A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
-
Habib Khan
and JaKeoung Koo
Abstract
The emergence of smart and nanobiosensor (NB) technologies has transformed the monitoring and management of bacterial infections. These developments offer remarkable accuracy and precision for detecting infectious pathogens. Smart artificial intelligence (AI)-assisted and NB-based methods are used as powerful tools in biomedicine for bacterial detection, combatting multidrug resistance, and diagnosing infections. In this study, we delve into the advancements in these technologies, focusing on AI-based techniques for NBs in detecting bacterial infections from 2019 to 2024. We analyze the contributions of machine learning and deep learning techniques to enhance performance and reliability. The new approaches to improve the effectiveness and versatility of antibacterial treatments are critically analyzed. Our study includes the observations of carbon nanoparticles that selectively target bacteria using photothermal properties and the production of hybrid hydrogel composites with capabilities. Furthermore, the study emphasizes the crucial significance of NBs in propelling the progress of diagnostic methods, biosensing technologies, and treatments, thereby transforming the healthcare industry and the way diseases are managed. In addition, we explore pathogen-based infections, bacterial diagnosis, and treatment using engineered NBs enhanced with various modalities such as electrochemistry, acoustics, electromagnetism, and photothermal resonance. Our comprehensive review highlights the potential and throws light on future research directions for effective management and control of bacterial infections.
1 Introduction
Bacterial resistance to antibiotics has emerged as a critical contemporary concern in human health, posing significant challenges to disease management and treatment outcomes. To present historical information on bacterial losses, the research determined the number of fatalities linked to 33 bacterial genera or species spanning 11 infectious syndromes in 2019. Furthermore, estimations demonstrate that the prevalence of infections caused by bacteria will continue to rise to almost 10 million cases yearly in the next years, more than cancer cases [1]. This trend emphasizes the urgent need for innovative technologies to detect and control bacterial infections. Bacterial infections harm patients and pose significant economic consequences for medical facilities. Continuous antibiotic therapy is frequently necessary to treat diseases caused by multidrug-resistant bacteria, especially in serious cases when tissue ablation may also be desired. Nevertheless, the successful outcome of these treatment options is hindered by disproportionate healthcare expenditures and poor patient compliance, culminating in a projected yearly total of social and health expenses surpassing USD 55 billion in the United States alone [2].
Antibiotic resistance leads to higher antibiotic use, which complicates the outbreak by promoting the spread of resilient varieties of bacteria. Studies indicate a high degree of antimicrobial resistance, with a significant percentage of bacterial types, such as Staphylococcus aureus (S. aureus), displaying resistance to several antimicrobial agents, including methicillin, vancomycin, and carbapenems [3]. Bacterial resistance to frequently employed antibiotics is a complex phenomenon that demands the contribution of several processes, including suppression of cell walls, production of proteins, and alterations in DNA and structures. Bacteria possess inherent mechanisms to endure the negative consequences of antimicrobials, such as change and spreading of genetic material. The emergence of genes such as NDM-1, which have been linked to high levels of resistance to particular beta-lactam antibiotics, poses substantial obstacles to the efficacy of antibiotics [4]. Furthermore, it is well established that microorganisms that cause diseases such as tuberculosis exhibit multidrug resistance, hence complicating the growth of effective treatment strategies [5]. Considering these challenges, there is an urgent requirement to find robust smart and nanobiosensor (NB)-based effective antibiotic strategies to successfully combat diseases caused by highly resistant bacteria [6].
Bacterial infections, drug resistance, and biofilm development present significant barriers, emphasizing the need for innovative identification, tracking, and treatment methods. Conventional approaches are often time-consuming, resource-intensive, and less effective due to emerging multidrug-resistant bacterial strains. Overuse and misuse of antibiotics further exacerbate resistance, prolong hospital stays, increase medical costs, and pose global public health threats. Biofilms add complexity by protecting bacteria from immune responses and antibiotics, leading to chronic infections. Advanced biosensor technologies, ingenious and NBs, offer promising solutions by providing rapid, sensitive, and specific pathogen detection, allowing timely and targeted treatment. Integrating artificial intelligence (AI) specifically machine learning (ML) and deep learning (DL) enhances these sensors’ capabilities with real-time data analysis and accurate bacterial monitoring and control. These intelligent sensors can detect specific biomarkers and patterns, monitor treatment effectiveness, and prevent biofilm formation. Nanotechnology in biosensing enables multifunctional platforms for diagnostic and therapeutic use, releasing antimicrobial agents in response to pathogen detection. Advanced smart and NB tools can revolutionize bacterial infection management by providing rapid, accurate, and cost-effective options, mitigating bacterial infections and resistance, and improving public health. The traditional dependence on these approaches necessitates significant financial and human capital and might unintentionally worsen antibiotic resistance due to overuse and improper usage [7].
This research investigates the significant capacity for change that smart NBs possess, particularly with or without cutting-edge technologies like ML- and DL-based smart approaches. These innovative approaches provide a potential substitute for conventional approaches, offering improved sensitivity, specificity, and efficiency in the treatment of bacterial infections. The integration of AI into the monitoring of bacterial infections and NBs represents significant progress, allowing rapid and precise analysis that surpasses conventional diagnostic methods. This facilitates the development of more precise forecasts about infection patterns and treatment outcomes and the availability of real-time and ongoing monitoring data [8]. Intelligent NBs can provide customized therapy interventions and real-time surveillance, leading to a significant influence on the management of bacterial infections. These technological advancements make previous approaches outdated by enabling accurate identification of bacterial infections even at very low levels [9]. These technologies provide enhanced data collection and management, facilitating more precise identification and tracking of bacterial infections, hence resulting in more effective treatment approaches. To efficiently control illnesses caused by various bacterial strains, address contamination issues, and overcome antibiotic-resistant microorganisms, it is essential to use this strategy [10]. The potential use of photothermal effects as well as electromagnetic resonances is being explored to enhance the diagnostic and treatment processes for NB. The advanced methods shown here illustrate the adaptability and capacity of intelligent NBs to transform the treatment. The aim is to emphasize the substantial potential of smart NBs in enhancing diagnostic accuracy, customizing treatments, decreasing healthcare expenses, and ultimately addressing the growing problem of antibiotic resistance [11]. This will be critically analyzed and achieved by thoroughly examining these cutting-edge technologies and their practical uses. In this study, we address several critical questions to understand the advancements and challenges in integrating AI, biosensor technology, and nanotechnology for bacterial infection management. Our research questions are as follows.
1.1 Research questions
What are the main developments in integrating AI, biosensor technology, and nanotechnology that have improved the ability to monitor, identify, and manage bacterial infections?
In what ways have AI methods, especially ML and DL approaches, enhanced the capability of sensors to deal with bacterial infections efficiently?
How have integrated biosensor technologies and nanotechnology-based treatments advanced the detection and control of bacterial infections, considering factors such as contamination by bacterial agents, resistance mechanisms, and biofilm formation?
How does the combination of biosensor technologies and nanotechnology-based solutions improve the identification and management of bacterial infections, taking into account aspects such as bacterial contamination, resistance mechanisms, and the emergence of biofilms?
How have several improvements, such as the use of photodynamics, electrochemistry, acoustics, electromagnetism, and photothermal resonance, enhancing the diagnostic and therapeutic powers of designed NBs in the treatment of bacterial infections?
What are the challenges and future paths in advancing and utilizing intelligent nanobots for managing bacterial infections, and how can these improvements be applied to overcome these challenges for more accurate and customized diagnostic and therapeutic interventions?
1.2 Contributions
This article offers an in-depth and comprehensive analysis of the latest advancements in AI-integrated technology and nanotechnology tools that significantly enhanced the monitoring, detection, and control of bacterial infections. By examining the specific applications of smart NBs, the study offers a detailed understanding of how these technologies have evolved and their overall impact on bacterial infection management.
Our study explores how AI-based techniques, particularly ML and DL approaches, have improved the performance of sensors in effectively monitoring bacterial infections inside the specified period.
We investigate how integrated biosensor and nanotechnology-based treatments advance the control and detection of bacterial infections, addressing contamination, resistance, and biofilm formation. A detailed analysis of contamination by bacterial agents, resistance mechanisms, and microorganism bio-curtains, offering insights into pathogen-based infection complexities and dynamics.
We examined various enhancements, including photodynamics, electrochemistry, acoustics, electromagnetism, and photothermal resonance, that have improved the diagnostic and therapeutic capabilities of engineered NBs in bacterial infection management. This analysis demonstrates the multifaceted approaches used to enhance the effectiveness and functionality of NBs.
The article identifies the current challenges in the development and application of smart NBs. We also discuss potential solutions and future directions, emphasizing the advancements that can be leveraged to address the highlighted challenges. We provided a roadmap for future research and development, aiming for more precise and tailored diagnostic and therapeutic interventions.
2 Overview of biosensor technologies in bacterial infection management
Biosensors offer innovative approaches to efficiently detect, monitor, and control bacterial pathogens. This overview encompasses conventional methods and cutting-edge advancements such as AI-integrated biosensor technology and the utilization of smart NBs. In addition, it explores the role of nanotechnology in biosensing applications, underscoring its significance in enhancing sensitivity, specificity, and overall performance in bacterial infection management.
2.1 Conventional methods
The challenge of treating infections is significantly compounded by bacterial survival in biofilms, where populations are encased in a protective matrix, leading to heightened resistance against antimicrobial agents. Biofilms contribute to the persistence and severity of bacterial infections, particularly in healthcare settings. Despite extensive research on bacterial resistance mechanisms, the complex dynamics of biofilm formation and development have not been thoroughly explored. Traditional techniques for identifying and managing bacterial infections, such as smear microscopy, isolation cultures, biochemical tests, and histiocyte cultures, are becoming less prevalent due to their limitations. Synthetic peptides derived from natural host-defence peptides demonstrate antibiofilm activity. Combined with traditional antibiotics, these peptides exhibit a synergistic effect, reducing the required antibiotic concentration to eradicate specific bacterial strains effectively [12,13]. These methods often suffer from restricted sensitivity, prolonged processing times, and complex handling procedures, leading to inaccurate diagnoses and ineffective treatments [14]. As a result, healthcare-associated infections are frequently mismanaged. There is an urgent need to develop innovative techniques to address the challenges posed by bacterial infections, biofilm formation, and antibiotic resistance. Advanced tools and methodologies are required to combat bacterial infections and mitigate the global impact of antibiotic resistance. Researchers are leveraging AI tools, nanotechnology, and bioinformatics to develop more effective diagnostic procedures and treatments for bacterial infections [15]. Integrating advanced technologies and multidisciplinary research efforts is essential in this endeavor. Collaboration between academia, industry, and healthcare sectors is crucial for the development and implementation of specific strategies to combat bacterial infections and safeguard public health.
2.2 AI-integrated biosensor technology
The integration of AI with biosensor technology is becoming an influential factor in the constantly shifting healthcare industry, transforming the way diseases get diagnosed and managed. The use of computer-aided diagnostics and AI technologies in clinical practice is an important achievement, though it comes with major challenges [16]. It is observable that medical imaging approaches [17], such as X-ray, magnetic resonance imaging, computed tomography, and positron emission tomography, are crucial methods in detecting different diseases [18]. They offer important information about different pathological situations. The diversity of openly available imaging and biological resources improves the abilities of AI systems, making it simpler to adapt them for medical purposes. Pioneering platforms like PathAI [19], Viz.ai, and Freenome harness the power of AI to augment the diagnostic capabilities of pathologists, enhance clinical diagnostics, aid clinical trial support, and advance translational research. Moreover, entities like Freenome revolutionize cancer screening, diagnostics, prevention, and overall disease management. The pervasive adoption of AI-powered tools underscores a commitment to leveraging cutting-edge technologies across various medical domains, from diagnostic imaging to biosensors [20,21]. In tandem with the burgeoning utilization of AI in medical imaging, biosensor technology emerges as a pivotal frontier in disease detection and management. The amalgamation of AI algorithms with biosensors heralds a paradigm shift in disease diagnostics, empowering clinicians with enhanced sensitivity, specificity, and accuracy in detecting biomarkers indicative of diverse health conditions. This joint coordination not only enhances diagnostic capabilities but also paves the way for personalized healthcare, offering tailored treatment methods adapted to the unique features of each patient [22].
Applications of biosensors in conjunction with AI Figure 1 enable lightning-fast detection of diseases, enabling proactive therapy prior to the appearance of clinical symptoms. AI-enabled NBs have continuous monitoring capabilities and offer up-to-the-minute data on the progression of diseases and the effectiveness of treatments. This enables doctors to enhance therapeutic tactics and improve patient outcomes. Furthermore, the use of AI-powered inspection of biosensor data enables clinicians to promptly evaluate the efficacy of medicines in real-time [23]. This allows clinicians to promptly modify treatment regimens, and the likelihood of antibiotic resistance may be reduced, leading to enhanced patient care. The development of powered by AI biosensors that easily connect with current healthcare systems represents a new age of improved communication among medical professionals and efficient clinical operations. AI researchers may provide tailored solutions to boost diagnostic precision, optimize workflow effectiveness, and improve patient care by comprehending the specific challenges and requirements encountered by doctors in various clinical roles. The combination of AI and biosensor technology is poised to significantly alter the management of illnesses and the delivery of healthcare. This confluence has a chance to result in a substantial transformation toward more efficient, customized, and patient-centric care models. The combination of AI with biosensor innovations in health care has the promise to usher in a new age of precise treatment. This would include timely identification, customized treatment, and enhanced results for patients, eventually reshaping the criteria for achieving healthcare performance.

Key nanotechnology techniques in biosensing for bacterial infections.
2.3 Smart NBs in bacterial infection management
The smart NB solution is a modern invention that revolutionizes the detection and management of infections caused by bacteria. These sensors are designed to instantly and accurately detect the existence and behavior of microorganisms. Smart NBs are pivotal in diagnosing infectious diseases, offering sensitivity, selectivity, and rapid detection capabilities. Combining nanomaterials and biosensing technology, these sensors provide versatile tools for disease diagnosis, including point-of-care methods for swift pathogen detection. While offering promise in controlling disease spread, the ongoing research focuses on optimizing these sensors and exploring new opportunities for enhanced diagnosis using nanotechnology [24]. Smart NB methods are frequently employed to detect bacteria instantaneously, enabling ongoing surveillance of their development [25,26]. Smart nanobots for bacterial infection prevention can be grouped into many broad categories and have an extensive list of functions. Table 1 presents an overview of many different uses together with the corresponding NB technology. It is observable that fluorescent NBs can quickly detect the existence of microorganisms, allowing medical professionals to react immediately as necessary. Point-of-care diagnostics use paper-based NBs to swiftly and precisely identify infections caused by bacteria at the patient’s bedside. Smart nanobots play a vital role in preventing antimicrobial resistance as they examine the variables that contribute to resistance in strains of bacteria. DNA-based NBs demonstrate exceptional accuracy in detecting such markers, enabling accurate administration of antibiotics and monitoring developments in resistance. Furthermore, they suggest potential methods for destroying bacterial biofilms, which are notoriously tough to eliminate and are frequently associated with chronic infections and resistance to antibiotics. Electrochemical nanobots are designed specifically to detect and eliminate features unique to biofilms, with the capacity to avoid the emergence of permanent diseases by addressing these resilient bacterial populations as highlighted by [27]. In addition, these small devices enable remote detection of bacterial infections in individuals, utilizing functional NBs that have wireless connectivity for transmitting real-time data to medical professionals. This allows for swift intervention and personalized treatment plans tailored to the unique attributes of each patient. The researchers deeply focused on this era, however, on enhancing the versatility and specificity of detection methods, as well as exploring novel approaches for targeted treatment and prevention strategies that need to be tailored to individual patient needs. In addition, research efforts could aim to further integrate wireless connectivity and remote monitoring capabilities into smart NBs for real-time disease surveillance and personalized healthcare interventions.
