Abstract
1,2-Azoles comprising pyrazoles, isoxazoles, and isothiazoles have occupied a distinctive position in the design and synthesis of novel biologically active compounds that exert remarkable pharmaceutical activities as medicines and agrochemicals. As a consequence, there has been an increasing interest among synthetic organic/medicinal chemists in the development of methodologies for the rapid access to these heterocycles. In this review, we have summarized the most significant advances in the construction of functionalized pyrazoles, isoxazoles, and isothiazoles reported in the literature up to 2012.
Introduction
Heterocycles are of immense importance in the design and discovery of new compounds for pharmaceutical applications. Among them, functionalized pyrazoles, isoxazoles, and isothiazoles are present in a myriad of medicinally important natural and synthetic compounds that have a broad and interesting range of biological activities including antibacterial [1], antiviral [2], antitumor [3], antidiabetic [4], anticancer [5], anti-inflammatory [6], antipyretic [7], and HIV-1 protease inhibitory activity [8]. Some representative molecules of this structural motif are shown in Figures 1 and 2. Therefore, synthetic methodologies allowing the rapid access to these heterocycles are highly desirable in organic synthesis and chemical biology/medicinal chemistry. Some review articles have been published on pyrazole chemistry in earlier literature [9, 10]; however, to the best of our knowledge there is no recent literature comprising all three 1,2-azoles, that is, pyrazoles, isoxazoles, and isothiazoles. This review is intended to provide updated information regarding the available methodologies (focusing on the period 2002–2012) in the synthesis of functionalized pyrazoles, isoxazoles, and isothiazoles.

Biologically important 1,2-azoles.

1,2-Azoles in natural products and pharmaceutical drugs.
1H-Pyrazoles
A simple methodology for the one-pot synthesis of 1,3,5-trisubstituted pyrazoles was reported by Armstrong et al. [11] in 2005. The protocol involves the electrophilic amination of primary amines with N-Boc-oxaziridine under metal-free conditions that affords synthetically versatile, mono-Boc-protected hydrazines. Condensation of the hydrazines with symmetrical 1,3-diketons affords the 1,3,5-trisubstitutes pyrazoles (Scheme 1).

In 2006, Boruah and coworkers [12] reported on a novel one-pot method for pyrazoles by the reaction of β-formyl enamides 4 with hydroxylamine hydrochloride catalyzed by potassium dihydrogenphosphate in acid medium. The reactions of a broad range of β-formyl enamides such as steroidal, alicyclic, aliphatic, and aromatic β-formyl enamides give rise to the corresponding pyrazoles in high yields (Scheme 2).

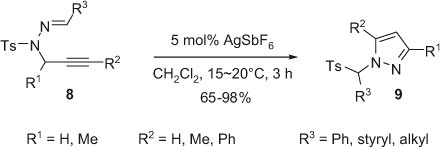
Silver(I)-catalyzed regioselective synthesis of 1,3- and 1,5-substituted pyrazoles was reported by the Chung group in 2008 [13]. Under mild reaction conditions, intramolecular cyclization of propargyl N-sulfonylhydrazones 8 in the presence of 5 mol% AgSbF6 as catalyst in dry dichloromethane affords 1,3,5-substituted pyrazoles 9 at room temperature. The process involves an intermolecular migration of the sulfonyl group through the formation of a tosyl anion and an electron-deficient pyrazolyl iminium cation as anion pair. N-Monosubstituted pyrazoles (R1=R2=H) are obtained in high yield from a variety of aryl, alkenyl, and styryl hydrazones. The introduction of a methyl group at the propargylic position in the styryl hydrazine 8 slightly decreases the yield of the reaction. Moreover, 1,3,5-substituted pyrazoles 9 are obtained in acceptable yield starting from 4-methoxystyryl hydrazone (Scheme 3).
An efficient, rapid, and general one-pot synthetic protocol of 3,5-, 1,3,5-, 3,4,5-, and fused ring-substituted pyrazoles 13 was reported by Heller and Natarajan in 2006 (Scheme 4) [14], starting from enolizable ketones 10, acid chlorides 11, and hydrazines. In the methodology, the 1,3-diketones (not isolated) so produced are converted in situ into pyrazoles by treatment with the appropriate hydrazines. A wide range of functional groups such as nitriles, alkyl halides, and electrophiles with enolizable moieties are tolerated in the reaction. The reaction between cyclic saturated ketones and acid chlorides affords fused pyrazoles in good yields. Moreover, both benzyl p-chlorophenyl ketone and n-butyl p-chlorophenyl ketone are coupled with p-methoxybenzoic acid chloride to give the corresponding 1,3-diketones, which are then condensed with hydrazine to afford the corresponding 3,4,5-substituted pyrazole derivatives.

