Abstract
Alkanoic acid derivatives bearing a nitroxyl moiety 3a–e were synthesized from 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (1) and the corresponding 2-bromoalkane carboxylic acids 2a–e. The herbicidal and antifungal activity of 3a–e was tested. No herbicidal activity of the tested compounds was found. The 2-[(1-oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]butanoic acid 3c revealed a strong antifungal activity against the pathogenic fungus Phytophthora cactorum.
Introduction
Aryloxy herbicides such as 2,4-D, MCPA, MCPB are widely known commercial products against many weeds [1–3]. Herein (as a continuation of the investigations of the research concerning nitroxides [4]), we present the synthesis and both herbicidal and fungicidal screening of the nitroxyl derivatives of alkanoic acids with the nitroxyl moiety instead of the aryl group found in the typical aryloxy herbicides. These compounds are schematically presented below as structures Ar and N-O.
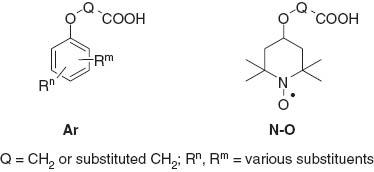
To synthesize the compounds generally abbreviated above as N-O, alkylation of 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (1) with a bromoalkanoic acid in the presence of a base is required. Alkylation of the hydroxyl group in 1 is usually performed under basic conditions. Examples are phase transfer catalysis (PTC) method (50% aqueous sodium hydroxide as a base) with tetrabutylammonium bromide [5, 6], hydrogen sulfate [7, 8] or triethylbenzylammonium chloride [9] as PTC catalysts and the reactions conducted in the presence of sodium hydride [10–14], sodium amide, cesium fluoride or potassium carbonate [15].
Results and discussion
Alkanoic acid derivatives bearing the nitroxyl moiety 3a–e were obtained by the alkoxylation of 2-bromoalkanecarboxylic acids 2a–e with an alkoxide anion of 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl 1 generated in the presence of sodium hydride (Scheme 1). Briefly, 2-bromocarboxylic acid 2 was added to the excess of sodium hydride in THF, and then a nitroxyl alcohol 1 was added. The mixture was heated under reflux for 24 h.
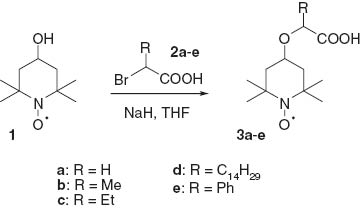
Synthesis of alkanoic acid derivatives bearing a nitroxyl moiety 3a–e.
The nitroxyl radicals 3a–e were identified using spectroscopic methods (EI MS, ESI MS, HRMS and IR). The 1H NMR spectra are not informative in these cases because signals of protons are paramagnetically broadened and unresolved owing to the presence of nitroxide radicals [16–20].
The herbicidal and antifungal activity of 3a–c, 3e was assayed. Compound 3d was not tested, due to its negligible yield. The compounds 3a,b,e show medium activity against four species of pathogenic fungi Botrytis cinerea, Fusarium culmorum, Phytophthora cactorum and Rhizoctonia solani. The butanoic acid derivative 3c shows strong antifungal activity against pathogenic fungus P. cactorum (Table 1).
Antifungal activity of 3a–e at a concentration of 200 ppm.