Broad categories and applications of smart NBs for bacterial infection management
| Category/application | Description | NBs technologies |
|---|---|---|
| Real-time detection | Continuous monitoring of bacterial presence and activity in real time. | Fluorescent |
| Point-of-care diagnostics | Rapid detection of bacterial infections at the point of care. | Paper based |
| Antibiotic resistance monitoring | Detection of antimicrobial resistance markers. | DNA based |
| Biofilm detection and disruption | Detection and disruption of bacterial biofilms. | Electrochemical |
| Remote monitoring | Remote monitoring of bacterial infections in patients. | Wearable |
| Environmental surveillance | Monitoring bacterial contamination in various environmental settings. | Microfluidic |
| Smart medical devices | Detection of bacterial infections in medical implants. | Implantable |
| Personalized treatment strategies | Tailoring treatment strategies based on individual characteristics. | Smartphones |
2.4 Nanotechnology for biosensing
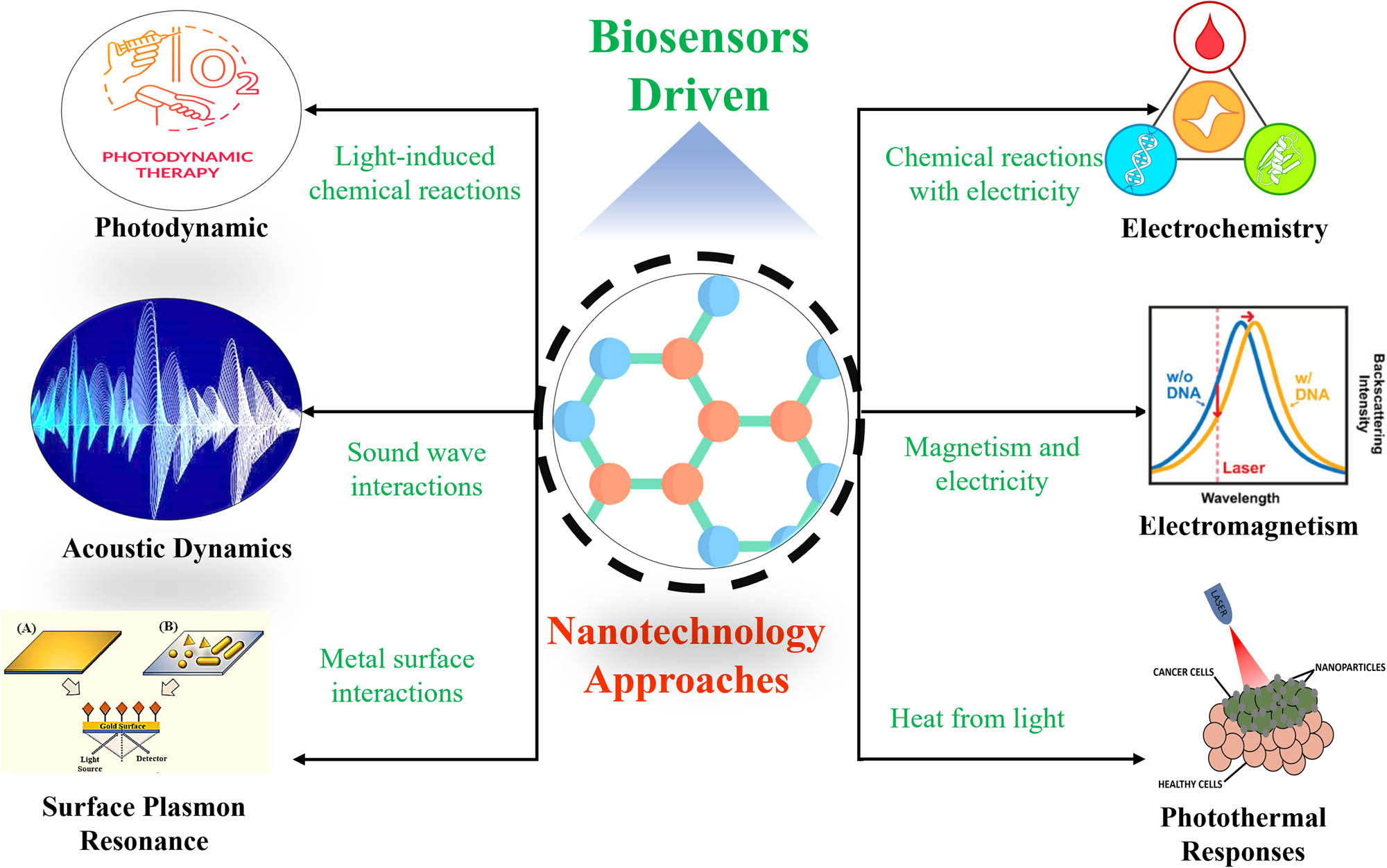
The rapid evolution of molecular biological science has propelled nanotechnology into a dynamic field characterized by continuous innovation and refinement. Leveraging nanotechnology, the scientific community has achieved remarkable breakthroughs, particularly in the realms of biomedical engineering, food safety management, and environmental health monitoring [28]. Nanotechnology presents a promising avenue for addressing challenges associated with bacterial infections, antimicrobial resistance, and biofilms [29]. With its unique chemical, biological, and physical properties, nanotechnology has emerged as an indispensable tool across various medical domains [30,31]. In light of the widespread application of nanomaterials and nanotechnology in diagnosing and treating bacterial infections, a comprehensive examination of recent research findings becomes imperative. This article endeavors to scrutinize the latest advancements in crafting tailored NBs for the detection and management of bacterial diseases. These innovative biosensors harness a diverse array of nanotechnology methodologies, encompassing electrochemistry, acoustic dynamics, electromagnetism, photothermal responses, and surface plasmon resonance. The multidisciplinary nature of NB techniques holds pivotal importance in unraveling intricate facets of bacterial responses and guiding innovative approaches in diagnosis and therapy. Ongoing research endeavors in nanotechnology are dedicated to exploring novel frontiers and enhancing the efficacy of NBs for precise and efficient diagnosis and treatment of bacterial infections. Through rigorous scientific inquiry and technological advancement, the field of nanotechnology continues to pave the way for transformative solutions in biomedical science and healthcare (Table 2).
Application of ML and DL for bacterial infection monitoring and control
| References & year | ML/DL technique | Detection mechanism | Target bacteria | Applications | Advantages |
|---|---|---|---|---|---|
| Rawson et al. [32] 2019 | SVM | Classification using six blood parameters (C-reactive protein, white cell count, bilirubin, creatinine, ALT, and alkaline phosphatase). | Community-acquired bacterial infections | Diagnosing bacterial infection in hospital-admitted patients. | Utilizes routinely available blood parameters for accurate diagnosis with high ROC AUC 84% |
| Agbaria et al. [33] 2019 | SVM, principal component analysis, LDA | Infrared spectroscopy of white blood cell (WBC) samples | Differentiation between bacterial and viral infections | Rapid identification of infection type within less than 1 h after blood sample collection | High sensitivity (93%) and specificity (85%); fast, accurate, sensitive, and low-cost method |
| Gadalla et al. [34] 2019 | Random forest, SVM coupled with recursive feature elimination. | Identification of clinical and urinary immunological predictors | Urinary tract infections (UTIs) | Diagnosis of uncomplicated UTIs in women | Improved accuracy in ruling in/out UTIs using urine cloudiness and specific urinary biomarkers. potential development of a point-of-care test |
| Oh et al. [35] 2019 | Convolutional neural network (CNN) | Embedding disease diagnosis and antibiotic prescriptions in electronic health records (EHR) and using a DL model for prediction | Antibiotic-resistant bacteria | Prediction and estimation of antibiotic resistance for preemptive measures | Improved f1-score from 0.525 to 0.617 by incorporating disease and antibiotic information; DL model outperformed traditional ML models |
| Ho et al. [36] 2019. | CNN, logistic regression and SVM | Raman optical spectroscopy for label-free bacterial detection, identification, and antibiotic susceptibility testing | 30 common bacterial pathogens, including S. aureus (both methicillin resistant and methicillin susceptible) | Culture-free pathogen identification and antibiotic susceptibility testing | Achieves high accuracies even on low signal-to-noise spectra; distinguishes between methicillin-resistant and -susceptible isolates with high accuracy |
| Hattori et al. [37] 2020 | Ensemble-learning algorithm for data classification in high-dimensional feature space | Oulter principle measuring transient drops in ionic current as bacteria translocate through a low aspect ratio pore | S. aureus, Pseudomonas fluorescens, Salmonella enterica, Escherichia coli, and Bacillus cereus | Rapid screening of pathogens for infection control in healthcare and environmental settings | Label-free single-cell identifications, high-spatiotemporal resolution, real-time detection of pathogenic bacteria |
| Nehal et al. [38] 2020 | CNN | Photonic crystal-based optical biosensor detecting spectral changes due to bacterial presence | E. coli | Prediction of bacterial contaminants in water | High accuracy (95%) in detecting bacterial presence; lightweight, small, portable, and low-noise optical biosensors that work without electric power |
| Iriya et al. [39] 2020 | Long short-term memory (LSTM) network | Optical imaging-based method to track motion patterns of single bacterial cells | E. coli | Rapid antibiotic susceptibility testing | Accurately determines antibiotic susceptibility within 30 min for five commonly used antibiotics |
| Brown et al. [40] 2020 | Neural network | Optical intensity information from an array of fiber optic cables to detect bacterial growth | S. aureus | Rapid and automated antimicrobial susceptibility testing (AST) | Faster results (within 5.72–10.5 h), cost-effective, eliminates human error, compatible with standard phenotypic assays, meets FDA criteria for essential and categorical agreements |
| Draz et al. [41] 2020 | CNN | Nanoparticle-enabled smartphone system with platinum nanoprobes inducing gas bubble formation | Hepatitis B virus (HBV), HCV, and Zika virus (ZIKV) | Rapid and sensitive virus detection | High sensitivity (98.97%) for detecting viral-infected samples, no need for optical hardware smartphone attachment |
| Alafeef et al. [42] 2020 | SVM in ML is used with wireless communication | Nanotechnology-based smart sensors using nanomaterials like carbon nanoparticles, metallic nanoparticles, metal oxide nanoparticles, and nanocomposites | Pathogenic bacteria, including antibiotic-resistant bacteria | Early and rapid detection of bacterial pathogens at the point-of-care | Enables real-time and continuous monitoring, potentially providing valuable information for outbreak prevention and containment |
| Zhang et al. [43] 2021 | CNN, SVM, PCA | Noninvasive biosensors collecting physiological signals | Physiological signals and bacterial data | Clinical practice, health monitoring, and food safety | Improved accuracy and efficiency in healthcare, bringing a digital revolution |
| Ding et al. [44] 2021 | Multi-scale CNN | SERS | Salmonella enteritidis, Salmonella typhimurium, and Salmonella paratyphi | Efficient detection and distinction of Salmonella serovars | High recognition accuracy (over 97%) for serovar identification |
| Yu et al. [45] 2021 | LSTM neural network and CNN | Raman spectroscopy | Pathogens isolated from the marine organism Urechis unicinctus | Efficient and accurate identification of pathogens in seafood and the environment | LSTM methods achieved average isolation-level accuracies exceeding 94%, faster and more accurate than normal CNN models |
| Li et al. [46] 2022. | MRDP was applied to LDA algorithm | Interaction-induced release of ssDNA from 2D-n | Pathogenic microorganisms including E. coli, S. aureus, methicillin-resistant S. aureus (MRSA) | Rapid and accurate microbial taxonomic identification | High overall accuracy of 97.9% |
| Yang et al. [47] 2022 | SVM, k-nearest neighbor, random forest | SERS with SiO2 coated silver nanorod array substrates | Thirteen respiratory virus species, including SARS-CoV-2, common human coronaviruses, and influenza viruses | Rapid and accurate detection and classification of respiratory viruses, aiding medical diagnosis and therapeutic intervention | High differentiation accuracy (
|
| Arano-Martinez et al. [48] 2022. | Linear regression, SVM, navie Bayes, decision trees, and KNN | Optical biosensors enhanced by nonlinear optics and multiphoton interactions | SARS-CoV-2 and other viruses | Detecting biological information, predicting urgent situations, and identifying viruses and harmful entities in living organisms | Enhanced sensitivity, expanded application range, and improved identification of complex agents |
| Verma et al. [49] 2022 | SVM, RF | Biosensors utilize ML to interpret environmental changes, detect biomarkers, and integrate sensing modalities for system understanding | Lung cancer-related exosomes or prostate cancer biomarkers | ML-enhanced biosensors monitor physiological parameters, diagnose diseases, and detect pathogens in healthcare settings | These biosensors offer improved accuracy, sensitivity, and personalized diagnostics |
| Guo et al. [50] 2022 | Deep neural network | EIS data from carbon nanotube thin film (CNT-TF) biosensors | BNP for heart failure diagnosis | Diagnosis and management of heart failure in point-of-care settings | High sensitivity, fast response, enhanced reliability, and applicability without advanced hardware |
| Zhou et al. [51] 2022 | CNN | Nonenzymatic electrochemical biosensing with chronoamperometry data analysis | Glucose and lactate | Point-of-care detection for glucose and lactate in biological samples | High sensitivity, low cost, long-term stability, enhanced selectivity without complex material synthesis, accurate prediction in real samples |
| Noreldeen et al. [52] 2022 | DNN, CNN | Intrinsic fingerprint of 3D fluorescence spectra | Five Vitamin B6 derivatives (VB6Ds) | Qualitative and quantitative analysis of VB6Ds | High accuracy in identifying and quantifying VB6Ds, effective transfer learning, broad concentration range detection |
| Wang et al. [53] 2022 | Neural networks and PCA | MEMS microcantilever structures | Various MEMS biosensors for automation, consumer electronics, industrial manufacturing, defence, medical equipment, | Development of high-sensitivity sensors and intelligent sensing systems | Small size, high sensitivity, mass production capability, simple arraying, and integration |
| Kumar et al. [54] 2022 | LSTM, Bi-LSTM with Bahdanau attention, CNN-LSTM, Convolutional-LSTM | Prediction of respiratory rate using ECG, PPG, and sEMG data | Respiratory rate prediction in a body sensor network | Monitoring and predicting respiratory rates for health monitoring | High prediction accuracy; Bi-LSTM with Bahdanau attention showed best results |
| Rahmani et al. [55] 2023 | CNN | Near-infrared (NIR) and RGB imaging analyzed with a SWCNT sensor and DNA aptamer | Bacteria detection using biosensor imaging | Early detection and prevention of crop diseases in agriculture | High accuracy (98.72%) and computational efficiency; effective classification of biosensor images |
| Ramalingam et al. [56] 2023 | KNN, SVM, NB, DT, GBT, RF, feedforward ANN, RNN and CNN | Nanotechnology-enhanced biosensors leveraging nanomaterials for improved performance | Viruses and pathogens detection | Virus detection, improving biosensor performance, facilitating efficient detection of viruses and pathogens | Enhanced sensitivity, high surface-to-volume ratio, quantum size effects, improved biosensor performance |
| Kang et al. [10] 2024 | 1D CNN with binary labeling | 3D nanostructure swab captures pathogens, and portable Raman instrument collects Raman signals for classification by the DL algorithm | Multiple foodborne bacterial species | Rapid identification of foodborne pathogens on contaminated surfaces and kitchen utensils for food safety monitoring | Rapid detection (
|
| Zhou et al. [57] 2024 | YOLOv7 | Portable lens-free holography microscope for digital single-particle counting and nucleic acid detection without pre-amplification | Salmonella typhimurium | Point-of-care testing for nucleic acids, food safety inspection, environmental monitoring, and in vitro diagnostics | High sensitivity (72 CFU/mL), low cost ($70), lightweight (
|
3 ML and DL analysis for in bacterial monitoring and control
Researchers utilized different applications of ML and DL techniques in improving nano biosensors for detecting bacterial infections. Subsection 2.1 analyzes ML methods, while Subsection 2.2 focuses on DL approaches, highlighting their contributions to enhancing performance.
3.1 ML approaches for detecting bacterial infections
ML methods have greatly improved biosensor technologies by increasing their sensitivity, accuracy, and efficiency. Significant progress has been made in the development of customized integrated sensor arrays that combine 2D nanomaterials with fluorescently labeled single-stranded DNA. The arrays can precisely identify different bacteria, including those that are resistant to drugs, by examining the unique patterns of response that occur when bacteria contact with the sensing components. The technique has a remarkable level of accuracy, with a detection rate of 97.9%, in recognizing pathogens such as S. aureus and Escherichia at various levels of concentration. Moreover, the integration of Surface-Enhanced Raman Scattering (SERS) with ML techniques like support vector machine (SVM) and random forest has greatly enhanced the ability to identify viruses [32,33].
This combination enables the rapid and accurate identification of respiratory viruses, such as SARS-CoV-2, common human coronaviruses, and influenza viruses. By using the enhanced sensitivity of SiO2-coated silver nanorod substrates, these systems can accurately differentiate and classify virus species and strains with an accuracy above 99%. This makes them a powerful tool for managing viral epidemics. In addition, the combination of optical biosensors, nonlinear optics, and ML enhances viral detection by using multiphoton interactions to boost both sensitivity and specificity. This makes them very effective in identifying a variety of viruses, including the SARS-CoV-2 virus [47]. Through the use of ML Figure 2, these biosensors can examine complex biological data and detect mobile entities inside the human body, facilitating rapid detection of possible health problems and quick intervention [43]. ML-enhanced biosensor technologies have revolutionized pathogen detection and virus identification, offering unprecedented levels of sensitivity and accuracy. Customized integrated sensor arrays, combining nanomaterials with DNA labeling, enable precise identification of bacteria, including drug-resistant strains, with high detection accuracy.
