Abstract
The attractiveness of heterocyclic compounds in medicinal chemistry has increased significantly in the past few decades as they have been proven to be highly active for a number of purposes. More specifically, the benzothiazole-containing heterocyclic compounds have shown great promise in the pharmaceutical industry. As continuation of our first review article of the synthesis and specific applications of various benzothiazole cyanine dyes (Henary, M.; Paranjpe, S.; Owens, E. A. Synthesis and application of benzothiazole containing cyanine dyes. Heterocycl. Commun. 2013, 19, 1–11), we will focus in this review on the synthesis and medical applications of alternate compounds that utilize the benzothiazole scaffold as a part of their molecular structure. Benzothiazole derivatives encompass an attractive heterocyclic class that exhibits exciting medicinal properties. The applicability of these heterocyclic structures includes positron emission tomography probes for monitoring Alzheimer’s disease progression; furthermore, these compounds exhibit antimicrobial, anti-inflammatory, anticancer, antidiabetic and anti-HIV activity. Benzothiazole-containing heterocyclic structures are prominent throughout the literature and it is very important to acknowledge their efficacy and applicability. Herein, we review the recent developments covering the past few years concerning the advancements in synthetic methodology for the preparation of medicinally relevant benzothiazole-containing heterocyclic structures.
Introduction
In the past few years, there has been an increasing interest in the chemistry of benzothiazole related compounds. These types of compounds have shown significant biological activities with a wide range of practical applications in the medical field. The following is a brief review of heterocyclic compounds containing the benzothiazole moiety and the corresponding advancements in the medical field utilizing this heterocyclic scaffold. Benzothiazole-containing heterocycles are becoming ever more prevalent within pharmacological research due to their amenability for diverse modifications with the potential for structurally tailoring the heterocycle towards exciting medical purposes. The broad spectrum of medical applications includes uses as anti-inflammatory agents and therapeutic compounds for combating cancer at very effective nanomolar concentrations; to begin, we introduce thioflavin-T and the synthesis of corresponding analogs for radiolabeled imaging of β-amyloid plaques in Alzheimer’s disease patients for determining the presence, progression and prognosis of the disease.
Positron emission tomography (PET) probes
Benzothiazole derivatives are recognized as a promising class of compounds for the imaging of β-amyloid (Aβ) plaques. Thioflavin-T is a benzothiazole-containing imaging agent which shows fluorescence enhancement upon binding to aggregates of protein amyloids [1, 2].
Patients with Alzheimer’s disease (AD) show the accumulation of β-amyloid fibrils in the brain [3, 4] and these fragments are then converted into hard plaques which directly contribute to the progression of AD. In recent studies, it has been suggested that by imaging these plaques, the severity and prognosis of AD can be estimated [5]. Among the existing molecular imaging technologies, PET possesses several advantages such as high intrinsic sensitivity, increased penetration depth and excellent spatial resolution while being fully quantitative [5]. PET is a widely recognized nuclear imaging technique employed to determine the severity of AD which makes use of nuclear probes containing positron emitters; specifically, radiolabeled benzothiazole derivatives are finding great applicability as potential PET probes. Several synthetic approaches have been explored for synthesizing various derivatives of thioflavin-T (Figure 1), including uncharged derivatives [6]. The synthesis of an uncharged derivative, compound 1, is shown in Equation 1. Commercially available substrate 6-Me-BTA-0 is allowed to react with methyl iodide in the presence of potassium carbonate to furnish compound 1 in 18% yield (the acronym BTA refers to benzothiazole-aniline backbone).

Structure of thioflavin-T.

The neutral derivatives of thioflavin-T exhibit higher affinity towards Aβ than the cationic thioflavin-T as evident by the affinity for the inhibitor (Ki) values (Table 1). The 6-Me-BTA-1 was further radiolabeled with 11C at the aniline nitrogen atom to form N-methyl-11C analog of 6-Me-BTA-2 which demonstrated high uptake and improved clearance from the rodent brain during animal experimentation (Figure 2).
Ki values of various benzothiazole derivatives.
| Compound | Ki (nm) |
|---|---|
| Thioflavin-T | 820±92 |
| 6-Me-BTA-2 (R1 = R2= Me) | 143±19 |
| 6-Me-BTA-1 (R1 = H; R2= Me) (1) | 20.3±3.0 |
| 6-Me-BTA-0 (R1 = R2= H) | 30.3±5.9 |