| No. | Botrytis cinerea | Fusarium culmorum | Phytophthora cactorum | Rhizoctonia solani |
|---|---|---|---|---|
| 3a | 32 | 44 | 9 | 0 |
| 3b | 32 | 46 | 16 | 20 |
| 3c | 36 | 34 | 100 | 0 |
| 3e | 24 | 40 | 29 | 26 |
Experimental
General
4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (1) was synthesized by oxidation of 2,2,6,6-tetramethyl-4-piperidinol with 30% hydrogen peroxide (76% yield, mp 71–73°C), as previously described [21–23]. THF was distilled over sodium under argon in the presence of benzophenone. The experiments were performed in a two-necked round-bottom flask of 25 mL capacity, equipped with a magnetic stir bar, reflux condenser protected against humidity, under an argon atmosphere. TLC was carried out on silica gel Merck Alurolle 5562 or Alufolien 5554. Column chromatography was performed using Merck silica gel, 230–400 mesh. TLC visualization was performed using UV 254 nm light and/or iodine vapor. EI MS data were recorded on an AMD 604 and Agilent Technologies 5975 B mass spectrometers. EI mass spectra were obtained at 70 eV. ESI mass spectra (negative ionisation, CH3OH as solvent) were recorded on a Micromass LCT apparatus. IR spectra were recorded on an FT/IR Jasco 420 spectrophotometer. Conditions of the fungicidal bioassay in vitro are identical to those described previously [24].
General procedure for 3a–e
Anhydrous THF (5 mL) and 2-bromoalkanecarboxylic acid 2a–e (1 mmol) were placed in a flask and the resultant solution was treated with a 60% slurry of sodium hydride in mineral oil (0.9 g). After the evolution of hydrogen ceased, compound 1 (0.17 g, 1 mmol) was added. The mixture was stirred while it foamed again, then heated under gentle reflux under argon for 24 h. After cooling, water (20 mL) was carefully added and the resultant dark orange solution was transferred to a separation funnel. The aqueous mixture was washed with diethyl ether (2 × 25 mL), with dichloromethane (2 × 20 mL) and then acidified with 1 N hydrochloric acid to pH 2. The acidified aqueous layer was extracted with dichloromethane (3 × 20 mL). The extract was dried with anhydrous magnesium sulfate, filtered and concentrated. The residue was subjected to silica gel chromatography eluting with benzene/methanol (9:1) to give product 3a–e as red oils.
[(1-Oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]acetic acid (3a)
Yield 51%; IR (ν, cm-1, film): 2977, 2938 (C-H, stretch), 1737 (C=O, stretch), 1177, 1123 (C-O, stretch); EI MS: m/z 277 (4), 231 (7), 230 (7, M+), 216 (100), 200 (40), 160 (6), 143 (18), 140 (87), 129 (11), 124 (51), 109 (19), 108 (28), 107 (34), 102 (33), 98 (39), 85 (61), 84 (21), 83 (20), 82 (22), 81 (25), 74 (28), 71 (91), 69 (27), 67 (28), 58 (43), 57 (29), 56 (44), 55 (59), 45 (23), 43 (57), 42 (57), 41 (97); ESI MS: m/z 230 (15, M-·), 229 (100, [M-H]-), 171 (5); HR ESI MS. Calcd for C11H19NO4 ([M-H]-): m/z 229.1314, found: m/z 229.1325.
2-[(1-Oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]propanoic acid (3b)
Yield 46%; IR (ν, cm-1, film): 3395, 2976, 2950 (C-H, stretch), 1732 (C=O, stretch), 1460 (CH3, bend), 1365 (CH2, CH3, bend), 1178, 1120, 1065 (C-O, stretch); EI MS: m/z 244 (4, M+), 230 (2), 172 (2), 157 (4), 154 (4), 149 (7), 143 (6), 140 (6), 139 (6), 124 (13), 109 (16), 98 (11), 85 (37), 71 (100), 69 (18), 57 (27), 56 (20), 55 (29), 45 (29), 43 (68), 42 (41), 41 (93); ESI MS: m/z 311 (3), 244 (20, M-·), 243 (100, [M-H]-), 171 (3); HR ESI MS. Calcd for C12H21NO4 [M-H]-: m/z 243.1471, found: m/z 243.1474.