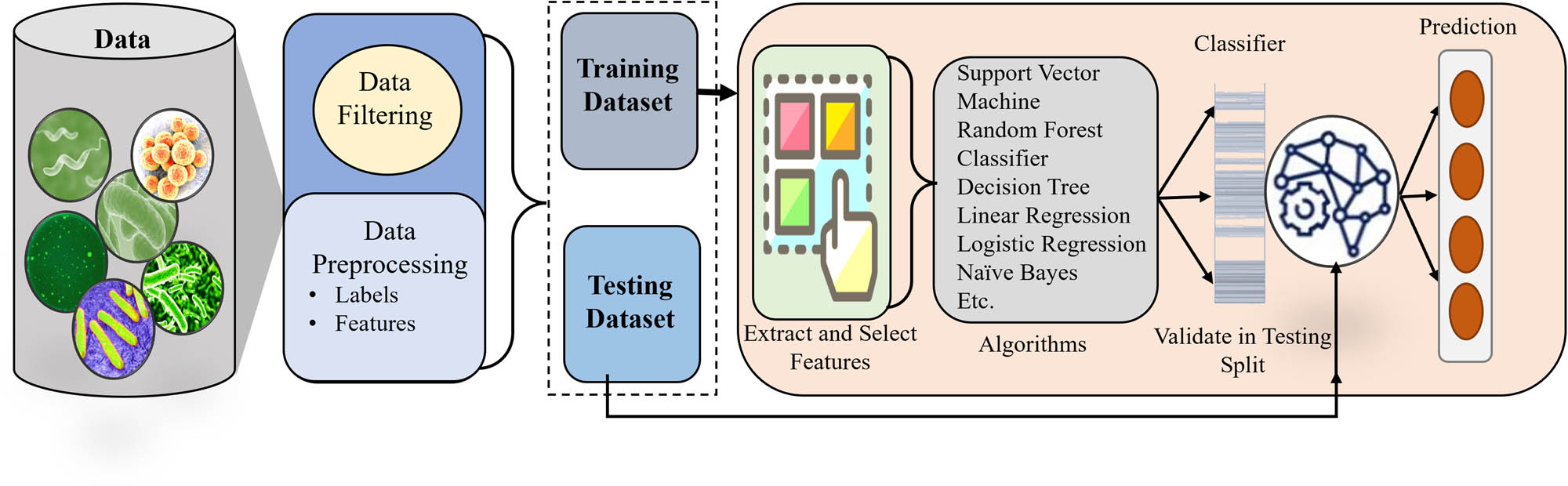
Generic overflow of ML approaches for bacteria diagnosis.
3.2 DL techniques for detecting bacterial infections
DL algorithms have significantly transformed biosensor technology by offering advanced data analysis and prediction capabilities. Carbon nanotube (CNT)-based biosensors, when combined with deep neural networks (DNNs), have shown substantial promise in the realm of medical diagnostics, namely, for the detection of heart failure [39]. These biosensors utilize electrochemical impedance spectroscopy (EIS) data to accurately forecast B-type natriuretic peptide (BNP) levels, hence eliminating the necessity for conventional error-prone analytical methods. This method improves both the accuracy of diagnosis and the availability of the technology for point-of-care testing. Nonenzymatic electrochemical biosensors, employing a back-propagation neural network, exhibit exceptional selectivity in the detection of glucose and lactate. These sensors utilize specialized working electrodes that are incorporated into arrays of electrochemical microdroplets. When combined with neural network analysis, they can accurately forecast the levels of analytes, even in complicated biological samples, simplifying the detection process and reducing the need for sophisticated material production and selection [58].
Figure 3 illustrates the general features of DL models, such as DNN and CNNs, which have achieved great success in recognizing and measuring vitamin B6 molecules through 3D fluorescence spectra [52]. These models may attain accuracy levels of up to 100%, showing their effectiveness in distinguishing between molecules with very similar chemical structures. Possessing this skill is essential for precise medical diagnosis and treatment. In addition, advanced DL models such as LSTM networks and bidirectional LSTM (Bi-LSTM) with Bahdanau attention have demonstrated high accuracy in predicting respiratory rate using data from bio-sensors such as ECG, PPG, and surface electromyogram (sEMG) [54]. The Bi-LSTM with attention mechanisms offers very precise predictions, which are essential for monitoring patient health in real time [37]. A novel lens-free holography microscope, which incorporates DL techniques, has been created for digital single-particle counting in nucleic acid detection, without the need for pre-amplification. This cost-effective and portable technology collects three-dimensional images of small spherical objects and uses an enhanced YOLOv7-based algorithm to effectively and precisely identify minuscule items [57,59].
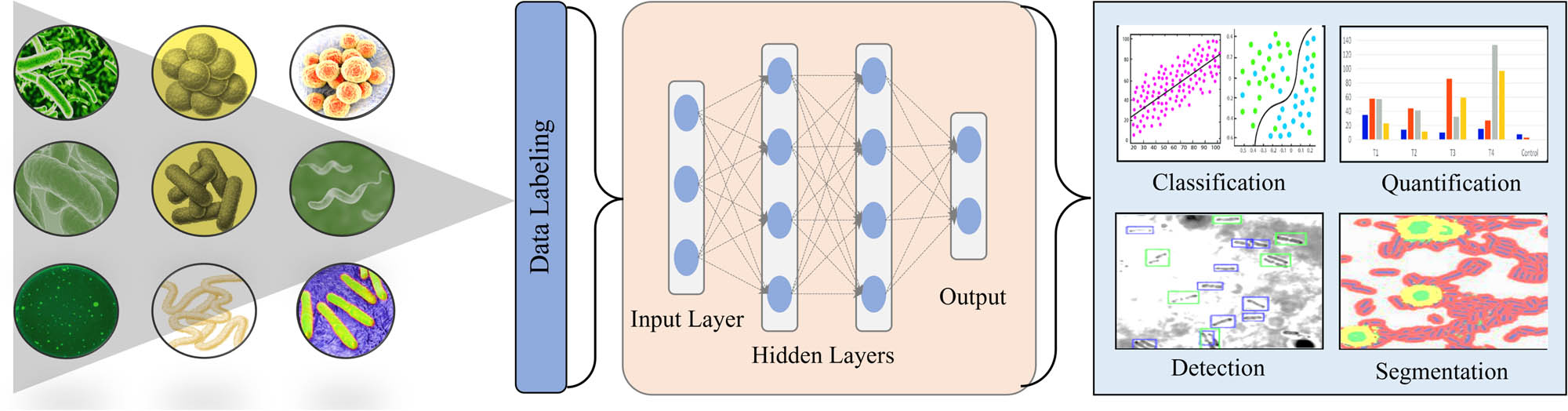
Technical overflow of DL techniques for the identification and monitoring of bacteria.
The combination of powerful ML and DL algorithms with state-of-the-art biosensor technology is resulting in significant progress in pathogen detection. ML approaches improve the sensitivity and accuracy of biosensors in identifying different pathogens, such as viruses and bacteria, by evaluating intricate data patterns and enhancing the efficiency of detection procedures. However, the ML-based approaches are not robust in complex problems as the features engineering is often manual. DL algorithms provide advanced data processing and prediction capabilities, allowing for precise diagnoses and real-time health monitoring. These technological advancements are improving the accuracy, efficiency, and accessibility of diagnostic techniques, which are vital for managing public health, detecting diseases early, and providing timely medical treatment. The integration of machine learning, deep learning, and nanotechnology is revolutionizing biosensor development, leading to diagnostic instruments that are more efficient, precise, and accessible. This advancement has substantial implications for healthcare, food safety, and disease prevention. The progress in this discipline is enhancing the existing state of pathogen detection and also creating opportunities for future study and application in other areas, such as environmental monitoring, food safety inspection, and clinical diagnostics.
4 Pathogen-based infection
Infectious diseases, including the outbreak of COVID-19, represent a constant and widespread danger to public health. According to the World Health Organization, there have been over 76,382,044 confirmed cases of COVID-19 and more than 1,702,128 deaths in over 200 countries or territories worldwide as of 23 December 2020 [60,61]. The lack of adequate point-of-care detection tools is a significant factor contributing to the high incidence of these disorders. Hence, the development of fast and dependable health technologies for the diagnosis of numerous viruses is crucial to protect ourselves from serious dangers. The current approaches for diagnosing viral infections involve detecting viruses or viral antigens in human bodily fluids and conducting lung computerized tomography scans of patients, focusing on specific imaging abnormalities. Common virus detection techniques encompass viral culture, a method called reverse transcription-polymerase chain reaction (RT-PCR), and enzyme-linked immunosorbent test (ELISA) [62–64]. However, the majority of these diagnostic procedures have disadvantages in terms of being time-consuming and requiring a lot of effort. Table 3 presents a comprehensive analysis of the advantages and limitations of current virus detection techniques. Virus culture is a laborious task that requires skilled individuals and particular procedures. To conduct the RT-PCR experiment, the viral RNA is initially extracted and then converted into complementary DNA (cDNA) using reverse transcription. The cDNA is subsequently amplified by PCR, or polymerase chain reaction, to facilitate detection.
Frequently employed methods for identifying pathogenic viruses
| Concept | Methodology | Benefits | Time Span | References |
|---|---|---|---|---|
| Nuclic acid | PCr | Clearly established limited quantity of test specimens | Hourly | [67] |
| Viral defense | Elisa | Extremely particular | Hourly | [68] |
| Infectiousness test | Cellular culture | Appropriate for virus subtyping and divergent strain recovery | Conversion from days to weeks | [69] |
| Quite affordable | ||||
| Viral components | The use of electron microscopes | Expeditious technique | Hourly | [70] |
| Thoracic imaging | Computerized tomography (CT) | Solid foundation for clinical diagnosis and therapy | Hourly | [71] |
The RT-PCR approach exhibits a high level of sensitivity; however, it requires costly specialist equipment, intricate primer design for sequence alignment, and tuning of assay conditions. In addition, the testing procedure of the RT-PCR technology is time consuming because it involves multiple cycles of heating. ELISA is a fast detection technique that uses a solid-phase enzyme immunoassay to identify viral antigens. However, its usefulness for on-site detection is limited due to its relatively poor sensitivity and the requirement for high-quality sample preparation [65]. Due to the initial shortage of testing kits and the high probability of false negative results from RT-PCR, chest CT scans were temporarily employed as a means of clinically diagnosing COVID-19 during the early stages of the illness outbreak. The CT scan is a non-invasive technology that requires the patient to lie still. The patient’s chest is subjected to several X-ray scans from various angles to generate cross-sectional images that vary depending on the stage of infection following the start of symptoms. CT scan for COVID-19 detection has a significant drawback in terms of low specificity (25%), as its imaging features are similar to those of other viral pneumonia. Molecular diagnostic techniques are more appropriate for precise diagnoses compared to syndromic testing and CT scanning. This is because they can directly focus on and identify specific infections [66].
4.1 Contamination by bacterial agents
It is generally acknowledged that once bacteria enter the body, they reproduce, multiply, and release toxic substances. Infection with bacteria or, in extreme circumstances, intoxication caused by germs can manifest in various pathogenic ways in the body. Bacterial infections commonly appear in two main forms: acute and persistent. The effects of different pathogenic bacteria on the host organism can differ [72]. During acute infections, bacteria trigger an immediate inflammatory reaction in the host, typically marked by symptoms such as redness, swelling, increased temperature, discomfort, and reduced functionality. In contrast, chronic infections develop slowly as bacteria gradually create biofilms, which make them more resistant to antimicrobial treatments and the host immune system. It is important to mention that although self-limiting bacterial infections might happen, most bacterial infections present considerable difficulties in terms of therapy [73]. The outcome and intensity of bacterial infections depend on the interaction between human immunity and bacterial pathogenicity. Several variables significantly impact the infection’s progression, including the infecting agent’s dosage, environmental conditions, co-infections, and associated factors. A thorough comprehension of these aspects is essential in ascertaining the result of bacterial infections and directing efficient approaches for prevention and therapy. Current research efforts strive to understand the complicated dynamics of bacterial infections, leading to progress in developing treatments and improving our capacity to deal with the intricacies of bacterial pathogenesis (Table 4).
Possible nanotechnology-based treatments for the control of bacterial infections
| References | Microorganisms | Nanoparticles | Anti-bacterial solution | Operations |
|---|---|---|---|---|
| [74] | E. coli and S. aureus | PLGA-poly(L-histidine)- PEG | Vancomycin | Increasing binding of antibiotic to bacteria |
| [75] | Multidrug-resistant P. aeruginosa | AgNPs | Polymyxin B | Three-fold higher biofilm reduction than the neat Ag nanoparticles |
| [76] | P. aeruginosa | PLGA/chitosan nanoparticles | Colistin | Inhibition of biofilm with a 90% reduction in biomass |
| [77] | Methicillin-resistant S. aureus (MRSA) | Chitosan | Daptomycin | Antibacterial effect |
| [78] | E. coli | Chitosan/heparin | Ciprofloxacin | A synergistic effect of drug and biopolymer on the disruption of bacterial membranes |
| [79] | P. aeruginosa | AuNPs | Esculentin-1a (antimicrobial pep- tide) | Fifteen-fold increase in anti-P. aeruginosa activity of free esculentin-1a without toxic effect on human keratinocytes |
| [80] | Gram-negative bacteria | Graphene oxide, Ag | Ciprofloxacin | Eradicating of Gram-negative bacteria |
| [81] | M. tuberculosis | PLGA | Rifampicin | Bactericidal activity against M. tuberculosis |
| [82] | MRSA | TiO2 | Erythromycin | Decreasing the MIC of erythromycin |
4.2 Resistance to bacterial agents
Antibiotic use is known to promote the evolution of bacteria that are resistant to their effects, which in turn leads to their eventual dominance. A major global health concern, the rise of antibiotic-resistant microorganisms has made the significant shift toward viable therapies seem like a distant dream. It is crucial to acknowledge that antibiotic resistance is an inherent and unavoidable occurrence. Bacteria have undergone ongoing evolution over billions of years, acquiring adaptations to counteract the effects of antimicrobial treatments. Bacterial resistance is influenced by a confluence of environmental and intrinsic factors. The quick spread of bacterial drug resistance is greatly influenced by environmental mechanisms, specifically the protracted process of ecological evolution. Alterations to the DNA sequence, such as genetic transfer across bacterial species, can occur when bacteria colonize or infect hosts in the environment [83,84]. Gaining a deep comprehension of the complex mechanisms behind antibiotic resistance is essential for formulating comprehensive approaches to tackle this rapidly developing worldwide health crisis. Current research efforts are focused on discovering innovative strategies to reduce antibiotic resistance, providing possible solutions to the difficulties posed by this intricate problem.
4.3 Microorganism bio-curtain
The process of bacterial biofilm formation might be described as a gripping narrative, resembling a symphony with four distinct stages. In Figure 4, microorganisms adhere to surfaces using mechanisms such as the cell’s surface charge of everything, the van der Waals force, its hydrophobicity, as well as electrostatic force [85], similar to artists using charged brushes. The story then transitions to a complex dance, as microorganisms move through liquid environments or gather on solid surfaces, resembling artists on a canvas, using the coordinated movements of rotating flagella [86]. The climax occurs during the maturity phase, where biofilm micro-colonies, resembling masterpieces, are surrounded by water transport channels. These channels facilitate the movement of nutrients and coordinate quorum sensing and gene regulation [87]. In the last stage, known as dispersion, bacteria elegantly transition between floating states and biofilm states within a complex community of many cells [88]. The fully developed biofilm serves as a channel for the transfer of energy, chemicals, and information, strengthening microorganisms underneath against unpredictable external conditions. This biofilm development pattern serves as evidence of the ability of cells to thrive in challenging conditions and spread out into new ecological habitats, similar to innovative explorers [89,90]. The composition’s subtleties are meticulously adjusted by factors such as bacterial density, duration of life, temperature, fluid flow characteristics, nutrient concentration, and the interplay of surface material qualities. The complex flagella and fimbriae on the bacterial canvas are additional decorations that play important functions in the development of biofilms. Continuing research efforts, similar to innovative composers, aim to enhance our comprehension and develop tactics for modulating or regulating the formation of biofilms in various environments. The life cycle of a microbial biofilm is illustrated with relevant features (Figure 4). where the process begins with initial attachment, where planktonic bacteria temporarily adhere to a surface through weak interactions. This progresses to irreversible attachment, where bacteria produce extracellular polymeric substances (EPS) to secure permanent adhesion. In the microcolony formation phase, bacteria proliferate, forming small clusters within the EPS matrix. During maturation, the biofilm evolves into a complex, three-dimensional structure with channels facilitating nutrient and waste flow. Finally, in the dispersal phase, cells from the mature biofilm are released as planktonic bacteria, ready to colonize new surfaces. This iterative process guarantees the persistence, expansion, and dissemination of bacterial populations across many habitats.

Phases overview of the biofilm cycle.
