Abstract
The measurement uncertainty budget should combine the uncertainty of higher order references, the uncertainty of commercial system calibration, the system imprecision and individual laboratory performance in terms of variability. Here we recommend that no more than one third of the total uncertainty budget, established by appropriate analytical performance specifications, is consumed by the uncertainty of references and approximately 50% of the total budget consumed by the manufacturer’s calibration and value transfer protocol. The remaining 50% should be available for the commercial system imprecision (including the batch to batch variation of the reagents) and individual laboratory performance in order to fulfil the uncertainty goal. For commercial systems to work properly, in vitro diagnostics (IVD) manufacturers will need to take more responsibility and ensure the traceability of the combination of platform, reagents, calibrators and control materials for system alignment verification that only as such (as a whole) are certified (“CE marked”) by the manufacturer itself in terms of traceability to the selected reference measurement system. Particularly, IVD manufacturers should report the combined (expanded) uncertainty associated with their calibrators when used in conjunction with other components of their analytical system (platform and reagents). This is more than what they are currently providing as traceability and uncertainty information.
Introduction
The laboratory measurement paradigm is based on the assumption that assays claiming to measure the same analyte should provide equivalent results for long-term and within clinically meaningful limits. Time, laboratory location and the assay system used to produce them should not influence patient results. There is now an international agreement on the fact that, to become equivalent for long-term, results must be traceable to higher order references [1]. In this regard, it is essential to build an unbroken metrological traceability chain whereby in vitro diagnostics (IVD) manufacturers can implement a reliable transfer of the measurement trueness from the highest level of the metrological hierarchy to calibrators of commercial analytical systems used in clinical laboratories and, finally, to the patient’s results. Only in this way results obtained by the calibrated commercial procedure are expressed in terms of the values obtained at the highest available level of the calibration hierarchy [1]. The European Union (EU) Directive 98/78 on IVD medical devices, supported by a specific standard of the International Standardization Organization (ISO) [2], requires manufacturers to ensure traceability of their analytical systems to recognized higher order references and the compliance with the IVD Directive is indicated through the CE (“Communautés Européennes”) marking of conformity on diagnostic products [3].
For establishing traceability some basic requirements should be fulfilled. Firstly, it is essential to establish a calibration hierarchy starting from the unequivocal definition of the measurand as the quantity subject to measurement [4]. The assay selectivity for the measurand at each level of the traceability chain is a crucial aspect and in a standardization project correlation studies should preliminarily be performed to test the relationship among methods. By definition, results on different measurands are indeed not comparable and cannot be standardized [5]. Another basic concept behind the theory of metrological traceability is that the measurement bias along all the traceability steps, if any, should be appropriately eliminated [6]. The applied implementation strategy should reliably transfer the measurement trueness from the highest level of the hierarchy to values of commercial calibrators, permitting to obtain unbiased results on clinical samples. Finally, an adequate estimation of measurement uncertainties should be performed [7]. Even when measurement procedures operate under unbiased conditions, results produced on biological samples have an associated uncertainty that derives both from uncertainties accumulated along the previous steps of the metrological chain and from random effects [8].
The temple of laboratory standardization
Classical key elements of the reference measurement system are higher order reference materials, reference measurement procedures and accredited reference laboratory services performing them [9]. Over time, it was however realized that three additional components are essential to correctly implement the metrological traceability concept in clinical practice [7]. First, only the availability of traceable reference intervals/decision limits will permit the definitive implementation of standardization projects, filling the gap between the production of traceable results and their correct use for patient care [10, 11]. Second, an analytical (internal and external) quality control program meeting metrological criteria has to be organized, becoming the fifth pillar of laboratory standardization [1, 8]. Finally, the sixth pillar sustaining the “temple” of laboratory standardization is the setting of targets for uncertainty and error of measurement that fit for purpose [7, 8].
