Abstract
In this study, the nanocomposites from biomass (soybean straw) and layered double hydroxides (LDHs), denoted as B/LDHs, were fabricated using the mechanical-hydrothermal method. The obtained B/LDHs nanocomposites were characterized by TEM, SEM, FT-IR, and N2 adsorption–desorption techniques. Adsorption of the heavy-metal ions Pb(ii) on the B/LDHs was determined at 25°C and pH 6.0 using a batch technique. The experimental results demonstrated that biomass contributed to the sorption process. The pseudo-second-order, Langmuir, and Freundlich models well fitted the sorption process, indicating chemisorption and monolayer adsorption were the main adsorption mechanisms. Meanwhile, it is found that there is an obvious effect of adsorbent concentration in the studied adsorption system. In comparison with soybean straw and Mg–Al LDHs, the B/LDHs nanocomposites exhibit significantly enhanced sorption capacities. It is evident from this study that the construction of B/LDHs nanocomposites is an effective strategy for improving the sorption capacity of LDHs, and the modified LDH-based adsorbent shows a good potential in the removal of heavy metals from water. More importantly, it solves the problem of a large number of agricultural waste disposals. And, it achieved the goal of a win-win situation.
1 Introduction
Biomass [1,2,3,4,5] refers to all kinds of organisms produced by photosynthesis through the use of atmosphere, water, and land. Its advantages are renewable, low pollution, and wide distribution. Representative biomass includes crops, crop waste (wheat straw, corn cob, soybean stalk, orange peel, coconut shell, rice husk, etc.), wood, wood waste, and animal waste. Straw, as a kind of agricultural waste, is produced in large quantities every year. At present, its use is still at a very inefficient level in China. It is of great strategic significance to pay attention to the development and utilization of renewable biomass resources in the face of rapid resource consumption and environmental degradation.
Layered double hydroxides (LDHs) [6,7,8,9,10] are commonly used adsorbents. And, its chemical composition is as follows: [MII (1−x)MIII x (OH)2] x+[A n− x/n ] x−·mH2O, where MII and MIII refer to divalent and trivalent metal cations, respectively; A stands for the interlayer anion; x is for per mole of LDHs MIII moles; and m is the number of moles of crystal water in the middle layer per mole LDHs. LDHs have a lamellar crystal structure, and the lamellar plates are positively charged with exchangeable anions. Different types of metal ions (MII and MIII) and interlayer anions (A n−) lead to different LDHs properties. Therefore, LDHs are a kind of new inorganic lamellar materials. It has broad application prospects in the fields of catalyst, adsorbent, ion exchange agent, combustion promoter, pesticide slow-release preparation, and liquid flowtype regulator, especially in the fields of pharmaceutical transport carrier and sewage treatment, etc.
In recent years, the pollution of global freshwater resources is one of the main environmental problems faced by human beings. In particular, heavy metal ions have aroused widespread concern. With the rapid development of petrochemical, printing, dyeing, textile, plastic, leather, food, and other industries, industrial wastewater containing heavy metal ions are discharged into soil and water in large quantities, which seriously threatens the living space of human beings. To ensure the supply of freshwater resources, reducing water pollution or the treatment and recycling of polluted water resources are paid more and more attention by various countries.
At present, there are ion-exchange [8], membrane filtration [11], chemical precipitation [12], biological methods [13], and adsorption [14,15,16,17,18,19,20,21] to treat heavy metals and organic dyes in industrial wastewater. Among these treatment methods, the adsorption method has its place with the advantages of good treatment effect, simple operation, and good selectivity, especially in the field of heavy metal pollution, and organic pollution wastewater with strong pollution, low concentration, and difficult to be effectively treated by other treatment methods [14,15,18,22]. The commonly used adsorbents are activated carbon [23,24], polymers [25], graphene-based nanomaterials [26,27,28], some industrial wastes [29,30], etc., but most of these adsorbents are of high price and low adsorption capacity. It is imperative to develop new highly effective adsorbents.
Recently, the synthesis of biomass/LDH materials from LDH and biomass provides a win-win strategy. There are two main reasons for explaining the rationale of adding LDH to straw: first, cellulose contained in soybean straw can provide substrate for LDH growth. Second, lignin contained in soybean straw has abundant hydroxyl, methoxy, and carbonyl groups on its surface, which forms hydrogen bond with hydroxyl groups and water molecules on and between LDH laminates. Therefore, combining biomass with synthetic materials is oftentimes quite advantageous [31]. It not only solves the problem of a large number of agricultural waste treatments but also has a certain breakthrough in the preparation of new adsorbent. Due to its particle stability, pore characteristics, and surface activity, more and more attention has been paid to these kind of materials [32,33,34,35]. For example, Wang et al. [32] found that the maximum As(v) sorption capacity of Ni/Fe-LDHs-biochar composites was 4.38 mg/g, which was approximately three times as large as the mechanical mixture of Ni/Fe-LDHs and biochar. Xue et al. [33] reported that the biochar/MgFe-LDHs composite has a strong sorption ability to nitrate with the maximum adsorption capacity of 24.8 mg/g. Wan et al. [34] indicated that the biochar/MgAl-LDHs composite containing 40% MgAl-LDHs exhibited the highest phosphate removal rate with >95%. Huang et al. [35] examined that the BC@EDTA-LDHs’ nano-adsorbent was used to remove Cr(vi) with the maximum adsorption capacity of 38 mg/g. However, the above biomass materials are calcined or lye washed before being used for adsorption. Calcining brings a series of problems, such as adsorption capacity decreases, greenhouse effect, and air pollution. More importantly, the pretreatment of biomass will consume more resources. To achieve the full utilization of resources and environmental protection, we used un-calcined or un-lye washing biomass to obtain a new adsorbent.