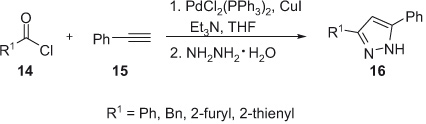
Subsequently, Jiang et al. [15] reported on an efficient and general one-pot procedure for the synthesis of pyrazoles from acid chlorides, terminal alkynes, and hydrazines. Acid chlorides 14 undergo coupling with terminal alkynes 15 to give α,β-unsaturated ynones which, in situ, are converted into pyrazoles by the cycloaddition of hydrazines catalyzed by Pd(PPh3)2Cl2/CuI. 3,5-Diaryl-1H-pyrazoles 16 are obtained in moderate to good yields from aryl chlorides and aryl terminal alkynes. However, the use of aliphatic alkyne, 1-octyne, leads to its corresponding pyrazole derivative in low yield (Scheme 5).
Fully substituted pyrazoles have received a great deal of attention as they exhibit useful pharmacological and agrochemical properties. In 2011, Wang et al. reported on a methodology in which tetrasubstituted pyrazole 19 has been obtained from 2-azidoacrylate 17 and hydrazine 18 via [3 + 2] cycloaddition reaction in the presence of triethylamine in moderate to good yields with high regioselectivity (Scheme 6) [16].

Soon after, Zora and coworkers [17] reported on the synthesis of highly substituted 4-iodopyrazole derivatives by electrophilic cyclization of acetylenic aldehydes and ketones through the intermediary of hydrazones in the presence of molecular iodine. The mechanism proposed by the authors involves an electrophilic addition of I+ to the alkyne, leading to an iodonium ion that undergoes an electrophilic cyclization via nucleophilic attack of the secondary nitrogen atom to furnish protonated pyrazole 23. Finally, deprotonation with base affords 4-iodopyrazole derivative 24 (Scheme 7).

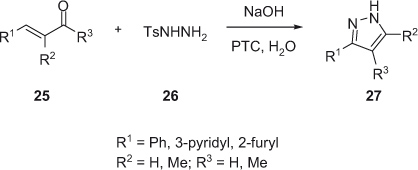
In recent years, interest in greener technologies has favored the development of using alternative reaction media that circumvent the problems associated with many of the traditional volatile organic solvents. Adopting this eco-friendly technology, Yu and coworkers in 2011 [18] developed a protocol for the synthesis of highly functionalized pyrazole 27. Thus, condensation of α,β-unsaturated carbonyl compound 25 with tosyl hydrazide 26 in water affords trisubstituted pyrazole 27 in high yield (Scheme 8).
Another strategy based on an environmentally benign, eco-friendly approach for the synthesis of pyrazoles has been developed by Chandak et al. [19]. The method involves the condensation of hydrazine/hydrazide 29 with 1,3-diketone 28 using Amberlyst-70 as a recyclable catalyst which proceeds efficiently at room temperature in aqueous medium without the use of any organic solvent affording substituted pyrazole 30 (Scheme 9).

Very recently, Dissanayake and Odom [20] reported on an efficient three-component, one-pot reaction for the preparation of substituted pyrazoles. The strategy involves a simple titanium complex-catalyzed three-component coupling of alkynes 32, isonitriles 33, and monosubstituted hydrazines 31 to provide substituted pyrazoles 34 in a single step. This is the first reported protocol where transition metal efficiently catalyzes the coupling to produce a single regioisomer in high yield (Scheme 10).