6-Me-BTA compounds.
Klunk et al. at the University of Pittsburgh developed another benzothiazole-based compound, N-methyl-[11C]2-[4′-(methylamino)phenyl]-6-hydroxybenzothiazole (compound 8 in Scheme 1), more popularly known as Pittsburgh compound B (PIB) that exhibits high binding affinity towards Aβ plaques [5]. Commercially available 4-methoxyaniline (2) was treated with p-nitrobenzoyl chloride in the presensce of pyridine to give compound 3 that was subsequently reacted with Lawesson’s reagent. The resulting compound 4 was cyclized to the benzothiazole derivative 5 through the Jacobsen synthesis using potassium ferricyanide and aqueous sodium hydroxide. The methoxy group of compound 5 was demethylated in the subsequent step and the resultant hydroxy group was then protected by using the labile methoxymethyl group (product 6), which on reduction with sodium borohydride in the presence of cupric acetate yielded compound 7. The radiolabeling was then achieved by treatment with sodium hydride in N,N-dimethylformamide (DMF), then with 11MeI followed by hydrolysis of the methoxymethyl group to furnish final compound 8 in approximately 15% radiochemical yield.

PIB retention in the brain of AD patients was found to be selectively higher than in non-AD patients. PIB is considered as a popular choice as radiotracer for PET imaging of Aβ plaques in AD patients [7].
A new and improved route for the synthesis of [11C] PIB is shown in Equation 2 [8]. The reaction was carried out using [11C] methyl triflate in the absence of base in acetone on the unprotected hydroxybenzothiazole base. This reaction resulted in increasing radiochemical yield for the compound to approximately 60% at the end of the synthesis; this important report is significant when designing compounds for clinical use to maximize the radiolabeled output for imaging.

As shown above, several PET probes containing the benzothiazole core structure have been developed in the past few years. These probes have been modified based on the thioflavin-T structure and these derivatives were successfully demonstrated to bind Aβ plaques in the brain [9, 10].
Pharmacological activities of benzothiazole compounds
The general benzothiazole structure is an important scaffold for drug development, and the corresponding derivatives have been extensively studied for their pharmacological applications. This idea of incorporating benzothiazole in designing medicinal compounds will be further expanded in the following sections, especially nuanced towards the ability to combat dangerous microbes, reduce inflammation, fight diabetes and improve the prognosis of patients diagnosed with HIV [11].
Antimicrobial activity
Ouyang et al. (Scheme 2) reported the synthesis of various benzothiazole derivatives bearing secondary amine functionalities as potential antimicrobial agents [12]. The synthesized compounds were evaluated for their antibacterial activity against American Type Culture Collection (ATCC) strains of Staphylococcus aureus, Enterococcus faecalis and Escherichia coli using the standard broth dilution method. Compound 13 demonstrated exciting inhibition properties towards Gram-positive bacteria as well as displayed promising behavior against drug-resistant bacteria such as methicillin-resistant S. aureus and vancomycin-resistant E. faecalis. Active compound 13 was synthesized as shown in Scheme 2. Commercially available propanediamine (9) was treated with Boc anhydride in tetrahydrofuran (THF), and the resulting mono-Boc protected compound 10 was then allowed to react with 2-chloro-5,6-difluorobenzothiazole in the presence of potassium carbonate to afford compound 11. Compound 11 in the next step was hydrolyzed with trifluoroacetic acid to the corresponding primary amine 12 which, using the coupling strategy shown, was transformed into compound 13.

Bandyopadhyay et al. synthesized a series of 2-substituted benzothiazoles using an Al2O3-Fe2O3 nanocatalyst as shown in Equation 3 [13]. The antibacterial activity of the synthesized compounds was evaluated using the Kirby-Bauer disc diffusion method. Compound 14 exhibited enhanced inhibitory activity against Vibrio cholerae, Bacillus cereus and Shigella dysenteriae compared with the well-known antibacterial drug ciprofloxacin. Compound 15 showed complete bactericidal activity within 24 h compared with 48 h for ciprofloxacin.

