Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
-
Muhammad Ikram
, Bilal Javed
, Zia-ur-Rehman Mashwani
Abstract
The present study was aimed to biosynthesize selenium nanoparticles (SeNPs) and assess their foliar applications to improve the growth of wheat plants under controlled irrigation and drought stress. Bud aqueous extract of Allium sativum L. was used as a reducing and stabilizing agent of SeNPs followed by their optical and morphological characterization by using ultraviolet-visible spectroscopy, scanning electron microscopy, Fourier-transform infrared, and energy dispersive X-ray analysis. Various concentrations of SeNPs (10, 20, 30, and 40 mg/L) were applied exogenously to drought-tolerant (V1) and drought-susceptible (V2) wheat varieties at the trifoliate stage. Under the positive control conditions, plants were irrigated with 450 mL of water/pot (100% field capacity); and under water-deficit environment, plants were irrigated with 160 mL of water/pot (35% field capacity). Remarkable increase in plant height, shoot length, shoot fresh weight, shoot dry weight, root length, root fresh weight, root dry weight, leaf area, leaf number, and leaf length has been observed when 30 mg/L concentration of SeNPs was used. However, the plant morphological parameters decreased gradually at higher concentrations (40 mg/L) in both selected wheat varieties. Therefore, 30 mg/L concentration of SeNPs was found most preferable to enhance the growth of selected wheat varieties under normal and water-deficient conditions.
1 Introduction
Good agriculture practices of an area help economic growth, ensure food security, reduce inflation, and improve the livelihood of farmers and the associated people. Poor agriculture practices include wrong seed selection and the use of low-grade pesticides and fertilizers along with drastic environmental conditions, affecting crop production and yield greatly [1,2]. Drought is an abiotic stress that affects crops worldwide. Drought is considered a main cause to affect the crop yield and grain quality and results in the accumulation of various heavy metals into plant bodies that make them unusable for humans and cause various health issues [3,4,5]. The other drastic abiotic environmental factors include extreme salinity, severe water-deficit soil, and harsh temperature that adversely affect the development and growth of the wheat crop. It also causes pollen sterility, produces shriveled seeds in wheat, disturbs photosynthetic and respiratory enzymes, and leads to the excessive production of reactive oxygen species (ROS) that pose detrimental impacts on plasma cell membrane lipids, deoxyribonucleic acids, carbohydrates, and protein contents and finally induce oxidative stress in plant species [1,6,7].
Wheat is considered an important staple food worldwide and is ranked as the third most important crop after rice and corn. Wheat and its products are used in various countries of the world including Pakistan, India, Sri Lanka, Bangladesh, and Nepal. Among all other abiotic stresses, drought is one of the destructive natural disasters for terrestrial ecosystems with the characteristics of severe influence, high frequency, and extensive coverage [6,8]. It indirectly results in economic losses worldwide. Extreme dry conditions due to increasing temperature could be an essential concern for the production of the wheat crop in South Asian countries such as India, Pakistan, Bangladesh, and Nepal [4,6,8,9]. A prolonged and severe water deficit results in a significant decline in the production of crops and disturbs food supply maintenance [8,10]. Drought stress brings about depletion in plant growth rate, stomatal movements, leaf expansion, and stem elongation. Furthermore, it changes several biochemical and physiological processes governing plant growth and productivity [6].
To overcome the unfavorable impacts of biotic and abiotic stress on wheat plants, different approaches, i.e., genetic engineering, quantitative trait locus mapping, molecular marker-assisted selection, and hybridization, are usually in use [11]. These innovative techniques have some disadvantages that require technical expertise and operation procedures and are not cost-effective. The current situation requires a reasonable, viable, and practical strategy having the ability to outperform these inadequacies. Among these technological innovations, nanobiotechnology has attained a prominent position due to its various applications in agricultural ecosystem maintenance [12].
Nanobiotechnology has extensive implementations in the discipline of climate change, sustainability, crop productivity and food security and is being utilized for creating various tools sets, i.e., nano-pesticides, nano-fertilizers, nano-herbicides, nano-sensor, and smart nanomachines for controlled and maintained discharge of agrochemicals [13]. Among various NPs, biosynthesized SeNPs are nontoxic and biocompatible compared to the counterparts, selenate (SeO42−) and selenite (SeO32−) [14,15]. Synthesis of SeNPs by using plant extract is safe and very inexpensive and employs environment-friendly nontoxic materials [16,17]. Additionally, SeNPs stimulate root growth and organogenesis. Trace amounts of selenium have been reported to stimulate growth in lettuce, ryegrass, Brassica oleracea, and potato plants [18]. However, no report is available on the differential effects of biosynthesized SeNPs on different wheat varieties under control irrigation and drought stress. The present investigation aimed to study the effects of different concentrations of biosynthesized SeNPs on morphological or agronomic parameters of the selected wheat varieties under controlled irrigation and drought stress.
2 Materials and methods
2.1 Preparation of Allium sativum aqueous extract
Buds of the Allium sativum were collected and washed with the distilled water to eliminate dust particles. After drying at the room temperature, 20 g of buds was crushed and a thick paste was formed with the distilled water [19]. This paste was filtered by using the Whatman No. 1 filter paper. The resulting liquid was used to reduce the selenium salt into Se° [20,21].
2.2 Biosynthesis of SeNPs by using Allium sativum extract
A flask containing 25 mL of Na2SeO3 solution (5 mM) was kept on a magnetic stirrer. Followed by the dropwise addition of Allium sativum aqueous extract until the color of the reaction mixture changed to the brick red which is a characteristic of SeNPs synthesis. After the synthesis of SeNPs, the resultant reaction mixture was centrifuged at 14,000 × g for 15 min to separate the NPs from crude plant extract. The NPs were resuspended in distilled water and recentrifuged thrice. After purification, the NPs were collected and dried on filter paper for further experimentation [16].