Isoxazoles
The electrophilic cyclization of functionally substituted alkynes has attracted much attention due to its wide utility in the preparation of a range of useful, functionally substituted heterocycles. In 2005, Waldo and Larock [21] reported on an efficient protocol in which the reaction of 2-alkyn-1-one O-methyl oximes 38 with ICl,I2,Br2, or PhSeBr affords 3,5-disubstituted 4-halo(seleno)isoxazoles 39 in good to excellent yields under mild reaction conditions (Scheme 11).

Currently, one-pot reactions are gaining prominence due to their environmental advantages and cost effectiveness. In 2006, Yavari and Moradi [22] reported on a one-pot strategy for the synthesis of isoxazoles 43 from dialkyl acetylenedicarboxylates or dibenzoylacetylene with alkyl 2-nitroethanoates 42 in the presence of triphenylphosphine (Scheme 12).

Microwave-assisted multicomponent reactions are efficient and effective methods in the sustainable and diversity-oriented one-pot synthesis of heterocycles. Based on consecutive coupling-cycloaddition sequencing starting with room temperature coupling and subsequent dielectric heating for completion of the cycloaddition, Müller and coworkers [23], in 2008, reported on new avenues to the one-pot synthesis of isoxazole frameworks in a multicomponent reaction manner. Thus, the Sonogashira coupling of acid chloride 44 with terminal alkyne 45, followed by 1,3-dipolar cycloaddition under dielectric heating of in situ generated nitrile oxide from hydroximinoyl chloride 47 furnished isoxazole 48 in moderate to good yields (Scheme 13).

A dipolar cycloaddition strategy for the synthesis of substituted isoxazoles with complete regioselectivity at room temperature was developed by Grecian and Fokin in 2008 [24]. Thus, the cycloaddition reaction of nitrile oxides (generated in situ from hydroximoyl chlorides by treatment with Et3N) and terminal or internal alkynes 15 in the presence of ruthenium(II)-catalyst affords 3,5-di- and 3,4,5-trisubstituted isoxazoles in excellent yields and with complete regioselectivity at room temperature (Scheme 14).

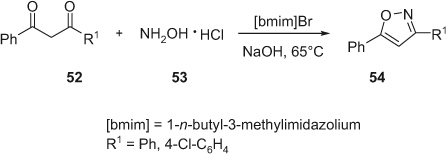
A first environmentally benign protocol using ionic liquid (IL) as noble solvent for the construction of the isoxazole ring was reported by the Valizadeh group in 2009 [25]. The novel method involves the reaction of β-diketone and hydroxylamine hydrochloride in IL [bmim]Br that provides biologically active isoxazoles in excellent isolated yields. The moderate reaction conditions, easy workup procedure, and recyclability of the nonvolatile IL make this protocol an environmentally friendly method amenable for scale-up synthesis (Scheme 15).
A versatile and efficient synthesis of a broad library of isoxazoles 58 was reported by Bhuniya and coworkers in 2009 [26] via 1,3-dipolar cycloaddition (1,3-DC) of aldoximes 55 with dipolarophile 56 or 57 in the presence of Magtrieve™ (CrO2), which is an efficient reagent for the oxidation of aldoximes to nitrile oxides, the intermediate products in this cycloaddition reaction (Scheme 16).

Campagne and coworkers [27] reported on an exquisite, efficient one-pot synthesis methodology for the construction of substituted isoxazoles 61. Using N-sulfonyl-protected hydroxylamines 59 as binucleophiles, the propargylic substitutions catalyzed by iron allows the selective, in situ formation of propargylic oxime 60 from starting propargylic alcohols 58. The substituted isoxazoles 61 are obtained in good isolated yields (Scheme 17).

A series of 3,4,5-trisubstituted isoxazoles exemplified by 64 were prepared by Chen and coworkers [28] in 2012 from O-methyl oxime substrates through a cascade process involving a Pd(II)-catalyzed 5-endo-dig cyclization to form the heteroaryl palladium intermediate, which is then transformed into another intermediate product after the loss of a methyl group. The Heck coupling between the last intermediate product and an alkene affords the trisubstituted isoxazole 64. Electron-withdrawing substituents on the alkenes increase yields of the final products 64 (Scheme 18).

