Abstract
Herein, we elucidate the potential role of ANO6 (TMEM16F) in gastrointestinal stromal tumors (GISTs). ANO6 expression in GIST and adjacent normal tissues was determined using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting. Cell proliferation, apoptosis, and pyroptosis were examined utilizing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, terminal deoxynucleotidyl transferase dUTP Nick-End Labeling staining, and flow cytometry. In addition, the total iron and Fe2+ levels were assessed. IL-18 and IL-1β levels were also evaluated. Lipid reactive oxygen species (ROS), cystine (Cys), glutathione (GSH), and glutathione peroxidase 4 (GPX4) levels were evaluated using appropriate kits. Ferroptotic markers, including Ptgs2, Chac1, SLC7A11, and SLC3A2, were analyzed by RT-qPCR, western blotting, and immunohistochemistry. ANO6 expression decreased in GIST tissues. ANO6-plasmid inhibits proliferation, induces apoptosis, and promotes pyroptosis in GIST-T1 and GIST-T1 IR cells. The ANO6-plasmid induced ferroptosis, as confirmed by enhanced lipid ROS levels, increased intracellular concentrations of total iron and Fe2+, promoted Ptgs2 and Chac1 expression, reduced Cys, GSH, and GPX4 levels, and downregulated SLC7A11 and SLC3A2 expression after in vitro and in vivo treatment with ANO6-plasmid. Moreover, the ANO6-plasmid inhibited GIST growth in vivo. Therefore, ANO6 may be a promising therapeutic target for blocking the development of GIST via the induction of apoptosis, pyroptosis, and ferroptosis.
1 Introduction
Gastrointestinal stromal tumor (GIST) is a type of tumor originating from the mesenchymal tissue of the gastrointestinal tract, accounting for the majority of gastrointestinal mesenchymal tumors [1,2]. GIST, as a special type of tumor, is not sensitive to traditional chemotherapy and radiotherapy [3,4]. Surgical resection is considered the most effective treatment method [5]. Moreover, cancer immunotherapy is increasingly receiving attention due to breakthroughs in immune checkpoint inhibitors [6,7], and the immunotherapeutic strategies for GIST are growing fast [8]. Yeh et al. identified aurora kinase A as an unfavorable prognostic factor and a potential treatment target for metastatic GIST [9]. However, at present, the recurrence rate of GIST is high, and the survival rate of patients is poor. Therefore, it is necessary to develop new and more effective treatment strategies for patients with GIST.
Recently, there have been reports that several new types of programmed cell death, such as ferroptosis and pyroptosis, play important roles in regulating cancer progression and are considered a promising strategy for cancer treatment. Ferroptosis and cell pyroptosis are highly correlated with immune regulation in the tumor microenvironment. Ferroptosis is a newly discovered mode of cell death, and its development depends on intracellular free iron (Fe2+). Abnormal regulation of iron levels in cells can result in an imbalance between cell membrane redox and lipid peroxidation, eventually resulting in cell death [10]. Ferroptosis plays a vital role in the progression and development of several diseases [11,12]. In addition, antioxidants and glutathione peroxidase 4 (GPX4) are involved in the progression of ferroptosis. For instance, Zhou et al. found that ferroptosis is initiated by glutathione (GSH) depletion or GPX4 inactivation [13]. The cystine (Cys)/glutamate antiporter system Xc−, which consists of SLC7A11 and SLC3A2, is closely associated with ferroptosis [14]. Li et al. verified that inhibition of the SLC7A11-GPX4 signaling pathway is involved in aconitine-induced ferroptosis in vivo and in vitro [15]. In recent years, ferroptosis has garnered enormous interest in cancer research communities. Ferroptosis can be seen in radiotherapy, chemotherapy, and tumor immunotherapy. Therefore, activation of ferroptosis may be a potential strategy to overcome the resistance mechanisms of traditional cancer treatments [10]. In addition, as a form of programmed cell death mediated by gasdermin, cell pyroptosis is a product of continuous cell expansion until cell membrane rupture, leading to the release of cell contents and activation of strong inflammatory and immune responses [16]. Pyroptosis is an innate immune response that can be triggered by various influencing factors that activate inflammasomes. Evidence has demonstrated that pyroptosis exerts benefits on cancer immunotherapies [17]. However, the specific mechanisms underlying ferroptosis and pyroptosis in GIST require further investigations.
ANO6 (TMEM16F) is a protein with ten transmembrane segments that exists in various tissues and cells [18]. Studies have shown that ANO6 (TMEM16F) plays important roles in cell growth and migration [19]. Zhao et al. found that ANO6 (TMEM16F) inhibition limited pain-associated behavior and improved motor function by promoting microglial M2 polarization in mice [20]. Bricogne et al. found that ANO6 (TMEM16F) activation by Ca(2+) triggers plasma membrane expansion and directs PD-1 trafficking [21]. Lin et al. found that TMEM16F/ANO6 is negatively regulated by the actin cytoskeleton and intracellular MgATP [22]. Moreover, evidence indicates that activation of ANO6 (TMEM16F) contributes to various forms of regulated cell death [23]. However, the expression and role of ANO6 (TMEM16F) in GISTs remains unclear.
Thus, our study was designed to illustrate the functions of ANO6 (TMEM16F) in GIST ferroptosis and elucidate its potential mechanism. Our findings provide the first evidence that ANO6 (TMEM16F) inhibits GIST growth and induces ferroptosis by regulating SLC7A11 and SLC3A2C expression, thereby providing a therapeutic basis for GIST treatment.
2 Methods
2.1 Clinical specimen collection
GIST and adjacent normal tissues were collected from 15 patients with GIST at the The Yangzhou School of Clinical Medicine of Nanjing Medical University. All specimens were rapidly frozen and stored in liquid nitrogen and preserved at −80°C for further analysis.
2.2 Cell culture
GIST-T1 cells were bought from the American Type Culture Collection and cultured in Dulbecco’s modified Eagle’s medium (Thermo Fisher) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA) and 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a humid atmosphere containing 5% CO2.
2.3 Construction of drug-resistant cell lines
Intermittent imatinib (IM) administration was performed on cells in the logarithmic growth phase. After 48 h of treatment, a drug-free culture medium was used instead of the culture medium. Specific IM concentrations were administered until normal cell growth was observed. Subsequently, the concentration was increased, and the above process was repeated to obtain drug-resistant cell lines.
2.4 Cell transfection
GIST-T1 and GIST-T1 IR cells were induced by 1 µg control-plasmid (sc-437275; Santa Cruz Biotechnology) and 1 µg ANO6-plasmid (sc-402736-ACT Santa Cruz Biotechnology) using Lipofectamine 2000 (11668019; Thermo Fisher) for 48 h following the manufacturer’s protocol. The cells were harvested after transfection.
2.5 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
After treatment, GIST-T1 and GIST-T1 IR cells were implanted into 96-well plates and cultured for 24 h at 37°C. Then, cells were treated with 10 μL of MTT solution and continuously incubated for a further 4 h. Afterward, the solution was removed and 100 μL of dimethyl sulfoxide was added to each well to solubilize the formazan product. Finally, the optical density at 570 nm was measured using a microplate reader (BioTek, Richmond, USA) after 15 min of vibration mixing, following the manufacturer’s instructions.
2.6 Flow-cytometry analysis
After treatment, GIST-T1 and GIST-T1 IR cells were implanted into 96-well plates and cultured for 24 h at 37°C. The cells were collected by centrifugation at 4°C for 5 min. The cells were then washed with phosphate-buffered saline (PBS). Apoptosis was detected using the Annexin V-FITC/PI Apoptosis Detection Kit (Beyotime, Beijing, China) following the manufacturer’s instructions. Apoptosis was assessed using a BD Aria III flow cytometer (BD Technologies). Pyroptosis in GIST-T1 and GIST-T1 IR cells was determined by flow cytometry according to the manufacturer’s instructions.
2.7 Reactive oxygen species (ROS) assay
2'-7'-Dichlorodihydrofluorescein diacetate (DCFH-DA) was used to quantify the ROS levels according to the protocol of the ROS fluorescence assay kit (E-BC-K138-F; Elabscience). GIST-T1 and GIST-T1 IR cells were plated in six-well plates and incubated for 24 h. After washing with PBS for three times, cells were labeled with 5 μM DCFH-DA under standard conditions for 30 min, cells were collected, and fluorescence intensity of DCF was detected using a microplate reader (SMR16.1, USCNK).
2.8 Iron assay
The Iron Assay Kit (E-BC-K772-M/E-BC-K773-M; Elabscience) was used to detect the total iron or Fe2+ levels in GIST-T1 and GIST-T1 IR cells. According to the manufacturer’s protocol, the iron assay buffer and iron reducer were sequentially added to the cells. The samples were thoroughly mixed in the dark, added to an iron-reducing agent and iron probe, and cultured for 30 min. Intracellular ferrous levels were quantified using a kit, and the absorbance at 593 nm was calculated as the intracellular Fe2+ level.
2.9 Cys, GSH level, and GPX4 activity measurements
GIST-T1 and GIST-T1 IR cells were collected after treatment with dimethyl sulfoxide or a specified concentration of the drug for 24 h, according to the manufacturer’s instructions. Cys and GSH levels were detected using a human Cys enzyme-linked immunosorbent assay (ELISA) kit (ELK9092; ELK Biotechnology) and a GSH Assay Kit (A006-2; Nanjing Jiancheng Biotechnology). The GPX4 activity was measured using a Human GPX4 ELISA Kit (ELK4775; ELK Biotechnology).
2.10 ELISA
After GIST-T1 and GIST-T1 IR cells were transfected with the control plasmid and ANO6-plasmid, we centrifuged and collected the supernatant of the cells for ELISA. Then, the levels of IL-18 (ELK1245; ELK Biotechnology) and IL-1β (ELK1270; ELK Biotechnology) were assessed following the manufacturer’s instructions.
2.11 Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from GIST cells and GIST-T1 IR lines or tissues using the TRIzol Total RNA Extraction Reagent (EP013; ELK Biotechnology) according to the manufacturer’s protocol. The levels of ANO6, Bax, Bcl-2, SLC7A11, SLC3A2, Ptgs2, and Chac1 were measured using RT-qPCR. Total RNA was reverse transcribed into cDNA following the instructions of EntiLink 1st Strand cDNA Synthesis Super Mix (EQ031; ELK Biotechnology), and RT-qPCR analysis was conducted using EnTurbo SYBR Green PCR SuperMix (EQ001; ELK Biotechnology) with an ABI 7500 Real-Time PCR System (Applied Biosystems). Target gene expressions were calculated using the 2−ΔΔCt method [24]. Primer sequences for PCR are listed in Table 1.
Primer sequences for PCR
| Gene | Sequences (5′–3′) | |
|---|---|---|
| GAPDH | sense | CATCATCCCTGCCTCTACTGG |
| antisense | GTGGGTGTCGCTGTTGAAGTC | |
| Ptgs2 | sense | AGATTATGTGCAACACTTGAGTGG |
| antisense | ATTCCTACCACCAGCAACCCT | |
| Chac1 | sense | GTGGTGACGCTCCTTGAAGAT |
| antisense | GCCTCTCGCACATTCAGGTAC | |
| SLC7A11 | sense | TGTGGGGTCCTGTCACTATTTG |
| antisense | GATATCACAGCAGTAGCTGCAGG | |
| Bax | sense | TCTGAGCAGATCATGAAGACAGG |
| antisense | ATCCTCTGCAGCTCCATGTTAC | |
| Bcl-2 | sense | AGGATTGTGGCCTTCTTTGAG |
| antisense | AGCCAGGAGAAATCAAACAGAG | |
| SLC3A2 | sense | CGATTACCTGAGCTCTCTGAAG |
| antisense | TAAGGTCCAGAATGACACGGAT | |
2.12 Western blot assay
GIST-T1 and GIST-T1 IR cell proteins were extracted using radioimmunoprecipitation assay buffer (AS1004; ASPEN) and evaluated using a bicinchoninic acid assay kit (AS1086; ASPEN). Average protein concentrations were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. Then, the membrane was blocked in 5% skim milk for 2 h at room temperature and incubated in specific antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ab181602, 1:10,000 dilution; Abcam), ANO6 (20784-1-AP, 1:1,000 dilution; Wuhan Sanying Biotechnology), Bax (50599-2-Ig, 1:2,000 dilution; Wuhan Sanying Biotechnology), Bcl-2 (ab321124, 1:1,000 dilution; Abcam), SLC7A11 (26864-4-AP, 1:1,000 dilution; Wuhan Sanying Biotechnology), SLC3A2 (15193-1-AP, 1:5,000 dilution; Wuhan Sanying Biotechnology), GSDMD-N (A22523, 1:1,000 dilution; abclonal), or cleaved-Caspase1 (AF4005, 1:500 dilution; affbiotech) overnight at 4°C. Membranes were then incubated with secondary antibodies (AS1107, 1:10,000 dilution; ASPEN) for 1 h. Finally, signals were developed using electrochemiluminescence detection system reagents (AS1059; ASPEN) according to the manufacturer’s instructions.
2.13 Animal studies
All mice were placed in a specific pathogen-free environment with a standard light–dark cycle of 25°C for 12 h and had free access to food and water. Then, GIST-T1 cells were injected subcutaneously into nude mice with 100 μL of normal saline. The tumor volume (V) was calculated using the formula: V = L × W 2/2, where L is the tumor length and W is the tumor width. Tumors were collected and weighed from all mice after euthanasia. This study is reported in accordance with ARRIVE guidelines. Animal care and experimental procedures were approved by the Animal Ethics Committee of Yangzhou University (Approval number: 202111020).
2.14 Immunohistochemical analysis
Tumor samples were fixed with 4% paraformaldehyde at room temperature for 24 h, dehydrated, and waxed. Then, the sample was cut into 2–3 μm slices. The slices were placed in 0.01 M citrate buffer (pH 6.0), heated in a microwave oven for antigen repair on medium heat for 2–8 min. The slices were exposed to 3% H2O2 for 15–30 min to bleach the endogenous peroxidase and then rinsed in PBS for three times. Then, a sufficient amount of diluted primary antibodies against ANO6, SLC7A11, or SLC3A2 were added to each slice and incubated overnight at 4°C. These sections were then incubated with the secondary antibody for 30 min. The slices were examined using light microscopy (Olympus).
2.15 Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) analysis
The tissue of GIST was fixed with 4% paraformaldehyde for more than 24 h, and 2–3 μm paraffin sections were taken after dehydration. The slices were then dewaxed in xylene for 5–10 min, washed with anhydrous ethanol for 5 min, soaked in 0.2% Triton X-100 for 15 min, and soaked in 3,3′-diaminobenzidine solution for 30 min. Images were analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.), and the slices were photographed and counted under an optical microscope.
2.16 Statistical analysis
Statistical analyses were conducted using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). We used the Kolmogorov–Smirnov test to determine the normality of the data in SPSS. All findings are displayed by mean ± standard deviation from three independent experiments. Mean differences among groups were estimated using the unpaired Student’s t-test or one-way analysis of variance with Tukey’s post hoc test. Statistical significance was set at P < 0.05.
-
Ethics approval and consent to participate: This study is reported in accordance with ARRIVE guidelines. The research procedure was approved by the ethics committee of The Yangzhou School of Clinical Medicine of Nanjing Medical University (Approval number: 2022ky152) in accordance with the Declaration of Helsinki. Animal care and experiment procedures are approved by the Animal Ethics Committee of Yangzhou University (Approval number. 202111020).
-
Informed consent: All patients signed an informed consent form and approved the use of their tissues in the study.
3 Results
3.1 ANO6 (TMEM16F) (ANO6) was low-expressed in the stromal tumor tissues of patients with GISTs
Stromal tumors and adjacent normal tissues were obtained from 15 patients with GISTs. The levels of ANO6 (TMEM16F) were analyzed using RT-qPCR and western blotting. As presented in Figure 1a and b, the levels of ANO6 (TMEM16F) were remarkably lower in the stromal tumor tissues of patients with GIST than in the adjacent normal tissues, indicating a regulatory role of ANO6 (TMEM16F) in GIST.
