Abstract
Three functionalized ionic liquids (ILs) of [HeMIM]Cl, [CeMIM]Cl, and [AeMIM]Br that can dissolve corn stalk were synthesized and characterized via Fourier transform infrared spectroscopy (FTIR) and 1H NMR. The dissolved corn stalk was in situ blended with phenol and formaldehyde to produce modified phenolic resin composites. The resulting composites were characterized via FTIR, differential scanning calorimetry, and X-ray diffraction analysis, and tested for their mechanical properties. In addition, the effects of ILs on the dissolution rate of corn stalks and on the mechanical properties of the modified phenolic resin were investigated as well. The results showed that the synthesized ILs presented good solubility toward corn stalk at the optimum temperature of 90°C. After modification with corn stalk dissolved in ILs, the mechanical properties of phenolic resin were significantly improved. At the same conditions, the phenolic resin modified with [AeMIM]Br presented the lowest concentration of free formaldehyde and the best mechanical properties, in which the tensile strength and impact strength were improved from 3.28 MPa and 0.93 kJ/m2 to 9.36 MPa and 5.74 kJ/m2, respectively, but the hardness only changed slightly.
1 Introduction
In recent years, ionic liquids (ILs) have become popular as green solvents with excellent application prospects (1, 2). ILs have been widely utilized in the fields of electrochemistry, organic synthesis, and chemical separation, as well as in materials preparation, because of their unique characteristics such as low vapor pressure, absence of pollution, possibility of easy recycling, good electrical conductivity, high thermal and chemical stability, designable structure, etc. (3–7). Recently, the use of ILs has also received increasing attention in polymer chemistry, which is focused on dissolving cellulose and natural polymers (8–12), catalysis of polymerization system (13, 14), and the preparation of polymerization medium (15–17) and polymer function materials (3, 18, 19). Pretreatment of ILs has been widely studied as a promising pretreatment technique but is too expensive to be commercialized. Therefore, Qing et al. (20) researched and developed an efficient, acid-catalyzed, aqueous IL pretreatment process to optimize the total sugar conversion of pretreated biomass and to reduce IL usage. The research of Xu et al. (21) indicated that the dissolution of cypress in ILs in a microwave-irradiated environment is a potential alternative method for the pretreatment of lignocellulosic materials. Both microwave irradiation and [Emim]Ac dissolution, which was screened as the best medium for this pretreatment, were found to significantly increase the rate and yield of enzymatic hydrolysis. Emiko and Hisashi (22) investigated the reaction behavior of cellulose in an IL, which can dissolve cellulose. The results showed that, aside from functioning as a solvent for cellulose, IL functioned as a reagent for both depolymerization to produce various low-molecular-weight compounds and the subsequent polymerization of those compounds.
With the development of technologies, pure phenolic resin has failed to meet the requirements of many high-tech fields. Hence, the modifications of phenolic resin with different methods have been the main focus in its study, and these remarkable achievements obtained can lay a theoretical basis for further studies on phenolic resin modification (23–27). Wen et al. (28) reported that larch bark was liquefied in the presence of phenol and that the obtained liquefied resultant was reacted with formaldehyde to prepare the liquefied bark-modified phenol formaldehyde resin (BPF) in an application attempt toward preparing straw boards. Moreover, the curing kinetics of the BPF resin was also investigated via dynamic differential scanning calorimetry. Streckova et al. (29) investigated in detail soft magnetic composites based on Fe powder and phenol formaldehyde resin (PFR) modified with tetraethyl orthosilicate. The chemical synthesis of PFR, including its modification with nanometer-sized SiO2 particles created via the sol-gel method and subsequent coating, enables the preparation of an insulating PFR-SiO2 (PFRT) layer on the surface of Fe particles.
The continuous development and permanent application of biomass have been a subject of significant importance in the 21st century because of its rich resource and possibility of regeneration. Corn stalks are rich in plant cellulose with a supramolecular structure and have been wasted without being fully developed and utilized. Therefore, dissolution of corn stalks is an important step and key to its application. In this study, we therefore attempted to synthesize three functional ILs of 1-(2-hydroxyethyl)-3-methyl imidazole chloride ([HeMIM]Cl), 1-ethylamine-3-methyl imidazole bromide ([AeMIM]Br), and 1-carboxyethyl-3-methylimidazole chloride ([CeMIM]Cl) to destroy the supramolecular structure of this cellulose. With NaOH as a catalyst, the dissolved corn stalk further reacted with phenol and formaldehyde to synthesize the combined materials of modified phenolic resin. The synthesized materials were characterized via various analytical methods and were tested for their mechanical properties.