Dadiboyena and Nefzi [29] have developed an efficient parallel solid phase methodology to construct the diversity oriented substituted isoxazoles via 1,3-dipolar cycloaddition reaction. Thus, the cycloaddition of resin-bound alkenes or alkynes, synthesized from the corresponding resin-bound carboxylic acids, with in situ generated nitrile oxides, leads to the formation of the corresponding disubstituted isoxazoles (Scheme 19).

Very recently Suzuki et al. [30] developed a two-step protocol for efficient synthesis of highly functionalized polycyclic isoxazoles. The method involves 1,3-dipolar cycloaddition of stable benzonitrile oxides to p-quinone monoacetals 72 in a regioselective manner, favoring the formation of 4-acyl cycloadducts 73, followed by MnO2-mediated dehydrogenation to highly functionalized polycyclic isoxazoles 74 (Scheme 20).
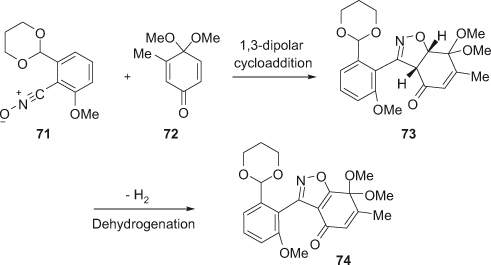
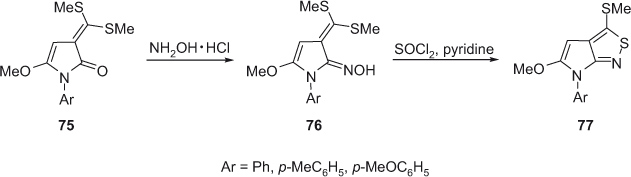
Isothiazoles
In 2002, Abd El-Nabi [31] reported on a methodology for the synthesis of highly substituted pyrrolo[2,3-c]isothiazoles. The method involves the treatment of 1-aryl-5-methoxypyrole-2-one with CS2 followed by treatment with dimethyl sulfate in the presence of NaOH and dimethyl sulfoxide that gives 3-bis(methylsulfonyl)methylenepyrrol-2-one 75. Compounds 75 are transformed into oximes 76 that are precursors to functionalized fused isothiazoles 77 (Scheme 21).
Shibata et al. [32] have developed a procedure for the synthesis of 3,3-disubstituted benzosultams 79 from benzenesulfonamide 77. The regiospecific o-lithiation of N-t-butylbenzenesulfonamide 77 followed by reaction with ketones afforded the carbinol sulfonamides 78, which underwent cyclization to the final products 79 (Scheme 22).

A facile synthesis of 3-substituted isothiazoles fused to a benzene ring has been developed by Devarie-Baez and Xian [33]. The protocol involves the nitrosation of o-mercaptoacylphenone 80 with isopentyl nitrite to form an unstable S-nitrosothio intermediate which undergoes an intramolecular aza-Wittig reaction to furnish the desired benzisothiazole 81 (Scheme 23).

In 2011, the Shibasaki group [34] developed a distinct approach to enantio-enriched fused isothiazoles through a cascade of C-C and S-N bond formation reactions promoted by Cu catalysis. The unprecedented route involves a Cu-catalyzed asymmetric conjugate addition of allyl cyanide 83 to α,β-unsaturated thioamides 82 by soft Lewis acid/hard Brønsted base/hard Lewis base cooperative catalysis followed by Cu-catalyzed cascade cyclization to afford isothiazole 84 (Scheme 24).

An efficient one-pot strategy for the synthesis of steroidal and non-steroidal isothiazoles found in some natural products and pharmaceuticals has been reported by Boruah et al. [35]. Thus, the reaction of ring annelated β-bromo-α,β-unsaturated aldehydes 86 with a mixture of sodium thiocyanate and urea as an environmentally benign source of ammonia under microwave irradiation (MWI) affords corresponding steroidal and non-steroidal isothiazole derivatives. These β-bromo-α,β-unsaturated aldehydes derivatives are efficiently synthesized from corresponding cyclic ketones 85 using the Vilsmeier formylation reaction (Scheme 25).

