Abstract
Background and aims
Preclinical studies have reported that activation of peripheral γ-aminobutyric acid A (GABAA) receptors may result in analgesia. The current study was conducted in young healthy men (n = 30) and women (n = 28) to determine whether injections of GABA into the masseter muscle reduce pain in a sex-related manner.
Methods
The effect of injection of GABA alone, or in combination with the non-inflammatory algogen glutamate, was assessed in two separate studies. Lorazepam, a positive allosteric modulator of the GABAA-receptor, was co-injected with GABA in both studies to explore the role of this receptor in muscle pain responses of healthy human volunteers. Masticatory muscle mechanical pain intensity was recorded on an electronic visual analogue scale (VAS) while muscle pain sensitivity was assessed by determining the pressure pain threshold (PPT), tolerance and maximal jaw opening (MJO) of the subjects prior to, and again after the various intramuscular injections.
Results
Intramuscular injection of GABA alone was reported to be significantly more painful, in a concentration related manner, than saline control injections, and this pain was further increased by co-injection of lorazepam with GABA. Co-injection of GABA with glutamate was found to significantly increase glutamate-evoked masseter muscle pain in men, but not in women. There was no effect of injections of either GABA alone, or GABA with glutamate, on PPT, tolerance or maximum jaw opening.
Conclusions
Injection of GABA into the human masseter muscle appears to excite nociceptors to produce muscle pain without a longer term effect on mechanical pain sensitivity in the muscle. The findings suggest that GABA-mediated pain in humans is produced through peripheral GABAA receptor activation. The mechanism underlying the sex-related difference in the effect of GABA on glutamate-evoked muscle pain was speculated to be due to a methodological artifact.
Implications
This study was designed to detect analgesic rather than algesic effects of peripherally administered GABA, and as a result, the concentration of glutamate chosen for injection was close to the maximal pain response for healthy women, based on previously determined pain-concentration response relationships for glutamate. This may explain the finding of greater pain in men than women, when GABA and glutamate were co-injected. Overall, the findings suggest that activation of peripheral GABAA receptors in human masticatory muscle produces pain, possibly due to depolarization of the masticatory muscle afferent fibers.
1 Introduction
In the central nervous system, γ-amino-butyric acid (GABA) decreases neuronal excitability by acting on a ligand-gated chloride channel, the GABAA receptor and a G-protein coupled receptor, the GABAB receptor [1], [2], [3], [4]. Activation of pre-synaptic GABAA receptors depolarizes the central endings of afferent fibers; a process that results in decreased release of neurotransmitters from terminal endings in the central nervous system [1], [5], [6], [7], [8], [9], [10]. Whether GABA depolarizes or hyperpolarizes the peripheral endings of nerve fibers is not known, however, limited evidence suggests that GABA is much less effective than the excitatory amino acid glutamate at exciting rat masseter muscle afferent fibers [11].
Activation of peripheral GABAA receptors in the rat can attenuate nociceptive input [12], [13], [14]. Injections of glutamate made into the rat temporomandibular joint evoke reflex jaw muscle activity [15] which can be attenuated by co-injection of GABA in a concentration dependent manner [12]. The effect of GABA was inhibited by bicuculline, a GABAA receptor antagonist. Subcutaneous administration of low concentration muscimol, a selective GABAA receptor agonist, to the rat paw also suppressed nocifensive responses, while a dose five times higher resulted in increased nocifensive responses to formalin [14]. Together, these results in animals suggest that activation of peripheral GABAA receptors may result in a local analgesic or hyperalgesic effects in a concentration related manner.
Intramuscular injection of glutamate (0.5 M, 0.2 mL) into the masticatory muscles of healthy human subjects produces pain of moderate intensity (4–6/10) that lasts for 10–15 min [13], [16], [17], [18], [19], [20], [21], [22], [23]. This pain is reported as being more intense by women than by men [11], [22]. Injection of glutamate at a higher concentration (1.0 M, 0.2 mL) can also produce a longer-lasting (~90 min) mechanical sensitization of the masseter muscle which is similar in both sexes [22]. In humans, glutamate-induced pain and mechanical sensitization can be attenuated by local injection of ketamine, which indicates that they are mediated, in part, through activation of peripheral N-methyl-D-aspartate (NMDA) receptors [17], [19], [20], [24]. Masseter muscle biopsies from healthy subjects have identified NMDA receptor expression in a subgroup of sensory nerve fibers [25]. As a result of these properties of intramuscular glutamate injection, it has been used to model acute masseter muscle pain and sensitivity reported by patients suffering from a myofascial temporomandibular disorder [19].
The purpose of the present study was to see if findings in rats of a GABAA mediated antinociceptive effect could be translated into healthy human subjects. Human subjects were given injections of GABA at concentrations that had been shown to reduce nociceptive input from the rat temporomandibular joint [12], [13]. However, since injection of these substances into the healthy human temporomandibular joint was not feasible for ethical reasons, the study was instead conducted using intramuscular injections into the masseter muscle. It was hypothesized that injections of GABA alone into the muscle would produce no more pain than injection of saline, and that injection of GABA with glutamate would attenuate glutamate-evoked muscle pain in a concentration-related manner that was enhanced by the GABAA receptor positive allosteric modulator, lorazepam.
2 Methods
The experimental protocol was approved by the North Denmark Ethics Committee (reference no. N-20160037) and carried out according to the Helsinki Declaration and the IASP guidelines. Written informed consent was obtained from all participants.
Thirty healthy volunteers (15 men and 15 women) were recruited for study one, and 30 additional healthy volunteers (15 men, 15 women) for study two. Two subjects, both women, withdrew from study two. Baseline demographics for subjects in both studies are provided (Table 1). Both studies were randomized, placebo-controlled, double-blinded, and had a crossover design. Subjects were eligible to participate in the study if they were between 20 and 40 years of age and free from ongoing or chronic pain. Subjects who were pregnant, or intended to become pregnant, were breast feeding, had signs or symptoms of any serious systemic diseases including malignancies or high blood pressure, required chronic administration of psychiatric, analgesic or other medications that might influence their response to pain, reported any recreational drug or alcohol use, reported a previous neurologic, musculoskeletal or mental illnesses or lacked the ability to cooperate, were excluded from participation in the study.
Baseline parameters.
| Study | Subjects | Number | Age (years) | PPT (kPa) | PPTOL (kPa) | MJO (mm) |
|---|---|---|---|---|---|---|
| 1 | Men | 15 | 26±1 | 161±23 | 411±45 | 53±2 |
| 1 | Women | 15 | 28±1 | 188±13 | 377±22 | 49±2 |
| 2 | Men | 15 | 25±1 | 203±16 | 478±50 | 52±2 |
| 2 | Women | 13 | 27±1 | 153±9 | 350±48 | 51±2 |
-
The table indicates the mean (± SE) baseline pressure pain threshold (PPT), pressure pain tolerance (PPTOL) and maximum jaw opening (MJO) averaged over all sessions for subjects in study 1 (GABA alone) and study 2 (GABA and glutamate). The only significant difference in baseline values was for PPT in study 2, where the men had a significantly higher PPT value than women. Bold text: Students t-test, p<0.05.
2.1 Study one
This project was designed to test whether intramuscular injection of GABA alone is painful and/or alters responses to mechanically-induced muscle pain. Each subject attended two sessions with a minimum interval of 1 week between sessions (Fig. 1A). Treatments were assigned randomly, and neither the subject nor the tester was aware of the content of the injections.
