Abstract
Synthesis of N-(5-methylisoxazol-3-yl)-2-(5-aryl-1,3,4-oxadiazol-2-yl)acetamides 5a–k was achieved from readily available materials. The compounds were screened for their in vitro antimicrobial activity against representative bacterial and fungal strains. Compounds 5b, 5d and 5f exhibit good activity.
Introduction
The emergence of microbial resistance to drugs is a widespread problem in the treatment of various infections. The identification of novel antibiotics for effective treatment of infections still remains a major challenge to medicinal chemists. The 1,3,4-oxadiazole moiety has become an important structural motif for the development of new drugs because of its biological activities including HIV integrase inhibition [[1], anti-inflammatory [2], anticancer [3], antibacterial [4], anticonvulsant [5], analgesic [6], antitubercular [7], antifungal [8] and anti-allergic [9] activities. Some compounds having 1,3,4-oxadiazole derivatives currently used as drugs are raltegravir, an antiretroviral drug 10], zibotentan, an anticancer agent [11], fenadiazole, a hypnotic drug [12], nesapidil, an antihypertensive agent [13] and furamizole, an antibiotic [14], among others [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. In continuation of our work on biologically active isoxazoles [25], [26], [27], we now report the synthesis of new compounds bearing 2,5-disubstituted 1,3,4-oxadiazoles that may be developed into practical drugs.
Results and discussion
Chemistry
Synthesis of N-(5-methylisoxazol-3-yl)-2-(5-phenyl-1,3,4-oxadiazol-2-yl)acetamides 5a–k is shown in Scheme 1. Condensation of 3-amino-5-methylisoxazole (1) with diethyl malonate in ethanol under reflux afforded ethyl 2-(5-methyl-3-isoxazolylcarbamoyl)acetate [28] (2). Treatment of ester 2 with excess of hydrazine hydrate in ethanol furnished 3-hydrazinyl-N-(5-methyl-3-isoxazolyl)-3-oxopropanamide (3). The hydrazide 3 was condensed with aromatic aldehydes in methanol to furnish (E/Z)-3-(2-benzylidenehydrazinyl)-N-(5-methylisoxazol-3-yl)-3-oxopropanamides 4a–k. Compounds 4a–k on treatment with chloramine-T underwent oxidative cyclization to give N-(5-methylisoxazol-3-yl)-2-(5-aryl-1,3,4-oxadiazol-2-yl)acetamides 5a–k.
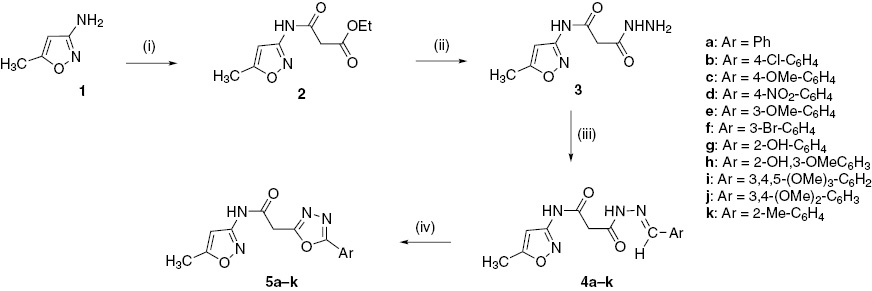
Reagents and conditions: (i) diethyl malonate, ethanol, reflux 12 h, 80%; (ii) N2H4H2O, ethanol, reflux 6 h, 92%; (iii) Ar-CHO, methanol, glacial AcOH, reflux 4–6 h, 90–95%; (iv) chloramine-T, ethanol, reflux 4–6 h, 68–76%.
The structures of compounds 3–5 were established based on IR, 1H NMR, 13C NMR, ESI-MS and analytical data. In particular, 1H NMR spectra of N-acylhydrazones 4a–k show characteristic double signals for each of the proton around the C=N bond, suggesting that these compounds exist in two isomeric forms. Two geometric isomers may be present in the ratio of 3:1; based on the integration values of proton signals. The results of the NMR experiments are discussed as follows.
Compound 4a was used for the analysis of nuclear Overhauser effect (nOe) and two-dimensional (2D)-1H NMR-13C heteronuclear multiple bond correlation (HMBC) spectra. In the nOe experiment, irradiation of the methylene protons (10-CH2) at δH 3.72 gives rise to an enhancement for both NH protons, which demonstrates that the methylene group is flanked by two amidic NH groups (Figure 1). The difference in nOe enhancement observed for the two amidic NH protons is due to their spatial arrangement. On irradiation of the NHCO proton at δH 11.07, only a signal for methylene protons is enhanced and irradiation of the olefinic proton NN=CH at δH 7.93 gives rise to nOe enhancement for phenyl ring protons and a hydrazine NH proton. Furthermore, irradiation of the other NHN=C proton at δH 11.49 gives nOe enhancement for both the methylene protons of the major and minor isomers. There is also a strong enhancement for the olefinic proton of the major isomer while the proton of the minor isomer is unaffected. These results demonstrate that the compounds exist as a mixture of E- and Z-isomeric forms.
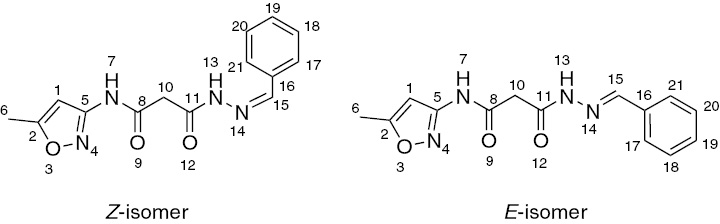
Z (minor) and E (major) isomers of compound 4a.
This conclusion was further confirmed by 2D-1H-13C HMBC experiment. HMBC data unambiguously show that the connectivity, as expected, between protons and carbons of the major and minor isomers is intact, and the assignments of chemical shift values for compound 4a are as follows: correlation of H-6 (δH 2.3, 2.4) with C2 (δc 170.0, 170.1) and C1 (δc 96.7); H7 (δH 11.0, 11.1) with C1 (δc 96.6) and C8 (δc 166.3, 165.9); H13 (δH 11.5, 11.6) with C15 (δc 143.5, 147.3) and C10 (δc 43.0, 43.7) and C11 (δc 163.0 and 169.1) and H15 (δH 7.9, 8.2) with C16 (δc 134.5) and C17 and C21 (δc 127.3, 127.6). The key points from HMBC data are the correlations between H7-C1, H13-C15 and H10-C8 and H10-C11, which indicate the absence of keto-enol tautomerism. On the basis of both 1D and 2D NMR data, it can be concluded that these compounds exist in the mixture of Z and E geometrical isomers.
