Concordance between the updated Elecsys cerebrospinal fluid immunoassays and amyloid positron emission tomography for Alzheimer’s disease assessment: findings from the Apollo study
-
Henrik Schinke
, Magnus Förnvik Jonsson
Abstract
Objectives
The Apollo study was designed to support the clinical performance verification of the adjusted cutoffs of the Elecsys® β-Amyloid(1–42) (Aβ42) cerebrospinal fluid (CSF) II, β-Amyloid(1–40) (Aβ40) CSF, Phospho-Tau (181P) (pTau) CSF and Total-Tau (tTau) CSF immunoassays (Roche Diagnostics International Ltd) for measuring fresh CSF samples, and assess the concordance of the Elecsys CSF pTau/Aβ42, tTau/Aβ42 and Aβ42/Aβ40 ratios, as well as Aβ42 alone, with amyloid positron emission tomography (PET) visual read status.
Methods
The primary study endpoint was to assess the concordance of the Elecsys CSF ratios and Aβ42 alone with amyloid PET visual read status using fresh CSF samples collected from individuals with subjective cognitive decline or mild cognitive impairment, handled with a new routine-use pre-analytical procedure and measured with the Elecsys CSF immunoassays. The sample stability after 1- to 13-week storage at −20 °C was also investigated in an exploratory analysis.
Results
Of 108 screened individuals, 91 met the eligibility criteria, of whom 44.0 % were amyloid PET-positive and 56.0 % amyloid PET-negative. Positive percent agreement (PPA) and negative percent agreement, respectively, were 0.800 and 0.882 for pTau/Aβ42, 0.775 and 0.902 for tTau/Aβ42, and 0.950 and 0.824 for Aβ42/Aβ40. For Aβ42, PPA was 0.975 and negative likelihood ratio was 0.039. Overall, 33 samples (36.3 %) were frozen at −20 °C for 1–13 weeks. All concentration recoveries were within 100 ± 10 % when stored at −20 °C for ≤8 weeks.
Conclusions
Elecsys CSF ratios and Aβ42 alone may be reliable alternatives to amyloid PET for identifying amyloid positivity in clinical practice.
Introduction
Alzheimer’s disease (AD) is a progressive brain disease accounting for 60–80 % of dementia cases in the United States [1], 2]. Globally, the prevalence of AD and other dementias is estimated to increase from 57.4 million cases in 2019, to 152.8 million by 2050 [3].
AD pathology involves accumulating amyloid-β (Aβ) plaques and the hyperphosphorylation of tau proteins (pTau) [4]. The recent development of disease-modifying treatments targeting the pathophysiology of AD, such as donanemab and lecanemab, has highlighted the need for accurate diagnostic tests [5], [6], [7], [8]. Recommendations provided by the International Working Group on the clinical diagnosis of AD suggest that the assessment of biological parameters, such as cerebrospinal fluid (CSF) biomarkers and amyloid positivity by positron emission tomography (PET) imaging, may help detect biological changes before symptom onset and aid in early AD diagnosis [9].
According to recent AD diagnostic criteria, the levels of β-amyloid(1–42) (Aβ42), tau phosphorylated at a threonine residue at position 181 (pTau181) and total tau (tTau) in CSF play a crucial role in the timely and accurate diagnosis of AD [10], 11]. Aβ42 levels are inversely correlated with amyloid plaque burden, while pTau181 and tTau levels are markers for tangle formation and neuronal degeneration, respectively [4]. Early-stage studies have shown that the ratios of Aβ42 with pTau181 and tTau may have increased performance in predicting clinical decline and cognitive impairment in AD, compared with each biomarker alone [12], [13], [14], [15]. Although the levels of β-amyloid(1–40) (Aβ40) have been found to remain unaltered in AD [16], the CSF Aβ42/Aβ40 ratio has also demonstrated better diagnostic performance than Aβ42 alone [16], [17], [18], [19], [20].
The fully automated Elecsys® β-Amyloid (1–42) CSF, Elecsys Phospho-Tau (181P) CSF and Elecsys Total-Tau CSF immunoassays (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) are in vitro diagnostic (IVD)-certified electrochemiluminescence immunoassays that employ a quantitative sandwich principle and were developed to aid amyloid pathology detection [21]. Since the initial clinical validation, all three immunoassays have been updated resulting in second-generation immunoassays (Elecsys β-Amyloid (1–42) CSF II [Aβ42 Gen2], Elecsys Phospho-Tau (181P) CSF [pTau] and Elecsys Total-Tau CSF [tTau]), which are IVD-certified for their intended use, have higher thresholds for biotin interference and run on a broader range of analyzers than previously [21]. The updated immunoassays have also been recently approved by the US Food and Drug Administration (FDA) due to their concordance with amyloid PET visual read status and ability to identify the presence of amyloid pathology [22], 23].
The initial Elecsys CSF immunoassay clinical cutoff values were established using CSF samples stored at −80 °C in a research setting [21]. To suit clinical routine testing requirements, a new, simplified pre-analytical procedure has been developed to ensure standardization and reduce pre-analytical variability when handling fresh CSF samples [24]. The new handling procedure and updated Elecsys CSF immunoassays, when used in combination, offer improved robustness in measuring CSF biomarkers [21]. However, due to the susceptibility of Aβ42 to differences in pre-analytical handling, a shift in Aβ42 levels is expected when different protocols are applied [25]. Therefore, the clinical cutoff values for the updated Elecsys Aβ42 Gen2 immunoassay and its ratios with pTau, tTau and Aβ40 were adjusted accordingly, as previously published [21].
The present study aimed to support clinical performance verification of the adjusted cutoffs of the Elecsys immunoassays in terms of their ability to correctly identify patients with subjective cognitive decline (SCD) and mild cognitive impairment (MCI) based on amyloid PET results.
Materials and methods
Study design
The Apollo study was a prospective, supportive verification study for the updated Aβ42 Gen2 immunoassay and its ratios with the updated Elecsys pTau and tTau CSF immunoassays as well as the Aβ40 immunoassay, used to measure fresh CSF samples handled according to the new routine-use pre-analytical procedure.
Individuals diagnosed as SCD/MCI were recruited for the Swedish BioFINDER-2 study (NCT03174938) based on previously described eligibility criteria at baseline or at the 2-year follow-up visit [26]. From this population, SCD/MCI individuals who had available amyloid PET scans and valid biomarker measurements in fresh CSF were eligible for Apollo. More details on the eligibility criteria for the Apollo study are described in the Supplementary Material.
The primary objective of the study was to investigate the concordance of the Elecsys CSF pTau/Aβ42 and tTau/Aβ42 ratios, as well as Aβ42 alone, with amyloid PET visual read status (positive vs. negative). An exploratory analysis was conducted to demonstrate the concordance of amyloid status based on the Elecsys CSF Aβ42/Aβ40 ratio with amyloid PET visual read status. The Aβ42/Aβ40 ratio was determined using the updated Aβ42 Gen2 immunoassay and an Elecsys Aβ40 CSF assay, which was in early development during this study. Additionally, the stability of frozen CSF samples after storage at −20 °C for 1–13 weeks was explored.
Elecsys CSF immunoassays
The original clinical cutoff values for Elecsys pTau/Aβ42, tTau/Aβ42 and Aβ42 alone were determined in frozen samples from the Swedish BioFINDER-1 study and their concordance with amyloid PET visual read status was validated in samples from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study [15]. The immunoassays were then updated to eliminate potential interference and improve analytical performance [21]. Additionally, the Elecsys Aβ42 immunoassay was re-standardized using updated certified reference material recently introduced by the International Federation of Clinical Chemistry and Laboratory Medicine, the Aβ42 measuring range was extended from 200–1,700 ng/L to 150–2,500 ng/L and the calibrator levels and control samples (PreciControl level 2) were updated.
The Elecsys β-Amyloid (1–40) CSF assay used in this study was at an early development stage, to be used for exploratory study measurements only. More details on the cutoff determination for Aβ42/Aβ40 are provided in the Supplementary material.