The objectives of this study were as follows: (1) to synthesize the compounds of Biomass (soybean straw)/MgAl-LDHs (B/LDHs) using the mechanical-hydrothermal method [36]; (2) to analyze the properties of B/LDHs using various characterization methods; and (3) to study the adsorption performance of B/LDHs for heavy metal ions Pb(ii).
2 Experimental
2.1 Materials
Al(OH)3, Pb(NO3)2, NaNO3, and HNO3 were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Mg(OH)2 was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. All chemicals were of analytical grade and used as received. Soybean straw was from local agricultural waste. Soybean straw was cleaned and dried. Then, it was crushed to obtain the powder. At last, the powder was passed through a 100-mesh sieve and standby. Water was purified with a Direct-Pure UP water purification system (Rephile, China).
2.2 Preparation of B/LHDs nanocomposites
The B/LHDs nanocomposites were prepared by the mechanical-hydrothermal method in our previous studies [36]. Different from previous studies, soybean straw (1.0 g) powder was added in the first step. After a series of reaction treatments, it finally obtained the B/LDHs’ nanocomposite sample. This sample was abbreviated as 10% B/LDHs (see supporting information for specific preparation).
Under the same conditions, the 50% B/LDHs and the Mg–Al LDHs without the soybean straw were prepared.
2.3 Characterization
Under the conditions of 10 kV acceleration voltage and gold spraying, the morphology of the samples were analyzed using a JSM-6700F scanning electron microscopy (SEM, JEOL, Japan) and a JEM-2100 transmission electron microscopy (TEM, JEOL, Japan). Fourier transform infrared spectra (FTIR) (Nicolet 5700 Spectrometer, USA) of adsorbents were recorded under the conditions that the resolution is better than 0.09 cm−1, the wavenumber accuracy is 0.01 cm−1, and the quick scanning speed is 65 times/second. FTIR of adsorbents was recorded in the range of 4,000–400 cm−1. The samples were pressed with KBr pellets. The N2 adsorption-desorption isotherms were determined using an Autosorb IQ-MP system (Quantachrome Instruments, USA), and the test samples were degassed at 120°C for 5 h under vacuum before measurement. The specific surface area (A s) and pore volume (V p) of the samples were calculated using the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively.
2.4 Adsorption experiments
The heavy metal ion Pb(ii) was used as the target to test the adsorption capacity of B/LDHs. The sorption experiments were conducted under a batch of equilibration techniques at a temperature of 25.0°C, solubilizing Pb(ii) solutions at different concentrations ranging 0–500 mg/L in water containing 0.010 M of NaNO3 to get Pb(NO3)2 solutions. NaNO3 was applied as a maintainer for the constant ionic strength of the solutions. With the use of 0.1 M HNO3 and NaOH solutions, the pH values of the Pb(ii) solutions were adjusted to 6.0. The mass-given adsorbent sample with 25 mL of the pollutant solutions was blended in polyethylene centrifuge tubes. The centrifuge tubes were allowed to swing in a thermostatic water bath vessel (Jiangsu Medical Instrument Factory, China) at a temperature of 25.0 ± 0.2°C for 24 h, and then, the supernatant solution was filtered through a 0.45 µm membrane. The residual pollutant concentrations in the filtrates were measured using the AAS-3600 flame atomic absorption spectrometry (Shanghai Metash Instruments Co., Ltd., China) for Pb(ii).
The sorption amount (Γ t ) was determined by the difference between the initial and the residual concentrations of Pb(ii):
where Γt (mg/g) is the sorption amount at time t, C 0 (mg/L) and C t (mg/L) are the initial and the remaining concentrations at time t, respectively, and C s (g/L) is the sorbent dosage.
Sorption kinetic tests showed that t = 10 h was required to reach equilibrium. To ensure sorption equilibrium, t = 24 h was selected in the equilibrium sorption tests.
The adsorption capacity and the removal rates (E R) in this study were calculated as follows:
where the C e refers to the equalized one and Γe indicates the equilibrium adsorption capacity.
The tests shall be conducted three times, and the final values were taken as the average of overall measurements, with a relative error of less than 5%.
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
3.1 Characterizations
Figure 1 shows the SEM and TEM images of the 50% B/LDHs, 10% B/LDHs, Mg–Al LDHs, and soybean straw samples. The B/LDHs samples featured irregular flaky particles (Figure 1a–d). Furthermore, with the decrease of soybean straw contents, a large number of flake structures could be observed growing on the soybean straw (Figure 1c and d). In addition, hexagonal crystals, typical of LDHs, were observed, and the lateral size of the LDH crystals was ∼200 nm, which has been reported in our previous studies [36]. Furthermore, the soybean straw showed irregular block structures.