Conclusion
The past decade has seen a considerable expansion in research on the chemistry of 1,2-azoles due to numerous applications of these heterocyclic compounds in various fields such as medicine, agrochemistry, natural product synthesis, and catalysis. This review critically demonstrates the synthetic methodologies developed within the past 10 years for access to these heterocycles. Although the classical cyclocondensation methods are still the most commonly used procedures for their construction, new approaches need to be developed with the aim of increasing both yields and regioselectivities in the preparation of substituted 1,2-azoles. We hope that this review will be helpful to those who have a keen interest in the chemistry of 1,2-azoles.
S.C. sincerely acknowledges CSIR, New Delhi, India for providing financial support through an SRF fellowship, and C.B. sincerely acknowledges UGC, ERO, Kolkata, India for providing financial support through Teacher Fellow Grant.
References
[1] Pathak, R. B.; Chovatia, P. T.; Parekh, H. H. Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorg. Med. Chem. Lett.2012, 22, 5129–5133.Search in Google Scholar
[2] Yan, S.; Appleby, T.; Gunic, E.; Shim, J. H.; Tasu, T.; Kim, H.; Rong, F.; Chen, H.; Hamatake, R.; Wu, J. Z.; et al. Isothiazoles as active-site inhibitors of HCV NS5B polymerase. Bioorg. Med. Chem. Lett.2007, 17, 28–33.Search in Google Scholar
[3] Ruan, B.-F.; Liang, Y.-K.; Liu, W.-D.; Wu, J.-Y.; Tian, Y.-P. Synthesis, characterization, and antitumor activities of two copper(II) complexes with pyrazole derivatives. J. Coordinat. Chem.2012, 65, 2127–2134.Search in Google Scholar
[4] Cottineau, B.; Toto, P.; Marot, C.; Pipaud, A.; Chenault, J. Synthesis and hypoglycemic evaluation of substituted pyrazole-4-carboxylic acids. Bioorg. Med. Chem. Lett.2002, 12, 2105–2108.Search in Google Scholar
[5] Shaw, J.; Chen, B.; Bourgault, J. P.; Jiang, H.; Kumar, N.; Mishra, J.; Andreana, P. R. Synthesis and biological evaluation of novel N-phenyl-5-carboxamidyl isoxazoles as potential chemotherapeutic agents for colon cancer. Am. J. Biomed. Sci.2012, 4, 14–25.Search in Google Scholar
[6] Waldo, J. P.; Larock, R. C. The synthesis of highly substituted isoxazoles by electrophilic cyclization: an efficient synthesis of valdecoxib. J. Org. Chem.2007, 72, 9643–9647.Search in Google Scholar
[7] Uramaru, N.; Shigematsu, H.; Toda, A.; Eyanagi, R.; Kitamura, S.; Ohta, S. Design, synthesis, and pharmacological activity of nonallergenic pyrazolone-type antipyretic analgesics. J. Med. Chem.2010, 53, 8727–8733.Search in Google Scholar
[8] Cutri, C. C. C.; Garozzo, A.; Pannecouque, C.; Castro, A.; Guerrera, F.; De Clercq, E. Isothiazole derivatives as novel HIV replication inhibitors. Antiviral Chem. Chemother.2004, 15, 201–206.Search in Google Scholar
[9] Fustero, S.; Simón-Fuentes, A.; Sanz-Cervera, J. F. Recent advances in the synthesis of pyrazoles. A review. Org. Prep. Proc. Int.2009, 41, 253–290.Search in Google Scholar
[10] Fustero, S.; Sanchez-Rosello, M.; Barrio, P.; Simon-Fuentes, A. From 2000 to mid-2010: a fruitful decade for the synthesis of pyrazoles. Chem. Rev.2011, 111, 6984–7034.Search in Google Scholar
[11] Armstrong, A.; Jones, L. H.; Knight, J. D.; Kelsey, R. D. Oxaziridine-mediated amination of primary amines: scope and application to a one-pot pyrazole synthesis. Org. Lett.2005, 7, 713–716.Search in Google Scholar