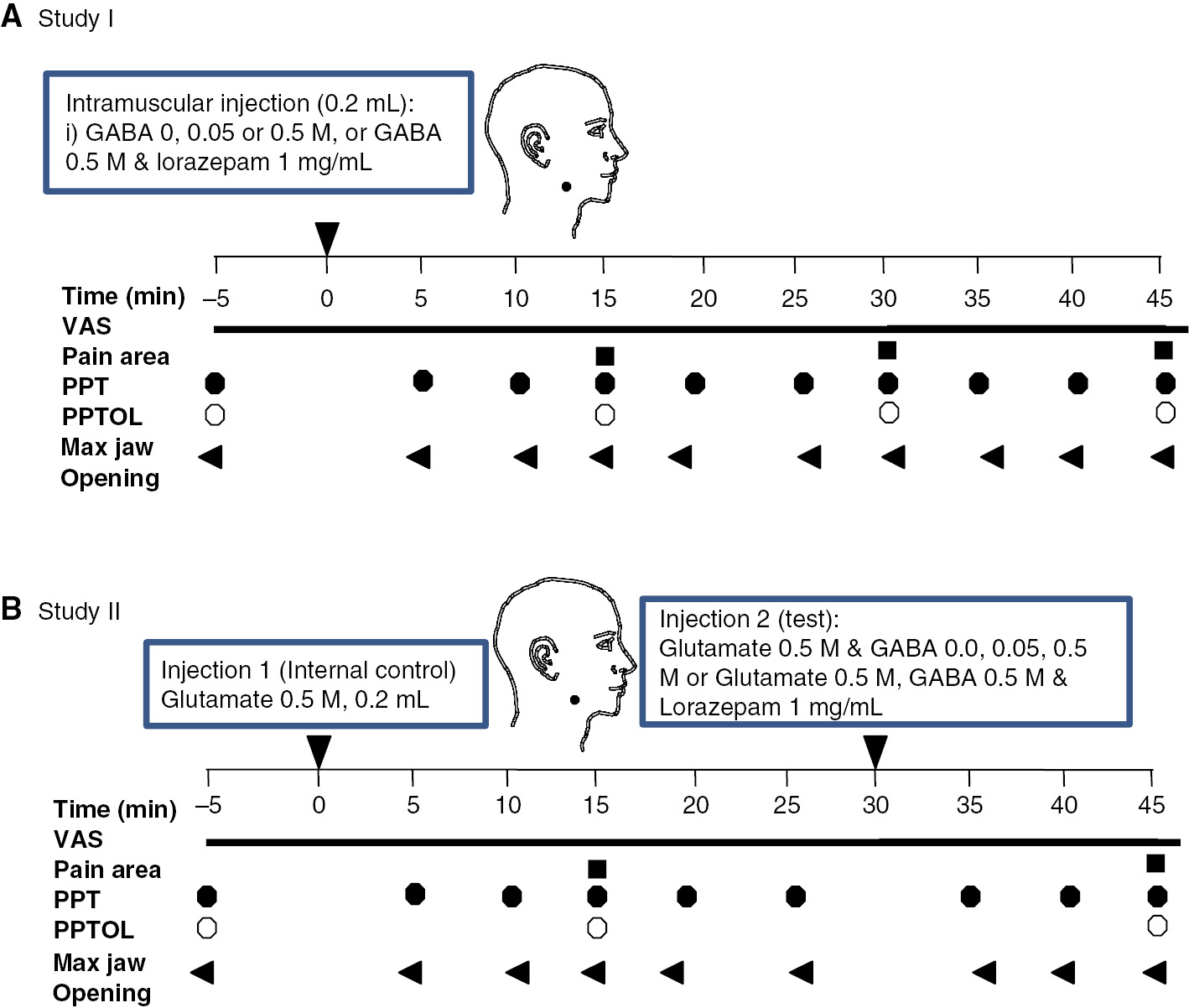
(A) The drawing shows the structure of study I. (B) The drawing shows the structure of study II.
Two injections were given in each session with an interval of at least 1 h between injections. Injections were made into the right, then subsequently into the left masseter muscle in each session. Subjects were instructed to continuously rate their pain after the injections. Bilateral masseter muscle pressure pain threshold (PPT) and pressure pain tolerance (PPTOL) as well as maximal jaw-opening (MJO) were assessed at baseline and periodically after injections (Fig. 1A). After each injection, subjects were also asked to draw their perceived distribution of pain on a picture of the profile of the face.
2.2 Study two
This study investigated whether GABA can modulate pain evoked by injection of glutamate into the masseter muscle. Subjects attended four sessions in total, each lasting 1 h and with an interval at least 1 week between sessions (Fig. 1B). During each session, two injections into the right masseter muscle were made at a 30 min interval. The first injection was glutamate 0.5 M alone, and served as an internal control. The second injection was of glutamate 0.5 M randomly combined with various concentrations of GABA (0, 0.05, or 0.5 M) alone or with lorazepam (GABA 0.5 M, lorazepam 1 mg/mL) to determine how GABA modulates pain and mechanical sensitivity induced by injection of glutamate.
Subjects were instructed to continuously rate their pain after each injection. PPT and MJO were measured 5 min before, and then again 5, 10 and 15 min after each injection. PPTOL was assessed 5 min before, and then again 15 min after each injection, after the assessment of PPT. After each injection, subjects were also asked to draw their perceived distribution of pain on a picture of the profile of the face.
2.3 Measurement of pain and mechanical sensitivity
Subjects were instructed to continuously rate their pain after injections on an electronic 10-cm computerized visual analogue scale (VAS; sampling rate 0.2 Hz). The lower endpoint of the VAS scale is labeled “no pain at all” and the upper endpoint labeled “the worst pain imaginable”.
Masseter muscle PPT (mean of three trials per side per time point) and PPTOL were measured with a Somedic Algometer (1 cm2 probe). During these assessments, the subjects were asked to keep their jaw at rest and not to clench their teeth. The algometer probe was pressed against the testing site with a constant advancing rate of 50 (Study 1) or 30 (Study 2) kPa/s and subjects push a button to stop the stimulation as soon as they felt pain (PPT) or could no longer tolerate pain (PPTOL). MJO was measured with a ruler in millimeters (mm). At the end of each experiment, subjects were asked to their perceived region of pain on a paper containing an image of the face in profile.
2.4 Injections
Injections (0.2 mL) were made into the deep masseter muscle midway between its upper and lower border and approximately 1 cm posterior to its anterior border over a 5–10 s period with a 27-gauge hypodermic needle and disposable syringe. Sterile stock solutions of pH neutral GABA (1 M) and glutamate (1 or 2 M) for injection were manufactured for the study by a hospital pharmacy (Skanderborg Apotek, Denmark). Lorazepam (4 mg/mL, Temesta) and buffered sterile saline were purchased from the same hospital pharmacy. Solutions for injection were made up prior to injection according to the randomization table by a member of the research team (SL) who did not further participate in the data collection. Sterile solutions of GABA (0.05 or 0.5 M), GABA 0.5 M and lorazepam (1 mg/mL) were made by diluting stock solutions in phosphate buffered saline (PBS). Sterile solutions of glutamate 0.5 M with GABA (0, 0.05, or 0.5 M) alone or with lorazepam (GABA 0.5 M, lorazepam 1 mg/mL) were made by diluting stock solutions in phosphate buffered saline (PBS).
2.5 Statistics
The sample size for each study was calculated with a risk of type I and type II errors of 5% and 20%, respectively, and a conservative estimate of the intra-individual variation of 30% on the VAS with the minimal relevant difference to detect as 25%. A total of 24 subjects were estimated to be required for each study paradigm (12 men, 12 women). However, it was anticipated that as many as 20% of subjects might drop out, and thus 30 subjects for each study were recruited.
Data from the recorded electronic VAS was used to determine the following pain parameters: peak pain, pain duration and area under the VAS curve. Pain area was calculated by summing the VAS scores after each injection. The VAS data was assessed using a 2-way repeated-measures ANOVA, with sex and treatment as factors run on the program Sigma Plot (Sigma Plot 12, Systat Inc., CA, USA). In study one, raw VAS parameters were assessed. In study two, the response to the second injection was normalized to the response to the first injection to control for intersession variability in raw pain ratings, and the normalized data assessed for statistical significance.
Data from measurements of PPT, PPTOL and MJO was recorded on an Excel spreadsheet. PPT and MJO data was assessed using a 2-way repeated-measures ANOVA, with time and treatment as factors run on the program Sigma Plot. PPTOL data was assessed with a 1-way-repeated measures ANOVA, with treatment as the factor. Drawings of pain area were scanned, and the digital images imported into the image-processing program ImageJ (National Institutes of Health, USA). Area (in arbitrary units) was obtained using this software program. Pain area was assessed using a 2-way repeated-measures ANOVA, with sex and treatment as factors with Sigma Plot.
The Holm-Sidak method was used for post-hoc assessments as appropriate. For all statistical tests employed, a p-value of less than 0.05 was considered significant.
3 Results
3.1 Study 1
Injection of GABA was more painful than injection of isotonic saline, but overall, pain evoked by GABA was mild (<3/10). Injection of GABA 0.5 M was rated more painful than injection of either isotonic saline or GABA 0.05 M (Fig. 2A). This was reflected in significantly higher peak and AUC values for GABA 0.5 M with or without lorazepam (Fig. 2B). The addition of lorazepam with GABA 0.5 M further increased pain ratings compared with GABA 0.5 M. Peak pain ratings for the combination of GABA and lorazepam were significantly higher than GABA 0.5 M alone (Fig. 2B). There were no statistically significant differences between male and female subjects in these parameters.