CSF measurements
All CSF samples in Apollo were collected for the Swedish BioFINDER-2 study at the Memory Clinic, Skåne University Hospital (Malmö, Sweden) [27] and handled according to the new routine-use pre-analytical procedure for fresh CSF samples [24]. The measurements of fresh and frozen CSF samples were performed using the updated Elecsys Aβ42 Gen2, pTau and tTau CSF immunoassays as well as the Elecsys Aβ40 CSF immunoassay on the Cobas® e 601 module (Roche Diagnostics International Ltd) at the Department of Clinical Chemistry and Pharmacology, Skåne University Hospital. No additional sample collections or measurements were performed under the Apollo study protocol.
Amyloid PET imaging and analysis
Amyloid PET scans for visual evaluation were collected under the Swedish BioFINDER-2 study, as previously described [27]. No additional PET scans were performed for the Apollo study. Further details on the expert amyloid PET visual read process can be found in the Supplementary material. The primary endpoint for all CSF biomarkers was the amyloid PET visual read outcome, determined as the majority vote from three independent readers, blinded to subject diagnosis and all other clinical and biomarker data.
Exploratory analysis of frozen samples (sample stability)
For the exploratory sample stability analysis, a subset of samples from individuals with available CSF samples were frozen at −20 °C and re-measured after storage for 1–13 weeks. For Aβ42, pTau and tTau, six and 27 samples were stored for 1–8 and >8–13 weeks, respectively; for Aβ40, six and 22 samples were stored for 1–8 and >8–13 weeks, respectively. After freezing, samples were thawed at a temperature between 20–25 °C for 30 min on a roller mixer. During rolling, the tube caps were placed slightly higher than the bottoms to prevent Aβ42 from sticking to the tube lids and ensure measurement accuracy.
Statistical analysis
Primary analysis – concordance of pTau/Aβ42, tTau/Aβ42 and Aβ42 in fresh CSF samples with amyloid PET visual read status
The primary analysis aimed to verify the performance at the pre-specified (adjusted) cutoffs (pTau/Aβ42>0.023; tTau/Aβ42>0.28; Aβ42≤1,030 ng/L) for the new routine-use pre-analytical protocol using the updated Elecsys CSF immunoassays, by demonstrating the concordance of the CSF biomarker status (positive or negative), determined by the pTau/Aβ42 and tTau/Aβ42 ratios and Aβ42 alone in fresh CSF, with amyloid PET visual read status (positive or negative). The minimum sample size for the analysis was determined to be at least 40 PET-positive and 40 PET-negative individuals with confirmed SCD/MCI to ensure a joint power of 90 % to meet the positive percent agreement (PPA), negative percent agreement (NPA) and negative likelihood ratio (LR–) acceptance criteria for an expected underlying performance of 0.85.
CSF biomarker concordance was tested using a fixed sequence approach based on the FDA Draft Guidance ‘Multiple Endpoints in Clinical Trials’ for the hypothesis testing of [28]: sensitivity (PPA) and specificity (NPA) for pTau/Aβ42 and tTau/Αβ42; PPA and the negative likelihood ratio (LR–=[1 – PPA]/NPA) for Aβ42 alone.
For each biomarker, two joint hypotheses for PPA and NPA (or LR– for Aβ42) had to be rejected (each with alpha level 0.05), so that the hypothesis testing was fulfilled. If a hypothesis for a biomarker was not rejected (e.g., the null hypothesis of non-concordance was accepted), hypothesis testing was terminated, and the subsequent biomarkers were considered non-concordant. For the test on PPA and NPA, the two-sided 95 % confidence interval (CI) was computed, and the acceptance criterion was met if the point estimate was >0.75 and the lower confidence limit was >0.60. For the test on LR–, the two-sided 95 % CI was computed, and the acceptance criterion was met if the upper confidence limit was <1.00.
Exploratory analysis – concordance of Aβ42/Aβ40 in fresh CSF samples with amyloid PET visual read status
The concordance of the dichotomized Aβ42/Aβ40 ratio values, measured with the Aβ42 Gen2 and Aβ40 immunoassays, with visual amyloid PET readout status was investigated in an exploratory analysis.
Exploratory analysis – sample stability
The influence of storage at −20 °C was investigated in an exploratory analysis using a regression approach and description of concentration recoveries after freezing and storage. Concentration recoveries were described using boxplots and descriptive tables. Concentration measurements in fresh samples and frozen samples at baseline were compared after storage using scatter plots and Passing–Bablok regression analysis.
Ethics
This study was conducted according to the principles of the Declaration of Helsinki. All samples used were collected under the Swedish BioFINDER-2 study. Written informed consent was obtained from each participant prior to enrollment into the Swedish BioFINDER-2 study. All samples and required clinical information were pseudonymized. Ethics approval was received for the Swedish BioFINDER-2 study, including data shared in the Apollo study, from the Swedish Ethical Review Authority, Sweden.
Results
Concordance of pTau/Aβ42, tTau/Aβ42, Aβ42/Aβ40 and Aβ42 in fresh CSF samples with amyloid PET visual read status
Baseline demographics and clinical characteristics
The Apollo study initially included 108 individuals selected from the BioFINDER-2 cohort based on the criteria described in the Supplementary material. Of the 108 individuals, 16 were excluded due to missing CSF biomarker measurement data and one was excluded during the monitoring process due to not fulfilling the inclusion criterion for Mini-Mental State Examination score (≥24) (Figure 1). Thus, data from 91 individuals were included in the primary analysis.

Enrollment summary. aThe low number of frozen samples fulfilling the required conditions was due to many samples being excluded for exceeding 13 weeks of storage during the COVID-19 pandemic. Aβ40, β-amyloid(1–40); Aβ42, β-amyloid(1–42); CSF, cerebrospinal fluid; PET, positron emission tomography; pTau, phosphorylated tau; tTau, total tau.
Individuals were enrolled from the Swedish BioFINDER-2 study at baseline (61/91; 67.0 %) or at the 2-year follow-up visit (30/91; 33.0 %). The demographic and clinical characteristics of the primary analysis population are summarized in Table 1. The primary analysis population comprised 40 (44.0 %) amyloid PET-positive and 51 (56.0 %) amyloid PET-negative individuals according to the majority vote of three independent readers.
Demographic and clinical characteristics of the primary analysis population, total and split by amyloid PET visual read status.
| PET (visual)-positive (n=40) | PET (visual)-negative (n=51) | Total (n=91) | |
|---|---|---|---|
| Age, years, mean (min–max) | 72.9 (55.0–90.0) | 68.2 (43.0–85.0) | 70.3 (43.0–90.0) |
| Education, years, mean (min–max) | 13.2 (7.0–31.0) | 12.8 (7.0–22.0) | 13.0 (7.0–31.0) |
| MMSE score, mean (min–max) | 28.2 (25.0–30.0) | 28.7 (24.0–30.0) | 28.5 (24.0–30.0) |
| Sex, n (%) Female Male |
15 (37.5) 25 (62.5) |
23 (45.1) 28 (54.9) |
38 (41.8) 53 (58.2) |
| SCD/MCI, n (%) SCD MCI Missing |
20 (50.0) 15 (37.5) 5 (12.5) |
11 (21.6) 26 (51.0) 14 (27.5) |
31 (34.1) 41 (45.1) 19 (20.9) |
| APOE genotype, n (%) E2/E2 E2/E3 E2/E4 E3/E3 E3/E4 E4/E4 |
1 (2.5) 2 (5.0) 0 (0.0) 9 (22.5) 21 (52.5) 7 (17.5) |
1 (2.0) 3 (5.9) 1 (2.0) 29 (56.9) 16 (31.4) 1 (2.0) |
2 (2.2) 5 (5.5) 1 (1.1) 38 (41.8) 37 (40.7) 8 (8.8) |
| Family history, n (%) Yes No Missing |
17 (42.5) 20 (50.0) 3 (7.5) |
25 (49.0) 23 (45.1) 3 (5.9) |
42 (46.2) 43 (47.3) 6 (6.6) |
| Visit during the BioFINDER-2 study, n (%) Baseline visit 2-year follow-up |
30 (75.0) 10 (25.0) |
31 (60.8) 20 (39.2) |
61 (67.0) 30 (33.0) |
-
APOE, apolipoprotein E; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; PET, positron emission tomography; SCD, subjective cognitive decline.