2 Results and discussion
2.1 Thermal stability of ionic liquids
The thermal stability of ILs was characterized using a TGA-4000 thermogravimetric instrument (Perkin Elmer, Shanghai, China) over a temperature range from 25°C to 500°C (Figure 1).
![Figure 1: Thermal stability of the ionic liquids: (1) [HeMIM]Cl, (2) [CeMIM]Cl, and (3) [AeMIM]Br.](/document/doi/10.1515/epoly-2014-0195/asset/graphic/epoly-2014-0195_fig1.jpg)
Thermal stability of the ionic liquids: (1) [HeMIM]Cl, (2) [CeMIM]Cl, and (3) [AeMIM]Br.
The thermal stabilities of three ILs ([HeMIM]Cl, [CeMIM]Cl, and [AeMIM]Br) are shown in Figure 1. The TGA curves indicated that the initial decomposition temperatures of these ILs were 294°C, 255°C, and 298°C, respectively, whereas the DTG curves suggested that the maximum decomposition temperatures were 317°C, 286°C, and 333°C, respectively. Moreover, [CeMIM]Cl was the most easily decomposed IL. The IL was completely decomposed when the temperature reached 315°C. [AeMIM]Br was difficult to be decomposed, and its thermal decomposition was the slowest of the three. Therefore, [AeMIM]Br possessed better thermal stability than [HeMIM]Cl and [CeMIM]Cl.
2.2 Effects of ILs and temperature on the dissolution rate of corn stalk
The influence of ILs and temperature on corn stalk dissolution rate was studied. The mass ratio of ILs and corn stalk was 20:1 (Figure 2).
![Figure 2: Effects of ILs and temperature on the dissolution rate of corn stalk: (1) [HeMIM]Cl, (2) [CeMIM]Cl, and (3) [AeMIM]Br.](/document/doi/10.1515/epoly-2014-0195/asset/graphic/epoly-2014-0195_fig2.jpg)
Effects of ILs and temperature on the dissolution rate of corn stalk: (1) [HeMIM]Cl, (2) [CeMIM]Cl, and (3) [AeMIM]Br.
These experiments indicated that all ILs presented good dissolution ability toward corn stalks. Increasing the temperature resulted in an increased dissolution rate. When the temperature was lower than 90°C, [CeMIM]Cl presented the best dissolution performance. When the temperature exceeded 90°C, the dissolution rate of corn stalks in [CeMIM]Cl evidently decreased, whereas that in [HeMIM]Cl decreased slightly and that in [AeMIM]Cl increased slowly with temperature. The reason for these findings was that, at temperatures exceeding 90°C, the dissolution performance was affected by the degradation of [CeMIM]Cl, whereas [AeMIM]Cl possessed good high temperature resistance. Even at 110°C, the dissolution rate of corn stalk in this IL still increased gradually, reaching the highest value of 31.5%. At a temperature below 100°C, [CeMIM]Cl and [AeMIM]Cl showed better performance, which may be due to the strong polar terminated-COOH and terminated-NH2 groups in their cations. In comparison with ILs with terminated-OH groups, [CeMIM]Cl and [AeMIM]Cl more easily formed coordination complexation with the oxygen atom of the hydroxyl group in the cellulose structure of corn stalks, which can weaken the intermolecular and intramolecular hydrogen bonds in the cellulose, destroy the aggregation structure of the cellulose, and then promote cellulose dissolution. Therefore, the optimum dissolution temperature is approximately 90°C.
2.3 Effects of ILs and its weight ratio on the corn stalk
Phenolic resin composites were prepared from the corn stalks dissolved in three IL products with phenol and formaldehyde via an in situ blending method. The effects of ILs and their weight ratio to corn stalks on the mechanical properties of the composite were investigated, and the results are shown in Table 1.