Defining analytical performance goals for each analyte is essential to make their determinations clinically usable and to ensure that the measurement error does not prevail on the result [7, 12]. In the conference held in 1999 in Stockholm, a hierarchy of sources for deriving the analytical goals of a laboratory measurement and defining quality specifications in laboratory medicine was established [13]. After 15 years, a new conference recently held in Milan has revisited the Stockholm consensus, investigating to what extent the advocated hierarchy is still valid or if it has to be changed or expanded [14]. Although the essence of the hierarchy established in Stockholm was supported, new perspectives have been forwarded prompting simplification and explanatory additions. According to the new consensus statement, the recommended approaches for defining analytical performance goals should rely on the effect of analytical performance on clinical outcomes or on the biological variation of the measurand [14]. The most innovative aspect of this consensus is that it is recognized that some models are better suited for certain measurands than for others; the attention is therefore primarily directed towards the measurand and its biological and clinical characteristics. For instance, the model based on biological variation is probably not appropriate for analytes showing high intra-individual CV (CVI) (i.e., >33%) [15]: for those analytes, outcome data or, in their absence, the state-of-art of the measurement should be the models to derive analytical goals.
Definition of the measurement uncertainty budget
Once the analytical goals are established, it is essential to understand how they can be fulfilled in practice. Focusing on the measurement uncertainty, the relevant goal that should be considered is that classically related to the allowable analytical variability (“imprecision”) [16–18], as the correct trueness transfer along the entire metrological traceability chain should allow the achievement of unbiased (or negligibly biased) results. For instance, using the biological variation model the analytical quality specification for measurement uncertainty is 0.25, 0.50 or 0.75 CVI, depending on the quality level one want to reach [18]. The (expanded) uncertainty goal (GU) represents the budget that should be fulfilled when combining the uncertainty of the analytical system employed in the individual laboratory (i.e., the random effects) to that accumulated along all the steps of metrological traceability chain, and then expanding it by a coverage factor. It is, therefore, essential that contributions to measurement uncertainty are defined across the entire traceability chain, starting with the reference materials, extending through the IVD manufacturers and their processes for assignment of calibrator values and, ultimately, to the final results reported to clinicians by clinical laboratories [1, 19].
The reference provider, the IVD manufacturer and the clinical laboratory have different roles along the metrological traceability chain. Although independent in their tasks, their activities contribute together to the total measurement uncertainty budget. Basically, the value assigned to the measurement standard(s) at each level in the calibration hierarchy shall have an uncertainty of measurement that includes the uncertainty contributions from each higher calibration step in the hierarchy. Accordingly, the combined uncertainty must be calculated as the square root of the sum of the squares of each uncertainty component [i.e., uncertainty of references, uncertainty of commercial calibrator and random uncertainty (analytical system imprecision plus individual laboratory performance)] and then converted to expanded uncertainty by multiplying the value by a coverage factor k (k=2 is recommended for a confidence level of approx. 95% for a normal distribution). Once the employed traceability chain and the corresponding (expanded) combined uncertainty are drawn, it will be possible to compare the latter with the appropriate GU selected for that specific measurand. We previously reported some examples of this approach, which is useful for establishing if the current status of the uncertainty budget of an analyte measurement is suitable for clinical application of the test [1, 7, 19, 20]. Hopefully, it should be applied to every analyte measured in the clinical laboratory.
Allowable limits for measurement uncertainty across the traceability chain
To obtain that a given measurement is able to fulfil the established GU when patient samples are determined, it is necessary to define what part of the GU should be used across the different steps of metrological traceability chain, by establishing combined uncertainty limits at different chain levels as a proportion of GU.
Uncertainty limits for references
The higher order references represent the first contribution to the overall measurement uncertainty budget. Their weight in terms of uncertainty should be sufficiently tight to allow, when IVD calibrator and random uncertainties have been added, to fulfil the GU. We recently reported the serum albumin example to illustrate this aspect, by highlighting that the expanded combined uncertainty associated to the currently available reference material (ERM-DA470k/IFCC) for this measurand (3.22%) [21], to be used as common calibrator to transfer the measurement trueness to commercial calibrators and patient results, is probably by itself much greater than the GU for serum albumin results on patient samples, allowed for clinical application of this test [1, 7, 20]. This is, therefore, representative of a measurand for which it should be a priority to significantly reduce the uncertainty associated to the upper levels of its metrological chain. The preparation by a reference institution of stable and commutable secondary reference materials, accurately characterized in agreement with the ISO 15194 standard [22], would be desirable to reduce the uncertainty associated with the upper part of metrological chain. In conjunction, a performance improvement of the reference procedures used to assign values to these materials should be achieved. It is, however, clear that it may not be possible to lower the uncertainty of a higher order reference below 1%. In that case alternative strategies focused on the contribution of the lowest parts of the traceability chain to the total uncertainty budget should be envisaged.