Anticancer properties
Shi et al. reported the synthesis of benzothiazole-2-thiol derivatives as potential anticancer agents. These compounds were evaluated against various cancer cell lines [14]. Synthesis was carried out as shown in Scheme 3. Commercially available amines 16–18 were reacted with 2-chloroacetyl chloride in the presence of potassium carbonate, and the resulting compounds 19–21 were reacted with 6-aminobenzothiazole-2-thiol to afford compounds 22–24. In the last step of the synthetic scheme, compounds 22–24 were treated with 2-chloroacetyl chloride in the presence of triethylamine to furnish final compounds 25–27 in 70–90% yield. Compound 26 demonstrated promising activity against SKRB-3 human breast cancer cells (IC50 = 1.2 nm), SW620 colon cancer cells (IC50 = 4.3 nm), A549 (IC50 = 44 nm) and HepG2 hepatic carcinoma cells (IC50 = 48 nm) as well as induced apoptosis in HepG2 cancer cells.

A series of 2-phenylbenzothiazoles was synthesized by Mortimer et al. and evaluated in vitro against breast and colon cancer cell lines [15]. The synthesis of the most efficient compound 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (29) was performed as shown in Scheme 4; the 2-amino-5-fluoro-benzothiazole was converted to compound 28 by treatment with an aqueous potassium hydroxide solution followed by acidification and air oxidation. The disulfide 28 was then treated with 3,4-dimethoxybenzaldehyde in the presence of triphenylphosphine and a catalytic amount of p-toluenesulfonic acid in refluxing toluene to yield final compound 29 in 88% yield. Compound 29 possesses antiproliferative activity against a breast cancer cell line (GI50 < 0.1 nm) and a colon cancer cell line (GI50 < 0.25 nm).

Activation of phosphoinositide 3-kinase (PI3K) is assumed to be one of the major causes for tumor growth in humans. PI3K signaling leads to activation of the mammalian target of rapamycin (mTOR) which in turn promotes uncontrollable cell growth. D’Angelo et al. reported the synthesis of aminobenzothiazole derivatives that have dual PI3K and mTOR inhibitory properties [16]. The synthesis of the most promising compound 32 is shown in Scheme 5. The starting compound, 2-amino-6-bromobenzothiazole, was first acylated using acetic anhydride in the presence of 4-dimethylaminopyridine (DMAP) and then the product was converted to boronate 30. Commercially available 5-bromo-2-chloropyridin-3-amine was reacted with compound 30 utilizing Suzuki coupling to furnish compound 31. Compound 31 in the last step was reacted with 4-fluorosulfonyl chloride in the presence of pyridine and 4-dimethylaminopyridine to prepare the final compound 32.

Compound 32 not only displays potent inhibitory activity against PI3Kα (Ki = 1.2±0.9) mTOR (Ki = 2.0±4.8) but also inhibits tumor growth in mouse xenograft models (tumor type: glioblastoma, lung adenocarcinoma and colorectal).
Anti-inflammatory activity
In addition to antimicrobial and chemotherapeutic properties, benzothiazole-based compounds have shown promising activity towards the development of anti-inflammatory agents. Specifically, Shafi et al. reported the synthesis of 2-mercaptobenzothiazole and 1,2,3-triazole based bis-heterocyclic compounds as potential anti-inflammatory agents [17]. The synthetic scheme for the synthesis of bis-heterocyclic compounds is shown in Scheme 6. Commercially available benzothiazole-2-thiol was reacted with propargyl bromide in dioxane in the presence of triethylamine to obtain compound 33 which was then treated with aromatic azides using click chemistry to afford final compounds 34–37.

Compounds 34–37 were tested for anti-inflammatory activity via biochemical cyclooxygenase (COX) activity assays and carrageenan-induced hind paw edema. Compound 35 exhibited selective COX-2 inhibition with an IC50 ratio of 0.44 of COX-2/COX-1 and displayed a better in vivo anti-inflammatory activity profile when compared with the industry standard, ibuprofen. Compound 35 showed increased analgesic activity compared with ibuprofen when tested using the Writhing method. Importantly, the synthesized compounds 34–37 displayed no gastric ulceration which has been a major downside to the clinical use of ibuprofen.
Gilani et al. synthesized a series of N-(6-chlorobenzo[d]thiazol-2-yl)hydrazine carboxamide benzothiazole derivatives and evaluated these compounds for corresponding anti-inflammatory activity [18]. The synthetic scheme for the compound 41 with best activity is shown in Scheme 7 beginning with the treatment of 4-chloroaniline with potassium thiocyanate in glacial acetic acid followed by the addition of a bromine solution to furnish compound 38. Compound 39 was furnished by heating precursor 38 with a solution of sodium cyanate in glacial acetic acid. Reaction of compound 39 with a hydrazine hydrate solution in the presence of concentrated sodium hydroxide solution afforded compound 40. In the final step, compound 40 was heated under reflux with 2,4-dichlorobenzoic acid in phosphorus oxychloride to furnish product 41 in 67% yield.

