Abstract
Synthesis, characterization and biological activity of novel 4,5-diaryl/heteroaryl thiophene-2-carboxylic acid derivatives are described. Aryl/heteroaryl esters were converted to substituted thiophene esters via a Vilsmeier-Haack reaction, which were then hydrolyzed to 4,5-diaryl/heteroaryl thiophene-2-carboxylic acid derivatives 8a–h. All products were characterized by 1H NMR, 13C NMR, IR and MS. Antibacterial activity of the synthesized compounds against two Gram-positive and two Gram-negative bacteria, as well as their anticancer activity against PC-3 cell line (human prostate cancer cell lines) were evaluated.
Introduction
Sulfur-containing heterocycles possess diverse properties such as antibacterial [1], antifungal [2], anti-HIV [3], antimicrobial [4] and antipsoriatic [5] activities. In particular, a thiophene system plays a crucial role in drug discovery of BACE1 inhibitory [6], antibacterial [7], anticancer [8], antitubercular [9], anti-inflammatory [10] and anti-HIV agents [11]. Currently, several thiophene-containing non-steroidal anti-inflammatory drugs (NSAIDs) such as tiaprofenic acid [12] (1) and tenidap [13] (2) (Figure 1) are used for treatment of pain and inflammatory disorders. The thiophenyl group mimics the phenyl group [14].

Thiophene based non-steroidal anti-inflammatory drugs 1 and 2.
According to our recent review, 3,5-disubstituted thiophene-2-carboxylic acid derivatives [15] and 2,5-disubstituted thiophene-3-carboxylic acid derivatives [16] have been synthesized and their anti-HCV and anti-TB activities evaluated. Furthermore, such derivatives have been developed as antibacterial agents [17]. However, synthesis and biological evaluation of 4,5-diaryl/heteroarylthiophene-2-carboxylic acid derivatives remain largely unexplored. In the present study, we report the synthesis of novel 4,5-diaryl/heteroarylthiophene-2-carboxylic acid derivatives through the Vilsmeier-Haack reaction, their spectral characterization and biological activity.
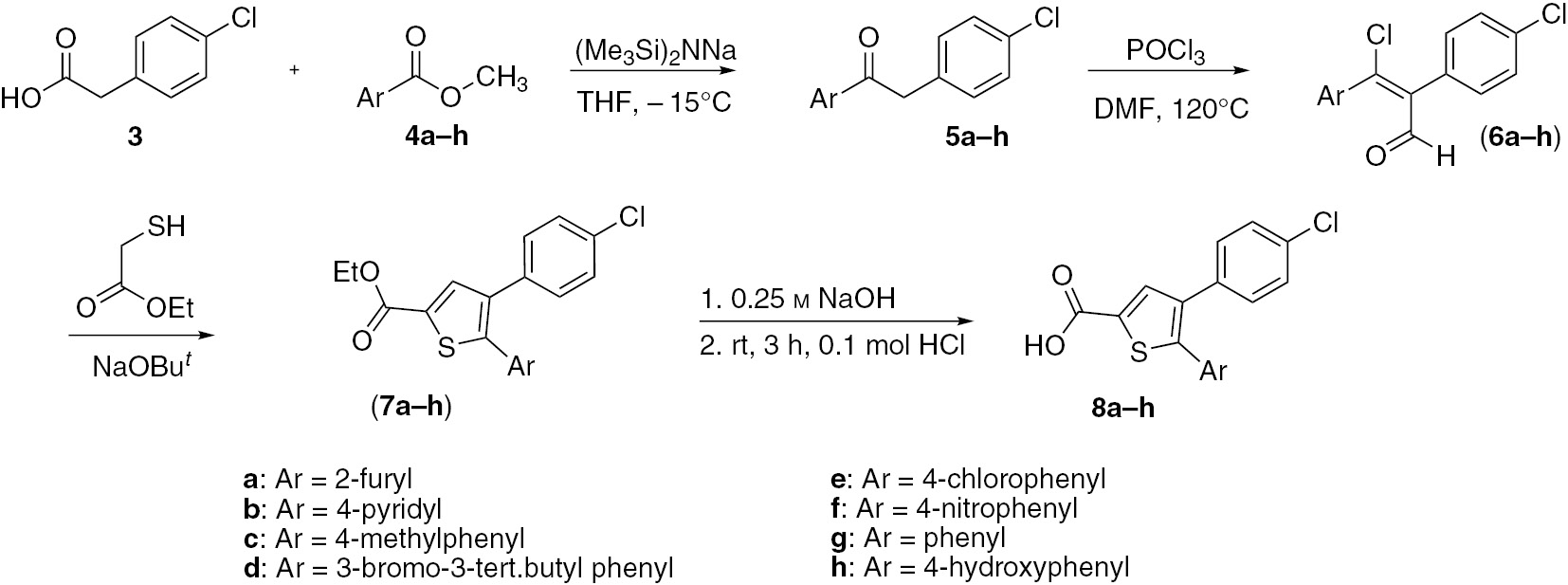
Initially, aromatic ketones 5a–h were obtained from 4-chlorophenylacetic acid (3) and aromatic esters 4a–h (Scheme 1). The Vilsmeier-Haack reaction is typically used for formylation of aromatic and heteroaromatic compounds [18, 19]. In this study, the ethanones 5a–h were allowed to react with phosphorus oxychloride and N,N-dimethylformamide to yield chloro acrylaldehyde derivatives 6a–h. In the next step, these products were allowed to react with ethyl mercaptoacetate in the presence of a sodium tert-butoxide to yield substituted thiophene esters 7a–h, which were then hydrolyzed to the desired products 8a–h.