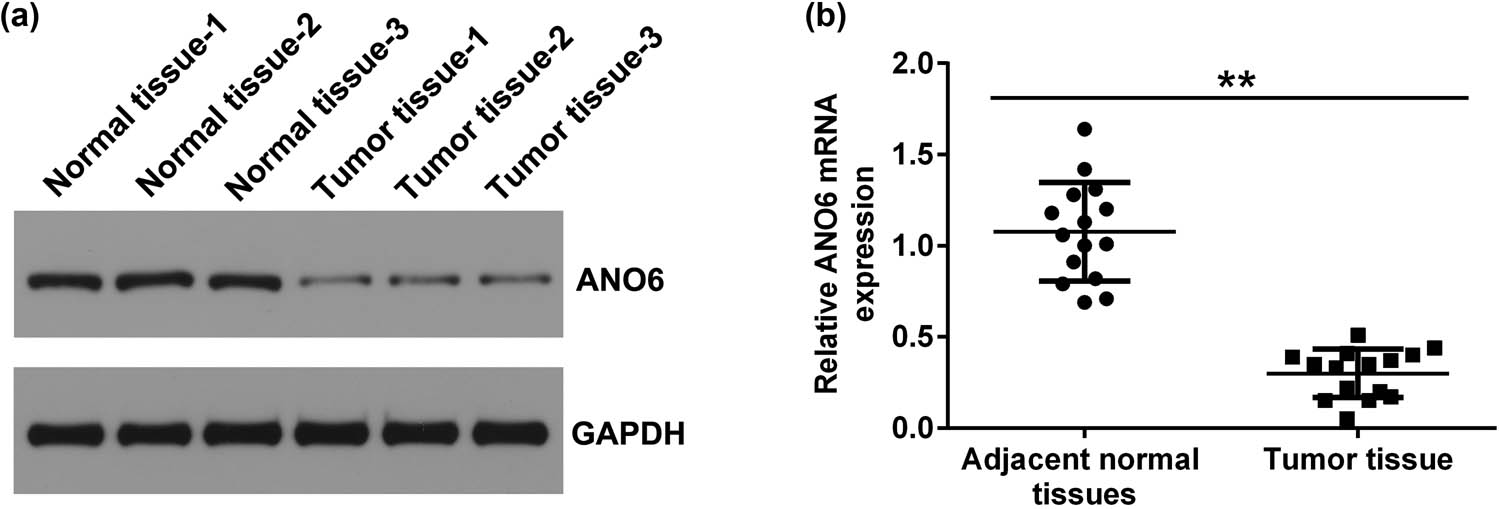
Expression of ANO6 (TMEM16F) in stromal tumor tissues and adjacent normal tissues of patients with GIST. Relative levels of ANO6 (TMEM16F) in stromal tumor tissues and adjacent normal tissues from patients with GISTs were evaluated by (a) western blot assay and (b) RT-qPCR. **P < 0.01.
3.2 ANO6 (TMEM16F) was downregulated in the IM‑resistant GIST‑T1 IR cell line
Moreover, we detected ANO6 (TMEM16F) expression in GIST-T1 IR and GIST-T1 cells. The RT-qPCR and western blotting results suggested that the level of ANO6 (TMEM16F) was lower in GIST-T1 IR cells than in GIST-T1 cells (Figure 2a and b). Our data indicate that ANO6 (TMEM16F) is involved in the regulation of IM-resistant GIST-T1 IR cell line.
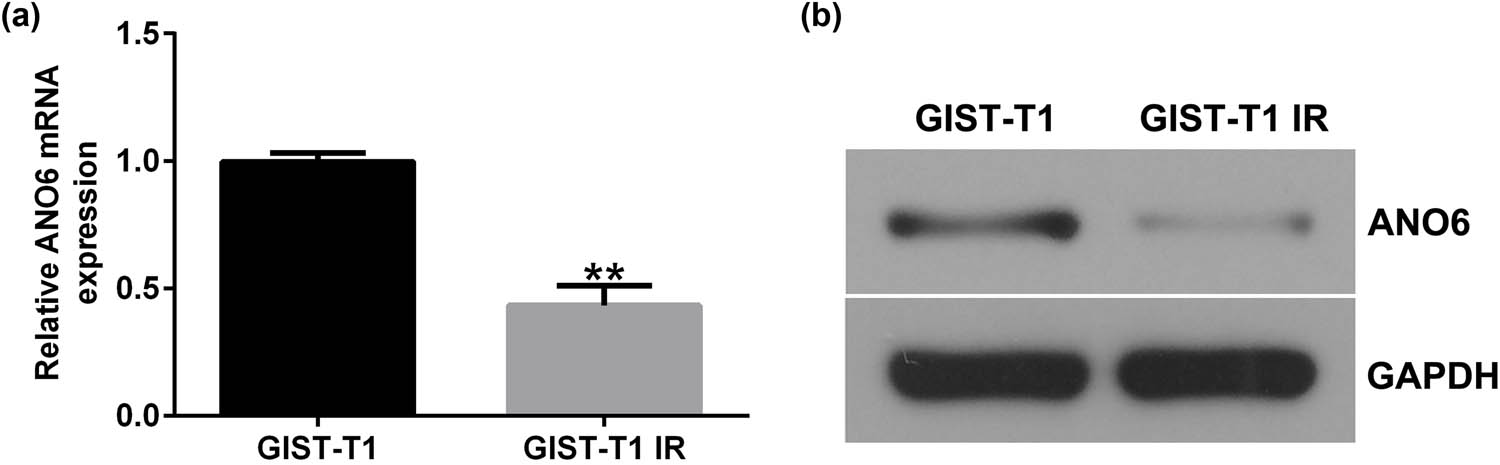
Expression of ANO6 (TMEM16F) in GIST-T1 and GIST-T1 IR cells. The relative levels of ANO6 (TMEM16F) in GIST-T1 and GIST-T1 IR cells were evaluated by (a) RT-qPCR and (b) western blotting, respectively. **P < 0.01.
3.3 Upregulation of ANO6 (TMEM16F) inhibited GIST-T1 cell proliferation and induced cell apoptosis
To further illustrate the mechanism of ANO6 (TMEM16F) (ANO6) in GIST, we transfected GIST-T1 cells with a control-plasmid and ANO6-plasmid. Results from RT-qPCR and western blot assays suggested that ANO6 (TMEM16F) was upregulated in ANO6-plasmid transfected GIST-T1 cells compared to the control plasmid group (Figure 3a and b). In addition, the results of MTT and flow cytometry assays revealed that the upregulation of ANO6 (TMEM16F) inhibited the proliferation of GIST-T1 cells (Figure 3c) and increased the number of apoptotic GIST-T1 cells (Figure 3d and e). We also determined the levels of apoptosis-related proteins, including Bax and Bcl-2, by RT-qPCR and western blotting. The ANO6-plasmid increased Bax expression (Figure 3f and g) and reduced Bcl-2 expression (Figure 3f and h). Our data revealed that ANO6 (TMEM16F) was involved in GIST progression by regulating GIST-T1 cell proliferation and apoptosis.
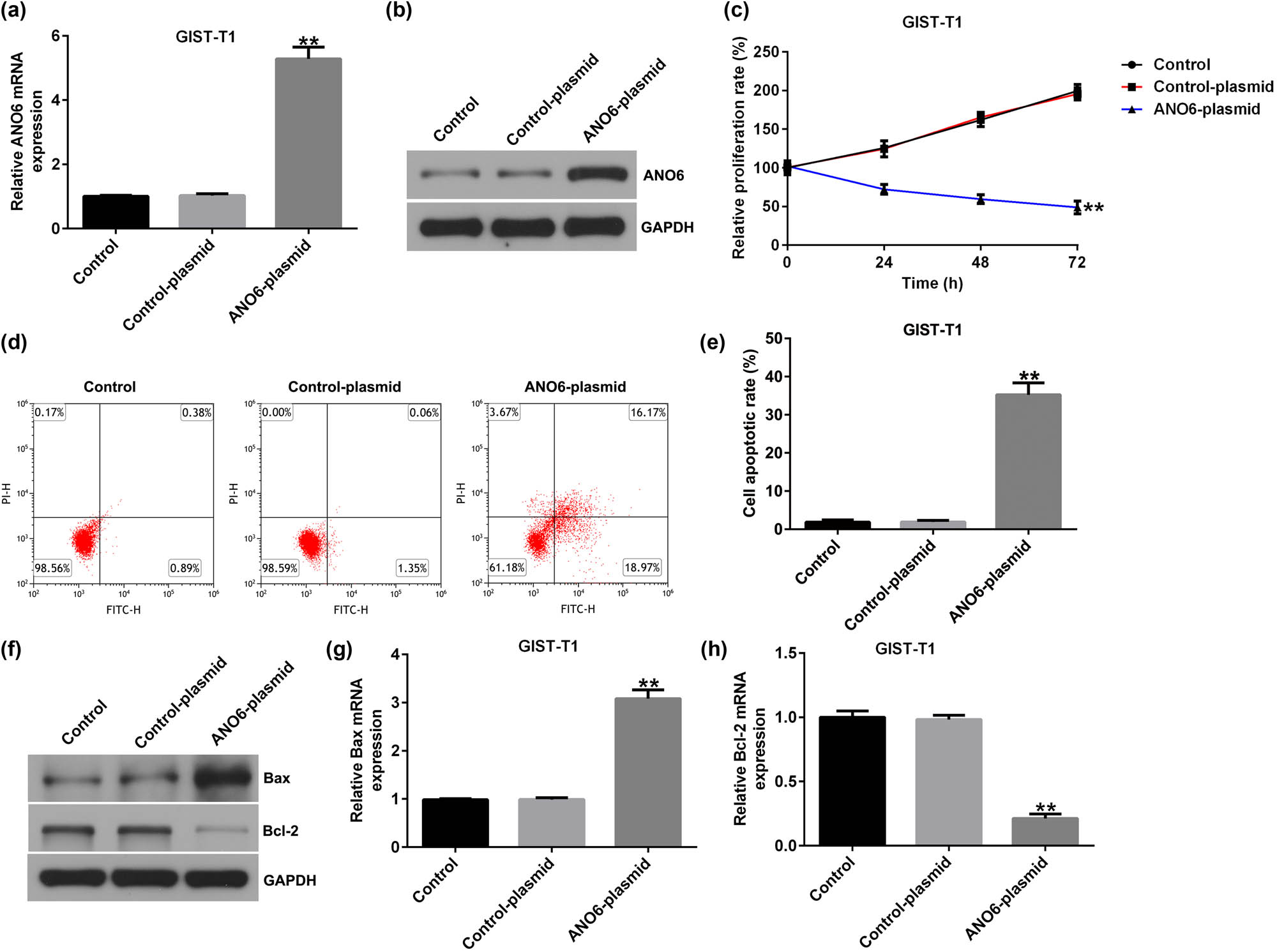
Effects of ANO6 (TMEM16F) on GIST-T1 cell proliferation and apoptosis. GIST-T1 cells were transfected with the control-plasmid and the ANO6-plasmid. (a) and (b) RT-qPCR and western blot analysis of ANO6 (TMEM16F) levels in the control-plasmid and ANO6-plasmid groups. (c) The growth of GIST-T1 cells was assessed by MTT. (d) Apoptosis was determined by flow cytometry. (e) Quantification of apoptotic GIST-T1 cells. (f) Western blotting analysis of Bax and Bcl-2 expression. (g) and (h) Bax and Bcl-2 mRNA levels were determined using RT-qPCR. **P < 0.01.
3.4 Overexpression of ANO6 (TMEM16F) suppressed GIST-T1 IR cell proliferation and promoted cell apoptosis
We also investigated the roles of the ANO6-plasmid in GIST-T1 IR cell proliferation and apoptosis. We found that the ANO6-plasmid upregulated ANO6 (TMEM16F) expression in GIST-T1 IR cells, compared to the control-plasmid group (Figure 4a and b). Moreover, the ANO6-plasmid led to suppressed GIST-T1 IR cell proliferation (Figure 4c) and enhanced apoptosis (Figure 4d and e). Furthermore, RT-qPCR and western blotting assays revealed that the ANO6-plasmid increased Bax expression (Figure 4f and g) and reduced Bcl-2 expression (Figure 4f and h) in GIST-T1 IR cells, indicating that ANO6 (TMEM16F) is a vital regulator of GIST progression.
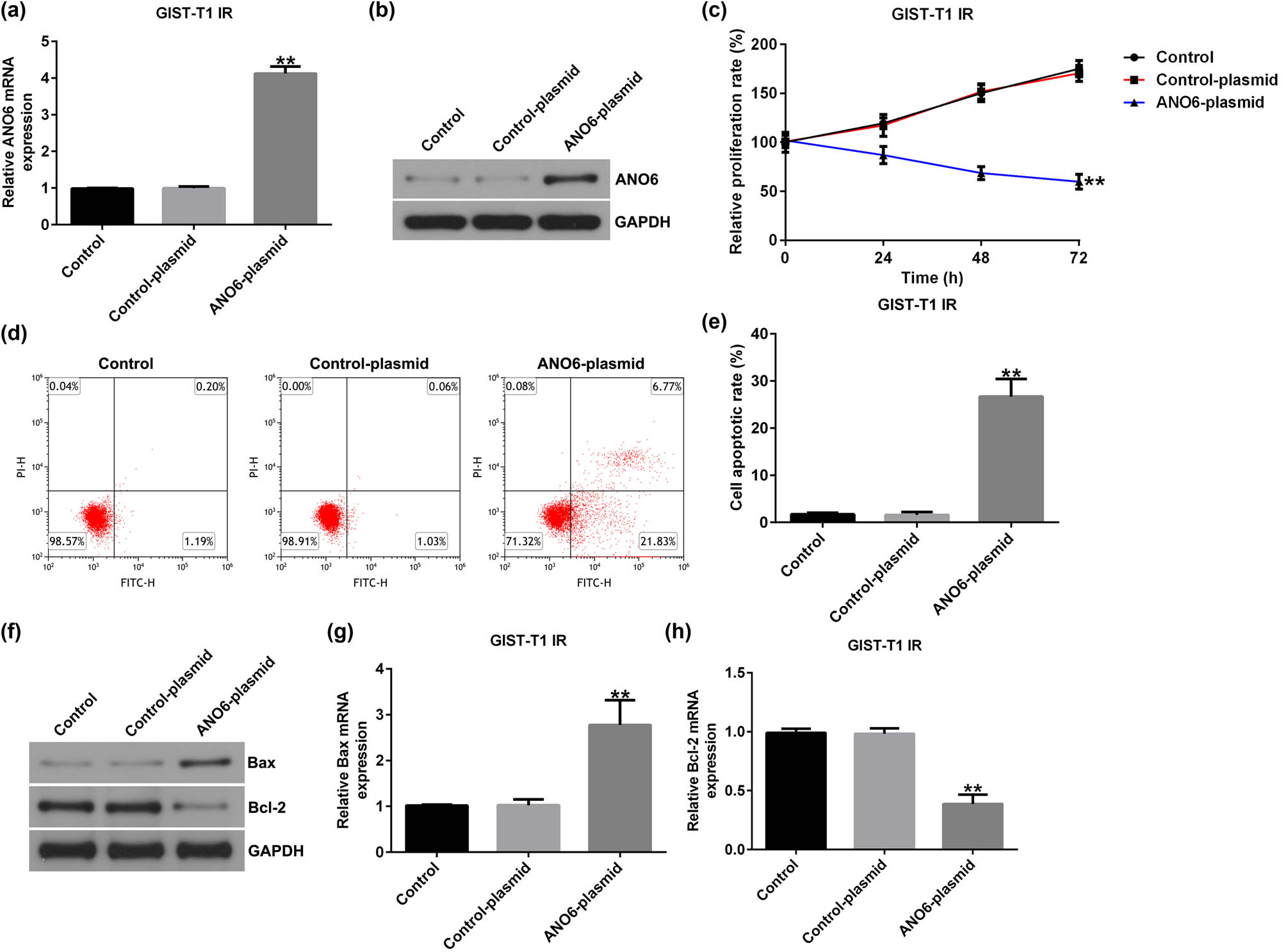
Effects of ANO6 (TMEM16F) on GIST-T1 IR cell proliferation and apoptosis. Control-plasmid and ANO6-plasmid were transfected into GIST-T1 IR cells. (a) and (b) ANO6 (TMEM16F) levels were determined by RT-qPCR and western blot analysis. (c) GIST-T1 IR cell viability was assessed by MTT. (d) Apoptosis was determined by flow cytometry. (e) Quantification of apoptotic GIST-T1 IR cells. (f) Western blotting analysis of Bax and Bcl-2 expression. (g) and (h) Bax and Bcl-2 mRNA levels were determined using RT-qPCR. **P < 0.01.
3.5 ANO6-plasmid promotes pyroptosis in GIST-T1 and GIST-T1 IR cells
Mechanistically, we explored the effects of ANO6 on GIST-T1 and GIST-T1 IR cell pyroptosis. As shown in Figure 5a and b, the ANO6-plasmid induced GIST-T1 and GIST-T1 IR cells. Furthermore, the effector molecules of pyroptosis, including GSDMD-N and cleaved caspase 1, were determined by western blotting. We observed that GSDMD-N and cleaved caspase 1 density in the ANO6-plasmid group was remarkably increased relative to the control-plasmid group (Figure 5c). ELISA for IL-18 and IL-1β expression levels were also evaluated. Results from Figure 5d and e revealed that the ANO6-plasmid increased IL-18 and IL-1β expressions, as opposed to the control-plasmid. These results suggested that the ANO6-plasmid increased pyroptosis-related markers, indicating a promotional effect on pyroptosis.
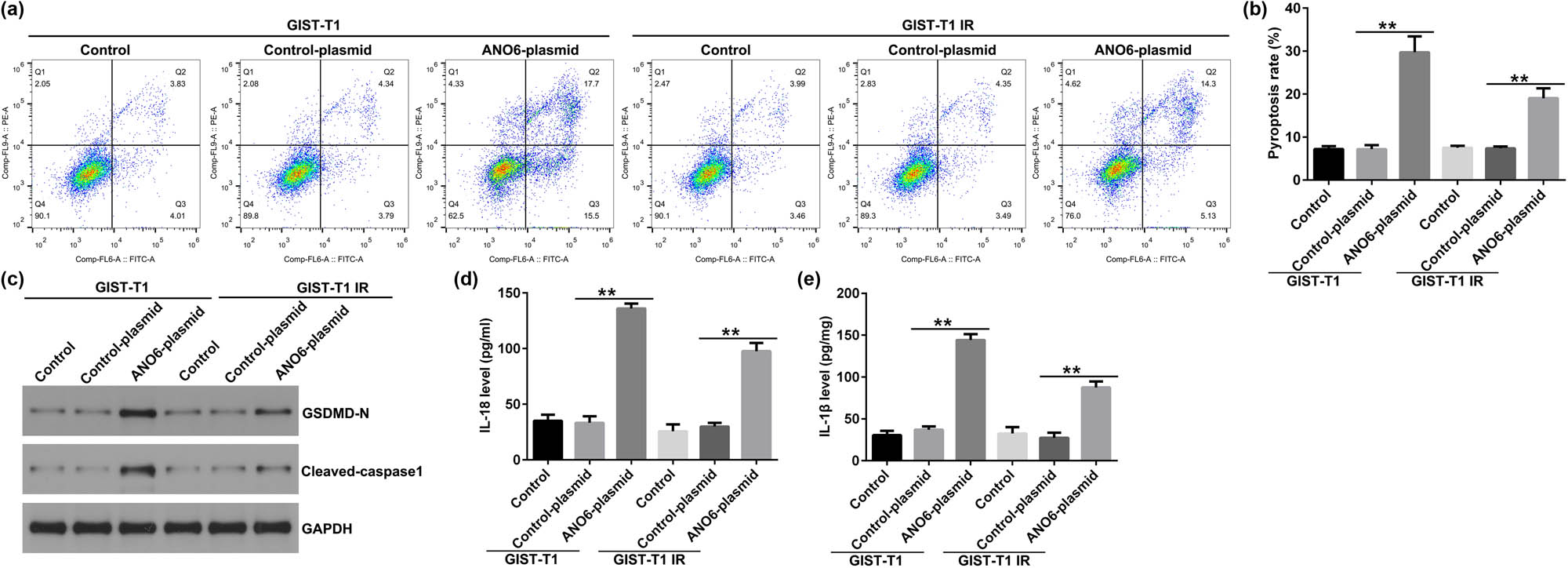
Effects of ANO6 (TMEM16F) on GIST-T1 and GIST-T1 IR cell pyroptosis. (a) and (b) Pyroptosis of GIST-T1 and GIST-T1 IR cells was confirmed by flow cytometry. (c) Western blot analysis of GSDMD-N and cleaved-caspase 1 expression. (d) and (e) The levels of IL-18 and IL-1β were assessed using ELISA assay. **P < 0.01.
3.6 ANO6 (TMEM16F) induced ferroptosis by regulating SLC7A11 and SLC3A2 in GIST-T1 cells
Ferroptosis is an iron-dependent process that is different from necrosis and apoptosis. An increasing number of studies have suggested that ferroptosis is a critical modality in cancer-related deaths [25]. We explored the effects of the ANO6-plasmid on ferroptosis in GIST-T1 cells. We found that the upregulation of ANO6 (TMEM16F) enhanced lipid ROS levels (Figure 6a) and increased the intracellular concentrations of total iron (Figure 6b) and Fe2+ (Figure 6c) in GIST-T1 cells. In addition, we determined the markers of ferroptosis, including Ptgs2 and Chac1. Our data revealed that Ptgs2 and Chac1 were upregulated in ANO6-plasmid transfected GIST-T1 cells (Figure 6d and e). Further mechanistic experiments indicated that the ANO6-plasmid reduced Cys, GSH, and GPX4 levels as opposed to the control-plasmid group (Figure 6f–h), indicating that ANO6 (TMEM16F) inhibits GIST growth and induces ferroptosis in GIST-T1 cells.
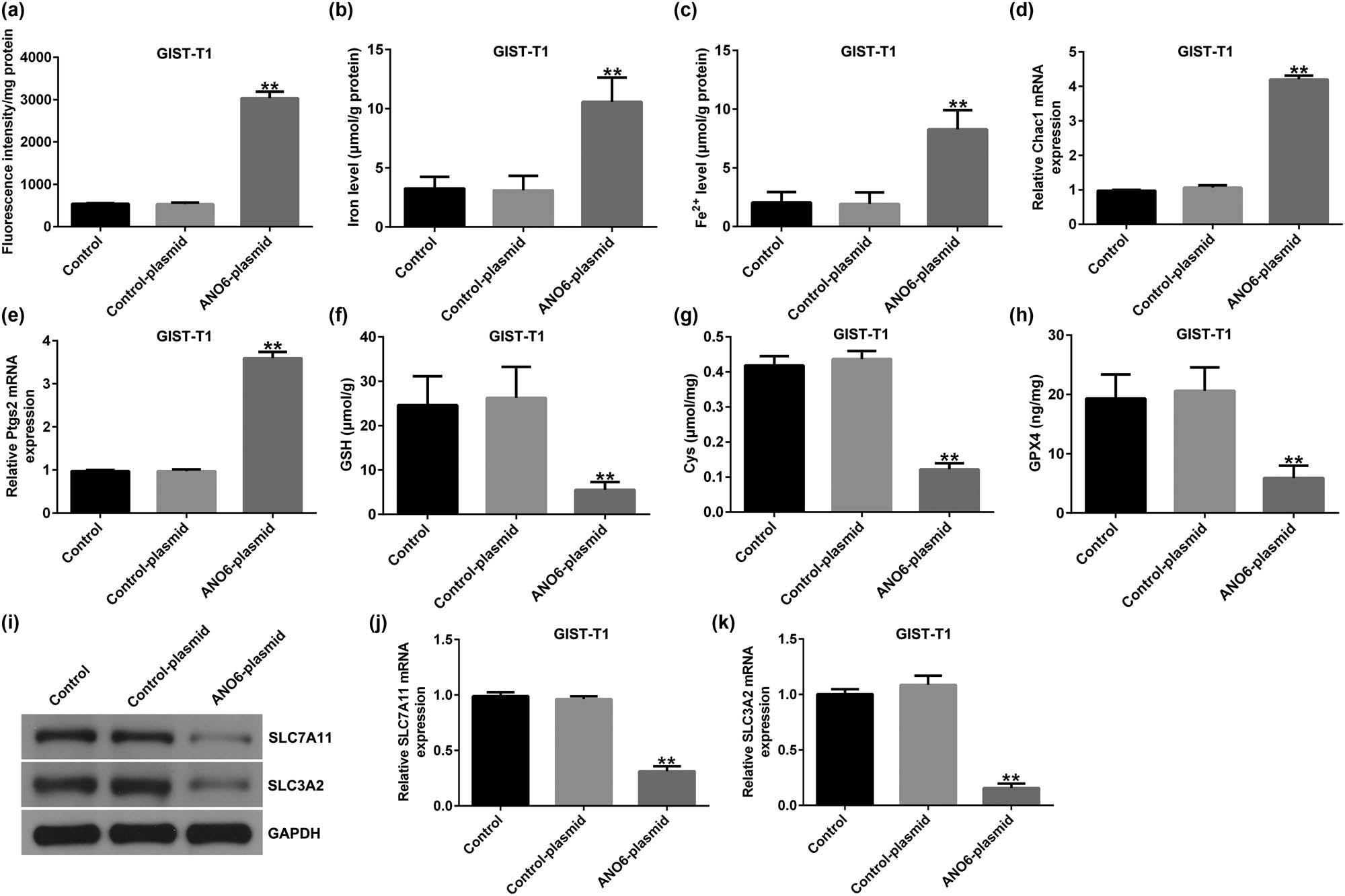
Effects of ANO6 (TMEM16F) on GIST-T1 cell ferroptosis. GIST-T1 cells were transfected with the control-plasmid and the ANO6-plasmid. (a) Generation of lipid ROS in GIST-T1 cells was detected by flow cytometry. Total iron (b) and ferrous iron (c) levels were evaluated in GIST-T1 cells after treatment with the control-plasmid or the ANO6-plasmid. (d) and (e) Levels of Ptgs2 and Chac1 were detected by RT-qPCR. Determination of Cys (f), GSH (g), and GPX4 (h) levels in GIST-T1 cells. (i) Detection of SLC7A11 and SLC3A2 expression in the control, control-plasmid, and ANO6-plasmid groups. (j) and (k) mRNA levels of SLC7A11 and SLC3A2 were assessed using RT-qPCR. **P < 0.01 vs control-plasmid.
Previous reports have revealed that ferroptosis is linked to multiple diseases and results from iron-dependent lipid peroxidation after inactivation of SLC7A11 and SLC3A2 [26]. Next, we evaluated whether ANO6 (TMEM16F) affected SLC7A11 and SLC3A2 expression in GIST-T1 cells. As shown in Figure 6I-K, SLC7A11 and SLC3A2 were downregulated in ANO6-plasmid transfected cells. Our data indicate that ANO6 (TMEM16F)-induced ferroptosis is mediated by SLC7A11 and SLC3A2.
3.7 ANO6 (TMEM16F) stimulated ferroptosis by regulating SLC7A11 and SLC3A2 in GIST-T1 IR cells
Furthermore, we investigated the roles of the ANO6-plasmid in the ferroptosis of GIST-T1 IR cells. Our data revealed that the ANO6-plasmid increased lipid ROS (Figure 7a), iron (Figure 7b), and Fe2+ levels (Figure 7c) in GIST-T1 IR cells. Furthermore, qRT-PCR analysis suggested that Ptgs2 and Chac1 expression was enhanced after ANO6-plasmid transfection (Figure 7d and e). Moreover, we detected ferroptosis-related gene expression in ANO6-plasmid transfected GIST-T1 IR cells, and our data demonstrated that the upregulation of ANO6 inhibited Cys, GSH, and GPX4 levels (Figure 7f–h), as well as SLC7A11 and SLC3A2 expression (Figure 7i–k), compared to the control-plasmid group. Our data revealed that ANO6 (TMEM16F) suppresses GIST growth and induces ferroptosis by regulating SLC7A11 and SLC3A2.
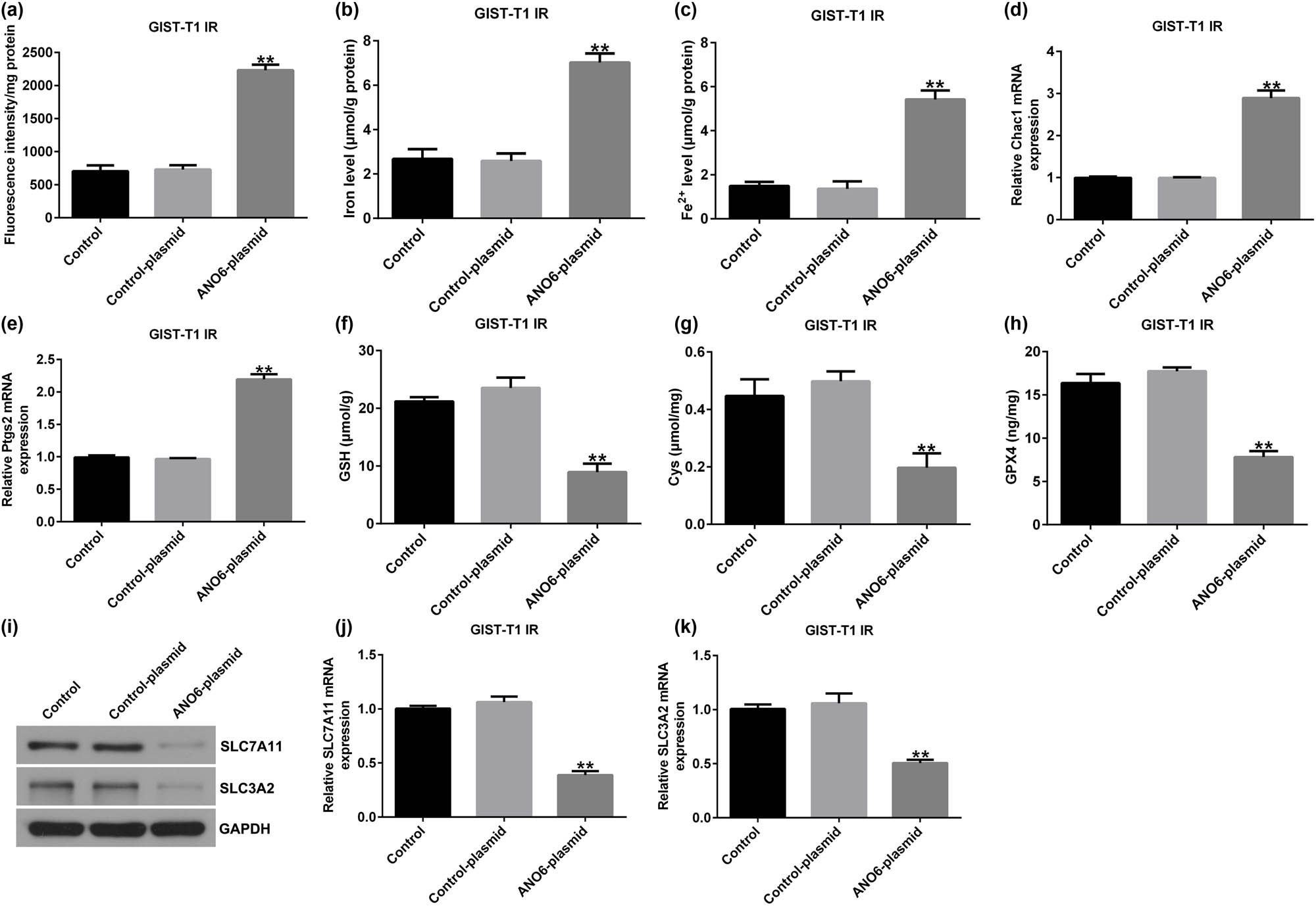
Effects of ANO6 (TMEM16F) on GIST-T1 IR cell ferroptosis. GIST-T1 IR cells were transfected with the control-plasmid and the ANO6-plasmid. (a) The generation of lipid ROS was detected using flow cytometry. Total iron (b) and ferrous iron (c) were evaluated in GIST-T1 IR cells after treatment with the control-plasmid or the ANO6-plasmid. (d) and (e) Levels of Ptgs2 and Chac1 were detected by RT-qPCR. Determination of Cys (f), GSH (g), and GPX4) (h) levels in GIST-T1 IR cells. (i) Detection of SLC7A11 and SLC3A2 expression in the control, control-plasmid, and ANO6-plasmid groups. (j) and (k) mRNA levels of SLC7A11 and SLC3A2 were assessed using RT-qPCR. **P < 0.01 vs control-plasmid.
3.8 ANO6-plasmid inhibited GIST growth in vivo
We performed in vivo experiments to analyze the regulatory role of ANO6 (TMEM16F) in GIST progression. The xenograft tumor models were treated with the ANO6-plasmid, and Figure 8a shows a representative diagram of the xenografts. Furthermore, we calculated tumor volumes and weights of the xenografts. We found that tumor volumes and weights were reduced in nude mice inoculated with GIST-T1 cells after ANO6-plasmid treatment (Figure 8b and c). In summary, our data suggested that the ANO6-plasmid blocked GIST growth.
