Abstract
(CH3NH3)2PdSe16, tetragonal,
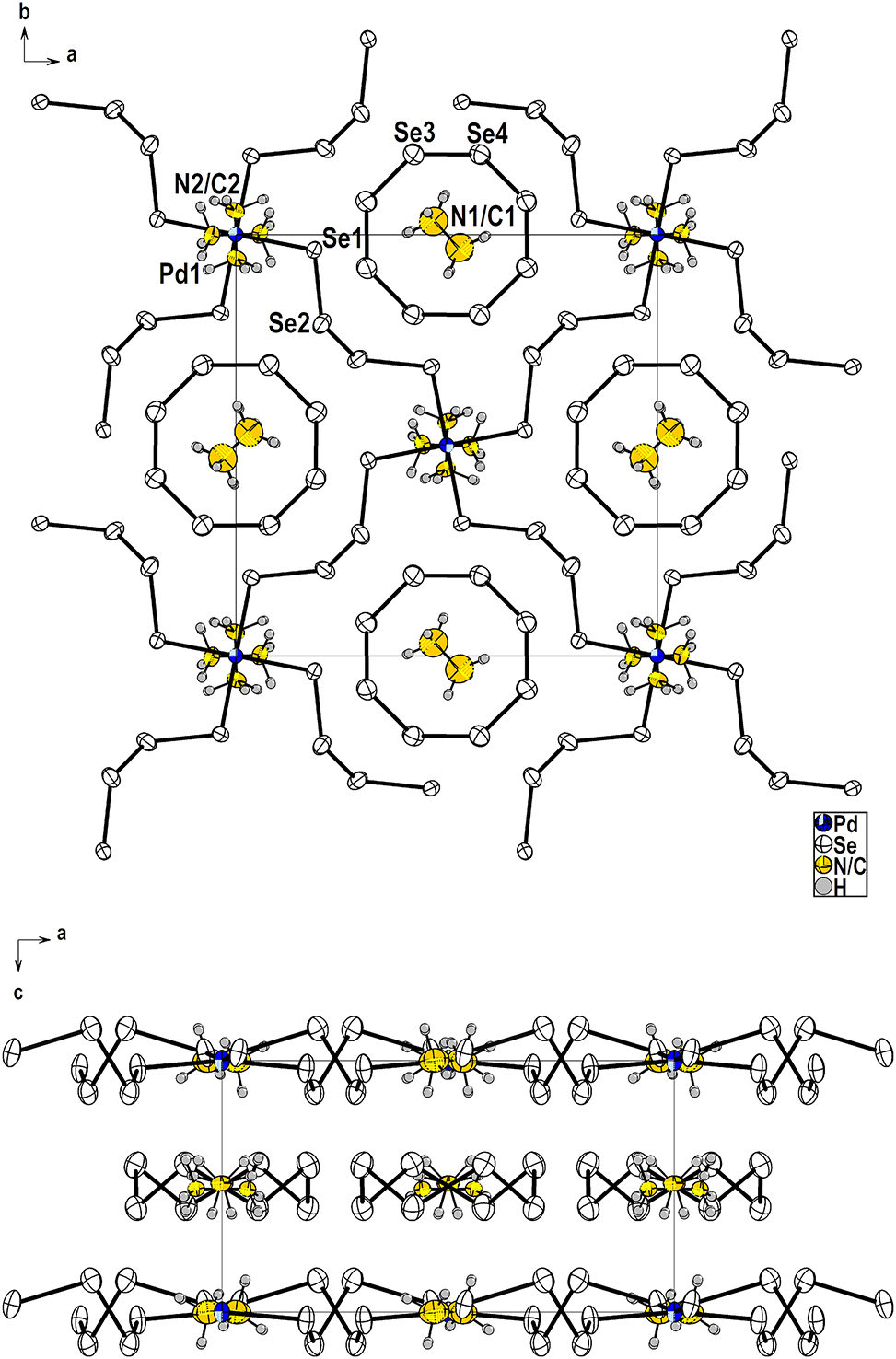
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Black chunk |
| Size: | 0.18 × 0.15 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 25.9 mm−1 |
| Diffractometer, scan mode: | PHOTON 100 CMOS, φ and ω |
| θ max, completeness: | 28.3°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 40,693, 1457, 0.099 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 1377 |
| N(param)refined: | 54 |
| Programs: | Bruker [1], SHELX [2], WinGX/ORTEP [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Pd1 | 1.000000 | 1.000000 | 1.000000 | 0.0246 (2) |
| Se1 | 0.96361 (6) | 0.81405 (6) | 0.96910 (16) | 0.0383 (2) |
| Se2 | 0.78848 (8) | 0.79594 (7) | 0.87366 (14) | 0.0390 (2) |
| Se3 | 0.57833 (8) | 0.80950 (8) | 0.58501 (16) | 0.0457 (3) |
| Se4 | 0.42052 (8) | 0.81025 (7) | 0.41248 (16) | 0.0433 (3) |
| N1a | 0.5309 (17) | 0.9691 (17) | 1.000000 | 0.156 (15) |
| H1Ab | 0.586293 | 0.991373 | 1.067024 | 0.234* |
| H1Bb | 0.550881 | 0.955961 | 0.881013 | 0.234* |
| H1Cb | 0.505206 | 0.910286 | 1.051963 | 0.234* |
| C1a | 0.5309 (17) | 0.9691(17) | 1.000000 | 0.156 (15) |
| H1Db | 0.590771 | 0.993270 | 1.071608 | 0.234* |
| H1Eb | 0.552140 | 0.954639 | 0.871705 | 0.234* |
| H1Fb | 0.503341 | 0.905839 | 1.056687 | 0.234* |
| N2b | 0.502 (4) | 0.5567 (14) | 0.489 (4) | 0.060 (5) |
| H2Ab | 0.438078 | 0.582056 | 0.459337 | 0.090* |
| H2Bb | 0.548755 | 0.578798 | 0.403637 | 0.090* |
| H2Cb | 0.520246 | 0.579637 | 0.603819 | 0.090* |
| C2b | 0.502 (4) | 0.5567 (14) | 0.489 (4) | 0.060 (5) |
| H2Db | 0.433056 | 0.584030 | 0.457575 | 0.090* |
| H2Eb | 0.552195 | 0.580523 | 0.396614 | 0.090* |
| H2Fb | 0.521989 | 0.581412 | 0.612604 | 0.090* |
-
aOccupancy: 0.5, bOccupancy: 0.25.
1 Source of material
K2PdCl4 (0.020 g, 0.061 mmol), K2Se4 (0.096 g, 0.24 mmol) and CH3NH3Cl (0.008 g, 0.12 mmol) were charged to a Pyrex tube with diameter of 9 mm under an argon atmosphere and about 0.5 mL methanol was added as a solvent. While the solvent was being frozen, the Pyrex tube was evacuated under vacuum and sealed with the use of a flame. The sealed tube was placed in an oven and heated at 80 °C for 3 days, then cooled to room temperature. Black chunky crystals were isolated by filtration and washed with methanol and diethyl ether several times. Crystals of (CH3NH3)2PdSe16 were obtained in 11 % yield, based on the Pd metal used.
2 Experimental details
H atoms were positioned geometrically and treated as riding, with N–H = 0.90 Å and C–H = 0.97 Å; U iso(H) = 1.2U eq(N) and 1.5U eq(C). H atoms of NH3 and CH3 were positioned to be staggered with respect to the shortest other bond to the atom to which NH3 and CH3 is attached.
3 Comment
The structures of Pd polyselenide anions have proven to be highly sensitive to the size and shape of their counterions [5, 6]. As one of Pd polyselenide compounds, A2PdSe16 (A = Rb, Cs) is featured to possess a 2D layered Pd polyselenide anion, [Pd(Se4)2] n 2n− with encapsulated Se8 rings [7], [8], [9]. Smaller alkali metal ion such as K+ has been found to be inappropriate for the A2PdSe16 (A = Rb, Cs) compound, instead, to stabilize K4[Pd(Se4)2][Pd(Se6)2] with two independent interpenetrating 3D frameworks of [Pd(Se x )2] n 2n− (x = 4, 6), K6[Pd(Se5)4] with a discrete anion of [Pd(Se5)4]6−, and K2[Pd(Se5)2] with a 1D polymeric [Pd(Se5)2]2− anion [5, 10, 11]. Compared to the alkali metal cations, organic ammonium cations have the advantage of being removed with less damage to the chalcogenidometallate anion framework by thermal treatment at lower temperatures. Among organic ammonium cations, the smallest cation, CH3NH+ 3 is known to be in possession of a close ionic radius to those of Cs+ and Rb+. Successful replacement of Cs+ or Rb+ with CH3NH+ 3 has already been demonstrated for the preparation of organic–inorganic hybrid perovskite material such as CH3NH3PbI3 [12, 13].
(CH3NH3)2PdSe16 was prepared by the methanothermal reaction of K2PdCl4, K2Se4 and CH3NH3Cl in a molar ratio of 1:4:2. In the case of A2PdSe16 (A = Rb, Cs), the mixture of A2Se (A = Na, Cs) and elemental Se served as the source of Se4 2- ligands and Se8 rings, but K2Se4 was used instead for the preparation of (CH3NH3)2PdSe16.
The structure of (CH3NH3)2PdSe16 is isostructural to those of A2PdSe16 (A = Rb, Cs), as all three compounds are crystallized in the same space group
Thermal stability and decomposition behaviour of (CH3NH3)2PdSe16 was examined by the TGA experiments. TGA results show that there are three decomposition steps upon heating to 900 °C under N2 flow. Based on the amount of weight loss during the periods of decomposition steps, CH3NH3 + cations were lost in the first step and seleniums were lost in the following two steps to leave PdSe as a final residue. The first decomposition step begins at about 150 °C and ends at about 200 °C. The other two steps occur between 300 and 420 °C and between 460 and 870 °C, respectively. While decomposition of both Rb2PdSe16 and Cs2PdSe16 analogues did not occur up to around 300 °C, (CH3NH3)2PdSe16 can lose its organic cation below 200 °C, and start its second decomposition at about 300 °C. When crystals of (CH3NH3)2PdSe16 were calcined at 250 °C under N2 flow for a day, the shape and morphology of the crystals remained intact, but numerous holes formed on the surface. The XRD pattern of the calcined crystals indicates a loss of crystallinity in (CH3NH3)2PdSe16 with no prominent crystalline peaks.
Acknowledgements
This work was supported by the Incheon National University Research Grant in 2023.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Incheon National University Research Grant in 2023.
References
1. Bruker. APEX2 and SAINT; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
3. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Kim, K.-W., Kanatzidis, M. G. Counterion effects in Pd polyselenides: evolution from molecular to three-dimensional framework structures. J. Am. Chem. Soc. 1998, 120, 8124–8135; https://doi.org/10.1021/ja981297s.Search in Google Scholar
6. Sheldrick, W. S. Polychalcogenide anions: structural diversity and ligand versatility. Z. Anorg. Allg. Chem. 2012, 638, 2401–2424; https://doi.org/10.1002/zaac.201200241.Search in Google Scholar
7. Wachhold, M., Kanatzidis, M. G. Powerful templating effect in Rb/Pd/Sex promoted by crown ether-like [Rb(Se8)]+ coordination. Formation of Rb2[Pd(Se4)2]·Se8: a layered Pd polyselenide with ”encapsulated” eight-membered selenium rings. J. Am. Chem. Soc. 1999, 121, 4189–4195; https://doi.org/10.1021/ja984053g.Search in Google Scholar
8. Chen, Z., Wang, R.-J., Li, J. Solvothermal synthesis of alkline metal selenides Cs2PdSe16 and studies of thermal stabilities. Chem. J. Chinese Univ. 2001, 22, 1091–1094.Search in Google Scholar
9. Goldbach, A., Fayon, F., Vosegaard, T., Wachhold, M., Kanatzidis, M. G., Massiot, D., Saboungi, M.-L. 77Se and 87Rb solid state NMR study of the structure of Rb2[Pd(Se4)2]·Se8. Inorg. Chem. 2003, 42, 6996–7000; https://doi.org/10.1021/ic030074m.Search in Google Scholar PubMed
10. Kim, K.-W., Kanatzidis, M. G. Hydrothermal synthesis of K2PdSe10. Coexistence of two large interpenetrating three-dimensional frameworks of [Pd(Se4)2]2− and [Pd(Se6)2]2−. J. Am. Chem. Soc. 1992, 114, 4878–4883; https://doi.org/10.1021/ja00038a063.Search in Google Scholar
11. Kim, K.-W. Crystal structure of potassium bis(pentaselenido-κ2Se1,Se5)palladate(II), K2[Pd(Se5)2]. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 551–553.10.1515/ncrs-2023-0081Search in Google Scholar
12. Dang, Y., Liu, Y., Sun, Y., Yuan, D., Liu, X., Lu, W., Liu, G., Xia, H., Tao, X. Bulk crystal growth of hybrid perovskite material CH3NH3PbI3. CrystEngComm 2015, 17, 665–670; https://doi.org/10.1039/c4ce02106a.Search in Google Scholar
13. Stoumpos, C. C., Malliakas, C. D., Kanatzidis, M. G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038; https://doi.org/10.1021/ic401215x.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of tris((Z)-2-hydroxy-N-((E)-pyridin-2-ylmethylene)benzohydrazonato-k2O,N)europium(III), C39H30N9O6Eu
- Crystal structure of (E)-3-(benzylideneamino)-2-phenylthiazolidin-4-one, C16H14N2OS
- The crystal structure of (E)-4-fluoro-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H15FN2O
- Crystal structure of (6-chloropyridin-3-yl)methyl 2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- Crystal structure of methyl 3-methoxy-4-(2-methoxy-2-oxoethoxy)benzoate, C12H14O6
- The crystal structure of bis[(4-methoxyphenyl)(picolinoyl)amido-κ2 N:N′]copper(II), C26H22CuN4O4
- The crystal structure of poly[di(μ2-aqua)-diaqua-bis(3-aminopyridine-4-carboxylate-κ2 O: O′)-tetra(μ2-3-aminopyridine-4-carboxylate-κ2 O: O′)-dineodymium(III), [Nd2(C6H5N2O2)6(H2O)4] n
- The crystal structure of t-butyl 7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoate, C28H34FNO4
- Crystal structure of catena-poly[(benzylamine-κ1 N)-(sorbato-κ1 O)-(μ2-sorbato-κ2 O,O′)-copper(II), C19H23CuNO4
- Crystal structure of (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl) methanone, C21H16ClFeNO
- The crystal structure of N-[4-(4-bromophenyl)-1,3-thiazol-2-yl]-3-(2-methylphenyl)-2-sulfanylprop-2-enamide hydrate, C19H17BrN2O2S2
- The crystal structure of N′-{5-[2-(2,6-dimethylphenoxy) acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide hydrate
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C26H24O3
- Crystal structure of naphthalen-1-ylmethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C25H22O3
- Crystal structure of poly[diaqua- (μ4-5-(1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ5N:O,O’:O’’:O’’’)calcium(II), C10H9CaN3O6
- Crystal structure of (E)-N′-(4-((E)-3-(dimethylamino)acryloyl)-3-hydroxyphenyl)-N, N-dimethylformimidamide, C14H19N3O2
- Crystal structure of (E)-3-(dimethylamino)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, C13H17NO4
- Crystal structure of (2-chloropyridin-3-yl)methyl-2-(6-methoxynaphthalen-2-yl)propanoate, C20H18ClNO3
- The crystal structure of diethyl 4-(3,4-dimethylphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C21H27NO4
- Crystal structure of (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-17-((4-(2-phenylpropyl)phenyl)ethynyl)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H42O2
- Synthesis and crystal structure of 4-(4-cyclopropylnaphthalen-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione, C15H13N3S
- Crystal structure of catena-poly[aqua-(2,6-di-(2-pyridyl)-pyridine-κ3 N,N′, N″)(μ2-1,4-naphthalene dicarboxylato-κ2 O,O′)nickel(II)], C27H19NiN3O5
- Crystal structure of 3-(diphenylphosphoryl)-3-hydroxy-1-phenylpropan-1-one, C21H19O3P
- The crystal structure of R,S-{N-[(2-oxidonaphthalen-1-yl)methylidene]phenylglycinato}divinylsilicon, C23H19NO3Si
- The crystal structure of 1,2,4-tris(bromomethyl)benzene, C9H9Br3
- Crystal structure of chlorido-[4-(pyridin-2-yl)benzaldehyde-κ2 N,C]-(diethylamine-κ1 N)platinum(II), C16H18ClN2OPt
- Crystal structure of 3-(methoxycarbonyl)-1-(4-methoxyphenyl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indol-2-ium chloride hydrate, C40H48Cl2N4O9
- The crystal structure of 1-(2-chlorobenzyl)-3-(3-chlorophenyl)urea, C14H12Cl2N2O
- Hydrothermal synthesis and crystal structure of aqua-tris(4-acetamidobenzoato-κ2 O,O′)-(1,10-phenanthroline-κ2 N,N′)terbium(III) hydrate C39H36N5O11Tb
- The crystal structure of zwitterionic 3-aminoisonicotinic acid, C6H6N2O2
- The crystal structure of bis{[monoaqua-μ2-4-[(pyridine-4-carbonyl)-amino]-phthalato-κ3 N:O,O′-(2,2′-bipyridine κ2 N,N′)copper(II)]}decahydrate, C48H56N8O22Cu2
- Crystal structure of poly[μ10-4,4′-methylene-bis(oxy)benzoatodipotassium], C15H10K2O6
- The crystal structure of catena-poly[[tetraaqua[(μ2-1,4-di(4-methyl-1-imidazolyl)benzene] cobalt(II)]bis(formate)], C16H24CoN4O8
- The crystal structure of (E)-2-chloro-5-((2-(nitromethylene)imidazolidin-1-yl)methyl)pyridine, C10H11ClN4O2
- The crystal structure of (E)-1-(((2-amino-4,5-dimethylphenyl)iminio)methyl)naphthalen-2-olate, C19H18N2O
- Crystal structure of N-(acridin-9-yl)-2-(4-methylpiperidin-1-yl) acetamide monohydrate, C21H25N3O2
- The crystal structure of dichlorido-bis(3-methyl-3-imidazolium-1-ylpropionato-κ2 O,O′)-zinc(II), C14H20Cl2N4O4Zn
- The crystal structure of 2,8-diethyl-1,3,7,9-tetramethyl-4λ4,5λ4-spiro[dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine-5,2′-naphtho[1,8-de][1,3,2]dioxaborinine], C25H29BN2O2
- The crystal structure of 5-tert-butyl-2-(5-tert-butyl-3-iodo-benzofuran-2-yl)-3-iodobenzofuran, C24H24I2O2
- Synthesis and crystal structure of methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio} acetate, C18H17N3O2S
- The crystal structure of n-propylammonium bis(2,3-dimethylbutane-2,3-diolato)borate-boric acid (1/1), [C3H10N][C12H24BO4]·B(OH)3
- Crystal structure of methyl 1-(2-bromophenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C19H17BrN2O2
- Crystal structure of (4-bromobenzyl)triphenylphosphonium bromide ethanol solvate, C52H48Br4OP2
- The crystal structure of unsymmetrical BOPHY C26H27BN4
- The crystal structure of Tb3B5O11(OH)2
- The crystal structure of (Z)-4-ethyl-2-((4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methylene)-3,5-dimethyl-2H-pyrrol-1-ium 2,2'-spirobi[naphtho[1,8-de][1,3,2]dioxaborinin]-2-uide, C37H37BN2O4
- Crystal structure of bis(methylammonium) hexadecaselenidopalladate(II), (CH3NH3)2PdSe16
- The crystal structure of (2-diphenylphosphanylphenyl) 2-[7-(dimethylamino)-2-oxochromen-4-yl]acetate, C31H26NO4P
- Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O
- The structure of RUB-56, (C6H16N)8 [Si32O64(OH)8]·32 H2O, a hydrous layer silicate (2D-zeolite) that contains microporous levyne-type silicate layers
- Crystal structure of 4-amino-3,5-dibromobenzonitrile, C7H4Br2N2
- Crystal structure of 2-(naphthalen-1-yl)ethyl 2-acetoxybenzoate, C21H18O4
- Single-crystal structure determination of Tm3B12O19(OH)7
- Crystal structure determination of NdB3.6O7
- The crystal structure of NdB6O8(OH)5·H3BO3
- Crystal structure of 2-(5-ethylpyridin-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H25NO3
- Crystal structure of N-(1-(3,4-dimethoxyphenyl)-2-methylpropyl)aniline, C18H23NO2
- Crystal structure of Ba6Cd12Mn4SiF48
- Synthesis and crystal structure of 5-fluoro-1-methyl-2-oxo-3-(2-oxochroman-4-yl)indolin-3-yl acetate, C20H16FNO5
- The crystal structure of 6-methacryloylbenzo[d][1,3]dioxol-5-yl 4-nitrobenzenesulfonate, C17H13NO8S
- Crystal structure of ethyl 2-(3-benzyl-4-oxo-3,4-dihydrophthalazin-1-yl)- 2,2-difluoroacetate, C19H16F2N2O3
- The crystal structure of tetrakis(μ 2-(1H-benzimidazole-2-methoxo-κ2 N,O:O:O)-(n-butanol-κO)-chlorido)-tetranickel(II), C48H68Cl4N8O8Ni4
- Synthesis and crystal structure of trans-tetraaqua-bis((1-((7-hydroxy-3-(4-methoxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carbonyl)oxy-κO)zinc(II)hexahydrate, C46H64N2O28S2Zn
- The crystal structure of 1-(4-carboxybutyl)-3-methyl-1H-imidazol-3-ium hexafluoridophosphate, C9H15F6N2O2P
- Crystal structure of 1-(4-chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O3
- Crystal structure of dimethyl (R)-2-(3-(1-phenylethyl)thioureido)-[1,1′-biphenyl]-4,4′-dicarboxylate, C25H24N2O4S
- The crystal structure of 1-(3-carboxypropyl)-1H-imidazole-3-oxide, C7H10N2O3
- Synthesis and crystal structure of dimethyl 4,4′-(propane-1,3-diylbis(oxy))dibenzoate, C19H20O6
- Crystal structure of methyl-1-(p-tolyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate, C20H20N2O2
- The crystal structure of 1-(1-adamantan-1-yl)ethyl-3-(3-methoxyphenyl)thiourea, C20H28N2OS
- The crystal structure of N,N′-carbonylbis(2,6-difluorobenzamide), C15H8F4N2O3