[12] Saikia, A.; Barthakur, M. G.; Borthakur, M.; Saikia, C. J.; Bora, U.; Boruah, R. C. Conjugate base catalysed one-pot synthesis of pyrazoles from β-formyl enamides. Tetrahedron Lett.2006, 47, 43–46.Search in Google Scholar
[13] Lee, Y. T.; Chung, Y. K. Silver(I)-catalyzed facile synthesis of pyrazoles from propargyl N-sulfonylhydrazones. J. Org. Chem.2008, 73, 4698–4701.Search in Google Scholar
[14] Heller, S. T.; Natarajan, S. R. 1,3-Diketones from acid chlorides and ketones: a rapid and general one-pot synthesis of pyrazoles. Org. Lett.2006, 8, 2675–2678.Search in Google Scholar
[15] Liu, H.-L.; Jiang, H.-F.; Zhang, M.; Yao, W.-J.; Zhu, Q.-H.; Tang, Z. One-pot three-component synthesis of pyrazoles through a tandem coupling-cyclocondensation sequence. Tetrahedron Lett.2008, 49, 3805–3809.Search in Google Scholar
[16] Li, Y.; Hong, D.; Lu, P.; Wang, Y. Synthesis of pyrazoles from 2-azidoacrylates and hydrazonyl chlorides. Tetrahedron Lett.2011, 52, 4161–4163.Search in Google Scholar
[17] Zora, M.; Kivrak, A.; Yazici, C. Synthesis of pyrazoles via electrophilic cyclization. J. Org. Chem.2011, 76, 6726–6742.Search in Google Scholar
[18] Wen, J.; Fu, Y.; Zhang, R.-Y.; Zhang, J.; Chen, S.-Y.; Yu, X.-Q. A simple and efficient synthesis of pyrazoles in water. Tetrahedron2011, 67, 9618–9621.Search in Google Scholar
[19] Chandak, H. S.; Lad, N. P.; Dange, D. S. Greener and facile aqueous synthesis of pyrazoles using Amberlyst-70 as a recyclable catalyst. Green Chem. Lett. Rev.2011, 5, 135–138.Search in Google Scholar
[20] Dissanayake, A. A.; Odom, A. L. Single-step synthesis of pyrazoles using titanium catalysis. Chem. Commun.2012, 48, 440–442.Search in Google Scholar
[21] Waldo, J. P.; Larock, R. C. Synthesis of isoxazoles via electrophilic cyclization. Org. Lett.2005, 7, 5203–5205.Search in Google Scholar
[22] Yavari, I.; Moradi, L. A synthesis of isoxazoles through the reaction of activated acetylenes and alkyl 2-nitroethanoates in the presence of triphenylphosphine. Tetrahedron Lett.2006, 47, 1627–1629.Search in Google Scholar
[23] Willy, B.; Rominger, F.; Müller, T. J. J. Novel microwave-assisted one-pot synthesis of isoxazoles by a three-component coupling-cycloaddition sequence. Synthesis2008, 2008 (EFirst), 293–303.10.1055/s-2007-1000856Search in Google Scholar
[24] Grecian, S.; Fokin, V. V. Ruthenium-catalyzed cycloaddition of nitrile oxides and alkynes: practical synthesis of isoxazoles. Angew. Chem. Int. Ed. Engl.2008, 47, 8285–8287.Search in Google Scholar
[25] Valizadeh, H.; Amiri, M.; Gholipur, H. Efficient and convenient method for the synthesis of isoxazoles in ionic liquid. J. Heterocycl. Chem.2009, 46, 108–110.Search in Google Scholar
[26] Bhosale, S.; Kurhade, S.; Prasad, U. V.; Palle, V. P.; Bhuniya, D. Efficient synthesis of isoxazoles and isoxazolines from aldoximes using Magtrieve™ (CrO2). Tetrahedron Lett.2009, 50, 3948–3951.Search in Google Scholar
[27] Debleds, O.; Gayon, E.; Ostaszuk, E.; Vrancken, E.; Campagne, J.-M. A versatile iron-catalyzed protocol for the one-pot synthesis of isoxazoles or isoxazolines from the same propargylic alcohols. Chemistry2010, 16, 12207–12213.Search in Google Scholar
[28] She, Z.; Niu, D.; Chen, L.; Gunawan, M. A.; Shanja, X.; Hersh, W. H.; Chen, Y. Synthesis of trisubstituted isoxazoles by palladium(II)-catalyzed cascade cyclization-alkenylation of 2-alkyn-1-one O-methyl oximes. J. Org. Chem.2012, 77, 3627–3633.Search in Google Scholar