The tumor necrosis factor-α (TNF-α) is associated with several inflammatory diseases, and designing small molecule inhibitors of TNF-α activity is considered a potential target for developing anti-inflammatory therapeutics [19]. The mitogen-activated protein kinase activated protein kinase 2 (MAPKAP-K2) gene is associated with inflammatory response as it regulates the TNF-α. A series of pyrazolo[1,5-a]pyrimidine based compounds was synthesized and evaluated as MAPKAP-K2 inhibitors [19]. The most potent inhibitor 46 was synthesized as depicted in Scheme 8. Commercially available 3-aminopyrazole was treated with diethyl methylmalonate in a solution of sodium ethoxide in ethanol to yield compound 42. Compound 43 was obtained by refluxing a solution of 42 with N,N-dimethylaniline and phosphorus oxychloride. Compound 43 in the next step was treated with 6-amino-2-methylbenzothiazole in tetrahydrofuran in the presence of sodium hydride in DMF to afford compound 44. The amino group of 44 was protected using Boc anhydride, and the resultant derivative 45 was coupled with (S)-3-amino-1-Boc-piperidine. Removal of the Boc groups using trifluoroacetic acid furnished the final product 46.

Antidiabetic activity
Estimates predict that over 150 million people suffer from diabetes, and through synthetic efforts, medicinally relevant heterocyclic compounds containing the benzothiazole moiety show promising activity towards combating diabetes. Van Zandt et al. synthesized a series of conjugated indole-N-acetic acid with benzothiazoles and evaluated these compounds for antidiabetic activity [20]. The synthetic scheme for various benzothiazole derivatives is shown in Scheme 9. The starting material, 2,3,5,6-tetrafluoroaniline, was first acylated with acetic anhydride in pyridine and then the product was treated with phosphorus pentasulfide in benzene to yield thioamide compound 48. Thioamide was then cyclized in the presence of sodium hydride in toluene. The resulting compound 49 was hydrolyzed using a sodium hydroxide solution followed by treatment with hydrochloric acid to afford 2-aminothiophenol hydrochloride 50. Indole-3-acetonitrile and its 5-substituted analogs were alkylated with ethyl bromoacetate in the presence of sodium hydride to furnish compounds 51–54. Compound 50, in the final step, was condensed with individual compounds 51–54 in the presence of trifluoroethanol followed by treatment with aqueous sodium hydroxide to furnish final products 55–58.

Compounds 55–58 inhibited aldose reductase with IC50 values in the range of 5–13 nm. Compound 55 was the most potent aldose reductase inhibitor with IC50 of 5 nm and displayed promising features such as the normalization of nerve sugars and prevention of diabetic cataracts in STZ-induced diabetic rat models.
The enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) is responsible for glucocorticoid activity at the tissue level primarily in adipose and liver tissues [21]. Glucocorticoids are insulin action antagonists and are the possible cause for the commonly elevated blood sugar concentration in diabetes patients. Moreno-Díaz et al. reported a series of benzothiazole benzenesulfonamides as shown in Equation 4 as potential 11β-HSD1 inhibitors for the treatment of noninsulin-dependent diabetes mellitus [21]. Starting material 2-amino-6-methoxybenzothiazole was condensed in dichloromethane with arylsulfonyl chlorides in the presence of triethylamine and a catalytic amount of DMAP to furnish compounds 59–62. Compounds 59 and 60 displayed effective inhibition activity of 11β-HSD1 in an in vitro cell-based assay with 38% and 53% inhibition, respectively.

As shown above, benzothiazole-based heterocyclic compounds are becoming prevalent when discussing active drugs that combat various diseased states; however, discovering antiviral agents, specifically anti-HIV agents, remains of paramount importance in pharmaceutical research.
Anti-HIV activity
Towards the goal of designing novel anti-HIV agents, Massari et al. synthesized 1,8-naphthyridone benzothiazole derivative 67 as a potential inhibitor of the HIV-1 Tat-mediated transcription and antiviral activity in HIV-infected cells (Scheme 10) [22]. Acrylamide 63 was treated with methylamine in a diethyl ether/ethanol mixture to yield compound 64. Compound 65 was obtained by cyclization of the precursor 64 in the presence of potassium carbonate in DMF. The intermediate product 65 was then condensed with 1-(benzothiazol-2-yl)piperazine in DMF to furnish compound 66 which was then hydrolyzed to afford the final product 67. Compound 67 displayed EC50 = 0.03 μg/mL and 0.02 μg/mL with HIV-1 and HIV-2 met allothionein 4 (MT-4) cells.