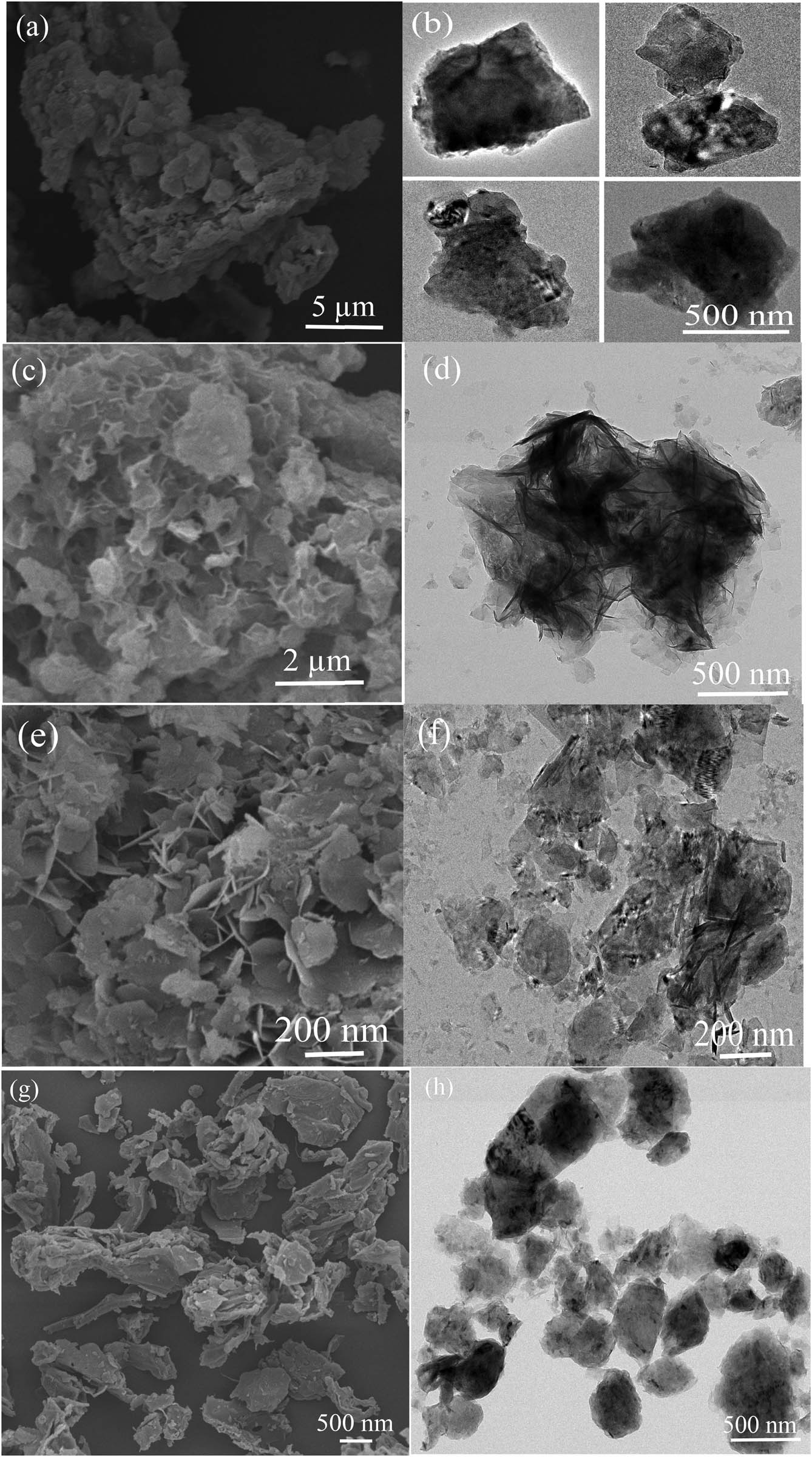
(a, c, e, and g) SEM and (b, d, f, and h) TEM images of (a and b) 50% B/LDHs, (c and d) 10% B/LDHs, (e and f) Mg–Al LDHs, and (g and h) soybean straw.
Figure 2 shows the FT-IR spectra of 50% B/LDHs, 10% B/LDHs, Mg–Al LDHs (LDHs), and soybean straw (B) samples. In the spectrum of soybean straw (B), the bands at 3,464 and 1,620 cm−1 were attributed to the hydroxyl group and water deformations, the band at 2,880 cm−1 arose from the characteristic peak of methylene on the molecular chain of total cellulose, and 1,080 cm−1 arose from the stretching vibration peak of C-H on total cellulose. In the spectrum of the LDH sample, the strong broad band centered at approximately 3,700 cm−1 was assigned to the O–H stretching modes of the hydroxyl groups in the LDH layers and the interlayer water, and another band corresponding to water deformation was recorded at approximately 1,620 cm−1; the band at 1,362 was attributed to the ν
3 vibrations of
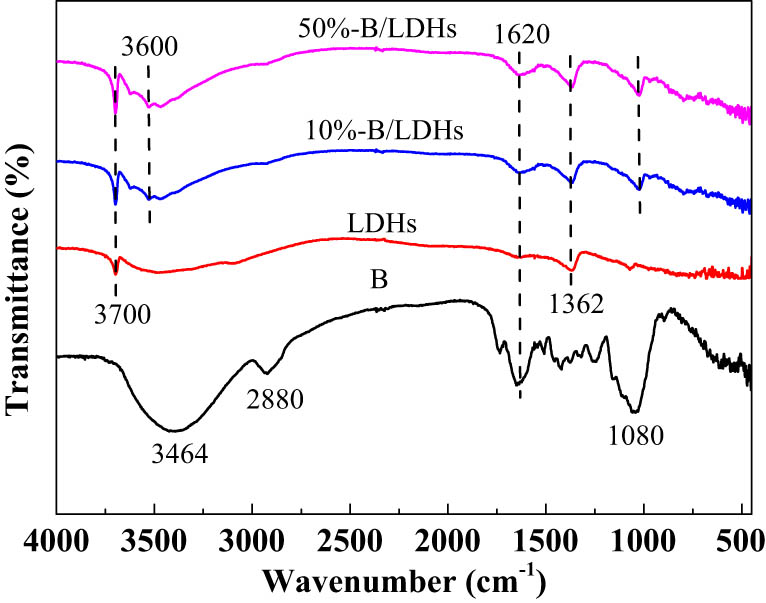
FT-IR spectra of 50% B/LDHs, 10% B/LDHs, Mg–Al LDHs (LDHs), and soybean straw (B).
