Abstract
Oil-impregnated paper is the most widely used insulating material for power transformers. Power transformers inevitably generate a lot of heat during operation. Among them, thermal aging is one of the main forms of aging for insulating paper. In this study, the ab initio molecular dynamics method based on the density functional theory is used to simulate the cracking mechanism of cellobiose under transient high temperature. The results show that the cellobiose is relatively stable at 343 K, the motility of the cellobiose is enhanced at 1,800 K, the cellobiose starts to decompose at 2,400 K, and new characteristic products are formed at 3,000 K. The characteristic products include CO, H2O, CH3OH, H2, and CH4. These characteristic products can represent the degree of cracking of insulating paper. Therefore, it is necessary to explore the mechanism of cracking of insulating paper caused by transient high temperature.
1 Introduction
Power transformer is one of the main power equipment in the power system that plays a role in transformation of electrical energy (1), and oil-paper insulation is the main insulation medium in today’s high-voltage transformers (2,3). In actual operation, insulating paper inside the transformer will generate a lot of heat (4), and thermal aging is the main form of deterioration of insulating paper in various aging forms (5). A large number of studies on the thermal aging of transformer oil-paper insulation have focused on the long-term aging process of the insulating paper, such as extracting characteristic parameters to generalize the aging law based on macroscopic accelerated aging experiments. The International Electrotechnical Commission points out that the physical and chemical performance parameters of oil-paper insulation system itself can be used as the characteristic quantity of aging evaluation during the aging process of transformer (6). The main characteristic parameters include dissolved gas analysis in transformer oil (7), tensile strength and degree of polymerization (DP) of insulation paper, and furfural content in oil (8,9). The DP is often used in previous studies to describe the structural integrity of the insulating paper, with a DP of 500, indicating that the paper is in the middle of its life, and a DP as low as 250 requiring immediate replacement with new paper (10,11,12). The process of thermal aging of insulating paper is complex, and the mechanism of chemical reactions in the condensed phase of pyrolysis is a key to a comprehensive understanding of cellulose pyrolysis (13). In recent years, a number of scholars have attempted to explore the stability of cellulose itself, complementing experimental data on cellulose pyrolysis with electronic structure calculations and molecular simulations (14,15,16,17,18). This allows the capture of individual chemical reactions that occur during decomposition (19,20,21,22). To explore the properties of cellulose, numerous studies have been carried out by scholars using molecular dynamics methods based on conventional mechanics. Due to the different physical and chemical properties of the crystalline and amorphous regions of cellulose (23), each part of the cellulose has different stability (24,25). In addition to the properties of cellulose itself, which affect the stability of insulating paper, temperature is also a major factor (26,27). To further understand the stability of insulating cellulose at the microscopic level, previous authors have investigated the cracking mechanism of insulating cellulose based on quantum mechanical methods. The formation and disappearance of chemical bonds, which act as bridges connecting atoms, directly affect the stability of cellulose itself. Moreover, the interaction of the aging product with its own atoms will directly lead to the formation and loss of chemical bonds (28,29). The integrity of the chemical bond between the glycosidic bond and the pyran ring in the cellobiose directly determines the DP of cellulose in the insulating paper (15,23).
However, the mechanism of cracking at transient high temperature has not yet been elucidated. Transient high temperature can easily cause local damage to the insulating paper. When the insulation paper has a fault similar to the point defect, the local insulation performance of the insulation paper will be greatly reduced. The point defects are the beginning of the cracking of the insulating paper. Therefore, it is necessary to carry out a theoretical study of the mechanism of thermal cracking at transient high temperature. In this article, the radial distribution function (RDF) method is used to verify the effect of instantaneous high temperature on the cellulose of insulating paper. The mechanism of thermal cracking at transient high temperature is explained by the ab initio molecular dynamics from the perspective of bond breaking and atom bonding. As the earliest characteristic product, CH3OH can be used as an important indicator of the early aging of insulating paper (DP > 900) (30,31). The content of CO and H2O can be used as the characteristic gas to detect the degree of decomposition inside the insulating paper (32). This also makes up for the theoretical gap that the furfural can only be used to monitor the aging state of the insulating paper at intermediate and late stages, but not at early stages (33).
2 Theory and simulation details
2.1 Calculation theory
The calculation of electronic ground state is a complex quantum many-body problem. Density functional theory can reduce the calculation amount on the basis of ensuring the calculation accuracy (34). The Ab initio molecular dynamics method is based on density functional theory to transform complex multibody problems into a set of self-consistent single-electron orbital equations (35,36). Compared with conventional molecular dynamics simulations, ab initio molecular dynamics is computationally more accurate, takes longer to compute, and is computationally more expensive. Therefore, ab initio molecular dynamics is more suitable for the calculation of small molecular systems. However, insulating paper cellulose is a type of polymer, and the aging process can take years under natural conditions. To reduce the simulation timescale and ensure the accuracy of the simulation, a reasonably high temperature should be set during the simulation to accurately predict the pyrolytic behavior of cellulose molecules in insulator paper under transient high temperature.
2.2 Simulation details settings
Cellobiose molecules are the basic skeleton of cellulose in insulating paper, which is polymerized from cellulose. The cellobiose model is constructed by Materials Studio, and the periodic boundary conditions are established with a box size of 18 × 18 × 18 Ǻ. As shown in Figure 1, the configuration is first geometrically optimized to approximate the exchange-correlation potential using generalized gradient correction and Perdew–Bruke–Ernzerhof (PBE) function (37,38). In view of the importance of the long-range dispersion correction for weakly interacting systems, the Grimme dispersion correction is added to the PBE generalization function. Ab initio molecular dynamics simulation is performed when the geometric optimization converged. The 5 ps kinetic simulations are performed for the cellobiose model under an isothermal-isochoric (NVT) ensemble systems as well as a Nose–Hoover thermostat to bring the system to equilibrium at 298.15 K (39). The equilibrated system is then subjected to 15 ps Ab initio molecular dynamics simulation at 343, 1,800, 2,400, and 3,000 K, respectively.