Jonckers et al. synthesized benzothiazole amides 74 and 75 as shown in Scheme 11 [23]. Commercially available epoxide 68 was treated with an excess of isobutylamine in isopropanol to yield compound 69. Compound 69 was coupled with a benzothiazole-6-carboxylic acid using O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU) as an activating agent in the presence of triethylamine to afford compounds 70 and 71, the subsequent treatment of which with trimethylsilyl chloride and sodium iodide furnished derivatives 72 and 73. In the final step, a triethylamine-mediated coupling of compounds 72 and 73 with 2,5-dioxopyrrolidin-1-yl thiazol-5-ylmethyl carbonate furnished final products 74 and 75 in approximately 85% yield. Compounds 74 and 75 were evaluated as pharmacokinetic enhancers of HIV protease inhibitors.

Miscellaneous activities
Spadaro et al. described a synthesis of various hydroxybenzothiazole compounds and evaluated them as potential agents for the treatment of estrogen-related diseases [24]. The synthesis of the most potent compound 80 is depicted in Scheme 12. 2-Amino-6-methoxybenzothiazole (76) was deaminated in two steps to afford compound 77. Compound 77 was treated with n-butyllithium and the resulting in situ formyl anion was then allowed to react with 4-methyl-3-methoxybenzaldehyde to yield compound 78. Compound 79 was obtained by treatment of 78 with iodoxybenzoic acid in anhydrous THF. Demethylation of 79 to the final product 80 in the next step was accomplished by heating with pyridinium hydrochloride. Compound 80 inhibits 17β-hydroxysteroid dehydrogenase 1 (17β-HSD1) with IC50 = 27 nm and shows selectivity towards 17β-HSD2.

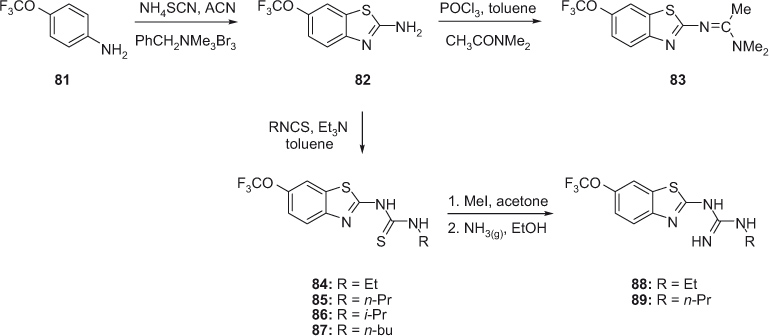
Anzini et al. developed various amidine, guanidine and thiourea derivatives of 2-amino-(6-trifluoromethoxy)benzothiazole 83–89 that were structurally related to riluzole, a neuroprotective drug, as illustrated in Scheme 13 [25]. These compounds were evaluated as neuroprotective agents via an in vitro procedure of ischemia/reperfusion injury. Condensation of 4-(trifluoromethoxy)aniline (81) with ammonium thiocyanate and benzyltrimethylammonium tribromide in acetonitrile yielded 2-amino-6-(trifluoromethoxy)benzothiazole (82). Treatment of 82 with N,N-dimethylacetamide and phosphorus oxychloride in toluene furnished amidine 83. The reaction of 82 with various isothiocyanates in the presence of triethylamine yielded various thiourea derivatives 84–87. Compounds 84 and 85 were further treated with methyl iodide in acetone followed by the reaction with gaseous ammonia to afford guanidine derivatives 88 and 89. Compounds 83–87 displayed a significant reduction in neuronal injury.
Ugale et al. reported the synthesis of quinazoline-substituted benzothiazoles 93–95 as shown in Equation 5. These compounds were evaluated as anticonvulsant agents by maximal electroshock and PTZ-induced seizure methods [26]. In the preparation of 93–95, substrates 90–92 and 6-bromo-2-ethyl-4H-benzo[d][1,3]oxazin-4-one in dry pyridine were heated under reflux. Compound 93 showed remarkable activity against tonic seizure, whereas compound 95 showed promising results against clonic seizure. Significantly, none of the compounds exhibited neurotoxicity or hepatotoxicity.