The IR spectrum of N-(5-methylisoxazol-3-yl)-2-(5-phenyl-1,3,4-oxadiazol-2-yl)acetamide (5a) shows a characteristic band at 1237 cm−1 due to C-O-C stretching vibration confirming the formation of 1,3,4-oxadiazole ring. 1H NMR spectrum of 5a does not exhibit signals due to the CH=N proton, and the NH protons of the hydrazone, which are present in its precursor 4a at δ 7.93, 8.16, 11.49 and 11.55, confirming the oxadiazole ring formation. The absence of azomethine carbon signals at δ 143.5 and 147.3 in 13C NMR spectrum of 5a also supports the formation of the oxadiazole ring. The mass spectrum of 5a also agrees with its structure by exhibiting the protonated molecular ion [M+H]+ peak at m/z 285.
Antibacterial activity
Compounds 5a–k exhibit good antibacterial activity in comparison to the activity of the standard drug ciprofloxacin (Table 1). Some of the compounds exhibit excellent minimum inhibitory concentration values (MIC). Compounds 5b and 5f are highly active. The exceptional activity of compound 5d may be due to the presence of a nitro group on the phenyl ring. The remaining compounds 5a, 5c, 5e, 5g, 5h, 5i, 5j and 5k show moderate activity. However, the degree of inhibition varies both with the test compound and with the bacteria used in the present investigation.
Antibacterial activity of N-(5-methylisoxazol-3-yl)-2-(5-aryl-1,3,4-oxadiazol-2-yl)acetamides 5a–k.
| Compound | Minimum inhibitory concentration (MIC)a,b | |||||
|---|---|---|---|---|---|---|
| Bacterial strains | ||||||
| P. aeruginosa | K. aerogenes | C. violaceum | B. subtilis | B. sphaericus | S. aureus | |
| 5a | 20 | 18 | 18 | 19 | 17 | 15 |
| 5b | 11 | 11 | 8 | 9 | 8 | 7 |
| 5c | 17 | 15 | 13 | 16 | 18 | 14 |
| 5d | 10 | 8 | 7 | 6 | 8 | 6 |
| 5e | 10 | 16 | 15 | 15 | 13 | 14 |
| 5f | 13 | 11 | 14 | 11 | 12 | 10 |
| 5g | 18 | 14 | 16 | 16 | 15 | 15 |
| 5h | 19 | 16 | 20 | 17 | 18 | 21 |
| 5i | 21 | 18 | 21 | 19 | 17 | 18 |
| 5j | 23 | 20 | 22 | 18 | 16 | 15 |
| 5k | 22 | 23 | 20 | 18 | 17 | 16 |
| Ciprofloxacin | 30 | 25 | 25 | 20 | 20 | 25 |
aNegative control (acetone) – no activity. bConcentration in μg/mL.
Antifungal activity
Compounds 5a–k are significantly toxic toward all five pathogenic fungi and are lethal even at 100 μg/mL concentration when compared to the standard drug clotrimazole (Table 2). The activity data are indicated as a zone of inhibition at 100 μg/mL concentration. Compounds 5b and 5f exhibit high activity and they inhibit the growth of fungi to a remarkable extent, which may be due to the presence of chloro, nitro and bromo substituents on the benzene ring, besides the presence of isoxazole and oxadiazole skeletons. Compound 5d bearing a nitro group on the benzene ring shows good toxicity against the fungi used. Compounds 5a, 5c, 5e, 5g, 5h, 5i, 5j and 5k are moderate in their toxicity and they are less active compared to other compounds in the present study, but better than the standard drug clotrimazole. The degree of spore germination inhibition varies with test compounds and with the type of fungi.
Antifungal activity of N-(5-methylisoxazol-3-yl)-2-(5-aryl-1,3,4-oxadiazol-2-yl)acetamides 5a–k.
| Compound | Zone of inhibition (mm)a,b | ||||
|---|---|---|---|---|---|
| Fungal strains | |||||
| A. niger | C. tropicum | R. oryzae | F. moniliforme | C. lunata | |
| 5a | 52.5 | 46.5 | 51.0 | 41.2 | 50.5 |
| 5b | 69.1 | 70.1 | 72.5 | 69.8 | 65.5 |
| 5c | 56.0 | 57.0 | 58.0 | 61.0 | 59.5 |
| 5d | 75.0 | 77.2 | 80.5 | 73.2 | 69.5 |
| 5e | 48.0 | 51.0 | 48.5 | 53.2 | 57.2 |
| 5f | 63.2 | 65.0 | 72.5 | 60.5 | 73.5 |
| 5g | 55.8 | 58.3 | 59.1 | 60.0 | 61.1 |
| 5h | 47.2 | 42.5 | 40.2 | 38.5 | 31.5 |
| 5i | 39.0 | 38.3 | 31.2 | 37.0 | 51.0 |
| 5j | 51.0 | 55.2 | 49.8 | 48.1 | 59.0 |
| 5k | 45.5 | 60.1 | 55.3 | 48.1 | 39.5 |
| Clotrimazole | 26.5 | 30.6 | 33.5 | 25.5 | 35.8 |
aNegative control (acetone) – no activity. bConcentration 100 μg/mL.
Conclusions
A simple and efficient protocol for the synthesis of isoxazolyl-2,5-disubstituted 1,3,4-oxadiazoles 5a–k with potential pharmacological properties is described. Compounds 4a–k exhibit characteristic 1H NMR spectra indicating the presence of E and Z geometrical isomers. The title compounds 5a–k were evaluated for antimicrobial activity. Compounds 5a, 5d and 5f show excellent antimicrobial activity.
Experimental
Melting points were determined on a Cintex melting point apparatus and are uncorrected. Analytical thin-layer chromatography (TLC) analysis was performed on Merck precoated 60F254 silica gel plates. Visualization was done by exposure to UV light. IR spectra were recorded in KBr pellets on a Perkin-Elmer BX series Fourier-transform infrared (FT-IR) spectrometer. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker spectrometer in CDCl3 or DMSO-d6 with tetramethylsilane (TMS) as internal standard. ESI mass spectra were recorded on an Agilent liquid chromatography-mass selective detector (LC-MSD). EA were performed on Carlo Erba 106 and Perkin-Elmer model 240 analyzers.
Synthesis of ethyl 2-(5-methyl-3-isoxazolylcarbamoyl)acetate (2)
A mixture of 3-amino-5-methylisoxazole (1, 0.1 mmol) and diethyl malonate (0.1 mmol) in ethanol (20 mL) was heated under reflux for 12 h. The reaction was monitored by TLC analysis. After the mixture was concentrated and cooled, the separated solid product was filtered under suction, dried and crystallized from ethanol; yield 80%; mp 112–113°C; IR: υmax 3266, 1743, 1700 cm−1 ; 1H NMR (CDCl3): δ 1.30 (t, J=8.0 Hz, 3H, OCH2-CH3), 2.41 (s, 3H, isoxazole-CH3), 3.52 (s, 2H, CH2), 4.25 (q, J=8.0 Hz, 2H, OCH2-CH3), 6.70 (s, 1H, isoxazole-H), 10.19 (s, 1H, NH); 13C NMR (CDCl3): δ 12.6, 14.0, 42.0, 62.0, 96.6, 157.7, 163.4, 168.2, 170.1; MS: m/z 213, (M+H)+. Anal. Calcd for C9H12N2O4: C, 50.94; H, 5.70; N, 13.20. Found: C, 50.92; H, 5.68; N, 13.21.
Synthesis of 3-hydrazino-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (3)
A mixture of compound 2 (0.1 mmol) in ethanol (15 mL) and hydrazine hydrate (0.5 mmol) (98%) was heated under reflux for 6 h, and the progress of the reaction was monitored by TLC. The excess ethanol was removed under reduced pressure and the residue of 3 was washed with cold water and cold methanol and crystallized from methanol; yield 92%; mp 152–153°C; IR: υmax 3211–3337, 3141, 1652 cm−1; 1H NMR (DMSO-d6): δ 2.35 (s, 3H, isoxazole-CH3), 3.19 (s, 2H, CH2), 4.26 (s, 2H, NH2), 6.57 (s, 1H, isoxazole-H), 9.13 (s, 1H, NH), 10.94 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.5, 42.5, 96.6, 158.3, 165.9, 166.1, 170.0; MS: m/z 199.15 (M+H)+. Anal. Calcd for C7H10N4O3: C, 42.42; H, 5.09; N, 28.27. Found: C, 42.40; H, 5.08; N, 28.25.
General procedure for the synthesis of 3-(2-arylidenehydrazino)-N-5-(methyl-3-isoxazolyl)-3-oxopropanamides 4a–k
A mixture of hydrazide 3 (0.1 mmol) and an aromatic aldehyde (0.1 mmol) was heated under reflux in methanol (20 mL) in the presence of a catalytic amount of glacial acetic acid for 4–6 h. The progress of the reaction was monitored by TLC. The reaction mixture was cooled, and the separated solid was filtered and crystallized from methanol.
(E/Z)-3-(2-Benzylidenehydrazino)-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (4a)
Yield 90%; mp 160–161°C; IR: υmax 3257, 3220, 1680, 1621 cm−1; 1H NMR (DMSO-d6): δ 2.33 and 2.36 (s, 3H, H6), 3.40 and 3.72 (s, 2H, H10), 6.57 and 6.60 (s, 1H, H1), 7.34 and 7.42 (m, 3H, H18, H19, H20), 7.61 and 7.68 (m, 2H, H17, H21), 7.93 and 8.17 (s, 1H, H15), 11.07 and 11.10 (s, 1H, H7), 11.49 and 11.55 (s, 1H, H13); 13C NMR (DMSO-d6): δ 12.6 (C6), 43.0 and 43.7 (C10), 96.6 (C1), 127.3, and 127.6 (C17 and C21), 129.2 and 129.3 (C18 and C20), 130.3 and 130.6 (C19), 134.5 (C16), 143.5 and 147.3 (C15), 158.4 and 158.6 (C5), 163.0 and 169.1 (C11), 165.9 and 166.3 (C8), 170.0 and 170.1 (C2); MS: m/z 287.15 (M+H)+. Anal. Calcd for C14H14N4O3: C, 58.73; H, 4.93; N, 19.57. Found: C, 58.72; H, 4.92; N, 19.56.
(E/Z)-3-(2-(4-Chlorobenzylidene)hydrazino)-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (4b)
Yield 92%; mp 178–180°C; IR: υmax 3225, 1678, 1618 cm−1; 1H NMR (DMSO-d6): δ 2.34 and 2.38 (2s, 3H, isoxazole-CH3), 3.41 and 3.70 (2s, 2H, CH2), 6.59 and 6.61 (2s, 1H, isoxazole-H), 6.80–7.30 (m, 4H, Ar-H), 8.01 and 8.20 (2s, 1H, N=CH), 10.92 and 10.96 (2s, 1H, NH), 11.20 and 11.28 (2s, 1H, NH); 13C NMR (DMSO-d6): δ 12.7, 43.1, 43.9, 95.7, 130.2, 131.9, 132.9, 133.2, 139.8, 143.2, 147.1, 157.8, 158.2, 162.2, 165.6, 165.8, 169.9, 170.2, 171.4; MS: m/z 321 (M+H)+. Anal. Calcd for C14H13ClN4O3: C, 52.43; H, 4.09; N, 17.47. Found: C, 52.41; H, 4.08; N, 17.45.
(E/Z)-3-(2-(4-Methoxybenzylidene)hydrazino)-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (4c)
Yield 93%; mp 172–173°C; IR: υmax 3230, 1675, 1622 cm−1; 1H NMR (DMSO-d6): δ 2.33 and 2.36 (2s, 3H, isoxazole-CH3), 3.40 and 3.72 (2s, 2H, CH2), 3.78 and 3.80 (2s, 3H, OCH3), 6.57 and 6.60 (2s, 1H, isoxazole-H), 6.91–7.36 (m, 4H, Ar-H), 7.90 and 8.13 (2s, 1H, N=CH), 11.07 and 11.10 (2s, 1H, NH), 11.10 and 11.56 (2s, 1H, NH); 13C NMR (DMSO-d6): δ 12.4, 43.0, 43.9, 55.4, 55.6, 96.5, 110.9, 111.2, 116.7, 116.9, 120.3, 120.6, 130.3, 130.4, 135.7, 143.8, 147.5, 158.2, 159.8, 162.0, 163.8, 165.8, 166.3, 169.1, 170.2, 170.4; MS: m/z 317 (M+H)+. Anal. Calcd for C15H16N4O4: C, 56.96; H, 5.10; N, 17.71. Found: C, 56.94; H, 5.09; N, 17.69.
(E/Z)-N-(5-Methylisoxazol-3-yl)-3-(2-(4-nitrobenzylidene)hydrazino)-3-oxopropanamide (4d)
Yield 95%; mp 185–186°C; IR: υmax 3190, 1679, 1617 cm−1; 1H NMR (DMSO-d6): δ 2.32 and 2.35 (2s, 3H, isoxazole-CH3), 3.41 and 3.77 (2s, 2H, CH2), 6.52 and 6.55 (2s, 1H, isoxazole-H), 7.78–7.89 (m, 4H, Ar-H), 8.25 and 8.36 (2s, 1H, N=CH), 9.60 and 9.62 (2s, 1H, NH), 11.22 and 11.25 (2s, 1H, NH); 13C NMR (DMSO-d6) δ 12.2, 42.9, 43.5, 96.1, 120.8, 131.2, 140.5, 142.9, 146.0, 149.7, 157.2, 163.0, 164.9, 165.2, 168.2, 169.0, 169.8, 170.0; MS: m/z 354 (M+Na)+. Anal. Calcd for C14H13N5O5: C, 50.76; H, 3.96; N, 21.14. Found: C, 50.75; H, 3.95; N, 21.12.
(E/Z)-3-(2-(3-Methoxybenzylidene)hydrazino)-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (4e)
Yield 90%; mp 174–176°C; IR: υmax 3257, 3219, 1677, 1619 cm−1; 1H NMR (DMSO-d6): δ 2.30 and 2.34 (2s, 3H, isoxazole-CH3), 3.40 and 3.76 (2s, 2H, CH2), 3.79 and 3.80 (2s, 3H, OCH3), 6.58 and 6.60 (2s, 1H, isoxazole-H), 6.95–7.40 (m, 4H, Ar-H), 8.00 and 8.30 (2s, 1H, N=CH), 9.98 and 10.02 (2s, 1H, NH), 11.21 and 11.26 (2s, 1H, NH); 13C NMR (DMSO-d6): δ 12.2, 43.2, 43.7, 56.0, 96.4, 114.9, 117.2, 122.3, 130.0, 135.0, 144.0, 147.1, 158.0, 159.9, 163.5, 165.2, 165.4, 169.2, 170.2; MS: m/z 317 (M+H)+, m/z 339 (M+Na)+. Anal. Calcd for C15H16N4O4: C, 56.96; H, 5.10; N, 17.71. Found: C, 56.98; H, 5.09; N, 17.72.
(E/Z)-3-(2-(3-Bromobenzylidene)hydrazino)-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (4f)
Yield 90%; mp 197–199°C; IR: υmax 3221, 1650, 1600 cm−1; 1H NMR (DMSO-d6): δ 2.33 and 2.36 (2s, 3H, isoxazole-CH3), 3.41 and 3.78 (2s, 2H, CH2), 6.51 and 6.53 (2s, 1H, isoxazole-H), 7.22–7.70 (m, 3H, Ar-H), 8.18 and 8.31 (2s, 1H, N=CH), 11.60 and 11.62 (2s, 1H, NH), 11.78 and 11.80 (2s, 1H, NH); 13C NMR (DMSO-d6): δ 11.9, 42.9, 43.6, 96.2, 122.0, 127.2, 130.9, 131.9, 132.8, 135.6, 144.3, 146.9, 157.2, 157.9, 163.9, 164.7, 165.1, 169.9, 170.6, 170.9; MS: m/z 365 (M+H)+; Anal. Calcd for C14H13BrN4O3: C, 46.05; H, 3.59; N, 15.34. Found: C, 46.02; H, 3.58; N, 15.32.
(E/Z)-3-(2-(2-Hydroxybenzylidene)hydrazino)-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (4g)
Yield 90%; mp 167–169°C; IR: υmax 3308, 3219, 1665, 1617 cm−1; 1H NMR (DMSO-d6): δ 2.30 and 2.34 (2s, 3H, isoxazole-CH3), 3.42 and 3.72 (2s, 2H, CH2), 6.50 and 6.52 (2s, 1H, isoxazole-H), 7.00–7.41 (m, 4H, Ar-H), 8.01 and 8.30 (2s, 1H, N=CH), 9.98 and 10.00 (2s, 1H, NH), 9.38 and 10.80 (2s, 1H, Ar-OH), 11.98 and 12.01 (2s, 1H, NH); 13C NMR (DMSO-d6): δ 11.7, 42.8, 43.2, 95.4, 117.1, 119.2, 122.5, 130.9, 133.2, 143.2, 145.9, 158.0, 160.2, 163.8, 164.5, 165.3, 169.2, 170.4; MS: m/z 303 (M+H)+, m/z 325 (M+Na)+. Anal. Calcd for C14H14N4O4: C, 55.63; H, 4.67; N, 18.53. Found: C, 55.65; H, 4.68; N, 18.52.
(E/Z)-3-(2-(2-Hydroxy-3-methoxybenzylidene)hydrazino)-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (4h)
Yield 94%; mp 180–182°C; IR: υmax 3448, 3197, 3155, 1697, 1616 cm−1; 1H NMR (DMSO-d6): δ 2.35 and 2.38 (2s, 3H, isoxazole-CH3), 3.40 and 3.71 (2s, 2H, CH2), 3.79 and 3.80 (2s, 3H, OCH3), 6.59 and 6.62 (2s, 1H, isoxazole-H), 6.69–7.20 (m, 3H, Ar-H), 8.29 and 8.40 (2s, 1H, N=CH), 9.29 and 10.69 (2s, 1H, Ar-OH), 11.09 (2s, 1H, NH), 11.46 and 11.78 (2s, 1H, NH); 13C NMR (DMSO-d6): δ 12.5, 42.9, 43.4, 56.3, 96.6, 113.2, 114.2, 118.0, 119.3, 119.5, 121.0, 140.8, 146.3, 147.3, 147.4, 148.4, 158.3, 158.5, 162.7, 165.7, 166.2, 168.7, 169.9, 170.1; MS: m/z 333 (M+H)+. Anal. Calcd for C15H16N4O5: C, 54.21; H, 4.85; N, 16.86. Found: C, 54.19; H, 4.84; N, 16.85.
(E/Z)-N-(5-Methylisoxazol-3-yl)-3-oxo-3-(2-(3,4,5-trimethoxybenzylidene)hydrazino)propanamide (4i)
Yield 94%; mp 199–200°C; IR: υmax 3179, 1679, 1619 cm−1; 1H NMR (DMSO-d6): δ 2.32 and 2.35 (2s, 3H, isoxazole-CH3), 3.41 and 3.72 (2s, 2H, CH2), 3.78 and 3.80 (2s, 9H, (OCH3)3), 6.51 and 6.52 (2s, 1H, isoxazole-H), 6.80 (s, 2H, Ar-H), 8.09 and 8.29 (2s, 1H, N=CH), 10.97 and 11.11 (2s, 1H, NH), 11.92 and 11.94 (2s, 1H, NH); 13C NMR (DMSO-d6): δ 11.9, 43.0, 44.0, 56.1, 95.9, 108.2, 108.9, 129.2, 129.4, 142.0, 143.2, 146.0, 151.6, 152.0, 157.2, 157.9, 163.6, 164.2, 164.9, 169.7, 170.1; MS: m/z 377 (M+H)+. Anal. Calcd for C17H20N4O6: C, 54.25; H, 5.36; N, 14.89. Found: C, 54.23; H, 5.35; N, 14.88.
(E/Z)-3-(2-(3,4-Dimethoxybenzylidene)hydrazino)-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (4j)
Yield 90%; mp 190–191°C; IR: υmax 3197, 3155, 1648, 1598 cm−1; 1H NMR (DMSO-d6): δ 2.32 and 2.36 (2s, 3H, isoxazole-CH3), 3.40 and 3.71 (2s, 2H, CH2), 3.75 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 6.52 and 6.54 (2s, 1H, isoxazole-H), 6.90–7.30 (m, 3H, Ar-H), 7.92 and 8.10 (2s, 1H, N=CH), 11.32 and 11.34 (2s, 1H, NH), 11.50 and 11.53 (2s, 1H, NH); 13C NMR (DMSO-d6): δ 12.2, 43.2, 44.1, 56.0, 96.2, 116.4, 116.9, 123.7, 128.2, 128.5, 143.9, 146.8, 151.2, 157.4, 157.8, 158.7, 163.4, 164.7, 165.2, 169.2, 169.9, 170.4; MS: m/z 347 (M+H)+; Anal. Calcd for C16H18N4O5: C, 55.49; H, 5.24; N, 16.18. Found: C, 55.46; H, 5.25; N, 16.20.
