearch in Google Scholar
[3] Marti, C.; Carreia, E. M. Construction of spiro[pyrrolidine-3,3′-oxindoles]−recent applications to the synthesis of oxindole alkaloids. Eur. J. Org. Chem.2003, 2003, 2209–2219.10.1002/ejoc.200300050Search in Google Scholar
[4] Osman, F. H.; El-Samahy, F. A.; Ahmed Farag; I. S. A Nucleophilic addition of acetone enolate to (E)-alkyloxindolylideneacetates. Monatsh. Chem.2004, 135, 823–831.10.1007/s00706-003-0059-4Search in Google Scholar
[5] Williams, R. M.; Cox, R. J. Paraherquamides, brevianamides, and asperparalines: Laboratory synthesis and biosynthesis. An interim report. Acc. Chem. Res.2003, 36, 127–139.10.1021/ar020229eSearch in Google Scholar PubMed
[6] Cao, Z. Y.; Wang, Y. H.; Zeng, X. P.; Zhou, J. Catalytic asymmetric synthesis of 3,3-disubstituted oxindoles: diazooxindole joins the field. Tetrahedron Lett.2014, 55, 2571–2584.10.1016/j.tetlet.2014.01.084Search in Google Scholar
[7] Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Strategies for the enantioselective synthesis of spirooxindoles. Org. Biomol. Chem.2012, 10, 5165–5181.10.1039/c2ob25184aSearch in Google Scholar PubMed
[8] Singh, G. S.; Desta, Z. Y. Isatins As Privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev.2012, 112, 6104–6155.10.1021/cr300135ySearch in Google Scholar PubMed
[9] Hong, L.; Wang, R. Recent advances in asymmetric organocatalytic construction of 3,3′-spirocyclic oxindoles. Adv. Synth. Catal.2013, 355, 1023–1030.10.1002/adsc.201200808Search in Google Scholar
[10] Cheng, D. Q.; Ishihara, Y.; Tan, B.; Barbas, C. B. III Organocatalytic asymmetric assembly reactions: synthesis of spirooxindoles via organocascade strategies. ACS Catalysis2014, 4, 743–762.10.1021/cs401172rSearch in Google Scholar
[11] Santos, M. Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 2014, 70, 9735–9757.10.1016/j.tet.2014.08.005Search in Google Scholar
[12] Drouhin, P.; Hurst, T. E.; Whitwood, A. C.; Taylor, R. J. K. Copper-mediated construction of spirocyclic bis-oxindoles via a double C-H, Ar-H coupling process. Org. Lett.2014, 16, 4900–4903.10.1021/ol5024129Search in Google Scholar PubMed
[13] Chen, R. S.; Xu, S. L.; Fan, X.; Li, H. Y.; Tang, Y. H.; He, Z. J. Construction of dispirocyclohexanes via amine-catalyzed [2+2+2] annulations of Morita–Baylis–Hillman acetates with exocyclic alkenes. Org. Biomol. Chem.2015, 13, 398–408.10.1039/C4OB01927JSearch in Google Scholar PubMed
[14] Milanesio, M.; Viterbo, D.; Albini, A.; Fasani, E.; Bianchi, R.; Barzaghi, M. Structural study of the solid-state photoaddition reaction of arylidenoxindoles. J. Org. Chem.2000, 65, 3416–3425.10.1021/jo991873iSearch in Google Scholar PubMed
[15] Zhou, R.; Yang, C. J. ; Liu, Y. Y.; Li, R. F.; He, Z. J. Diastereoselective synthesis of functionalized spirocyclopropyl oxindoles via P(NMe2)3-mediated reductive cyclopropanation. J. Org. Chem.2014, 79, 10709–10715.10.1021/jo502106cSearch in Google Scholar PubMed
[16] Wu, L.; Sun, J.; Yan, C. G. Facile synthesis of spiro[indoline-3,3′-pyrrolo[1,2-a]quinolines] and spiro-[indoline-3,1′-pyrrolo[2,1-a]isoquinolines] via 1,3-dipolar cycloaddition reactions of heteroaromatic ammonium salts with 3-phenacylideneoxindoles. Org. Biomol. Chem.2012, 10, 9452–9463.10.1039/c2ob26849cSearch in Google Scholar PubMed
[17] Fu, Q.; Yan, C. G. Molecular diversity of cycloaddition reactions of the functionalized pyridinium salts with 3-phenacylideneoxindoles. Tetrahedron2013, 69, 5841–5849.10.1016/j.tet.2013.05.034Search in Google Scholar
[18] Gong, H.; Sun, J.; Yan, C. G. Povarov reaction of beta-enamino esters and isatin-3-imines for diastereoselective synthesis of spiro[indoline-3,2′-quinolines]. Synthesis2014, 46, 489–495.10.1055/s-0033-1340459Search in Google Scholar
[19] Gao, H.; Sun, J.; Yan, C. G. Selective synthesis of functionalized spiro[indoline-3,2′-pyridines] and spiro[indoline-3,4′-pyridines] by Lewis acid catalyzed reactions of acetylenedicarboxylate, arylamines, and isatins. J. Org. Chem.2014, 79, 4131–4136.10.1021/jo500144zSearch in Google Scholar PubMed
[20] Han, Y.; Sheng, Y. J.; Yan, C. G. Convenient synthesis of triphenylphosphanylidene spiro[cyclopentane-1,3′-indolines] and spiro[cyclopent[2]ene-1,3′-indolines] via three-component reactions. Org. Lett.2014, 16, 2654–2657.10.1021/ol5008394Search in Google Scholar PubMed
[21] Yang, F.; Zhang, L. J.; Yan, C. G. Four-component reaction of N-alkylimidazoles (N-alkylbenzimidazoles), dialkyl but-2-ynedioate, N-alkylisatins and malononitrile. RSC Adv.2014, 4, 64466–64475.10.1039/C4RA12278JSearch in Google Scholar
[22] Jiang, Y. H.; Yang, R. Y.; Sun, J.; Yan, C. G. Diastereoselective synthesis of dispiro[indoline-3,1′-cyclobutane-2′,3″-indolines] via visible light catalyzed cyclodimerization of 3-phenacylideneoxindoles. Heterocycl. Commun.2016, 22, 151–156.10.1515/hc-2016-0022Search in Google Scholar
[23] Sun, J.; Chen, L.; Gong, H.; Yan, C. G. Convenient synthesis of functionalized spiro[indoline-3,2′-pyrrolizines] or spiro[indoline-3,3′-pyrrolidines] via multicomponent reactionsof α-amino acids, dialkyl acetylenedicarboxylates and 3-methyleneoxindoles. Org. Biomol. Chem.2015, 13, 5905–5917.10.1039/C5OB00437CSearch in Google Scholar
[24] Shanthi, G.; Perumal, P. T. An InCl3 catalyzed facile one-pot synthesis of novel dispiro[cyclopent-3′-ene]bisoxindoles. Tetrahedron Lett.2008, 49, 7139–7142.10.1016/j.tetlet.2008.09.152Search in Google Scholar
[25] Lingam, K. A. P.; Shanmugam, P.; Selvakumar, K. Stereoselective synthesis of geometrically strained, oxindole-appended vinyl cyclopropanes and highly substituted cyclopentenes via sulfur ylide cyclopropanation and vinyl cyclopropane rearrangement. Synlett2012, 23, 278–284.10.1002/chin.201220113Search in Google Scholar
[26] Lu, L. J.; Fu, Q. Sun, J.; Yan, C. G. Synthesis of complex dispirocyclopentanebisoxindoles via cycloaddition reactions of 4-dimethylamino-1-alkoxycarbonylmethylpyridinium bromides with 2-oxoindolin-3-ylidene derivatives. Tetrahedron2014, 70, 2537–2545.10.1016/j.tet.2014.02.050Search in Google Scholar
[27] Lu, L. J.; Yan, C. G. Synthesis of dispirocyclopentyl-3,30-bisoxindoles via base promoted cyclization reaction of 3-phenacylideneoxindoles with nitromethane. Tetrahedron2014, 70, 9587–9591.10.1016/j.tet.2014.11.028Search in Google Scholar
[28] Lu, L. J.; Yan, C. G. Synthesis of dispirocyclopentyl-3,3′-bisoxindoles via domino cycloaddition reactions of 4-dimethylaminopyridinium bromides with 3-phenacylideneoxindoles. Chin. J. Chem.2015, 33, 1178–1188.10.1002/cjoc.201500438Search in Google Scholar
[29] Shen, G. L.; Sun, J.; Yan, C. G. Construction of dispirocyclohexyl-3,3′-bisoxindole and dispirocyclopentyl-3,3′-bisoxindole via domino cycloaddition reactions of N-benzylbenzimidazolium salts with 2-(2-oxoindolin-3-ylidene)acetates. RSC Advances2015, 5, 4475–4483.10.1039/C4RA13760DSearch in Google Scholar
[30] Tan, B.; Candeias, N. R.; Barbas III, C. F. Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst. Nat. Chem.2011, 3, 473–477.10.1038/nchem.1039Search in Google Scholar PubMed