Effects of ILs and dissolved corn stalks on the mechanical properties of the phenolic resin composite.
| No. | ILs | Weight ratio of IL to corn stalk | Weight ratio of IL to phenol | Tensile strength (MPa) | Impact strength (kJ/m2) | Relative hardness (%) | Free formaldehyde (%) |
|---|---|---|---|---|---|---|---|
| 0 | – | – | – | 3.28 | 0.93 | 95 | 3.64 |
| 1 | [HeMIM]Cl | 10:1 | 1:5 | 7.52 | 3.68 | 94 | 2.35 |
| 2 | [HeMIM]Cl | 15:1 | 1:5 | 7.83 | 4.06 | 90 | 2.28 |
| 3 | [HeMIM]Cl | 20:1 | 1:5 | 8.34 | 5.39 | 92 | 1.07 |
| 4 | [HeMIM]Cl | 10:1 | 1:10 | 8.47 | 4.02 | 98 | 0.98 |
| 5 | [HeMIM]Cl | 20:1 | 1:10 | 8.89 | 5.66 | 96 | 0.78 |
| 6 | [CeMIM]Cl | 20:1 | 1:5 | 9.04 | 5.28 | 95 | 0.92 |
| 7 | [AeMIM]Br | 20:1 | 1:5 | 9.36 | 5.74 | 94 | 0.88 |
Sample 0 was the original phenolic resin prepared at the same conditions without any modification.
According to Table 1, the mechanical properties of the modified phenolic resin were considerably improved and the concentration of free formaldehyde in the composite was decreased by ILs and dissolved corn stalks. By comparing samples 1–5, at a weight ratio of [HeMIM]Cl to the corn stalk of 20:1 and a ratio of ILs to phenol of 1:10, the tensile strength and the impact strength reached the highest values of 8.89 MPa and 5.66 kJ/m2, respectively. Meanwhile, the free formaldehyde achieved the lowest value, decreasing from 3.64% to 0.78%, and hardness only slightly changed. By comparing samples 3, 6, and 7 at the same conditions, the phenolic resin modified with corn stalks dissolved in [AeMIM]Br, in which the concentration of the free formaldehyde was the lowest, presented the best mechanical properties, i.e., the tensile strength and impact strength were improved from 3.28 MPa and 0.93 kJ/m2 to 9.36 MPa and 5.74 kJ/m2, respectively, whereas hardness slightly changed and the concentration of free formaldehyde decreased from 3.64% to 0.88%.
These results may be due to hydrophilic functionalized polar groups and hydrophobic alkyl side chains in the structure, which showed good compatibility of ILs toward phenolic resin. Considering that they are well dispersed in the polymer and the difficulty in transferring them to the surface during the curing process, ILs presented a specific coupling effect during phenolic resin preparation, which was beneficial in improving the mechanical properties of the modified phenolic resin. Moreover, the functionalized end groups in ILs reacted with phenolic resin at specific conditions and formed a more stable polar conjugate structure, which was beneficial to the mechanical properties of phenolic resin. By contrast, [AeMIM]Br, which possessed amino end groups, showed better high temperature resistance, i.e., corn stalks dissolved better at temperatures exceeding 90°C, with better coupling effect and a more stable polar conjugate structure. Hence, the modified phenolic resin from [AeMIM]Br and the corresponding dissolved corn stalk presented the best mechanical properties and the lowest concentration of free formaldehyde, which is in accordance with the result in Figure 2.
2.4 FTIR spectra of corn stalks and phenolic resin
The chemical structures of the original phenolic resin (sample 0 in Table 1), the corn stalks, and the phenolic resin modified with the corn stalk dissolved in [HeMIM]Cl (sample 3 in Table 1) were characterized using a MAGNA-IR750 Fourier infrared spectrum instrument (Thermo Nicolet Corporation, Beijing, China) (Figure 3).
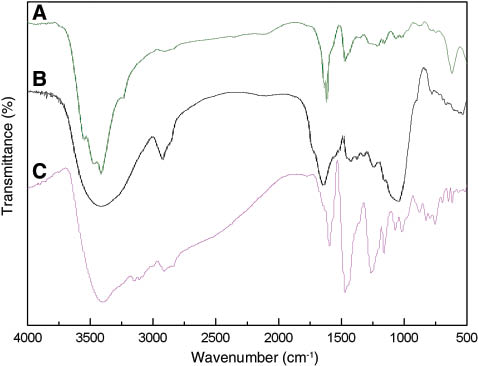
FTIR spectra of (A) the original phenolic resin, (B) the corn stalk, and (C) the modified phenolic resin.