[29] Dadiboyena, S.; Nefzi, A. Solid phase synthesis of isoxazole and isoxazoline-carboxamides via [2+3]-dipolar cycloaddition using resin-bound alkynes or alkenes. Tetrahedron Lett.2012, 53, 2096–2099.Search in Google Scholar
[30] Hashimoto, Y.; Takada, A.; Takikawa, H.; Suzuki, K. Synthesis of isoxazoles en route to semi-aromatized polyketides: dehydrogenation of benzonitrile oxide-para-quinone acetal cycloadducts. Org. Biomol. Chem.2012, 10, 6003–6009.Search in Google Scholar
[31] Abd El-Nabi, H. A. 1-Aryl-5-methoxypyrrolones as synthons for fused heterocycles. Tetrahedron2002, 58, 135–141.10.1016/S0040-4020(01)01029-8Search in Google Scholar
[32] Liu, Z.; Shibata, N.; Takeuchi, Y. Facile synthesis of disubstituted and spiro five-membered benzosultams mediated by TMSCl-NaI-MeCN reagent. J. Chem. Soc. Perkin Trans.2002, 1, 302–303.Search in Google Scholar
[33] Devarie-Baez, N. O.; Xian, M. Facile preparation of 3-substituted benzisothiazoles from o-mercaptoacylphenones. Org. Lett.2010, 12, 752–754.Search in Google Scholar
[34] Yanagida, Y.; Yazaki, R.; Kumagai, N.; Shibasaki, M. Asymmetric synthesis of isothiazoles through Cu catalysis: direct catalytic asymmetric conjugate addition of allyl cyanide to α,β-unsaturated thioamides. Angew. Chem. Int. Ed. Engl.2011, 50, 7910–7914.Search in Google Scholar
[35] Bezbaruah, P.; Gogoi, J.; Rao, K. S.; Gogoi, P.; Boruah, R. C. Microwave-assisted novel and efficient one-pot synthesis of fused steroidal and non-steroidal isothiazoles. Tetrahedron Lett.2012, 53, 4389–4392.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- A fascinating decade for the synthesis of 1,2-azoles
- Substituted benzothiazoles: synthesis and medicinal characteristics
- Preliminary Communication
- Synthesis of thiazolo[4,5-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives
- Research Articles
- Ultrasonic-assisted Cu-catalyzed multicomponent synthesis of furo[3,4-b]pyrazolo[4,3-f]quinolinones
- Synthesis of dihydropyrrolo[2,1-a]isoquinolines via isocyanide-based four-component reaction
- Efficient oxidative cyclization of N-acylhydrazones for the synthesis of 2,5-disubstituted 1,3,4-oxadiazoles using t-BuOI under neutral conditions
- Synthesis and transformations of 1-[2-(toluene-4-sulfonamido)ethyl]thiourea
- Synthesis of new heterocyclic compounds containing benzimidazole moiety as inhibitors of breast cancer cell growth
- An approach to C-glycosidic conjugates of isoflavones
- Ionic liquid catalyzed one-pot synthesis of spiropyran derivatives via three-component reaction in water
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- A fascinating decade for the synthesis of 1,2-azoles
- Substituted benzothiazoles: synthesis and medicinal characteristics
- Preliminary Communication
- Synthesis of thiazolo[4,5-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives
- Research Articles
- Ultrasonic-assisted Cu-catalyzed multicomponent synthesis of furo[3,4-b]pyrazolo[4,3-f]quinolinones
- Synthesis of dihydropyrrolo[2,1-a]isoquinolines via isocyanide-based four-component reaction
- Efficient oxidative cyclization of N-acylhydrazones for the synthesis of 2,5-disubstituted 1,3,4-oxadiazoles using t-BuOI under neutral conditions
- Synthesis and transformations of 1-[2-(toluene-4-sulfonamido)ethyl]thiourea
- Synthesis of new heterocyclic compounds containing benzimidazole moiety as inhibitors of breast cancer cell growth
- An approach to C-glycosidic conjugates of isoflavones
- Ionic liquid catalyzed one-pot synthesis of spiropyran derivatives via three-component reaction in water