2.3 Characterization of biosynthesized SeNPs
The morphological and optical characterization of SeNPs was performed by using various material characterization techniques, e.g., ultraviolet-visible (UV-Vis) spectrophotometer, scanning electron microscopy (SEM) Fourier-transform infrared spectroscopy (FTIR), and energy dispersive X-ray (EDX) analysis. The UV-Vis spectrophotometry (Systronics HALO DB-20, Dynamica Scientific Ltd.) was used to confirm the synthesis of SeNPs by measuring the wavelength of the reaction sample in the range of 200–800 nm of the light wavelength [22]. FTIR investigation was performed in the range of 400–4,000 cm−1 by employing an FTIR spectrometer with a resolution of 0.15 cm−1 to identify the functional groups that are responsible to stabilize SeNPs [23]. Morphological analysis of the synthesized SeNPs was done by using an SEM (SIGMA model, Zeiss, Germany) at 15 kV. The sample was prepared on copper grids and dried in a vacuum chamber before placing it in an SEM holder. The EDX analysis was carried out on EDX XL-30 working at 15–25 keV [24].
2.4 Selection of plant material, growth conditions, and site description
The healthy seeds of the selected wheat varieties (Pakistan-2013 and NARC-2011) were acquired from the National Agriculture and Research Council of Pakistan. The wheat variety Pakistan-2013 is drought tolerant (V1) and NARC-2011 is drought susceptible (V2). The seeds were sterilized by using a 10% of sodium hypochlorite solution for 15 min followed by washing with double-distilled water [25]. The research experiment was carried out from November 2019 to February 2020 within an experimental zone (Glasshouse) at PMAS-Arid Agriculture University Rawalpindi. The temperature was maintained at 22 ± 2.4°C, a midday photosynthetic photon flux density of 398 ± 7.2 µmol m−2 s−2 while the relative humidity was maintained between 32% and 61%.
The plants were grown in special pots (24 cm in diameter and 19 cm height) filled with sandy loam soil (sand: 44.2%, silt: 4.6%, and clay: 51.2%). To measure pot’s water-holding capacity all pots were drenched with the tap water and drained overnight. The soil relative water content was calculated by using the following formula [4]:
2.5 Experimental design and application of treatments
Plants of both selected wheat varieties were divided into the following 10 major groups: control irrigation (positive control condition) without foliar application of selenium NPs (T1+), drought condition (negative control condition) without foliar application of SeNPs (T2−), control irrigation with 10 mg/L foliar application of SeNPs (T3+), control irrigation with 20 mg/L foliar application of SeNPs (T4+), control irrigation with 30 mg/L foliar application of SeNPs (T5+), control irrigation with 40 mg/L foliar application of SeNPs (T6+), drought condition with 10 mg/L foliar application of SeNPs (T7−), drought condition with 20 mg/L foliar application of SeNPs (T8−), drought condition with 30 mg/L foliar application of SeNPs (T9−), and drought condition with 40 mg/L foliar application of SeNPs (T10−) [8]. Each group was comprised of four plants. Under the positive control conditions, plants were irrigated with 450 mL of water/pot (100% field capacity); while under the negative control conditions, they were irrigated with 160 mL of water/pot (35% field capacity). Various concentrations of biosynthesized SeNPs (10, 20, 30, and 40 mg/L) were applied exogenously to the selected wheat varieties at the trifoliate stage (Table 1) [10].
The layout of the treatments of the wheat plants under drought and controlled irrigation
| Treatment code | Conditions |
|---|---|
| T1+ | Control irrigation + 0 mg/L of SeNPs |
| T2− | Drought condition + 0 mg/L of SeNPs |
| T3+ | Control irrigation + 10 mg/L of SeNPs |
| T4+ | Control irrigation + 20 mg/L of SeNPs |
| T5+ | Control irrigation + 30 mg/L of SeNPs |
| T6+ | Control irrigation + 40 mg/L of SeNPs |
| T7− | Drought condition + 10 mg/L of SeNPs |
| T8− | Drought condition + 20 mg/L of SeNPs |
| T9− | Drought condition + 30 mg/L of SeNPs |
| T10− | Drought condition + 40 mg/L of SeNPs |
2.6 Collection of data for growth parameters
Different morphological or agronomic parameters were examined after 3 months through a random sampling strategy. The morphological attributes included root, leaf and shoot length, root and shoot dry and fresh weight, plant height, leaf number, and leaf area. Almost four plants from every treatment were used for the measurement of growth parameters [8].
2.7 Analysis of selected growth parameters
To analyze the plant height and root, shoot, and leaf length, nearly four plants were collected randomly from every treatment and washed carefully. The length of different plant parts was measured in centimeter (cm) by using a ruler. The plant’s root and shoot fresh weight were measured and then oven-dried at 64°C for 12 h to analyze the dry weight. The leaf area meter (CID, CI-202) was used to calculate the leaf area of the respective sample from each treatment. Similarly, four plants from all respective treatments of both wheat varieties were chosen and the number of leaves per plant was examined carefully [8].
2.8 Statistical analysis
Two-way analysis of variance (ANOVA) was applied to analyze the difference between treatments and varieties using SPSS software (SPSS 20.1). Significant differences in resulting data were recognized at P < 0.05 level by utilizing Duncan’s multiple range test [8].
3 Results and discussion
3.1 Optical and morphological characterization of biosynthesized SeNPs
SeNPs were synthesized from Na2SeO3 through an eco-friendly approach by using Allium sativum bud’s aqueous extract as an effective stabilizing and reducing agent. The dropwise addition of Allium sativum bud extract in the Na2SeO3 solution resulted in the color change of the reaction mixture to light yellow and finally attained a brick red color (Figure 1a and b), which indicates the biosynthesis of SeNPs. Our observations are following the findings of Satgurunathan et al. [16].
