Abstract
C12H15N3S, orthorhombic, Pbca (no. 61), a = 11.9612(5) Å, b = 8.1215(3) Å, c = 27.5865(12) Å, V = 2679.83(19) Å3, Z = 8, Rgt(F) = 0.0520, wRref(F2) = 0.1514, T = 293(2) K.
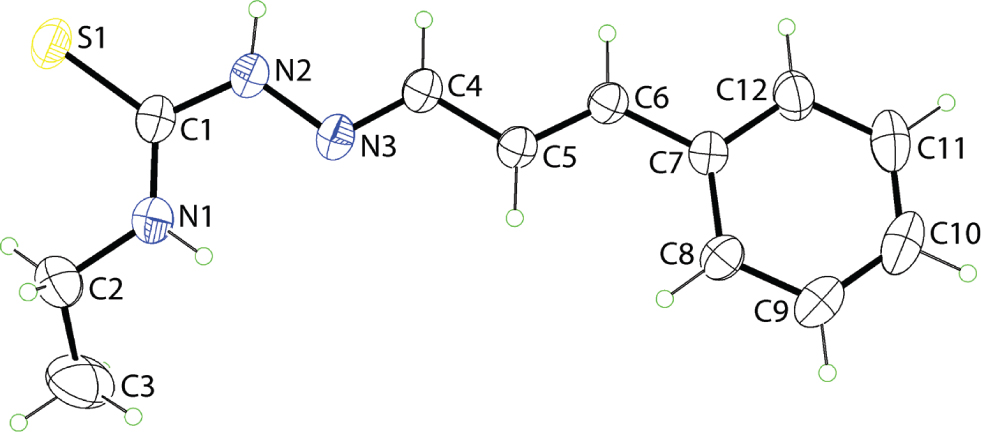
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless slab |
| Size: | 0.50 × 0.50 × 0.20 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.96 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 74.2°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 5668, 2612, 0.024 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2077 |
| N(param)refined: | 153 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.40769(5) | 1.07994(8) | 0.56349(2) | 0.0808(3) |
| N1 | 0.20400(17) | 0.9676(3) | 0.54422(8) | 0.0840(6) |
| H1N | 0.161(2) | 0.895(3) | 0.5314(10) | 0.101* |
| N2 | 0.33773(15) | 0.9067(2) | 0.48914(6) | 0.0625(4) |
| H2N | 0.4060(10) | 0.908(3) | 0.4787(8) | 0.075* |
| N3 | 0.25787(14) | 0.82382(19) | 0.46276(6) | 0.0607(4) |
| C1 | 0.30945(17) | 0.9790(2) | 0.53135(7) | 0.0626(5) |
| C2 | 0.1582(3) | 1.0259(5) | 0.58985(11) | 0.1101(10) |
| H2A | 0.197919 | 1.124233 | 0.599923 | 0.132* |
| H2B | 0.168847 | 0.942470 | 0.614581 | 0.132* |
| C3 | 0.0386(3) | 1.0632(5) | 0.58535(17) | 0.1391(14) |
| H3A | 0.028524 | 1.153114 | 0.563184 | 0.209* |
| H3B | 0.009184 | 1.092909 | 0.616522 | 0.209* |
| H3C | −0.000141 | 0.967978 | 0.573391 | 0.209* |
| C4 | 0.28904(16) | 0.7701(2) | 0.42132(6) | 0.0570(4) |
| H4 | 0.362028 | 0.787942 | 0.410951 | 0.068* |
| C5 | 0.21236(16) | 0.6825(2) | 0.39066(6) | 0.0551(4) |
| H5 | 0.141266 | 0.659052 | 0.402428 | 0.066* |
| C6 | 0.23898(15) | 0.6335(2) | 0.34599(6) | 0.0557(4) |
| H6 | 0.310561 | 0.659404 | 0.335262 | 0.067* |
| C7 | 0.16775(15) | 0.5435(2) | 0.31210(6) | 0.0541(4) |
| C8 | 0.06033(17) | 0.4929(2) | 0.32368(7) | 0.0636(5) |
| H8 | 0.030544 | 0.519669 | 0.353807 | 0.076* |
| C9 | −0.0029(2) | 0.4039(3) | 0.29140(10) | 0.0829(7) |
| H9 | −0.074344 | 0.369578 | 0.300014 | 0.099* |
| C10 | 0.0386(3) | 0.3657(4) | 0.24700(12) | 0.1029(9) |
| H10 | −0.004487 | 0.305024 | 0.225343 | 0.123* |
| C11 | 0.1430(3) | 0.4160(4) | 0.23407(10) | 0.1037(9) |
| H11 | 0.170577 | 0.391110 | 0.203416 | 0.124* |
| C12 | 0.2087(2) | 0.5047(3) | 0.26659(8) | 0.0773(6) |
| H12 | 0.280185 | 0.537972 | 0.257693 | 0.093* |
Source of material
4-Ethyl-3-thiosemicarbazide (1.192 g, 0.01 mol) was dissolved in heated ethanol (50 mL). Cinnamaldehyde (1.50 mL, 0.01 mol) was added into heated ethanolic 4-ethyl-3-thiosemicarbazide while stirring and heating for around 30 mins. The yellow precipitate was filtered, washed with cold ethanol and dried in vacuo. Single crystals were grown at room temperature from the slow evaporation of a mixture of ethanol and acetonitrile (1:2 v/v). Yield: 74%; M. Pt: 446 K. FT-IR (ATR (solid) cm−1): 3317 ν(N—H), 3133 ν(Ar C—H), 3000 ν(=C—H), 2971, 2873 ν(C—H), 1625 ν(C=N), 1545, 1523 ν(Ar C=C), 1084 ν(C=S). 1H NMR (500 MHz, CDCl3): δ 10.00 (s, 1H, N—NH), 7.71 (d, 1H, 4CH), 7.45 (d, 2H, 8CH and 12CH), 7.37–7.30 (m, 4H, C—NH, 9CH, 10CH and 11CH), 6.90 (d, 1H, 6CH), 6.81 (dd, 1H, 5CH), 3.77–3.70 (m, 2H, 2CH2), 1.30 (t, 3H, CH3). 13C{1H} NMR (500 MHz, CDCl3): δ 176.71 (C1), 144.57 (C4), 140.24 (C7), 135.89 (C6), 129.21 (C10), 128.96 (C9 and C11), 127.13 (C8 and C12), 124.39 (C5), 39.40 (CH2), 14.60 (CH3).
Experimental details
The C-bound H atoms were geometrically placed (C—H = 0.93–0.97 Å) and refined as riding with Uiso(H) = 1.2–1.5Ueq(C). The N-bound H atoms were refined with N—H = 0.86 ± 0.01 Å, and with Uiso(H) = 1.2Ueq(N). An extinction correction was applied with the coefficent refining to 0.0055(5).
Comment
Cinnamaldehyde is major component of cinnamon essential oil extracted from a number of cinnamon bark species and along with derivatives is known to exhibit potential biological activities [5], for example, anti-diabetic [6], anti-fungal [7] and anti-microbial activity against various foodborne pathogenic bacteria such as Escherichia coli and Staphylococcus aureus [8]. However, the application of cinnamaldehyde as an anti-microbial agent remains challenging owing to its strong odour and high volatility [9]. The presence of the conjugated double bond in cinnamaldehyde allows for the formation of stable Schiff base compounds after reaction with an amine, chemistry motivated by the desire to decrease the drawbacks associated with cinnamaldehyde’s odour and volatility [10]. In the present report, the synthesis and crystal structure determination of a cinnamaldehyde-based Schiff base compound EtN(H)C(=S)N(H)N=C(H)C(H)=C(H)Ph, (I), is described.
The molecular structure of (I) is shown in the figure (25% displacement ellipsoids) and adopts an extended [all trans] conformation. This is seen in the sequence of C1—N2—N3—C4 [174.56(17)°], N2—N3—C4—C5 [−179.71(15)°], N3—C4—C5—C6 [175.63(17)°] and C4—C5—C6—C7 [179.69(17) Å] torsion angles with the major twist in this part of the molecule observed about the N2—N3 bond. The greatest deviation from planarity in the molecule is observed for the terminal methyl group with the C1—N1—C2—C3 torsion angle being 154.8(3)°. The conformation about each of the C4=N3 [1.279(2) Å] and C5=C6 [1.333(2) Å] double bonds is E. The amide-H atoms lie to either side of the molecule which enables the formation of an intramolecular amide-N—H⋯N(imine) hydrogen bond [N1—H1n⋯N3: H1n⋯N3 = 2.29(3) Å, N1⋯N3 = 2.613(3) Å with angle at H1n = 102(2)°] to close a S(6) loop.
In the crystal of (I), centrosymmetric dimers are formed via amide-N—H⋯S(thione) hydrogen bonds [N2—H2n⋯S1i: H2n⋯S1i = 2.516(15) Å, N2⋯S1i = 3.3752(19) Å with angle at H2n = 171.7(19)° for symmetry operation (i) 1 − x, 2 − y, 1 − z] which lead to the formation of an eight-membered {⋯HNCS}2 synthon. The connections between the aggregates thus formed to form a supramolecular layer in the ab-plane are of the type amide-N—H⋯S(thione) [N1—H1n⋯S1ii: H1n⋯S1ii = 2.83(2) Å, N1⋯S1ii = 3.461(2) Å with the angle at H1n = 132(2)° for (ii) 1 − x, 2 − y, 1 − z] involving the same amide-N—H atom that participates in the intramolecular N—H⋯N hydrogen bond which, therefore, may be considered bifurcated. Additional stability to the layers are provided by a phenyl-C—H⋯S(thione) [C8—H8⋯S1iii: H8⋯S1iii = 2.83 Å, C8⋯S1iii = 3.657(2) Å with angle at H8 = 148° for (iii) −1/2 + x, 3/2 − y, 1 − z] interaction. Despite the phenyl rings projecting to either side of the layers, which stack along the c-axis, no specific interactions between molecules are noted along the c-axis. In order to investigate the nature of the supramolecular association further, the Hirshfeld surfaces were calculated along with the full and delineated two-dimensional fingerprint plots using Crystal Explorer 17 [11] following published procedures [12].
The fingerprint plot delineated into H⋯S/S⋯H contacts show sharp spikes corresponding to the N—H⋯S hydrogen bonds and overall, contribute 14.7% of all contacts to the surface. Reflecting the overall molecular packing, including the nature of the inter-layer region, H⋯H [55.9%] and H⋯C/C⋯H [19.6%] contacts make greater contributions to the surface. The remaining contributions are all small, for example, 3.5% for H⋯N/N⋯H and 2.5% for C⋯C contacts.
Acknowledgements
Sunway University Sdn Bhd is thanked for financial support of this work through Grant No. STR-RCTR-RCCM-001-2019.