In agreement with previous discussion [19, 23], to assure that the expanded combined uncertainty associated with patient results may fulfil the GU, the higher order references should display expanded uncertainty at most equal to one third of the GU. This means that the specifications of a certified reference material for a given measurand in terms of uncertainty should be defined by the performance needs of the clinical assays for that measurand. As an example, if one accepts that, according to an outcome-based study on diagnostic misclassification rates [24], the maximum allowable expanded combined uncertainty for cardiac troponin I (cTnI) clinical results is 13%, which allows for <2% misclassification (as minimum quality goal) [25], it may be anticipated that the expanded uncertainty associated with a reference material to be used as common calibrator for commercial cTnI systems should be no more than (approximating) 4.5%.
Uncertainty limits for commercial system calibration
The role of IVD manufacturers is to define a calibration hierarchy to assign traceable values to their system calibrators and to fulfil during this process uncertainty limits, which represent a proportion of the uncertainty budget allowed for clinical laboratory results [1, 7, 8]. The manufacturer assumes, therefore, total responsibility for supplying products of acceptable quality in terms of traceability and uncertainty of the system [7]. According to this concept, it is no longer possible to consider separately the components of each analytical system (i.e., platform, reagents, calibrators and control materials for testing the system alignment), which in terms of performance can only be guaranteed and certified by the manufacturer as a whole (“CE marked”). It should be clear that any change introduced by users or third parties (e.g., the use of reagents, calibrators or control materials from other suppliers) may alter the performance of the analytical system, as certified by the manufacturer, removing any responsibility from the manufacturer itself and depriving the system (and, consequently, the produced results) of the certification originally provided through CE marking.
IVD manufacturers occupy a critical position along the metrological traceability chain as they are in the middle between reference providers and end-users. If their task seems theoretically rather simple [i.e., to identify the higher order material or method, to establish metrological traceability to them and to assign values (and uncertainty) to assay calibrators], in practice IVD manufacturers have to carry out a complex and tricky process. Particularly, they have to select suitable (i.e., commutable) reference materials and/or identify a reference laboratory performing the reference procedure and to establish the acceptability for the calibration uncertainty. The Joint Committee for Traceability in Laboratory Medicine (JCTLM) database may, at least partly, gives information on available higher order reference materials and measurement procedures and on accredited laboratory reference measurement services, assisting IVD industry in implementing traceability of commercial systems [9]. More difficult for IVD manufacturers is to correctly derive the expanded uncertainty associated with their calibrators, when used in conjunction with other components of the analytical system (platform and reagents), and to establish acceptable limits for its validation. We recently showed that in doing this, manufacturers usually do not include the uncertainty associated with higher levels of the selected metrological traceability chain, declaring an uncertainty for their commercial calibrators sometimes much lower than that associated to the upper part of the traceability chain used by the same company to transfer the measurement trueness [7]. However, it is clear that for a proper evaluation of analytical performance and associated uncertainty of the commercial systems, these earlier steps cannot be ignored and uncertainty estimates provided by manufacturers should include (combine) the uncertainty associated with higher levels of the metrological traceability chain.
Also at this intermediate level of the traceability chain there is a need to define criteria for manufacturers that can be achieved for their calibrators leaving enough uncertainty budget for the clinical laboratories to produce clinically acceptable results. In agreement with the concept by Bais et al. [18], IVD manufacturers should produce calibrators displaying combined uncertainty at most equal to 50% of the total budget goal.
Uncertainty margins for clinical laboratories
With regard to the contribution to measurement uncertainty, the role of clinical laboratory is to monitor the reliability of the employed commercial system through an internal quality control (IQC) program estimating the uncertainty due to the random effects, which includes the analytical system imprecision together with the individual laboratory performance in terms of variability [8]. This program should provide, through mechanisms of retrospective evaluation, data useful to the knowledge of variability of analytical system (e.g., the reagent lot-to-lot variation) and of its daily use by the individual laboratory. We already emphasized how this IQC component II should be appropriately designed and keep completely separated by the other IQC component I addressed to check the alignment of analytical system [7, 8]. Table 1 reports the most important characteristics for a control material to be used in the IQC component II program.
Main characteristics for a control material to be used in the internal quality control component II program in order to derive the uncertainty of the analytical system due to the random effects.
| Characteristic | Remarks |
|---|---|
| Matrixed material from a third-party independent source should be used (e.g., fresh-frozen pool) | Material must be different from the system control material used for checking its alignment |
| Material should closely resemble to authentic patient samples (fulfil commutability) | Commercial non-commutable controls may provide a different impression of imprecision performance |
| Material concentrations should be appropriate to the clinical application of the analyte | When clinical decision cut-points are employed for a given analyte, samples around these concentrations should preferentially be selected |
As reported above, if for being acceptable, the degree of uncertainty (expanded) of a measurand results in the clinical setting (including the accumulated uncertainty of the corresponding traceability chain) should stay within GU, decisions need to be made on what proportion of this budget can be used up in the traceability chain to ensure enough budget is left for use in clinical analysis. In principle, to allow that diagnostic laboratory provides clinically reliable patient results fulfilling the performance analytical goals it is necessary that uncertainties accumulated at level of reference providers and IVD manufacturers be sufficiently confined. Figure 1 summarizes the recommended limits for combined uncertainty budget (expressed as percentage of total budget GU) in traceability implementation.
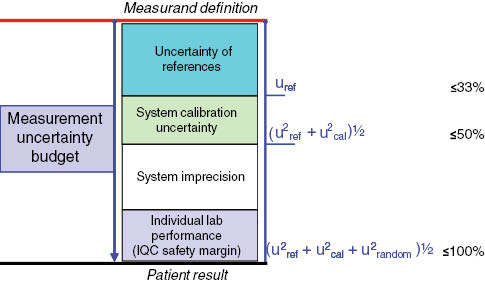
Recommended limits for sources of combined uncertainty budget (expressed as percentage of total budget uncertainty goal) in traceability implementation.
IQC, internal quality control.
Why uncertainty limits matter
The CE marking on IVD products by itself does not guarantee that manufacturer has transferred trueness successfully as at present no normative verification or validation of the manufacturer’s statement by a third party is provided [1]. However, even when the traceability of calibration is successfully implemented, this does not ensure acceptable trueness and/or uncertainty for individual patient results. Using the example of glucose, we recently showed that the selection of different types of traceability chains (all officially recognized in the JCTLM database) may lead to different combined uncertainties at the level of commercial calibrators, not always permitting to fulfil the GU [7]. Unfortunately, the information regarding uncertainty associated with commercial calibrators is currently very poor when consulting calibrator package inserts. Manufacturers only provide the name of higher order reference material and/or procedure to which the assay calibration is traceable, without any description of implementation steps and their corresponding uncertainty or, if any, the use of acceptable limits for calibrator uncertainty assignment. Table 2 lists the information that, in our opinion, laboratory users should be able to access, ideally in the assay or calibrator package inserts. In this regard, it is expected that the ISO Technical Committee 212 working on the revision of the ISO 17511 standard will consider these requirements, asking manufacturers to make them easily available, in line with the concept that the traceability implementation by IVD manufacturers should necessarily be associated to the demonstration that the commercial system meets the analytical goals established for its clinical use.
The information that in vitro diagnostics manufacturers should provide to laboratory users about the implementation of metrological traceability of their commercial systems. Adapted from [7].
| a) | An indication of higher order references (materials and/or procedures) used to assign traceable values to calibrators; |
| b) | Which internal calibration hierarchy has been applied by the manufacturer, and |
| c) | A detailed description of each step; |
| d) | The (expanded) combined uncertainty value of commercial calibrators, and |
| e) | Which, if any, acceptable limits for uncertainty of calibrators were applied in the validation of the analytical system. |
Another aspect that may compromise the patient’s result accuracy regardless of successful implementation of calibration traceability is the too large uncertainty (including assay imprecision) of the available traceability chain that does not permit to meet clinical needs. By analyzing the uncertainty of the current traceability chain for HbA1c, it was clear that the (expanded) combined uncertainty associated with the measurement of a human sample in even optimal conditions (approx. 4.0%) is still >2 times the minimum acceptable target for the clinical application of the test [19]. Our conclusion was that further advances are needed to reduce the uncertainty associated with higher order metrological references and to improve the precision of commercial HbA1c assays [19].