Azam et al. synthesized a series of benzothiazole-urea derivatives as potential agents to combat Parkinson’s disease. The synthesis is shown in Scheme 14 [27]. The reaction of aniline with potassium thiocyanate and bromine afforded 2-aminobenzothiazole (96), the treatment of which with appropriate isocyanate furnished compounds 97–101. Compounds 97–101 exhibited reduction in catalepsy by more than 70% in a standard bar test.

Karalı et al. synthesized benzothiazole containing spiroindolinones and screened these compounds for antioxidant activity [28]. The synthetic scheme is shown in Scheme 15. 1H-Indole-2,3-dione (102) was alkylated with methyl iodide to furnish compound 103. Compounds 102 and 103 were then allowed to react with 2-aminothiophenol in ethanol to afford final compounds 104–111. The methyl analog 107 was found to be the most potent antioxidant in the whole set of compounds compared with α-tocopherol and ascorbic acid.

As discussed above and in our other recent review [1], benzothiazole-based compounds have widespread medicinal applicability when conjugated with various heterocyclic and cyanine moieties. The extensive literature presentations in the preceding sections show that the benzothiazole heterocyclic structure is effective in the preparation of novel heterocyclic compounds and functions as the basic scaffold for determining the prognosis of patients suffering from AD through PET scans and generating anti-HIV medication. We have previously shown that benzothiazole-containing cyanine dyes have extensive medicinal applications and as synthetic and medicinal chemists further develop these compounds, it is likely that the benzothiazole system will remain an integral portion of the design of compounds in the future.
References
[1] Henary, M.; Paranjpe, S.; Owens, E. A. Synthesis and application of benzothiazole containing cyanine dyes. Heterocycl. Commun.2013, 19, 1–11.Search in Google Scholar
[2] Robbins, K.; Liu, G.; Lin, G.; Lazo, N. Detection of strongly bound thioflavin T species in amyloid fibrils by ligand-detected 1H NMR. J. Phys. Chem. Lett.2011, 2, 735–740.Search in Google Scholar
[3] Sisodia, S.; Price, D. Role of the β-amyloid protein in Alzheimer’s disease. FASEB J.1995, 9, 366–370.Search in Google Scholar
[4] Yan, P.; Bero, A.; Cirrito, J.; Xiao, Q.; Hu, X.; Wang, Y.; Gonzales, E.; Holtzman, D.; Lee, J. -M. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J. Neurosci.2009, 29, 10706–10714.Search in Google Scholar
[5] Klunk, W.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.; Bergström, M.; Savitcheva, I.; Huang, G.-F.; Estrada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann. Neurol.2004, 55, 306–319.Search in Google Scholar
[6] Klunk, W.; Wang, Y.; Huang, G.-F.; Debnath, M.; Holt, D.; Mathis, C. Uncharged thioflavin-T derivatives bind to amyloid-β protein with high affinity and readily enter the brain. Life Sciences2001, 69, 1471–1484.Search in Google Scholar
[7] Landau, S.; Breault, C.; Joshi, A.; Pontecorvo, M.; Mathis, C.; Jagust, W.; Mintun, M. For the Alzheimer’s Disease Neuroimaging Initiative. Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J. Nucl. Med.2013, 54, 476–490.Search in Google Scholar
[8] Wilson, A.; Garcia, A.; Chestakova, A.; Kung, H.; Houle, S. A rapid one-step radiosynthesis of the β-amyloid imaging radiotracer N-methyl-[11C]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole ([11C]-6-OH-BTA-1). J. Label. Compd. Rad.2004, 47, 679–682.Search in Google Scholar
[9] Yousefi, B.; Manook, A.; Drzezga, A.; Reutern, B.; Schwaiger, M.; Wester, H.-J.; Henriksen, G. Synthesis and evaluation of 11C-labeled imidazo[2,1-b]benzothiazoles (ibts) as PET tracers for imaging β-amyloid plaques in Alzheimer’s disease. J. Med. Chem.2011, 54, 949–956.Search in Google Scholar
[10] Ribeiro Morais, G.; Paulo, A.; Santos, I. A synthetic overview of radiolabeled compounds for β-amyloid targeting. Eur. J. Org. Chem.2012, 7, 1279–1293.Search in Google Scholar
[11] Yadav, P.; Devprakash; Senthilkumar, G. Benzothiazole: different methods of synthesis and diverse biological activities. Int. J. Pharm. Sci. Drug Res.2011, 3, 1–7.Search in Google Scholar
[12] Ouyang, L.; Huang, Y.; Zhao, Y.; He, G.; Xie, Y.; Liu, J.; He, J.; Liu, B.; Wei, Y. Preparation, antibacterial evaluation and preliminary structure-activity relationship (SAR) study of benzothiazol- and benzoxazol-2-amine derivatives. Bioorg. Med. Chem.2012, 22, 3044–3049.Search in Google Scholar
[13] Bandyopadhyay, P.; Sathe, M.; Ponmariappan, S.; Sharma, A.; Sharma, P.; Srivastava, A.; Kaushik, M. Exploration of in vitro time point quantitative evaluation of newly synthesized benzimidazole and benzothiazole derivatives as potential antibacterial agents. Bioorg. Med. Chem.2011, 21, 7306–7309.Search in Google Scholar
[14] Shi, X.-H.; Wang, Z.; Xia, Y.; Ye, T.-H.; Deng, M.; Xu, Y.-Z.; Wei, Y.-Q.; Yu, L.-T. Synthesis and biological evaluation of novel benzothiazole-2-thiol derivatives as potential anticancer agents. Molecules2012, 17, 3933–3944.Search in Google Scholar
[15] Mortimer, C.; Wells, G.; Crochard, J.-P.; Stone, E.; Bradshaw, T.; Stevens, M.; Westwell, A. Antitumor benzothiazoles. 26.1 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. J. Med. Chem.2006, 49, 179–185.Search in Google Scholar
[16] D’Angelo, N.; Kim, T.-S.; Andrews, K.; Booker, S.; Caenepeel, S.; Chen, K.; D’Amico, D.; Freeman, D.; Jiang, J.; Liu, L.; et al. Discovery and optimization of a series of benzothiazole phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors. J. Med. Chem.2011, 54, 1789–1811.Search in Google Scholar
[17] Shafi, S.; Mahboob Alam, M.; Mulakayala, N.; Mulakayala, C.; Vanaja, G.; Kalle, A.; Pallu, R.; Alam, M. Synthesis of novel 2-mercapto benzothiazole and 1,2,3-triazole based bis-heterocycles: their anti-inflammatory and anti-nociceptive activities. Eur. J. Med. Chem.2012, 49, 324–333.Search in Google Scholar
[18] Gilani, S.; Khan, S. Synthesis and pharmacological evaluation of N-(6-chlorobenzo[d]-thiazol-2-yl)hydrazine carboxamide derivatives of benzothiazole. Med. Chem. Res.2012, 19, 1–13.Search in Google Scholar
[19] Kosugi, T.; Mitchell, D.; Fujino, A.; Imai, M.; Kambe, M.; Kobayashi, S.; Makino, H.; Matsueda, Y.; Oue, Y.; Komatsu, K.; et al. Mitogen-activated protein kinase-activated protein kinase 2 (MAPKAP-K2) as an antiinflammatory target: discovery and in vivo activity of selective pyrazolo[1,5-a]-pyrimidine inhibitors using a focused library and structure-based optimization approach. J. Med. Chem.2012, 55, 6700–6715.Search in Google Scholar
[20] Van Zandt, M.; Jones, M.; Gunn, D.; Geraci, L.; Jones, J.; Sawicki, D.; Sredy, J.; Jacot, J.; DiCioccio, A.; Petrova, T.; et al. Discovery of 3-[(4,5,7-trifluorobenzo-thiazol-2-yl)methyl]indole-N-acetic acid (Lidorestat) and congeners as highly potent and selective inhibitors of aldose reductase for treatment of chronic diabetic complications. J. Med. Chem.2005, 48, 3141–3152.Search in Google Scholar
[21] Moreno-Díaz, H.; Villalobos-Molina, R.; Ortiz-Andrade, R.; Díaz-Coutiño, D.; Medina-Franco, J.; Webster, S.; Binnie, M.; Estrada-Soto, S.; Ibarra-Barajas, M.; León-Rivera, I.; et al. Antidiabetic activity of N-(6-substituted-1,3-benzothiazol-2-yl)benzen-esulfonamides. Bioorg. Med. Chem.2008, 18, 2871–2877.Search in Google Scholar
[22] Massari, S.; Daelemans, D.; Barreca, M.; Knezevich, A.; Sabatini, S.; Cecchetti, V.; Marcello, A.; Pannecouque, C.; Tabarrini, O. A 1,8-naphthyridone derivative targets the HIV-1 Tat-mediated transcription and potently inhibits the HIV-1 replication. J. Med. Chem.2010, 53, 641–648.Search in Google Scholar
[23] Jonckers, T.; Rouan, M.-C.; Haché, G.; Schepens, W.; Hallenberger, S.; Baumeister, J.; Sasaki, J. Benzoxazole and benzothiazole amides as novel pharmacokinetic enhancers of HIV protease inhibitors. Bioorg. Med. Chem.2012, 22, 4998–5002.Search in Google Scholar
[24] Spadaro, A.; Frotscher, M.; Hartmann, R. Optimization of hydroxybenzothiazoles as novel potent and selective inhibitors of 17β-HSD1. J. Med. Chem.2012, 55, 2469–2473.Search in Google Scholar
[25] Anzini, M.; Chelini, A.; Mancini, A.; Cappelli, A.; Frosini, M.; Ricci, L.; Valoti, M.; Magistretti, J.; Castelli, L.; Giordani, A.; et al. Synthesis and biological evaluation of amidine, guanidine, and thiourea derivatives of 2-amino(6-trifluoromethoxy)-benzothiazole as neuroprotective agents potentially useful in brain diseases. J. Med. Chem.2010, 53, 734–744.Search in Google Scholar
[26] Ugale, V.; Patel, H.; Wadodkar, S.; Bari, S.; Shirkhedkar, A.; Surana, S. Quinazolino-benzothiazoles: fused pharmacophores as anticonvulsant agents. Eur. J. Med. Chem.2012, 53, 107–113.Search in Google Scholar
[27] Azam, F.; Prasad, M.; Thangavel, N. Structure-based design, synthesis, and molecular modeling studies of 1-(benzo[d]thiazol-2-yl)-3-(substituted aryl)urea derivatives as novel anti-Parkinsonian agents. Med. Chem. Res.2012, 21, 2630–2643.Search in Google Scholar
[28] Karalı, N.; Güzel, Ö.; Özsoy, N.; Özbey, S.; Salman, A. Synthesis of new spiroindolinones incorporating a benzothiazole moiety as antioxidant agents. Eur. J. Med. Chem.2010, 45, 1068–1077.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- A fascinating decade for the synthesis of 1,2-azoles
- Substituted benzothiazoles: synthesis and medicinal characteristics
- Preliminary Communication
- Synthesis of thiazolo[4,5-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives
- Research Articles
- Ultrasonic-assisted Cu-catalyzed multicomponent synthesis of furo[3,4-b]pyrazolo[4,3-f]quinolinones
- Synthesis of dihydropyrrolo[2,1-a]isoquinolines via isocyanide-based four-component reaction
- Efficient oxidative cyclization of N-acylhydrazones for the synthesis of 2,5-disubstituted 1,3,4-oxadiazoles using t-BuOI under neutral conditions
- Synthesis and transformations of 1-[2-(toluene-4-sulfonamido)ethyl]thiourea
- Synthesis of new heterocyclic compounds containing benzimidazole moiety as inhibitors of breast cancer cell growth
- An approach to C-glycosidic conjugates of isoflavones
- Ionic liquid catalyzed one-pot synthesis of spiropyran derivatives via three-component reaction in water
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- A fascinating decade for the synthesis of 1,2-azoles
- Substituted benzothiazoles: synthesis and medicinal characteristics
- Preliminary Communication
- Synthesis of thiazolo[4,5-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives
- Research Articles
- Ultrasonic-assisted Cu-catalyzed multicomponent synthesis of furo[3,4-b]pyrazolo[4,3-f]quinolinones
- Synthesis of dihydropyrrolo[2,1-a]isoquinolines via isocyanide-based four-component reaction
- Efficient oxidative cyclization of N-acylhydrazones for the synthesis of 2,5-disubstituted 1,3,4-oxadiazoles using t-BuOI under neutral conditions
- Synthesis and transformations of 1-[2-(toluene-4-sulfonamido)ethyl]thiourea
- Synthesis of new heterocyclic compounds containing benzimidazole moiety as inhibitors of breast cancer cell growth
- An approach to C-glycosidic conjugates of isoflavones
- Ionic liquid catalyzed one-pot synthesis of spiropyran derivatives via three-component reaction in water