The peaks at 3410–3450 cm-1 were attributed to the stretching frequency of -OH in the phenolic resin. Compared with the band in curve (A), the one in curve (B) was stronger and wider because of a large amount of -OH existing in the corn stalks. The bands at 1615 and 1470 cm-1 were both assigned to the vibration frequency of the aromatic skeleton. The bands at 1216 and 878 cm-1 were assigned to the stretching of the aromatic ring and the out-of-plane bending of -CH on the benzene ring, respectively. All these peaks are typical characteristic adsorption peaks of the phenolic resin.
The bands at 2923 and 1644 cm-1 were attributed to the stretching of the -CH2 and the adsorption vibration of C=C in the cellulose, respectively. The strong and wide band at 1043 cm-1 was assigned to the adsorption vibration of C-O-C, which was different from that in curve (A). All these bands are typical characteristic adsorption peaks of the cellulose in the corn stalks.
More bands appeared in curve (C). Aside from the characteristic adsorption peaks of phenolic resin in curve (A), those of corn stalks in curve (B) were also observed. Furthermore, the typical characteristic adsorption bands of [HeMIM]Cl appeared at 3151, 1594, 1015, and 756 cm-1. The stronger band at 1466 cm-1 of the modified phenolic resin indicated the reaction between the phenolic hydroxyl and the aldehyde groups, whereas the bands at 1044 and 3397 cm-1, which were assigned to the characteristic peaks of C-O-C and -OH- in cellulose, respectively, significantly became weaker. All these results indicate that the cellulose in the corn stalks reacted with aldehyde.
2.5 XRD patterns of the corn stalk and composite phenolic resin
The crystal structures of the corn stalks and the phenolic resin modified with the dissolved corn stalk in [HeMIM]Cl (sample 3 in Table 1) were characterized using a D/max-2400 X-ray diffractometer (Shenyang Xing Dao Scientific Instrument Co., Ltd, Shenyang, China) (Figure 4).

XRD patterns of the original phenolic resin and the modified phenolic resin: (A) corn stalk and (B) modified phenolic resin.
In Figure 4, the diffraction peaks of the corn stalks at 20.7°, 26.5°, 27.7°, and 49.9° indicated the crystal area existing in them. After dissolution in [HeMIM]Cl, a large amount of bare -OH in cellulose reacted with formaldehyde, which destroyed the crystal structure of the cellulose in corn stalks and resulted in the modified phenolic resin. Thus, no obvious diffraction peak was found in the diffraction angle range.
2.6 DSC analysis of the composite phenolic resin
The curing temperatures of the original phenolic resin (sample 0 in Table 1) and the modified phenolic resin (sample 3 in Table 1) were tested using a Mettler 821e/400 differential scanning calorimetry instrument (Mettler Toledo Company, Shanghai, China) (Figure 5).
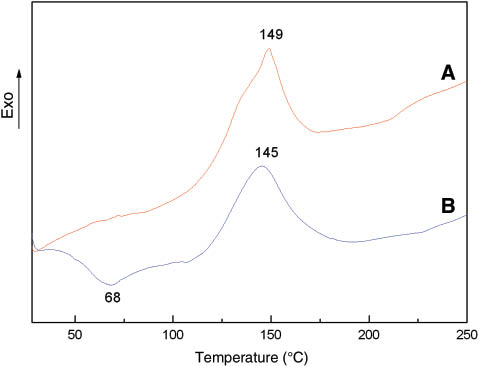
DSC curves of the original phenolic resin and the modified phenolic resin: (A) original phenolic resin and (B) modified phenolic resin.
As seen in Figure 5, the curing temperature of the original phenolic resin was 149°C. The addition of ILs exerted a particular effect on the thermal property of the phenolic resin. An endothermic peak was observed at 68°C, which may be due to the remaining solvent used in the preparation of ILs evaporating at this temperature. The exothermic peak at 145°C indicated the curing temperature of the modified phenolic resin. Furthermore, no other peak was observed in this range, which proved that the functionalized groups in both ILs and corn stalk cellulose reacted with formaldehyde and produced single homogeneous phenolic resin composites with curing temperatures that were slightly lower than that of the original phenolic resin.
3 Conclusions
In this work, the composite material of phenolic resin was in situ prepared with corn stalk dissolved in synthesized ILs. According to the results of the Fourier transform infrared spectroscopy (FTIR) and 1H NMR analyses, three functionalized ILs of [HeMIM]Cl, [AeMIM]Br, and [CeMIM]Cl were prepared with high purities that exceeded 90%. All prepared ILs presented good dissolution abilities toward the corn stalks. When the temperature was below 90°C, [CeMIM]Cl showed the best dissolution effect, whereas [AeMIM]Cl showed the best dissolution performance, with a dissolution rate that reached 31.5% when the temperature exceeded 90°C. To avoid the thermal degradation of the ILs, the optimal temperature was approximately 90°C.
The mechanical properties of the phenolic resin were significantly improved by modification with the corn stalk dissolved in ILs. At the same conditions, the concentration of free formaldehyde in the phenolic resin modified with the corn stalk dissolved in [AeMIM]Br was the lowest and the mechanical properties were the best, in which tensile strength and impact strength improved from 3.28 MPa and 0.93 kJ/m2 to 9.36 MPa and 5.74 kJ/m2, respectively, whereas hardness slightly changed and the concentration of free formaldehyde decreased from 3.64% to 0.88%. The characterization results of the modified phenolic resin via FTIR, X-ray diffraction analysis (XRD) and differential scanning calorimetry (DSC) indicated that both the structure of cellulose in the corn stalk and the active end groups of ILs reacted with formaldehyde.
4 Experimental
4.1 Experimental reagents
All reagents used were of analytical grade and used without further purification. N-Methylimidazole, chloroethanol, and chloroacetic acid were procured from Shenyang Licheng Chemical Reagent Factory (Shenyang city, Liaoning Province, China). 2-Bromoethylamine salt, phenol, NaOH, N-ethylinidazole, ethanol, and ethyl acetate were purchased from National Medicine Chemical Reagent Co. Ltd. (China). Corn stalks were provided by Tieling Farm (Tieling, China).
4.2 Analysis method
IR spectra were recorded in KBr pellets by using a MAGNAIR750 Fourier infrared spectrum instrument operating in the region of 4000 to 400 cm-1. Proton NMR spectra were recorded at room temperature by using an FT-NMR Bruker Avance II 400-MHz spectrometer (Bruker, Fällanden, Switzerland) with DMSO-d6 and CDCl3 as solvents. The thermal stability of ILs was investigated using a TGA-4000 thermogravimetric instrument with temperatures ranging from 25°C to 500°C. The curing temperature of the phenolic resin was determined using a Mettler821e/400 differential scanning calorimeter in a temperature region from room temperature to 250°C at a heating rate of 10°C/min. XRD measurements for the crystal structures of wood powder and the phenolic resin were performed using a D/max-2400 X-ray diffractometer, employing Cu Kα radiation (12 kW, λ=10.1541 nm). The mechanical properties of the materials were tested using the following machines: XLB-D 400×400×2 plate vulcanization machine (Qingdao Yaxing Machinery Co., Ltd, Qingdao, China), CMT4304 electronic universal testing machine (Shanghai Jiehu Instrument Co., Ltd, Shanghai, China), DJF-20 dynamic impaction analyzer (Changchun Hengyue Electronic Technology Instrument Co., Ltd, Changchun, China), and SFX-2L rotary evaporator (Gongyi Yuhua Instrument Co., Ltd, Zhengzhou, China).
4.3 Preparation and characterization of functionalized ionic liquids
In a round-bottom flask with a reflux condenser, N-methylimidazole was respectively added with chloroethanol, chloroacetic acid, and 2-bromoethylamine salt at a molar ratio 1.2:1 and heated with stirring at 80°C for 8–36 h. With the reaction, the mixture turned into a colorless and slightly thickened liquid. Upon reaction completion, the obtained mixture was transferred to a separating funnel and washed with ether to remove the unreacted methylimidazole. The residue was evaporated in a vacuum drying oven at 80°C at 0.08 MPa for 24 h to produce three functionalized ILs. The products were colorless and slightly thick. After weighing, the yields of ILs all exceeded 93%. The structures of the ILs were characterized via FTIR and 1H NMR. The results are shown in Scheme 1.

Chemical structures of the three functionalized imidazolium ionic liquids.
[HeMIM]Cl IR (KBr, cm-1): 3410 (O-H), 3146 (C-H), 2956 (C-H), 2876 (C-H), 1644 (C=C), 1574 (C=N), 1167 (C-O), 755 (imidazole ring). 1H NMR (400 MHz, DMSO): 5.40 (d, 1H, OH-a), 3.88 (d, 3H, CH3-b), 7.76 (d, 1H, CH-c), 7.71 (s,1H,CH-d), 9.23 (s,1H,CH-e), 4.23 (s, 2H,CH2-f), 3.71 (s, 2H, CH2-g), 2.50 (DMSO).
[CeMIM]Cl IR (KBr, cm-1): 3426 (O-H), 3079 (C-H), 2976 (C-H), 2850 (C-H), 1732 (C=O), 1635 (C=C), 1560 (C=N), 1166 (C-O), 763 (imidazole ring). 1H NMR (400 MHz, CDCl3): 2.58 (d, 1H, COOH-a), 3.81 (d, 3H, CH3-b), 7.16 (d, 1H, CH-c), 6.96 (s, 1H, CH-d), 8.01 (s, 1H, CH-e), 3.72 (s, 2H, CH2-f), 7.26 (CDCl3).
[AeMIM]Br IR (KBr, cm-1): 3455 (N-H), 3163 (C-H), 2957 (C-H), 2870 (C-H), 1616 (C=C), 1579 (C=N), 744 (imidazole ring). 1H NMR (400 MHz, CDCl3): 4.01 (d, 2H, NH2-a), 4.03 (d, 3H, CH3-b), 7.10 (d, 1H, CH-c), 7.37 (s, 1H, CH-d), 9.09 (s, 1H, CH-e), 4.02 (s, 2H, CH2-f), 3.75 (d, 2H, CH2-g), 7.26 (CDCl3).
4.4 Preparation and application of dissolved corn stalk in ILs
After crushing and screening, the corn stalks at mesh 80 were washed with an alkali solution to remove impurities and small molecular amounts of fats and then washed with water until neutral. After oven drying at 70°C for 12 h, the corn stalks were collected and reserved.
In a round-bottom flask, the corn stalks were added with ILs prepared individually at a specific weight ratio and dissolved at 90°C with stirring. Timing samples were obtained, and the morphology was observed using a polarizing microscope. After 3 h of dissolution and cooling to room temperature, the dissolved system was centrifuged for 3 min. The supernatant was then removed, and the residue in the lower layer was washed with methanol for several times. After drying and weighing, the dissolution rates of the corn stalks in ILs were calculated according to the method reported in the literature (30).
4.5 Preparation and testing of the mechanical properties of the phenolic resin composites
Known amounts of phenol, formaldehyde, and NaOH at a molar ratio of 1:2:0.5 were added into the previously mentioned dissolved systems, in which the molar ratio of phenol to ILs was 5:1. The temperature of the mixture was increased to 70°C in 20 min, maintained for 30 min, and then gradually increased to 95°C and kept for 1–2 h. When the solution became brownish red and stinky, the temperature was decreased to 70°C for decompression dehydration. After adjusting the viscosity to 380–400 mPa s with ethanol, the solution was poured into a mold and cured at 80°C–130°C for 4–5 h by using a plate vulcanization machine (Qingdao Yaxing Machinery Co. Ltd, Qingdao, China) to obtain the final product. The concentration of free formaldehyde in the phenolic resin was determined via a method reported in the literature (31). The tensile strength and impact strength of the phenolic resin before and after modification were tested using a CMT4304 electronic universal testing machine and a DJF-20 dynamic impaction analyzer, whereas the relative hardness values of the resins were tested using a Shore hardness meter.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (51203091) and Liaoning Province Education Department Project of China (L2014037).
References
1. Seddon KR, Stark A, Torres MJ. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl Chem. 2000;72:2275.10.1351/pac200072122275Search in Google Scholar
2. Earle MJ, Seddon KR. Ionic liquids. Green solvents for the future. Pure Appl Chem. 2000;72:1391–8.10.1351/pac200072071391Search in Google Scholar
3. Lu XB, Zhang Q, Zhang L, Li J. Direct electron transfer of horseradish peroxidase and its biosensor based on chitosan and room temperature ionic liquid. Electrochem Commun. 2006;8:874–8.10.1016/j.elecom.2006.03.026Search in Google Scholar
4. Saha S, Brahman D, Sinha B. Cu(II) complexes of an ionic liquid-based Schiff base 1-{2-(2-hydroxy benzylidene amino) ethyl}-3-methyl-imidazolium]PF6: Synthesis, characterization and biological activities. J Serb Chem Soc. 2014;79:5945.Search in Google Scholar
5. Zhao W, He GH, Zhang LL, Ju J, Dou H, Nie F, Li GN, Liu HJ. Effect of water in ionic liquid on the separation performance of supported ionic liquid membrane for CO2/N2. J Membr Sci. 2010;350:279–85.10.1016/j.memsci.2010.01.002Search in Google Scholar
6. Yang XJ, Fang YX, Li XM, Zhang K, Cui YD, Zhang BN, Yin GQ. Synthesis of two AMPS-based polymerizable room temperature ionic liquids and swelling difference between their co-polymeric gels with HEMA. e-Polymers 2014;14:335–43.10.1515/epoly-2014-0070Search in Google Scholar
7. Alamdari RF, Zamani FG, Zekri N. An efficient and highly selective ortho-tert-butylation of p-cresol with tert-butyl methyl ether catalyzed by sulfonated ionic liquids. J Serb Chem Soc. 2014;79:5764.10.2298/JSC130717072ASearch in Google Scholar
8. Emiko O, Hisashi M. Decomposition of cellulose in an ionic liquid, 1-ethyl-3-methylimidazolium chloride. J Wood Sci. 2014;60:428–37.10.1007/s10086-014-1421-3Search in Google Scholar
9. Swatloski RP, Spear SK, Holbrey JD. Dissolution of cellulose with ionic liquids. J Am Chem Soc. 2002;124:4974–5.10.1021/ja025790mSearch in Google Scholar PubMed
10. Zhang H, Wu J, Zhang J, He JS. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005;38:8272–7.10.1021/ma0505676Search in Google Scholar
11. Meng ZJ, Zheng XJ, Tang KY, Liu J, Qin SF. Dissolution of natural polymers in ionic liquids: a review. e-Polymers 2012;12:317–45.10.1515/epoly.2012.12.1.317Search in Google Scholar
12. Guo LY, Shi TJ, Li Z, Duan YP, Wang YG. Synthesis of novel and functionalized ionic liquid [HeEIM]Cl and its solubility for cotton fiber. Chem J Chin U. 2008;29:1901.Search in Google Scholar
13. Weuster BD. Process intensification of whole-cell biocatalysis with ionic liquids. Chem Rec. 2007;7:334–40.10.1002/tcr.20130Search in Google Scholar
14. Joan FD, Khadidja B, Mustapha R. Catalysed esterifications in room temperature ionic liquids with acidic counteranion as recyclable reaction media. Catal Commun. 2002;3:185–90.10.1016/S1566-7367(02)00087-0Search in Google Scholar
15. Guo LY, Shi TJ, Li Z. Dissolution of microcrystalline cellulose and its graft copolymer in ionic liquid of 1-allyl-3-ethylimidazolium chloride. e-Polymers 2009;9:1378–88.10.1515/epoly.2009.9.1.1378Search in Google Scholar
16. Guo LY, Shi TJ, Li Z. Dissolution of fir powder and its graft copolymer in ionic liquid. Holz Roh Werkst. 2011;69:383–9.10.1007/s00107-010-0438-6Search in Google Scholar
17. Song J, Cheng BW, Jie XJ, Liang Y, Lu F, Zhang FN. Study on the coagulation process of cellulose nascent fibers with ionic liquid AMIMCl as a solvent during dry-wet spinning. e-Polymers 2011;11:401–11.10.1515/epoly.2011.11.1.401Search in Google Scholar
18. Li Z, Shi TJ, Guo LY. Preparation and morphology of porous SIO2 ceramics derived from fir flour templates. J Serb Chem Soc. 2010;75:385.10.2298/JSC090410010ZSearch in Google Scholar
19. Zein ES, Endres F. Electrodeposition of metals and semiconductors in air- and water-stable ionic liquids. Chemphyschem. 2006;7:58–61.10.1002/cphc.200500288Search in Google Scholar PubMed
20. Qing Q, Hu R, He YC, Zhang Y, Wang LQ. Investigation of a novel acid-catalyzed ionic liquid pretreatment method to improve biomass enzymatic hydrolysis conversion. Appl Microbiol Biotechnol. 2014;98:5275–86.10.1007/s00253-014-5664-0Search in Google Scholar PubMed
21. Xu JK, Sun YC, Sun RC. Structural and hydrolysis characteristics of cypress pretreated by ionic liquids in a microwave irradiation environment. Bioenerg Res. 2014;7:1305–16.10.1007/s12155-014-9464-2Search in Google Scholar
22. Emiko O, Hisashi M. Reaction behavior of cellulose in an ionic liquid, 1-ethyl-3-methylimidazolium chloride. J Wood Sci. 2013;59:221–8.10.1007/s10086-013-1322-xSearch in Google Scholar
23. Wang JG, Guo QG, Liu L. High temperature bonding properties of phenol formaldehyde resin modified by ceramic particles. Mater Mech Eng. 2005;29:24.10.3901/JME.2005.01.024Search in Google Scholar
24. Jin HY, Wu YQ, Hou SE, Li YL, Liu M, Ji ZJ, Yuan J. The effect of spherical silica powder on the tribological behavior of phenolic resin-based friction materials. Tribol Lett. 2013;51:65–72.10.1007/s11249-013-0146-6Search in Google Scholar
25. Naderi A, Mazinani S, Ahmadi SJ, Sohrabian M, Arasteh R. Modified thermo-physical properties of phenolic resin/carbon fiber composite with nano zirconium dioxide. J Therm Anal Calorim. 2014;117:393–401.10.1007/s10973-014-3742-2Search in Google Scholar
26. Cheng SN, Yuan ZS, Leitch M, Anderson M, Xua CB. Highly efficient de-polymerization of organosolv lignin using a catalytic hydrothermal process and production of phenolic resins/adhesives with the depolymerized lignin as a substitute for phenol at a high substitution ratio. Ind Crop Prod. 2013;44:315–22.10.1016/j.indcrop.2012.10.033Search in Google Scholar
27. Tabarsa T, Jahanshahi S, Ashori A. Mechanical and physical properties of wheat straw boards bonded with a tannin modified phenol-formaldehyde adhesive. Composites: Part B 2011;42:176–80.10.1016/j.compositesb.2010.09.012Search in Google Scholar
28. Wen MY, Shi JY, Park HJ. Dynamic wettability and curing characteristics of liquefied bark-modified phenol formaldehyde resin (BPF) on rice straw surfaces. J Wood Sci. 2013;59:262–8.10.1007/s10086-013-1329-3Search in Google Scholar
29. Streckova M, Fuzer J, Medvecky L, Bures R, Kollar P, Faberova M, Girman V. Characterization of composite materials based on Fe powder (core) and phenol-formaldehyde resin (shell) modified with nanometer-sized SiO2. Bull Mater Sci. 2014;37:167–77.10.1007/s12034-014-0644-7Search in Google Scholar
30. Guo LY, Shi TJ, Li Z. Solubilities of two kinds of imidazolium ionic liquids for fir powder. J Chem Ind Eng. 2008;59:1299.Search in Google Scholar
31. Guo LY, Shi TJ, Li Z. Influence of ionic liquid and fir powder on properties of phenol-formaldehyde adhesive. Chin J Mater Res. 2009;62:1177.Search in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Editorial May 2015
- Full length articles
- Synthesis, characterization, and theoretical study of an acrylamide-based magnetic molecularly imprinted polymer for the recognition of sulfonamide drugs
- Polyethylene glycol and iron oxide nanoparticles blended polyethersulfone ultrafiltration membrane for enhanced performance in dye removal studies
- Applications of chelating resin for heavy metal removal from wastewater
- Commingled composites of polypropylene/coir-sisal yarn: effect of chemical treatments on thermal and tensile properties
- Hydrothermal synthesis and characterization of carbon spheres using citric-acid-catalyzed carbonization of starch
- Biodegradation of chemically modified lignocellulosic sisal fibers: study of the mechanism for enzymatic degradation of cellulose
- Synthesis and application of functionalized ionic liquids as solvent to corn stalk for phenolic resin modification
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Editorial May 2015
- Full length articles
- Synthesis, characterization, and theoretical study of an acrylamide-based magnetic molecularly imprinted polymer for the recognition of sulfonamide drugs
- Polyethylene glycol and iron oxide nanoparticles blended polyethersulfone ultrafiltration membrane for enhanced performance in dye removal studies
- Applications of chelating resin for heavy metal removal from wastewater
- Commingled composites of polypropylene/coir-sisal yarn: effect of chemical treatments on thermal and tensile properties
- Hydrothermal synthesis and characterization of carbon spheres using citric-acid-catalyzed carbonization of starch
- Biodegradation of chemically modified lignocellulosic sisal fibers: study of the mechanism for enzymatic degradation of cellulose
- Synthesis and application of functionalized ionic liquids as solvent to corn stalk for phenolic resin modification