The last important aspect that may influence the accuracy of individual patient’s results is the selectivity (“specificity”) of commercial assays for the measurand as the quantity subject to measurement. A classic example is that of creatinine, whose determination in daily laboratory work can be performed by alkaline picrate-based and enzymatic methods. It is well known that the alkaline picrate-based assays are unable to measure solely creatinine and some endogenous and exogenous substances in serum, particularly proteins, may significantly interfere causing significant overestimation [26]. Similarly to the previously reported example of glucose [7], by analyzing IVD manufacturer statements we were able to show that at least four different types of metrological traceability chain are currently employed to assign values to commercial calibrators of marketed creatinine assays (Figure 2). The (expanded) combined uncertainty associated with the selected chain ranged from 1.5% to 2.8% showing, like for glucose, that IVD manufacturers may spend substantially different amounts of the total uncertainty budget just by selecting one or the other of the available traceability chains (Table 3). Differently from glucose, however, the same calibrator on the same platform (e.g., Roche Cobas, Modular or Siemens Advia) shows different uncertainty depending on which method is employed, with alkaline picrate assays displaying markedly higher calibrator uncertainties than enzymatic assays (Table 3). It is well known that traceability implementation is unable to correct for analytical non-specificity issues [26, 28] and the manufacturers’ approach trying to mitigate this problem inevitably leads to higher uncertainties of commercial calibrators when used for alkaline picrate methods.
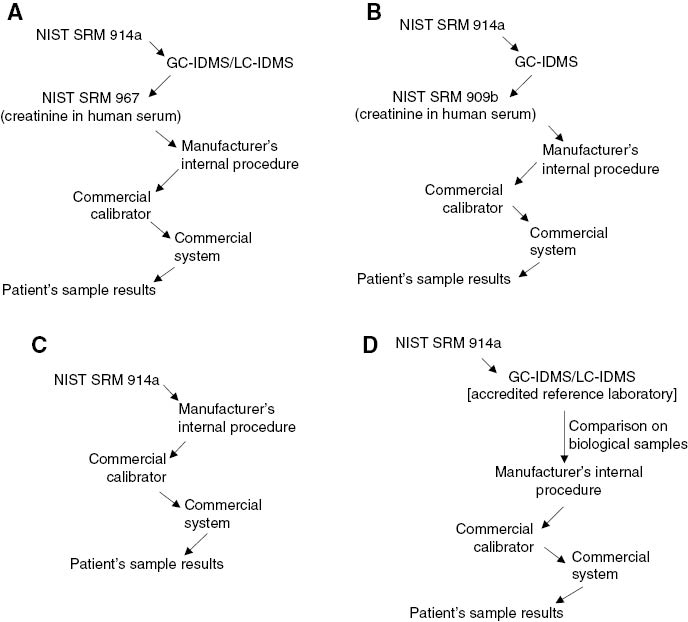
Types of metrological chains that can be used to implement the traceability of blood creatinine results.
GC-IDMS, isotope dilution-mass spectrometry coupled to gas chromatography; LC-IDMS, isotope dilution-mass spectrometry coupled to liquid chromatography; NIST, National Institute of Standards and Technology; SRM, standard reference material.
Metrological traceability and uncertainty information derived from calibrator package inserts of commercial systems measuring serum creatinine marketed by four in vitro diagnostics companies.