Experimental
All reactions were monitored by thin-layer chromatography analysis using silica gel plates (Kieselgel 60F254 Merck). Compounds were visualized by irradiation with UV light (254 nm) after spraying with a 10% sulfuric acid/methanol solution. Melting points were determined in open capillary tubes on a Boetius melting point apparatus and are uncorrected. The IR spectra were recorded using KBr pellets on a Bruker spectrophotometer. 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were obtained at room temperature on a Varian Gemini spectrometer. Mass spectra were recorded on a JEOL SX102 mass spectrometer coupled with a liquid chromatography system in electrospray time of flight (TOF) mode. Elemental analysis was performed on a Perkin Elmer CHNS elemental analyzer. The purity of compounds was determined using a reverse-phase Waters HPLC system equipped with a C18 analytical column (4.6×75 mm). Peaks were detected by UV absorption (254 nm) using a diode array detector.
General procedure for synthesis of compounds 5a–h
A solution of 2-(4-chlorophenyl)acetic acid (3, 1 g, 5.8 mmol) in THF (10 mL) was cooled to −60°C and stirred for 10 min before treatment with sodium bis(trimethylsilyl)amide (NaHMDS, 2.90 g, 10 mmol) and then stirred for 1 h at −20°C. An aryl ester 4a–h (5.8 mmol) was added and the mixture was stirred for 30 min at −15°C and then at room temperature for an additional 30 min. The reaction progress was monitored by TLC. The reaction mass was quenched with NH4Cl and extracted with ethyl acetate. The product was purified by silica gel column chromatography eluting with ethyl acetate.
2-(4-Chlorophenyl)-1-(furan-2-yl)ethanone (5a)
Yield 54%; white solid; mp 196°C; 1H NMR (CDCl3): δ 7.6 (d, 1H, J=7.5 Hz, Ar-H), 7.2–7.5 (m, 5H, Ar-H), 6.45 (d, 1H, J=7.8 Hz, Ar-H), 4.05 (s, 2H, -CH2); 13C NMR (CDCl3): δ 188.6, 162.0, 150.7, 138.4, 132.9, 122.7, 114.0, 38.7; IR: υ 3072.1 (Ar=CH str), 2882.7 (CH str), 1709.7 (CO str), 1518.2, 1475.7 (Ar C=C str), 1182.0 (C-O-C str), 862.9 (C-Cl str) cm−1; LC-MS (m/z): 220 (M)+, 221, 222 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C12H9ClO2: C, 65.32; H, 4.11. Found: C, 65.22; H, 4.06.
2-(4-Chlorophenyl)-1-(pyridin-4-yl)ethanone (5b)
Yield 47%; white solid; mp 210°C; 1H NMR (DMSO-d6): δ 8.8 (m, 2H, Ar-H), 7.95 (m, 2H, Ar-H), 7.4 (m, 2H, Ar-H), 7.25 (m, 2H, Ar-H), 4.25 (s, 2H, -CH2); 13C NMR (CDCl3, 75 MHz): δ 194.8, 152.8, 150.8, 146.4, 138.5, 133.7, 130.8, 122.7, 118.0, 46.7; IR: υ 3068.3 (Ar=CH str), 2973.8 (CH str), 1710.8 (CO str), 1520.9, 1482.6 (Ar C=C str), 1422.3 (CN str), 826.4 (C-Cl str) cm−1; LC-MS: m/z 231 (M)+, 232, 233 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C13H10ClNO: C, 67.39; H, 4.35; N, 6.06. Found: C, 67.41; H, 4.29; N, 5.97.
2-(4-Chlorophenyl)-1-(p-tolyl)ethanone (5c)
Yield 53%; white solid; mp 1215°C; 1H NMR (DMSO-d6): δ 7.92 (m, 2H, Ar-H), 7.38 (m, 4H, Ar-H), 7.24 (m, 2H, Ar-H), 4.20 (s, 2H, -CH2), 2.20 (s, 3H, -CH3); 13C NMR (CDCl3): δ 196.2, 148.8, 138.6, 137.2, 135.4, 122.7, 118.0, 46.2, 25.3; IR: υ 3088.7 (Ar=CH str), 2943.2 (CH str), 2847.0 (CH str), 1698.7 (CO str), 1540.9, 1452.4 (Ar C=C str), 796.3 (C-Cl str) cm−1; LC-MS (m/z): 244 (M+), 245, 246 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C15H13ClO: C, 73.62; H, 5.35. Found: C, 73.56; H, 5.29.
1-(3-Bromo-5-(tert-butyl)phenyl)-2-(4-chlorophenyl) ethanone (5d)
Yield 27%; white solid; mp 223°C; 1H NMR (DMSO-d6): δ 8.02 (d, 1H, J=7.6 Hz, Ar-H), 7.82 (d, 1H, J=8.2 Hz, Ar-H), 7.80 (s, 1H, Ar-H), 7.42 (m, 2H, Ar-H), 7.26 (m, 2H, Ar-H), 4.22 (s, 2H, -CH2), 1.32 (s, 9H, -CH3); 13C NMR (CDCl3): δ 188.8, 148.8, 140.8, 135.2, 132.4, 128.8, 124.7, 118.8, 42.7, 36.7, 30.2; IR: υ 3048.7 (Ar=CH str), 2842.3 (CH str), 2758.4 (CH str), 1688.5 (CO str), 1540.9, 1452.4 (Ar C=C str), 845.2 (C-Cl str) cm−1; LC-MS (m/z): (M)+ at 364 and (M+2)+ at 366 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C18H18BrClO: C, 59.12; H, 4.96. Found: C, 59.11; H, 4.88.
2-(4-Chlorophenyl)-1-(2-hydroxyphenyl)ethanone (5e)
Yield 52%; white solid; mp 197°C; 1H NMR (CDCl3): δ 7.89 (d, 2H, J=7.8 Hz, Ar-H), 7.20–7.65 (m, 4H, Ar-H), 7.05 (dd, 1H, J=7.6 Hz, Ar-H), 6.75 (d, 1H, J=7.5 Hz, Ar-H), 6.05 (s, 1H, OH), 3.95 (s, 2H, -CH2); 13C NMR (CDCl3): δ 189.2, 166.4, 148.3, 139.4, 133.0, 126.4, 119.0, 112.7, 36.7; IR: υ 3426.8 (OH str), 3089.2 (Ar=CH str), 2972.7 (CH str), 1714.9 (CO str), 1574.1, 1462.0 (Ar C=C str), 871.0 (C-Cl str) cm−1; LC-MS (m/z): 246 (M+), 247, 248 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C14H11ClO2: C, 68.16; H, 4.49. Found: C, 68.09; H, 4.41.
2-(4-Chlorophenyl)-1-(4-nitrophenyl)ethanone (5f)
Yield 48%; White solid, mp: 199°C; 1H NMR (DMSO-d6): δ 8.60 (d, 2H, J=7.9 Hz, Ar-H), 7.92 (m, 4H, Ar-H), 7.57 (d, 2H, J=8.2 Hz, Ar-H), 4.05 (s, 2H, -CH2); 13C NMR (CDCl3): δ 192.0, 153.3, 149.2, 145.4, 136.7, 133.6, 129.5, 121.6, 117.0, 44.3; IR: υ 3052.8 (Ar=CH str), 2890.2 (CH str), 1702.3 (CO str), 1603.1, 1522.8 (Ar C=C str), 826.4 (C-Cl str) cm−1; LC-MS (m/z): 275 (M+), 277 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C14H10ClNO3: C, 60.99; H, 3.66; N, 5.08. Found: C, 60.91; H, 3.61; N, 4.99.
2-(4-Chlorophenyl)-1-phenylethanone (5g)
Yield 50%; white solid; mp 189°C; 1H NMR (CDCl3): δ 7.94 (d, 2H, J=7.8 Hz, Ar-H), 7.52 (dd, 1H, J=7.1 Hz, Ar-H), 7.32 (d, 2H, J=7.9 Hz, Ar-H), 7.08 (m, 4H, Ar-H), 4.08 (s, 2H, -CH2); 13C NMR (CDCl3): δ 188.0, 143.6, 137.1, 135.3, 122.0, 118.0, 46.3; IR: υ 3059.2 (Ar=CH str), 2896.2 (CH str), 1696.5 (CO str), 1578.9, 1478.9 (Ar C=C str), 810.9 (C-Cl str) cm−1; LC-MS (m/z): 230 (M+), 232 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C14H11ClO: C, 72.89; H, 4.81. Found: C, 72.81; H, 4.71.
2-(4-Chlorophenyl)-1-(4-hydroxyphenyl)ethanone (5h)
Yield 43%; white solid; mp 198°C; 1H NMR (CDCl3): δ 8.34 (d, 2H, J=7.2 Hz, Ar-H), 7.28 (m, 4H, Ar-H), 7.04 (d, 2H, J=8.2 Hz, Ar-H), 5.81 (s, 1H, OH), 3.82 (s, 2H, -CH2); 13C NMR (CDCl3): δ 192.6, 160.2, 142.1, 134.8, 134.0, 128.0, 128.7, 119.2, 40.2; IR: υ 3409.7 (OH str), 3053.7 (Ar=CH str), 2925.3 (CH str), 1708.9 (CO str), 1549.2, 1473.0 (Ar C=C str), 840.0 (C-Cl str) cm−1; LC-MS (m/z): 246 (M+), 248 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C14H11ClO2: C, 68.16; H, 4.49. Found: C, 68.09; H, 4.41.
General procedure for synthesis of compounds 6a–h
POCl3 (0.86 g, 5.65 mmol) was added at 0°C to DMF (0.49 g, 6.78 mmol) and the mixture was allowed to warm to room temperature and stirred for 30 min. The mixture was cooled again to 0°C, treated with compound 5a–h (0.5 g, 2.26 mmol) and the resulting reaction mass was stirred for 1 h at 0°C and then at 70°C for 4 h, quenched with sodium acetate and extracted with dichloromethane. The extract was dried over sodium sulfate and concentrated. The residue of 6a–h was used for the next step without purification. As a representative example, the structure of 3-chloro-2-(4-chlorophenyl)-3-(furan-2-yl)acrylaldehyde (6a) was confirmed on the basis of the following analytical data: yield 45%; white solid; mp 203°C; 1H NMR (CDCl3): δ 9.2 (s, 1H, CHO), 7.6 (d, 1H, J=7.6 Hz, Ar-H), 7.2–7.5 (m, 5H, Ar-H), 6.45 (d, 1H, J=8.3 Hz, Ar-H); 13C NMR (CDCl3): δ 210.3, 162.0, 150.7, 138.4, 135.5, 132.9, 122.7, 112.0; IR (KBr): υ 3062.2 (Ar=CH str), 1752.8 (CO str), 1518.2, 1475.7 (Ar-C=C str), 1164.2 (C-O-C str), 862.9 (C-Cl str) cm−1; LC-MS (m/z): 265 (M)+, 267.23 [relative intensity M+/(M+2)+=3:1].
General procedure for the preparation of compounds 7a–h
Ethyl mercaptoacetate (0.112 g, 9.3 mmol) was added at 0°C to a solution of sodium tert-butoxide (0.06 g, 9.3 mmol) in ethanol (1.5 mL) and the mixture was stirred at the same temperature for 30 min, then treated with compound 6a–h and the stirring was continued overnight followed by reflux for an additional 2 h. The reaction mass was quenched with water and extracted with dichloromethane. The extract was concentrated affording compound 7a–h that was used without further purification. As a representative example, the structure of ethyl 4-(4-chlorophenyl)-5-(furan-2-yl)thiophene-2-carboxylate (7a) was confirmed based on the following analytical data: yield 45%; yellowish solid; mp 198°C; 1H NMR (CDCl3): δ 7.74 (d, 1H, J=7.5 Hz, Ar-H), 7.62 (s, 1H, Ar-H), 7.48 (m, 2H, Ar-H), 7.40 (m, 2H, Ar-H), 6.52 (dd, 1H, Ar-H), 6.23 (d, 1H, J=7.7 Hz, Ar-H), 2.95 (q, 2H, J=13.1 Hz, OCH2), 1.72 (t, 3H, J=8.1 Hz, CH3); 13C NMR (CDCl3): δ 165.7, 154.3, 148.8, 146.8, 140.8, 135.2, 132.4, 128.8, 124.7, 118.8, 106.2; IR: υ 3097.9 (Ar=CH str), 1761.9 (CO str), 1568.7, 1530.2 (Ar C=C str), 1059.6 (C-S-C str), 838.1 (C-Cl str) cm−1; LC-MS (m/z): 332 (M+), 334 [relative intensity M+/(M+2)+=3:1].
General procedure for hydrolysis of compounds 7a–h (preparation of 8a–h)
The ester 7a–h (0.3 g, 0.9 mmol) was dissolved in methanol (3 mL) and the solution was treated with water (0.6 mL) and solid NaOH (0.25 mol). The mixture was stirred for 3 h at ambient temperature, acidified with HCl (0.1 m), concentrated and the residue was extracted with ethyl acetate. The acid 8a–h was purified by silica gel chromatography eluting with ethyl acetate and petroleum ether.
4-(4-Chlorophenyl)-5-(furan-2-yl)thiophene-2-carboxylic acid (8a)
Yield 54%; white solid; mp 201°C; 1H NMR (DMSO-d6): δ 13.41 (s, 1H, COOH), 7.74 (d, 1H, J=7.4 Hz, Ar-H), 7.62 (s, 1H, Ar-H), 7.48 (m, 2H, Ar-H), 7.40 (m, 2H, Ar-H), 6.52 (dd, 1H, J=8.1 Hz, Ar-H), 6.23 (d, 1H, J=7.9 Hz, Ar-H); 13C NMR (DMSO-d6): δ 187.9, 159.4, 149.1, 146.7, 137.7, 136.0, 135.5, 134.5, 130.8, 129.4, 127.6, 122.3, 119.7, 114.6; IR: υ 3417.4 (OH str), 3057.5 (Ar=CH str), 1739.5 (CO str), 1621.8, 1520.4, 1478.4 (Ar C=C str), 1139.0 (C-S-C str), 857.0 (C-Cl str) cm−1; LC-MS (m/z): 303.36 (M+), 305.33 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C15H9ClO3S: C, 59.12; H, 2.98; S, 10.52. Found: C, 59.11; H, 2.87; S, 10.51.
4-(4-Chlorophenyl)-5-(pyridin-4-yl)thiophene-2-carboxylic acid (8b)
Yield 55%; light yellowish solid; mp 210°C; 1H NMR (DMSO-d6): δ 13.41 (s, 1H, COOH, D2O exchangeable), 8.54 (dd, 2H, J=7.4 Hz, Ar-H), 7.82 (s, 1H, Ar-H), 7.42 (d, 2H, J=8.2 Hz, Ar-H), 7.30 (m, 2H, Ar-H), 7.20 (m, 2H, Ar-H); 13C NMR (CDCl3): δ 187.9, 159.4, 153.2, 149.4, 146.9, 136.3, 136.0, 135.8, 134.1, 132.6, 130.6, 129.9, 129.8, 123.8, 121.5, 120.7, 114.1, 113.8, 55.2; IR: υ 3417.4 (OH str), 3057.5 (Ar=CH str), 1739.3 (CO str), 1622.31, 1564.93, 1479.72 (Ar C=C str), 1138.8 (C-S-C str), 829.4 (C-Cl str) cm−1; LC-MS (m/z): 315.78 (M+), 316.17, 317.35 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C16H10ClNO2S: C, 60.86; H, 3.19; N, 4.44; S, 10.15. Found: C, 60.81; H, 3.1; N, 4.39; S, 10.11.
4-(4-Chlorophenyl)-5-(p-tolyl)thiophene-2-carboxylic acid (8c)
Yield 57% white solid; mp 213°C; 1H NMR (DMSO-d6): δ 13.21 (s, 1H, COOH, D2O exchangeable), 7.76 (s, 1H, Ar-H), 7.38 (d, 2H, J=7.8 Hz, Ar-H), 7.26 (d, 2H, J=7.9 Hz, Ar-H), 7.18 (m, 4H, Ar-H) 2.2 (s, 3H, CH3); 13C NMR (CDCl3): δ 174.0, 162.4, 145.3, 135.1, 132.5, 130.6, 129.4, 128.5, 128.3, 126.4, 127.2, 127.9, 126.4, 30.0, 23.2; IR: υ 3484.26 (OH str), 3048.4 (Ar=CH str), 1739.4 (CO str), 1621.7, 1556.9, 1478.4 (Ar C=C str), 1139.0 (C-S-C str), 830.8 (C-Cl str) cm−1; LC-MS (m/z): 328.82 (M)+, 329.37, 330.37 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C18H13ClO2S: C, 65.75; H, 3.99; S, 9.75. Found: C, 65.69; H, 3.91; S, 9.69.
5-(3-Bromo-5-(tert-butyl)phenyl-4-(4-chlorophenyl)thiophene-2-carboxylic acid (8d)
Yield 61%; white solid; mp 216°C; 1H NMR (DMSO-d6): δ 13.25 (s, 1H, COOH, D2O exchangeable), 7.78 (s, 1H, Ar-H), 7.52 (s, 1H, Ar-H), 7.40 (m, 3H, Ar-H), 7.26 (dd, 2H, J=7.6 Hz, Ar-H), 7.08 (d, 1H, J=8.4 Hz, Ar-H), 1.05 (s, 9H, (CH3)3); 13C NMR (CDCl3): δ 175.0, 162.1, 148.3, 145.4, 141.6, 139.6, 135.1, 132.3, 128.9, 128.5, 127.8, 127.7, 126.9, 115.3, 29.9, 23.9; IR: υ 3496.2 (OH str), 3058.4 (Ar=CH str), 1724.4 (CO str), 1547.9, 1459.4 (Ar C=C str), 1048.9 (C-S-C str), 820.7 (C-Cl str) cm−1; LC-MS (m/z): 449.8 (M+), 451.3 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C21H18BrClO2S: C, 56.08; H, 4.03; S, 7.13. Found: C, 55.98; H, 4.07; S, 7.09.
4-(4-Chlorophenyl)-5-(2-hydroxyphenyl)thiophene-2-carboxylic acid (8e)
Yield 56%; white solid; mp 218°C; 1H NMR (DMSO-d6): δ 11.56 (s, 1H, COOH), 8.12 (s, 1H, Ar-H), 7.62 (d, 2H, J=7.4 Hz, Ar-H), 7.37 (m, 3H, Ar-H), 7.14 (dd, 2H, J=7.1 Hz, Ar-H), 6.85 (d, 1H, J=7.8 Hz, Ar-H), 6.44 (s, 1H, Ar-OH); 13C NMR (CDCl3): δ 174.3, 162.3, 145.3, 142.3, 135.7, 132.9, 130.2, 128.7, 128.0, 126.4, 125.9, 122.9, 118.3; IR: υ 3476.2 (OH str), 3436.0 (OH str), 3068.4 (Ar=CH str), 1704.1 (CO str), 1558.9, 1452.4 (Ar C=C str), 1099.4 (C-S-C str), 864.1 (C-Cl str) cm−1; LC-MS (m/z): 330.8 (M+), 332.3 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C17H11ClO3S: C, 61.73; H, 3.35; S, 9.69. Found: C, 61.69; H, 3.36; S, 9.61.
4-(4-Chlorophenyl)-5-(4-nitrophenyl)thiophene-2-carboxylic acid (8f)
Yield: 59%. Light yellow solid, mp 220°C; 1H NMR (CDCl3): δ 13.26 (s, 1H, COOH), 8.06 (s, 1H, Ar-H), 7.73 (d, 2H, J=7.6 Hz, Ar-H), 7.24 (d, 2H, J=8.3 Hz, Ar-H), 7.12 (m, 2H, Ar-H) 6.87 (m, 2H, Ar-H); 13C NMR (75 MHz, CDCl3): δ 178.8, 160.2, 152.5, 143.2, 140.5, 137.3, 134.7, 131.7, 127.8, 123.8, 117.8; IR: υ 3424.4 (OH str), 3078.1 (Ar=CH str), 1724.8 (CO str), 1558.8, 1464.1 (Ar C=C str), 1145.3 (C-S-C str), 845.4 (C-Cl str) cm−1. LC-MS (m/z): 359 (M+), 360, 361 [relative intensity M+/(M+2)+=3:1]. Anal. Calcd for C17H10 ClNO4S: C, 56.75; H, 2.80; N, 3.89; S, 8.91. Found: C, 56.68; H, 3.81; N, 3.81; S, 8.89.
4-(4-Chlorophenyl)-5-phenylthiophene-2-carboxylic acid (8g)
Yield 61%; white solid; mp 208°C; 1H NMR (DMSO-d6): δ 13.41 (s, 1H, COOH, D2O exchangeable), 8.54 (dd, 2H, J=7.4 Hz, Ar-H), 7.82 (s, 1H, Ar-H), 7.42 (d, 2H, J=8.1 Hz, Ar-H), 7.30 (m, 2H, Ar-H), 7.20 (m, 2H, Ar-H); 13C NMR (CDCl3): δ 172.4, 160.5, 150.7, 139.8, 139.3, 135.6, 131.6, 128.7, 122.5, 115.4; IR: υ 3452.5 (OH str), 3027.7 (Ar=CH str), 1717.4 (CO str), 1579.7, 1482.7 (Ar C=C str), 1126.7 (C-S-C str), 864.9 (C-Cl str) cm−1; LC-MS (m/z): 314 (M)+, 316 [relative intensity of M+/(M+2)+=3:1]. Anal. Calcd for C17H11ClO2S: C, 64.86; H, 3.52; S, 10.19. Found: C, 64.81; H, 3.49; S, 10.11.
4-(4-Chlorophenyl)-5-(4-hydroxyphenyl)thiophene-2-carboxylic acid (8h)
Yield 59%; white solid; mp 213°C; 1H NMR (DMSO-d6): δ 13.41 (s, 1H, COOH, D2O exchangeable), 8.54 (dd, 2H, J=7.2 Hz, Ar-H), 7.82 (s, 1H, Ar-H), 7.42 (d, 2H, J=8.2 Hz, Ar-H), 7.30 (m, 2H, Ar-H), 7.20 (m, 2H, Ar-H); 13C NMR (CDCl3): δ 186.7, 164.1, 148.7, 146.4, 141.3, 139.4, 131.7, 130.4, 127.2, 116.7, 112.8; IR: υ 3486.8 (OH str), 3067.1 (Ar=CH str), 1708.5 (CO str), 1564.9, 1462.8 (Ar C=C str), 1052.0 (C-S-C str), 840.0 (C-Cl str) cm−1; LC-MS (m/z): 330 (M+), 331, 332 [relative intensity M+/(M+2) +=3:1]. Anal. Cacld for C17H11ClO3S: C, 61.73; H, 3.35; S, 9.69. Found: C, 61.69; H, 3.29; S, 9.61.
Antibacterial activity
Two Gram-positive (Bacillus sphaericus and Staphylococcus aureus) and two Gram-negative (Pseudomonas vulgaris and Escherichia coli) organisms were used for this study. Pseudomonas aeruginosa (MTCC 6642) and E. coli were obtained from Microbial Type Culture Collection, IMTECH, Chandigarh, India. Clinical isolates of S. aureus and B. sphaericus, were obtained from Microbiology laboratory of Global Hospital, Hyderabad, India. Ciprofloxacin was used as standard reference. Compounds 8a–h were tested for antibacterial activity in vitro by agar well diffusion method [20]. The bacterial strains were reactivated from stock cultures by incubating the inoculated broths at 37°C for 24 h in an incubator. Final inoculums containing 106 colonies (1×106 CFU/mL) were added aseptically to Mueller-Hinton agar medium and poured into sterile Petri dishes. One hundred microliters of each test extract from stock concentrations (1 mg/mL) were added to the wells (8 mm) punched on agar surface. Plates were incubated in a bacterial incubator overnight at 37°C. The activity was evaluated by measuring the diameter of inhibition zone (Table 1). Compounds 8a and 8b show considerable activity against E. coli.
Antibacterial activity.
| No. | Compounds | Zone of inhibition (diameter, mm) 0.4 mg/mL | |||
|---|---|---|---|---|---|
| Bacillus sphaericus (+ve) | Staphylococcus aureus (+ve) | Pseudomonas vulgaris (−ve) | Escherichia coli (−ve) | ||
| 1 | 5a | 15±0.2 | 07±0.3 | 17±0.4 | 20±0.4 |
| 2 | 5b | 11±0.2 | NI | 09±0.2 | 25±0.5 |
| 3 | 5c | 18±0.4 | 10±0.2 | NI | 18±0.3 |
| 4 | 5d | 14±0.2 | 12±0.3 | NI | 09±0.2 |
| 5 | 5e | 16±0.3 | 11±0.2 | 12±0.3 | NI |
| 6 | 5f | NI | 08±0.2 | 11±0.2 | 10±0.2 |
| 7 | 5g | 16±0.4 | NI | 09±0.2 | NI |
| 8 | 5h | 12±0.2 | 11±0.3 | 07±0.2 | 11±0.2 |
| 9 | Ciprofloxacin | 28±0.2 | 36±0.5 | 35±0.4 | 31±0.5 |
NI, No inhibition.
Anticancer activity
Cisplatin and MTT (3,4,5-dimethylthiazol-2-yl-2-5-diphenyltetrazolium bromide) were procured from Sigma Aldrich (USA). PC-3 (human prostate cancer cell line) was procured from NCCS (Pune, India). Compounds 8a–h were screened for anticancer activity following a reported protocol [21] and the results are summarized in Table 2. The cell line was grown in the RPMI-1640 with 2 mmol L-glutamine, supplemented with 10% FBS, penicillin (100 IU/mL) and streptomycin (100 μg/mL) at 37°C in a humidified incubator with 5% CO2 atmosphere. The cell line was passaged twice weekly for maintaining subconfluent state. MTT was used as cell viability assay [22]. Cisplatin, a known anticancer drug, was used as a reference. The treated and untreated cells were washed with PBS (phosphate buffer saline) and MTT (100 μg/mL) was added and the mixture was incubated at 37°C for 5 h. The MTT was then removed and the formazan crystals were dissolved by adding DMSO. The absorbance at 540 nm for viable cells was measured. The absorbance from the untreated cells was defined as 100% viable cells. The % viable cells were plotted (Y-axis) against concentration (X-axis). The IC50 values was interpolated from the graph. Compounds 8c and 8h show good activity and further work is in progress.
Anticancer activity of title compounds against PC-3.
| Compounds | % of cell death at various concentrations (μg/mL) | IC50 (μg/mL) | |||
|---|---|---|---|---|---|
| 2.0 | 5.0 | 10.0 | 25.0 | ||
| 8a | 28.9 | 37.72 | 50.73 | 61.27 | 10 |
| 8b | 29.72 | 34.62 | 41.35 | 60.27 | 17 |
| 8c | 47.28 | 56.38 | 58.65 | 59.71 | 6 |
| 8d | 29.98 | 37.48 | 38.24 | 36.37 | 18 |
| 8e | 26.47 | 38.24 | 49.27 | 62.48 | 9.6 |
| 8f | 20.34 | 36.37 | 40.31 | 41.91 | 4.5 |
| 8g | 30.88 | 35.04 | 44.11 | 61.76 | 15.2 |
| 8h | 33.33 | 42.89 | 51.27 | 61.27 | 6.8 |
| Cisplatin | 22.38 | 44.24 | 49.36 | 64.17 | 25 |
Acknowledgments
We acknowledge the financial support of Dr. B. R. Ambedkar University, Srikakulam. The financial support of DST-FIST program to Department of Chemistry, GITAM University is also acknowledged.
References
[1] Li, J. R.; Li, D. D.; Wang, R. R.; Sun, J.; Dong, J. J.; Du, Q. R.; Fang, F.; Zhang, W. M.; Zhu, H. L. Design and synthesis of thiazole derivatives as potent FabH inhibitors with antibacterial activity. Eur. J. Med. Chem.2014, 75, 438–447.10.1016/j.ejmech.2013.11.020Search in Google Scholar PubMed
[2] Cemil, I.; Zeliha, O. G.; Amac, F. T.; Sibel, S. A.; Hakan, B.; Maryna, V. S.; Rostyslav, Y. M.; Olena, K. P.; Volodymyr, N. Synthesis, antibacterial and antifungal evaluation of thio- or piperazinyl-substituted 1,4-napthaquinone derivatives. J. Sulfur Chem.2016, 37, 477–487.10.1080/17415993.2016.1187734Search in Google Scholar
[3] Kim, J.; Kwon, J.; Lee, D.; Jo, S.; Park, E.; Hwang, J. Y.; Ko, Y.; Chor, I.; Ju, M. K.; Ahn, J.; et al. Serendipitous discovery of 2-((phenylsulfonyl)methyl)-thieno[3,2-d]pyrimidine derivatives as novel HIV-1 replication inhibitors. Bioorg. Org. Med. Chem. Lett. 2014, 24, 5473–5477.10.1016/j.bmcl.2014.10.007Search in Google Scholar PubMed
[4] Pravinkumar, N. S.; Swastika, G.; Pravin, D. C. An efficient one-pot three-component synthesis and antimicrobial evaluation of tetra substituted thiophene derivatives. Chin. Chem. Lett.2014, 25, 1099–1103.10.1016/j.cclet.2014.03.044Search in Google Scholar
[5] Moriyama, H.; Tsukida, T.; Inoue, Y.; Yokota, K.; Yoshino, K.; Kondo, H.; Miura, N.; Nishimura, S. Azasugar-based MMP/ADAM inhibitors as antipsoriatic agents. J. Med. Chem. 2004, 47, 1930–1938.10.1021/jm0304313Search in Google Scholar PubMed
[6] Ying, Z. X.; Shendong, Y.; Simeon, B.; Roy, K. H.; Wayman, C.; Hing, L. S.; Yong, L. Z.; Paul, B. H. P.; Eric, B. N. Y.; Julie, L.; et al. Design and synthesis of thiophene dihydroisoquinolines as novel BACE1 inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 3075–3080.10.1016/j.bmcl.2013.03.009Search in Google Scholar PubMed
[7] Mamta, R.; Yusuf, M. Synthesis, studies and in vitro antibacterial activity of some 5-(thiophene-2-yl)-phenyl pyrazoline derivatives. J. Saudi Chem. Soc.2014, 18, 411–417.10.1016/j.jscs.2011.09.002Search in Google Scholar
[8] Rakesh, K. S.; Jagaadish, S.; Swaroop, T. R.; Mohan, C.; Ashwin, N.; Harsha, K. B.; Zamer, F.; Girish, K. S.; Rangappa, K. S. Anti-cancer activity of 2,4-disubstituted thiphene derivatives: dual inhibitors of lipoxygenase and cycloxygenase. Med. Chem. 2015, 11, 462–472.10.2174/1573406411666141210141918Search in Google Scholar PubMed
[9] Xiaoyun, L.; Baojie, W.; Scott, G. F.; Qidong, Y. Synthesis and anti-tubercular evaluation of new 2-acylated and 2-alkylated amino-5-(4-(benzyloxy)phenyl) thiophene-3-carboxylic acid derivatives. Eur. J. Med. Chem. 2011, 46, 3551–3563.10.1016/j.ejmech.2011.05.018Search in Google Scholar PubMed
[10] Helal, M. H.; Salem, M. A.; Gouda, M. A.; Ahmed, N. S.; El-Sherif, A. A. Design, synthesis, characterization, quantum-chemical calculations and anti-inflammatory activity of novel series of thiophene derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.2015, 147, 73–83.10.1016/j.saa.2015.03.070Search in Google Scholar PubMed
[11] Dongwel, K.; Zengjun, F.; Zhenyu, L.; Boshi, H.; Heng, Z.; Xueyi, L.; Haoran, X.; Zhonfxia, Z.; Xiao, D.; Dirk, D.; et al. Design, synthesis and evaluation of thiophene[3,2-d]pyrimidine derivatives as HIV-1 non-nucleoside reverse transcriptase inhibitors with significantly improved drug resistance profiles. J. Med. Chem.2016, 59, 7991–8007.10.1021/acs.jmedchem.6b00738Search in Google Scholar PubMed
[12] Zhang, S.; Huang, S.; Feng, C.; Cai, J.; Chen, J.; Ji, M. Novel preparation of tiaprofenic acid. J. Chem. Res. 2013, 37, 385–443.10.3184/174751913X13709565021526Search in Google Scholar
[13] Xing-Hua, T.; Xun-Yi, W.; Lan, X.; Zhen, H. Tenidap is neuroprotective in a pilocarpine rat model of temporal lobe epilepsy. Chinese Med. J. 2013, 126, 1900–1905.Search in Google Scholar
[14] Parvesh, S.; Parul, S.; Krishna, B.; Mohinder, P. M. Synthesis and docking studies of thiophene scaffolds in COX-2. ARKIVOC2011, X, 55–70.10.3998/ark.5550190.0012.a05Search in Google Scholar
[15] Laval, C.; Sanjoy, K. D.; Reddy, J. T.; Carl, P.; Melanie, P.; Marc, C.; Caroline, R.; Wuyi, W.; Arshad, S.; Constantin, G. Y.; et al. Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Bioorg. Med. Chem. Lett.2004, 14, 793–796.10.1016/j.bmcl.2003.10.067Search in Google Scholar PubMed
[16] Xiaoyun, L.; Baojje, W.; Franzblu, S. G.; Qidong, Y. Design, synthesis and anti-tubercular evaluation of new 2-acylated and 2-alkylated amino-5-(4-(benzyloxy)phenyl) thiophene-3-carboxylic acid derivatives. Eur. J. Med. Chem. 2011, 46, 3551–3563.10.1016/j.ejmech.2011.05.018Search in Google Scholar PubMed
[17] Yahia, N. M.; Fatima, A.; Nahed, N. E. E. S.; Salim, A. S.; Nablia, A. K.; Abdul, W.; Abdur, R.; Saud, B.; Taibi, B. H. Antimicrobial activity of some novel armed derivatives and Petra/Osiris/Molinspiration (POM) analyses. Molecules2016, 21, 222.10.3390/molecules21020222Search in Google Scholar PubMed PubMed Central
[18] Vilsmeier, A.; Haack, A. The effect of halogen phosphor on alkyl formanilide – a new method for the characterisation of secondary and tertiary p-alkylamino-benzaldehyde. Ber. Dt. Chem. Ges.1927, 60, 119–122.10.1002/cber.19270600118Search in Google Scholar
[19] Gowda, G. B.; Charnaraj, T. P.; Kumara, C. S. P.; Ramesh, N.; Thomas, S. P.; Sadashiva, M. P.; Junjappa, H. Synthesis of novel β-aryl-β-(methylthio)acroleins via Vilsmeier-Haack protocol as potential 1,3-dielectrophilic three-carbon building blocks. Tetrahedron Lett. 2014, 55, 4475–4479.10.1016/j.tetlet.2014.06.065Search in Google Scholar
[20] Mounyr, B.; Moulay, S.; Saad, K. I. Method for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 2016, 6, 71–79.10.1016/j.jpha.2015.11.005Search in Google Scholar PubMed PubMed Central
[21] McCauley, J.; Zivanovic, A.; Skropeta, D. Bioassays for anticancer activities. Methods Mol. Biol. 2013, 1055, 191–205.10.1007/978-1-62703-577-4_14Search in Google Scholar PubMed
[22] Juan, C. S.; Alfonso, B. C.; Magdalena, C.; Richar, W. H.; Angele, V. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem.2012, 114, 785–796.10.1016/j.acthis.2012.01.006Search in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Tandem hetero-Diels–Alder-hemiacetal reaction in the synthesis of new chromeno[4′,3′:4,5]thiopyrano[2,3-d]thiazoles
- A new method for the reaction of cross-coupling: preparation of 5,5′-bi(1,10-phenanthroline)
- Design and synthesis of 4,5-diaryl/heteroarylthiophene-2-carboxylic acid derivatives and evaluation of their biological activities
- Research Articles
- Synthesis and properties of dicarbazolyltriphenylethylene-substituted fluorene derivatives exhibiting aggregation-induced emission enhancement
- Synthesis of a new polycyclic heterocyclic ring system. Part III. Benzo[b]imidazo[1,5-d][1,4]oxazepine-1,4(2H,5H)-diones
- Br2- or HBr-catalyzed synthesis of asymmetric 3,3-di(indolyl)indolin-2-ones
- Mono- and bis-dipicolinic acid heterocyclic derivatives – thiosemicarbazides, triazoles, oxadiazoles and thiazolidinones as antifungal and antioxidant agents
- Synthesis and antimicrobial activity of new piperazine-based heterocyclic compounds
- Synthesis and evaluation of chromene-based compounds containing pyrazole moiety as antimicrobial agents
- Ultrasonic synthesis, characterization, and antibacterial evaluation of novel heterocycles containing hexahydroquinoline and pyrrole moieties
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Tandem hetero-Diels–Alder-hemiacetal reaction in the synthesis of new chromeno[4′,3′:4,5]thiopyrano[2,3-d]thiazoles
- A new method for the reaction of cross-coupling: preparation of 5,5′-bi(1,10-phenanthroline)
- Design and synthesis of 4,5-diaryl/heteroarylthiophene-2-carboxylic acid derivatives and evaluation of their biological activities
- Research Articles
- Synthesis and properties of dicarbazolyltriphenylethylene-substituted fluorene derivatives exhibiting aggregation-induced emission enhancement
- Synthesis of a new polycyclic heterocyclic ring system. Part III. Benzo[b]imidazo[1,5-d][1,4]oxazepine-1,4(2H,5H)-diones
- Br2- or HBr-catalyzed synthesis of asymmetric 3,3-di(indolyl)indolin-2-ones
- Mono- and bis-dipicolinic acid heterocyclic derivatives – thiosemicarbazides, triazoles, oxadiazoles and thiazolidinones as antifungal and antioxidant agents
- Synthesis and antimicrobial activity of new piperazine-based heterocyclic compounds
- Synthesis and evaluation of chromene-based compounds containing pyrazole moiety as antimicrobial agents
- Ultrasonic synthesis, characterization, and antibacterial evaluation of novel heterocycles containing hexahydroquinoline and pyrrole moieties