The N2 adsorption–desorption isotherms of the 50% B/LDHs, 10% B/LDHs, Mg–Al LDHs (LDHs), and soybean straw (B) samples (Figure 3) showed a type IV adsorption isotherm with a type H3 hysteresis loops when P/P0 ratio was greater than ∼0.4 [37], which was manifested as fractured pores formed by the accumulation of flap-like particles. The A s, V p, and average pore size (D p) of the samples are listed in Table 1. With the increase of soybean straw contents, A s and pore data (D p, V p) of the B/LDHs samples decreased significantly. In addition, the mesoporous structure of D p of ∼3.4‒9.3 nm remained in the nanocomposites, which is conducive to its application as an adsorbent.
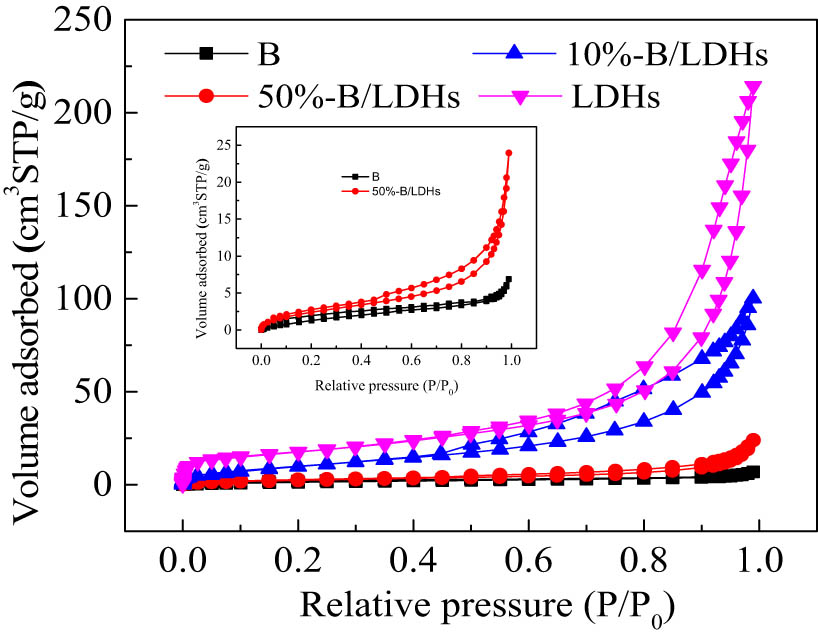
N2 adsorption–desorption isotherms of 50% B/LDHs, 10% B/LDHs, Mg–Al LDHs (LDHs), and soybean straw (B).
Specific surface area (A s) and pore data (D p, V p) of B, B/LDHs, and LDHs samples
| Sample | A s (m2/g) | D p (nm) | V p (cm3/g) |
|---|---|---|---|
| B | 6.508 | 3.416 | 0.008 |
| 50% B/LDHs | 9.726 | 3.828 | 0.037 |
| 10% B/LDHs | 39.93 | 3.815 | 0.169 |
| LDHs | 62.95 | 9.324 | 0.342 |
3.2 Removal performance of B/LDHs composites for Pb(ii)
3.2.1 Effects of pH
The pH value of the solution is one of the important parameters affecting the adsorption process. Under the conditions of C
0 = 50 mg/L, C
s = 1.0 g/L,
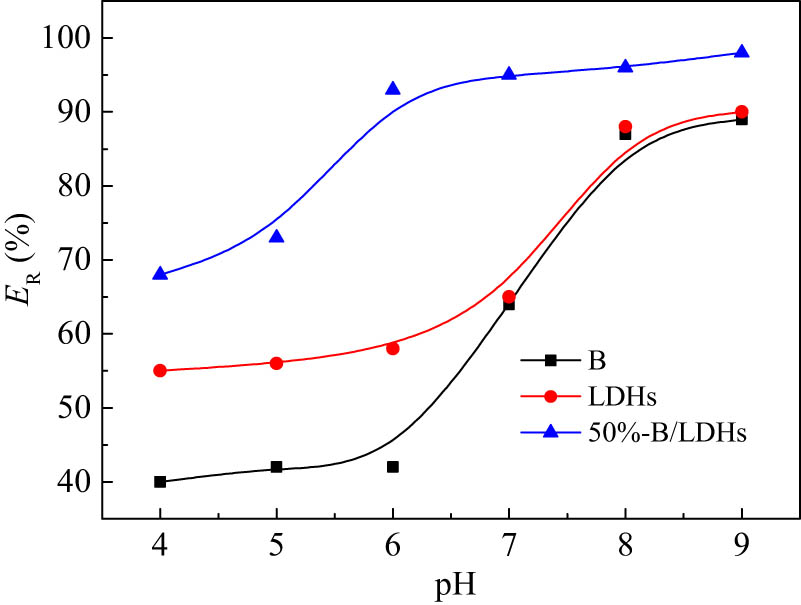
Effects of pH on the removal efficiency of B, LDHs, and 50% B/LDHs for Pb(ii). C
0 = 50 mg/L, C
s = 1.0 g/L,
3.2.2 Adsorption kinetics and removal efficiency
First, B/LDHs adsorption kinetics test for Pb(ii) (C
0 = 100 mg/L, pH = 6.0) was performed to evaluate the contact time required for adsorption equilibrium, where the sorbent dosage (C
s) was 1.0 g/L, the concentration of NaNO3 (
Under the conditions of C
s = 1.0 g/L,
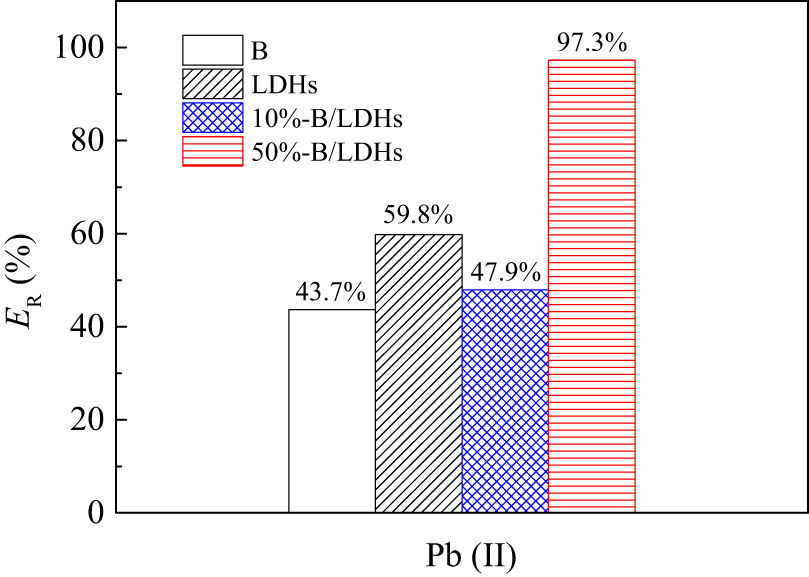
Removal rates of Pb(ii) on B, B/LDHs and LDHs samples. (C
0 = 50 mg/L, pH = 6.0). C
s = 1.0 g/L, 25°C, and
