Abstract
Psoriasis is a chronic, immune-mediated skin condition marked by excessive cell growth and inflammation. Current therapies frequently have limitations, such as side effects and insufficient efficacy, emphasizing the need for safer, more effective options. Erianin (ERN), a naturally occurring bioactive molecule produced from Dendrobium chrysotoxum, has anti-inflammatory and antioxidant characteristics, although its therapeutic potential in psoriasis has not been fully investigated. The purpose of this investigation was to look into the protective benefits of ERN against psoriasis-like skin inflammation utilizing laboratory-based cell models and an imiquimod-induced psoriasis animal model. Human keratinocytes were subjected to pro-inflammatory cytokines in vitro to simulate psoriasis disease, and cell survival and proliferation were measured. In vivo, mice given ERN for 6 days demonstrated a significant decrease in skin thickness, inflammatory cell infiltration, and overall histopathological alterations. ERN reduced pro-inflammatory substances (IL-6, IL-17, IL-23, IL-1β), TNF-α, COX-2, and inducible nitric oxide synthase, while increasing anti-inflammatory cytokine IL-10 and antioxidant-related molecules. Additionally, ERN stimulated the Kelch-like ECH-associated protein 1- nuclear factor erythroid 2-related factor 2 signaling pathway, which is essential for cellular antioxidant defense. The results presented here demonstrate that ERN reduces psoriasis-like inflammation by modifying immunological responses and increasing antioxidant protection, pointing to its potential as a viable therapeutic agent for psoriasis treatment.
Graphical abstract
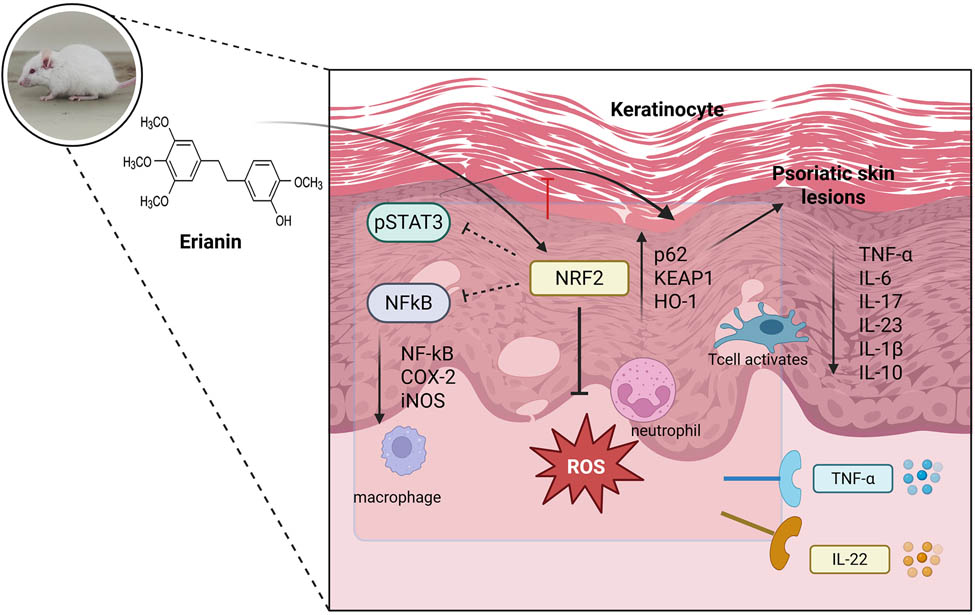
1 Introduction
Psoriasis affects millions of people around the world, a chronic, immune-driven skin disorder that has serious effects on both the body and the mind [1,2]. Psoriasis, which is distinguished by increased keratinocyte growth and chronic inflammation, mostly affects the epidermis, the outermost layer of the skin, and presents as red, scaly plaques [3,4]. This disorder includes intricate relationships between immune cells and keratinocytes at the molecular level, and the illness progresses due to the release of pro-inflammatory substances, including TNF-α, IL-17, and IL-23 [5,6]. Although the results of moderate-to-severe situations have benefited from biopharmaceutical drugs that target certain cytokines, these therapies are sometimes expensive and come with concerns of systemic immunosuppression [7]. Because of this, there is still a requirement for topical therapies that are safe, efficient, and affordable, particularly for individuals with mild-to-moderate illness [8,9,10].
The etiology of psoriasis appears to be linked to oxidative damage and inflammation signaling, according to the latest study, which has also identified potential treatment targets for these pathways [11,12,13]. Of them, the KEAP1-NRF2 pathway has attracted special attention because of its crucial role in shielding cells from oxidative damage and inflammation [14]. A gene nuclear factor erythroid 2-related factor 2 (NRF2), when triggered, coordinates the production of cytoprotective and antioxidant-related genes that aid in redox balance restoration and the suppression of inflammation-related signals [15,16]. Normally, NRF2 is appropriated in the cytoplasm and is destroyed by proteasomes under the control of its negative regulator, KEAP1 (Kelch-like ECH-associated protein 1) [17,18]. NRF2 parts from KEAP1 transfer into the nucleus and stimulate genes encoding anti-oxidants along with additional protective molecules in reaction to oxidative stress or certain chemical stimulants [19,20]. Thus, altering this system has become a viable treatment approach for inflammatory conditions, such as psoriasis, where immunological dysregulation and cellular oxidative stress are major causes [21,22].
Recent studies have underlined that psoriasis is more than just a skin disorder; it also has systemic effects on other essential organs. As an instance, psoriasis-like inflammation has been demonstrated to cause kidney dysfunction by upregulating NADPH oxidases and inducible nitric oxide synthase (iNOS), demonstrating that oxidative stress is a key contributor to organ damage beyond the skin [23]. Furthermore, psoriatic inflammation has been linked to hepatic inflammation as well as marked dysregulation in liver metabolism via IL-17A/IL-17 receptor signaling, emphasizing the importance of Th17 cytokines in extrapolating psoriatic pathologists to the livers [24]. Furthermore, modification of immunological signaling pathways, notably the IL-23/IL-17A axis, has emerged as a potential therapeutic target. Additionally, a BTK inhibitor was discovered to decrease imiquimod (IMQ)-induced psoriasis-like inflammation by modulating IL-23/IL-17A in innate immune cells [25], establishing a physiological link involving immune control and symptom alleviation. The results obtained highlight the systemic aspect of psoriasis and encourage more research into immune-modulatory treatments.
Natural products have a variety of pharmaceutical properties that may allow them to target key inflammatory factors, signaling pathways, and physiological activities associated with psoriasis [26,27]. The KEAP1-NRF2 pathway can be modulated by natural chemicals, providing a multi-targeted and frequently less hazardous therapy strategy [28,29]. Erianin (ERN), a naturally occurring isoflavone that comes from Dendrobium chrysotoxum, has shown anti-inflammatory, antioxidant, and anti-proliferative implications, among other pharmacological qualities [30,31,32,33,34,35]. According to preliminary research, by regulating proliferation and preserving the stability of the skin barrier, ERN can control keratinocyte behavior, something that is essential to the pathophysiology of psoriasis [34]. However, the precise ways in which ERN affects psoriatic inflammation specifically, via the KEAP1-NRF2 pathway, remain unclear. In the scenario of psoriasis, ERN is an intriguing option for more research because of the therapeutic potential of NRF2 induction in lowering the levels of oxidative and inflammatory pathways.
The persistence of the current investigation was to examine how ERN could affect psoriasiform inflammation by activating the KEAP1-NRF2 pathway. To evaluate ERN’s influence on cell proliferation and proinflammatory marker expression, we examined its effects on psoriatic skin cells in vitro using HaCaT cells treated with cytokines linked to psoriasis. To assess the in vivo effects of ERN on skin inflammation, antioxidant stress indicators, and important elements of the KEAP1-NRF2 pathway, we also used a mouse model of psoriasis produced by IMQ. Based on our research, ERN promotes a healthy intracellular context in psoriatic skin by lowering inflammation and promoting keratinocyte growth while also strengthening antioxidant defenses.
The therapeutic potential of ERN as a new topical medication for the treatment of psoriasis is highlighted by this study. ERN may provide a focused strategy for lowering oxidative stress, promoting skin health, and easing psoriasiform inflammation by modifying the KEAP1-NRF2 pathway. To deal with the demand for more effective and convenient choices for controlling mild-to-moderate psoriasis, this investigation offers important insights into the underlying mechanisms that govern the effectiveness of ERN and establishes the groundwork for its progress as a topical drug.
2 Materials and methods
2.1 Cell culture
The human differentiated keratinocytes (HaCaT; Procell, Wuhan, China) were cultivated in a moist incubator at 37°C and 5% CO2 using DMEM supplied with 10% FBS and 1% penicillin-streptomycin. At 37°C and 5% CO2, an in vitro cell model for psoriasis was created by inducing 10 ng/mL TNF-α in a logarithmic growth phase of HaCaT cells [36], for 48 h.
2.2 CCK8 assay
To ascertain the antiproliferative effect of ERN on HaCaT, the CCK-8 reagent was used. For a brief duration, HaCaT cells were seeded at a rate of 5 × 103 cells/well in 96-well dishes, administered ERN at various doses (0, 5, 10, 20, 40, and 80 μM) for 24 h, and then maintained with CCK-8 reagent for 1 h at 37°C in the dark. ERN was administered to HaCaT cells at doses ranging from 5 to 20 μM following a 48 h TNF-α treatment at 37°C and 5% CO2. Utilizing a Microplate Reader, the absorption coefficient of the assessments was measured at an apparent wavelength of 450 nm.
2.3 EdU assessment
The HaCaT cells were cultivated in 24-well plates and exposed to varying doses of combined TNF-α and ERN. As directed by the manufacturer, the cell proliferation EdU (5-ethynyl-2′-deoxyuridine) assay kit (Thermo Fisher Scientific, Catalog No. C10337) was utilized to measure the proliferative growth rate of HaCaT cells. Immunofluorescence was used to evaluate EdU accumulation.
2.4 ELISA
ERN (5–10 μM) was applied to the TNF-α-persuaded HaCaT cells for 24 h. The ELISA kit (BlueGene Biotech, Catalog No. E03P0661) was then used to identify the various inflammatory cytokines (IL-6, IL-8, IL-22, and IL-1β) in the supernatant following the directions provided by the maker.
2.5 Reverse transcription-polymerase chain reaction (RT-PCR) analysis
After seeding HaCaT keratinocytes in 12-well plates, they were either triggered using TNF-α (10 ng/mL) or without it for 2 h after being treated with ERN (5, 10, and 20 μM) or not for 24 h. RNA was collected from cells and used for qRT-PCR investigation of Keratin 1 (KRT1) and Keratin 6 (KRT6) mRNA levels. As directed by the supplier, the TRIzolTM reagent was used for extracting total RNA from mouse skin tissue or HaCaT keratinocytes. 2 µg of total RNA was used to create the first strand of cDNA using the cDNA Synthesis Kit (Servicebio, Catalog No. G3331). Using the SYBR Green PCR Master Mix kit (Thermo Fisher Scientific, Catalog No. 4309155), GAPDH was used as an internal control gene, and the relative mRNA expression levels of the target genes were calculated using the 2−ΔΔCt method. This method allowed for the comparison of gene expression across different experimental groups. All reactions were performed in triplicate, and data were analyzed using the CFX96 Touch™ Real-Time PCR Detection System software. To target the genes in HaCaT keratinocytes, the following primer sequences were used (Table 1).
Forward and reverse primer sequencing for RT-PCR analysis
| Genes | Forward Primer (5′ → 3′) |
|---|---|
| Reverse Primer (5′ → 3′) | |
| KRT6 | F 5′-GGGTTTCAGTGCCAACTCAG-3′ |
| R 5′-CCAGGCCATACAGACTGC GG-3′ | |
| KRT1 | F 5′-CTTTTCTGCTGTTTCCCAATGAA-3′ |
| R 5′-GGAAAGAACAAAGCAGGG TCATAG-3′ | |
| GAPDH | F 5′-CACATGGCCTCCAAGGAGTAA-3′ |
| R 5′-TGAGGGTCTCTCTCTTCCTCT TGT-3′ |
2.6 Animal studies
All experiments on animals were conducted on male BALB/c mice in optimal condition, evaluating between 24 and 28 g at 5 weeks of age. The mice were housed in the animal house facility of the Department of Dermatology, The Second Children and Healthcare of Jinan, China, for approximately 7 days to allow them to become used to the laboratory environment (CPCSEA No: LL2024250001). A regular pellet meal and unlimited access to clean water were provided to the animals. For the duration of the investigative protocol, animals were kept in typical laboratory settings with a 12 h light/dark cycle, a temperature of 22 ± 2°C, and a moisture content of 40–70%. All that was possible has been taken to minimize the discomfort that the animals went through and to use only a few in totals for the assessment.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals, and has been approved by the Animal Care and Use Committee of the Department of Dermatology, The Second Children and Healthcare of Jinan, China (Approval No: CPCSEA No: LL2024250001).
2.7 Fabrication of an experimental psoriasis system and therapy strategy in mice activated by IMQ
An animal model of IMQ-induced testing psoriasis was created [37], by topically treating mice with IMQ 5% (w/w) for 6 days in a row (days 1–6). A 2.5 cm × 2.5 cm section of every mouse’s dorsal skin was shaved to remove mouse hairs. IMQ and ERN were not applied topically to the control group. Based on day 1 to day 6, all the animals aside from the control group, were topically administered with commercially accessible IMQ, at an administration of 62.5 mg lotion on their smooth-shaven rear skin (50 mg of IMQ lotion), every morning. This equates to a daily dose of 3.125 mg of the effective IMQ. For the conventional clobetasol group of controls, 40 mg of commercially accessible clobetasol cream (0.05% w/w) was administered topically on all the animals’ trimmed back skin (equivalent to 32 mg of clobetasol cream) every day, resulting in a daily supply of 20 μg of the influential substance.
External administration of ERN was performed in an anionic suspension base containing 100 mg at three distinct concentrations of 0.5, 1, and 2% w/w per animal. Each animal's shaved dorsal skin was topically treated with 100 mg of the produced anionic emulsifying cream containing ERN (0.5, 1, and 2% w/w) for six days in a row. This amounts to 80 mg of ERN cream each application. This equates to a daily allowance of 0.5, 1, and 2 mg of the ERN dose for each animal, Accordingly, every aspect concentration was directed at 12 h intervals after the use of IMQ for 6 successive days, except for control and IMQ control. Six groups of six mice were chosen at random from among the mice (n = 6).
