Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
-
Hind Ibork
, Zakaria Ait lhaj
, Ousman B. Mahamat
, Khalid Taghzouti
Abstract
The legalization of cannabis for industrial and medicinal purposes has significantly expanded worldwide. This study delves into the analgesic potential toxicity study of chloroformic extract from the Moroccan Cannabis sativa L. (C. sativa) cultivar, Khardala (KH extract). Our findings reveal that the lethal dose of KH extract is ≥5,000 mg/kg, with mice given 2,000 mg/kg exhibiting neurotoxic symptoms, including piloerection, aggressiveness, and fear, along with marked hepato-renal toxicity indicated by elevated levels of alanine aminotransferase, aspartate aminotransferase, total bilirubin, and creatinine in both male and female subjects. Importantly, no toxicity was observed at 250 mg/kg and 500 mg/kg doses. Remarkably, at a dose of 500 mg/kg, the KH extract demonstrated a potent analgesic effect superior to cannabidiol (CBD), suggesting a synergistic interaction among the extract’s bioactive compounds, such as CBD, cannabidivarin (CBDV), Delta 9 tetrahydrocannabinol (THC), cannabigerol (CBG), Delta 9 tetrahydrocannabivarin (THCV), and β-caryophyllene. In silico analysis supports these findings, showing the strong binding potential of THC, THCV, CBG, and CBDV to delta opioid receptors, with G-scores >−5.0 kcal/mol, highlighting the promising analgesic efficacy of this cannabis cultivar extract. This study underscores the therapeutic potential of the KH cultivar, positioning it as a promising candidate for pain management therapies.
Graphical abstract
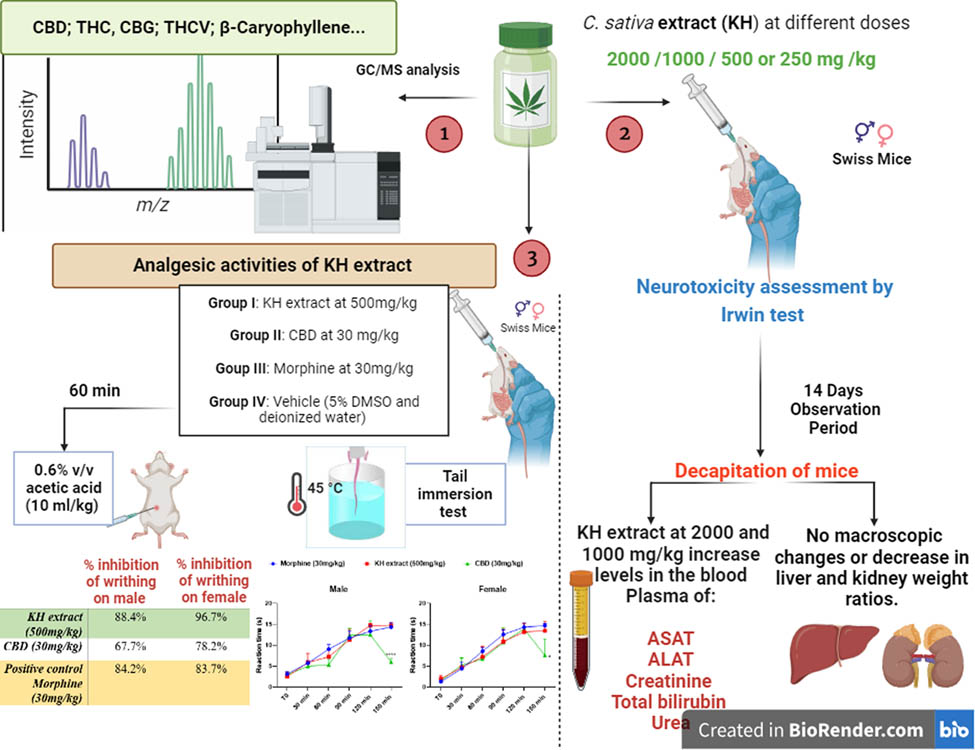
1 Introduction
Morocco, widely recognized as the leading global producer of Cannabis sativa L. (C. sativa), experienced a transformative shift following the legalization of cannabis for medicinal and industrial purposes in 2021, leading to a notable increase in cultivation and utilization. Among the diverse C. sativa cultivars, Khardala (KH) has emerged as the predominant strain, occupying over 65% of cannabis fields [1,2]. This cultivar is particularly esteemed for its high Delta 9 tetrahydrocannabinol (THC) content and its rich profile of over 400 cannabinoid compounds with profound therapeutic potential, primarily concentrated in the trichome cavity of female flowers [3].
Exploring bioactive compounds from C. sativa, including cannabinoids, terpenoids, flavonoids, and alkaloids, has garnered considerable interest for their pharmacological effects, particularly as analgesics [4,5]. While cannabinoids traditionally interact with the endocannabinoid system, recent studies suggest their interaction with other systems such as the opioidergic system, particularly through delta opioid receptors, elucidating potential analgesic effects [6–8]. This multifaceted interaction hints at the complex neuropharmacological mechanisms underlying cannabis’s therapeutic effects.
Among extraction methods, used to explore phytochemical plant contents, chloroform extraction has proven to be particularly effective for isolating a wide array of compounds from C. sativa, including cannabinoids, with minimal degradation and higher concentrations compared to alternative methods like supercritical CO2 or ethanol extraction, offering cost-effectiveness and accessibility for scientific investigations.
However, despite its widespread use, data on toxicity and pharmacokinetics of orally administered natural cannabinoids in humans and laboratory animals remain scarce [9]. Prior toxicity studies have predominantly focused on individual cannabinoids such as THC and cannabidiol (CBD), rather than investigating the entire spectrum of compounds present in the plant. This oversight is significant, as the synergistic interactions between the various molecules found in the cannabis plant may yield different outcomes, emphasizing the need for comprehensive evaluations of the plant’s entirety. In Morocco, the Poison and Pharmacovigilance Center reports an annual average of approximately 221 cases of drug intoxication and 430 requests for toxicological analyses, of which over 70 cases are recorded as intoxication caused by cannabinoids [10]. Hence, assessing the acute toxicity and neurotoxicity of Moroccan cannabis, particularly the KH cultivar is imperative.
CBD exhibits analgesic effects through multiple mechanisms, including modulating cannabinoid receptors: cannabinoid type-1 and type-2 receptors (CB1 and CB2), and engaging non-cannabinoid pathways [11]. CBD acts as a negative allosteric modulator of CB1 receptors, influencing receptor internalization, G protein-dependent signaling, and arrestin2 protein recruitment, particularly in regions of the central nervous system (CNS) associated with pain perception [12,13]. It also exerts an indirect effect on CB2 receptors involved in immune and inflammatory responses [14,15]. Beyond these, CBD interacts with G protein-coupled receptors like GPR3 [16], which are implicated in pain reception and neuropathic pain regulation [16]. Additionally, CBD influences serotonergic pathways via the 5-HT1A receptor [17], modulates transient receptor potential vanilloid subtype 1 (TRPV1) channels, μ and δ receptors for pain regulation, and affects the activity of enzymes like cyclooxygenases and lipoxygenases involved in inflammation [18–21]. Notably, the combination of CBD with THC is suggested to enhance CBD’s ability to interact with CB1 and CB2 receptors to elicit therapeutic responses and potentiate its analgesic properties [22]. Thus, elucidating the analgesic potential and establishing an effective dose devoid of toxicity or neurotoxicity is essential for the therapeutic optimization of specific KH extract, rich in CBD as the main non-psychoactive compound, along with THC, minor phytocannabinoids, and β-caryophyllene. Molecular docking represents a pivotal tool in drug discovery to study drug–receptor interactions and guide the development of novel therapeutics. In this study, we utilized the SWISS ADME application to investigate the potential interaction of identified cannabinoids in C. sativa L. KH extract against delta opioid receptors, to provide insights into the potential mechanism that may underlie their analgesic effects.
2 Material and methods
2.1 Plant material and extract preparation
The plant C. sativa (Khardala cultivar) was harvested from the Touanate region in Morocco (34°43′54.7″N 4°52′02.8″W; 269A) during autumn (October), and authenticated by the Scientific Institute of Rabat under the code UPOV: CANNB_SAT_SAT-68T. The fresh inflorescences were air-dried until a stable weight was achieved. A total of 2,000 g of fresh inflorescences were used, which resulted in 500 g of dried powder after air-drying, reflecting a fresh-to-dried ratio of 5:1. The dried plant material was then ground into a fine powder with a particle size of 1 µm for subsequent analysis.
The extraction of the KH extract was carried out through hot reflux extraction using a Soxhlet apparatus. To initiate the process, 20 g of the dry inflorescence powder was meticulously transferred into a cellulose extraction cartridge and inserted into the Soxhlet apparatus. Subsequently, extraction was conducted using chloroform (at a ratio of 1:10 w/v) for approximately 6 h following a defatting step with hexane. Post-extraction, the obtained extract underwent freeze-drying to eliminate any remaining solvent traces, resulting in the retention of the crude extract. The crude extract was then stored at −8°C until further analysis. The extraction yield was calculated as follows:
where M 0 is the weight of the initial powder and M is the weight of the extract.
2.2 Identification of the chemical composition of KH extract by gas chromatography-mass spectrometry (GC-MS)
The chemical composition of the KH extract was determined through GC-MS analysis using a Thermo Scientific GC system (TRACE GC ULTRA), coupled with a mass spectrometer (model ISQ) and a TriPlus RSH auto-sampler. Separation of extract metabolites was performed using a TG-5MS capillary column (30 m × 0.25 mm × 0.25 μm). The injector, interface line, and detector temperatures were all set at 250°C. Helium served as the carrier gas with a flow rate of 1.5 mL/min. The acquisition mass range was configured at m/z 50–550, employing the electron-impact ionization mode (EI, 70 eV). The oven temperature was programmed to start at 80°C for 2 min, followed by a programmed rate of 8°C/min until reaching the final temperature of 280°C, which was held steady for 3 min. The extract, dissolved in chloroform, was injected into the GC-MS system without any derivatization, with a 1 μL solution injected using split mode (ratio of 10:100). Identification of the MS spectra of the separated components was conducted through comparison with the National Institute of Standards and Technology spectral library (version 2.2).
2.3 Animal housing and experimental design
One hundred eight male and female SWISS mice, aged 12 weeks and weighing 29 ± 0.84 g, were utilized in this study. The mice were bred in the central animal care facilities of the Faculty of Sciences at Mohammed V University in Rabat, Morocco. All animal experiments were conducted in strict accordance with the guidelines of the European Council Directive (EU2010/63), with ethical clearance number PPL/70/8647.
Before starting our experimental design, the bioactivity and drug-likeness properties of the phytocannabinoids in the C. sativa extract were assessed based on the published bioactivity scores and drug-likeness models of phytocannabinoids as described by Desa et al. [23]. Importantly, all compounds in the C. sativa extract adhered to Lipinski’s Rule of Five (molecular weight ≤500 Daltons, Log P ≤ 5, hydrogen bond donors ≤5, and hydrogen bond acceptors ≤10), suggesting favorable pharmacokinetic properties, including good oral bioavailability. Based on these data, we conclude that the phytocannabinoids present in our extract likely exhibit potential therapeutic properties and warrant further investigation. The only exception noted was the cannabidiol acid, which showed moderate activity in some bioactive scores.
Both sexes were included in the study as toxicity and analgesic responses can demonstrate sex-dependent variations. The 108 animals were distributed as follows.
For the toxicity assessment, 60 animals were randomly divided into one control group (n = 6 per sex) and four experimental groups (Group I: 2,000 mg/kg, n = 6 per sex; Group II: 1,000 mg/kg, n = 6 per sex; Group III: 500 mg/kg, n = 6 per sex; and Group IV: 250 mg/kg, n = 6 per sex).
For the analgesic potential study, 48 animals were randomly divided into one control group (n = 6 per sex) and three experimental groups: CBD group (n = 6 per sex), KH extract group (n = 6 per sex), and morphine group (n = 6 per sex).
Mice were housed in Plexiglas cages with wood shavings bedding and provided with standard diet and water ad libitum. Housing conditions were maintained constant, with a 12:12 light/dark cycle and a temperature of 22 ± 2°C. Each experiment involved two groups: a test group and a control group, each consisting of six mice. Female and male mice were housed separately, and female mice were non-pregnant and nulliparous. Before the experiments began, mice underwent a 1-week acclimatization period.
Animals were orally administered doses of 250, 500, 1,000, and 2,000 mg/kg of chloroform extract of KH inflorescence dissolved in a solution of deionized water and 5% dimethyl sulfoxide (DMSO). This solvent combination was chosen to prevent chemical reactions among dissolved chemicals, ensuring a purer and homogenized extract solution. The preparation of these doses followed the guidelines outlined in the OECD (2008, Document No. 425) [24,25].
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals, and has been approved by the Ethical Committee, of the Moroccan Association for Research and Ethics (PPL/70/8647).
2.4 Assessment of acute toxicity
Acute oral toxicity was evaluated following the OECD Test Guideline 425 [25], known as the Up-and-Down Procedure (UDP). The initial dose of KH extract administered was 2,000 mg/kg, as per the protocol’s recommended starting point. A control group received the vehicle (5% DMSO and deionized water). No mortality occurred at this dose, prompting the use of a step-down approach to further explore the neurotoxic and behavioral effects at lower doses. Subsequent doses of 1,000, 500, and 250 mg/kg were administered to additional groups of animals (6 per dose, equally distributed by sex) to determine the lowest dose at which no toxicity was observed. Behavioral and neurotoxic effects were recorded over a 24 h observation period using the Irwin procedure [26].
Before the gavage, the mice underwent fasting for 6 h. Their weights were then measured, and the test substance was administered. Food was withheld for 1.5 h following extract administration. The mice were monitored continuously during the initial 4 h, as well as at 24 h and 14 days post-exposure. During this observation period, changes in body weight (BW) and mortality were documented.