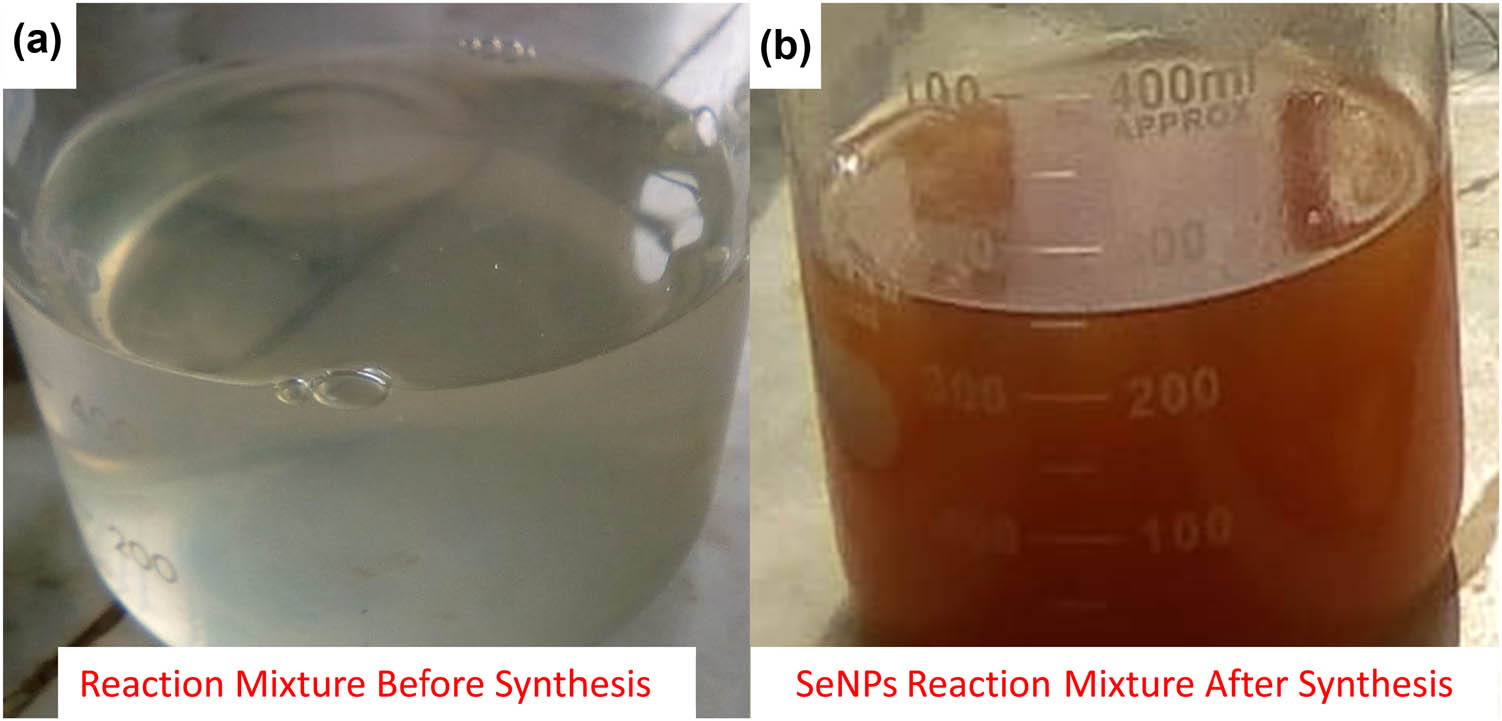
(a) Change in the color of reaction mixture before synthesis and (b) after the synthesis of SeNPs.
3.1.1 Spectrophotometric analysis of SeNPs
The visual color change was considered as an evidence of the reduction of the selenium ions into SeNPs. The change in the color of the reaction mixture is the response of the interaction of the SeNPs with the wavelength of light which was measured in the form of surface plasmon resonance (SPR) band by using spectrophotometry [26]. The spectral measurement was performed within the range of about 200–800 nm of light wavelength. The characteristic peak was acquired between 200 and 400 nm of the light wavelength. The maximum absorption peak was obtained at 357 nm with another peak at 257 nm which indicates the SPR characteristic of SeNPs (Figure 2a). The peak at 257 nm can be attributed to the small-sized SeNPs and the resulting decline in the absorption peak suggests the aggregation of synthesized NPs. Our findings are in accordance with Lin et al. [17]. According to the work of another researcher, the plant extract (Terminalia arjuna L.) was used and the SPR band has been observed at 390 nm which is in favor of our findings [27].
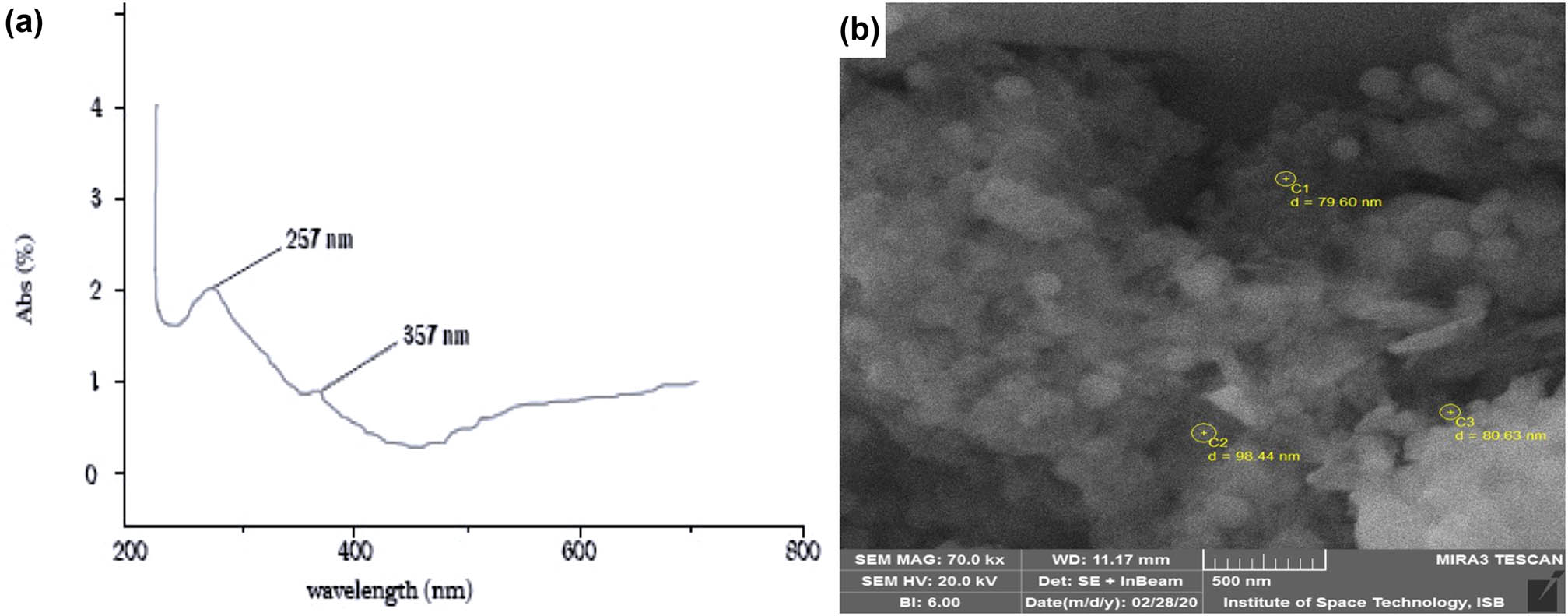
(a) UV-Visible spectrum showing the characteristic SPR band at 257 nm confirming the synthesis of SeNPs and (b) SEM image is showing that nanoparticles are anisotropic and irregular in shape and have a size range of 50–150 nm.