2.8 Experimental groups
Group I: control (without IMQ and ERN)
Group II: IMQ control (IMQ alone; without ERN)
Group III: IMQ + 0.05% w/w Clobetasol (standard drug)
Group IV: IMQ + 0.5% w/w ERN (IMQ + ERN 0.5% w/w)
Group V: IMQ + 1% w/w ERN (IMQ + ERN 1% w/w)
Group VI: IMQ + 2% w/w ERN (IMQ + ERN 2% w/w)
The psoriasis area and severity index (PASI), which is rated using successive days’ aggregate scores for scaling, skin redness, and thickness, was used to track the infection’s progression. Day 7 saw the aseptic collection of the animals’ blood, spleen, skin, and ears, as well as their CO2 asphyxia death. The sections of the epidermis and ear tissues that were extracted were preserved in 10% formalin, and for later analysis, the remaining tissues were stored at −80°C. Blood was extracted to determine the hematological and biochemical features. The skin tissue in the IMQ-treated region provided the expected parameters for the entire investigation.?A3B2 tpb -13pt?>
2.9 Evaluation of the PASI
Animals were examined from day 1 to day 6 to calculate the PASI (for instance, following IMQ administration). The PASI was rated by the days that followed. On a scale of 0–4, erythema, skin thickness, and scales were rated distinctly as follows: Erythema: 0 (None), 1 (Weak), 2 (Moderate), 3 (Severe), 4 (Very severe), Skin thickness: 0 (None), 1 (Slightly thickened), 2 (Moderately thickened), 3 (Markedly thickened), 4 (Very markedly thickened), and Scales: 0 (None), 1 (Few scales), 2 (Moderate scales), 3 (Numerous scales), and 4 (Very numerous scales). The overall PASI score, which ranged from 0 (no inflammation) to 3 (severe inflammation), was computed by adding the distinct scores for erythema, skin thickness, and scales for every animal. This score was used to measure the level of inflammation involved with the psoriasis-like erythema on each day of the examination [38]. An aerospace digimatic micrometer Screw gauge was used to measure the thickness of the ear folds. On different days, the dorsal skin photos were captured to investigate the complete macroscopic structure in IMQ-induced animals.
2.10 Spleen to the mass ratio of the body
The animals’ weights were recorded before their killing and continued throughout the conclusion of the research, till the moment when the spleens were removed, cleansed, and evaluated. The spleen masses were regulated using body weight to compute the organ index. The results that were obtained were expressed in grams per gram.
2.11 Determining parameters for hematology
On the seventh day, blood from every testing animal was collected via the retroorbital plexus and placed into containers containing the anticoagulant substance EDTA. To ascertain the hematological features, a blood cell counter was employed. Hematological cell counts, measured in WBC, have been defined as (×103 cell/μL).
2.12 Histopathological evaluation
Before being embedded in paraffin, the rear skin of the assassinated animals had been eliminated and treated in 4% paraformaldehyde. H & E staining was applied to the paraffin-embedded portions after they had been cut to a thickness of 5 μm for histological assessment.
2.13 Measuring oxidative stress markers and antioxidant defenses
Commercial kits for malondialdehyde (MDA) (Catalog No. A003-1), NO (Catalog No. A012-1-1), glutathione (GSH) (Catalog No. A006-2), glutathione s-transferases (GST) (Catalog No. A004), total superoxide dismutase (SOD; A001-1), and catalase (CAT; A007-1), Vitamin C (Catalog No. A009), and thiobarbituric acid reactive substances (TBARS) (Catalog No. A003-1) were purchased from Nanjing Jiancheng Bio Ins (Nanjing, China). Protein samples from skin tissues or cell supernatants were isolated and utilized to evaluate the contents of the aforementioned oxidative stress components using commercially available kits following the manufacturer’s instructions.
2.14 Myeloperoxidase (MPO) activity assay
MPO activity was measured using Bradley et al.’s [39] methodology. MPO activity is an indication of neutrophils’ infiltration of inflammatory cells in the dermis layer of psoriasis. Skin tissues were dissolved in ice-cold 0.1 M potassium phosphate buffer (pH 6.5) with 0.5% hexadecyltrimethylammonium bromide and 10 mM EDTA. Tissue homogenates have been centrifuged at 13,100 × g for 20 min at 4°C. An aliquot of the supernatant (0.1 mL) was mixed with 2.9 mL of 50 mM phosphate buffer containing 0.167 mg o-dianisidine hydrochloride and 0.0005% hydrogen peroxide. The absorbance change over 5 min was measured at 460 nm. The data were presented as U/g of tissue.
2.15 Western blot analysis
BCA kit (Sigma-Aldrich, Catalog No. 71285-M) was used to homogenize and measure the cellular and tissue homogenates that are used to measure the protein fractions. Polyvinylidene fluoride membrane were equally loaded with the protein samples after they had been separated on 10% SDS polyacrylamide gels. The membranes were treated with primary antibodies overnight at 4°C following a 1 h incubation period with 5% bovine serum albumin. All primary (Rabbit IgG) and secondary antibodies were purchased from Cell Signaling Technology, USA, and were employed at the specified dilutions. The expressions of Nf-κB p65 (1:500), NRF2 (1:1,000), COX (1:1,000), iNOS (1:1,000) p62 (1:1,000), and KEAP1 (1:1,000) were studied. β-actin (1:1,000) was used as a housekeeping protein for whole-tissue extracts, and Lamin B (1:1,000) was used as a housekeeping protein for nuclear extracts. Horseradish-peroxidase (HRP)-conjugated anti-mouse or anti-rabbit antibodies (1:5,000) were used as secondary antibodies. Digital imaging devices used ECL to identify protein expression on blots after they were treated for 1 h with secondary antibodies coupled with HRP. The western blot findings’ intensity was examined using ImageJ software.
2.16 Examining data statistically
All statistical analyses were performed using GraphPad Prism software. Data were expressed as mean value ± standard error of the mean (SEM). To assess statistical significance, one-way analysis of variance was used, followed by Tukey’s post hoc test to compare differences between groups. A p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Effect of ERN on keratinocyte viability and TNF-α-induced proliferation
The molecular structure of ERN is shown in Figure 1a. The effects of ERN on human keratinocyte viability were assessed using the CCK8 assay. Following ERN therapy, no discernible toxicity was seen at dosages lower than 40 μM (Figure 1b). The inhibitory effect was particularly pronounced at higher concentrations (above 40 µM), suggesting that ERN exerts cytotoxic effects on keratinocytes at elevated doses. Thus, for the following investigations, keratinocytes were exposed to 5, 10, and 20 μM ERN.
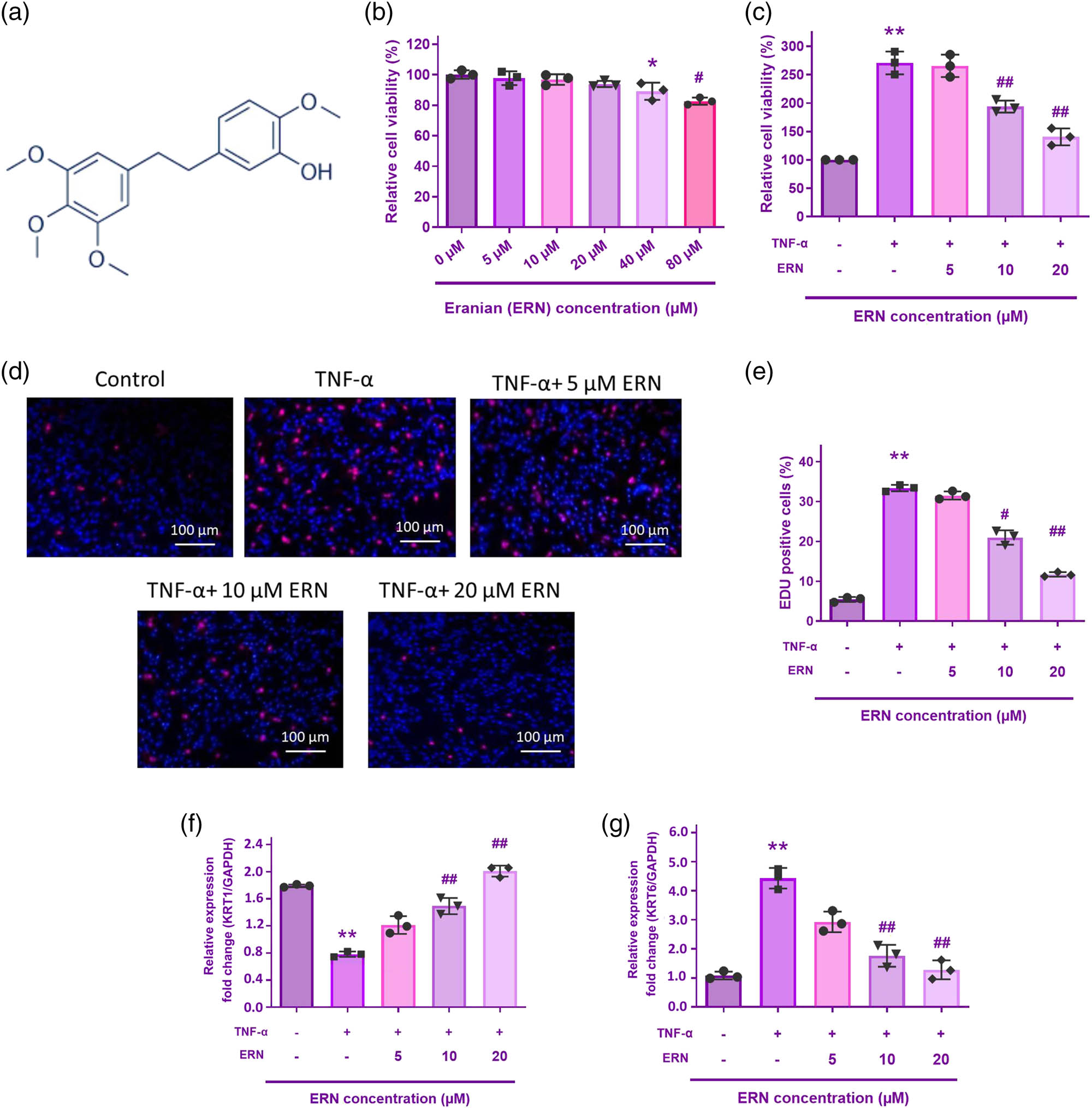
The impact of ERN on TNF-α-stimulated HaCaT cell proliferation. (a) ERN molecular structure. (b) and (c) Cell viability was assessed using the CCK-8 assay for 24 h. (d) The EdU assay was utilized to quantify the proliferation rate. (e) Quantification of EdU-positive cells in HaCaT cells treated with TNF-α (10 ng/mL) and increasing concentrations of ERN (5, 10, and 20 μM) for 24 h. The bar graph represents the percentage of proliferating (EdU-positive) cells. Data are expressed as mean value ± SD (n = 3). (f) and (g) KRT6 and KRT1 mRNA levels were analyzed by qRT-PCR. The internal reference is GAPDH. **P < 0.01 vs control. ## P < 0.01 vs TNF-α group. Data are expressed as mean value ± SD.
To confirm the impact of ERN on TNF-α-induced proliferation of HaCaT cells, the growth of HaCaT cells was measured using the CCK-8 and EdU assays. Proliferating cells were triggered by TNF-α, but this effect was mitigated by ERN administration at 5, 10, and 20 μM (Figure 1c). The findings of EdU staining verified that the proliferation of HaCaT cells was significantly increased by TNF-α therapy. Intriguingly, ERN reduced the rate of proliferation brought on by TNF-α induction in a manner that was dose-related (Figure 1d).
KRT6 is recognized as a marker of hyperproliferation in psoriatic keratinocytes. qRT-PCR analysis demonstrated that TNF-α exposure significantly upregulated KRT6 mRNA expression (Figure 1e), indicating increased keratinocyte proliferation. Conversely, KRT1, a marker of keratinocyte differentiation, was significantly downregulated following TNF-α treatment (Figure 1f). Cotreatment with ERN effectively reduced KRT6 expression in a dose reliant on the way, suggesting that ERN inhibits TNF-α-persuaded keratinocyte hyperproliferation. However, KRT1 expression was also increased upon ERN treatment, which implies that ERN restores keratinocyte differentiation. These findings suggest that ERN exerts an anti-proliferative effect on TNF-α-stimulated keratinocytes, supporting the successful establishment of an in vitro psoriasis model.
3.2 ERN reduces inflammatory cytokine stimulation
Measurement of various inflammatory cytokines linked to the emergence of inflammation, including IL-6, IL-8, IL-22, and IL-1β, was done using ELISA to ascertain the effect of ERN (5, 10, and 20 μM) on TNF-α-induced inflammation of HaCaT cells (Figure 2). According to the ELISA results, the TNF-α monotherapy group’s expressions of inflammatory factors were considerably higher than those of the untreated group. However, in a dose-dependent manner, ERN significantly decreased them. In a dose-responsive way, the data showed that ERN reduced the inflammation of HaCaT cells caused by TNF-α.