At the end of the 14-day observation period, the animals were fasted overnight (approximately 18 h) on the last day of the experimental protocol before blood samples were collected in clot-activator vacutainer tubes for biochemical analysis. The animals were then anesthetized and sacrificed. Subsequently, their livers and kidneys were dissected and weighed.
2.5 Assessment of neurotoxicity of KH extract according to Irwin procedure
The evaluation of KH extract’s impact on CNS activity and physiological function was assessed following the Irwin procedure. This standardized protocol is employed to determine the minimum lethal dose of KH extract, identify the dose range for CNS responses, and observe primary effects on behavior and physiological functions [26]. During the assessment, animals receiving various doses of KH extract, alongside a control group, were monitored for neurotoxicity-specific behaviors, including seizures and tremors. Additionally, behaviors associated with CNS stimulation, such as excitation, Straub tail response, jumping, hypersensitivity to external stimuli, stereotypical behaviors, and aggression, were observed. Conversely, behaviors indicative of CNS depression, such as sedation, rolling gait, loss of balance, impaired traction, motor incoordination, reduced sensitivity to external stimuli, decreased muscle tone, akinesia, and catalepsy, were recorded. Furthermore, the evaluation encompassed observations of autonomous functions, including breathing patterns, pupil diameter, salivation, and defecation, to comprehensively assess the neurotoxic effects induced by the KH extract.
2.6 Biochemical parameters analysis
Following collection, the blood samples underwent centrifugation at 3,000 rpm for 10 min at 4°C. The resulting plasma was then stored at −4°C until further analysis. A comprehensive panel of biochemical parameters was evaluated, including urea, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, total protein, cholesterol, uric acid, and albumin. Additionally, the ionogram profile, encompassing sodium (Na+), calcium (Ca+), chloride (Cl−), and potassium (K+), was determined. Analysis of these parameters was conducted using the Abbott Architect c8000 autoanalyzer, following the manufacturer’s instructions (Abbott, USA) [27].
2.7 Analgesic activities of KH extract
2.7.1 Determination of the analgesic activity of KH extract
To investigate the peripheral analgesic effect of the KH extract, a dose of 500 mg/kg was selected based on the toxicity and neurotoxicity study findings. The evaluation was conducted using the acetic acid-induced writhing method described by Rashid et al. [28]. In this setup, experimental mice were randomly assigned to four groups: Group I, received vehicle (5% DMSO and deionised water); Group II, received an oral treatment of 500 mg/kg of KH extract; Group III, received an oral administration of CBD at 30 mg/kg; and Group IV, received oralmorphine at 30 mg/kg BW as positive control.
2.7.2 Writhing test
Writhing was induced in mice by administering an intraperitoneal injection of 0.6% v/v acetic acid (10 mL/kg) 60 min after drug treatment. Each animal was closely monitored, and the number of writhes was recorded for 30 min, starting 5 min after writhing induction. Subsequently, the average number of writhes and the percentage inhibition of writhing were calculated, serving as indicators of analgesic activity. The calculation followed the equation outlined by Rashid et al. [28]:
where W c is the mean number of writhes in the control group and W is the mean number in the experimental group (KH extract, CBD, or morphine).
2.7.3 Tail immersion test
This test consists of immersing the tail of a mouse in warm water at 45°C so that the tail whips or a whole-body twitch occurs. During the experiment, the tail of the mice was placed in a water bath heated to 45 ± 0.5°C with a cut-off time of 15 s to avoid damage to the tail skin tissue [29–32]. We first measured the baseline latency (baseline latency response) for the mouse to remove the distal half of the tail after immersion in the heated water. The mice were dosed with the extract, CBD, or morphine by gavage, and the post-treatment latency responses were determined 150 min after gavage at 30 min intervals (30, 60, 90, 120, and 150 min). Furthermore, a negative control group of male and female mice was included, administered orally with vehicle (5% DMSO and deionized water), in this tail immersion test to avoid potential confounding effects and ensure that any observed variations in response could be attributed to the treatments rather than the handling or administration procedures.
2.8 Statistical analysis and data management
GraphPad 8.0 was used for statistical analysis. The normality of the data was evaluated using the Kolmogorov–Smirnov test. As all data were normally distributed, results were subjected to two-way ANOVA or two-way ANOVA repeated measures, followed by Bonferroni’s multiple comparisons when significance was observed. The level of significance was set at p < 0.05. Data from anti-analgesic activities were expressed as mean ± standard error of the mean (SEM) of biological replicates (n = 6 per sex), following OECD guidelines [25]. For the analgesia experiment the sample size (n = 6 per sex) was defined based on resources [33,34].
Acute oral toxicity data were qualitatively and quantitatively analyzed according to OECD guideline document No. 425 [25].
2.9 Molecular docking methodology
To investigate the analgesic activity, the X-ray crystal structure of the active delta-opioid receptor in complex with agonist molecule DPI-287 (PDB ID: 6PT3) with a resolution of 3.30 Å [35] was imported onto the workspace of Schrodinger (http://www.schrodinger.com). The protein structure was optimized at pH 7 coupled with restrained minimization using OPLS-2005 force field by employing Maestro’s protein preparation wizard. The receptor grid file, centered on the crystallized ligand, was generated with the Receptor Grid Generation module to define the receptor’s active site precisely.
The 2D structures of investigated ligands were retrieved in Structure-Data File format from the PubChem database and were prepared with the LigPrep module. The ionization states were generated at pH 7.0 ± 2.0 using Maestro’s Epick tool, employing the Hammet and Taft methodologies for accuracy. The ligands were then subjected to an energy minimization process by utilizing the OPLS-2005 forcefield to achieve the most energetically favorable geometry. The prepared ligands were docked using Glide SP scoring with an OPLS-2005 force field to find each ligand’s optimal conformational pose [36]. The G-score and E-model scores were calculated and then juxtaposed with the scores of crystallized ligands to evaluate the binding affinities of investigated ligands.
3 Results
3.1 Extraction yield and chemical composition of the KH extract evaluated by GC-MS
The extract yield obtained from the hot reflux extraction by Soxhlet was calculated to be 2.45%. As illustrated in Figure 1 and Table 1, the GC-MS analysis revealed a diverse array of phytoconstituents in this extract. These include terpenoids such as neophytadiene and β-caryophyllene, alongside several cannabinoids like cannabidivarin (CBDV), THC, Delta 9 tetrahydrocannabivarin (THCV), cannabigerol (CBG), cannabinol (CBN), and CBD. Notably, CBD emerged as the predominant non-psychoactive compound, constituting approximately 60% of the peak area in the total chromatogram (with a retention time [RT] of 23.08 min).
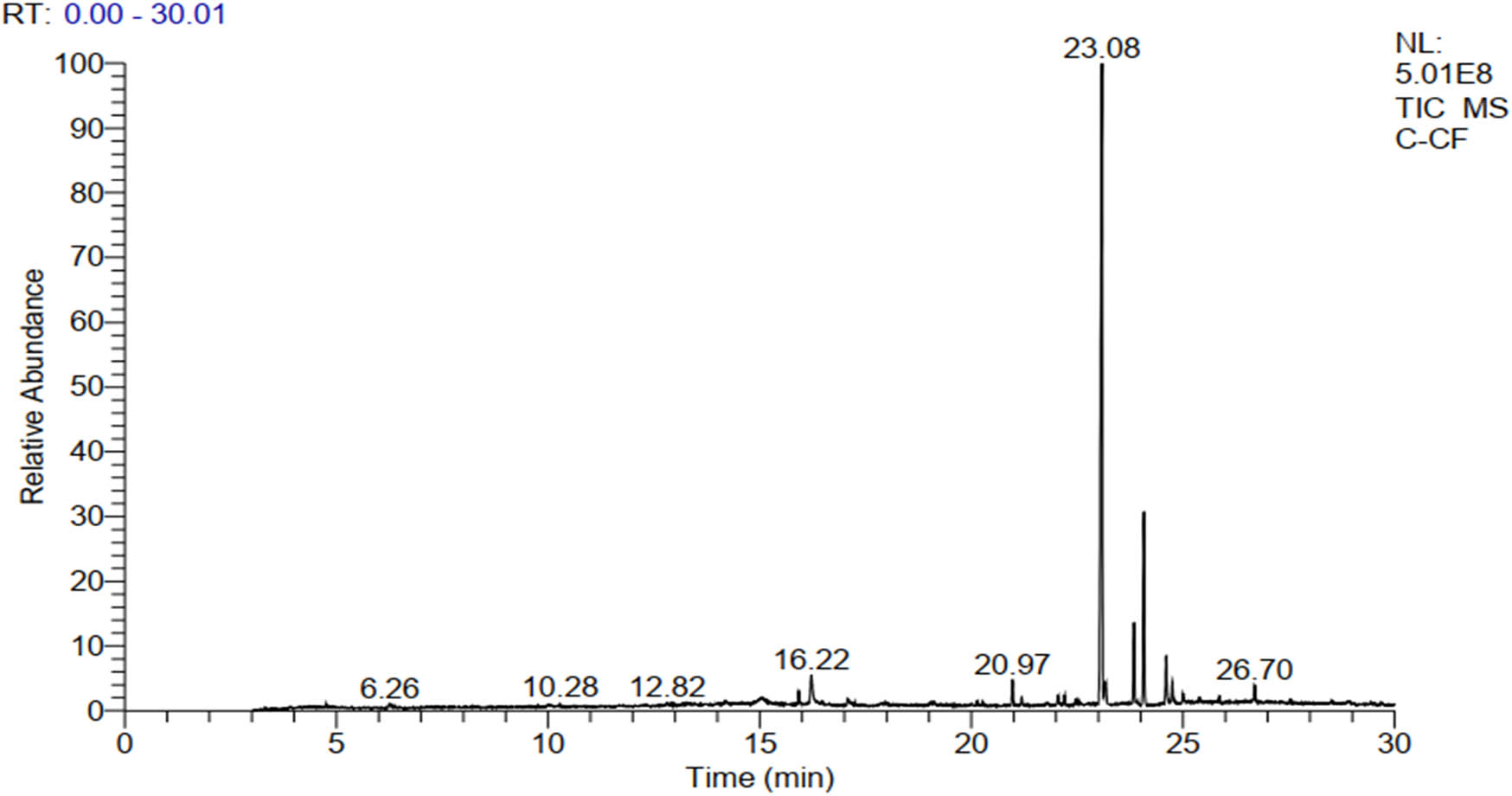
GC-MS chromatogram showing the major bioactive compounds of the KH extract.
Compounds identified in the KH extract
| Sr. no. | Identified compounds | RT | SI/RSI | MS (m/z) | Formula |
|---|---|---|---|---|---|
| 1 | Undecane | 4.77 | 880/940 | 156 | C11H24 |
| 2 | 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl | 6.26 | 829/846 | 144 | C6H8O4 |
| 3 | β-Caryophyllene | 10.28 | 826/845 | 204 | C15H24 |
| 4 | 4-(6,6-Dimethyl-2-methylenecyclohex-3-enylidene) pentan-2-ol | 14.20 | 711/728 | 206 | C14H22O |
| 5 | Neophytadiene | 15.92 | 898/928 | 278 | C20H38 |
| 6 | CBDV | 20.97 | 722/732 | 286 | C19H26O2 |
| 7 | THC isomer | 22.01 | 873/874 | 314 | C21H30O2 |
| 8 | THCV | 22.05 | 713/798 | 286 | C19H26O2 |
| 9 | Heneicosane | 22.20 | 854/891 | 296 | C21H44 |
| 10 | CBD | 23.08 | 901/904 | 314 | C21H30O2 |
| 11 | Delta-9-THC | 24.08 | 851/852 | 314 | C21H30O2 |
| 12 | Heptacosane | 24.52 | 868/896 | 380 | C27H56 |
| 13 | CBG | 24.60 | 835/843 | 316 | C21H32O2 |
| 14 | CBN | 24.75 | 723/751 | 310 | C21H26O2 |
3.2 Acute toxicity and neurotoxicity effect of KH extract
As we reported, the acute toxicity test was conducted following the protocol described in the acute oral toxicity – UDP 425 protocol, specifically the limit test at 2,000 mg/kg. One animal was initially dosed, and upon survival, five additional animals were sequentially dosed, totaling six animals. Since all six animals survived without any fatalities, we concluded that the LD50 of KH extract is greater than 2,000 mg/kg. Based on the absence of mortality at this limited dose, we determined that the LD50 of the KH extract is ≥5,000 mg/kg.
Even though no lethality was observed at 2,000 mg/kg, subsequent doses of 1,000, 500, and 250 mg/kg were administered to additional groups to determine the lowest non-toxic dose, with behavioral and neurotoxic effects recorded over 24 h. As demonstrated in Figures 2 and 3, a range of neurotoxic and behavioral changes were recorded using Irwin’s procedure. At higher doses of 2,000 and 1,000 mg/kg, the KH extract induced pronounced signs of CNS stimulation, including aggressive behavior and excitation, as well as sensorimotor deficits such as ptosis and fear. Additionally, it impacted autonomic function and peripheral activity, resulting in lacrimation, alterations in respiration rate, myosis, and mydriasis, while also demonstrating CNS depressant activity leading to sedation. Moreover, administration of KH extract at 2,000 mg/kg resulted in a motor deficit in males, characterized by a loss of traction. Based on these Irwin test results, the dose of 500 mg/kg of KH extract was selected for evaluating its potential analgesic effect.