| Company | Platform | Principle of commercial method | Calibrator | Declared standard uncertaintya | Higher order reference employed | Type of traceability chain usedb | Combined standard uncertainty associated with the used chainc | |
|---|---|---|---|---|---|---|---|---|
| Method | Material | |||||||
| Abbott | Architect | Enzymatic | Multigent clin chem calibrator | 1.48% | IDMS | NIST SRM 967 | A | 2.12%–2.79%d |
| ND | Multiconstituent calibrator | 2.7% | IDMS | NIST SRM 967 | A | 2.12%–2.79%d | ||
| Beckman | AU | Enzymatic | System calibrator | ND | ND | NIST SRM 967 | A | 2.12%–2.79%d |
| Alkaline picrate | System calibrator | ND | IDMS | NIST SRM 967 | A | 2.12%–2.79%d | ||
| Uncompensated alkaline picrate | System calibrator | ND | ND | NIST SRM 909b L2 | B | 1.51% | ||
| Synchron | ND | LX aqua calibrator | ND | IDMS | NIST SRM 914a | D | 1.5%e | |
| Roche | Cobas c | Enzymatic | C.f.a.s. | 0.91% | IDMS | ND | D | 1.5%e |
| Alkaline picrate compensated | C.f.a.s. | 1.62% | IDMS | ND | D | 1.5%e | ||
| Alkaline picrate rate–blanked and compensated | C.f.a.s. | 1.42% | IDMS | ND | D | 1.5%e | ||
| Integra/Cobas c111 | Enzymatic | C.f.a.s | 1.06% | IDMS | ND | D | 1.5%e | |
| Integra400/Cobas c111 | Alkaline picrate compensated | C.f.a.s | 0.30% | IDMS | ND | D | 1.5%e | |
| Integra800 | Alkaline picrate compensated | C.f.a.s | 0.72% | IDMS | ND | D | 1.5%e | |
| Modular | Enzymatic | C.f.a.s | 0.91% | IDMS | ND | D | 1.5%e | |
| Alkaline picrate compensated | C.f.a.s | 1.38% | IDMS | ND | D | 1.5%e | ||
| Alkaline picrate rate-blanked and compensated | C.f.a.s | 0.79% | IDMS | ND | D | 1.5%e | ||
| Siemens | Dimension Vista | Enzymatic | ECREA calibrator A | 5.08%f | ND | NIST SRM 914a | C | NA |
| ECREA calibrator B | 3.16%f | ND | NIST SRM 914a | C | NA | |||
| Alkaline picrate | Chemistry calibrator | 1.6% | GC-IDMS | NIST SRM 914a | D | 1.5%e | ||
| Advia | Enzymatic | Chemistry calibrator | 0.45% | IDMS | NIST SRM 914a NIST SRM 967 | A | 2.12%–2.79%d | |
| Alkaline picrate rate-blanked and compensated | Chemistry calibrator | 1.6% | IDMS | NIST SRM 967 | A | 2.12%–2.79%d | ||
GC-IDMS, IDMS coupled to gas chromatography; IDMS, isotopic dilution-mass spectrometry; NA, not available; ND, not declared; NIST, National Institute of Standards and Technology; SRM, standard reference material. aExpanded with a coverage factor of 2 (except for Siemens Advia, undeclared), not combined with uncertainty reported in the last column (exceptf); bSee Figure 2 for more information; cExpanded with a coverage factor of 2; dUncertainty depends on the concentration level of NIST SRM 967 [27]; eDerived from Laboratory for Analytical Chemistry, University of Gent (Belgium), GC-IDMS data, available at http://www.dgkl-rfb.de:81/4Daction/g_search_RELA?selectAnalyte=Creatinine&submitplot=show+result+plot&session_id=00000000000000000000&cur_year=2013&LabCode=000 (Accessed December 2014); fCombined with uncertainty of previous steps of the selected traceability chain.
Conclusions
In addition to the correct implementation of calibration traceability, the definition of the total expanded uncertainty budget is essential to make sure that laboratory measurements are clinically usable. In particular, it is necessary to define combined uncertainty budget limits across the entire metrological traceability chain. We recommend that no more than one third of the total uncertainty budget, established by appropriate analytical performance specifications, is consumed by the uncertainty of references and approximately 50% of the total budget consumed by the manufacturer’s calibration and value transfer protocol. The remaining 50% should be available for the commercial system imprecision and individual laboratory performance as a safety margin in order to fulfil the GU.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Financial support: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Panteghini M. Implementation of standardization in clinical practice: not always an easy task. Clin Chem Lab Med 2012;50:1237–41.10.1515/cclm.2011.791Search in Google Scholar PubMed
2. International Organization for Standardization (ISO) 17511:2003. In vitro diagnostic medical devices – measurement of quantities in biological samples – metrological traceability of values assigned to calibrators and control materials. Geneva: ISO, 2003.Search in Google Scholar
3. Directive 98/97/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Off J Eur Communities 1998;L331:1–7.Search in Google Scholar
4. Panteghini M. Traceability, reference systems and result comparability. Clin Biochem Rev 2007;28:97–104.Search in Google Scholar