Amyloid PET concordance analysis
Amyloid PET concordance analysis showed that the performance of the pTau/Aβ42 and tTau/Aβ42 ratios at the adjusted cutoffs was as expected and the pre-defined acceptance criteria were met (pTau/Aβ42: PPA 0.800, NPA 0.882; tTau/Aβ42: PPA 0.775, NPA 0.902; Figure 2; Table 2). The observed concordance between Aβ42/Aβ40, dichotomized at the previously published adjusted cutoff, and amyloid PET status was comparable with a PPA of 0.950 and an NPA of 0.824 (Figure 2).
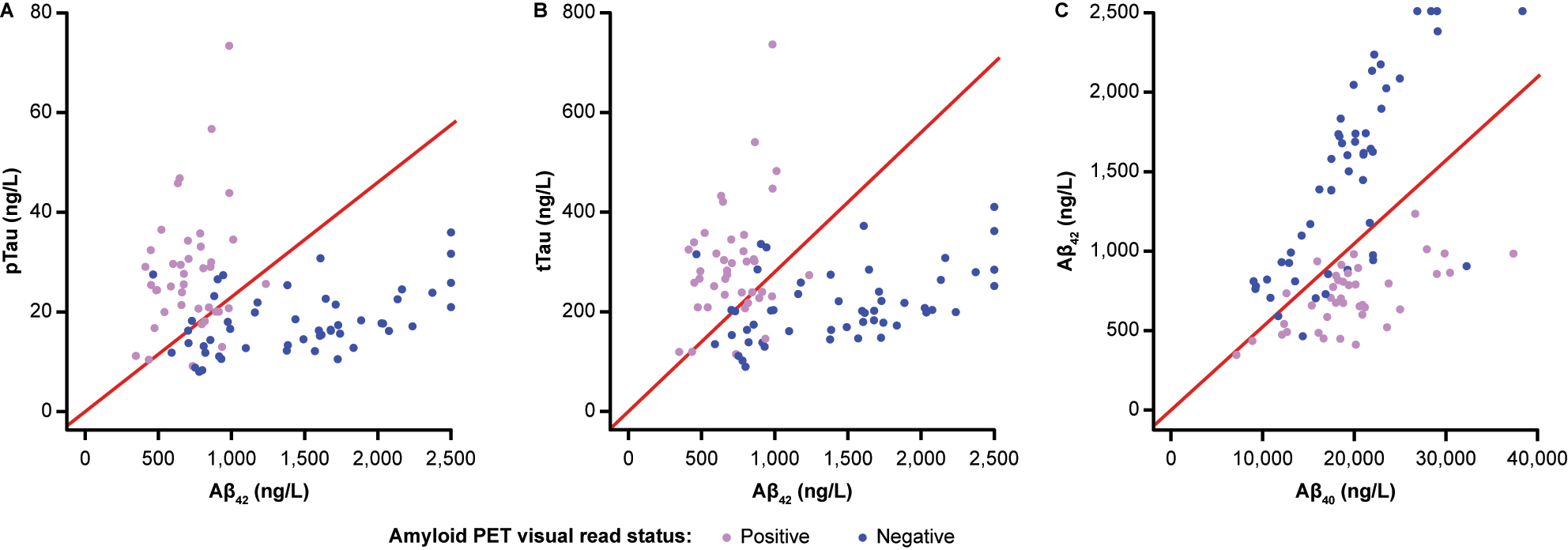
Joint distributions of the single biomarkers (A) pTau and Aβ42, (B) tTau and Aβ42 and (C) Aβ42 and Aβ40. Red lines indicate the respective cutoffs (pTau/Aβ42>0.023; tTau/Aβ42>0.28; Aβ42/Aβ40<0.052). Points are coloured by amyloid PET visual read status. Aβ40, β-amyloid(1–40); Aβ42, β-amyloid(1–42); PET, positron emission tomography; pTau, phosphorylated tau; tTau, total tau.
Hypothesis testing of the pre-specified acceptance criteria for pTau/Aβ42, tTau/Aβ42 and Aβ42.
| Performance measure | Point estimate (95 % CI) | Acceptance criteria | Testing result |
|---|---|---|---|
| pTau/Aβ42 PPA NPA |
0.800 (0.652–0.895) 0.882 (0.766–0.945) |
PPA >0.75 & LCL >0.60 PPA >0.75 & LCL >0.60 |
Successful Successful |
| tTau/Aβ42 PPA NPA |
0.775 (0.625–0.877) 0.902 (0.790–0.957) |
PPA >0.75 & LCL >0.60 PPA >0.75 & LCL >0.60 |
Successful Successful |
| Aβ42 PPA LR– |
0.975 (0.871–0.996) 0.039 (0.006–0.270) |
PPA >0.75 & LCL >0.60 UCL <1 |
Successful Successful |
-
Aβ42, β-amyloid(1–42); CI, confidence interval; LCL, lower confidence limit; LR–, negative likelihood ratio; NPA, negative percent agreement; PPA, positive percent agreement; pTau, phosphorylated tau; tTau, total tau; UCL, upper confidence limit.
Using the CSF pTau/Aβ42-based classification, 38 individuals were scored as CSF-positive, of whom 32 were concordant with a positive PET result; 53 individuals were scored as CSF-negative, of whom 45 were concordant with a negative PET result. In total, 77/91 (84.6 %) individuals showed concordant CSF and amyloid PET visual read results (Table 3). Of the 14 individuals with discordant results, eight were CSF-negative with a positive PET result and six were CSF-positive with a negative PET result (Supplementary Table 1); for 6/8 and 2/6 individuals, biomarker values were within ±10 % of the cutoff value (0.023), respectively (Supplementary Table 2). Similar results were observed using the CSF tTau/Aβ42-based classification, where in total 77/91 (84.6 %) individuals showed concordant CSF and amyloid PET visual read results, while 9/14 were CSF-negative with a positive PET result and 5/14 were CSF-positive with a negative PET result (Table 3; Supplementary Table 1). For 5/9 and 1/5 individuals with discordant results, biomarker values were within ±10 % of the cutoff value (0.28), respectively (Supplementary Table 2). Using the exploratory Aβ42/Aβ40-based classification, in total 80/91 (87.9 %) individuals showed concordant CSF and amyloid PET visual read results, while 2/11 were CSF-negative with a positive PET result and 9/11 were CSF-positive with a negative PET result (Table 3; Supplementary Table 1). Two of the nine individuals with CSF-positive and PET-negative results had biomarker values within ±10 % of the cutoff value (0.052) (Supplementary Table 2).
Concordance tables of classification based on pTau/Aβ42, tTau/Aβ42, Aβ42/Aβ40 and Aβ42 vs. amyloid PET visual read status.
| PET (visual)-positive (n=40) | PET (visual)-negative (n=51) | Total (n=91) | |
|---|---|---|---|
| pTau/Aβ42 CSF-positive, n (%) CSF-negative, n (%) |
32 (35.2) 8 (8.8) |
6 (6.6) 45 (49.5) |
38 (41.8) 53 (58.2) |
| tTau/Aβ42 CSF-positive, n (%) CSF-negative, n (%) |
31 (34.1) 9 (9.9) |
5 (5.5) 46 (50.5) |
36 (39.6) 55 (60.4) |
| Aβ42/Aβ40 CSF-positive, n (%) CSF-negative, n (%) |
38 (41.8) 2 (2.2) |
9 (9.9) 42 (46.2) |
47 (51.6) 44 (48.4) |
| Aβ42 CSF-positive, n (%) CSF-negative, n (%) |
39 (42.9) 1 (1.1) |
18 (19.8) 33 (36.3) |
57 (62.6) 34 (37.4) |
-
Aβ40, β-amyloid(1–40); Aβ42, β-amyloid(1–42); CSF, cerebrospinal fluid; PET, positron emission tomography; pTau, phosphorylated tau; tTau, total tau. The cutoffs for CSF-positivity were as follows: pTau/Aβ42 >0.023; tTau/Aβ42 >0.28; Aβ42/Aβ40 <0.052; Aβ42 ≤1,030 ng/L.
The concordance analysis also showed that the performance of Αβ42 as a single biomarker at the adjusted cutoff was as expected and met the pre-defined acceptance criteria (PPA 0.975, LR– 0.039; Figure 3; Table 2). Of the 91 individuals tested, 57 individuals were scored as CSF-positive, of whom 39 were concordant with a positive PET result; 34 individuals were scored as CSF-negative, of whom 33 were concordant with a negative PET result. In total, 72/91 (79.1 %) individuals had concordant CSF and amyloid PET visual read results using the updated Αβ42 Gen2 immunoassay (Table 3). The NPA observed for the Αβ42 Gen2 immunoassay was lower than the NPA of the pTau/Aβ42 and tTau/Aβ42 ratios, as expected, and 18/91 (35.3 %) individuals with negative PET scans were misclassified as positive by the Αβ42 immunoassay (Table 3; Supplementary Tables 1 and 2). Nevertheless, the performance of pTau/Aβ42, tTau/Aβ42 and Aβ42 met the pre-specified acceptance criteria for all three biomarkers (Table 2).

Box plot of Aβ42 concentration (ng/L) by visual PET status. Red lines indicate the respective cutoff (≤1,030 ng/L). Aβ42, β-amyloid(1–42); PET, positron emission tomography.
Stability analysis in frozen CSF samples
Of the 91 CSF samples, 33 samples (36.3 %) were frozen at −20 °C for 1–13 weeks and used to explore the effect of storage and one freeze-thaw cycle on the stability of frozen CSF samples; for Aβ40, measurements were available for 28/33 samples. For all four biomarkers, the measurements in samples before and after freezing were highly correlated, with Pearson’s R >0.99, and slope estimates were close to 1.000 (pTau: 0.973; tTau: 0.965; Aβ42: 1.000; Aβ40: 1.050; Figure 4, Supplementary Table 3). The largest bias estimate at the pre-specified concentration was observed for tTau (bias: −2.74 % [95 % CI –3.42; −1.17]) at a concentration of 300 ng/L, followed by Aβ42 (bias: −2.64 % [95 % CI –6.08; −1.11]), Aβ40 (bias: −1.6 % [95 % CI –5.6; 0.4]) and pTau (bias: −1.15 % [95 % CI –3.94; 0.62]) (Figure 4, Supplementary Table 3). The concentration recoveries for pTau and tTau were within 100 ± 10 % in all samples stored for 1–13 weeks (Figure 5). Aβ42 and Aβ40 recoveries were within 100 ± 10 % in all samples stored at −20 °C for 1–8 weeks (n=6). For Aβ42 and Aβ40, concentration recoveries for 6/27 and 2/22 samples, respectively, stored at the same temperature for >8–13 weeks, were below 90 %.
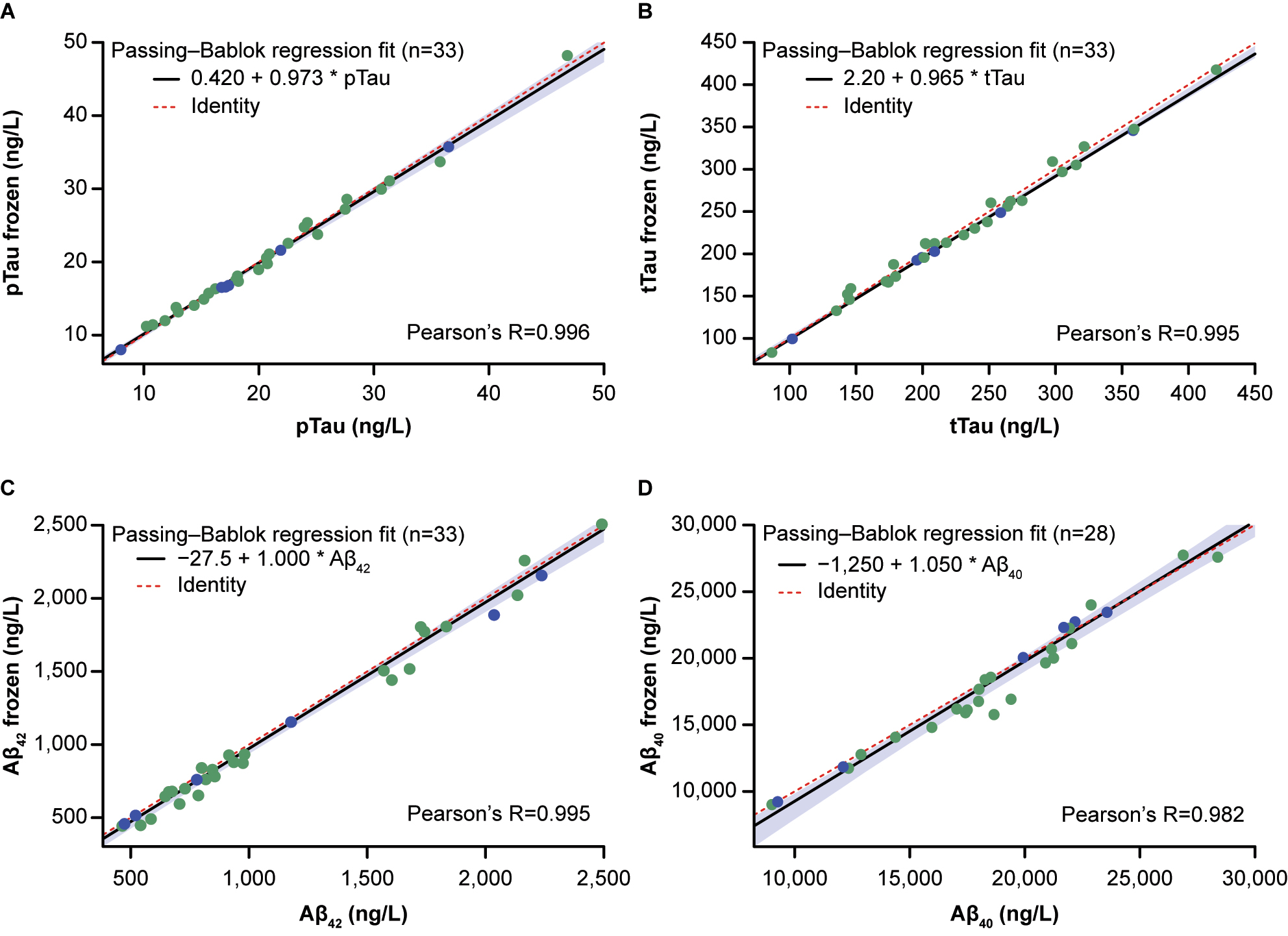
Stability of (A) pTau, (B) tTau, (C) Aβ42 and (D) Aβ40 at −20 °C for 1–8 and >8–13 weeks. Passing–Bablok regression fit is shown as a black line with 95 % confidence bounds (light blue shaded area). X-axes show concentrations in fresh samples and y-axes concentrations in frozen samples. Red dashed lines represent identity lines. Blue points indicate storage for 1–8 weeks and green points storage for >8–13 weeks. Aβ40, β-amyloid(1–40); Aβ42, β-amyloid(1–42); pTau, phosphorylated tau; tTau, total tau.