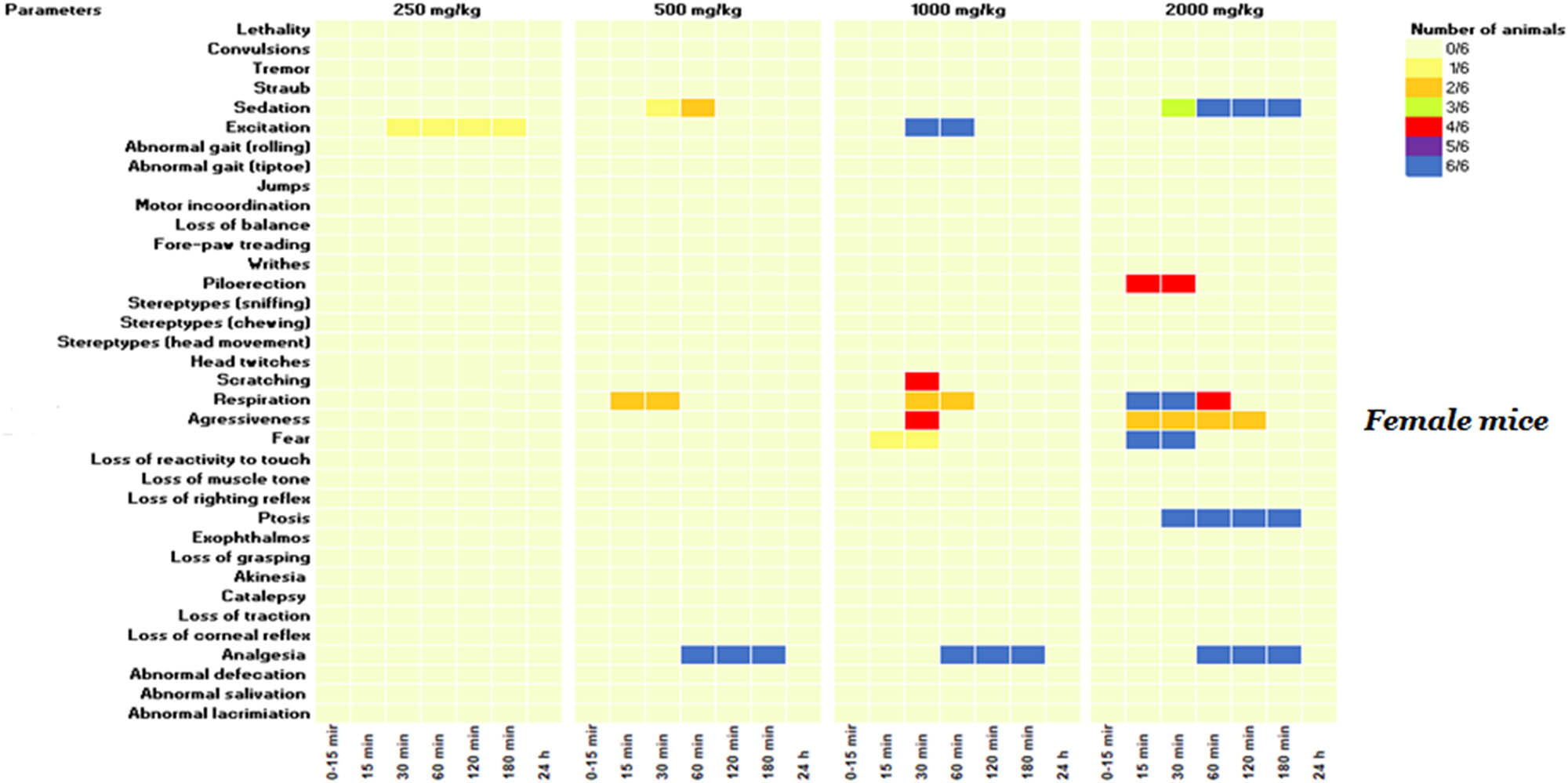
KH extract neurotoxic effects in the primary observations according to Irwin’s procedure in female mice. Data are presented as the number of animals showing neurotoxic symptoms during the test evaluated by comparison of the mean scores obtained in treated and control animals. (X/N) indicates the number of mice showing the symptoms. Observations were performed at 15, 30, 60, 120, 180 min, and 24 h after administration.
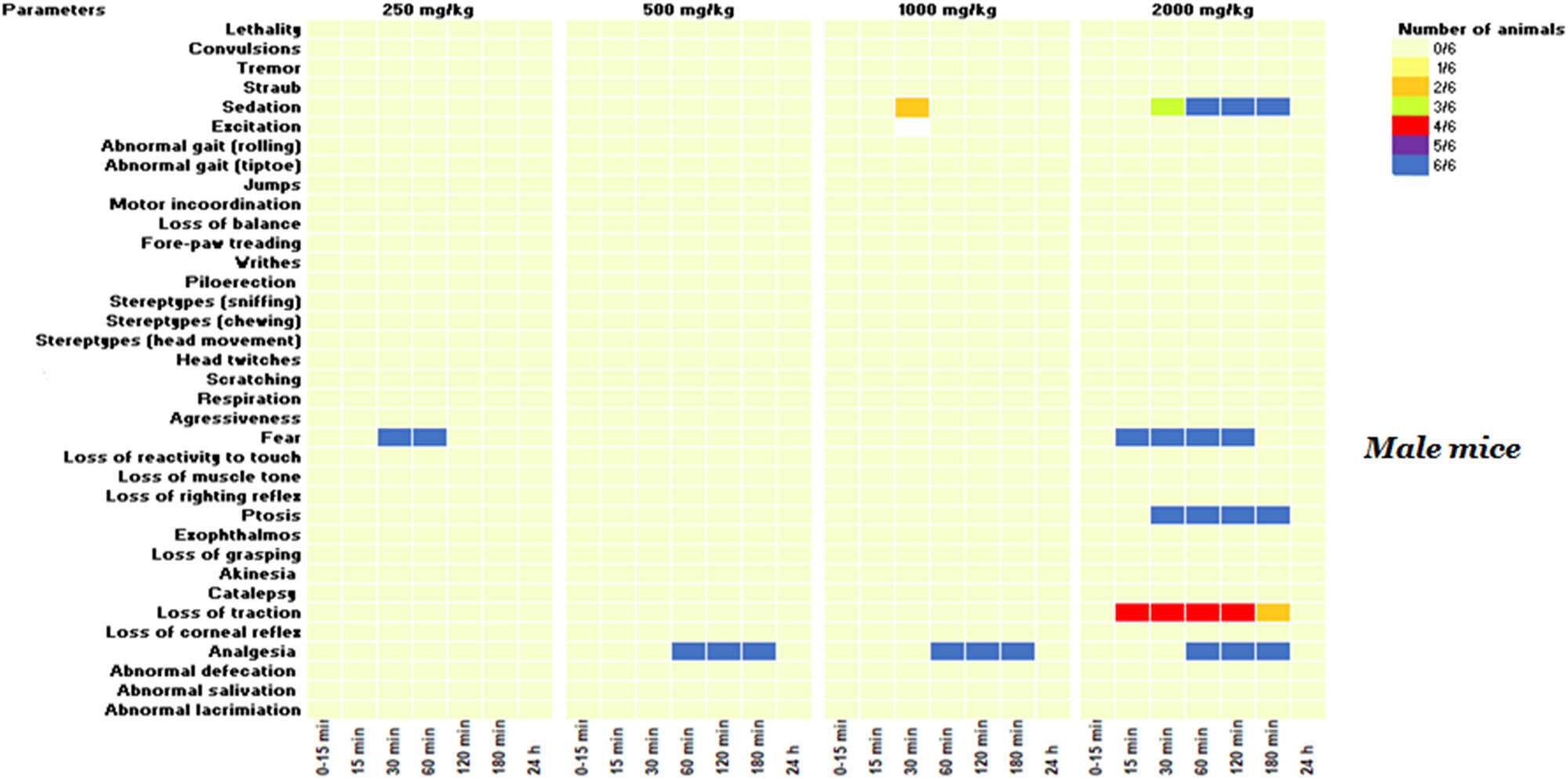
KH extract neurotoxic effects in the primary observations according to Irwin’s procedure in male mice. Data are presented as the number of animals showing neurotoxic symptoms during the test evaluated by comparison of the mean scores obtained in treated and control animals. (X/N) indicates the number of mice showing the symptoms. Observations were performed at 15, 30, 60, 120, 180 min, and 24 h after administration.
3.3 Effect of KH extract on BW
In this study, we monitored the average BW of mice from Day 1 to Day 14. A two-way ANOVA repeated measures analysis revealed that both treatment and time significantly influenced the BW of male and female mice (F (52,299) = 5.962, p < 0.0001). There were no notable changes in BW observed in untreated mice or those treated with doses of 1,000, 500, or 250 mg/kg (p > 0.05 vs control). However, administration of the KH extract at a dose of 2,000 mg/kg significantly decreased the BW of both male and female mice, particularly evident from the fourth and third days of the observation period (p = 0.002 and p = 0.0074 vs control, respectively) (Figure 4).
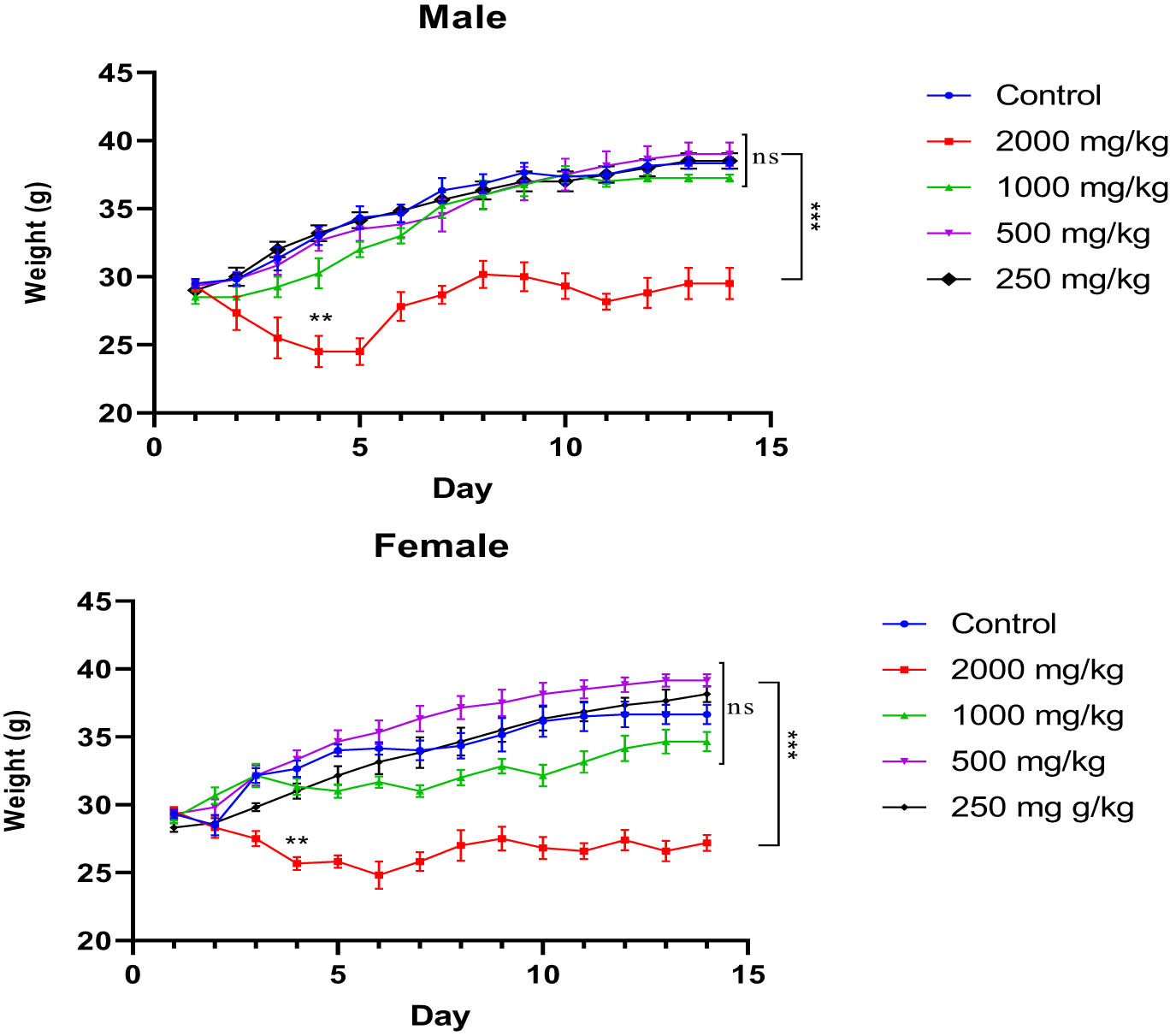
Evolution of the average BW of untreated and treated mice with the different doses (2,000, 1,000, 500, and 250 mg/kg) of KH extract of both sexes during the 14 days of experimentation. Data are expressed as mean ± SEM (n = 6). Data are analyzed via Bonferroni post-hoc; ***p < 0.001 compared to control and other dose groups.
3.4 Effect of KH extract on liver and kidneys weight
Analysis of the ratio of organ weights (liver and kidney) to the BW of mice, conducted using two-way ANOVA, did not reveal any significant interaction effect between treatment and the sex of the animals (F (4,47) = 0.8694, p = 0.4893 and F (4,47) = 1.477, p = 0.2242 for liver and kidney, respectively). Furthermore, the results indicated no significant independent effect of treatment with KH extract at different doses on the liver and kidney ratio (F (4,47) = 1.426, p = 0.2401 and F (4,47) = 1.310, p = 0.2799, respectively) (Figure 5). These findings suggest that the administration of KH extract, even at its highest dose (2,000 mg/kg), does not influence the ratio of these organs to BW.