5. Boulo S, Hanisch K, Bidlingmaier M, Arsène CG, Panteghini M, Auclair G, et al. Gaps in the traceability chain of human growth hormone measurements. Clin Chem 2013;59:1074–82.10.1373/clinchem.2012.199489Search in Google Scholar PubMed
6. Kallner A. Measurement performance goals: how they can be estimated and a view to managing them. Scand J Clin Lab Invest 2010;70(Suppl 242):34–9.10.3109/00365513.2010.493364Search in Google Scholar PubMed
7. Braga F, Panteghini M. Verification of in vitro medical diagnostics (IVD) metrological traceability: responsibilities and strategies. Clin Chim Acta 2014;432:55–61.10.1016/j.cca.2013.11.022Search in Google Scholar PubMed
8. Panteghini M. Application of traceability concepts to analytical quality control may reconcile total error with uncertainty of measurement. Clin Chem Lab Med 2010;48:7–10.10.1515/CCLM.2010.020Search in Google Scholar PubMed
9. Panteghini M. Traceability as a unique tool to improve standardization in laboratory medicine. Clin Biochem 2009;42:236–40.10.1016/j.clinbiochem.2008.09.098Search in Google Scholar PubMed
10. Ceriotti F, Hinzmann R, Panteghini M. Reference intervals: the way forward. Ann Clin Biochem 2009;46:8–17.10.1258/acb.2008.008170Search in Google Scholar PubMed
11. Panteghini M, Ceriotti F. Obtaining reference intervals traceable to reference measurement systems: it is possible, who is responsible, what is the strategy? Clin Chem Lab Med 2012;50:813–7.10.1515/cclm.2011.828Search in Google Scholar PubMed
12. Thienpont LM, Van Uytfanghe K, Rodriguez Cabaleiro D. Metrological traceability of calibration in the estimation and use of common medical decision-making criteria. Clin Chem Lab Med 2004;42:842–50.10.1515/CCLM.2004.138Search in Google Scholar PubMed
13. Petersen PH, Fraser CG, Kallner A, Kenny D, editors. Strategies to set global analytical quality specifications in laboratory medicine. Scand J Clin Lab Invest 1999;59:475–585.Search in Google Scholar
14. Sandberg S, Fraser C, Horvath AR, Jansen R, Jones G, Oosterhuis W, et al. 1st EFLM strategic conference on ‘Defining analytical performance goals 15 years after the Stockholm Conference on Quality Specifications in Laboratory Medicine’ – Consensus agreement. Clin Chem Lab Med 2015;53:833–5.10.1515/cclm-2015-0067Search in Google Scholar PubMed
15. Braga F, Ferraro S, Lanzoni M, Szoke D, Panteghini M. Estimate of intraindividual variability of C-reactive protein: a challenging issue. Clin Chim Acta 2013;419:85–6.10.1016/j.cca.2013.02.004Search in Google Scholar PubMed
16. Fraser CG, Petersen PH. The importance of imprecision. Ann Clin Biochem 1991;28:207–11.10.1177/000456329102800301Search in Google Scholar PubMed
17. Fraser CG, Petersen PH, Ricos C, Haeckel R. Proposed quality specifications for the imprecision and inaccuracy of analytical systems for clinical chemistry. Eur J Clin Chem Clin Biochem 1992;30:311–7.Search in Google Scholar
18. Bais R, Armbruster D, Jansen RT, Klee G, Panteghini M, Passarelli J, et al. Defining acceptable limits for the metrological traceability of specific measurands. Clin Chem Lab Med 2013;51:973–9.10.1515/cclm-2013-0122Search in Google Scholar PubMed
19. Braga F, Panteghini M. Standardization and analytical goals for glycated haemoglobin measurement. Clin Chem Lab Med 2013;51:1719–26.10.1515/cclm-2013-5001Search in Google Scholar
20. Infusino I, Panteghini M. Serum albumin: accuracy and clinical use. Clin Chim Acta 2013;419:15–8.10.1016/j.cca.2013.01.005Search in Google Scholar PubMed
21. Zegers I, Keller T, Schreiber W, Sheldon J, Albertini R, Blirup-Jensen S, et al. Characterization of the new serum protein reference material ERM-DA470k/IFCC: value assignment by immunoassay. Clin Chem 2010;56:1880–8.10.1373/clinchem.2010.148809Search in Google Scholar PubMed
22. ISO 15194:2009. In vitro diagnostic medical devices – Measurement of quantities in samples of biological origin – Requirements for certified reference materials and content of supporting documentation, 2nd ed. Geneva: ISO, 2009.Search in Google Scholar
23. Bunk D. Requirements of a reference measurement procedure and how they relate to a certified reference material for cTnI that is fit for purpose. 5th International Scientific Meeting “Standardization of cardiac troponin I: the ongoing international efforts”. November 30, 2011, Milano, Italy. Available from: http://users.unimi.it/barb/public/UploadAttach/2011Bunk.pdf Accessed December, 2014.Search in Google Scholar
24. Sheehan P, Blennerhassett J, Vasikaran SD. Decision limit for troponin I and assay performance. Ann Clin Biochem 2002;39:213–6.10.1258/0004563021902161Search in Google Scholar PubMed