To evaluate the recyclability of 50% B/LDHs composite materials, the recovery tests were carried out under the conditions of C
s = 1.0 g/L,
3.2.3 Sorption isotherm
To evaluate the adsorption capacity of B, B/LDHs, and LDHs samples to Pb(ii), the adsorption isotherm was determined at C
s = 1 g/L,
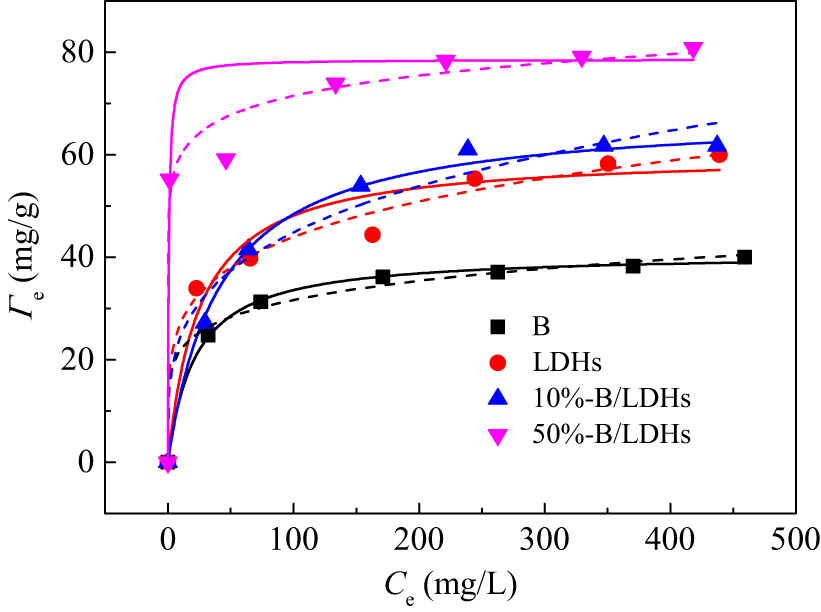
Sorption isotherms of Pb(ii) on B, B/LDHs, and LDHs samples. C
s = 1.0 g/L,
Adsorption isotherms are usually described by Langmuir or Freundlich isotherms. Langmuir model can be expressed as follows:
or in a linear form as
The Freundlich model can be expressed in a nonlinear form as
or in a linear form as
In these equations, the maximum sorption amount is Γm, the Langmuir equilibrium constant is K L, and the Freundlich constants are K F and n F.
The linear and nonlinear regression Langmuir and Freundlich isotherms were used to fit the adsorption data of different composites. The nonlinear model diagram is consistent with the experimental data (Figure 6), and the linear model diagram is a straight line (Figure S3 in the supporting information). The best values of the model parameters, Γm, K L, K F, n F, and the correlation coefficient (R 2), are listed in Table 2 (and Table S1 in the supporting information). The correlation coefficient (R 2) of each model fitting curve was high, indicating that both Langmuir and Freundlich models could well describe the adsorption isotherms of different composites. In addition, the optimal fitting values of model parameters using nonlinear regression and linear regression are very similar. Furthermore, it can be seen from Table 2, Γm values increase with the increase of the content of B. This is due to the availability of more adsorption sites (or functional groups) from straw lignin and LDH nurturing substrate effect of straw cellulose. It is worth noting that the Γm value of 50% B/LDHs increased by 15% over 10% B/LDHs composites. From the point of view of saving resources, it is necessary to replace hydroxides with a large amount of soybean straw.