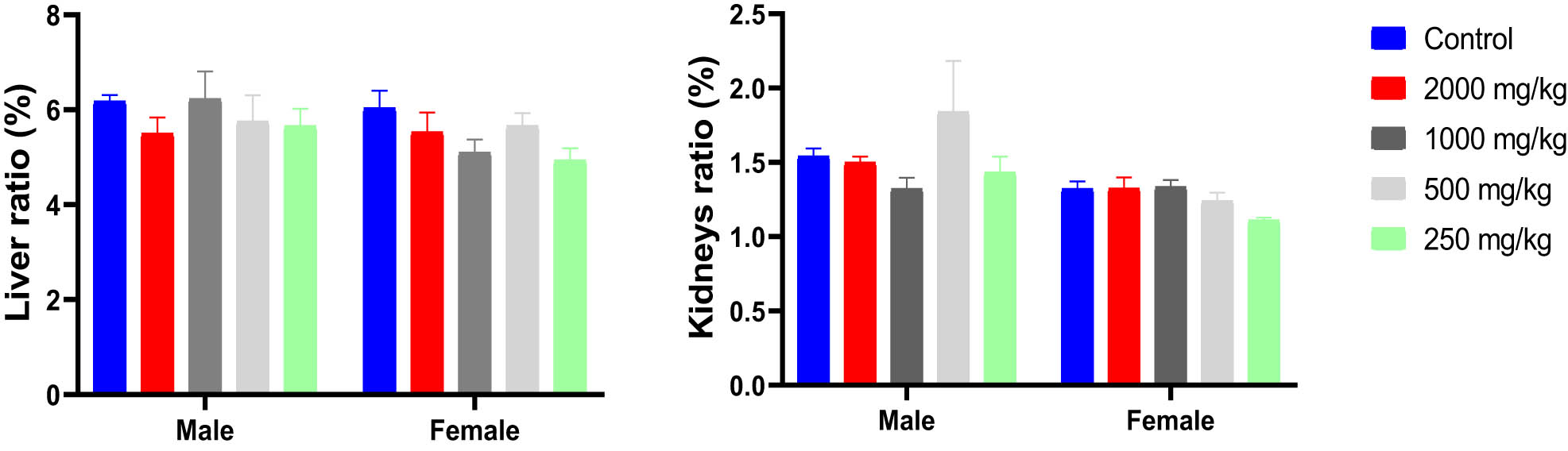
Ratio of liver and kidney weights/BWs (%) of male and female mice treated with the KH extract at 2,000, 1,000, 500, and 250 mg/kg.
3.5 Effect of KH extract on biochemical parameters in mice
The results of the acute toxicity test revealed that oral administration of a single dose of 2,000 mg/kg of KH extract induced various signs of toxicity in both male and female mice. However, no deaths were recorded during the 14-day observation period. Conversely, administration of 250 and 500 mg/kg doses of KH extract to both groups of mice did not show any signs of toxicity throughout the entire 14-day observation period. To further confirm these results, the biochemical parameters of the animals’ plasma were analyzed, and the findings are depicted in Figure 6. However adverse biochemical impairments were observed in animals that received 2,000 and 1,000 mg/kg doses of KH extract. Two-way ANOVA analysis indicated a significant interaction between sex and treatment effects on the biochemical parameters, including creatinine (F (4,49) = 2.562, p = 0.0499), AST (F (4,47) = 7.230, p = 0.0001), ALT (F (4,47) = 2.611, p = 0.0472), and total bilirubin (F (4,47) = 4.008, p = 0.0070). Bonferroni multiple comparisons test indicated a significant increase in creatinine, ALT, AST, urea, and total bilirubin in male and female animals treated with 2,000 and 1,000 mg/kg KH extract compared to the untreated and treated group with 500 and 250 mg/kg of KH extract. However, no significant interactions were revealed for total protein (F (4,47) = 0.7274, p = 0.5777), uric acid (F (4,47) = 2.212, p = 0.0820), albumin (F (4,46) = 1.026, p = 0.4038), and cholesterol (F (4,47) = 2.212, p = 0.0820).
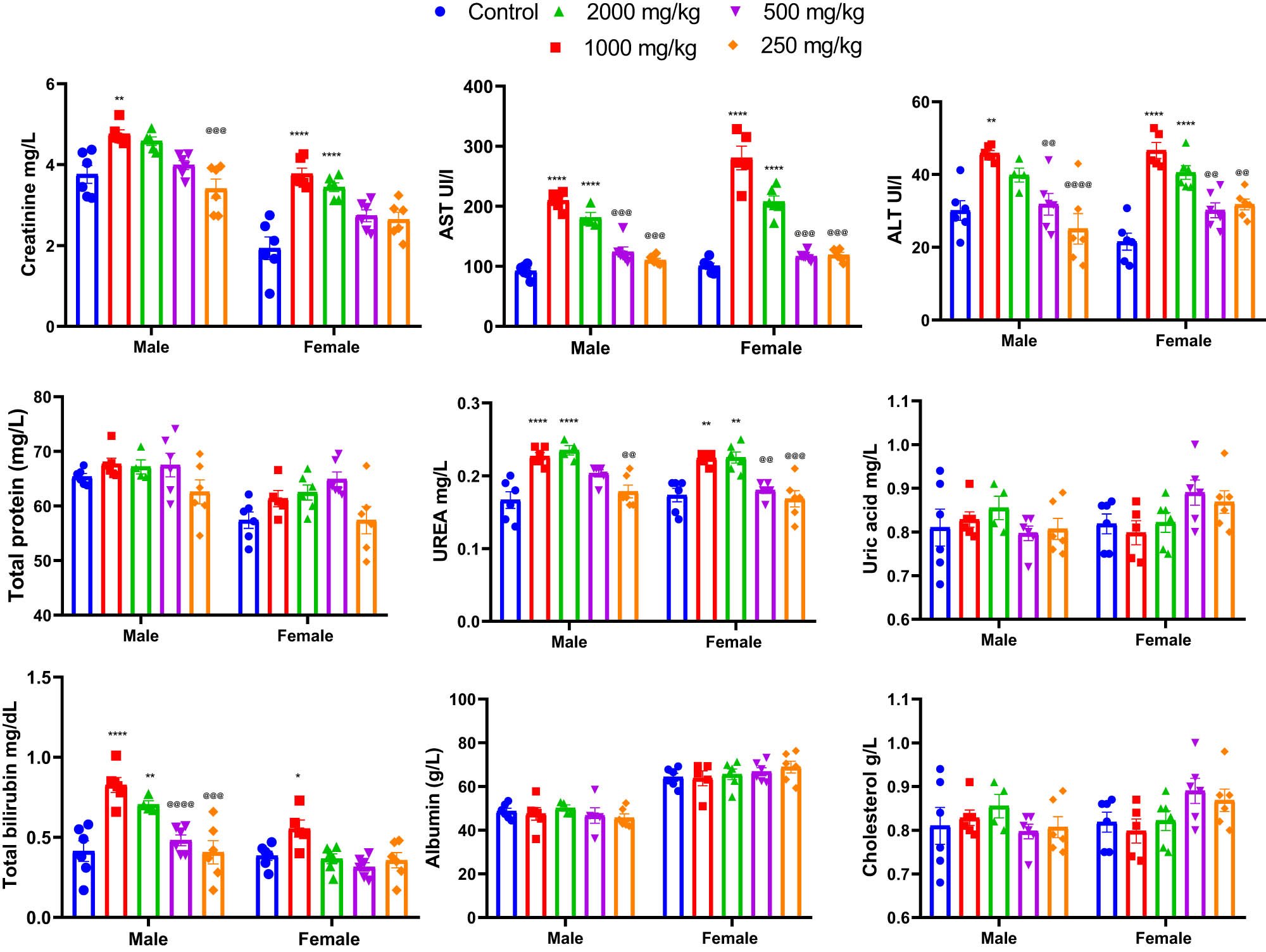
Effects of acute oral administration of KH chloroform inflorescence extract (KH extract at 2,000, 1,000, 500, and 250 mg/kg) on biochemical parameters of male and female mice (n = 6). Data expressed as mean ± SEM. Data are analyzed via two-way ANOVA, then Bonferroni post-hoc; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to control; @@ p < 0.01, @@@ p < 0.001, @@@@ p < 0.0001 compared to 2,000 and 1,000 mg/kg KH treated groups. Alanine aminotransferase: ALT; aspartate aminotransferase: AST.
Additionally, the potential effect of KH extract was also evaluated by assessing the ionogram profile in the blood plasma of untreated and treated mice. As illustrated in Figure 7, two-way ANOVA analysis revealed no significant interaction between sex and treatment effects on the ionogram profile of mice, indicating no effect on blood plasma levels of Na+ (F (4,47) = 2.006, p = 0.1090), Ca+ (F (4,47) = 1.661, p = 0.1749), K+ (F (4,47) = 0.4032, p = 0.8054), and Cl− (F (4,47) = 0.3517, p = 0.8415).
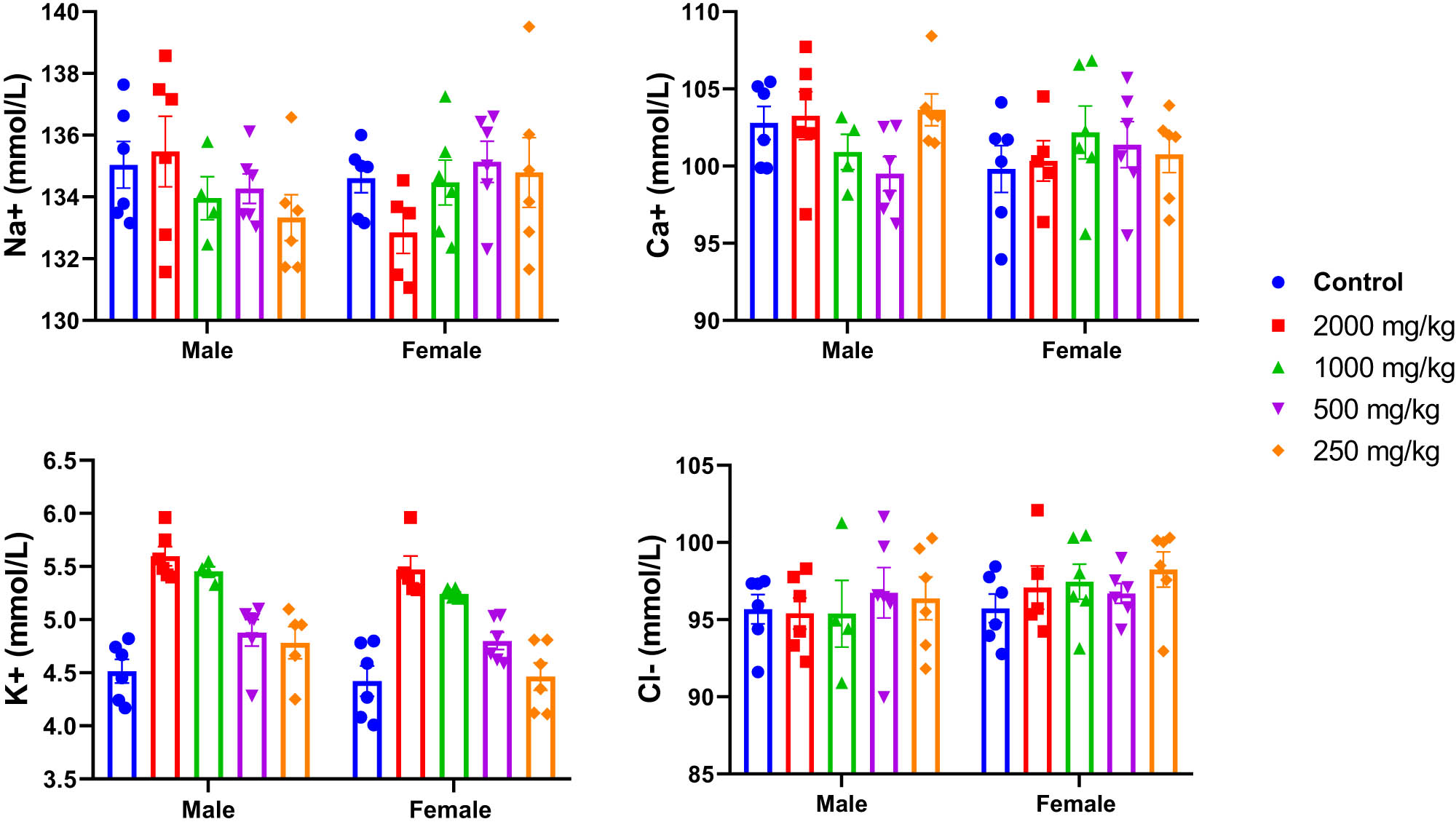
Effects of acute oral administration of KH chloroform inflorescence extract (KH extract at 2,000, 1,000, 500, and 250 mg/kg) on blood ionogram of male and female mice (n = 6). Data expressed as mean ± SEM.
3.6 Analgesic activities
3.6.1 Effect of KH extract on writhing frequency in mice
The impact of KH extract on the acetic acid-induced writhing test was assessed using two-way ANOVA. The results revealed a significant interaction between the sex of the animals and the treatment effect (F (3,40) = 4.291, p = 0.0102) (Table 2). Female and male mice administered KH extract at a dose of 500 mg/kg BW displayed a notable reduction in writhing compared to both the negative control and CBD experimental groups (p < 0.0001 vs both groups, Table 2). Notably, the percentage inhibition of acetic acid-induced writhing was higher in female and male mice treated with KH extract compared to the positive control group treated with morphine.
Effect of the chloroform KH extract on acetic acid-induced writhing in mice; data expressed as mean ± SEM (n = 6, per sex)
| Negative control | KH extract (500 mg/kg) | CBD (30 mg/kg) | Positive control morphine (30 mg/kg) | |
|---|---|---|---|---|
| Male | 27.33 ± 1.05 | 3.16 ± 1.07***@@ | 8.83 ± 1.07*** | 4.33 ± 0.66***@@ |
| Female | 30.66 ± 1.14 | 1.00 ± 0.25***@@ | 6.66 ± 0.80*** | 5.00 ± 0.77***@@ |
| % Inhibition on male | — | 88.4% | 67.7% | 84.2% |
| % Inhibition on female | — | 96.7% | 78.2% | 83.7% |
***p < 0.0001 vs control group.
@@ p < 0.001 vs CBD group.