3.1.2 SEM analysis of SeNPs
The SEM images of the biosynthesized SeNPs showed that they are nearly spherical, rectangular, or cylindrical (Figure 2b). Some nanostructures were anisotropic and irregular in shape. Few nanostructures were fused and appeared in the form of small nanoclusters. Most of the nanostructures were observed in the size range of 50–150 nm. Our results are in accordance with the previously published work [16,28].
3.1.3 EDX spectroscopic analysis of SeNPs
EDX spectroscopic analysis gives the qualitative and quantitative detail of the elements that may be involved in the synthesis of SeNPs. The strong signal of the atomic selenium between characteristic 2.5 and 3.0 KeV confirmed the presence of the Se while the intensity of the signal was recorded as 50%. Other elements such as sodium, carbon, oxygen, aluminum, silicon, and copper were found in the trace amounts. Figure 3a shows the elemental profile of the biosynthesized SeNPs by using the Allium sativum aqueous extract.
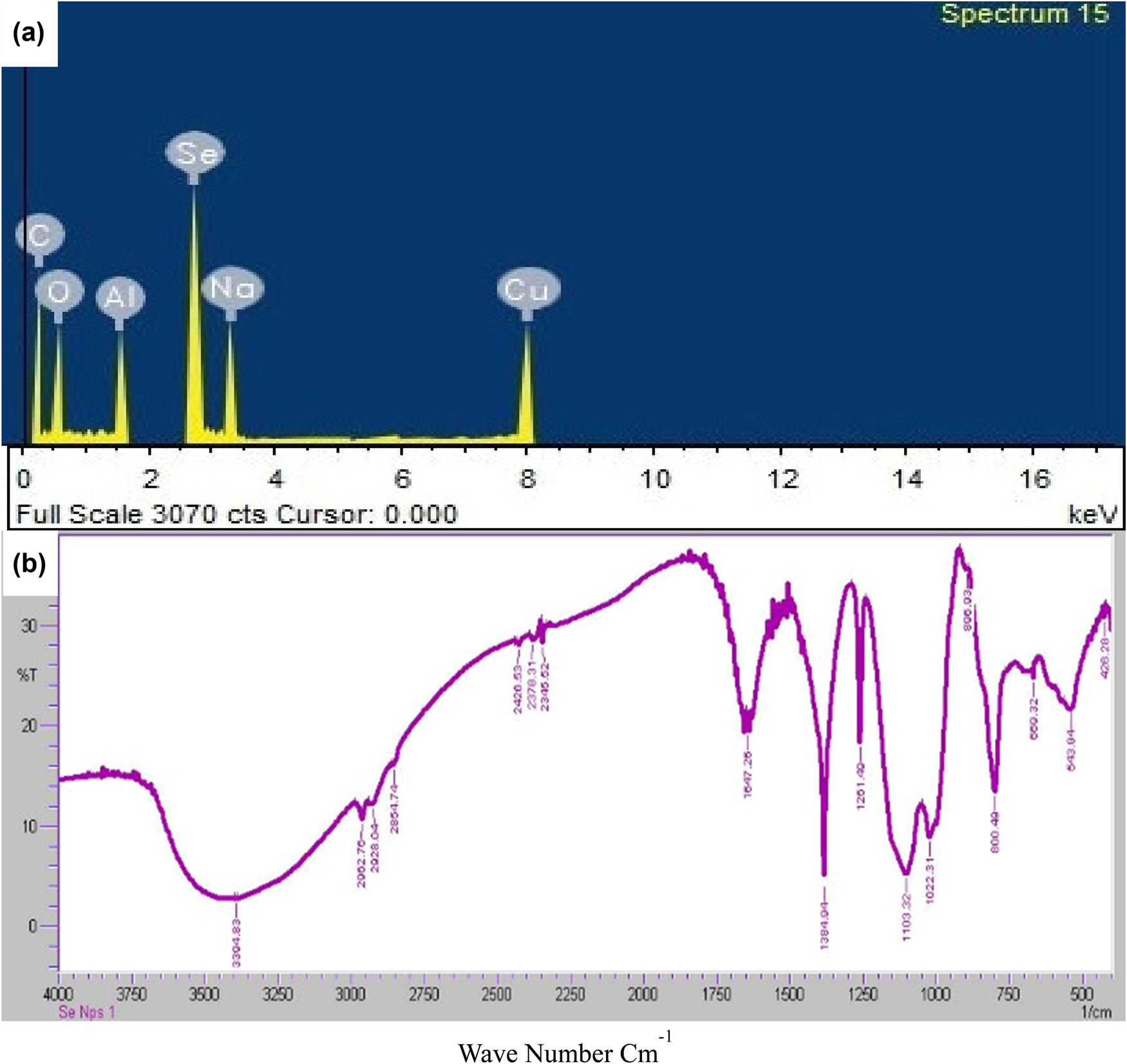
(a) Elemental composition analysis of SeNPs; (b) FTIR spectrum is showing different potential functional groups responsible to cap or stabilize SeNPs.
3.1.4 FTIR spectroscopic analysis of SeNPs
The FTIR analysis was used to characterize the presence of potential functional groups responsible for the synthesis and stability of the biosynthesized SeNPs. Figure 3b showed the transmittance of the peak at 3,200–3,400 cm−1 of wave number that may be due to the presence of N–H and O–H groups. Essentially, the peak at 2,854.74 cm−1 can be assumed as the absorption peak of carbon and hydrogen stretch (C–H). The peaks around 1,500–1,800 cm−1 can be allotted as the peaks of C═N, C═O, and C═C. The spectrum absorption peak at 1,384.94 cm−1 may show the presence of the nitrogen and oxygen groups. The peaks around 1,100 to 1,200 cm−1 were ascribed to the carboxyl group (C═O). The peaks at 1,000 and 1,100 cm−1 may be due to the presence of carbon and oxygen groups. Peaks near 700 cm−1 showed the occurrence of carbon and hydrogen medium bending. The peaks around 500 and 600 cm−1 may be due to the halfway deuteriation of the amine or carboxyl group. The FTIR results indicate that the CO carboxyl group and NO– a nitro group of Allium sativum extract are responsible for the reduction in biosynthesized SeNPs. Our findings are in collaboration with ref. [29] who illustrated similar findings. In another study, the authors described that C═O, N–H, O–H, and C–H functional groups are responsible for the reduction and stabilization of SeNPs [30].
3.2 Effect of SeNPs on morphological parameters of selected varieties of wheat
During this study, various concentrations of biosynthesized SeNPs were used on different wheat varieties under drought stress and controlled irrigation. Effects of SeNPs on the morphological parameters of the wheat plant have been studied and represented as root-, leaf-, shoot-length, root-, shoot-fresh and dry weight, leaf number, leaf area, and plant height. The water-deficit conditions are imperative nonliving factors that can alter plants’ physiological attributes that affect the plant morphology and have degradative effects on the developmental and growth [4,10]. Drought is responsible for hampering shoot development and invigorating root growth and decreases chlorophyll content [15,31]. Additionally, the stunted growth of wheat plants under water-deficit conditions may well be related to an increased level of ROS and accumulation of ROS leads to lipid oxidation, pigment bleaching, and protein degradation [6,31]. However, the foliar application of biosynthesized SeNPs has positive or growth-enhancing effects on wheat varieties against drought stress by improving plant height, shoot length, root length and root fresh weight, root dry weight, leaf area, leaf number, leaf length, shoot fresh weight, and shoot dry weight compared to the control crop plants. Our results revealed significant increase in plant height (9.10% and 12.94%), shoot length (34.9% and 26.12%), shoot fresh weight (13.62% and 16.25%), shoot dry weight (12.37% and 30.62%), root length (34.16% and 25.34%), root fresh weight (7.8% and 8.92%), root dry weight (25.21% and 17.85%), leaf area (22.32% and 52.12%), leaf number (44.33% and 38.33%), and leaf length (22.6% and 18.09%) by using 30 mg/L of SeNPs as compared to negative control condition (drought stress) in selected wheat varieties (Pakistan-2013 (V1) and NARC-2011 (V2)), respectively. Moreover, remarkable increases in plant height (3.59% and 5.55%), shoot length (3.25% and 5.70%), shoot fresh weight (9.21% and 8.47%), shoot dry weight (9% and 5.93%), root length (24.18% and 19.35%), root fresh weight (16.68% and 12.24%), root dry weight (19.23% and 16.11%), leaf area (5.24% and 1.54%), leaf number (21.8% and 16.37%), and leaf length (4.73% and 2.01%) have been observed by using 30 mg/L of SeNPs as compared to positive control condition in both wheat varieties (Pakistan-2013 (V1) and NARC-2011 (V2)), respectively (Figures 4 and 5). No change was observed in the plant agronomic parameters on control irrigation conditions; while a significant increase was observed in morphological parameters when 10 and 20 mg/L of SeNPs were used. However, the increase in concentration beyond 30 mg/L resulted in declining the morphological parameters of the wheat plant (40 mg/L).