25. Panteghini M. Quality requirements for troponin assays – An overview. In: Tate J, Johnson R, Jaffe A, Panteghini M, editors. Laboratory and clinical issues affecting the measurement and reporting of cardiac troponins: a guide for clinical laboratories. Alexandria, NSW: Australasian Association of Clinical Biochemists, 2012:53–61.Search in Google Scholar
26. Panteghini M. Enzymatic assays for creatinine: time for action. Clin Chem Lab Med 2008;46:567–72.10.1080/00365510802149978Search in Google Scholar PubMed
27. National Institute of Standard and Technology. Certificate of analysis. Standard reference material 967. Creatinine in frozen human serum. Certificate issue date: January 24, 2007.Search in Google Scholar
28. Carobene A, Ceriotti F, Infusino I, Frusciante E, Panteghini M. Evaluation of the impact of standardization process on the quality of serum creatinine determination in Italian laboratories. Clin Clin Acta 2014;427:100–6.10.1016/j.cca.2013.10.001Search in Google Scholar PubMed
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- Editorial
- Defining analytical performance specifications 15 years after the Stockholm conference
- Consensus Statement
- Defining analytical performance specifications: Consensus Statement from the 1st Strategic Conference of the European Federation of Clinical Chemistry and Laboratory Medicine
- Opinion Papers
- The 1999 Stockholm Consensus Conference on quality specifications in laboratory medicine
- Setting analytical performance specifications based on outcome studies – is it possible?
- Performance criteria based on true and false classification and clinical outcomes. Influence of analytical performance on diagnostic outcome using a single clinical component
- Analytical performance specifications based on how clinicians use laboratory tests. Experiences from a post-analytical external quality assessment programme
- Rationale for using data on biological variation
- Reliability of biological variation data available in an online database: need for improvement
- A checklist for critical appraisal of studies of biological variation
- Optimizing the use of the “state-of-the-art” performance criteria
- Are regulation-driven performance criteria still acceptable? – The German point of view
- Performance criteria for reference measurement procedures and reference materials
- Performance criteria for combined uncertainty budget in the implementation of metrological traceability
- How to define a significant deviation from the expected internal quality control result
- Analytical performance specifications for EQA schemes – need for harmonisation
- Proposal for the modification of the conventional model for establishing performance specifications
- Before defining performance criteria we must agree on what a “qualitative test procedure” is
- Performance criteria and quality indicators for the pre-analytical phase
- Performance criteria of the post-analytical phase
Articles in the same Issue
- Frontmatter
- Editorial
- Defining analytical performance specifications 15 years after the Stockholm conference
- Consensus Statement
- Defining analytical performance specifications: Consensus Statement from the 1st Strategic Conference of the European Federation of Clinical Chemistry and Laboratory Medicine
- Opinion Papers
- The 1999 Stockholm Consensus Conference on quality specifications in laboratory medicine
- Setting analytical performance specifications based on outcome studies – is it possible?
- Performance criteria based on true and false classification and clinical outcomes. Influence of analytical performance on diagnostic outcome using a single clinical component
- Analytical performance specifications based on how clinicians use laboratory tests. Experiences from a post-analytical external quality assessment programme
- Rationale for using data on biological variation
- Reliability of biological variation data available in an online database: need for improvement
- A checklist for critical appraisal of studies of biological variation
- Optimizing the use of the “state-of-the-art” performance criteria
- Are regulation-driven performance criteria still acceptable? – The German point of view
- Performance criteria for reference measurement procedures and reference materials
- Performance criteria for combined uncertainty budget in the implementation of metrological traceability
- How to define a significant deviation from the expected internal quality control result
- Analytical performance specifications for EQA schemes – need for harmonisation
- Proposal for the modification of the conventional model for establishing performance specifications
- Before defining performance criteria we must agree on what a “qualitative test procedure” is
- Performance criteria and quality indicators for the pre-analytical phase
- Performance criteria of the post-analytical phase


