Abstract
Chalcogenide glasses are emerging as important enabling materials for low-cost infrared imaging by virtue of their transparency in the key short-wave infrared (SWIR) to long-wave infrared (LWIR) bands and the ability to be mass produced and molded into near-net shape lenses. In this paper, we introduce a new family of chalcogenide glasses, which offer visible as well as infrared transmission and improved thermal and mechanical properties. These glasses are based on Ga2S3-La2S3 with added Ga2Se3-up to complete the substitution of Ga2S3 for Ga2Se3. The samples are prepared via the melt-quench method in an argon-purged atmosphere. All the studied compositions showed a lower glass transition temperature and an extended transmission window. Particularly, the LWIR transmission was extended from about 9 μm for gallium lanthanum sulfide (Ga-La-S) glass to about 15 μm for Se-added Ga-La-S retaining visible transmission from around 463 nm. The thermal and mechanical properties were investigated to prove the suitability of these novel materials for the production of optical components such as visible to LWIR lenses. Their suitability for drawing into optical fibers is also discussed.
1 Introduction
Devices for long- and short-wave infrared (SWIR and LWIR) imaging are largely demanded for a number of applications such as defense, security, and healthcare [1]. A great improvement of infrared imaging systems was given after uncooled detectors became available in the market [2]. In particular, HgCdTe and InGaAs were two key materials for detectors included in devices for thermal imaging in the range between 8 and 14 μm [3]. One of the effects of this improvement was that thermal cameras and devices could conveniently work at room temperature. However, due to the lower sensitivity of these detectors compared to low-temperature devices, particular attention to the design of optical components is required in order to collect a larger amount of signal [2], [4].
The production of devices that can combine visible and LWIR imaging in a single solution is currently the main technological challenge [5]. The products currently commercialized rely on two separate combination of optical components to capture signals from both visible and LWIR. The materials used to produce optical components are the principal limitation. Indeed, a material transparent from the visible to the LWIR range is not yet available in the market.
Chalcogenide glasses are widely used to produce parts such as lenses and prisms for thermal imaging apparatuses. These materials are made of chalcogen elements (S, Se, Te) bonded with a covalent bond to heavy metals and metalloids [6]. The infrared transparency can be observed up to 13 μm for sulfide glasses, 17 μm for selenide glasses, and 20 μm for telluride glasses [7]. However, the transmission window cuts-off around 700–900 nm due to the electronic band gap absorption, which prevents the transmission of visible radiation [8]. Extrinsic losses due to impurities have a large impact on the transparency of the glass. Dedicated facilities for the purification of the precursors and for melting the glass can address this issue, improving the quality of the glass and insuring repeatable results.
Previous studies on multispectral chalcogenide glasses led to the production of Ga2S3-GeS2-CsCl glasses, which transmitted from 500 nm to 11 μm [9], [10], [11]. However, the presence of CsCl resulted in high water uptake and subsequently large losses at the O-H vibration wavelengths. Therefore, a material that combines a large transmission window, low losses, good thermal stability, and small expansion coefficient is desirable for the production of multispectral optical components.
In this paper, we report our work on the development of a chalcogenide material that can satisfy the optical, thermal, and mechanical requirements of multispectral devices. Gallium lanthanum sulfide (Ga-La-S) has been reported as a robust glass with a low thermal expansion and capable to transmit from visible to 8 μm [12]. The modification of this glass with selenium could give a solution to address the specific requirements for multispectral materials.
The addition of selenium to Ga-La-S glass resulted in a material that can successfully transmit from 463 nm to 15 μm. On the other hand, the thermal and mechanical characterization revealed the material to keep a low expansion coefficient and thermal stability. These features make this material a promising solution for the design and production on a large scale of components for visible to LWIR imaging devices.
2 Experimental
The samples were produced from mixtures of La2S3 (3 N purity), Ga2S3 (5 N purity), and Ga2Se3 (5 N purity) batched in a nitrogen-purged glovebox. The mixtures were homogenized using a roller mixer for 1 h. Each of the mixtures was placed in a vitreous carbon crucible and melted in an argon-purged silica tube. A horizontal split-tube furnace was used to produce the glasses with a melt-quench process lasting for 24 h in total. The mixtures were initially baked for 3 h at 350°C to eliminate moisture and volatile oxides of selenium and sulfur. During the melting step, the temperature was ramped by 10°C/min up to 1150°C for 21 h to melt the precursors.