[31] Cao, Y. M.; Shen, F. F.; Zhang, F. T.; Wang, R. Catalytic asymmetric Michael addition/cyclization of isothiocyanato oxindoles: Highly efficient and versatile approach for the synthesis of 3,2′-pyrrolidinyl mono- and bi-spirooxindole Frameworks. Chem. Eur. J.2013, 19, 1184–1188.10.1002/chem.201204114Search in Google Scholar PubMed
[32] Sun, W. S.; Hong, L.; Zhu, G. M.; Wang, Z. L.; Wei, X. J. Ni, J. M.; Wang, R. An organocatalytic Michael–Michael cascade for the enantioselective construction of spirocyclopentane bioxindoles: control of four contiguous stereocenters. Org. Lett.2014, 16, 544−547.10.1021/ol4034226Search in Google Scholar PubMed
[33] Sun, J.; Xie, Y. J.; Yan, C. G. Construction of dispirocyclopentanebisoxindoles via self-domino Michael-Aldol reactions of 3-phenacylideneoxindoles. J. Org. Chem.2013, 78, 8354–8365.10.1021/jo4010603Search in Google Scholar PubMed
[34] Shen, G. L.; Sun, J.; Xie, Y. J.; Yan, C. G. Synthesis of densely substituted dispirocyclopentanebisoxindoles by base promoted sequential reaction of two different 3-methyleneoxindoles with thiol. Chem. Select,2016, 1, 1447–1451.10.1002/slct.201600209Search in Google Scholar
[35] Suman, K.; Thennarasu, S. Base catalysed domino and self-domino Michael–Aldol reactions: one-pot synthesis of dispirocyclopentaneoxindoles containing multiple chiral stereocenters. RSC Adv.2015, 5, 23291–23302.10.1039/C5RA00283DSearch in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Chemical and pharmacological research on the plants from genus Ajuga
- Research Articles
- One-pot synthesis of annulated 1,8-naphthyridines
- Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone
- Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids
- Regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole
- A simple one-pot synthesis of 2,4-diaryl- 9H-pyrido[2,3-b]indoles under solvent-free conditions
- Cyclodimerization of 3-phenacylideneoxindolines with amino esters for the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines]
- An efficient approach to the synthesis of coumarin-fused dihydropyridinones
- Halogenoheterocyclization of terminally substituted 2-allylthio(seleno)quinolin- 3-carbaldehydes
- A new synthetic route to benzophenone derivatives
- Design, synthesis, docking and in vitro antifungal study of 1,2,4-triazole hybrids of 2-(aryloxy)quinolines
- Synthesis, antimicrobial activity and anti-biofilm activity of novel tetrazole derivatives
Articles in the same Issue
- Frontmatter
- Review
- Chemical and pharmacological research on the plants from genus Ajuga
- Research Articles
- One-pot synthesis of annulated 1,8-naphthyridines
- Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone
- Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids
- Regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole
- A simple one-pot synthesis of 2,4-diaryl- 9H-pyrido[2,3-b]indoles under solvent-free conditions
- Cyclodimerization of 3-phenacylideneoxindolines with amino esters for the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines]
- An efficient approach to the synthesis of coumarin-fused dihydropyridinones
- Halogenoheterocyclization of terminally substituted 2-allylthio(seleno)quinolin- 3-carbaldehydes
- A new synthetic route to benzophenone derivatives
- Design, synthesis, docking and in vitro antifungal study of 1,2,4-triazole hybrids of 2-(aryloxy)quinolines
- Synthesis, antimicrobial activity and anti-biofilm activity of novel tetrazole derivatives