2-[(1-Oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]butanoic acid (3c)
Yield 42%; IR (ν, cm-1, KBr): 2974, 2930 (C-H, stretch), 1726 (C=O, stretch), 1460 (CH3, bend), 1389 (CH2, CH3, bend), 1178, 1130, 1080 (C-O, stretch); EI MS: m/z 258 (30, M+), 244 (32), 228 (4), 213 (2), 202 (4), 171 (14), 157 (21), 155 (15), 154 (17), 140 (26), 139 (24), 124 (60), 116 (7), 109 (48), 98 (24), 85 (100), 71 (26), 69 (34), 57 (13), 56 (19), 55 (27), 43 (15), 41 (43); ESI MS: 258 (5, M-·), 257 (100, [M-H]-); HR ESI MS. Calcd for C13H23NO4 [M-H]-: 257.1627, found: m/z 257.1622.
2-[(1-Oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]hexadecanoic acid (3d)
Yield 3%; IR (ν, cm-1, KBr): 2925, 2854 (C-H, stretch), 1717 (C=O, stretch), 1462 (CH3, bend), 1378 (CH2, CH3, bend), 1109 (C-O, stretch); EI MS: m/z 426 (7, M+), 412 (100), 396 (57), 156 (16), 155 (11), 154 (9), 140 (43), 124 (43), 109 (11), 100 (14), 98 (17), 87 (10), 85 (21), 83 (19), 74 (20), 71 (22), 69 (21), 67 (9), 58 (19), 57 (20), 56 (16), 55 (30), 43 (43), 41 (25); ESI MS: 426 (100, M-·), 313 (5), 221 (10); HR ESI MS. Calcd for C25H48NO4 (M-·): 426.3583, found: m/z 426.3569.
[(1-Oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy](phenyl)acetic acid (3e)
Yield 53%; IR (ν, cm-1, KBr): 3430, 2976, 2960 (C-H, stretch), 1736 (C=O, stretch), 1460 (CH3, bend), 1389 (CH2, CH3, bend), 1177, 1099 (C-O, stretch); EI MS: m/z 306 (33, M+), 292 (55), 276 (10), 261 (10), 155 (23), 154 (24), 140 (28), 139 (18), 136 (80), 135 (100), 124 (52), 118 (18), 109 (31), 107 (42), 105 (10), 100 (13), 98 (16), 85 (17), 83 (12), 82 (13), 81 (14), 79 (44), 77 (27), 74 (11), 69 (23), 67 (12), 57 (17), 56 (19), 55 (25), 41 (34); ESI MS: 306 (30, M-·), 305 (100, [M-H]-), 261 (10), 239 (8), 171 (35); HR ESI MS. Calcd for C17H23NO4 [M-H]-: m/z 305.1627, found: m/z 305.1640.
Acknowledgment
This work was supported by the Polish Ministry of Science and Higher Education, 4180/E-142/S/2013.
References
[1] Krawczyk, M.; Legocki, J. Badania estrów alkilowych kwasu 2,4-dichlorofenoksyoctowego (2,4-D) i kwasu 4-chloro-2-metylofenoksyoctowego (MCPA). Przem. Chem. 2007, 86, 506–509.Search in Google Scholar
[2] Bovey, R. W.; Young, A. L. The Science of 2,4,5-T and Associated Phenoxy Herbicides; John Wiley & Sons: Hoboken, NJ, 1980.Search in Google Scholar
[3] Schulz, C. O.; Segal, S. A.; Reichardt, W. D. Review of literature on herbicides, including phenoxy herbicides and associated dioxins. Chemosphere1990, 20, 1001–1004.Search in Google Scholar
[4] Zakrzewski, J. Reactions of nitroxides XIII: synthesis of the Morita-Baylis-Hillman adducts bearing a nitroxyl moiety using 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl as a starting compound, and DABCO and quinuclidine as catalysts. Beilstein J. Org. Chem.2012, 8, 1515–1522.Search in Google Scholar
[5] Wunderlich, W.; Clauss, M.; Roth, M.; Fuso, F.; Ciba Specialty Chemicals Corp. Use of 2,2,6,6 tetraalkylpiperidine-N-oxyl radicals having long alkyl chains as polymerization regulators. US Patent 6864313, 2005.Search in Google Scholar
[6] Luston, J.; Smieskova, E.; Vass, F. 4-(2,3-Epoxypropoxy)-2,2,6,6-tetrametylpiperidin-1-oxyl a sposob jeho priprawy. Czech. Patent 254699, 1987; Chem. Abstr.1987, 111, 115143.Search in Google Scholar