The quenching step consisted of a rapid cooling of the mixtures down to room temperature. It was done by sliding the tube furnace away from the melt to allow the samples to exchange heat with air. The samples were annealed at 490°C for 48 h to release the internal stress in the glasses. The glass formation was investigated over a range of compositions summarized in Figure 1.

La2S3-Ga2S3-Ga2Se3 system phase diagram.
The samples were cut into rectangular flats and polished to optical finish to investigate the optical characteristics. The transmission window of the samples was registered using a nitrogen-purged Varian 670-IR FTIR spectrometer (Varian, Palo Alto, CA, USA) and Cary 50 UV-Vis spectrophotometer (Agilent, Santa Clara, CA, USA) scanning over a range of wavelengths between 450 nm and 16 μm. The refractive index was measured with a Woollam M-2000 ellipsometer (Woollam, Lincoln, NE, USA) over a range of wavelengths between 0.193 and 1.690 μm. The effect of selenium on the transparency in both visible and infrared range between 7 and 14 μm was probed taking pictures with a FLIR C2 camera. The camera was equipped with two separate objectives for visible and thermal imaging. Flats of GLS and GLSSe glasses with thickness of 1 mm were placed in front of the objectives, and then the pictures were taken. The software included in the camera was then employed to combine visible and thermal pictures.
Thermal and mechanical investigations were carried out to test the stability of the glass.
Thermal studies were carried out with the aid of a Perkin-Elmer Diamond TG-DTA to determine the glass transition temperature (Tg), crystallization temperature (Tx), and melting temperature (Tm).
The viscosity and the coefficients of thermal expansion (CTE) were determined using a Perkin-Elmer Diamond TMA. The viscosity was determined following the three-point-bending procedure ISO 7887-4. It relates the position of a bending probe to the viscosity as:
where η is the viscosity (dPa·s), ls is the span of the three-point-bending stage (mm), Δt is the measured time (s), m is the mass of the sample in (g), Ic is the cross-sectional moment of inertia in (mm4), and Δf is the change in position of the probe measured (mm).
The coefficients of thermal linear expansion were determined with an expansion probe placed on top of a rod. The position of the probe was related to the CTE using the relation:
where h is the height of the rod (mm), Δh is the variation of height (cm), and ΔT is the variation of temperature (°C).
3 Results and discussion
The glass-forming compositions of the ternary system La2S3-Ga2S3-Ga2Se3 were investigated, and the results are summarized in Figure 1. The bottom line of the plot refers to previous studies carried out on a regular Ga-La-S glass [12]. The samples were divided into glassy, crystalline, and glassy with particles. The melt-quench process adopted was a novel method to produce selenide glasses. Conventional selenide glasses are produced involving sealed systems to avoid losses of materials. The glasses made with these systems have the same amount of impurities as the initial precursors because compounds such as volatile oxides cannot be eliminated during the melt. However, due to the high melting temperature of La2S3, Ga2S3, and Ga2Se3, the samples could be conveniently melted in an open system purged with argon. In this way, the oxides contained in the precursors can be eliminated. The level of oxygen in the glass could be as low as the purity of the purge gas, currently 10 ppm/m3 in our facilities. The losses of mass are around 2% mainly due to volatile oxide impurities.
A large glass formation region was found ranging from 20 to 45 mol% of La2S3 (blue and red dots, Figure 1). The region 30 mol% La2S3:70 mol% Ga2Ch3(Ch=S,Se) exhibited the largest range of glassy samples probably due to the matching of the La/Ga ratio to the available S and Se. In fact, the complete replacement of Ga2S3 for Ga2Se3 was achieved forming a glass-ceramic.
The formation of crystalline samples was observed for content of La2S3 over 50 mol% and below 20 mol%. A minimum quantity of La2S3 is necessary in order to form the glass. However, an excessive quantity did not allow the Ga2S3 main network to be formed.
The large number of glass-forming compositions allow the transmission window properties, in particular refractive index, to be tailored according to the application. For instance, Figure 2 shows the refractive index dispersion for samples containing an increasing amount of Ga2Se3 between 0.6 and 1.69 μm. As expected, the addition of selenium results in a growth of the refractive index. This parameter spanned from 2.30 for 20 mol% Ga2Se3 to 2.47 60 mol% Ga2Se3. This gives the possibility to control the refractive index according to the amount of selenium added, tailoring the properties of the glass to the application of interest.