Nonlinear-fit data of model parameters for Pb(ii) sorption on B, LDHs, and B/LDHs samples
| Sample | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| Γm (mg/g) | K L × 10−2 (L/mg) | R 2 | K F (L nF mg1−nF/g) | n F | R 2 | |
| B | 40.8 | 4.66 | 0.999 | 15.1 | 0.161 | 0.993 |
| LDHs | 60.3 | 3.96 | 0.955 | 16.7 | 0.211 | 0.987 |
| 10% B/LDHs | 68.3 | 2.44 | 0.997 | 13.3 | 0.264 | 0.968 |
| 50% B/LDHs | 78.6 | 143 | 0.927 | 50.0 | 0.0776 | 0.979 |
3.2.4 Effect of sorbent dosage
The amount of adsorbent used (C
s) in adsorption test is an important factor affecting the adsorption capacity of solid adsorbent in aqueous solutions [39,40]. It is of great theoretical and practical significance to study the effect of C
s on adsorption properties. Taking 50% B/LDHs as an example, the adsorption isotherm of Pb(ii) at different C
s values was measured. Using the 50% B/LDHs as an example, the sorption isotherms for Pb(ii) at different C
s values were measured under the conditions of
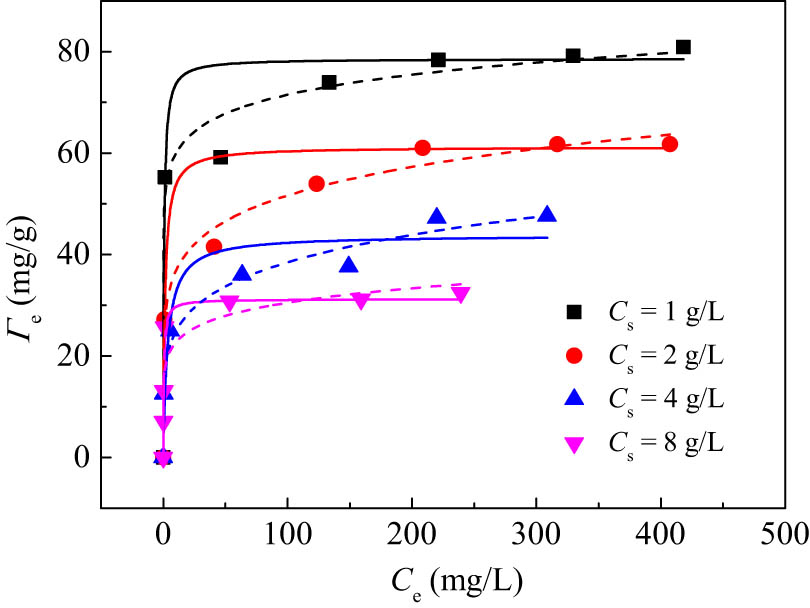
Sorption isotherms of Pb(ii) on 50% B/LDHs at different sorbent dosages.
Langmuir and Freundlich isotherms were used for nonlinear and linear regression to fit adsorption data under different C s values. The nonlinear model diagram was consistent with the experimental data (Figure 7), and the linear model diagram was a straight line (Figure S4 in the supporting information). The best values of the model parameters, Γm, K L, K F, n F, and R 2, are listed in Table 3 (and Table S2 in the supporting information). The high R 2 values of various model fitting curves indicated that the Langmuir and Freundlich models fully described the adsorption isotherms of any given C s value. In addition, the optimal fitting values of model parameters using nonlinear regression and linear regression are very similar. Furthermore, it can be seen from Table 3 that the Γm values decreased with increasing C s. In fact, all Langmuir and Freundlich parameters change with the change of C s. Similar results have been reported in the literature. The C s-dependence of the model parameters violates the predictions of the Langmuir and Freundlich models.
Nonlinear fit data of model parameters for Pb(ii) sorption on 50% B/LDHs at different C s
| C s (g/L) | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| Γm (mg/g) | K L (L/mg) | R 2 | K F (L nF mg1−nF/g) | n F | R 2 | |
| 1.00 | 78.6 | 1.43 | 0.927 | 50.0 | 0.0776 | 0.979 |
| 2.00 | 61.2 | 0.698 | 0.889 | 26.0 | 0.149 | 0.989 |
| 4.00 | 43.8 | 0.0283 | 0.933 | 16.0 | 0.190 | 0.790 |
| 8.00 | 31.2 | 1.92 | 0.981 | 16.9 | 0.129 | 0.851 |
4 Conclusion
The B/LDHs nanocomposites were successfully fabricated using a mechanical-hydrothermal route. The so-obtained nanocomposites were efficient adsorbents to remove heavy metal ions. The experimental results demonstrated that biomass was donated in the sorption process. The pseudo-second-order, Langmuir and Freundlich models well fitted the sorption process, indicating chemisorption and monolayer adsorption were the main adsorption mechanisms. Meanwhile, it is found that there is an obvious effect of adsorbent concentration in the studied adsorption system. Since biomass was from local agricultural waste, the use of biomass as a carrier for LDHs was environmentally friendly. The B/LDHs nanocomposites are potential sorbents for wastewater treatment. Furthermore, the synthesis of biomass/LDHs materials from LDHs and biomass provides a win-win strategy.
Acknowledgments
The authors are grateful to Zheng Niu, Qingqing Guo, and Shaokang Fang for sample preparations for the experiments.
-
Funding information: This work is supported financially by the Science and Technology Program for Colleges and Universities of Shandong Province (No. J18KA104).