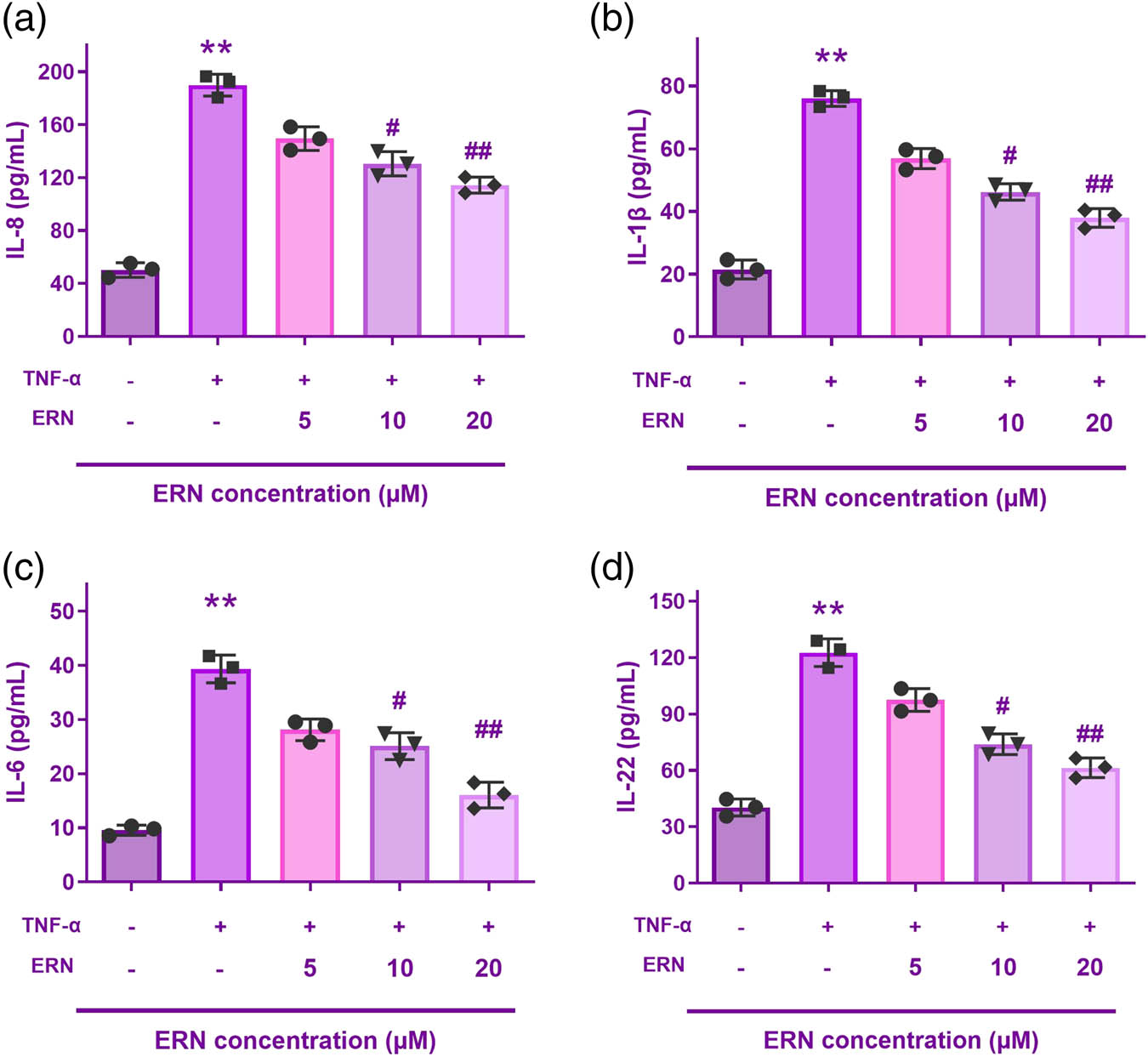
ERN reduced the inflammation of HaCaT cells caused by TNF-α. (a–d) Interleukin-8 (IL-8), IL-1β, IL-6, and IL-22 levels are all reduced by ERN. **P < 0.01 vs control. ## P < 0.01 vs TNF-α group. Data are expressed as mean value ± SD.
To confirm its promise as a treatment, we expanded our research to include an in vivo psoriasis model. We used an IMQ-induced psoriasis approach, which particularly resembles human psoriasiform inflammatory processes, to assess ERN’s effectiveness in a physiologically appropriate environment. The implications of ERN on oxidative stress, inflammatory release of cytokines, and keratin hyperplasia can be evaluated in vivo using this paradigm.
3.3 Therapeutic impact of ERN on psoriasis severity, body weight, and spleen index
To determine the degree of psoriasis lesions in the IMQ-induced psoriasis model, the PASI was measured regularly from day 1 to day 6 following IMQ administration. Erythema, skin thickness, and scaling were individually recorded on a scale from 0 to 4: 0, none; 1, weak; 2, modest; 3, noticeable; and 4, very noticeable. The scores for each parameter were then summed to obtain a composite PASI score, which could range from 0 (no inflammation) to 12 (severe inflammation). Here are the specific findings based on the PASI and other measures such as erythema, skin thickness, scaling, composite PASI score, and macroscopic morphology (Figure 3).
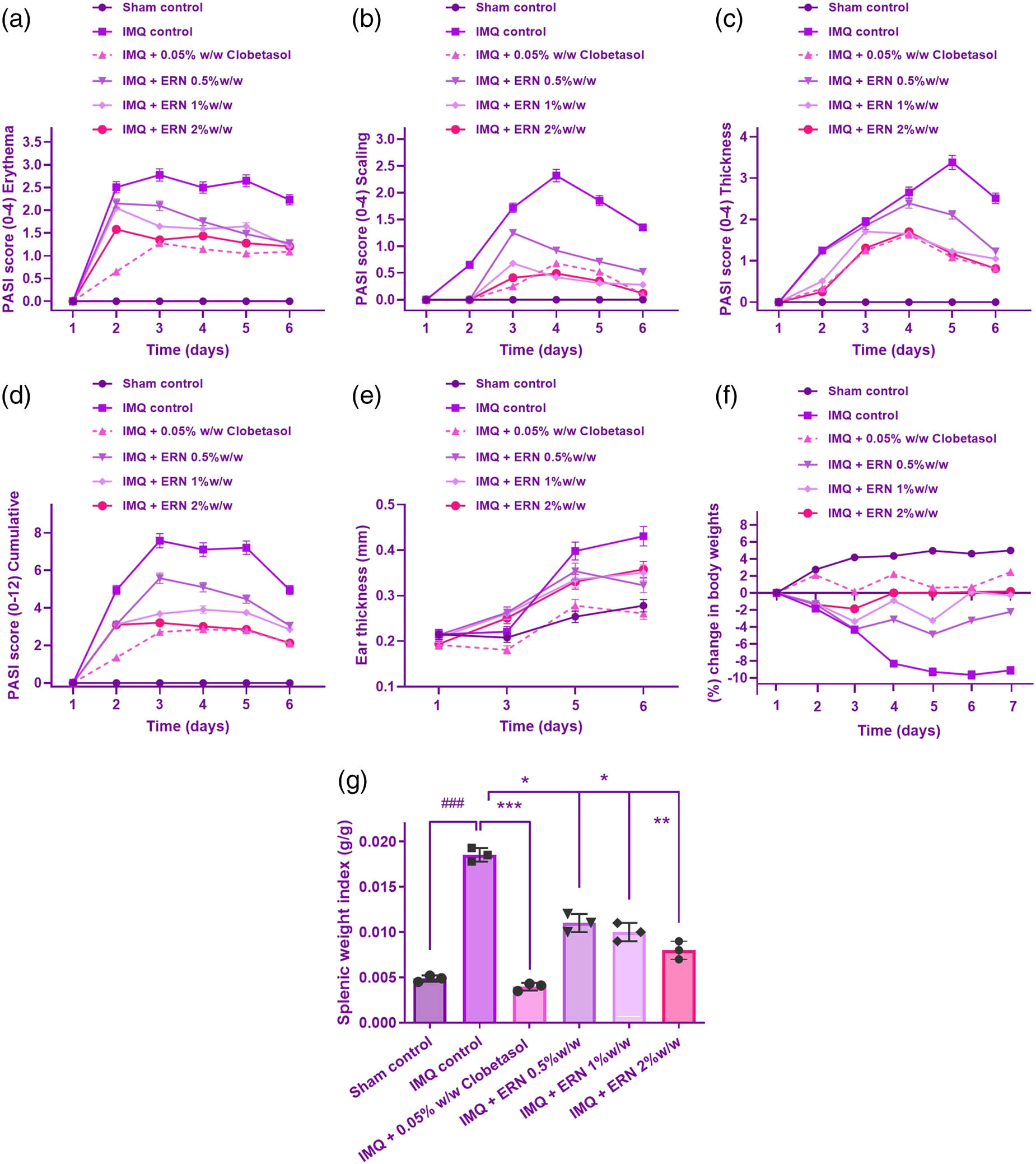
Effect of ERN on IMQ-induced psoriasiform severity in mice. ERN treatment significantly alleviated psoriatic skin changes in mice after 6 days, resembling human psoriasis. The severity of psoriasiform lesions was evaluated using the PASI based on erythema (redness), scaling, and skin thickness, scored from 0 to 4. The following parameters were assessed: (a) Erythema, (b) scaling, (c) thickness, (d) cumulative PASI score, (e) ear thickness, measured using a screw gauge on days 1, 3, 5, and 6, (f) changes in body weight induced by IMQ and its modulation by ERN treatment, (g) spleen mass, calculated as the spleen-to-body weight ratio, comparing ERN-treated and IMQ control groups. Results are expressed as mean value ± SEM (n = 8). ### p < 0.001; ## p < 0.01, # p < 0.05 compared with the sham control group; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the IMQ alone treated group; ns, non-significant.
IMQ administration induced a progressive increase in erythema from day 1, peaking around day 4 and remaining at a high level through day 6. Animals treated with ERN showed an important drop in erythema related to the IMQ-only group, with erythema scores in the ERN-treated group remaining 1–2 points lower throughout the treatment period (Figure 3a).
IMQ-induced scaling became evident by day 2 and intensified through day 6 (Figure 3b). In the IMQ group, scales were thick and widespread, with scores reaching 3–4 by day 4. ERN-treated animals showed a marked reduction in scaling, with scores approximately 1–1.5 points lower than the IMQ-only group. On Day 6, the 1% ERN group had marginally higher PASI scaling scores than the 0.5% group. These data point to a potential plateau or nonlinear connection in ERN’s therapeutic impact at increasing dosages. By the end of the observation period, scaling was significantly reduced in ERN-treated animals, suggesting ERN’s effectiveness in controlling hyperkeratosis.
Measurement of skin thickness using an Aerospace digimatic micrometer screw gauge demonstrated that the IMQ-treated animals developed substantial epidermal thickening by day 2, which continued to increase until day 6. ERN treatment significantly attenuated this increase, with skin thickness measurements consistently showing a lower degree of thickening compared to the IMQ-only group. By day 6, ERN-treated animals displayed skin thickness scores that were approximately 30–40% lower than untreated IMQ animals (Figure 3c).
The overall PASI score, obtained by summing the erythema, skin thickness, and scaling scores, provided a cumulative measure of psoriasis severity. In IMQ-only animals, the PASI score increased from an average of 2 on day 1 to around 10 by day 6, indicating severe psoriasiform inflammation. ERN treatment consistently reduced the PASI score across all days, with a final average PASI score of approximately 5 by day 6. This reduction in PASI score indicates a substantial alleviation of psoriasis-like symptoms with ERN treatment (Figure 3d). ERN considerably decreased and maintained the ear’s IMQ-induced epidermal thickness, as seen in Figure 3e. Also, it was observed that the animals in the common drug group demonstrated a reduction in their psoriatic symptoms.
Macroscopic morphology of the dorsal skin on different days revealed visible differences between IMQ-only and ERN-treated animals (Figure S1). IMQ-treated animals exhibited progressively more pronounced erythema, thickening, and scaling over time. In contrast, the ERN-treated animals maintained relatively smooth and less inflamed skin, with a reduced severity of scales and erythema, supporting the quantitative PASI findings.
Body weight measurements were made daily to analyze the consequences of ERN therapy (0.5, 1, and 2% w/w) and IMQ treatment. The IMQ-treated category’s body weight steadily dropped for 6 days, which was probably due to systemic inflammation and stress caused by psoriasis-like skin lesions. At all dosages (0.5, 1, and 2%), ERN treatment reduced IMQ-induced weight loss, with higher doses showing a more pronounced prophylactic effect (Figure 3f). There existed no notable distinctions between the ERN-treated category and the untreated group, indicating that ERN therapy had no adverse systemic effects.
The weight of every animal’s spleen was divided by its total weight to determine the spleen-to-bodyweight ratio, often known as the organ’s index. In the IMQ-only group, the organ index was notably elevated, with a mean increase of approximately 40–50% compared to control animals. This elevated organ index reflects the systemic inflammatory response triggered by IMQ treatment.
In contrast, ERN treatment led to a substantial decline in the organ index associated with the IMQ-only group. ERN-treated animals displayed an organ index that was approximately 20–30% lower than the IMQ group, indicating mitigation of spleen enlargement and suggesting an anti-inflammatory effect of ERN on systemic immune responses (Figure 3g). Statistical comparisons between groups revealed that the organ index in the IMQ-only group was significantly higher than in the control group (p < 0.05). ERN treatment significantly condensed the organ index associated with the IMQ-only group (p < 0.05), indicating that ERN treatment attenuated the systemic inflammatory response. The enhanced immunological activity brought on by psoriasiform inflammation is consistent with the higher organ index in the IMQ-only group. The spleen-to-bodyweight ratio decreased in the group that received ERN treatment, indicating that ERN has an immune-regulating impact that lowers the level of systemic inflammation. These results indicate that ERN may help to control not only local skin inflammation but also the broader immune activation associated with psoriasis.
3.4 Effect of ERN on oxidative stress markers in IMQ-induced skin inflammation in animal tissue
Figure 4 shows that treatment with IMQ alone substantially exacerbated oxidative stress, as demonstrated by elevated levels of MDA and nitric oxide (NO), as well as a substantial decline in antioxidant defense markers such as GSH, SOD, CAT, and vitamin C when compared to the control group. These data demonstrate that IMQ weakens the antioxidant barrier, resulting in increased oxidative damage.
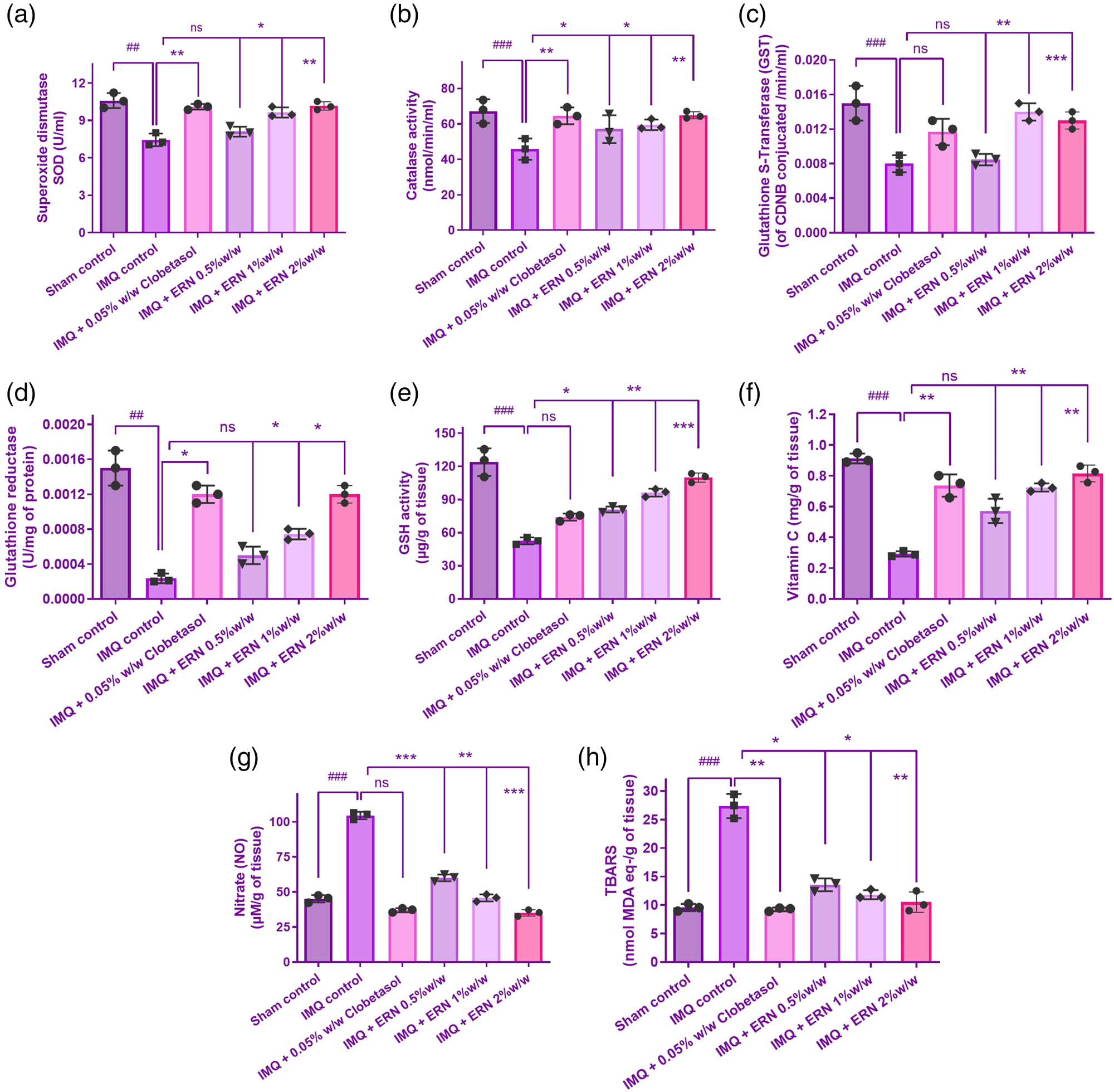
Effects of ERN treatment on antioxidant and oxidative stress markers in IMQ-induced psoriasis- skin tissue. (a)–(h) Biochemical analysis of antioxidant enzyme activity and oxidative stress markers in all experimental groups. Results are presented as mean values ± SEM (n = 6). ### p < 0.001, ## p < 0.01, # p < 0.05 compared with the sham control group; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the IMQ alone treated group; ns, non-significant.
On the other hand, ERN therapy dramatically reduced the oxidative damage caused by IMQ. This was demonstrated by reduced MDA and NO levels, as well as the restoration of antioxidant indices such as GSH, SOD, CAT, and vitamin C activities (Figure 4a–h). These findings imply that ERN improves the endogenous antioxidant defence system and might defend against IMQ-induced oxidative damage. The antioxidant enzyme levels (SOD and CAT) in the 2% ERN group were not substantially higher than those in the 1% group (Figure 4c and d). The results indicate an expected threshold or nonlinear connection in ERN’s therapeutic impact as doses increase. More research is needed to better understand the molecular processes behind ERN’s antioxidant capability.
3.5 Histopathological and clinical assessment of skin inflammation
Skin tissues were histologically examined with hematoxylin and eosin (H&E) staining to determine the therapeutic potential of ERN in IMQ-induced psoriatic mice (Figure 5a and b). Histological depiction of the IMQ-only group exhibited classic psoriatic characteristics such as extensive acanthosis, hyperkeratosis, parakeratosis, rete ridge elongation, and extensive dermal inflammatory infiltrates. In comparison, the control group’s skin had normal epidermal thickness and was free of inflammation.
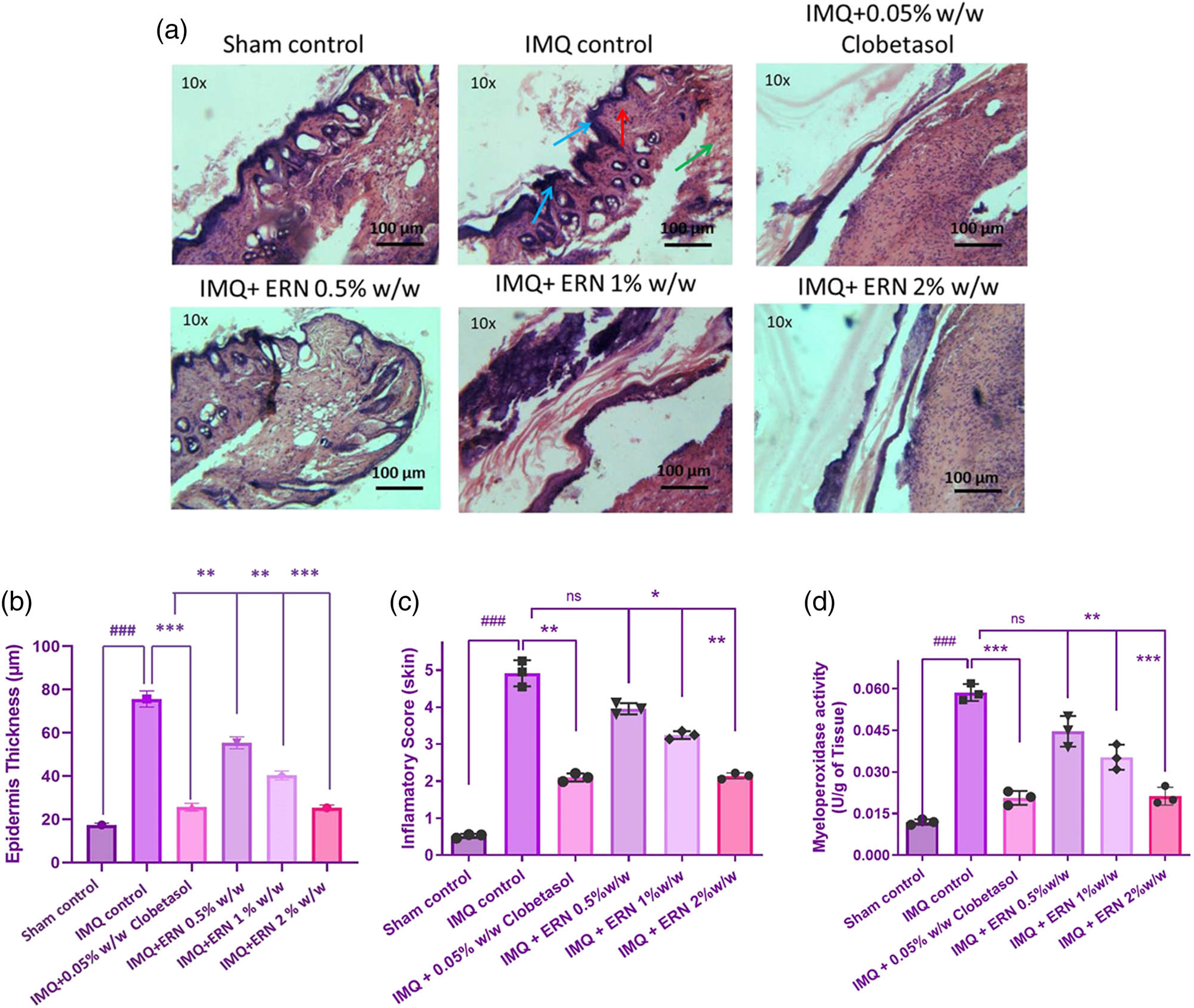
Impact of ERN treatment on histopathological alterations in IMQ tempted psoriasis. Representative H&E-stained photomicrographs of (a) skin tissue for all experimental groups. Histopathological assessments include blue arrows indicating hyperkeratosis, red arrows denoting acanthosis, and green arrows highlighting the infiltration of neutrophils. (b) Quantification of epidermal thickness from H&E-stained dorsal skin sections. Epidermal thickness was measured at five randomly selected sites per section using ImageJ software. IMQ-induced epidermal thickening was significantly reduced following ERN treatment in a dose-dependent manner. (c) Inflammatory score of the skin and (d) MPO activity in skin tissue. Blue arrows indicate hyperkeratosis, red arrows indicate acanthosis, and green arrows indicate infiltration of neutrophils in histopathology images. Data are expressed as mean values ± SD. n = 6 animals per group, experiments were repeated three times. ### p < 0.001; ## p < 0.01, # p < 0.05 compared with the sham control group; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the IMQ alone treated group; ns, non-significant.
ERN-treated groups (0.5, 1, and 2% w/w) showed dose-dependent enhancement in histopathological characteristics. The 2% ERN group had the greatest loss in epidermal thickness and immune cell infiltration, closely mirroring the skin architecture seen in the clobetasol-treated standard group. ERN treatment significantly reduced inflammatory severity, epidermal hyperplasia, and keratinocyte proliferation compared to IMQ alone (p < 0.001; Figure 5b). These refined histological data highlight the capacity of ERN to dramatically restore close to typical skin architecture in psoriatic mice. The combined visual and quantitative evaluations show strong evidence for ERN’s anti-psoriatic effectiveness.
3.6 Effect of ERN on skin tissue MPO
The skin tissue data showed that the IMQ monotherapy recipients had significantly greater MPO activity, a marker of neutrophil infiltration, than those who were not treated. As opposed to the IMQ monotherapy group of cures, MPO activities significantly decreased MPO levels during exposure to increased ERN doses (Figure 5c).
3.7 Determining parameters for hematology
The blood cell count data revealed differential responses between the experiments, IMQ and control groups (Figure 6). Outcomes exhibited that the levels of WBC in the blood were noticeably greater in the IMQ individually treated group than those of the control group, indicating an inflammatory or immunological response to the therapy. Compared to the group that was obtaining IMQ alone, the treatment groups (IMQ + ERN) showed a substantial drop in WBC count (Figure 6a), which may indicate immunological suppression. The RBC count, hemoglobin, and hematocrit values did not significantly differ across groups, indicating that the experimental treatments did not affect these parameters. Consequently, our results demonstrate that ERN therapy effectively declined the quantity of WBCs in the distribution of whole blood, this has thus decreased the blood’s production of inflammatory chemicals, resulting in an anti-inflammatory impact.
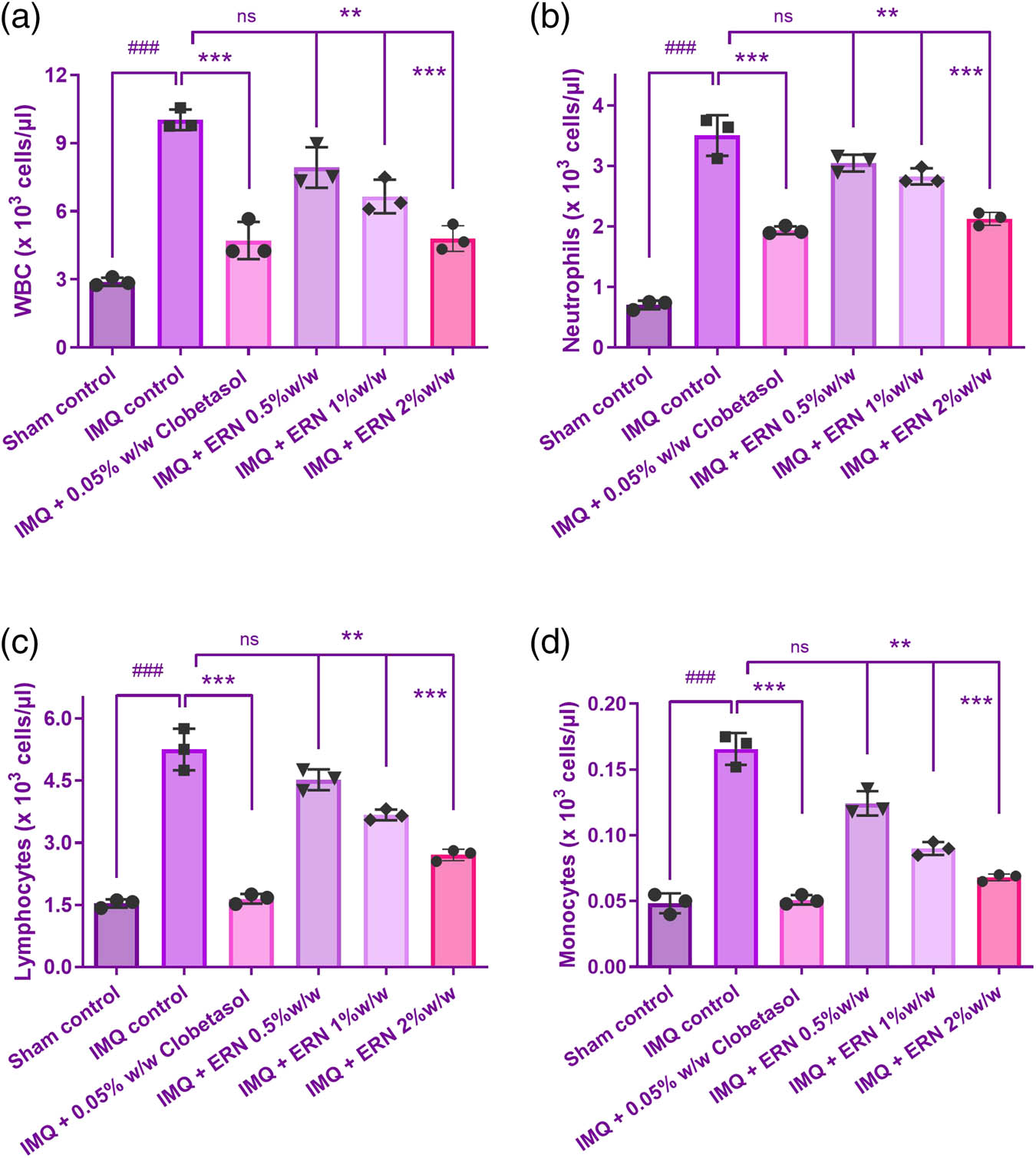
Effect of ERN treatment on hematological parameters in IMQ-exposure mice. Hematological analysis of whole blood from all experimental groups on day 7 post-treatment. (a) WBC count, (b) neutrophil count, (c) lymphocyte count, and (d) monocyte count. Results are expressed as mean value ± SEM (n = 6). ### p < 0.001, ## p < 0.01, and # p < 0.05 compared with the sham control group; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the IMQ alone treated group; ns, non-significant.
The IMQ-only group showed a significant increase in neutrophils, monocytes, and lymphocyte count indicating an inflammatory response (Figure 6b–d). ERN treatment (0.5, 1, and 2% w/w) significantly modulated these changes in a dose-dependent manner. Neutrophil and monocyte levels were notably decreased, while lymphocyte counts were restored toward normal levels, suggesting an immunomodulatory and anti-inflammatory effect of ERN.
3.8 ERN-mediated modulation of pro-inflammatory and anti-inflammatory cytokines
The proportions of pro-inflammatory (TNF-α, IL-6, IL-17, IL-23, and IL-1β) and anti-inflammatory (IL-10) cytokines were assessed in the serum and cutaneous tissue of psoriatic animals produced by IMQ to assess the immunomodulatory impact of ERN in psoriasis-like inflammation. Pro-inflammatory cytokines were significantly reduced in the skin tissue and serum in dissimilarity to the subjects treated with IMQ (Figure 7a–g), suggesting that ERN effectively modulates systemic and localized inflammation in psoriasis.