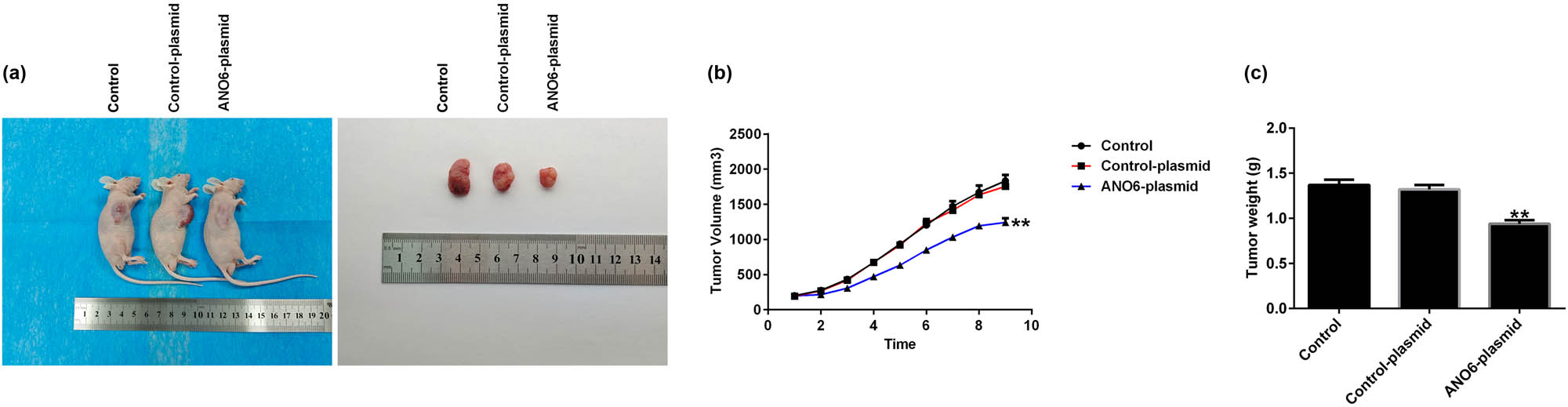
Effects of ANO6 (TMEM16F) on the growth of xenograft in vivo. (a) Gross appearance of tumor. (b) and (c) Average tumor volume and body weight changes in each group were calculated. **P < 0.01 vs control-plasmid.
3.9 ANO6 (TMEM16F) regulated SLC7A11 and SLC3A2 expression in GIST in vivo
We assessed the effect of ANO6 (TMEM16F) on SLC7A11 and SLC3A2 expression in GIST in vivo. Immunohistochemistry suggested that the ANO6-plasmid prominently increased ANO6 (TMEM16F) expression (Figure 9a) and obviously reduced SLC7A11 and SLC3A2 expression (Figure 9a) compared to the control-plasmid group. Similar results were obtained using RT-qPCR (Figure 9b–d). These findings demonstrate that ANO6 (TMEM16F) inhibits GIST growth by regulating SLC7A11 and SLC3A2 expression.
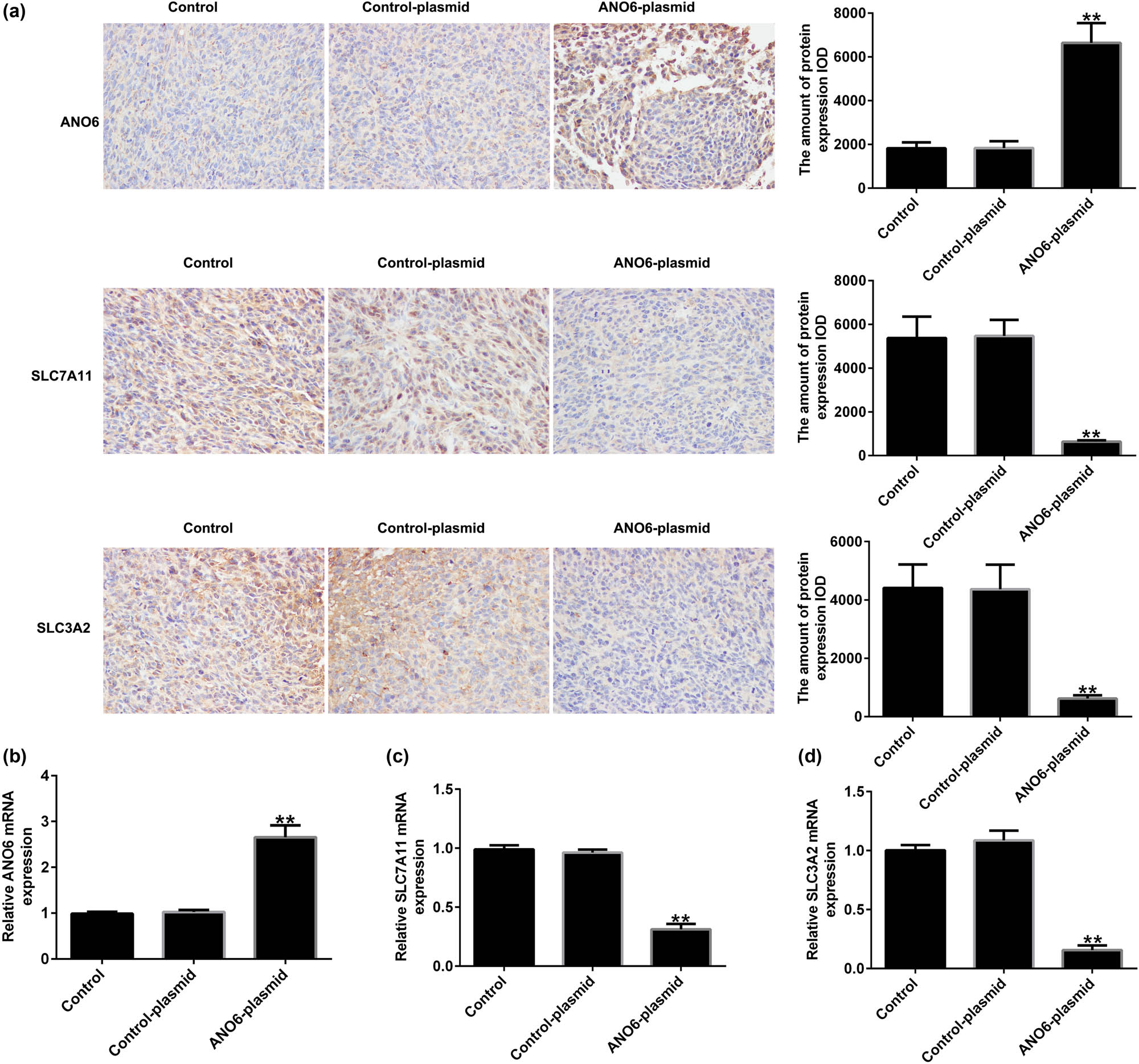
Effects of ANO6 (TMEM16F) on SLC7A11 and SLC3A2 expression in GIST in vivo. (a) The expression of ANO6 (TMEM16F), SLC7A11, and SLC3A2 in GIST tissues was determined using IHC assays. (b)–(d) mRNA expression of ANO6 (TMEM16F), SLC7A11, and SLC3A2 in GISTs tissues was determined by RT-qPCR. **P < 0.01 vs control-plasmid.
3.10 ANO6 (TMEM16F) induced cell apoptosis in GIST in vivo
To further illustrate the mechanism by which ANO6 (TMEM16F) inhibited tumor growth in vivo, we determined the number of apoptotic cells in GIST. As shown in Figure 10, TUNEL staining suggested that the ANO6-plasmid significantly increased TUNEL-positive GIST-T1 cells compared to control-plasmid transfected cells, suggesting that ANO6 (TMEM16F) plays a pro-apoptotic role in GIST.
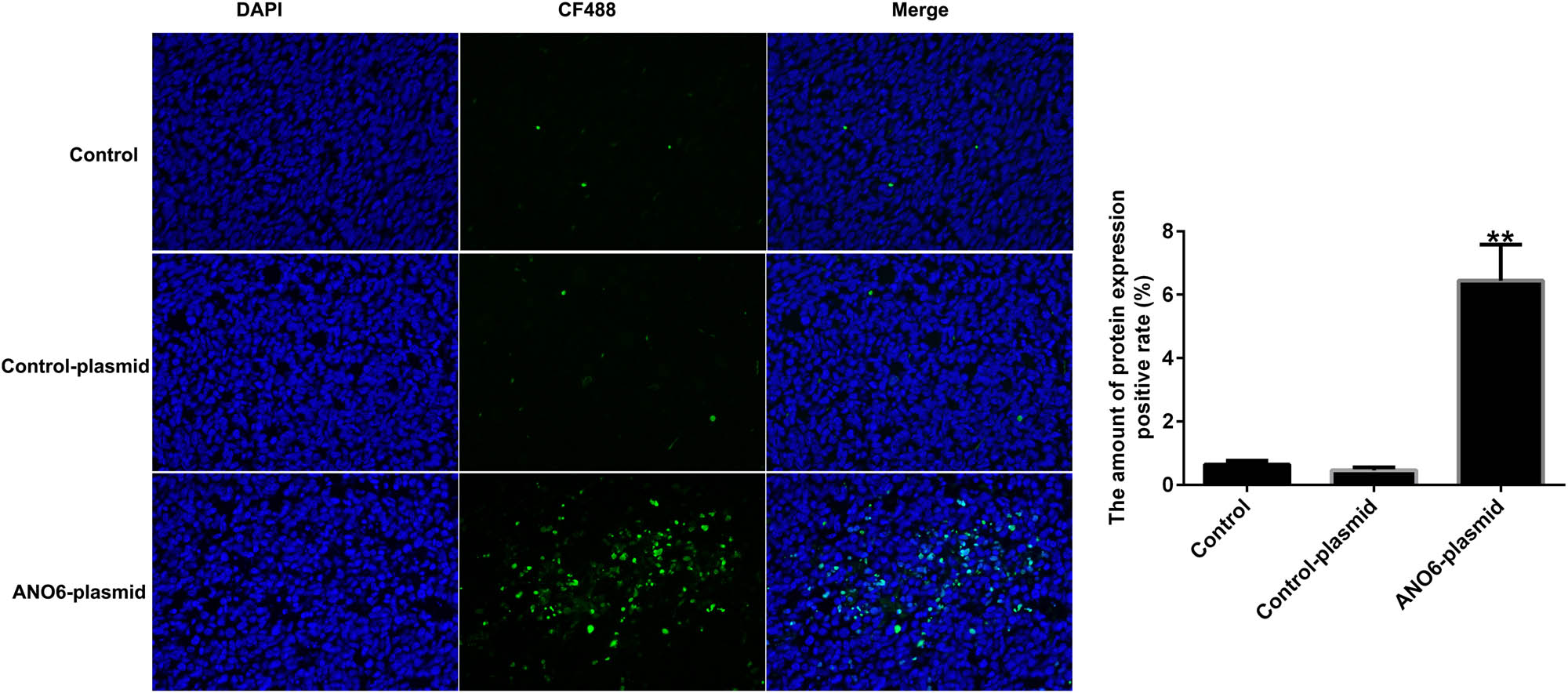
Effects of ANO6 (TMEM16F) on GIST cell apoptosis in vivo. TUNEL images of tumor samples and quantitative analysis of apoptosis rates. **P < 0.01 vs control-plasmid.
3.11 ANO6 (TMEM16F) inhibited GIST growth by inducing ferroptosis in vivo
Finally, we evaluated the effect of ANO6 (TMEM16F) on the ferroptosis of GIST in vivo. Our data demonstrated that the ANO6-plasmid remarkably enhanced lipid ROS levels (Figure 11a), intracellular concentrations of total iron (Figure 11b), and Fe2+ levels (Figure 11c). RT-qPCR analysis revealed that the ANO6-plasmid enhanced Ptgs2 and Chac1 expression levels as opposed to the control-plasmid group (Figure 11d and e). In addition, reduced Cys, GSH, and GPX4 expression levels were observed in ANO6-plasmid treated xenograft tumor models (Figure 11f–h). Our findings revealed that ANO6 (TMEM16F) inhibited GIST growth and induced ferroptosis by regulating SLC7A11 and SLC3A2 expression.
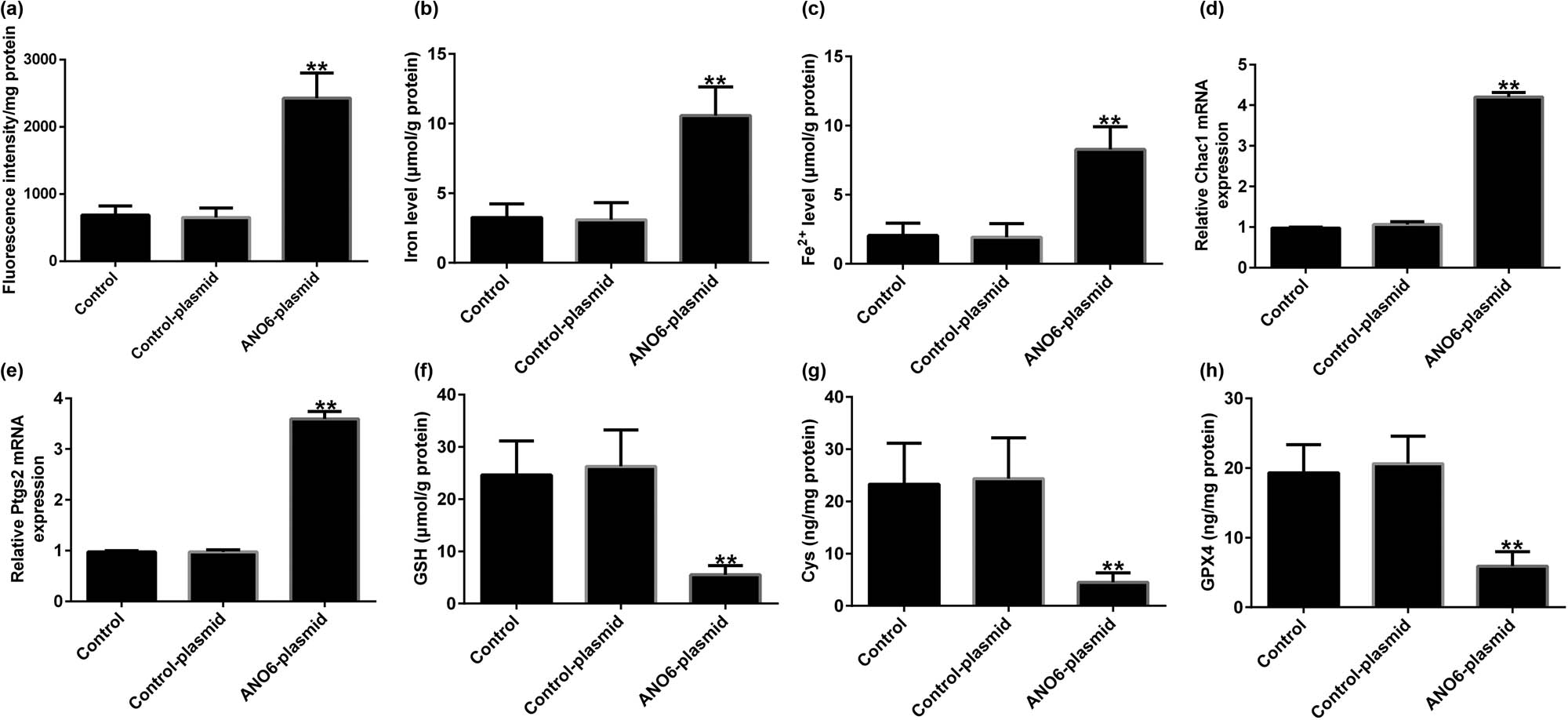
Effects of ANO6 (TMEM16F) on GIST cell ferroptosis in vivo. (a) Lipid ROS stimulation was detected using an ROS fluorescence assay kit. Total iron (b) and ferrous iron (c) were analyzed after treatment with the control-plasmid or the ANO6-plasmid. (d) and (e) RT-qPCR analysis of Ptgs2 and Chac1 mRNA levels. Detection of Cys (f), GSH (g), and GPX4 (h) in GISTs. **P < 0.01 vs control-plasmid.
4 Discussion
This study indicated that ANO6 (TMEM16F) is abnormally low expressed in GIST, which can inhibit the growth of GIST in vitro and in vivo, inducing cell pyroptosis and ferroptosis. It is a potential therapeutic target for GIST.