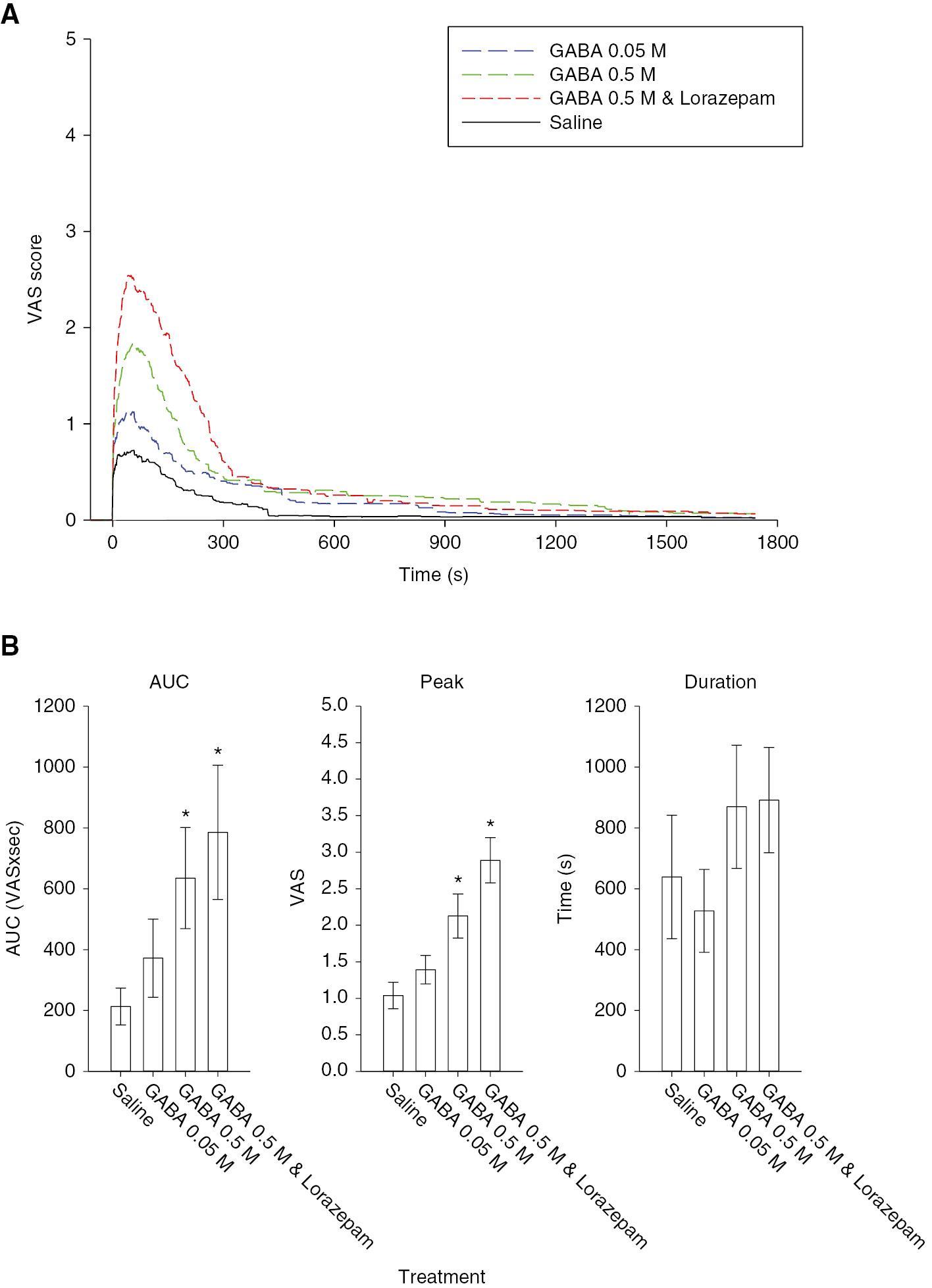
(A) The line graphs illustrate the mean masseter muscle pain intensity produced by injection of GABA with or without lorazepam, compared to saline in the 30 subjects. (B) The bar graphs indicate the mean area under the pain curve (AUC), peak and duration of pain produced by injection of the substances indicated. There was a significant concentration-related increase in GABA-evoked overall (F=6.223, p<0.001) and peak (F=19.237, p<0.001) muscle pain, and a non-significant increase in the duration of pain. The addition of lorazepam to GABA 0.5 M injections resulted in a significantly higher peak pain rating than GABA 0.5 M alone, which suggests that pain was being mediated through activation of GABAA receptors. Asterisks: p<0.05 compared to saline control; Error bars: SE.
There were no significant effects of any of the injected substances on PPT, PPTOL or MJO over the time course of the experiment (Fig. 3). There were no significant sex-related differences in the baseline values of these three parameters (Table 1).
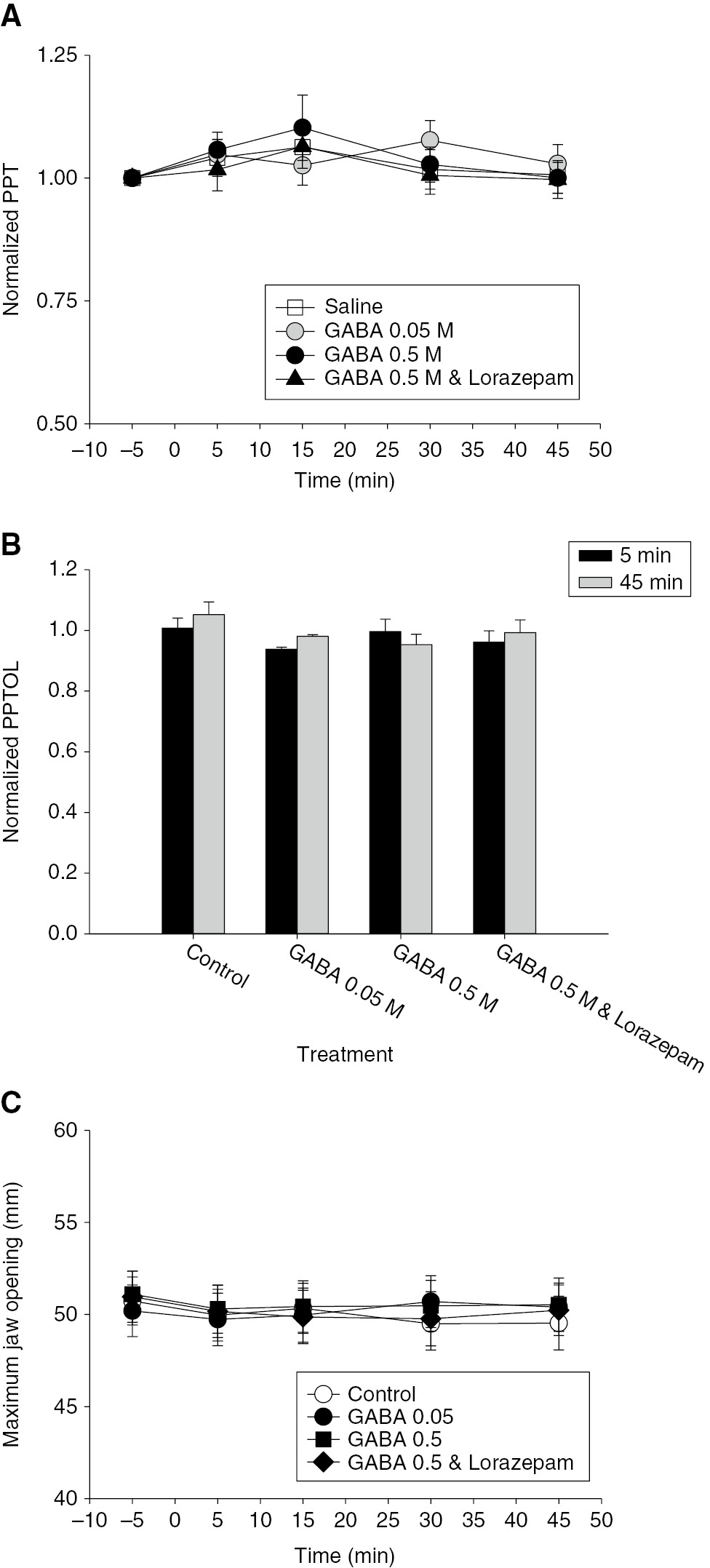
(A) The line and scatter plot shows the mean relative pressure pain threshold (PPT) normalized to baseline (−5 min). There was no significant effect of any of the injections on PPT. (B) The bar graphs indicate the mean relative pressure pain tolerance (PPTOL) 5 and 45 min after masseter muscle injections. There was no significant effect of any of the injections on the PPTOL. (C) The line and scatter plot shows the mean maximal jaw opening (MJO). There was a significant decrease in MJO over time (F=3.426, p=0.011), but no significant effect of treatment or treatment time interaction. Error bars: SE.