3.6.2 Effect of KH extract on nociceptive-like responses of mice to hot water
The results of the tail immersion test demonstrated a time-dependent increase in nociceptive-like thresholds for both male and female mice following morphine, KH extract, or CBD treatments. Two-way ANOVA revealed significant interactions, characterized by an elongation of tail flick latency (Figure 8, F (15,100) = 30.67, P < 0.0001 and F (15,100) = 15.90, P < 0.0001, respectively). Notably, as demonstrated by the Bonferroni post-hoc multiple comparisons tests, the KH extract, CBD, and morphine-treated groups exhibited a significant antinociceptive effect during the early observation period (30 min) compared to the negative control group (p = 0.0025, p = 0.0058, and p < 0.0001, respectively). Moreover, both the KH and morphine groups reached their maximum effect (latency = 15 s) after 150 min, the final phase of observation. In contrast, the CBD analgesic effect notably decreased after 120 min (p < 0.0001 vs KH extract and morphine in male groups and p = 0.0147 and p = 0.0308 vs KH extract and morphine in female groups, respectively). These findings suggest that the KH extract possesses a potent and long-lasting antinociceptive activity compared to CBD alone.
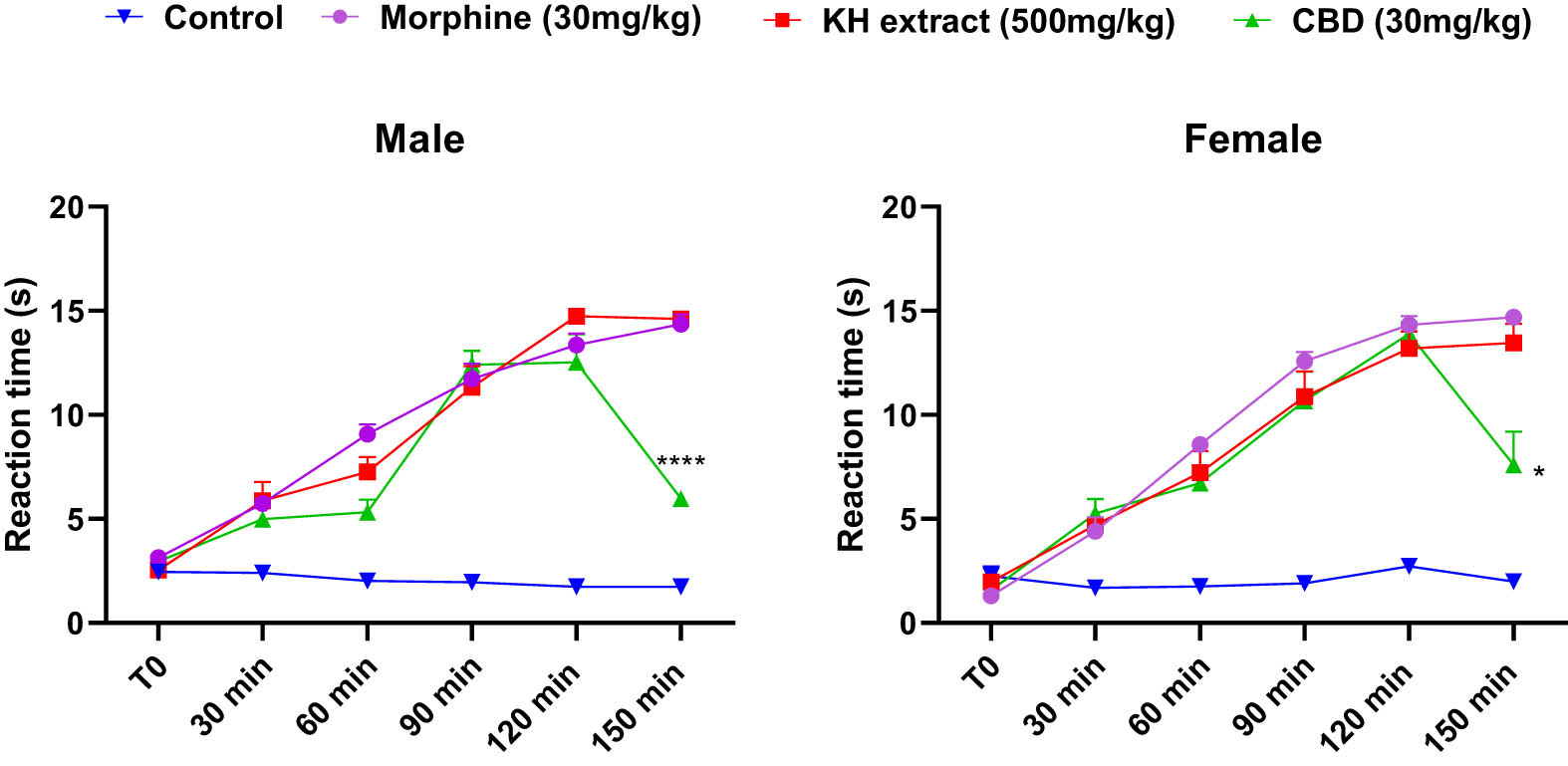
Analgesic effects of acute oral administration of KH chloroform inflorescence extract, CBD, assessed by mice reaction time in tail immersion test. Data expressed as mean ± SEM (n = 6). Data are analyzed via two-way ANOVA repeated measures, then Bonferroni post-hoc; *p < 0.05 and ****p < 0.0001 compared to morphine group and KH extract group.
3.7 Molecular docking analysis
Molecular docking analysis was performed to predict the formation of the ligand–receptor complex. The docking score was utilized to evaluate the ligand’s ability to adopt appropriate conformations within the active site of the receptor protein [37]. The analgesic activity of investigated ligands was assessed based on binding affinity with δ-opioid receptor. To function as an agonist the ligand must exhibit electrostatic or H-bonding interactions with ASP 128 deeper within the binding pocket, specifically in the extracellular region (ECL3). The interaction with ASP128 resulted in polar network rearrangements required for receptor activation and it has been observed that ligands that lack this interaction are unable to activate the receptor [35]. The Delta-9-THC demonstrated the highest binding affinity (G-score = −5.9 kcal/mol) and established two hydrogen bonds with the amino acid scaffold ASP 128. CBDV, THCV, and CBG also displayed H-bonding interactions with ASP128 and ILE 304 at distances ranging from 1.60 to 2.68 Å, accompanied by G-scores >5.0 kcal/mol. Despite the substantial binding interactions with a G-score of −5.2 kcal/mol, β-caryophyllene showed no interaction with ASP128, indicating that it may not activate the receptor. The detailed information on G-score, E-model score, H-bonding, and hydrophobic interacting residues for top hit ligands is presented in Table 3.
Molecular glide score, hydrogen bonding, hydrophobic, and other interactions with distances in Angstrom for top hit ligands with δ-opioid receptor (PDB ID: 6PT3)
| Ligands | Name of ligands | G-score (kcal/mol) | Emodel | H.B.I residue (distance, Å) | Hydrophobic and other interacting residues |
|---|---|---|---|---|---|
| 03 | β-Caryophyllene | −5.2 | −28.71 | — | ALA98, TYR129, SER131, MET132, TRP274, ILE277, CYS303, ILE304, GLY307, TYR308, SER311 |
| 06 | CBDV | −5.4 | −43.72 | ASP128 (2.47) | ALA98, GLN105, TYR129, SER131, MET132, SER135, VAL217, TRP274, ILE277, VAL281, GLY307, TYR308, SER311 |
| ASP128 (2.68) | |||||
| ILE304 (2.37) | |||||
| 08 | THCV | −5.7 | −42.20 | ASP128 (1.60) | ALA98, GLN105, TYR129, MET132, LYS214, VAL217, TRP274, ILE277, VAL281, ILE304, GLY307, TYR308, SER311 |
| ASP128 (2.34) | |||||
| 11 | Delta-9-THC | −5.9 | −49.29 | ASP128 (1.63) | ALA98, GLN105, TYR129, SER131, MET132, LYS214, VAL217, TRP274, ILE277, HIS278, VAL281, ILE304, GLY307, TYR308, SER311 |
| ASP128 (2.38) | |||||
| 13 | CBG | −5.1 | −51.99 | ASP128 (1.63) | ALA98, GLN105, TYR129, SER131, MET132, LYS214, VAL217, TRP274, ILE277, PHE280, VAL281, TRP284, LEU300, ILE304, GLY307, TYR308, SER311 |
| ASP128 (2.38) | |||||
| CL | Crystallized ligand | −8.2 | −97.43 | ASP128 (2.62) | ALA98, GLN105, MET132, LYS214, VAL217, TRP274, ILE277, HIS278, VAL281, TRP284, LEU300, ILE304, TYR308, SER311 |
| ASP128 (2.40) | |||||
| ASP128 (1.94) | |||||
| ASP128 (3.55) | |||||
| TYR129 (2.67) | |||||
| SER131 (2.53) | |||||
| GLY307 (2.43) |
CL: crystallized ligand (DPI-287); H.B.I: hydrogen bonding interacting residues.
A significant rotation of TRP284 was observed upon ligand interaction, leading to the opening of a hydrophobic pocket, comprised ILE277, HIS 278, PHE280, VAL281, TRP284, and LEU300 thereby stabilizing ligand–receptor complex through π–π stacking interactions within the binding pocket. The crystallized agonist, DPI-287, exhibited salt bridge and H-bonding interaction with ASP 128 along with other polar network aminoacid residue TYR 129 with G-score −8.2 kcal/mol. The aforementioned residues are involved in interactions with crystallized ligands (DPI-287), affirming a uniform binding pattern between top-hit ligands and the reference co-crystallized ligand. The visual representations of 2D and 3D interactions for ligands 03, 06, 08, and 11 are shown in Figure 9, while those for ligand 13 and the crystallized ligand DPI-287 are shown in Figure 10.
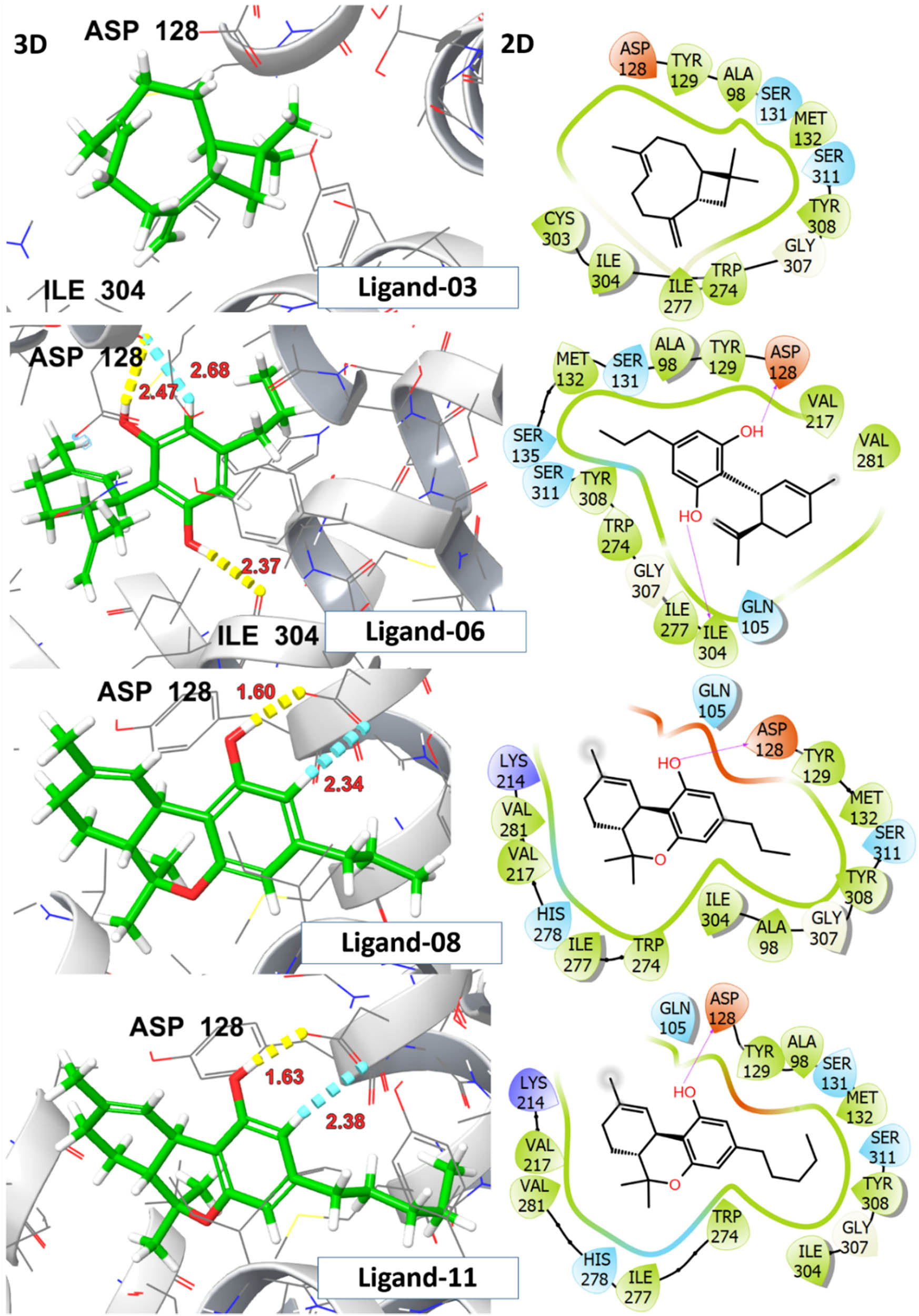
2D and 3D representation of investigated ligands 03, 06, 08, and 11 with δ-opioid receptor (PDB ID:6PT3).