GISTs are a type of mesenchymal tumors that originate from the precursors of gastrointestinal connective histiocytes and often occur in middle-aged and elderly individuals [27]. IM has been used as a first-line treatment for patients with GIST with metastatic recurrence or unresectability [28,29]. Although many patients benefit from IM, some exhibit drug resistance within 18–24 months of treatment, leading to disease progression and even death [30]. However, GISTs have a high recurrence rate and poor survival rate, and there is currently no effective method for treating advanced metastatic diseases. Drug resistance remains unclear. Therefore, there is an urgent need to identify novel therapeutic targets against GIST. According to reports, TMEM16A/ANO1 and TMEM16F/ANO6 are regulated by intracellular Ca2+ and plasma membrane phospholipids, while TMEM16F/ANO6 is a phospholipid scramjet enzyme that also generates Cl− current [31]. In addition, activation of ANO6 (TMEM16F) contributes to various forms of regulatory cell death in diseases. Cui et al. suggested that ANO6 (TMEM16F) may be a new therapeutic target for Alzheimer’s disease [32]. Research has confirmed the important regulatory role of ANO6 (TMEM16F) in cell growth and migration [19]. However, the expression and role of ANO6 (TMEM16F) in GIST and GIST-T1 IR cells remain unclear.
First, we determined the ANO6 (TMEM16F) expression levels in stromal tumor tissues and adjacent normal tissues. Our data revealed that ANO6 (TMEM16F) was expressed at low levels in stromal tumor tissues from patients with GISTs compared to those in adjacent normal tissues, indicating that ANO6 (TMEM16F) is related to the progression of GIST. Further mechanistic experiments suggested that the ANO6-plasmid upregulated ANO6 (TMEM16F) levels compared with the control-plasmid group. Moreover, the ANO6-plasmid inhibited the proliferation of GIST-T1 and GIST-T1 IR cells and increased the number of apoptotic cells. Zhao et al. reported that ligustrazine suppresses neuronal apoptosis via the Bax/Bcl-2 and caspase-3 pathways in PC-12 cells and rats with vascular dementia [33]. Wei et al. suggested that borax-induced apoptosis in HepG2 cells involves p53, Bcl-2, and Bax [34]. Therefore, we determined the effects of the ANO6-plasmid on Bax and Bcl-2. Our data revealed that the ANO6-plasmid increased Bax expression and reduced Bcl-2 expression, indicating that ANO6 (TMEM16F) is involved in GIST progression by regulating GIST-T1 and GIST-T1 IR cell proliferation and apoptosis. Pyroptosis is another form of programmed inflammatory cell death associated with various pathologies [35–37]. We further determined the effects of the ANO6-plasmid on GIST-T1 and GIST-T1 IR cells. Our data revealed that the ANO6-plasmid enhances pyroptosis, as confirmed by increased GSDMD-N, cleaved-caspase 1, IL-18, and IL-1β expressions.
Further in vivo experiments confirmed the regulatory role of ANO6 (TMEM16F) in GIST progression, as evidenced by the reduction in tumor volume and weights after ANO6-plasmid treatment. Moreover, TUNEL staining suggested that the ANO6-plasmid significantly increased TUNEL-positive GIST-T1 cells compared to control-plasmid transected cells, further indicating the pro-apoptotic role of ANO6 (TMEM16F) in GIST. Ferroptosis is a new type of non-apoptotic programmed cell death caused by the loss of GPX4 activity and subsequent accumulation of lipid-based ROS and is considered an effective target for cancer treatment [38]. Balachander and Paramasivam identified ferroptosis as an emerging therapeutic target in oral cancer [39]. Delvaux et al. demonstrated that ferroptosis induction and YAP inhibition are new therapeutic targets for GISTs [40]. A previous study found that iron is an important executor of ferroptosis. Intracellular iron levels are regulated by iron regulatory transporters, and Fe2+ is particularly important in iron deficiency anemia [41]. Therefore, we explored the effect of ANO6 (TMEM16F) on ferroptosis in GIST-T1 cells, in vivo models, and GIST-T1 IR cells. We found that the ANO6-plasmid increased the stimulation of lipid ROS and increased the intracellular concentrations of total iron and Fe2+ in GIST-T1 cells, GIST-T1 IR cells, and tissues. We also determined the expression of ferroptosis markers including Ptgs2 and Chac1. Our data revealed that Ptgs2 and Chac1 were upregulated in ANO6-plasmid transfected cells and tissues compared to the control-plasmid group. The Cys/glutamate reverse transport system Xc− plays an important role in ferroptosis [42]. GSH is one of the most abundant free radical scavengers in cells and one of the most effective regulatory factors of iron deficiency [43]. GPX4 is a central mediator of cancer cell death and is associated with the production of lipid ROS for ferroptosis. Yang et al. revealed the involvement of the GSH-GPX4 pathway in ferroptosis of the retinal pigment epithelium ferroptosis [44]. Moreover, Yang et al. suggested that Maresin1 protects against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation [45]. Therefore, we examined the ferroptosis-related pathway regulatory factors, including Cys, GSH, and GPX4. Our findings indicated that the ANO6-plasmid reduced Cys, GSH, and GPX4 levels compared to the control-plasmid group.
The Cys/glutamate acid reverse transport system, Xc-, consists of two subunits, SLC7A11 and SLC3A2, which are closely associated with ferroptosis. SLC7A11 transports Cys into cells, promotes GSH synthesis, and accelerates the inhibition of GPX4 on ferroptosis. Yang et al. have suggested that STAT6 inhibits ferroptosis and alleviates acute lung injury by regulating the P53/SLC7A11 pathway [26]. In addition, Wu et al. revealed that SLC3A2 inhibits ferroptosis in laryngeal carcinoma via the mTOR pathway [46]. RT-qPCR and western blotting were used to determine the expression levels of SLC7A11 and SLC3A2 in ANO6-plasmid transfected GIST-T1 cells. The results suggested that the ANO6-plasmid obviously reduced the expression of SLC7A11 and SLC3A2 compared to the control-plasmid group. In addition, several studies have shown that pharmacological inhibition of SLC7A11 plays a vital role in both in vitro and in vivo models. Based on these findings, we clarified the latent regulatory mechanism of the ANO6-plasmid, SLC7A11, and SLC3A2. Immunohistochemistry assays suggested that the ANO6-plasmid prominently increased ANO6 (TMEM16F) expression and reduced SLC7A11 and SLC3A2 expression, demonstrating that ANO6 (TMEM16F) inhibited GIST growth by regulating SLC7A11 and SLC3A2.
There were also some limitations of this study. First, the specific molecular mechanisms by which ANO6 (TMEM16F) regulates the biological behavior of GIST cells (involved signaling pathways) still require further analysis and exploration. In addition, the correlation between the expression of ANO6 (TMEM16F) and pathological parameters in patients with GIST needs to be elucidated. In the future, we will conduct in-depth research on these issues.
5 Conclusion
Taken together, our findings provide strong evidence for the mechanisms by which ANO6 (TMEM16F) induces ferroptosis by regulating SLC7A11 and SLC3A2 expression in GIST. This report provides new insights into the treatment of GIST.
Abbreviations
- Cys
-
cystine
- ELISA
-
enzyme-linked immunosorbent assay
- GIST
-
gastrointestinal stromal tumors
- GPX4
-
glutathione peroxidase 4
- GSH
-
glutathione
- IM
-
imatinib
- MTT
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
-
phosphate-buffered saline
- ROS
-
reactive oxygen species
- RT-qPCR
-
reverse transcription-quantitative polymerase chain reaction
- TUNEL
-
terminal deoxynucleotidyl transferase dUTP nick-end labeling
Acknowledgements
Not applicable.
-
Funding information: No funding was received.
-
Author contributions: Hao Wang contributed to conceptualization data curation, investigation, methodology, project administration, writing – original draft, and writing – review & editing. Wei Zhao contributed to data curation, resources, and supervision. Daorong Wang and Jin Chen contributed to conceptualization, supervision, and writing – review & editing. All authors read and approved the final manuscript.
-
Conflict of interest: The authors declare that they have no competing interests.
-
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Nishida T, Gotouda N, Takahashi T, Cao H. Clinical importance of tumor rupture in gastrointestinal stromal tumor. J Dig Dis. 2023. 10.1111/1751-2980.13190.Search in Google Scholar PubMed
[2] Schaefer IM, DeMatteo RP, Serrano C. The GIST of advances in treatment of advanced gastrointestinal stromal tumor. Am Soc Clin Oncol Educ Book. 2022;42:1–15.10.1200/EDBK_351231Search in Google Scholar PubMed PubMed Central
[3] Mikuni M, Wakuta M, Masaki T, Hirose Y, Takasu H, Kawano H, et al. Surgical resection of intraorbital metastasis of a gastrointestinal stromal tumor resistant to chemotherapy. Am J Ophthalmol Case Rep. 2022;25:101353.10.1016/j.ajoc.2022.101353Search in Google Scholar PubMed PubMed Central
[4] Knowlton CA, Brady LW, Heintzelman RC. Radiotherapy in the treatment of gastrointestinal stromal tumor. Rare Tumors. 2011;3:e35.10.4081/rt.2011.e35Search in Google Scholar PubMed PubMed Central
[5] Kwak HV, Tardy KJ, Allbee A, Stashek K, DeMatteo RP. Surgical management of germline gastrointestinal stromal tumor. Ann Surg Oncol. 2023;30:4966–74.10.1245/s10434-023-13519-ySearch in Google Scholar PubMed
[6] Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther. 2023;8:104.10.1038/s41392-023-01365-zSearch in Google Scholar PubMed PubMed Central
[7] Liu Y, Lu S, Sun Y, Wang F, Yu S, Chen X, et al. Deciphering the role of QPCTL in glioma progression and cancer immunotherapy. Front Immunol. 2023;14:1166377.10.3389/fimmu.2023.1166377Search in Google Scholar PubMed PubMed Central
[8] Arshad J, Costa PA, Barreto-Coelho P, Valdes BN, Trent JC. Immunotherapy strategies for gastrointestinal stromal tumor. Cancers. 2021;13:3525.10.3390/cancers13143525Search in Google Scholar PubMed PubMed Central
[9] Yeh CN, Yen CC, Chen YY, Cheng CT, Huang SC, Chang TW, et al. Identification of aurora kinase A as an unfavorable prognostic factor and potential treatment target for metastatic gastrointestinal stromal tumors. Oncotarget. 2014;5:4071–86.10.18632/oncotarget.1705Search in Google Scholar PubMed PubMed Central
[10] Zhao L, Zhou X, Xie F, Zhang L, Yan H, Huang J, et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. 2022;42:88–116.10.1002/cac2.12250Search in Google Scholar PubMed PubMed Central
[11] Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, et al. Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther. 2021;6:49.10.1038/s41392-020-00428-9Search in Google Scholar PubMed PubMed Central
[12] Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82.10.1038/s41580-020-00324-8Search in Google Scholar PubMed PubMed Central
[13] Zhou J, Zhang L, Yan J, Hou A, Sui W, Sun M. Curcumin induces ferroptosis in A549 CD133(+) Cells through the GSH-GPX4 and FSP1-CoQ10-NAPH Pathways. Discov Med. 2023;35:251–63.10.24976/Discov.Med.202335176.26Search in Google Scholar PubMed
[14] Li FJ, Long HZ, Zhou ZW, Luo HY, Xu SG, Gao LC. System X(c) (-)/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front Pharmacol. 2022;13:910292.10.3389/fphar.2022.910292Search in Google Scholar PubMed PubMed Central
[15] Li Q, Peng F, Yan X, Chen Y, Zhou J, Wu S, et al. Inhibition of SLC7A11-GPX4 signal pathway is involved in aconitine-induced ferroptosis in vivo and in vitro. J Ethnopharmacol. 2023;303:116029.10.1016/j.jep.2022.116029Search in Google Scholar PubMed
[16] Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T, et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19:971–92.10.1038/s41423-022-00905-xSearch in Google Scholar PubMed PubMed Central
[17] Hsu SK, Li CY, Lin IL, Syue WJ, Chen YF, Cheng KC, et al. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics. 2021;11:8813–35.10.7150/thno.62521Search in Google Scholar PubMed PubMed Central
[18] Lin H, Roh J, Woo JH, Kim SJ, Nam JH. TMEM16F/ANO6, a Ca(2+)-activated anion channel, is negatively regulated by the actin cytoskeleton and intracellular MgATP. Biochem Biophys Res Commun. 2018;503:2348–54.Search in Google Scholar
[19] Jacobsen KS, Zeeberg K, Sauter DR, Poulsen KA, Hoffmann EK, Schwab A. The role of TMEM16A (ANO1) and ANO6 (TMEM16F) (ANO6) in cell migration. Pflugers Arch. 2013;465:1753–62.10.1007/s00424-013-1315-zSearch in Google Scholar PubMed PubMed Central
[20] Zhao J, Gao QY. ANO6 (TMEM16F) inhibition limits pain-associated behavior and improves motor function by promoting microglia M2 polarization in mice. Biochem Biophys Res Commun. 2019;517:603–10.10.1016/j.bbrc.2019.07.070Search in Google Scholar PubMed
[21] Bricogne C, Fine M, Pereira PM, Sung J, Tijani M, Wang Y, et al. ANO6 (TMEM16F) activation by Ca(2 +) triggers plasma membrane expansion and directs PD-1 trafficking. Sci Rep. 2019;9:619.10.1038/s41598-018-37056-xSearch in Google Scholar PubMed PubMed Central
[22] Lin H, Roh J, Woo JH, Kim SJ, Nam JH. TMEM16F/ANO6, a Ca(2 +)-activated anion channel, is negatively regulated by the actin cytoskeleton and intracellular MgATP. Biochem Biophys Res Commun. 2018;503:2348–54.10.1016/j.bbrc.2018.06.160Search in Google Scholar PubMed
[23] Ousingsawat J, Schreiber R, Kunzelmann K. TMEM16F/anoctamin 6 in ferroptotic cell death. Cancers (Basel). 2019;11:625.10.3390/cancers11050625Search in Google Scholar PubMed PubMed Central
[24] Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8.10.1006/meth.2001.1262Search in Google Scholar PubMed
[25] Li D, Wang Y, Dong C, Chen T, Dong A, Ren J, et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene. 2023;42:83–98.10.1038/s41388-022-02537-xSearch in Google Scholar PubMed PubMed Central
[26] Yang Y, Ma Y, Li Q, Ling Y, Zhou Y, Chu K, et al. STAT6 inhibits ferroptosis and alleviates acute lung injury via regulating P53/SLC7A11 pathway. Cell Death Dis. 2022;13:530.10.1038/s41419-022-04971-xSearch in Google Scholar PubMed PubMed Central
[27] Long ZW, Wu JH, Cai-Hong, Wang YN, Zhou Y. MiR-374b promotes proliferation and inhibits apoptosis of human GIST cells by inhibiting PTEN through activation of the PI3K/Akt Pathway. Mol Cells. 2018;41:532–44.Search in Google Scholar
[28] Garcia DMX. Nilotinib, imatinib, and GIST therapy. Lancet Oncol. 2015;16:483–4.10.1016/S1470-2045(15)70179-8Search in Google Scholar PubMed
[29] Eriksson M, Joensuu H. Adjuvant imatinib for GIST: duration likely matters. Ann Oncol. 2021;32:434–6.10.1016/j.annonc.2021.01.073Search in Google Scholar PubMed
[30] Zhou Y, Chen J, Weng X, Lin G, Huang Z, Shui H. Establishment of a GIST-T1 gastrointestinal stromal tumour cell line resistant to imatinib mesylate. Oncol Lett. 2018;15:7589–94.10.3892/ol.2018.8283Search in Google Scholar PubMed PubMed Central
[31] Schreiber R, Ousingsawat J, Wanitchakool P, Sirianant L, Benedetto R, Reiss K, et al. Regulation of TMEM16A/ANO1 and TMEM16F/ANO6 ion currents and phospholipid scrambling by Ca(2+) and plasma membrane lipid. J Physiol. 2018;596:217–29.10.1113/JP275175Search in Google Scholar PubMed PubMed Central
[32] Cui ZQ, Hu XY, Yang T, Guan JW, Gu Y, Li HY, et al. ANO6 (TMEM16F) may be a new therapeutic target for Alzheimer’s disease. Neural Regen Res. 2023;18:643–51.10.4103/1673-5374.350211Search in Google Scholar PubMed PubMed Central
[33] Zhao T, Fu Y, Sun H, Liu X. Ligustrazine suppresses neuron apoptosis via the Bax/Bcl-2 and caspase-3 pathway in PC12 cells and in rats with vascular dementia. IUBMB Life. 2018;70:60–70.10.1002/iub.1704Search in Google Scholar PubMed
[34] Wei Y, Yuan FJ, Zhou WB, Wu L, Chen L, Wang JJ, et al. Borax-induced apoptosis in HepG2 cells involves p53, Bcl-2, and Bax. Genet Mol Res. 2016;15(2). 10.4238/gmr.15028300.Search in Google Scholar PubMed
[35] Wu C, Chen H, Zhuang R, Zhang H, Wang Y, Hu X, et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci. 2021;17:1138–52.10.7150/ijbs.57825Search in Google Scholar PubMed PubMed Central
[36] Yan H, Luo B, Wu X, Guan F, Yu X, Zhao L, et al. Cisplatin induces pyroptosis via activation of MEG3/NLRP3/caspase-1/GSDMD pathway in triple-negative breast cancer. Int J Biol Sci. 2021;17:2606–21.10.7150/ijbs.60292Search in Google Scholar PubMed PubMed Central
[37] Zhuang Y, Wang L, Ji C, Sun Y, Shao F. Construction of a novel pyrotosis-related prognostic model of esophageal square cell carcinoma and determination of the anti-tumor effect of WFDC12. Funct Integr Genomics. 2023;23:177.10.1007/s10142-023-01103-2Search in Google Scholar PubMed
[38] Yang S, Wong KH, Hua P, He C, Yu H, Shao D, et al. ROS-responsive fluorinated polyethyleneimine vector to co-deliver shMTHFD2 and shGPX4 plasmids induces ferroptosis and apoptosis for cancer therapy. Acta Biomater. 2022;140:492–505.10.1016/j.actbio.2021.11.042Search in Google Scholar PubMed
[39] Balachander K, Paramasivam A. Ferroptosis: An emerging therapeutic target for oral cancer. Oral Oncol. 2022;131:105970.10.1016/j.oraloncology.2022.105970Search in Google Scholar PubMed
[40] Delvaux M, Hagué P, Craciun L, Wozniak A, Demetter P, Schöffski P, et al. Ferroptosis induction and YAP inhibition as new therapeutic targets in gastrointestinal stromal tumors (GISTs). Cancers (Basel). 2022;14:5050.10.3390/cancers14205050Search in Google Scholar PubMed PubMed Central