Significant treatment effects similar to those found for average pain were found for pain area (Fig. 4). Injections of GABA 0.5 M with and without lorazepam resulted in subject drawings of significantly greater pain area than injection of isotonic saline or GABA 0.05 M. Female subjects drew significantly larger areas of pain after injection of GABA 0.5 M than did male subjects.
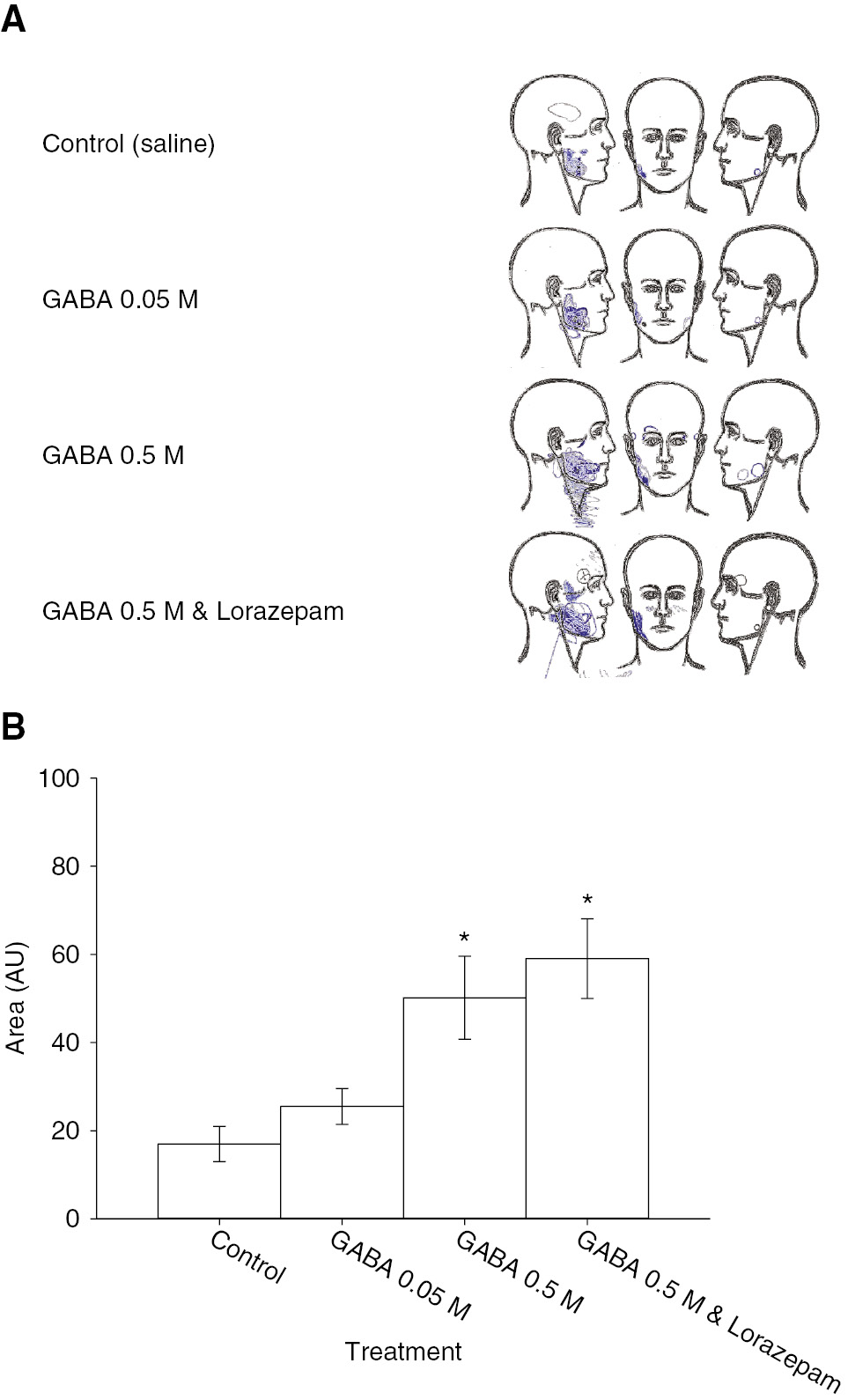
(A) The image shows composite pain area drawings from the 30 subjects. Pain was generally localized to the site of injection with all treatments. (B) The bar graphs show the mean pain area. There was a significant effect of treatment (F=11.489, p<0.001). The drawn pain areas for GABA 0.5 M with or without lorazepam were significantly larger than those drawn for saline. There was no significant difference between areas drawn for GABA 0.05 M and saline or between GABA 0.5 M with lorazepam and without lorazepam. Asterisks: p<0.05 compared to saline control; Error bars: SE.
3.2 Study 2
Injection of glutamate 0.5 M alone as an internal control produced moderate pain, with average ratings of just under 4. Overall and peak pain produced by the initial glutamate injection was reported as significantly greater by women than by men (Table 2).
Glutamate-evoked pain.
| VAS | Women | Men |
|---|---|---|
| AUC | 1,722±463 | 808±178 |
| Peak | 4.8±0.5 | 3.6±0.5 |
| Duration (s) | 571±120 | 395±75 |
-
The mean (± SE) area under the pain curve (AUC), peak and duration of muscle pain evoked by the first injection of glutamate in men and women over four sessions are shown. Women reported significantly greater overall pain (AUC) and higher intensity of pain (peak) than did men. Bold text: Students t-test, p<0.05.
Repeated injection of glutamate evoked pain of similar intensity and duration (Fig. 5A). The addition of GABA 0.5 M to the glutamate in the second injection significantly increased overall, peak and duration of pain reported (Fig. 5B). However, the addition of lorazepam to the glutamate/GABA 0.5 M injection did not further increase pain.