[7] Chang, C.; Zhu, J.; Zhang, Z.; Zhou, N.; Cheng, Z.; Zhu, X. Synthesizing and characterization of comb-shaped carbazole containing copolymer via combination of ring opening polymerization and nitroxide-mediated polymerization. Polymer2010, 51, 1947–1953.Search in Google Scholar
[8] Song, C.; Hu, K.-N.; Joo, C.-G.; Swager, T. M.; Griffin, R. G. TOTAPOL: a biradical polarizing agent for dynamic nuclear polarization experiments in aqueous media. J. Am. Chem. Soc.2006, 128, 11385–11390.Search in Google Scholar
[9] Zakrzewski, J. Synthesis of 2-hydroxybenzophenone derivatives bearing 2,2,6,6-tetramethylpiperidine moiety. Thesis, Technical University of Poznan, 1997.Search in Google Scholar
[10] Wagner, H.; Brinks, M. K.; Studer, A.; Hirtz, M.; Chi, L.; Schaefer, A. Chemical surface modification of self-assembled monolayers by radical nitroxide exchange reactions. Chem. Eur. J.2011, 17, 9107–9112.Search in Google Scholar
[11] Matsumoto, K.; Iwata, T.; Suenaga, M.; Okudomi, M.; Nogawa, M.; Nakano, M.; Sugahara, A.; Bannai, Y.; Baba, K. Mild oxidation of alcohols using soluble polymer-supported TEMPO in combination with oxone: effect of a basic matrix of TEMPO derivatives. Heterocycles2010, 81, 2539–2553.Search in Google Scholar
[12] Miyazawa, T.; Endo, T.; Shiihashi, S.; Okawara, M. Selective oxidation of alcohols by oxoaminium salts. J. Org. Chem.1985, 50, 1332–1334.Search in Google Scholar
[13] Debuigne, A.; Caille, J.-R.; Jerome, R. Synthesis of end-functional poly(vinyl acetate) by cobalt-mediated, radical polymerization. Macromolecules2005, 38, 5452–5458.Search in Google Scholar
[14] Endo, T.; Takuma, K.; Takata, T.; Hirose, C. Synthesis and polymerization of 4-(glycidyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl. Macromolecules1993, 26, 3227–3229.Search in Google Scholar
[15] Zeika, O.; Li, Y.; Jockusch, S.; Parkin, G.; Sattler, A.; Sattler, W.; Turro, N. J. Synthesis of polynitroxides based on nucleophilic aromatic substitution. Org. Lett.2010, 12, 3696–3699.Search in Google Scholar
[16] Einhorn, J.; Einhorn, C.; Ratajczak, F.; Durif, A.; Averbuch, M.-T.; Pierre, J.-L. Synthesis and resolution of a chiral analogue of 2,2,6,6-tetramethylpiperidine and of its corresponding nitroxide. Tetrahedron Lett. 1998, 39, 2565–2568.Search in Google Scholar
[17] Barrett, A. G. M.; Hanson, G. R.; White, A. J. P.; Williams, D. J.; Micallef, A. S. Synthesis of nitroxide-functionalized phthalocyanines. Tetrahedron2007, 63, 5244–5250.Search in Google Scholar
[18] Gubskaya, V. P.; Berezhnaya, L. Sh.; Gubaidullin, A. T.; Faingold, I. I.; Kotelnikova, R. A.; Konovalova, N. P.; Morozov, V. I.; Litvinov, I. A.; Nuretdinov, I. A. Synthesis, structure and biological activity of nitroxide malonate methanofullerenes. Org. Biomol. Chem.2007, 5, 976–981.Search in Google Scholar
[19] Yi, S.; Mileo, E.; Kaifer, A. E. EPR and NMR investigation on the interactions of nitroxide probes with resorcin[4]arene molecular capsules. Org. Lett.2009, 11, 5690–5693.Search in Google Scholar
[20] Fairfull-Smith, K. E.; Debele, E. A.; Allen, J. P.; Pfrunder, M. C.; McMurtrie, J. C. Direct iodination of isoindolines and isoindoline nitroxides as precursors to functionalized nitroxides. Eur. J. Org. Chem.2013, 4829–4835.10.1002/ejoc.201300313Search in Google Scholar
[21] Brière, R.; Lemaire, H.; Rassat, A. Nitroxides XV: Synthèse et étude de radicaux libres stables pipéridiniques et pyrrolidinique. Bull. Soc. Chim. Fr.1965, 3273–3283.Search in Google Scholar
[22] Sosnovsky, G.; Konieczny, M. Preparation of 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl. A reinvestigation of methods using hydrogen peroxide in the presence of catalysts. Z. Naturforsch. B1976, 31B, 1376–1378.10.1515/znb-1976-1017Search in Google Scholar
[23] Zakrzewski, J. A reaction of nitroxides with ethyl mercaptane: a mild method for the conversion of nitroxides into their corresponding amines. Monat. Chem.1990, 121, 803–808.Search in Google Scholar
[24] Zakrzewski, J.; Krawczyk, M. Reactions of nitroxides with sulphur-containing compounds, part IV: synthesis of novel nitroxide (thio)ureas. Heteroatom Chem. 2006, 17, 393–401.Search in Google Scholar
©2014 by Walter de Gruyter Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents of plants from the genus Neolitsea
- Preliminary Communication
- One-pot two-step synthesis of 2,5-dihydro-2-oxofuran-3-carboxamides
- Research Articles
- Synthesis of new 2- and 3-hydroxyquinoline-4-carboxylic acid derivatives as potential antioxidants
- Reactions of nitroxides XIV. Analogs of phenoxy carboxylic herbicides based on the piperidine scaffold; unexpected fungicidal activity of the 2-[(1-oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]butanoic acid
- A simple approach to fused pyrido[2,3-d]pyrimidines incorporating khellinone and trimethoxyphenyl moieties as new scaffolds for antibacterial and antifungal agents
- Synthesis and molecular docking of indole and carbazole derivatives with potential pharmacological activity
- An access to new N-pyrrolylcarboxylic acids as potential COX-2 inhibitors via Paal-Knorr cyclization
- Structural modification of isoalantolactone and biological activity against the hepatoma cell lines
- Three-component anti selective Mannich reactions in a tetrahydro-4-pyranone system by using PDAG-Co catalyst
- Synthesis of novel 7-(heteryl/aryl)chromones via Suzuki coupling reaction
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents of plants from the genus Neolitsea
- Preliminary Communication
- One-pot two-step synthesis of 2,5-dihydro-2-oxofuran-3-carboxamides
- Research Articles
- Synthesis of new 2- and 3-hydroxyquinoline-4-carboxylic acid derivatives as potential antioxidants
- Reactions of nitroxides XIV. Analogs of phenoxy carboxylic herbicides based on the piperidine scaffold; unexpected fungicidal activity of the 2-[(1-oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]butanoic acid
- A simple approach to fused pyrido[2,3-d]pyrimidines incorporating khellinone and trimethoxyphenyl moieties as new scaffolds for antibacterial and antifungal agents
- Synthesis and molecular docking of indole and carbazole derivatives with potential pharmacological activity
- An access to new N-pyrrolylcarboxylic acids as potential COX-2 inhibitors via Paal-Knorr cyclization
- Structural modification of isoalantolactone and biological activity against the hepatoma cell lines
- Three-component anti selective Mannich reactions in a tetrahydro-4-pyranone system by using PDAG-Co catalyst
- Synthesis of novel 7-(heteryl/aryl)chromones via Suzuki coupling reaction