[41] Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20:7–23.10.1038/s41569-022-00735-4Search in Google Scholar PubMed PubMed Central
[42] Jyotsana N, Ta KT, DelGiorno KE. The role of cystine/glutamate antiporter SLC7A11/xCT in the pathophysiology of cancer. Front Oncol. 2022;12:858462.10.3389/fonc.2022.858462Search in Google Scholar PubMed PubMed Central
[43] Liu T, Sun L, Zhang Y, Wang Y, Zheng J. Imbalanced GSH/ROS and sequential cell death. J Biochem Mol Toxicol. 2022;36:e22942.10.1002/jbt.22942Search in Google Scholar PubMed
[44] Yang M, Tsui MG, Tsang JKW, Goit RK, Yao KM, So KF, et al. Involvement of FSP1-CoQ(10)-NADH and GSH-GPx-4 pathways in retinal pigment epithelium ferroptosis. Cell Death Dis. 2022;13:468.10.1038/s41419-022-04924-4Search in Google Scholar PubMed PubMed Central
[45] Yang W, Wang Y, Zhang C, Huang Y, Yu J, Shi L, et al. Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation. Front Pharmacol. 2022;13:865689.10.3389/fphar.2022.865689Search in Google Scholar PubMed PubMed Central
[46] Wu F, Xiong G, Chen Z, Lei C, Liu Q, Bai Y. SLC3A2 inhibits ferroptosis in laryngeal carcinoma via mTOR pathway. Hereditas. 2022;159:6.10.1186/s41065-022-00225-0Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- EDNRB inhibits the growth and migration of prostate cancer cells by activating the cGMP-PKG pathway
- STK11 (LKB1) mutation suppresses ferroptosis in lung adenocarcinoma by facilitating monounsaturated fatty acid synthesis
- Association of SOX6 gene polymorphisms with Kashin-Beck disease risk in the Chinese Han population
- The pyroptosis-related signature predicts prognosis and influences the tumor immune microenvironment in dedifferentiated liposarcoma
- METTL3 attenuates ferroptosis sensitivity in lung cancer via modulating TFRC
- Identification and validation of molecular subtypes and prognostic signature for stage I and stage II gastric cancer based on neutrophil extracellular traps
- Novel lumbar plexus block versus femoral nerve block for analgesia and motor recovery after total knee arthroplasty
- Correlation between ABCB1 and OLIG2 polymorphisms and the severity and prognosis of patients with cerebral infarction
- Study on the radiotherapy effect and serum neutral granulocyte lymphocyte ratio and inflammatory factor expression of nasopharyngeal carcinoma
- Transcriptome analysis of effects of Tecrl deficiency on cardiometabolic and calcium regulation in cardiac tissue
- Aflatoxin B1 induces infertility, fetal deformities, and potential therapies
- Serum levels of HMW adiponectin and its receptors are associated with cytokine levels and clinical characteristics in chronic obstructive pulmonary disease
- METTL3-mediated methylation of CYP2C19 mRNA may aggravate clopidogrel resistance in ischemic stroke patients
- Understand how machine learning impact lung cancer research from 2010 to 2021: A bibliometric analysis
- Pressure ulcers in German hospitals: Analysis of reimbursement and length of stay
- Metformin plus L-carnitine enhances brown/beige adipose tissue activity via Nrf2/HO-1 signaling to reduce lipid accumulation and inflammation in murine obesity
- Downregulation of carbonic anhydrase IX expression in mouse xenograft nasopharyngeal carcinoma model via doxorubicin nanobubble combined with ultrasound
- Feasibility of 3-dimensional printed models in simulated training and teaching of transcatheter aortic valve replacement
- miR-335-3p improves type II diabetes mellitus by IGF-1 regulating macrophage polarization
- The analyses of human MCPH1 DNA repair machinery and genetic variations
- Activation of Piezo1 increases the sensitivity of breast cancer to hyperthermia therapy
- Comprehensive analysis based on the disulfidptosis-related genes identifies hub genes and immune infiltration for pancreatic adenocarcinoma
- Changes of serum CA125 and PGE2 before and after high-intensity focused ultrasound combined with GnRH-a in treatment of patients with adenomyosis
- The clinical value of the hepatic venous pressure gradient in patients undergoing hepatic resection for hepatocellular carcinoma with or without liver cirrhosis
- Development and validation of a novel model to predict pulmonary embolism in cardiology suspected patients: A 10-year retrospective analysis
- Downregulation of lncRNA XLOC_032768 in diabetic patients predicts the occurrence of diabetic nephropathy
- Circ_0051428 targeting miR-885-3p/MMP2 axis enhances the malignancy of cervical cancer
- Effectiveness of ginkgo diterpene lactone meglumine on cognitive function in patients with acute ischemic stroke
- The construction of a novel prognostic prediction model for glioma based on GWAS-identified prognostic-related risk loci
- Evaluating the impact of childhood BMI on the risk of coronavirus disease 2019: A Mendelian randomization study
- Lactate dehydrogenase to albumin ratio is associated with in-hospital mortality in patients with acute heart failure: Data from the MIMIC-III database
- CD36-mediated podocyte lipotoxicity promotes foot process effacement
- Efficacy of etonogestrel subcutaneous implants versus the levonorgestrel-releasing intrauterine system in the conservative treatment of adenomyosis
- FLRT2 mediates chondrogenesis of nasal septal cartilage and mandibular condyle cartilage
- Challenges in treating primary immune thrombocytopenia patients undergoing COVID-19 vaccination: A retrospective study
- Let-7 family regulates HaCaT cell proliferation and apoptosis via the ΔNp63/PI3K/AKT pathway
- Phospholipid transfer protein ameliorates sepsis-induced cardiac dysfunction through NLRP3 inflammasome inhibition
- Postoperative cognitive dysfunction in elderly patients with colorectal cancer: A randomized controlled study comparing goal-directed and conventional fluid therapy
- Long-pulsed ultrasound-mediated microbubble thrombolysis in a rat model of microvascular obstruction
- High SEC61A1 expression predicts poor outcome of acute myeloid leukemia
- Comparison of polymerase chain reaction and next-generation sequencing with conventional urine culture for the diagnosis of urinary tract infections: A meta-analysis
- Secreted frizzled-related protein 5 protects against renal fibrosis by inhibiting Wnt/β-catenin pathway
- Pan-cancer and single-cell analysis of actin cytoskeleton genes related to disulfidptosis
- Overexpression of miR-532-5p restrains oxidative stress response of chondrocytes in nontraumatic osteonecrosis of the femoral head by inhibiting ABL1
- Autologous liver transplantation for unresectable hepatobiliary malignancies in enhanced recovery after surgery model
- Clinical analysis of incomplete rupture of the uterus secondary to previous cesarean section
- Abnormal sleep duration is associated with sarcopenia in older Chinese people: A large retrospective cross-sectional study
- No genetic causality between obesity and benign paroxysmal vertigo: A two-sample Mendelian randomization study
- Identification and validation of autophagy-related genes in SSc
- Long non-coding RNA SRA1 suppresses radiotherapy resistance in esophageal squamous cell carcinoma by modulating glycolytic reprogramming
- Evaluation of quality of life in patients with schizophrenia: An inpatient social welfare institution-based cross-sectional study
- The possible role of oxidative stress marker glutathione in the assessment of cognitive impairment in multiple sclerosis
- Compilation of a self-management assessment scale for postoperative patients with aortic dissection
- Left atrial appendage closure in conjunction with radiofrequency ablation: Effects on left atrial functioning in patients with paroxysmal atrial fibrillation
- Effect of anterior femoral cortical notch grade on postoperative function and complications during TKA surgery: A multicenter, retrospective study
- Clinical characteristics and assessment of risk factors in patients with influenza A-induced severe pneumonia after the prevalence of SARS-CoV-2
- Analgesia nociception index is an indicator of laparoscopic trocar insertion-induced transient nociceptive stimuli
- High STAT4 expression correlates with poor prognosis in acute myeloid leukemia and facilitates disease progression by upregulating VEGFA expression
- Factors influencing cardiovascular system-related post-COVID-19 sequelae: A single-center cohort study
- HOXD10 regulates intestinal permeability and inhibits inflammation of dextran sulfate sodium-induced ulcerative colitis through the inactivation of the Rho/ROCK/MMPs axis
- Mesenchymal stem cell-derived exosomal miR-26a induces ferroptosis, suppresses hepatic stellate cell activation, and ameliorates liver fibrosis by modulating SLC7A11
- Endovascular thrombectomy versus intravenous thrombolysis for primary distal, medium vessel occlusion in acute ischemic stroke
- ANO6 (TMEM16F) inhibits gastrointestinal stromal tumor growth and induces ferroptosis
- Prognostic value of EIF5A2 in solid tumors: A meta-analysis and bioinformatics analysis
- The role of enhanced expression of Cx43 in patients with ulcerative colitis
- Choosing a COVID-19 vaccination site might be driven by anxiety and body vigilance
- Role of ICAM-1 in triple-negative breast cancer
- Cost-effectiveness of ambroxol in the treatment of Gaucher disease type 2
- HLA-DRB5 promotes immune thrombocytopenia via activating CD8+ T cells
- Efficacy and factors of myofascial release therapy combined with electrical and magnetic stimulation in the treatment of chronic pelvic pain syndrome
- Efficacy of tacrolimus monotherapy in primary membranous nephropathy
- Mechanisms of Tripterygium wilfordii Hook F on treating rheumatoid arthritis explored by network pharmacology analysis and molecular docking
- FBXO45 levels regulated ferroptosis renal tubular epithelial cells in a model of diabetic nephropathy by PLK1
- Optimizing anesthesia strategies to NSCLC patients in VATS procedures: Insights from drug requirements and patient recovery patterns
- Alpha-lipoic acid upregulates the PPARγ/NRF2/GPX4 signal pathway to inhibit ferroptosis in the pathogenesis of unexplained recurrent pregnancy loss
- Correlation between fat-soluble vitamin levels and inflammatory factors in paediatric community-acquired pneumonia: A prospective study
- CD1d affects the proliferation, migration, and apoptosis of human papillary thyroid carcinoma TPC-1 cells via regulating MAPK/NF-κB signaling pathway
- miR-let-7a inhibits sympathetic nerve remodeling after myocardial infarction by downregulating the expression of nerve growth factor
- Immune response analysis of solid organ transplantation recipients inoculated with inactivated COVID-19 vaccine: A retrospective analysis
- The H2Valdien derivatives regulate the epithelial–mesenchymal transition of hepatoma carcinoma cells through the Hedgehog signaling pathway
- Clinical efficacy of dexamethasone combined with isoniazid in the treatment of tuberculous meningitis and its effect on peripheral blood T cell subsets
- Comparison of short-segment and long-segment fixation in treatment of degenerative scoliosis and analysis of factors associated with adjacent spondylolisthesis
- Lycopene inhibits pyroptosis of endothelial progenitor cells induced by ox-LDL through the AMPK/mTOR/NLRP3 pathway
- Methylation regulation for FUNDC1 stability in childhood leukemia was up-regulated and facilitates metastasis and reduces ferroptosis of leukemia through mitochondrial damage by FBXL2
- Correlation of single-fiber electromyography studies and functional status in patients with amyotrophic lateral sclerosis
- Risk factors of postoperative airway obstruction complications in children with oral floor mass
- Expression levels and clinical significance of serum miR-19a/CCL20 in patients with acute cerebral infarction
- Physical activity and mental health trends in Korean adolescents: Analyzing the impact of the COVID-19 pandemic from 2018 to 2022
- Evaluating anemia in HIV-infected patients using chest CT
- Ponticulus posticus and skeletal malocclusion: A pilot study in a Southern Italian pre-orthodontic court
- Causal association of circulating immune cells and lymphoma: A Mendelian randomization study
- Assessment of the renal function and fibrosis indexes of conventional western medicine with Chinese medicine for dredging collaterals on treating renal fibrosis: A systematic review and meta-analysis
- Comprehensive landscape of integrator complex subunits and their association with prognosis and tumor microenvironment in gastric cancer
- New target-HMGCR inhibitors for the treatment of primary sclerosing cholangitis: A drug Mendelian randomization study
- Population pharmacokinetics of meropenem in critically ill patients
- Comparison of the ability of newly inflammatory markers to predict complicated appendicitis
- Comparative morphology of the cruciate ligaments: A radiological study
- Immune landscape of hepatocellular carcinoma: The central role of TP53-inducible glycolysis and apoptosis regulator
- Serum SIRT3 levels in epilepsy patients and its association with clinical outcomes and severity: A prospective observational study
- SHP-1 mediates cigarette smoke extract-induced epithelial–mesenchymal transformation and inflammation in 16HBE cells
- Acute hyper-hypoxia accelerates the development of depression in mice via the IL-6/PGC1α/MFN2 signaling pathway
- The GJB3 correlates with the prognosis, immune cell infiltration, and therapeutic responses in lung adenocarcinoma
- Physical fitness and blood parameters outcomes of breast cancer survivor in a low-intensity circuit resistance exercise program
- Exploring anesthetic-induced gene expression changes and immune cell dynamics in atrial tissue post-coronary artery bypass graft surgery
- Empagliflozin improves aortic injury in obese mice by regulating fatty acid metabolism
- Analysis of the risk factors of the radiation-induced encephalopathy in nasopharyngeal carcinoma: A retrospective cohort study
- Reproductive outcomes in women with BRCA 1/2 germline mutations: A retrospective observational study and literature review
- Evaluation of upper airway ultrasonographic measurements in predicting difficult intubation: A cross-section of the Turkish population
- Prognostic and diagnostic value of circulating IGFBP2 in pancreatic cancer
- Postural stability after operative reconstruction of the AFTL in chronic ankle instability comparing three different surgical techniques
- Research trends related to emergence agitation in the post-anaesthesia care unit from 2001 to 2023: A bibliometric analysis
- Frequency and clinicopathological correlation of gastrointestinal polyps: A six-year single center experience
- ACSL4 mediates inflammatory bowel disease and contributes to LPS-induced intestinal epithelial cell dysfunction by activating ferroptosis and inflammation
- Affibody-based molecular probe 99mTc-(HE)3ZHER2:V2 for non-invasive HER2 detection in ovarian and breast cancer xenografts
- Effectiveness of nutritional support for clinical outcomes in gastric cancer patients: A meta-analysis of randomized controlled trials
- The relationship between IFN-γ, IL-10, IL-6 cytokines, and severity of the condition with serum zinc and Fe in children infected with Mycoplasma pneumoniae
- Paraquat disrupts the blood–brain barrier by increasing IL-6 expression and oxidative stress through the activation of PI3K/AKT signaling pathway
- Sleep quality associate with the increased prevalence of cognitive impairment in coronary artery disease patients: A retrospective case–control study
- Dioscin protects against chronic prostatitis through the TLR4/NF-κB pathway
- Association of polymorphisms in FBN1, MYH11, and TGF-β signaling-related genes with susceptibility of sporadic thoracic aortic aneurysm and dissection in the Zhejiang Han population
- Application value of multi-parameter magnetic resonance image-transrectal ultrasound cognitive fusion in prostate biopsy
- Laboratory variables‐based artificial neural network models for predicting fatty liver disease: A retrospective study
- Decreased BIRC5-206 promotes epithelial–mesenchymal transition in nasopharyngeal carcinoma through sponging miR-145-5p
- Sepsis induces the cardiomyocyte apoptosis and cardiac dysfunction through activation of YAP1/Serpine1/caspase-3 pathway
- Assessment of iron metabolism and iron deficiency in incident patients on incident continuous ambulatory peritoneal dialysis
- Tibial periosteum flap combined with autologous bone grafting in the treatment of Gustilo-IIIB/IIIC open tibial fractures
- The application of intravenous general anesthesia under nasopharyngeal airway assisted ventilation undergoing ureteroscopic holmium laser lithotripsy: A prospective, single-center, controlled trial
- Long intergenic noncoding RNA for IGF2BP2 stability suppresses gastric cancer cell apoptosis by inhibiting the maturation of microRNA-34a
- Role of FOXM1 and AURKB in regulating keratinocyte function in psoriasis
- Parental control attitudes over their pre-school children’s diet
- The role of auto-HSCT in extranodal natural killer/T cell lymphoma
- Significance of negative cervical cytology and positive HPV in the diagnosis of cervical lesions by colposcopy
- Echinacoside inhibits PASMCs calcium overload to prevent hypoxic pulmonary artery remodeling by regulating TRPC1/4/6 and calmodulin
- ADAR1 plays a protective role in proximal tubular cells under high glucose conditions by attenuating the PI3K/AKT/mTOR signaling pathway
- The risk of cancer among insulin glargine users in Lithuania: A retrospective population-based study
- The unusual location of primary hydatid cyst: A case series study
- Intraoperative changes in electrophysiological monitoring can be used to predict clinical outcomes in patients with spinal cavernous malformation
- Obesity and risk of placenta accreta spectrum: A meta-analysis
- Shikonin alleviates asthma phenotypes in mice via an airway epithelial STAT3-dependent mechanism
- NSUN6 and HTR7 disturbed the stability of carotid atherosclerotic plaques by regulating the immune responses of macrophages
- The effect of COVID-19 lockdown on admission rates in Maternity Hospital
- Temporal muscle thickness is not a prognostic predictor in patients with high-grade glioma, an experience at two centers in China
- Luteolin alleviates cerebral ischemia/reperfusion injury by regulating cell pyroptosis
- Therapeutic role of respiratory exercise in patients with tuberculous pleurisy
- Effects of CFTR-ENaC on spinal cord edema after spinal cord injury
- Irisin-regulated lncRNAs and their potential regulatory functions in chondrogenic differentiation of human mesenchymal stem cells
- DMD mutations in pediatric patients with phenotypes of Duchenne/Becker muscular dystrophy
- Combination of C-reactive protein and fibrinogen-to-albumin ratio as a novel predictor of all-cause mortality in heart failure patients
- Significant role and the underly mechanism of cullin-1 in chronic obstructive pulmonary disease
- Ferroptosis-related prognostic model of mantle cell lymphoma
- Observation of choking reaction and other related indexes in elderly painless fiberoptic bronchoscopy with transnasal high-flow humidification oxygen therapy
- A bibliometric analysis of Prader-Willi syndrome from 2002 to 2022
- The causal effects of childhood sunburn occasions on melanoma: A univariable and multivariable Mendelian randomization study
- Oxidative stress regulates glycogen synthase kinase-3 in lymphocytes of diabetes mellitus patients complicated with cerebral infarction
- Role of COX6C and NDUFB3 in septic shock and stroke
- Trends in disease burden of type 2 diabetes, stroke, and hypertensive heart disease attributable to high BMI in China: 1990–2019
- Purinergic P2X7 receptor mediates hyperoxia-induced injury in pulmonary microvascular endothelial cells via NLRP3-mediated pyroptotic pathway
- Investigating the role of oviductal mucosa–endometrial co-culture in modulating factors relevant to embryo implantation
- Analgesic effect of external oblique intercostal block in laparoscopic cholecystectomy: A retrospective study
- Elevated serum miR-142-5p correlates with ischemic lesions and both NSE and S100β in ischemic stroke patients
- Correlation between the mechanism of arteriopathy in IgA nephropathy and blood stasis syndrome: A cohort study
- Risk factors for progressive kyphosis after percutaneous kyphoplasty in osteoporotic vertebral compression fracture
- Predictive role of neuron-specific enolase and S100-β in early neurological deterioration and unfavorable prognosis in patients with ischemic stroke
- The potential risk factors of postoperative cognitive dysfunction for endovascular therapy in acute ischemic stroke with general anesthesia
- Fluoxetine inhibited RANKL-induced osteoclastic differentiation in vitro
- Detection of serum FOXM1 and IGF2 in patients with ARDS and their correlation with disease and prognosis
- Rhein promotes skin wound healing by activating the PI3K/AKT signaling pathway
- Differences in mortality risk by levels of physical activity among persons with disabilities in South Korea
- Review Articles
- Cutaneous signs of selected cardiovascular disorders: A narrative review
- XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis
- A narrative review on adverse drug reactions of COVID-19 treatments on the kidney
- Emerging role and function of SPDL1 in human health and diseases
- Adverse reactions of piperacillin: A literature review of case reports
- Molecular mechanism and intervention measures of microvascular complications in diabetes
- Regulation of mesenchymal stem cell differentiation by autophagy
- Molecular landscape of borderline ovarian tumours: A systematic review
- Advances in synthetic lethality modalities for glioblastoma multiforme
- Investigating hormesis, aging, and neurodegeneration: From bench to clinics
- Frankincense: A neuronutrient to approach Parkinson’s disease treatment
- Sox9: A potential regulator of cancer stem cells in osteosarcoma
- Early detection of cardiovascular risk markers through non-invasive ultrasound methodologies in periodontitis patients
- Advanced neuroimaging and criminal interrogation in lie detection
- Maternal factors for neural tube defects in offspring: An umbrella review
- The chemoprotective hormetic effects of rosmarinic acid
- CBD’s potential impact on Parkinson’s disease: An updated overview
- Progress in cytokine research for ARDS: A comprehensive review
- Utilizing reactive oxygen species-scavenging nanoparticles for targeting oxidative stress in the treatment of ischemic stroke: A review
- NRXN1-related disorders, attempt to better define clinical assessment
- Lidocaine infusion for the treatment of complex regional pain syndrome: Case series and literature review
- Trends and future directions of autophagy in osteosarcoma: A bibliometric analysis
- Iron in ventricular remodeling and aneurysms post-myocardial infarction
- Case Reports
- Sirolimus potentiated angioedema: A case report and review of the literature
- Identification of mixed anaerobic infections after inguinal hernia repair based on metagenomic next-generation sequencing: A case report
- Successful treatment with bortezomib in combination with dexamethasone in a middle-aged male with idiopathic multicentric Castleman’s disease: A case report
- Complete heart block associated with hepatitis A infection in a female child with fatal outcome
- Elevation of D-dimer in eosinophilic gastrointestinal diseases in the absence of venous thrombosis: A case series and literature review
- Four years of natural progressive course: A rare case report of juvenile Xp11.2 translocations renal cell carcinoma with TFE3 gene fusion
- Advancing prenatal diagnosis: Echocardiographic detection of Scimitar syndrome in China – A case series
- Outcomes and complications of hemodialysis in patients with renal cancer following bilateral nephrectomy
- Anti-HMGCR myopathy mimicking facioscapulohumeral muscular dystrophy
- Recurrent opportunistic infections in a HIV-negative patient with combined C6 and NFKB1 mutations: A case report, pedigree analysis, and literature review
- Letter to the Editor
- Letter to the Editor: Total parenteral nutrition-induced Wernicke’s encephalopathy after oncologic gastrointestinal surgery
- Erratum
- Erratum to “Bladder-embedded ectopic intrauterine device with calculus”
- Retraction
- Retraction of “XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis”
- Corrigendum
- Corrigendum to “Investigating hormesis, aging, and neurodegeneration: From bench to clinics”
- Corrigendum to “Frankincense: A neuronutrient to approach Parkinson’s disease treatment”
- Special Issue The evolving saga of RNAs from bench to bedside - Part II
- Machine-learning-based prediction of a diagnostic model using autophagy-related genes based on RNA sequencing for patients with papillary thyroid carcinoma
- Unlocking the future of hepatocellular carcinoma treatment: A comprehensive analysis of disulfidptosis-related lncRNAs for prognosis and drug screening
- Elevated mRNA level indicates FSIP1 promotes EMT and gastric cancer progression by regulating fibroblasts in tumor microenvironment
- Special Issue Advancements in oncology: bridging clinical and experimental research - Part I
- Ultrasound-guided transperineal vs transrectal prostate biopsy: A meta-analysis of diagnostic accuracy and complication rates
- Assessment of diagnostic value of unilateral systematic biopsy combined with targeted biopsy in detecting clinically significant prostate cancer
- SENP7 inhibits glioblastoma metastasis and invasion by dissociating SUMO2/3 binding to specific target proteins
- MARK1 suppress malignant progression of hepatocellular carcinoma and improves sorafenib resistance through negatively regulating POTEE
- Analysis of postoperative complications in bladder cancer patients
- Carboplatin combined with arsenic trioxide versus carboplatin combined with docetaxel treatment for LACC: A randomized, open-label, phase II clinical study
- Special Issue Exploring the biological mechanism of human diseases based on MultiOmics Technology - Part I
- Comprehensive pan-cancer investigation of carnosine dipeptidase 1 and its prospective prognostic significance in hepatocellular carcinoma
- Identification of signatures associated with microsatellite instability and immune characteristics to predict the prognostic risk of colon cancer
- Single-cell analysis identified key macrophage subpopulations associated with atherosclerosis
Articles in the same Issue
- Research Articles
- EDNRB inhibits the growth and migration of prostate cancer cells by activating the cGMP-PKG pathway
- STK11 (LKB1) mutation suppresses ferroptosis in lung adenocarcinoma by facilitating monounsaturated fatty acid synthesis
- Association of SOX6 gene polymorphisms with Kashin-Beck disease risk in the Chinese Han population
- The pyroptosis-related signature predicts prognosis and influences the tumor immune microenvironment in dedifferentiated liposarcoma
- METTL3 attenuates ferroptosis sensitivity in lung cancer via modulating TFRC
- Identification and validation of molecular subtypes and prognostic signature for stage I and stage II gastric cancer based on neutrophil extracellular traps
- Novel lumbar plexus block versus femoral nerve block for analgesia and motor recovery after total knee arthroplasty
- Correlation between ABCB1 and OLIG2 polymorphisms and the severity and prognosis of patients with cerebral infarction
- Study on the radiotherapy effect and serum neutral granulocyte lymphocyte ratio and inflammatory factor expression of nasopharyngeal carcinoma
- Transcriptome analysis of effects of Tecrl deficiency on cardiometabolic and calcium regulation in cardiac tissue
- Aflatoxin B1 induces infertility, fetal deformities, and potential therapies
- Serum levels of HMW adiponectin and its receptors are associated with cytokine levels and clinical characteristics in chronic obstructive pulmonary disease
- METTL3-mediated methylation of CYP2C19 mRNA may aggravate clopidogrel resistance in ischemic stroke patients
- Understand how machine learning impact lung cancer research from 2010 to 2021: A bibliometric analysis
- Pressure ulcers in German hospitals: Analysis of reimbursement and length of stay
- Metformin plus L-carnitine enhances brown/beige adipose tissue activity via Nrf2/HO-1 signaling to reduce lipid accumulation and inflammation in murine obesity
- Downregulation of carbonic anhydrase IX expression in mouse xenograft nasopharyngeal carcinoma model via doxorubicin nanobubble combined with ultrasound
- Feasibility of 3-dimensional printed models in simulated training and teaching of transcatheter aortic valve replacement
- miR-335-3p improves type II diabetes mellitus by IGF-1 regulating macrophage polarization
- The analyses of human MCPH1 DNA repair machinery and genetic variations
- Activation of Piezo1 increases the sensitivity of breast cancer to hyperthermia therapy
- Comprehensive analysis based on the disulfidptosis-related genes identifies hub genes and immune infiltration for pancreatic adenocarcinoma
- Changes of serum CA125 and PGE2 before and after high-intensity focused ultrasound combined with GnRH-a in treatment of patients with adenomyosis
- The clinical value of the hepatic venous pressure gradient in patients undergoing hepatic resection for hepatocellular carcinoma with or without liver cirrhosis
- Development and validation of a novel model to predict pulmonary embolism in cardiology suspected patients: A 10-year retrospective analysis
- Downregulation of lncRNA XLOC_032768 in diabetic patients predicts the occurrence of diabetic nephropathy
- Circ_0051428 targeting miR-885-3p/MMP2 axis enhances the malignancy of cervical cancer
- Effectiveness of ginkgo diterpene lactone meglumine on cognitive function in patients with acute ischemic stroke
- The construction of a novel prognostic prediction model for glioma based on GWAS-identified prognostic-related risk loci
- Evaluating the impact of childhood BMI on the risk of coronavirus disease 2019: A Mendelian randomization study
- Lactate dehydrogenase to albumin ratio is associated with in-hospital mortality in patients with acute heart failure: Data from the MIMIC-III database
- CD36-mediated podocyte lipotoxicity promotes foot process effacement
- Efficacy of etonogestrel subcutaneous implants versus the levonorgestrel-releasing intrauterine system in the conservative treatment of adenomyosis
- FLRT2 mediates chondrogenesis of nasal septal cartilage and mandibular condyle cartilage
- Challenges in treating primary immune thrombocytopenia patients undergoing COVID-19 vaccination: A retrospective study
- Let-7 family regulates HaCaT cell proliferation and apoptosis via the ΔNp63/PI3K/AKT pathway
- Phospholipid transfer protein ameliorates sepsis-induced cardiac dysfunction through NLRP3 inflammasome inhibition
- Postoperative cognitive dysfunction in elderly patients with colorectal cancer: A randomized controlled study comparing goal-directed and conventional fluid therapy
- Long-pulsed ultrasound-mediated microbubble thrombolysis in a rat model of microvascular obstruction
- High SEC61A1 expression predicts poor outcome of acute myeloid leukemia
- Comparison of polymerase chain reaction and next-generation sequencing with conventional urine culture for the diagnosis of urinary tract infections: A meta-analysis
- Secreted frizzled-related protein 5 protects against renal fibrosis by inhibiting Wnt/β-catenin pathway
- Pan-cancer and single-cell analysis of actin cytoskeleton genes related to disulfidptosis
- Overexpression of miR-532-5p restrains oxidative stress response of chondrocytes in nontraumatic osteonecrosis of the femoral head by inhibiting ABL1
- Autologous liver transplantation for unresectable hepatobiliary malignancies in enhanced recovery after surgery model
- Clinical analysis of incomplete rupture of the uterus secondary to previous cesarean section
- Abnormal sleep duration is associated with sarcopenia in older Chinese people: A large retrospective cross-sectional study
- No genetic causality between obesity and benign paroxysmal vertigo: A two-sample Mendelian randomization study
- Identification and validation of autophagy-related genes in SSc
- Long non-coding RNA SRA1 suppresses radiotherapy resistance in esophageal squamous cell carcinoma by modulating glycolytic reprogramming
- Evaluation of quality of life in patients with schizophrenia: An inpatient social welfare institution-based cross-sectional study
- The possible role of oxidative stress marker glutathione in the assessment of cognitive impairment in multiple sclerosis
- Compilation of a self-management assessment scale for postoperative patients with aortic dissection
- Left atrial appendage closure in conjunction with radiofrequency ablation: Effects on left atrial functioning in patients with paroxysmal atrial fibrillation
- Effect of anterior femoral cortical notch grade on postoperative function and complications during TKA surgery: A multicenter, retrospective study
- Clinical characteristics and assessment of risk factors in patients with influenza A-induced severe pneumonia after the prevalence of SARS-CoV-2
- Analgesia nociception index is an indicator of laparoscopic trocar insertion-induced transient nociceptive stimuli
- High STAT4 expression correlates with poor prognosis in acute myeloid leukemia and facilitates disease progression by upregulating VEGFA expression
- Factors influencing cardiovascular system-related post-COVID-19 sequelae: A single-center cohort study
- HOXD10 regulates intestinal permeability and inhibits inflammation of dextran sulfate sodium-induced ulcerative colitis through the inactivation of the Rho/ROCK/MMPs axis
- Mesenchymal stem cell-derived exosomal miR-26a induces ferroptosis, suppresses hepatic stellate cell activation, and ameliorates liver fibrosis by modulating SLC7A11
- Endovascular thrombectomy versus intravenous thrombolysis for primary distal, medium vessel occlusion in acute ischemic stroke
- ANO6 (TMEM16F) inhibits gastrointestinal stromal tumor growth and induces ferroptosis
- Prognostic value of EIF5A2 in solid tumors: A meta-analysis and bioinformatics analysis
- The role of enhanced expression of Cx43 in patients with ulcerative colitis
- Choosing a COVID-19 vaccination site might be driven by anxiety and body vigilance
- Role of ICAM-1 in triple-negative breast cancer
- Cost-effectiveness of ambroxol in the treatment of Gaucher disease type 2
- HLA-DRB5 promotes immune thrombocytopenia via activating CD8+ T cells
- Efficacy and factors of myofascial release therapy combined with electrical and magnetic stimulation in the treatment of chronic pelvic pain syndrome
- Efficacy of tacrolimus monotherapy in primary membranous nephropathy
- Mechanisms of Tripterygium wilfordii Hook F on treating rheumatoid arthritis explored by network pharmacology analysis and molecular docking
- FBXO45 levels regulated ferroptosis renal tubular epithelial cells in a model of diabetic nephropathy by PLK1
- Optimizing anesthesia strategies to NSCLC patients in VATS procedures: Insights from drug requirements and patient recovery patterns
- Alpha-lipoic acid upregulates the PPARγ/NRF2/GPX4 signal pathway to inhibit ferroptosis in the pathogenesis of unexplained recurrent pregnancy loss
- Correlation between fat-soluble vitamin levels and inflammatory factors in paediatric community-acquired pneumonia: A prospective study
- CD1d affects the proliferation, migration, and apoptosis of human papillary thyroid carcinoma TPC-1 cells via regulating MAPK/NF-κB signaling pathway
- miR-let-7a inhibits sympathetic nerve remodeling after myocardial infarction by downregulating the expression of nerve growth factor
- Immune response analysis of solid organ transplantation recipients inoculated with inactivated COVID-19 vaccine: A retrospective analysis
- The H2Valdien derivatives regulate the epithelial–mesenchymal transition of hepatoma carcinoma cells through the Hedgehog signaling pathway
- Clinical efficacy of dexamethasone combined with isoniazid in the treatment of tuberculous meningitis and its effect on peripheral blood T cell subsets
- Comparison of short-segment and long-segment fixation in treatment of degenerative scoliosis and analysis of factors associated with adjacent spondylolisthesis
- Lycopene inhibits pyroptosis of endothelial progenitor cells induced by ox-LDL through the AMPK/mTOR/NLRP3 pathway
- Methylation regulation for FUNDC1 stability in childhood leukemia was up-regulated and facilitates metastasis and reduces ferroptosis of leukemia through mitochondrial damage by FBXL2
- Correlation of single-fiber electromyography studies and functional status in patients with amyotrophic lateral sclerosis
- Risk factors of postoperative airway obstruction complications in children with oral floor mass
- Expression levels and clinical significance of serum miR-19a/CCL20 in patients with acute cerebral infarction
- Physical activity and mental health trends in Korean adolescents: Analyzing the impact of the COVID-19 pandemic from 2018 to 2022
- Evaluating anemia in HIV-infected patients using chest CT
- Ponticulus posticus and skeletal malocclusion: A pilot study in a Southern Italian pre-orthodontic court
- Causal association of circulating immune cells and lymphoma: A Mendelian randomization study
- Assessment of the renal function and fibrosis indexes of conventional western medicine with Chinese medicine for dredging collaterals on treating renal fibrosis: A systematic review and meta-analysis
- Comprehensive landscape of integrator complex subunits and their association with prognosis and tumor microenvironment in gastric cancer
- New target-HMGCR inhibitors for the treatment of primary sclerosing cholangitis: A drug Mendelian randomization study
- Population pharmacokinetics of meropenem in critically ill patients
- Comparison of the ability of newly inflammatory markers to predict complicated appendicitis
- Comparative morphology of the cruciate ligaments: A radiological study
- Immune landscape of hepatocellular carcinoma: The central role of TP53-inducible glycolysis and apoptosis regulator
- Serum SIRT3 levels in epilepsy patients and its association with clinical outcomes and severity: A prospective observational study
- SHP-1 mediates cigarette smoke extract-induced epithelial–mesenchymal transformation and inflammation in 16HBE cells
- Acute hyper-hypoxia accelerates the development of depression in mice via the IL-6/PGC1α/MFN2 signaling pathway
- The GJB3 correlates with the prognosis, immune cell infiltration, and therapeutic responses in lung adenocarcinoma
- Physical fitness and blood parameters outcomes of breast cancer survivor in a low-intensity circuit resistance exercise program
- Exploring anesthetic-induced gene expression changes and immune cell dynamics in atrial tissue post-coronary artery bypass graft surgery
- Empagliflozin improves aortic injury in obese mice by regulating fatty acid metabolism
- Analysis of the risk factors of the radiation-induced encephalopathy in nasopharyngeal carcinoma: A retrospective cohort study
- Reproductive outcomes in women with BRCA 1/2 germline mutations: A retrospective observational study and literature review
- Evaluation of upper airway ultrasonographic measurements in predicting difficult intubation: A cross-section of the Turkish population
- Prognostic and diagnostic value of circulating IGFBP2 in pancreatic cancer
- Postural stability after operative reconstruction of the AFTL in chronic ankle instability comparing three different surgical techniques
- Research trends related to emergence agitation in the post-anaesthesia care unit from 2001 to 2023: A bibliometric analysis
- Frequency and clinicopathological correlation of gastrointestinal polyps: A six-year single center experience
- ACSL4 mediates inflammatory bowel disease and contributes to LPS-induced intestinal epithelial cell dysfunction by activating ferroptosis and inflammation
- Affibody-based molecular probe 99mTc-(HE)3ZHER2:V2 for non-invasive HER2 detection in ovarian and breast cancer xenografts
- Effectiveness of nutritional support for clinical outcomes in gastric cancer patients: A meta-analysis of randomized controlled trials
- The relationship between IFN-γ, IL-10, IL-6 cytokines, and severity of the condition with serum zinc and Fe in children infected with Mycoplasma pneumoniae
- Paraquat disrupts the blood–brain barrier by increasing IL-6 expression and oxidative stress through the activation of PI3K/AKT signaling pathway
- Sleep quality associate with the increased prevalence of cognitive impairment in coronary artery disease patients: A retrospective case–control study
- Dioscin protects against chronic prostatitis through the TLR4/NF-κB pathway
- Association of polymorphisms in FBN1, MYH11, and TGF-β signaling-related genes with susceptibility of sporadic thoracic aortic aneurysm and dissection in the Zhejiang Han population
- Application value of multi-parameter magnetic resonance image-transrectal ultrasound cognitive fusion in prostate biopsy
- Laboratory variables‐based artificial neural network models for predicting fatty liver disease: A retrospective study
- Decreased BIRC5-206 promotes epithelial–mesenchymal transition in nasopharyngeal carcinoma through sponging miR-145-5p
- Sepsis induces the cardiomyocyte apoptosis and cardiac dysfunction through activation of YAP1/Serpine1/caspase-3 pathway
- Assessment of iron metabolism and iron deficiency in incident patients on incident continuous ambulatory peritoneal dialysis
- Tibial periosteum flap combined with autologous bone grafting in the treatment of Gustilo-IIIB/IIIC open tibial fractures
- The application of intravenous general anesthesia under nasopharyngeal airway assisted ventilation undergoing ureteroscopic holmium laser lithotripsy: A prospective, single-center, controlled trial
- Long intergenic noncoding RNA for IGF2BP2 stability suppresses gastric cancer cell apoptosis by inhibiting the maturation of microRNA-34a
- Role of FOXM1 and AURKB in regulating keratinocyte function in psoriasis
- Parental control attitudes over their pre-school children’s diet
- The role of auto-HSCT in extranodal natural killer/T cell lymphoma
- Significance of negative cervical cytology and positive HPV in the diagnosis of cervical lesions by colposcopy
- Echinacoside inhibits PASMCs calcium overload to prevent hypoxic pulmonary artery remodeling by regulating TRPC1/4/6 and calmodulin
- ADAR1 plays a protective role in proximal tubular cells under high glucose conditions by attenuating the PI3K/AKT/mTOR signaling pathway
- The risk of cancer among insulin glargine users in Lithuania: A retrospective population-based study
- The unusual location of primary hydatid cyst: A case series study
- Intraoperative changes in electrophysiological monitoring can be used to predict clinical outcomes in patients with spinal cavernous malformation
- Obesity and risk of placenta accreta spectrum: A meta-analysis
- Shikonin alleviates asthma phenotypes in mice via an airway epithelial STAT3-dependent mechanism
- NSUN6 and HTR7 disturbed the stability of carotid atherosclerotic plaques by regulating the immune responses of macrophages
- The effect of COVID-19 lockdown on admission rates in Maternity Hospital
- Temporal muscle thickness is not a prognostic predictor in patients with high-grade glioma, an experience at two centers in China
- Luteolin alleviates cerebral ischemia/reperfusion injury by regulating cell pyroptosis
- Therapeutic role of respiratory exercise in patients with tuberculous pleurisy
- Effects of CFTR-ENaC on spinal cord edema after spinal cord injury
- Irisin-regulated lncRNAs and their potential regulatory functions in chondrogenic differentiation of human mesenchymal stem cells
- DMD mutations in pediatric patients with phenotypes of Duchenne/Becker muscular dystrophy
- Combination of C-reactive protein and fibrinogen-to-albumin ratio as a novel predictor of all-cause mortality in heart failure patients
- Significant role and the underly mechanism of cullin-1 in chronic obstructive pulmonary disease
- Ferroptosis-related prognostic model of mantle cell lymphoma
- Observation of choking reaction and other related indexes in elderly painless fiberoptic bronchoscopy with transnasal high-flow humidification oxygen therapy
- A bibliometric analysis of Prader-Willi syndrome from 2002 to 2022
- The causal effects of childhood sunburn occasions on melanoma: A univariable and multivariable Mendelian randomization study
- Oxidative stress regulates glycogen synthase kinase-3 in lymphocytes of diabetes mellitus patients complicated with cerebral infarction
- Role of COX6C and NDUFB3 in septic shock and stroke
- Trends in disease burden of type 2 diabetes, stroke, and hypertensive heart disease attributable to high BMI in China: 1990–2019
- Purinergic P2X7 receptor mediates hyperoxia-induced injury in pulmonary microvascular endothelial cells via NLRP3-mediated pyroptotic pathway
- Investigating the role of oviductal mucosa–endometrial co-culture in modulating factors relevant to embryo implantation
- Analgesic effect of external oblique intercostal block in laparoscopic cholecystectomy: A retrospective study
- Elevated serum miR-142-5p correlates with ischemic lesions and both NSE and S100β in ischemic stroke patients
- Correlation between the mechanism of arteriopathy in IgA nephropathy and blood stasis syndrome: A cohort study
- Risk factors for progressive kyphosis after percutaneous kyphoplasty in osteoporotic vertebral compression fracture
- Predictive role of neuron-specific enolase and S100-β in early neurological deterioration and unfavorable prognosis in patients with ischemic stroke
- The potential risk factors of postoperative cognitive dysfunction for endovascular therapy in acute ischemic stroke with general anesthesia
- Fluoxetine inhibited RANKL-induced osteoclastic differentiation in vitro
- Detection of serum FOXM1 and IGF2 in patients with ARDS and their correlation with disease and prognosis
- Rhein promotes skin wound healing by activating the PI3K/AKT signaling pathway
- Differences in mortality risk by levels of physical activity among persons with disabilities in South Korea
- Review Articles
- Cutaneous signs of selected cardiovascular disorders: A narrative review
- XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis
- A narrative review on adverse drug reactions of COVID-19 treatments on the kidney
- Emerging role and function of SPDL1 in human health and diseases
- Adverse reactions of piperacillin: A literature review of case reports
- Molecular mechanism and intervention measures of microvascular complications in diabetes
- Regulation of mesenchymal stem cell differentiation by autophagy
- Molecular landscape of borderline ovarian tumours: A systematic review
- Advances in synthetic lethality modalities for glioblastoma multiforme
- Investigating hormesis, aging, and neurodegeneration: From bench to clinics
- Frankincense: A neuronutrient to approach Parkinson’s disease treatment
- Sox9: A potential regulator of cancer stem cells in osteosarcoma
- Early detection of cardiovascular risk markers through non-invasive ultrasound methodologies in periodontitis patients
- Advanced neuroimaging and criminal interrogation in lie detection
- Maternal factors for neural tube defects in offspring: An umbrella review
- The chemoprotective hormetic effects of rosmarinic acid
- CBD’s potential impact on Parkinson’s disease: An updated overview
- Progress in cytokine research for ARDS: A comprehensive review
- Utilizing reactive oxygen species-scavenging nanoparticles for targeting oxidative stress in the treatment of ischemic stroke: A review
- NRXN1-related disorders, attempt to better define clinical assessment
- Lidocaine infusion for the treatment of complex regional pain syndrome: Case series and literature review
- Trends and future directions of autophagy in osteosarcoma: A bibliometric analysis
- Iron in ventricular remodeling and aneurysms post-myocardial infarction
- Case Reports
- Sirolimus potentiated angioedema: A case report and review of the literature
- Identification of mixed anaerobic infections after inguinal hernia repair based on metagenomic next-generation sequencing: A case report
- Successful treatment with bortezomib in combination with dexamethasone in a middle-aged male with idiopathic multicentric Castleman’s disease: A case report
- Complete heart block associated with hepatitis A infection in a female child with fatal outcome
- Elevation of D-dimer in eosinophilic gastrointestinal diseases in the absence of venous thrombosis: A case series and literature review
- Four years of natural progressive course: A rare case report of juvenile Xp11.2 translocations renal cell carcinoma with TFE3 gene fusion
- Advancing prenatal diagnosis: Echocardiographic detection of Scimitar syndrome in China – A case series
- Outcomes and complications of hemodialysis in patients with renal cancer following bilateral nephrectomy
- Anti-HMGCR myopathy mimicking facioscapulohumeral muscular dystrophy
- Recurrent opportunistic infections in a HIV-negative patient with combined C6 and NFKB1 mutations: A case report, pedigree analysis, and literature review
- Letter to the Editor
- Letter to the Editor: Total parenteral nutrition-induced Wernicke’s encephalopathy after oncologic gastrointestinal surgery
- Erratum
- Erratum to “Bladder-embedded ectopic intrauterine device with calculus”
- Retraction
- Retraction of “XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis”
- Corrigendum
- Corrigendum to “Investigating hormesis, aging, and neurodegeneration: From bench to clinics”
- Corrigendum to “Frankincense: A neuronutrient to approach Parkinson’s disease treatment”
- Special Issue The evolving saga of RNAs from bench to bedside - Part II
- Machine-learning-based prediction of a diagnostic model using autophagy-related genes based on RNA sequencing for patients with papillary thyroid carcinoma
- Unlocking the future of hepatocellular carcinoma treatment: A comprehensive analysis of disulfidptosis-related lncRNAs for prognosis and drug screening
- Elevated mRNA level indicates FSIP1 promotes EMT and gastric cancer progression by regulating fibroblasts in tumor microenvironment
- Special Issue Advancements in oncology: bridging clinical and experimental research - Part I
- Ultrasound-guided transperineal vs transrectal prostate biopsy: A meta-analysis of diagnostic accuracy and complication rates
- Assessment of diagnostic value of unilateral systematic biopsy combined with targeted biopsy in detecting clinically significant prostate cancer
- SENP7 inhibits glioblastoma metastasis and invasion by dissociating SUMO2/3 binding to specific target proteins
- MARK1 suppress malignant progression of hepatocellular carcinoma and improves sorafenib resistance through negatively regulating POTEE
- Analysis of postoperative complications in bladder cancer patients
- Carboplatin combined with arsenic trioxide versus carboplatin combined with docetaxel treatment for LACC: A randomized, open-label, phase II clinical study
- Special Issue Exploring the biological mechanism of human diseases based on MultiOmics Technology - Part I
- Comprehensive pan-cancer investigation of carnosine dipeptidase 1 and its prospective prognostic significance in hepatocellular carcinoma
- Identification of signatures associated with microsatellite instability and immune characteristics to predict the prognostic risk of colon cancer
- Single-cell analysis identified key macrophage subpopulations associated with atherosclerosis