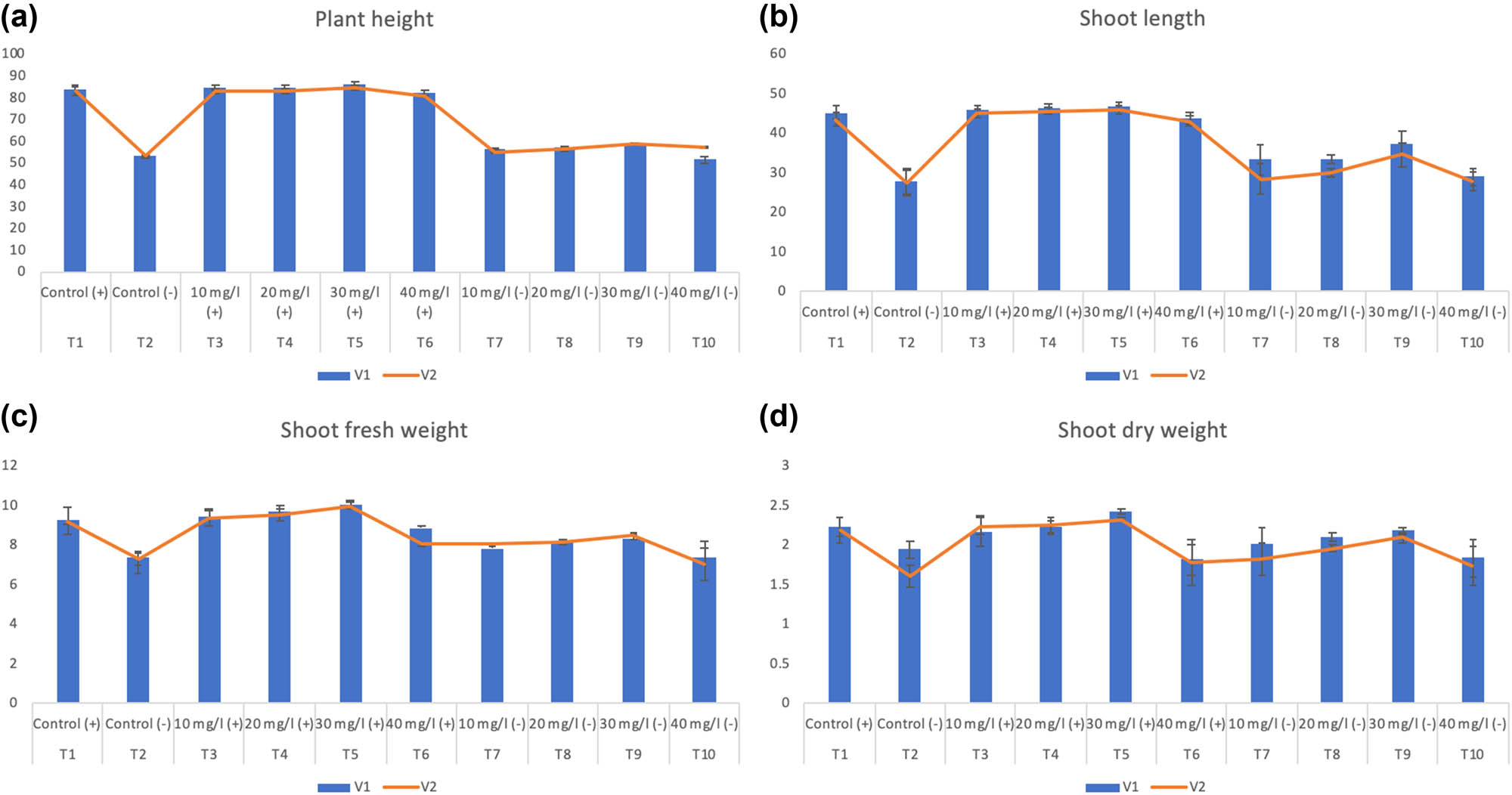
Effect of different treatments of SeNPs on (a) plant height, (b) shoot length, (c) shoot fresh weight, and (d) shoot dry weight.
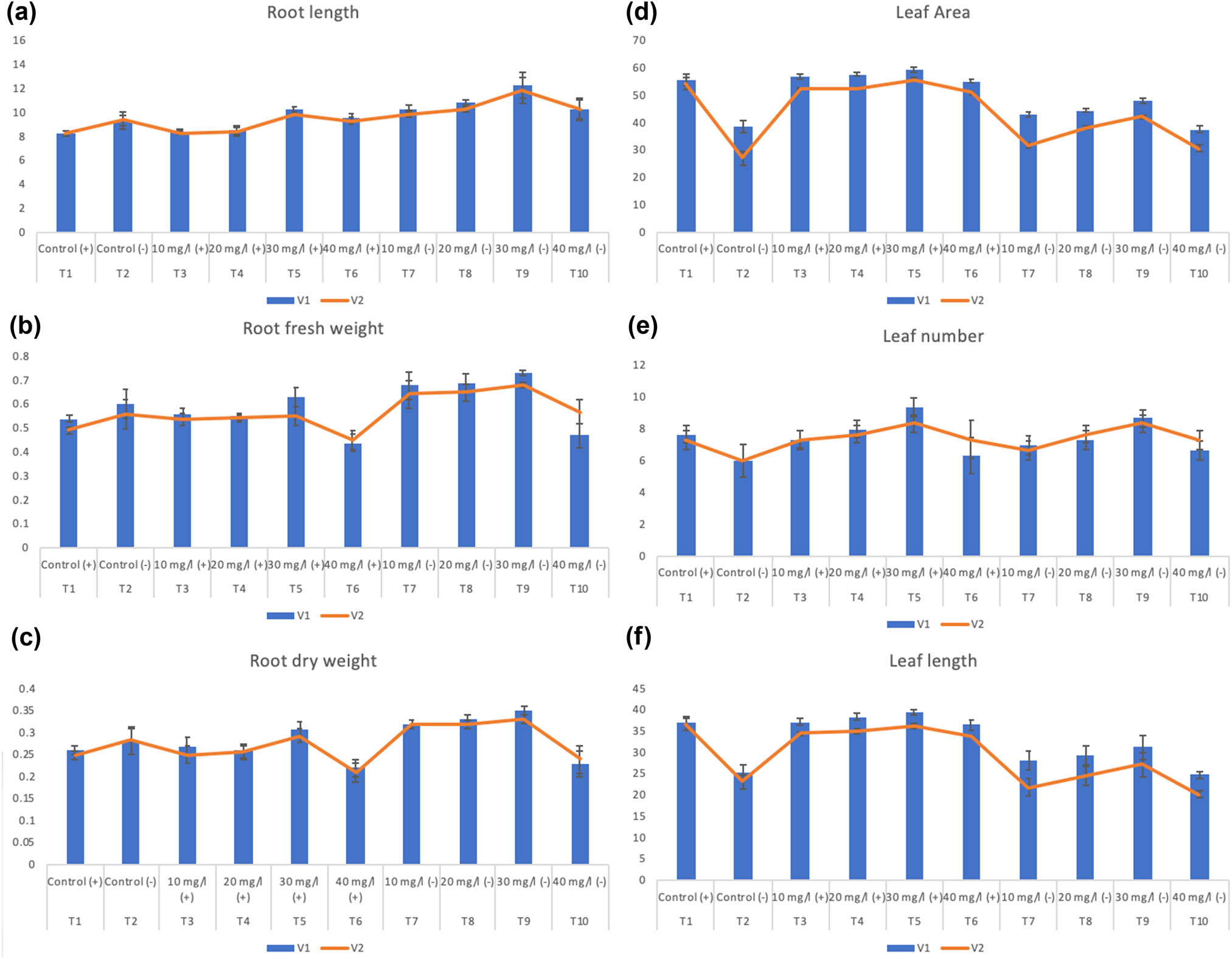
Effect of different treatments of SeNPs on (a) root length, (b) root fresh weight, (c) root dry weight, (d) leaf area (e), leaf number, and (f) leaf length.
Germ et al. [32] reported that the applications of selenium at low concentrations have beneficial effects and increases plants’ abilities to fight the drought stress. According to our study, a low-dose SeNP enhances wheat growth at both normal and water-deficit conditions. Moreover, the other researchers demonstrated that trace concentration of selenium enhances plant growth, delay senescence, and regulate plant water contents under water-deficit conditions [33]. In addition, our results are in corroboration with the findings of Shoeibi et al. [34] who demonstrated that biogenic SeNPs stimulate organogenesis and promote root growth in different plants. The foliar applications of SeNPs significantly improve the morphological features of wheat plants under drought conditions. Our findings revealed that 30 mg/L of SeNPs is more efficient in enhancing the growth of both wheat verities under normal and drought environments. Correspondingly, Bao-shan et al. [35] exogenously applied SiO2 NPs on Larix olgensis seedlings and observed a remarkable increase in height, the number of lateral roots, root length, leaf fresh, and dry weight when plants were exposed to severe saline conditions. Moreover, Boldrin et al. [36] illustrated that the trace level of selenium promotes plant growth.
4 Conclusion
Drought is the most important environmental factor that is responsible to limit crop production worldwide. Improving drought tolerance and elimination of harmful effects of drought stress in different crops are the major research challenges. Herein we reported the foliar applications of different concentrations of SeNPs bio-fabricated by using aqueous extract of Allium sativum buds. The foliar application of biosynthesized SeNPs fortifies wheat crops against drought stress and also improves the growth under controlled irrigation. It was observed that 30 mg/L of SeNPs is effective to enhance the morphological or agronomic attributes such as shoot, root length, plant height, root-, shoot-dry and fresh weight, leaf area, leaf length and leaf number of wheat crop. However, an increase in the concentration of SeNPs at 40 mg/L resulted in declining growth of the wheat plants. Thus, it is concluded that SeNPs play a proficient role in improving drought tolerance by promoting the development and growth of wheat plants under severe drought stress.
Author contributions: Naveed Iqbal Raja and Zia-Ur-Rehman Mashwani devised the study. Muhammad Ikram performed experiments and wrote the first draft. Bilal Javed edited, reviewed, and revised the manuscript. Mubashir Hussain, Maria Ehsan, Noman Rafique, Khafsa Malik and Tahira Sultana assisted in the characterization of NP samples. Mujahid Hussain helped in statistical analysis. Abida Akram contributed reagents and provided information. All authors reviewed and endorsed the final version of the manuscript for submission.
Conflict of interest: The authors declare no conflict of interest.
References
[1] Aissa N, Malagoli M, Radhouane L. An approach to alleviate the impact of drought stress with selenium amendment. Iran J Sci Technol Trans A Sci. 2018;42(1):283–8.10.1007/s40995-018-0511-2Suche in Google Scholar
[2] Barnabás B, Jäger K, Fehér A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008;31(1):11–38.10.1111/j.1365-3040.2007.01727.xSuche in Google Scholar PubMed
[3] Bhargava S, Sawant K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013;132(1):21–32.10.1111/pbr.12004Suche in Google Scholar
[4] Wang X, Vignjevic M, Liu F, Jacobsen S, Jiang D, Wollenweber B. Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul. 2015;75(3):677–87.10.1007/s10725-014-9969-xSuche in Google Scholar
[5] Keyantash J, Dracup JA. The quantification of drought: An evaluation of drought indices. Am Meteorol Soc. 2002;83(8):1167–80.10.1175/1520-0477-83.8.1167Suche in Google Scholar
[6] Caverzan A, Casassola A, Brammer SP. Antioxidant responses of wheat plants under stress. Genet Mol Biol. 2016;39(1):1–6.10.1590/1678-4685-GMB-2015-0109Suche in Google Scholar PubMed PubMed Central
[7] Mondal S, Singh RP, Crossa J, Huerta-Espino J, Sharma I, Chatrath R, et al. Earliness in wheat: a key to adaptation under terminal and continual high temperature stress in South Asia. F Crop Res. 2013;151:19–26.10.1016/j.fcr.2013.06.015Suche in Google Scholar
[8] Iqbal M, Raja NI, Mashwani ZUR, Hussain M, Ejaz M, Yasmeen F. Effect of silver nanoparticles on growth of wheat under heat stress. Iran J Sci Technol Trans A Sci. 2019;43(2):387–95.10.1007/s40995-017-0417-4Suche in Google Scholar
[9] Tadesse W, Sanchez-Garcia M, Tawkaz S, Baum M. Doubled haploid Prod Wheat. 2019:93–116.10.19103/AS.2019.0051.03Suche in Google Scholar
[10] Tadina N, Germ M, Kreft I, Breznik B, Gaberščik A. Effects of water deficit and selenium on common buckwheat (Fagopyrum esculentum Moench.) plants. Photosynthetica. 2007;45(3):472–6.10.1007/s11099-007-0080-7Suche in Google Scholar
[11] Kumar U, Joshi AK, Kumari M, Paliwal R, Kumar S, Röder MS. Identification of QTLs for stay green trait in wheat (Triticum aestivum L.) in the ‘Chirya 3’ × ‘Sonalika’ population. Euphytica. 2010;174(3):437–45.10.1007/s10681-010-0155-6Suche in Google Scholar
[12] Preet G, Sidhu S. Wheat production in changing environments. In: Wheat production in changing environments. 2019. 10.1007/978–81-13-6883-7.Suche in Google Scholar
[13] Rai V, Acharya S, Dey N. Implications of nanobiosensors in agriculture. J Biomater Nanobiotechnol. 2012;3(2):315–24.10.4236/jbnb.2012.322039Suche in Google Scholar
[14] Wadhwani SA, Shedbalkar UU, Singh R, Chopade BA. Biogenic selenium nanoparticles: current status and future prospects. Appl Microbiol Biotechnol. 2016;100(6):2555–66.10.1007/s00253-016-7300-7Suche in Google Scholar
[15] Seppänen M, Turakainen M, Hartikainen H. Selenium effects on oxidative stress in potato. Plant Sci. 2003;165(2):311–9.10.1016/S0168-9452(03)00085-2Suche in Google Scholar
[16] Satgurunathan T, Bhavan PS, Komathi S. Green synthesis of selenium nanoparticles from sodium selenite using garlic extract and its enrichment on Artemia nauplii to feed the freshwater prawn Macrobrachium rosenbergii post-larvae. Res J Chem Environ. 2017;21(10):1–12.Suche in Google Scholar
[17] Lin ZH, Wang CRC. Evidence on the size-dependent absorption spectral evolution of selenium nanoparticles. Mater Chem Phys. 2005;92(2–3):591–4.10.1016/j.matchemphys.2005.02.023Suche in Google Scholar
[18] Al-Saggaf MS, Tayel AA, Ghobashy MOI, Alotaibi MA, Alghuthaymi MA, Moussa SH. Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens. Green Process Synth. 2020;9(1):477–87.10.1515/gps-2020-0038Suche in Google Scholar
[19] Hussain A, Mehmood A, Murtaza G, Ahmad KS, Ulfat A, Khan MF, et al. Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities. Green Process Synth. 2020;9(1):451–61.10.1515/gps-2020-0047Suche in Google Scholar
[20] Javed B, Nadhman A, Mashwani Z. Optimization, characterization and antimicrobial activity of silver nanoparticles against plant bacterial pathogens phyto-synthesized by Mentha longifolia. Mater Res Express. 2020;7(8):085406.10.1088/2053-1591/abaf19Suche in Google Scholar
[21] Javed B, Raja NI, Nadhman A, Mashwani Z-R. Understanding the potential of bio-fabricated non-oxidative silver nanoparticles to eradicate Leishmania and plant bacterial pathogens. Appl Nanosci. 2020;10(6):2057–67.10.1007/s13204-020-01355-5Suche in Google Scholar
[22] Javed B, Mashwani ZUR. Synergistic effects of physicochemical parameters on bio-fabrication of mint silver nanoparticles: structural evaluation and action against HCT116 colon cancer cells. Int J Nanomed. 2020;15:3621–37.10.2147/IJN.S254402Suche in Google Scholar
[23] Javed B, Nadhman A, Mashwani ZUR. Phytosynthesis of Ag nanoparticles from Mentha longifolia: their structural evaluation and therapeutic potential against HCT116 colon cancer, Leishmanial and bacterial cells. Appl Nanosci. 2020;10(9):3503–15. 10.1007/s13204-020-01428-5.Suche in Google Scholar
[24] Javed B, Nadhman A, Razzaq A, Mashwani Z. One-pot phytosynthesis of nano-silver from Mentha longifolia L.: their characterization and evaluation of photodynamic potential. Mater Res Express. 2020;7(5):1–9.10.1088/2053-1591/ab903bSuche in Google Scholar
[25] Adrees M, Khan ZS, Ali S, Hafeez M, Khalid S, Ur Rehman MZ, et al. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere. 2020;238:124681. 10.1016/j.chemosphere.2019.124681.Suche in Google Scholar
[26] El Shafey AM. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process Synth. 2020;9(1):304–39.10.1515/gps-2020-0031Suche in Google Scholar
[27] Prasad KS, Selvaraj K. Biogenic synthesis of selenium nanoparticles and their effect on As(III)-induced toxicity on human lymphocytes. Biol Trace Elem Res. 2014;157(3):275–83.10.1007/s12011-014-9891-0Suche in Google Scholar
[28] Gates B, Mayers B, Cattle B, Xia Y. Synthesis and characterization of uniform nanowires of trigonal selenium. Adv Funct Mater. 2002;12(3):219–27.10.1002/1616-3028(200203)12:3<219::AID-ADFM219>3.0.CO;2-USuche in Google Scholar
[29] Yang F, Tang Q, Zhong X, Bai Y, Chen T, Zhang Y, et al. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int J Nanomed. 2012;7:835–44.10.2147/IJN.S28278Suche in Google Scholar
[30] Vyas J, Rana S. Antioxidant activity and green synthesis of selenium nanoparticles using allium sativum extract. Int J Phytomed. 2017;9(4):634.10.5138/09750185.2185Suche in Google Scholar
[31] Shen X, Dong Z, Chen Y. Drought and UV-B radiation effect on photosynthesis and antioxidant parameters in soybean and maize. Acta Physiol Plant. 2015;37(2):25. 10.1007/s11738-015-1778-y.Suche in Google Scholar
[32] Germ M, Kreft I, Osvald J. Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant Physiol Biochem. 2005;43(5):445–8.10.1016/j.plaphy.2005.03.004Suche in Google Scholar PubMed
[33] Rahmat S, Hajiboland R, Sadeghzade N. Selenium delays leaf senescence in oilseed rape plants. Photosynthetica. 2017;55(2):338–50.10.1007/s11099-016-0643-6Suche in Google Scholar
[34] Shoeibi S, Mozdziak P, Golkar-Narenji A. Biogenesis of selenium nanoparticles using green chemistry. Top Curr Chem. 2017;375(6):1–21.10.1007/s41061-017-0176-xSuche in Google Scholar PubMed
[35] Bao-shan L, Shao-qi D, Chun-hui L, Li-jun F, Shu-chun Q, Min Y. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J For Res. 2004;15(2):138–40.10.1007/BF02856749Suche in Google Scholar
[36] Boldrin PF, de Figueiredo MA, Yang Y, Luo H, Giri S, Hart JJ, et al. Selenium promotes sulfur accumulation and plant growth in wheat (Triticum aestivum). Physiol Plant. 2016;158(1):80–91.10.1111/ppl.12465Suche in Google Scholar PubMed
© 2020 Muhammad Ikram et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis
Artikel in diesem Heft
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis