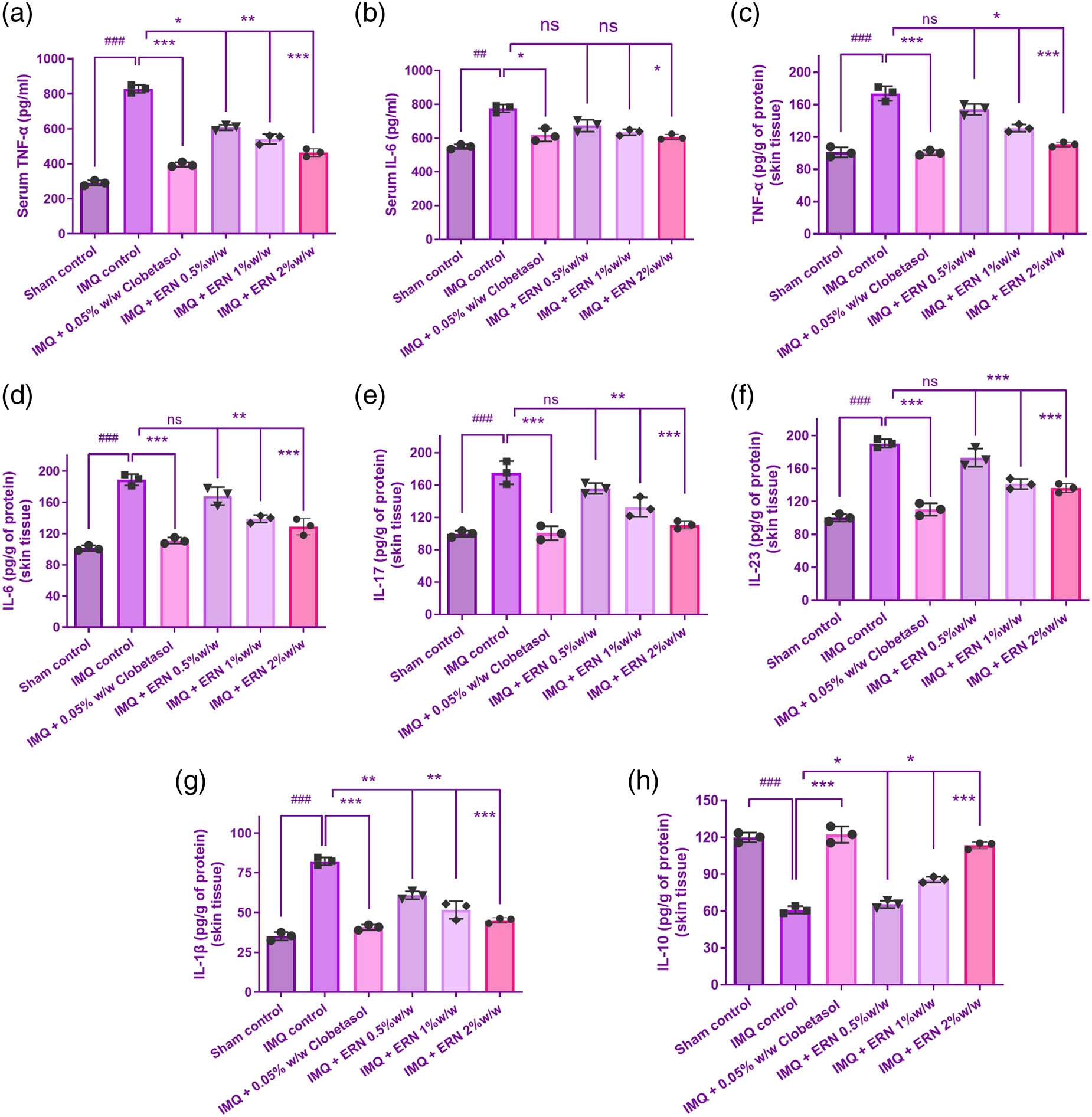
Proinflammatory and anti-inflammatory cytokine levels in IMQ-stimulated psoriatic skin tissue and serum. (a) TNF-α, (b) IL-6 levels, (c) TNF-α, (d) IL-6, (e) IL-17, (f) IL-23, and (g) IL-1β levels in mice skin tissue at 7 days post-ERN treatment. (h) IL-10 level in mouse skin tissue with ERN treatment, representing anti-inflammatory response. Cytokine levels were quantified using ELISA kits. The mean value ± SEM is used to present the data (n = 6). ### p < 0.001, ## p < 0.01, and # p < 0.05 compared with the sham control group; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the IMQ alone treated group; ns, non-significant.
The levels of anti-inflammatory cytokines elevated after ERN treatment, especially IL-10, and these had elevated levels in the treated animals’ blood and epidermis (Figure 7h). This supports ERN’s promise as a treatment for psoriasis by indicating that it encourages the skin to respond in a way that is less inflammatory.
3.9 Impact of ERN on NF-κB signaling
The present study assessed the expression profiles of key proteins involved in the NF-κB signaling pathway to evaluate the effect of ERN in IMQ-induced psoriatic skin. IMQ treatment markedly increased the total expression and nuclear localization of NF-κB p65, as demonstrated by Western blot (Figure 8a), indicating enhanced activation of the NF-κB pathway. In parallel, expression of downstream pro-inflammatory mediators iNOS and COX-2 was also significantly upregulated in IMQ-treated mice (Figure 8b). Treatment with ERN at concentrations of 0.5, 1, and 2% resulted in a dose-dependent reduction in nuclear NF-κB p65 expression (Figure 8c). Furthermore, this finding supported ERN’s function in preventing NF-κB-mediated inflammation by confirming lower nuclear translocation of NF-κB p65 in ERN-treated groups. This effect was accompanied by a substantial decrease in the expression of iNOS and COX-2 (Figure 8d and e), suggesting attenuation of the NF-κB-mediated inflammatory response. Taken together, these findings suggest that ERN mitigates IMQ-induced psoriasis-like inflammation by suppressing NF-κB signaling and its downstream inflammatory mediators.
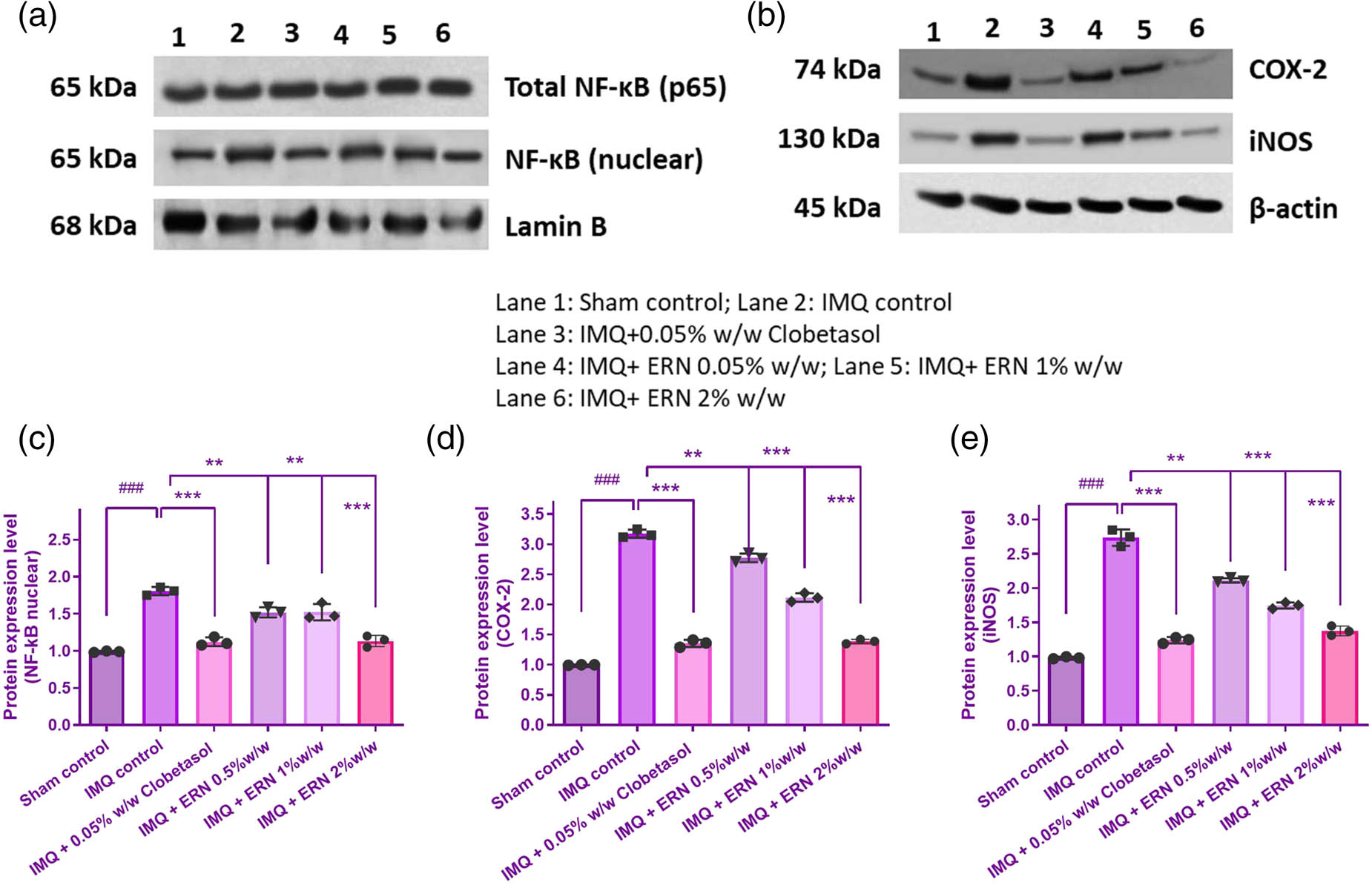
Impact of ERN therapy on the NF-κB signaling pathway in skin tissue produced by IMQ. The appearance levels of p65, COX-2, and iNOS in the skin tissue of IMQ compared to ERN are shown in (a) and (b), an illustrative immunological blot analysis. β-actin and Lamin B served as the internal reference. (c)–(e) shows the band intensities measured concerning protein expression using Image J software analyses. The findings were expressed using the mean value ± SEM (n = 3). ### p < 0.001 compared with the sham control group; **p < 0.01 and ***p < 0.001 compared with the IMQ alone treated group.
3.10 Activation of KEAP1-NRF2 pathway in animal models
The impact of ERN was then assessed by using immunoblotting techniques to influence the localization of HO-1 in the IMQ-induced skin and Nrf2 in the nuclear region. When comparing the IMQ-only treated group to the untreated control group, we discovered a substantial p < 0.001 drop in KEAP1 and nuclear deposition of NRF2 (Figure 9). On the contrary, western blot analysis demonstrated that ERN treatment expressively improved the levels of KEAP1, p62, HO-1, and NRF2 in the skin tissue (Figure 9a–e). Altogether, ERN progresses the antioxidant enzyme status in IMQ-induced psoriatic mice. These results propose that ERN modulates the KEAP1-NRF2 pathway in vivo, enhancing antioxidant defence mechanisms and reducing oxidative stress in psoriasis-like skin inflammation.
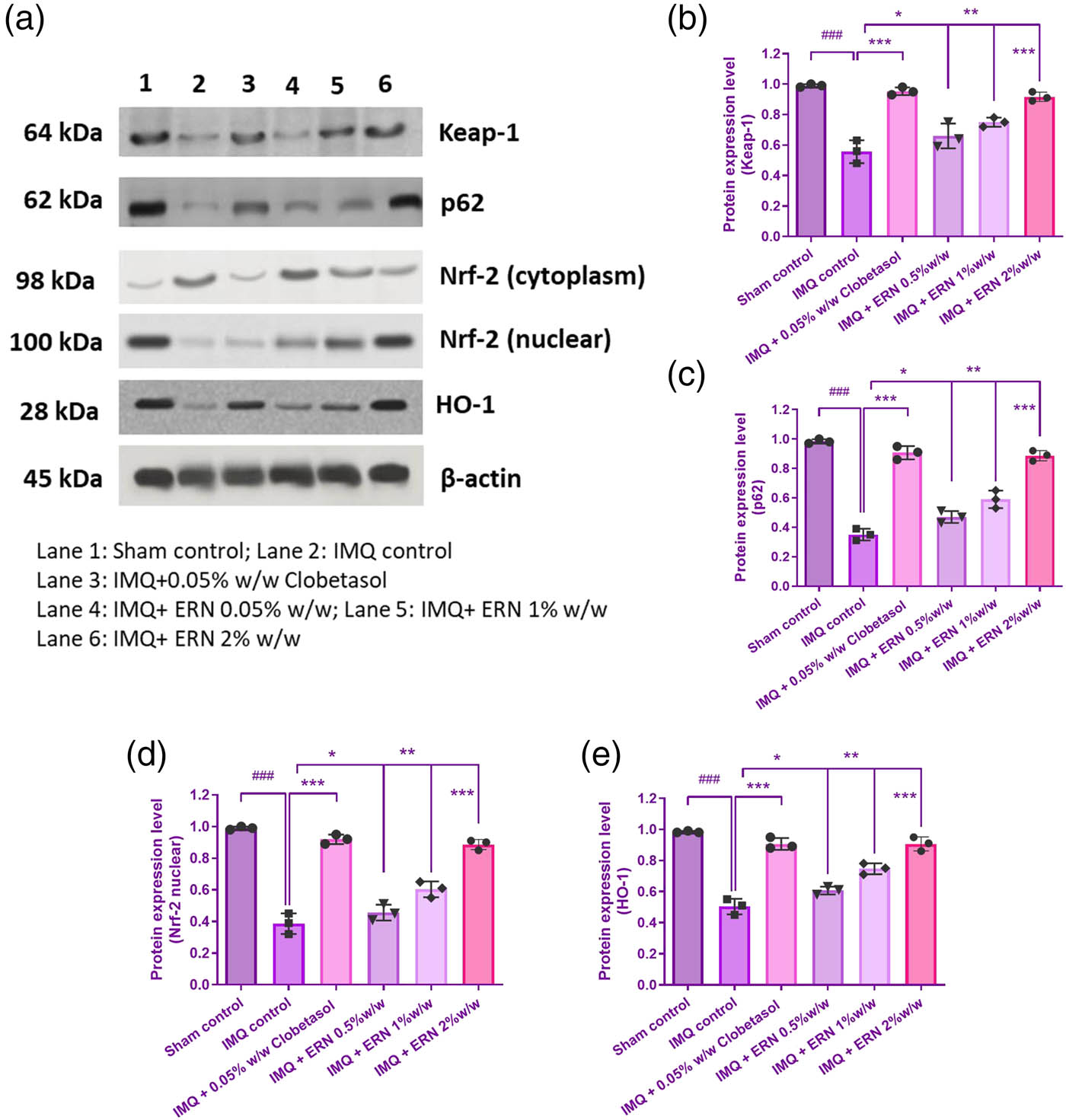
Impact of ERN therapy on the KEAP1-Nrf-2 signaling cascade in skin tissue that could be stimulated by IMQ. (a) The protein expression levels of Nrf2, p62, Keap1, and HO-1 in IMQ + ERN skin tissues are demonstrated by exemplary immunoblot studies. The internal control in this case was β-actin. (b)–(e) Shows various band intensities assessed by Image J software evaluation to protein expression levels graphically. Results are expressed as mean values ± SEM (n = 3). ### p < 0.001, ## p < 0.01, and # p < 0.05 compared with the sham control group; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the IMQ alone treated group; ns, non-significant.
4 Discussion
Psoriasis is a long-term autoimmune skin disorder caused by an overreactive defense system and an increase in the number of epithelial keratinocytes. Additionally, there is now no effective treatment for this illness; nevertheless, there are several treatment possibilities provided to control its symptoms, such as topical therapies that mostly include steroid medications. Steroid usage over time is known to have detrimental effects, including heightened susceptibility to infections, liver damage, and diabetes. Consequently, the development of new and safer psoriasis therapy drugs is crucial. Natural bioactive compounds have a great probability for treating illnesses and may be better than synthetic ones [40,41].
The most notable component is ERN, a naturally occurring bibenzyl molecule that has been utilized in traditional Chinese medicine as an analgesic and antipyretic [31,42]. ERN has been successfully demonstrated to have a therapeutic impact on decreasing tumor development and angiogenesis both in vivo and in vitro [43,44,45]. It shares structural similarities with combretastatin A-4, an attractive candidate for reducing the aberrant angiogenesis of cancer growth [46,47]. The possibility of ERN as a psoriasis treatment has not yet been investigated.
Therefore, the current study sought to evaluate the potential of ERN as a therapeutic agent in psoriasis by assessing its impacts on critical inflammatory pathways, oxidative stress control, and keratinocyte proliferation. In an in vivo IMQ-induced psoriasis model, ERN effectively reduces psoriatic symptoms via modulating NF-κB signaling, inflammatory cytokine release, and antioxidant response processes. Previous investigations [25,48,49] have linked NF-κB and oxidative stress to the etiology of psoriasis, indicating that ERN might be a potential therapy option. Furthermore, current research supports the regulation of the KEAP1-NRF2 pathway shown in our work, stressing its function in reducing oxidative damage and inflammation in diverse skin diseases.
Strong evidence linking ERN’s effectiveness as a psoriasis treatment is provided by this investigation’s findings. As a persistent inflammatory skin condition, psoriasis is characterized by pro-inflammatory cytokines being produced and uncontrolled keratinocyte growth [50]. The capacity of ERN to inhibit the growth of psoriasis-like keratinocytes and the inflammatory mediator process, which is a defining feature of psoriasis pathophysiology, serves as one of the main conclusions of this study. This effect was shown in both in vitro and in vivo settings, representing that ERN has a wide-ranging impact on pathways linked to psoriasis. AKT/mTOR and JNK/c-Jun signaling driven by ROS inhibited the proliferation of HaCaT cells and resulted in their death [51].
ERN dramatically decreased the stimulation of these mechanisms, which subsequently in turn suppressed the formation of inflammatory molecules such as TNF-α, IL-6, IL-1β, IL-23, and IL-17, according to this investigation. It is important to note that IL-8 and IL-22 were only assessed in the cell-based experiments. IL-8, a major chemokine involved in neutrophil recruitment, is not functionally conserved in mice and thus was not included in the animal experiments. Similarly, IL-22, while relevant to skin inflammation, was not evaluated in vivo due to the prioritization of cytokines more commonly associated with the IMQ-induced murine model, such as IL-17 and IL-23. These choices reflect methodological differences and highlight how the in vitro and in vivo models offer complementary insights into the anti-inflammatory potential of the treatment. Current evidence suggests that these cytokines play a role in initiating and maintaining the inflammation associated with psoriasis [52,53]. ERN may aid in the restoration of immunological homeostasis in psoriasis by inhibiting these pathways. Furthermore, the ability of ERN to increase IL-10 levels, an anti-inflammatory, inflammatory substance, implies that ERN usually promotes an anti-inflammatory milieu. Especially, the excessive production of IL-10, which may aid in rebuilding and restoring the epidermal obstacles, are frequently compromised in psoriasis, in conjunction with inhibiting cytokines that cause inflammation.
Our findings highlight the need to combine in vitro and in vivo approaches for understanding ERN’s anti-psoriatic properties. The in vitro model focused on keratinocyte reaction to TNF-α stimulation, whereas the in vivo model assessed the overall immunological dynamics, such as the IL-23/IL-17 axis. This divergence across keratinocyte-centric in vitro inflammation and immune-driven in vivo responses emphasizes our study’s translational implications. It reveals how ERN has diverse immunomodulatory effects at many stages of the psoriatic inflammatory process. This comprehensive approach not only validates ERN’s effectiveness but also demonstrates its potential application across different phases and processes of psoriasis etiology.
It is critical to recognize the distinction between the IMQ-induced mouse model of psoriasis and human psoriasis. Although the IMQ model accurately reproduces several inflammatory aspects of psoriasis, such as epidermal hyperplasia, immune cell infiltration, and skin thickness, it does not entirely imitate the complicated immunopathogenesis seen in actual psoriasis. Human psoriasis is predominantly caused by the IL-23/Th17 axis, with Th17 cells generating pro-inflammatory cytokines including IL-17 and IL-22, which play an important role in inflammation and tissue destruction [54]. Conversely, the IMQ-induced psoriasis paradigm primarily stimulates the IL-1 and TLR7 pathways, which, while contributing to inflammation, vary from the main IL-23/Th17-mediated inflammation reported in patients [55,56]. Consequently, while the results we obtained give useful insights into the potential therapeutic benefits of ERN, more advanced models that better mirror the actual disease pathophysiology are required to properly comprehend the therapeutic impact of ERN in human psoriasis.
The observed reduction in IL-23 and IL-17 in vivo may be due to ERN-mediated NRF2 activation, as evidenced by enhanced nuclear Nrf2 and HO-1 expression in psoriatic lesions. Previous investigation has connected NRF2 activation to the suppression of Th17-related cytokines via redox-sensitive pathways [15,57]. However, the current approach did not show a clear causal association, our findings indicate that the NRF2 pathway may play a role in controlling Th17-driven inflammation in psoriasis. Further research using NRF2-specific inhibition or gene knockdown will assist in elucidating this process.
ERN effectively decreased the histological characteristics of psoriasis, which includes excessive keratin and epidermis dimension, along with its clinical signs, characterized as erythema and scaling, in the context of preliminary animal experiments. A rise in blood indicators, namely, a drop in WBC count, additionally showed the comprehensive anti-inflammatory effects of ERN, which may eventually result in therapeutic advantages in individuals.
The organ index, also known as the spleen-to-body mass ratio, was used to quantify total inflammation in the IMQ-induced psoriasis theory. Spleen enlargement is often associated with psoriasis and other inflammatory diseases because of increased immune cell trafficking and immunological activation. A normalized spleen-to-body weight connection in grams per gram (g/g) [38], was obtained by calculating the organ index in the current research by matching the weight of a particular animal’s spleen to its entire weight. The organ index (spleen-to-body mass proportion) findings demonstrate that ERN treatment significantly reduces spleen enlargement associated with IMQ-induced systemic inflammation. This reduction in the spleen index supports ERN’s role as an anti-inflammatory agent, potentially beneficial in alleviating both local and systemic inflammatory responses in psoriatic conditions.
Multiple investigations indicate that oxidative stress plays a foremost part in the pathophysiology of psoriasis. Reduced activity of anti-oxidant enzymes, augmented ROS levels, and an imbalance between oxidants and antioxidants are particular factors that impact the etiology and development of psoriasis. Ma et al. [28], Pleńkowska et al. [11], Bakić et al. [58], and Klisic et al. [59] suggest that oxidative stress biomarkers can be used to measure the severity of psoriasis, predict the course of other medical problems, and assess the effectiveness of treatment. Some of the exogenous oxidative stressors that can exacerbate psoriasis symptoms include medications, alcohol, smoking, stress, and bacterial infections [60–62]. Our study confirmed that ERN has antioxidant properties, as it significantly reduced the oxidative stress damage associated with psoriasis.
The KEAP1/NRF2 cascade has anti-inflammatory and antioxidant activity properties and is crucial for maintaining skin homeostasis [29]. Research indicates that NRF2 may enhance keratinocyte proliferation and aid in the formation of skin epidermal barriers [63,64]. Dimethyl fumarate, an NRF2 activator, is used therapeutically to treat moderately severe and mild psoriasis [65–67], and some of its constituents have been investigated and proposed to relieve psoriasis [68]. However, excessive NRF2 stimulation might be detrimental. Consequently, additional study is needed to understand how NRF2 impacts psoriasis and to identify safe and effective NRF2 stimulants for psoriasis treatment.
The primary process behind ERN’s anti-inflammatory and antioxidant actions is its stimulation of the KEAP1-NRF2 cascade. Under normal conditions, NRF2 binds to KEAP1 to maintain its inactive state in the cytoplasm. NRF2 separates from KEAP1 and moves to the nucleus in response to signs of oxidative stress or inflammation. There, it triggers the production of protective enzymes that lessen cell damage [28,69]. In our investigation, we found that ERN therapy increased the expression of NRF2 and its subsequent targets, including having an impact on reducing oxidative stress and deactivating ROS, including SOD, CAT, and GST. This implies that ERN may lessen the pathophysiology of psoriasis by regulating inflammation and lowering oxidative stress in the skin. The present investigation supports earlier findings [28]. However, our observations suggest that the therapeutic benefits of ERN may not follow a precise linear dosage response trend. For instance, the 1% ERN dosage exceeded the 2% dose in certain outcome measures, such as skin scaling and antioxidant enzyme activity. This shows that raising the dose over a particular threshold may not increase efficacy correspondingly, emphasizing the necessity of determining the ideal therapeutic range. Additional research is needed to understand the pharmacodynamic (PD) ceiling of ERN and find the most effective and safe dose range. Such nonlinear dose-response relationships have been reported in other natural compounds, including curcumin and resveratrol, where high doses do not always equate to greater efficacy and may, in some cases, reduce bioactivity [70,71].
Evidence indicates that NRF2 may support the development of skin-epidermal obstacles and enhance cell differentiation [20,66,72]. The NRF2 stimulator dimethyl fumarate has been employed therapeutically to treat moderately severe psoriasis [73], and some of its derivatives may be able to alleviate psoriasis. On the other hand, activating NRF2 too much could be harmful [19]. Thus, more investigation into how NRF2 functions in psoriasis is required, as is the hunt for safe and efficient NRF2-activating drugs to treat psoriasis.
Our results are in line with the existing research, which indicates that healthy skin has high levels of NRF2 expression [19,74,75]. Many skin conditions may benefit from NRF2 activation as a treatment. Remarkably, we discovered that in the lesional skin of a rat model of psoriatic IMQ, NRF2 expression was markedly decreased. ERN therapy significantly raised NRF2 expression and decreased the psoriatic pathogenic phenotype by halting oxidative stress-induced skin tissue damage and inflammation. Since NRF2 depletion increased the mice’s inflammatory reaction, aberrant keratinocyte development, and psoriasis-like signs and symptoms, the curative properties of ERN appeared to be NRF2-dependent. This result confirms our theory that ERN operates through NRF2-related pathways and that NRF2 protects against the onset of psoriasis.
According to Silva et al. [76], the popular inflammatory cytokine TNF-α is the cause of numerous inflammatory diseases. Macrophages are the primary generators of this toxic material during inflammation [77,78]. The main way that TNF-α causes inflammation is by paracrine and autocrine stimulation of macrophages, which increases the production of inflammatory cytokines like IL-1β, IL-6, COX-2, and iNOS. This can result in a cascade caused by an inflammation cascade [79–81]. Moreover, anti-TNF-α treatment alleviates dermatitis, and TNF-α contributes to the inflammatory process of skin inflammatory diseases [82,83].
Additionally, ERN increased the levels of antioxidant enzymes while dramatically lowering nitrites and TBARS. In the reaction to IMQ-induced psoriasis, these results led to the defensive activity of ERN by increasing antioxidant enzymes, preventing the production of lipid peroxidation byproducts in macrophages via COX-2 expression, and lowering nitrite concentrations within iNOS expression. ERN-treated animals with IMQ-induced psoriasis showed markedly decreased amounts of COX-2 and iNOS protein expression. By activating the Nrf2 system in psoriatic mice, we explain how the number of plant-based constituents and associated structural analogues provide increased protection against oxidative stress [38,84]. The increase in phase II-detoxifying and antioxidant enzymes from oxidative damage is aided by Nrf2 modulation [85]. According to a prior study, Nrf2 expression interruptions used redox control to repress the pro-inflammatory and inflammation-related genes of mediators [86–88]. The impact of ERN on the pathway used by NF-B to signal in skin tissues was confirmed by our study. By improving the expression of KEAP1, p62, and HO-1, we additionally investigated how ERN effectively increased the nuclear translocation of NRF2. Thus, ERN protects against psoriasis caused by IMQ through the antioxidant enzyme’s defense mechanism, which is aided by KEAP1-Nrf2.
Dimethyl fumarate (DMF), a well-known NRF2 activator approved for clinical use in treating psoriasis and multiple sclerosis, has demonstrated significant efficacy by modulating oxidative stress and immune responses through NRF2 pathway activation [66,89]. However, DMF is often associated with side effects such as gastrointestinal discomfort, flushing, and lymphopenia, which may impact long-term patient adherence [90].
In contrast, ERN, as evidenced by our findings, exhibits strong antioxidant and anti-inflammatory properties by activating the KEAP1-NRF2 pathway, with potentially fewer adverse effects. Notably, preclinical studies suggest that ERN can suppress key pro-inflammatory mediators and restore redox homeostasis with high potency [91]. While clinical trials are still lacking, the favorable safety and efficacy profiles observed in our study suggest that ERN could be a promising alternative to traditional NRF2 activators like DMF. Comparative studies assessing efficacy, bioavailability, and safety are essential to validate ERN’s potential advantages in a clinical setting.
Although ERN displayed strong anti-inflammatory and antioxidant benefits in both in vitro and in vivo psoriasis models, it is crucial to note that the exact pharmacokinetic (PK) and PD profiles of ERN are still poorly understood. Existing preclinical studies show that ERN has low water solubility and is rapidly degraded, potentially limiting its bioavailability and systemic exposure. These qualities may limit its clinical applicability until addressed by formulation tactics such as nanoparticle delivery systems or prodrug approaches. Furthermore, no human clinical studies or thorough PK/PD investigations have been published thus far. Future research should focus on complete PK analysis and delivery system optimization to fully investigate ERN’s therapeutic potential and support its clinical adoption.
In addition, whereas our work demonstrates the beneficial effects of ERN on inflammation and oxidative stress, the long-term ramifications of ERN, notably chronic NRF2 activation, must be carefully considered [92,93]. NRF2 is essential in cellular responses to oxidative stress, but prolonged activation of NRF2 may have unexpected repercussions, such as changes in immune cell function or disruption of normal cellular processes in other organs. Future studies should extensively study these possible consequences to deeply comprehend ERN’s safety profile. Long-term studies are required to determine the chronic consequences of NRF2 activation in vivo, as well as the influence on immune cells and diverse organs, in order to ensure ERN’s safety for long-term usage in clinical settings.
5 Conclusion
The current investigation shows that ERN has substantial potential as a treatment agent for psoriasis-like lesions. ERN reduces psoriasis symptoms by altering key molecular mechanisms, such as the KEAP1-NRF2 signaling cascade, which is important for lowering inflammation and oxidative stress. ERN has been shown to diminish TNF-α-induced inflammation and keratinocyte hyperproliferation in both in vitro and in vivo models, indicating it could serve as a reliable and efficient treatment for psoriasis. However, it is crucial to emphasize that, though the IMQ-induced psoriasis model gives useful insights into the disease process, the pathophysiology of clinical psoriasis may vary. To completely grasp the therapeutic potential of ERN in human psoriasis, more research with clinical samples is required. These investigations will serve to confirm ERN’s clinical relevance and influence future psoriasis therapy alternatives.
-
Funding information: Authors state no funding involved.
-
Author contributions: HY: conceptualization, methodology, original draft preparation, and data curation and GW: validation and supervision.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Bu J, Ding R, Zhou L, Chen X, Shen E. Epidemiology of psoriasis and comorbid diseases: a narrative review. Front Immunol. 2022;13:880201.10.3389/fimmu.2022.880201Suche in Google Scholar PubMed PubMed Central
[2] Damiani G, Bragazzi NL, Karimkhani AC, Wu D, Alicandro G, McGonagle D, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. 2021;8:743180.10.3389/fmed.2021.743180Suche in Google Scholar PubMed PubMed Central
[3] Man AM, Orasan MS, Hoteiuc OA, Olanescu-Vaida-Voevod MC, Mocan T. Inflammation and psoriasis: a comprehensive review. Int J Mol Sci. 2023;24(22):16095.10.3390/ijms242216095Suche in Google Scholar PubMed PubMed Central
[4] Rajguru JP, Maya D, Kumar D, Suri P, Bhardwaj S, Patel ND. Update on psoriasis: A review. J Family Med Prim Care. 2020;9(1):20–4.10.4103/jfmpc.jfmpc_689_19Suche in Google Scholar PubMed PubMed Central
[5] Albanesi C, Madonna S, Gisondi P, Girolomoni G. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front Immunology. 2018;9:1549.10.3389/fimmu.2018.01549Suche in Google Scholar PubMed PubMed Central
[6] Ni X, Lai Y. Crosstalk between keratinocytes and immune cells in inflammatory skin diseases. Explor Immunol. 2021;1(5):418–31.10.37349/ei.2021.00028Suche in Google Scholar
[7] Davis JS, Ferreira D, Paige E, Gedye C, Boyle M. Infectious complications of biological and small molecule targeted immunomodulatory therapies. Clin Microbiol Rev. 2020;33(3):10–1128.10.1128/CMR.00035-19Suche in Google Scholar PubMed PubMed Central
[8] Gupta S, Garbarini S, Nazareth T, Khilfeh I, Costantino H, Kaplan D. Characterizing outcomes and unmet needs among patients in the United States with mild-to-moderate plaque psoriasis using prescription topicals. Dermatol Ther. 2021;11:2057–75.10.1007/s13555-021-00620-xSuche in Google Scholar PubMed PubMed Central
[9] Luger T, Augustin M, Lambert J, Paul C, Pincelli C, Torrelo A, et al. Unmet medical needs in the treatment of atopic dermatitis in infants: An expert consensus on safety and efficacy of pimecrolimus. Pediatr Allergy Immunol. 2021;32(3):414–24.10.1111/pai.13422Suche in Google Scholar PubMed
[10] Kuang Y, Li Y, Lv C, Li M, Zhang Z, Chen Y, et al. Unmet needs and treatment preference of systemic treatments for moderate-to-severe psoriasis from the perspectives of patients and dermatologists in China. Dermatol Ther. 2024;14(5):1245–57.10.1007/s13555-024-01159-3Suche in Google Scholar PubMed PubMed Central
[11] Pleńkowska J, Gabig-Cimińska M, Mozolewski P. Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int J Mol Sci. 2020;21(17):6206.10.3390/ijms21176206Suche in Google Scholar PubMed PubMed Central
[12] Khan AQ, Agha MV, Sheikhan KSA, Younis SM, Al Tamimi M, Alam M, et al. Targeting deregulated oxidative stress in skin inflammatory diseases: An update on clinical importance. Biomed Pharmacother. 2022;154:113601.10.1016/j.biopha.2022.113601Suche in Google Scholar PubMed
[13] Wojcik P, Łuczaj W, Zarkovic N, Skrzydlewska E. Modulation of oxidative stress in psoriasis: Pathophysiology and therapy. In Modulation of oxidative stress. Italy: Academic Press; 2023. p. 255–78.10.1016/B978-0-443-19247-0.00014-XSuche in Google Scholar
[14] Tu W, Wang H, Li S, Liu Q, Sha H. The anti-inflammatory and anti-oxidant mechanisms of the KEAP1/NRF2/ARE signaling pathway in chronic diseases. Aging Dis. 2019;10(3):637.10.14336/AD.2018.0513Suche in Google Scholar PubMed PubMed Central
[15] Xu WD, Yang C, Huang AF. The role of Nrf2 in immune cells and inflammatory autoimmune diseases: a comprehensive review. Expert Opin Ther Targets. 2024;28(9):789–806.10.1080/14728222.2024.2401518Suche in Google Scholar PubMed
[16] Helou DG, Martin SF, Pallardy M, Chollet-Martin S, Kerdine-Römer S. NRF2 involvement in chemical-induced skin innate immunity. Front Immunol. 2019;10:1004.10.3389/fimmu.2019.01004Suche in Google Scholar PubMed PubMed Central
[17] Tian W, de la Vega MR, Schmidlin CJ, Ooi A, Zhang DD. Kelch-like ECH-associated protein 1 (KEAP1) differentially regulates nuclear factor erythroid-2-related factors 1 and 2 (NRF1 and NRF2). J Biol Chem. 2018;293(6):2029–40.10.1074/jbc.RA117.000428Suche in Google Scholar PubMed PubMed Central
[18] Kamel NM, El-Sayed SS, El-Said YA, El-Kersh DM, Hashem MM, Mohamed SS. Unlocking milk thistle’s anti-psoriatic potential in mice: Targeting PI3K/AKT/mTOR and KEAP1/NRF2/NF-κB pathways to modulate inflammation and oxidative stress. Int Immunopharmacol. 2024;139:112781.10.1016/j.intimp.2024.112781Suche in Google Scholar PubMed
[19] Panieri E, Telkoparan‐Akillilar P, Saso L. NRF2, a crucial modulator of skin cells protection against vitiligo, psoriasis, and cancer. BioFactors. 2023;49(2):228–50.10.1002/biof.1912Suche in Google Scholar PubMed
[20] Boo YC. Natural Nrf2 modulators for skin protection. Antioxidants. 2020;9(9):812.10.3390/antiox9090812Suche in Google Scholar PubMed PubMed Central
[21] Wroński A, Wójcik P. Impact of ROS-dependent lipid metabolism on psoriasis pathophysiology. Int J Mol Sci. 2022;23(20):12137.10.3390/ijms232012137Suche in Google Scholar PubMed PubMed Central
[22] Yu Q, Ke J, Xie B, Li N, Zhang M, Tang L, et al. Yinxieling attenuates psoriasis in mice by regulating oxidative stress and lipid mediators to correct immune cell disorder through the NF-κB/Nrf2 signaling pathways. J Tradit Complementary Med. 2024. 10.1016/j.jtcme.2024.08.003.Suche in Google Scholar
[23] Al-Harbi NO, Nadeem A, Ansari MA, Al-Harbi MM, Alotaibi MR, AlSaad AM, et al. Psoriasis-like inflammation leads to renal dysfunction via upregulation of NADPH oxidases and inducible nitric oxide synthase. Int Immunopharmacol. 2017;46:1–8.10.1016/j.intimp.2017.02.018Suche in Google Scholar PubMed
[24] Al-Harbi NO, Nadeem A, Al-Harbi MM, Zoheir KM, Ansari MA, El-Sherbeeny AM, et al. Psoriatic inflammation causes hepatic inflammation with concomitant dysregulation in hepatic metabolism via IL-17A/IL-17 receptor signaling in a murine model. Immunobiology. 2017;222(2):128–36.10.1016/j.imbio.2016.10.013Suche in Google Scholar PubMed
[25] Nadeem A, Ahmad SF, Al-Harbi NO, Ibrahim KE, Alqahtani F, Sobeai HMA, et al. Inhibition of interleukin-2-inducible T-cell kinase causes reduction in imiquimod-induced psoriasiform inflammation through reduction of Th17 cells and enhancement of Treg cells in mice. Biochimie. 2020;179:146–56.10.1016/j.biochi.2020.09.023Suche in Google Scholar PubMed
[26] Burlec AF, Hăncianu M, Ivănescu B, Macovei I, Corciovă A. Exploring the therapeutic potential of natural compounds in psoriasis and their inclusion in nanotechnological systems. Antioxidants. 2024;13(8):912.10.3390/antiox13080912Suche in Google Scholar PubMed PubMed Central
[27] Lee YG, Jung Y, Choi HK, Lee JI, Lim TG, Lee J. Natural product-derived compounds targeting keratinocytes and molecular pathways in psoriasis therapeutics. Int J Mol Sci. 2024;25(11):6068.10.3390/ijms25116068Suche in Google Scholar PubMed PubMed Central
[28] Ma C, Gu C, Lian P, Wazir J, Lu R, Ruan B, et al. Sulforaphane alleviates psoriasis by enhancing antioxidant defense through KEAP1-NRF2 Pathway activation and attenuating inflammatory signaling. Cell Death Dis. 2023;14(11):768.10.1038/s41419-023-06234-9Suche in Google Scholar PubMed PubMed Central
[29] Ogawa T, Ishitsuka Y. The role of KEAP1-NRF2 system in atopic dermatitis and psoriasis. Antioxidants. 2022;11(7):1397.10.3390/antiox11071397Suche in Google Scholar PubMed PubMed Central
[30] Li G, Zhang H, Lai H, Liang G, Huang J, Zhao F, et al. Erianin: a phytoestrogen with therapeutic potential. Front Pharmacol. 2023;14:1197056.10.3389/fphar.2023.1197056Suche in Google Scholar PubMed PubMed Central
[31] Qiao Q, Du Y, Xie L. Research advances of erianin: Source, production, biological activities and pharmacological properties. Pharmacol Res-Modern Chin Med. 2022;2:100059.10.1016/j.prmcm.2022.100059Suche in Google Scholar
[32] Zhang X, Hu L, Xu S, Ye C, Chen A. Erianin: A direct NLRP3 inhibitor with remarkable anti-inflammatory activity. Front Immunol. 2021;12:739953.10.3389/fimmu.2021.739953Suche in Google Scholar PubMed PubMed Central
[33] Jia Z, Yue W, Zhang X, Xue B, He J. Erianin alleviates cerebral ischemia-reperfusion injury by inhibiting microglial cell polarization and inflammation via the PI3K/AKT and NF-κB pathways. Int Immunopharmacol. 2024;141:112915.10.1016/j.intimp.2024.112915Suche in Google Scholar PubMed
[34] Xiang Y, Chen X, Wang W, Zhai L, Sun X, Feng J, et al. Natural product erianin inhibits bladder cancer cell growth by inducing ferroptosis via NRF2 inactivation. Front Pharmacol. 2021;12:775506.10.3389/fphar.2021.775506Suche in Google Scholar PubMed PubMed Central
[35] Yang A, Li MY, Zhang ZH, Wang JY, Xing Y, Ri M, et al. Erianin regulates programmed cell death ligand 1 expression and enhances cytotoxic T lymphocyte activity. J Ethnopharmacol. 2021;273:113598.10.1016/j.jep.2020.113598Suche in Google Scholar PubMed
[36] Li J, Ren F, Yan W, Sang H. Kirenol inhibits TNF-α-induced proliferation and migration of HaCaT cells by regulating NF-κB pathway. Qual Assur Saf Crop Foods. 2021;13(4):44–53.10.15586/qas.v13i4.968Suche in Google Scholar
[37] Yue LU, Ailin W, Jinwei Z, Leng L, Jianan W, Li L, et al. PSORI-CM02 ameliorates psoriasis in vivo and in vitro by inducing autophagy via inhibition of the PI3K/Akt/mTOR pathway. Phytomedicine. 2019;64:153054.10.1016/j.phymed.2019.153054Suche in Google Scholar PubMed
[38] Sangaraju R, Alavala S, Nalban N, Jerald MK, Sistla R. Galangin ameliorates Imiquimod-Induced psoriasis-like skin inflammation in BALB/c mice via down regulating NF-κB and activation of Nrf2 signaling pathways. Int Immunopharmacol. 2021;96:107754.10.1016/j.intimp.2021.107754Suche in Google Scholar PubMed
[39] Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–9.10.1111/1523-1747.ep12506462Suche in Google Scholar PubMed
[40] Zhao Z, Liu T, Zhu S, Pi J, Guo P, Qi D, et al. Natural medicine combined with nanobased topical delivery systems: a new strategy to treat psoriasis. Drug Delivery Transl Res. 2022;12(6):1326–38.10.1007/s13346-021-01031-3Suche in Google Scholar PubMed
[41] Huang TH, Lin CF, Alalaiwe A, Yang SC, Fang JY. Apoptotic or antiproliferative activity of natural products against keratinocytes for the treatment of psoriasis. Int J Mol Sci. 2019;20(10):2558.10.3390/ijms20102558Suche in Google Scholar PubMed PubMed Central
[42] Wei X, Liu J, Xu Z, Wang D, Zhu Q, Chen Q, et al. Research progress on the pharmacological mechanism, in vivo metabolism and structural modification of Erianin. Biomed Pharmacother. 2024;173:116295.10.1016/j.biopha.2024.116295Suche in Google Scholar PubMed
[43] Yan L, Zhang Z, Liu Y, Ren S, Zhu Z, Wei L, et al. Anticancer activity of erianin: Cancer-specific target prediction based on network pharmacology. Front Mol Biosci. 2022;9:862932.10.3389/fmolb.2022.862932Suche in Google Scholar PubMed PubMed Central
[44] Zhang HQ, Xie XF, Li GM, Chen JR, Li MT, Xu X, et al. Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytother Res. 2021;35(8):4511–25.10.1002/ptr.7154Suche in Google Scholar PubMed
[45] Gong YQ, Fan Y, Wu DZ, Yang H, Hu ZB, Wang ZT. In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent. Eur J Cancer. 2004;40(10):1554–65.10.1016/j.ejca.2004.01.041Suche in Google Scholar PubMed
[46] Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000;66(8):709–23.10.1016/S0024-3205(99)00642-6Suche in Google Scholar PubMed
[47] Singh SB. Discovery, synthesis, activities, structure–activity relationships, and clinical development of combretastatins and analogs as anticancer drugs. A comprehensive review. Nat Product Rep. 2024;41(2):298–322.10.1039/D3NP00053BSuche in Google Scholar
[48] Ahmad SF, Zoheir KM, Ansari MA, Korashy HM, Bakheet SA, Ashour AE, et al. Stimulation of the histamine 4 receptor with 4-methylhistamine modulates the effects of chronic stress on the Th1/Th2 cytokine balance. Immunobiology. 2015;220(3):341–9.10.1016/j.imbio.2014.10.014Suche in Google Scholar PubMed
[49] Alzahrani KS, Nadeem A, Ahmad SF, Al-Harbi NO, Ibrahim KE, El-Sherbeeny AM, et al. Inhibition of spleen tyrosine kinase attenuates psoriasis-like inflammation in mice through blockade of dendritic cell-Th17 inflammation axis. Biomed Pharmacother. 2016;111:347–58.10.1016/j.biopha.2018.12.060Suche in Google Scholar PubMed
[50] Singh S, Ramani P, Jayakumar ND, Pannu SJ, Sharma RK, Gill SS. Role of pro-inflammatory and anti-inflammatory cytokines in pathophysiology of psoriasis. Curr Oral Health Rep. 2022;9(4):132–45.10.1007/s40496-022-00320-1Suche in Google Scholar
[51] Mo C, Shetti D, Wei K. Erianin inhibits proliferation and induces apoptosis of HaCaT cells via ROS-mediated JNK/c-Jun and AKT/mTOR signaling pathways. Molecules. 2019;24(15):2727.10.3390/molecules24152727Suche in Google Scholar PubMed PubMed Central
[52] Mohd Noor AA, Azlan M, Mohd Redzwan N. Orchestrated cytokines mediated by biologics in psoriasis and its mechanisms of action. Biomedicines. 2022;10(2):498.10.3390/biomedicines10020498Suche in Google Scholar PubMed PubMed Central
[53] Li YL, Du ZY, Li PH, Yan L, Zhou W, Tang YD, et al. Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int Immunopharmacol. 2018;64:319–25.10.1016/j.intimp.2018.09.015Suche in Google Scholar PubMed
[54] Sharma A, Upadhyay DK, Gupta GD, Narang RK, Rai VK. IL-23/Th17 axis: a potential therapeutic target of psoriasis. Curr Drug Res Rev Formerly: Curr Drug Abuse Rev. 2022;14(1):24–36.10.2174/2589977513666210707114520Suche in Google Scholar PubMed
[55] Köhler I. Innovative small-molecule therapies for psoriasis. Sweden: Linkopings Universitet; 2025.10.3384/9789180758390Suche in Google Scholar
[56] Fitch EL, Rizzo HL, Kurtz SE, Wegmann KW, Gao W, Benson JM, et al. Inflammatory skin disease in K5. hTGF-β1 transgenic mice is not dependent on the IL-23/Th17 inflammatory pathway. J Invest Dermatol. 2009;129(10):2443–50.10.1038/jid.2009.88Suche in Google Scholar PubMed PubMed Central
[57] Nadeem A, Ahmad SF, Al-Harbi NO, Attia SM, Bakheet SA, Ibrahim KE, et al. Nrf2 activator, sulforaphane ameliorates autism-like symptoms through suppression of Th17 related signaling and rectification of oxidant-antioxidant imbalance in periphery and brain of BTBR T + tf/J mice. Behav Brain Res. 2019;364:213–24.10.1016/j.bbr.2019.02.031Suche in Google Scholar PubMed
[58] Bakić M, Klisić A, Kocić G, Kocić H, Karanikolić V. Oxidative stress and metabolic biomarkers in patients with psoriasis. J Med Biochem. 2024;43(1):97.10.5937/jomb0-45076Suche in Google Scholar PubMed PubMed Central
[59] Klisic A, Bakic M, Karanikolic V. Comparative analysis of redox homeostasis biomarkers in patients with psoriasis and atopic dermatitis. Antioxidants. 2023;12(10):1875.10.3390/antiox12101875Suche in Google Scholar PubMed PubMed Central
[60] Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–3.10.1016/j.redox.2015.01.002Suche in Google Scholar PubMed PubMed Central
[61] Liu S, He M, Jiang J, Xiaoru D, Bao C, Jingyu Z, et al. Triggers for the onset and recurrence of psoriasis: a review and update. Cell Commun Signal. 2024;22:108.10.1186/s12964-023-01381-0Suche in Google Scholar PubMed PubMed Central
[62] Winiarska-Mieczan A, Mieczan T, Wójcik G. Importance of redox equilibrium in the pathogenesis of psoriasis – impact of antioxidant-rich diet. Nutrients. 2020;12(6):1841.10.3390/nu12061841Suche in Google Scholar PubMed PubMed Central
[63] Chaiprasongsuk A, Panich U. Role of phytochemicals in skin photoprotection via regulation of Nrf2. Front Pharmacol. 2022;13:823881.10.3389/fphar.2022.823881Suche in Google Scholar PubMed PubMed Central
[64] Wu R, Zhang H, Zhao M, Li J, Hu Y, Fu J, et al. Nrf2 in keratinocytes protects against skin fibrosis via regulating epidermal lesion and inflammatory response. Biochem Pharmacol. 2020;174:113846.10.1016/j.bcp.2020.113846Suche in Google Scholar PubMed
[65] Brück J, Dringen R, Amasuno A, Pau-Charles I, Ghoreschi K. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp Dermatology. 2018;27(6):611–24.10.1111/exd.13548Suche in Google Scholar PubMed
[66] Bresciani G, Manai F, Davinelli S, Tucci P, Saso L, Amadio M. Novel potential pharmacological applications of dimethyl fumarate – an overview and update. Front Pharmacol. 2023;14:1264842.10.3389/fphar.2023.1264842Suche in Google Scholar PubMed PubMed Central
[67] Reszke R, Szepietowski JC. A safety evaluation of dimethyl fumarate in moderate-to-severe psoriasis. Expert Opin Drug Saf. 2020;19(4):373–80.10.1080/14740338.2020.1736553Suche in Google Scholar PubMed
[68] Bojanowski K, Ibeji CU, Singh P, Swindell WR, Chaudhuri RK. A sensitization-free dimethyl fumarate prodrug, isosorbide di-(methyl fumarate), provides a topical treatment candidate for psoriasis. JID Innov. 2021;1(4):100040.10.1016/j.xjidi.2021.100040Suche in Google Scholar PubMed PubMed Central
[69] Ren J, Chen X, Wang HY, Yang T, Zhang KR, Lei SY. Gentiopicroside ameliorates psoriasis-like skin lesions in mice via regulating the Keap1-Nrf2 pathway and inhibiting keratinocyte activation. Acta Pharmacol Sin. 2025;46(5):1361–74.10.1038/s41401-024-01449-8Suche in Google Scholar PubMed PubMed Central
[70] Yang H, Du Z, Wang W, Song M, Sanidad K, Sukamtoh E, et al. Structure–activity relationship of curcumin: Role of the methoxy group in anti-inflammatory and anticolitis effects of curcumin. J Agric Food Chemistr. 2017;65(22):4509–15.10.1021/acs.jafc.7b01792Suche in Google Scholar PubMed
[71] Mousavi SM, Milajerdi A, Sheikhi A, Kord‐Varkaneh H, Feinle‐Bisset C, Larijani B, et al. Resveratrol supplementation significantly influences obesity measures: a systematic review and dose–response meta‐analysis of randomized controlled trials. Obes Rev. 2019;20(3):487–98.10.1111/obr.12775Suche in Google Scholar PubMed
[72] Rojo DLVM, Krajisnik A, Zhang DD, Wondrak GT. Targeting NRF2 for improved skin barrier function and photoprotection: focus on the achiote-derived apocarotenoid bixin. Nutrients. 2017;9(12):1371.10.3390/nu9121371Suche in Google Scholar PubMed PubMed Central
[73] Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. Jama. 2020;323(19):1945–60.10.1001/jama.2020.4006Suche in Google Scholar PubMed
[74] Salamito M, Gillet B, Syx D, Vaganay E, Malbouyres M, Cerutti C, et al. NRF2 shortage in human skin fibroblasts dysregulates matrisome gene expression and affects collagen fibrillogenesis. J Invest Dermatol. 2023;143(3):386–97.10.1016/j.jid.2022.07.034Suche in Google Scholar PubMed
[75] Petkovic M, Leal EC, Alves I, Bose C, Palade PT, Singh P, et al. Dietary supplementation with sulforaphane ameliorates skin aging through activation of the Keap1-Nrf2 pathway. J Nutr Biochem. 2021;98:108817.10.1016/j.jnutbio.2021.108817Suche in Google Scholar PubMed PubMed Central
[76] Silva LC, Ortigosa LC, Benard G. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy. 2010;2(6):817–33.10.2217/imt.10.67Suche in Google Scholar PubMed
[77] Bagameri L, Botezan S, Bobis O, Bonta V, Dezmirean DS. Molecular insights into royal jelly anti-inflammatory properties and related diseases. Life. 2023;13(7):1573.10.3390/life13071573Suche in Google Scholar PubMed PubMed Central
[78] Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-α. Nature. 1987;329(6140):630–2.10.1038/329630a0Suche in Google Scholar PubMed
[79] Qu R, Chen X, Hu J, Fu Y, Peng J, Li Y, et al. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-α/NF-κB signaling pathways. Sci Rep. 2019;9(1):1348.10.1038/s41598-018-38174-2Suche in Google Scholar PubMed PubMed Central
[80] Notarte KIR, Quimque MTJ, Macaranas IT, Khan A, Pastrana AM, Villaflores OB, et al. Attenuation of lipopolysaccharide-induced inflammatory responses through inhibition of the NF-κB pathway and the increased Nrf2 level by a flavonol-enriched n-butanol fraction from Uvaria alba. ACS Omega. 2023;8(6):5377–92.10.1021/acsomega.2c06451Suche in Google Scholar PubMed PubMed Central
[81] Shukla R, Pandey V, Vadnere GP, Lodhi S. Role of flavonoids in management of inflammatory disorders. In Bioactive food as dietary interventions for arthritis and related inflammatory diseases. United Kingdom: Academic Press; 2019. p. 293–322.10.1016/B978-0-12-813820-5.00018-0Suche in Google Scholar
[82] Oh JH, Kim SH, Kwon OK, Kim JH, Oh SR, Han SB, et al. Purpurin suppresses atopic dermatitis via TNF-α/IFN-γ-induced inflammation in HaCaT cells. Int J Immunopathol Pharmacol. 2022;36:03946320221111135.10.1177/03946320221111135Suche in Google Scholar PubMed PubMed Central
[83] Lee KS, Chun SY, Lee MG, Kim S, Jang TJ, Nam KS. The prevention of TNF-α/IFN-γ mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed Pharmacother. 2018;97:1331–40.10.1016/j.biopha.2017.11.056Suche in Google Scholar PubMed
[84] Sunkari S, Thatikonda S, Pooladanda V, Challa VS, Godugu C. Protective effects of ambroxol in psoriasis like skin inflammation: Exploration of possible mechanisms. Int Immunopharmacol. 2019;71:301–12.10.1016/j.intimp.2019.03.035Suche in Google Scholar PubMed
[85] Zhang JF, Bai KW, Su WP, Wang AA, Zhang LL, Huang KH, et al. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult Sci. 2018;97(4):1209–19.10.3382/ps/pex408Suche in Google Scholar PubMed
[86] Keleku-Lukwete N, Suzuki M, Yamamoto M. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Antioxid Redox Signal. 2018;29(17):1746–55.10.1089/ars.2017.7358Suche in Google Scholar PubMed
[87] Bardallo G, Panisello‐Roselló R, Sanchez‐Nuno A, Alva S, Roselló‐Catafau N, Carbonell T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. FEBS J. 2022;289(18):5463–79.10.1111/febs.16336Suche in Google Scholar PubMed
[88] Aladaileh SH, Abukhalil MH, Saghir SA, Hanieh H, Alfwuaires MA, Almaiman AA, et al. Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules. 2019;9(8):346.10.3390/biom9080346Suche in Google Scholar PubMed PubMed Central
[89] Hosseini A, Masjedi A, Baradaran B, Hojjat‐Farsangi M, Ghalamfarsa G, Anvari E, et al. Dimethyl fumarate: Regulatory effects on the immune system in the treatment of multiple sclerosis. J Cell Physiol. 2019;234(7):9943–55.10.1002/jcp.27930Suche in Google Scholar PubMed
[90] Phillips JT, Agrella S, Fox RJ. Dimethyl fumarate: a review of efficacy and practical management strategies for common adverse events in patients with multiple sclerosis. Int J MS Care. 2017;19(2):74–83.10.7224/1537-2073.2015-086Suche in Google Scholar PubMed PubMed Central
[91] Dinkova-Kostova AT, Copple IM. Advances and challenges in therapeutic targeting of NRF2. Trends Pharmacol Sci. 2023;44(3):137–49.10.1016/j.tips.2022.12.003Suche in Google Scholar PubMed
[92] Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98(3):1169–203.10.1152/physrev.00023.2017Suche in Google Scholar PubMed PubMed Central
[93] Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discovery. 2019;18(4):295–317.10.1038/s41573-018-0008-xSuche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- 10.1515/biol-2025-1168
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
Artikel in diesem Heft
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- 10.1515/biol-2025-1168
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”