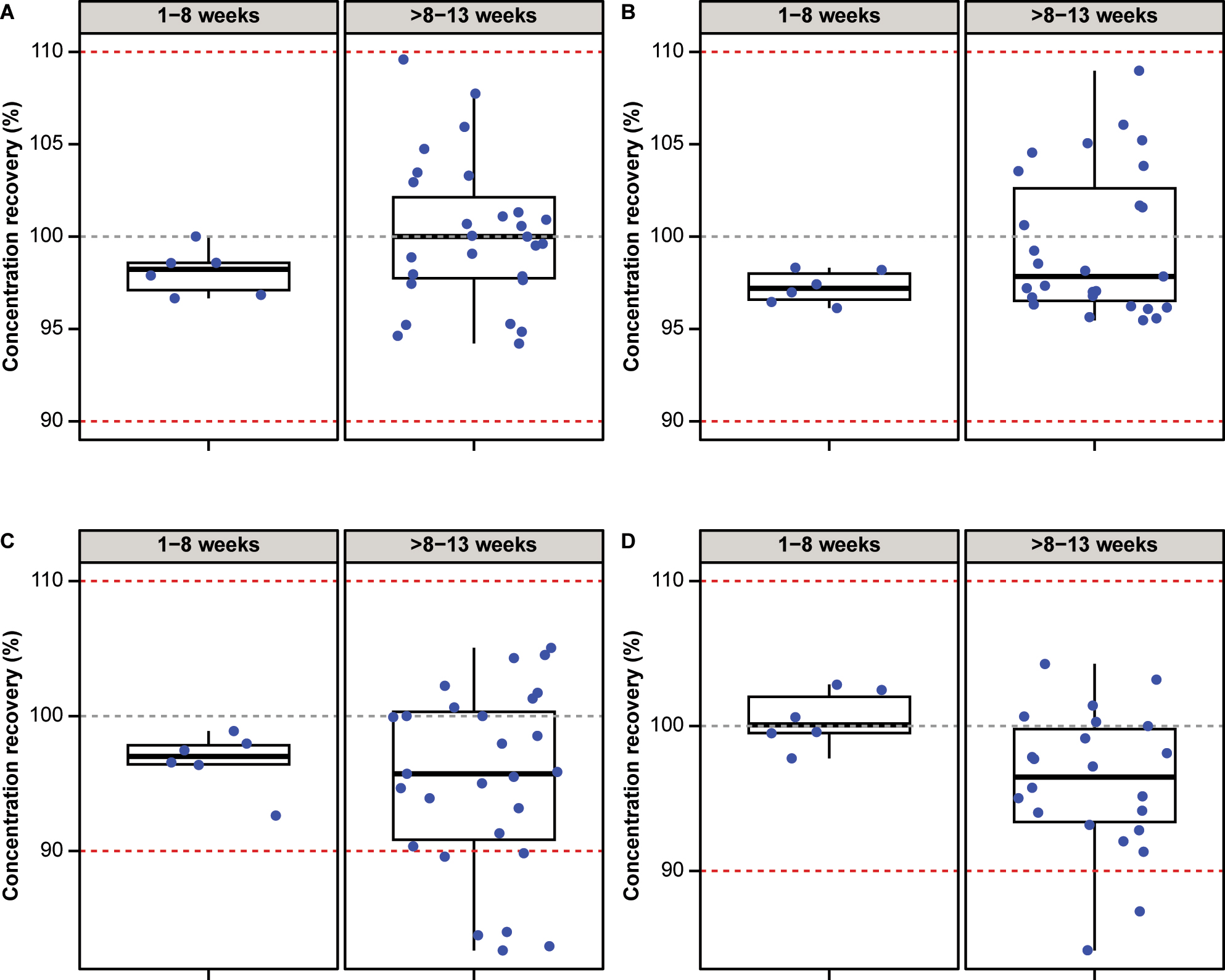
Concentration recoveries (%) for (A) pTau, (B) tTau, (C) Aβ42 and (D) Aβ40 observed after storage at −20 °C for 1–8 weeks and >8–13 weeks. Red dashed lines indicate 90 and 110 % recovery bounds. Aβ40, β-amyloid(1–40); Aβ42, β-amyloid(1–42); pTau, phosphorylated tau; tTau, total tau.
Discussion
This study supports the concordance of pTau/Aβ42, tTau/Aβ42 and Aβ42 with amyloid PET visual reads and verifies that the performance of the adjusted cutoffs for the Elecsys ratios is as expected in CSF samples handled with the new routine-use pre-analytical procedure and measured with the updated CSF immunoassays. These results suggest that both biomarker ratios plus Aβ42 alone could be used in clinical practice as reliable alternatives to amyloid PET imaging to aid in the diagnosis of amyloid pathology.
In this study, the pTau/Aβ42 and tTau/Aβ42 ratios met the pre-defined acceptance criteria for PPA and NPA and showed more than 80 % concordant positive and negative CSF and PET scan results, while only a low percentage (<16 %) were discordant. Aβ42 alone also met the pre-defined acceptance criteria, and the LR– value was low (0.039). This indicated that the likelihood of an amyloid PET-positive individual having an Aβ42 concentration greater than 1,030 ng/L is significantly smaller (by a factor of 0.039) compared with an amyloid PET-negative individual. Nevertheless, the NPA value of Αβ42 as a single biomarker was substantially lower than the ratios, as biomarkers alone typically perform worse than combination ratios, and the original Αβ42 cutoff value was set to fulfill a high PPA to ensure high sensitivity [29], [30], [31]. The exploratory analysis also indicated that normalization with Aβ40 improved the performance of Aβ42 alone, and the performance of the Aβ42/Aβ40 ratio was comparable to that of the pTau/Aβ42 and tTau/Aβ42 ratios, consistent with previously reported results [32], [33], [34], [35], since the CIs of PPA and NPA overlapped.
Storage at −20 °C for 1–8 weeks and one freeze-thaw cycle had no effect on any of the four biomarker concentration recoveries. Storage for >8–13 weeks also had no significant effect on pTau and tTau concentration recoveries, whereas a small effect was observed on Aβ42 and Aβ40, respectively, under the same conditions, with 6/27 and 2/22 samples showing concentration recoveries <90 %. It is therefore recommended to store CSF samples at −20 °C for ≤8 weeks to maintain stability.
This study supports the verification of the clinical performance of the updated Elecsys CSF immunoassays with the new routine-use pre-analytical procedure and their concordance with amyloid PET visual read status in distinguishing amyloid-positive individuals with early-stage AD, who are considered perhaps the most relevant but also the most diagnostically challenging group of the intended use population. Although early-stage disease PET scans are challenging to correctly classify as positive or negative, and biomarker levels are closer to the cutoff values, this study indicated a good performance of concordance with amyloid PET imaging. A better performance of the tests in terms of PPA and NPA is expected in individuals with Alzheimer’s dementia (not included here) due to the more advanced amyloid pathology, which is more clearly reflected in the CSF biomarker levels and PET scans.
This study’s findings are consistent with previous research using Elecsys and other platforms. Specifically, previous studies have shown that the Elecsys CSF pTau/Aβ42, tTau/Aβ42 and Aβ42/Aβ40 ratios, as well as Aβ42 alone, are strongly concordant with PET imaging assessing Aβ burden in AD, supporting the use of CSF biomarkers in early amyloid identification [15], 34]. For instance, Hansson et al. indicated that pTau/Aβ42 and tTau/Aβ42, measured with the first-generation Elecsys CSF immunoassays, were highly concordant with amyloid PET visual reads across two different cohorts (BioFINDER and ADNI) comprising different populations and PET radiotracers [15]. Schindler et al. reported high concordance between Pittsburgh compound B PET imaging and pTau/Aβ42, tTau/Aβ42 and Aβ42/Aβ40 ratios, measured using the first-generation Elecsys CSF immunoassays, in discriminating PET-positive from PET-negative individuals [35]. Campbell et al. showed agreement between pTau/Aβ42 and Aβ42/Aβ40 ratios, measured with the first-generation Elecsys and LUMIPULSE immunoassays, and amyloid PET classification, and the biomarker ratio results were superior to individual biomarkers [36]. In another study, Alcolea et al. reported that pTau/Aβ42, tTau/Aβ42 and Aβ42/Aβ40, measured on the fully automated, Conformité Européenne-marked and FDA-approved LUMIPULSE G600II platform (Fujirebio), had good diagnostic agreement with 18F-flutemetamol amyloid PET and the ratios were suggested to be more reliable in clinical practice than Aβ42 alone [31], 32], 37].