5 Bacterial diagnosis and treatment using engineered NBs
The era of bacterial diagnosis and treatment using engineered NBs involves the integration of nanotechnology with photodynamic therapy (PDT) to accurately and efficiently control bacterial infections. The incorporation of PD into novel NBs capitalizes on the ongoing advancements in optical technology and photosensitive compounds, enabling the generation of reactive oxygen species inside bacterial cells to eliminate infections. Nanomaterials are crucial in the delivery of phototherapeutic drugs, since they may specifically target and eliminate bacterial cells via PD mechanisms. Zeolite L-nanocrystals and other nanoparticles are particularly effective in this procedure. By combining the use of photonic synergy with drugs such as metronidazole (MNZ), the efficiency of PDT against biofilm infections is improved [91]. This is achieved by stimulating anaerobic metabolism in bacteria, namely, MRSA. NBs are activated by NIR light irradiation. These biosensors use nanocomposite films and hierarchically constructed nanoparticles to generate reactive oxygen species and nitric oxide, resulting in strong antibacterial effects. Moreover, the use of gold and silver nanocages in precision phototherapy exploits their specific surface plasmon reflection properties, allowing for the efficient elimination of drug-resistant biofilms and infections via the aggregation-induced photothermal (AIP) effect. Together, these categories emphasize the revolutionary capability of merging nanotechnology with PD principles to provide advanced biosensing technologies and therapeutic approaches for addressing bacterial resistance and infections.
5.1 Integration of photodynamics into innovative NBs
The emergence of PDT represents a notable advancement in the targeted treatment of bacteria, propelled by the continuous advancement of technology for optics. In recent years, there has been tremendous interest in the continual development of new photosensitive substances, which offer a novel and inventive approach to antibacterial treatments. This innovative antibacterial medication employs PD principles to induce the production of reactive oxygen compounds within microbial cells, leading to their eradication. The utilization of nanotechnologies in bacterial detection and therapy by PD is becoming increasingly popular due to its advantages, such as fewer adverse effects and decreased vulnerability to medication resistance [91,92]. PDT, as a burgeoning therapeutic method, exhibits immense potential in the accurate and efficient management of bacterial infections. As research progress continues, the combination of optical technologies and nanomaterials is increasingly enabling new opportunities, enhancing the field of bacterial therapy and strengthening human defenses against infectious pathogens.
5.1.1 Nanomaterials for phototherapeutic drug delivery
The use of phototherapeutic medicinal materials has significant therapeutic promise, and nanotechnology is crucial in exploiting this potential to develop multifunctional structures that can selectively target and destroy cells. The integration of these two domains presents new possibilities, especially in fighting microorganisms that are resistant to many drugs [93]. Scientists employ nanometer-scale particles derived from zeolite L-nanocrystals to affix a chemical component onto the bacterial coat using a simple and economical approach. These particles possess the capacity to incorporate dye molecules that generate a green fluorescence when examined under a microscope, permitting simple identification of the microorganisms. PD processing involves exposing bacteria to light to trigger a response that effectively kills them. In focused studies, researchers have enhanced micron-sized crystals with a third element. Because of their red light sensitivity, these crystals release specific molecules of reactive oxygen species. Oxygen molecules, even a single oxygen molecule, can set off a chain reaction that damages bacterial cells [94].
Integrating nanotechnology with phototherapeutic materials to improve bacterial therapy precision is the goal of the ongoing investigation in this area. The potential for these flexible structures to concentrate on specific markers and their adaptability bode well for the fight against bacterial infections, especially those that defy standard treatment methods. In the never-ending quest for effective treatments for bacterial infections, researchers are always looking for new ways to improve these procedures.
5.1.2 Photonic synergy in combined therapy
To treat bacterial biofilm infections, scientists are pioneering a method that combines PDT with the MNZ, which amplifies the effects of low oxygen levels. Hyaluronic acid (HA)-Ce6-MNZ nanoparticles (HCM NPs) are synthesized by combining chlorin e6 (Ce6) with MNZ in a unique way. The release of Ce6 and MNZ occurs when the HCM NPs are broken down by the hyaluronidase (Hyal) secreted by MRSA biofilm when they reach sites contaminated with this biofilm. Utilizing atmospheric pressure and the principles of PD treatment, laser irradiation of Ce6 starts a reaction that generates the singlet oxygen (1O2), effectively killing any bacteria in the biofilm. As PD therapy consumes oxygen, the oxygen deprivation within the biofilm intensifies, leading to the creation of nitroreductase by MRSA. The enzymatic response triggers the activation of MNZ, resulting in the elimination of bacteria in low-oxygen settings [95]. Table 5 summarizes the advantages, disadvantages, applications, and general usage of antimicrobial nanoparticles in this context.
Advantages, disadvantages, applications, and general usage of antimicrobial nanoparticles
| Usage | Advantages | Applications of antimicrobal nanoparticles | Disadvantages |
|---|---|---|---|
| Precision drug delivery to infection sites, reducing systemic side effects. | Nano-biosensors enhance drug delivery precision, ensuring antimicrobial nanoparticles accumulate at infection sites. | Enhanced precision in treating bacterial infections with NBs. | Risk of nanoparticle accumulation in nontarget tissues. |
| Localized drug release to specific sites, reducing off- target effects. | Smart biosensors control and localize drug release, minimizing side effects. | Smart biosensors offer solutions for multidrug-resistant bacteria. | Keeping nanoparticles localized is challenging. |
| Early detection and intervention of bacterial contamination, preventing device-associated infections. | Nano-biosensor techniques help reduce bacterial resistance by enabling timely interventions. | Nano-biosensors monitor and control bacterial contamination in devices. | Potential toxicity in vital organs. |
| Facilitates drug delivery to the brain, enabling the treatment of neurological infections. | Nano-biosensors facilitate nanoparticle transport across barriers like the blood-brain barrier. | Biosensors aid in delivering nanoparticles across the blood brain barrier. | Limited safety data; biosensors collect essential long-term information. |
| Real-time monitoring of therapeutic levels, optimizing treatment efficacy. | Biosensors provide real- time feedback for sustained therapeutic levels. | Continuous monitoring ensures effective chronic infection treatment. | Accurate nano-particle characterization is crucial. |
| Enhanced drug formulation and water purification, improving drug efficacy and environmental safety. | Nano-biosensors improve drug formulation, increasing solubility and bioavailability. | Nano-biosensors detect and eliminate bacterial contaminants in water. | Stability and environmental impact issues. |
| Safe administration of higher doses, improving treatment efficacy. | Biosensors ensure safe administration of higher nanoparticle doses. | Enhanced sterilization techniques with antimicrobial nanoparticles and biosensors. | Nanoparticles can induce stress. biosensors detect early responses. |
| Reduced immune response and improved compatibility, extending product shelf life. | Nano-biosensors minimize immune detection and improve compatibility. | Smart packaging with NBs controls bacterial contamination, extending shelf life. | Production is costly. |
This innovative incorporation of PD treatment not only enhances the oxygen-deficient microclimate but also eradicates this MRSA swarm even in the absence of hypoxic conditions. This method induces anaerobic metabolism in MRSA and enhances the antimicrobial effects of MNZ, showing the potential of this adaptable strategy in addressing complex bacterial infections. As scientific research progresses, these innovative methods create opportunities for the development of precise treatments that utilize the interaction between nanotechnology and PD principles to effectively address antibiotic-resistant biofilm infections.
5.1.3 NIR light irradiation activates NBs
The wide range of organic and inorganic nanomaterials that emit NIR light are being utilized in antibacterial approaches to address bacterial resistance. A inventive strategy entails creating nanocomposite films for NIR irradiation that utilize nitric oxide (NO)-assisted PD treatment. This approach is based on nanoparticles that are built hierarchically, namely composed of upconversion nanoparticles (UCNPs) and porphyrinic metal-organic frameworks (PCN-224). To enhance their efficiency, the particles are impregnated with the amino acid L- (LA) and subsequently bonded to a polyvinylidene fluoride (PVDF), structure, leading to the creation of an electrically spun nanocomposite material membrane (UCNP@PCN@LA-PVDF) [96]. When exposed to NIR light at a wavelength of 980 nm, this novel membrane enhances the production of reactive oxygen species (ROS). In addition to its ability to kill bacteria in PDT, the creation of ROS also activates loaded LA, leading to the formation of nitric oxide (No). This dynamic collaboration provides an innovative antibacterial effect through NO-assisted PDT. The employment of NIR light for irradiating nanoparticles holds potential for application in medical engineering biosensors, particularly for the detection and treatment of bacteria. The combination of nanotechnology and light-based therapies shows great potential for enhancing antibacterial interventions and biosensing technologies in the field of biomedical engineering. Current research efforts are focused on improving and broadening existing techniques, introducing innovative approaches to address bacterial resistance, and improving the functionality of biosensors in the field of biomedicine.
5.1.4 Nanocages for precise phototherapy using biosensing technology
In addressing bacterial biofilms, the utilization of tailored phototherapy technology is commonly employed. This approach is often paired with nanomaterials to induce microenvironmental regulation of the antimicrobial effects of drugs by photodynamic therapy. Extensive studies have been conducted in the past on metal nanoparticles, particularly gold and silver, because of their distinct local surface plasmon reflection (LSPR) characteristics. The utilization of gold and silver nanoparticles is credited to their remarkable durability, safety for biological systems, and adaptable capacity for modification, making them more helpful in various applications including highly sensitive detection, imaging, and diagnosis and therapy of bacterial infections. This technique involves the integration of abundant quantities of silver-loaded golden silver nanocages (GSNCs) with thiolate. The whole treatment utilizing the rapid release of silver from GSNCs and the thermal action of NIR radiation shows effectiveness in completely removing biofilms produced by bacteria that are resistant to several drugs in a controlled laboratory setting, as well as eradicating drug-resistant S. aureus in mice with the disease. This approach offers a hopeful pathway in the fight against stubborn multidrug-resistant bacterial illnesses [97].
Tan proposes a distinct method that utilizes red phosphorus and NIR irradiation to swiftly eliminate biofilms on phototube implants. This method makes use of the favorable biocompatibility and exceptionally effective photothermal properties of red phosphorus [98]. Continuing research is demonstrating the possibility of combining targeted phototherapy with nanomaterials to effectively tackle the challenges presented by bacterial biofilms and infections that are resistant to several drugs. These novel approaches signify crucial progress in the quest for efficacious remedies against resilient bacterial illnesses.
5.2 NBs enhanced with electrochemistry
There has been considerable interest in using bioelectric nanotechnology techniques to address resistance genes and biofilms of bacterial species. Specifically, researchers have focused on surface modification methods that disrupt the bio-electric balance within bacteria, both internally and externally [25,99]. Moreover, the inherent benefits of electrochemical sensors, which include affordability, little energy usage, lack of intricate automation, and micro-miniaturization, make them well suited for the quick and precise detection of bacteria. When combined with microfluidic technology and nanotechnology, this becomes especially important since it allows for rapid and extremely accurate identification of intricate bacterial samples directly at the location. Furthermore, the simplicity with which electrochemical detection can be scaled down and rendered portable increases its appropriateness for swift and economical identification of bacteria in areas with restricted medical facilities. The purpose of this flexibility is to avoid significant public health incidents caused by delays in detection, highlighting the essential contribution of bioelectric nanotechnology in improving accessible and prompt bacterial detection methods. Continual research efforts are being made to improve these methods, guaranteeing their effectiveness and availability for general use in various healthcare environments. While this section focuses on bacterial detection, Table 6 provides a summarized review of advancements in NBs for the detection of transmissible and nontransmissible diseases, highlighting the broader applications of this technology. Figure 5 illustrates the integrated biosensors technology for bacterial infection management.
Review on the advancements in NBs for the detection of nontransmissible diseases
| Various disorder | Mechanisms used for biosensors | Biomarkers of health | minimum detectable levels | Nanotechnologies and biological factors are incorporated | References |
|---|---|---|---|---|---|
| Diabetes | Amperometric | Glucose | 0.1 M | Glucose oxidase that has been fixed or attached to a material called rGO-Fe3O4 | [100] |
| Lung cancer | Electrochemical | Cancer miRNA-182 | 0.43 fM | The combination of MoS2 (molybdenum disulfide)/Ti3C2 nanohybrids and a modified glassy carbon electrode (GCE) | [101] |
| Second type diabetes | Fluorescence | Vaspin |
|
Upconverting nanoparticles | [102] |
| Jaundice | Voltammetric | Bilirubin |
|
Coating of reduced graphene oxide (rGO) and polystyrene sulfonate (PSS) on an electrode composed of glassy carbon | [103] |
| Alzheimer’s illness | Voltammetric analysis | Acetylcholine |
|
A gold electrode with a high level of porosity that has been modified with acetylcholinesterase | [104] |
| E. coli and Vibrio cholerae | Colorimetric assay | Bacterial receptor-binding protein | The detection limit is approximately 100 cells | AuNPs | [105] |
| Neoplasm of the breast | Electrochemical processes | HER-2 | Two cells in one milliliter | Functionalized graphene and nanostructured polyaniline (PANI) with attached AuNPs | [106] |
| Malaria | EIS stands for electrochemical impedance spectroscopy | pldh | 0.5 fm | A glassy carbon electrode is a type of electrode made from glassy carbon material | [107] |
Applications of integrated biosensors technology for bacterial infection management.
5.2.1 3D electrode scaffold design
It is observable that to understand disease mechanisms and drug responses, researchers have looked into the possibility of creating electrochemical cells that can identify bacteria with multidrug resistance. First, a glass carbon electrode is attached to a custom-made electrochemical cell. At the same time, as adding the soluble electron transfer agent phenazine methosulfate (PMS) to the growth medium, the bacterial culture is placed into the specified battery configuration. Electrons are generated during bacterial respiration, leading to a reduction in PMS and resulting in its oxidation on the surface of the electrode. This process allows the current to be recorded. The electrochemical antibiotic sensitivity test yielded consistent findings, accurately classifying bacteria as either antibiotic resistant or sensitive. This classification was accomplished not only within a 90 min timeframe of research on methods but also within a 150 min timeframe of blind testing. Future developments are expected to significantly reduce the detection time [108]. This study represents a significant achievement in the advancement and verification of electrochemical diagnostic methods for detecting the susceptibility of antibiotics. It allows for the rapid and precise classification of infections as either sensitive or resistant to antibiotics. The mechanism of action of three-dimensional electrode scaffolds involves their use in electrochemical coupling with intracellular metabolism and transforming extracellular redox. The three-dimensional electrode holder can host bacteria that are capable of producing a reference current density. The utilization of three-dimensional electrode scaffolds in solar-powered biochemical processes offers significant benefits. This adaptable platform can be utilized in the creation of environmentally friendly chemicals, forming a strong connection with the inherent physiological processes of bacteria, resembling a partially biological system. Because it contains a porous hydrophilic IO-ITO electrode structure, the three-dimensional electrode scaffold can easily integrate that produce electricity [109]. The incorporation allows for the real-time and ongoing tracking of bacterial infections, showing how this innovative method has the potential to improve our understanding and management of bacterial processes. Numerous three-dimensional electrode scaffolds are the subject of ongoing research due to their promising applications in green chemical processes and enhanced real-time bacterial infection monitoring.
5.2.2 Platforms for biosensing at a small scale
A novel microfluidic impedance biosensor has been created for bacterial isolation and detection. It integrates immunomagnetic nanoparticles for bacterial isolation, urease for bio-signal amplification, and microfluidic chips for electrochemical capacitance sensing. The aforementioned biosensor provides a cutting-edge platform for the rapid, uninterrupted, and responsive monitoring of E. coli O157:H7 in continuous flow systems. The sensor’s rate of impedance change was directly correlated with the frequencies of E. coli O157:H7, which were found in percentages ranging from
5.3 Acoustic signal-enhanced NBs
Sonodynamic therapy is a treatment method that is based on PDT. It primarily employs low-frequency ultrasound (LFU) to activate sensitizers and stimulate the production of reactive oxygen species (ROS). Sonodynamic treatment involves using ultrasonic sound stimulation to activate acoustic sensitizers, resulting in the production of reactive oxygen species. These ROS have strong lethal effects on certain multidrug-resistant microbes without the establishment of resistance [112,113]. Ultrasound, a noninvasive therapeutic technique, shows great potential for use in clinical settings. This is because it has the unique capacity to deeply penetrate tissues, even when faced with difficult obstacles [114,115]. Currently, the precise mechanism of sonodynamic treatment remains unidentified. However, the prevalent viewpoint highlights ROS as the key agent responsible, independent of the specific mechanism involved. Antimicrobial sonodynamic therapy (SDT) is an advanced and revolutionary technology that has the ability to effectively combat bacterial infections. The current study in this area seeks to decipher the complexities of the sonodynamic treatment mechanism, leading to better knowledge and a wider range of applications in the field of antimicrobial therapies. The inherent nonresistance characteristic of an SDT, along with its nonintrusive nature and exceptional tissue penetration capability, establishes it as a highly promising approach in the ongoing fight against bacterial diseases.
5.3.1 Sonodynamic nanozyme system
The nanoplatform, known as Pd@Pt-T790, is named after the enzymatic creation of a Pd@Pt nanoplate that is intimately linked to tetra-(4-carboxyphenyl) porphyrin, which acts as an organic acoustic sensitizer. Specifically, the conversion of the substance is carefully regulated during ultrasonic activation, which enables the efficient production of both catalytic oxygen and reactive oxygen species through the use of the acoustic sensitizer. The ongoing buildup of reactive species reduces obstacles associated with a lack of oxygen, ultimately improving the effectiveness of SDT. The ultrasound-switchable nanozyme system is notable for its active, adjustable, and precise features, as well as its enhanced acoustic properties. This technology effectively eliminates deeply entrenched bacterial infections [116].
The Pd@Pt-T790-based SDT nanosystems effectively employ ultrasound-switchable enzyme activity, together with substantial accumulation at the infection site and exceptional biological compatibility, to eradicate myositis caused by MRSA. Furthermore, they enable the unobtrusive monitoring of the progress of sonodynamic therapy by the utilization of both photoacoustic scanning and MRI [117]. The ultrasound switchable nano-enzyme system reported in this study presents a highly promising method to actively, precisely, and selectively boost the efficacy of sonodynamic therapy for eradicating bacterial infections situated in deep anatomical regions. Continuing research seeks to enhance and broaden the possible uses of this groundbreaking nanozyme technology in the field of therapeutic interventions.
5.3.2 Ultrasonic sterilization at low frequencies
Sonodynamic technology employs ultrasonography for stimulation allowing for deeper penetration into physiological tissues compared to the photodynamic technique. This can help overcome the limits typically associated with PD therapy. Acoustic power utilizes LFU, namely, in the range of 20 kHz to 3 MHz, to efficiently treat deep-seated lesions within the body. This technology has a maximum depth of tissue penetration of 10 cm. A LFU is defined as sound waves with frequencies ranging from 20 kHz to 1 MHz [118]. Further research on ultrasound capabilities has revealed that LFU exhibits robust penetration, precise targeting, and substantial efficacy, enabling the eradication of bacterial biofilms and the successful elimination of germs.
The established research research demonstrates that the use of LFU at specific parameters (40 kHz, 600 mW/cm2, 30 min, duty cycle 1:9) leads to a noteworthy reduction in the population of bacteria within biofilms, whether used alone or in conjunction with a single agent. This significantly improves the effectiveness of the treatment in killing microorganisms. Significantly, when colistin was used in conjunction with larger concentrations, the combination treatments exhibited a more pronounced ultrasound-enhanced antimicrobial impact. The time-kill curves conducted over 24 h showed that the combination of colistin (8 mg/mL) and vancomycin (4 mg/mL) with LFU. After 8 h, there was a substantial decrease in the total bacteria count in biofilms over time The decrease persisted and gradually diminished until reaching the 24 h point [119]. The results emphasize the capability of LFU to enhance antibacterial approaches, particularly in addressing bacterial biofilms and maximizing treatment results. The current study is exploring the various ways in which LFU can be used to improve therapeutic approaches.
5.3.3 UltraSonic activation of chemokinetic therapeutic response
Ultrasound-activated chemokinetic therapy (SCDT) is a method for using ultrasound to drive therapeutic interventions. SCDT possesses remarkable features that enable noninvasive operations and deep tissue penetration, making it a highly successful approach to combat bacterial resistance and diseases. This innovative therapy utilizes the catalytic production of superoxide anions, along with the harmful hydroxyl radicals [120].
The SCDT open platform was developed by incorporating Fe3+ over polyethylenimide-modified bismuth oxybromide (BiOBr) nanoplates. Simultaneously, ultrasonic catalysis efficiently segregated the positive holes (h+) and the minus electrons (
5.4 Acoustic-responsive nanostructure for bacterial capture
An innovative approach that combines antibacterial acoustic-dynamic treatment with antivirulent immunotherapy, drawing inspiration from biological processes. A experiment was conducted to modify an antibody that specifically targets the alpha-toxin of MRSA, enabling it to attach to the outer surface of cell membrane nanovesicles. These nanovesicles were then enclosed with a substance that is responsive to sound. This novel approach goes beyond traditional passive absorption of virulence by utilizing natural red blood cell (RBC) membranes [114]. It takes advantage of the strong antiboil-toxin interaction to enhance the efficiency of nanovesicles in capturing virulence in laboratory conditions.
When ultrasound is used, acoustic sensitizers are crucial in producing reactive oxygen species, which effectively eradicate microorganisms and accelerate the removal of harmful effects. The efficacy of our antibody-based nanotrap in detecting MRSA infections and distinguishing between lesions and sterility has been validated by in vivo optical imaging. This novel combination of antimicrobial sonodynamic therapy and antivirulent immunotherapy provides a potent and antibiotic-free nanotherapeutic approach to combat multidrug-resistant bacterial infections, thereby paving the way for cutting-edge medical interventions. The generic visual overflow of the three main parts of biosensor is shown in Figure 6. Continuing research endeavors to enhance and broaden the uses of this combined treatment approach to have a more extensive and significant effect in clinical settings.
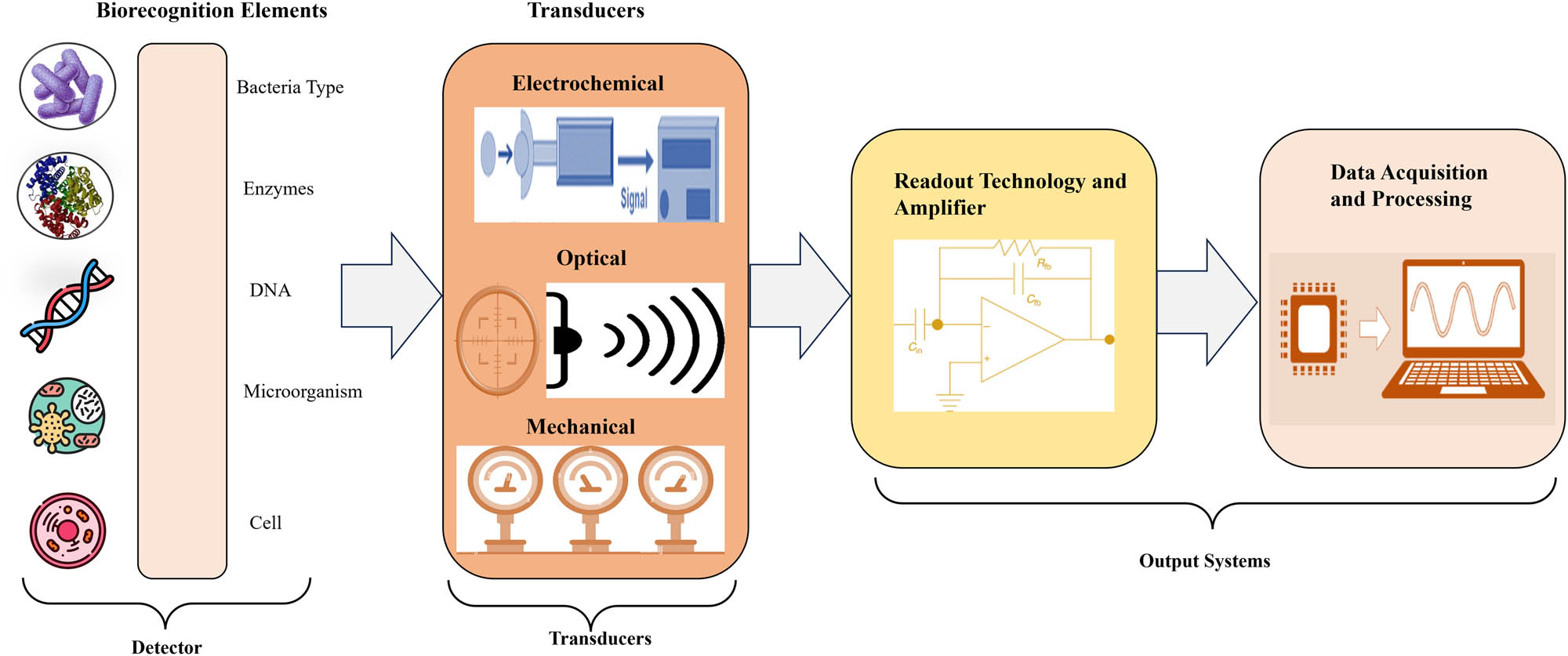
The three parts of a biosensor – the detector, the transducer, and the output system.
5.5 Nano-biotechnological sensors operating on electromagnetic forces
Magnetic nanoparticles are small, uniform particles with a size between 1 and 100 nm. Particles with these unusual properties exhibit features seen in other nanomaterials as well as those associated with quantum size and surface effects. Through the integration of superpara magnetization with the inherent magnetic conductivity of magnetic materials, and more especially magnetic nanoparticles, exhibit extraordinary characteristics. Their advantages also include a straightforward preparation process, high surface reactivity, and exceptional compatibility with living things. Conductive nanoparticles [122], magnetic iron oxide (Fe3O4) particles, for example, which are nanometer-sized, have lately garnered a lot of attention from scientists. The surface effects and superparamagnetism of magnetic Fe3O4 nanoparticles make them very interesting and have great potential for many uses in fields as diverse as biomedicine and sewage treatment. At room temperature, an external magnetic field improves the surface modification of Fe3O4 which allows it to incorporate distinct functionalities like adsorption and separation. In addition, Fe3O4 nanoparticles find widespread application in the realm of water and food safety monitoring. To aid in bacterial enrichment and separation in complex substrates, magnetic nanoparticles are frequently employed. In addition, they make it possible to directly test clinical specimens for microorganisms with low abundance. The distinctive properties of Fe3O4 nanoparticles make them stand out among the other magnetic nanophase materials. Their magnetic field reactivity and surface modifiability make them very flexible tools. Their adaptability and practicality in various scientific fields are evident from this [123,124].
5.5.1 Microscale motor governed by magnetism
An innovative tool for combating bacterial biofilms and illnesses, this micromotor is both magnetically controlled and extremely adaptable. The micromotor employs hydrogen peroxide (H2O2) as a fuel and manganese oxide (MnO2) as a catalyst to self-propel using oxygen microbubbles generated by the H2O2. The distinctive propulsion method enables the micromotor to infiltrate the EPSs of biofilms, resulting in their total eradication with the aid of bubbles. The process reaches its climax with the eradication of vulnerable bacteria, which succumb to the extremely poisonous (OH) generated. The focused strategy has exceptional efficacy, successfully eliminating microbial infections in a brief period of around 10 min. The micromotor holds promising implications in clinical medicine, particularly in tackling complicated infection sites on a large scale [125]. The micro/nano hybrid multifunctional motor demonstrates remarkable precision, manipulation, and profound infiltration, leading to improved antibacterial efficacy and potent effectiveness against challenging infections caused by biofilm.
5.5.2 Attractive cantilever structures
The attractive cantilever, which is essential for its operation, needs to be attached to a substrate that can be controlled from a distance. Based on the laws of electromagnetism, the magnetic cantilever experiences a self-driven and periodic vertical deflection from its original position when it is subjected to an alternating magnetic field. It is noteworthy that the frequency of this deflection is extremely modest, usually approximately 0.16 Hz. The deflection and periodic motion of the magnetic cantilever beam have demonstrated remarkable efficacy in preventing the adherence of bacterial biofilms and impeding their subsequent growth. The researchers’ liquid culture experiments with E. coli yielded empirical evidence showing a substantial decrease, reaching 70%, in the formation of microbe biofilms. The decrease in bacterial bio-film development is credited to the efficient magneto-mechanical driving of micro-structure construction [126].
5.5.3 Nano-magnetic bacterial navigators
The magnetotactic bacterium is a distinct category of bacteria that can align themselves with a magnetic field as a result of their magneto-sensitivity. The electron microscopy analysis demonstrates the existence of magnetite magnetosomes, a magnetic material, within these magnetotactic bacteria [127] developed a pioneering method to isolate S. aureus by altering the surface of magnetotactic bacteria MO-1 cells using a rabbit anti-MO-1 polyclonal antibody. This study is the initial evidence of bacterial microrobots that are capable of transporting infections, namely S. aureus, to a specified place by using a magnetic field. The utilization of magnetotactic bacteria in the creation of magnetic-guided, self-propelled microrobots for pathogen isolation highlights their significant potential. This achievement establishes a strong basis for future efforts in the detection of pathogenic bacteria. Further experiments were conducted to evaluate the deadly effects of S. aureus using MO-1. The utilization of both alternating electromagnetic-field erythema and the mechanical force generated by an oscillating magnetic field exhibited a substantial bactericidal effect in animal experiments. highlighting the versatility and effectiveness of this technique [128,129]. This research not only enhances our comprehension of magnetotactic bacteria but also creates opportunities for novel applications in pathogen manipulation and detection.
5.5.4 Nano-magnetic alloy fluid particles
A research team conducted investigation on the application of magneto-responsive gallium-based liquid metal (LM) droplets as antimicrobial agents to assess their ability to kill germs. The nanometer-sized droplets exhibited the capacity to physically harm, dissolve, and eradicate bacteria present in fully developed biofilms. When subjected to a weak magnetic field, the droplets experienced a change in shape, resulting in the creation of nanoscale sharp edges. Upon encountering bacterial biofilms, these droplets, with their motion and jagged contours, effectively perturbed the biofilm structure, resulting in the complete annihilation of bacterial cells. The technique’s effectiveness against two forms of bacterial biofilms, both Gram-positive and Gram-negative, was assessed in a recent study. After being exposed to the liquid metal nanoparticles for 90 min, both biofilms were completely eradicated, leading to a significant decrease of 99% in bacterial survival. Importantly, laboratory experiments verified that these droplets, which can kill bacteria, did not cause any harmful effects on human cells [130,131]. This novel methodology exhibits potential for creating antibacterial remedies that target specific bacteria while minimizing harm to human cells, demonstrating its versatility for various applications in biomedical research and healthcare.
5.6 Nano-biosensing with photothermal resonance
Photothermal therapy (PTT) is a highly regarded and extensively studied method for identifying and treating bacterial infections. It is noninvasive and has the ability to selectively target the disease. PTT, utilizes physical mechanisms along with chemical approaches [132,133]. Microorganisms, as living organisms with complex cellular structures, experience protein denaturation and subsequent death when exposed to high temperatures. The sterilizing process based on this idea is widely known as heat sterilization technology. This innovative approach has significant promise for revolutionizing bacterial diagnostic and treatment protocols, marking a pivotal advancement in the realm of medical technologies.
5.6.1 NBs exploiting photothermal effects
Many researchers demonstrated fast and comprehensive methods for killing germs using MXene and light, known as photothermal ablation. This refers to a rapid, thorough, and multilevel approach to using light-induced heat to destroy microorganisms. Ti3C2 MXenes had a highly effective antibacterial activity against 15 diverse bacterial strains when subjected to 808 nm light for only 20 min. The potential of this revolutionary photothermal technique is immense. In addition, the rapid antibacterial approach demonstrates efficacy against MRSA biofilms by disrupting their structures and eradicating bacteria present within the biofilms [134]. This innovative research not only broadens the possible uses of photothermography but also provides a method to completely eliminate bacteria and biofilms without promoting medication resistance, representing notable progress in antibacterial technologies.
5.6.2 Interwoven sheath
Devised a method to alter the surface of a substance with the aim of improving its ability to combat microorganisms and facilitate several functionalities. The surface of functionalized polyurethane (PU), a frequently used biomedical material for hernia recovery, was modified (PU-Au-PEG) to provide intrinsic properties that prevent fouling and can kill bacteria through photothermal means. The change was made possible by applying a coating that reacts to near-infrared (NIR) light and contains both organic and inorganic components. The coating is made up of polyethylene glycol and gold nanorods (Au NRs). When subjected to 808 nm NIR irradiation, the PU-Au-PEG material showed remarkable bactericidal properties, especially against MDR bacteria, and was highly effective in preventing bacterial attachment. In addition, PU-Au-PEG showed the ability to inhibit biofilm formation for an extended period of time. By conducting cytotoxicity and hemolysis assays, the biocompatibility of PU-Au-PEG was confirmed, demonstrating its potential as a versatile and biocompatible alternative for antibacterial uses [135,136].
5.6.3 Bacterial-selective photothermal carbon nanoparticles
A bacterial affinity is the propensity of bacteria to attach themselves to a particular surface or substance. Photothermal carbon dots (BAPTCDs) are specifically engineered to target MurD ligase, an enzyme that plays a crucial role in the manufacture of peptidoglycan (PG) in bacteria. These carbon dots demonstrate outstanding performance by effectively and accurately detecting bacterial organisms with enhanced specificity and sensitivity. BAPTCDs exhibit a unique chiral arrangement and display a particular attraction to bacteria. The enzyme’s biological activity is greatly diminished by the competitive interaction with D-glutamate and simultaneous attachment to MurD ligase. This dual mechanism hinders the production of bacterial cell walls, hence enhancing the effectiveness of therapy with lasers for microorganisms. The synergistic impact of these mechanisms enhances the remarkable antibacterial effectiveness of the photothermal carbon dots, especially when directed towards specific bacteria [137,138].
5.6.4 Smart hybrid hydrogel composites
Wang created a complex hydrogel that can both visually detect bacterial infections and provide PTT. The use of a conjugated polymer (PTDBD) that absorbs NIR light- and pH-sensitive bromothymol blue (BTB) is the major way that bacteria are killed using thermotherapy. A chitosan (CS) thermosensitive hydrogel incorporates these components. Biofilms and infected wounds can be more easily detected with the help of the BTB/PTDBD/CS hydrogel, which can visually detect the acidic microenvironment of S. aureus by going through noticeable color shifts. Upon rapid diagnosis, the hydrogel precisely targets the affected area by generating localized high temperatures in response to near-infrared laser (808 nm) light. This method efficiently eliminates even the most resistant biofilms that are persistent and hard to remove [139].
5.7 Bacterial disease theranostics facilitated by nano-sensors assembled in situ
One new way to image and identify cancer cells, tissues, and microbes is with in situ synthesis technology. Incorporating metal elements chemically into live organisms to form functional nanocluster probes accomplishes this [140]. Among these methods is fluorescence in situ hybridization (FISH), a new tool for determining the spatial distribution of microbes in biofilms and quantifying microbial communities. There is no need to extract nucleic acids while using FISH, which differs from previous approaches. To conduct in situ hybridization, it substitutes fluorescent oligonucleotide probe labeling. Multiple populations can be identified at once by marking the probes with fluorescent dyes with distinct excitation and emission spectra [141]. Using in situ synthesis extends beyond enhancing antimicrobial permeability in biofilms and has wider implications. It may cause biochemical or physical processes, like photothermal conversion, that compromise biofilm integrity. Chemically linking nanoclusters of gold with the antibacterial peptide daptomycin demonstrated the production of a very efficient antibacterial molecule. The synthesized conjugated structure significantly boosts the synergistic impact by fusing the two parts’ best qualities. The structure effectively eliminates bacterial biofilms by creating membrane holes through localized daptomycin action [142,143]. By using in situ chemical synthesis techniques, the authors created bioresponsive nanocomposite materials for disinfection and bacterial bioimaging. The novel biofilm microenvironment was used to construct a nanoheating platform with multiple applications. The nanoclusters, which were sensitive to the microenvironment, were able to detect bacteria and promote sterilization through electrostatic interactions, breakdown of the cell membrane, suppression of biofilm formation, and accumulation of reactive oxygen species (ROS) [144]. The particular interactions with bacteria emphasize the promise of in situ synthesis as a versatile instrument for accurate detection and treatment of bacterial infections [145].
6 Discussion and future directions
The increasing danger presented by bacterial infections, resistance to therapy, and the creation of biofilms requires the immediate development of novel treatment solutions. The significance of this progress is underscored by the growing occurrence of antibiotic-resistant bacterial strains and the creation of enduring biofilms, which complicate therapy and contribute to protracted illnesses. Conventional antimicrobial medications and diagnostic technologies, while necessary, frequently have limitations in terms of their sluggish procedures and unintentional promotion of antibiotic resistance. Improper use of these compounds may exacerbate the challenge of managing biofilms and also facilitate the establishment of resistance. Traditional techniques for identifying bacteria and managing infections with antibiotics need significant resources, in both terms of finances and personnel. These approaches usually need substantial time and resources, resulting in treatment delays and higher healthcare expenses. The dependence on conventional antibiotics further worsens the issue of resistance, as organisms adapt to tolerate these treatments, making them progressively less efficient.
However, recent breakthroughs in nanotechnology, including the creation of intelligent NBs, provide encouraging options. These state-of-the-art biosensors provide increased sensitivity, specificity, and effectiveness in the detection and treatment of germs. These sensors have the ability to identify bacterial infections at very low concentrations, making them far more efficient than traditional approaches. Currently, there are ongoing clinical evaluations of many innovative methods that include NBs. These evaluations have shown enhanced sensitivity, high specificity, and efficacy in both monitoring and therapy. This work also emphasizes the substantial potential of combining smart NBs with AI and nanotechnology to transform bacterial infection management. By using methods such as PDT, electrochemical sensors, acoustic dynamics, electromagnetic forces, photothermal responses, and surface plasmon resonance, these biosensors have the capability to provide individualized and accurate therapies. The use of modern methods allows for the creation of individualized treatment plans using nanotechnology, which are specifically designed for individual patients. This improves the accuracy of diagnosis and the efficiency of therapy.
Through a comprehensive examination of these cutting-edge technologies, we highlight the increasing danger of antibiotic resistance and its effect on healthcare expenses, emphasizing the need for proactive actions to tackle these difficulties. The article provides a comprehensive analysis of bacterial resistance to antibiotics and its impact on illness management, treatment, and overall results. It highlights the need for novel methodologies to precisely detect and treat bacterial infections. We emphasizes the need to promote interdisciplinary cooperation and undertake translational research, while also investigating the possibilities offered by advanced technologies like smart NBs and AI. The use of a collaborative and interdisciplinary approach is crucial in the development of efficient ways to address bacterial infections and antibiotic resistance.
To ensure the practical and widespread application of smart NBs, particularly in local and community settings, future research and development should focus on several key areas:
Develop AI and ML algorithms customized to the particular bacterial diseases patterns that are common in local populations. Improving the accuracy and efficacy of NBs would enable a quick and precise analysis of intricate data for immediate monitoring and predictive modeling of bacterial infections.
Develop affordable and easy-to-use NBs specifically tailored for use in nearby clinics and by community health practitioners. These devices should possess multifunctionality, enabling them to concurrently diagnose, monitor, and cure bacterial infections. Their design should guarantee that only a little training is necessary, thereby enabling the provision of sophisticated care at the community level.
Carry out comprehensive clinical studies in nearby communities to evaluate the safety, effectiveness, and cost-efficiency of NB technologies in real-life environments. Engaging in partnerships with local healthcare practitioners and community groups will guarantee that the results are relevant and can be implemented, therefore promoting the incorporation of new technologies into regular clinical procedures.
Tackle the obstacles associated with the large-scale manufacturing and dissemination of NBs to guarantee their affordability and broad accessibility, especially in distant and underserved regions. Establishing resilient distribution networks will be essential to aid this process, guaranteeing that the advantages of these advances are accessible to all communities.
Utilize nanomaterials to optimize medication delivery systems, ensuring precision, efficacy, and customization of therapies. This focused strategy strives to optimize therapeutic results while avoiding adverse effects, guaranteeing that therapies are both secure and efficacious for a wide range of patient demographics.
Evaluate the environmental impact of nanomaterials used in biosensors and address ethical considerations, particularly regarding data privacy and patient consent, within local communities. Ensuring that these technologies are developed and deployed responsibly will help mitigate potential negative impacts and build public trust.
Incorporate NB technology with community-based public health methods to actively monitor and manage bacterial outbreaks in realtime. Inform community members on the advantages and correct use of these technologies to promote acceptance and optimize their efficiency. Providing communities with information and tools will improve their capacity to proactively control bacterial illnesses.
7 Conclusion
The urgency of developing effective treatment strategies is highlighted by the threat posed by bacterial infections, medication resistance, and the formation of bacterial biofilms. Although traditional antimicrobial agents are frequently used, their incorrect usage can worsen the difficulty of controlling biofilms and contribute to the spread of resistance. Traditional methods for detecting microorganisms and treating infections with antibiotics require substantial resources, including financial and human resources. Nevertheless, recent progress in nanotechnology provides encouraging opportunities for addressing multidrug-resistant bacteria and eliminating antimicrobial biofilms. These novel methods demonstrate increased sensitivity, strong specificity, and effectiveness in monitoring and treatment, with several currently undergoing clinical evaluation. This study presents an in-depth examination of bacterial resistance to antibiotics and its effects on the management and treatment of diseases and their ultimate outcomes. By thoroughly analyzing the tools and state-of-the-art technologies accurately diagnose and manage infections caused by bacteria. The study emphasizes the increasing risk of antibiotic resistance and its effect on costs associated with healthcare, underscoring the need to take action to tackle highlighted limitations. In addition, we explore the potential of modern technologies such as smart NBs and AI to transform the management of bacterial infections. Our objective is to highlight the reliability of diagnosis and the developed nano-based personalized treatment strategies for individual patients by incorporating advanced techniques such as PDT, electrochemical sensors, acoustic dynamics, electromagnetic forces, photothermal responses, and surface plasmon resonance. By fostering collaboration across disciplines and conducting translational research, we technically analyze the techniques to control bacterial diseases and protect public health in the future.
-
Funding information: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2021R1I1A1A01055652).
-
Author contributions: Habib Khan: conceptualization, AI studies analysis, design, and writing original draft. Zahoor Jan: literature search, data collection, visualization, and analysis. Inam Ullah: visualization, synthesis and analysis. Abdullah Alwabli: investigation, literature search, and reviewing. Faisal Alharbi: Critical review, analysis, and design. Shabana Habib: investigation, formal analysis, and reviewing. Muhammad Islam: data validation, reviewing, and visualization. Byung-Joo Shin: writing, funding, and review. Mi Young Lee: study design, Conceptualization, and visualization. JaKeoung Koo: reviewing, editing, and supervision. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Cai P, Jia T, Zhang L, Jiang Y, Wang S, Liu G, et al. Multifunctional antibacterial materials for inhibiting bacterial surface growth and triggering release of bacterial resistance genes. Nature Commun. 2018;9:4865. https://www.nature.com/articles/s41467-018-07226-7. Search in Google Scholar
[2] Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathogens Global Health. 2015;109:309–18. 10.1179/2047773215Y.0000000030Search in Google Scholar PubMed PubMed Central
[3] Ventola CL. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm Ther. 2015;40:277. Search in Google Scholar
[4] Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed. 2017;12:1227. https://doi.org/10.2147/IJN.S121956. Search in Google Scholar PubMed PubMed Central
[5] Lee NY, Koooo WC, Hsueh PR. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front Pharmacol. 2019;10:1153. https://www.frontiersin.org/articles/10.3389/fphar.2019.01153. Search in Google Scholar
[6] Mubeen B, Ansar AN, Rasool R, Ullah I, Imam SS, Alshehri S, et al. Nanotechnology as a novel approach in combating microbes providing an alternative to antibiotics. Antibiotics. 2021;10:1473. https://www.mdpi.com/2079-6382/10/12/1473. 10.3390/antibiotics10121473Search in Google Scholar PubMed PubMed Central
[7] Singh A, Amod A, Pandey P, Bose P, Pingali MS, Shivalkar S, et al. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed Materials. 2022;17(2):022003. 10.1088/1748-605X/ac50f6Search in Google Scholar PubMed
[8] Darwish MA, Abd-Elaziem W, Elsheikh A, Zayed AA. Advancements in nanomaterials for nanosensors: a comprehensive review. Nanoscale Adv. 2024. 10.1039/D4NA00214HSearch in Google Scholar PubMed PubMed Central
[9] Ahangari A, Mahmoodi P, Mohammadzadeh A. Advanced nano biosensors for rapid detection of zoonotic bacteria. Biotechnol Bioeng. 2023;120(1):41–56. 10.1002/bit.28266Search in Google Scholar PubMed
[10] Kang H, Lee J, Moon J, Lee T, Kim J, Jeong Y, et al. Multiplex detection of foodborne pathogens using 3D nanostructure swab and deep learning-based classification of Raman spectra. Small. 2024:2308317. 10.1002/smll.202308317Search in Google Scholar PubMed
[11] Chauhan N, Saxena K, Tikadar M, Jain U. Recent advances in the design of biosensors based on novel nanomaterials: An insight. Nanotechnol Precision Eng (NPE). 2021;4(4):045003. 10.1063/10.0006524Search in Google Scholar
[12] De La Fuente-Núñez C, Cardoso MH, de Souza Cândido E, Franco OL, Hancock RE. Synthetic antibiofilm peptides. Biochim Biophys Acta, Biomembr. 2016;1858(5):1061–9. 10.1016/j.bbamem.2015.12.015Search in Google Scholar PubMed PubMed Central
[13] Rudilla H, Fusté E, Cajal Y, Rabanal F, Vinuesa T, Viñas M. Synergistic antipseudomonal effects of synthetic peptide AMP38 and carbapenems. Molecules. 2016;21(9):1223. 10.3390/molecules21091223Search in Google Scholar PubMed PubMed Central
[14] Tse BN, Adalja AA, Houchens C, Larsen J, Inglesby TV, Hatchett R. Challenges and opportunities of nontraditional approaches to treating bacterial infections. Clin Infect Diseases. 2017;65(3):495–500. 10.1093/cid/cix320Search in Google Scholar PubMed PubMed Central
[15] Anandaram H. Role of bioinformatics in nanotechnology: An initiation towards personalized medicine. In: Data analytics in medicine: concepts, methodologies, tools, and applications. Hershey, Pennsylvania, United States: IGI Global; 2020. p. 1875–94. 10.4018/978-1-7998-1204-3.ch094Search in Google Scholar
[16] Bhambri P, Khang A. AI-integrated biosensors and bioelectronics for healthcare. AI-driven innovations in digital healthcare: emerging trends, challenges, and applications. Hershey, Pennsylvania, United States: IGI Global; 2024. p. 82–96. 10.4018/979-8-3693-3218-4.ch004Search in Google Scholar
[17] Ullah N, Hassan M, Khan JA, Anwar MS, Aurangzeb K. Enhancing explainability in brain tumor detection: A novel DeepEBTDNet model with LIME on MRI images. Int J Imag Syst Technol. 2024;34(1):e23012. 10.1002/ima.23012Search in Google Scholar
[18] Rasheed Z, Ma YK, Ullah I, Ghadi YY, Khan MZ, Khan MA, et al. Brain tumor classification from MRI using image enhancement and convolutional neural network techniques. Brain Sci. 2023;13(9):1320. 10.3390/brainsci13091320Search in Google Scholar PubMed PubMed Central
[19] Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452–60. 10.1111/cas.14377Search in Google Scholar PubMed PubMed Central
[20] Nasir N, Kansal A, Barneih F, Al-Shaltone O, Bonny T, Al-Shabi M, et al. Multi-modal image classification of COVID-19 cases using computed tomography and X-rays scans. Intelligent Syst Appl. 2023;17:200160. 10.1016/j.iswa.2022.200160Search in Google Scholar
[21] Chatterjee A, Somayaji NR, Kabakis IM. Abstract WMP16: artificial intelligence detection of cerebrovascular large vessel occlusion-nine month, 650 patient evaluation of the diagnostic accuracy and performance of the Viz. ai LVO algorithm. Stroke. 2019;50(Suppl_1):AWMP16–AWMP16. 10.1161/str.50.suppl_1.WMP16Search in Google Scholar
[22] Antony A. Flexible and wearable biosensors: revolutionizing health monitoring. in: Biosensors: developments, challenges and perspectives. Singapore: Springer; 2024. p. 237–58. 10.1007/978-981-97-3048-3_12Search in Google Scholar
[23] Dave S, Dave A, Radhakrishnan S, Das J, Dave S. Biosensors for healthcare: an artificial intelligence approach. Biosensors Emerging Re-Emerging Infect Diseases. 2022:365–83. 10.1016/B978-0-323-88464-8.00008-7Search in Google Scholar
[24] Deng J, Zhao S, Liu Y, Liu C, Sun J. Nanosensors for diagnosis of infectious diseases. ACS Appl Bio Materials. 2020;4(5):3863–79. 10.1021/acsabm.0c01247Search in Google Scholar PubMed
[25] Xia Q, Jiang H, Liu X, Yin L, Wang X. Advances in engineered nano-biosensors for bacteria diagnosis and multidrug resistance inhibition. Biosensors. 2024;14(2):59. 10.3390/bios14020059Search in Google Scholar PubMed PubMed Central
[26] Ghafouri P, Kasaei B, Aghili S, Monirvaghefi A, Hosseini AM, Amoozegar H, et al. Application of nanobiosensors in detection of pathogenic bacteria: an update. Res Biotechnol Environ Sci. 2023;2(4):65–74. 10.58803/rbes.v2i4.22Search in Google Scholar
[27] Markandan K, Tiong YW, Sankaran R, Subramanian S, Markandan UD, Chaudhary V, et al. Emergence of infectious diseases and role of advanced nanomaterials in point-of-care diagnostics: a review. Biotechnol Genetic Eng Rev. 2022:1–89. 10.1080/02648725.2022.2127070Search in Google Scholar PubMed
[28] Ramesh M, Janani R, Deepa C, Rajeshkumar L. Nanotechnology-enabled biosensors: A review of fundamentals, design principles, materials, and applications. Biosensors. 2022;13(1):40. 10.3390/bios13010040Search in Google Scholar PubMed PubMed Central
[29] Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Delivery Reviews. 2013;65(13–14):1803–15. 10.1016/j.addr.2013.07.011Search in Google Scholar PubMed
[30] Munir MU, Ahmad MM. Nanomaterials aiming to tackle antibiotic-resistant bacteria. Pharmaceutics. 2022;14(3):582. 10.3390/pharmaceutics14030582Search in Google Scholar PubMed PubMed Central
[31] Liu H, Liu Z, Wang Y, Xiao J, Liu X, Jiang H, et al. Intracellular liquid-liquid phase separation induces tunable anisotropic nanocrystal growth for multidimensional analysis. Adv Funct Materials. 2023;33:2302136. 10.1002/adfm.202302136Search in Google Scholar
[32] Rawson T, Hernandez B, Moore L, Blandy O, Herrero P, Gilchrist M, et al. Supervised machine learning for the prediction of infection on admission to hospital: a prospective observational cohort study. J Antimicrob Chemotherapy. 2019;74(4):1108–15. 10.1093/jac/dky514Search in Google Scholar PubMed
[33] Agbaria AH, Salman A, Beck G, Lapidot I, Rich DH, Kapelushnik J, et al. Potential of bacterial infection diagnosis using infrared spectroscopy of WBC and machine learning algorithms. In: European Conference on Biomedical Optics. Optica Publishing Group; 2019. p. 11073_40. 10.1117/12.2525248Search in Google Scholar
[34] Gadalla AA, Friberg IM, Kift-Morgan A, Zhang J, Eberl M, Topley N, et al. Identification of clinical and urine biomarkers for uncomplicated urinary tract infection using machine learning algorithms. Sci Rep. 2019;9(1):19694. 10.1038/s41598-019-55523-xSearch in Google Scholar PubMed PubMed Central
[35] Oh SW, Lee H, Shin JY, Lee JH. Antibiotics-resistant bacteria infection prediction based on deep learning. J Soc e-Business Stud. 2019;24(1):105–20. Search in Google Scholar
[36] Ho CS, Jean N, Hogan CA, Blackmon L, Jeffrey SS, Holodniy M, et al. Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nature Commun. 2019;10(1):4927. 10.1038/s41467-019-12898-9Search in Google Scholar PubMed PubMed Central
[37] Hattori S, Sekido R, Leong IW, Tsutsui M, Arima A, Tanaka M, et al. Machine learning-driven electronic identifications of single pathogenic bacteria. Sci Rep. 2020;10(1):15525. 10.1038/s41598-020-72508-3Search in Google Scholar PubMed PubMed Central
[38] Nehal SA, Roy D, Devi M, Srinivas T. Highly sensitive lab-on-chip with deep learning AI for detection of bacteria in water. International J Inform Technol. 2020;12:495–501. 10.1007/s41870-019-00363-1Search in Google Scholar
[39] Iriya R, Jing W, Syal K, Mo M, Chen C, Yu H, et al. Rapid antibiotic susceptibility testing based on bacterial motion patterns with long short-term memory neural networks. IEEE Sensors J. 2020;20(9):4940–50. 10.1109/JSEN.2020.2967058Search in Google Scholar PubMed PubMed Central
[40] Brown C, Tseng D, Larkin PM, Realegeno S, Mortimer L, Subramonian A, et al. Automated, cost-effective optical system for accelerated antimicrobial susceptibility testing (AST) using deep learning. ACS Photonics. 2020;7(9):2527–38. 10.1021/acsphotonics.0c00841Search in Google Scholar
[41] Draz MS, Vasan A, Muthupandian A, Kanakasabapathy MK, Thirumalaraju P, Sreeram A, et al. Virus detection using nanoparticles and deep neural network-enabled smartphone system. Sci Adv. 2020;6(51):eabd5354. 10.1126/sciadv.abd5354Search in Google Scholar PubMed PubMed Central
[42] Alafeef M, Moitra P, Pan D. Nano-enabled sensing approaches for pathogenic bacterial detection. Biosensors Bioelectronics. 2020;165:112276. 10.1016/j.bios.2020.112276Search in Google Scholar PubMed
[43] Zhang K, Wang J, Liu T, Luo Y, Loh XJ, Chen X. Machine learning-reinforced noninvasive biosensors for healthcare. Adv Healthcare Materials. 2021;10(17):2100734. 10.1002/adhm.202100734Search in Google Scholar PubMed
[44] Ding J, Lin Q, Zhang J, Young GM, Jiang C, Zhong Y, et al. Rapid identification of pathogens by using surface-enhanced Raman spectroscopy and multi-scale convolutional neural network. Analytic Bioanalytic Chem. 2021;413(14):3801–11. 10.1007/s00216-021-03332-5Search in Google Scholar PubMed
[45] Yu S, Li X, Lu W, Li H, Fu YV, Liu F. Analysis of Raman spectra by using deep learning methods in the identification of marine pathogens. Analytic Chem. 2021;93(32):11089–98. 10.1021/acs.analchem.1c00431Search in Google Scholar PubMed
[46] Li Z, Jiang Y, Tang S, Zou H, Wang W, Qi G, et al. 2D nanomaterial sensing array using machine learning for differential profiling of pathogenic microbial taxonomic identification. Microchim Acta. 2022;189(8):273. 10.1007/s00604-022-05368-5Search in Google Scholar PubMed PubMed Central
[47] Yang Y, Xu B, Murray J, Haverstick J, Chen X, Tripp RA, et al. Rapid and quantitative detection of respiratory viruses using surface-enhanced Raman spectroscopy and machine learning. Biosens Bioelectron. 2022;217:114721. 10.1016/j.bios.2022.114721Search in Google Scholar PubMed
[48] Arano-Martinez JA, Martínez-González CL, Salazar MI, Torres-Torres C. A framework for biosensors assisted by multiphoton effects and machine learning. Biosensors. 2022;12(9):710. 10.3390/bios12090710Search in Google Scholar PubMed PubMed Central
[49] Verma S, Shukla RP, Dutta G. Machine learning-enabled biosensors in clinical decision making. In: Next-generation nanobiosensor devices for point-of-care diagnostics. Singapore: Springer; 2022. p. 163–94. 10.1007/978-981-19-7130-3_7Search in Google Scholar
[50] Guo Z, Tian R, Xu W, Yip D, Radyk M, Santos FB, et al. Highly accurate heart failure classification using carbon nanotube thin film biosensors and machine learning assisted data analysis. Biosens Bioelectron X. 2022;12:100187. 10.1016/j.biosx.2022.100187Search in Google Scholar
[51] Zhou Z, Wang L, Wang J, Liu C, Xu T, Zhang X. Machine learning with neural networks to enhance selectivity of nonenzymatic electrochemical biosensors in multianalyte mixtures. ACS Appl Mater Interfaces. 2022;14(47):52684–90. 10.1021/acsami.2c17593Search in Google Scholar PubMed
[52] Noreldeen HA, Huang KY, Wu GW, Peng HP, Deng HH, Chen W. Deep learning-based sensor array: 3D fluorescence spectra of gold nanoclusters for qualitative and quantitative analysis of vitamin B6 derivatives. Analytic Chem. 2022;94(26):9287–96. 10.1021/acs.analchem.2c00655Search in Google Scholar PubMed
[53] Wang J, Xu B, Shi L, Zhu L, Wei X. Prospects and challenges of AI and neural network algorithms in MEMS microcantilever biosensors. Processes. 2022;10(8):1658. 10.3390/pr10081658Search in Google Scholar
[54] Kumar AK, Ritam M, Han L, Guo S, Chandra R. Deep learning for predicting respiratory rate from biosignals. Comput Biol Med. 2022;144:105338. 10.1016/j.compbiomed.2022.105338Search in Google Scholar PubMed
[55] Rahmani MKI, Ghanimi HM, Jilani SF, Aslam M, Alharbi M, Alroobaea R, et al. Early pathogen prediction in crops using nano biosensors and neural network-based feature extraction and classification. Big Data Res. 2023;34:100412. 10.1016/j.bdr.2023.100412Search in Google Scholar
[56] Ramalingam M, Jaisankar A, Cheng L, Krishnan S, Lan L, Hassan A, et al. Impact of nanotechnology on conventional and artificial intelligence-based biosensing strategies for the detection of viruses. Discover Nano. 2023;18(1):58. 10.1186/s11671-023-03842-4Search in Google Scholar PubMed PubMed Central
[57] Zhou Y, Zhao J, Chen R, Lu P, Zhao W, Ma R, et al. A portable deep-learning-assisted digital single-particle counting biosensing platform for amplification-free nucleic acid detection using a lens-free holography microscope. Nano Today. 2024;56:102238. 10.1016/j.nantod.2024.102238Search in Google Scholar
[58] Iqbal S, Qureshi AN, Aurangzeb K, Alhussein M, Haider SI, Rida I. AMIAC: adaptive medical image analyzes and classification, a robust self-learning framework. Neural Comput Appl. 2023:1–29. 10.1007/s00521-023-09209-1Search in Google Scholar
[59] Wang Y, Wang Z, Shang Y, Wang J, Zhu Z, Xi L, et al. Next-generation pathogen detection: Exploring the power of nucleic acid amplification-free biosensors. Coordination Chem Rev. 2024;513:215895. 10.1016/j.ccr.2024.215895Search in Google Scholar
[60] Yi Y, Lagniton PNP, Ye S, Li E, Xu RH. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int J Biologic Sci. 2020;16:1753–66. 10.7150/ijbs.45134Search in Google Scholar PubMed PubMed Central
[61] Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatrics. 2020;87:281–6. 10.1007/s12098-020-03263-6Search in Google Scholar PubMed PubMed Central
[62] Deee la Rica R, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nature Nanotechnol. 2012;7:821–4. 10.1038/nnano.2012.186Search in Google Scholar PubMed
[63] Leland DS, Ginocchio CC. Role of cell culture for virus detection in the age of technology. Clin Microbiol Rev. 2007;20:49–78. 10.1128/CMR.00002-06Search in Google Scholar PubMed PubMed Central
[64] Killian ML. Hemagglutination assay for the avian influenza virus. In: Spackman E, editor. Avian Influenza Virus. Totowa, New Jersey, United States: Humana; 2008. p. 47–52. 10.1007/978-1-59745-279-3_7Search in Google Scholar PubMed
[65] Lequin RM. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem. 2005;51(12):2415–8. 10.1373/clinchem.2005.051532Search in Google Scholar PubMed
[66] Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VY, et al. Diagnosing COVID-19: the disease and tools for detection. ACS nano. 2020;14(4):3822–35. 10.1021/acsnano.0c02624Search in Google Scholar PubMed
[67] Payungporn S, Chutinimitkul S, Chaisingh A, Damrongwantanapokin S, Buranathai C, Amonsin A, et al. Single step multiplex real-time RT-PCR for H5N1 influenza A virus detection. J Virological Methods. 2006;131(2):143–7. 10.1016/j.jviromet.2005.08.004Search in Google Scholar PubMed
[68] Munch M, Nielsen LP, Handberg K, Jørgensen PH. Detection and subtyping (H5 and H7) of avian type A influenza virus by reverse transcription-PCR and PCR-ELISA. Arch Virol. 2001;146:87–97. 10.1007/s007050170193Search in Google Scholar PubMed
[69] Hematian A, Sadeghifard N, Mohebi R, Taherikalani M, Nasrolahi A, Amraei M, et al. Traditional and modern cell culture in virus diagnosis. Osong Public Health Res Perspectives. 2016;7(2):77–82. 10.1016/j.phrp.2015.11.011Search in Google Scholar PubMed PubMed Central
[70] Bishop R, Davidson G, Holmes I, Ruck B. Detection of a new virus by electron microscopy of faecal extracts from children with acute gastroenteritis. Lancet. 1974;303(7849):149–51. 10.1016/S0140-6736(74)92440-4Search in Google Scholar PubMed
[71] Xing Y, Mo P, Xiao Y, Zhao O, Zhang Y, Wang F. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Eurosurveillance. 2020;25(10):2000191. 10.2807/1560-7917.ES.2020.25.10.2000191Search in Google Scholar PubMed PubMed Central
[72] Sahu T, Ratre YK, Chauhan S, Bhaskar L, Nair MP, Verma HK. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J Drug Delivery Sci Technol. 2021;63:102487. 10.1016/j.jddst.2021.102487Search in Google Scholar
[73] Krishnan Y, Seeman NC. Introduction: nucleic acid nanotechnology. Washington DC, United States: ACS Publications; 2019. 10.1021/acs.chemrev.9b00181Search in Google Scholar PubMed
[74] Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 2012;6(5):4279–87. 10.1021/nn3008383Search in Google Scholar PubMed PubMed Central
[75] Lambadi PR, Sharma TK, Kumar P, Vasnani P, Thalluri SM, Bisht N, et al. Facile biofunctionalization of silver nanoparticles for enhanced antibacterial properties, endotoxin removal, and biofilm control. Int J Nanomed. 2015:2155–71. 10.2147/IJN.S72923Search in Google Scholar PubMed PubMed Central
[76] d’Angelo I, Casciaro B, Miro A, Quaglia F, Mangoni ML, Ungaro F. Overcoming barriers in Pseudomonas aeruginosa lung infections: engineered nanoparticles for local delivery of a cationic antimicrobial peptide. Colloids Surf B Biointerfaces. 2015;135:717–25. 10.1016/j.colsurfb.2015.08.027Search in Google Scholar PubMed
[77] Silva NC, Silva S, Sarmento B, Pintado M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Delivery. 2015;22(7):885–93. 10.3109/10717544.2013.858195Search in Google Scholar PubMed PubMed Central
[78] Kumar GV, Su CH, Velusamy P. Ciprofloxacin loaded genipin cross-linked chitosan/heparin nanoparticles for drug delivery application. Mater Lett. 2016;180:119–22. 10.1016/j.matlet.2016.05.108Search in Google Scholar
[79] Casciaro B, Moros M, Rivera-Fernandez S, Bellelli A, de la Fuente JM, Mangoni ML. Gold-nanoparticles coated with the antimicrobial peptide esculentin-1a (1-21) NH2 as a reliable strategy for antipseudomonal drugs. Acta Biomater. 2017;47:170–81. 10.1016/j.actbio.2016.09.041Search in Google Scholar PubMed
[80] Kooti M, Sedeh AN, Motamedi H, Rezatofighi SE. Magnetic graphene oxide inlaid with silver nanoparticles as antibacterial and drug delivery composite. Appl Microbiol Biotechnol. 2018;102:3607–21. 10.1007/s00253-018-8880-1Search in Google Scholar PubMed
[81] Rani S, Gothwal A, Pandey PK, Chauhan DS, Pachouri PK, Gupta UD, et al. HPMA-PLGA based nanoparticles for effective in vitro delivery of rifampicin. Pharmaceutic Res. 2019;36:1–12. 10.1007/s11095-018-2543-xSearch in Google Scholar PubMed
[82] Ullah K, Khan SA, Mannan A, Khan R, Murtaza G, Yameen MA. Enhancing the antibacterial activity of erythromycin with titanium dioxide nanoparticles against MRSA. Current Pharmaceutic Biotechnol. 2020;21(10):948–54. 10.2174/1389201021666200128124142Search in Google Scholar PubMed
[83] Feynman R. There is plenty of room at the bottom. In: Feynman and computation. Boca Raton, Florida, United States: CRC Press; 2018. p. 63–76. 10.1201/9780429500459-7Search in Google Scholar
[84] Al-Shuj’a O, Obeid A, El-Shekeil Y, Hashim M, Al-Washali Z, et al. New strategy for chemically attachment of imine group on multi-walled carbon nanotubes surfaces: synthesis, characterization and study of DC electrical conductivity. J Materials Sci Chem Eng. 2017;5(02):11. 10.4236/msce.2017.52002Search in Google Scholar
[85] Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2019;25(1):112. 10.3390/molecules25010112Search in Google Scholar PubMed PubMed Central
[86] Thostenson ET, Ren Z, Chou TW. Advances in the science and technology of carbon nanotubes and their composites: a review. Composites Sci Technol. 2001;61(13):1899–912. 10.1016/S0266-3538(01)00094-XSearch in Google Scholar
[87] Wang H, Agarwal P, Jiang B, Stewart S, Liu X, Liang Y, et al. Bioinspired one cell culture isolates highly tumorigenic and metastatic cancer stem cells capable of multilineage differentiation. Adv Sci. 2020;7(11):2000259. 10.1002/advs.202000259Search in Google Scholar PubMed PubMed Central
[88] Duuuuu G, Moulin E, Jouault N, Buhler E, Giuseppone N. Muscle-like supramolecular polymers: integrated motion from thousands of molecular machines. Angew Chem. 2012;124(50):12672–6. 10.1002/ange.201206571Search in Google Scholar
[89] Campbell EK, Holz M, Gerlich D, Maier JP. Laboratory confirmation of C60+ as the carrier of two diffuse interstellar bands. Nature. 2015;523(7560):322–3. 10.1038/nature14566Search in Google Scholar PubMed
[90] Song T, Cai X, Tu MWY, Zhang X, Huang B, Wilson NP, et al. Giant tunneling magnetoresistance in spin-filter van der Waals heterostructures. Science. 2018;360(6394):1214–8. 10.1126/science.aar4851Search in Google Scholar PubMed
[91] Larue L, Myrzakhmetov B, Ben-Mihoub A, Moussaron A, Thomas N, Arnoux P, et al. Fighting hypoxia to improve PDT. Pharmaceuticals. 2019;12(4):163. 10.3390/ph12040163Search in Google Scholar PubMed PubMed Central
[92] Liu H, Guo Z, Mo L, Sun Y, Zhang J, Liu X, et al. Quantitative label-free optical technique to analyze the ultrastructure changes and spatiotemporal relationship of enamel induced by Msx2 deletion. J Biophotonics. 2021;14(10):e202100165. 10.1002/jbio.202100165Search in Google Scholar PubMed
[93] Guo Z, Chen Y, Wang Y, Jiang H, Wang X. Advances and challenges in metallic nanomaterial synthesis and antibacterial applications. J Materials Chem B. 2020;8(22):4764–77. 10.1039/D0TB00099JSearch in Google Scholar PubMed
[94] Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kedzierska E, Knap-Czop K, et al. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–107. 10.1016/j.biopha.2018.07.049Search in Google Scholar PubMed
[95] Xiu W, Wan L, Yang K, Li X, Yuwen L, Dong H, et al. Potentiating hypoxic microenvironment for antibiotic activation by photodynamic therapy to combat bacterial biofilm infections. Nature Commun. 2022;13(1):3875. 10.1038/s41467-022-31479-xSearch in Google Scholar PubMed PubMed Central
[96] Sun J, Fan Y, Ye W, Tian L, Niu S, Ming W, et al. Near-infrared light triggered photodynamic and nitric oxide synergistic antibacterial nanocomposite membrane. Chem Eng J. 2021;417:128049. 10.1016/j.cej.2020.128049Search in Google Scholar
[97] Qin Z, Zheng Y, Du T, Wang Y, Gao H, Quan J, et al. Cysteamine: A key to trigger aggregation-induced NIR-II photothermal effect and silver release booming of gold-silver nanocages for synergetic treatment of multidrug-resistant bacteria infection. Chem Eng J. 2021;414:128779. 10.1016/j.cej.2021.128779Search in Google Scholar
[98] Tan L, Li J, Liu X, Cui Z, Yang X, Zhu S, et al. Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light. Adv Mater. 2018;30(31):1801808. 10.1002/adma.201801808Search in Google Scholar PubMed
[99] Sun J, Wang K, Hao R, Zhang Z, Feng Z, Shi Z, et al. Disregarded free chains affect bacterial adhesion on cross-linked polydimethylsiloxane surfaces. ACS Appl Mater Interfaces. 2023;15(30):36936–44. 10.1021/acsami.3c05477Search in Google Scholar PubMed
[100] Pakapongpan S, Poo-Arporn RP. Self-assembly of glucose oxidase on reduced graphene oxide-magnetic nanoparticles nanocomposite-based direct electrochemistry for reagentless glucose biosensor. Mater Sci Eng: C. 2017;76:398–405.10.1016/j.msec.2017.03.031Search in Google Scholar PubMed
[101] Liu L, Wei Y, Jiao S, Zhu S, Liu X. A novel label-free strategy for the ultrasensitive miRNA-182 detection based on MoS2/Ti3C2 nanohybrids. Biosens Bioelectron. 2019;137:45–51. Elsevier.10.1016/j.bios.2019.04.059Search in Google Scholar PubMed
[102] Ali M, Sajid M, Khalid MA, Kim SW, Lim JH, Huh D, et al. A fluorescent lateral flow biosensor for the quantitative detection of Vaspin using upconverting nanoparticles. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2020;226:117610. Elsevier.10.1016/j.saa.2019.117610Search in Google Scholar PubMed
[103] Balamurugan T, Berchmans S. Non-enzymatic detection of bilirubin based on a graphene–polystyrene sulfonate composite. RSC Adv. 2015;5(62):50470–7. Royal Society of Chemistry.10.1039/C5RA06681FSearch in Google Scholar
[104] Moreira FT, Sale M, Goreti F, Di Lorenzo M. Towards timely Alzheimer diagnosis: A self-powered amperometric biosensor for the neurotransmitter acetylcholine. Biosens Bioelectron. 2017;87:607–14.10.1016/j.bios.2016.08.104Search in Google Scholar PubMed
[105] Peng H, Chen IA. Rapid colorimetric detection of bacterial species through the capture of gold nanoparticles by chimeric phages. Acs Nano. 2018;13(2):1244–52. ACS Publications.10.1021/acsnano.8b06395Search in Google Scholar PubMed PubMed Central
[106] Salahandish R, Ghaffarinejad A, Naghib SM, Majidzadeh-A K, Zargartalebi H, Sanati-Nezhad A. Nano-biosensor for highly sensitive detection of HER2 positive breast cancer. Biosens Bioelectron. 2018;117:104–11. Elsevier.10.1016/j.bios.2018.05.043Search in Google Scholar PubMed
[107] Krampa FD, Aniweh Y, Kanyong P, Awandare GA. Recent advances in the development of biosensors for malaria diagnosis. Sensors. 2020;20(3):799. MDPI.10.3390/s20030799Search in Google Scholar PubMed PubMed Central
[108] Tibbits G, Mohamed A, Call DR, Beyenal H. Rapid differentiation of antibiotic-susceptible and-resistant bacteria through mediated extracellular electron transfer. Biosens Bioelectron. 2022;197:113754. 10.1016/j.bios.2021.113754Search in Google Scholar PubMed
[109] Fang X, Kalathil S, Divitini G, Wang Q, Reisner E. A three-dimensional hybrid electrode with electroactive microbes for efficient electrogenesis and chemical synthesis. Proc National Acad Sci. 2020;117(9):5074–80. 10.1073/pnas.1913463117Search in Google Scholar PubMed PubMed Central
[110] Yao L, Wang L, Huang F, Cai G, Xi X, Lin J. A microfluidic impedance biosensor based on immunomagnetic separation and urease catalysis for continuous-flow detection of E. coli O157: H7. Sensors Actuators B Chem. 2018;259:1013–21. 10.1016/j.snb.2017.12.110Search in Google Scholar
[111] Yang Y, Chu B, Cheng J, Tang J, Song B, Wang H, et al. Bacteria eat nanoprobes for aggregation-enhanced imaging and killing diverse microorganisms. Nature Commun. 2022;13(1):1255. 10.1038/s41467-022-28920-6Search in Google Scholar PubMed PubMed Central
[112] Qian X, Zheng Y, Chen Y. Micro/nanoparticle-augmented sonodynamic therapy (SDT): breaking the depth shallow of photoactivation. Adv Mater. 2016;28(37):8097–129. 10.1002/adma.201602012Search in Google Scholar PubMed
[113] Xuuuu M, Zhou L, Zheng L, Zhou Q, Liu K, Mao Y, et al. Sonodynamic therapy-derived multimodal synergistic cancer therapy. Cancer Lett. 2021;497:229–42. 10.1016/j.canlet.2020.10.037Search in Google Scholar PubMed
[114] Sun D, Pang X, Cheng Y, Ming J, Xiang S, Zhang C, et al. Ultrasound-switchable nanozyme augments sonodynamic therapy against multidrug-resistant bacterial infection. ACS Nano. 2020;14(2):2063–76. 10.1021/acsnano.9b08667Search in Google Scholar PubMed
[115] Gong Z, Dai Z. Design and challenges of sonodynamic therapy system for cancer theranostics: from equipment to sensitizers. Adv Sci. 2021;8(10):2002178. 10.1002/advs.202002178Search in Google Scholar PubMed PubMed Central
[116] Ouyang J, Tang Z, Farokhzad N, Kong N, Kim NY, Feng C, et al. Ultrasound mediated therapy: Recent progress and challenges in nanoscience. Nano Today. 2020;35:100949. 10.1016/j.nantod.2020.100949Search in Google Scholar
[117] Huang H, Ali A, Liu Y, Xie H, Ullah S, Roy S, et al. Advances in image-guided drug delivery for antibacterial therapy. Adv Drug Delivery Reviews. 2023;192:114634. 10.1016/j.addr.2022.114634Search in Google Scholar PubMed
[118] Mitragotri S, Kost J. Low-frequency sonophoresis: a review. Adv Drug Deliv Rev. 2004;56(5):589–601. 10.1016/j.addr.2003.10.024Search in Google Scholar PubMed
[119] Liu X, Yin H, Weng CX, Cai Y. Low-frequency ultrasound enhances antimicrobial activity of Colistin-Vancomycin combination against Pan-Resistant Biofilm of Acinetobacter baumannii. Ultrasound Med Biol. 2016;42(8):1968–75. 10.1016/j.ultrasmedbio.2016.03.016Search in Google Scholar PubMed
[120] Song M, Cheng Y, Tian Y, Chu C, Zhang C, Lu Z, et al. Sonoactivated chemodynamic therapy: a robust ROS generation nanotheranostic eradicates multidrug-resistant bacterial infection. Adv Funct Mater. 2020;30(43):2003587. 10.1002/adfm.202003587Search in Google Scholar
[121] Erriu M, Blus C, Szmukler-Moncler S, Buogo S, Levi R, Barbato G, et al. Microbial biofilm modulation by ultrasound: current concepts and controversies. Ultrason Sonochem. 2014;21(1):15–22. 10.1016/j.ultsonch.2013.05.011Search in Google Scholar PubMed
[122] Wu MC, Deokar AR, Liao JH, Shih PY, Ling YC. Graphene-based photothermal agent for rapid and effective killing of bacteria. ACS nano. 2013;7(2):1281–90. 10.1021/nn304782dSearch in Google Scholar PubMed
[123] Ibarra IS, Rodriguez JA, Galán-Vidal CA, Cepeda A, Miranda JM, et al. Magnetic solid phase extraction applied to food analysis. J Chem. 2015;2015. 10.1155/2015/919414Search in Google Scholar
[124] Wang H, Wang J, Xu L, Zhang Y, Chen Y, Chen H, et al. Selection and characterization of thioflavin T aptamers for the development of light-up probes. Anal Methods. 2016;8(48):8461–5. 10.1039/C6AY02890JSearch in Google Scholar
[125] Ji H, Hu H, Tang Q, Kang X, Liu X, Zhao L, et al. Precisely controlled and deeply penetrated micro-nano hybrid multifunctional motors with enhanced antibacterial activity against refractory biofilm infections. J Hazard Mater. 2022;436:129210. 10.1016/j.jhazmat.2022.129210Search in Google Scholar PubMed
[126] Leulmi Pichot S, Joisten H, Grant A, Dieny B, Cowburn R. Magneto-mechanically actuated microstructures to efficiently prevent bacterial biofilm formation. Sci Rep. 2020;10(1):15470. 10.1038/s41598-020-72406-8Search in Google Scholar PubMed PubMed Central
[127] Blakemore R, Maratea D, Wolfe R. Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. J Bacteriol. 1979;140(2):720–9. 10.1128/jb.140.2.720-729.1979Search in Google Scholar PubMed PubMed Central
[128] Chen CY, Chen CF, Yi Y, Chen LJ, Wu LF, Song T. Construction of a microrobot system using magnetotactic bacteria for the separation of Staphylococcus aureus. Biomed Microdevices. 2014;16:761–70. 10.1007/s10544-014-9880-2Search in Google Scholar PubMed
[129] Chen C, Chen L, Wang P, Wu LF, Song T. Magnetically-induced elimination of Staphylococcus aureus by magnetotactic bacteria under a swing magnetic field. Nanomed Nanotechnol Biol Med. 2017;13(2):363–70. 10.1016/j.nano.2016.08.021Search in Google Scholar PubMed
[130] Ye P, Li F, Zou J, Luo Y, Wang S, Lu G, et al. In situ generation of gold nanoparticles on bacteria-derived magnetosomes for imaging-guided starving/chemodynamic/photothermal synergistic therapy against cancer. Adv Funct Materials. 2022;32(17):2110063. 10.1002/adfm.202110063Search in Google Scholar
[131] Liu H, Chen C, Chen H, Mo L, Guo Z, Ye B, et al. 2D-PROTACs with augmented protein degradation for super-resolution photothermal optical coherence tomography guided momentary multimodal therapy. Chem Eng J. 2022;446:137039. 10.1016/j.cej.2022.137039Search in Google Scholar
[132] Han HS, Choi KY. Advances in nanomaterial-mediated photothermal cancer therapies: toward clinical applications. Biomedicines. 2021;9(3):305. 10.3390/biomedicines9030305Search in Google Scholar PubMed PubMed Central
[133] Chen Y, Gao Y, Chen Y, Liu L, Mo A, Peng Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J Controlled Release. 2020;328:251–62. 10.1016/j.jconrel.2020.08.055Search in Google Scholar PubMed
[134] Wu F, Zheng H, Wang W, Wu Q, Zhang Q, Guo J, et al. Rapid eradication of antibiotic-resistant bacteria and biofilms by MXene and near-infrared light through photothermal ablation. Sci China Mater. 2021;64(3):748–58. 10.1007/s40843-020-1451-7Search in Google Scholar
[135] Zhao YQ, Sun Y, Zhang Y, Ding X, Zhao N, Yu B, et al. Well-defined gold nanorod/polymer hybrid coating with inherent antifouling and photothermal bactericidal properties for treating an infected hernia. Acs Nano. 2020;14(2):2265–75. 10.1021/acsnano.9b09282Search in Google Scholar PubMed
[136] Liu H, Mo L, Chen H, Chen C, Wu J, Tang Z, et al. Carbon dots with intrinsic bioactivities for photothermal optical coherence tomography, tumor-specific therapy and postoperative wound management. Adv Healthcare Materials. 2022;11(6):2101448. 10.1002/adhm.202101448Search in Google Scholar PubMed
[137] Qie X, Zan M, Gui P, Chen H, Wang J, Lin K, et al. Design, synthesis, and application of carbon dots with synergistic antibacterial activity. Front Bioeng Biotech. 2022;10:894100. 10.3389/fbioe.2022.894100Search in Google Scholar PubMed PubMed Central
[138] Liu H, Chen H, Liu X, Mo L, Chen C, Guo Z, et al. Dual-responsive ultrathin 1T-phase niobium telluride nanosheet-based delivery systems for enhanced chemo-photothermal therapy. J Materials Chem B. 2021;9(38):8109–20. 10.1039/D1TB01469BSearch in Google Scholar PubMed
[139] Wang H, Zhou S, Guo L, Wang Y, Feng L. Intelligent hybrid hydrogels for rapid in situ detection and photothermal therapy of bacterial infection. ACS Appl Mater Interfaces. 2020;12(35):39685–94. 10.1021/acsami.0c12355Search in Google Scholar PubMed
[140] Wang M, Chen Y, Cai W, Feng H, Du T, Liu W, et al. In situ self-assembling Au-DNA complexes for targeted cancer bioimaging and inhibition. Proc National Acad Sci. 2020;117(1):308–16. 10.1073/pnas.1915512116Search in Google Scholar PubMed PubMed Central
[141] Singh MP, Singh P, Li HB, Song QQ, Singh RK. Microbial biofilms: development, structure, and their social assemblage for beneficial applications. In: New and future developments in microbial biotechnology and bioengineering: microbial biofilms. Amsterdam, Netherlands: Elsevier; 2020. p. 125–38. 10.1016/B978-0-444-64279-0.00010-4Search in Google Scholar
[142] Zheng Y, Liu W, Chen Y, Li C, Jiang H, Wang X. Conjugating gold nanoclusters and antimicrobial peptides: From aggregation-induced emission to antibacterial synergy. J Colloid Interface Sci. 2019;546:1–10. 10.1016/j.jcis.2019.03.052Search in Google Scholar PubMed
[143] Guo Z, Zeng J, Liu W, Chen Y, Jiang H, Weizmann Y, et al. Formation of bio-responsive nanocomposites for targeted bacterial bioimaging and disinfection. Chem Eng J. 2021;426:130726. 10.1016/j.cej.2021.130726Search in Google Scholar
[144] Zeng J, Guo Z, Wang Y, Qin Z, Ma Y, Jiang H, et al. Intelligent bio-assembly imaging-guided platform for real-time bacteria sterilizing and infectious therapy. Nano Res. 2022;15(5):4164–74. 10.1007/s12274-021-3998-3Search in Google Scholar
[145] Wang J, Xia Q, Huang K, Yin L, Jiang H, Liu X, et al. Ultrafast cancer cells imaging for liquid biopsy via dynamic self-assembling fluorescent nanoclusters. Biosensors. 2023;13(6):602. 10.3390/bios13060602Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy


