To the Editor,
Several lines of evidence now attest that spurious hemolysis is the leading source of blood samples’ non-conformance in the vast majority of clinical laboratories worldwide [1], [2]. Some previous studies have attempted to estimate the economic burden of sample hemolysis on healthcare budgets [3], [4], [5], but none of these has provided definitive data on the sustainable economic threshold for implementation of reliable hemolysis prevention tools, to the best of our knowledge. Therefore, we conducted an economic analysis at a large university hospital, aimed at identifying our willingness-to-pay threshold for preventing spurious hemolysis during drawing of blood samples.
The cumulative cost of a blood sample collection at the local facility (i.e. University Hospital of Verona) is 1.358 €, partitioned as follows: 1.047 € for nurse time (i.e. 5 min per venipuncture), 0.155 € for 21-gauge straight needle and holder (Kima Safety, Kima, Padova, Italy), 0.002 € for detergent (Neoxinal Alcolico, Nuova Farmec, Verona, Italy), 0.011 € for gauge pad (Luigi Salvadori, Florence, Italy), 0.006 € for two tube labels and 0.140 € for one clinical chemistry and one coagulation blood tube (Kima, Padova, Italy). This observational study was carried out in accordance with the Declaration of Helsinki and under the terms of all relevant local legislations.
During the entire year 2017, a total number of 782,479 blood tubes were received in the local laboratory for clinical chemistry (n=536,001) and coagulation (n=246,478) testing, of which 10,991 (1.40%) were classified as being significantly hemolyzed according to a local standard operating procedure (SOP) compliant with the International Organization for Standardization (ISO) 15189 document, and based on systematic assessment of serum/plasma indices on both clinical chemistry (Dimension Vista, Siemens Healthcare, Erlangen, Germany) and coagulation (ACL TOP 750, Instrumentation Laboratory, Bedford, MA, USA) analyzers. Sample rejection is set at a value of cell-free hemoglobin >0.5 g/L for potassium and >3.0 g/L for hemostasis testing. Test result suppression for the presence of clinically significant hemolysis is promptly notified to the hospital wards, and a second set of samples is recollected, whenever feasible and necessary.
According to local cost analysis, the overall expenditure for drawing blood for clinical chemistry and coagulation testing was 1,065,458 € during the entire year 2017 at our institution. Overall, recollection of all previously hemolyzed samples would have hence imposed a significant extra economic burden of 14,966 €. By associating this extra and potentially avoidable expenditure with the total number of blood tubes received in the year 2017, we can hence estimate the threshold cost of adopting reliable tools for avoiding sample hemolysis, and thus preventing sample recollection with a new venipuncture, at 0.019 € (i.e. ~2 cents of an €) per single tube in our healthcare facility, which corresponds to 1.4% of the total cost of a routine venipuncture. Whatever preventive measure costing more than this threshold per tube would hence be considered no longer economically viable. It is also worth mentioning here that diagnostic delays due to longer stay in the emergency department (ED) for recollecting blood samples will generate additional expenditures for the healthcare system. Considering that the average cost of the length of stay (LOS) in the ED can be comprised between 1540 and 2200 € per day [6], a 1-h delay for receiving suitable results of laboratory testing would generate an incremental cost of approximately 64–92 € per patient.
Albeit the real value of laboratory diagnostics lies far ahead of a simple balance between cost and profit [7], the results of our analysis show that the economic threshold for implementing reliable hemolysis prevention means [8], thus potentially including specific blood collection sets, needles, holders or even usage of devices allowing rapid hemolysis detection in uncentrifuged blood specimens (e.g. HemCheck, HemCheck Sweden AB, Karlstad, Sweden), is seemingly very narrow (Figure 1), at least in our healthcare facility.
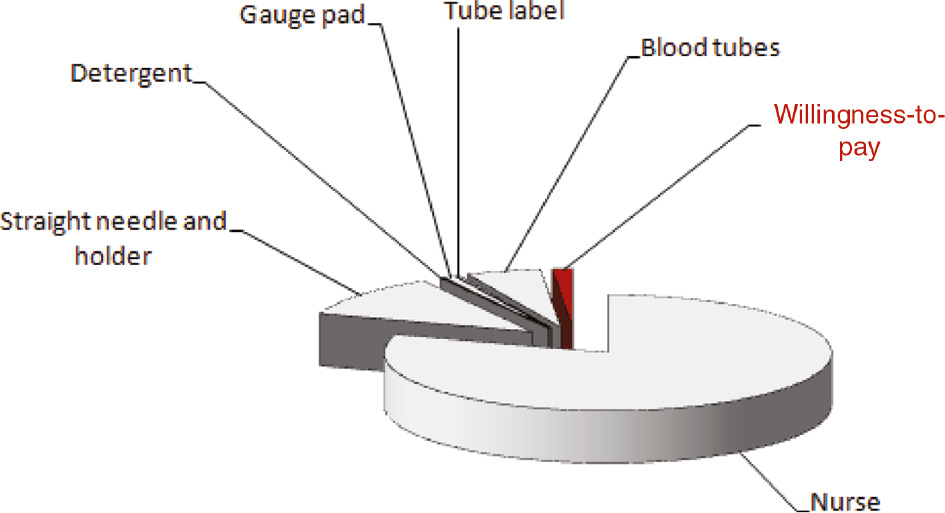
Willingness-to-pay threshold for preventing spurious hemolysis during blood sample collection related to the overall expenditure of a routine venipuncture.
Nevertheless, this conclusion is exclusively economical, and does not consider either patient inconvenience due to repeated venipuncture or the potential adverse consequences of diagnostic delay caused by the need of recollecting blood samples in urgent healthcare settings, such as EDs or intensive care units, where results of laboratory testing are crucial for managing patients with acute and often life-threatening conditions. This conclusion perhaps applies also to non-hospital laboratories, where the willingness-to-pay threshold may be even higher considering that blood sample recollection in non-hospital facilities is a magnified challenge in terms of turnaround time and delayed diagnosis. Indeed, public tenders for blood collection devices should hence be focused on these essential aspects other than simply relying on lower prices [9].
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Lippi G, Cadamuro J, von Meyer A, Simundic AM. Practical recommendations for managing hemolyzed samples in clinical chemistry testing. Clin Chem Lab Med 2018;56:718–27.10.1515/cclm-2017-1104Suche in Google Scholar PubMed
2. Lippi G, von Meyer A, Cadamuro J, Simundic A-M. Blood sample quality. Diagnosis 2019;6:25–31.10.1515/dx-2018-0018Suche in Google Scholar PubMed
3. Jacobs P, Costello J, Beckles M. Cost of haemolysis. Ann Clin Biochem 2012;49:412.10.1258/acb.2011.011232Suche in Google Scholar PubMed
4. Lippi G, Bonelli P, Cervellin G. Prevalence and cost of hemolyzed samples in a large urban emergency department. Int J Lab Hematol 2014;36:e24–6.10.1111/ijlh.12135Suche in Google Scholar PubMed
5. Cadamuro J, Wiedemann H, Mrazek C, Felder TK, Oberkofler H, Fiedler GM, et al. The economic burden of hemolysis. Clin Chem Lab Med 2015;53:e285–8.10.1515/cclm-2015-0363Suche in Google Scholar PubMed
6. Foley M, Kifaieh N, Mallon WK. Financial impact of emergency department crowding. West J Emerg Med 2011;12:192–7.Suche in Google Scholar
7. Lippi G, Plebani M. The add value of laboratory diagnostics: the many reasons why decision-makers should actually care. J Lab Precis Med 2017;2:100.10.21037/jlpm.2017.12.07Suche in Google Scholar
8. Lippi G, Cadamuro J. Novel opportunities for improving the quality of preanalytical phase. A glimpse to the future? J Med Biochem 2017;36:293–300.10.1515/jomb-2017-0029Suche in Google Scholar PubMed PubMed Central
9. Lippi G, Cornes MP, Grankvist K, Nybo M, Simundic AM. EFLM WG-Preanalytical phase opinion paper: local validation of blood collection tubes in clinical laboratories. Clin Chem Lab Med 2016;54:755–60.10.1515/cclm-2015-1274Suche in Google Scholar PubMed
©2019 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- The preanalytical phase – a field for improvement
- Articles
- Managing inappropriate utilization of laboratory resources
- The Choosing Wisely initiative and laboratory test stewardship
- Blood sample quality
- Blood sampling guidelines with focus on patient safety and identification – a review
- Sample transportation – an overview
- Values and stability of serum (or plasma) indices in uncentrifuged serum and lithium-heparin plasma
- Willingness-to-pay threshold for preventing spurious hemolysis during blood sample collection
- Preanalytical aspects on short- and long-term storage of serum and plasma
- Preanalytical considerations in therapeutic drug monitoring of immunosuppressants with dried blood spots
- Diagnostic error as a result of drug-laboratory test interactions
- Editorial
- The preanalytical phase in the era of high-throughput genetic testing. What the future holds
Artikel in diesem Heft
- Frontmatter
- Editorial
- The preanalytical phase – a field for improvement
- Articles
- Managing inappropriate utilization of laboratory resources
- The Choosing Wisely initiative and laboratory test stewardship
- Blood sample quality
- Blood sampling guidelines with focus on patient safety and identification – a review
- Sample transportation – an overview
- Values and stability of serum (or plasma) indices in uncentrifuged serum and lithium-heparin plasma
- Willingness-to-pay threshold for preventing spurious hemolysis during blood sample collection
- Preanalytical aspects on short- and long-term storage of serum and plasma
- Preanalytical considerations in therapeutic drug monitoring of immunosuppressants with dried blood spots
- Diagnostic error as a result of drug-laboratory test interactions
- Editorial
- The preanalytical phase in the era of high-throughput genetic testing. What the future holds