Cellobiose model with boundary conditions.
3 Results and discussion
3.1 Effect of transient high temperature on thermal cracking
The pyran rings are the main skeleton of the cytosol, where the C–C and C–O bonds are the main building blocks of the pyran rings, and the C–O bond is also the main chemical bond forming the glycosidic bond. At the same time, hydrogen bonding also plays a crucial role in the stability of cellulose, where hydrogen bonding is mainly caused by hydroxyl groups. In particular, it is important to investigate the stability of the C–C bond, the C–O bond, and the O–H bond.
RDFs are often used to describe the probability of occurrence of other particles around a certain particle in a dynamical problem. The stability of the chemical bonds of cellobiose molecules can be explored by plotting the RDF of different chemical bonds at the corresponding temperatures separately using VMD software. The equation used to calculate the RDF is shown in Eq. 1 (40), where g(r) is a function describing the density of particles at a certain distance from the reference particle, which reflects the probability of finding another particle in a spherical space with radius from r to r + δr, and V is the volume of the system; moreover, N is the number of atoms. We can see a summary diagram of the RDF in Figure 2.
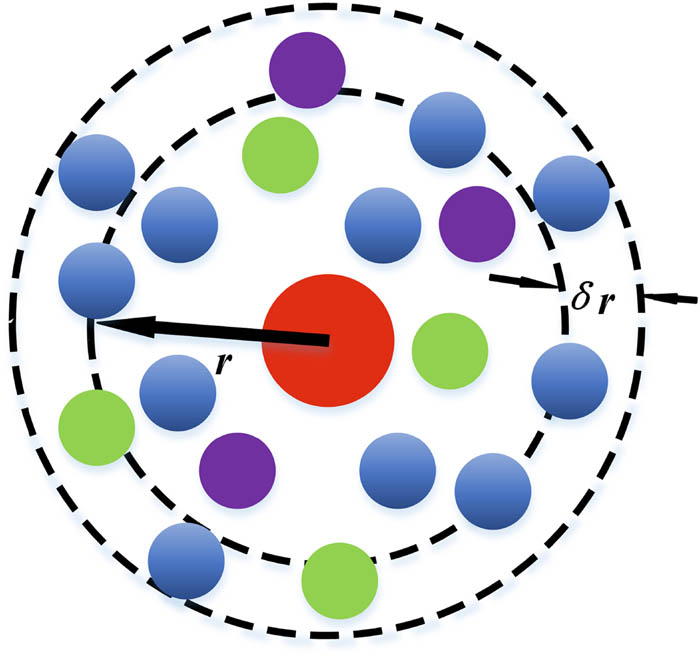
Summary diagram of radial distribution function.
As shown in Figure 3, the horizontal axis r in the four plots represents the interatomic distance and the vertical axis g(r) represents the particle distribution probability. The valence r corresponding to the peak of each curve is exactly the number of corresponding bond lengths corresponding to Table 1. As the temperature increases, the value of the award distribution function for each chemical bond decreases, and temperature is able to influence the composition of these three types of chemical bonds. Among the four temperatures, the RDF values of the three bonds are the largest at 343 K. Although the RDF values of the three bonds at 1,800 K change much compared with 343 K, the bonds are not broken. It can be seen that the transient high temperature enhances the kinetic activity of the cellobiose. The increasing distance between cellobiose molecules is not conducive to polymerization. At temperatures below 2,400 K, the same temperature basically follows the largest value of the RDF for the C–C bond, followed by the C–O bond, and the smallest value of the RDF for the O–H bond, which also indicates that the temperature below 2,400 K has less effect on the basic skeleton of the cellobiose. When the temperature reaches 3,000 K, the values of the RDFs for the different chemical bonds change, with the largest changes for the C–C bond and the C–O bond, indicating that the temperature affects the C–C bond and the C–O bond much more than the O–H bond. This is also related to the bond energy and length, as shown in Table 1. The C–C and C–O bonds have similar energies and are much smaller than the O–H bond. The smaller the bond energy, the easier it is to be broken.

Radial distribution functions of C–C, C–O, and C–H bonds at (a) 343, (b) 1,800, (c) 2,400, and (d) 3,000 K.
Bond energy and bond length of C–C, C–O, and O–H bonds
| C–C bond | C–O bond | O–H bond | |
|---|---|---|---|
| Length of bond (Å) | 1.54 | 1.43 | 0.96 |
| Energy of bonds (kJ‧mol−1) | 332 | 326 | 464 |
3.2 Analysis of cellobiose bond breaking and characteristic product generation process
3.2.1 Cellobiose bond-breaking process
From Figures 4a and 5a, it can be seen that the glycosidic bond breaking, pyran ring opening, and C–C bond breaking of the C atoms attached to the glycosidic alcohols mainly occur at 2,400 K in cellobiose. At 11.432 ps, the glycosidic bond C23–O31 is broken first and the cellobiose split. The C4–O5 bond and the pyran ring are broken at 11.542 ps. Two C–C chemical bonds, C10–C6 and C28–C32, are broken at 11.78 ps and 11.81 ps, respectively. When the simulation time increased to 12.788 ps, C32–H42 is broken. In summary, it is not difficult to find the order of bond breaking in this order, C–O bond, C–C bond, C–H bond, consistent with the law of variation for different bond energy sizes in Table 1. It is clear that the main structure of the cell body is fully cleaved. When the temperature reaches or even exceeds 2,400 K, the transient high temperature immediately causes the bonds of cellulose to break.

(a) Bond breaking of cellobiose at 2,400 K. (b) and (c) Bond breaking of two cellobiose molecules at 3,000 K. (d) Atomic serial number and corresponding atom.

(a) Diagram of cellobiose cleavage process at 2,400 K. (b) and (c) Diagram of cellobiose cleavage process at 3,000 K.
As shown in Figures 4b and 5b, the glycoside bond C23–O31 breaks at 3,000 K and 1.283 ps. The C4–O5 bond on the pyran ring breaks at 1.319 ps, which also marks the opening of the pyran ring. Then a lot of C–O, C–C bonds crack. Meanwhile, the cleavage process of another cellobiose at 3,000 K can be seen in Figures 4c and 5c. The glucoside bond C48–O75 breaks first at 0.874 ps. At a time of 0.974 ps, the C50–O49 bond on the pyran ring breaks. A large number of C–O bonds and many C–C bonds crack after 1.158 ps.
It is found that the glucoside bond, as the weakest chemical bond, breaks first by comparing the cleavage of cellobiose at 2,400 and 3,000 K. This is also one of the main factors that reduces the DP of insulating paper under extreme conditions. Second, the C–O bond on the pyrane ring also breaks immediately after the glycoside bond. The service life of insulating paper is determined by the opening of the pyran ring and the breaking of the glycoside bond.
3.2.2 Analysis of characteristic product generation process
Instantaneous high temperature not only causes thermal cracking of cellobiose but also produces new characteristic products. To explore the mechanism of cracking, the generation process of various products is described in detail at 3,000 K.
3.2.2.1 The formation of H2O molecules
The two sources of H2O molecules are shown in Figure 7a and b. Figure 6a shows that the C70–H82 chemical bond breaks at 3.434 ps and the H82 atom is shed. This is immediately followed by the C76–O77 bond breaking at 3.533 ps, and the primary alcohol hydroxyl O77–H88 is shed. The detached primary alcohol hydroxyl group O77–H82 forms a H2O molecule with the H82 atom at 3.592 ps. Figure 7a demonstrates the origin of the radicals in the H2O molecule, where the primary alcohol hydroxyl group combines with the H atom on the pyran ring to form H2O.

AIMD simulation of the variation of bond length with time for cellobiose broken bonds of insulating paper and H2O bonding.

(a) The primary alcohol hydroxyl group combines with the H atom on the pyran ring to form H2O. (b) The glycoside bond breaks and dehydrogenates the secondary alcohol hydroxyl group to form H2O.
The H2O molecule shown in Figure 7b is formed by the interaction of two cellobiose molecules. Figure 6b shows that C48–O75 breaks at 0.873 ps causing O75 to fall off, while the strong electronegativity of the oxygen atom attracts the H85 atom that has not yet broken. The O75–H85 bond breaking occurs at 0.894 ps. The broken H85 atom immediately combines with the O75 atom to form an O–H bond. The time increases to 2.773 ps when the O7–H17 bond breaks, dislodging the H17 atom. The C67–O75 bond is broken when the simulation time reaches 7.014 ps, allowing the O75–H85 to completely detach from the cellobiose. At 7.275 ps, O75–H17 binds to form a chemical bond, at which point the H2O molecule is fully formed. The H2O molecule formation process is dominated by C–O bonding, O–H bond breaking, and the formation of new O–H bonds. There is a direct link between the formation of H2O molecules and the breaking of one of the glycosidic bonds of the cytosol, which is completely broken. This also reflects the fact that the amount of dissolved H2O in oil in oil-immersed transformers is one of the measures to characterize the degree of aging of insulating paper. In the formation of both H2O molecules, it was found that when the C–O bond is broken, the O atoms in the secondary alcohol hydroxyl group are more electronegative and also more likely to cause hydrogen capture and dehydration of the O atoms.
3.2.2.2 The formation of CO
The formation process of CO is complex, and the three different pathways of CO cleavage are illustrated in Figure 9a–c. The bond breakage of C23–O31 at 1.274 ps shown in Figure 8a marks the glycosidic bond damage. Immediately, the bond breaking at 1.311 ps occurs at C4–O5, and since C4–O5 is a component of the pyran ring, the pyran ring opens at this point. The formation of CO is closely related to the glycosidic bond and the pyran ring as shown in Figure 9a, which requires both glycosidic bond breakage and pyran ring opening to form CO, and the C–O bond is derived from the glycosidic bond.

AIMD simulation of the variation of bond length with time for cellobiose broken bonds and CO bonding of insulating paper.

(a) The C–O bond on the glycoside bond dissociates to form CO. (b) The C atom on the pyran ring dissociates with the O atom on the hydroxyl group to form CO. (c) The C–O bond on the pyran ring dissociates to form CO.
The formation of CO is shown in Figure 8b, where the C1–C6 bond breaks at 1.378 ps causing the pyran ring to open. The C1–C2 bond is broken at 9.769 and 9.731 ps, respectively. The C1–H12 bond breaks when the simulation time reaches 10.626 ps, allowing C1–O9 to be free to form CO. It can also be seen from Figure 9b that the two C–C bonds on the pyran ring break first causing the pyran ring to open, followed by the H atom on the C atom of the secondary alcohol shedding the H atom on the attached hydroxyl group to eventually form CO. The formation of this type of CO is related to the O atom on the pyran ring and the hydroxyl group.
The chemical bonds C4–O5, C1–C6, C6–C10, and C6–H16 are broken within 1.317–1.416 ps in Figure 8c, respectively, and C6–O5 on the pyran ring broke off to form CO. As shown in Figure 9c, the C–O bonds on the pyran ring are broken successively with the chemical bonds formed by the surrounding atoms. Finally, the C–O bond on the pyran ring breaks free to form CO. In summary, there are three ways of CO formation, which are formed by the C–O bond on the glycosidic bond, the C atom of the secondary alcohol on the pyran ring with the O atom on the attached hydroxyl group, and the C–O bond on the pyran ring breaking free.
3.2.2.3 The formation process of H2
Figure 10 depicts the formation of H2. The C2–H13, O9–H19 chemical bond is broken at 9.737 ps when the cellobiose is continuously cleaved at a transient high temperature. The two free H atoms combine to form H2. Figure 11 shows a schematic diagram of the source of hydrogen H2, where the seco-alcohol C atom on the pyran ring is dehydrogenated; the adjacent seco-alcohol C atom is dehydrogenated by attachment to the hydroxyl group; and the two free H atoms combine to form H2.