-
Author contributions: Fengrong Zhang conceived and designed the experiment. She performed the experiments and carried out the data analysis. Binghan Zhang and Dandan Han carried out characterization experiments on the samples. Lishun Wu and Wanguo Hou have polished the grammar of the manuscript. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Fermanelli CS, Córdoba A, Pierella LB, Saux C. Pyrolysis and copyrolysis of three lignocellulosic biomass residues from the agro-food industry: a comparative study. Waste Manage. 2020;102:362–70.10.1016/j.wasman.2019.10.057Search in Google Scholar PubMed
[2] Abolghasemi MM, Amirifard H, Piryaei M. Bio template route for fabrication of a hybrid material composed of hierarchical boehmite, layered double hydroxides (Mg–Al) and porous carbon on a steel fiber for solid phase microextraction of agrochemicals. Microchim Acta. 2019;186:678–84.10.1007/s00604-019-3782-1Search in Google Scholar PubMed
[3] Bian H, Gao Y, Luo J, Jiao L, Wu W, Fang G, et al. Lignocellulosic nanofibrils produced using wheat straw and their pulping solid residue: from agricultural waste to cellulose nanomaterials. Waste Manage. 2019;91:1–8.10.1016/j.wasman.2019.04.052Search in Google Scholar PubMed
[4] Shen J, Huang G, An C, Xin X, Huang C, Rosendahl S. Removal of tetrabromobisphenol A by adsorption on pinecone-derived activated charcoals: synchrotron FTIR, kinetics and surface functionality analyses. Bioresour Technol. 2018;247:812–20.10.1016/j.biortech.2017.09.177Search in Google Scholar PubMed
[5] Saha D, Mirando N, Levchenko A. Liquid and vaporphase adsorption of BT Xin lignin derived activated carbon: equilibrium and kinetics study. J Clean Prod. 2018;182:372–8.10.1016/j.jclepro.2018.02.076Search in Google Scholar
[6] Zhang X, Gao J, Zhao S, Lei Y, Yuan Y, He C, et al. Hexavalent chromium removal from aqueous solution by adsorption on modified zeolites coated with Mg-layered double hydroxides. Environ Sci Pollut R. 2019;26:32928–41.10.1007/s11356-019-06410-5Search in Google Scholar PubMed
[7] Xiang Y, Xiang Y, Jiao Y. Simultaneous disintegration of municipal sludge and generation of ethanol with magnetic layered double hydroxides. Bioresour Technol. 2019;289:121654–62.10.1016/j.biortech.2019.121654Search in Google Scholar PubMed
[8] Li L, Qi G, Wang B, Yue D, Wang Y, Sato T. Fulvic acid anchored layered double hydroxides: A multifunctional composite adsorbent for the removal of anionic dye and toxic metal. J Hazard Mater. 2018;343:19–28.10.1016/j.jhazmat.2017.09.006Search in Google Scholar PubMed
[9] Shamsayei M, Yamini Y, Asiabi H. Fabrication of zwitterionic histidine/layered double hydroxide hybrid nanosheets for highly effcient and fast removal of anionic dyes. J Colloid Inter Sci. 2018;529:255–64.10.1016/j.jcis.2018.06.022Search in Google Scholar PubMed
[10] Wang N, Sun J, Fan H, Ai S. Anion-intercalated layered double hydroxides modified test strips for detection of heavy metal ions. Talanta. 2016;148:301–7.10.1016/j.talanta.2015.11.007Search in Google Scholar PubMed
[11] Goh PS, Ismail AF. A review on inorganic membranes for desalination and wastewater treatment. Desalination. 2018;434:60–80.10.1016/j.desal.2017.07.023Search in Google Scholar
[12] Hao J, Ji L, Li C, Hu C, Wu K. Rapid, efficient and economic removal of organic dyes and heavy metals from wastewater by zinc-induced in-situ reduction and precipitation of graphene oxide. J Taiwan Inst Chem Eng. 2018;88:137–45.10.1016/j.jtice.2018.03.045Search in Google Scholar
[13] Bhatia D, Sharma NR, Singh J, Kanwar RS. Biological methods for textile dye removal from wastewater: a review. Crit Rev Environ Sci Technol. 2017;47:1836–76.10.1080/10643389.2017.1393263Search in Google Scholar
[14] Sirviö JA, Visanko M. Lignin-rich sulfated wood nanofibers as high-performing adsorbents for the removal of lead and copper from water. J Hazard Mater. 2020;383:121174–81.10.1016/j.jhazmat.2019.121174Search in Google Scholar PubMed
[15] Yusof MSM, Othman MHD, Wahab RA, Jumbri K, Razak FIA, Kurniawan TA, et al. Arsenic adsorption mechanism on palm oil fuel ash (POFA) powder suspension. J Hazard Mater. 2020;383:121214–23.10.1016/j.jhazmat.2019.121214Search in Google Scholar PubMed
[16] Parvin S, Biswas BK, Rahman A, Rahman H, Anik S, Uddin R. Study on adsorption of Congo red onto chemically modified egg shell membrane. Chemosphere. 2019;236:124326–33.10.1016/j.chemosphere.2019.07.057Search in Google Scholar PubMed
[17] Zhang Y, Liu Q, Yang C, Wu S, Cheng J. Magnetic aluminum-based metal organic framework as a novel magnetic adsorbent for the effective removal of minocycline from aqueous solutions. Environ Pollut. 2019;255:113226–35.10.1016/j.envpol.2019.113226Search in Google Scholar PubMed
[18] Zare EN, Motaharib A, Sillanpää M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: a review. Environ Res. 2018;162:173–95.10.1016/j.envres.2017.12.025Search in Google Scholar PubMed
[19] Zou Y, Liu Y, Wang X, Sheng G, Wang S, Ai Y, et al. Glycerol-modified binary layered double hydroxide nanocomposites for uranium immobilization via extended X-ray absorption fine structure technique and density functional theory calculation. ACS Sustain Chem Eng. 2017;5;3583–95.10.1021/acssuschemeng.7b00439Search in Google Scholar
[20] Santhosh C, Velmurugan V, Jacob G, Jeong SK, Grace AN, Bhatnagar A. Role of nanomaterials in water treatment applications: a review. Chem Eng J. 2016;306:1116–37.10.1016/j.cej.2016.08.053Search in Google Scholar
[21] Dey T. Magnetic nanoparticles and cellulosic nanofibers to remove arsenic and other heavy metals from water, Nanotechnology for water purification. Florida: Universal-Publishers; 2012. p. 1–28.Search in Google Scholar
[22] Yu S, Wang X, Pang H, Zhang R, Song W, Fu D, et al. Boron nitride-based materials for the removal of pollutants from aqueous solutions: a review. Chem Eng J. 2018;333:343–60.10.1016/j.cej.2017.09.163Search in Google Scholar
[23] Li Y, Du Q, Liu T, Peng X, Wang J, Sun J, et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem Eng Res Des. 2013;91:361–8.10.1016/j.cherd.2012.07.007Search in Google Scholar
[24] Selmi T, Sanchez-Sanchez A, Gadonneix P, Jagiello J, Seffen M, Sammouda H, et al. Tetracycline removal with activated carbons produced by hydrothermal carbonisation of Agave americana fibres and mimosa tannin. Ind Crop Prod. 2018;115:146–57.10.1016/j.indcrop.2018.02.005Search in Google Scholar
[25] Sheng X, Shi H, Yang L, Shao P, Yu K, Luo X. Rationally designed conjugated microporous polymers for contaminants adsorption. Sci Total Env. 2020;750:141683–98.10.1016/j.scitotenv.2020.141683Search in Google Scholar PubMed
[26] Ren F, Li Z, Tan W, Liu X, Sun Z, Ren P, et al. Facile preparation of 3D regenerated cellulose/graphene oxide composite aerogel with high-effciency adsorption towards methylene blue. J Colloid Inter Sci. 2018;532:58–67.10.1016/j.jcis.2018.07.101Search in Google Scholar PubMed
[27] Zhang F, Song Y, Song S, Zhang R, Hou W. Synthesis of magnetite−graphene oxide-layered double hydroxide composites and applications for the removal of Pb(ii) and 2,4-dichlorophenoxyacetic acid from aqueous solutions. ACS Appl Mater Inter. 2015;7:7251–63.10.1021/acsami.5b00433Search in Google Scholar PubMed
[28] Shen Y, Fang Q, Chen B. Environmental applications of three-dimensional graphene-based macrostructures: adsorption, transformation, and detection. Environ Sci Technol. 2015;49:67–84.10.1021/es504421ySearch in Google Scholar PubMed
[29] Zhang W, Cheng H, Peng S, Li D, Gao H, Wang D. Performance and mechanisms of wastewater sludge conditioning with slag-based hydrotalcite-like minerals (Ca/Mg/Al-LDH). Water Res. 2020;169:115265–80.10.1016/j.watres.2019.115265Search in Google Scholar PubMed
[30] Ai J, Zhang W, Chen F, Liao G, Li D, Hua X, et al. Catalytic pyrolysis coupling to enhanced dewatering of waste activated sludge using KMnO4 single bond Fe(ii) conditioning for preparing multi-functional material to treat groundwater containing combined pollutants. Water Res. 2019;158:424–37.10.1016/j.watres.2019.04.044Search in Google Scholar PubMed
[31] Dey T. Properties of vinyl ester resins containing methacrylated fatty acid comonomer: the effect of fatty acid chain length. Polym Int. 2007;56:853–9.10.1002/pi.2215Search in Google Scholar
[32] Wang S, Gao B, Li Y, Zimmerman AR, Cao X. Sorption of arsenic onto Ni/Fe layered double hydroxide (LDH)-biochar composites. RSC Adv. 2016;6:17792–99.10.1039/C5RA17490BSearch in Google Scholar
[33] Xue L, Gao B, Wan Y, Fang J, Wang S, Li Y, et al. High effciency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions. J Taiwan Ins Chem Eng. 2016;63:312–7.10.1016/j.jtice.2016.03.021Search in Google Scholar
[34] Wan S, Wang S, Li Y, Gao B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J Ind Eng Chem. 2017;47:246–53.10.1016/j.jiec.2016.11.039Search in Google Scholar
[35] Huang D, Liu C, Zhang C, Deng R, Wang R, Xue W, et al. Cr(VI) removal from aqueous solution using biochar modified with Mg/Al layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresour Technol. 2019;276:127–32.10.1016/j.biortech.2018.12.114Search in Google Scholar
[36] Zhang F, Du N, Song S, Liu J, Hou W. Mechano-hydrothermal synthesis of Mg2Al-NO3 layered double hydroxides. J Solid State Chem. 2013;206:45–50.10.1016/j.jssc.2013.07.030Search in Google Scholar
[37] Hu W, Gu H, Wang J, Li Y, Wang Z. One-Step synthesis of silica hollow particles in a W/O inverse emulsion. Colloid Polym Sci. 2013;291:2697–704.10.1007/s00396-013-3003-0Search in Google Scholar
[38] Madadrang CJ, Kim HY, Gao G, Wang N, Zhu J, Feng H, et al. Adsorption behavior of EDTA-graphene oxide for Pb(ii) removal. ACS Appl Mater Inter. 2012;4:1186–93.10.1021/am201645gSearch in Google Scholar
[39] O’Connor DJ, Connolly JP. The effect of concentration of adsorbing solids on the partition coefficient. Water Res. 1980;14:1517–23.10.1016/0043-1354(80)90018-4Search in Google Scholar
[40] Voice TC, Weber WJ. Sorbent concentration effects in liquid/solid partitioning. Environ Sci Technol. 1985;19:789–96.10.1021/es00139a004Search in Google Scholar PubMed
[41] Zhao L, Hou W. The effect of sorbent concentration on the partition coefficient of pollutants between aqueous and particulate phases. Colloids Surf A. 2012;396:29–34.10.1016/j.colsurfa.2011.12.026Search in Google Scholar
[42] Zhao L, Song S, Du N, Hou W. A sorbent concentration-dependent Freundlich isotherm. Colloid Polym Sci. 2013;291:541–50.10.1007/s00396-012-2742-7Search in Google Scholar
© 2021 Fengrong Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation


