Abstract
Novel triazole Schiff-base derivative 3 bearing a coumarin unit was synthesized by the reaction of 4-formylbenzocoumarin (1) with 3,5-diphenyl-4-amino-1,2,4-triazole (2). The structure of the product was characterized by means of IR, MS, 1H NMR, 13C NMR, and elemental analysis. The UV-vis absorption and fluorescence emission spectra of compound 3 exhibit blue-shifted absorption and fluorescent enhancement upon chelation to cupric ion. Fluorometric titration revealed a 2:1 ligand to metal ratio in the complex and the binding constant of 4.62×106m-1.
Introduction
Selective recognition and sensing of transition metal ions have become a focus of numerous studies in supramolecular chemistry owing to their importance in chemical, biological, and environmental processes [1–4]. Among the essential heavy metal ions in the human body, Cu2+ is third in abundance after Fe3+ and Zn2+, and it plays important roles in many fundamental physiological processes. Either deficiency or overdose of copper can induce some serious diseases [5–7]. Consequently, much attention has been given to the development of selective Cu2+ sensors [8–10]. However, owing to the low concentrations at which this metal ion is present in biosystems, high-sensitivity probes are necessary for practical applications.
Schiff bases are known to form stable complexes with transition metal ions and to display different optical properties from the ligand itself. Recently, there have been some excellent studies on chemosensors and molecular logic gates based on Schiff bases [11–13], the nitrogen atoms which easily coordinate metal ions. Owing to their intrinsic high fluorescence quantum yield, good water solubility, and viability for chemical transformations, coumarin derivatives have attracted much attention as some of the most popular fluorophores amenable to a novel sensor design [14, 15]. In this paper, a novel Schiff-base derivative bearing a coumarin unit 3 was synthesized, characterized, and its recognition ability for Cu2+ was studied. The results showed that probe 3 is an excellent selective probe for Cu2+ in the presence of other metal ions.
Results and discussion
The target product 3 was synthesized using a three-step procedure as shown in Scheme 1. The preparation of the intermediate products 1 and 2 has been reported previously [16, 17]. The desired triazole Schiff-base derivative 3 was obtained by condensation of 1 with 2. The target compound was characterized by IR, MS, 1H NMR, 13C NMR, and elemental analysis.

Synthesis of the triazole Schiff-base derivative 3.
UV-vis spectroscopic changes of the prepared chemosensing ensemble upon addition of various cations (Ca2+, Cd2+, Cu2+, Hg2+, Mn2+, Ni2+, Pb2+, Zn2+, Mg2+, Na+, Al3+, Fe3+, Ag+) are shown in Figure 1. A significant change in the absorption intensity is observed upon addition of Cu2+. No influence on the ultraviolet absorption of 3 is observed in the presence of other tested ions. Thus, it can be concluded that compound 3 selectively recognizes Cu2+.

UV-vis spectra of compound 3 (10 μm) upon addition of various metal ions (20 μm) in a mixture of CH3 OH/DMF (9:1).
As shown in Figure 2, compound 3 exhibits a weak fluorescence emission at 551 nm in CH3 OH/DMF medium. After addition of Cu2+ to the solution of compound 3, the fluorescence intensity is enhanced remarkably, that is, the emission intensity is increased 12-fold. Fluorescence enhancement observed for 3 is attributed to the formation of the 3-Cu2+ complex, as a result of which the electron transfer from nitrogen atoms to the coumarin fluorophore is suppressed, resulting in fluorescent enhancement. Importantly, no change in fluorescence occurs in the presence of the other cations including Ca2+, Cd2+, Mn2+, Ni2+, Pb2+, Zn2+, Mg2+, Na+, Al3+, Fe3+, Hg2+, and Ag+ (not shown).

Fluorescence emission spectra of compound 3 upon addition of cupric ion (10 μm) in a mixture of CH3 OH/DMF (9:1).
The sensitivity of the fluorescent probe 3 was estimated by changing amounts of Cu2+ from 0 equiv to 3 equiv with regard to the ligand and quantitatively assessing the fluorescence emission intensity change (Figure 3). As already mentioned, before addition of Cu2+, the ligand is almost nonemissive. After the gradual addition of Cu2+ to the ligand, a significant increase in fluorescence intensity can be observed. From these titrations, the association constant of compound 3 for Cu2+, Ka =4.62×106, and the limit of detection, LOD =3.72×10-7m, were obtained.
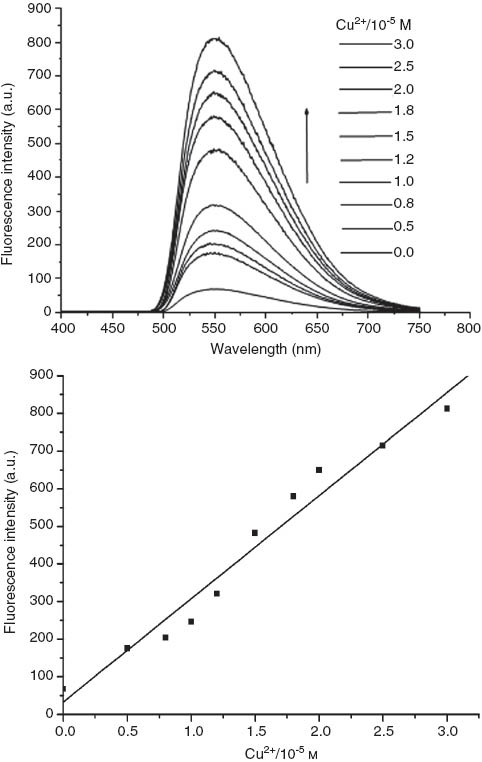
Fluorescence emission spectra of compound 3 (10 μm) for Cu2+ ion titration in a mixture of CH3 OH/DMF (9:1).
Job’s plot was used to analyze the complexation ratio between compound 3 and Cu2+ ion. As shown in Figure 4, the maximum point at the mole fraction of 0.33 indicates the complexation ratio of compound 3 and Cu2+ as 2:1.

Job’s plot for determining the stoichiometry of receptor 3 and Cu2+ ion in CH3 OH/DMF (9:1). I and I0 are the fluorescence intensity of 3 in the presence and absence of Cu2+, respectively. The total concentration of 3 and Cu2+ ion is 0.1 mm, λex=392 nm.
Conclusion
A new triazole Schiff-base derivative was designed and synthesized. Compound 3 displays selective UV-vis absorption and fluorescence changes upon the addition of cupric ion. The design of Schiff-base hosts for selective binding of other ions is currently under investigation.
Experimental
Coumarin aldehyde 1 [17] and 3,5-diphenyl-4-amino-1,2,4-triazole 2 [16] were prepared as previously described. Other chemicals were obtained from Aladdin and used without further purification.
Synthesis of (E)-4-[(3,5-diphenyl-4H-1,2,4-triazol-4-ylimino)methyl]-2H-benzo[h]chromen-2-one (3)
A mixture of 3,5-diphenyl-4-amino-1,2,4-triazole 2 (0.02 mol), coumarin aldehyde 1 (0.02 mol), and glacial acetic acid (20 mL) was heated under reflux for 8 h, then cooled and filtered. The solid product 3 thus obtained was crystallized from ethanol: yield 61%; mp 247–249°C (dec); FT-IR (KBr): 3022, 2994, 2897, 1682, 1605, 1497, 1466, 1377, 1229, 843, 720 cm-1; 1H NMR (500 MHz, CDCl3): δH 8.569 (1H, s, CH=N), 8.474 (1H, s, Ar-H), 7.909 (5H, m, Ar-H), 7.712 (2H, m, Ar-H), 7.590 (7H, m, Ar-H), 7.505(1H, d, J = 9.0 Hz, Ar-H), 6.677 (1H, s, Ar-H); 13C NMR (125 MHz, CDCl3): δC 162.2, 160.4, 152.8, 151.8, 144.2, 136.0, 131.5, 130.5, 130.2, 129.9, 128.6, 127.2, 125.7, 123.7, 120.8, 118.8; ESI-MS: m/z 442.6 (M + H+). Anal. Calcd for C28 H18 N4 O2: C 76.01, H 4.10, N 12.66. Found: C 76.07, H 4.08, N 12.62.
Acknowledgments
The project was supported by the Key Laboratory Project (2008S127) and the Initial Fund for Young Teachers of University of Science and Technology Liaoning (008131).
References
[1] Chandrasekhar, V.; Das, S.; Yadav, R.; Hossain, S.; Parihar, R.; Subramaniam, G.; Sen, P. Novel chemosensor for the visual detection of copper(II) in aqueous solution at the ppm level. Inorg. Chem.2012, 51, 8664–8666.Search in Google Scholar
[2] Zhang, J. F.; Zhou, Y.; Yoon, J.; Kim, J. S. Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem. Soc. Rev. 2011, 40, 3416–3429.Search in Google Scholar
[3] Zhao, B.; Zhou, Y. C.; Fan, M. J.; Li, Z. Y.; Wang, L. Y.; Deng, Q. G. Synthesis, fluorescence properties and selective Cr(III) recognition of tetraaryl imidazole derivatives bearing thiazole group. Chinese Chem. Lett.2013, 24, 257–259.Search in Google Scholar
[4] Huo, F. J.; Yin, C. X.; Yang, Y. T.; Su, J.; Chao, J. B.; Liu, D. S. Ultraviolet-visible light (UV-Vis)-reversible but fluorescence-irreversible chemosensor for copper in water and its application in living cells. Anal. Chem.2012, 84, 2219–2223.Search in Google Scholar
[5] Kumar, M.; Kumar, N.; Bhalla, V.; Sharma, P. R.; Kaur, T. Highly selective fluorescence turn-on chemodosimeter based on rhodamine for nanomolar detection of copper ions. Org. Lett.2012, 14, 406–409.Search in Google Scholar
[6] Barceloux, D. G. Copper. J. Toxicol. Clin. Toxicol.1999, 37, 217–230.Search in Google Scholar
[7] Thomas, T. G.; Sreenath, K.; Gopidas, K. R. Colorimetric detection of copper ions in submicromolar concentrations using a triarylamine-linked resin bead. Analyst2012, 137, 5358–5362.Search in Google Scholar
[8] Buck, K. N.; Ross, J. R. M.; Flegal, A. R.; Bruland, K. W. A review of total dissolved copper and its chemical speciation in San Francisco Bay. Environ. Res.2007, 105, 5–19.Search in Google Scholar
[9] Camargo, J. A.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ. Int.2006, 32, 831–849.Search in Google Scholar
[10] Guo, Z. Q.; Zhu, W. H.; Tian, H. Hydrophilic copolymer bearing dicyanomethylene-4H-pyran moiety as fluorescent film sensor for Cu2+ and pyrophosphate anion. Macromolecules2010, 43, 739–744.Search in Google Scholar
[11] Wang, L. N.; Qin, W. W.; Liu, W. S. A sensitive Schiff-base fluorescent indicator for the detection of Zn2+. Inorg. Chem. Commun.2010, 13, 1122–1125.Search in Google Scholar
[12] Gao, L.; Wang, Y.; Wang, J. Q.; Huang, L.; Shi, L. Y.; Fan, X. X.; Zou, Z. G.; Yu, T.; Zhu, M.; Li, Z. S. A novel ZnII-sensitive fluorescent chemosensor assembled within aminopropyl-functionalized mesoporous SBA-15. Inorg. Chem. 2006, 45, 6844–6850.Search in Google Scholar
[13] Li, G. B.; Fang, H. C.; Cai, Y. P.; Zhou, Z. Y.; Thallapally, P. K.; Tian, J. Construction of a novel Zn-Ni trinuclear Schiff base and a Ni2+ chemosensor. Inorg. Chem. 2010, 49, 7241–7243.Search in Google Scholar
[14] Gocmen, A.; Bulut, M.; Erk, C. A synthesis and characterization of coumarin-crown ethers. Pure Appl. Chem. 1993, 65, 447–450.Search in Google Scholar