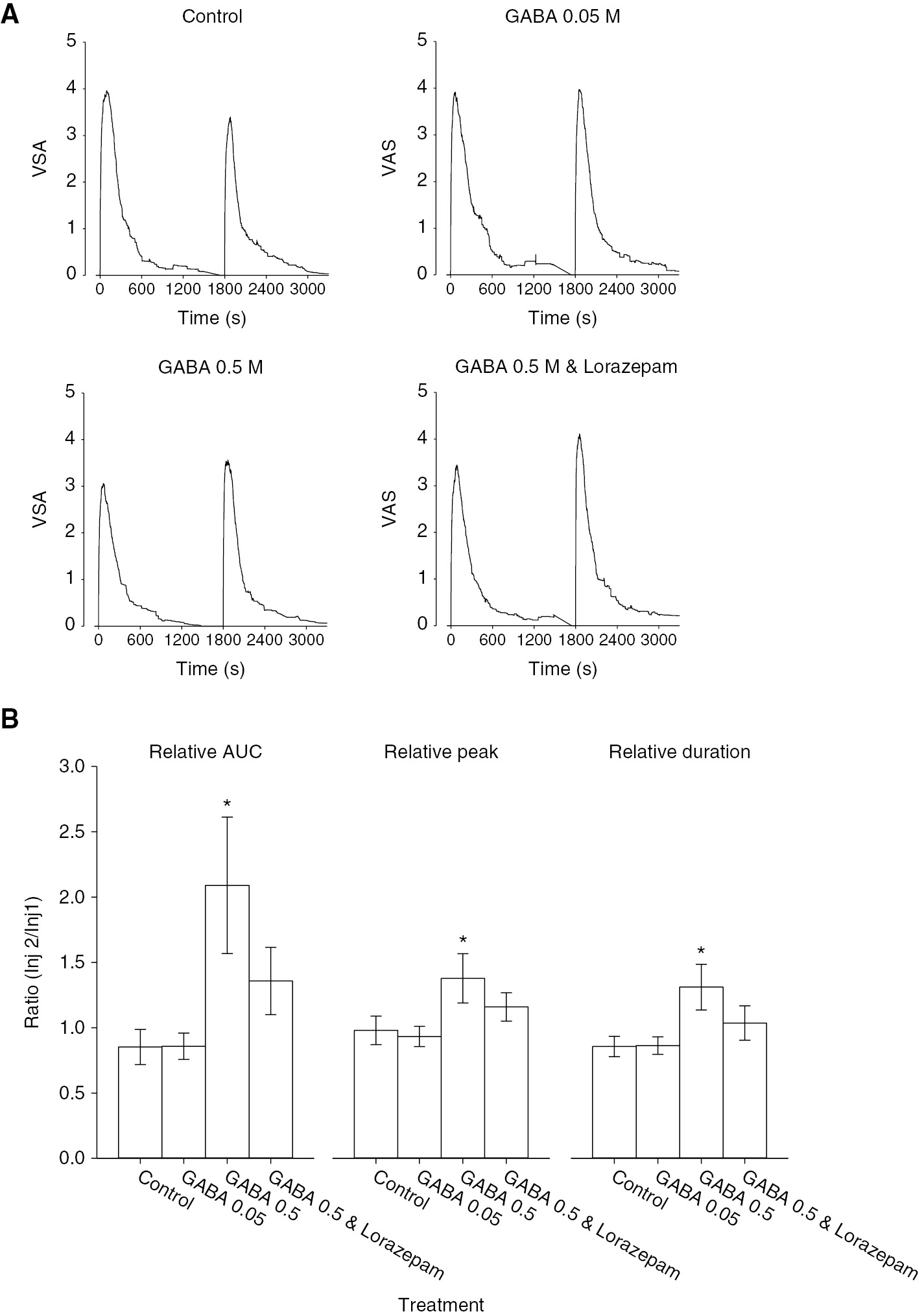
(A) The line graphs illustrate the mean masseter muscle pain intensity produced by injection of glutamate (time 0) followed 30 min later by glutamate with GABA (0.05 or 0.5 M) or GABA 0.5 M with lorazepam in 28 healthy subjects. Repeat injection of glutamate alone (control) evoked relatively reproducible pain responses. The addition of GABA 0.5 M, with or without lorazepam, in the second injection increased the pain intensity compared to injection of glutamate alone. (B) The bar graphs indicate the mean relative area under the pain curve (AUC), relative peak and relative duration of pain produced by injection of the substances indicated. There was a significant effect of treatment on overall pain (AUC) (F=4.094, p=0.009), peak pain (F=3.021, p=0.035), and duration of pain (F=3.444, p=0.021). The addition of GABA 0.5 M significantly increased pain compared to control injections. The addition of lorazepam lowered pain ratings compared to GABA 0.5 M without lorazepam. GABA 0.05 M had no effect on glutamate-evoked muscle pain. Asterisks: p<0.05 compared to saline control; Error bars: SE.
Analysis of the pain parameters also revealed a significant interaction between sex and treatment. In men, the addition of GABA 0.5 M with glutamate in the second injection evoked significantly greater overall and peak pain than glutamate alone (Fig. 6). However, in women, the addition of GABA 0.5 M with or without lorazepam in the second injections did not significantly alter pain ratings compared to glutamate alone. When men and women were compared, men reported a significantly greater enhancement of their pain by GABA 0.5 M added to glutamate than women (Fig. 6B).
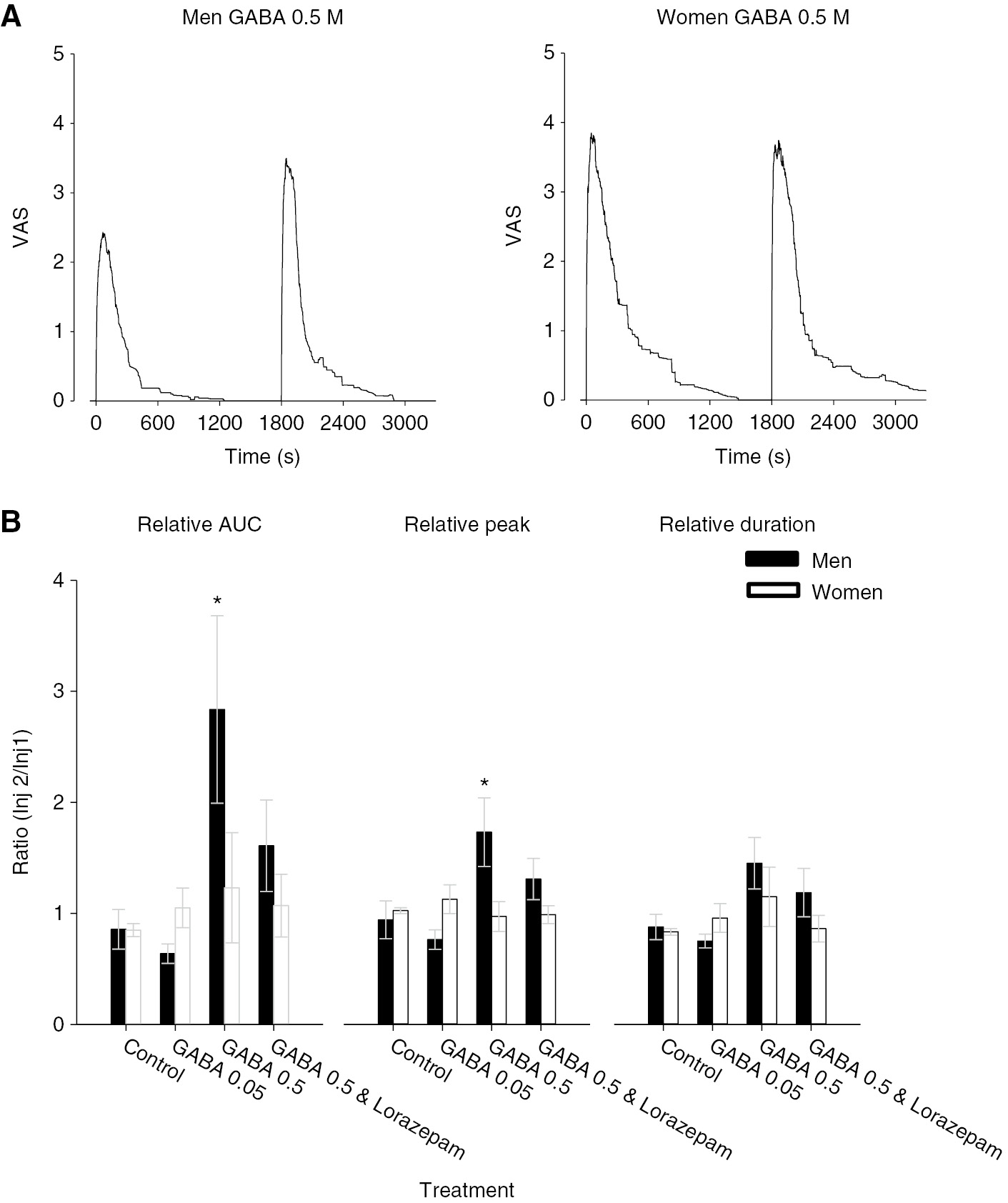
(A) The line graphs illustrate the mean masseter muscle pain intensity produced by injection of glutamate (time 0) followed 30 min later by glutamate with GABA 0.5 M in men (n=15) and women (n=13). Note that the initial injection of glutamate alone produced substantially less pain in men than in women (see Table 2). Further, the graphs illustrate that in men, there was a substantial increase in pain ratings when glutamate and GABA were injected together, whereas in women, the pain responses appear almost identical. (B) The bar graphs indicate the mean relative area under the pain curve (AUC), relative peak and relative duration of pain produced by injection of the substances indicated in men (black) and women (white). There was a significant interaction between sex and treatment for AUC (F=5.005, p=0.003). In men, the combination of GABA 0.5 M with glutamate significantly increased AUC, peak pain, and duration of pain compared to glutamate alone. In women, none of these injections had significantly altered pain ratings compared with glutamate alone. Asterisks: p<0.05 men compared with women; Error bars: SE.
There were no significant treatment effects on PPT, PPTOL or MJO over the time course of the experiment (Fig. 7). The only significant sex-related difference found was in the baseline PPT value, which was significantly higher in men than in women. No other sex-related differences in these parameters were identified.