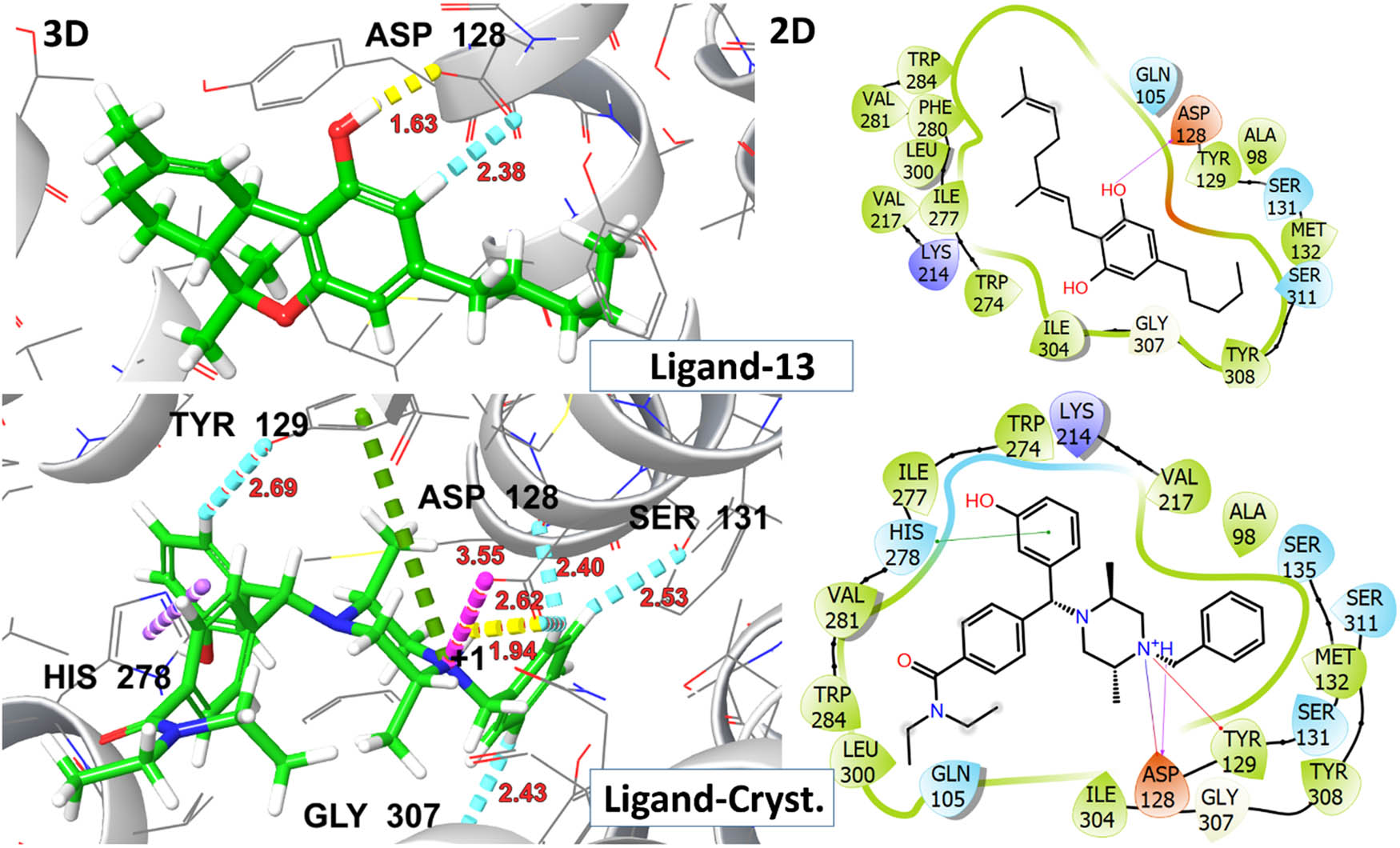
2D and 3D representation of ligands 13 and DPI-278 (co-crystallized ligand) with δ-opioid receptor (PDB ID: 6PT3).
4 Discussion
Building upon previous studies, the chemical composition of C. sativa is intricate and diverse, spanning various compounds including cannabinoids, steroids, terpenoids, flavonoids, glycosides, and alkaloids [38,39]. Therefore, in this study, we specifically focused on the KH extract of C. sativa. Analysis conducted via GC-MS revealed the presence of diverse phytochemical metabolites. Notably, among these metabolites, we identified a diterpene neophytadiene, a sesquiterpene β-caryophyllene, and several cannabinoids such as CBDV, THC, THCV, CBG, CBN, and CBD, with CBD being the predominant non-psychoactive compound, constituting approximately 60% of the total chromatogram area.
Treatment of animals with this CBD-rich extract at various doses (2,000, 1,000, 500, and 250 mg/kg) induced differential side effects in male and female mice, which were transient and lasted less than 24 h. Notably, the high dose of 2,000 mg/kg of KH extract elicited neurotoxic side effects in males, characterized by sedation, fear, ptosis, and loss of traction. However, in addition to these effects, the extract induced piloerection and aggression in 66 and 33% of females, respectively, along with abnormal respiration rates, highlighting the greater susceptibility of female mice to the extract’s toxicity compared to males. These effects might be primarily mediated by the interaction between cannabinoids and the endocannabinoid system. THC, for example, may bind to CB1 receptors, inhibiting the release of neurotransmitters like anandamide and 2-arachidonoylglycerol (2-AG), subsequently leading to an increase in dopamine, glutamate, and acetylcholine in certain brain regions. This cascade of events influences behavioral symptoms and physiological functions such as cognition and motor movements [40,41]. Furthermore, THC induces a conformational change in the receptor, inhibiting the release of neurotransmitters like glutamate and gamma-aminobutyric acid (GABA), potentially heightening the likelihood of aggression [42]. This could explain the observed fear and aggression in mice administered with KH extract at doses of 2,000 and 1,000 mg/kg. Additionally, CBD may interact with various physiological targets, as it has been shown to inhibit CYP3A4 and CYP2D6 enzymes, resulting in reduced drug concentrations through enhanced metabolism. This interaction could amplify the effects of the molecules in the KH extract and potentially lead to adverse reactions [43,44]. Clinical observations have indeed revealed that common side effects of CBD include tiredness, diarrhea, nausea, and hepatotoxicity. It is noteworthy that these side effects are generally low in incidence and depend on the duration of exposure to CBD [45]. On another note, the neurotoxic effects induced by the KH extract (1,000 and 2,000 mg/kg) differed significantly from those reported for EU-GMP-certified C. sativa [46]. The certified C. sativa, besides its sedative impact, elicited various effects, including writhing, Straub phenomena, mild motor incoordination, stereotypes, short-duration facial tremor, and abortive seizures at doses of 300 and 2,000 mg/kg during the initial 8 h monitoring period [46]. These differences seem to be mostly mediated by the biochemical extract profile, delineating more particularly the absence of cannabinoids in their acidic forms. The authors reported that typically, cannabigerol acid (CBGA), cannabidivarinic acid, and cannabichromevarinic acid presented in the certified C. sativa were responsible for the Straub phenomena and writhing observed in mice [46]. Effects mediated by the pro-convulsive effect of CBGA, by acting on GABA and TRPV1 receptors [47].
Moreover, numerous studies have explored the effects of C. sativa extracts on neurotransmitter systems, offering a robust foundation for understanding its neurochemical impact. For example, peroral administration of ethanolic C. sativa extract at doses between 10 and 100 mg/kg in rats elevated glutamate and dopamine levels in the prefrontal cortex, without affecting acetylcholine levels, suggesting potential changes in emotional and cognitive performance [48]. Similarly, at a dose of 60 mg/kg, ethanolic extracts significantly increased hippocampal dopaminergic D1 receptor expression [49] Consumption of whole cannabis edibles (100 mg/kg/day every other day for 2 weeks) showed diverse neurotransmitter effects, including increased dopamine, phenylalanine, and 3,4-dihydroxyphenylacetic acid, while tryptophan decreased consistently in both brain and plasma. Additionally, 2-AG levels varied, decreasing at low doses but increasing at higher doses of complex cannabis extracts [50]. Furthermore, oral administration of chloroformic extract (50 mg/kg) in mice increased noradrenaline levels and reduced behavioral signs of stress and anxiety [51,52]. Supercritical CO2 extracts rich in CBD at doses of 300 mg/kg administered orally in rats modulated serotonin pathways, reducing anxiety-like behavior [53–55]. Additionally, aqueous C. sativa extract (200 mg/kg) administered orally in mice increased dopamine levels in the striatum, indicating potential neuroprotective properties [56–58]. These findings underscore the significant role of cannabis extracts in influencing neurotransmitter systems, with variations depending on extraction methods, doses, and routes of administration.
Moreover, to assess the extract’s toxicity, we monitored the mice’s BW daily for 14 days after the KH extract administration. Unlike the first group treated with KH extract at a dose of 2,000 mg/kg, which showed a decrease in BW from Day 4 for both males and females, the average BW of the groups treated with doses of 1,000, 500, and 250 mg/kg did not significantly differ from the control group. This effect on BW of KH extract at high doses could be attributed to the presence of THCV, as it was previously reported to decrease weight in obese mice by decreasing both body fat and leptin serum levels [59]. In addition, our findings are consistent with a recent study that reported the weight-loss effect of C. sativa at 10% in Sprague Dawley rats. This effect is attributed to the ability of THCV and CBD to enhance the animals’ metabolism [60].
However, during the 14 days of observation, no lethality was detected, suggesting that the LD50 of our extract was higher than 2,000 mg/kg. This finding is consistent with Filipiuc et al. [46] reporting that the LD50 value for oral administration of a certified C. sativa inflorescence extract (Cannabixir® Medium Flos) in rats is estimated to be equal to or higher than 5,000 mg/kg. Also, Weinstein et al. [61] showed that doses of 17 and 13 mg of THC, the primary psychoactive compound, did not cause any lethality, but only affected coordination and motor skills.
The assessment of biochemical parameters, such as liver and kidney enzymes, can provide valuable insights into the potential toxicity of cannabis extract on the physiological functions of mice [62]. ALT, AST, and bilirubin levels are important indicators for assessing and monitoring healthy liver function and cytolysis [62,63].
Our results demonstrated that exposure of mice to higher cannabis extract doses (2,000 and 1,000 mg/kg) significantly increased AST, ALT, and bilirubin levels in blood plasma, similar to effects observed in rats consuming a diet with 10% C. sativa [60]. This hepatotoxicity may primarily be attributed to THC, as most other components found in the KH extract, such as CBD, minor cannabinoids, flavonoids, and terpenoids, are generally considered safe. In contrast, CBD, for instance, has exhibited potential hepatoprotective effects in certain animal studies. However, THC, undergoing first-pass metabolism in the liver before entering circulation [64], could lead to heightened AST and ALT levels in treated mice compared to the control group. Additionally, there was no significant increase in the biochemical parameters in the blood of mice administered with KH extract at doses of 500 and 250 mg/kg.
Additionally, this study evaluated kidney function by analyzing urea and creatinine levels. The results revealed elevated creatinine levels in the blood of mice treated with KH extract at a dose of 2,000 mg/kg compared to the control group. Also, oral administration at this dose led to a significant increase in blood urea levels, suggesting potential acute deleterious effects on the liver and kidneys [65]. Nevertheless, in line with Filipiuc et al. [66] the oral administration of KH did not result in significant alterations in total cholesterol, uric acid, albumin, and in total protein levels. This indicates that both metabolic and hepatic synthesis functions, as well as renal filtration and purification functions, remained normal, without any indications of organic damage or functional abnormalities [67].
In this respect, and based on the neurotoxicity results, we selected the 500 mg/kg BW dose to evaluate the therapeutic effects of the KH extract on mice, particularly in terms of its efficacy on pain [68]. Furthermore, according to the Irwin procedures conducted, the results demonstrated an analgesic effect in all the treated groups, suggesting the potential of KH extract as an analgesic agent [69]. To assess the analgesic effect of the KH extract, we employed two different pain assessment tests: the tail immersion test and the acid acetic-induced writhing test in mice, reflecting nociceptive and visceral pain, respectively [70–72].
Our results revealed a significant effect of KH extract in relieving acute thermal stimulus pain and reducing writhing behavior. The analgesic effect of KH extract at 500 mg/kg was comparable to that of morphine at 30 mg/kg in both male and female mice, with the effect persisting for more than 150 min (Table 2 and Figure 8). These findings are consistent with other earlier studies on the analgesic effects of cannabinoids isolated from KH extract, such as THC, CBD, CBG, and CBN [73]. Utilizing molecular docking procedures, we visualized the dynamic interactions between ligands and δ-opioid receptors, a pivotal approach in unraveling the intricate molecular mechanisms and identifying key amino acid residues (such as ASP128) responsible for δ-opioid receptor activation. Notably, ligand 11 (THC) exhibited the highest binding affinity with the target receptor, boasting a G-score of −5.9 kcal/mol. The cumulative analgesic effect might stem from a synergistic interaction observed among ligands 08 (THCV), 06 (CBDV), and 13 (CBG). This methodological approach not only facilitated interpreting binding preferences but also laid the groundwork for elucidating the pharmacological effects and potential analgesic applications of identified ligands targeting δ-opioid receptors. Similarly, other studies revealed that those phytocannabinoids had a higher binding affinity to opioid receptors, including µ-opioid receptor and δ-opioid receptor, with corresponding Ki (nM) values of 7,000 and 10,000, respectively, as well as with other CB receptors (CB1, CB2), [74].
The analgesic prowess of the KH extract is not solely efficient but also enduring, persisting beyond 150 min, corroborating recent research indicating that orally administered cannabinoids offer prolonged relief from allodynia in a mouse model of chronic neuropathic pain [75]. Unlike the effect of pure CBD, which decreased significantly after 150 min, this result may be attributed to the weak binding of CBD to the orthosteric site of cannabinoid receptors with a Ki in the micromolar range [76,77]. These findings were corroborated by Abraham et al. [75] demonstrating that the THC-containing extract mirrored the effect of morphine without inducing tolerance, whereas pure CBD showed a comparatively diminished effect.
This enduring effect likely owes itself to various analgesic components of KH extract, particularly CBG, previously recognized for its potent analgesic action on α-2 adrenoreceptors [78,79]. Furthermore, THCV displayed a CB2-mediated capacity to inhibit carrageenan-induced hyperalgesia and inflammation, along with both phases of formalin-induced pain behavior, achieved through the activation of CB1 and CB2 receptors in mice [80,81]. Moreover, the potential synergy between cannabinoids and terpenoids as natural remedies could bolster the KH extract’s analgesic potential. Recent studies have demonstrated that the combination of CBD and β-caryophyllene exhibits analgesic potential in alleviating chronic pain while avoiding the psychoactive effects typically associated with THC by acting on CB1 and CB2 receptors, which explains the absence of the binding of CBD and β-caryophyllene with δ-opioid receptors in our docking procedure [82–84].
Moreover, preclinical and clinical studies suggest that the combination of CBD with other compounds is more effective in reducing pain-related diseases than pure CBD alone [84,85]. In this regard, they developed medications called nabiximols such as Sativex® where the THC concentration was more than CBD (10.8–16.2 mg THC, 10.0–15 mg CBD) to help provide the best analgesic effect. However, subsequent findings indicated that using a higher co-administration dose (ranging from 29.7 to 43.2 mg THC and 27.5 to 40 mg CBD) led to side effects resembling those observed with THC alone [86,87].
Taking into account the side effects of THC, coupled with the ability of CBD to reduce them, we can conclude that the combination of THC, CBG, THCV, and β-caryophyllene with a high amount of CBD is a promising therapeutic option, which is in the line with a recent study conducted by Eeswara et al. [84]. Assuming that in this study, KH extract contains a significantly higher level of CBD than THC (60% of the total peak area of KH extract chromatogram) alongside these compounds had provided an efficient and long-lasting analgesic effect.
5 Conclusion
In summary, this study contributes to the growing body of research on C. sativa, highlighting the potential therapeutic applications of KH extract in pain management. The acute oral toxicity assessment revealed a high LD50 (>5,000 mg/kg) with no mortality at 2,000 mg/kg, though transient neurotoxic effects and mild hepato-renal toxicity were observed at this dose, emphasizing the need for further toxicological evaluations. At a moderate dose (500 mg/kg), KH extract exhibited significant analgesic effects in neuropathic and nociceptive pain models. This effect is likely mediated by the synergistic action of its phytoconstituents, particularly the high CBD/THC ratio with CBDV, CBG, and THCV. Molecular docking studies further supported its potential interaction with δ-opioid receptors, providing mechanistic insights into its analgesic activity.
While this study provides valuable insights into the analgesic effects of the KH extract, it is important to acknowledge certain limitations and suggest future research directions. One notable limitation is the use of mammalian models for toxicological evaluation. Although the UDP 425 protocol was rigorously followed and no animals died at the 2,000 mg/kg dose, future studies could benefit from incorporating alternative models such as in vitro cell cultures, computer simulations, or non-mammalian organisms (e.g., zebrafish). These alternatives could provide additional toxicological data and align with the principles of the 3Rs in animal research. Additionally, the KH extract demonstrates potential advantages over morphine concerning dependence and tolerance due to its higher CBD level and reduced THC content, along with other non-psychoactive phytocannabinoids. Hence, further research to support the current evidence base, and to feature the potential mechanisms of action related to this analgesic effect are underway. In light of this, it would be valuable to explore the crosstalk between cannabinoids and opioid receptors as we had revealed that KH extract provided the same curve slope as morphine by increasing pain thresholds in mice. Moreover, exploring the potential for reduced tolerance and dependence compared to traditional opioids could provide significant benefits for clinical pain management.
Acknowledgments
The authors would like to thank the Physiology and Physiopathology Team, Faculty of Sciences, Rabat, Morocco. They would also like to acknowledge Laboratory Du Nord, Tetaoun, Morocco for the technical assistance. The authors would like to extend their sincere appreciation to the Ongoing Research Funding Program (ORF-2025-437), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: This work is financially supported by the Ongoing Research Funding Program (ORF-2025-437), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results and approved the final version of the manuscript. H.I., Z.A.L., O.B.M., and O.A. conceptualized the study; M.B., O.A., F.S., and H.K. developed the methodology; O.A. and H.I. performed the software analysis; O.A., K.T., and L.H. validated the findings; H.I., F.S., and Z.A.L. conducted the formal analysis; S.E., A.M.S., and R.E. carried out the investigation; A.M.S., H.K., F.K., and L.H. provided resources; H.I. curated the data; H.I., M.B., F.S., and Z.A.L. prepared the original draft; O.A., F.K., and H.K. reviewed and edited the manuscript; and H.I., Z.A.L., and O.A. handled visualization.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Afsahi K. The commodification of nature and the construction of a contested international market1. The Routledge Handbook of Post-Prohibition Cannabis Research. London, UK: Routledge; 2021. p. 241.Search in Google Scholar
[2] Afsahi K. Maroc: quand la Khardala et les hybrides bouleversent le Rif. JWAPS. 2017;87:21–5.Search in Google Scholar
[3] Badrana F, El Fahime EM, Mokhtari A, Soulaymani A, Safini N, Chaouni B, et al. In silico and in vitro analysis of THCA synthase gene in Moroccan Cannabis sativa. L F1000 Res. 2022;11:840.10.12688/f1000research.123384.1Search in Google Scholar
[4] Piluzza G, Delogu G, Cabras A, Marceddu S, Bullitta S. Differentiation between fiber and drug types of hemp (Cannabis sativa L.) from a collection of wild and domesticated accessions. Genet Resour Crop Evol. 2013;60:2331–42.10.1007/s10722-013-0001-5Search in Google Scholar
[5] Bonini SA, Premoli M, Tambaro S, Kumar A, Maccarinelli G, Memo M, et al. Cannabis sativa: a comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol. 2018;227:300–15.10.1016/j.jep.2018.09.004Search in Google Scholar PubMed
[6] Ozdemir E. The role of the cannabinoid system in opioid analgesia and tolerance. Mini-Rev Med Chem. 2020;20:875–85.10.2174/1389557520666200313120835Search in Google Scholar PubMed
[7] Auh Q-S, Chun YH, Melemedjian OK, Zhang Y, Ro JY. Peripheral interactions between cannabinoid and opioid receptor agonists in a model of inflammatory mechanical hyperalgesia. Brain Res Bull. 2016;125:211–17.10.1016/j.brainresbull.2016.07.009Search in Google Scholar PubMed PubMed Central
[8] Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–403.10.1038/sj.npp.1301243Search in Google Scholar PubMed
[9] Poyatos L, Pérez-Acevedo AP, Papaseit E, Pérez-Mañá C, Martin S, Hladun O, et al. Oral administration of cannabis and Δ-9-tetrahydrocannabinol (THC) preparations: a systematic review. Medicina. 2020;56:309.10.3390/medicina56060309Search in Google Scholar PubMed PubMed Central
[10] Kharchoufa L, Bouhrim M, Bencheikh N, Addi M, Hano C, Mechchate H, et al. Potential toxicity of medicinal plants inventoried in northeastern Morocco: an ethnobotanical approach. Plants. 2021;10:1108.10.3390/plants10061108Search in Google Scholar PubMed PubMed Central
[11] Urits I, Gress K, Charipova K, Habib K, Lee D, Lee C, et al. Use of cannabidiol (CBD) for the treatment of chronic pain. Best Pract Res Clin Anaesthesiol. 2020;34:463–77.10.1016/j.bpa.2020.06.004Search in Google Scholar PubMed
[12] Laprairie R, Bagher A, Kelly M, Denovan‐Wright. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–805.10.1111/bph.13250Search in Google Scholar PubMed PubMed Central
[13] Silva-Cardoso GK, Lazarini-Lopes W, Hallak JE, Crippa JA, Zuardi AW, Garcia-Cairasco N, et al. Cannabidiol effectively reverses mechanical and thermal allodynia, hyperalgesia, and anxious behaviors in a neuropathic pain model: possible role of CB1 and TRPV1 receptors. Neuropharmacology. 2021;197:108712.10.1016/j.neuropharm.2021.108712Search in Google Scholar PubMed
[14] Narouze S. Antinociception mechanisms of action of cannabinoid-based medicine: an overview for anesthesiologists and pain physicians. Reg Anesth Pain Med. 2021;46:240–50.10.1136/rapm-2020-102114Search in Google Scholar PubMed
[15] Manzanares J, Julian M, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4:239–57.10.2174/157015906778019527Search in Google Scholar PubMed PubMed Central
[16] Laun AS, Shrader SH, Brown KJ, Song Z-H. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol Sin. 2019;40:300–8.10.1038/s41401-018-0031-9Search in Google Scholar PubMed PubMed Central
[17] Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–43.10.1007/s11064-005-6978-1Search in Google Scholar PubMed
[18] Bruni N, Della Pepa C, Oliaro-Bosso S, Pessione E, Gastaldi D, Dosio F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules. 2018;23:2478.10.3390/molecules23102478Search in Google Scholar PubMed PubMed Central
[19] Dieterle M, Zurbriggen L, Mauermann E, Mercer-Chalmers-Bender K, Frei P, Ruppen W, et al. Pain response to cannabidiol in opioid-induced hyperalgesia, acute nociceptive pain, and allodynia using a model mimicking acute pain in healthy adults in a randomized trial (CANAB II). Pain. 2022;163:1919–28.10.1097/j.pain.0000000000002591Search in Google Scholar PubMed PubMed Central
[20] Bosquez-Berger T, Gudorf JA, Kuntz CP, Desmond JA, Schlebach JP, VanNieuwenhze MS, et al. Structure–activity relationship study of cannabidiol-based analogs as negative allosteric modulators of the μ-opioid receptor. J Med Chem. 2023;66:9466–94.10.1021/acs.jmedchem.3c00061Search in Google Scholar PubMed PubMed Central
[21] Grogan G, Stephens K, Chou J, Timko MP, Cottler P, DeGeorge Jr BR. The mechanism of cannabichromene and cannabidiol alone versus in combination in the alleviation of arthritis-related inflammation. Ann Plast Surg. 2023;90:S408–15.10.1097/SAP.0000000000003547Search in Google Scholar PubMed
[22] Thomas A, Baillie G, Phillips A, Razdan R, Ross RA, Pertwee R. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–23.10.1038/sj.bjp.0707133Search in Google Scholar PubMed PubMed Central
[23] Desa S, Osman A. Hyslop RJEJoS, mathematics, technology. in silico assessment of drug-like properties of phytocannabinoids in Cannabis sativa. 2017;4:1–7.10.37134/ejsmt.vol4.2.1.2017Search in Google Scholar
[24] Luechtefeld T, Maertens A, Russo DP, Rovida C, Zhu H, Hartung T. Analysis of public oral toxicity data from REACH registrations 2008–2014. J Altex. 2016;33:111.10.14573/altex.1510054Search in Google Scholar PubMed PubMed Central
[25] Development OfEC-o. Test No. 425: acute oral toxicity: up-and-down procedure. Paris, France: OECD Publishing; 2008.Search in Google Scholar
[26] Roux S, Sablé E, Porsolt RD. Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effects on behavior and physiological function. Curr Protoc Pharmacol. 2004;27:10.10.1002/0471141755.ph1010s27Search in Google Scholar PubMed
[27] Pauli D, Seyfarth M, Dibbelt L. The Abbott Architect c8000: analytical performance and productivity characteristics of a new analyzer applied to general chemistry testing. Clin Lab. 2005;51:31–42.Search in Google Scholar
[28] Rashid M, Biswas S, Abdullah-Al-Mamun M, Huque A, Bhuiyan JR. Phytochemical screening and analgesic, anti-bacterial and cytotoxic activity evaluation of ethanol extract of Pithcellobium dulce (Roxb.) benth leaf. Asian J Pharm Clin Res. 2015;8:451–7.Search in Google Scholar
[29] Luttinger D. Determination of antinociceptive efficacy of drugs in mice using different water temperatures in a tail-immersion test. J Pharmacol Methods. 1985;13:351–7.10.1016/0160-5402(85)90017-8Search in Google Scholar PubMed
[30] Sun L, Liao L, Wang B. Potential antinociceptive effects of Chinese propolis and identification on its active compounds. J Immunol Res. 2018;2018:5429543.10.1155/2018/5429543Search in Google Scholar PubMed PubMed Central
[31] Jain M, Nema P, Jain S, Khan R, Dixena S, Jain PK, et al. To evaluate and compare the analgesic activity of piperine and extracts of Piper nigrum L. fruit. J Drug Delivery Ther. 2024;14:49–53.10.22270/jddt.v14i8.6731Search in Google Scholar
[32] Luo L, Wang Y, Li B, Xu L, Kamau PM, Zheng J, et al. Molecular basis for heat desensitization of TRPV1 ion channels. Nat Commun. 2019;10:2134.10.1038/s41467-019-09965-6Search in Google Scholar PubMed PubMed Central
[33] Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–6.10.4103/0253-7176.116232Search in Google Scholar PubMed PubMed Central
[34] Festing MF, Altman, Douglas G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002;43:244–58.10.1093/ilar.43.4.244Search in Google Scholar PubMed
[35] Claff T, Yu J, Blais V, Patel N, Martin C, Wu L, et al. Elucidating the active δ-opioid receptor crystal structure with peptide and small-molecule agonists. Sci Adv. 2019;5:eaax9115.10.1126/sciadv.aax9115Search in Google Scholar PubMed PubMed Central
[36] Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–49.10.1021/jm0306430Search in Google Scholar PubMed
[37] Meng X-Y, Zhang H-X, Mezei M, Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput drug Des. 2011;7:146–57.10.2174/157340911795677602Search in Google Scholar PubMed PubMed Central
[38] Richter G, Hazzah T, Hartsel JA, Eades J, Hickory B, Makriyannis A. Cannabis sativa: an overview. Nutraceuticals. Academic Press; 2021 p. 603–24.10.1016/B978-0-12-821038-3.00038-0Search in Google Scholar
[39] Odieka AE, Obuzor GU, Oyedeji OO, Gondwe M, Hosu YS, Oyedeji AO. The medicinal natural products of Cannabis sativa Linn.: a review. Molecules. 2022;27:1689.10.3390/molecules27051689Search in Google Scholar PubMed PubMed Central
[40] López‐Moreno JA, González‐Cuevas G, Moreno G, Navarro M. The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addict Biol. 2008;13:160–87.10.1111/j.1369-1600.2008.00105.xSearch in Google Scholar PubMed
[41] Mechoulam R, Hanuš L. A historical overview of chemical research on cannabinoids. Chem Phys Lipids. 2000;108:1–13.10.1016/S0009-3084(00)00184-5Search in Google Scholar
[42] Copas G, Amazonas E, Brandon S. The pharmacology of cannabinoids. In Cannabis therapy in veterinary medicine: a complete guide. Cham: Springer; 2021. p. 17–59.10.1007/978-3-030-68317-7_2Search in Google Scholar
[43] Kowalczyk K, Lasek P, Trąbka N, Binkowska P, Demidowicz G, Lasota N, et al. Medical cannabis: mechanisms of action and therapeutic targets. J Educ Health Sport. 2024;58:176–90.10.12775/JEHS.2024.58.013Search in Google Scholar
[44] Chayasirisobhon S. Mechanisms of action and pharmacokinetics of cannabis. Perm J. 2020;25:1–3.10.7812/TPP/19.200Search in Google Scholar PubMed PubMed Central
[45] Maldonado C, Peyraube R, Fagiolino P, Oricchio F, Cuñetti L, Vázquez M. Human data on pharmacokinetic interactions of cannabinoids: a narrative review. Curr Pharm Des. 2024;30:241–54.10.2174/0113816128288510240113170116Search in Google Scholar PubMed
[46] Filipiuc L-E, Ştefănescu R, Solcan C, Ciorpac M, Szilagyi A, Cojocaru D, et al. Acute toxicity and pharmacokinetic profile of an EU-GMP-Certified Cannabis sativa L. in rodents. Pharmamaceuticals. 2023;16:694.Search in Google Scholar
[47] Anderson LL, Low IK, Banister SD, McGregor IS, Arnold JC. Pharmacokinetics of phytocannabinoid acids and anticonvulsant effect of cannabidiolic acid in a mouse model of Dravet syndrome. J Nat Prod. 2019;82:3047–55.10.1021/acs.jnatprod.9b00600Search in Google Scholar PubMed
[48] Yinka OS, Olubunmi OP, Zabdiel AA, Oladele OJ, Taiye AS, Ayodele A, et al. Peroral exposure to Cannabis sativa a ethanol extract caused neuronal degeneration and astrogliosis in wistar rats’ prefrontal cortex. Ann Neurosci. 2023;30:84–95.10.1177/09727531221120988Search in Google Scholar PubMed PubMed Central
[49] Haghparast E, Sheibani V, Komeili G, Chahkandi M, Rad NS. The effects of chronic marijuana administration on 6-OHDA-induced learning & memory impairment and hippocampal dopamine and cannabinoid receptors interaction in male rats. Neurochem Res. 2023;48:2220–9.10.1007/s11064-023-03899-8Search in Google Scholar PubMed
[50] Reisdorph N, Doenges K, Levens C, Manke J, Armstrong M, Smith H, et al. Oral cannabis consumption and intraperitoneal THC: CBD dosing results in changes in brain and plasma neurochemicals and endocannabinoids in mice. J Cannabis Res. 2024;6:10.10.1186/s42238-024-00219-xSearch in Google Scholar PubMed PubMed Central
[51] Burggren AC, Shirazi A, Ginder N, London ED. Cannabis effects on brain structure, function, and cognition: considerations for medical uses of cannabis and its derivatives. Am J Drug Alcohol Abuse. 2019;45:563–79.10.1080/00952990.2019.1634086Search in Google Scholar PubMed PubMed Central
[52] ElShebiney S, El-Denshary ES, Abdel-Salam O, Salem NA, El-Khyat ZA, El N. Cannabis resin extract in Parkinson’s disease: behavioral, neurochemical, and histological evaluation. Cell Biol Res Ther. 2014;10:12.Search in Google Scholar
[53] Dosumu OA, Taiwo OA, Akinloye OA, Obadina AO, Rotimi SO, Owolabi OP, et al. Implications of Cannabis sativa on serotonin receptors 1B (HTR1B) and 7 (HTR7) genes in modulation of aggression and depression. Vegetos. 2022;35:19–25.10.1007/s42535-021-00308-9Search in Google Scholar
[54] Wang M, Wang Y-H, Avula B, Radwan MM, Wanas AS, van Antwerp J, et al. Decarboxylation study of acidic cannabinoids: a novel approach using ultra-high-performance supercritical fluid chromatography/photodiode array-mass spectrometry. Cannabis Cannabinoid Res. 2016;1:262–71.10.1089/can.2016.0020Search in Google Scholar PubMed PubMed Central
[55] Abdel-Salam OM, El-Shamarka ME-S, Salem NA, Gaafar AE-DM. Effects of Cannabis sativa extract on haloperidol-induced catalepsy and oxidative stress in the mice. Excli J. 2012;11:45.Search in Google Scholar
[56] Amaza DS, Maidugu F, Zirahei J, Numan A, Mari H. The effect of Cannabis sativa leaves aqueous extract on cerebral cortex in albino rats. J Dental Med Sci. 2013;6:53–58.10.9790/0853-0625358Search in Google Scholar
[57] Schwarz AM, Keresztes A, Bui T, Hecksel RJ, Peña A, Lent B, et al. Terpenes from Cannabis sativa induce antinociception in mouse chronic neuropathic pain via activation of spinal cord adenosine A(2A) receptors. bioRxiv. 2023.10.1101/2023.03.28.534594Search in Google Scholar PubMed PubMed Central
[58] Demshimeno P, Ukoha U, Aguwa U. Effect of aqueous extract of Cannabis sativa leaf on the oxidative stress markers in the brain of male wistar rats. Asian J Appl Chem Res. 2024;15:53–62.10.9734/ajacr/2024/v15i3291Search in Google Scholar
[59] Riedel G, Fadda P, McKillop‐Smith S, Pertwee RG, Platt B, Robinson L. Synthetic and plant‐derived cannabinoid receptor antagonists show hypophagic properties in fasted and non‐fasted mice. Br J Pharmacol. 2009;156:1154–66.10.1111/j.1476-5381.2008.00107.xSearch in Google Scholar PubMed PubMed Central
[60] Abey NO. Cannabis sativa (Marijuana) alters blood chemistry and the cytoarchitecture of some organs in Sprague Dawley rat models. Food Chem Toxicol. 2018;116:292–97.10.1016/j.fct.2018.04.023Search in Google Scholar PubMed
[61] Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, et al. A study investigating the acute dose–response effects of 13 mg and 17 mg Δ 9-tetrahydrocannabinol on cognitive–motor skills, subjective and autonomic measures in regular users of marijuana. J Psychopharmacol. 2008;22:441–51.10.1177/0269881108088194Search in Google Scholar PubMed
[62] Ahmed Z, Isaq H, Khan ZS, Noureen H, Khan MA, Ishaq M. Effect of Cannabis sativa extract on the liver and cardic enzymes of normal healthy mice. Khyber Med Univ J. 2016;9:117.Search in Google Scholar
[63] Mobashar A, Akbar Z, Barkat K, Hussain K, Nadeem H, Kharl HAA, et al. Evaluation of anti-inflammatory and anti-arthritic activities of benzimidazole derivative 2-((1H-benzo [d] imiadazol-2-yl) thio)-1-3, 5-diphenyl-1h-pyrazol-1-yl) ethanone. Atl J Life Sci. 2025;2025.10.71005/z815ma53Search in Google Scholar
[64] Kumar R, Chambers W, Pertwee R. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia. 2001;56:1059–68.10.1046/j.1365-2044.2001.02269.xSearch in Google Scholar PubMed
[65] Scherberich JE, Gruber R, Nockher WA, Christensen EI, Schmitt H, Herbst V, et al. Serum uromodulin—a marker of kidney function and renal parenchymal integrity. Nephrol Dialysis Transplant. 2018;33:284–95.10.1093/ndt/gfw422Search in Google Scholar PubMed PubMed Central
[66] Filipiuc L-E, Ştefănescu R, Solcan C, Ciorpac M, Szilagyi A, Cojocaru D, et al. Acute toxicity and pharmacokinetic profile of an EU-GMP-certified Cannabis sativa L. in rodents. Pharmaceuticals. 2023;16:694.10.3390/ph16050694Search in Google Scholar PubMed PubMed Central
[67] de Faria Viana E, de Carvalho Mello HH, Carvalho FB, Café MB, Leandro NSM, Arnhold E, et al. Blood biochemical parameters and organ development of brown layers fed reduced dietary protein levels in two rearing systems. Anim Biosci. 2022;35:444.10.5713/ab.21.0145Search in Google Scholar PubMed PubMed Central
[68] Groce E. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research; 2018.10.30770/2572-1852-104.4.32Search in Google Scholar
[69] Hill KP, Palastro MD, Johnson B, Ditre JW. Cannabis and pain: a clinical review. Cannabis Cannabinoid Res. 2017;2:96–104.10.1089/can.2017.0017Search in Google Scholar PubMed PubMed Central
[70] Deng J, Han J, Chen J, Zhang Y, Huang Q, Wang Y, et al. Comparison of analgesic activities of aconitine in different mice pain models. PLoS One. 2021;16:e0249276.10.1371/journal.pone.0249276Search in Google Scholar PubMed PubMed Central
[71] Doncheva ND, Vasileva L, Saracheva K, Dimitrova D, Getova D. Study of antinociceptive effect of ketamine in acute and neuropathic pain models in rats. Adv Clin Exp Med. 2019;28:573–79.10.17219/acem/94143Search in Google Scholar PubMed
[72] Rapacz A, Rybka S, Obniska J, Jodłowska A, Góra M, Koczurkiewicz P, et al. Analgesic and antiallodynic activity of novel anticonvulsant agents derived from 3-benzhydryl-pyrrolidine-2, 5-dione in mouse models of nociceptive and neuropathic pain. Eur J Pharmacol. 2020;869:172890.10.1016/j.ejphar.2019.172890Search in Google Scholar PubMed
[73] Sofia RD, Vassar HB, Knobloch LC. Comparative analgesic activity of various naturally occurring cannabinoids in mice and rats. J Psychopharmacol. 1975;40:285–95.10.1007/BF00421466Search in Google Scholar PubMed
[74] Mlost J, Bryk M, Starowicz K. Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. Int J Mol Sci. 2020;21:8870.10.3390/ijms21228870Search in Google Scholar PubMed PubMed Central
[75] Abraham AD, Leung EJ, Wong BA, Rivera ZM, Kruse LC, Clark JJ, et al. Orally consumed cannabinoids provide long-lasting relief of allodynia in a mouse model of chronic neuropathic pain. J Neuropsychopharmacol. 2020;45:1105–14.10.1038/s41386-019-0585-3Search in Google Scholar PubMed PubMed Central
[76] Tham M, Yilmaz O, Alaverdashvili M, Kelly ME, Denovan‐Wright EM, Laprairie RB. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol‐dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol. 2019;176:1455–69.10.1111/bph.14440Search in Google Scholar PubMed PubMed Central
[77] Martínez-Pinilla E, Varani K, Reyes-Resina I, Angelats E, Vincenzi F, Ferreiro-Vera C, et al. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front Pharmacol. 2017;8:744.10.3389/fphar.2017.00744Search in Google Scholar PubMed PubMed Central
[78] Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent α2‐adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol. 2010;159:129–41.10.1111/j.1476-5381.2009.00515.xSearch in Google Scholar PubMed PubMed Central
[79] Nachnani R, Raup-Konsavage WM, Vrana KE. The pharmacological case for cannabigerol. J Pharmacol Exp Ther. 2021;376:204–12.10.1124/jpet.120.000340Search in Google Scholar PubMed
[80] Bolognini D, Costa B, Maione S, Comelli F, Marini P, Di Marzo V, et al. The plant cannabinoid Δ9‐tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br J Pharmacol. 2010;160:677–87.10.1111/j.1476-5381.2010.00756.xSearch in Google Scholar PubMed PubMed Central
[81] Kalvala AK, Bagde A, Arthur P, Surapaneni SK, Ramesh N, Nathani A, et al. Role of cannabidiol and tetrahydrocannabivarin on paclitaxel-induced neuropathic pain in rodents. Int Immunopharmacol. 2022;107:108693.10.1016/j.intimp.2022.108693Search in Google Scholar PubMed PubMed Central
[82] Aguilar-Avila DS, Flores-Soto ME, Tapia-Vázquez C, Pastor-Zarandona OA, López-Roa RI, Viveros-Paredes JM. β-Caryophyllene, a natural sesquiterpene, attenuates neuropathic pain and depressive-like behavior in experimental diabetic mice. J Med Food. 2019;22:460–8.10.1089/jmf.2018.0157Search in Google Scholar PubMed
[83] Aly E, Khajah MA, Masocha W. β-Caryophyllene, a CB2-receptor-selective phytocannabinoid, suppresses mechanical allodynia in a mouse model of antiretroviral-induced neuropathic pain. Molecules. 2019;25:106.10.3390/molecules25010106Search in Google Scholar PubMed PubMed Central
[84] Eeswara A, Pacheco-Spiewak A, Jergova S, Sagen J. Combined non-psychoactive Cannabis components cannabidiol and β-caryophyllene reduce chronic pain via CB1 interaction in a rat spinal cord injury model. PLoS One. 2023;18:e0282920.10.1371/journal.pone.0282920Search in Google Scholar PubMed PubMed Central
[85] Sofia RD, Barry H. Acute and chronic effects of Δ 9-tetrahydrocannabinol on food intake by rats. Psychopharmacology. 1974;39:213–22.10.1007/BF00421028Search in Google Scholar PubMed
[86] Boyaji S, Merkow J, Elman RNM, Kaye AD, Yong RJ, Urman RD. The role of cannabidiol (CBD) in chronic pain management: an assessment of current evidence. Curr Pain Headache Rep. 2020;24:1–6.10.1007/s11916-020-0835-4Search in Google Scholar PubMed
[87] Casey SL, Atwal N, Vaughan CW. Cannabis constituent synergy in a mouse neuropathic pain model. Pain. 2017;158:2452–60.10.1097/j.pain.0000000000001051Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- 10.1515/biol-2025-1168
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- 10.1515/biol-2025-1168
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”