[15] Kasapbasi, E.; Yurtsever, M. A DFT study on the structural and opticalp and cation selectivities of some metal-coumarin-crown ether complexes. Turk.J. Chem.2012, 36, 147–158.Search in Google Scholar
[16] Yukio, I.; Naoto, H.; Akikazu, K. A facile and solvent-free synthesis of 3,5-disubstituted-4-amino-1,2,4-triazoles by reactions of aromatic nitriles with hydrazine. Heterocycl.Commun. 2002, 8, 439–442.Search in Google Scholar
[17] Bender, D. R.; Kanne, D.; Frazier, J. D.; Rapoport, H. Synthesis and derivatization of 8-acetylpsoralens. Acetyl migrations during Claisen rearrangement. J. Org. Chem. 1983, 48, 2709–2719.Search in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Reviews
- Recent advances in dibenzo[b,f][1,4]oxazepine synthesis
- Recent trends in synthesis of five- and six-membered heterocycles using dimethyl N-cyanodithioiminocarbonate
- Preliminary Communication
- 12T061C, a new Julichrome family radical scavenger from Streptomyces species
- Research Articles
- Synthesis of pyrimido[4′,5′:2,3][1,4]thiazepino[7,6-b]quinolines, derivatives of a novel ring system
- Solvent-free multicomponent assembling of aldehydes, N,N′-dialkyl barbiturates and malononitrile: fast and efficient approach to pyrano[2,3-d]pyrimidines
- One-pot, three-component synthesis of pyranocoumarins containing an aroyl group
- Selective fluorescence sensor for Cu2+ with a novel triazole Schiff-base derivative with coumarin units
- Microwave-assisted synthesis of (E)-7-[(1-benzyl-1H-1,2,3-triazol-4-yl)methoxy]-8-(3-arylacryloyl)-4-methyl-2H-chromen-2-ones and their antimicrobial activity
- One-pot synthesis of 3,4-dihydro-3-hydroxyisochroman-1-one and evaluation of acetal derivatives as antibacterial and antifungal agents
- Copper-mediated ligand-free Ullmann reaction approach to substituted s-triazines: rationale, synthesis, and biological evaluation
Articles in the same Issue
- Frontmatter
- Reviews
- Recent advances in dibenzo[b,f][1,4]oxazepine synthesis
- Recent trends in synthesis of five- and six-membered heterocycles using dimethyl N-cyanodithioiminocarbonate
- Preliminary Communication
- 12T061C, a new Julichrome family radical scavenger from Streptomyces species
- Research Articles
- Synthesis of pyrimido[4′,5′:2,3][1,4]thiazepino[7,6-b]quinolines, derivatives of a novel ring system
- Solvent-free multicomponent assembling of aldehydes, N,N′-dialkyl barbiturates and malononitrile: fast and efficient approach to pyrano[2,3-d]pyrimidines
- One-pot, three-component synthesis of pyranocoumarins containing an aroyl group
- Selective fluorescence sensor for Cu2+ with a novel triazole Schiff-base derivative with coumarin units
- Microwave-assisted synthesis of (E)-7-[(1-benzyl-1H-1,2,3-triazol-4-yl)methoxy]-8-(3-arylacryloyl)-4-methyl-2H-chromen-2-ones and their antimicrobial activity
- One-pot synthesis of 3,4-dihydro-3-hydroxyisochroman-1-one and evaluation of acetal derivatives as antibacterial and antifungal agents
- Copper-mediated ligand-free Ullmann reaction approach to substituted s-triazines: rationale, synthesis, and biological evaluation