(E/Z)-3-(2-(2-Methylbenzylidene)hydrazino)-N-(5-methylisoxazol-3-yl)-3-oxopropanamide (4k)
Yield 90%; mp 204–205°C; IR: υmax 3216, 1650, 1600 cm−1; 1H NMR (DMSO-d6): δ 2.20 and 2.24 (2s, 3H, Ar-CH3), 2.32 and 2.35 (2s, 3H, isoxazole-CH3), 3.41 and 3.72 (2s, 2H, CH2), 6.50 and 6.51 (2s, 1H, isoxazole-H), 7.20–7.45 (m, 4H, Ar-H), 8.21 and 8.30 (2s, 1H, N=CH), 10.52 and 10.55 (2s, 1H, NH), 11.38 and 11.40 (2s, 1H, NH); 13C NMR (DMSO-d6): δ 12.2, 19.9, 42.8, 43.9, 95.9, 125.6, 126.4, 126.7, 128.6, 128.9, 130.8, 139.1, 144.0, 147.2, 158.2, 163.1, 165.1, 165.4, 169.6, 170.5; MS: m/z 301 (M+H)+. Anal. Calcd for C15H16N4O3: C, 59.99; H, 5.37; N, 18.66: Found: C, 59.97: H, 5.36: N, 18.64.
General procedure for the synthesis of N-(5-methylisoxazol-3-yl)-2-(5-aryl-1,3,4-oxadiazol-2-yl)acetamides 5a–k
A mixture of hydrazone 4 (0.1 mmol), chloramine-T (0.5 mmol) and ethanol (15 mL) was heated under reflux for 4–6 h. The progress of the reaction was monitored with TLC. Afterward, the mixture was poured into water and extracted with ethyl acetate. The extract was washed with water, dried over anhydrous sodium sulfate and concentrated under reduced pressure. After removal of the solvent, the crude product was passed over silica gel column. The product was eluted with 40% ethyl acetate in n-hexane.
N-(5-Methylisoxazol-3-yl)-2-(5-phenyl-1,3,4-oxadiazol-2-yl)acetamide (5a)
Yield 72%; mp 220–221°C; IR: υmax 3215, 1655, 1237 cm−1; 1H NMR (DMSO-d6): δ 2.30 (s, 3H, isoxazole-CH3), 3.42 (s, 2H, CH2), 6.58 (s, 1H, isoxazole-H), 7.13–7.17 (m, 1H, Ar-H), 7.31–7.35 (m, 2H, Ar-H), 7.47–7.49 (m, 2H, Ar-H), 9.50 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.3, 42.6, 96.4, 126.8, 127.9, 128.1, 128.9, 129.2, 129.9, 156.2, 164.2, 168.0, 169.4, 169.6; MS: m/z 285 (M+H)+. Anal. Calcd for C14H12N4O3: C, 59.15; H, 4.25; N, 19.71. Found: C, 59.13; H, 4.23; N, 19.69.
2-(5-(4-Chlorophenyl)-1,3,4-oxadiazol-2-yl)-N-(5-methylisoxazol-3-yl)acetamide (5b)
Yield 74%; mp 231–232°C; IR: υmax 3220, 1659, 1235 cm−1; 1H NMR (DMSO-d6): δ 2.56 (s, 3H, isoxazole-CH3), 3.41 (s, 2H, CH2), 6.48 (s, 1H, isoxazole-H), 7.15–7.40 (m, 4H, Ar-H), 9.52 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.5, 42.2, 96.0, 125.6, 129.2, 130.2, 134.8, 155.8, 164.0, 167.8, 169.0, 169.8; MS: m/z 319 (M+H)+; Anal. Calcd for C14H11ClN4O3: C, 52.76; H, 3.48; N, 17.58. Found: C, 52.74; H, 3.47; N, 17.56.
2-(5-(4-Methoxyphenyl)-1,3,4-oxadiazol-2-yl)-N-(5-methylisoxazol-3-yl)acetamide (5c)
Yield 74%; mp 226–227°C; IR: υmax 3215, 1685, 1261 cm−1; 1H NMR (DMSO-d6): δ 2.41 (s, 3H, isoxazole-CH3), 3.80 (s, 3H, OCH3), 4.19 (s, 2H, CH2), 6.55 (s, 1H, isoxazole-H), 6.70 (d, J=8.0 Hz, 2H, Ar-H), 7.29 (s, J=8.2 Hz, 2H, Ar-H), 9.25 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.7, 42.1, 56.1, 96.0, 114.2, 116.8, 128.9, 155.9, 160.9, 163.3, 167.8, 169.4, 169.9; MS: m/z 315 (M+H)+. Anal. Calcd for C15H14N4O4: C, 57.32; H, 4.49; N, 17.83. Found C, 57.34; H, 4.50; N, 17.85.
N-(5-Methylisoxazol-3-yl)-2-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)acetamide (5d)
Yield 70%; mp 250–251°C; IR: υmax 3230, 1656, 1230 cm−1; 1H NMR (DMSO-d6): δ 2.40 (s, 3H, isoxazole-CH3), 4.05 (s, 2H, CH2), 6.40 (s, 1H, isoxazole-H), 7.40 (d, J=8.0 Hz, 2H, Ar-H), 8.10 (d, J=8.0 Hz, 2H, Ar-H), 9.32 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.8, 41.9, 95.6, 122.8, 129.2, 135.2, 147.9, 155.8, 163.7, 167.2, 168.9, 170.0; MS: m/z 330 (M+H)+. Anal. Calcd for C14H11N5O5: C, 51.07; H, 3.37; N, 21.27. Found: C, 51.09; H, 3.38; N, 21.29.
2-(5-(3-Methoxyphenyl)-1,3,4-oxadiazol-2-yl)-N-(5-methylisoxazol-3-yl)acetamide (5e)
Yield 74%; mp 236–237°C; IR: υmax 3225, 1659, 1232 cm−1; 1H NMR (DMSO-d6): δ 2.51 (s, 3H, isoxazole-CH3), 3.80 (s, 3H, OCH3), 4.00 (s, 2H, CH2), 6.38 (s, 1H, isoxazole-H), 6.95–7.02 (m, 3H, Ar-H), 7.20–7.25 (m, 1H, Ar-H), 9.25 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.6, 42.5, 56.0, 94.9, 111.6, 115.1, 120.6, 128.5, 130.9, 155.6, 160.2, 164.2, 166.8, 169.2, 169.8; MS: m/z 315 (M+H)+. Anal. Calcd for C15H14N4O4: C, 57.32; H, 4.49; N, 17.83. Found: C, 57.30; H, 4.48; N, 17.82.
2-(5-(3-Bromophenyl)-1,3,4-oxadiazol-2-yl)-N-(5-methylisoxazol-3-yl)acetamide (5f)
Yield 72%; mp 240–241°C; IR: υmax 3215, 1650, 1228 cm−1; 1H NMR (DMSO-d6): δ 2.54 (s, 3H, isoxazole-CH3), 4.02 (s, 2H, CH2), 6.42 (s, 1H, isoxazole-H), 7.42–7.70 (m, 4H, Ar-H) 9.56 (s, 1H, NH); 13C NMR (DMSO-d6): δ 13.0, 42.3, 95.7, 124.2, 125.2, 129.0, 132.6, 132.8, 134.0, 155.8, 163.0, 165.9, 169.1, 169.9; MS: m/z 363 (M+H)+. Anal. Calcd for C14H11BrN4O3 C, 46.30; H, 3.05; N, 15.43. Found: C, 46.32; H, 3.06; N, 15.44.
2-(5-(2-Hydroxyphenyl)-1,3,4-oxadiazol-2-yl)-N-(5-methylisoxazol-3-yl)acetamide (5g)
Yield 68%; mp 208–210°C; IR: υmax 3221, 1660, 1236 cm−1; 1H NMR (DMSO-d6): δ 2.31 (s, 3H, isoxazole-CH3), 4.10 (s, 2H, CH2), 6.20 (s, 1H, isoxazole-H), 6.50–7.30 (m, 4H, Ar-H), 8.51 (s, 1H, Ar-OH), 9.62 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.2, 42.0, 95.3, 114.2, 117.1, 122.5, 130.2, 132.0, 154.8, 156.2, 163.5, 165.6, 169.8, 170.1; MS: m/z 301 (M+H)+. Anal. Calcd for C14H12N4O4: C, 56.00; H, 4.03; N, 18.66. Found: C, 56.03; H, 4.01; N, 18.65.
2-(5-(2-Hydroxy-3-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-N-(5-methylisoxazol-3-yl)acetamide (5h)
Yield 75%; mp 248–249°C; IR: υmax 3231, 1652, 1235cm−1; 1H NMR (DMSO-d6): δ 2.48 (s, 3H, isoxazole-CH3), 3.80 (s, 3H, OCH3), 3.99 (s, 2H, CH2), 6.45 (s, 1H, isoxazole-H), 6.90–7.18 (m, 3H, Ar-H), 8.71 (s, 1H, Ar-OH), 9.22 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.6, 42.4, 56.1, 95.2, 114.2, 116.5, 122.2, 124.0, 146.2, 152.2, 156.1, 163.9, 165.2, 168.9, 169.8; MS: m/z 331 (M+H)+. Anal. Calcd for C15H14N4O5: C, 54.55; H, 4.27; N, 16.96. Found: C, 54.58; H, 4.29; N, 16.98.
2-(5-(3,4,5-Trimethoxyphenyl)-1,3,4-oxadiazol-2-yl)-N-(5-methylisoxazol-3-yl)acetamide (5i)
Yield 70%; mp 216–217°C; IR: υmax 3229, 1669, 1222 cm−1; 1H NMR (DMSO-d6): δ 2.52 (s, 3H, isoxazole-CH3), 4.04 (s, 2H, CH2), 3.79–3.80 (s, 9H, (OCH3)3), 6.54 (s, 1H, isoxazole-H), 6.82 (s, 2H, Ar-H), 8.90 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.4, 41.9, 56.0, 56.2, 56.3, 95.5, 106.1, 106.2, 122.0, 141.2, 150.2, 150.4, 155.9, 163.5, 166.9, 168.9, 169.2; MS: m/z 375 (M+H)+. Anal. Calcd for C17H18N4O6: C, 54.54; H, 4.85; N, 14.97; Found: C, 54.52; H, 4.83; N, 14.95.
2-(5-(3,4-Dimethoxyphenyl)-1,3,4-oxadiazol-2-yl)-N-(5-methylisoxazol-3-yl)acetamide (5j)
Yield 71%; mp 228–229°C; IR: υmax 3232, 1665, 1232 cm−1; 1H NMR (DMSO-d6): δ 2.51 (s, 3H, isoxazole-CH3), 4.02 (s, 2H, CH2), 3.80 (s, 6H, (OCH3)2), 6.49 (s, 1H, isoxazole-H), 6.80 (m, 2H, Ar-H), 6.99 (d, J=8.0 Hz, 1H, Ar-H), 9.00 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.6, 42.0, 56.1, 56.2, 95.4, 114.2, 116.5, 121.2, 122.0, 150.2, 150.4, 155.8, 163.8, 166.5, 169.8, 170.1; MS: m/z 345 (M+H)+. Anal. Calcd for C16H16N4O5: C, 55.81; H, 4.68; N, 16.27. Found: C, 55.79; H, 4.67; N, 16.25.
N-(5-Methylisoxazol-3-yl)-2-(5-o-tolyl-1,3,4-oxadiazol-2-yl)acetamide (5k)
Yield 76%; mp 250–254°C; IR: υmax 3222, 1668, 1240 cm−1; 1H NMR (DMSO-d6): δ 2.20 (s, 3H, Ar-CH3), 2.52 (s, 3H, isoxazole-CH3), 4.00 (s, 2H, CH2), 6.45 (s, 1H, isoxazole-H), 7.05–7.40 (m, 4H, Ar-H), 9.02 (s, 1H, NH); 13C NMR (DMSO-d6): δ 12.6, 20.2, 42.2, 95.9, 127.2, 128.1, 129.0, 130.2, 138.0, 139.1, 156.0, 162.5, 165.8, 168.8, 170.8; MS: m/z 299 (M+H)+. Anal. Calcd for C15H14N4O3: C, 60.40; H, 4.73; N, 18.78. Found: C, 60.42: H, 4.74; N, 18.80.
Antibacterial activity
The antibacterial activity was assayed by the broth dilution method [29] and expressed as minimum inhibitory concentration. The nutrient broth medium (HiMedia, 24 g) was suspended in distilled water (100 mL) and heated to boiling until it dissolved completely. The medium and test tubes were autoclaved at a pressure of 15 lb/inch2 for 20 min. A set of sterilized test tubes with nutrient broth medium was capped with cotton plugs. The test compound 5a–k was dissolved in acetone and the concentration of 100 μg/mL of the test compound was added to the first test tube, which was serially diluted. A fixed volume of 0.5 mL was added to all test tubes, and the tubes were incubated at 37°C for 24 h. Then, the tubes were measured for turbidity. Bacterial strains used were Pseudomonas aeruginosa (MTCC 741), Klebsiella aerogenes (MTCC 39) Chromobacterium violaceum (MTCC 2656) (Gram-negative) and Bacillus subtilis (MTCC 441), Bacillus sphaericus (MTCC 511) and Staphylococus aureus (MTCC 96) (Gram-positive). Ciprofloxacin was used as the standard drug for comparison.
Antifungal activity
The antifungal activity was assayed using agar cup bioassay method [30]. The potato dextrose agar (PDA) medium (HiMedia, 39 g) was suspended in distilled water (1 L) and the mixture was heated to boiling until a solution was formed. The medium and Petri dishes were autoclaved at a pressure of 15 lb/inch2 for 20 min. The medium was poured into sterile Petri dishes under aseptic conditions in a laminar flow chamber. When the medium in the plates solidified, 0.5 mL of (week old) culture of the test organism was inoculated and uniformly spread over the agar surface with a sterile L-shaped rod. A solution was prepared by dissolving the compound 5a–k in acetone (100 μg/mL). Agar inoculated cups were scooped out with a 6-mm sterile cork borer, and the lids of the dishes were scooped out with a 6-mm sterile cork borer and the lids of the dishes were replaced. To each cup, 100 μg/mL concentration of the test solution 5a–k was added. Controls were maintained with acetone and clotrimazole (100 μg/mL). The treated mixtures and the controls were kept at room temperature for 72–96 h. The inhibition zone was measured as a diameter (mm). Three to four replicates were maintained for each treatment. The fungal strains Aspergillus niger (MTCC 282), Chrysosporium tropicum (MTCC 2821), Rhizopus oryzae (MTCC 262), Fusarium moniliforme (MTCC 1848) and Curvularia lunata (MTCC 2030) were used.
Acknowledgments
The authors are thankful to Department of Chemistry, University College of Science, Satavahana University, Karimnagar and Department of Chemistry, Siddhartha Degree and P.G. College, Narsampet for providing laboratory facilities. All authors are thankful to Prof. E. Rajanarendar for his valuable suggestions.
References
[1] Johns, B. A. Naphthyridine integrase inhibitors. PCT International Application WO patent 2004101512, 2004.Search in Google Scholar
[2] Moth, C. W.; Prusakiewicz, J. J.; Marnett, L. J.; Lybrand, T. P. Stereo selective binding of indomethacin ethanolamide derivatives to cyclooxygenase-1. J. Med. Chem.2005, 48, 3613–3620.10.1021/jm0494164Search in Google Scholar
[3] Dalip, K.; Swapna, S.; Emmanuel, O. J.; Kavitha, S.; Kumar, D.; Sundaree, S.; Johnson, E. O.; Shah, K. An efficient synthesis and biological study of novel indolyl-1,3,4-oxadiazoles as potent anticancer agents. Bioorg. Med. Chem. Lett.2009, 19, 4492–4494.10.1016/j.bmcl.2009.03.172Search in Google Scholar
[4] Holla, B. S.; Gonsalves, R.; Shenoy, S. Synthesis and antibacterial studies of a new series of 1,2-bis(1,3,4-oxadiazol-2-yl)ethanes and 1,2-bis(4-amino-1,2,4-triazol-3-yl)ethanes. Eur. J. Med. Chem.2000, 35, 267–271.10.1016/S0223-5234(00)00154-9Search in Google Scholar
[5] Barghi, A.; Tabatabai, S. A.; Faizi, M.; Ahadian, A.; Navabi, A.; Zanganeh, V.; Shafiee, A. Synthesis and anticonvulsant activity of new 2-substituted-5-(2-benzyloxyphenyl)-1,3,4-oxadiazole. Bioorg. Med. Chem. Lett.2005, 15, 1863–1865.10.1016/j.bmcl.2005.02.014Search in Google Scholar
[6] Husain, A.; Ajmal, M. Synthesis of novel 1,3,4-oxadiazole derivatives and their biological properties. Acta Pharm.2009, 59, 223–233.10.2478/v10007-009-0011-1Search in Google Scholar
[7] Kucukguzel, S. G.; Orue, E. E.; Rollas, S.; Sahin, F.; Ozbek, A. Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem.2002, 37, 197–206.10.1016/S0223-5234(01)01326-5Search in Google Scholar
[8] Zou, X. J.; Lai, L. H.; Jin, G. Y.; Zhang, Z. X. Synthesis, fungicidal activity, and 3D-QSAR of pyridazinone-substituted 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. J. Agric. Food Chem.2002, 50, 3757–3760.10.1021/jf0201677Search in Google Scholar PubMed
[9] Reddy, G. D.; Park, S.; Cho, H. M.; Kim, T. J.; Lee, M. E. Antiallergic activity profile in vitro RBL-2H3 and in vivo passive cutaneous anaphylaxis mouse model of new sila-substituted 1,3,4-oxadiazoles. J. Med. Chem.2012, 55, 6438–6444.10.1021/jm300421hSearch in Google Scholar PubMed
[10] Savarino, A. A historical sketch of the discovery and development of HIV-1 integrase inhibitors. Expert Opin. Investig. Drugs2006, 15, 1507–1522.10.1517/13543784.15.12.1507Search in Google Scholar PubMed
[11] James, N. D.; Growcott, J. W. Zibotentan. Drugs Future.2009, 34, 624–633.10.1358/dof.2009.034.08.1400202Search in Google Scholar
[12] Brandenburger, H.; Maes, R. A. A. Clinical Biochemistry: Analytical Toxicity for Clinical, Forensic and Pharmaceutical Chemists; 5th Edition Walter de Gruyter: Berlin, 1997.10.1515/9783110881615Search in Google Scholar
[13] Adlstein, G. W.; Yen, C. H.; Dajani, E. Z.; Bianchi, R. G. 3,3-Diphenyl-3-(2-alkyl-1,3,4-oxadiazol-5-yl)propylcycloalkylamines, a novel series of antidiarrheal agents. J. Med. Chem.1976, 19, 1221–1225.10.1021/jm00232a010Search in Google Scholar
[14] Ogata, M.; Atobe, H.; Kushida, H.; Yamamoto, K. In vitro Sensitivity of mycoplasmas isolated from various animals and sewage to antibiotics and nitrofurans. J. Antibiot.1971, 24, 443–451.10.7164/antibiotics.24.443Search in Google Scholar
[15] Daidone, G.; Raffa, D.; Maggio, B.; Plescia, F.; Cutuli, V. M. C.; Mangano, N. G.; Caruso, A. Synthesis and pharmacological activities of novel 3-(isoxazol-3-yl)-quinazolin-4(3H)-one derivatives. Arch.Pharm. Med. Chem.1999, 332, 50–54.10.1002/(SICI)1521-4184(19993)332:2<50::AID-ARDP50>3.0.CO;2-SSearch in Google Scholar
[16] Rajanarendar, E.; Nagi Reddy, M.; Rama Krishna, S.; Govardhan Reddy, K.; Reddy, Y. N.; Rajam, M. V. Design, synthesis, in vitro antimicrobial and anticancer activity of novel methylenebis-isoxazolo[4,5-b]azepines derivatives. Eur. J. Med. Chem.2012, 50, 344–349.10.1016/j.ejmech.2012.02.013Search in Google Scholar
[17] Hirpara, K.; Patel, S.; Joshi, A.; Parekh, H. Synthesis and biological evaluation of some cyanopyridines and isoxazoles. Indian J. Heterocycl. Chem.2004, 13, 221–224.Search in Google Scholar
[18] Uno, H.; Kurokawa, M.; Masuda, Y.; Nishimura, H. Studies on 3-substituted 1,2-benzisoxazole derivatives. 6. Syntheses of 3-(sulfamoylmethyl)-1,2-benzisoxazole derivatives and their anticonvulsant activities. J. Med. Chem.1979, 22, 180–183.10.1021/jm00188a011Search in Google Scholar
[19] Li, W.-T.; Hwang, D.-R.; Chen, C.-P.; Shen, C.-W.; Huang, C.-L.; Chen, T.-W.; Lin, C.-H.; Chang, Y.-L.; Chang, Y.-Y.; Lo, Y.-K. et al. Synthesis and biological evaluation of N-heterocyclic indolyl glyoxylamides as orally active anticancer agents. J. Med. Chem.2003, 46, 1706–1715.10.1021/jm020471rSearch in Google Scholar
[20] Randall, L. O.; Bagdon, R. E. Pharmacology of iproniazid and other amine oxidase inhibitors. Ann. N. Y. Acad. Sci.1959, 80, 626: Chem. Abstr.1959, 53, 11630b.10.1111/j.1749-6632.1959.tb49241.xSearch in Google Scholar
[21] Satoda, I.; Fukui, T.; Mori, K. Synthesis of 3,4-tetramethylene-5-sulfanilamidoisoxazol and its derivatives. Yakugaku Zasshi1959, 79, 961: Chem. Abstr.1959, 53, 21885h.10.1248/yakushi1947.79.7_961Search in Google Scholar
[22] Talley, J. A.; Brown, D. L.; Carter, J. S.; Mafferrer, M. J.; Perkins, W. E.; Rogers, R. S.; Shaffer, A. F.; Zhang, Y. Y.; Zweifel, B. S. Seibert. 4-[5-Methyl-3-phenylisoxazol-4-yl]-benzenesulfonamide, Valdecoxib: a potent and selective inhibitor of COX-2. J. Med. Chem.2000, 43, 775–777.10.1021/jm990577vSearch in Google Scholar
[23] Miranada, N. G.; Leanos, M. B. E.; Vilchis, P. M.; Solorzano, S. F. In vitro activity effects of combinations of cephalothin, dicloxacillin, imipenem, vancomycin and amikacin against methicillin-resistant Staphylococcus spp. Strains. Ann. Clin. Microbial Antimicrob.2006, 5, 25.10.1186/1476-0711-5-25Search in Google Scholar PubMed PubMed Central
[24] Thakkar, M. M.; Winston, S.; McCarley, R. W. Effect of microdialysis perfusion of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol in the perifornical hypothalamus on sleep-wakefulness: role of -subunit containing extrasynaptic GABAA receptors. Neuroscience2008, 153, 551–553.10.1016/j.neuroscience.2008.02.053Search in Google Scholar PubMed PubMed Central
[25] Ramu, K.; Srinivas, M.; Murali Krishna, M. P. S.; Parusharamulu, M.; Reddy, Y. N. Synthesis and biological evaluation of 3,4-dihydro-3-(3-methylisoxazol-5-yl)-2H-benzo[e][1,3]oxazine derivatives as anticancer agents. Lett. Org. Chem.2018, 15, 124–132.10.2174/1570178614666170623121207Search in Google Scholar
[26] Rajanarendar, E.; Ramu, K.; Shiva Rami Reddy, A.; Shaik, F. P. Synthesis and in vitro study of novel isoxazolyl benzamides, acrylamides and propanamides as antifungal agents. Indian J. Chem.2008, 47B, 1284–1290.Search in Google Scholar
[27] Rajanarendar, E.; Ramu, K.; Karunakar, D.; Ramesh, P. Microwave-assisted synthesis of new isoxazolyl triazinethiones and isoxazolyl oxadiazinethiones in dry media. J. Heterocycl. Chem.2005, 42, 711–715.10.1002/jhet.5570420437Search in Google Scholar
[28] Rajanarender, E.; Ramu, K.; Ramesh, P. Synthesis of 2-oxo-2H-chromene-3-carboxylic acid (5-methyl-3-isoxazolyl) and (3-methyl-5-styryl-4-isoxazolyl) amides as potential bioactive compounds. Indian J. Chem.2004, 43B, 1790–1793.10.1002/chin.200450100Search in Google Scholar
[29] National Committee for Clinical Laboratory Standards (NCCLS). Standard methods for dilution antimicrobial susceptibility tests for bacteria, which grows aerobically. Nat. Comm. Clin. Lab Standards, Villanova, 1982, 242.Search in Google Scholar
[30] Margery Linday, E. Practical Introduction to Microbiology; E & FN Spon Ltd.: UK, 1962, pp 177.Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Antioxidant, α-glucosidase inhibitory and in vitro antitumor activities of coumarin-benzothiazole hybrids
- Synthesis and properties of tetracyanoquinodimethane derivatives
- Research Articles
- Synthesis, characterization and computational studies of 2-cyano-6-methoxybenzothiazole as a firefly-luciferin precursor
- Synthesis of fluorine-containing phthalocyanines and investigation of the photophysical and photochemical properties of the metal-free and zinc phthalocyanines
- Copper-catalyzed synthesis of 2,3-disubstituted quinazolin-4(3H)-ones from benzyl-substituted anthranilamides
- Synthesis and mass spectrometric fragmentation pattern of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines
- An efficient cascade synthesis of substituted 6,9-dihydro-1H-pyrazolo[3,4-f]quinoline- 8-carbonitriles
- Synthesis and antimicrobial evaluation of isoxazole-substituted 1,3,4-oxadiazoles
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Antioxidant, α-glucosidase inhibitory and in vitro antitumor activities of coumarin-benzothiazole hybrids
- Synthesis and properties of tetracyanoquinodimethane derivatives
- Research Articles
- Synthesis, characterization and computational studies of 2-cyano-6-methoxybenzothiazole as a firefly-luciferin precursor
- Synthesis of fluorine-containing phthalocyanines and investigation of the photophysical and photochemical properties of the metal-free and zinc phthalocyanines
- Copper-catalyzed synthesis of 2,3-disubstituted quinazolin-4(3H)-ones from benzyl-substituted anthranilamides
- Synthesis and mass spectrometric fragmentation pattern of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines
- An efficient cascade synthesis of substituted 6,9-dihydro-1H-pyrazolo[3,4-f]quinoline- 8-carbonitriles
- Synthesis and antimicrobial evaluation of isoxazole-substituted 1,3,4-oxadiazoles