References
1. Agilent Technologies. CrysAlisPRO. Agilent Technologies, Santa Clara, CA, USA (2014).Suche in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
4. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 45 (2012) 849–854.10.1107/S0021889812029111Suche in Google Scholar
5. Gruenwald, J.; Freder, J.; Armbruester, N.: Cinnamon and Health. Crit. Rev. Food Sci. Nutr. 50 (2010) 822–834.10.1080/10408390902773052Suche in Google Scholar PubMed
6. Subash Babu, P.; Prabuseenivasan, S.; Ignacimuthu, S.: Cinnamaldehyde – a potential antidiabetic agent. Phytomedicine 14 (2007) 15–22.10.1016/j.phymed.2006.11.005Suche in Google Scholar PubMed
7. Shreaz, S.; Wani, W. A.; Behbehani, J. M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W. A.; Hun, L. T.: Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 112 (2016) 116–131.10.1016/j.fitote.2016.05.016Suche in Google Scholar PubMed
8. Becerril, R.; Gómez-Lus, R.; Goñi, P.; López, P.; Nerín, C.: Combination of analytical and microbiological techniques to study the antimicrobial activity of a new active food packaging containing cinnamon or oregano against E. coli and S. aureus. Anal. Bioanal. Chem. 388 (2007) 1003–1011.10.1007/s00216-007-1332-xSuche in Google Scholar PubMed
9. Feng, K.; Wen, P.; Yang, H.; Li, N.; Lou, W. Y.; Zong, M. H.; Wu, H.: Enhancement of the antimicrobial activity of cinnamon essential oil-loaded electrospun nanofilm by the incorporation of lysozyme. RSC Adv. 7 (2017) 1572–1580.10.1039/C6RA25977DSuche in Google Scholar
10. Arulmurugan, S.; Kavitha, H. P.; Venkatraman, B. R.: Biological activities of schiff base and its complexes: a review. Rasayan J. Chem. 3 (2010) 385–410.Suche in Google Scholar
11. Turner, M. J.; McKinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Spackman, P. R.; Jayatilaka, D.; Spackman, M. A.: Crystal Explorer v17. The University of Western Australia, Australia (2017).Suche in Google Scholar
12. Tan, S. L.; Jotani, M. M.; Tiekink, E. R. T.: Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. E75 (2019) 308–318.10.1107/S2056989019001129Suche in Google Scholar PubMed PubMed Central
©2020 Ming Yueh Tan et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of poly[tetraaqua-bis(μ4-5-(4-carboxy-benzylamino)-isophthalato-κ4O,O′:O′′:O′′′)-(μ2-4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl-κ2N:N′)dicadmium(II)], C25H22N3O8Cd
- The crystal structure of 2-(2-(2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-ium-1-yl)phenoxy)acetate, C19H18N2O3
- Crystal structure of poly[aqua-μ2-4,4′-bipyridine-κ2N:N′)-μ2-bis(2-(2-((2,6-dichlorophenyl)amino)phenyl)acetato-κ2O,O′)zinc(II)], C38H28Cl4N4O4Zn
- Crystal structure of 1-(2-(1H-indol-3-yl)ethyl)-4-benzyl-3-hydroxy-3,6-diphenylpiperazine-2,5-dione, C33H29N3O3
- The crystal structure 2,2′-bipyridine-κ2N,N′-(2-(3-amino-4-chlorobenzoyl)benzoato-κ1O)-(2-(3-amino-4-chlorobenzoyl)benzoato-κ2O,O′)zinc(II) — ethanol (1/1), C40H32Cl2N4O7Zn
- Crystal structure of catena-poly[(μ3-2-carboxy-4-(3-carboxy-5-carboxylatophenoxy)benzoato-κ3O:O′:O′′)-bis(μ2-4,4′-bis(pyrid-4-yl)biphenyl-k1N)copper(II)], C60H40N4O9Cu
- The crystal structure of dimethylammonium catena-[di(μ-aqua)-bis(μ9-benzene-1,3,5-tricarboxylato)pentalithium], C20H16Li5NO13
- Crystal structure of tetraaqua-bis(3,5-di(pyridin-4-yl)-1,2,4-triazol-1-ido-κ1N)nickel(II) dihydrate, C24H28O6N10Ni
- The crystal structure of tetrakis(1-methylimidazole-κ1N)-oxido-(sulfato-κ1O)vanadium(IV), C16H24N8O5SV
- Crystal structure of methyl 2-(6,11-dioxo-2,3,6,11-tetrahydro-1H-benzo[f]pyrrolo[2,1-a]isoindole-5-carbonyl)benzoate, C24H17NO5
- Crystal structure of (E)-N′-(2-hydroxy-4-(2-(piperidin-1-yl)ethoxy)benzylidene) nicotinohydrazide monohydrate, C20H24N4O3 ⋅ H2O
- Crystal structure of poly[bis(μ3-(1-(3,5-di(1H-imidazol-1-yl)phenyl)-1H-imidazole-κ3N:N′:N′′)cobalt(II)] dinitrate — N,N-dimethylformamide (1/4), C42H52N18O10Co
- The crystal structure bis{hexakis(1-methyl-1H-imidazole-κ1N)cobalt(II)} tetrakis(μ3-oxido)-octakis(μ2-oxido)-tetradecaoxido-octamolybdate(VI), C24H36CoMo4N12O13
- Crystal structure of di-μ-nicotinato-κ2N:O; κ2O:N-bis-[aqua-bis(benzyl)(nicotinato-κ2O,O′)tin(IV)], C52H48N4O10Sn2
- Crystal structure of dichlorido-bis[2-(2-(3-(pyridin-2-yl)-1H-1,2,4-triazol-5-yl)phenoxy)benzoic acidmanganese(II) monohydrate, C40H30N8O7MnCl2
- The crystal structure of benzyl 3β-acetylglycyrrhetate, C39H54O5
- Synthesis and crystal structure of (E)-1-benzyl-3-(4-methoxystyryl)quinoxalin-2(1H)-one, C24H20N2O2
- Crystal structure of trans-dichloridobis(4-chlorophenyl-κC1)(1,10-phenanthroline-κ2N,N′)tin(IV) dimethylsulphoxide solvate, C26H22Cl4N2OSSn
- Crystal structure of phenyl(1,3,4a-triphenyl-4a,5,6,10b-tetrahydro-1H-[1,4]oxazino[2,3-c]quinolin-5-yl)methanone, C36H28N2O2
- Crystal structure of (4aS,5S,6aS,6a1S, 10aS)-4a,5,6a,6a1,9,10-hexahydro-7H-4,5-methanocyclobuta[4,5]naphtho[8a,1-b]pyran-6(2H)-one, C15H16O2
- Crystal structure of [(Z)-O-isopropyl N-(4-chlorophenyl)thiocarbamato-κS]-(triphenylphosphine-κP)-gold(I), C28H26AuClNOPS
- Crystal structure of (μ2-1,1′-bis(diphenylphosphino)ferrocene-P,P′)-bis[(Z)-O-isopropyl N-(4-chlorophenyl)thiocarbamato-S]-di-gold(I) acetonitrile di-solvate, C54H50Au2Cl2FeN2O2P2S2⋅2(C2H3N)
- Crystal structure of (6aR,6a1S,10aS)-2,4a,6a,6a1,9,10-hexahydro-7H-4,5-methanocyclobuta[4,5]naphtho[8a,1-b]pyran, C15H16O
- Crystal structure of 5,17-diformyl-25,26,27,28-tetrahydroxycalix[4]arene- dichloromethane, C31H26Cl2O6
- Crystal structure of 2-tert-butyl 1-methyl 5-{4-[(methoxycarbonyl)amino]phenyl}-2,5-dihydro-1H-pyrrole-1,2-dicarboxylate, C19H24N2O6
- Crystal structure of [2-carboxybenzene-1-thiolato-S]-(triethylphosphane-P)-gold(I), C13H20AuO2PS
- Synthesis and crystal structure of bis(5-methyl-2-aldehyde-phenolato-κ2O1,O2)copper(II), C16H14CuO4
- Crystal structure of poly[triaqua-(di(2,2′-bipyridine-κ2N,N′)-μ4-silanetetrayltetrakis(benzene-4,1-diyl)tetrakis (hydrogen phosphonato)-κ4O:O′:O′′:O′′′) dicadmium(II)], C44H42N4O15P4Cd2Si
- Crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)]-bis(triethylphosphine-P)-di-silver(I), C22H50Ag2N2P2S4
- Crystal structure of bis[μ2-(pyrrolidine-1-carbodithioato-κS:κS,κS′)]-bis(triethylphosphine-κP)disilver(I), C22H46Ag2N2P2S4
- Crystal structure of bis[μ2-(N-(2-hydroxyethyl)-N-methylcarbamodithioato-κS:κS,κS′)]-bis(triethylphosphine-P)-di-silver(I), C20H46Ag2N2O2P2S4
- The crystal structure of (2E,2′E)-,2,2′-bis[1-(2-pyrazinyl)ethylidene]carbonimidic dihydrazide, C13H15N9
- The crystal structure of (E)-1-(quinolin-2-ylmethyl)-2-((1-(quinolin-2-ylmethyl)pyridin-2(1H)-ylidene)amino)pyridin-1-ium, C30H25BrN5
- Crystal structure of catena-poly[(μ2-1-((benzotriazol-1-yl)methyl)-1H-1,3-imdazole-κ2N:N′)-(1-((benzotriazol-1-yl)methyl)-1H-1,3-imdazole-κ1N)-(methanol-κ1O)mercury(II)] dinitrate, C21H22N12O7Hg
- Crystal structure of 1-(6-hydroxy-2-phenylbenzofuran-5-yl)ethan-1-one, C16H12O3
- The crystal structure of oxonium hexaquaaluminium disulfate hexahydrate
- Crystal structure of catena{(μ2-1,10-phenanthroline-κ4N,N,N′,N′)-(μ2-1,10-phenanthroline-κ3N,N,N′)potassium(I) {[bis(2-hydroxyethyl)iminiumyl](sulfanidyl)methyl}sulfanide hemi(1,10-phenanthroline)}, {C24H16KN4, 0.5(C12H8N2), C5H10NO2S2}
- Crystal structure of chlorido-[(N,N-di-isobutyl)dithiocarbamato-κ2S,S′]-di(4-methylbenzyl-κC)tin(IV), C25H36ClNS2Sn
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-(4-chloro-4-pyridyl-2,2′:6′,2′′-terpyridine-κ2N,N′) rhodium(III) hexaflourophosphate, C31H29Cl2F6N3PRh
- The crystal structure of catena-poly[bis-(3,5-dinitro-1,2,4-triazolato-κ2N:O)-(μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′)copper(II)], C16H10CuN14O8
- Crystal structure of poly[triaqua-bis(μ3-3,3′-((5-carboxylato-1,3-phenylene)bis(oxy))dibenzoato)-tris(1,10-phenanthroline)cobalt(II)], C78H46N6O20Co3
- The crystal structure of 2,4-dihydroxybenzoic acid–nicotinamide–methanol (1/1/1), C15H18N2O6
- The crystal structure of aqua{N,N,N′,N′-tetrakis[(1H-benzimidazol-κN3) methyl]cyclohexane-1,2-diamine}lead(II) diacetate–methanol (1/2), C44H54N10O7Pb
- Crystal structure of (2-amino-5-bromo-3-iodophenyl)(3-(4-chlorophenyl)oxiran-2-yl)methanone, C15H10BrClINO2
- Synthesis and crystal structure of 3-octyl-5,5-diphenylimidazolidine-2,4-dione, C23H28N2O2
- Synthesis and crystal structure of 2-azido-N-(4-nitrophenyl)acetamide, C8H7N5O3
- Crystal structure of tert-butyl (1S,2R,5R)-2-(hydroxymethyl)-4-(4-methoxyphenyl)-6-oxa-3-azabicyclo[3.1.0]hexane-3-carboxylate, C17H23NO5
- Crystal structure of 4-[(4-methoxy-2-nitrophenyl)carbamoyl]butanoic acid, C12H14N2O6
- Crystal structure of 3-ethyl-1-[(E)-[(2E)-3-phenylprop-2-en-1-ylidene]amino]thiourea, C12H15N3S
- Crystal structure of 4,4′-bipyridin-1,1′-dium poly[bis(μ4-benzene-1,3,5-triyltris(hydrogen phosphonato-κ4O:O′:O′′:O′′′))zinc(II)], C11H11NO9P3Zn
- Crystal structure of (μ2-1,1′-bis(diphenylphosphino)butane-κ2P,P′)-bis[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-κS]-di-gold(I), C44H42Au2F2N2O2P2S2
- Crystal structure of (μ2-1,1′-bis(diphenylphosphino)hexane-κ2P,P′)-bis[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-κS]digold(I), C46H46Au2F2N2O2P2S2
- Crystal structure of tetrakis (N-(2-hydroxyethyl)-N-isopropylcarbamodithioato-κS,S′)-(μ2(2-(pyridin-4-yl)vinyl)pyridine-κN,N′)dicadmium(II), C36H58Cd2N6O4S8
- Crystal structure of 4-(2-(benzo[b]thiophen-2-yl)-3,3,4,4,5,5-hexafluorocyclopent-1-en-1-yl)-1,5-dimethyl-1H-pyrrole-2-carbonitrile, C20H12F6N2S
- Crystal structure of bis(octahydrocyclopenta[c]pyrrolium)pentachlorobismuthate(III), (C7NH14)2BiCl5
- The crystal structure of diaqua-tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(p-pyridyl)imidazoline-1-oxyl 3-oxide-κN)samarium(III), C24H36N9O15Sm
- Synthesis and crystal structure of methyl 2-(2-((tert-butoxycarbonyl)amino)phenyl)-2-(4-oxo-4H-chromen-3-yl)acetate, C23H23NO6
- Crystal structure of O-hexyl benzoylcarbamothioate, C14H19NO2S
- Crystal structure of chlorido-(O-methyl phenylcarbamothioamide-κS)-bis(triphenylphosphane-κP)silver(I), C44H39AgClNOP2S
- Crystal structure of chlorido-(O-ethyl phenylcarbamothioamide-κS)-bis(triphenylphosphane-κP)-silver(I), C45H41AgClNOP2S
- Crystal structure of 4-[(2-methoxyphenyl)carbamoyl]butanoic acid, C12H15NO4
- Crystal structure of ethyl 4-methyl-2-oxo-5-phenyl-1,3,4-oxadiazinane-3-carboxylate, C13H16N2O4
- Crystal structure of catena-poly[diaqua(μ2-2-(hydroxymethyl)-1H-imidazole-4,5-dicarboxylato)cadmium(II)], C6H8CdN2O7
- Crystal structure of (1S)-N-(chloromethyl)-1-((4S,6aR,8aS, 8bR,9aR)-4-methoxy-6a,8a-dimethyl-1,3,4, 5,6,6a,6b,7,8,8a,9a,10,10a,10b-tetradecahydro-8bH-naphtho[2′,1′:4,5] indeno[1,2-b]oxiren-8b-yl)-N-methylethan-1-amine, C24H46ClNO5
- Crystal structure of 4-[(3,5-dichlorophenyl)carbamoyl]butanoic acid, C11H11Cl2NO3
- Crystal structure of (2Z)-2-amino-3-[(E)-[(2,4-dihydroxyphenyl)methylidene]-amino]but-2-enedinitrile, C11H8N4O2
- Crystal structure of 3-methyl-1-[(E)-(4-phenylbutan-2-ylidene)amino]thiourea, C12H17N3S
- Crystal structure of carbonyl{hydridotris[3-phenyl-5-methylpyrazol-1-yl]borato-κ3N,N′N′′}copper(I), C31H28BCuN6O
- Crystal structure of ethane-1,2-diylbis(diphenylphosphine oxide) – dihydrogenperoxide (1/2), C26H28O6P2
- Crystal structure of 2-(pyridin-2-ylamino)pyridinium chloride dibenzyldichlorostannane, [C10H10N3]Cl, C14H14Cl2Sn
- Crystal structure of 4-[(3-methoxyphenyl)carbamoyl]butanoic acid, C12H15NO4
- Crystal structure of dichlorido-bis(tri-4-tolylphosphane oxide-κO)-di(4-chlorophenyl-κC)tin(IV), C54H50Cl4O2P2Sn
- Crystal structure of dichloridodimethylbis(tri-4-tolylphosphane oxide-κO)-tin(IV), C44H48Cl2O2P2Sn
- Crystal structure of chlorido(2-methylquinolin-8-olato-κ2N,O)-bis(4-tolyl-κC)tin(IV), C24H22ClNOSn
- Crystal structure of (E)-dichloro(1-chloro-3-methoxyprop-1-en-2-yl)(4-methoxyphenyl)-λ4-tellane, C11H13Cl3O2Te
- Crystal structure of bis{N-methyl-N′-[3-(4-methoxyphenyl)-1-methylpropane-1-ylidene]carbamohydrazonothioato}zinc(II), C26H36N6O2S2Zn
- Crystal structure of (2-carboxy-4-(3-carboxy-5-carboxylatophenoxy)benzoato-κ2O,O′)bis(1,10-phenantroline-κ2N,N′)cobalt(II), C40H24N4O9Co
- The crystal structure of (3S,8R,10R,14R)-17-((2S,5S)-5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)-4,4,8,10,14-pentamethyl-12-oxohexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H52O5
- Crystal structure of (μ2-1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-bis[(Z)N-(3-fluorophenyl)-O-methylthiocarbamato-S]digold(I) chloroform solvate, C50H42Au2F2FeN2O2P2S2, CHCl3
- Crystal structure of poly[bis(μ2-1,4-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)cobalt(II)], C24H20N8O4MoCo
Artikel in diesem Heft
- Frontmatter
- Crystal structure of poly[tetraaqua-bis(μ4-5-(4-carboxy-benzylamino)-isophthalato-κ4O,O′:O′′:O′′′)-(μ2-4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl-κ2N:N′)dicadmium(II)], C25H22N3O8Cd
- The crystal structure of 2-(2-(2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-ium-1-yl)phenoxy)acetate, C19H18N2O3
- Crystal structure of poly[aqua-μ2-4,4′-bipyridine-κ2N:N′)-μ2-bis(2-(2-((2,6-dichlorophenyl)amino)phenyl)acetato-κ2O,O′)zinc(II)], C38H28Cl4N4O4Zn
- Crystal structure of 1-(2-(1H-indol-3-yl)ethyl)-4-benzyl-3-hydroxy-3,6-diphenylpiperazine-2,5-dione, C33H29N3O3
- The crystal structure 2,2′-bipyridine-κ2N,N′-(2-(3-amino-4-chlorobenzoyl)benzoato-κ1O)-(2-(3-amino-4-chlorobenzoyl)benzoato-κ2O,O′)zinc(II) — ethanol (1/1), C40H32Cl2N4O7Zn
- Crystal structure of catena-poly[(μ3-2-carboxy-4-(3-carboxy-5-carboxylatophenoxy)benzoato-κ3O:O′:O′′)-bis(μ2-4,4′-bis(pyrid-4-yl)biphenyl-k1N)copper(II)], C60H40N4O9Cu
- The crystal structure of dimethylammonium catena-[di(μ-aqua)-bis(μ9-benzene-1,3,5-tricarboxylato)pentalithium], C20H16Li5NO13
- Crystal structure of tetraaqua-bis(3,5-di(pyridin-4-yl)-1,2,4-triazol-1-ido-κ1N)nickel(II) dihydrate, C24H28O6N10Ni
- The crystal structure of tetrakis(1-methylimidazole-κ1N)-oxido-(sulfato-κ1O)vanadium(IV), C16H24N8O5SV
- Crystal structure of methyl 2-(6,11-dioxo-2,3,6,11-tetrahydro-1H-benzo[f]pyrrolo[2,1-a]isoindole-5-carbonyl)benzoate, C24H17NO5
- Crystal structure of (E)-N′-(2-hydroxy-4-(2-(piperidin-1-yl)ethoxy)benzylidene) nicotinohydrazide monohydrate, C20H24N4O3 ⋅ H2O
- Crystal structure of poly[bis(μ3-(1-(3,5-di(1H-imidazol-1-yl)phenyl)-1H-imidazole-κ3N:N′:N′′)cobalt(II)] dinitrate — N,N-dimethylformamide (1/4), C42H52N18O10Co
- The crystal structure bis{hexakis(1-methyl-1H-imidazole-κ1N)cobalt(II)} tetrakis(μ3-oxido)-octakis(μ2-oxido)-tetradecaoxido-octamolybdate(VI), C24H36CoMo4N12O13
- Crystal structure of di-μ-nicotinato-κ2N:O; κ2O:N-bis-[aqua-bis(benzyl)(nicotinato-κ2O,O′)tin(IV)], C52H48N4O10Sn2
- Crystal structure of dichlorido-bis[2-(2-(3-(pyridin-2-yl)-1H-1,2,4-triazol-5-yl)phenoxy)benzoic acidmanganese(II) monohydrate, C40H30N8O7MnCl2
- The crystal structure of benzyl 3β-acetylglycyrrhetate, C39H54O5
- Synthesis and crystal structure of (E)-1-benzyl-3-(4-methoxystyryl)quinoxalin-2(1H)-one, C24H20N2O2
- Crystal structure of trans-dichloridobis(4-chlorophenyl-κC1)(1,10-phenanthroline-κ2N,N′)tin(IV) dimethylsulphoxide solvate, C26H22Cl4N2OSSn
- Crystal structure of phenyl(1,3,4a-triphenyl-4a,5,6,10b-tetrahydro-1H-[1,4]oxazino[2,3-c]quinolin-5-yl)methanone, C36H28N2O2
- Crystal structure of (4aS,5S,6aS,6a1S, 10aS)-4a,5,6a,6a1,9,10-hexahydro-7H-4,5-methanocyclobuta[4,5]naphtho[8a,1-b]pyran-6(2H)-one, C15H16O2
- Crystal structure of [(Z)-O-isopropyl N-(4-chlorophenyl)thiocarbamato-κS]-(triphenylphosphine-κP)-gold(I), C28H26AuClNOPS
- Crystal structure of (μ2-1,1′-bis(diphenylphosphino)ferrocene-P,P′)-bis[(Z)-O-isopropyl N-(4-chlorophenyl)thiocarbamato-S]-di-gold(I) acetonitrile di-solvate, C54H50Au2Cl2FeN2O2P2S2⋅2(C2H3N)
- Crystal structure of (6aR,6a1S,10aS)-2,4a,6a,6a1,9,10-hexahydro-7H-4,5-methanocyclobuta[4,5]naphtho[8a,1-b]pyran, C15H16O
- Crystal structure of 5,17-diformyl-25,26,27,28-tetrahydroxycalix[4]arene- dichloromethane, C31H26Cl2O6
- Crystal structure of 2-tert-butyl 1-methyl 5-{4-[(methoxycarbonyl)amino]phenyl}-2,5-dihydro-1H-pyrrole-1,2-dicarboxylate, C19H24N2O6
- Crystal structure of [2-carboxybenzene-1-thiolato-S]-(triethylphosphane-P)-gold(I), C13H20AuO2PS
- Synthesis and crystal structure of bis(5-methyl-2-aldehyde-phenolato-κ2O1,O2)copper(II), C16H14CuO4
- Crystal structure of poly[triaqua-(di(2,2′-bipyridine-κ2N,N′)-μ4-silanetetrayltetrakis(benzene-4,1-diyl)tetrakis (hydrogen phosphonato)-κ4O:O′:O′′:O′′′) dicadmium(II)], C44H42N4O15P4Cd2Si
- Crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)]-bis(triethylphosphine-P)-di-silver(I), C22H50Ag2N2P2S4
- Crystal structure of bis[μ2-(pyrrolidine-1-carbodithioato-κS:κS,κS′)]-bis(triethylphosphine-κP)disilver(I), C22H46Ag2N2P2S4
- Crystal structure of bis[μ2-(N-(2-hydroxyethyl)-N-methylcarbamodithioato-κS:κS,κS′)]-bis(triethylphosphine-P)-di-silver(I), C20H46Ag2N2O2P2S4
- The crystal structure of (2E,2′E)-,2,2′-bis[1-(2-pyrazinyl)ethylidene]carbonimidic dihydrazide, C13H15N9
- The crystal structure of (E)-1-(quinolin-2-ylmethyl)-2-((1-(quinolin-2-ylmethyl)pyridin-2(1H)-ylidene)amino)pyridin-1-ium, C30H25BrN5
- Crystal structure of catena-poly[(μ2-1-((benzotriazol-1-yl)methyl)-1H-1,3-imdazole-κ2N:N′)-(1-((benzotriazol-1-yl)methyl)-1H-1,3-imdazole-κ1N)-(methanol-κ1O)mercury(II)] dinitrate, C21H22N12O7Hg
- Crystal structure of 1-(6-hydroxy-2-phenylbenzofuran-5-yl)ethan-1-one, C16H12O3
- The crystal structure of oxonium hexaquaaluminium disulfate hexahydrate
- Crystal structure of catena{(μ2-1,10-phenanthroline-κ4N,N,N′,N′)-(μ2-1,10-phenanthroline-κ3N,N,N′)potassium(I) {[bis(2-hydroxyethyl)iminiumyl](sulfanidyl)methyl}sulfanide hemi(1,10-phenanthroline)}, {C24H16KN4, 0.5(C12H8N2), C5H10NO2S2}
- Crystal structure of chlorido-[(N,N-di-isobutyl)dithiocarbamato-κ2S,S′]-di(4-methylbenzyl-κC)tin(IV), C25H36ClNS2Sn
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-(4-chloro-4-pyridyl-2,2′:6′,2′′-terpyridine-κ2N,N′) rhodium(III) hexaflourophosphate, C31H29Cl2F6N3PRh
- The crystal structure of catena-poly[bis-(3,5-dinitro-1,2,4-triazolato-κ2N:O)-(μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N′)copper(II)], C16H10CuN14O8
- Crystal structure of poly[triaqua-bis(μ3-3,3′-((5-carboxylato-1,3-phenylene)bis(oxy))dibenzoato)-tris(1,10-phenanthroline)cobalt(II)], C78H46N6O20Co3
- The crystal structure of 2,4-dihydroxybenzoic acid–nicotinamide–methanol (1/1/1), C15H18N2O6
- The crystal structure of aqua{N,N,N′,N′-tetrakis[(1H-benzimidazol-κN3) methyl]cyclohexane-1,2-diamine}lead(II) diacetate–methanol (1/2), C44H54N10O7Pb
- Crystal structure of (2-amino-5-bromo-3-iodophenyl)(3-(4-chlorophenyl)oxiran-2-yl)methanone, C15H10BrClINO2
- Synthesis and crystal structure of 3-octyl-5,5-diphenylimidazolidine-2,4-dione, C23H28N2O2
- Synthesis and crystal structure of 2-azido-N-(4-nitrophenyl)acetamide, C8H7N5O3
- Crystal structure of tert-butyl (1S,2R,5R)-2-(hydroxymethyl)-4-(4-methoxyphenyl)-6-oxa-3-azabicyclo[3.1.0]hexane-3-carboxylate, C17H23NO5
- Crystal structure of 4-[(4-methoxy-2-nitrophenyl)carbamoyl]butanoic acid, C12H14N2O6
- Crystal structure of 3-ethyl-1-[(E)-[(2E)-3-phenylprop-2-en-1-ylidene]amino]thiourea, C12H15N3S
- Crystal structure of 4,4′-bipyridin-1,1′-dium poly[bis(μ4-benzene-1,3,5-triyltris(hydrogen phosphonato-κ4O:O′:O′′:O′′′))zinc(II)], C11H11NO9P3Zn
- Crystal structure of (μ2-1,1′-bis(diphenylphosphino)butane-κ2P,P′)-bis[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-κS]-di-gold(I), C44H42Au2F2N2O2P2S2
- Crystal structure of (μ2-1,1′-bis(diphenylphosphino)hexane-κ2P,P′)-bis[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-κS]digold(I), C46H46Au2F2N2O2P2S2
- Crystal structure of tetrakis (N-(2-hydroxyethyl)-N-isopropylcarbamodithioato-κS,S′)-(μ2(2-(pyridin-4-yl)vinyl)pyridine-κN,N′)dicadmium(II), C36H58Cd2N6O4S8
- Crystal structure of 4-(2-(benzo[b]thiophen-2-yl)-3,3,4,4,5,5-hexafluorocyclopent-1-en-1-yl)-1,5-dimethyl-1H-pyrrole-2-carbonitrile, C20H12F6N2S
- Crystal structure of bis(octahydrocyclopenta[c]pyrrolium)pentachlorobismuthate(III), (C7NH14)2BiCl5
- The crystal structure of diaqua-tris(nitrato-κ2O,O′)-bis(4,4,5,5-tetramethyl-2-(p-pyridyl)imidazoline-1-oxyl 3-oxide-κN)samarium(III), C24H36N9O15Sm
- Synthesis and crystal structure of methyl 2-(2-((tert-butoxycarbonyl)amino)phenyl)-2-(4-oxo-4H-chromen-3-yl)acetate, C23H23NO6
- Crystal structure of O-hexyl benzoylcarbamothioate, C14H19NO2S
- Crystal structure of chlorido-(O-methyl phenylcarbamothioamide-κS)-bis(triphenylphosphane-κP)silver(I), C44H39AgClNOP2S
- Crystal structure of chlorido-(O-ethyl phenylcarbamothioamide-κS)-bis(triphenylphosphane-κP)-silver(I), C45H41AgClNOP2S
- Crystal structure of 4-[(2-methoxyphenyl)carbamoyl]butanoic acid, C12H15NO4
- Crystal structure of ethyl 4-methyl-2-oxo-5-phenyl-1,3,4-oxadiazinane-3-carboxylate, C13H16N2O4
- Crystal structure of catena-poly[diaqua(μ2-2-(hydroxymethyl)-1H-imidazole-4,5-dicarboxylato)cadmium(II)], C6H8CdN2O7
- Crystal structure of (1S)-N-(chloromethyl)-1-((4S,6aR,8aS, 8bR,9aR)-4-methoxy-6a,8a-dimethyl-1,3,4, 5,6,6a,6b,7,8,8a,9a,10,10a,10b-tetradecahydro-8bH-naphtho[2′,1′:4,5] indeno[1,2-b]oxiren-8b-yl)-N-methylethan-1-amine, C24H46ClNO5
- Crystal structure of 4-[(3,5-dichlorophenyl)carbamoyl]butanoic acid, C11H11Cl2NO3
- Crystal structure of (2Z)-2-amino-3-[(E)-[(2,4-dihydroxyphenyl)methylidene]-amino]but-2-enedinitrile, C11H8N4O2
- Crystal structure of 3-methyl-1-[(E)-(4-phenylbutan-2-ylidene)amino]thiourea, C12H17N3S
- Crystal structure of carbonyl{hydridotris[3-phenyl-5-methylpyrazol-1-yl]borato-κ3N,N′N′′}copper(I), C31H28BCuN6O
- Crystal structure of ethane-1,2-diylbis(diphenylphosphine oxide) – dihydrogenperoxide (1/2), C26H28O6P2
- Crystal structure of 2-(pyridin-2-ylamino)pyridinium chloride dibenzyldichlorostannane, [C10H10N3]Cl, C14H14Cl2Sn
- Crystal structure of 4-[(3-methoxyphenyl)carbamoyl]butanoic acid, C12H15NO4
- Crystal structure of dichlorido-bis(tri-4-tolylphosphane oxide-κO)-di(4-chlorophenyl-κC)tin(IV), C54H50Cl4O2P2Sn
- Crystal structure of dichloridodimethylbis(tri-4-tolylphosphane oxide-κO)-tin(IV), C44H48Cl2O2P2Sn
- Crystal structure of chlorido(2-methylquinolin-8-olato-κ2N,O)-bis(4-tolyl-κC)tin(IV), C24H22ClNOSn
- Crystal structure of (E)-dichloro(1-chloro-3-methoxyprop-1-en-2-yl)(4-methoxyphenyl)-λ4-tellane, C11H13Cl3O2Te
- Crystal structure of bis{N-methyl-N′-[3-(4-methoxyphenyl)-1-methylpropane-1-ylidene]carbamohydrazonothioato}zinc(II), C26H36N6O2S2Zn
- Crystal structure of (2-carboxy-4-(3-carboxy-5-carboxylatophenoxy)benzoato-κ2O,O′)bis(1,10-phenantroline-κ2N,N′)cobalt(II), C40H24N4O9Co
- The crystal structure of (3S,8R,10R,14R)-17-((2S,5S)-5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)-4,4,8,10,14-pentamethyl-12-oxohexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H52O5
- Crystal structure of (μ2-1,1′-bis(diphenylphosphino)ferrocene-κ2P,P′)-bis[(Z)N-(3-fluorophenyl)-O-methylthiocarbamato-S]digold(I) chloroform solvate, C50H42Au2F2FeN2O2P2S2, CHCl3
- Crystal structure of poly[bis(μ2-1,4-di(1H-imidazol-1-yl)benzene-κ2N:N′)-(μ2-tetraoxidomolybdato(VI)-κ2O:O′)cobalt(II)], C24H20N8O4MoCo