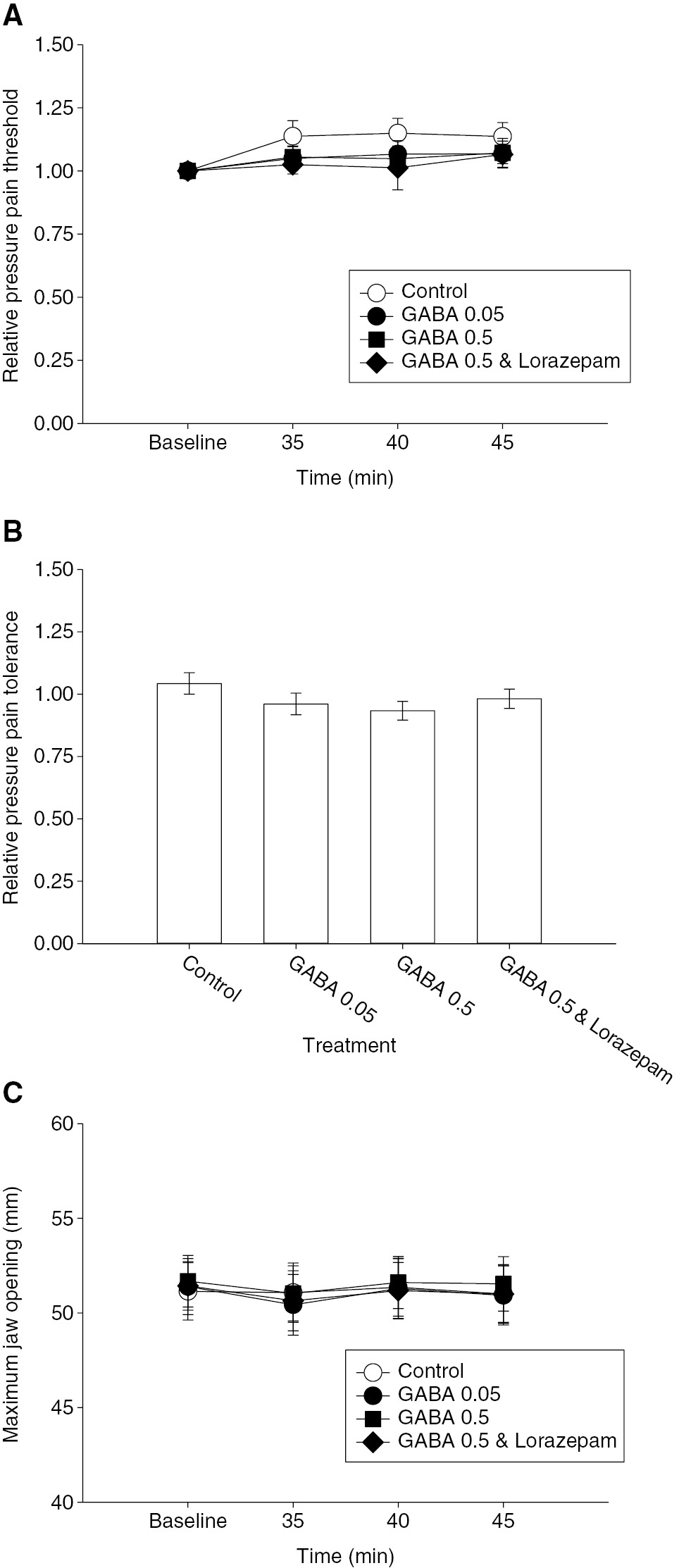
(A) The line and scatter plot shows the mean relative pressure pain threshold (PPT) normalized to the pre-injection baseline. There was no significant effect of any of the second injections on PPT. (B) The bar graphs indicate the mean relative pressure pain tolerance (PPTOL) 45 min after the second masseter muscle injections. There was no significant effect of any of the injections on the PPTOL. (C) The line and scatter plot shows the mean maximum jaw opening (MJO). There was no significant effect of any of the second injections on MJO. Error bars: SE.
There were no significant treatment effects on pain area. There was also no sex-related difference in average pain area for the initial glutamate injection when areas from men and women were compared.
4 Discussion
Preclinical studies have reported that activation of peripheral GABAA receptors can exert either analgesic or algesic effects, depending on the concentration used [12], [13], [14]. The present study found that injection of GABA alone into the masseter muscle of healthy humans resulted in concentration-related reports of mild pain that was further increased by lorazepam, a GABAA receptor positive allosteric modulator. These results suggest that intramuscular injection of GABA excites muscle nociceptors through activation of GABAA receptors. Subsequent experiments examined the effect of GABA administration on pain evoked by intramuscular injection of glutamate, which provokes pain responses through activation of peripheral NMDA receptors [17]. These experiments indicated that GABA can, in a concentration-related manner, increase glutamate-evoked masseter muscle pain. This pro-algesic effect of GABA was significantly greater in men than in women. The effect of GABA injections in either sex are apparently short lasting, as they did not significantly affect mechanical nociception within 5 min of an intramuscular injection. Taken together, these results suggest that GABA can act through the peripheral GABAA receptor in humans to provoke and enhance masseter muscle pain.
The peripheral effect of elevated GABA concentrations on nociception has previously been investigated in the skin, joint and oral cavity of rats. Injection of GABA into the temporomandibular joint of male rats resulted in a concentration related declination in the magnitude of the glutamate-evoked TMJ-jaw muscle reflex in male rats [12], [13]. This effect could be reversed by bicuculline, a GABAA receptor antagonist, but not phaclofen, a GABAB receptor antagonist, which indicates it was mediated through activation of GABAA receptors. However, subcutaneous injection of the GABAA receptor agonist muscimol was shown to differentially modulate responses in the formalin model of cutaneous inflammatory pain which depended on concentration [14]. At low concentration, muscimol exerted analgesic effects, whereas at increased concentration it enhanced formalin-evoked nocifensive behavior. More recently, oral administration of muscimol to the tongue was shown to significantly increase the mechanical thresholds of tongue afferent fibers compared to vehicle, but only after the tongue had been heated with 60 °C water [26]. It was also shown in this study that 95% of afferent fibers innervating the rat tongue mucosa express GABAA receptors [26]. GABA containing oral rinses have been recently employed in humans to test their effect on burning pain induced by topical application of capsaicin [27]. In these experiments, capsaicin was applied to the tongue to provoke burning pain, and the effect GABA mouthwashes examined. GABA rinses did not alter the peak pain, but did shorten the time for healthy human subjects to stop feeling the burning pain [27]. Taken together, these previous results demonstrate that peripheral GABAA receptor activation can exert very different effects that are dependent on the tissue to which it is applied and the concentration used.
In the spinal cord and trigeminal sensory nucleus, GABA acts via the GABAA receptor to mediate primary afferent depolarization; a presynaptic inhibitory mechanism [5], [6], [7], [8], [9]. This is thought to occur because the chloride reversal potential in central endings of primary afferent fibers is more depolarized than the resting membrane potential. Indeed, in vivo experiments in the rat have shown that sustained application of GABA to the dorsal root ganglion neurons results in biphasic depolarization as long as GABA is present [28], [29], [30]. However, the environment that central terminals and sensory ganglion neurons are in may be quite different from that of their peripheral endings in various tissues, and thus it can only be speculated that the resting membrane potential in these endings is similar to that measured in sensory ganglion neurons. The finding that intramuscular injection of GABA was painful, and that GABA could enhance glutamate-evoked muscle pain is suggestive that GABA can depolarize peripheral afferent endings, at least in masticatory muscle, and that this mechanism contributes to its algesic effects. Why then, in other tissues at different concentrations, does GABA administration result in analgesia? The analgesic effect of GABAA receptor activation in preclinical studies has been speculated to be due to a current shunt or depolarization block [12], [14], [26]. It has been shown that GABA produces greater depolarizations of Aβ and Aδ afferent fibers, than of C fibers [6]. There is a good temporal relationship between the firing of a population of Aδ afferent fibers in rats and the change in pain in human subjects, after injection of glutamate into the masseter muscle [31]. This may mean that much of the acute pain response evoked by glutamate when injected into the human masseter muscle is coded by the firing of Aδ afferent fibers and thus that GABA, through its stronger depolarizing action on myelinated fibers acts to increase pain intensity reports. A recent report also indicated that GABA has the ability to activate neuromuscular nicotinic receptors at high concentration, which could lead to low level muscle contraction [32]. In the present study, subjects commonly reported that the muscle felt tight after GABA and glutamate were co-injected. If GABA induces low levels of muscle contraction, this may also contribute to increased pain reports [33]. Indeed, this difference might also help explain why GABA increased glutamate-evoked pain when injected into the human masseter muscle, but decreased glutamate-evoked nocifensive response when injected into the rat temporomandibular joint.
The effects of GABA can also be mediated through activation of G-protein linked GABAB receptors, which are expressed by around one third of trigeminal ganglion neurons that innervate the rat masseter muscle (Cairns, unpublished results). Baclofen, a GABAB receptor selective agonist has been shown to exert suppressive effects on the responses of vagal and pelvic afferent fibers to mechanical stimulation in ferrets and rats, respectively [34], [35]. Baclofen has also been shown to increase a transient and a sustained potassium current in trigeminal ganglion neurons, which results in membrane hyperpolarization and decreased excitability [36]. However, GABA injection into the human masseter muscle did not alter PPT or PPTOL, either alone or in combination with glutamate. This suggests that if sensory afferent fibers that innervate the human masseter muscle express GABAB receptors, their activation by injection of GABA does not result in a detectable change in mechanical sensitivity.
Both healthy men and women were recruited into the present study to test whether there are sex-related differences in the effect of intramuscularly injected GABA. While no sex-related differences were found for pain AUC, duration or intensity after injection of GABA alone into the masseter muscle, it was found that GABA significantly enhanced glutamate-evoked masseter muscle pain in men but not in women. It is a consistent finding that injection of glutamate 0.5 M into the masseter muscle evokes significantly more pain in women than in men (Table 2) [19], [22], [37]. However, the combination of GABA 0.5 M with glutamate evoked muscle pain of relatively similar intensity in men and women (Fig. 5). If one simply adds the intensity of pain produced by GABA alone (Fig. 1B) to the pain produced by glutamate alone (Table 1) for men, it is a pretty good estimate of the pain reported when GABA 0.5 M and glutamate were injected together in men. A similar estimation for women, suggests that peak pain intensity produced by the combination GABA 0.5 M and glutamate should have been around 6. It is not clear why pain produced by injection of glutamate and GABA together were not also additive in women. It is possible this sex-related difference in the enhancement of glutamate-evoked pain by GABA is merely a methodological artifact.
In conclusion, the present study was unable to translate findings of analgesic actions of activation of peripheral GABAA receptors from animal models into healthy human subjects. Indeed, the findings support the currently accepted concept that activation of GABAA receptors on sensory primary afferent fibers leads to depolarization and increased excitability. However, it is important to recognize that the actions of peripherally administered GABA, with regard to algesic versus analgesic effect, appear to differ depending on the tissue tested (oral mucosa versus skeletal muscle). The mechanistic basis for these differences in the effect of GABA remain to be determined.
Acknowledgements
We would like to thank Dr. Enrico De Martino for his valuable assistance.
-
Author’s statements
-
Research funding: Funded by the Danish National Research Foundation (DNRF121) for the Center for Neuroplasticity and Pain (CNAP).
-
Conflict of interest: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The study was approved by the Scientific Ethics Committee for the North Jutland Region of Denmark (reference no. N-20160037) and was performed in accordance with the tenets of the Helsinki Declaration.
References
[1] Bowery NG, Smart TG. GABA and glycine as neurotransmitters: a brief history. Br J Pharmacol 2006;147(Suppl 1):S109–19.10.1038/sj.bjp.0706443Search in Google Scholar PubMed PubMed Central
[2] McCarson KE, Enna SJ. GABA pharmacology: the search for analgesics. Neurochem Res 2014;39:1948–63.10.1007/s11064-014-1254-xSearch in Google Scholar PubMed
[3] Bormann J. The ‘ABC’ of GABA receptors. Trends Pharmacol Sci 2000;21:16–9.10.1016/S0165-6147(99)01413-3Search in Google Scholar
[4] Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology 2002;96:1161–7.10.1097/00000542-200205000-00020Search in Google Scholar PubMed
[5] Curtis DR, Lodge D, Bornstein JC, Peet MJ, Leah JD. The dual effects of GABA and related amino acids on the electrical threshold of ventral horn group Ia afferent terminations in the cat. Exp Brain Res 1982;48:387–400.10.1007/BF00238615Search in Google Scholar PubMed
[6] Desarmenien M, Santangelo F, Loeffler JP, Feltz P. Comparative study of GABA-mediated depolarizations of lumbar A delta and C primary afferent neurones of the rat. Exp Brain Res 1984;54:521–8.10.1007/BF00235477Search in Google Scholar PubMed
[7] Gallagher JP, Higashi H, Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol 1978;275: 263–82.10.1113/jphysiol.1978.sp012189Search in Google Scholar PubMed PubMed Central
[8] Levy RA. The effect of intravenously administered gamma-aminobutyric acid on afferent fiber polarization. Brain Res 1975;92:21–34.10.1016/0006-8993(75)90525-9Search in Google Scholar PubMed
[9] Lovick TA. Primary afferent depolarization of tooth pulp afferents by stimulation in nucleus raphe magnus and the adjacent reticular formation in the cat: effects of bicuculline. Neurosci Lett 1981;25:173–8.10.1016/0304-3940(81)90327-XSearch in Google Scholar
[10] Matthews G, Ayoub GS, Heidelberger R. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci 1994;14:1079–90.10.1523/JNEUROSCI.14-03-01079.1994Search in Google Scholar PubMed PubMed Central
[11] Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, SvenssonP. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol 2001;86:782–91.10.1152/jn.2001.86.2.782Search in Google Scholar PubMed
[12] Cairns BE, Sessle BJ, Hu JW. Activation of peripheral GABA(A) receptors inhibits temporomandibular joint-evoked jaw muscle activity. J Neurophysiol 1999;81:1966–9.10.1152/jn.1999.81.4.1966Search in Google Scholar PubMed
[13] Cai BBY, Cairns BE, Sessle BJ, Hu JW. Sex-related suppression of reflex jaw muscle activity by peripheral morphine but not GABA. Neuroreport 2001;12:3457–60.10.1097/00001756-200111160-00016Search in Google Scholar PubMed
[14] Carlton SM, Zhou S, Coggeshall RE. Peripheral GABA(A) receptors: evidence for peripheral primary afferent depolarization. Neuroscience 1999;93:713–22.10.1016/S0306-4522(99)00101-3Search in Google Scholar PubMed
[15] Cairns BE, Sessle BJ, Hu JW. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J Neurosci 1998;18:8056–64.10.1523/JNEUROSCI.18-19-08056.1998Search in Google Scholar PubMed PubMed Central
[16] Arendt-Nielsen L, Svensson P, Sessle BJ, Cairns BE, Wang K. Interactions between glutamate and capsaicin in inducing muscle pain and sensitization in humans. Eur J Pain 2008;12:661–70.10.1016/j.ejpain.2007.10.013Search in Google Scholar PubMed PubMed Central
[17] Cairns BE, Svensson P, Wang KL, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol 2003;90: 2098–105.10.1152/jn.00353.2003Search in Google Scholar PubMed
[18] Cairns BE, Wang KL, Hu JW, Sessle BJ, Arendt-Nielsen L, Svensson P. The effect of glutamate-evoked masseter muscle pain on the human jaw-stretch reflex differs in men and women. J Orofac Pain 2003;17:317–25.Search in Google Scholar
[19] Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle B, Arendt-Nielsen L, Svensson P. Glutamate-evoked jaw muscle pain as a model of persistent myofascial TMD pain? Arch Oral Biol 2008;53:666–76.10.1016/j.archoralbio.2008.01.008Search in Google Scholar PubMed PubMed Central
[20] Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle BJ, Arendt-Nielsen L, Svensson P. Effect of a peripheral NMDA receptor antagonist on glutamate-evoked masseter muscle pain and mechanical sensitization in women. J Orofac Pain 2007;21:216–24.Search in Google Scholar
[21] da Silva LB, Kulas D, Karshenas A, Cairns BE, Bach FW, Arendt-Nielsen L, Gazerani P. Time course analysis of the effects of botulinum neurotoxin type A on pain and vasomotor responses evoked by glutamate injection into human temporalis muscles. Toxins (Basel) 2014;6:592–607.10.3390/toxins6020592Search in Google Scholar PubMed PubMed Central
[22] Svensson P, Cairns BE, Wang KL, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain 2003;101:221–7.10.1016/S0304-3959(02)00079-9Search in Google Scholar PubMed
[23] Svensson P, Wang K, Arendt-Nielsen L, Cairns BE, Sessle BJ. Pain effects of glutamate injections into human jaw or neck muscles. J Orofac Pain 2005;19:109–18.10.1016/j.pain.2003.12.031Search in Google Scholar PubMed
[24] Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, Arendt-Nielsen L. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res 2006;169:467–72.10.1007/s00221-005-0158-zSearch in Google Scholar PubMed
[25] Wong H, Kang I, Dong XD, Christidis N, Ernberg M, Svensson P, Cairns BE. NGF-induced mechanical sensitization of the masseter muscle is mediated through peripheral NMDA receptors. Neuroscience 2014;269:232–44.10.1016/j.neuroscience.2014.03.054Search in Google Scholar PubMed
[26] Tan SN, Song E, Dong XD, Somvanshi RK, Cairns BE. Peripheral GABAA receptor activation modulates rat tongue afferent mechanical sensitivity. Arch Oral Biol 2014;59:251–7.10.1016/j.archoralbio.2013.11.015Search in Google Scholar PubMed
[27] Zhang Y, Wang K, Arendt-Nielsen L, Cairns BE. gamma-Aminobutyric acid (GABA) oral rinse reduces capsaicin-induced burning mouth pain sensation: an experimental quantitative sensory testing study in healthy subjects. Eur J Pain 2018;22:393–401.10.1002/ejp.1128Search in Google Scholar PubMed
[28] Deschenes M, Feltz P, Rouzaire-Dubois B, Kelly JS. Preliminary investigation of a process of desensitization on mammalian presynaptic gamma-aminobutyric (GABA) receptors [proceedings]. Br J Pharmacol 1977;59:487P.Search in Google Scholar
[29] Deschenes M, Feltz P, Lamour Y. A model for an estimate in vivo of the ionic basis of presynaptic inhibition: an intracellular analysis of the GABA-induced depolarization in rat dorsal root ganglia. Brain Res 1976;118:486–93.10.1016/0006-8993(76)90318-8Search in Google Scholar PubMed
[30] Deschenes M, Feltz P. GABA-induced rise of extracellular potassium in rat dorsal root ganglia: an electrophysiological study in vivo. Brain Res 1976;118:494–9.10.1016/0006-8993(76)90319-XSearch in Google Scholar
[31] Cairns BE. Physiological properties of thin-fiber muscle afferents: Excitation and modulatory effects. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, editors. Fundamentals of Musculoskeletal Pain. Seattle: IASP Press, 2008:19–31.Search in Google Scholar
[32] Dionisio L, Berge I, Bravo M, Esandi Mdel C, Bouzat C. Neurotransmitter GABA activates muscle but not alpha7 nicotinic receptors. Mol Pharmacol 2015;87:391–400.10.1124/mol.114.095539Search in Google Scholar PubMed
[33] Torisu T, Wang K, Svensson P, De Laat A, Fujii H, Arendt-Nielsen L. Effect of low-level clenching and subsequent muscle pain on exteroceptive suppression and resting muscle activity in human jaw muscles. Clin Neurophysiol 2007;118:999–1009.10.1016/j.clinph.2006.11.311Search in Google Scholar PubMed
[34] Page AJ, Blackshaw LA. GABA(B) receptors inhibit mechanosensitivity of primary afferent endings. J Neurosci 1999;19: 8597–602.10.1523/JNEUROSCI.19-19-08597.1999Search in Google Scholar PubMed PubMed Central
[35] Sengupta JN, Medda BK, Shaker R. Effect of GABA(B) receptor agonist on distension-sensitive pelvic nerve afferent fibers innervating rat colon. Am J Physiol Gastrointest Liver Physiol 2002;283:G1343–51.10.1152/ajpgi.00124.2002Search in Google Scholar PubMed
[36] Takeda M, Tanimoto T, Ikeda M, Kadoi J, Matsumoto S. Activaton of GABAB receptor inhibits the excitability of rat small diameter trigeminal root ganglion neurons. Neuroscience 2004;123:491–505.10.1016/j.neuroscience.2003.09.022Search in Google Scholar PubMed
[37] Castrillon EE, Cairns BE, Wang K, Arendt-Nielsen L, Svensson P. Comparison of glutamate-evoked pain between the temporalis and masseter muscles in men and women. Pain 2012;153:823–9.10.1016/j.pain.2012.01.003Search in Google Scholar PubMed
© 2020 Scandinavian Association for the Study of Pain. Published by Walter de Gruyter GmbH, Berlin/Boston. All rights reserved.
Articles in the same Issue
- Frontmatter
- Editorial
- Change in Editorship: A Tribute to the Outgoing Editor-in-Chief
- Editorial comments
- Laboratory biomarkers of systemic inflammation – what can they tell us about chronic pain?
- Considering the interpersonal context of pain catastrophizing
- Systematic review
- Altered pain processing and sensitisation is evident in adults with patellofemoral pain: a systematic review including meta-analysis and meta-regression
- Topical reviews
- Pain revised – learning from anomalies
- Role of the immune system in neuropathic pain
- Clinical pain research
- Cryoneurolysis for cervicogenic headache – a double blinded randomized controlled study
- Interpersonal problems as a predictor of pain catastrophizing in patients with chronic pain
- Pain and small-fiber affection in hereditary neuropathy with liability to pressure palsies (HNPP)
- Predicting the outcome of persistent sciatica using conditioned pain modulation: 1-year results from a prospective cohort study
- Observational studies
- Revised chronic widespread pain criteria: development from and integration with fibromyalgia criteria
- The relationship between patient factors and the refusal of analgesics in adult Emergency Department patients with extremity injuries, a case-control study
- Chronic neuropathic pain after traumatic peripheral nerve injuries in the upper extremity: prevalence, demographic and surgical determinants, impact on health and on pain medication
- Tramadol prescribed use in general and chronic noncancer pain: a nationwide register-based cohort study of all patients above 16 years
- Changes in inflammatory plasma proteins from patients with chronic pain associated with treatment in an interdisciplinary multimodal rehabilitation program – an explorative multivariate pilot study
- Original experimental
- The pro-algesic effect of γ-aminobutyric acid (GABA) injection into the masseter muscle of healthy men and women
- The relationship between fear generalization and pain modulation: an investigation in healthy participants
- Experimental shoulder pain models do not validly replicate the clinical experience of shoulder pain
- Computerized quantification of pain drawings
- Head repositioning accuracy is influenced by experimental neck pain in those most accurate but not when adding a cognitive task
- Short communications
- Dispositional empathy is associated with experimental pain reduction during provision of social support by romantic partners
- Superior cervical sympathetic ganglion block under ultrasound guidance promotes recovery of abducens nerve palsy caused by microvascular ischemia
Articles in the same Issue
- Frontmatter
- Editorial
- Change in Editorship: A Tribute to the Outgoing Editor-in-Chief
- Editorial comments
- Laboratory biomarkers of systemic inflammation – what can they tell us about chronic pain?
- Considering the interpersonal context of pain catastrophizing
- Systematic review
- Altered pain processing and sensitisation is evident in adults with patellofemoral pain: a systematic review including meta-analysis and meta-regression
- Topical reviews
- Pain revised – learning from anomalies
- Role of the immune system in neuropathic pain
- Clinical pain research
- Cryoneurolysis for cervicogenic headache – a double blinded randomized controlled study
- Interpersonal problems as a predictor of pain catastrophizing in patients with chronic pain
- Pain and small-fiber affection in hereditary neuropathy with liability to pressure palsies (HNPP)
- Predicting the outcome of persistent sciatica using conditioned pain modulation: 1-year results from a prospective cohort study
- Observational studies
- Revised chronic widespread pain criteria: development from and integration with fibromyalgia criteria
- The relationship between patient factors and the refusal of analgesics in adult Emergency Department patients with extremity injuries, a case-control study
- Chronic neuropathic pain after traumatic peripheral nerve injuries in the upper extremity: prevalence, demographic and surgical determinants, impact on health and on pain medication
- Tramadol prescribed use in general and chronic noncancer pain: a nationwide register-based cohort study of all patients above 16 years
- Changes in inflammatory plasma proteins from patients with chronic pain associated with treatment in an interdisciplinary multimodal rehabilitation program – an explorative multivariate pilot study
- Original experimental
- The pro-algesic effect of γ-aminobutyric acid (GABA) injection into the masseter muscle of healthy men and women
- The relationship between fear generalization and pain modulation: an investigation in healthy participants
- Experimental shoulder pain models do not validly replicate the clinical experience of shoulder pain
- Computerized quantification of pain drawings
- Head repositioning accuracy is influenced by experimental neck pain in those most accurate but not when adding a cognitive task
- Short communications
- Dispositional empathy is associated with experimental pain reduction during provision of social support by romantic partners
- Superior cervical sympathetic ganglion block under ultrasound guidance promotes recovery of abducens nerve palsy caused by microvascular ischemia