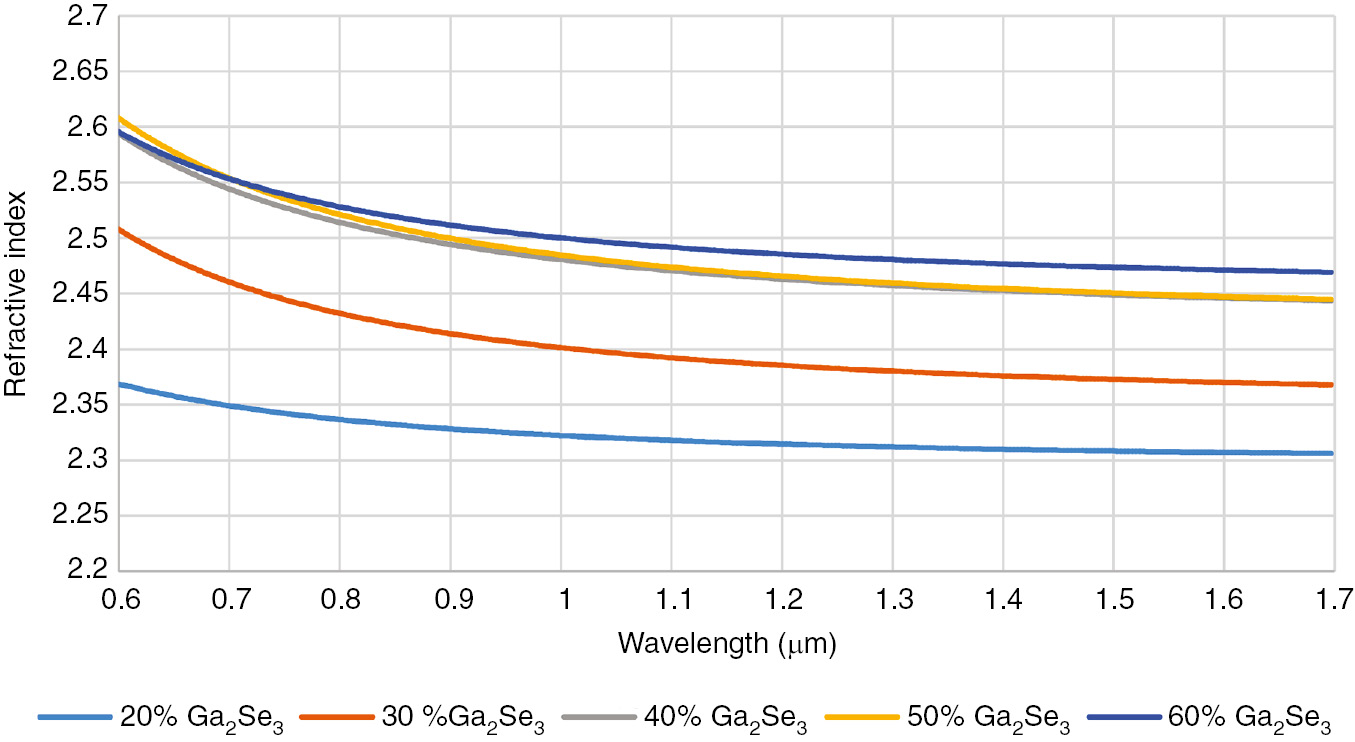
Refractive index dispersion of samples containing an increasing amount of Ga2Se3.
In addition, as shown in Figure 3, the samples with composition 30 mol% La2S3:70 mol% Ga2Ch3(Ch=S,Se) exhibit a larger transmission in the band between 8 and 14 μm even for a low content of Se. In particular, the 50% transmission threshold shifted from 7.85 μm for Ga-La-S to 10.42 μm for the sample containing 60 mol% Ga2Se3. This result is due to the effect of selenium on the structure of the glass. Indeed, the substitution of S for the heavier Se results in molecular vibration with lower energy and larger transmission in the LWIR, but the addition of selenium did not result in a decreased transparency in the visible range. In fact, the cut-off edge was registered at 463 nm for the sample containing 20 mol% Ga2Se3 and 504 nm for the sample with 60 mol% Ga2Se3. Therefore, the addition of an appropriate amount of Se to the regular Ga-La-S glass produced samples with transmission in both visible range and infrared between 8 and 14 μm.

Transmission spectra of Ga-La-S (light-blue line), 20 mol% Ga2Se3 sample (red line), 60 mol% Ga2Se3 sample (black line). Data measured with Cary 50 UV-Visible spectrometer between 0.485 and 1.5 μm and with Varian 670-IR FTIR spectrometer between 1.66 and 16 μm. The thickness of the flats was 1 mm. The plots are not corrected for Fresnel reflection.
The effect of selenium on thermal and visible transparency was investigated using a FLIR C2 camera, which operates in the wavelength band from 7 to 12 μm, with a separate, but integrated, conventional optical digital camera. A sample of regular Ga-La-S and a sample containing 35 mol% Ga2Se3were placed in front of both lenses of the camera in order to evaluate their ability to simultaneously transmit the visible and LWIR radiation. The results of this experiment are show in Figure 3.
The comparison in visible imaging is highlighted in Figure 4A–C. In particular, Figure 4A is a picture taken without any addition of samples in front of the objectives, while Figure 4B and C was taken using a Ga-La-S glass and a sample containing 35 mol% Ga2Se3 as filters, respectively. The addition of the filters excludes the blue radiation from the pictures. However, due to the different cut-off edge in the visible range, picture 4C is missing part of the green features as well.
Figure 5C was taken using the thermal-imaging apparatus of the camera. Figure 5A reports the picture without addition of any filter. The addition of Ga-La-S as filter (Figure 5B) induces the loss of most of the thermal features from the picture, while no substantial changes emerged after the addition of the Ga2Se3 glass as filter (Figure 5C). This remarkable improvement on the quality of the thermal pictures can be explained by the wider LWIR transmission of the 35 mol% Ga2Se3. In fact, the infrared cut-off wavelength of the selenide glasses is included in a range between 13 and 15 μm, while the transmission fell around 11 μm for Ga-La-S. As a consequence, a larger extent of LWIR light reached the camera detector producing a detailed picture.

Visible range pictures with no filter (4A), Ga-La-S glass filter (4B), and 35 mol% Ga2Se3 sample filter (4C).
The three figures on the bottom (Figure 6C) show the combination of thermal and visible pictures taken with the camera. Figure 6B lacks thermal features due to the addition of the Ga-La-S glass disc (1-mm thick) in front of the camera lens and which acts as a filter to wavelengths beyond about 8 μm, while a good similarity can be observed between Figure 6A and C wherein a 35 mol% Ga2Se3 glass covers the camera aperture. Therefore, the optical characteristics of Ga2Se3-added GLS glass may be promising for multispectral imaging in a range from visible to 14 μm.
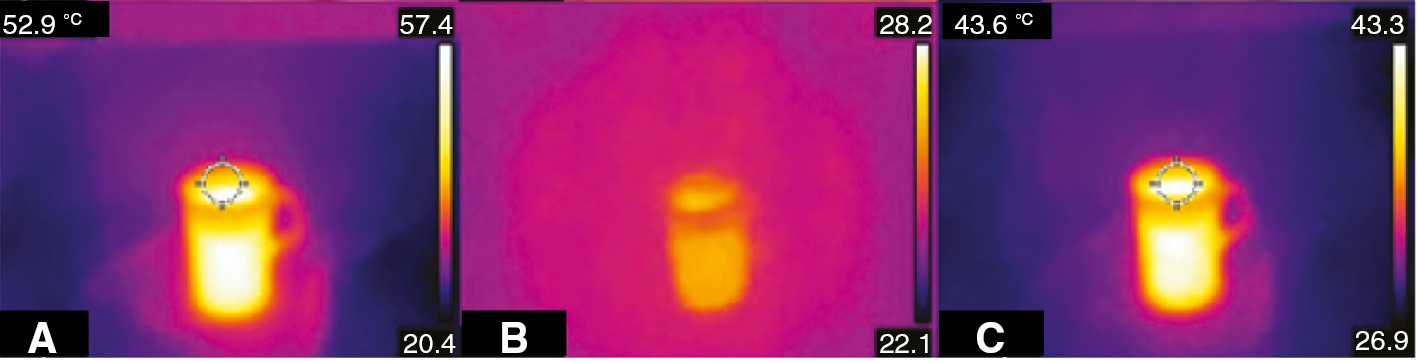
Thermal pictures with no filter (5A), Ga-La-S glass filter (5B), and 35 mol% Ga2Se3 sample filter (5C).

Hybrid visible-thermal range pictures with no filter (6A), Ga-La-S glass filter (6B), and 35 mol% Ga2Se3 sample filter (6C).
The thermal and mechanical characterization were carried out to determine the effect of selenium on the stability of the sample. Table 1 summarizes the results for each composition having 30 mol% La2S3:70 mol% Ga2Ch3(Ch=S,Se).
Thermal and mechanical features of samples containing 30 mol% La2S3:70 mol% Ga2Ch3(Ch=S,Se).
| Composition | Characteristic temperatures | Mechanical features | ||||||
|---|---|---|---|---|---|---|---|---|
| Ga2S3 (mol%) | La2S3 (mol%) | Ga2Se3 (mol%) | Tg±3 (°C) | Tx±3 (°C) | Tm±3 (°C) | CTE×10−6±0.01×10−6 (°C−1) | Tη=107.6Pa±3 (°C) | |
| 50 | 30 | 20 | 512 | 674 | 788 | 9.94 | 623 | |
| 40 | 30 | 30 | 516 | 661 | 785 | 9.92 | 581 | |
| 30 | 30 | 40 | 500 | 655 | 773 | 1.06 | 597 | |
| 20 | 30 | 50 | 494 | 634 | 769 | 9.78 | 585 | |
| 10 | 30 | 60 | 497 | 627 | 767 | 9.56 | 565 | |
| 0 | 30 | 70 | 466 | 602 | 766 | – | – | |
As could be seen, the addition of Ga2Se3 resulted in higher characteristic temperatures when compared with other conventional chalcogenide glasses [13]. A change in Tg as low as 46°C was registered between samples with low and high content of selenium (20 and 70 mol% Ga2Se3). In addition, the minimum Tx−Tg difference was found to be as high as 130°C for a content of 60 mol% Ga2Se3 spanning up to 162°C for 20 mol% Ga2Se3. This large Tg−Tx span is desired for glass molding and fiber drawing in order to avoid crystallization during the process.
The melting temperature of all the samples was observed well above 700°C reaching 766°C as minimum value for 70 mol% Ga2Se3 content. These observations prove the thermal stability of the whole range of compositions which may be suitable for processing at relatively high temperatures.
The coefficient of thermal expansion (CTE) was investigated between 35°C and 385°C to probe the behavior of the materials before the occurrence of the glass transition. A low expansion coefficient is preferable to achieve small and uniform shrinking during the shaping processes. The materials exhibited coefficients similar to other chalcogenide materials and much lower than ionic materials [14], [15]. In fact, the ionic La-S bond represents a small portion of the glassy networks mostly made of covalent Ga-S and Ga-Se bonds.
The softening point of the materials was also determined for consideration in applications such as molded lenses and glass fiber drawing. It is usually defined as the temperature at which the viscosity reaches 107.6 Pa. For all the compositions, the softening point was found at least 50°C below the crystallization temperature. This large gap could be flexibly exploited to tailor the shape of the glass for different applications. In addition, the coefficients of thermal expansion of the samples were found to be similar to those of other commercial materials currently used to produce LWIR lenses (Table 2). This may represent the evidence of the suitability of these materials for the production of optical components for both visible and LWIR optics and imaging.
Comparison of Tg, softening point, and CTE for GLSSe glass and other commercial chalcogenides.
| Composition | Tg (°C) | Softening point (°C) | CTE×10−5 (°C−1) | References |
|---|---|---|---|---|
| 70 mol% Ga2S3:30 mol% La2S3 | 553 | 630 | 0.604 | [12], [16] |
| 20 mol% Ga2Se3:50 mol% Ga2S3:30 mol% La2S3 | 512 (±3) | 623 (±3) | 0.994 (±0.010) | This work |
| 60 mol% Ga2Se3:10 mol% Ga2S3:30 mol% La2S3 | 497 (±3) | 565 (±3) | 0.996 (±0.010) | This work |
| IG2 (Ge33As12Se55) | 368 | 445 | 1.21 | [17] |
| IG3 (Ge30As13Se32Te25) | 275 | 360 | 1.34 | [18] |
| IG4 (Ge10As40Se50) | 225 | 310 | 2.04 | [19] |
| IG5 (Ge28Sb12Se60) | 285 | 348 | 1.4 | [20] |
| IG6 (As40Se60) | 185 | 236 | 2.07 | [21] |
| AMTIR-1 (Ge-As-Se) | 368 | 405 | 1.2 | [22] |
| AMTIR-2 (As-Se) | 167 | 188 | 22.4 | [22] |
| AMTIR-3 (Ge-Sb-Se) | 278 | 295 | 1.4 | [22] |
| AMTIR-4 (As-Se) | 103 | 131 | 2.7 | [22] |
| AMTIR-5 (As-Se) | 143 | 170 | 2.37 | [22] |
| AMTIR-6 (As-S) | 187 | 210 | 21.6 | [22] |
| C1 (As-Se-Te) | 133 | 154 | 23 | [22] |
| GASIR-1 | 292 | – | 1.7 | [23] |
| GASIR-5 | 180 | – | 2.35 | [24] |
4 Conclusions
The optical, thermal, and mechanical characterization of novel selenide glasses were carried out for possible optical applications. The glass was produced as a result of the addition of Ga2Se3 to the well-known Ga-La-S glass. For an appropriate extent of substitutions, the transmission window of the glasses covered both visible range and thermal radiation range. The characteristic temperatures of the samples were found to be higher than the commercially available chalcogenides. On the other hand, the mechanical properties are comparable to those chalcogenides used to produce lenses.
Future studies will be addressed to produce active and passive optical components for multispectral applications.
Funding: Engineering and Physical Sciences Research Council, (Grant/Award Number: ‘EP/M015130/1’)
References
[1] L. Dick, S. Risse and A. Tünnermann, Adv. Opt. Technol. 5, 277 (2016).10.1515/aot-2016-0023Search in Google Scholar
[2] X. H. Zhang, Y. Guimond and Y. Bellec, J. Non. Cryst. Solids 326–327, 519–523 (2003).10.1016/S0022-3093(03)00464-2Search in Google Scholar
[3] A. Rogalski, Prog. Quantum Electron. 27(2–3), 59–210 (2003).10.1016/S0079-6727(02)00024-1Search in Google Scholar
[4] C. D. Tran, Anal. Lett. 38(5) 735–752 (2005).10.1081/AL-200047754Search in Google Scholar
[5] D. Gibson, S. Bayya, V. Nguyen, J. Sanghera, M. Kotov, et al., Proc. SPIE 9451, 94511P (2015).10.1117/12.2177136Search in Google Scholar
[6] B. J. Eggleton, B. Luther-Davies and K. Richardson, Nat. Photonics 5, 725–725 (2011).10.1038/nphoton.2011.311Search in Google Scholar
[7] I. D. Aggarwal and J. S. Sanghera, J. Optoelectron. Adv. Mater. 4, 665–678 (2002).Search in Google Scholar
[8] A. Zakery and S. R. Elliott, in Optical Nonlinearities in Chalcogenide Glasses and their Applications, (Springer Berlin Heidelberg, Berlin, Heidelberg, 2007), pp. 1–28.Search in Google Scholar
[9] A. Brehault, L. Calvez, T. Pain, P. Adam, J. Rollin, et al., Proc. SPIE 9070, 90702F–90702F–12 (2014).10.1117/12.2048319Search in Google Scholar
[10] A. Bréhault, L. Calvez, T. Pain, H. L. Ma, D. Bigou, et al., Proc. SPIE 9822, 982202 (2016).10.1117/12.2222900Search in Google Scholar
[11] Y. Ledemi, M. El-Amraoui, L. Calvez, X.-H. Zhang, B. Bureau, et al., Photonic Fiber Cryst. Devices Adv. Mater. Innov. Device Appl. VII 8847, 884704 (2013).10.1117/12.2024461Search in Google Scholar
[12] P. Bastock, C. Craig, K. Khan, E. Weatherby, J. Yao, et al., Properties of Gallium Lanthanum Sulphide Glass, in CLEO ‘15, San Jose, US, 10–15 May 2015, pp. 2.10.1364/CLEO_SI.2015.STh1G.1Search in Google Scholar
[13] A. B. Seddon, J. Non. Cryst. Solids 184, 44–50 (1995).10.1016/0022-3093(94)00686-5Search in Google Scholar
[14] A. S. M. Rao, J. Mod. Phys. 4, 208–214 (2013).10.4236/jmp.2013.42029Search in Google Scholar
[15] F. a S. Al-Alamy, A. A. Balchin and M. White, J. Mater. Sci. 12, 2037–2042 (1977).10.1007/BF00561976Search in Google Scholar
[16] D. W. Hewak, D. Brady, R. J. Curry, G. Elliott, C. C. Huang, et al., in ‘Photonic Glasses and Glass-Ceramics’, Ed. By G. S. Murugan (Research Signpost, Kerala, India, 2010) pp. 29–102.Search in Google Scholar
[17] “IG2 (Ge33As12Se55).” [Online]. Available: http://www.vitron.de/english/IR-Glaeser/Daten-Infrarotglaeser.php.Search in Google Scholar
[18] “IG3 (Ge30As13Se32Te25).” [Online]. Available: http://www.vitron.de/english/IR-Glaeser/Daten-Infrarotglaeser.php.Search in Google Scholar
[19] “IG4 (Ge10As40Se50).” [Online]. Available: http://www.vitron.de/english/IR-Glaeser/Daten-Infrarotglaeser.php.Search in Google Scholar
[20] “IG5 (Ge28Sb12Se60).” [Online]. Available: http://www.vitron.de/english/IR-Glaeser/Daten-Infrarotglaeser.php.Search in Google Scholar
[21] “IG6 (As40Se60).” [Online]. Available: http://www.vitron.de/english/IR-Glaeser/Daten-Infrarotglaeser.php.Search in Google Scholar
[22] “AMTIR.” [Online]. Available: http://www.amorphousmaterials.com/products/.Search in Google Scholar
[23] “GASIR-1.” [Online]. Available: http://eom.umicore.com/en/infrared-optics/blanks/GASIR1-for-infrared-optics.pdf.Search in Google Scholar
[24] “GASIR-5.” [Online]. Available: http://eom.umicore.com/en/infrared-optics/blanks/GASIR5-for-infrared-optics.pdf.Search in Google Scholar
©2017, Andrea Ravagli et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Community
- Conference Notes
- News from the European Optical Society EOS
- Topical Issue: Photonics in Security Systems
- Editorial
- Photonics in security systems
- Review Article
- Hyperspectral imaging: future applications in security systems
- Letter
- Development of an open-path gas analyser for plume detection in security applications
- Research Articles
- Standoff detection and classification of bacteria by multispectral laser-induced fluorescence
- Hyperspectral data acquisition and analysis in imaging and real-time active MIR backscattering spectroscopy
- High peak-power laser system tuneable from 8 to 10 μm
- Mid-infrared laser beam steering based on Fourier transform OPO
- Adaptive holographic interferometer based on optically addressed spatial light modulator for high-sensitivity optical fiber sensing
- IPS – a vision aided navigation system
- Ga-La-S-Se glass for visible and thermal imaging
- Picosecond imaging of signal propagation in integrated circuits
Articles in the same Issue
- Cover and Frontmatter
- Community
- Conference Notes
- News from the European Optical Society EOS
- Topical Issue: Photonics in Security Systems
- Editorial
- Photonics in security systems
- Review Article
- Hyperspectral imaging: future applications in security systems
- Letter
- Development of an open-path gas analyser for plume detection in security applications
- Research Articles
- Standoff detection and classification of bacteria by multispectral laser-induced fluorescence
- Hyperspectral data acquisition and analysis in imaging and real-time active MIR backscattering spectroscopy
- High peak-power laser system tuneable from 8 to 10 μm
- Mid-infrared laser beam steering based on Fourier transform OPO
- Adaptive holographic interferometer based on optically addressed spatial light modulator for high-sensitivity optical fiber sensing
- IPS – a vision aided navigation system
- Ga-La-S-Se glass for visible and thermal imaging
- Picosecond imaging of signal propagation in integrated circuits