The future clinical application of these findings is expected to aid earlier diagnosis of patients with AD, giving them and their caregivers time to plan for the future and access potential treatments for early symptom management. Implementing the new pre-analytical procedure and recommended storage conditions for handling fresh CSF samples is expected to reduce the variability of assay measurements and enable comparison of CSF biomarker levels between different laboratories, thus increasing the utility of CSF biomarkers in research and routine clinical practice [24].
This study had some limitations, such as the relatively small number of individuals enrolled, which suggests that the results should be confirmed in a wider population. The enrolled population was not randomly selected from the intended use population, but was based on the Swedish BioFINDER-2 study cohort. Thus, the results of this study may be biased due to the inclusion and exclusion criteria of the BioFINDER-2 study. However, the BioFINDER-2 study includes participants from secondary care specialized memory clinics, and therefore does not differ substantially from an intended use population. Additionally, the concordance of Aβ42/Aβ40 with amyloid PET visual reads was assessed using an early version of the Elecsys CSF Aβ40 immunoassay and the acceptance criteria as well as the adjusted cutoff for the Aβ42/Aβ40 ratio were not pre-specified. Moreover, the objectives for the frozen sample analysis were limited to exploratory due to the low number of frozen samples available. The number of samples stored up to 8 weeks was small, suggesting that the results of the exploratory analysis under these storage conditions will need to be confirmed in a larger sample size. It is also worth noting that although the pTau/Aβ42, tTau/Aβ42, Aβ42/Aβ40 ratios and Aβ42 alone can successfully identify individuals with positive amyloid PET results, their performance does not establish a diagnosis of AD or other cognitive disorder and cannot be used for predicting the development of dementia or other neurological conditions, or to monitor responses to therapies.
Conclusions
CSF biomarker status, determined by the pTau/Aβ42, tTau/Aβ42 and Aβ42/Aβ40 ratios and Aβ42 alone in fresh CSF, is concordant with amyloid PET visual read status. All three ratios can be used to identify amyloid PET positivity in individuals with SCD/MCI with high sensitivity and specificity, and Αβ42 alone can distinguish amyloid PET-positive individuals with high sensitivity. As a conservative approach, CSF samples should be stored at −20 °C for ≤8 weeks to maintain stability before testing. The new routine-use pre-analytical procedure and the updated Elecsys Aβ42 Gen2, pTau and tTau CSF immunoassays could be used in clinical practice as alternatives to amyloid PET imaging to identify amyloid positivity in SCD/MCI individuals, thus contributing to the accurate and timely diagnosis of AD.
Funding source: Roche Diagnostics International Ltd
Award Identifier / Grant number: n/a
Acknowledgments
The authors would like to thank the patients for their participation in this study. The authors would also like to thank Chad Logan for contributing to study design; Andreas Franke and Sabine Wizemann for contributing to study management; and Gwendlyn Kollmorgen for managing the Roche-sponsored study, which provided the CSF measurements to the Swedish BioFINDER-2 study and subsequently to the Apollo study, and additionally for her contribution in supporting the Apollo study. ELECSYS and COBAS are trademarks of Roche. All other product names and trademarks are the property of their respective owner. Elecsys β-Amyloid (1–42) CSF II, Elecsys Phospho-Tau (181P) CSF and Elecsys Total-Tau CSF assays are approved for clinical use.
-
Research ethics: The study was conducted according to the principles of the Declaration of Helsinki. All samples used were prospectively collected for the Swedish BioFINDER-2 study. Ethics approval was received for the Swedish BioFINDER-2 study, including the data shared in the Apollo study, from the Swedish Ethical Review Authority, Sweden.
-
Informed consent: Written informed consent was obtained from each participant prior to enrollment into the Swedish BioFINDER-2 study. All sample information and all required clinical information were pseudonymized.
-
Author contributions: Henrik Schinke: data curation, formal analysis, software, supervision, visualization, writing – original draft, writing – review & editing. Magnus Förnvik Jonsson: investigation, resources, supervision, validation, writing – review & editing. Mayme Gummesson: investigation, resources, software, validation, writing – review & editing. Rikard Nilsson: investigation, resources, software, validation, writing – review & editing. Stefanie Gaupp: supervision, writing – review & editing. Ekaterina Manuilova: conceptualization, methodology, validation, writing – review & editing. Silja McIlwrick: conceptualization, investigation, methodology, supervision, writing – review & editing. Jan-Philipp Weinberger: conceptualization, software, validation, writing – review & editing. Sandra Rutz: investigation, resources, writing – review & editing. Margherita Carboni: conceptualization, writing – original draft, writing – review & editing. Erik Stomrud: conceptualization, methodology, project administration, supervision, writing – review & editing. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: HS, EM, SM and SR are full-time employees of Roche Diagnostics GmbH, Penzberg, Germany, and shareholders of F. Hoffmann-La Roche Ltd. MFJ, MG, RN and ES have no conflicts of interest. SG is an employee of TRIGA-S GmbH contracted by Roche Diagnostics GmbH, Penzberg, Germany. J-PW is a full-time employee of Roche Diagnostics GmbH, Penzberg, Germany. MC is a full-time employee of Roche Diagnostics International Ltd, Rotkreuz, Switzerland and a shareholder of F. Hoffmann-La Roche Ltd.
-
Research funding: The study was funded by Roche Diagnostics International Ltd. Third-party medical writing assistance under the direction of the authors was provided by Dimitra Pournara, PhD (Thessaloniki, Greece) and Tiffany Blythe, BSc (London, UK), of Ashfield MedComms, an Inizio company, and was funded by Roche Diagnostics International Ltd (Rotkreuz, Switzerland).
-
Data availability: Requests concerning the data supporting the findings of this study can be directed to rotkreuz.datasharingrequests@roche.com for consideration.
References
1. Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 2023;19:1598–695.10.1002/alz.13016Search in Google Scholar PubMed
2. Zvěřová, M. Clinical aspects of Alzheimer’s disease. Clin Biochem 2019;72:3–6. https://doi.org/10.1016/j.clinbiochem.2019.04.015.Search in Google Scholar PubMed
3. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022;7:e105-25. https://doi.org/10.1016/S2468-2667(21)00249-8.Search in Google Scholar PubMed PubMed Central
4. Paraskevas, GP, Kapaki, E. Cerebrospinal fluid biomarkers for Alzheimer’s disease in the era of disease-modifying treatments. Brain Sci 2021;11:1258. https://doi.org/10.3390/brainsci11101258.Search in Google Scholar PubMed PubMed Central
5. Bjerke, M, Engelborghs, S. Cerebrospinal fluid biomarkers for early and differential Alzheimer’s disease diagnosis. J Alzheimers Dis 2018;62:1199–209. https://doi.org/10.3233/jad-170680.Search in Google Scholar PubMed PubMed Central
6. Cummings, J, Osse, AML, Cammann, D, Powell, J, Chen, J. Anti-amyloid monoclonal antibodies for the treatment of Alzheimer’s disease. BioDrugs 2024;38:5–22. https://doi.org/10.1007/s40259-023-00633-2.Search in Google Scholar PubMed PubMed Central
7. Eisai Inc. US prescribing information: LEQEMBITM (lecanemab-irmb) injection, for intravenous use. 2023. Nutley, NJ, USA: Eisai R&D Management Co., Ltd; 2023.Search in Google Scholar
8. Eli Lilly. US prescribing information: KISUNLA (donanemab-azbt) injection, for intravenous use. 2024. Indianapolis, IN, USA: Eli Lilly and Company; 2024.Search in Google Scholar
9. Dubois, B, Villain, N, Frisoni, GB, Rabinovici, GD, Sabbagh, M, Cappa, S, et al.. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol 2021;20:484–96. https://doi.org/10.1016/s1474-4422(21)00066-1.Search in Google Scholar
10. Dulewicz, M, Kulczyńska-Przybik, A, Mroczko, P, Kornhuber, J, Lewczuk, P, Mroczko, B. Biomarkers for the diagnosis of Alzheimer’s disease in clinical practice: the role of CSF biomarkers during the evolution of diagnostic criteria. Int J Mol Sci 2022;23:8598. https://doi.org/10.3390/ijms23158598.Search in Google Scholar PubMed PubMed Central
11. Jack, CR Jr., Andrews, JS, Beach, TG, Buracchio, T, Dunn, B, Graf, A, et al.. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement 2024;20:5143–69. https://doi.org/10.1002/alz.13859.Search in Google Scholar PubMed PubMed Central
12. Fagan, AM, Roe, CM, Xiong, C, Mintun, MA, Morris, JC, Holtzman, DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 2007;64:343–9. https://doi.org/10.1001/archneur.64.3.noc60123.Search in Google Scholar PubMed
13. Prakash, RS, McKenna, MR, Gbadeyan, O, Andridge, R, Scharre, DW. For the Alzheimer’s Disease Neuroimaging Initiative. p-tau/Aβ42 ratio associates with cognitive decline in Alzheimer’s disease, mild cognitive impairment, and cognitively unimpaired older adults. Preprint from medRxiv 2020. https://doi.org/10.1101/2020.10.13.20211375.Search in Google Scholar
14. Blennow, K, Shaw, LM, Stomrud, E, Mattsson, N, Toledo, JB, Buck, K, et al.. Predicting clinical decline and conversion to Alzheimer’s disease or dementia using novel Elecsys Aβ(1–42), pTau and tTau CSF immunoassays. Sci Rep 2019;9:19024. https://doi.org/10.1038/s41598-019-54204-z.Search in Google Scholar PubMed PubMed Central
15. Hansson, O, Seibyl, J, Stomrud, E, Zetterberg, H, Trojanowski, JQ, Bittner, T, et al.. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018;14:1470–81. https://doi.org/10.1016/j.jalz.2018.01.010.Search in Google Scholar PubMed PubMed Central
16. Hansson, O, Lehmann, S, Otto, M, Zetterberg, H, Lewczuk, P. Advantages and disadvantages of the use of the CSF amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s disease. Alzheimers Res Ther 2019;11:34. https://doi.org/10.1186/s13195-019-0485-0.Search in Google Scholar PubMed PubMed Central
17. Lewczuk, P, Matzen, A, Blennow, K, Parnetti, L, Molinuevo, JL, Eusebi, P, et al.. Cerebrospinal fluid Aβ42/40 corresponds better than Aβ42 to Amyloid PET in Alzheimer’s disease. J Alzheimers Dis 2017;55:813–22. https://doi.org/10.3233/jad-160722.Search in Google Scholar
18. Janelidze, S, Zetterberg, H, Mattsson, N, Palmqvist, S, Vanderstichele, H, Lindberg, O, et al.. CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 2016;3:154–65. https://doi.org/10.1002/acn3.274.Search in Google Scholar PubMed PubMed Central
19. Janelidze, S, Pannee, J, Mikulskis, A, Chiao, P, Zetterberg, H, Blennow, K, et al.. Concordance between different amyloid immunoassays and visual amyloid positron emission tomographic assessment. JAMA Neurol 2017;74:1492–501. https://doi.org/10.1001/jamaneurol.2017.2814.Search in Google Scholar PubMed PubMed Central
20. Hansson, O, Zetterberg, H, Buchhave, P, Andreasson, U, Londos, E, Minthon, L, et al.. Prediction of Alzheimer’s disease using the CSF Ab42/Ab40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord 2007;23:316–20. https://doi.org/10.1159/000100926.Search in Google Scholar PubMed
21. Blennow, K, Stomrud, E, Zetterberg, H, Borlinghaus, N, Corradini, V, Manuilova, E, et al.. Second-generation Elecsys cerebrospinal fluid immunoassays aid diagnosis of early Alzheimer’s disease. Clin Chem Lab Med 2023;61:234–44. https://doi.org/10.1515/cclm-2022-0516.Search in Google Scholar PubMed
22. Roche, Diagnostics.: Roche receives FDA clearance for additional Alzheimer’s disease cerebrospinal fluid (CSF) assays, supporting timely diagnosis and treatment decision-making [Online]. https://diagnostics.roche.com/us/en/news-listing/2023/roche-fda-clearance-additional-alzheimers-disease-cerebrospinal-fluid-ttau.html [Accessed 15 November 2024].Search in Google Scholar
23. Roche Diagnostics, GmbH. Press release: Roche Alzheimer’s disease Cerebrospinal Fluid (CSF) assays receive FDA clearance, supporting more accurate and timely diagnosis [Online]. https://www.roche.com/media/releases/med-cor-2022-12-08 [Accessed 20 November 2024].Search in Google Scholar
24. Hansson, O, Rutz, S, Zetterberg, H, Bauer, E, Hähl, T, Manuilova, E, et al.. Pre-analytical protocol for measuring Alzheimer’s disease biomarkers in fresh CSF. Alzheimers Dement (Amst) 2020;12:e12137. Erratum in: Alzheimers Dement (Amst). 2021;13(1):e76. https://doi.org/10.1002/dad2.12137.Search in Google Scholar PubMed PubMed Central
25. Toombs, J, Foiani, MS, Wellington, H, Paterson, RW, Arber, C, Heslegrave, A, et al.. Amyloid β peptides are differentially vulnerable to preanalytical surface exposure, an effect incompletely mitigated by the use of ratios. Alzheimers Dement (Amst) 2018;10:311–21. https://doi.org/10.1016/j.dadm.2018.02.005.Search in Google Scholar PubMed PubMed Central
26. THE SWEDISH BioFINDER STUDY. Population & study design [Online]. https://biofinder.se/two/population-study-design/ [Accessed 27 November 2024].Search in Google Scholar
27. Palmqvist, S, Janelidze, S, Quiroz, YT, Zetterberg, H, Lopera, F, Stomrud, E, et al.. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 2020;324:772–81. https://doi.org/10.1001/jama.2020.12134.Search in Google Scholar PubMed PubMed Central
28. U.S. Food & Drug Administration (FDA). Multiple endpoints in clinical trials [Online]. Malvern, PA, USA: U.S. Food and Drug Administration. https://www.fda.gov/media/162427/download?attachment [Accessed 27 November 2024].Search in Google Scholar
29. van Harten, AC, Wiste, HJ, Weigand, SD, Mielke, MM, Kremers, WK, Eichenlaub, U, et al.. Detection of Alzheimer’s disease amyloid beta 1-42, p-tau, and t-tau assays. Alzheimers Dement 2022;18:635–44. https://doi.org/10.1002/alz.12406.Search in Google Scholar PubMed PubMed Central
30. Mattsson-Carlgren, N, Grinberg, LT, Boxer, A, Ossenkoppele, R, Jonsson, M, Seeley, W, et al.. Cerebrospinal fluid biomarkers in autopsy-confirmed Alzheimer disease and frontotemporal lobar degeneration. Neurology 2022;98:e1137–50. https://doi.org/10.1212/wnl.0000000000200040.Search in Google Scholar PubMed PubMed Central
31. Iaccarino, L, Burnham, SC, Dell’Agnello, G, Dowsett, SA, Epelbaum, S. Diagnostic biomarkers of amyloid and tau pathology in Alzheimer’s disease: an overview of tests for clinical practice in the United States and Europe. J Prev Alzheimers Dis 2023;10:426–42. https://doi.org/10.14283/jpad.2023.43.Search in Google Scholar PubMed
32. Alcolea, D, Pegueroles, J, Muñoz, L, Camacho, V, López-Mora, D, Fernández-León, A, et al.. Agreement of amyloid PET and CSF biomarkers for Alzheimer’s disease on lumipulse. Ann Clin Transl Neurol 2019;6:1815–24. https://doi.org/10.1002/acn3.50873.Search in Google Scholar PubMed PubMed Central
33. Amft, M, Ortner, M, Eichenlaub, U, Goldhardt, O, Diehl-Schmid, J, Hedderich, DM, et al.. The cerebrospinal fluid biomarker ratio Aβ42/40 identifies amyloid positron emission tomography positivity better than Aβ42 alone in a heterogeneous memory clinic cohort. Alzheimers Res Ther 2022;14:60. https://doi.org/10.1186/s13195-022-01003-w.Search in Google Scholar PubMed PubMed Central
34. Doecke, JD, Ward, L, Burnham, SC, Villemagne, VL, Li, QX, Collins, S, et al.. Elecsys CSF biomarker immunoassays demonstrate concordance with amyloid-PET imaging. Alzheimers Res Ther 2020;12:36. https://doi.org/10.1186/s13195-020-00595-5.Search in Google Scholar PubMed PubMed Central
35. Schindler, SE, Gray, JD, Gordon, BA, Xiong, C, Batrla-Utermann, R, Quan, M, et al.. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimer Dement 2018;14:1460–9. https://doi.org/10.1016/j.jalz.2018.01.013.Search in Google Scholar PubMed PubMed Central
36. Campbell, MR, Ashrafzadeh-Kian, S, Petersen, RC, Mielke, MM, Syrjanen, JA, van Harten, AC, et al.. P-tau/Aβ42 and Aβ42/40 ratios in CSF are equally predictive of Amyloid PET status. Alzheimer Dement 2021;13:e12190. https://doi.org/10.1002/dad2.12190.Search in Google Scholar PubMed PubMed Central
37. Fujirebio. Fujirebio Diagnostics receives FDA breakthrough device designation for Lumipulse® G β-amyloid ratio (1-42/1-40) quantitative in vitro diagnostic test [Online]. https://www.fujirebio.com/en/news-events/fujirebio-diagnostics-receives-fda-breakthrough-device-designation-for-lumipulser-g [Accessed December 02, 2024].Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cclm-2024-1476).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Setting analytical performance specification by simulation (Milan model 1b)
- Reviews
- Unveiling the power of R: a comprehensive perspective for laboratory medicine data analysis
- Clostebol detection after transdermal and transmucosal contact. A systematic review
- Opinion Papers
- A value-based score for clinical laboratories: promoting the work of the new EFLM committee
- Digital metrology in laboratory medicine: a call for bringing order to chaos to facilitate precision diagnostics
- Perspectives
- Supporting prioritization efforts of higher-order reference providers using evidence from the Joint Committee for Traceability in Laboratory Medicine database
- Clinical vs. statistical significance: considerations for clinical laboratories
- Genetics and Molecular Diagnostics
- Reliable detection of sex chromosome abnormalities by quantitative fluorescence polymerase chain reaction
- Targeted proteomics of serum IGF-I, -II, IGFBP-2, -3, -4, -5, -6 and ALS
- Candidate Reference Measurement Procedures and Materials
- Liquid chromatography tandem mass spectrometry (LC-MS/MS) candidate reference measurement procedure for urine albumin
- General Clinical Chemistry and Laboratory Medicine
- Patient risk management in laboratory medicine: an international survey to assess the severity of harm associated with erroneous reported results
- Exploring the extent of post-analytical errors, with a focus on transcription errors – an intervention within the VIPVIZA study
- A survey on measurement and reporting of total testosterone, sex hormone-binding globulin and free testosterone in clinical laboratories in Europe
- Quality indicators in laboratory medicine: a 2020–2023 experience in a Chinese province
- Impact of delayed centrifugation on the stability of 32 biochemical analytes in blood samples collected in serum gel tubes and stored at room temperature
- Concordance between the updated Elecsys cerebrospinal fluid immunoassays and amyloid positron emission tomography for Alzheimer’s disease assessment: findings from the Apollo study
- Novel protocol for metabolomics data normalization and biomarker discovery in human tears
- Use of the BIOGROUP® French laboratories database to conduct CKD observational studies: a pilot EPI-CKD1 study
- Reference Values and Biological Variations
- Consensus instability equations for routine coagulation tests
- Hematology and Coagulation
- Flow-cytometric lymphocyte subsets enumeration: comparison of single/dual-platform method in clinical laboratory with dual-platform extended PanLeucogating method in reference laboratory
- Cardiovascular Diseases
- Novel Mindray high sensitivity cardiac troponin I assay for single sample and 0/2-hour rule out of myocardial infarction: MERITnI study
- Infectious Diseases
- Cell population data for early detection of sepsis in patients with suspected infection in the emergency department
- Letters to the Editor
- Lab Error Finder: A call for collaboration
- Cascading referencing of terms and definitions
- Strengthening international cooperation and confidence in the field of laboratory medicine by ISO standardization
- Determining the minimum blood volume required for laboratory testing in newborns
- Performance evaluation of large language models with chain-of-thought reasoning ability in clinical laboratory case interpretation
- Vancomycin assay interference: low-level IgM paraprotein disrupts Siemens Atellica® CH VANC assay
- Dr. Morley Donald Hollenberg. An extraordinary scientist, teacher and mentor
Articles in the same Issue
- Frontmatter
- Editorial
- Setting analytical performance specification by simulation (Milan model 1b)
- Reviews
- Unveiling the power of R: a comprehensive perspective for laboratory medicine data analysis
- Clostebol detection after transdermal and transmucosal contact. A systematic review
- Opinion Papers
- A value-based score for clinical laboratories: promoting the work of the new EFLM committee
- Digital metrology in laboratory medicine: a call for bringing order to chaos to facilitate precision diagnostics
- Perspectives
- Supporting prioritization efforts of higher-order reference providers using evidence from the Joint Committee for Traceability in Laboratory Medicine database
- Clinical vs. statistical significance: considerations for clinical laboratories
- Genetics and Molecular Diagnostics
- Reliable detection of sex chromosome abnormalities by quantitative fluorescence polymerase chain reaction
- Targeted proteomics of serum IGF-I, -II, IGFBP-2, -3, -4, -5, -6 and ALS
- Candidate Reference Measurement Procedures and Materials
- Liquid chromatography tandem mass spectrometry (LC-MS/MS) candidate reference measurement procedure for urine albumin
- General Clinical Chemistry and Laboratory Medicine
- Patient risk management in laboratory medicine: an international survey to assess the severity of harm associated with erroneous reported results
- Exploring the extent of post-analytical errors, with a focus on transcription errors – an intervention within the VIPVIZA study
- A survey on measurement and reporting of total testosterone, sex hormone-binding globulin and free testosterone in clinical laboratories in Europe
- Quality indicators in laboratory medicine: a 2020–2023 experience in a Chinese province
- Impact of delayed centrifugation on the stability of 32 biochemical analytes in blood samples collected in serum gel tubes and stored at room temperature
- Concordance between the updated Elecsys cerebrospinal fluid immunoassays and amyloid positron emission tomography for Alzheimer’s disease assessment: findings from the Apollo study
- Novel protocol for metabolomics data normalization and biomarker discovery in human tears
- Use of the BIOGROUP® French laboratories database to conduct CKD observational studies: a pilot EPI-CKD1 study
- Reference Values and Biological Variations
- Consensus instability equations for routine coagulation tests
- Hematology and Coagulation
- Flow-cytometric lymphocyte subsets enumeration: comparison of single/dual-platform method in clinical laboratory with dual-platform extended PanLeucogating method in reference laboratory
- Cardiovascular Diseases
- Novel Mindray high sensitivity cardiac troponin I assay for single sample and 0/2-hour rule out of myocardial infarction: MERITnI study
- Infectious Diseases
- Cell population data for early detection of sepsis in patients with suspected infection in the emergency department
- Letters to the Editor
- Lab Error Finder: A call for collaboration
- Cascading referencing of terms and definitions
- Strengthening international cooperation and confidence in the field of laboratory medicine by ISO standardization
- Determining the minimum blood volume required for laboratory testing in newborns
- Performance evaluation of large language models with chain-of-thought reasoning ability in clinical laboratory case interpretation
- Vancomycin assay interference: low-level IgM paraprotein disrupts Siemens Atellica® CH VANC assay
- Dr. Morley Donald Hollenberg. An extraordinary scientist, teacher and mentor