AIMD simulation of the variation of bond length with time for cellobiose broken bonds and H2 bond formation in insulating paper.

The H atom on the hydroxyl group combines with the H atom attached to the C atom in the pyran ring to form H2.
3.2.2.4 The formation process of CH4
The methane formation process is represented in Figure 12. The C32–O33 bond is broken when the cleavage time reaches 5.5 ps. The hydrogen atom H78 then forms a new bond with the carbon atom C32 at 6.566 ps. The C32–C28 bond is broken at 9.315 ps, when the C32 valence electron is in an unsaturated state. The free methyl group forms a new C–H bond with the hydrogen atoms H58 and H78 at 11.173 and 6.601 ps, respectively. The methane molecule is formed. The CH4 radical shown in Figure 13 is associated with the source of the constituent atoms. The C–O bond formed by the C atom of the primary alcohol with the attached hydroxyl group and the C–C bond formed with the C atom on the pyran ring break at high temperatures. Afterward, the C atom of the glycosidic bond of the other cellobiose sheds an H atom, the C atom on the pyran ring sheds an H atom, and the free two H atoms combine with the C atom of the secondary alcohol to form methane CH4.

AIMD simulation of the variation of bond length with time for cellobiose broken bonds and CH4 bond formation in insulating paper.

The free radical CH2 from the primary alcohol C atom combines with the H atom from the secondary alcohol C atom to form CH4.
3.2.2.5 The formation of CH3OH
The formation process of CH3OH can be divided into two categories, one is the shedding and reorganization of the cellobiose own atoms and radicals. From Figure 14a, it can be seen that the simulation time breaks the C10–C6 and C6–H16 chemical bonds at 1.357 ps and 1.403 ps, respectively, and the free H16 forms a new chemical bond with C10. Figure 15a shows that the C–C bond formed by the C atom of the primary alcohol with the C atom on the pyran ring breaks, and the C–H bond formed by the H atom attached to the C atom on the pyran ring breaks, and the shed methanol group CH2OH combines with the H atom to produce CH3OH.

AIMD simulation of the variation of bond length with time for cellobiose broken bonds and CH3OH bond formation in insulating paper.

(a) After the C–C bond of primary alcohol is broken and dissociated, a free radical is generated and bonded with its own hydrogen atom to form CH3OH. (b) The primary alcohol C–C bond breaks and dissociates, resulting in a free radical that combines with the hydrogen atom of another cellobiose to form CH3OH.
The other category is the free combination of two cellobiose molecules with their own atoms shedding free radicals. Figure 14b shows the formation process of the second type of methanol molecule. The first to break the bond is C50–C54, and the valence electron layer, which is originally saturated with C54, is no longer stable. With time, several chemical bonds are broken, and new bonds are formed between 7.1 and 7.2 ps. H66 on O55 is freed and bonds with C54, and the valence electron layer of C54 reaches stable saturation again. O55 captures the H39 atom on C28, and a new O–H bond is formed after the C–H bond is broken. Figure 15b shows the origin of the composition of this type of methanol molecule. The C–C bond consisting of the C atom of the primary alcohol and the C atom on the pyran ring breaks, along with a break in the primary alcohol hydroxyl group. The C atom on the pyran ring of another cellobiose breaks the C–H bond with the attached H atom, and a bond break occurs in the own primary alcohol hydroxyl group. The free radical CH2O combines with the detached H atom to form a new chemical bond, allowing the formation of the methanol molecule CH3OH.
The main products of cellulose at instantaneous high temperatures are shown in Figure 16. The highest content of H2O and CO is followed by hydrogen H2 and CH3OH, and the lowest content is methane CH4. Insulating paper is the only source of CH3OH in oiled paper insulation system. Even though there is less CH3OH after thermal cracking of insulating paper, CH3OH is the first to be produced by insulating paper under instantaneous high temperature. Therefore, the content of CO, H2O, and CH3OH, and the rate of CH3OH production can be used to monitor partial discharge faults.

Diagram of product content.
4 Conclusions
In this study, the influence of transient high temperature on thermal cracking of insulating paper is explored. In this simulation, it is found that when the temperature is higher than 2,400 K, the glycosidic bond and pyran ring would first crack in a short time. The primary source of H2O is the glycosidic bond. The strong electronegativity of the O atom is also the main driving force for the formation of H2O. Dehydration will cause a rapid decline in the DP of insulating paper. CO is mainly derived from the C–O bond on the glycosidic bond and the C–O bond on the pyran ring. The production of a large amount of CO proves that the pyranoid ring is heavily cleaved. Therefore, the content of H2O and CO can directly explain the degree of thermal cracking of insulating paper. H2 and CH4 are produced in a similar process, both of which are formed by the recombination of atoms and chemical bonds connected to the pyran ring. CH3OH is formed by breaking the bond between C atom of primary alcohol on the pyran ring and C atom on the pyran ring and combining with H atom. The presence of CH3OH confirms that the chemical bond to the pyran ring is not stable. In addition to cracking of the glycosidic bond and the main body of the pyran ring, the part connected to the main body will also be largely cracked. Finally, the insulating paper loses its insulation property at instantaneous high temperature. As a result, transient high temperature will cause thermal cracking of insulating paper. Glycosidic bond, pyran ring, and the broken bond recombination of atoms and chemical bonds connected with the pyran ring result in thermal cracking of insulating paper. This study also shows that the content of CO, H2O, and CH3OH as well as the CH3OH production rate is a feasible method to detect partial point defects. CH3OH is a unique cracking product of insulating paper. By monitoring CH3OH, the operating conditions of the insulating paper can be determined earlier. This will be conducive to the improvement of power quality and the long-term stable operation of the power system.
-
Funding information: This work was supported by The National Natural Science Foundation of China (52007138) and Key Research and Development Program of Shaanxi Province (2023-YBGY-070).
-
Author contributions: Hao Yang: writing – review and editing; Xin Wang: writing – original draft and formal analysis; Yimeng Duan: data curation and methodology; Haotian Zhang: data curation and methodology; Miaomiao Chen: conceptualization; Xinyu Wang: investigation. All the authors have read the paper and commented on the text.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data in this article can be obtained by contacting the corresponding author.
References
(1) Bakar NA, Abu-Siada A, Islam S. A review on chemical diagnosis techniques for transformer paper insulation degradation. IEEE Power Engineering Conference; 2014. 10.1109/AUPEC.2013.6725476.Search in Google Scholar
(2) Cui HZ, Yang LQ, Zhu YW, Li ST, Abu-Siada A, Islam S. A comprehensive analyses of aging characteristics of oil-paper insulation system in HVDC converter transformers. IEEE Trans Dielectr Electr Insulation. 2020;27(5):1707–14. 10.1109/TDEI.2020.008788.Search in Google Scholar
(3) Murugan R, Ramasamy R. Understanding the power transformer component failures for health index-based maintenance planning in electric utilities. Eng Fail Anal. 2018;96:274–88. 10.1016/j.engfailanal.2018.10.011.Search in Google Scholar
(4) Hussain MR, Refaat SS, Abu-Rub H. Overview and partial discharge analysis of power transformers: A literature review. IEEE Access. 2021;9:64587–605. 10.1109/ACCESS.2021.3075288.Search in Google Scholar
(5) Liao RJ, Lin YD, Guo P, Liu HB, Xia HH. Thermal aging effects on the moisture equilibrium curves of mineral and mixed oil-paper insulation systems. IEEE Trans Dielectr & Electr Insulation. 2015;22(2):842–50. 10.1109/TDEI.2015.7076783.Search in Google Scholar
(6) International Electrotechnical Commission. Ageing Procedures and Evaluation of Test Results. Geneva: IX-IEC; 2001.Search in Google Scholar
(7) Xia GQ, Wu GN, Gao B, Yin HJ, Yang FB. A new method for evaluating moisture content and aging degree of transformer oil-paper insulation based on frequency domain spectroscopy. Energies. 2017;10(8):1195. 10.3390/en10081195.Search in Google Scholar
(8) Ghoneim S, Taha I. A new approach of DGA interpretation technique for transformer fault diagnosis. Int J Electr Power & Energy Syst. 2016;81:265–74. 10.1016/j.ijepes.2016.02.018.Search in Google Scholar
(9) Duval M, Pablo DA, Atanasova-Hoehlein I, Grisaru M. Significance and detection of very low degree of polymerization of paper in transformers. IEEE Electr Insulation Mag. 2017;33(1):31–8. 10.1109/MEI.2017.7804314.Search in Google Scholar
(10) Oommen TV. Cellulose insulation materials evaluated by degree of polymerization measurements. In: Proc.elect./electron.insul.conf; 1981. p. 257–61.Search in Google Scholar
(11) Ali M, Eley C. Measuring and understanding the ageing of kraft insulating paper in power transformers. IEEE Electr Insulation Mag. 1996;12(3):28–34. 10.1109/57.509922.Search in Google Scholar
(12) Shang Y, Yang L, Guo ZJ, Yan Z. Assessing aging of large transformers by furfural investigation. In: ICSD'01. Proceedings of the 20001 IEEE 7th International Conference on Solid Dielectrics; 2001. p. 272–4.10.1109/ICSD.2001.955620Search in Google Scholar
(13) Mettler MS, Vlachos DG, Dauenhauer PJ. Top ten fundamental challenges of biomass pyrolysis for biofuels. Energy & Environ Sci. 2012;5(7):7797–809. 10.1039/c2ee21679e.Search in Google Scholar
(14) Liu C, Huang J, Huang X, Li H, Zhang Z. Theoretical studies on formation mechanisms of CO and CO2 in cellulose pyrolysis. Computational Theor Chem. 2011;964(1–3):207–12. 10.1016/j.comptc.2010.12.027.Search in Google Scholar
(15) Zhang X, Li J, Yang W, Blasiak W. Formation Mechanism of Levoglucosan and Formaldehyde during cellulose pyrolysis. Energy & Fuels. 2011;25(Jul–Aug):3739–46. 10.1021/ef2005139.Search in Google Scholar
(16) Mayes HB, Broadbelt LJ. Unraveling the reactions that unravel cellulose. J Phys Chem A. 2012;116(26):7098–106. 10.1021/jp300405x.Search in Google Scholar PubMed
(17) Agarwal V, Dauenhauer PJ, Huber GW, Auerbach SM. Ab initio dynamics of cellulose pyrolysis: Nascent decomposition pathways at 327 and 600 ℃. J Am Chem Soc. 2012;134(36):14958–72. 10.1021/ja305135u.Search in Google Scholar PubMed
(18) Hosoya T, Skaki S. Levoglucosan formation from crystalline cellulose: importance of a hydrogen bonding network in the reaction. ChemSusChem. 2013;6(12):2356–68. 10.1002/cssc.201300338.Search in Google Scholar PubMed
(19) Zhang Y, Liu C, Xie H. Mechanism studies on β-d-glucopyranose pyrolysis by density functional theory methods. J Anal Appl Pyrolysis. 2014;105(Jan):23–34. 10.1016/j.jaap.2013.09.016.Search in Google Scholar
(20) Murillo JD, Moffet M, Biernacki JJ, Northrup S. High‐temperature molecular dynamics simulation of cellobiose and maltose. AIChE J. 2015;61(8):2562–70. 10.1002/aic.14854.Search in Google Scholar
(21) Zhang Y, Liu C, Chen X. Unveiling the initial pyrolytic mechanisms of cellulose by DFT study. J Anal Appl Pyrolysis. 2015;113:621–9. 10.1016/j.jaap.2015.04.010.Search in Google Scholar
(22) Zheng M, Wang Z, Li X, Qiao X, Song W, Guo L. Initial reaction mechanisms of cellulose pyrolysis revealed by ReaxFF molecular dynamics. Fuel. 2016;177(Aug.1):130–41. 10.1016/j.fuel.2016.03.008.Search in Google Scholar
(23) Zhang M, Geng Z, Yu Y. Density functional theory (DFT) study on the pyrolysis of cellulose: the pyran ring breaking mechanism. Computational Theor Chem. 2015;1067:13–23. 10.1016/j.comptc.2015.05.001.Search in Google Scholar
(24) Li X, Tang C, Wang J, Tian W, Hu D. Analysis and mechanism of adsorption of naphthenic mineral oil, water, formic acid, carbon dioxide, and methane on meta-aramid insulation paper. J Mater Sci. 2019;54(11):8556–70. 10.1007/s10853-019-03476-x.Search in Google Scholar
(25) Miyamoto H, Yamane C, Ueda K. Molecular dynamics simulation of dehydration in cellulose/water crystals. Cellulose. 2015;22:2899–910. 10.1007/s10570-015-0716-x.Search in Google Scholar
(26) Ishikawa T, Hayakawa D, Miyamoto H, Ozawa M, Ozawa T, Ueda K. Ab initio studies on the structure of and atomic interactions in cellulose IIII crystals. Carbohydr Res. 2015;417:72–7. 10.1016/j.carres.2015.09.006.Search in Google Scholar PubMed
(27) Wang YY, Yang T, Liao RJ. Molecular dynamic simulations of glass transition temperature and mechanical properties in the amorphous region of oil-immersed transformer insulation paper. Int J Mod Phys B. 2012;26(19):9–479. 10.1142/S0217979212501007.Search in Google Scholar
(28) Li Y, Lin M, Davenport JW. Ab Initio Studies of Cellulose I: Crystal Structure, Intermolecular Forces, and Interactions with Water. JPhysChemC. 2011;115(23):11533–9. 10.1021/jp2006759.Search in Google Scholar
(29) Maurer RJ, Sax AF. Molecular dynamics of cellulose crystal surfaces with ChemShell. Procedia Computer Sci. 2010;1(1):1149–54. 10.1016/j.procs.2010.04.128.Search in Google Scholar
(30) Gilbert R, Jalbert J, Pierre TétreaultMorin, Denos B, Y. Kinetics of the production of chain-end groups and methanol from the depolymerization of cellulose during the ageing of paper/oil systems. Part 1: Standard wood kraft insulation. Cellulose. 2009;16(2):327–38. 10.1007/s10570-008-9261-1.Search in Google Scholar
(31) Laurichesse D, Bertrand Y, Tran-Duy C, Murin V. Ageing diagnosis of MV/LV distribution transformers via chemical indicators in oil. 2013 IEEE Electrical Insulation Conference (EIC). IEEE; 2013.10.1109/EIC.2013.6554289Search in Google Scholar
(32) Sergei L, John S, Giovanni C, Wander. Depolymerization processes in the thermal degradation of cellulosic paper insulation in electrical transformers. Polym Degrad & Stab. 1998;61(3):507–11. 10.1016/S0141-3910(97)00249-8.Search in Google Scholar
(33) Madhavan K, Murthy T, Sethuraman R. Estimation of degree of polymerisation and residual age of transformers based on furfural levels in insulating oil through generalized regression neural networks. Springer Berl Heidelb. 2006. 10.1007/3-540-34783-6_7.Search in Google Scholar
(34) Wang S, Guo X, Liang T, Zhou Y, Luo Z. Mechanism research on cellulose pyrolysis by Py-GC/MS and subsequent density functional theory studies. Bioresour Technol. 2012;104:722–8. 10.1016/j.biortech.2011.10.078.Search in Google Scholar PubMed
(35) Engel E, Dreizler RM. Density functional theory. Theoretical and Mathematical Physics; 2011.10.1007/978-3-642-14090-7Search in Google Scholar
(36) Hohenberg P, Kohn W. Density functional theory (DFT). Phys Rev. 1964. vol. 136, No. 1964, p. B864.10.1103/PhysRev.136.B864Search in Google Scholar
(37) Kohn W, Sham LJ. Self-consistent equations including exchange and correlation effects. Phys Rev. 1965;140(4A):A1133.10.1103/PhysRev.140.A1133Search in Google Scholar
(38) Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys Rev B. 1992;46(11):6671. 10.1103/PHYSREVB.46.6671.Search in Google Scholar PubMed
(39) Bosko JT, Todd BD, Sadus RJ. Molecular simulation of dendrimers and their mixtures under shear: Comparison of isothermal-isobaric (NPT) and isothermal-isochoric (NVT) ensemble systems. J Chem Phys. 2005;123(3):541. 10.1063/1.1946749.Search in Google Scholar PubMed
(40) Ji J, Wang K, Zhu S, Zhu W. Structure, intermolecular interactions, and dynamic properties of NTO crystals with impurity defects: a computational study. CrystEngComm. 2021;23(12):2455–68. 10.1039/D0CE01670E.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites

